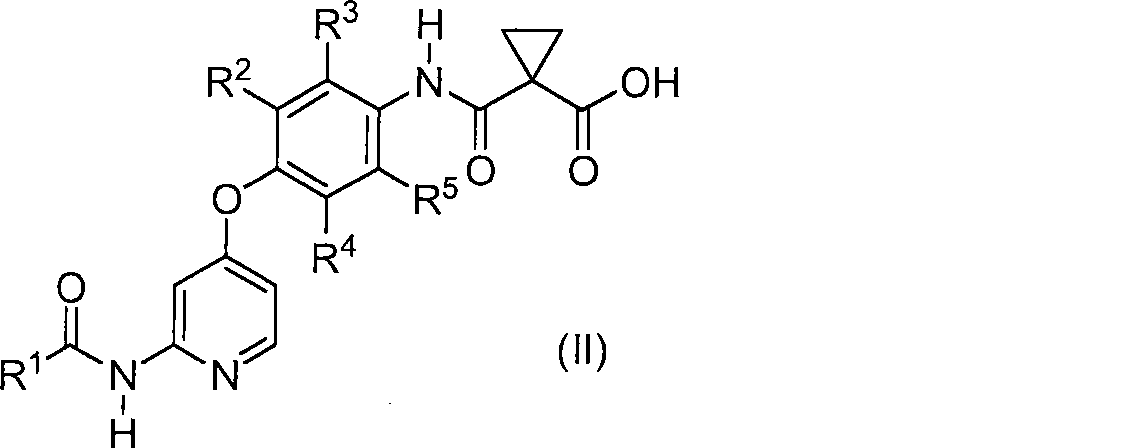
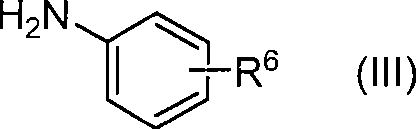
Method for producing phenoxypyridine derivative
A technology of piperidine and methyl, which is applied in the directions of medical preparations, drug combinations, and pharmaceutical formulations containing active ingredients, can solve the problems such as preparation of intermediates that are not disclosed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
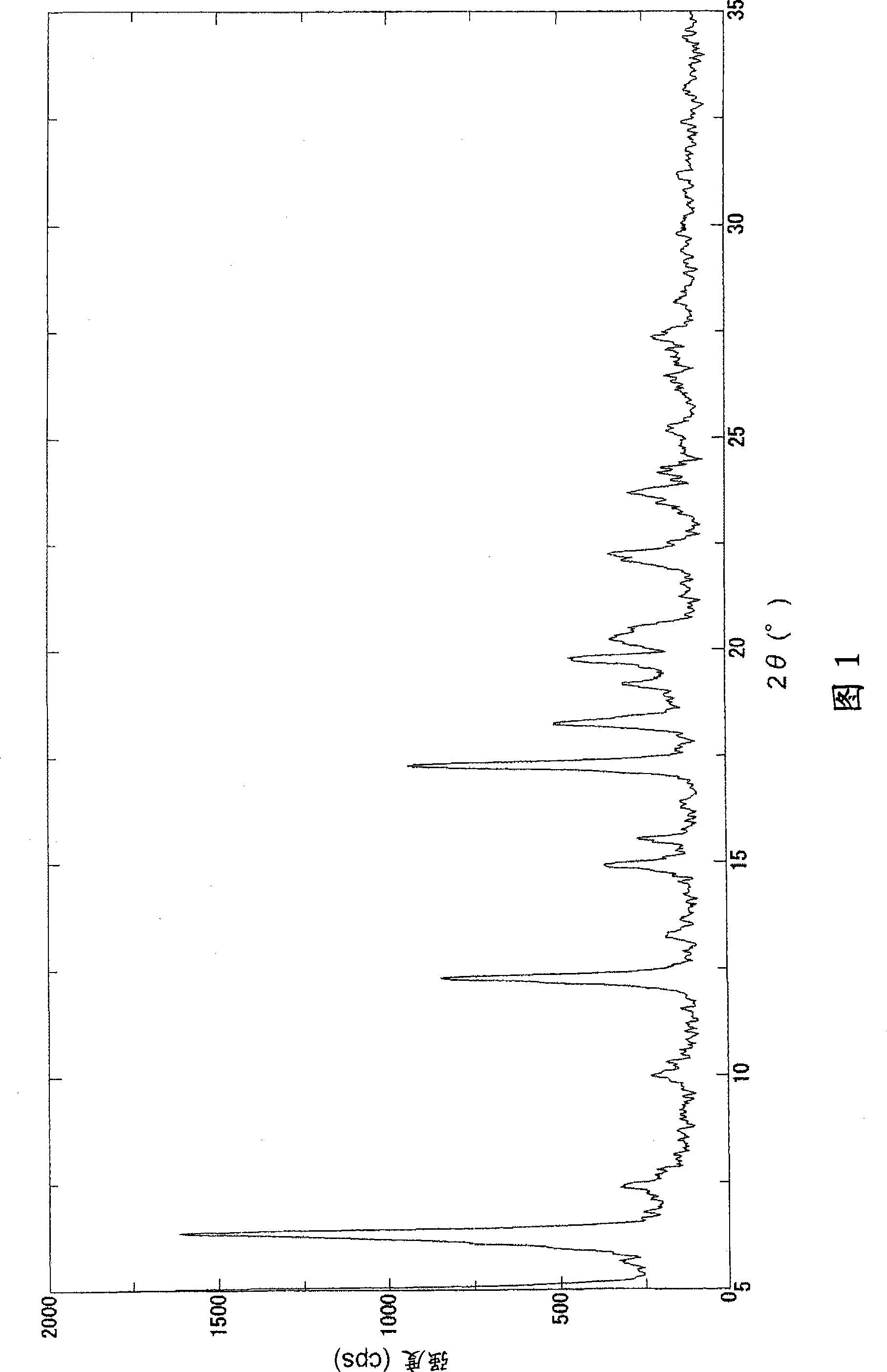
Image
Examples
preparation example Construction
[0148] The preparation method of the present invention is described in detail below.
[0149]
[0150] [In the formula, each symbol has the same meaning as defined above. 〕
[0151] [step 1]
[0152] This step is a step of preparing compound (VIII) by reacting compound (IX) and compound (X) in the presence of a halogenating agent or a condensing agent.
[0153] As the compound (IX), there can be used compounds described in the following Examples, known compounds, commercially available compounds, or compounds that can be easily prepared from commercially available compounds by methods generally performed by those skilled in the art.
[0154] As the compound (X), there can be used compounds described in the following Examples, known compounds, commercially available compounds, or compounds that can be easily prepared from commercially available compounds by methods generally performed by those skilled in the art.
[0155] The solvent used in this step is not particularly ...
preparation example 1
[0222] (Preparation Example 1) [1-(2-Dimethylaminoacetyl)piperidin-4-yl]amino tert-butyl formate
[0223]
