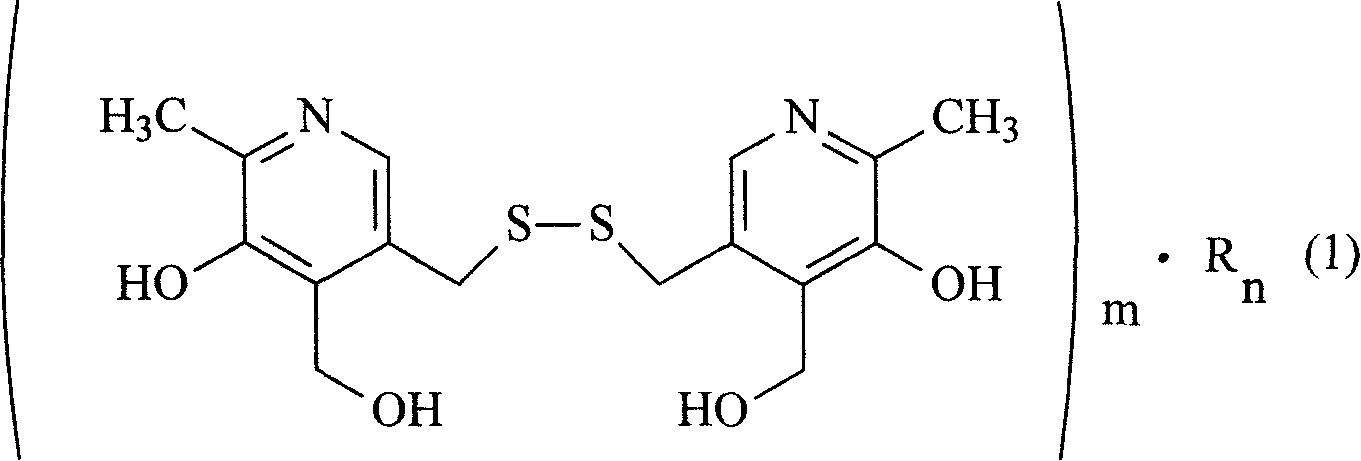
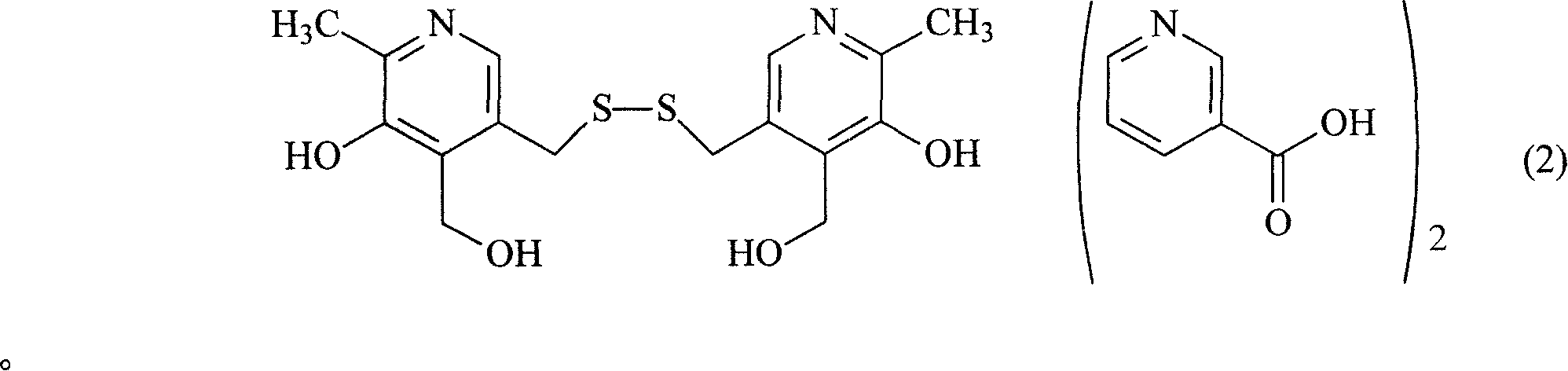
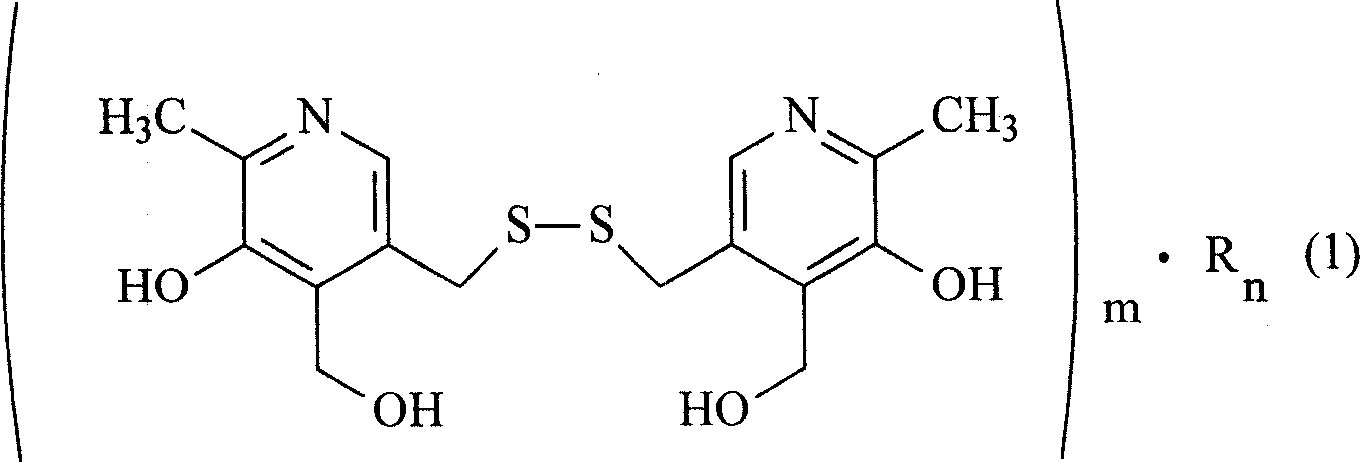
Organic salt of pyritinol and its prepn process
A technology of organic acid salt and pyrithione, which is applied in the field of preparation of pyrithione organic acid salt, and can solve the problems such as the use limitation of pyrithione hydrochloride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: the synthesis of nicotinic acid pyrithione salt
[0017] Take 24.6g of nicotinic acid, fully dissolve in 300ml of anhydrous benzene, heat and stir until completely dissolved, add 40.5g of pyrithione under the state of complete dissolution, heat and reflux the mixture for 3 hours, TLC thin layer identification reaction is complete (developing agent: acetic acid Ethyl ester: ethanol: glacial acetic acid = 5: 6: 0.6), after cooling slightly, add 200ml of absolute ethanol, put the mixture into the refrigerator to fully cool, and filter out white crystals by suction filtration, wash the solid with a small amount of cold anhydrous ether. Vacuum-dried at 65°C to obtain 62.1 g of nicotinic acid pyrithione salt with a yield of 89.7%. The contents of niacin and pyrithione were determined by acid-base titration, and the water content was determined by Karl Fischer method. The result is: niacin 37.2%, pyrithione 62.0%, water 5.8%, close to the theoretical value, cont...
Embodiment 2
[0018] Embodiment 2: the synthesis of pyrithione fumarate
