Adamts13-mdtcs fusion protein with extended half-life in vivo and its application
A fusion protein and protein technology, which is applied in the field of medical bioengineering, can solve the problem that the biological activity consistency of protein drugs cannot be guaranteed, and achieve the effect of increasing the half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
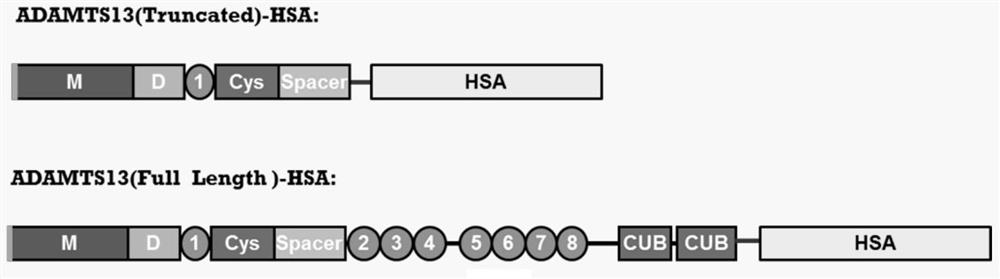
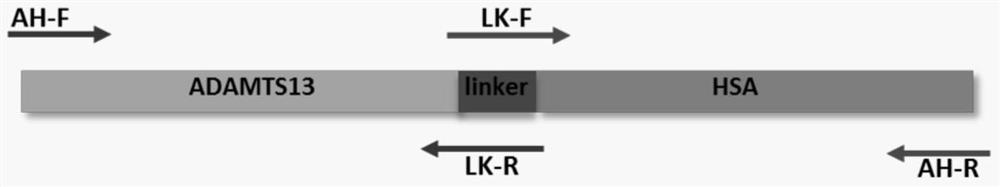
[0063] The construction of embodiment 1 ADAMTS13-MDTCS-HSA fusion protein and its plasmid
[0064] 1.1 Materials and methods
[0065] Reagent: HEK293-Free style cell line (Life tech);
[0066] Cell culture reagents: high sugar DMEM, Opti-MEM, 293-F expression Medium, FBS, 0.25% trypsin-EDTA solution, antibiotic G418 for cell resistance screening (Life tech); strain: Top10 (Tiangen Biochemical); vector : The pCEP4 vector is preserved by ourselves; kits: gel recovery kit; PCR purification kit, small amount of plasmid extraction kit, large amount of endotoxin-free plasmid extraction kit (Tiangen Biochemical);
[0067] Lipofectamine 2000 (Life tech) for transfection liposome; polymerase for PCR (TAKARA); restriction endonuclease (NEB); all sequencing was done by Life tech; HSA antibody for Western-blot (Santa Cruz); ADAMTS13 antibody , HRP secondary antibody (Abcam); Ultra-15 ultrafiltration tube (Millipore); Superdex purification column (GEhealth).
[0068] 1.2 Construction of...
Embodiment 2
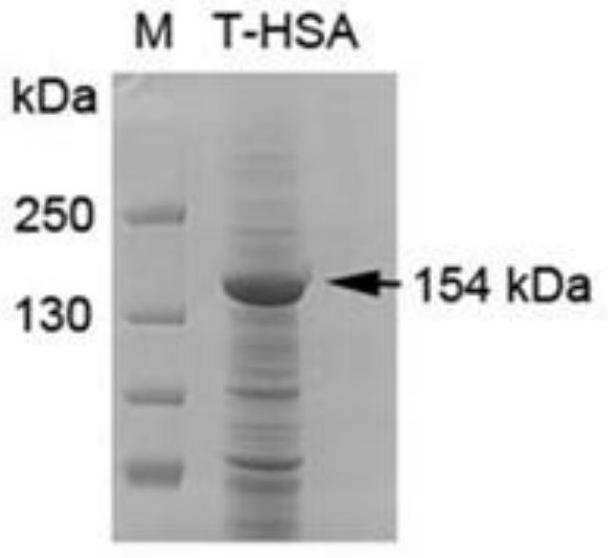
[0086] Example 2 Transient expression and protein identification of ADAMTS13-MDTCS fusion protein
[0087] a) Inoculation of cells. The day before transfection, inoculate the cells into a 10cm plate (try not to use cells that grow to 90% in two days, if the growth is slow, please spread more cells). When transfecting, the cell confluence is required to be 90-95%;
[0088] b) For each plate, dilute 50 μl of LIP 2000 with 700 μl Opti-MEM Medium (the volume of LIP is not higher than 1 / 10 of the total volume), and incubate at room temperature for 5 minutes;
[0089] c) Dilute 70 μl of DNA with 680 μl Opti-MEM Medium per plate (the volume of DNA should not be higher than 1 / 10 of the total volume);
[0090] d) Add the diluted DNA to the diluted LIP2000 (1:1 mix), and incubate at room temperature for 20 minutes;
[0091] e) Carefully wash the prepared 10cm plate cells once with 3-5ml sterile PBS, gently add the Bath pipette along the wall, so as not to wash up the 293F cells, do n...
Embodiment 3
[0096] Example 3 Screening of cell lines stably expressing ADAMTS13-MDTCS HSA fusion protein
[0097] a) Inoculation of cells. Cells were seeded into 10 cm dishes one day before transfection. When transfecting, the cell confluence is required to be 90-95%;
[0098] b) For each plate, dilute 50 μl of LIP2000 with 700 μl Opti-MEM Medium (the volume of LIP is not higher than 1 / 10 of the total volume), and incubate at room temperature for 5 minutes;
[0099] c) Dilute 70 μl of DNA with 680 μl Opti-MEM Medium per plate (the volume of DNA should not be higher than 1 / 10 of the total volume);
[0100] d) Add the diluted DNA to the diluted LIP2000 (1:1 mix), and incubate at room temperature for 20 minutes;
[0101] e) Carefully wash the prepared 10cm plate cells once with 3-5ml sterile PBS, add the Bath pipette gently along the wall, so as not to wash up the 293F cells, and do not shake vigorously;
[0102] f) Add the incubated DNA+LIP2000 mixed solution, 1.5ml / dish, directly into ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com