[0224] Add N,N-dimethylglycine (2.97g), 1-hydroxybenzene Triazole (3.89g), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (5.27g) were stirred at room temperature for 46 hours under a nitrogen atmosphere. Ethyl acetate (400 ml), saturated brine (200 ml), and 1N aqueous sodium hydroxide solution (50 ml) were added to the reaction solution, stirred at room temperature for 30 minutes, and then partitioned. The aqueous layer was extracted with ethyl acetate. The organic layer was collected, washed successively with 1N aqueous sodium hydroxide solution and saturated brine, and dried over anhydrous sodium sulfate. The dried organic layer was concentrated under reduced pressure to obtain the title compound (8.03 g, quantitative) as colorless crystals.
[0225] ESI-MS(m / z): 286[M+H] + .
preparation example 2
[0226] (Preparation Example 2) N-[1-(2-Dimethylaminoethyl)piperidin-4-yl] -N-Methylamine
[0227]
[0228] A solution of tert-butyl [1-(2-dimethylaminoacetyl)piperidin-4-yl]carbamate (7.07 g) in tetrahydrofuran (100 ml) was stirred with ice-cooling under a nitrogen atmosphere. Lithium aluminum hydride (280 mg) was added thereto, followed by stirring on an ice bath for 15 minutes and at room temperature for 15 minutes. Under a nitrogen atmosphere, the reaction solution was heated to reflux at 100° C. for 11 hours. Ice cold reaction solution. Water (2.8 ml), 5N aqueous sodium hydroxide solution (2.8 ml), and water (14.0 ml) were successively added thereto, and it was stirred for 2 hours. Insolubles were filtered. The filtrate was concentrated to give the title compound (4.65 g, quantitative) as a yellow oil.
[0229] 1 H-NMR spectrum (CDCl 3 )δ (ppm): 1.34-1.43 (2H, m), 1.87-1.90 (2H, m), 2.02-2.08 (2H, m), 2.25 (6H, s), 2.31-2.50 (7H, m), 2.90 (2H, m), 3.14-3.27 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com