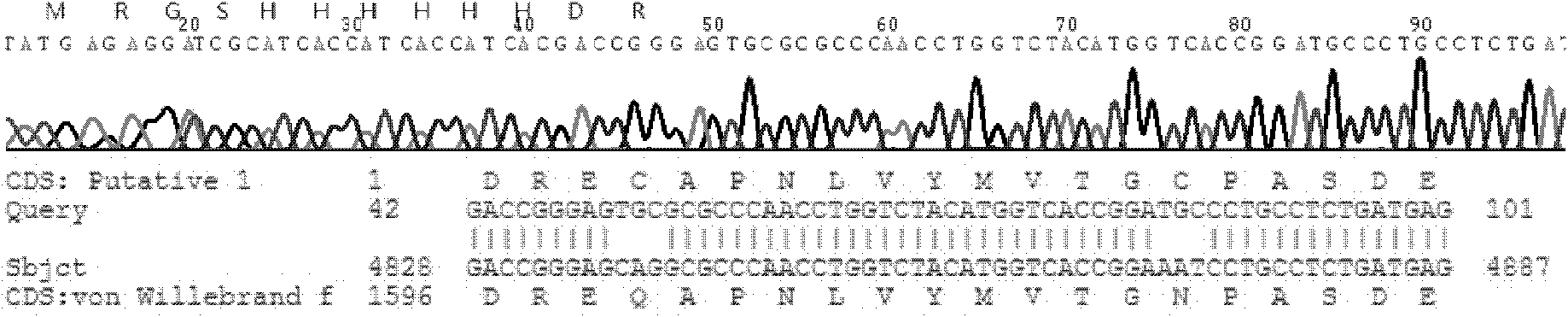Patents
Literature
36 results about "ADAMTS13" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
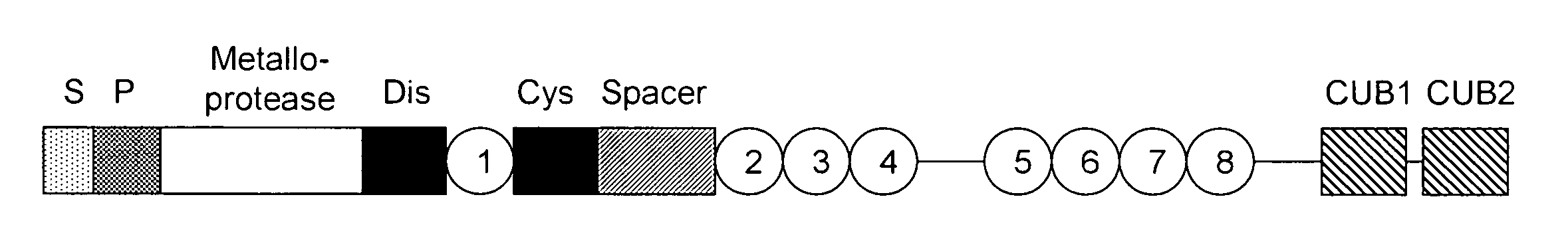
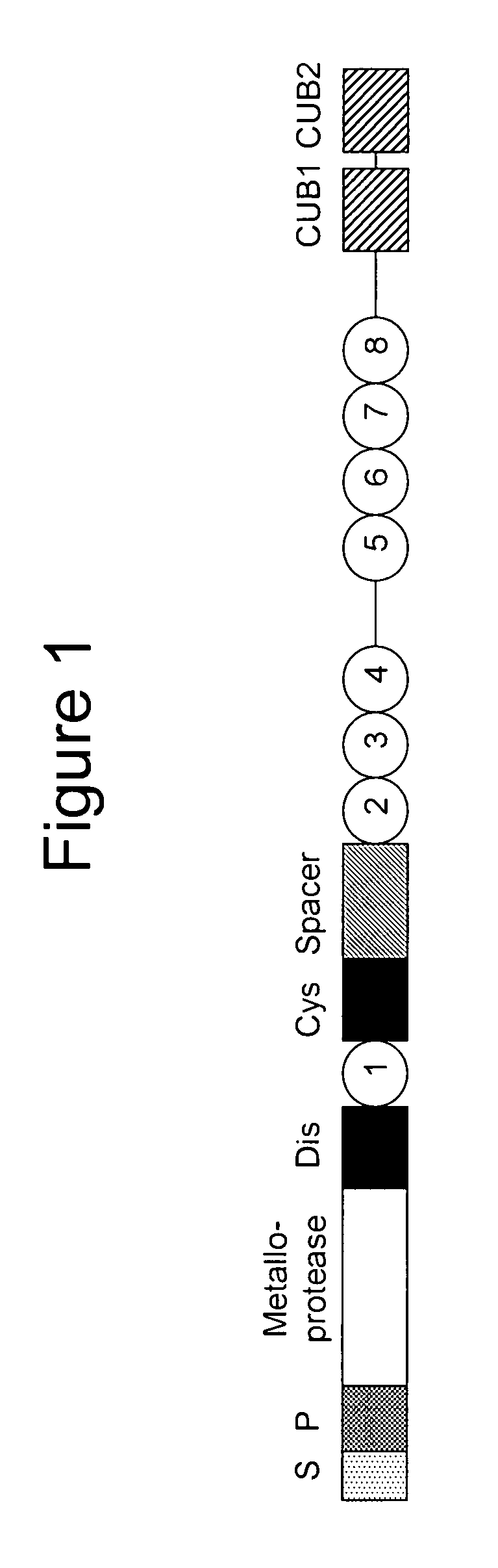
ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13)—also known as von Willebrand factor-cleaving protease (VWFCP)—is a zinc-containing metalloprotease enzyme that cleaves von Willebrand factor (vWf), a large protein involved in blood clotting. It is secreted into the blood and degrades large vWf multimers, decreasing their activity.
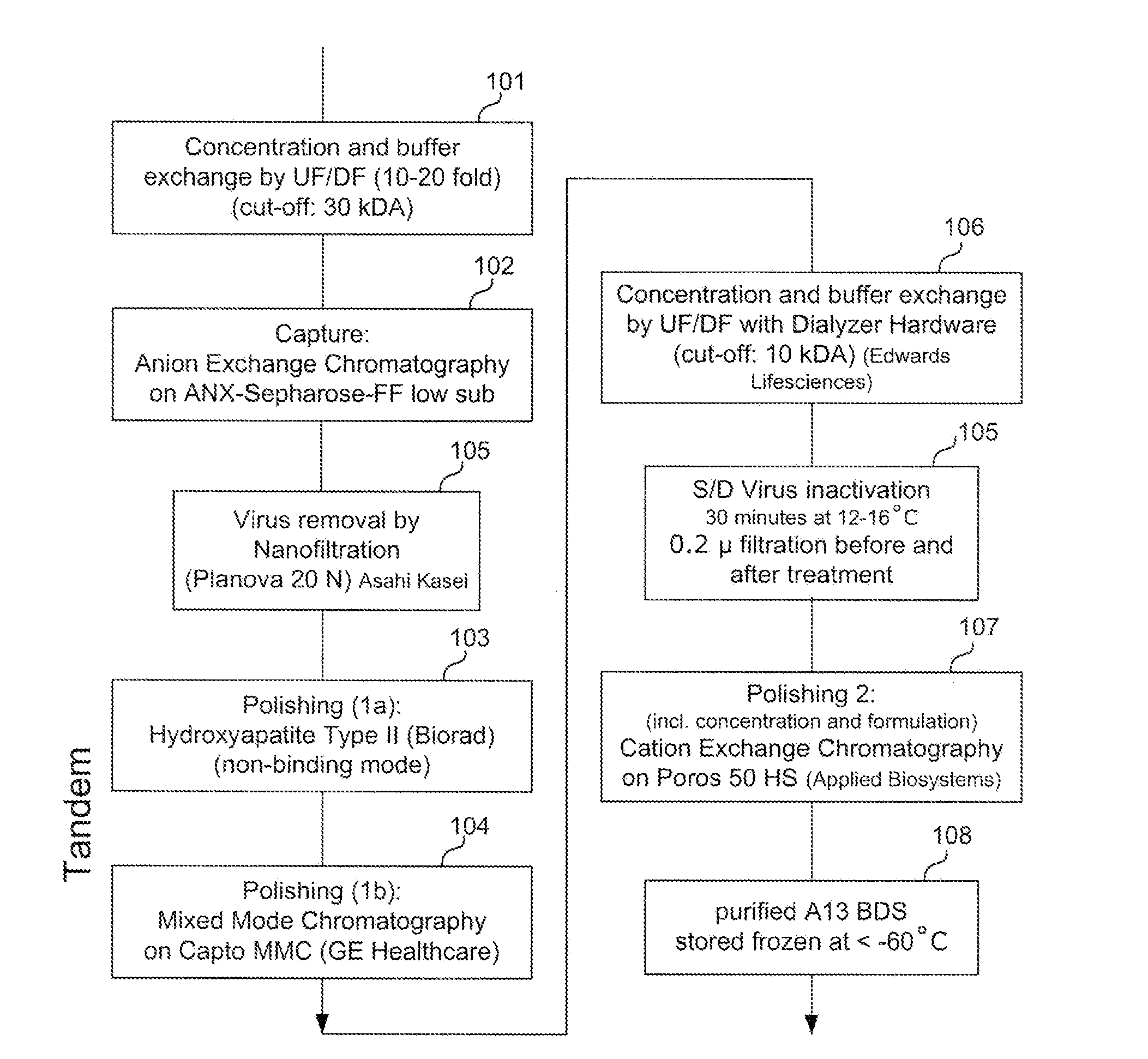
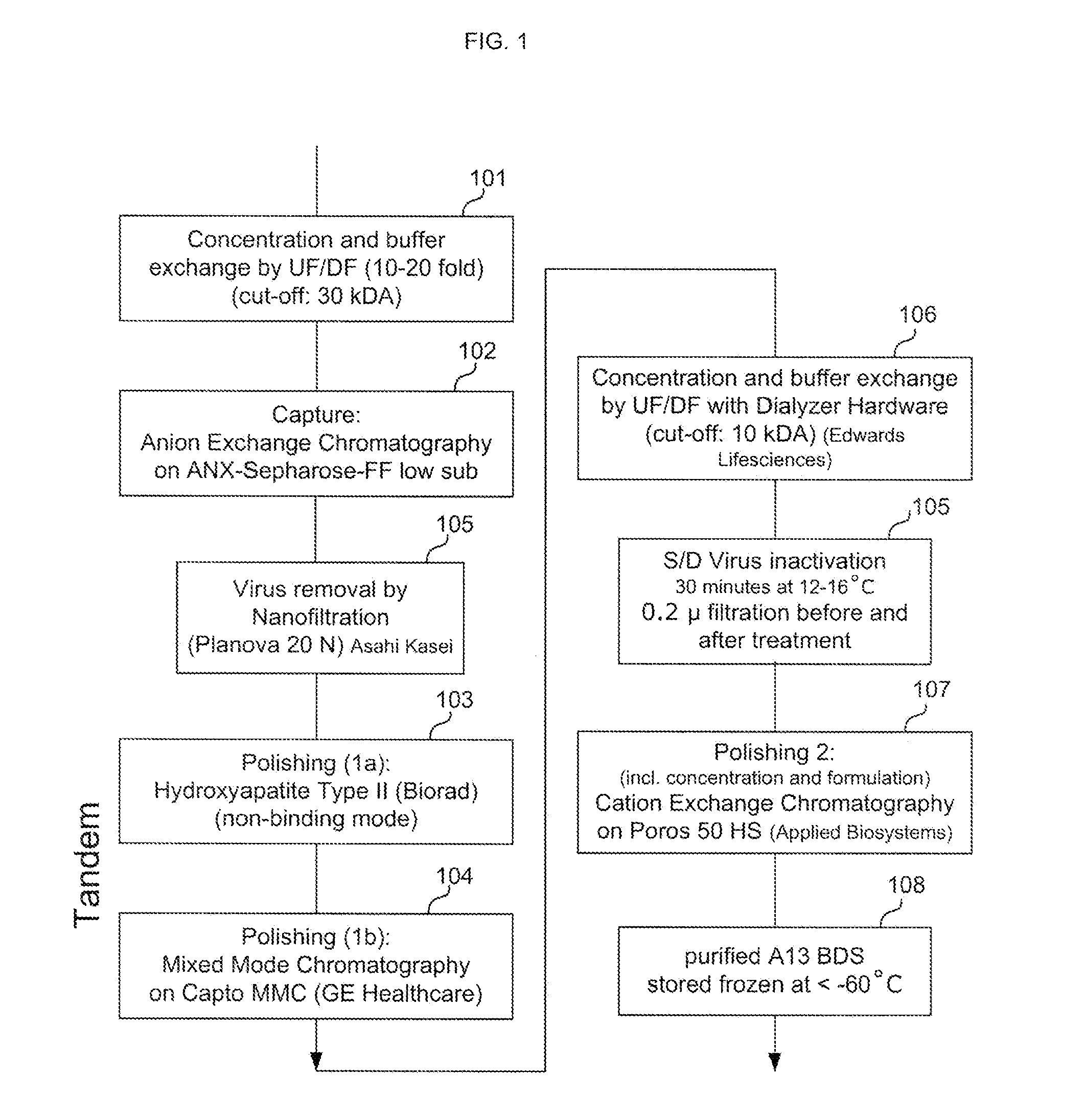
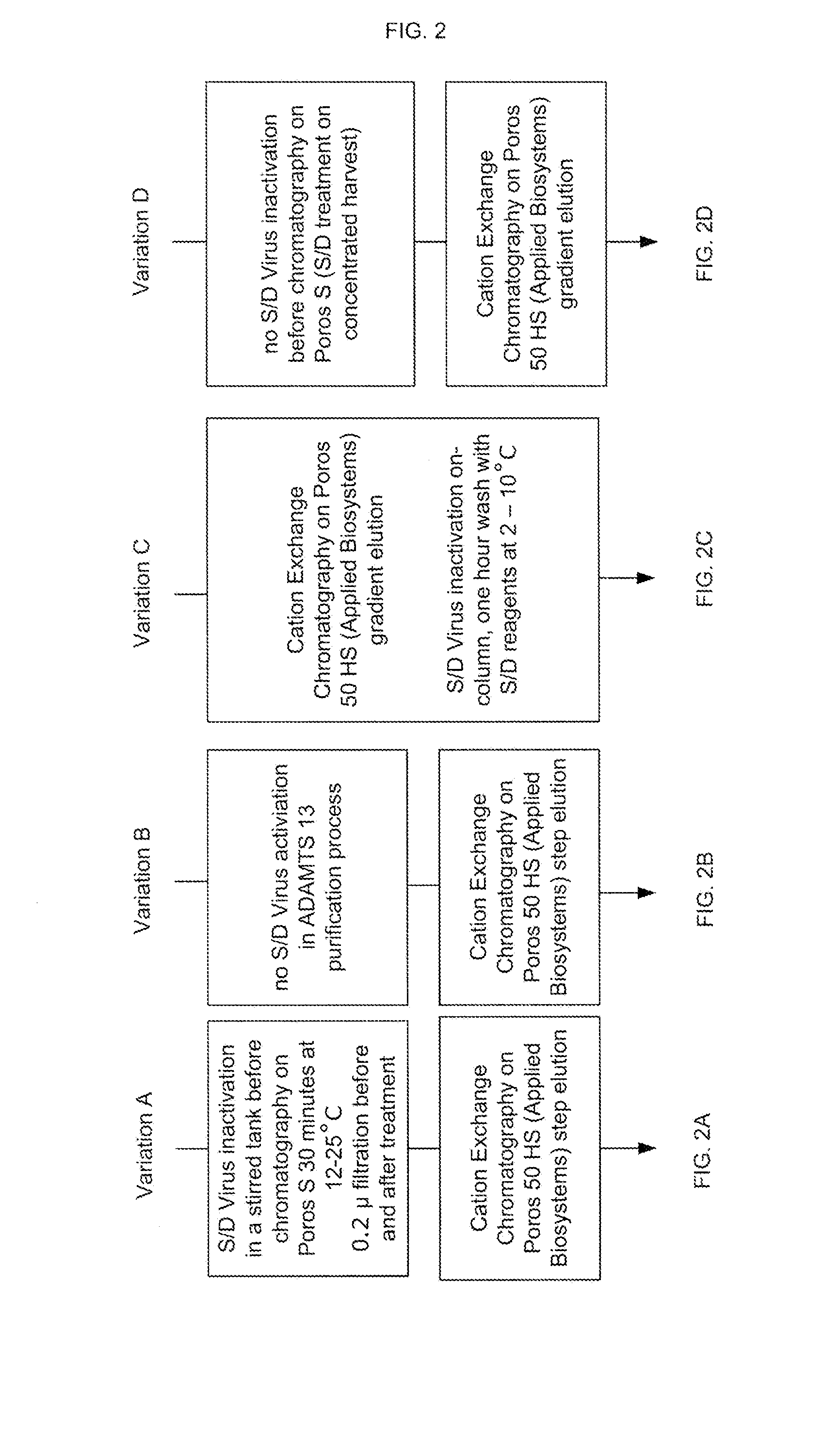
Methods of Purifying Recombinant Adamts13 and Other Proteins and Compositions Thereof
ActiveUS20110081700A1Reduce formationExtension of timeInactivation/attenuationBiomass after-treatmentHydroxylapatiteAnion-exchange chromatography
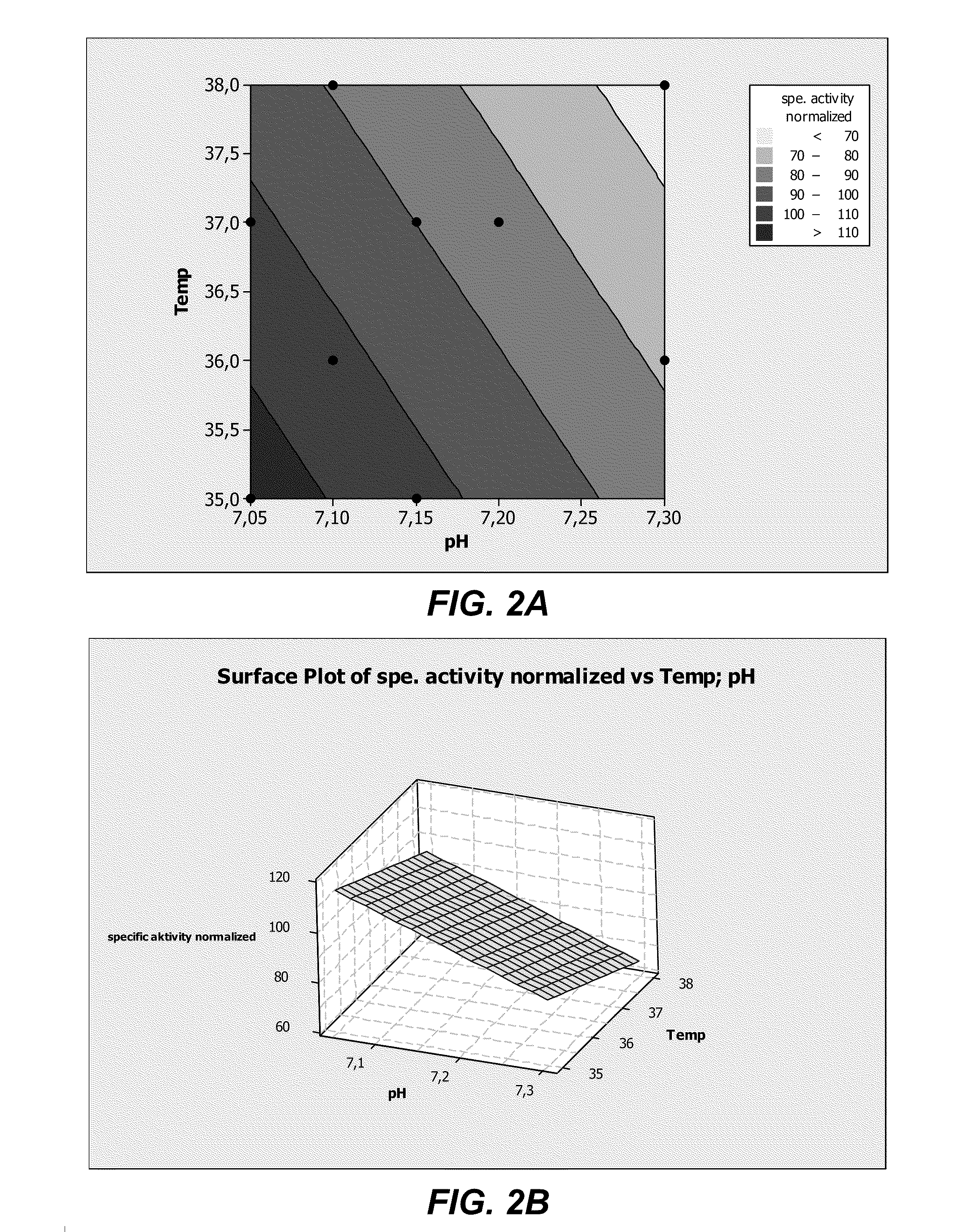
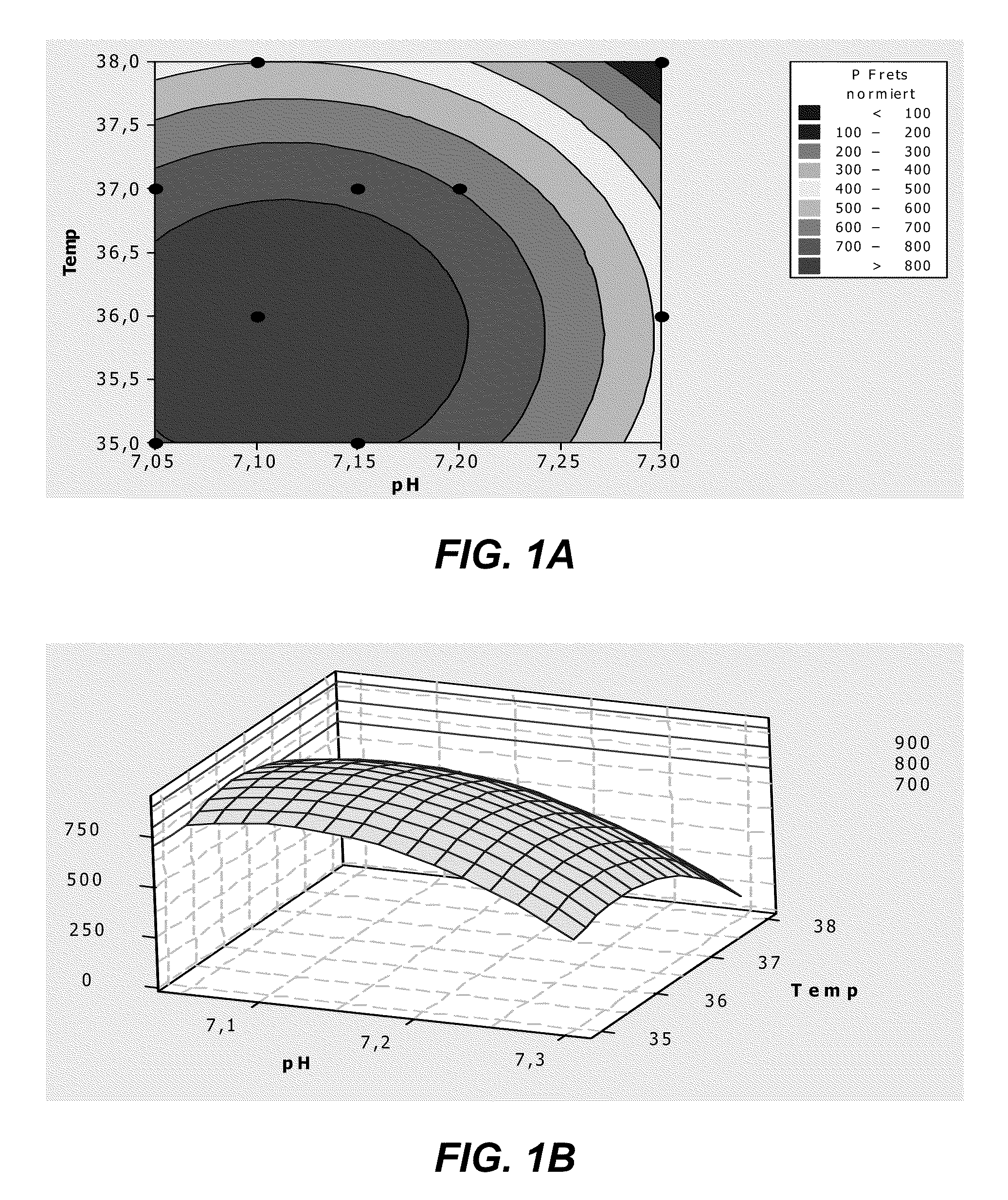
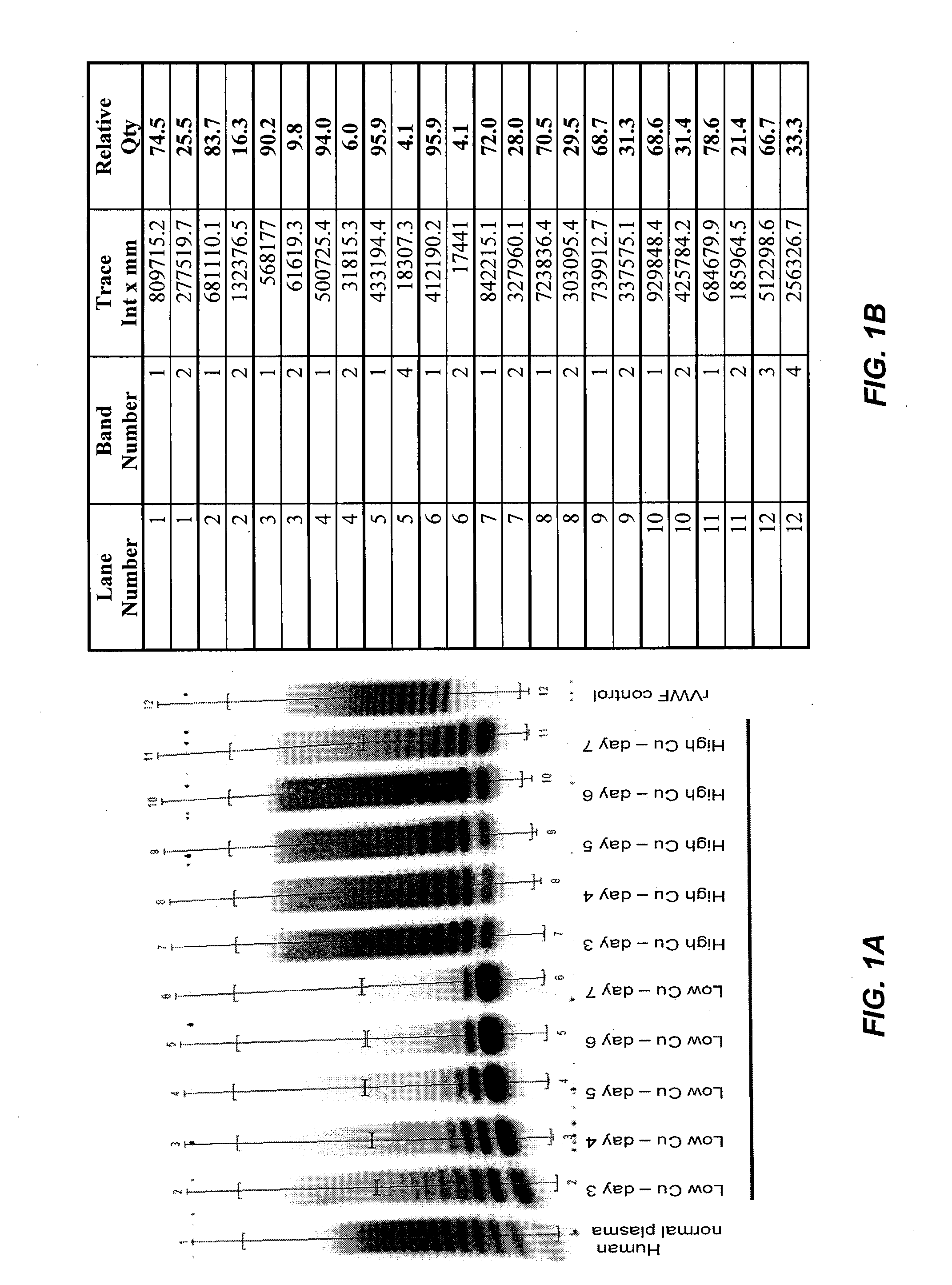
Provided herein are methods for purifying recombinant A Disintegrin-like and Metallopeptidase with Thrombospondin Type 1 Motif 13 (ADAMTS13) protein from a sample. The method comprises enriching for ADAMTS13 protein by chromatographically contacting the sample with hydroxyapatite under conditions that allow ADAMTS13 protein