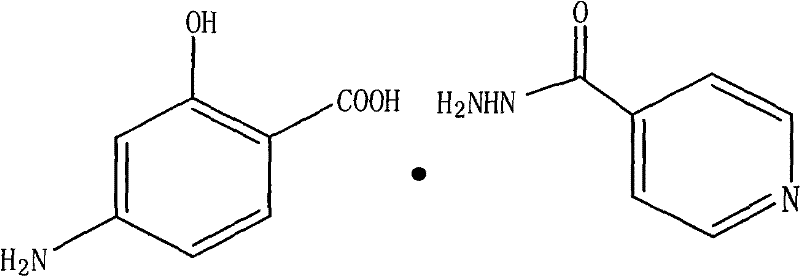
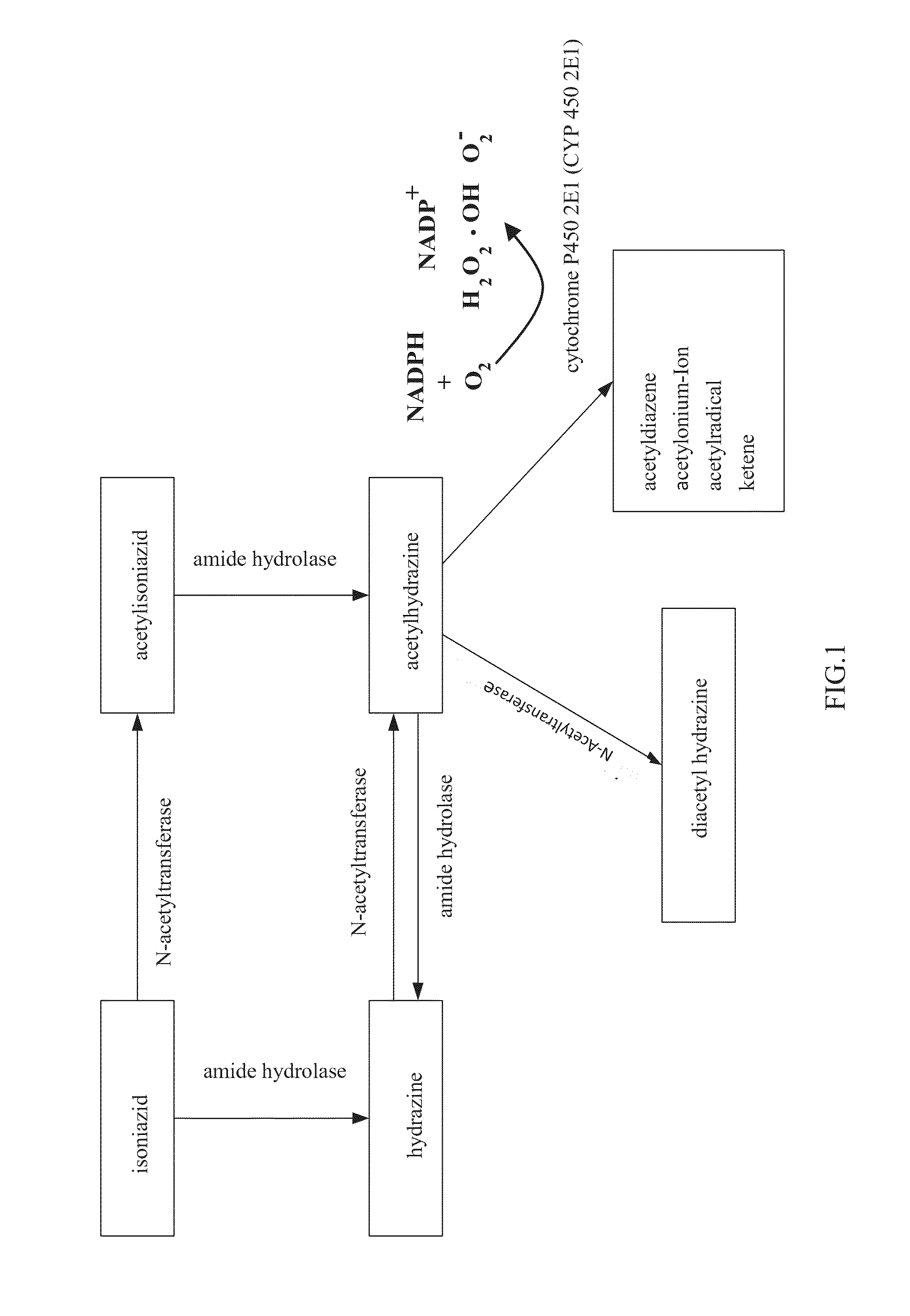
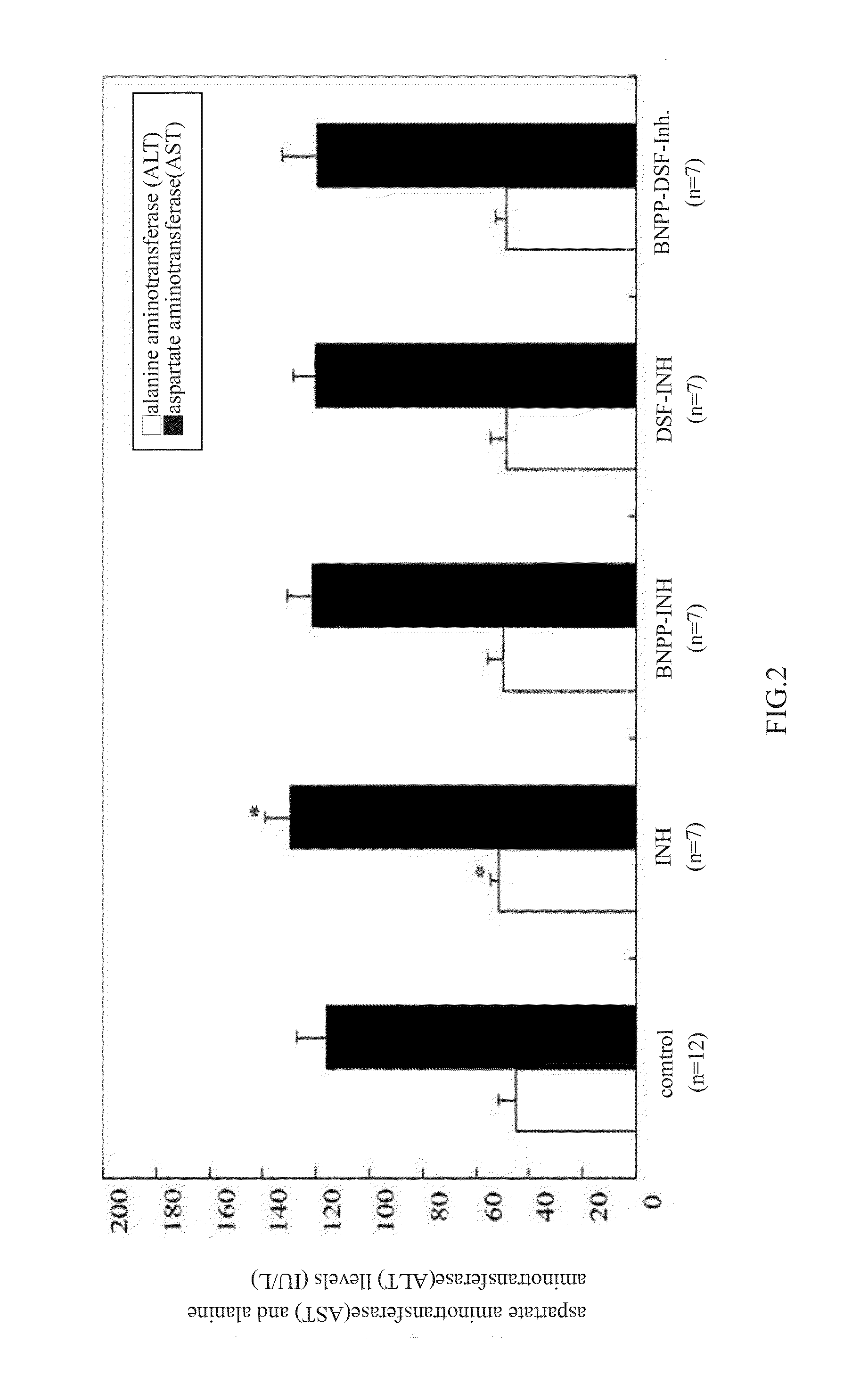
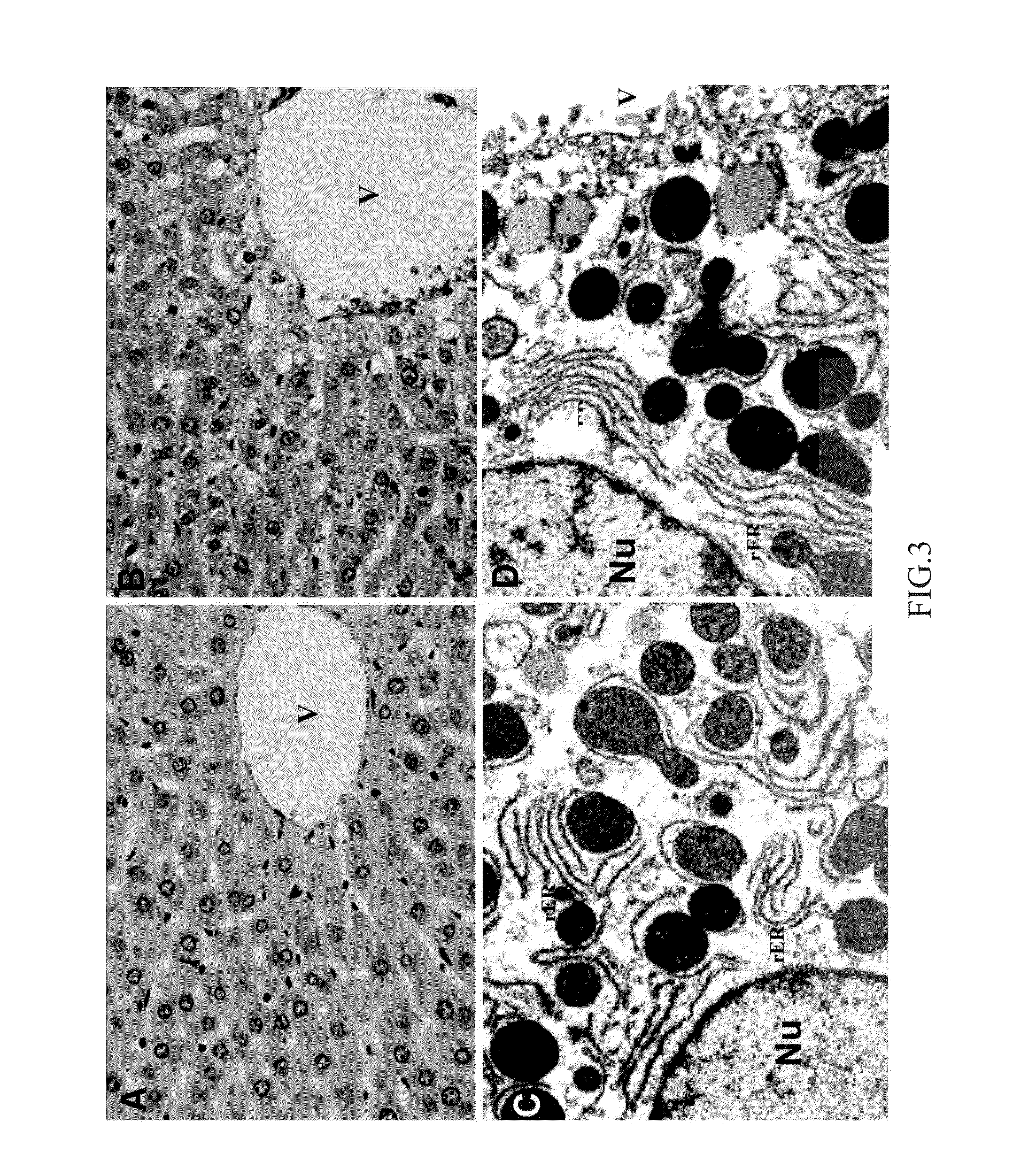
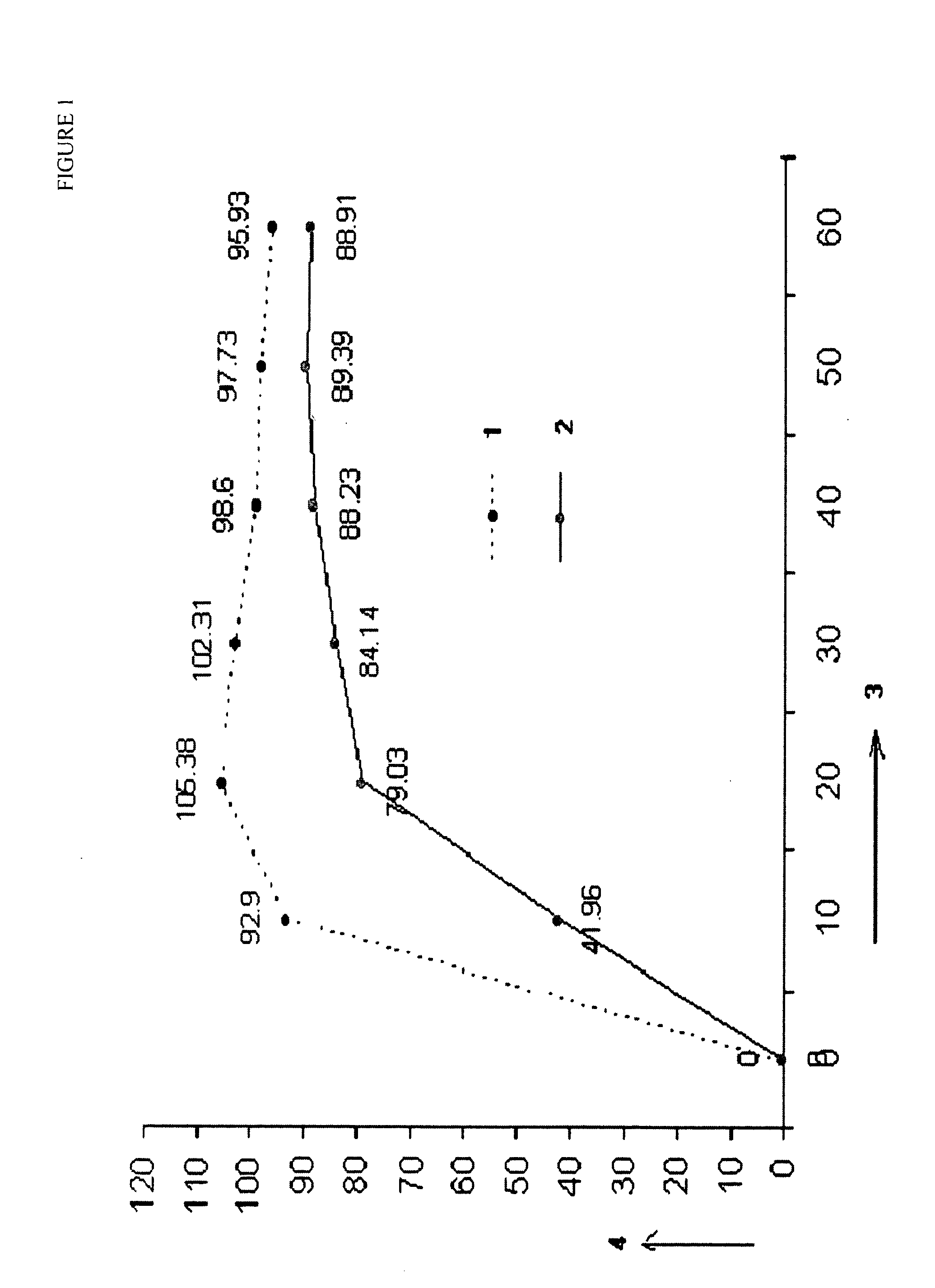
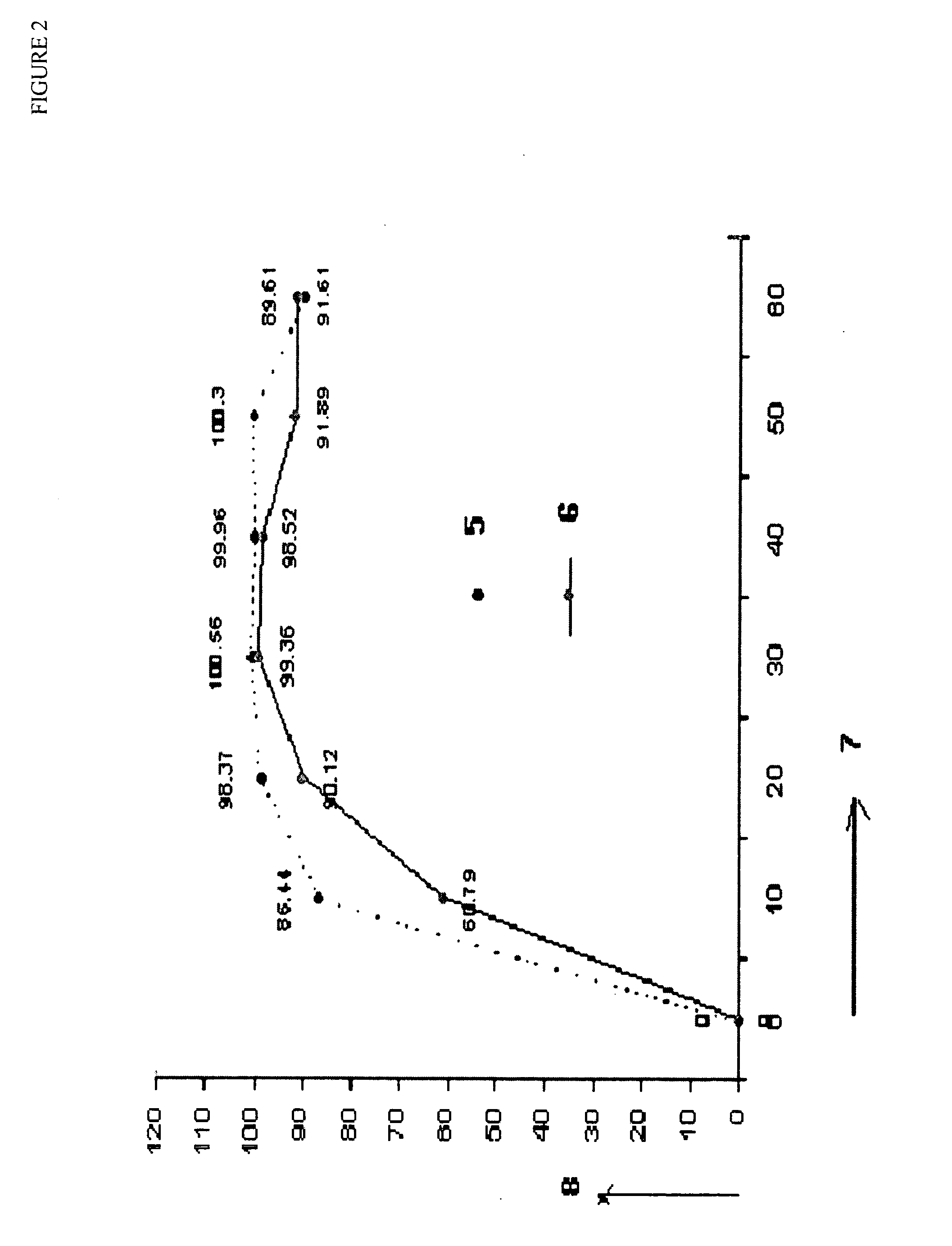
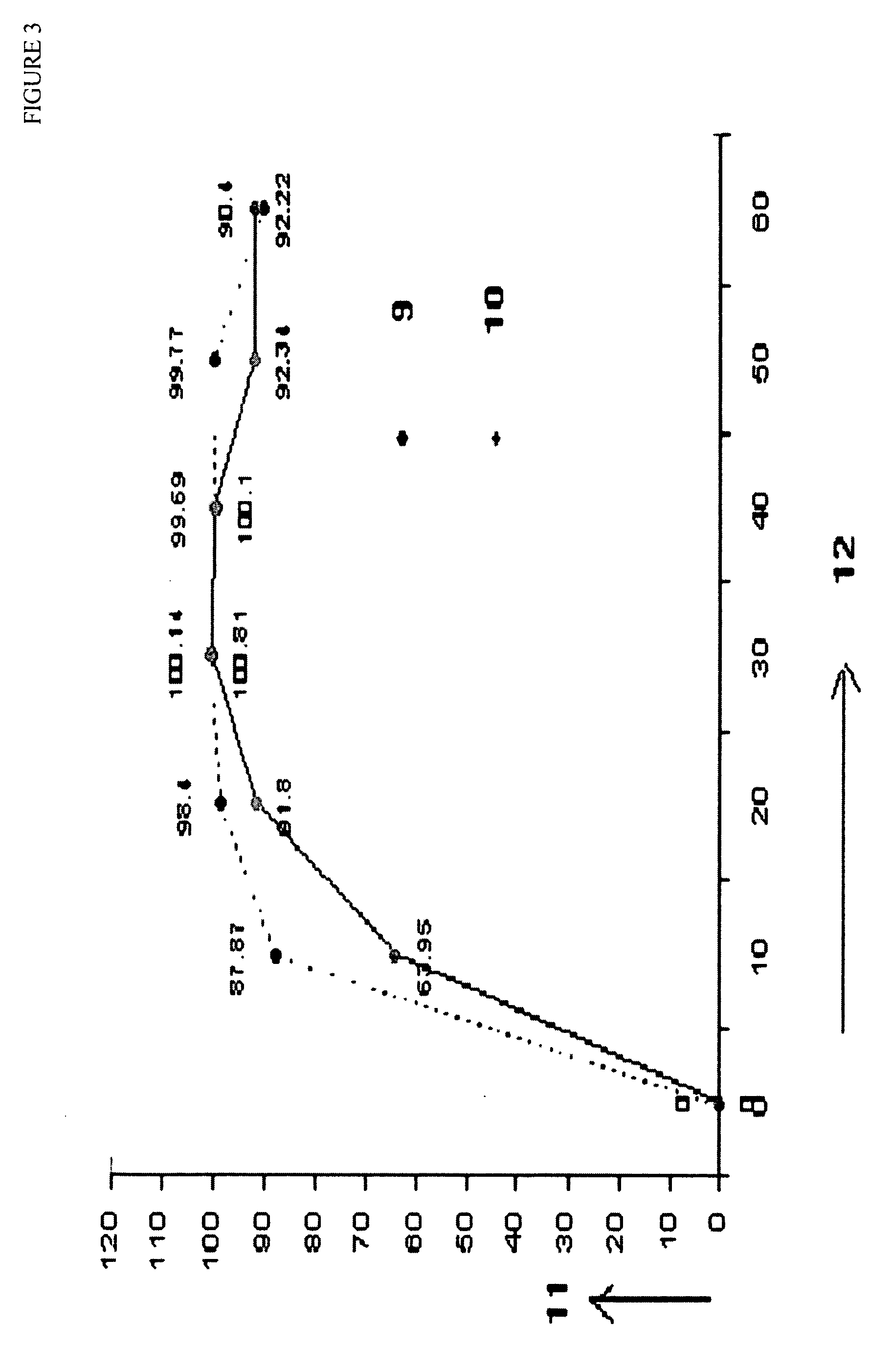
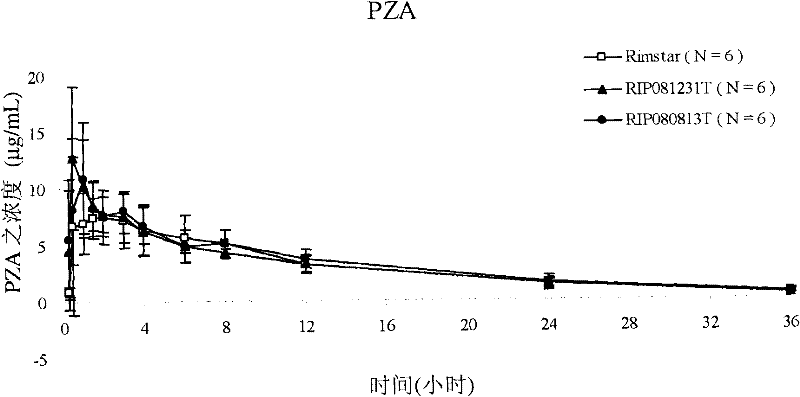
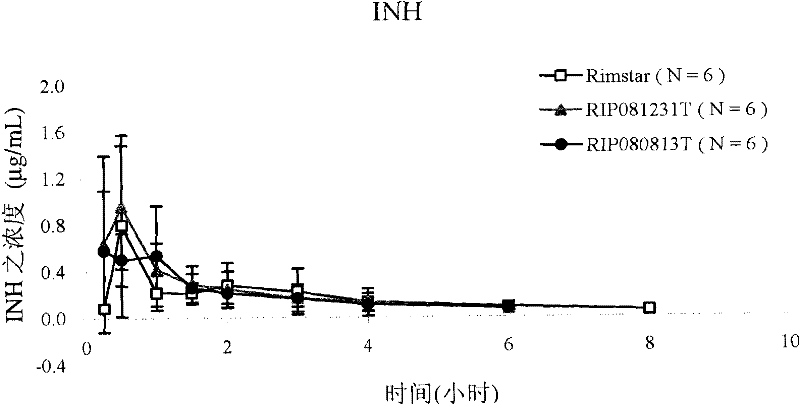
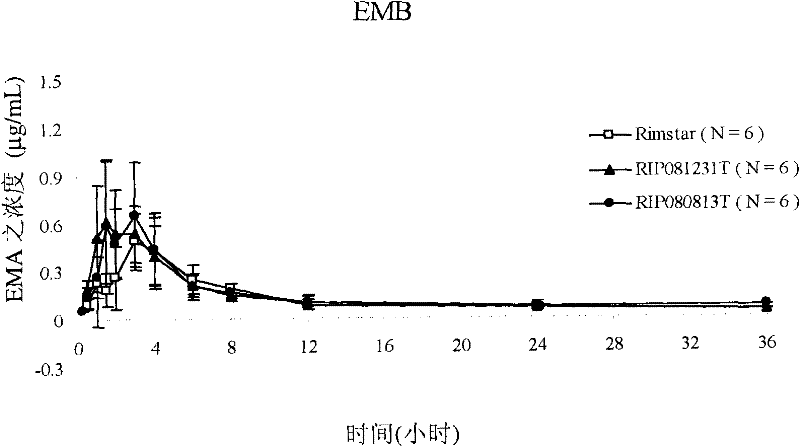
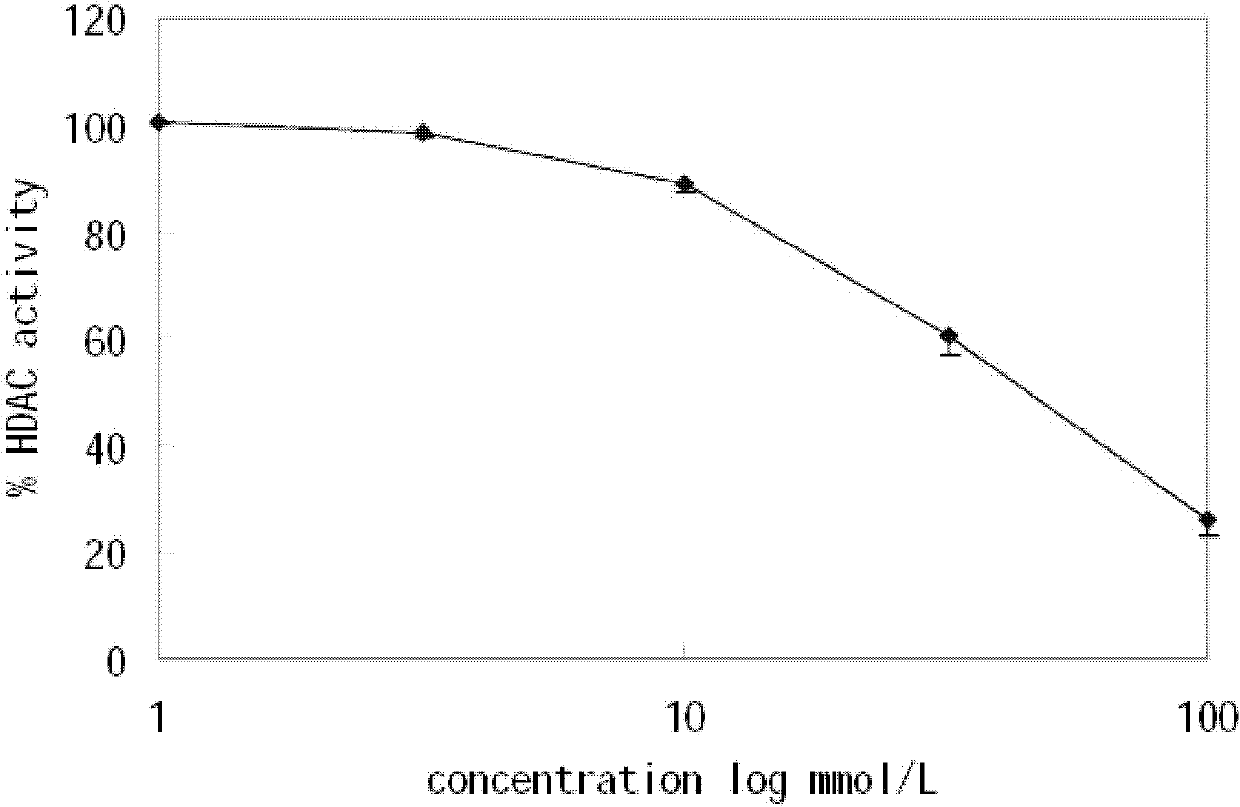
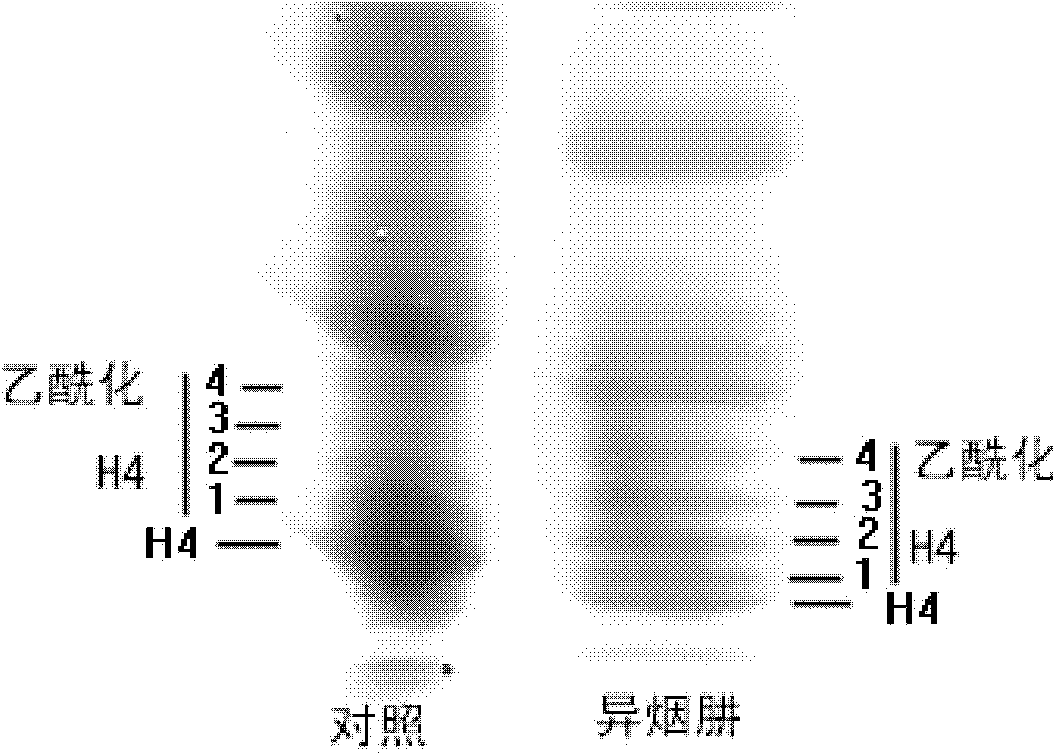
