Anthranilamide insecticides
A technology of alkyl amino and alkyl, which is applied in the field of preventing and controlling invertebrate pests such as arthropods in agronomic and non-agronomic environments, and can solve problems such as endangering productivity, reducing, and increasing consumer costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 11C
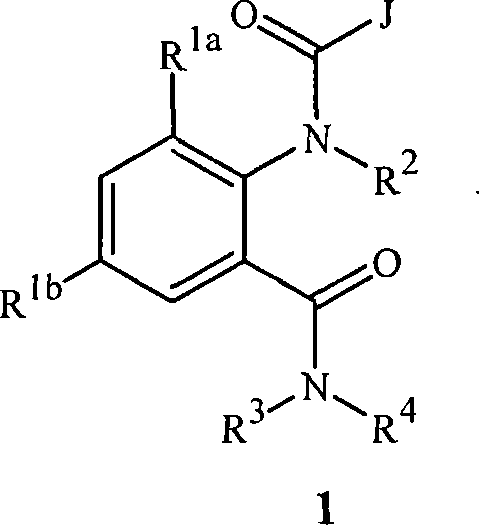
[0140] Embodiment 11C. A compound of Formula 1 wherein R 7 yes
[0141]
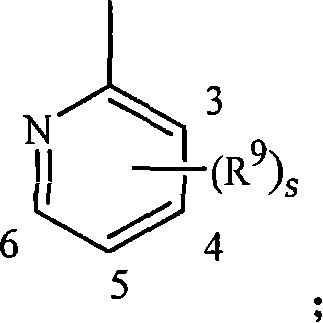
[0142] Embodiment 11D. A compound of Formula 1 wherein each R 9 are independently H, CH 3 、CF 3 , CN or halogen.
Embodiment approach 12A
[0143] Embodiment 12A. Compounds of Formula 1 wherein R 7 yes
[0144]
[0145] Embodiment 12B. A compound of Formula 1 wherein each R 9 independently is C 1 -C 4 Alkyl, C 1 -C 4 haloalkyl, halo or CN; and s is 0, 1 or 2.
Embodiment approach 12C
[0146] Embodiment 12C. A compound of Formula 1 wherein R 7 yes
[0147]
[0148] Embodiment 12D. A compound of Formula 1 wherein each R 9 are independently H, CH 3 、CF 3 , CN or halogen.
[0149] Embodiment 13A. Compounds of Formula 1 wherein R 8 is C 1 -C 4 Alkyl or C 1 -C 4 Haloalkyl.
[0150] Embodiment 13B. A compound of Formula 1 wherein R 8 is CH 2 CF 3 or CHF 2 .
[0151] Embodiment 14A. A compound of Formula 1 wherein J is optionally replaced by 1-4 R 5 Substituted phenyl.
[0152] Embodiment 14B. A compound of Formula 1 wherein each R 5 independently is C 1 -C 4 Alkyl, C 1 -C 4 Haloalkyl, C 1 -C 2 Haloalkoxy, halogen or CN.
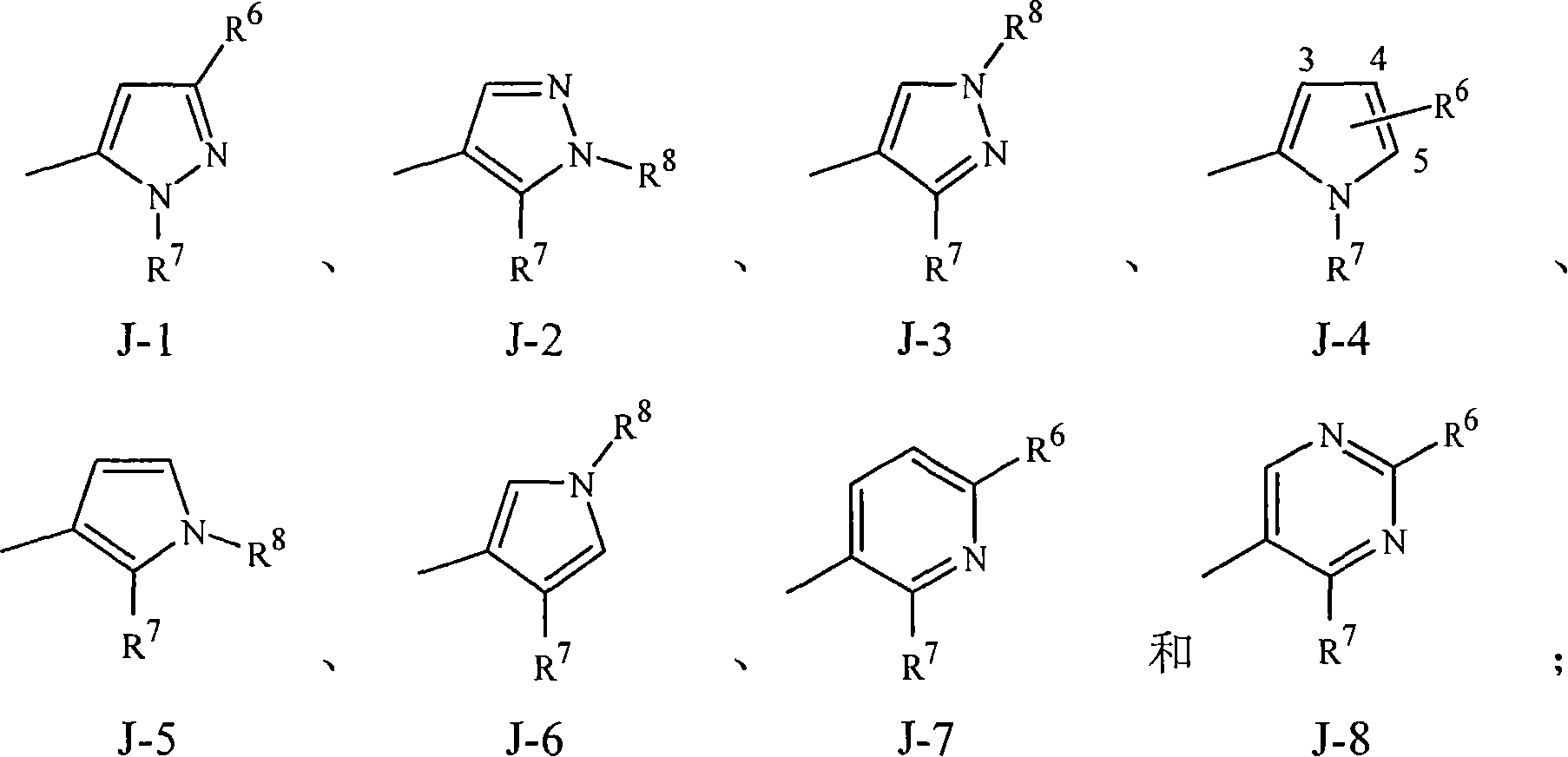
[0153] Embodiment 15A. A compound of Formula 1 wherein J is a heterocycle selected from the group consisting of J-1, J-2, J-3, J-4, J-5, J-6, J-7 and J-8.
[0154] Embodiment 15B. A compound of Formula 1 wherein J is J-1, J-2, J-4, J-7 or J-8.
[0155] Embodiment 15C. A compound of Formula 1 wherein J is J-1, J-2 or J-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com