to appear in the eluate or supernatant from the hydroxylapatite. The methods may further comprise tandem chromatography with a mixed mode cation exchange / hydrophobic interaction resin that binds ADAMTS13 protein. Additional optional steps involve ultrafiltration / diafiltration, anion exchange chromatography, cation exchange chromatography, and viral inactivation. Also provided herein are methods for inactivating virus contaminants in protein samples, where the protein is immobilized on a support. Also provided herein are compositions of ADAMTS13 prepared according to said methods.
Owner:TAKEDA PHARMA CO LTD
Cell Culture Medium For ADAMTS Protein Expression
ActiveUS20110086413A1Improve expression levelHigh activitySugar derivativesGenetically modified cellsProtein compositionADAMTS Proteins
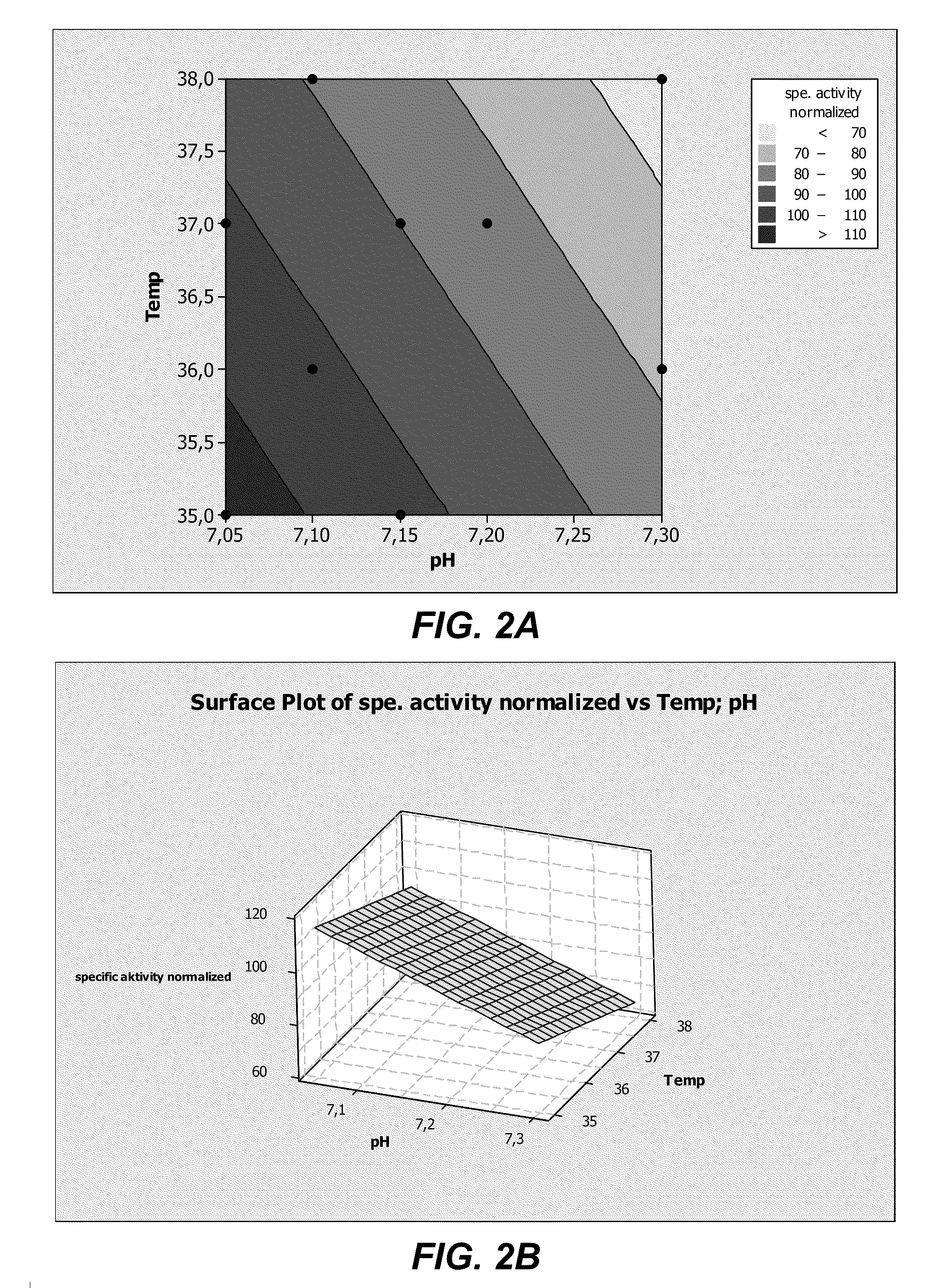
The present invention provides culture mediums that are useful for the expression of ADAMTS proteins, such as ADAMTS13. Methods for the expression and purification of ADAMTS proteins are also provided. In some embodiments, the mediums and methods of the invention are useful for the expression of ADAMTS proteins having high specific activities. Also provided are ADAMTS, e.g., ADAMTS13, protein compositions with high specific activities, which are expressed and purified according to the methods provided herein.