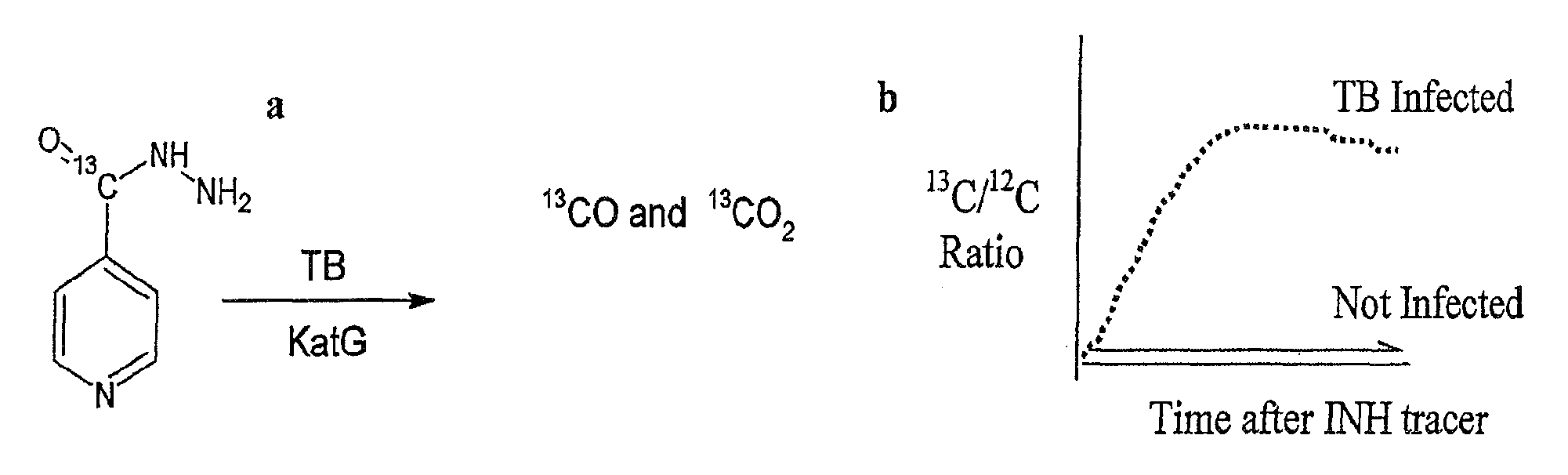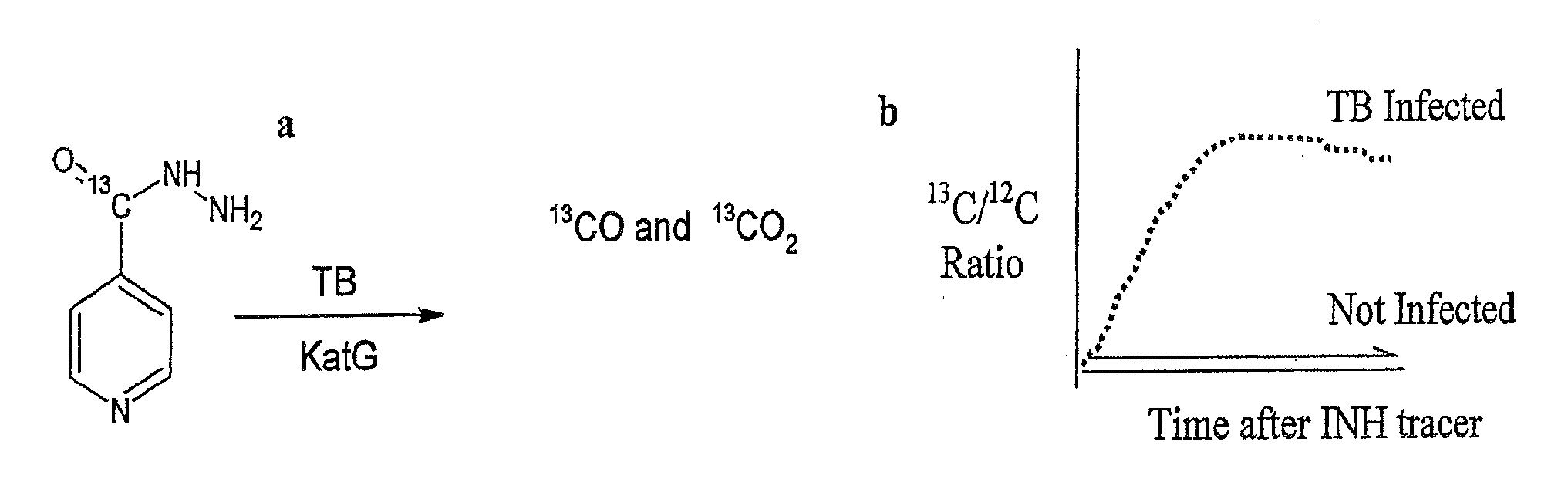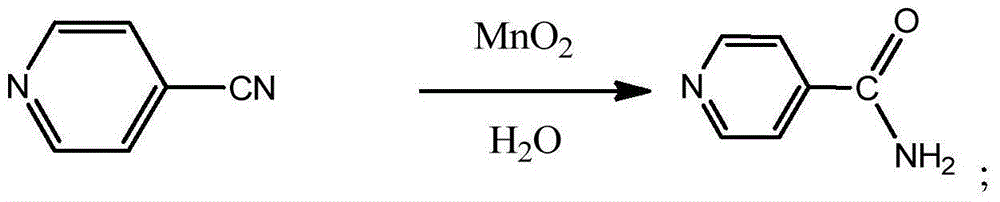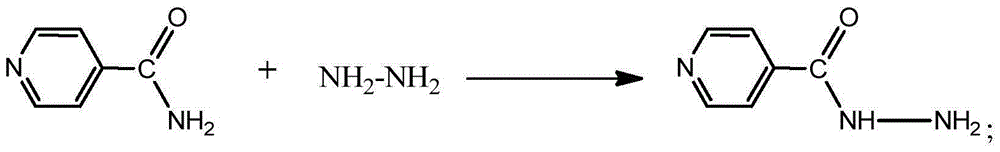Patents
Literature
89 results about "ISONIAZID/PYRIDOXINE" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Isoniazid is associated with pyridoxine deficiency due to the increased excretion of pyridoxine. Pyridoxal phosphate (a derivative of pyridoxine) is required for d-aminolevulinic acid synthase, the enzyme responsible for the rate-limiting step in heme synthesis.
