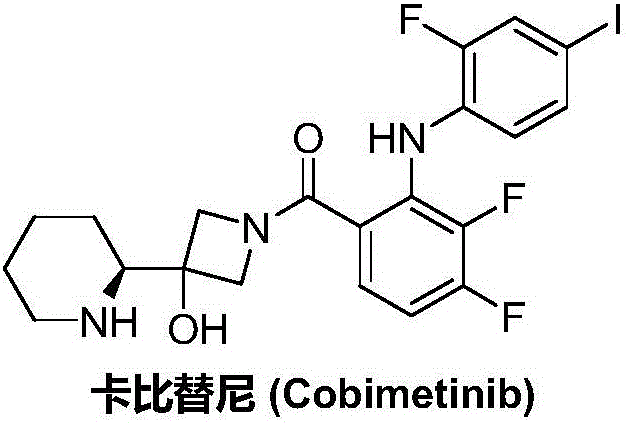
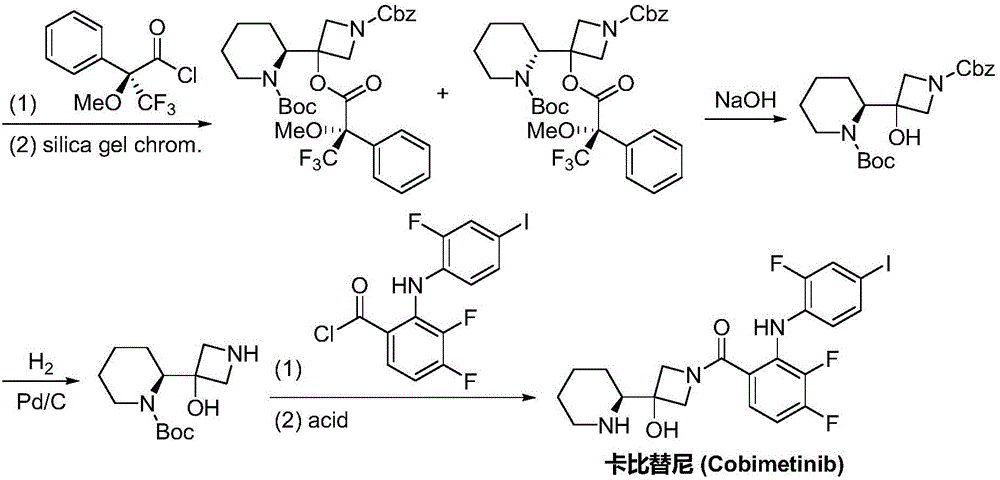
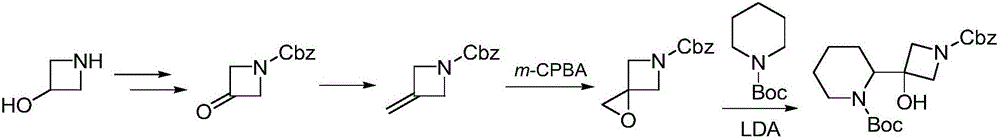Synthesis method of cobimetinib
A technology of cabitinib and a synthesis method, which is applied in the field of synthesis of a new anti-tumor drug cabitinib, can solve problems such as increasing the difficulty of industrialized production operation, unfavorable industrialized production promotion and application, long synthesis route, etc., and achieves less impurities and simplification. Manipulate, simplify the effect of compositing steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A) Preparation of (R)-N-Boc-2-bromopiperidine:
[0039] (R)-N-Boc-2-piperidine carboxylic acid (10.0g, 0.04mol) was dissolved in carbon tetrachloride (70mL), potassium hydroxide (2.7g, 0.05mol) and silver nitrate (8.2g, 0.05mol) were added ), the reaction mixture was stirred at 25°C for 10 minutes, bromine water (8.4g, 0.05mol) was slowly added, the reaction mixture was stirred and reacted at 70°C for 15 hours, TLC was spotted to confirm that the reaction was complete, the rotary evaporation was concentrated to dryness, and water (20mL) was slowly added , cooled to -10°C and crystallized for 3 hours, filtered, recrystallized from isopropanol to obtain (R)-N-Boc-2-bromopiperidine, off-white solid (9.9g), yield 85.5%, the step The reaction formula is as follows:
[0040]
[0041] B) Preparation of 1-benzyloxycarbonyl-3-hydroxy-3-[(2S)-N-Boc-2-piperidinyl]-azetidine:
[0042] (R)-N-Boc-2-bromopiperidine (9.9g, 0.037mol) was dissolved in tetrahydrofuran (40mL), magnesi...
Embodiment 2
[0054] A) Preparation of (R)-N-Boc-2-bromopiperidine:
[0055] (R)-N-Boc-2-piperidine formic acid (22.927g, 0.08mol) was dissolved in chloroform (120mL), sodium hydroxide (3.8g, 0.094mol) and mercuric oxide (20.4g, 0.094mol) were added, and the reaction The mixture was stirred at 25°C for 10 minutes, bromine water (16.3g, 0.10mol) was slowly added, the reaction mixture was stirred and reacted at 80°C for 12 hours, TLC was spotted to confirm that the reaction was complete, concentrated to dryness by rotary evaporation, slowly added water (30mL), cooled to Crystallize at -10°C for 3 hours, filter, and recrystallize from isopropanol to obtain (R)-N-Boc-2-bromopiperidine, off-white solid (17.2g), yield 82.9%, the reaction formula of this step is the same as Embodiment 1;
[0056] B) Preparation of 1-benzyloxycarbonyl-3-hydroxy-3-[(2S)-N-Boc-2-piperidinyl]-azetidine:
[0057] (R)-N-Boc-2-bromopiperidine (17.0g, 0.064mol) was dissolved in methyl tert-butyl ether (70mL), magnesium ...
Embodiment 3
[0065] A) Preparation of (R)-N-Boc-2-bromopiperidine:
[0066](R)-N-Boc-2-piperidine carboxylic acid (18.5g, 0.08mol) was dissolved in dichloromethane (160mL), lithium hydroxide (2.4g, 0.10mol) and silver nitrate (17.1g, 0.10mol) were added , the reaction mixture was stirred at 25°C for 10min, bromine water (17.4g, 0.11mol) was slowly added, the reaction mixture was stirred at 90°C for 10 hours, TLC was spotted to confirm that the reaction was complete, concentrated to dryness by rotary evaporation, slowly added water (35mL), cooled Crystallize at -10°C for 3 hours, filter, and recrystallize from isopropanol to obtain (R)-N-Boc-2-bromopiperidine, off-white solid (18.4g), yield 86.1%, the reaction formula of this step With embodiment 1;
[0067] B) Preparation of 1-benzyloxycarbonyl-3-hydroxy-3-[(2S)-N-Boc-2-piperidinyl]-azetidine:
[0068] (R)-N-Boc-2-Bromopiperidine (26.416g, 0.07mol) was dissolved in ether (70mL), magnesium sticks (2.2g, 0.09mol) were added, and the reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com