Owner:TAKEDA PHARMA CO LTD
Methods for expressing ADAMTS proteins in cell culture medium supplemented with zinc
ActiveUS8313926B2Improve expression levelHigh activitySugar derivativesGenetically modified cellsProtein compositionCell culture media
The present invention provides culture mediums that are useful for the expression of ADAMTS proteins, such as ADAMTS13. Methods for the expression and purification of ADAMTS proteins are also provided. In some embodiments, the mediums and methods of the invention are useful for the expression of ADAMTS proteins having high specific activities. Also provided are ADAMTS, e.g., ADAMTS13, protein compositions with high specific activities, which are expressed and purified according to the methods provided herein.
Owner:TAKEDA PHARMA CO LTD
Von willebrand factor (VWF) inhibitors for treatment or prevention of infarction
InactiveUS20090317375A1Reduce capacitySuppress activityPeptide/protein ingredientsCardiovascular disorderFactor VIII vWFFactor ii
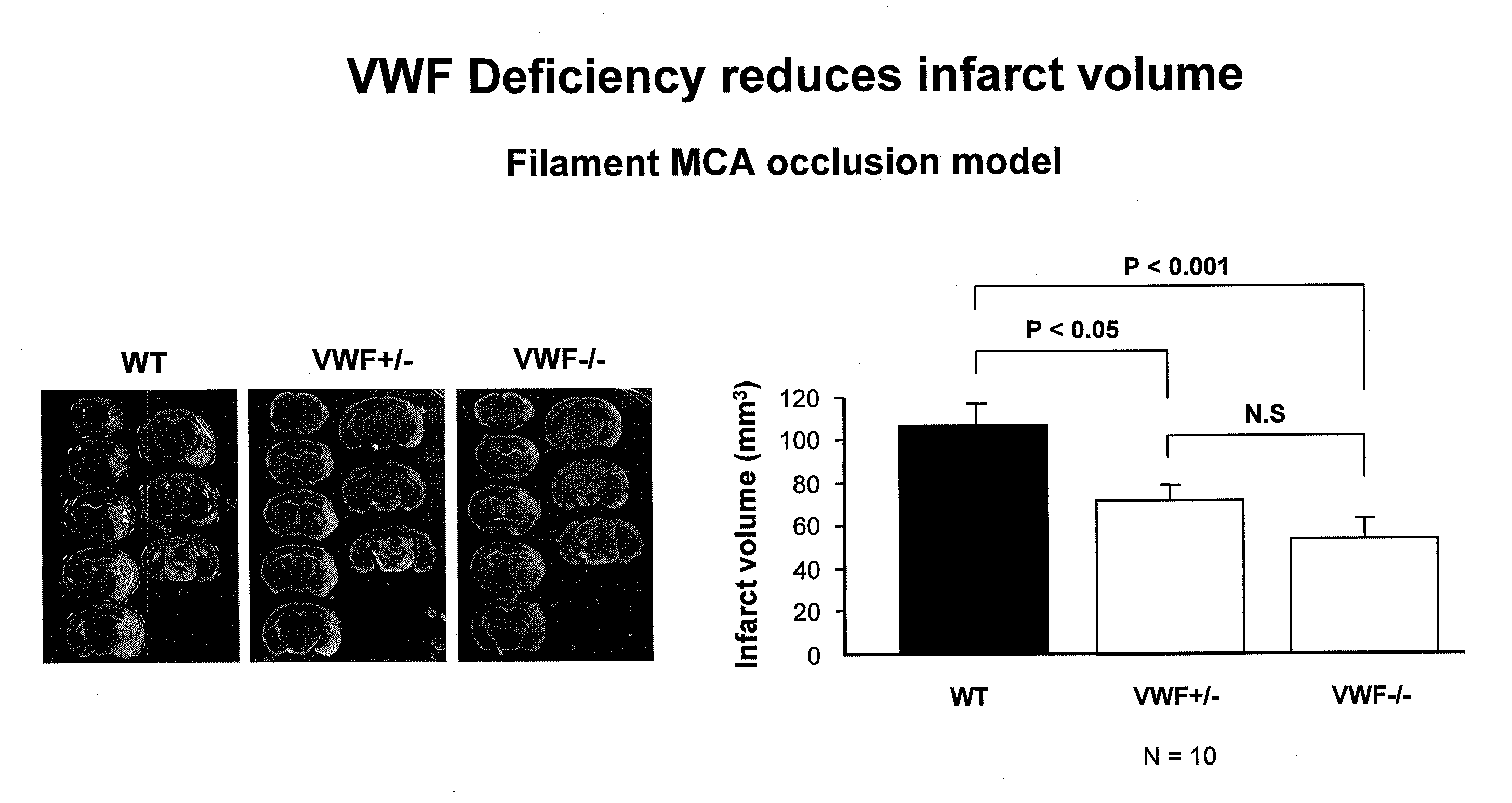
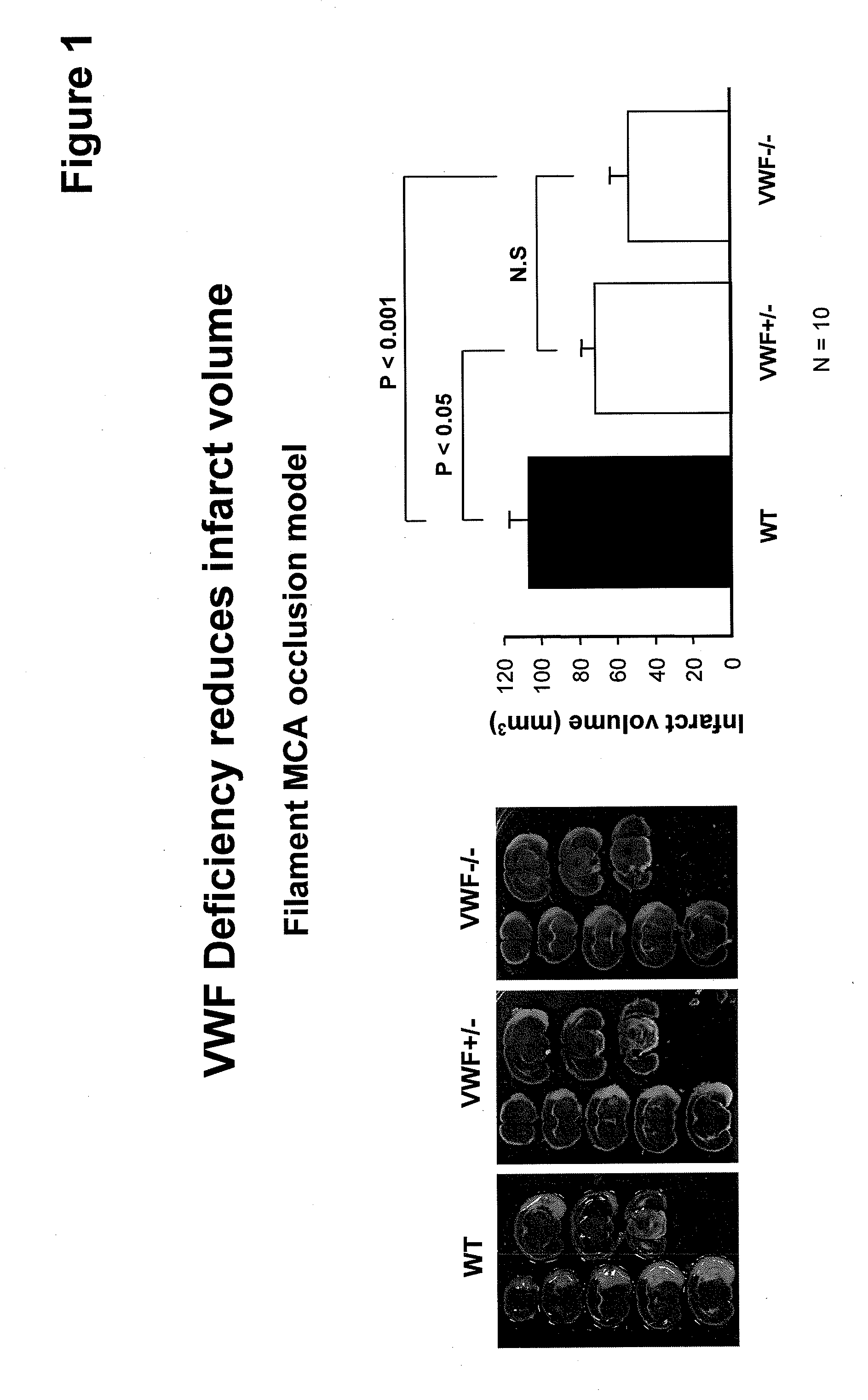
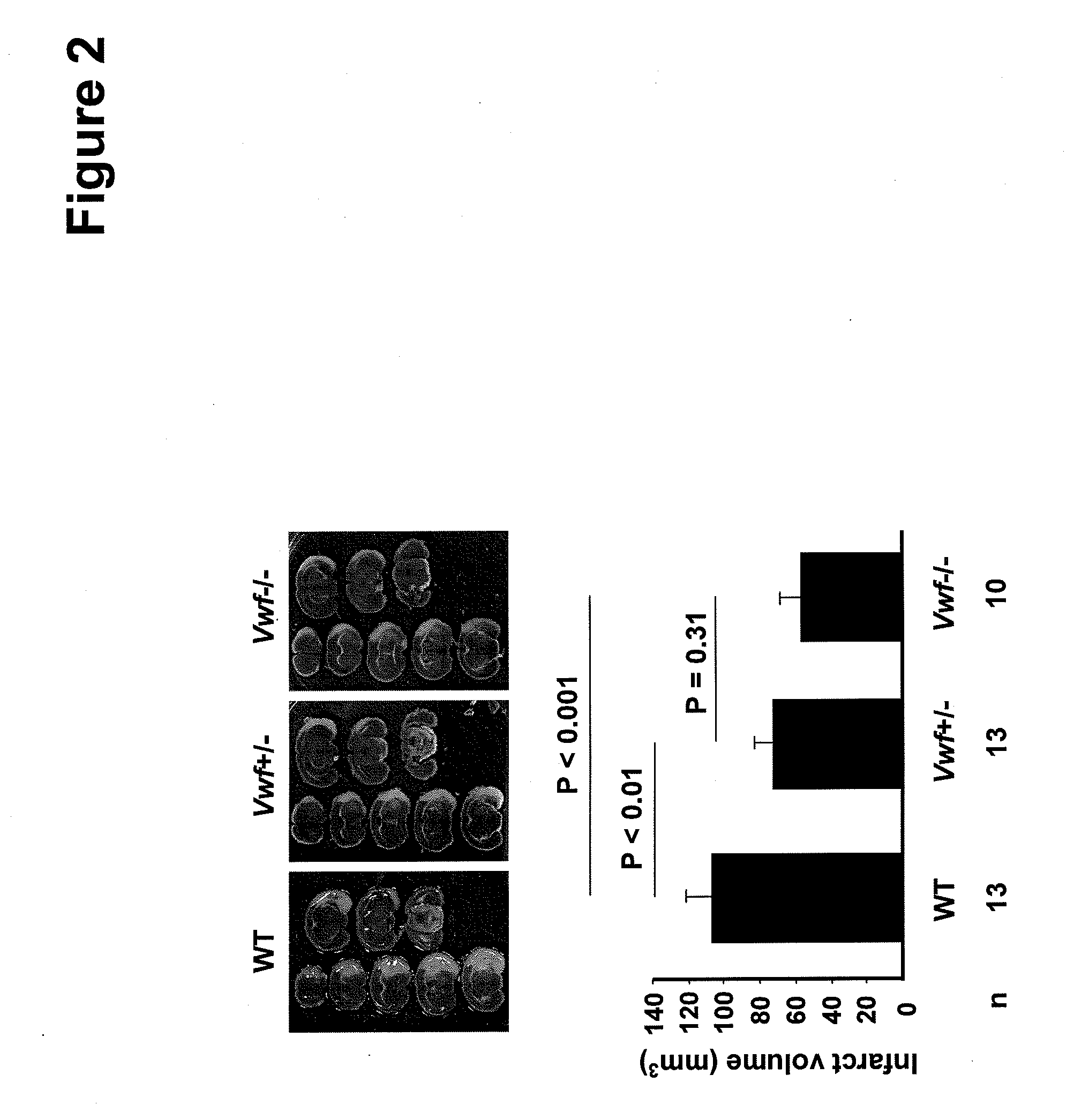
This invention relates to methods for treating or preventing an infarction by administering to a patient in need thereof a compound capable of suppressing the expression or activity of the von Willebrand Factor (VWF). Thus, the invention relates to the use of a pharmaceutically effective amount of a VWF inhibitor, such as ADAMTS13, for the preparation of a medicament for treating conditions known to involve infarction to reduce or eliminate the symptoms and effect of an infarction.
Owner:CHILDRENS MEDICAL CENT CORP
Method of producing recombinant adamts13 in cell culture
ActiveUS20120034674A1High activityHigh expressionFactor VIIPeptide/protein ingredientsCell culture mediaADAMTS13
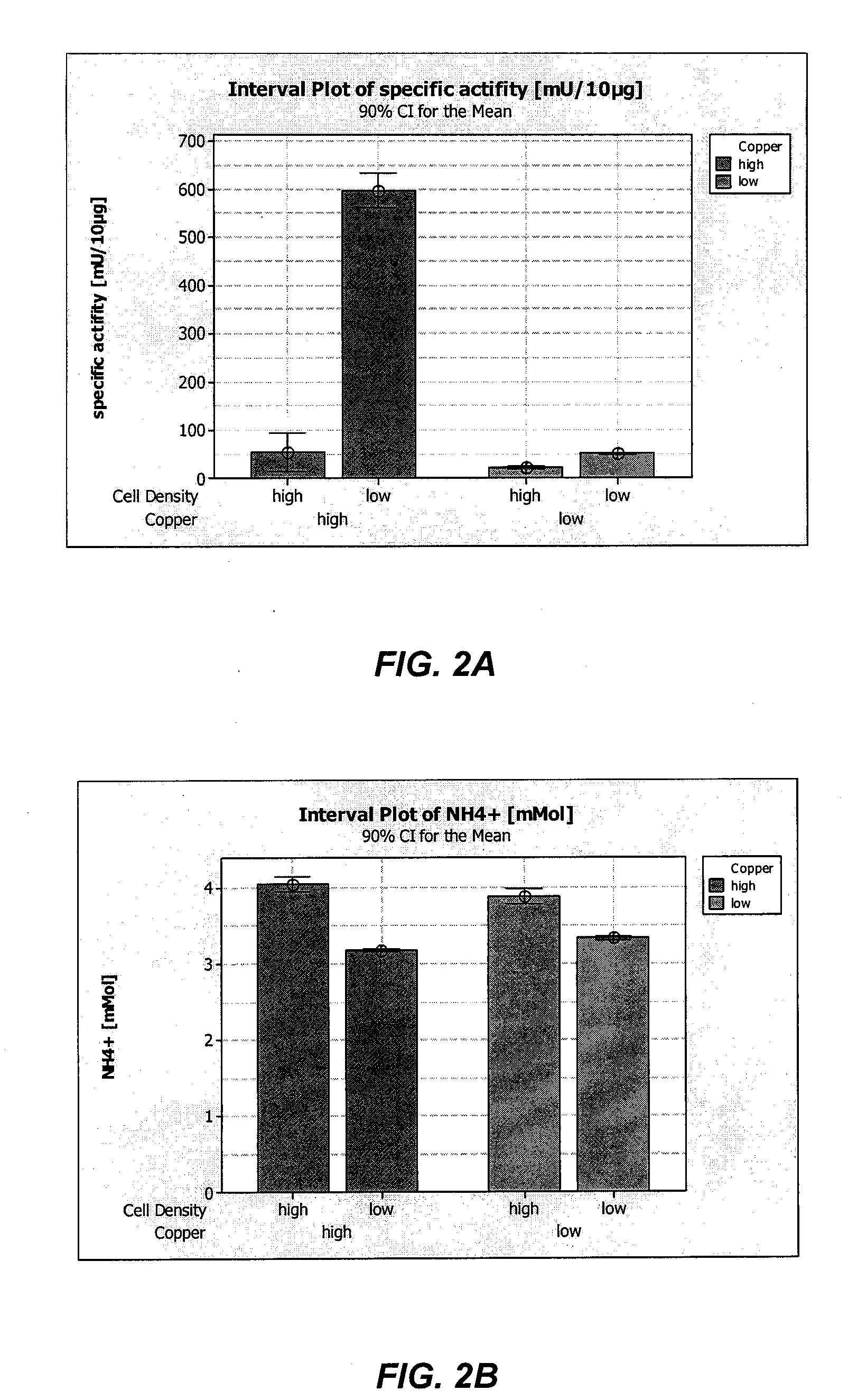
Among other aspects, the present invention relates to cell culture conditions for producing high molecular weight vWF, in particular, highly multimericWF with a high specific activity and ADAMTS13 with a high specific activity. The cell culture conditions of the present invention can include, for example, a cell culture medium with an increased copper concentration and / or cell culture supernatant with a low ammonium (NH4+) concentration. The present invention also provides methods for cultivating cells in the cell culture conditions to express high molecular weight vWF and rA13 having high specific activities.