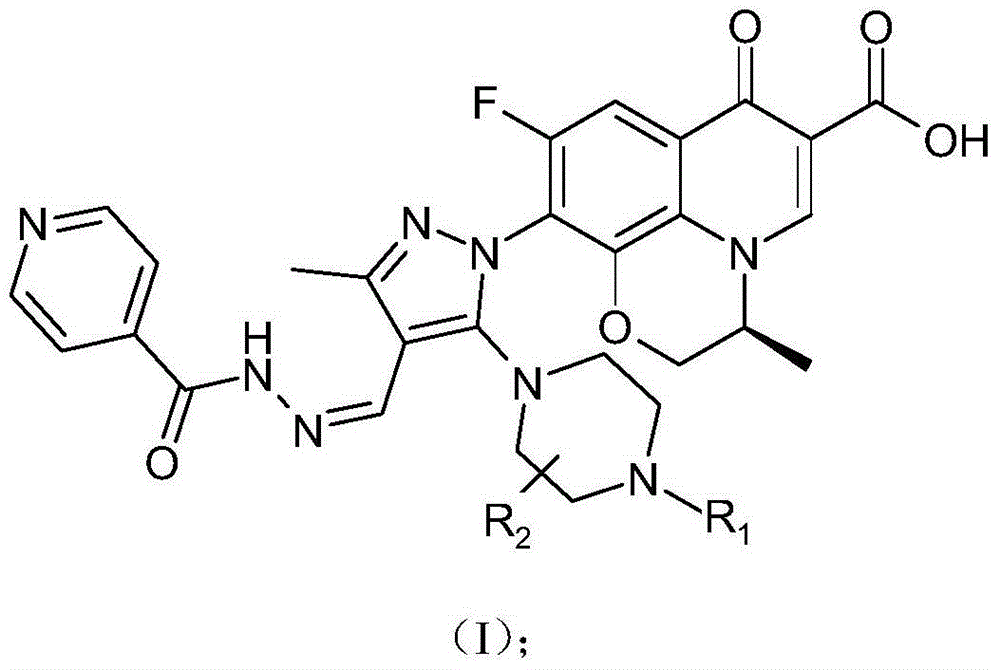
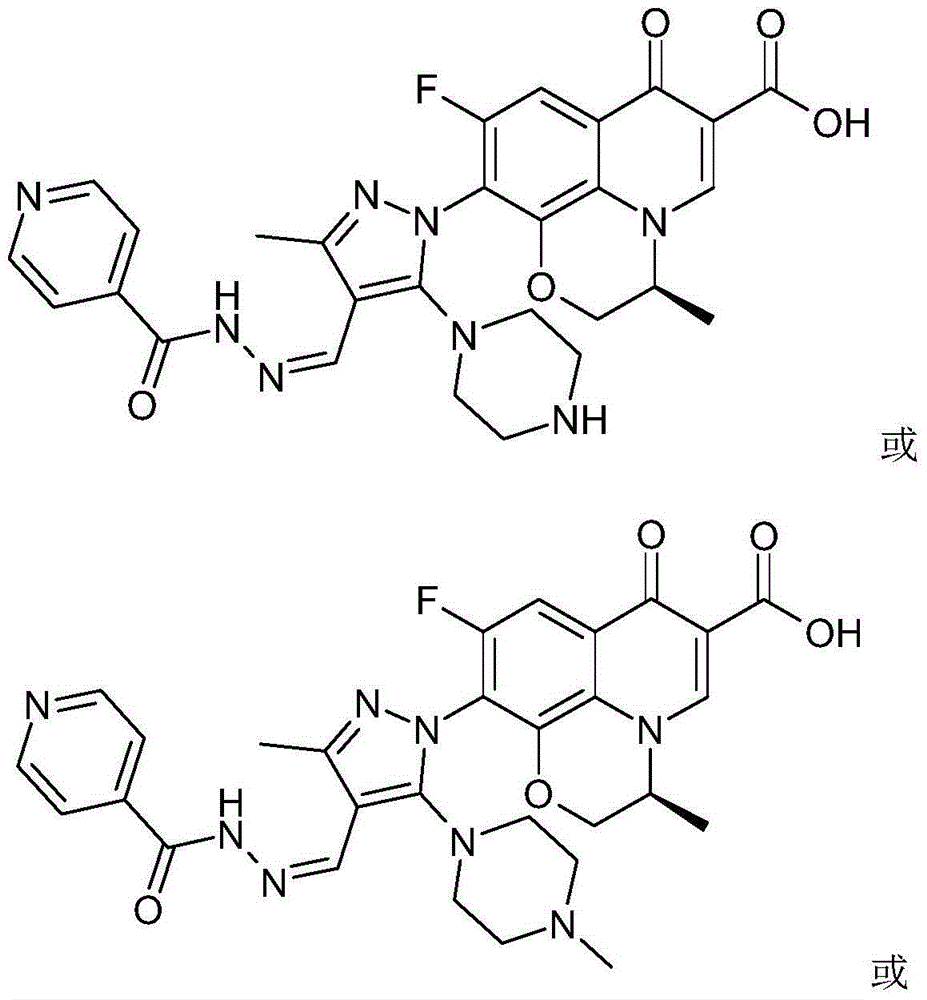
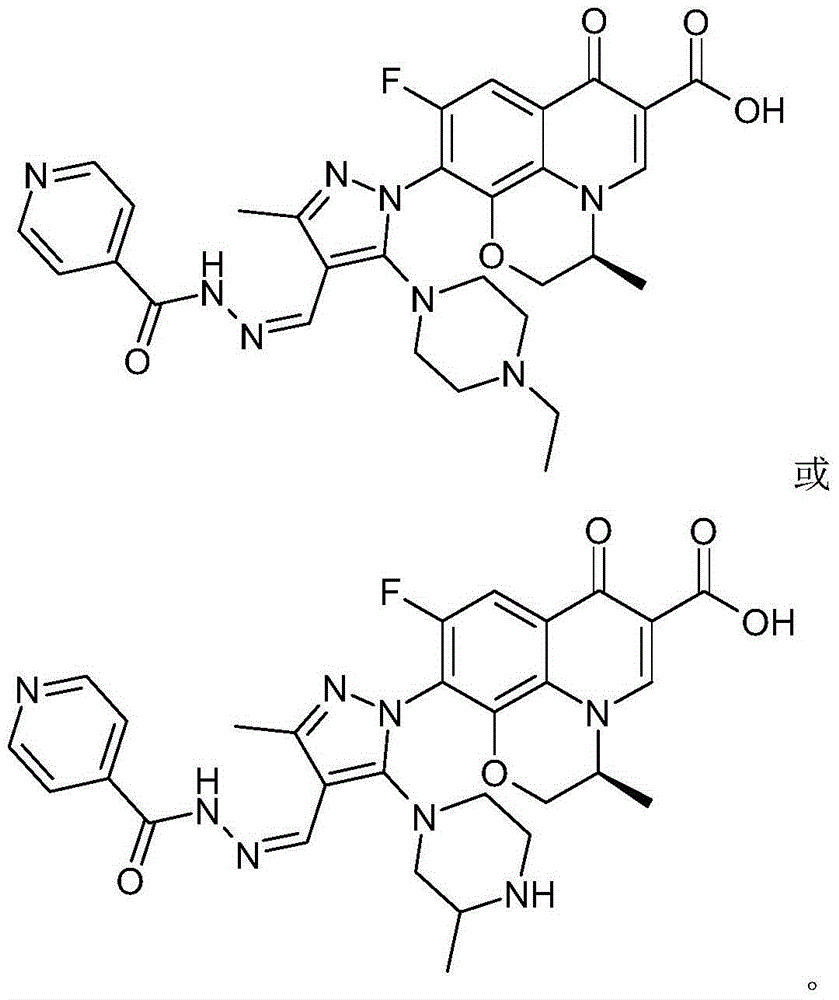
Chirality 7-(piperazine-substituted pyrazol aldehyde condensation isoniazide) fluoroquinolone carboxylic acid derivative as well as preparation method and application thereof
InactiveCN104402902AAchieve overlayReduce chance of drug resistanceAntibacterial agentsOrganic chemistryIsoniazidSide effect
The invention discloses a chirality 7-(piperazine-substituted pyrazol aldehyde condensation isoniazide) fluoroquinolone carboxylic acid derivative as well as a preparation method and application thereof. The chirality 7-(piperazine-substituted pyrazol aldehyde condensation isoniazide) fluoroquinolone carboxylic acid derivative is a compound with the general structural formula (I), wherein R1 is H, methyl or ethyl, and R2 is H or methyl. According to the chirality 7-(piperazine-substituted pyrazol aldehyde condensation isoniazide) fluoroquinolone carboxylic acid derivative provided by the invention, fluoroquinolone, isoniazide and pyrazole aldehyde hydrazone are effectively combined to form a compound with a new structure; superposition and cooperation of activity are achieved; superposition of the three pharmacophores of fluoroquinolone, isoniazide and pyrazole aldehyde hydrazone is realized, the antituberculosis activity is improved, the toxic and side effects of fluoroquinolone and isoniazide to normal cells are decreased, and meanwhile, the probability that mycobacterium tuberculosis resists such drugs can be lowered; the chirality 7-(piperazine-substituted pyrazol aldehyde condensation isoniazide) fluoroquinolone carboxylic acid derivative can serve as an antituberculous active substance used for development of an antituberculous drug with a new structure.
Owner:HENAN UNIVERSITY +1
Medicament for treating pulmonary tuberculosis
The invention provides a medicament for treating pulmonary tuberculosis. Adenophora root, Tuber of Dwarf Lilyturf, asparagi radix, rehmanniae radix, Rehmannia Glutinosa, stemona, tendril-leaved fritillary bulb, donkey-hide gelatin, pseudo-ginseng, Poria cocos, Chinese yam, polyghace seche, lily, bletilla striata, hairyvein agrimony, cortex lycii, isoniazid and vitamin B6 are mixed uniformly, and crushed into a medicinal powder of 80-120 mesh; and the medicinal powder is disinfected and filled into capsules to obtain the capsule product. The medicament is composed of traditional Chinese medicines and Western medicines, has efficacies of resisting tuberculosis, disinfesting, supporting right, securing the root, nourishing yin, reliving cough, reducing phlegm and stopping bleeding, and can play fast curative effect without side effect on treatment of pulmonary tuberculosis.
Owner:赵军海
Biologically active substance on the basis of tetracyclic nitrogen heterocycles of pyrimidine row
PCT No. PCT / RU97 / 00098 Sec. 371 Date Mar. 17, 1998 Sec. 102(e) Date Mar. 17, 1998 PCT Filed Apr. 2, 1997 PCT Pub. No. WO98 / 43982 PCT Pub. Date Aug. 10, 1998Biologically active substance on the basis of tetracyclicnitrogen heterocycles of pyrimidine row for treating tuberculosis, mycobacteriousis, viral diseases, infections caused by chlamydias, and also diseases which are accompanied by immunodeficiency, in particular malignant neoplasm, has high antimicrobial activity, in particular to strains of mycobacteria which are resistant to the prototype-isoniazid, and simultaneously possess antiviral activity (relative to herpes simplex viruses), antichylamidial activity and also stimulate production of endogenic interferons in organisms. It represents a derivative of 5-oxo-5H-[1]-benzopyrano-[5,6-b]-4-oxo-4H-[1,2]-pyrimido-1,4,5,6-tetrahydro-1,3-thiazine (1) of general formula(1). (I-X) where: R1-H or halogen; R2-H, or halogen, or nitro-group, or hydroxy-group or methoxy-group.
