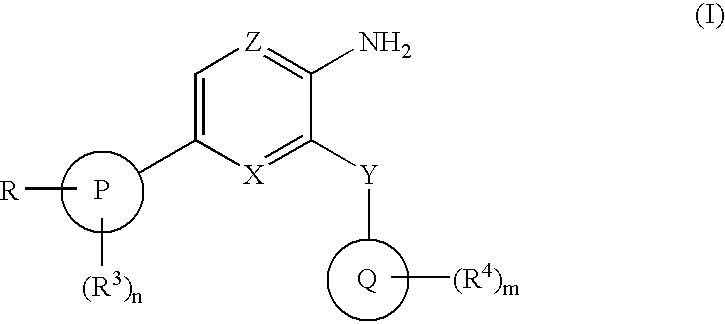
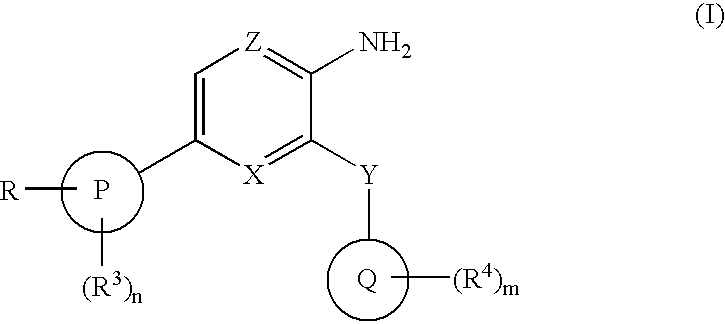
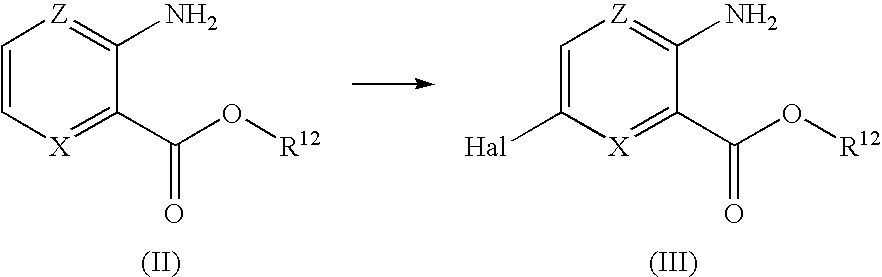
Compounds having selective inhibiting effect at GSK3
a selective inhibitor and compound technology, applied in the field of compounds, can solve the problems of lithium intoxication, the back of axons and neuritis, and the inability to inhibit gsk3, and achieve good bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
3-Amino-6-bromo-N-pyridin-3-ylpyrazine-2-carboxamide
[0049]To 3-aminopyridine (10 g, 106 mmol) at 70° C. were added methyl 3-amino-6-bromo-2-pyrazinecarboxylate (1.0 g, 4.3 mmol) and 1,8-diazabicyclo[5.4.0]undec-7-ene (645 μL, 4.3 mmol). The reaction solution was stirred for 4 h, diluted with water (75 mL) and extracted with methylene chloride. The combined organic layers were washed with a saturated ammonium chloride solution, dried (MgSO4), filtered and evaporated in vacuo. The crude product was purified on a silica gel column using methylene chloride / ethanol, (9:1), as the eluent to give 750 mg (59% yield) of the title compound as a yellow solid: 1H NMR (CDCl3, 400 MHz) δ 9.50 (br s, 1H), 8.82 (d, J=3 Hz, 1H), 8.43 (dd, J=5 and 1 Hz, 1H), 8.31 (s, 1H), 8.23 (ddd, J=8, 3 and 2 Hz, 1H), 7.34 (dd, J=8, 5 Hz, 1H); MS (TSP) m / z 294 (M++1).
example 2
N-[3-(4-Bromophenyl)propyl]-N,N-dicyclobutylamine
[0050]3-(4-Bromophenyl)propan-1-amine (0.50 g, 2.34 mmol; described in: Davies, R. V. et al. J. Chem. Soc. Perkin Trans. 1 1977, 2357-2364), cyclobutanone (0.393 g, 5.61 mmol) and acetic acid (0.140 mL, 2.34 mmol) were mixed in dichloroethane (6 mL) and stirred for 30 min. Sodium triacetoxy borohydride was added and the reaction mixture was stirred for 15 h. The reaction was quenched with water (15 mL) and extracted with methylene chloride (50 mL). The organic phase was separated, dried and evaporated. Purification by column chromatography using methylene chloride to methylene chloride / methanol, (2:1), gradient as the eluent gave 0.32 g (42% yield) of the title compound as colorless oil: MS (ESI) m / z 322 and 324 (M++1).
example 3
3-Amino-6-{4-[3-(dicyclobutylamino)propyl]phenyl}-N-pyridin-3-ylpyrazine-2-carboxamide Hydrochloride
[0051]n-Butyllithium (0.97 mL, 1.55 mmol) was added dropwise over 20 min to a cooled (−78° C.) solution of N-[3-(4-bromophenyl)propyl]-N,N-dicyclobutylamine (0.10 g, 0.31 mmol) and triisopropyl borate (0.21 mL, 0.93 mmol) in anhydrous tetrahydrofuran (2 mL) under a nitrogen atmosphere. The reaction mixture was stirred for 2 h at −78° C. HCl (aq 3 M, 0.6 mL) was added to the reaction mixture and the mixture was allowed to warm to room temperature. Tetrahydrofuran (2 mL) was added, followed by sodium carbonate (1.1 g, 10.8 mmol), Pd(dppf)Cl2 (0.010 g, 12.2 μmol) and 3-amino-6-bromo-N-pyridin-3-ylpyrazine-2-carboxamide (0.10 g, 0.34 mmol). The mixture was heated to 65° C. for 15 h. The solvent was removed and purification by column chromatography on silica using a gradient methylene chloride to methylene chloride / methanol, (2:1), as the eluent gave a yellow solid. The solid was dissolved...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com