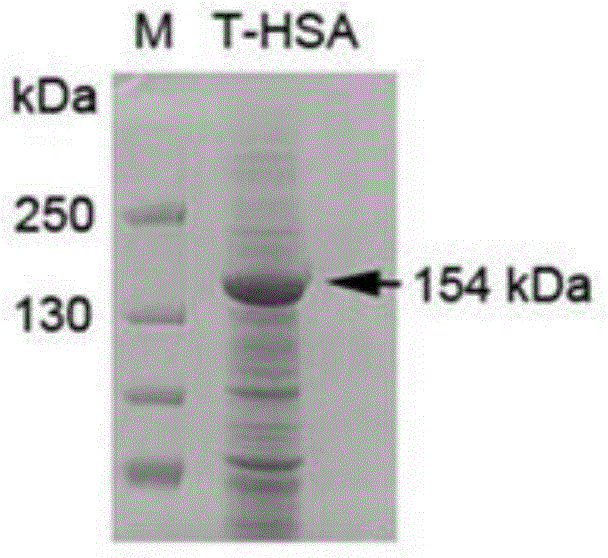
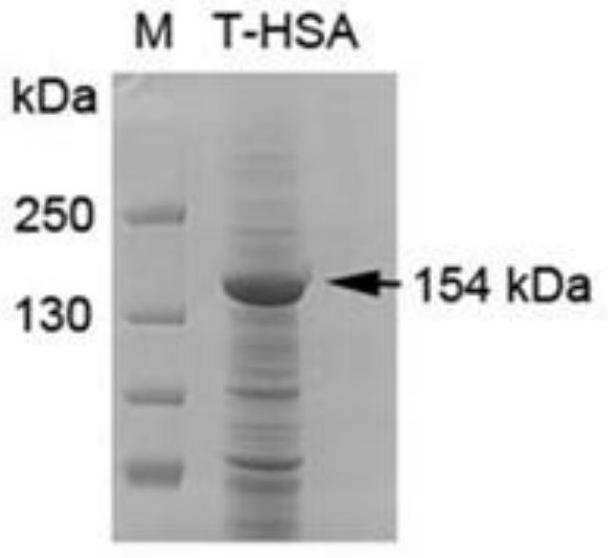
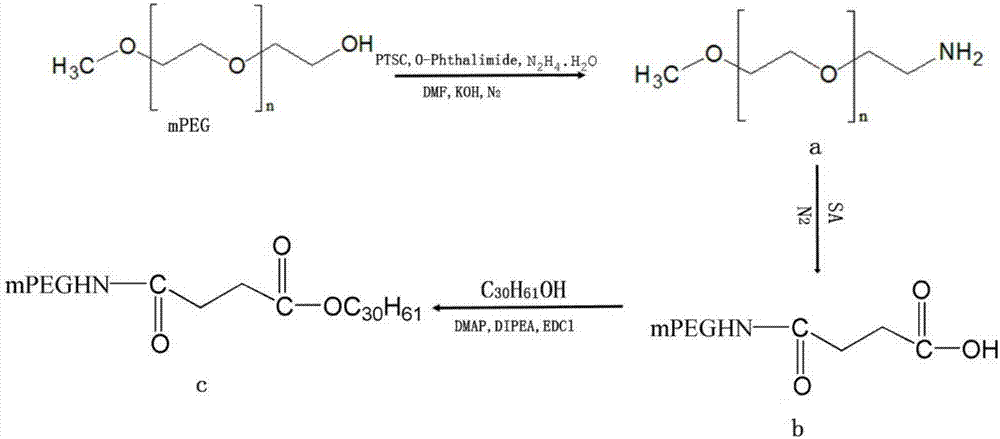
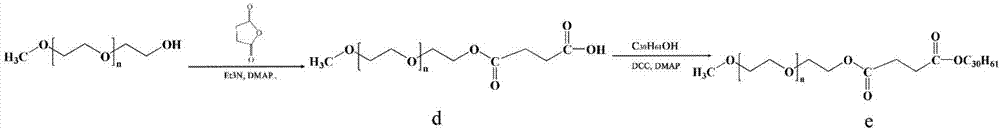
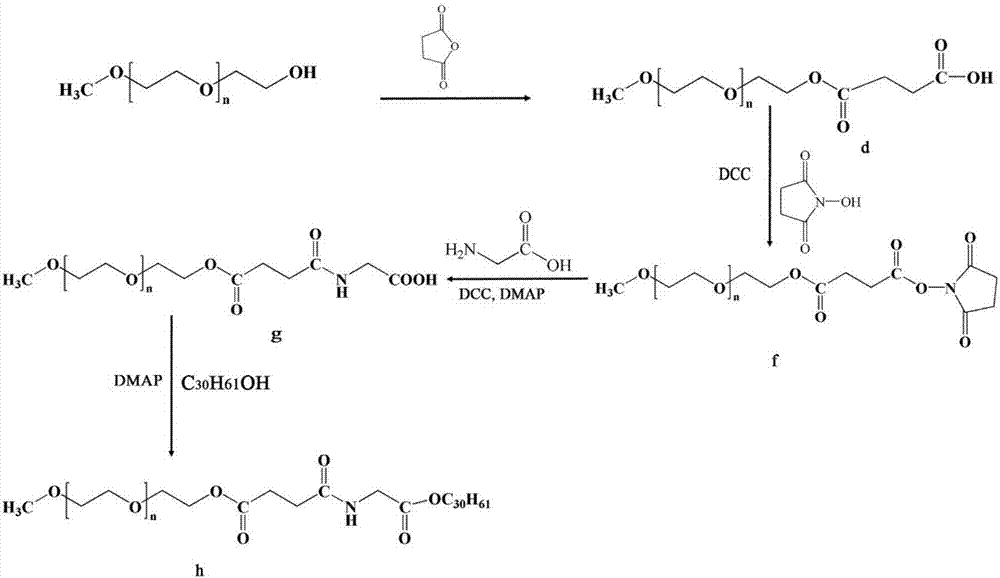
Patents
Literature
33results about How to "No biological activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Traditional Chinese medicine placebo, as well as preparation process and evaluation method thereof
InactiveCN103110962AAchieve objective quantificationHigh similarityIn-vivo testing preparationsTesting medicinal preparationsHuman bodyExperimental research
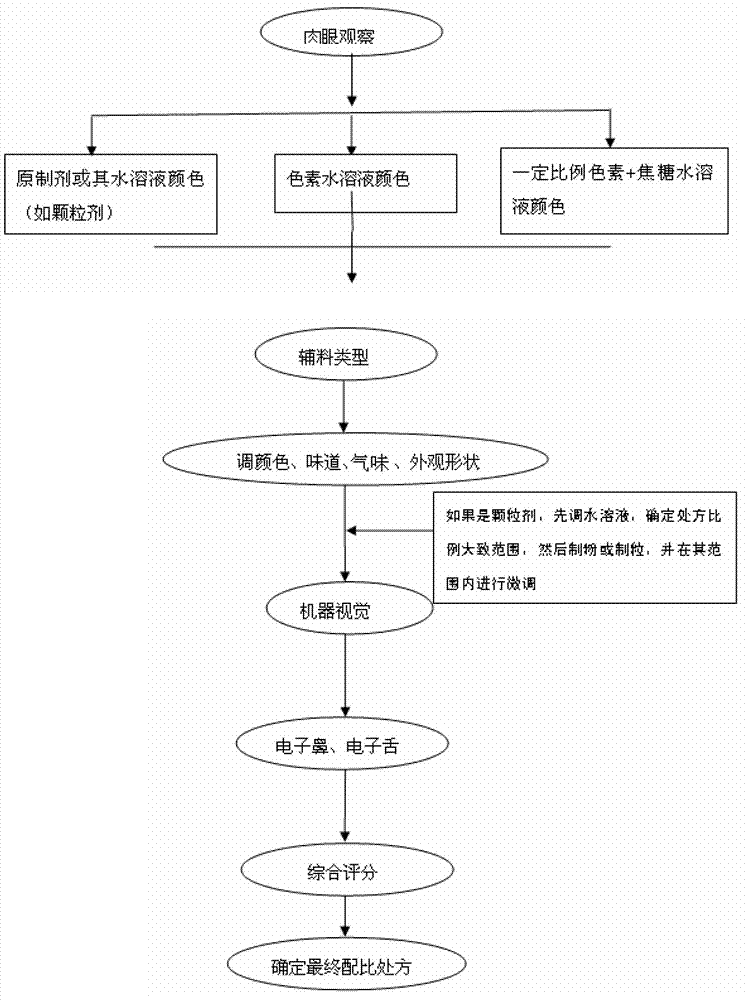
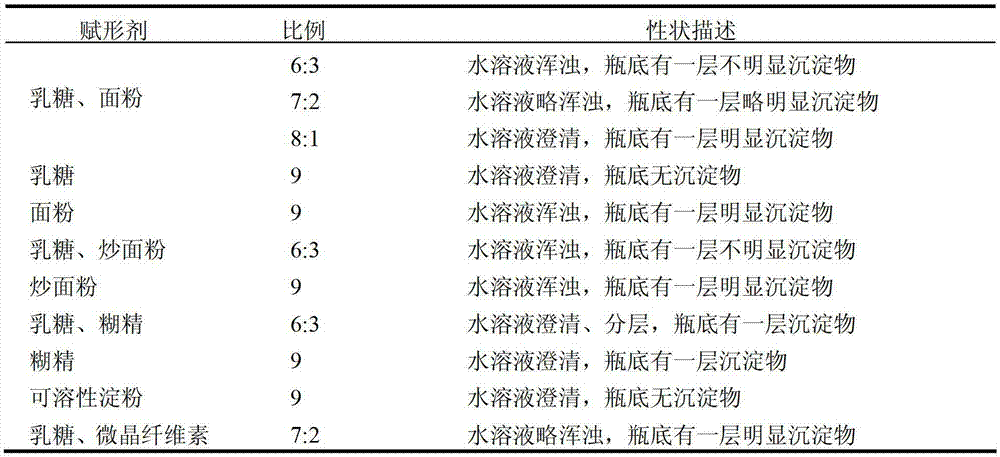
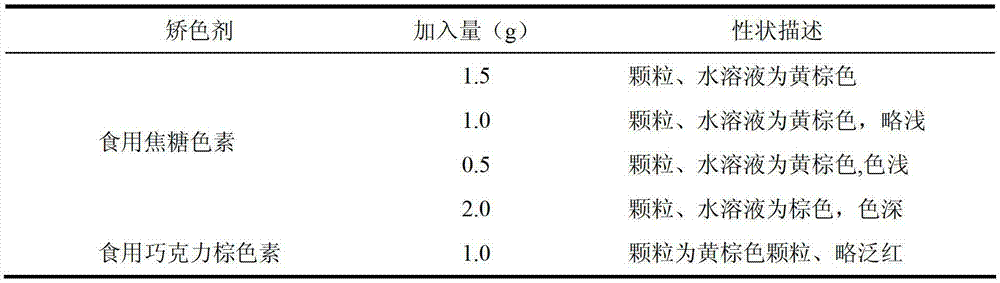
The invention provides a traditional Chinese medicine placebo preparation process. The invention also provides a traditional Chinese medicine placebo and an evaluation method of the placebo. According to the invention, a small amount of an original preparation (granule, mixture, tablet, capsule, pill, external preparation, and the like) is taken and prepared into a traditional Chinese medicine placebo, the similarity of properties of the placebo and the original preparation is high, and the placebo has no physiological activity, therefore, the placebo is more suitable for clinical and experimental research; meanwhile, the invention also provides a multi-sensing information fusion based intelligent sensory evaluation method for the traditional Chinese medicine placebo, which is implemented through simulating three sensory organs, namely, eye, nose and tongue of the human body respectively by using a machine vision (visual sensor), an electronic nose (smell sensor) and an electronic tongue (taste sensor), so that the objective quantification of properties evaluation of the traditional Chinese medicine placebo is realized, and the subjective deviation caused by the experiential sensory evaluation of people is avoided.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
Nasal in situ gel delivery system, preparation and applications thereof
ActiveCN104997724AImprove performanceQuality is easy to controlPeptide/protein ingredientsAerosol deliveryNasal cavityGel preparation
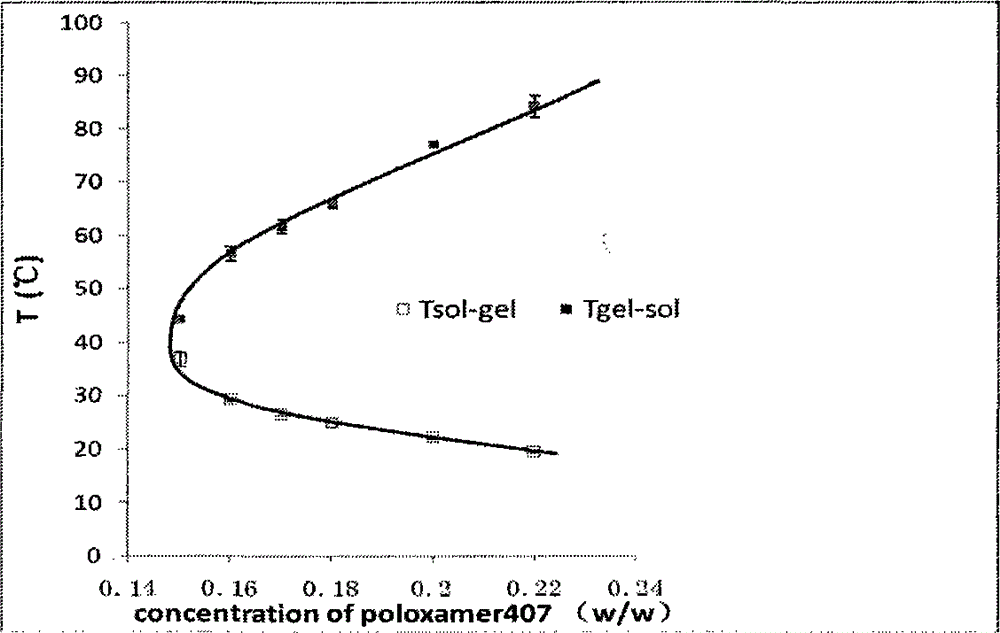
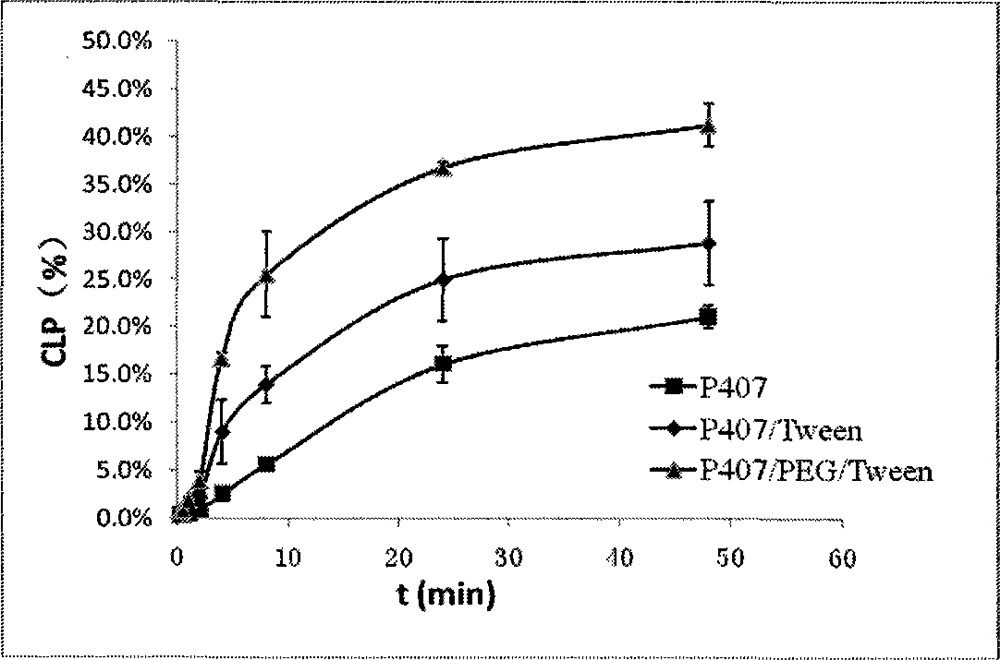
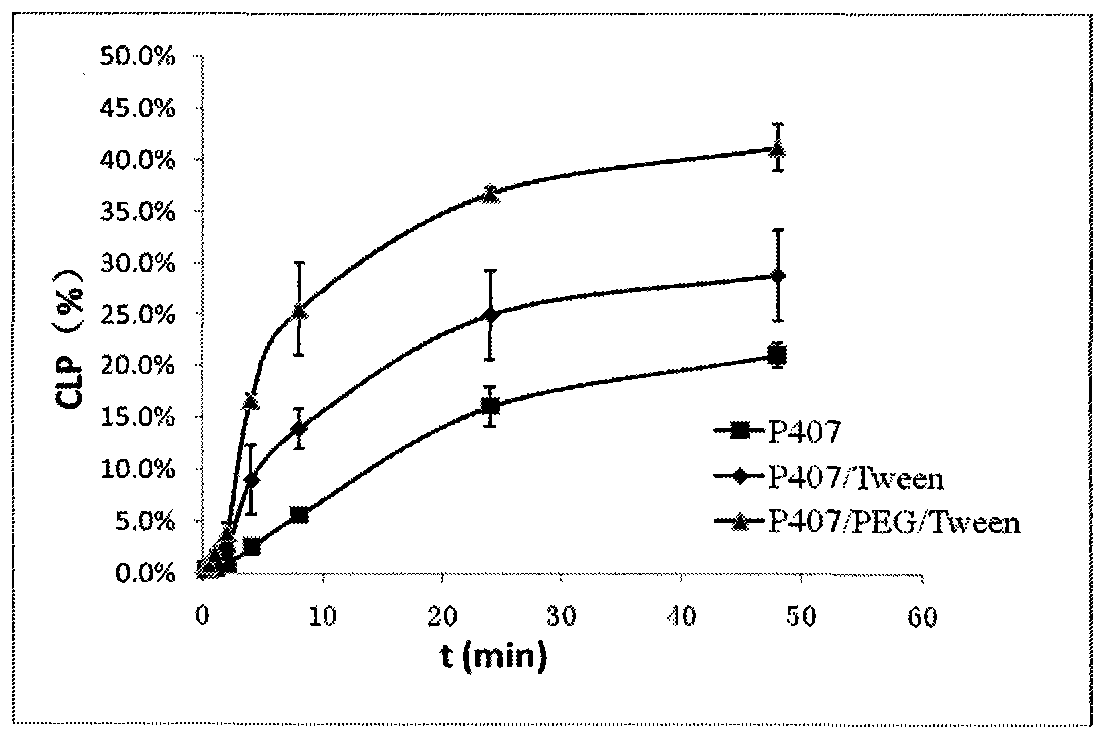