Owner:NATURAL DRUG SCI
Non-invasive rapid diagnostic test for M. tuberculosis infection
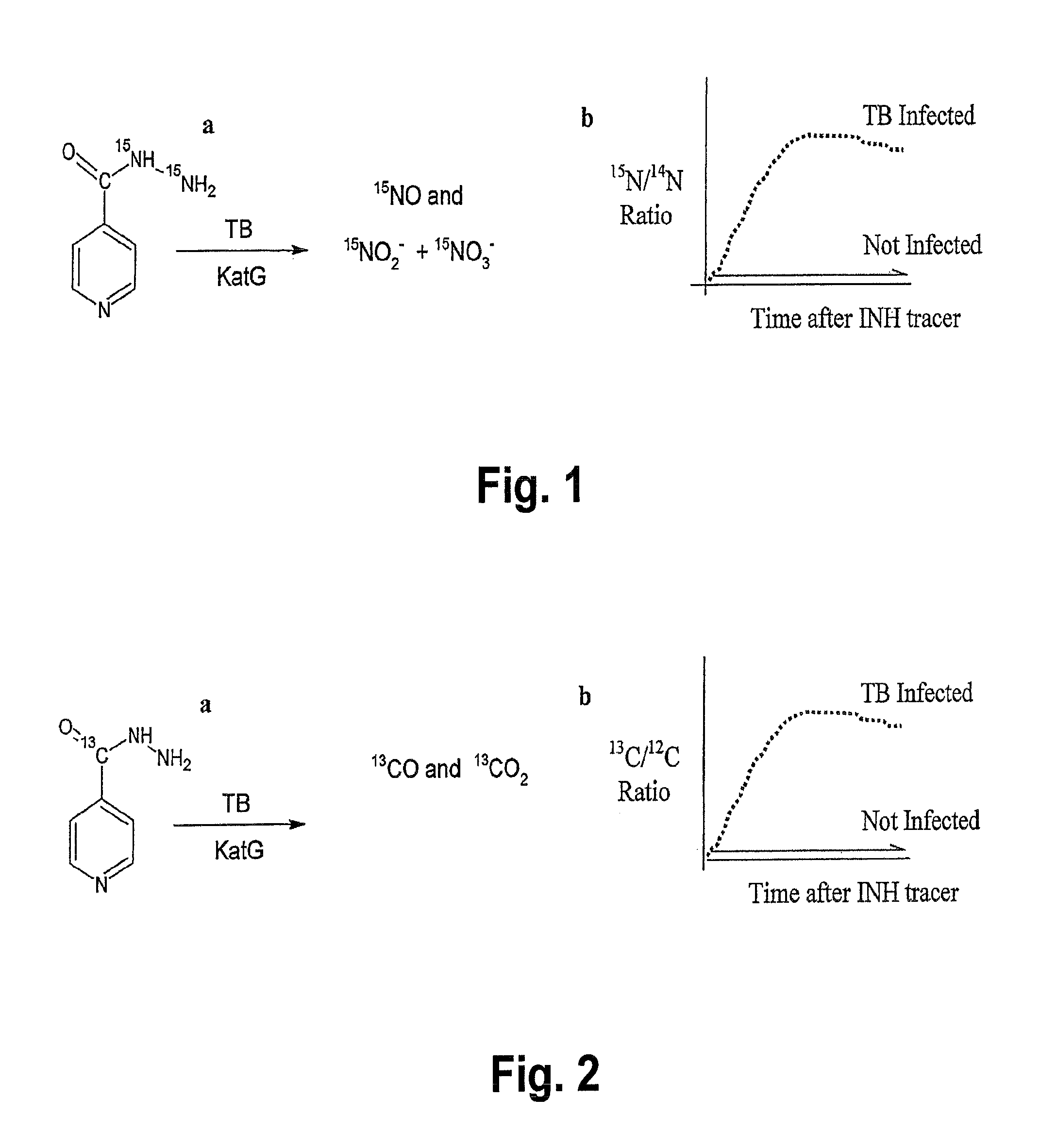
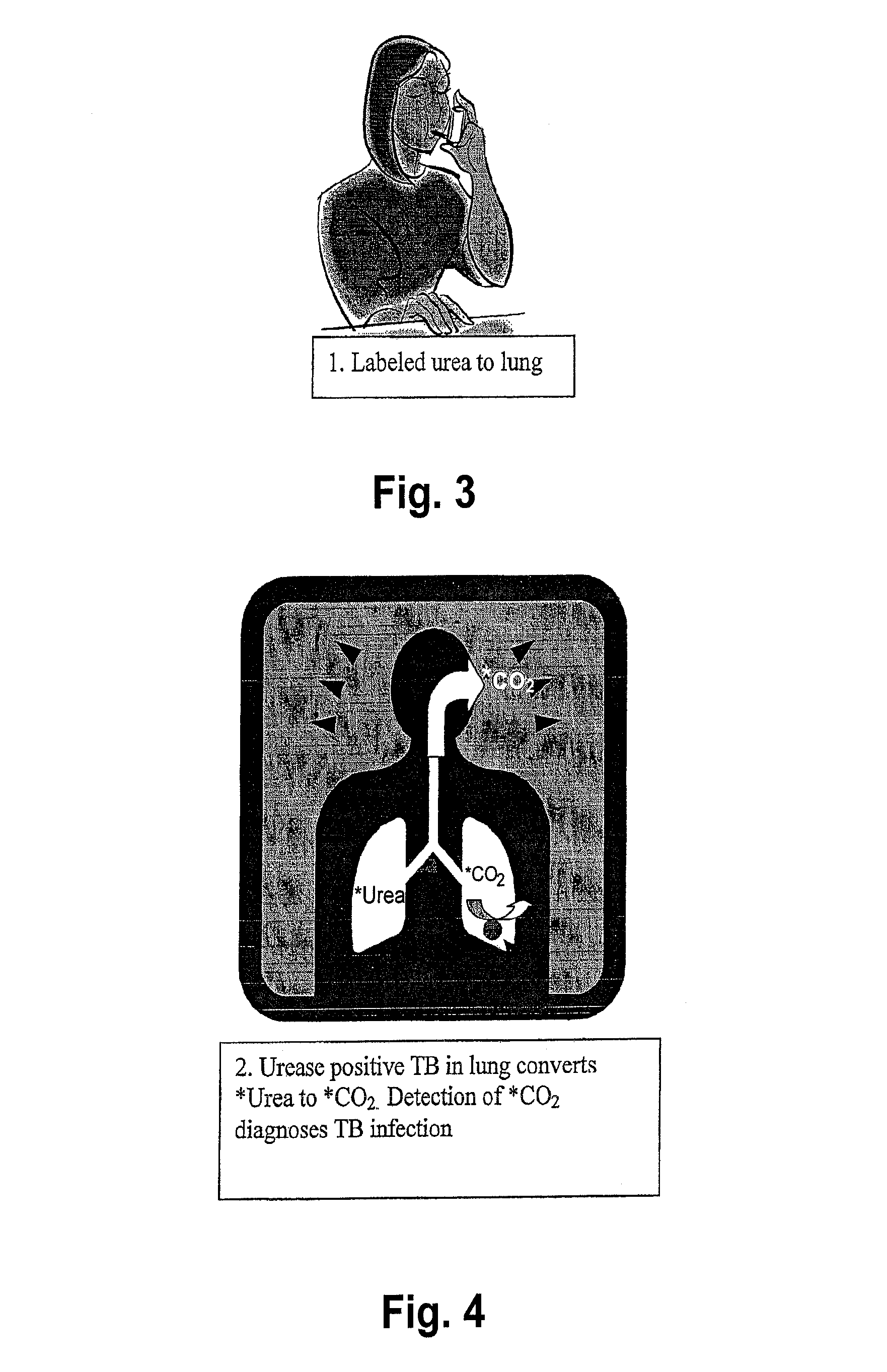
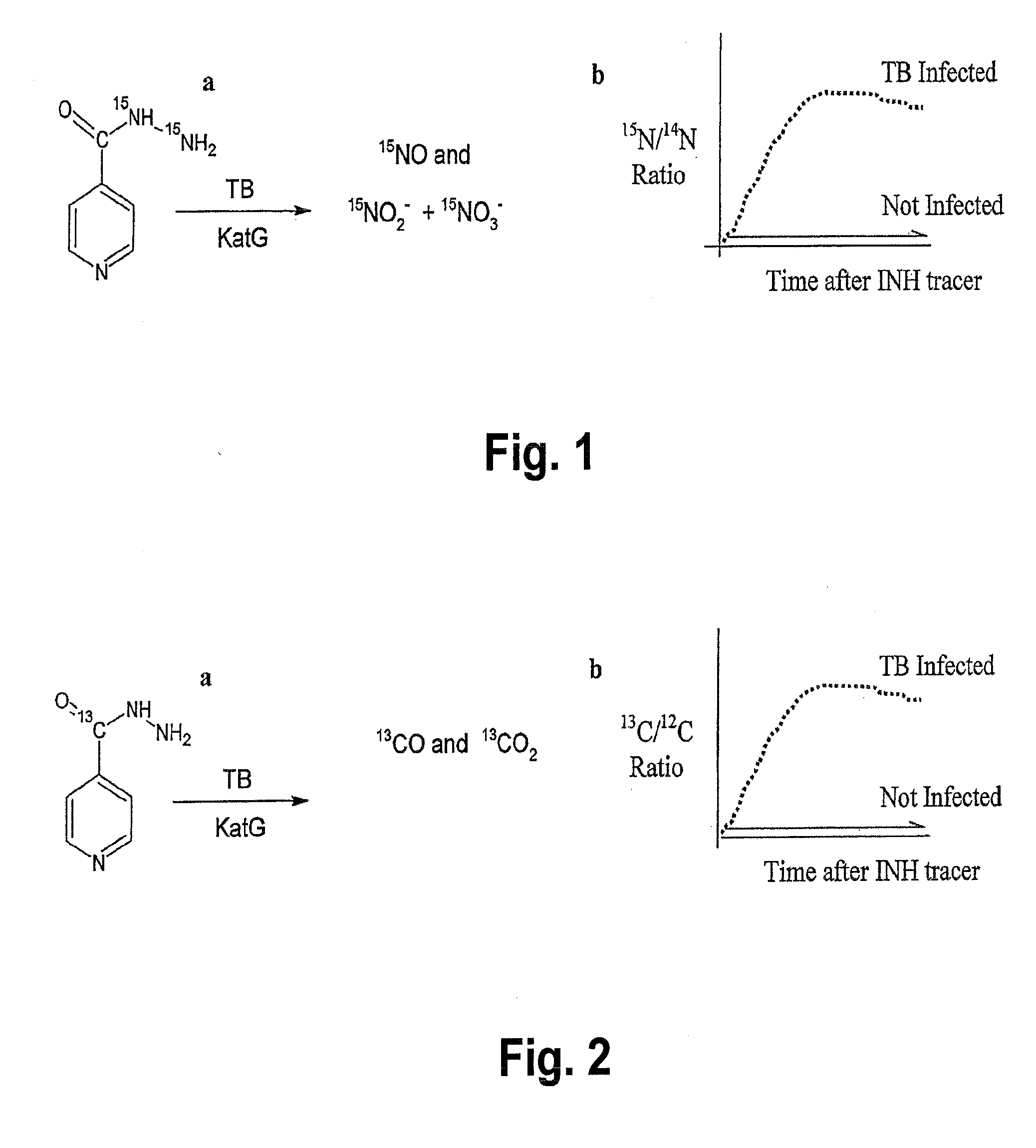
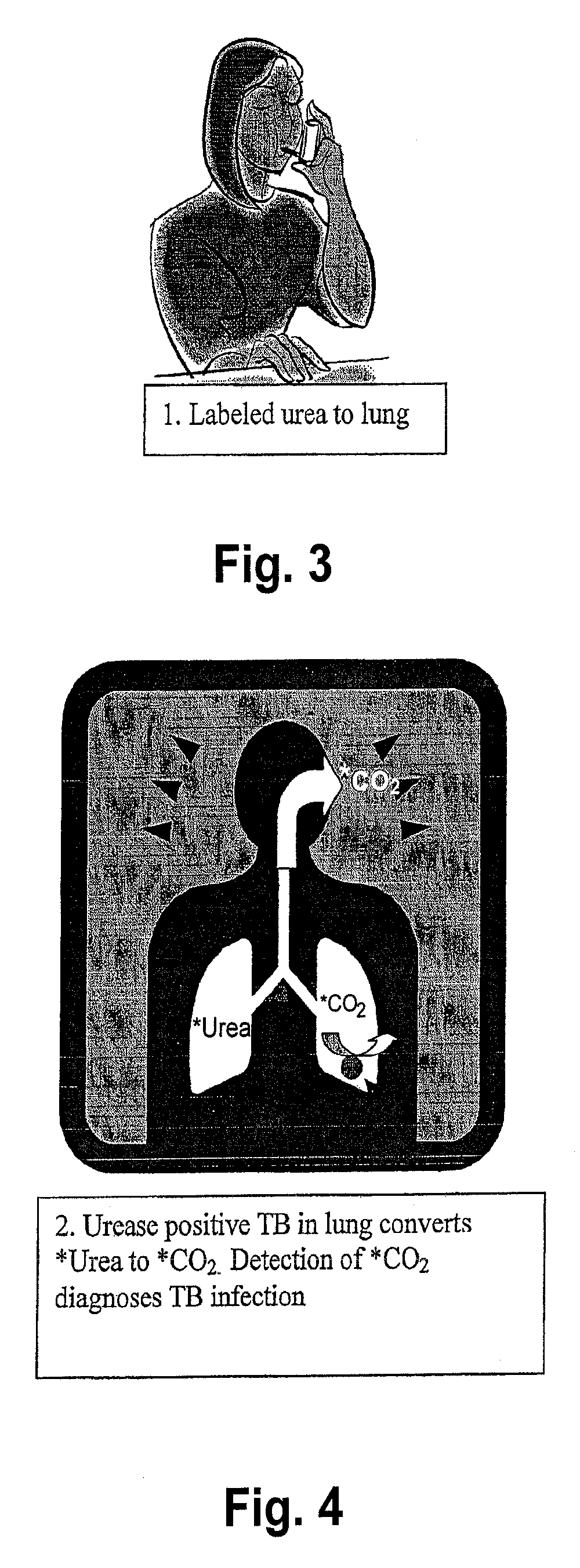
This invention relates to a test for detecting a Mycobacterium tuberculosis (tuberculosis or TB) infection in a patient or subject, specifically a diagnostic test, including a breath test, whereby patients are provided a small dose of an isotopically labeled TB drug, Isoniazid (INH) orally or directly to the lungs of the patient or subject. If TB is present, a TB enzyme mycobacterial peroxidase KatG oxidizes the INH; and KatG specific metabolites, in particular, isotopically labeled nitric oxide (NO), nitrites, nitrates, carbon monoxide (CO) or carbon dioxide converted from carbon monoxide of INH cleavage are measured. Other embodiments relate to a diagnostic breath test for detecting TB utilizing isotopically labeled urea (preferably, carbon-13 labeled urea), alone or in combination with isotopically labeled isoniazid (preferably, nitrogen-15 labeled isoniazid), wherein M. tuberculosis organism, if present in the patient or subject's lungs (or other tissues), will metabolize the isotopically labeled urea to isotopically labeled carbon dioxide (CO2) such that a determination of the residence of M. tuberculosis, including residence of an isoniazid resistant strain of M. tuberculosis, may be made.
Owner:STC UNM
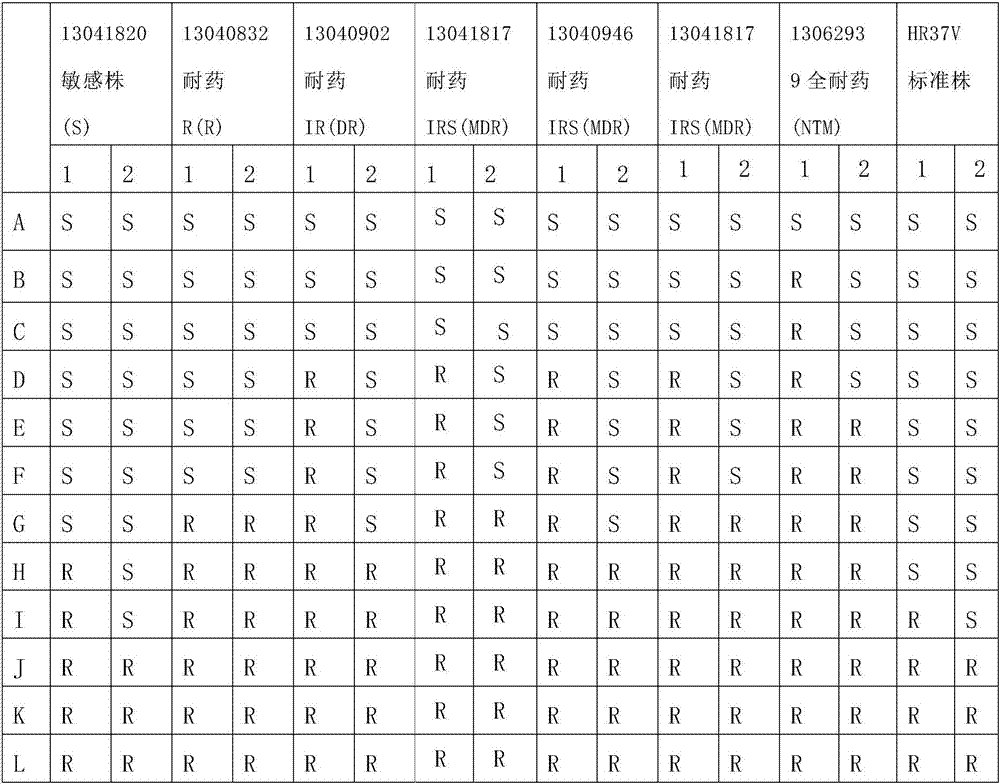
Method and material for simultaneously detecting mycobacterium tuberculosis compound and identifying its medicament resistance
InactiveCN101210265AMicrobiological testing/measurementBiological material analysisIsoniazidFluorescence
The present invention relates to molecular biology, microbiology and medicine, and provides a method for detecting nodule offshoot bacilli complex body in clinic samples and evaluating the sensitivity of strain to rifampicin as well as isoniazid in distinguish biochip. The method obtains fluorescence DNA segments based on two-step multiple PCR, then to hybridize said segments on a micro-array containing differential distinguished oligonucleotide group. To determine the resistance of concretion offshoot bacilli to rifampicin and isoniazid by means of evaluating point nucleotide replacement in microbiology DNA. The present invention allows analyze directly clinic sample to evaluate mass mutation at one time, so as to reduce analysis cost and implement time. The present invention also relates to primer group, biochip and oligonucleotide probe group for implementing said method.