Owner:TAKEDA PHARMA CO LTD
Assays for detection of von willebrand factor (vWF) multimers and for degradation of vWF by agents such as ADAMTS13 and related methods
Owner:NORTH CAROLINA AT CHAPEL HILL THE UNIV OF
Methods and kits for detecting and measuring ADAMTS13/FXI complexes
InactiveUS20080138837A1Inhibit and enhance formationMicrobiological testing/measurementDisease diagnosisHemostatic DisordersPeptide substrate
Provided are assays and kits to detect ADAMTS13 activity using peptide substrates and ADAMTS13 / Factor XI complexes using ELISA. These assays and kits can be used for diagnostic applications and to evaluate treatment of thrombotic or hemostatic disorders, for example, thrombotic thrombocytopenic purpura (TTP). Also provided is a novel form of ADAMTS13 found on platelets, and anti-ADAMTS13 antibodies.
Owner:AMERICAN DIAGNOSTICA
Assays for detection of von willebrand factor (vWF) multimers and for degradation of vWF by agents such as ADAMTS13 and related methods
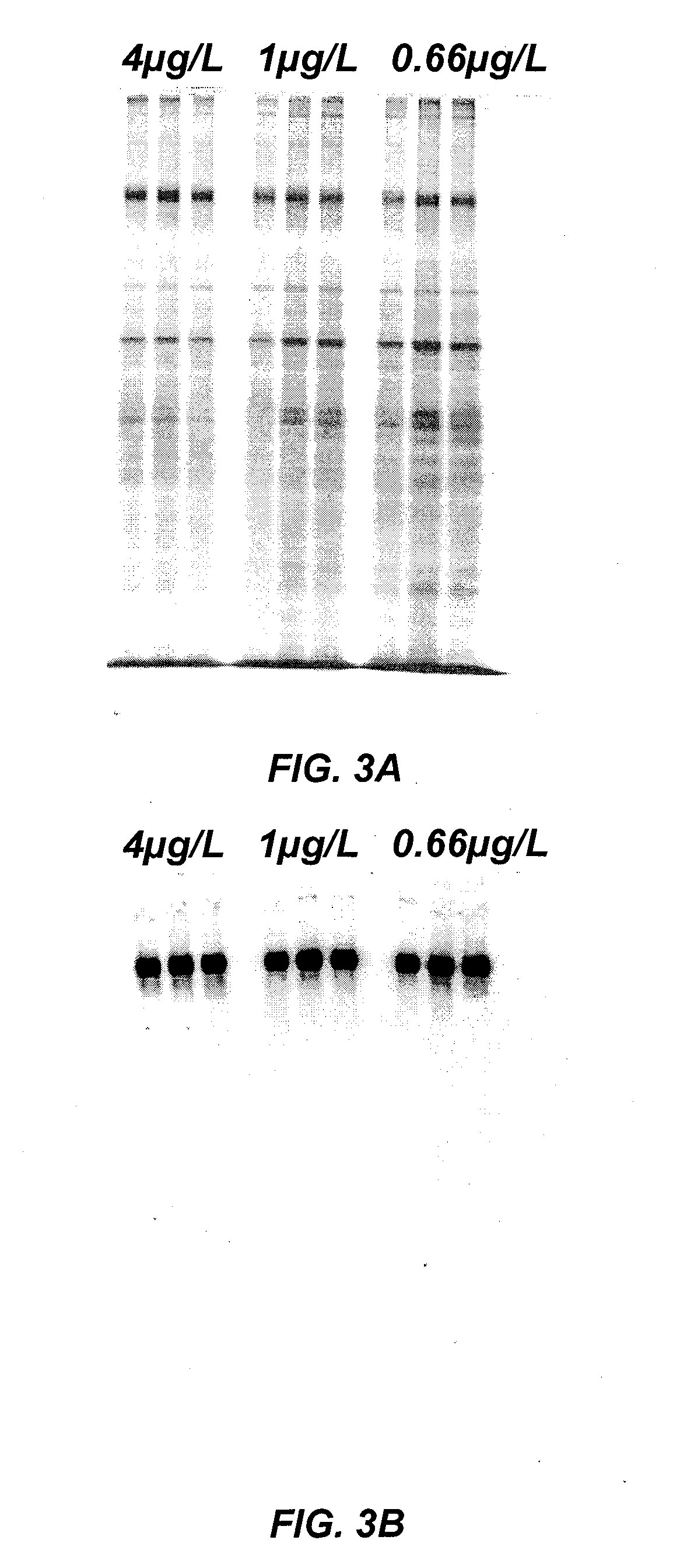
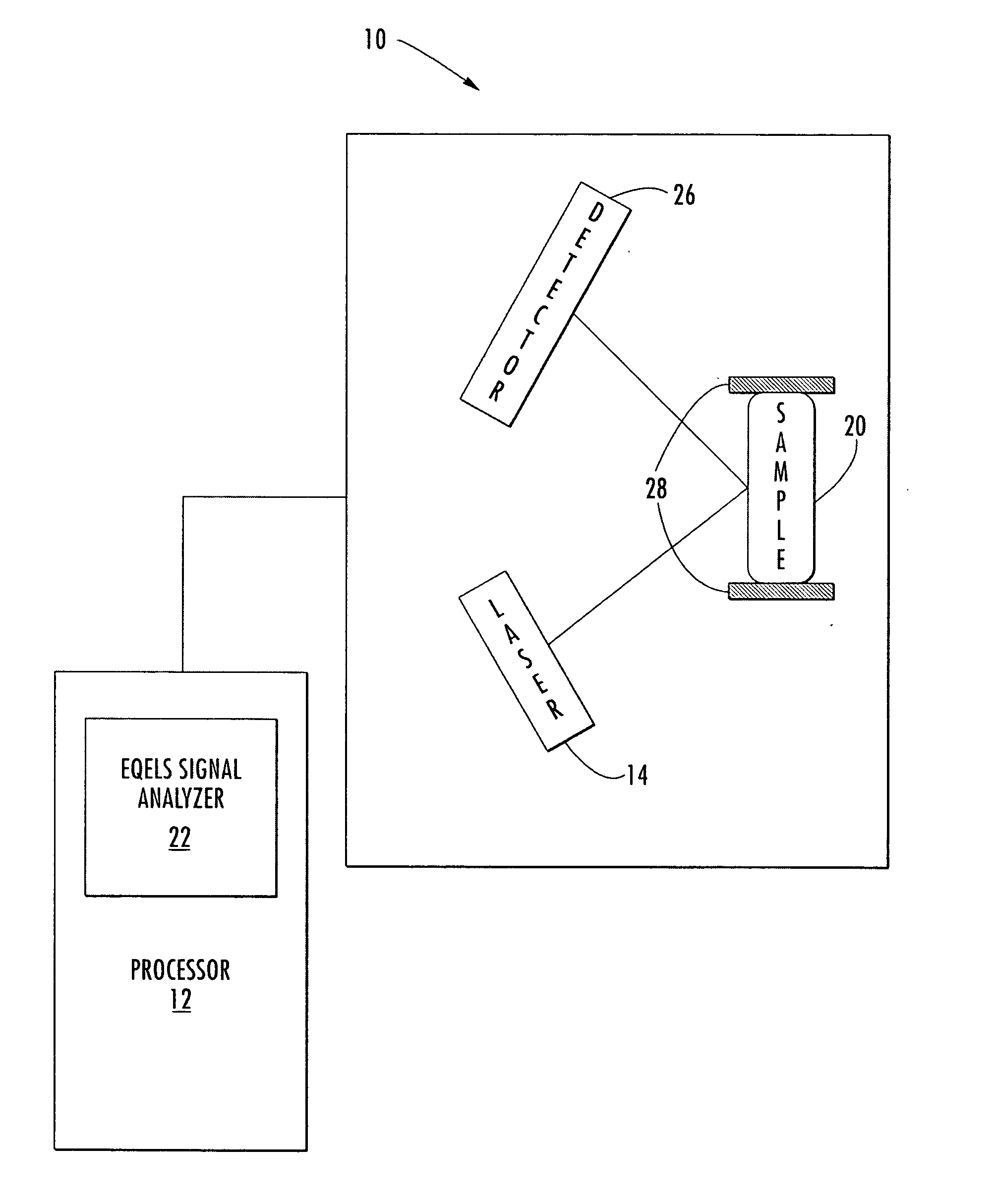
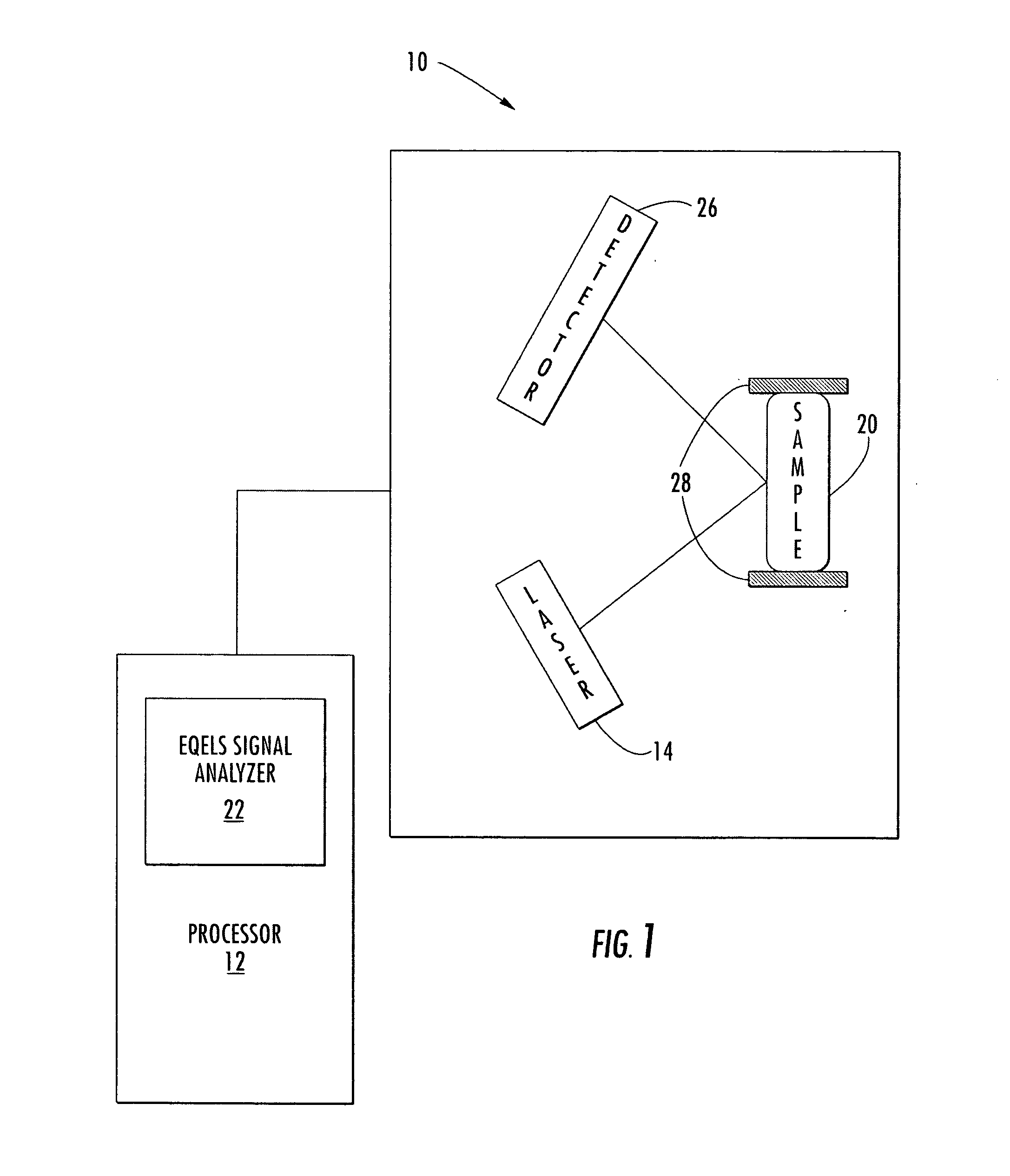
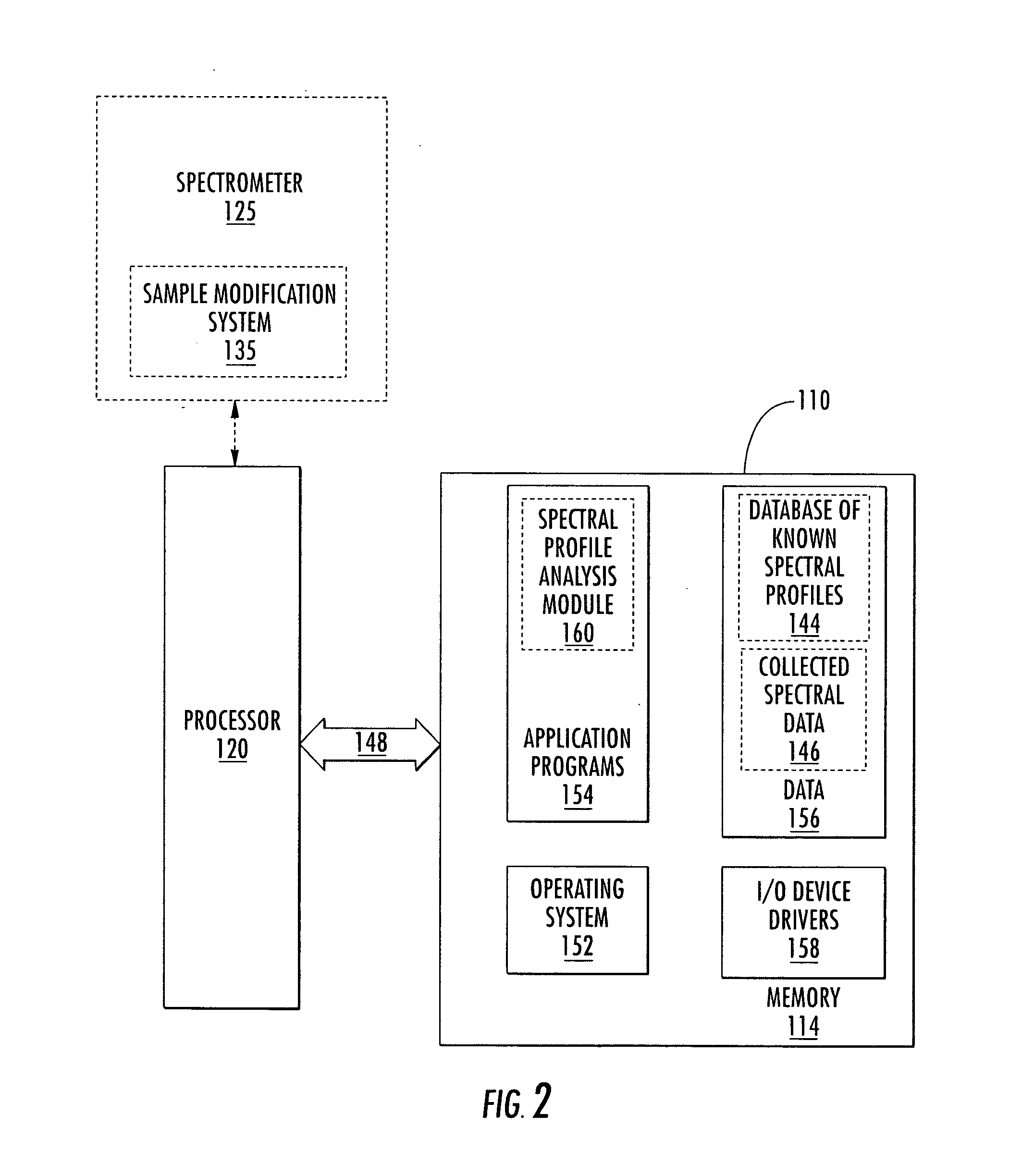
Methods of analyzing the electrophoretic mobility distribution of von Willebrand factor (vWF) multimers include providing a sample medium comprising a plurality vWF multimers. The vWF multimers are electrophoretically separated by electrohoretic mobility in the sample medium by subjecting said sample medium to an electric field to provide separated vWF multimers. The separated vWF multimers in the sample medium are exposed to a light source to produce scattered light. The scattered light is detected, and the electrophoretic mobility distribution of the separated vWF multimers is determined from the detected scattered light.