The present invention belongs to the field of pharmaceutical formulations in medicine, and discloses a nasal in situ gel delivery system, preparation and applications thereof, wherein particularly the system has great advantages in the fields of central nervous system drugs and protein peptide drugs. According to the present invention, the process for preparing the NGF nasal in situ gel preparation is a two-bottle method, wherein one bottle is an in situ gel solution, wherein poloxamer is preferably poloxamer P407 and the content is 16-16.8%, the polyethylene glycol is preferably PEG10000 and the content is 0.2-0.6%, the polysorbate is preferably Tween80 and the content is 1-3%, the preservative is preferably benzalkonium chloride and the content is 0.001-0.002%, the other bottle is NGF lyophilized powder preparation, the NGF content is 0.05-0.1%, the albumin content is 0.1-0.2%, the mannitol content is 5%, and before the use, the gel solution is added to the NGF lyophilized powder and uniform mixing is performed so as to obtain the system. According to the present invention, the disclosed drug delivery system can be used in any nasal administration dosage forms, preferably nasal drops and sprays; and with the nasal in situ gel delivery system, the difficult problem that the protein peptide drugs are used for the central nervous system is solved, and especially the application range for the nerve growth factor is expanded.
Owner:PEKING UNIV +1
Polyurethane potting glue for household water purification hollow fiber membranes, and preparation method thereof
PendingCN109111893APromote infiltrationControl creepNon-macromolecular adhesive additivesPolyureas/polyurethane adhesivesPolyolCore product
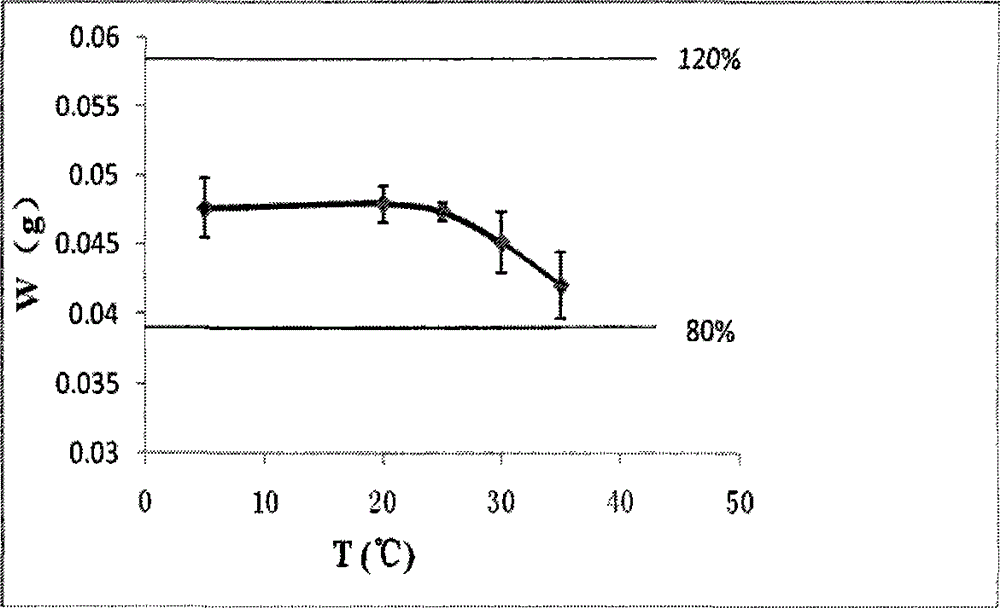
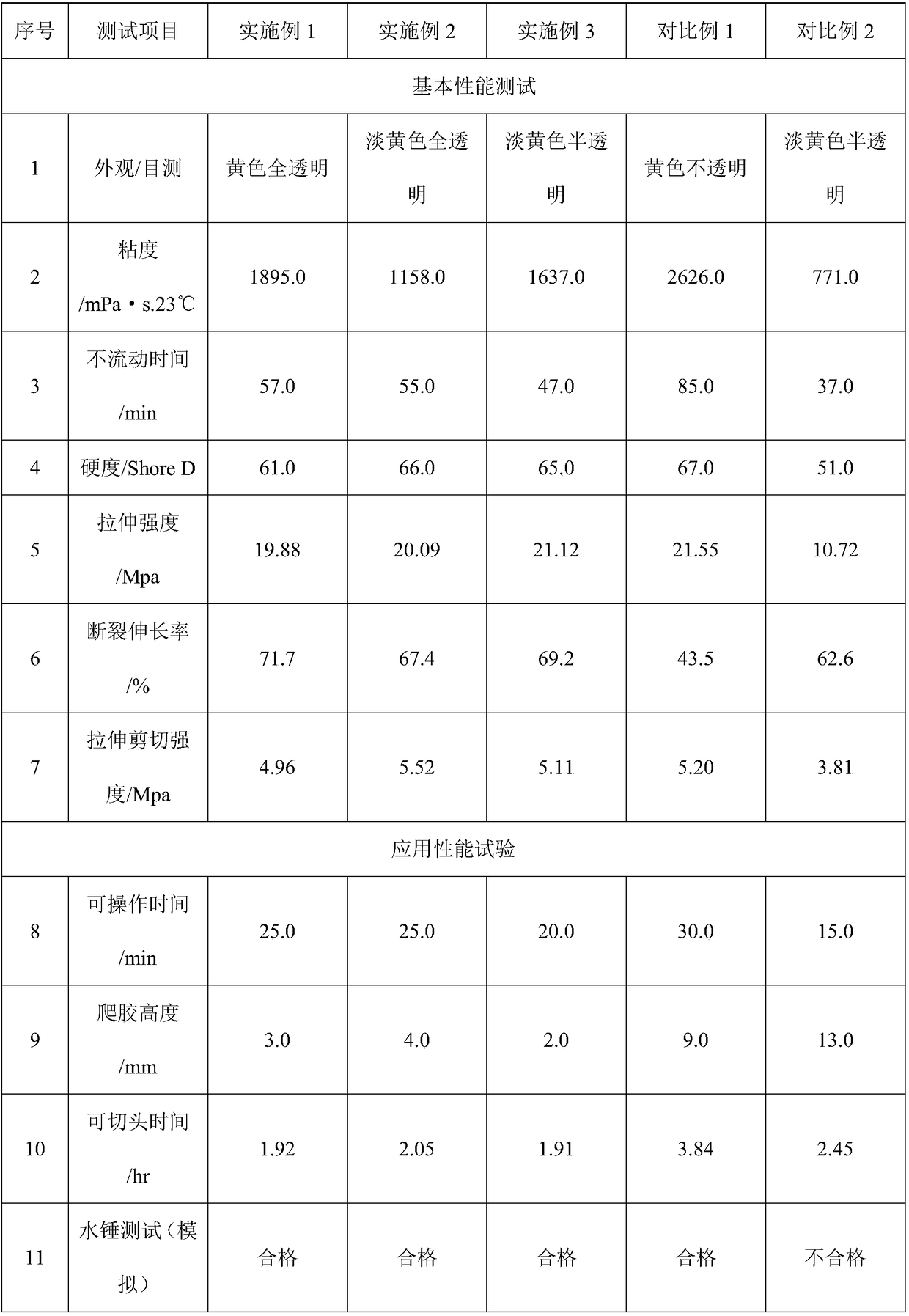
The invention discloses a polyurethane potting glue for household water purification hollow fiber membranes, wherein the polyurethane potting glue comprises two components A and B, wherein the component A is the mixture of a variety of polyols, the component B is one or a variety of polyol modified isocyanate prepolymers, and during the use, the component A and the component B are mixed accordingto a weight ratio of 100:50-100. According to the present invention, the polyurethane potting glue has the following advantages that the mixing viscosity is moderate, the polyurethane potting glue easily infiltrates most of hollow fiber membrane filaments, and the glue extending is not easily generated; the hardening and the strength of the glue are high after the curing, the adhesion to the ABS shell is strong, and the water hammer simulation test results show that the problems of cracking, separation from the shell and the like are not generated; the curing rate is moderate, and the cured filtering core end head can be cut within 2 h in the case of the operable time of 20-25 min; and the problems caused by the use on the water filtering core product can be solved, and the quality of thewater filtering core product and the production efficiency can be improved.