Owner:RUSN ACAD V A ENGERHARDT MOLECULAR BIOLOGY INST
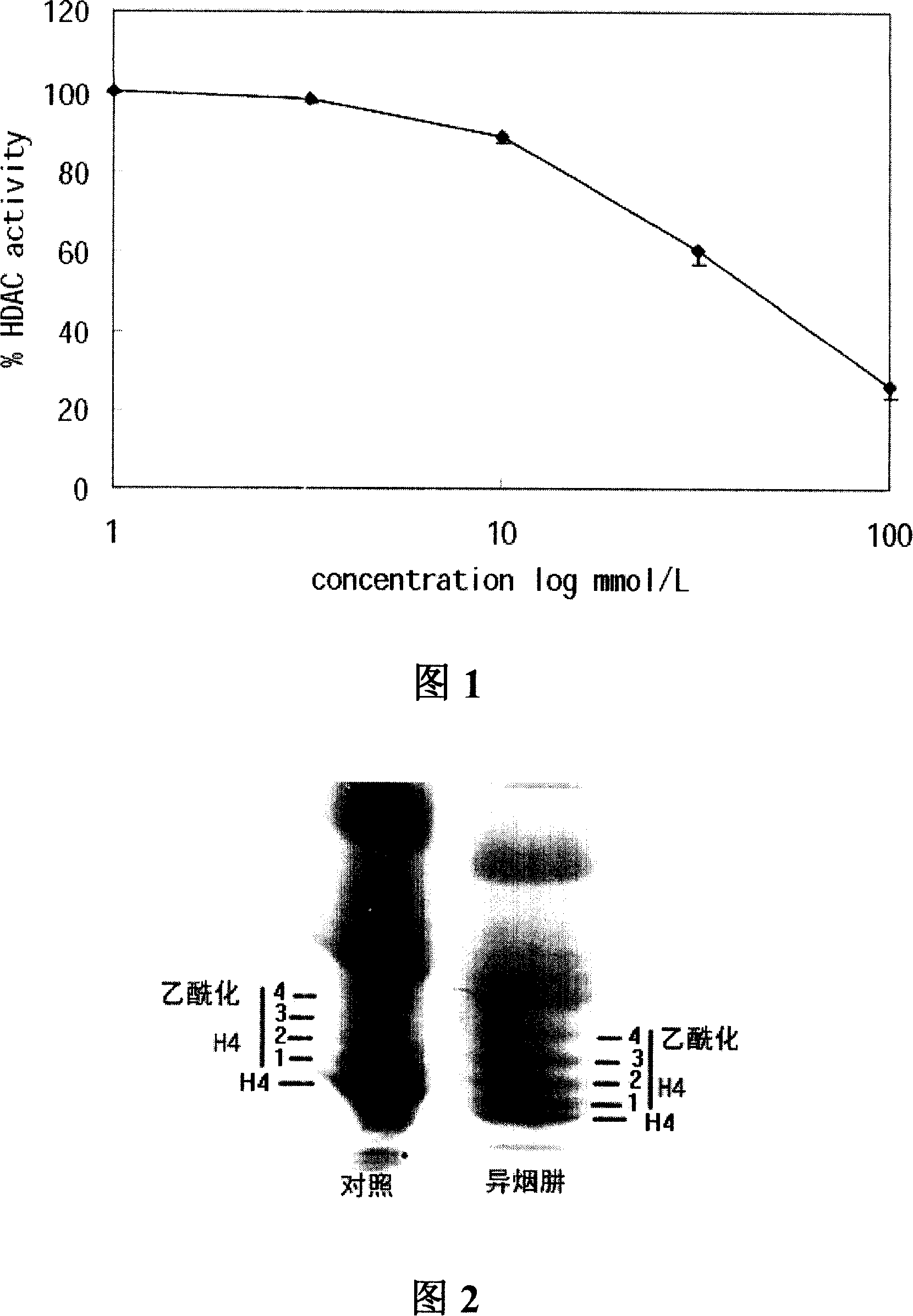
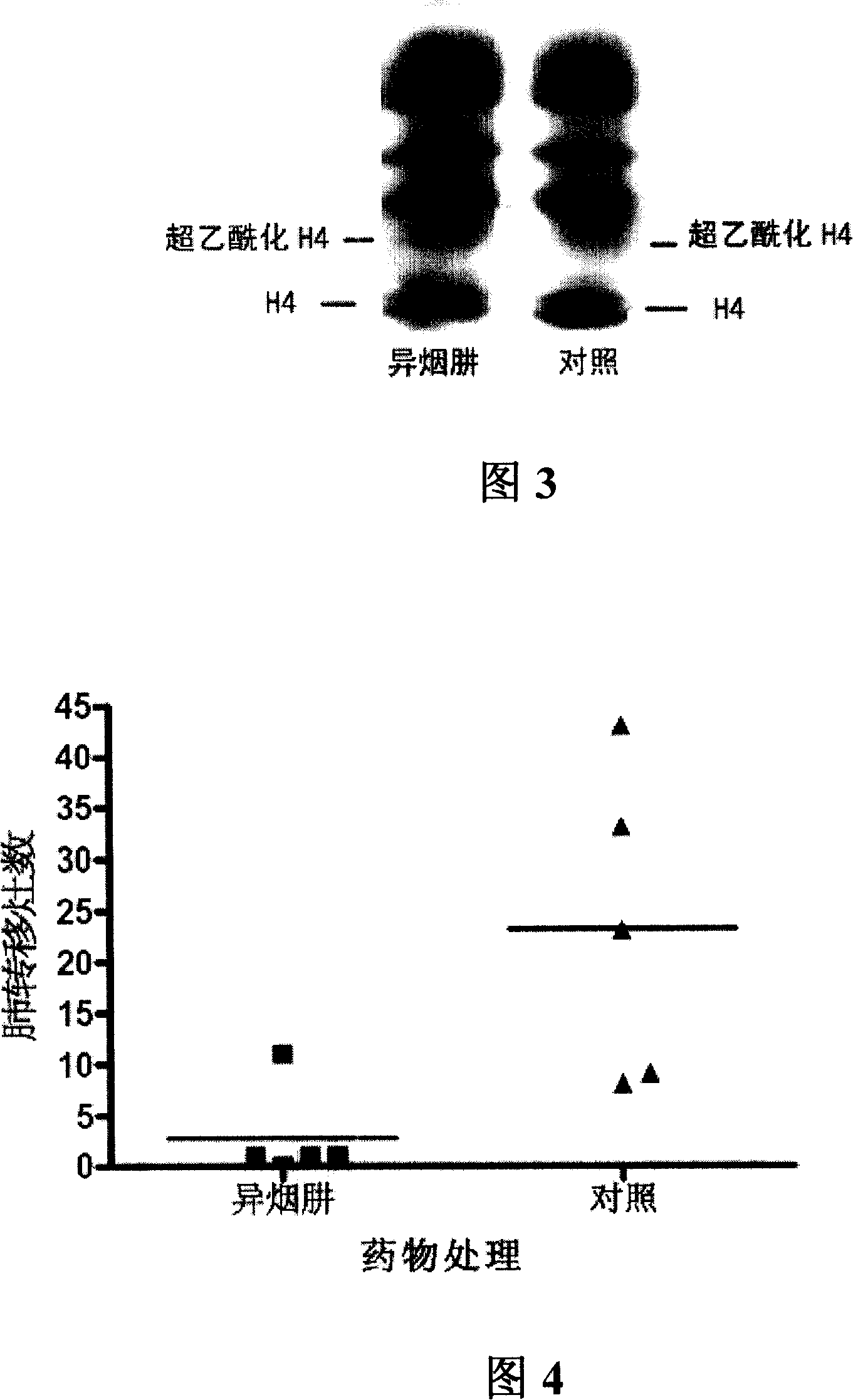
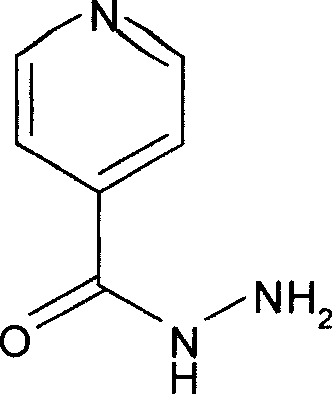
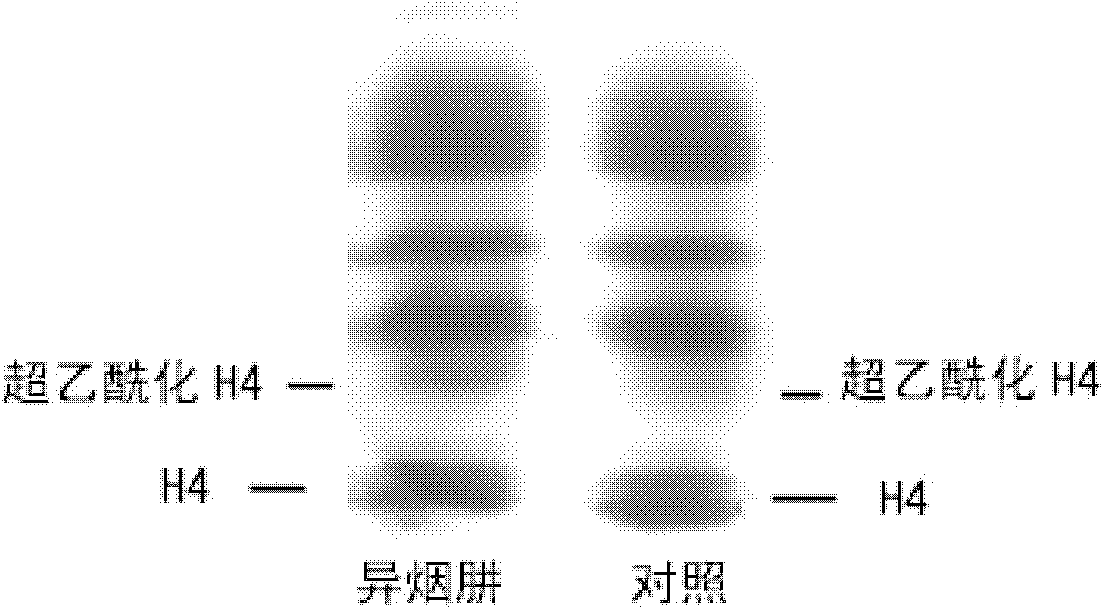
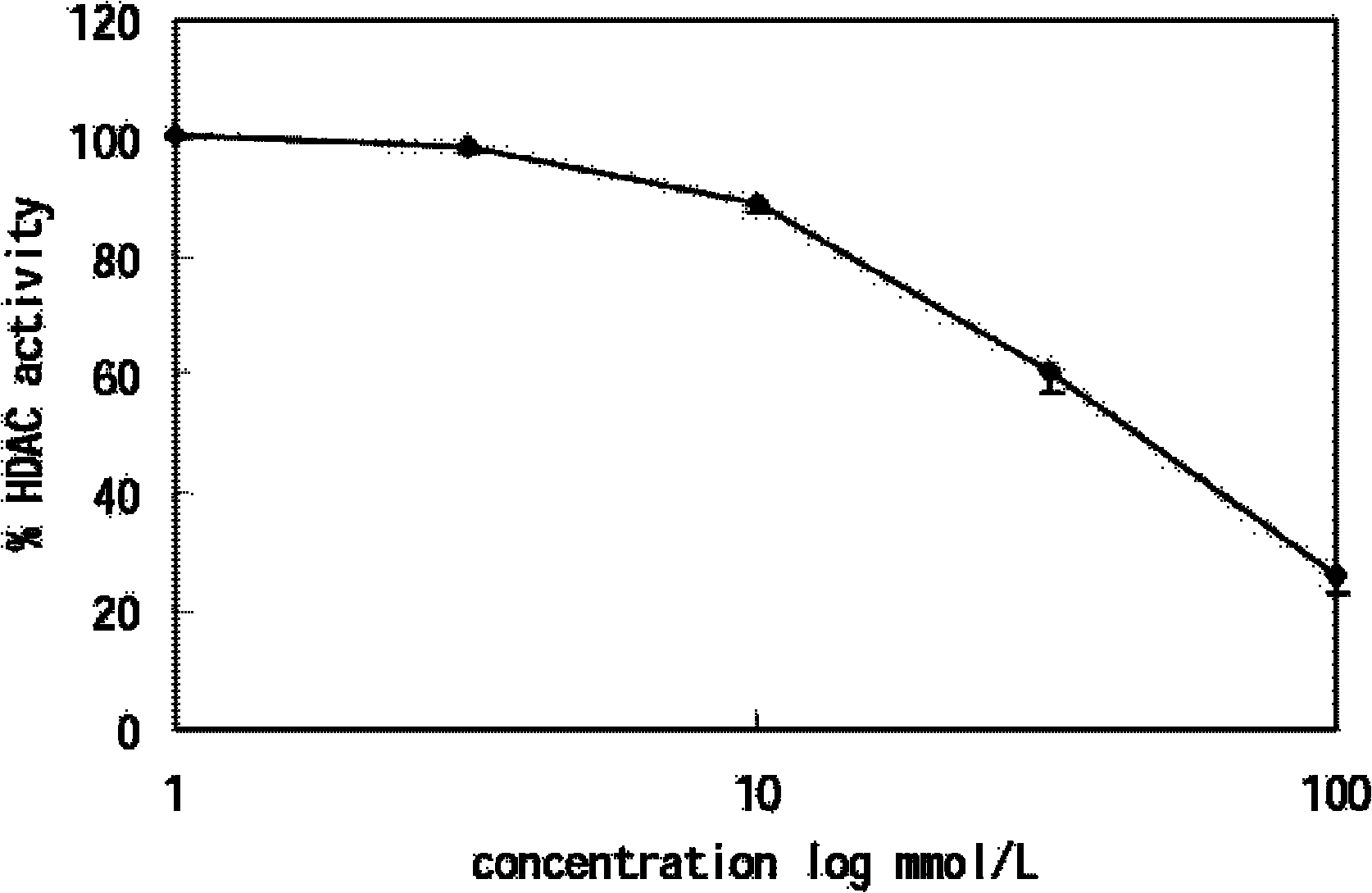
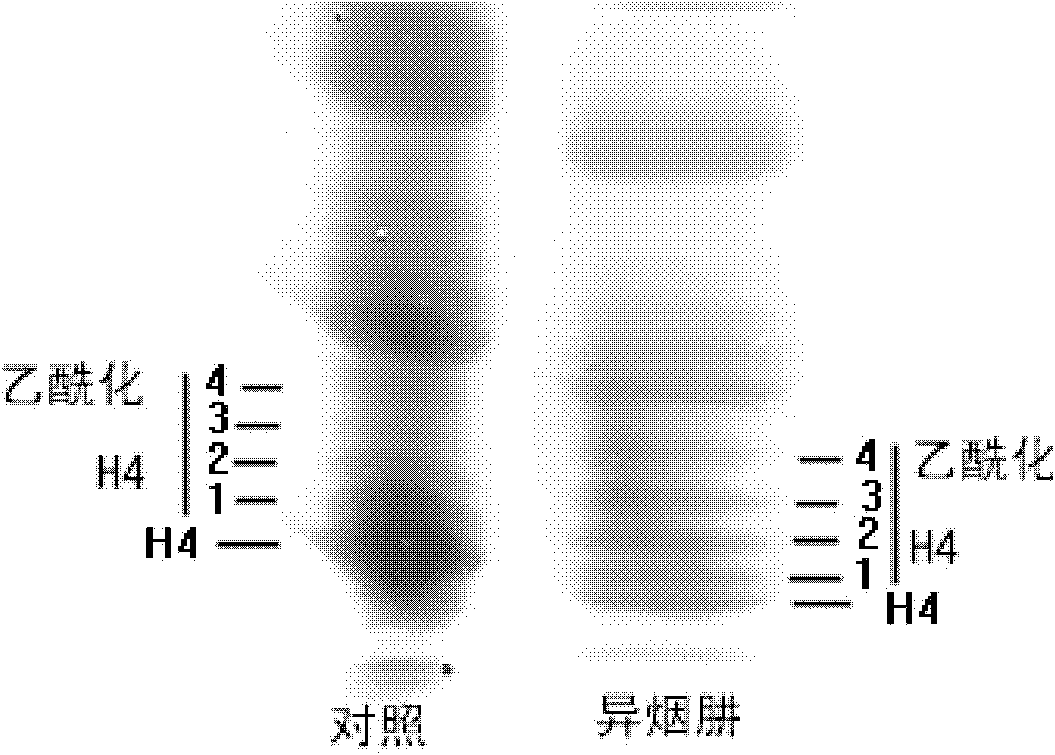
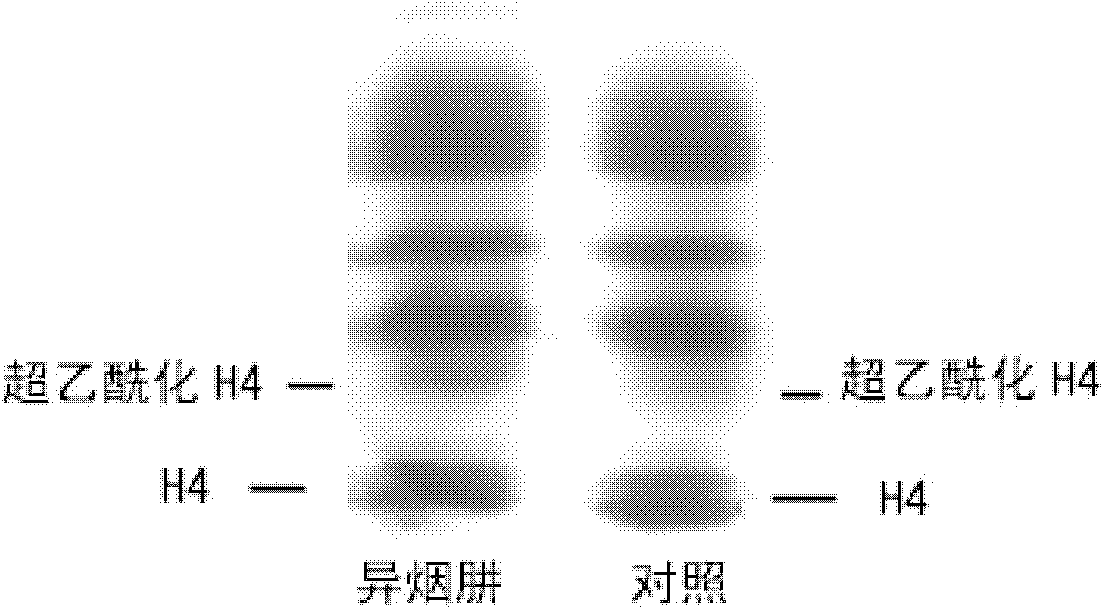
Application of isoniazide as histone deacetylase inhibitors
InactiveCN101152179APromote acetylationPrevent transplant rejectionOrganic active ingredientsAntipyreticDiseaseIsoniazid
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
Polyacid compound for resisting penicillin streptococcus pneumoniae (PRSP) and its preparing method
InactiveCN1616466AAntibacterial agentsPhosphorous compound active ingredientsChemical synthesisMoroxydine
The present invention belongs to the field of chemically synthesized medicine and its preparation process. The medicine of the present invention is synthesized with amantadine, moroxydine, 5-Fu (5-Fc), isoniazid and other clinical medicine and polyoxometallate, and through self-assembling. The said polyoxometallate includes heteropoly acid salts of Keggin type with chemical expression XM12O40n-, Dowson type X2M18O62n- and of chemical expression LnW10O36n-. The combined use of said medicines and polyoxometallate has obvious curative effect on intractable streptococcus pneumoniae infection.
Owner:NORTHEAST NORMAL UNIVERSITY
Non-Invasive Rapid Diagnostic Test For M.Tuberculosis Infection
ActiveUS20090191639A1Improve protectionImprove the immunityCompounds screening/testingBiocideRapid screening testMetabolite
This invention relates to a test for detecting a Mycobacterium tuberculosis (tuberculosis or TB) infection in a patient or subject, specifically a diagnostic test, including a breath test, whereby patients are provided a small dose of an isotopically labeled TB drug, Isoniazid (INH) orally or directly to the lungs of the patient or subject. If TB is present, a TB enzyme mycobacterial peroxidase KatG oxidizes the INH; and KatG specific metabolites, in particular, isotopically labeled nitric oxide (NO), nitrites, nitrates, carbon monoxide (CO) or carbon dioxide converted from carbon monoxide of INH cleavage are measured. Other embodiments relate to a diagnostic breath test for detecting TB utilizing isotopically labeled urea (preferably, carbon-13 labeled urea), alone or in combination with isotopically labeled isoniazid (preferably, nitrogen-15 labeled isoniazid), wherein M. tuberculosis organism, if present in the patient or subject's lungs (or other tissues), will metabolize the isotopically labeled urea to isotopically labeled carbon dioxide (CO2) such that a determination of the residence of M. tuberculosis, including residence of an isoniazid resistant strain of M. tuberculosis, may be made.
Owner:STC UNM
Topiroxostat impurity synthesis method
InactiveCN106008465APrecise positioningQualitative highOrganic chemistryIsoniazidTrimethylsilyl cyanide
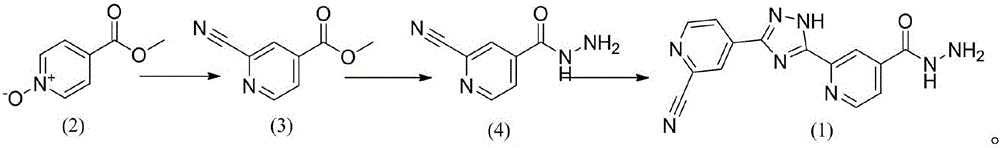
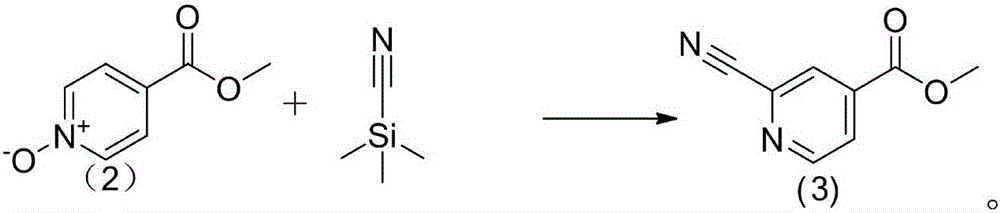
The invention discloses a topiroxostat impurity synthesis method, and belongs to the chemical pharmaceutical technical field. The method comprises the steps: with methyl isonicotinic acid-N-oxide (2) as a starting material, generating 2-cyano-4-pyridine carboxylic acid methyl ester from methyl isonicotinic acid-N-oxide (2) and trimethylsilyl cyanide; carrying out hydrazinolysis of the compound (3) to generate 2-cyano-isoniazid (4); and carrying out ring self-formation of the compound (4) to generate a target 2-(3-(2-amino pyridine-4-yl)-1H-1,2,4-triazole-5-yl)isoniazide (1). The synthesized high-purity topiroxostat impurity can be used as an impurity standard in topiroxostat finished product detection analysis, so as to enhance accurate positioning and qualitation on the impurity in the topiroxostat finished product detection analysis, be conducive to strengthening of the control of the impurity, and improve the quality of the topiroxostat finished product; the method provided by the invention has the advantages of cheap and easily obtained raw materials, and simple operation; the yield of the obtained product is 85%+ / -5%, and the HPLC purity is not less than 98%.
Owner:JIANGSU YUEXING PHARMA
Preparation method of isoniazid
The invention discloses a preparation method of isoniazid. The method comprises the following steps: 1) performing esterification reaction to isonicotinic acid, alcohol and acylating reagent to obtain isonicotinic acid ester; 2) performing condensation reaction to isonicotinic acid ester and hydrazine hydrate and performing post-treatment to reaction liquid to obtain isoniazid finished products. The isoniazid is prepared firstly through esterification of isonicotinic acid and alcohol and then condensation of the obtained ester and hydrazine hydrate. The method can well control the content of impurities in the target product isoniazid, high-purity isoniazid can be obtained, the purity is above 99.9 percent and the content of individual impurity is smaller than 0.10 percent. The method is simple to operate, is easy to control and is applicable to industrial operation. In addition, by using recovered isonicotinic acid to prepare isoniazid, the cyclic utilization of resources can be realized, the waste emission is reduced, the cost is reduced and the method is very suitable for industrial production.