Owner:NORTH CAROLINA AT CHAPEL HILL THE UNIV OF
Fluorogenic substrate for detecting ADAMTS13 enzymatic activity and detection method
InactiveCN102533937ASimple and fast operationThe test result is accurateMicrobiological testing/measurementPeptide preparation methodsFluoresceinBlood plasma
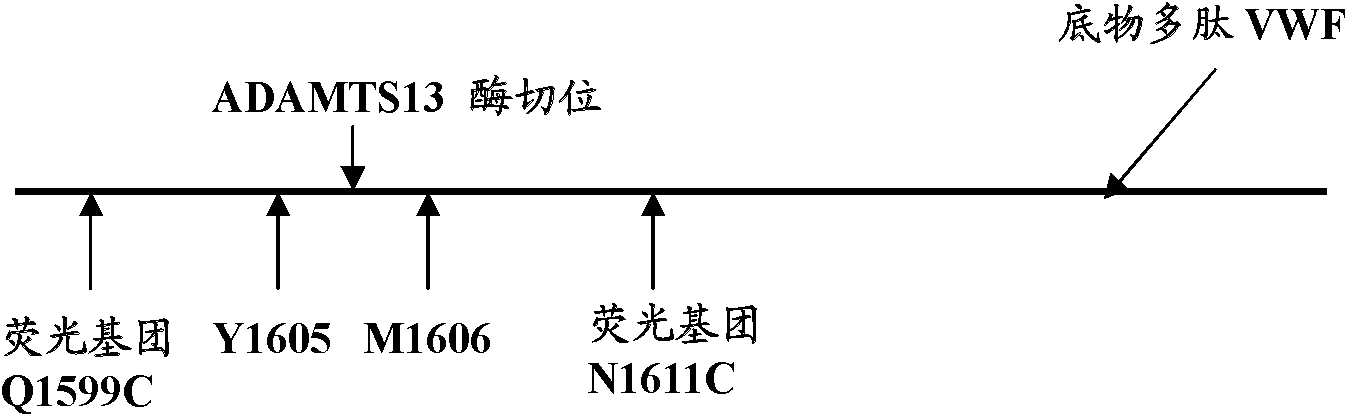
The invention belongs to the technical field of biology, in particular to a fluorogenic substrate for detecting ADAMTS13 enzymatic activity and a detection method. The fluorogenic substrate for detecting the ADAMTS13 enzymatic activity comprises an amino acid sequence shown as SEQ ID NO:2, and sulfydryl of cysteine at the 4 position and the 15 position at the N end of the amino acid sequence is combined with fluorescein-5- maleimide. Q1599C of the fluorogenic substrate is closer to N1611C modified site, simultaneously the N-end sequence of the fluorogenic substrate does not contain extra amino acid GS, and enzymolysis can be conducted on the fluorogenic substrate through ADAMTS13 more effectively. Therefore, sensitivity for detecting ADAMTS13 enzymatic activity can be improved. The method for detecting the ADAMTS13 enzymatic activity is convenient to operate, accurate in detection result, high in sensitivity and suitable for quick detection of the ADAMTS13 enzymatic activity in blood plasma in hospitals, health departments and medical research and development institutions at different levels.
Owner:THE FIRST AFFILIATED HOSPITAL OF SOOCHOW UNIV
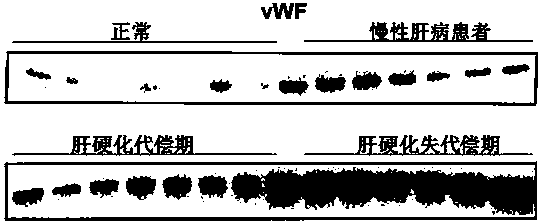
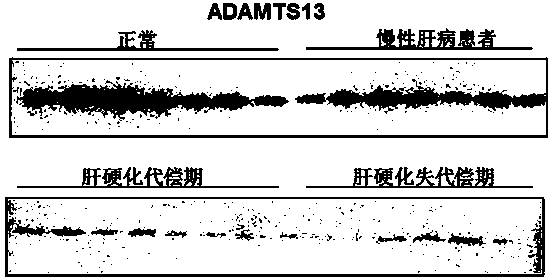
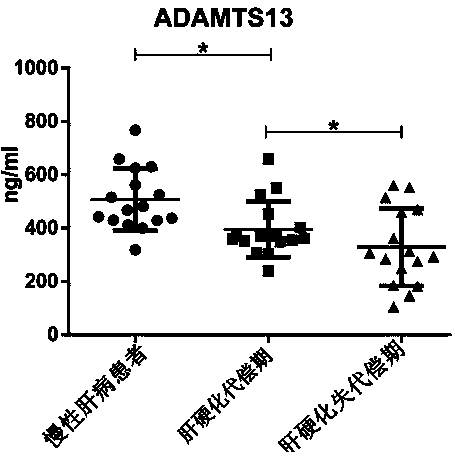
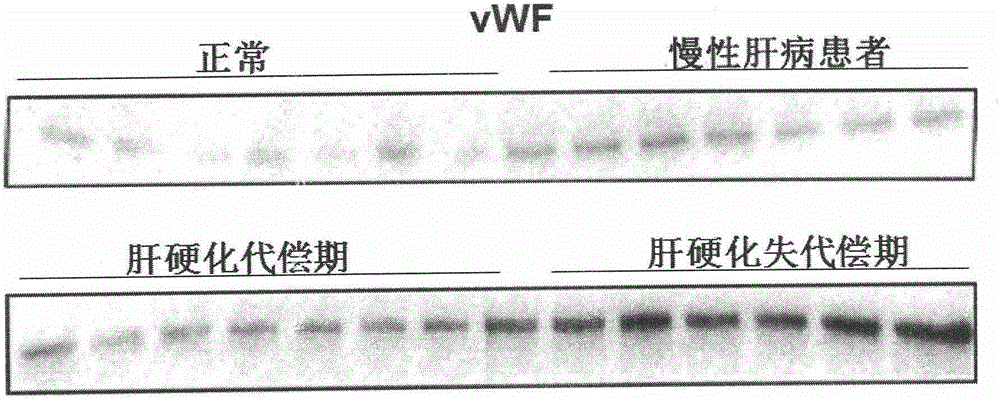
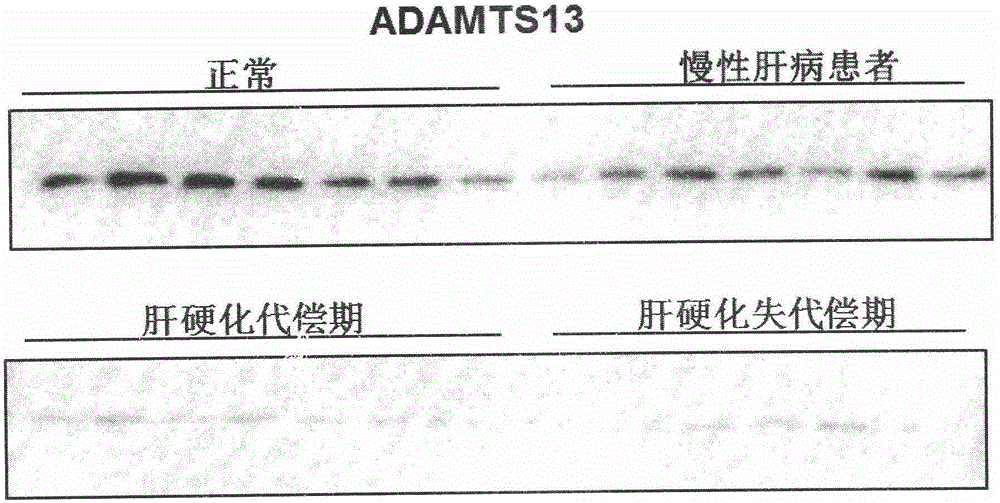
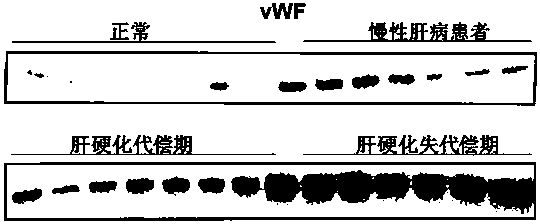
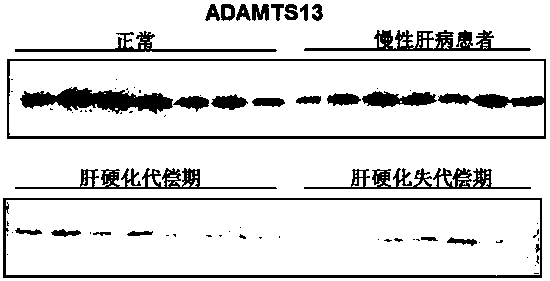
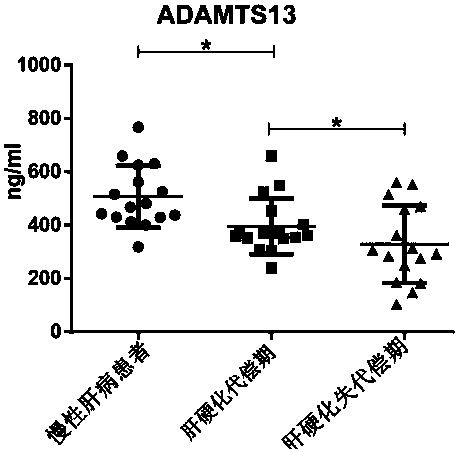
Biomarkers von willebrand factor (VWF) and ADAMTS13 and use thereof in cirrhosis diagnosis reagent
ActiveCN103808944AEasy to operateEasy screening and selectionDisease diagnosisBiological testingDiseaseProtein composition
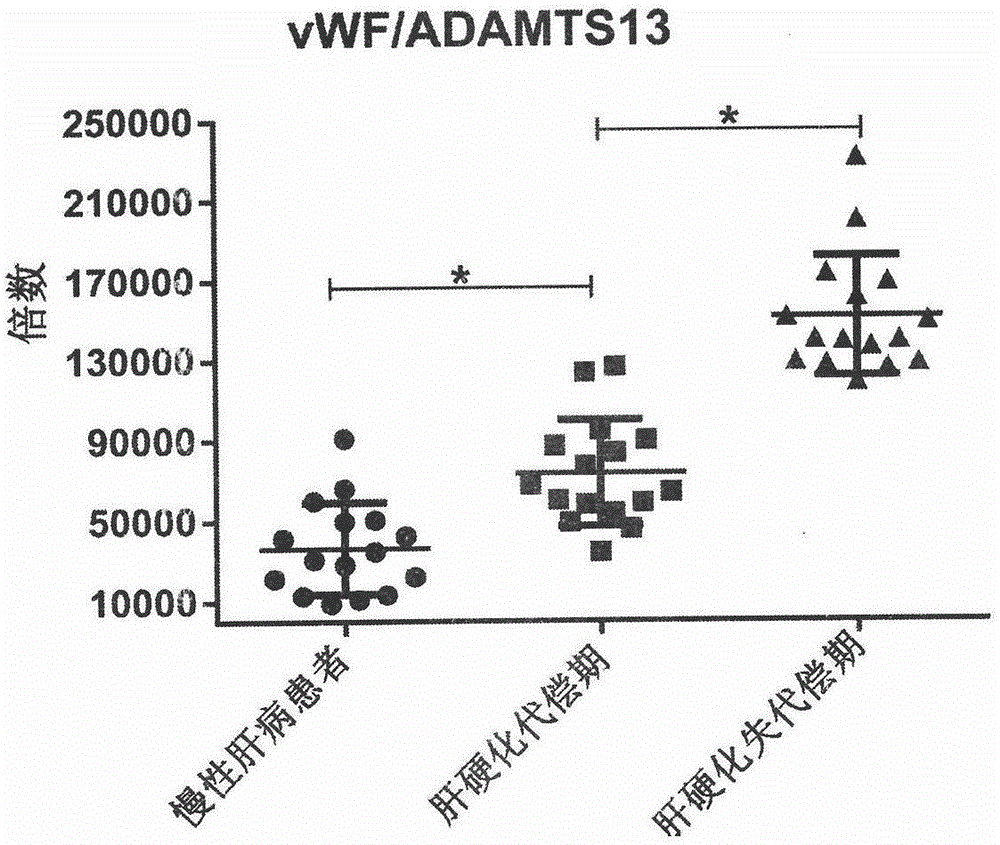
The invention relates to the field of use of a biomarker for a disease, and discloses biomarkers von willebrand Factor (VWF) and ADAMTS13 and use thereof in a cirrhosis diagnosis reagent. The VWF disclosed by the invention is formed by protein of which the accession number of an amino acid sequence in a database for the national center of biotechnology information (NCBI) is GI317373549; the ADAMTS13 is formed by protein of which the accession number in the database for the NCBI is GI74749836. A testee is subjected to cirrhosis diagnosis by the biomarkers VWF and ADAMTS13, and the biomarkers have the advantages of being simple and feasible, safe and free of a wound in the diagnosis process, easy to be accepted by a patient, small in effect on diagnostic criteria unification caused by personal subjective factors, high in specificity and sensitivity on diagnosis result, easy to carry out a lot of screening for selection, and the like.