[0019] Take 11.6g of fumaric acid, fully dissolve it in 300ml of anhydrous benzene, heat and stir until it is completely dissolved, add 40.5g of pyrithione in the fully dissolved state, heat and reflux the mixture for 3 hours, TLC thin layer identification reaction is complete (developing agent: Ethyl acetate: ethanol: glacial acetic acid = 5: 4: 0.8), after cooling slightly, add 200ml of absolute ethanol, put the mixture in the refrigerator to fully cool, and filter out white crystals by suction filtration, wash the solid with a small amount of cold anhydrous ether. Vacuum-dried at 65°C to obtain 49.9 g of pyrithione fumarate, with a yield of 88.9%. The content of fumaric acid and pyrithione was determined by acid-base titration, and the moisture content was determined by Karl Fischer method. The result is: 20.8% fumaric acid, 72.7% pyrithione, 6.5% water, close to the theoretical value, containing 2 crystal waters....
Embodiment 3
[0020] Embodiment 3: the synthesis of glucuronic acid pyrithione salt
[0021] Get 42.4g of glucuronic acid, fully dissolve in 300ml of anhydrous benzene, heat and stir until fully dissolved, add 40.5g of pyrithione under fully dissolved state, heat and reflux the mixture for 3 hours, TLC thin layer identification reaction is complete (developing agent: Ethyl acetate: ethanol: glacial acetic acid = 6: 3: 0.8), after cooling slightly, add 200ml of absolute ethanol, put the mixture into the refrigerator to fully cool, and filter out white crystals by suction filtration, wash the solid with a small amount of cold anhydrous ether. Vacuum-dried at 65°C to obtain 75.9 g of pyrithione glucuronate, with a yield of 87.8%. The content of glucuronic acid and pyrithione was determined by acid-base titration, and the moisture content was determined by Karl Fischer method. The result is: glucuronic acid 49.0%, pyrithione 46.8%, water 4.2%, close to theoretical value, containing 2 crystal w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com