Owner:上海汉司实业有限公司
Process for producing freeze-dried lactic acid bacteria powder
InactiveCN104673690APromote dissolutionNo biological activityBacteriaMicroorganism based processesBacilliFreeze dry
The invention belongs to the technical field of microbes or enzymes and compositions thereof and specifically relates to a process for producing freeze-dried lactic acid bacteria powder. By adopting the process for producing the freeze-dried lactic acid bacteria powder, the freeze-dried powder can be applied to the food industry more conveniently. The process for producing the freeze-dried lactic acid bacteria powder comprises the following steps: 1, preparing raw materials; 2, activating lactobacillus; 3, collecting strains; and 4, freeze drying bacterial sludge.
Owner:杨树才
Organic salt of pyritinol and its prepn process
InactiveCN101066266ASolve solubilityResolve irritationOrganic active ingredientsNervous disorderHydroxybutyric acidSenile dementia
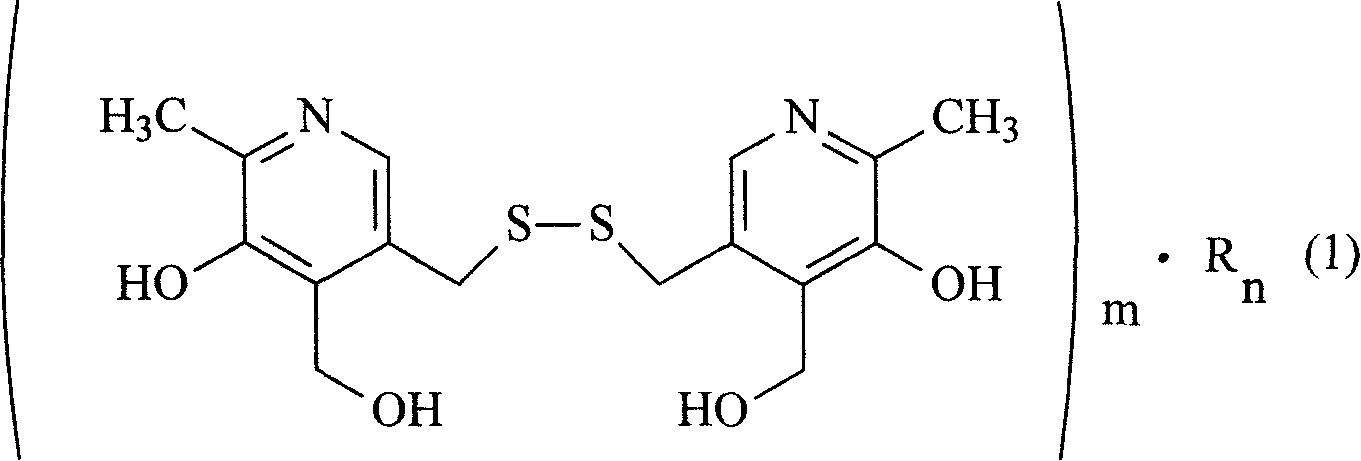
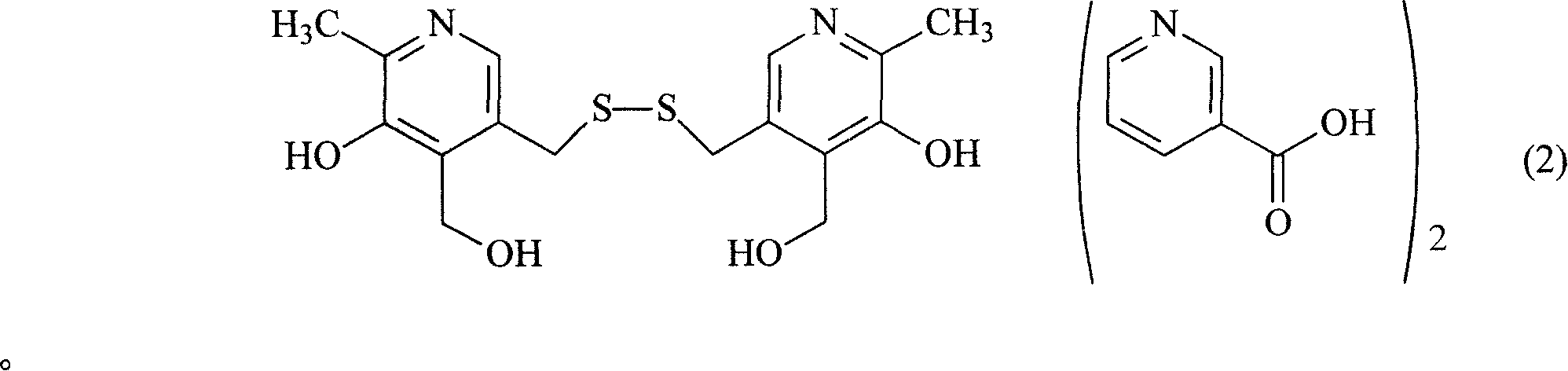
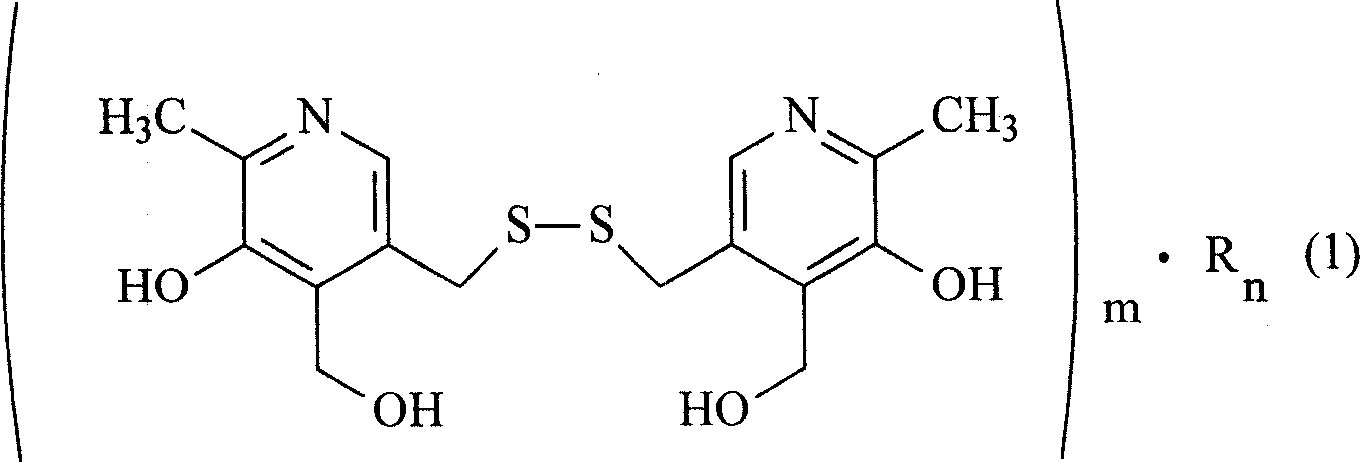
The present invention relates to organic salt of pyritinol as shown for treating cerebral concussion, brain trauma, encephalitis, meningitis sequelae and senile dementia. The organic salt exists in hydrate and is named as 3, 3-(dithio methylene) bis(5-hydroxy-6-methyl-pyridyl methane) organic salt chemically. The organic salt is salt of nicotinic acid, lactobionic acid, malic acid, etc. Experiments show that the organic acid and pyritinol have synergistic effect of protecting cardiac muscle, and the nicotinate of pyritinol is especially hopeful in developing pyritinol medicine.