Owner:重庆早柒天生物科技股份有限公司
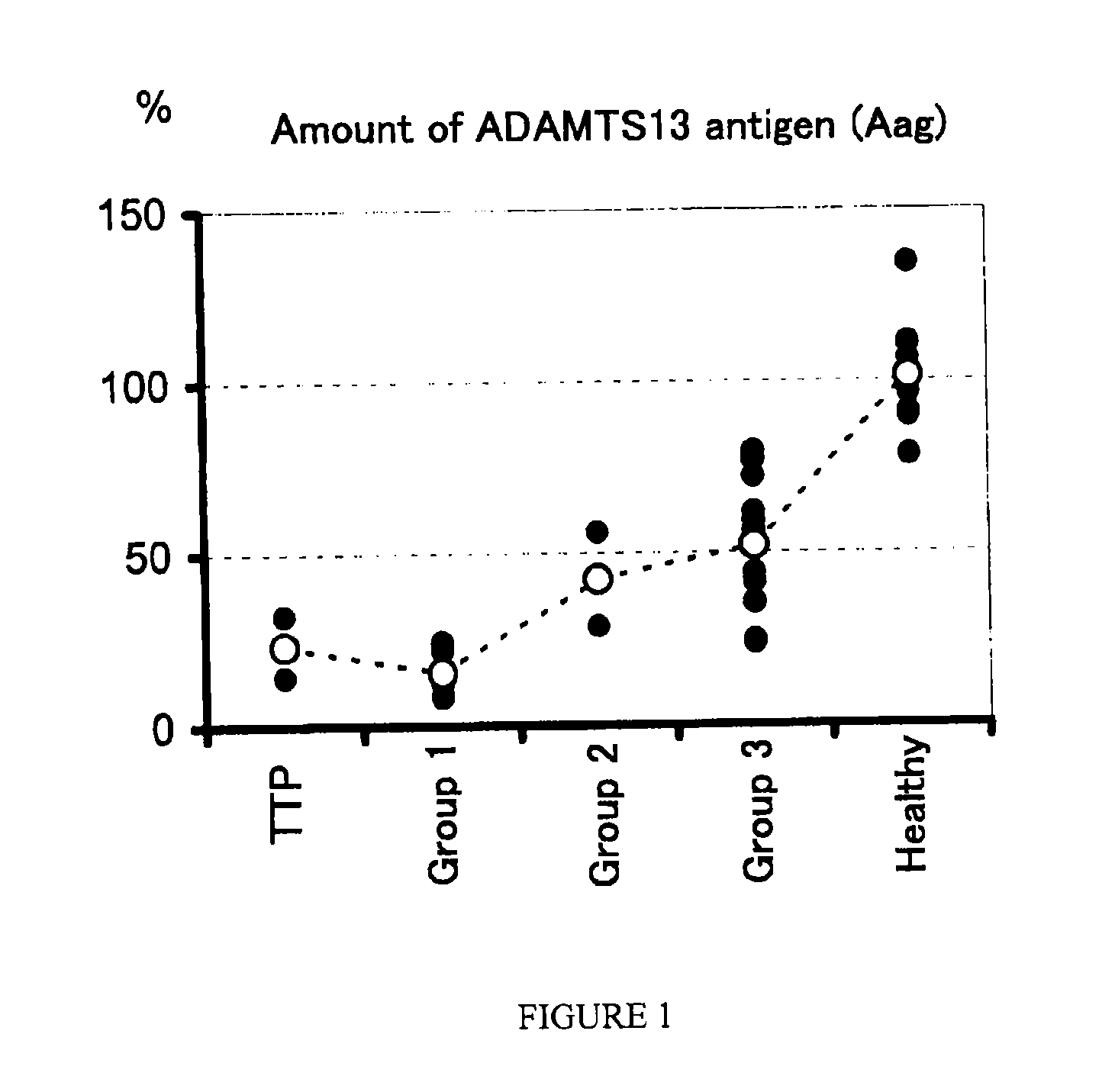
Method for Determining Condition of Disseminated Intravascular Coagulation
InactiveUS20090004673A1Difficult to accuratelyImprove survival rateMicrobiological testing/measurementDisease diagnosisProteinase activityFactor VIII vWF
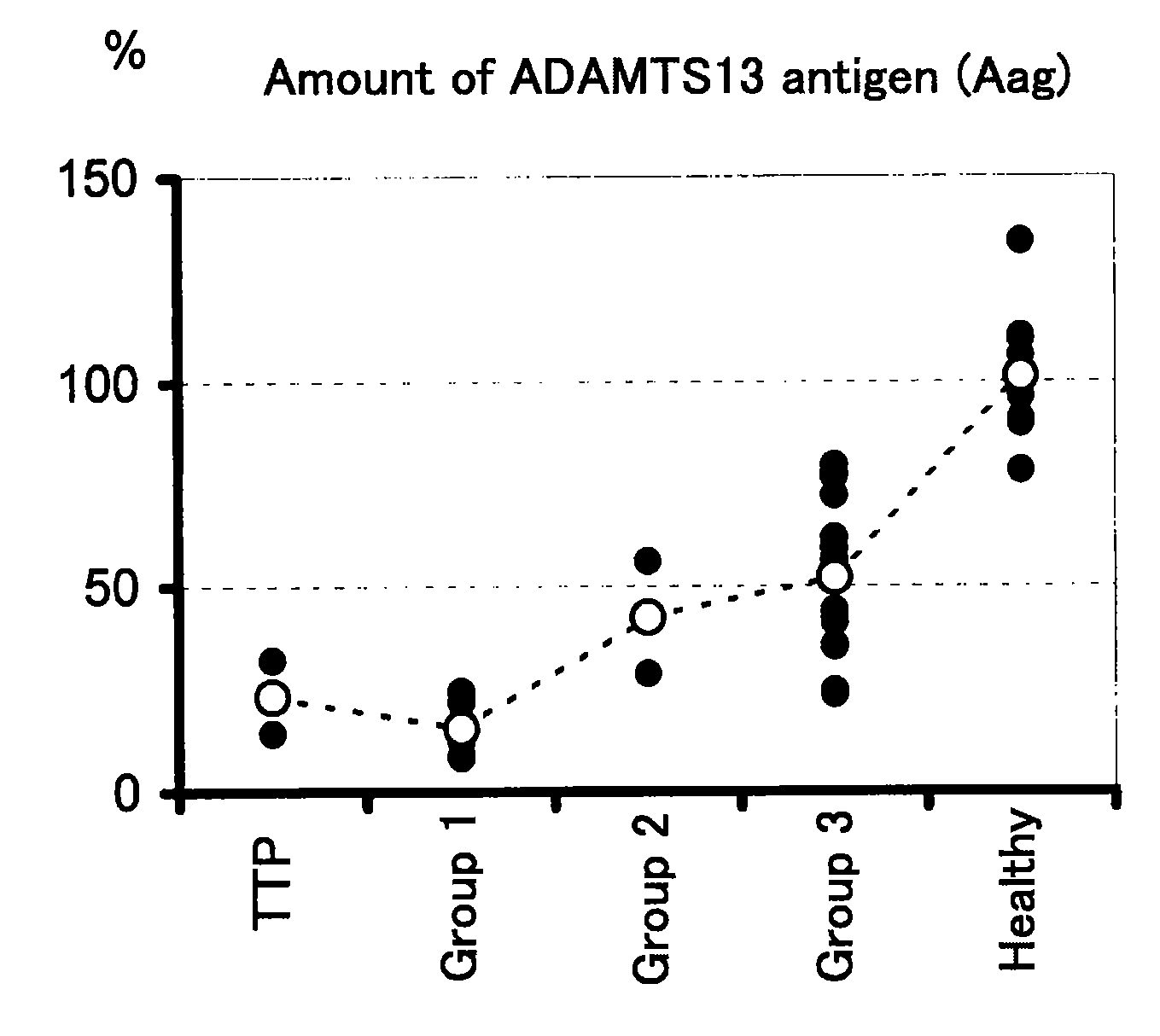
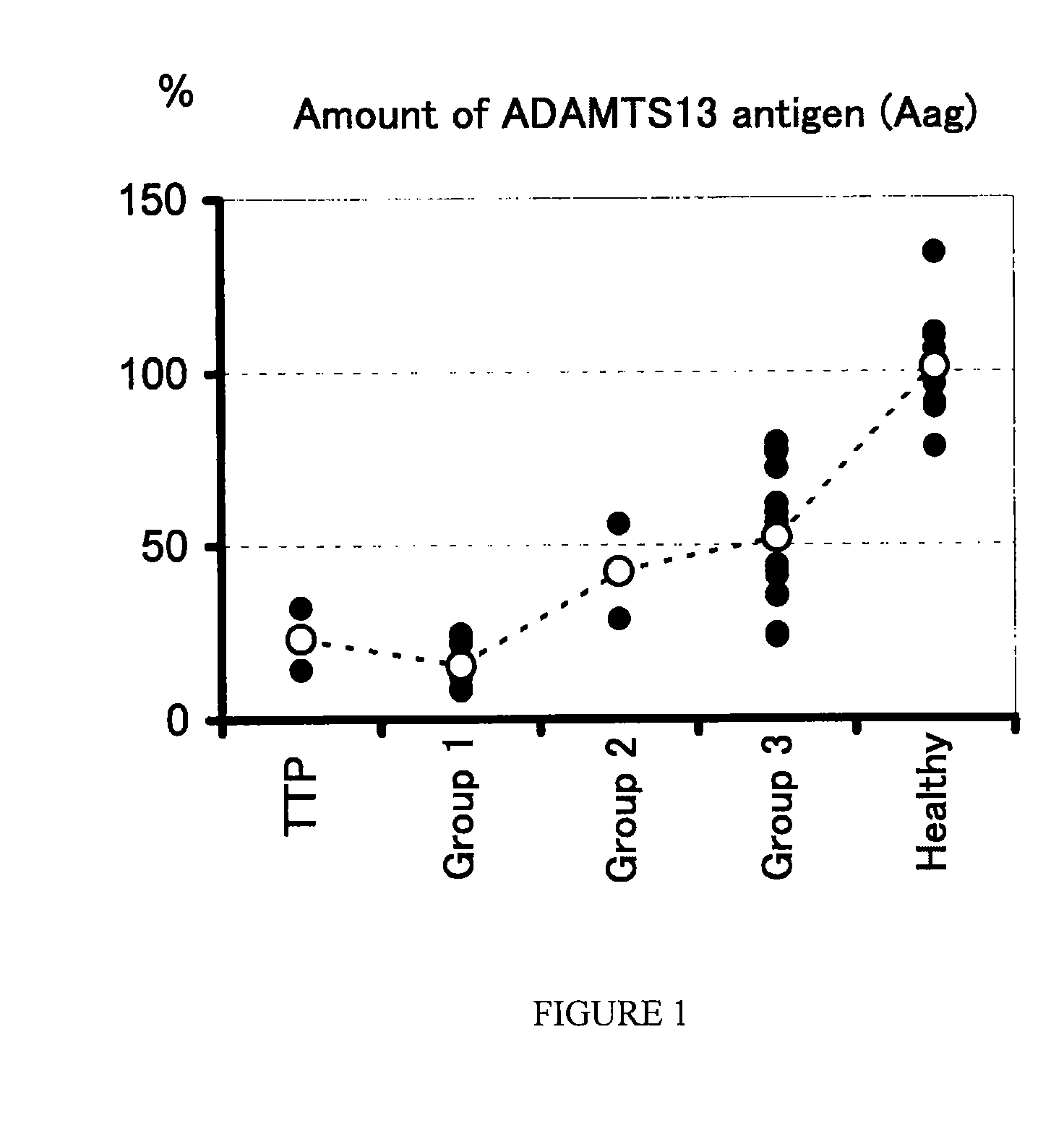
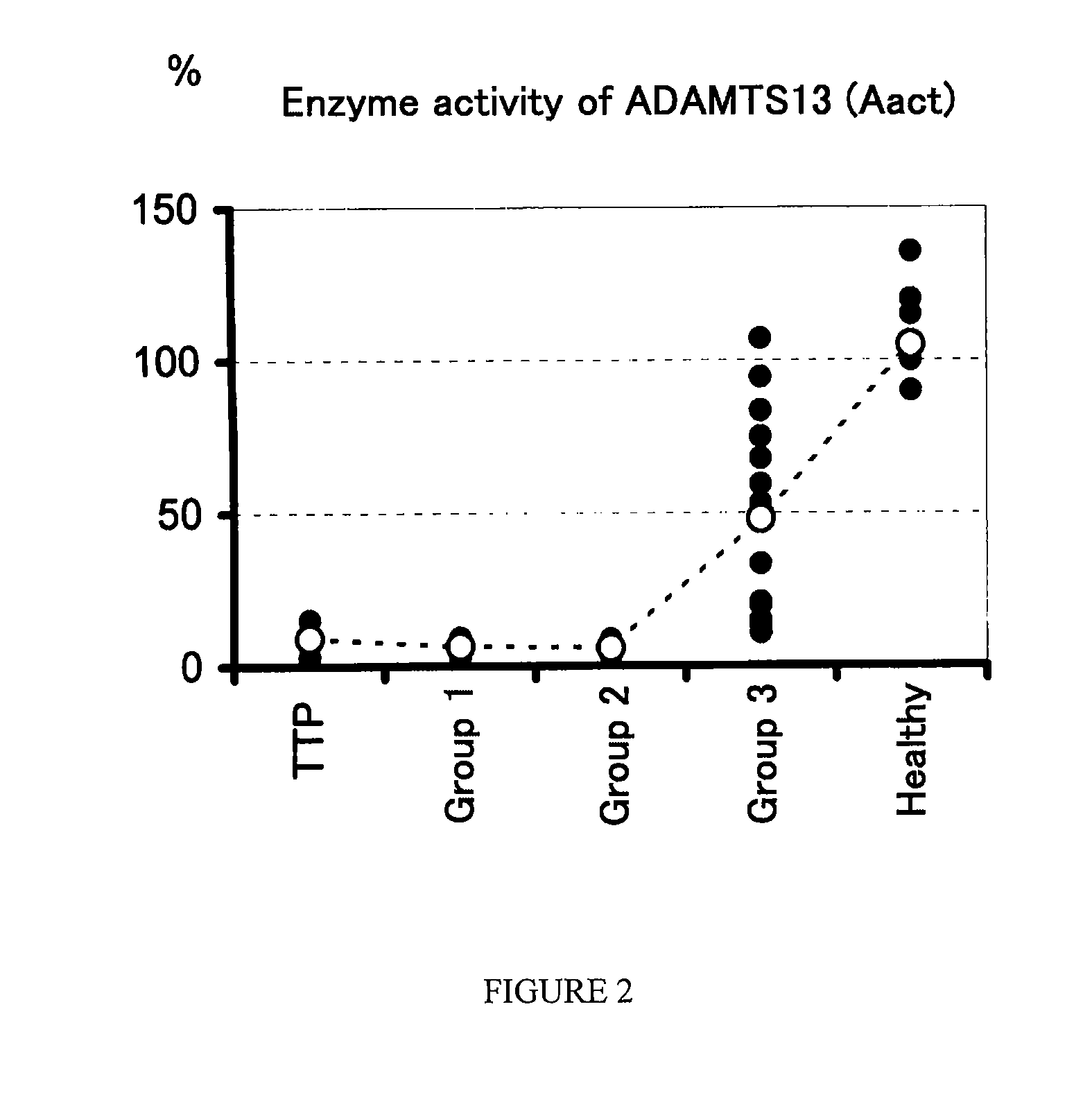
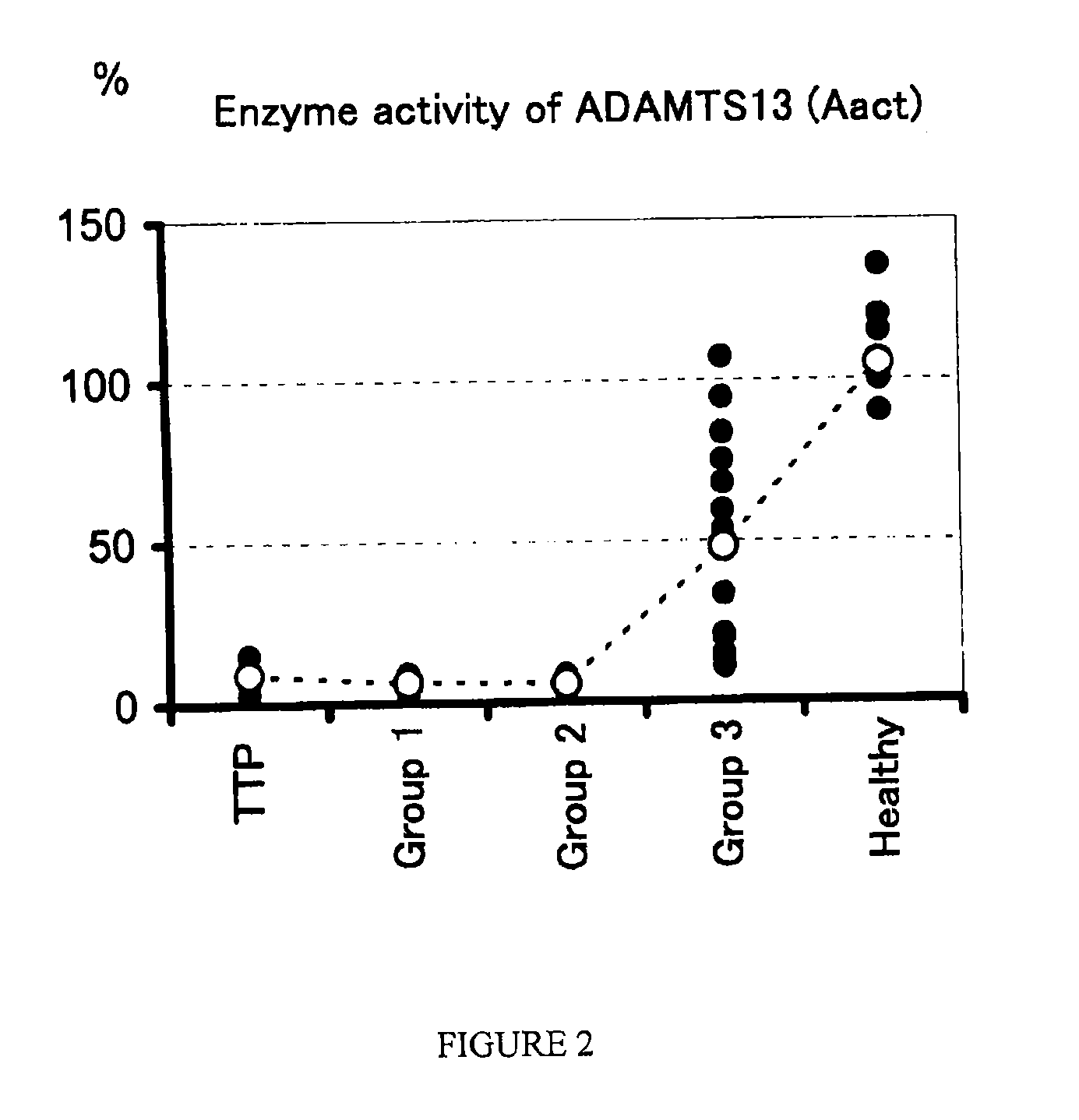
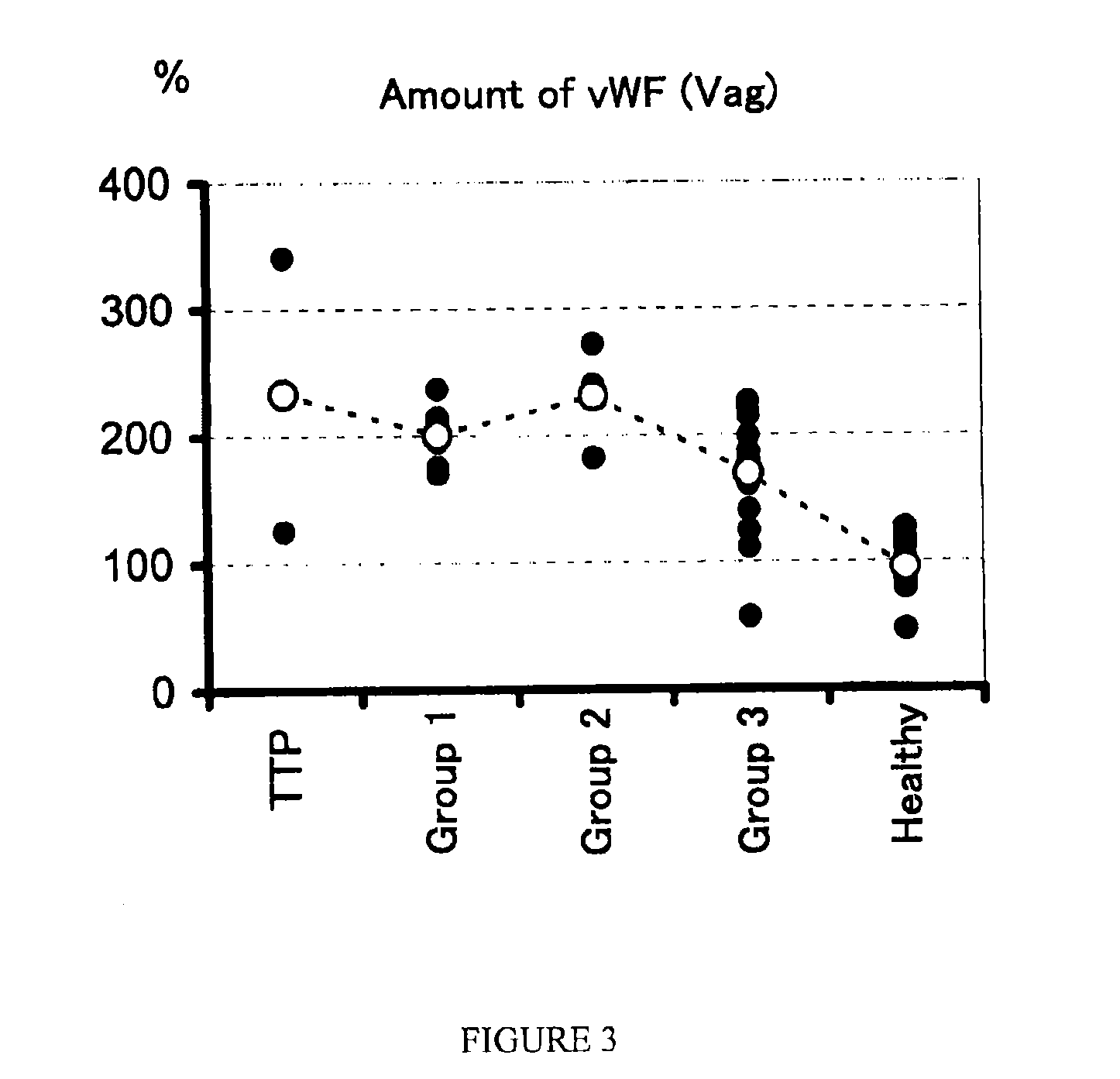
A method for determining a condition of disseminated intravascular coagulation (DIC), by analyzing the amount and / or enzyme activity of a von Willebrand factor (vWF)-cleaving protease (ADAMTS13) (preferably also the amount of vWF) in a patient suffering from DIC, and a kit for determining a condition of DIC, comprising an antibody or a fragment thereof which specifically binds to ADAMTS13, are disclosed. According to the present invention, a differential diagnosis of patients with thrombotic thrombocytopenic purpura (TTP) can be carried out from among patients with DIC, which could not be distinguished on the basis of only clinical findings or known markers.