Owner:浙江新赛科药业有限公司
Preparation method of isoniazid para-aminosalicylate
ActiveCN102344412AGood water solubilityHigh purityOrganic compound preparationAmino-carboxyl compound preparationIsoniazidOrganic solvent
The invention discloses a preparation method of isoniazid para-aminosalicylate. The preparation method comprises that a sodium para-aminosalicylate solution is added with an acid and then is extracted by an organic solvent and isoniazid is added into the extract to produce a desired product. The isoniazid para-aminosalicylate product prepared by the preparation method has isoniazid para-aminosalicylate purity above 98%. Through the preparation method, a yield of 90 to 98% is obtained. The preparation method adopts simple and environmentally friendly processes, realizes recycle of an extraction solvent and saves greatly costs.
Owner:CHONGQING HUABANGSHENGKAI PHARM
New low side effect pharmaceutical composition containing antituberculosis drugs
ActiveUS20140038921A1Reduce isoniazidReduce pyrazinamide induced hepatotoxicityAntibacterial agentsBiocideDiseaseSide effect
A pharmaceutical composition for treating tuberculotic diseases with no side effect / low side effect is provided by the present invention, which pharmaceutically effective amount of one or more compounds chosen from isoniazid, rifampin, pyrazinamide and ethambutol, and pharmaceutically effective amount of substances which can reduce the side effect of the antituberculosis agents.
Owner:INT EDUCATION FOUND
Mycobacterium tuberculosis drug-resistant mutant gene detection kit
ActiveCN102424859AReduce spreadGuaranteed therapeutic effectMicrobiological testing/measurementMicroorganism based processesIsoniazidNucleotide
The invention discloses a mycobacterium tuberculosis drug-resistant mutant gene detection kit comprising: (1) a gene chip which has (i) a nucleotide probe, wherein the probe is a sequence of SEQIDNos: 1-31 or a complementary sequence of SEQIDNos: 1-31, (ii) a DNA sequence labeled with biotin points, wherein the sequence is SEQIDNO. 32, (iii) an IS6110DNA sequence of a mycobacterium tuberculosis complex, wherein the sequence is SEQIDNo. 33; (2) various primers used for amplifying DNA sequences in clinical samples, wherein the DNA sequences of the primers are SEQIDNos. 34-41. The kit of the invention detects the drug resistance of mycobacterium tuberculosis to rifampin and isoniazid, provides reference basis for the prevention and treatment of tuberculosis, and has significant meaning.
Owner:GUANGDONG HYBRIBIO BIOTECH CO LTD
Stabilized short-course chemotherapy (SCC) anti-tuberculosis drug compositions
A stabilized oral powder or granule mixture made from at least two different anti-microbial tuberculosis drugs (e.g., rifampacin, isoniazid, ethambutol, pyrazinamide), for a short-course therapy; the powder can be consumed by mixing in a glass of water or juice and assures that each of the various drugs is in fact consumed by the tuberculosis patient.
Owner:SAPTE VINAY RAMAKANT
Process for preparation of anti-tubercular combination and pharmaceutical composition prepared therefrom
This invention relates to a process for preparing a pharmaceutical composition comprising four antitubercular drugs: rifampin or a pharmaceutically acceptable salt thereof, isoniazid or a pharmaceutically acceptable salt thereof, pyrazinamide or a pharmaceutically acceptable salt thereof and ethambutol or a pharmaceutically acceptable salt thereof, wherein rifampin and isoniazid are in separate layers. The invention also provides a pharmaceutical composition prepared therefrom having advantageous stability and bioavailability.
Owner:TAIWAN BIOTECH +1
Application of isoniazid to preparation of medicament for preventing or treating lung cancer and colorectal carcinoma
InactiveCN102389424APromote acetylationPrevent transplant rejectionOrganic active ingredientsAntineoplastic agentsDiseaseIsoniazid
The invention is a divisional application of 200610113436.7. The invention discloses novel purpose of isoniazid shown as a formula (I) as histone deacetylases inhibitor and application of isoniazid to preparation of medicaments for preventing and / or treating a plurality of diseases correlative to histone deacetylases.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
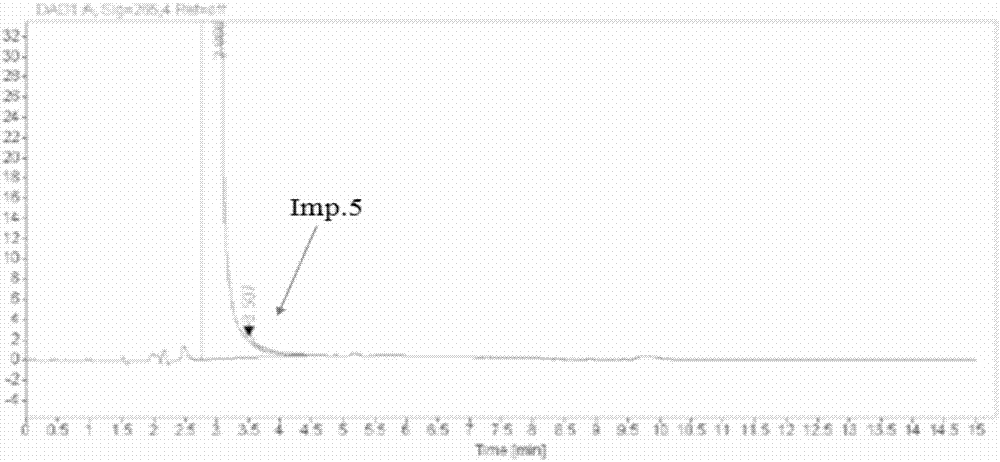
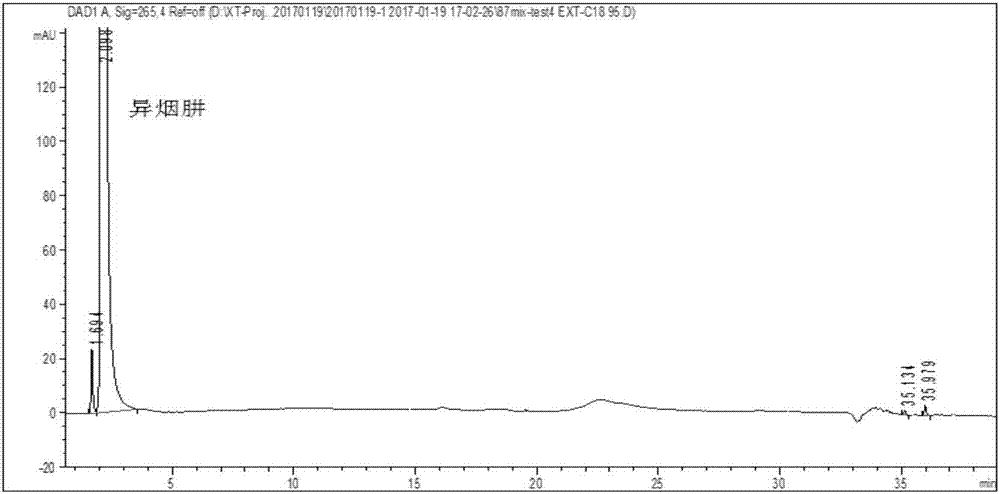
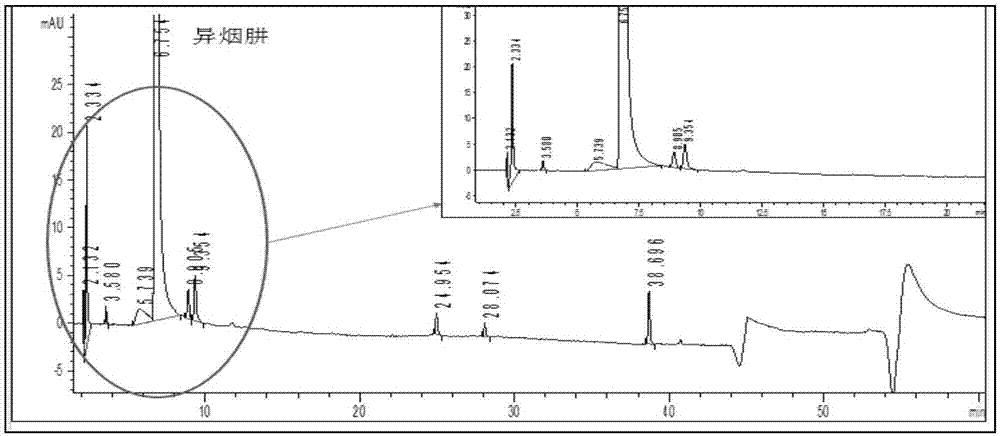
Method for detecting content of impurities in isoniazid or medicinal composition thereof
The invention relates to a method for detecting the content of impurities in isoniazid or a medicinal composition thereof. The method comprises the following steps: 1, preparing an impurity reference substance solution, namely precisely weighing a proper amount of isonicotinic acid and an isonicotinic acid reference substance, dissolving with water, and quantitatively diluting to serve as isonicotinic acid and the isonicotinic acid reference substance solution; 2, preparing a test substance solution, namely weighing a proper amount of a test substance, adding water to dissolve isoniazid, diluting, filtering and taking subsequent filtrate as a test substance solution; 3, preparing a reference solution, namely precisely weighing the test substance solution, and diluting for 100 times to serve as a reference solution; and 4, carrying out a detection method, namely precisely weighing 10mu l of the impurity reference substance solution, 10mu l of the test sample solution and 10mu l of the reference solution respectively, respectively injecting into a liquid chromatograph, recording a chromatogram map, and calculating contents of isonicotinic acid, pyrazinamide and other impurities by adopting a peak area method according to the chromatogram map.
Owner:SHENYANG HONGQI PHARMA
Pharmaceutical composition comprising rifamoin and isoniazid
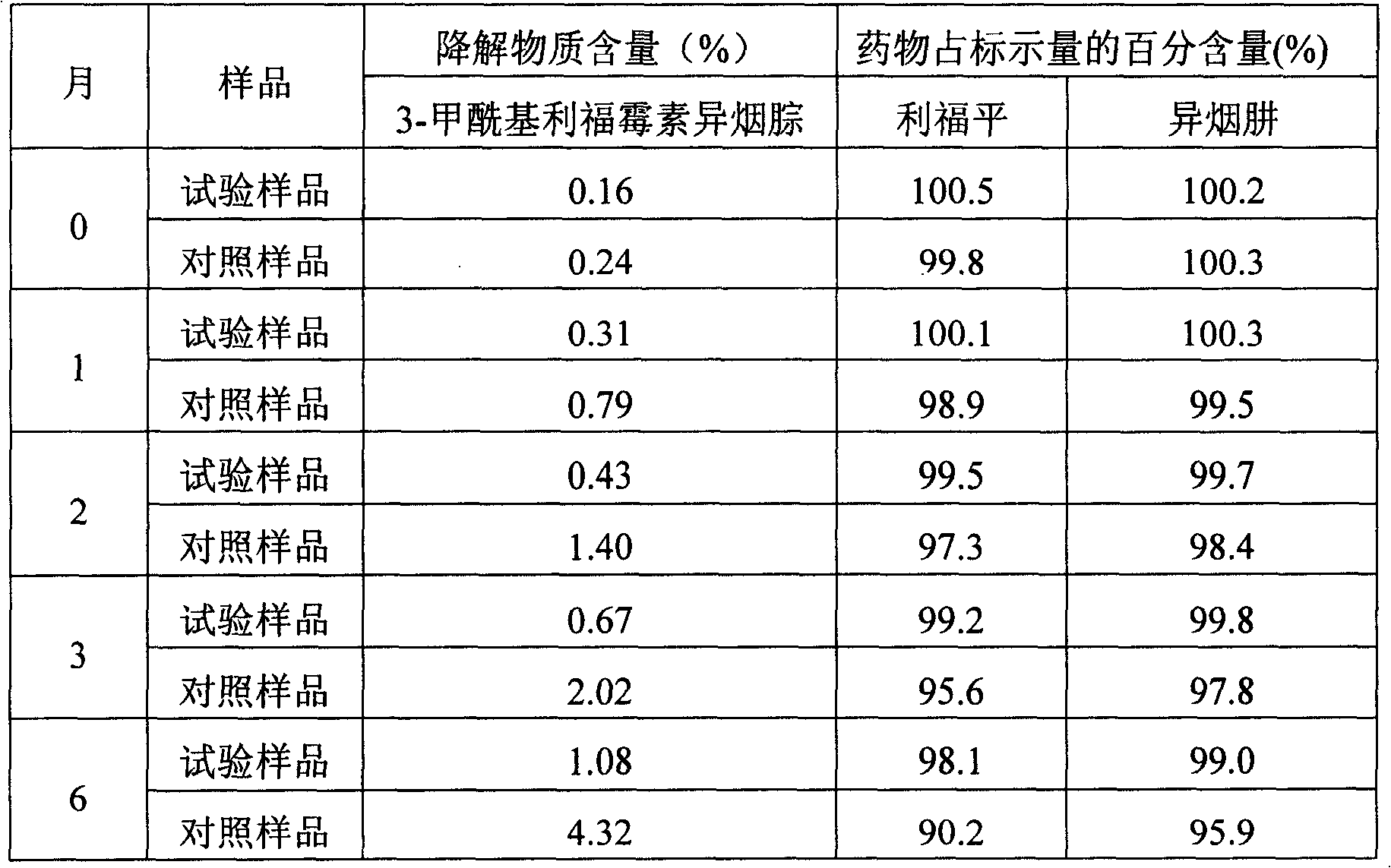
InactiveCN1989966AImprove stabilityReduce degradationAntibacterial agentsPowder deliveryIsoniazidMedicine
The invention involves a drug combinations including rifampin and isoniazid, the combination contains pH modifier of 0.1% ~ 10% (w / w). Said pH regulator is selected from weak acid- weak acid salt buffer system, weak alkali- weak alkali salt buffer system or weak acid salt- weak alkali salt buffer system. The drug combination in the invention can effectively reduce the product of polymer-3-formyl rifamycin SV isoniazone caused by rifampin and isoniazid degradation, and increased drug stability. The method of the invention has advantages as: operation is convenient, process is simple, equipment needs not improvment and the cost is low.
Owner:CHONGQING HUAPONT PHARMA
Preparation method of isoniazid para-aminosalicylate
ActiveCN103539733AAdequate responseHigh purityOrganic compound preparationAmino-carboxyl compound preparationCarbon numberOrganic Ester
The invention relates to a preparation method of isoniazid para-aminosalicylate. The preparation method of isoniazid para-aminosalicylate comprises the following steps: (1) respectively weighting water and an organic solvent, mixing, adding para-aminosalicylic acid and isoniazid to a solvent of the water and the organic solvent, heating and stirring; (2) cooling and growing grains, and filtering to obtain isoniazid para-aminosalicylate, wherein the organic solvent is an organic ester with the carbon number of less than or equal to 6. By using the preparation method, the preparation of isoniazid para-aminosalicylate is realized at low cost and high efficiency in a water and ester two-phase solvent system under a simple, convenient and feasible technological condition. The content of isoniazid para-aminosalicylate prepared by the method is higher than 99%. Isoniazid para-aminosalicylate has good appearance crystal form, standard color and luster and good stability. The preparation method has the advantages of few reaction operating units, simple technological process, short time consumption, low production cost, environmental friendliness, simple operation and easiness for control on the implementation process and is suitable for the industrial production of isoniazid para-aminosalicylate.
