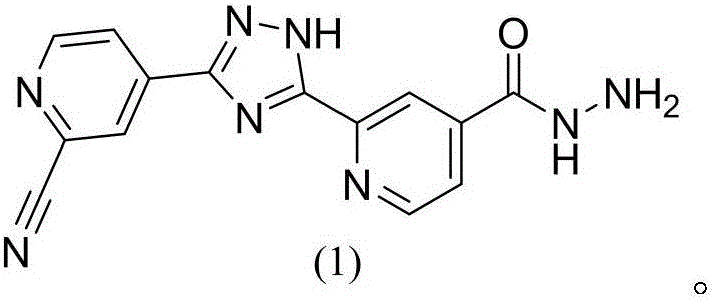
Topiroxostat impurity synthesis method
A technology of topinostat and synthetic method, applied in the field of chemical pharmacy, to achieve the effect of cheap raw materials, simple operation and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
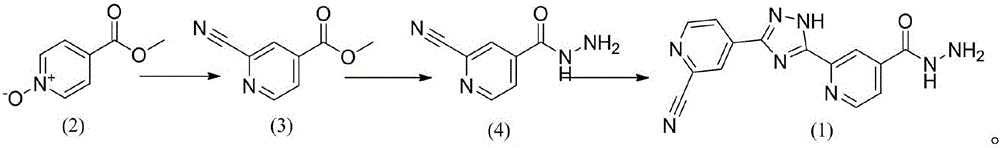
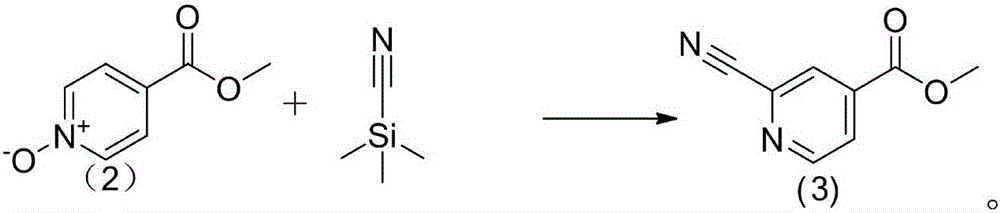
[0020] Example 1: Preparation of methyl 2-cyano-4-pyridinecarboxylate
[0021]
[0022] Add methylisonicotinic acid-N-oxide (2) (15.31g, 0.1mol) and 200ml of acetonitrile into a 250ml three-necked flask and stir to completely dissolve, then add triethylamine (15g, 0.15mol), trimethylsilyl cyanide (29.76g, 0.3mmol) after nitrogen protection reflux reaction for 20 hours, TLC detection reaction ended. The solvent was distilled to dryness under reduced pressure, and the residue was subjected to silica gel column chromatography, eluting with dichloromethane-methanol (50:1), to obtain a white crystal with a yield of 13.48 g, 83.14%.
[0023] 1 HNMR (600 MHz, DMSO-d6, δppm): 4.03 (s, 3H,), 8.11 (d, 1H,), 7.76 (s, 1H), 8.26 (s, 1H), 8.89 (d, 1H).
Embodiment 2
[0024] Embodiment two: the preparation of 2-cyanoisoniazid
[0025]
[0026] Add methyl 2-cyano-4-pyridinecarboxylate (3) (13.48g, 0.083mmol) and 130ml methanol into a 250ml three-necked flask, stir, cool to -5°C in an ice-salt bath, and dropwise add hydrazine hydrate (80%) 13.48 g, react at -5°C for 6 h, and TLC detects that the reaction is complete. Recrystallized with ethanol to obtain 11.12 g of white crystals, with a yield of 82.46%.
[0027] 1 HNMR (600 MHz, DMSO-d6, δppm): 4.77 (s, 2H,), 8.03 (d, 1H,), 8.33 (s, 1H), 8.89 (s, 1H), 10.24 (s, 1H).
Embodiment 3
[0028] Example 3: Preparation of 2-(3-(2-aminopyridin-4-yl)-1H-1,2,4-triazol-5-yl)isoniazid
[0029]
[0030] Add 2-cyanoisoniazid (4) (5.56g, 0.034mmol) and 50ml of methanol into a 100ml three-necked flask and stir, then add sodium methoxide (0.37g, 0.0068mmol), stir at room temperature for 1 hour, then add another part of 2-cyano Isoniazid (4) (5.56g, 0.034mmol) was heated to reflux for 26 hours, and the reaction was detected by TLC. After cooling to room temperature, it was filtered and dried to obtain 9.21 g of a light yellow solid with a yield of 88.42%.
[0031] 1 HNMR (600MHz, DMSO-d6, δppm): 3.49 (s, 2H,), 7.60 (s, 1H,), 7.76 (s, 1H), 8.14 (d, 1H), 8.32 (s, 1H), 8.55 ( s, 1H), 8.80(s, 1H), 8.93(s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com