Owner:MITSUBISHI CHEM MEDIENCE +1
Biomarkers VWF and ADAMTS13 and use thereof in liver cirrhosis diagnostic reagents
ActiveCN106066401AEasy to operateEasy screening and selectionDisease diagnosisBiological testingProtein compositionAmino acid
Owner:CHONGQING ZAOQITIAN BIOTECHNOLOGY CO LTD
Biomarkers vwf and adamts13 and their use in diagnostic reagents for liver cirrhosis
ActiveCN103808944BEasy to operateEasy screening and selectionDisease diagnosisBiological testingDiseaseProtein composition
The invention relates to the field of use of a biomarker for a disease, and discloses biomarkers von willebrand Factor (VWF) and ADAMTS13 and use thereof in a cirrhosis diagnosis reagent. The VWF disclosed by the invention is formed by protein of which the accession number of an amino acid sequence in a database for the national center of biotechnology information (NCBI) is GI317373549; the ADAMTS13 is formed by protein of which the accession number in the database for the NCBI is GI74749836. A testee is subjected to cirrhosis diagnosis by the biomarkers VWF and ADAMTS13, and the biomarkers have the advantages of being simple and feasible, safe and free of a wound in the diagnosis process, easy to be accepted by a patient, small in effect on diagnostic criteria unification caused by personal subjective factors, high in specificity and sensitivity on diagnosis result, easy to carry out a lot of screening for selection, and the like.
Owner:重庆早柒天生物科技股份有限公司