Owner:GUANGZHOU BAIYUNSHAN PHARM CO LTD
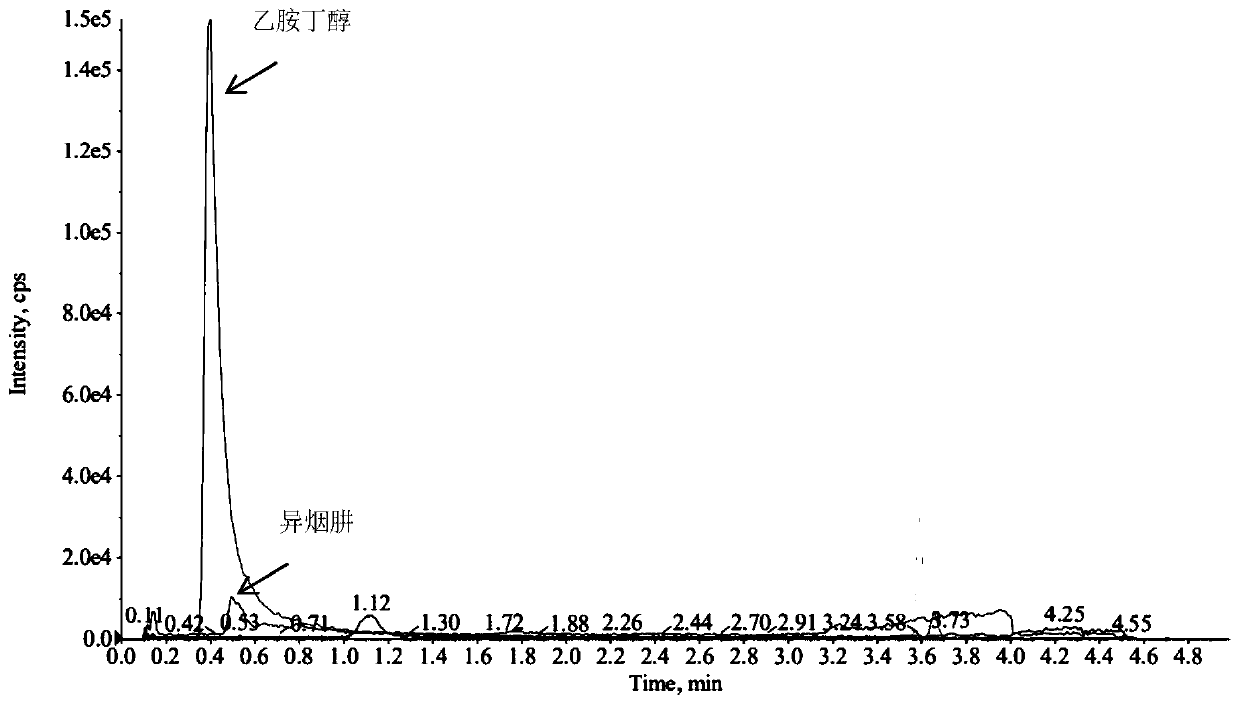
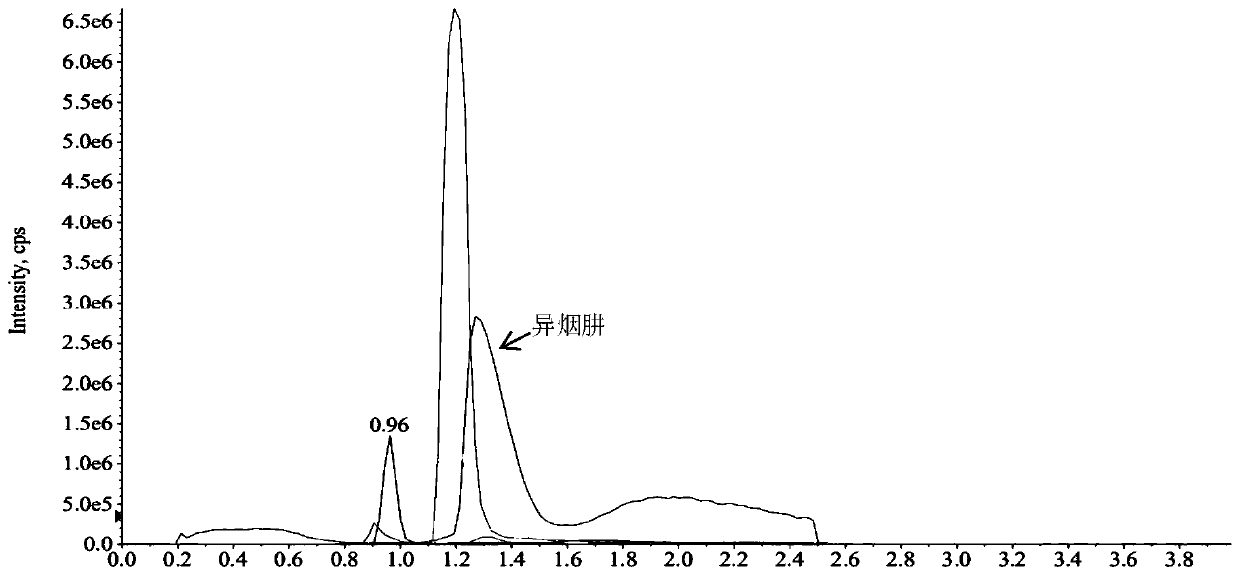
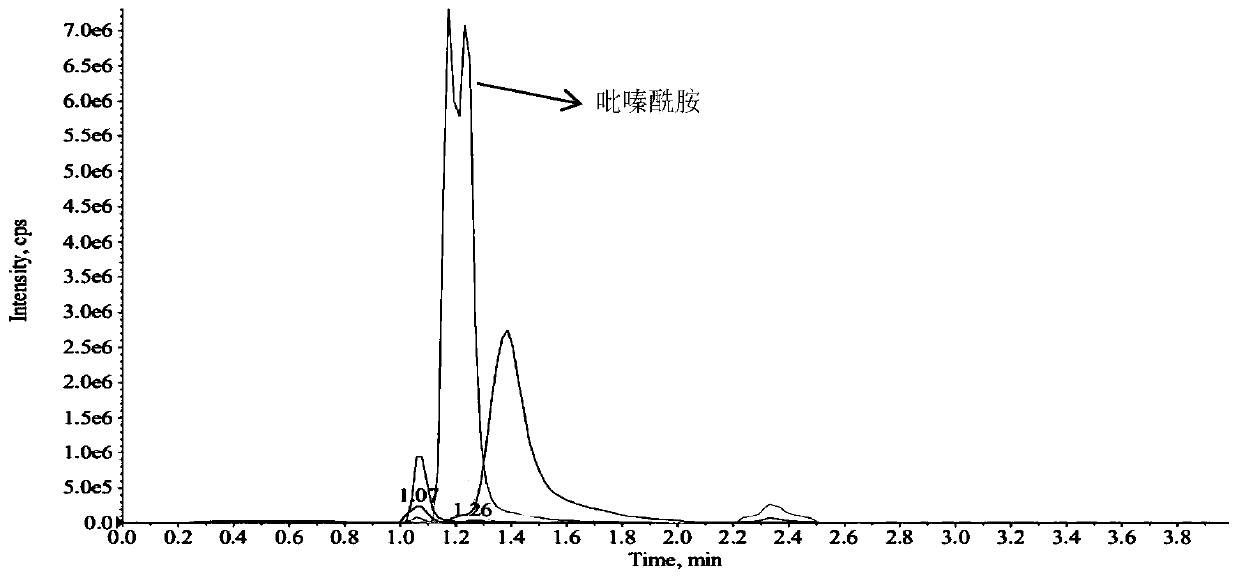
Method for simultaneously detecting five anti-tuberculosis drugs in blood plasma via UPLC-MS/MS method
The invention discloses a method for simultaneously detecting five anti-tuberculosis drugs (including rifampin, rifabutin, Pyrazinamide, ethambutol and isoniazid) in blood plasma via a UPLC-MS / MS method. Blank blood plasma is weighed precisely, and added with standard working solutions mixed with a series of standard substances, standard working solutions of isotope internal standards in one to one correspondence are added, pre-treatment is carried out in a protein precipitation method, the UPLC-MS / MS method is used for analysis, chromatograms of different samples are obtained, and a standardcurve is established by taking the ratio of an object to be measured and the corresponding internal standard peak area as the abscissa and the concentration of the object to be measured as the ordinate; and blood plasma to be measured is weighed precisely, the standard working solutions of isotope internal standards in one to one correspondence are added, pre-treatment is carried out in the protein precipitation method, the UPLC-MS / MS method is used for analysis, chromatograms of different samples are obtained, and the concentration of the blood plasma sample is calculated by using the standard curve. The method is simple and rapid in operation and high in sensitivity, accuracy and precision, the matrix effect is low, and can satisfy requirements for monitoring the drug concentration of five anti-tuberculosis drugs in clinical application.
Owner:MENGCHAO HEPATOBILIARY HOSPITAL OF FUJIAN MEDICAL UNIV
Chinese medicine powder for treating tuberculosis and its usage
InactiveCN1739737ACure tuberculosisHeal the bleedingAntibacterial agentsAnthropod material medical ingredientsRegimenBletilla striata
The present invention discloses one kind of Chinese medicine powder for treating tuberculosis and its treating method via combination with Western medicines. The Chinese medicine powder consists of bletilla tuber, notoginseng, gallnut and pangolin scales, and is orally taken together with Western medicines isoniazid, ethambutol, metronidazole and vitamin B6. It is taken with pork stomach and lung soup or pork trotter soup. It has high curative effect, short treatment period, and other advantages, and may be industrial produced.
Owner:曹国钧
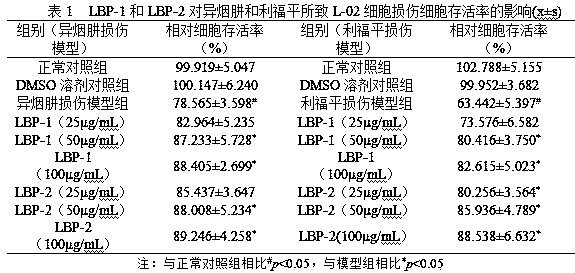
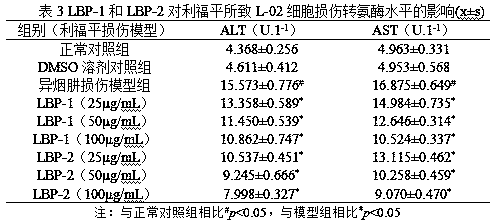
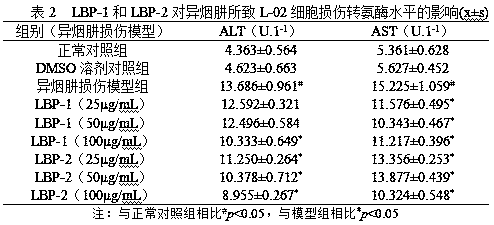
Preparation method of lycium barbarum glycopeptide with effect of repairing and preventing liver injury induced by anti-tuberculosis drugs
ActiveCN110183511AHigh purityImprove qualityDigestive systemPeptide preparation methodsFreeze-dryingRifampicin
The invention discloses a preparation method of lycium barbarum glycopeptide with effect of repairing and preventing liver injury induced by anti-tuberculosis drugs. The method subjects lycium barbarum fruits to crushing, sieving, subcritical degreasing, high-speed shearing and low-temperature wall-breaking water extraction, ethanol precipitation, hot water redissolution, high-speed centrifugation, microfiltration membrane purification, nanofiltration membrane concentration, trichloroacetic acid deproteinization, active carbon decolorization and freeze drying so as to obtain the lycium barbarum glycopeptide with effect of repairing and preventing liver injury induced by anti-tuberculosis drugs. The main component analysis shows that the molecular weight of the lycium barbarum glycopeptideis 10kD-5kD, the polysaccharide content is 85% or above, and the uronic acid content is 10% or above. Activity experiments show that the lycium barbarum glycopeptide can enhance the activity of livercells, obviously regulate down the activity of glutamic-pyruvic transaminase and the level of glutamic-oxaloacetic transaminase caused by isoniazid and rifampicin, has better prevention and repair effects on liver injury caused by anti-tuberculosis drugs of rifampicin and isoniazid, and can be used as an effective component for preparing health-care foods and pharmaceutical preparations.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Anti-tumor drug and application of isoniazid in preparation of anti-tumor drug
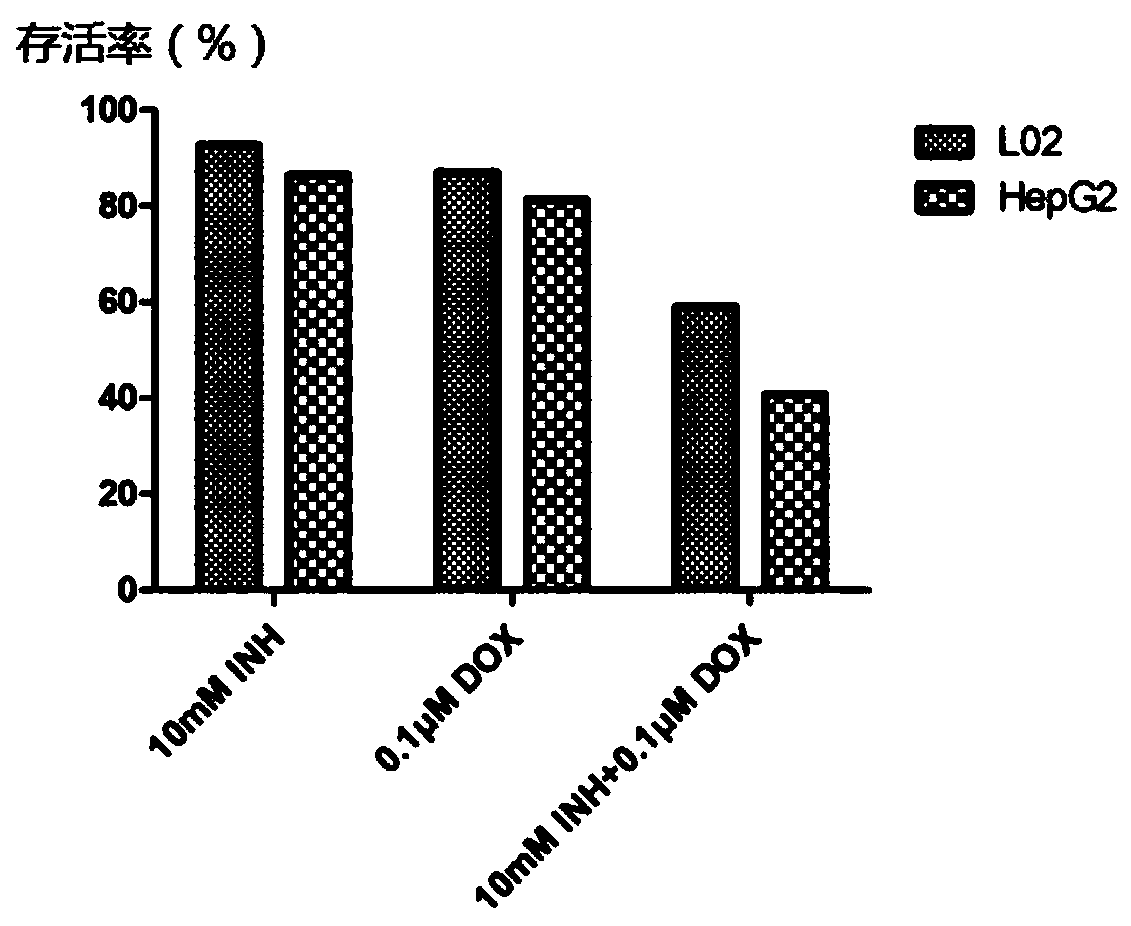
InactiveCN110075106AIncreased sensitivityReduce sensitivityOrganic active ingredientsAntineoplastic agentsIsoniazidSide effect
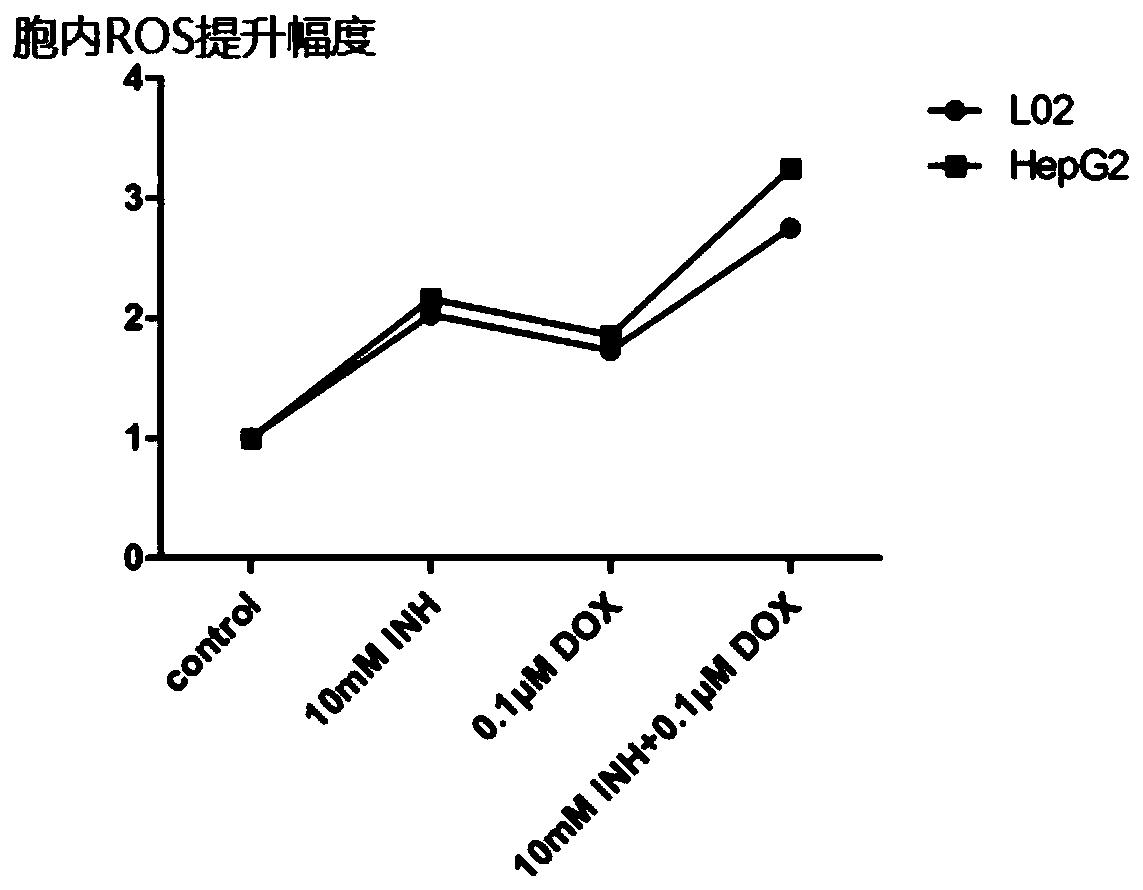
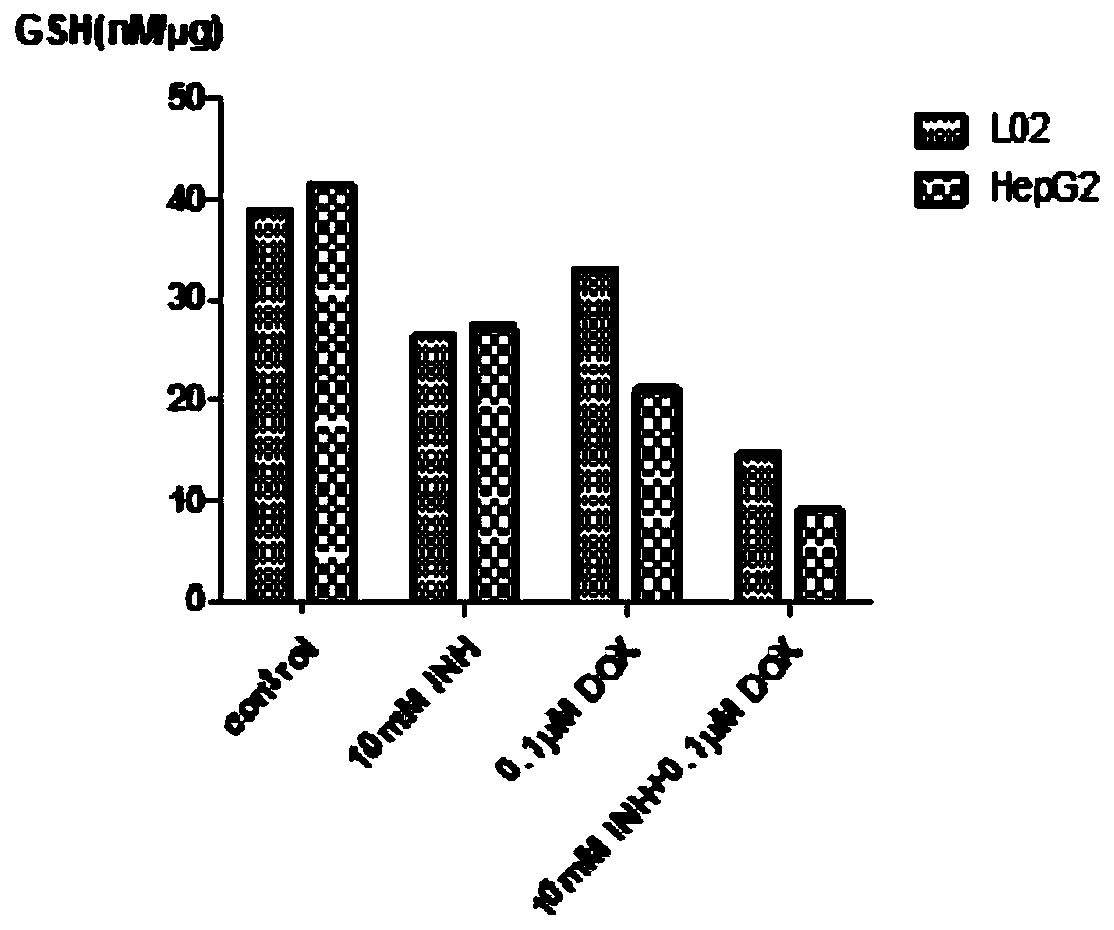
The invention discloses an anti-tumor drug and application of isoniazid in preparation of the anti-tumor drug, and belongs to the technical field of biomedicine. According to the application of the isoniazid in preparation of the anti-tumor drug, the anti-tumor drug is a compound preparation of a chemotherapy drug and an enhancer thereof, and the isoniazid is used as the enhancer of the chemotherapy drug. The compound preparation clears away a path through promoting oxidation of tumor cells and blocking cell active oxygen, so as to improve the level of the active oxygen in the tumor cells, thereby enhancing the ability of the chemotherapy drug to kill the tumor cells. According to the anti-tumor drug and the application of the isoniazid in preparation of the anti-tumor drug, the usage amount of the anti-tumor chemotherapy drug is effectively reduced under the same curative effect, the inhibitory effect on the tumor cells is stronger and selectivity is realized, that is, normal cells are less damaged, and the toxic and side effects of doxorubicin are effectively reduced; a safer and more effective chemotherapy drug administration method is provided; a relatively high popularizationvalue is realized.