Owner:GUANGDONG ZHONGKE DRUG R&D
Bismuth potassium citrate buccal adhesive tablet and preparation method thereof
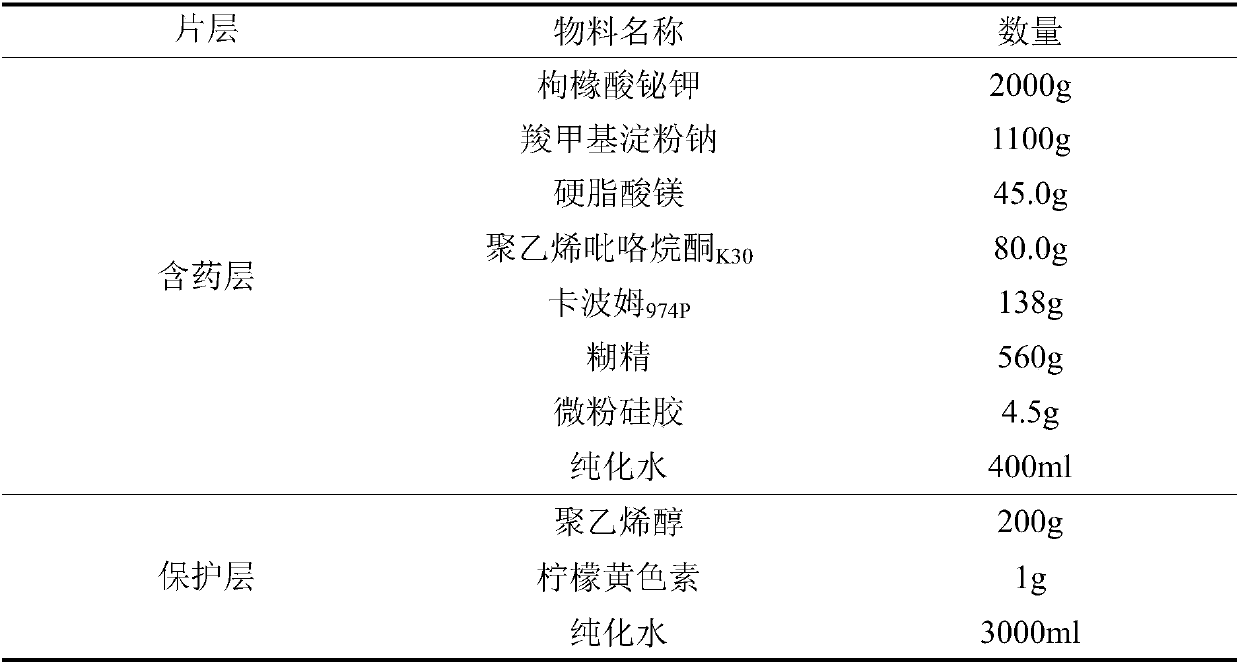
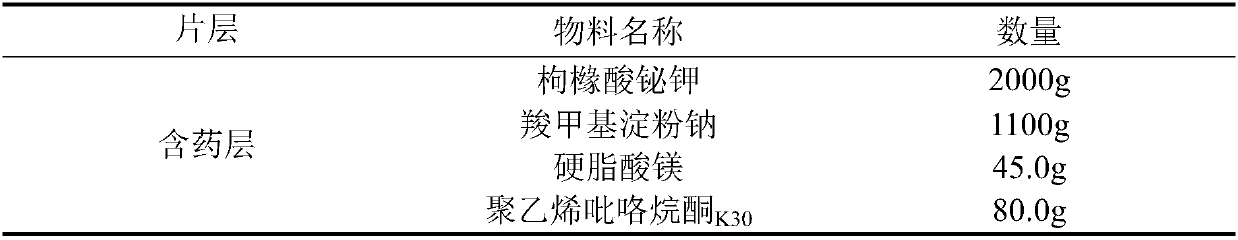
ActiveCN107582536ASmall doseReactivity NoneOrganic active ingredientsDigestive systemAdhesivePolyvinyl alcohol
The invention relates to a bismuth potassium citrate buccal adhesive tablet and a preparation method thereof, and belongs to the technical field of medicinal preparation. For every 100,000 bismuth potassium citrate buccal adhesive tablets, the drug containing layer is prepared from the following components in parts by weight: 1000 to 2500 parts of bismuth potassium citrate, 1000 to 1100 parts of disintegrating agent, 120 to 152 parts of film forming agent, 60 to 100 parts of adhesive, 4.5 to 6.5 parts of flow aid, 25 to 45 parts of lubricant, and 440 to 560 parts of diluent, and the protectivelayer is prepared from the following components in parts by weight: 150 to 250 parts of polyvinyl alcohol and 0.8 to 1.5 parts of lemon yellow pigment. The provided bismuth potassium citrate buccal adhesive tablet enriches the kinds of conventional drugs for treating dental ulcer and is suitable for infants and people with allergic physique.
Owner:SHIJIAZHUANG UNIVERSITY
Fat-soluble drug submicron capsule glucose injection and preparation method thereof
ActiveCN107693488AEvenly wrappedUniform particle sizeEmulsion deliveryMacromolecular non-active ingredientsPolyvinyl alcoholPolyethylene glycol
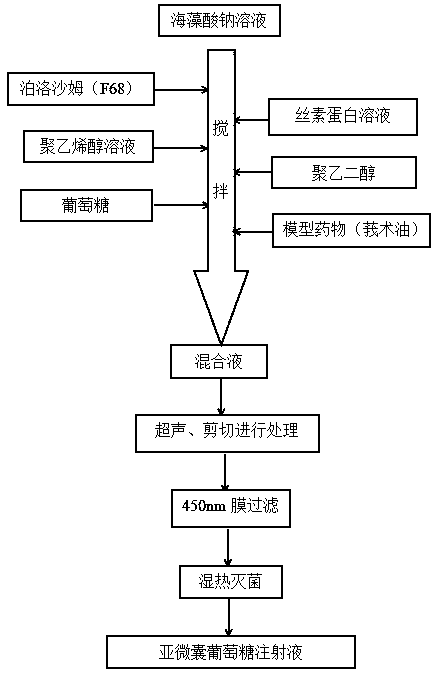
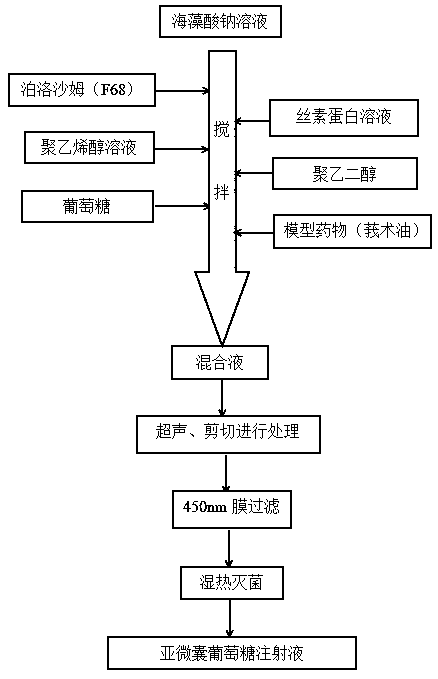
The invention discloses a fat-soluble drug submicron capsule glucose injection and a preparation method thereof. The invention is characterized in that the capsule material comprises the following components in parts by weight: 15-45 parts of sodium alginate, 2.5-9 parts of poloxamer 188, 1.5-5 parts of silk fibroin, 0.5-3 parts of polyvinyl alcohol and 0.2-3 parts of polyethylene glycol. The preparation method comprises the following steps of: sequentially adding the poloxamer, a silk fibroin aqueous solution, a polyvinyl alcohol aqueous solution, the polyethylene glycol and glucose into a sodium alginate aqueous solution at intervals under stirring, and finally dropwise adding a corresponding fat-soluble drug; adopting an ultrasonic and shearing treatment mode; and finally, carrying outfiltration with a 450nm membrane and damp-heat sterilization to obtain the submicron capsule glucose injection. The preparation process of the submicron capsule glucose injection provided by the invention is simple and economic, the particle size of submicron capsules is controllable and uniform, and the biological safety is high.
Owner:CHONGQING UNIV OF TECH
Preparation and application in pharmacy field of silica medical microsphere
InactiveCN106177968AHigh hardnessReduce breakage rateOrganic active ingredientsDispersion deliveryControlled releaseMicrosphere
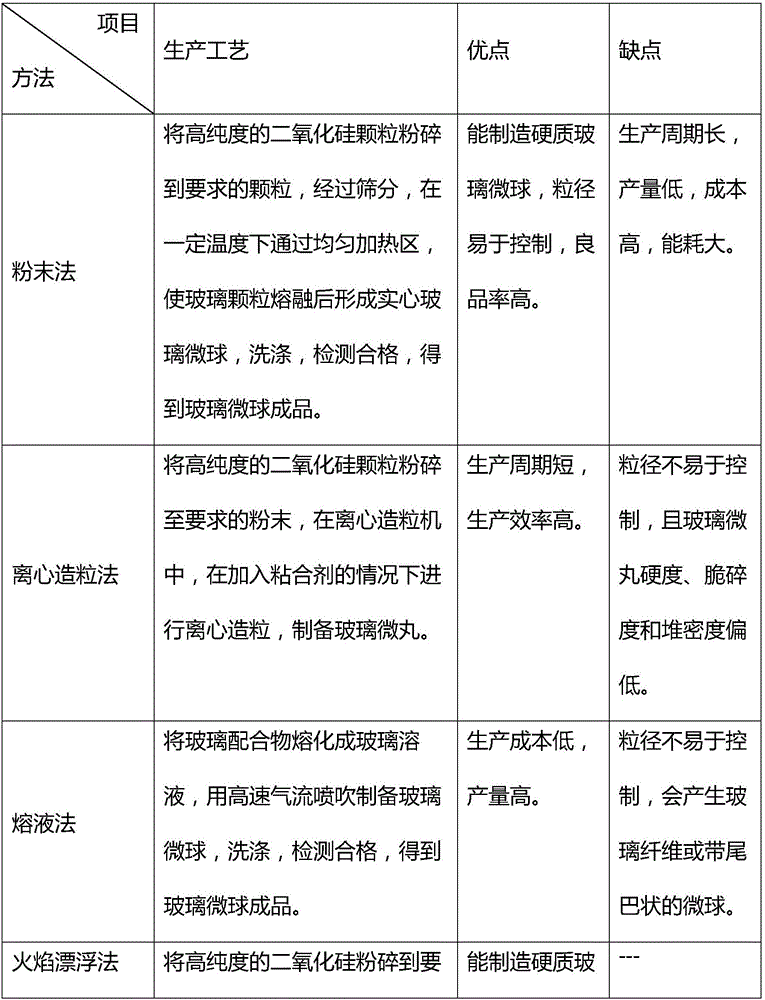
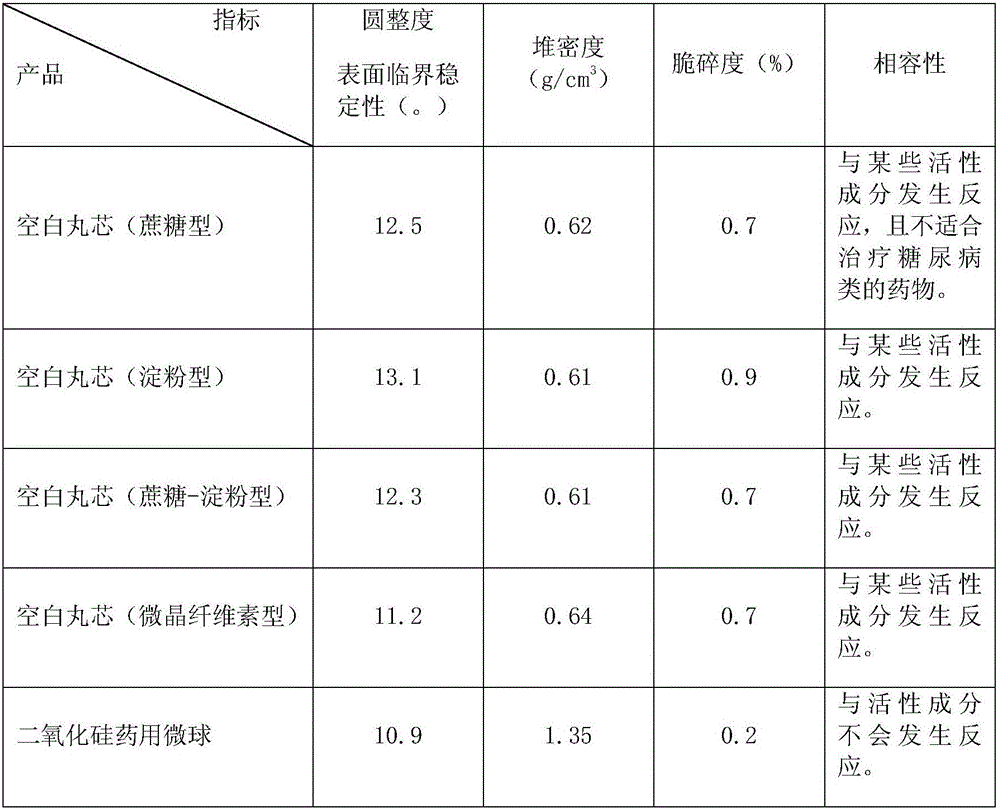
A silica microsphere is prepared by melting or grinding high purity silica; the silica microsphere is purified then to reach the requirements of pharmaceutical adjuvant and has good roughness and mechanical strength; the particle size is in a range of 40 to 250 [mu]m, and the bulk density is in a range of 1.2 to 1.6 g / cm3. The silica medical microsphere can be applied to the pharmacy field, is taken as a blank pill core for wuster coating, can be used to coat particles with a size smaller than 150 [mu]m, can cover the odor, and is capable of achieving the sustained-release and control-release functions. Based on the proper particle size distribution, high bulk density and mechanical strength, and good roughness, the yield of coated particles with a size smaller than 150 [mu]m is largely increased, and the coating yield is more than 90%.
