Rifamycin-isoniazide hybrid drug and preparation method thereof
A technology of rifamycin and rifamycin sodium, applied in antibacterial drugs, organic chemistry and other directions, can solve the problems of complex post-processing, difficult production methods, etc., achieve in vitro stability, improve pharmaceutical and pharmacokinetic properties, Enhanced therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
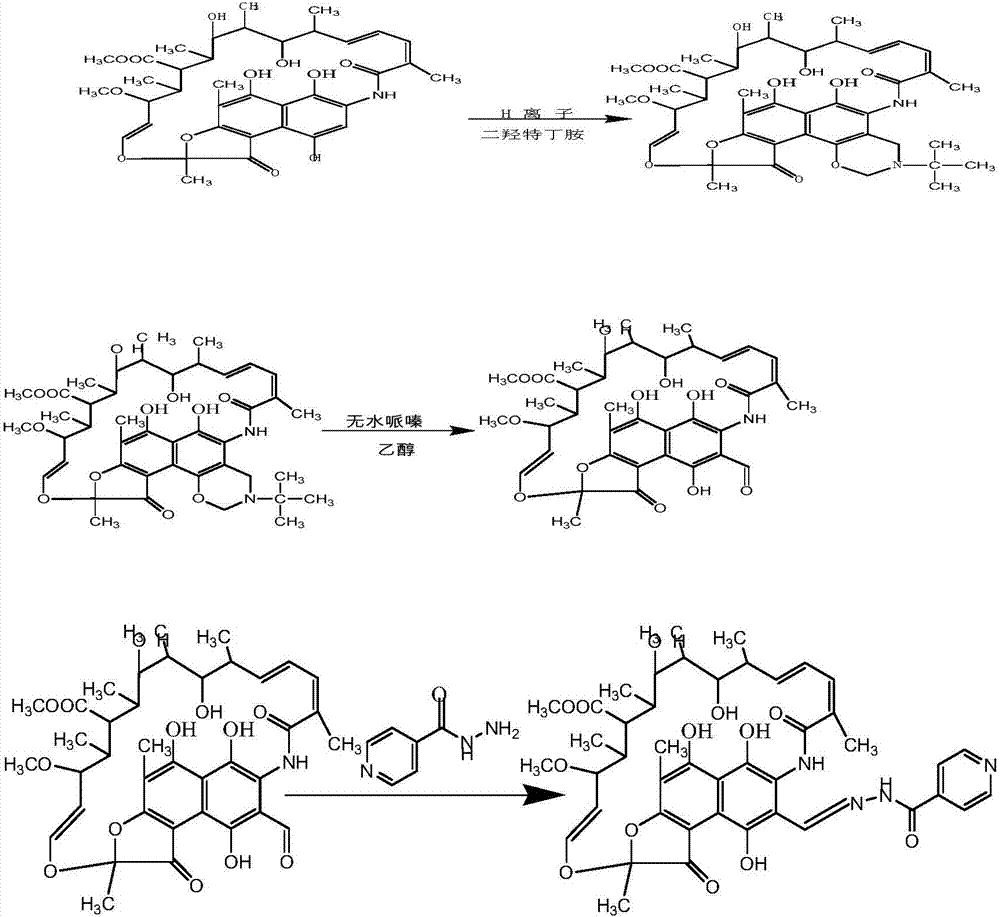
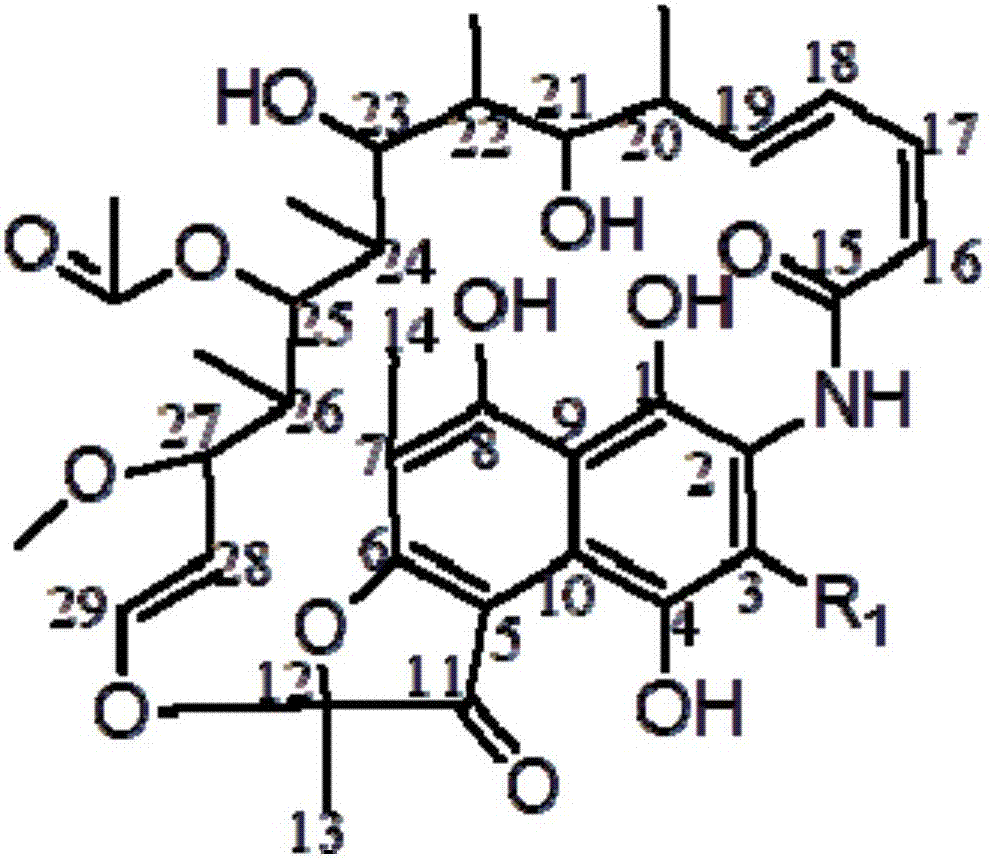
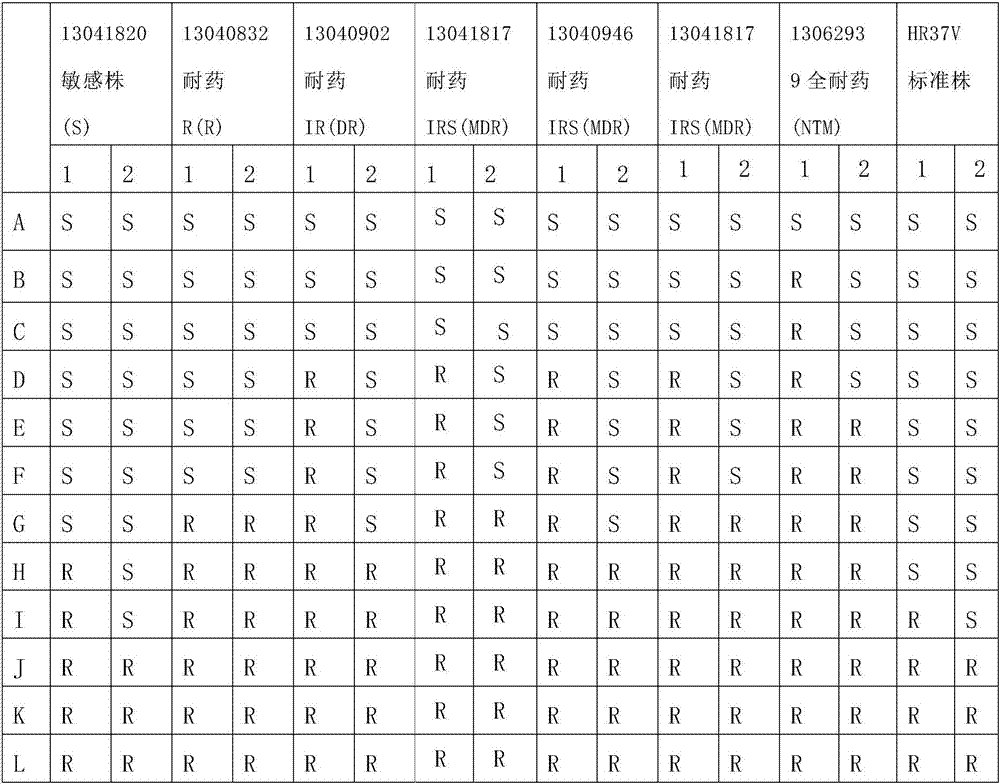
[0027] Synthesis of oxazine rifamycin: put 1.00 g (0.0014 mol) of rifamycin sodium in a 100 mL three-necked bottle, add 20 mL of N,N-dimethylformamide to dissolve it, put it into a stirring magnet, and put it into a stirring magnet at room temperature. Stir for 30 minutes, after fully dissolving, add 0.072g concentrated sulfuric acid (95%) (0.0007mol) for acidification, stir at room temperature for 30 minutes, the reaction solution changes from dark blue to dark red, then heat up to 35-50 ° C, slowly Slowly add N,N-dimethylol tert-butylamine 0.352g (0.0026mol) dropwise, after the completion of dropping, the reaction solution changes from dark red to dark blue, and continue to keep the reaction for 2 hours (use thin-layer chromatography silica gel plate to track the reaction), After the reaction was completed, it was cooled to room temperature, 200 mL of an aqueous solution with 2 drops of glacial acetic acid added dropwise to the reaction solution, stirred intermittently, a lar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com