Owner:HUAZHONG UNIV OF SCI & TECH
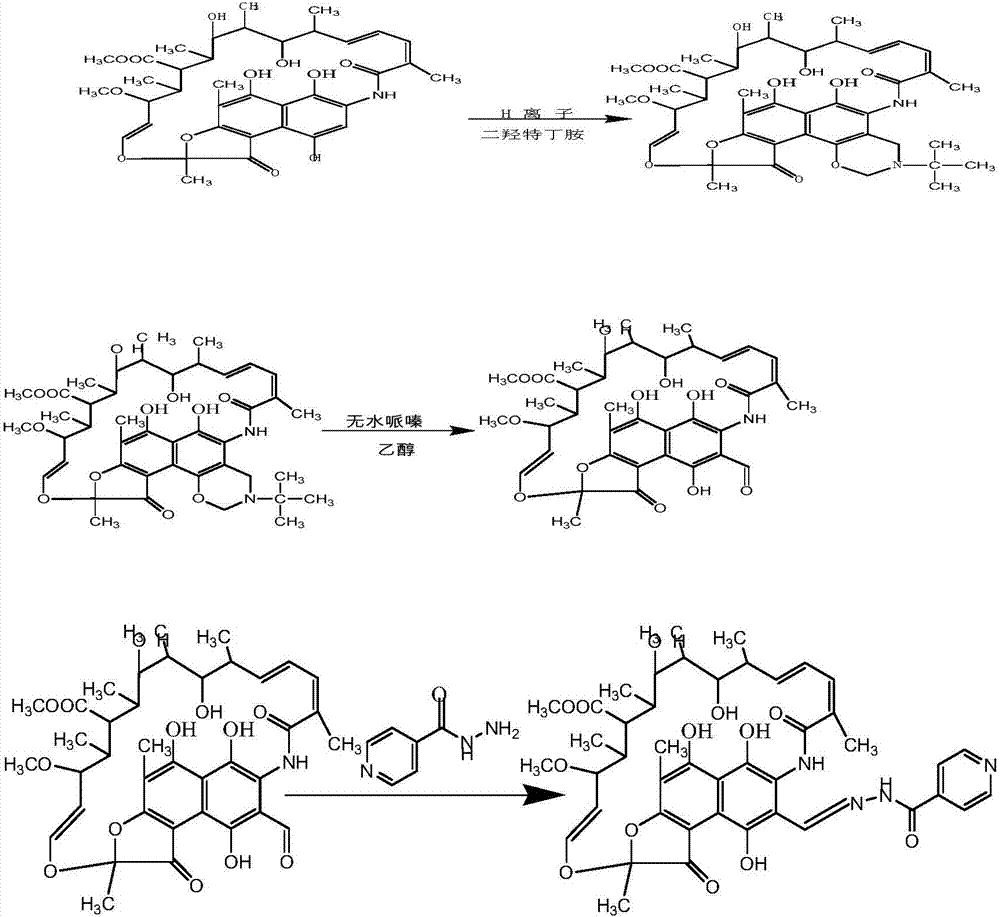
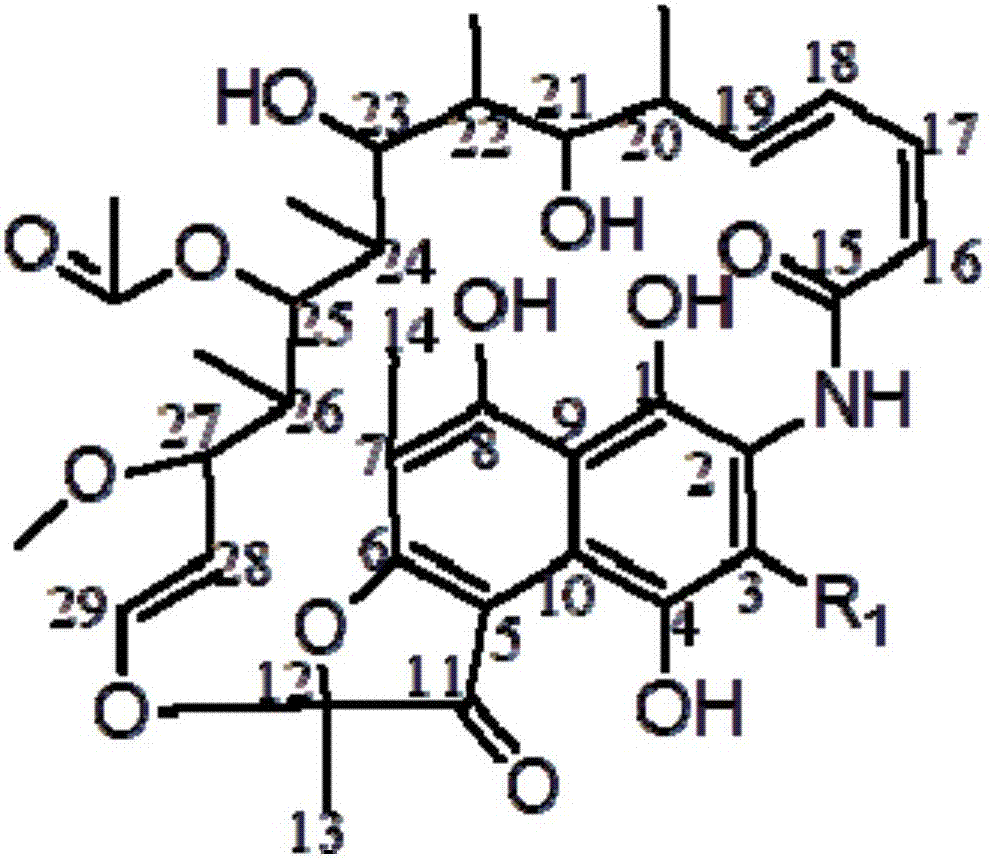
Rifamycin-isoniazide hybrid drug and preparation method thereof
InactiveCN107216343ANot easy to decomposeNo biological activityAntibacterial agentsOrganic active ingredientsIsoniazidMedicine
The invention relates to a rifamycin-isoniazide hybrid drug and a preparation method thereof. The rifamycin-isoniazide hybrid drug is prepared according to the preparation method which comprises the following steps: acidizing rifamycin sodium and then generating cyclisation with N,N-dihydroxymethyl tertbutylamine, thereby compounding oxazine rifamycin, and then performing condensation reaction on the oxazine rifamycin, piperazine and isoniazide, thereby acquiring the rifamycin-isoniazide hybrid drug. The rifamycin-isoniazide hybrid drug prepared according to the invention is stable in vitro, is difficult to degrade, is free from biological activity and can be dosed at multiple target points. The hybrid drug has higher biological activity, sterilizing effect equivalent to that of rifampicin and wide development prospect.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Process for preparing a catalyst for conversion of cyanopyridines to nicotinamides
InactiveUS7455827B2Increase conversionsHigh selectivityOrganic chemistryManganese oxides/hydroxidesIsoniazidAntituberculous drugs
Nicotinamides and isonicotinamides, used in the preparation of anti-TB drugs i.e. isoniazid and as an intermediate of vitamin B12 are prepared from cyanopyridines and nicotinamides. Catalysts useful for the preparation of nicotinamide and isonicotinamide.
Owner:COUNCIL OF SCI & IND RES
Pharmaceutical composition comprising rifamoin and isoniazid
InactiveCN1989966BImprove stabilityReduce degradationAntibacterial agentsOrganic active ingredientsIsoniazidMedicine
The invention involves a drug combinations including rifampin and isoniazid, the combination contains pH modifier of 0.1% ~ 10% (w / w). Said pH regulator is selected from weak acid- weak acid salt buffer system, weak alkali- weak alkali salt buffer system or weak acid salt- weak alkali salt buffer system. The drug combination in the invention can effectively reduce the product of polymer-3-formyl rifamycin SV isoniazone caused by rifampin and isoniazid degradation, and increased drug stability. The method of the invention has advantages as: operation is convenient, process is simple, equipmentneeds not improvment and the cost is low.
Owner:CHONGQING HUAPONT PHARMA
Compound isoniazid tablet
The invention relates to a compound isoniazid tablet used for treating tuberculosis, which comprises isoniazid and vitamin B6, wherein the isoniazid and the vitamin B6 are mixed together and prepared into tablets when preparing the compound isoniazid tablet. Patients with stomach upset for taking the isoniazid can directly take the compound isoniazid tablet to achieve the function of treatment without having any stomach upset, and the invention lays a good foundation for treating the tuberculosis because of being more accepted by patients for reduced total amount of medicines taken.
Owner:张徐红
Application of isoniazid to preparation of medicament for preventing or treating lung cancer and colorectal carcinoma
InactiveCN102389424BPromote acetylationOrganic active ingredientsAntineoplastic agentsDiseaseIsoniazid
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI
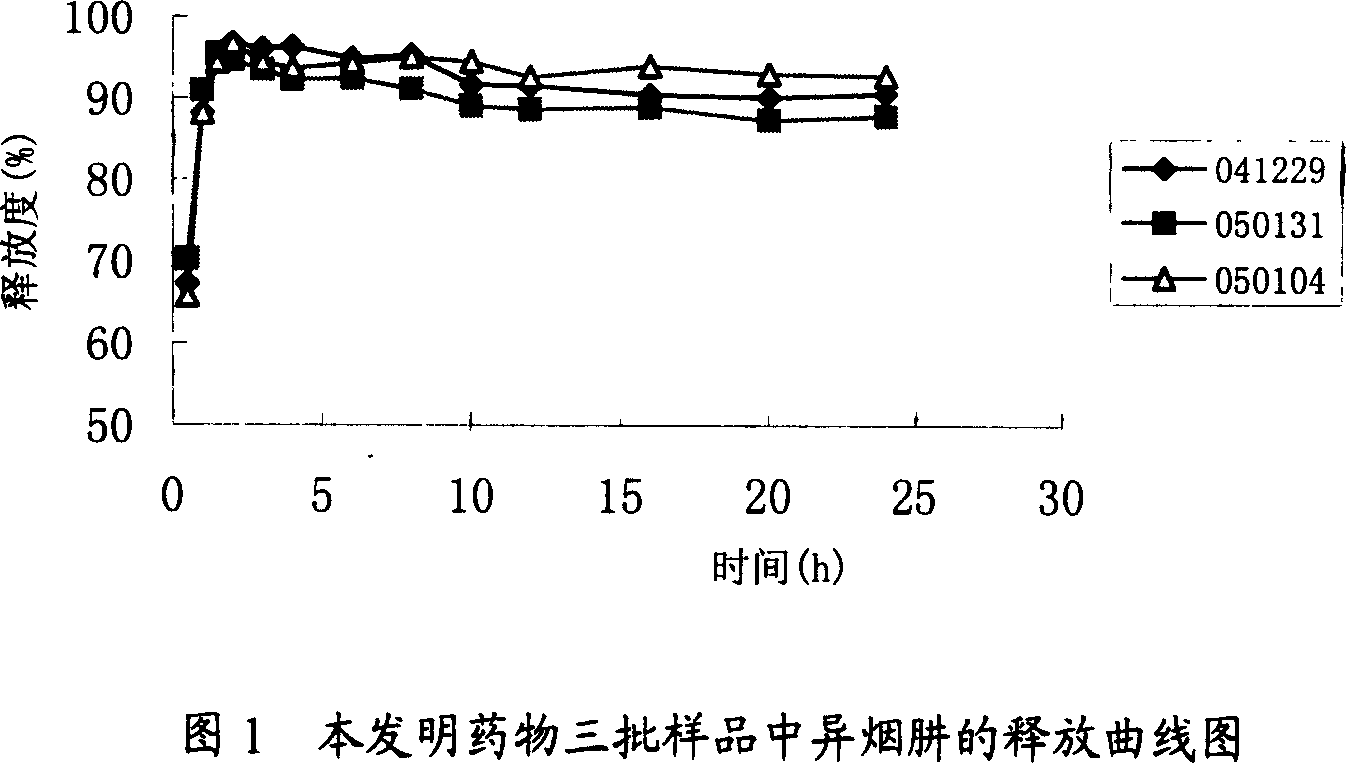
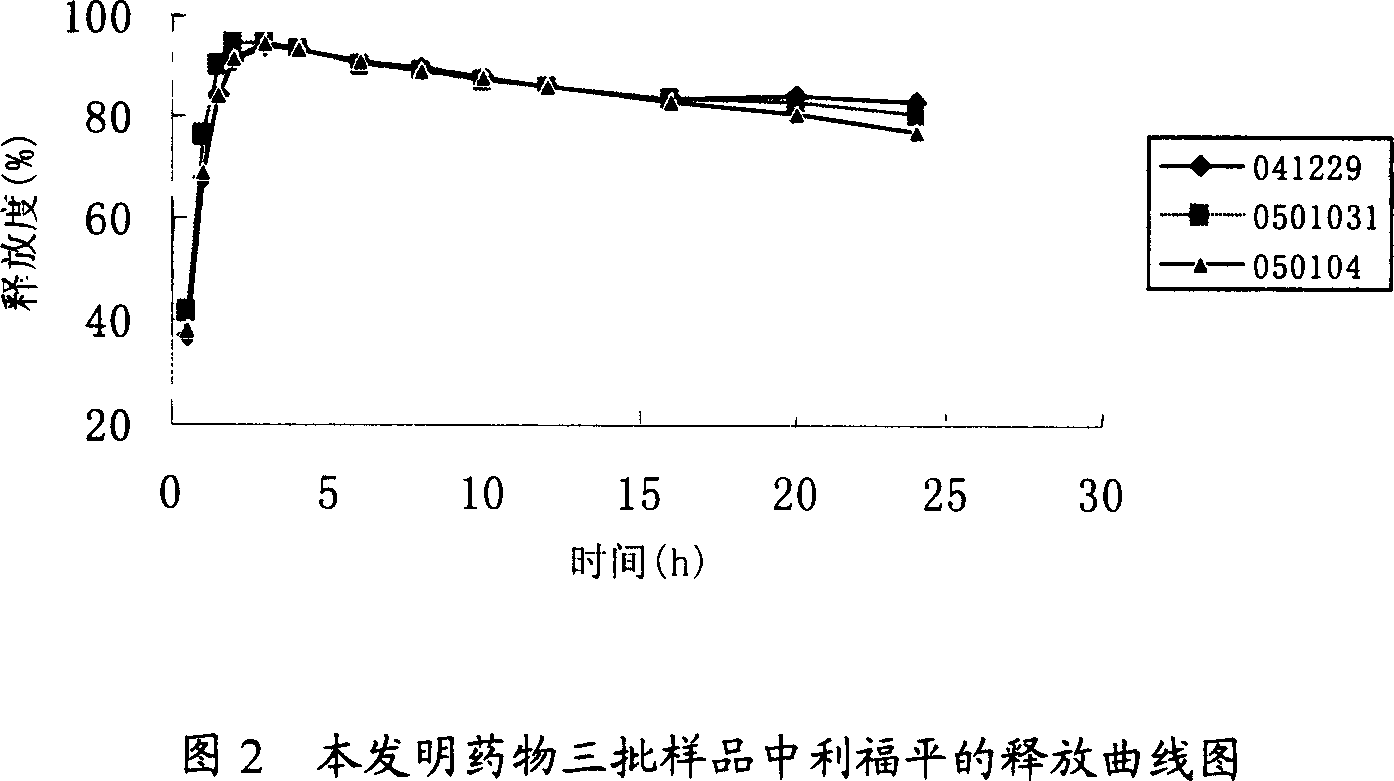
Quick-slow compound medicine of anti-tuberculosis
InactiveCN1954816AAchieve fast-sustained release effectShort onset timeAntibacterial agentsOrganic active ingredientsDiseaseIsoniazid
A both fast-release and slow-release compound medicine for preventing and treating tuberculosis (TB) is prepared from rifampin, isoniazid in the ratio of 2:1, and the pharmacologically acceptable auxiliary consisting of skeleton material and disintegrant. Its preparing process is also disclosed.
Owner:江西万基药物研究院药业有限责任公司
Compound preparation containing rifampicin and isoniazid and its preparing method
The invention discloses a compound preparation containing rifampicin isoniazid and a preparation method thereof. The compound preparation containing rifampicin and isoniazid of the present invention is a double-layered chip containing rifampicin in a chip and an outer layer containing isoniazid and pyrazinamide. The preparation of the invention not only greatly reduces the polymer (3-methylrifamycin SV isoniazone) produced by rifampicin and isoniazid, but also statistical analysis and bioequivalence verification show that isoniazid, The above pharmacokinetic parameters of rifampicin and pyrazinamide were not significantly different between the two tablets (p>0.05), and the two tablets were bioequivalent.
Owner:SHANGHAI SINE PHARMA LAB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com