Owner:ZHONGSHUAI PHARMA SCI & TECH CO LTD
Molecular imprinting sensor for detecting antibiotic pefloxacin and preparation method thereof
InactiveCN102967638AEasy to prepareStrong specificityMaterial electrochemical variablesBinding siteTetrabutylammonium perchlorate
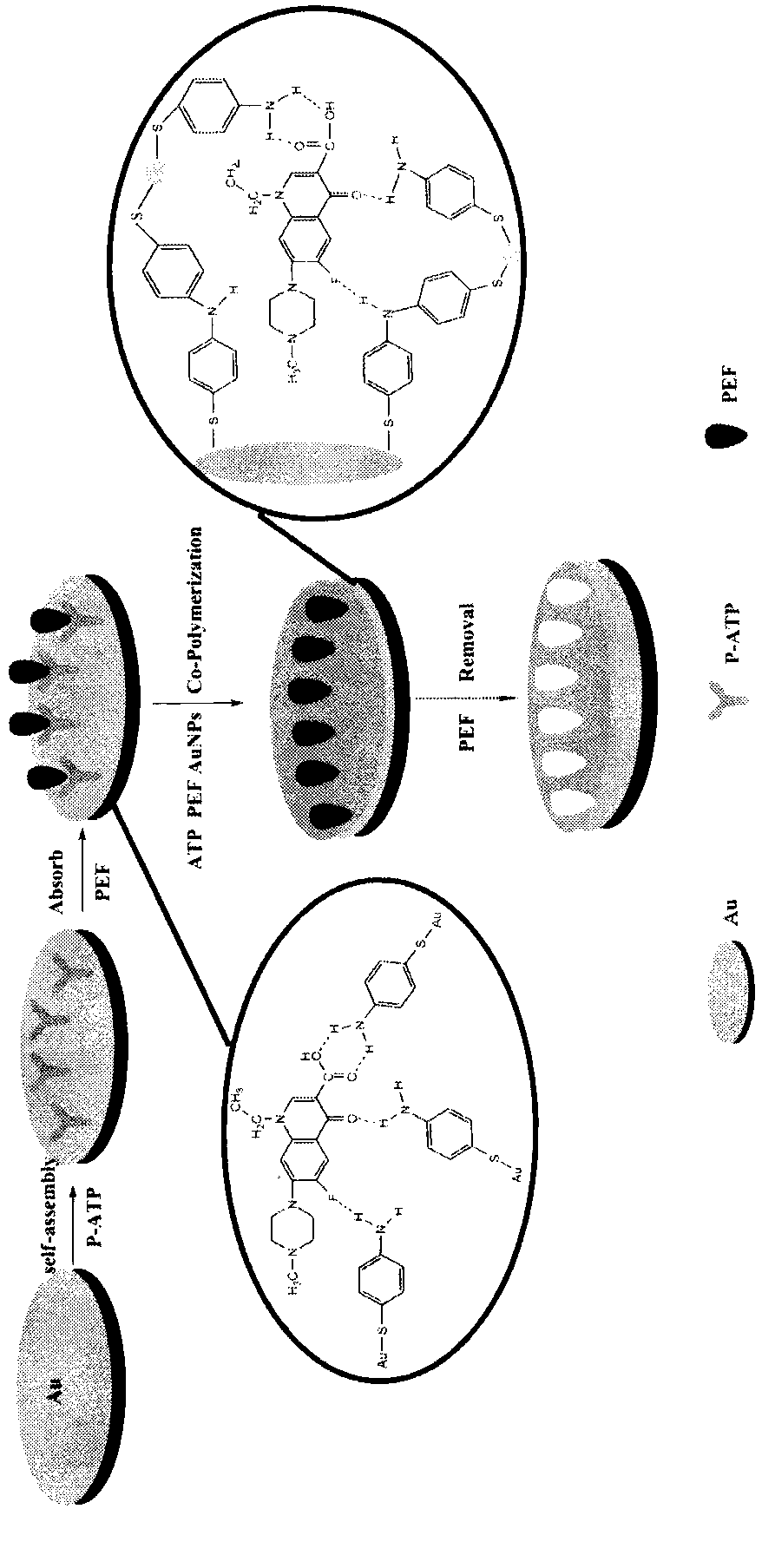
The invention relates to a molecular imprinting sensor for detecting antibiotic pefloxacin and a preparation method thereof. A molecular imprinting electrochemical sensor which can specifically identify the pefloxacin is prepared based on an electroplating process. According to the molecular imprinting electrochemical sensor, a gold electrode serves as a working electrode and is modified by p-aminothiophenol, so that binding sites of the pefloxacin are increased, mixed solution of chloroauric acid, p-aminothiophenol and tetrabutylammonium perchlorate serves as electroplating solution, a molecular imprinting identification film is prepared through electroplating, and the template pefloxacin in the molecular imprinting identification film is removed by employing an electrolytic dissociation method of hydrochloric acid. The prepared molecular imprinting electrochemical sensor is stable in structure, simple in preparation method, high in sensitivity and high in specificity and can be repeatedly used. The trace detection of pefloxacin in milk is realized, the detection sensitivity is improved, and the method is low in cost and has a good application prospect.
Owner:JIANGNAN UNIV
Levofloxacin hydrochloride capsule and preparation method thereof
InactiveCN105687157ASimple preparation processSuitable for large-scale productionAntibacterial agentsOrganic active ingredientsPharmaceutical drugLevofloxacin
The invention provides a levofloxacin hydrochloride capsule and a preparation method thereof. The levofloxacin hydrochloride capsule is characterized by being prepared from the following ingredients in percentage by the medicine composition recipe weight: 20 to 70 percent of levofloxacin hydrochloride, 20 to 65 percent of filling agents and disintegrating agents and 1 to 15 percent of lubricating agents. The levofloxacin hydrochloride capsule and the preparation method have the advantages that the recipe matching is reasonable; the preparation method is simple; the operation is easy; the production requirements can be met by conventional equipment; and the levofloxacin hydrochloride capsule and the preparation method are suitable for industrial mass production.
Owner:KAMP PHARMA
Clindamycin hydrochloride medicine composition and preparation method thereof
InactiveCN105687224ASimple preparation processNo biological activityAntibacterial agentsOrganic active ingredientsClindamycin HydrochlorideBiomedical engineering
The invention provides a clindamycin hydrochloride medicine composition and a preparation method thereof. The clindamycin hydrochloride medicine composition is characterized by being prepared from the following ingredients in percentage by the medicine composition recipe weight: 20 to 70 percent of clindamycin hydrochloride, 20 to 70 percent of filling agents and disintegrating agents, a proper amount of bonding agents (removal in the drying process) and 1 to 10 percent of lubricating agents. The clindamycin hydrochloride medicine composition and the preparation method have the advantages that the recipe is reasonable; the preparation is simple; the operation is easy; and the production requirements can be met by conventional equipment.
Owner:KAMP PHARMA
Application of Pluronic F-127 solution in chick embryo chorioallantoic membrane experiment
InactiveCN103134910ADoes not affect growthEasy to observeTesting medicinal preparationsMedicineLiquid state
The invention relates to application of Pluronic F-127 solution in chick embryo chorioallantoic membrane experiment as medicine carrying material. The application includes the steps of setting the Pluronic F-127 solution at 4 DEG C through still-setting method to dissolve the solution, configurating biological isotonic solution of certain concentration, and thus the medicine carrying material is obtained. The Pluronic F-127 solution is in liquid state in low temperature, and thus the solution can be mixed with tested medicine conveniently, and possibility of temperature-sensitive medicine degeneration is reduced to the greatest extent. The Pluronic F-127 solution is transparent when the solution is in solid state in high temperature, and thus chick embryo chorioallantoic membrane does not deform when the Pluronic F-127 solution is used and observation is convenient.
Owner:SHANXI UNIV
Preparation method of synaptic vesicle protein SV2A
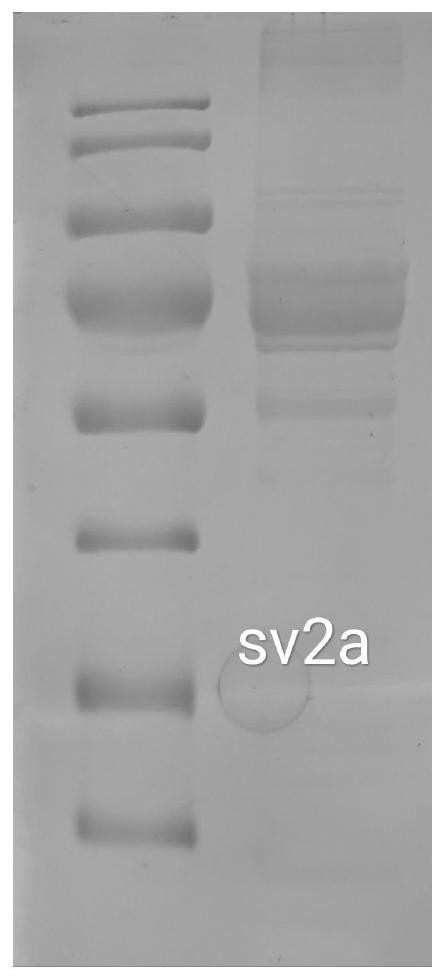
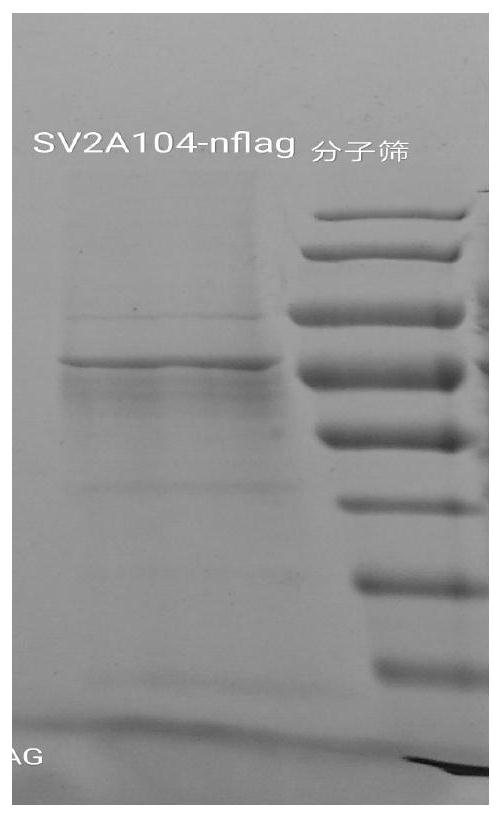
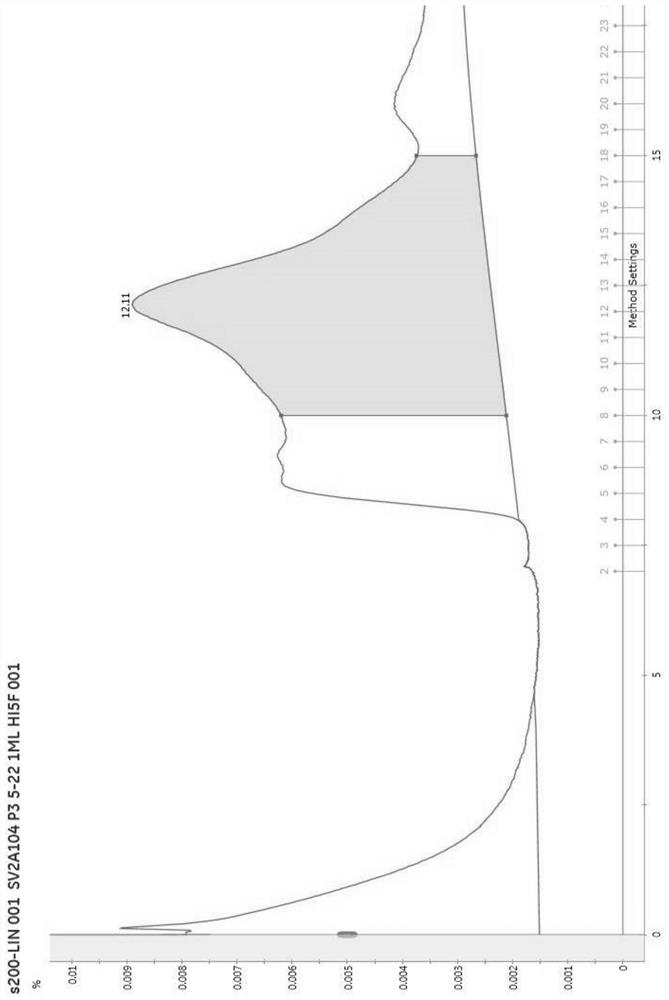
PendingCN112625111AConformational stabilityNo biological activityPeptide preparation methodsFermentationProtein solutionCholesterol
The invention provides a preparation method of synaptic vesicle protein SV2A. The preparation method comprises the following steps: 1) overexpression: transferring the 104th-742nd basic groups of a human SV2A gene with a label for protein affinity purification into an animal cell through a virus vector to overexpress the animal cell; (2) dissolving: cracking the animal cell, and dissolving membrane protein by using an excessive mixed solution of n-dodecyl beta-D-maltitoside and cholesterol hemicellulose succinate relative to the membrane protein, wherein the mass concentration ratio of the n-dodecyl beta-D-maltitoside to the cholesterol hemicellulose succinate is 10: 1; and 3) purification: performing centrifuging, taking supernate, performing purifying by affinity chromatography, replacing n-dodecyl beta-D-maltitoside and cholesterol hemicellulose succinate trisalt in the protein solution by using a digitonin solution which is excessive relative to the membrane protein, and performing purifying again by using a molecular sieve to obtain the SV2A protein. According to the method, the SV2A protein with stable configuration can be successfully separated.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
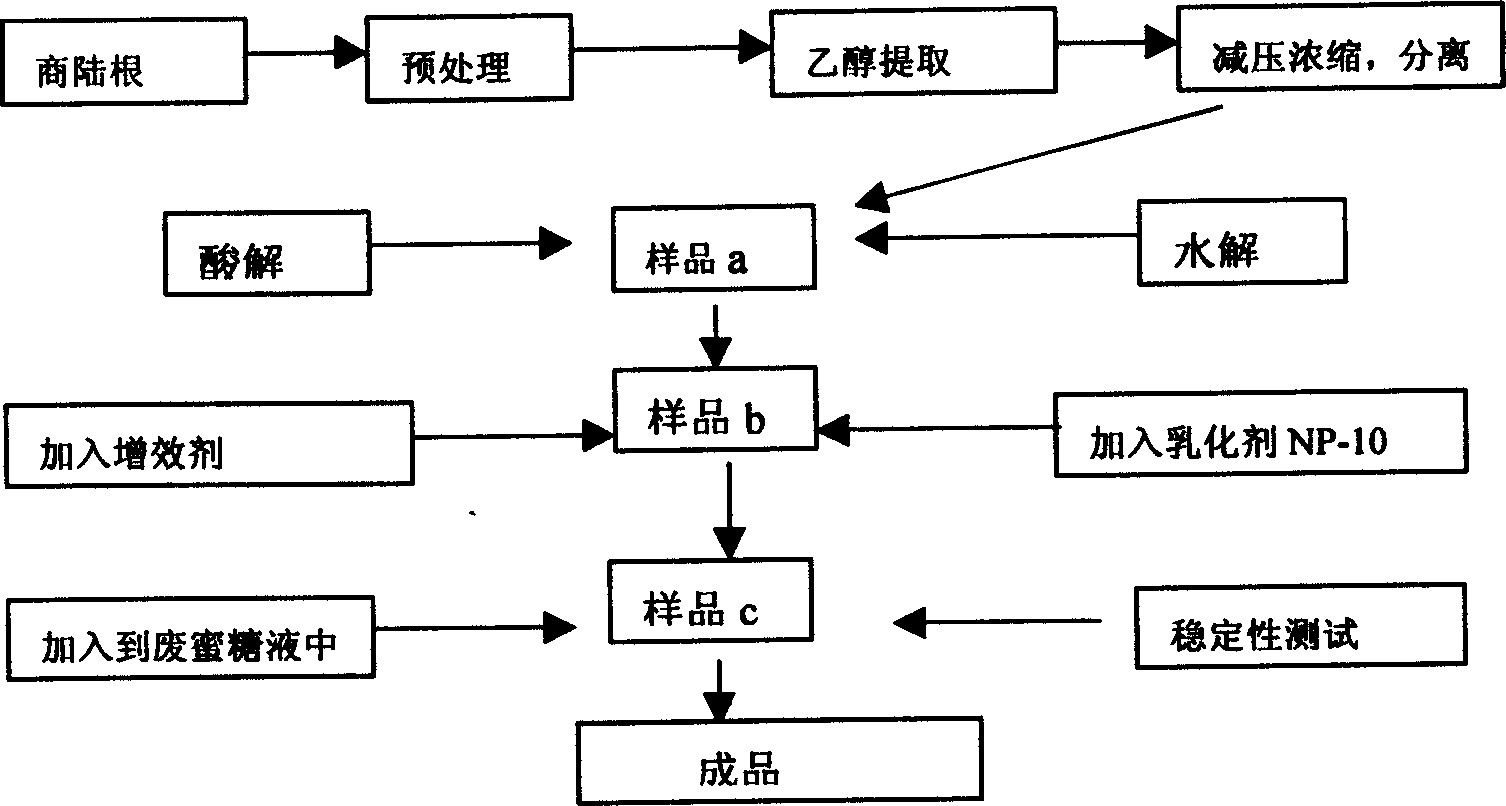
Fruit preserving agent produced from extract of plant of phytolaccaceae, its preparation and use
InactiveCN1582683APrevent decayImprove control effectFruits/vegetable preservation using chemicalsPEARAdditive ingredient
Owner:CHONGQING ACAD OF CHINESE MATERIA MEDICA
Flame retardant thermal insulation negative ion wood lacquer additive
InactiveCN109971238ANot easy to produceImprove protectionFireproof paintsAntifouling/underwater paintsLife qualityThermal insulation
The invention discloses a flame retardant thermal insulation negative ion wood lacquer additive, which comprises following components: water, a negative ion additive, an antifoaming agent, a wetting agent, a dispersant, a polyurethane thickening agent, a film forming polymer, a cosolvent, a film forming aid, tea oil, tea seed shells, kaolin, tea residues, and diatomite. In the prior art, a conventional wood lacquer additive only has a single function, multiple additives should be added, the cost is increased, the resources are wasted, the air is polluted, the human health is damaged, the conventional wood lacquer is not environmentally friendly, and the shortages mentioned above are overcome. Lacquer cracking and shredding are prevented; the substrate covered by the lacquer is better protected; the cost is reduced, and the drying time is shortened. The work efficiency is improved, and at the same time, the wood lacquer additive has a flame retardant function and a thermal insulation function, is capable of continuously releasing negative ions, quickly removes odor of wood lacquer, and improves the life quality.
Owner:安徽名士达新材料有限公司
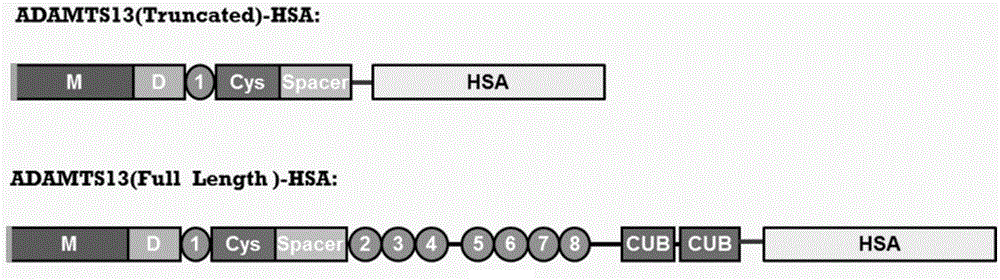
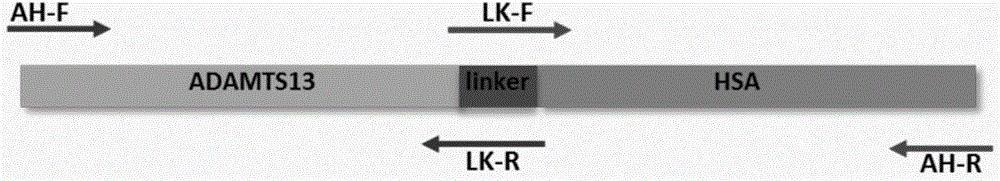
ADAMTS13-MDTCS fusion protein with function of prolonging half life in vivo and application thereof
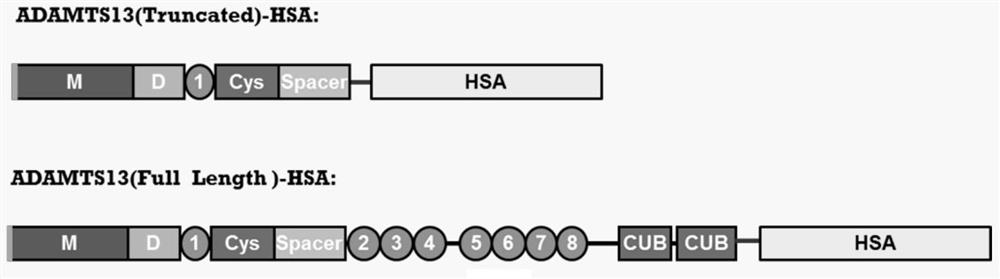
ActiveCN104926946AExtended half-lifeProlonged survival half-lifePeptide/protein ingredientsSerum albuminChemistryHalf-life
The invention belongs to the technical field of medical bioengineering technology, and discloses a mutant ADAMTS13-MDTCS fusion protein of human von willebrand factor lyase and the application of the mutant ADAMTS13-MDTCS fusion protein for preparing a drug for treating thrombotic thrombocytopenic purpura (TTP). According to the mutant ADAMTS13-MDTCS fusion protein, an ADAMTS13-MDTCS and human serum albumin are combined through a connecting peptide to form the fusion protein, the fusion protein ensures the biological activity of original ADAMTS13-MDTCS protein, the half-life period of the ADAMTS13-MDTCS is remarkably prolonged, the problem of existing ADAMTS13-MDTCS degradation is solved, and the mutant ADAMTS13-MDTCS fusion protein has the long-acting biological activity, is high in protein expression and can be used for industrial production.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
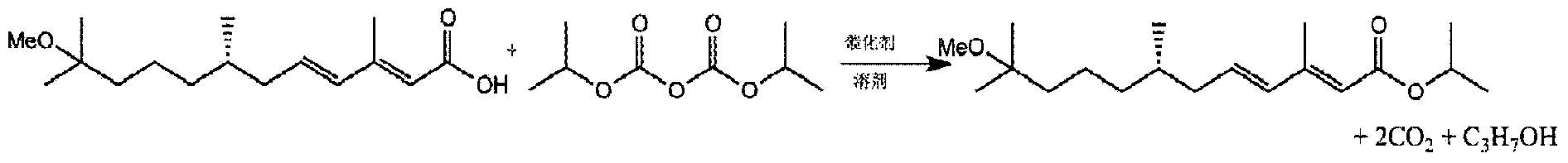
Preparation method of juvenile hormone analogue
ActiveCN104276946ACleverly and Effectively PreparedHigh purityOrganic compound preparationOrganic chemistry methodsOrganic solventHydrogen
The invention relates to the field of insecticides, and particularly relates to a preparation method of a juvenile hormone analogue (which comprises S-methoprene and S-hydroprene). The preparation method of the juvenile hormone analogues comprises the following steps of: A. adding (2E, 4E)-11-R-3,7,11-trimethyl-2,4-dodecadienoic acid (R is hydrogen or methoxyl) serving as raw material, an organic alkali catalyst and an organic solvent to a reaction vessel, and starting stirring; B. dropwise adding an organic solvent (the same as the organic solvent in the step A) solution of pyrocarbonate; C. after the addition is completed, reacting at 0-30 DEG C for 0-3 hours; D. adding water to stop reaction, and splitting phases of reaction liquid to obtain an organic phase; E. carrying out reduced pressure concentration and distillation to obtain a product, wherein the steps can also be carried out under the condition that the addition sequences of the pyrocarbonate and an organic strongly-alkaline catalyst are exchanged. The preparation method disclosed by the invention has the advantages of mild reaction condition, high product purity, high yield, environment-friendly and safe route and good industrial prospect.
Owner:常州胜杰生命科技股份有限公司
Oil field industry defoaming agent
The invention discloses an oil field industry defoaming agent comprising poly dimethyl siloxane, methoxy poly ether, 1-dodecene, hexamethyl disiloxane, octamethylcyclosiloxane and a catalyst. The preparation method is as follows: polyether and long chain alkyl co-modified silicone oil is prepared by solvent-free hydrosilation of poly dimethyl siloxane, methoxy poly ether and 1-dodecene under the catalysis effects of chloroplatinic acid, the catalyst and an allyl polyether and long chain olefin mixture are added dropwise for reaction, and unreacted excess polyether and olefin mixture is removed under reduced pressure to obtain the product. The oil field industry defoaming agent enhances the antifoaming and defoaming performance, reduces the defoaming agent system surface tension so as to improve the defoaming agent overall performance, and at the same time improves the defoaming agent corrosion resistance, solvent resistance and oil resistance.
Owner:QINGDAO HAOTAI WATER
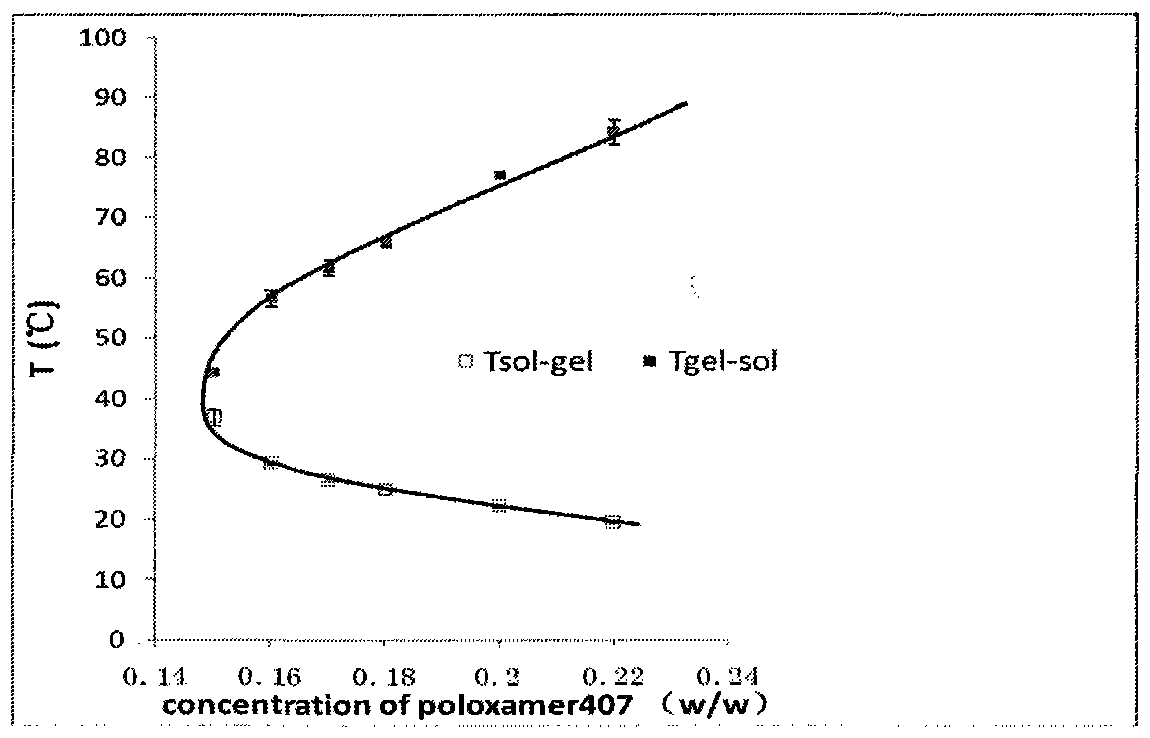
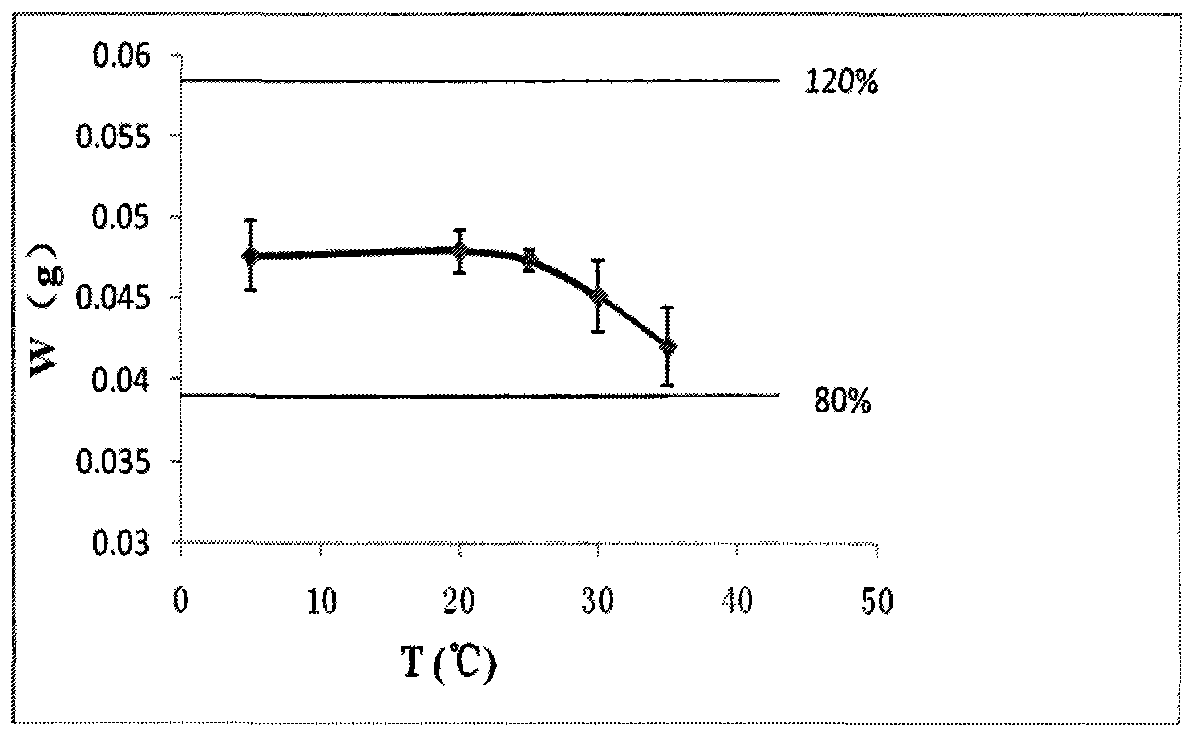
Nasal in situ gel drug delivery system and its preparation and application
ActiveCN104997724BExtended stayControl releasePeptide/protein ingredientsAerosol deliveryNasal cavityGel preparation
The invention belongs to the field of pharmaceutical preparations in medical technology. Disclosed are a nasal in-situ gel drug delivery system and its preparation and application. Especially in central nervous system drugs and protein peptide drugs have great advantages. The process for preparing the NGF nasal in-situ gel preparation of the present invention is a two-bottle method. One of the bottles is in situ gel solution, in which poloxamer is preferably poloxamer P407: 16%-16.8%, polyethylene glycol is preferably PEG10000: 0.2%-0.6%, polysorbate is preferably Tween80: 1%- 3%, and the preservative is preferably benzalkonium chloride: 0.001% to 0.002%. The other bottle is NGF freeze-dried powder preparation, wherein the content of NGF is 0.05%-0.1%, the content of albumin is 0.1%-0.2%, and the content of mannitol is 5%. Add the gel solution to the NGF freeze-dried powder before use, and mix well. The disclosed drug delivery system can be used in any nasal administration dosage forms, preferably sprays and nasal drops. The nasal in situ gel drug delivery system provided by the invention solves the problem of using protein and polypeptide drugs in the central nervous system, especially for nerve growth factors, expanding its application range.
Owner:PEKING UNIV +1
Clarithromycin capsule
InactiveCN108685869AReasonable compositionSafe to takeAntibacterial agentsOrganic active ingredientsGranularityMedicine
The invention relates to the technical field of clarithromycin preparations, in particular to a clarithromycin capsule. The clarithromycin capsule comprises clarithromycin, a filling agent, a binder,a flow aid, a medicinal hollow capsule shell and gastric-soluble coating premix. The clarithromycin capsule has the advantages that the clarithromycin, the filling agent and the binder are prepared into pellets with certain granularity, the stability and flowability of the pellets are increased through the gastric-soluble coating premix and the flow aid, the quality of the clarithromycin capsule is increased, and drug use safety is increased.
Owner:江苏黄河药业股份有限公司
Silodosin film-coated tablet and preparation method thereof
InactiveCN108685867ADissolution requirementsAchieve commercial productionOrganic active ingredientsUrinary disorderDissolutionMedical prescription
The invention provides a completely-new silodosin tablet composition and a preparation method thereof, wherein the particle size D90 of the silodosin raw material is 20-70 [mu]m, and the auxiliary material comprises pregelatinized starch. According to the present invention, the formula uses the commonly used inert auxiliary material, the commonly used preparation process is used, and the raw material does not need micro-powder, such that the dissolution of the tablet can be effectively improved, the commercial production can be achieved, and the use amount of the added lubricant is not sensitive; and by using the specific prescription, the lubricant content in the silodosin tablet can be significantly increased so as to avoid the sticking problem easily generated in the actual production.
Owner:KUNMING JIDA PHARMA
Anti-ultraviolet and anti-tarnish industrial gear oil
PendingCN112940830AImprove poor suspension stabilityHigh bonding strengthLubricant compositionAntioxidantUltraviolet lights
The invention discloses anti-ultraviolet and anti-tarnish industrial gear oil. The gear oil is prepared from, by weight, base oil, bright stock, an antioxidant, nano silicon dioxide, a UV531 anti-ultraviolet agent, talcum powder, a gear oil additive, a pour point depressant, a defoaming agent and a cosolvent. The anti-ultraviolet and anti-tarnish industrial gear oil solves the problems that industrial gear oil in the existing market does not have an anti-ultraviolet effect, normal use of the industrial gear oil is affected, meanwhile, gears may discolor after being used for a long time, maintenance personnel cannot conveniently distinguish the working state of the gears, and unpleasant smell and unfavorable health and environmental protection exist in the use process of the industrial gear oil due to addition of additional additives in the industrial gear oil, carries out better processing and production work, improves the ultraviolet resistance and tarnish resistance of the product, and can also efficiently resist aging and absorb ultraviolet light.
Owner:马鞍山中集瑞江润滑油有限公司
A kind of fat-soluble drug submicrocapsule glucose injection and preparation method thereof
ActiveCN107693488BEvenly wrappedUniform particle sizeEmulsion deliveryMacromolecular non-active ingredientsPolyvinyl alcoholPolythylene glycol
The invention discloses a fat-soluble drug submicron capsule glucose injection and a preparation method thereof. The invention is characterized in that the capsule material comprises the following components in parts by weight: 15-45 parts of sodium alginate, 2.5-9 parts of poloxamer 188, 1.5-5 parts of silk fibroin, 0.5-3 parts of polyvinyl alcohol and 0.2-3 parts of polyethylene glycol. The preparation method comprises the following steps of: sequentially adding the poloxamer, a silk fibroin aqueous solution, a polyvinyl alcohol aqueous solution, the polyethylene glycol and glucose into a sodium alginate aqueous solution at intervals under stirring, and finally dropwise adding a corresponding fat-soluble drug; adopting an ultrasonic and shearing treatment mode; and finally, carrying outfiltration with a 450nm membrane and damp-heat sterilization to obtain the submicron capsule glucose injection. The preparation process of the submicron capsule glucose injection provided by the invention is simple and economic, the particle size of submicron capsules is controllable and uniform, and the biological safety is high.
Owner:CHONGQING UNIV OF TECH
Azithromycin dispersible tablet
InactiveCN108619105AReasonable compositionNo biological activityAntibacterial agentsOrganic active ingredientsAzithromycinActive component
The invention relates to the technical field of azithromycin preparations and particularly relates to an azithromycin dispersible tablet prepared from azithromycin, a disintegrating agent, a binder, and a lubricant; by organically combining the mentioned components, the tablet can be disintegrated quickly and stably with the disintegrating agent, thus quickly releasing active components, increasing bioavailability and reducing pain of patients.
Owner:江苏黄河药业股份有限公司
Fruit preserving agent produced from extract of plant of phytolaccaceae, its preparation and use
InactiveCN1299583CPrevent decayImprove control effectFruits/vegetable preservation using chemicalsBiotechnologyPhytolaccatoxin
Owner:CHONGQING ACAD OF CHINESE MATERIA MEDICA
Adamts13-mdtcs fusion protein with extended half-life in vivo and its application
ActiveCN104926946BExtended half-lifeNo biological activityPeptide/protein ingredientsSerum albuminLyaseIn vivo
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
PEG-modified triacontanol water-soluble prodrug
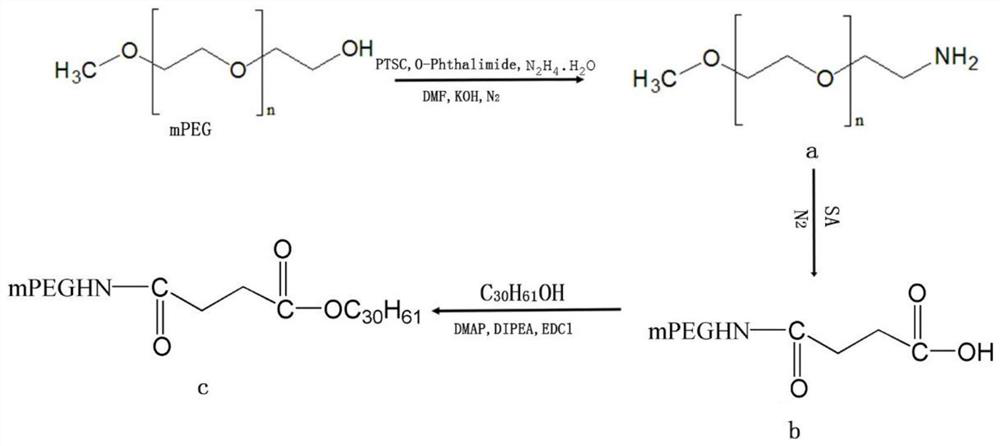
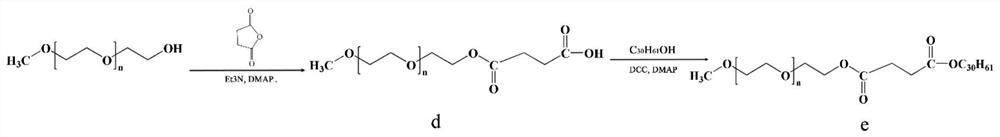
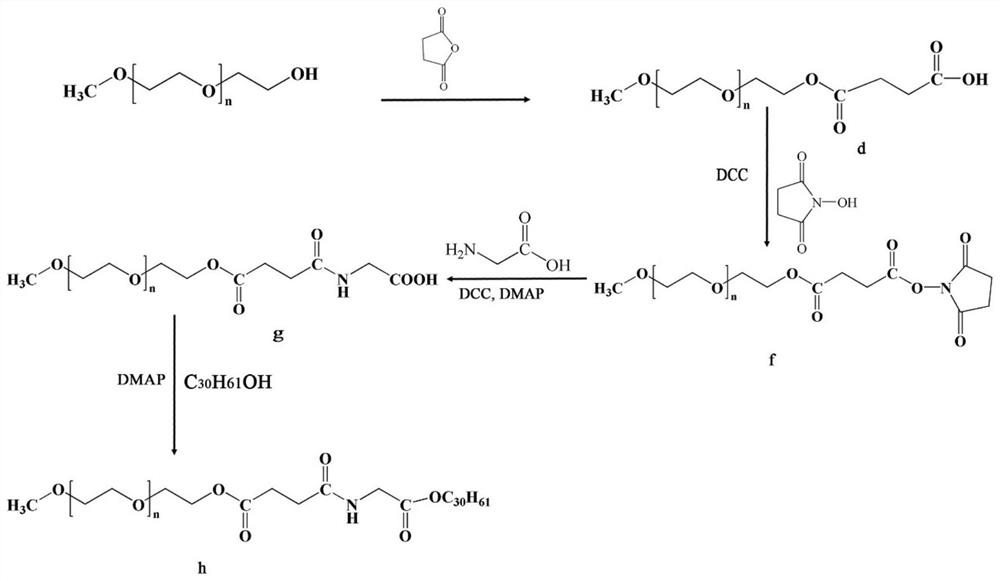
ActiveCN107998403AGood water solubilityIncrease exposureHydroxy compound active ingredientsMetabolism disorderSolubilityTumor targeting
The invention relates to a preparation method and anticancer therapeutic effects of a triacontanol water-soluble macromolecular prodrug based on polyethyleneglycol. According to the prodrug, triacontanol is taken as a model drug, and the water-soluble prodrug of the triacontanol is prepared by chemically bonding PEG with the triacontanol through covalent bonds by means of easily degradable ester bonds. The PEG-triacontanol prodrug can effectively improve water-solubility, tumor targeting and stability of the triacontanol and prolong half-life in vivo of the triacontanol, is convenient to use,and meanwhile has good anti-cancer effects. The preparation method of the triacontanol water-soluble macromolecular prodrug has the advantages that the preparation method is simple and convenient andapplicable to large-scale production, so that application range of the triacontanol is wider.
Owner:陈西敬
peg-modified water-soluble prodrugs of triacontanol
ActiveCN107998403BGood water solubilityIncrease exposureHydroxy compound active ingredientsMetabolism disorderWater soluble prodrugTumor targeting
The invention relates to a preparation method and anticancer curative effect of a polyethylene glycol-based water-soluble triacontanol macromolecular prodrug. In the present invention, triacontanol is used as a model drug, and PEG and triacontanol are covalently linked through an easily degradable ester bond to prepare a water-soluble prodrug of triacontanol. PEG-triacontanol prodrug can effectively improve the water solubility, tumor targeting and stability of triacontanol, prolong its half-life in vivo, is convenient to use, and has good anticancer effect at the same time. The preparation method of the present invention is simple and convenient, is suitable for large-scale production, and makes the application range of triacontanol wider.
Owner:陈西敬
Novel coronavirus antibody colloidal gold immunochromatography detection card and application thereof
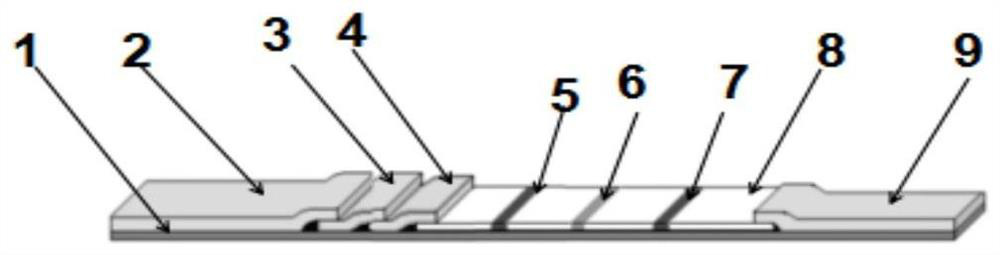
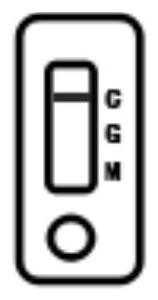
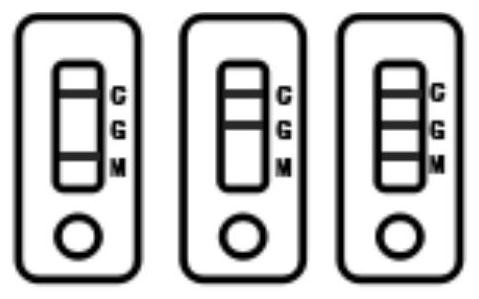
PendingCN114113587AThe detection method is accurateSimple samplingImmunoassaysBlood filteringCoronavirus antibody
The invention relates to a novel coronavirus antibody colloidal gold immunochromatography detection card and application, and belongs to the technical field of virus colloidal gold immunochromatography detection cards. The detection card comprises a bottom plate, a sample pad, a gold-labeled combination pad, a blood filter membrane, a nitrocellulose membrane and an absorption pad, the sample pad, the gold-labeled combination pad, the blood filtering membrane, the nitrocellulose membrane and the absorption pad are sequentially adhered to the bottom plate from left to right, the sample pad is connected with the gold-labeled combination pad, the gold-labeled combination pad is connected with the blood filtering membrane, the blood filtering membrane is connected with the nitrocellulose membrane, and the nitrocellulose membrane is connected with the absorption pad; a detection line G line, a detection line M line and a quality control line are distributed on the nitrocellulose membrane; according to the detection card, a capture method is adopted, novel coronavirus IgM and IgG antibodies in human whole blood are detected through an IgG secondary antibody and an IgM secondary antibody which are coated on a nitrocellulose membrane and a novel coronavirus antigen which is coated on a gold-labeled combination pad by utilizing a colloidal gold immunochromatography principle, and the detection method is rapid and accurate.
Owner:HUAZHONG UNIV OF SCI & TECH +1
Levofloxacin hydrochloride capsule
InactiveCN108619157AReasonable compositionSafe to takeAntibacterial agentsCapsule deliveryWestern medicineTherapeutic effect
The invention relates to the technical field of levofloxacin preparations and particularly relates to a levofloxacin hydrochloride capsule which is prepared from levofloxacin hydrochloride, an additive mixture, assistant antibiotics, and a Chinese herbal medicine mixture. By organically integrating the components, the capsule has effect of treating both exterior and interior by compounding Chineseand Western medicines, and antibacterial range of the capsule is broadened. Meanwhile, drug resistance of bacteria against the levofloxacin hydrochloride can be reduced, so that the capsule is a preparation having high effect and broad-spectrum antibacterial function. The capsule enlarges the antibacterial spectrum and improves treatment effect of the levofloxacin hydrochloride.
Owner:江苏黄河药业股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com