Detection of circulating adamts13-antibody complexes
The present invention relates to methods and means for detecting ADAMTS13 immune complexes in a sample. The methods include the steps of capturing and labelling immune complexes of anti-ADAMTS13 antibodies. Capturing and labelling may be achieved by two different binding units targeting the immune complexes. The invention further relates to diagnosing diseases associated with immunologic ADAMTS13 dysfunction like TTP (thrombotic thrombocytopenic purpura).
Owner:BAXALTA GMBH
Haemorrhagic disorder due to ventricular assist device
ActiveUS10829562B2Avoid complicationsIncrease shear stressAntibody ingredientsBlood disorderDiseaseDevice implant
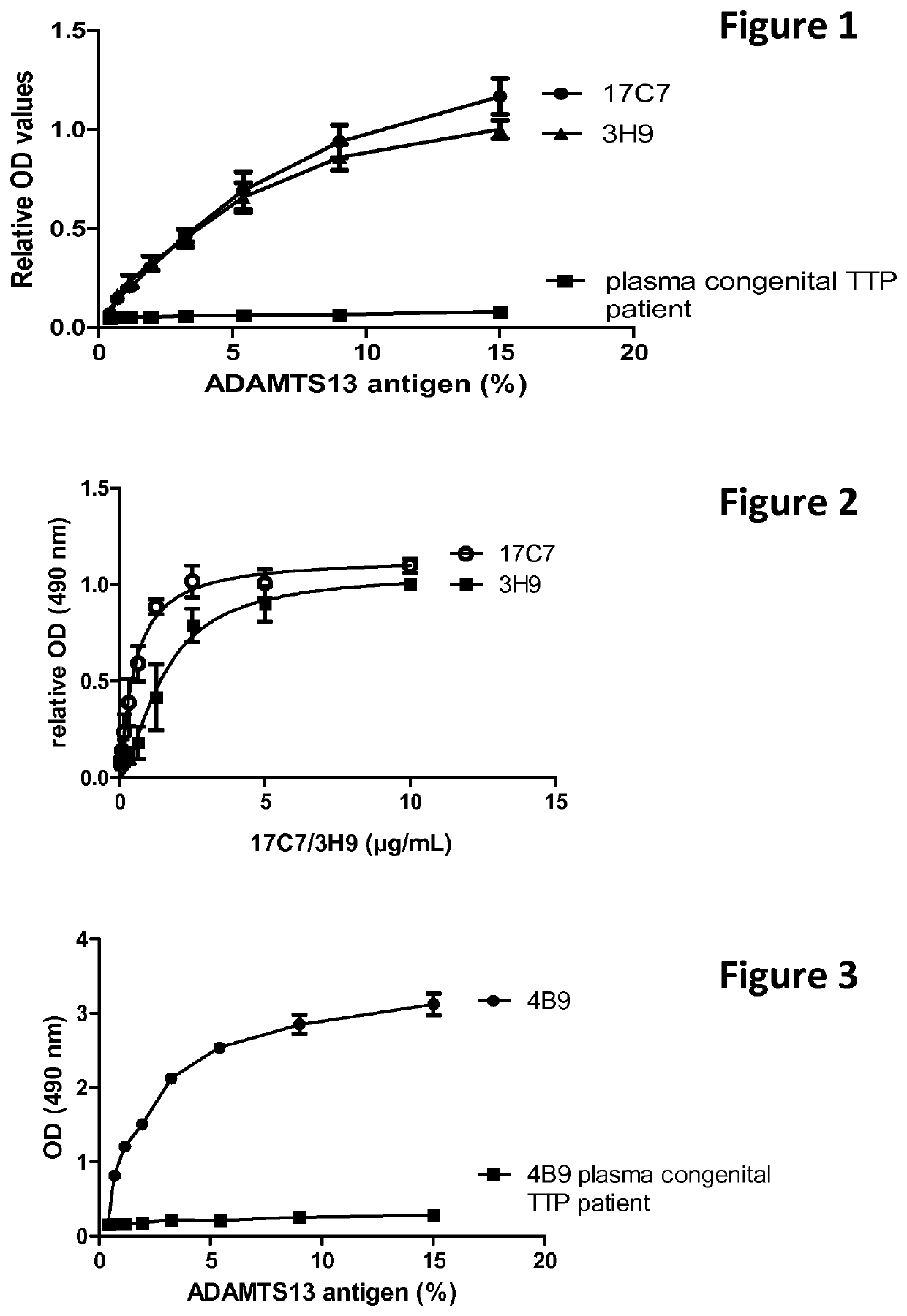
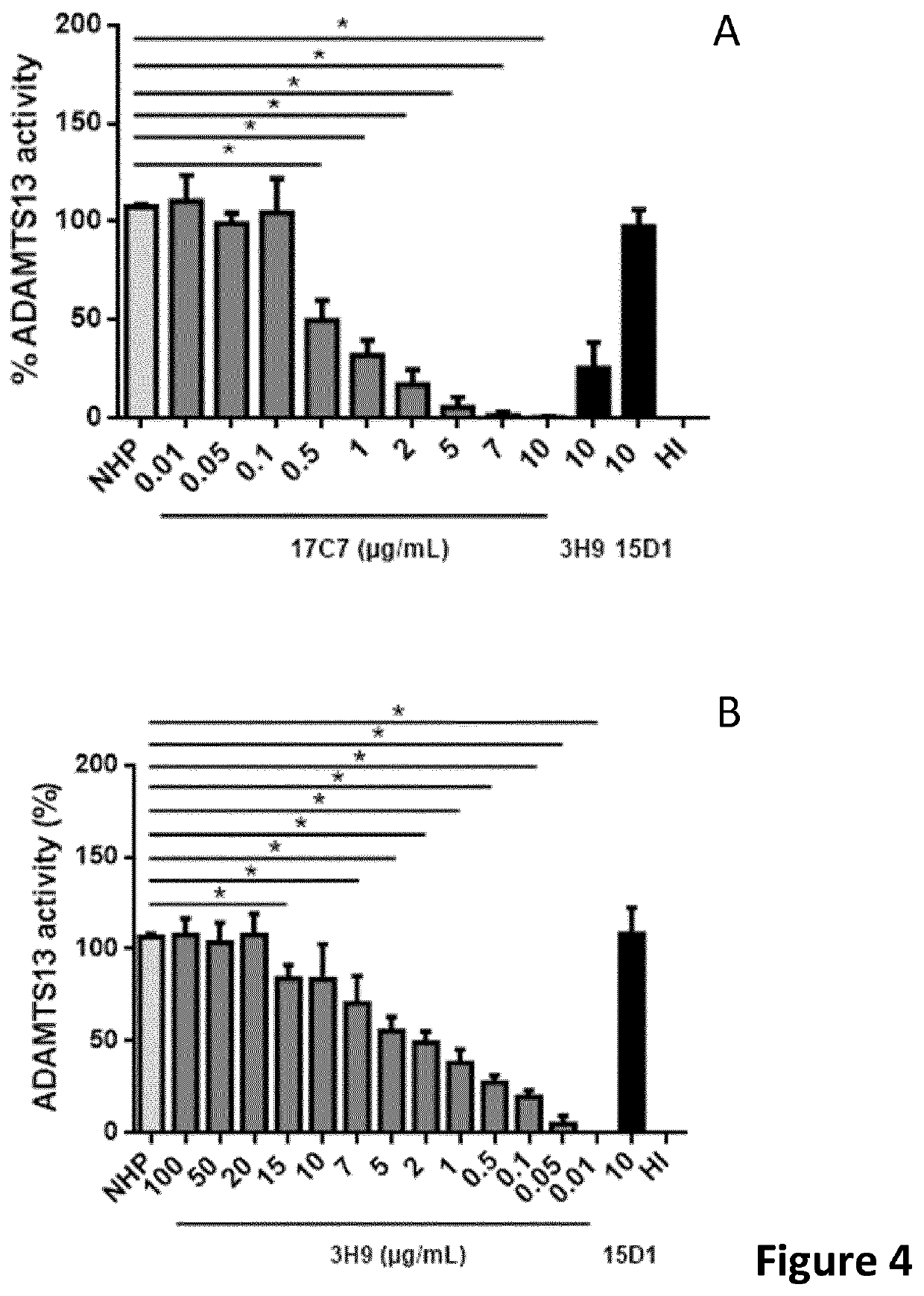
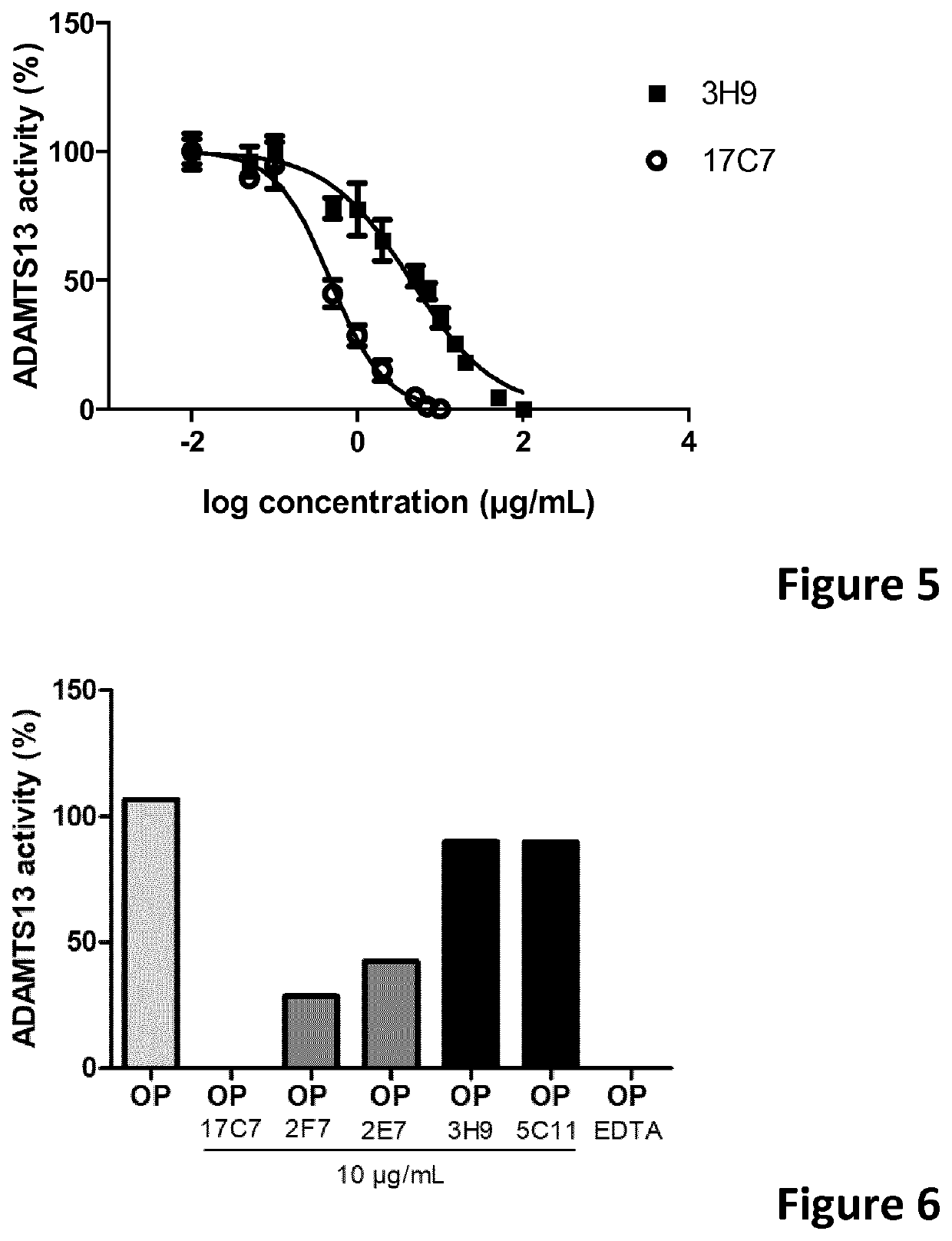
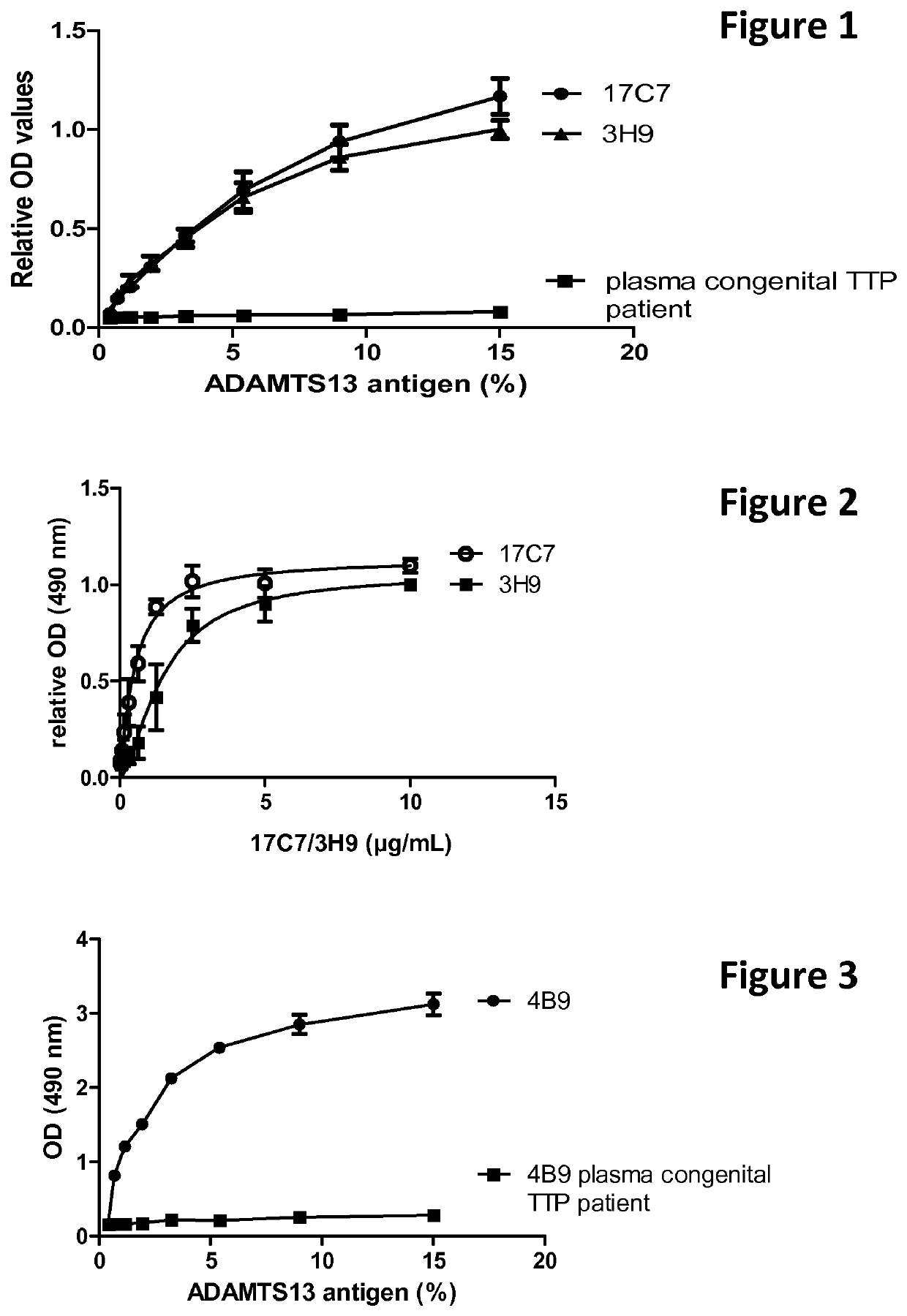
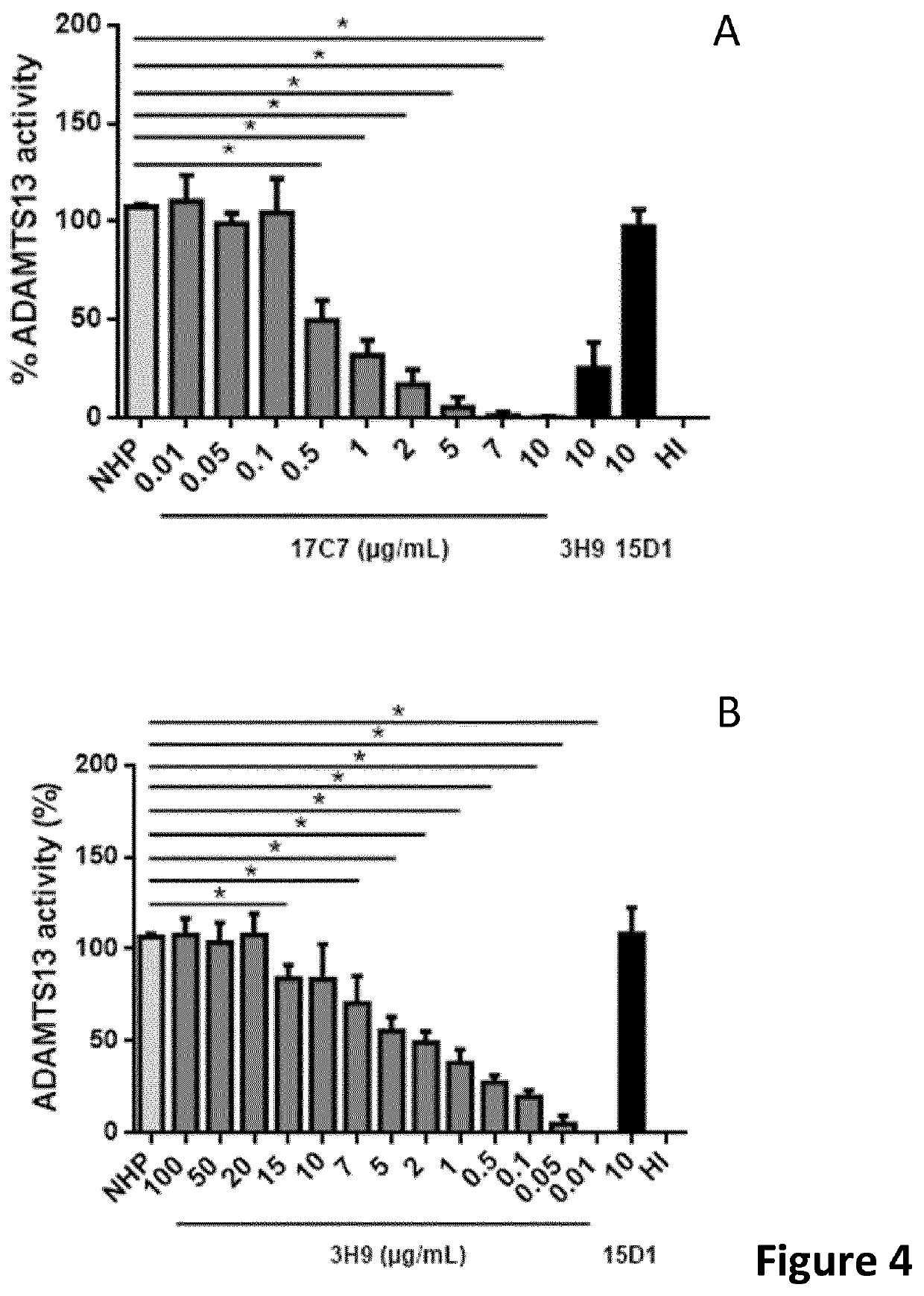
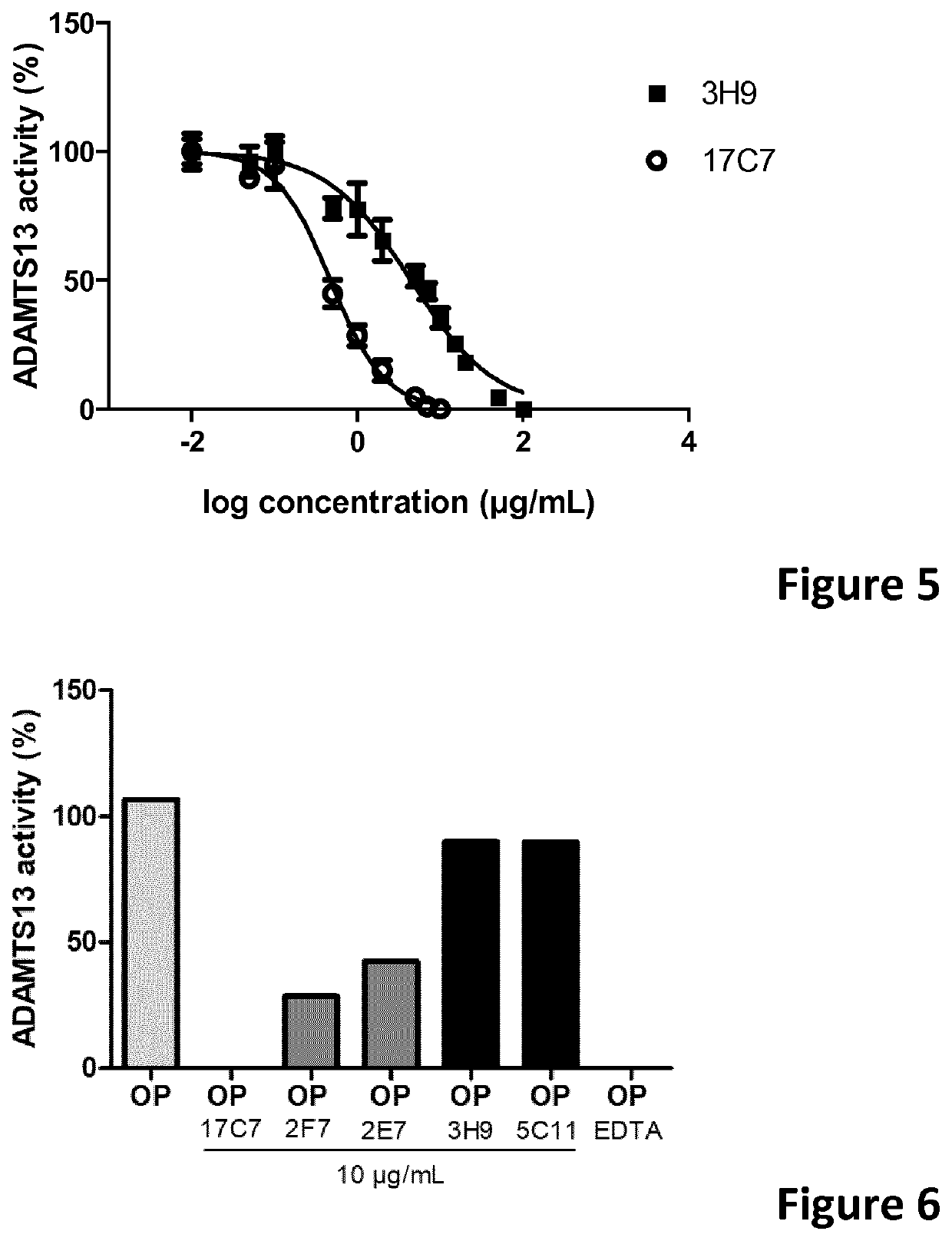
The present invention relates to methods of treatment by human ADAMTS13 inhibition in circulatory assist device induced haemorrhagic complication such as a bleeding disorder, in particular, bleeding after left ventricular assist device implantation. The present invention further relates to specific monoclonal antibodies inhibiting ADAMTS13 function.
Owner:KATHOLIEKE UNIV LEUVEN
Application of ADAMTS13 to preparation of drugs used for promoting vascular remodeling after stroke
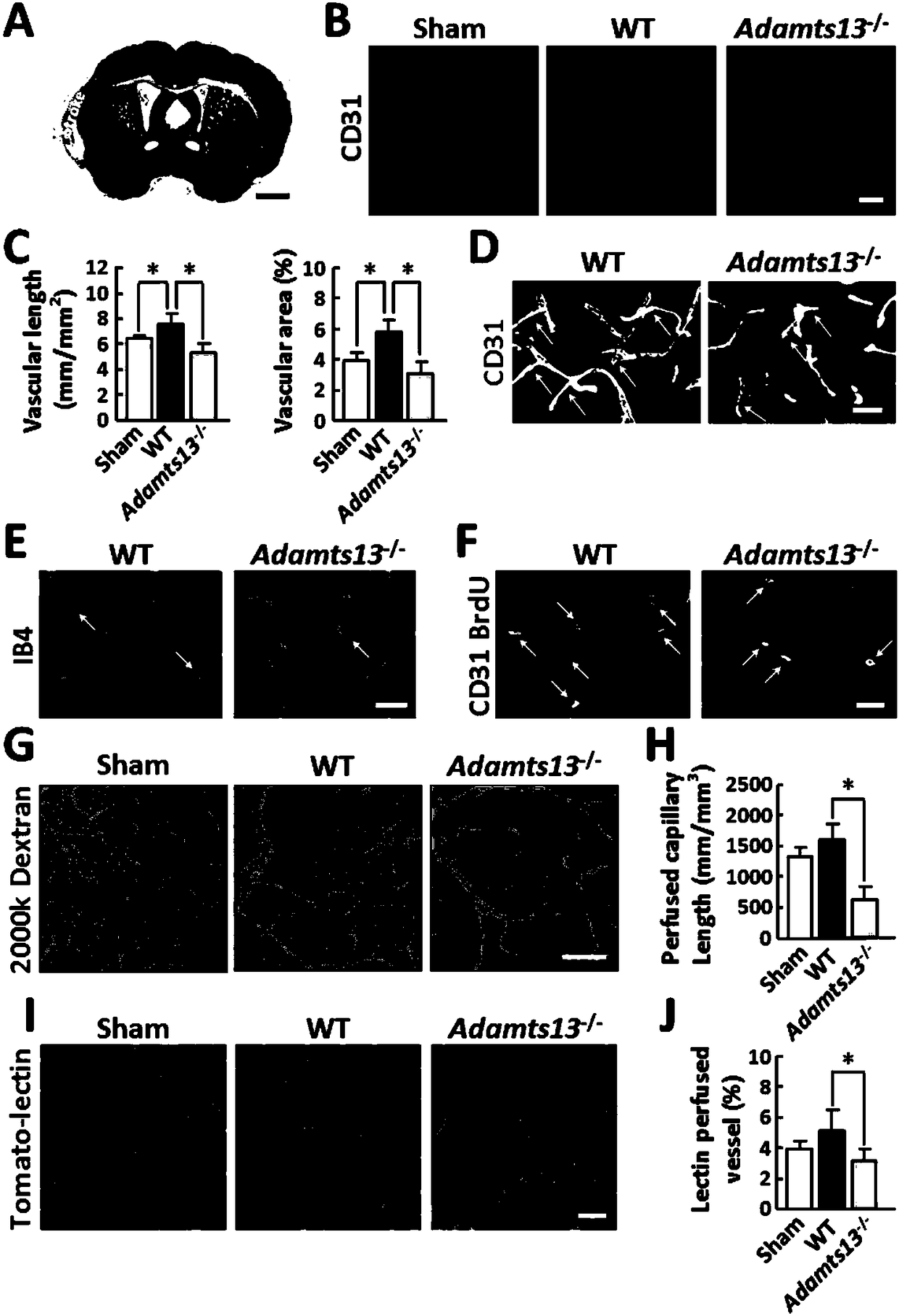
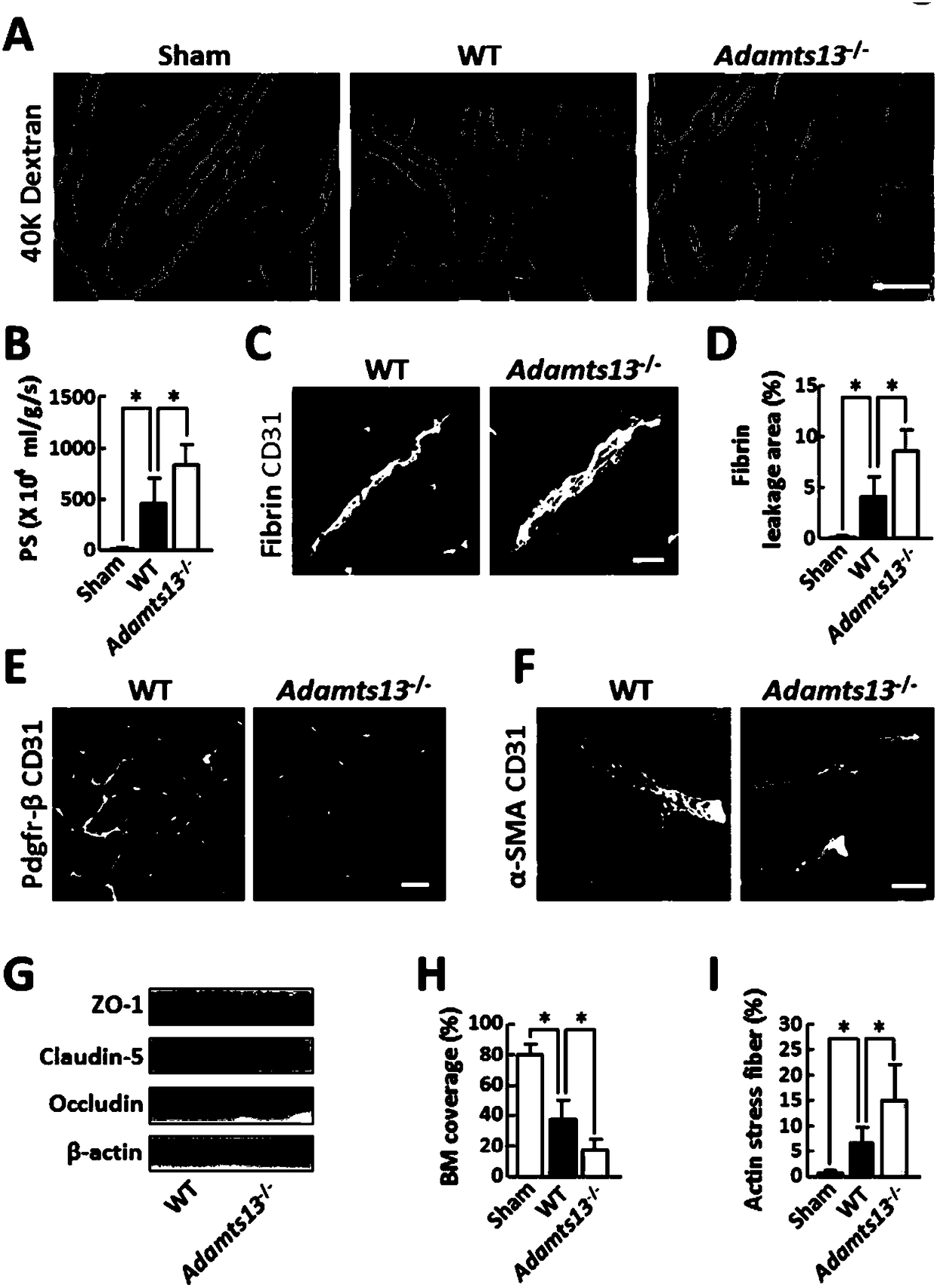
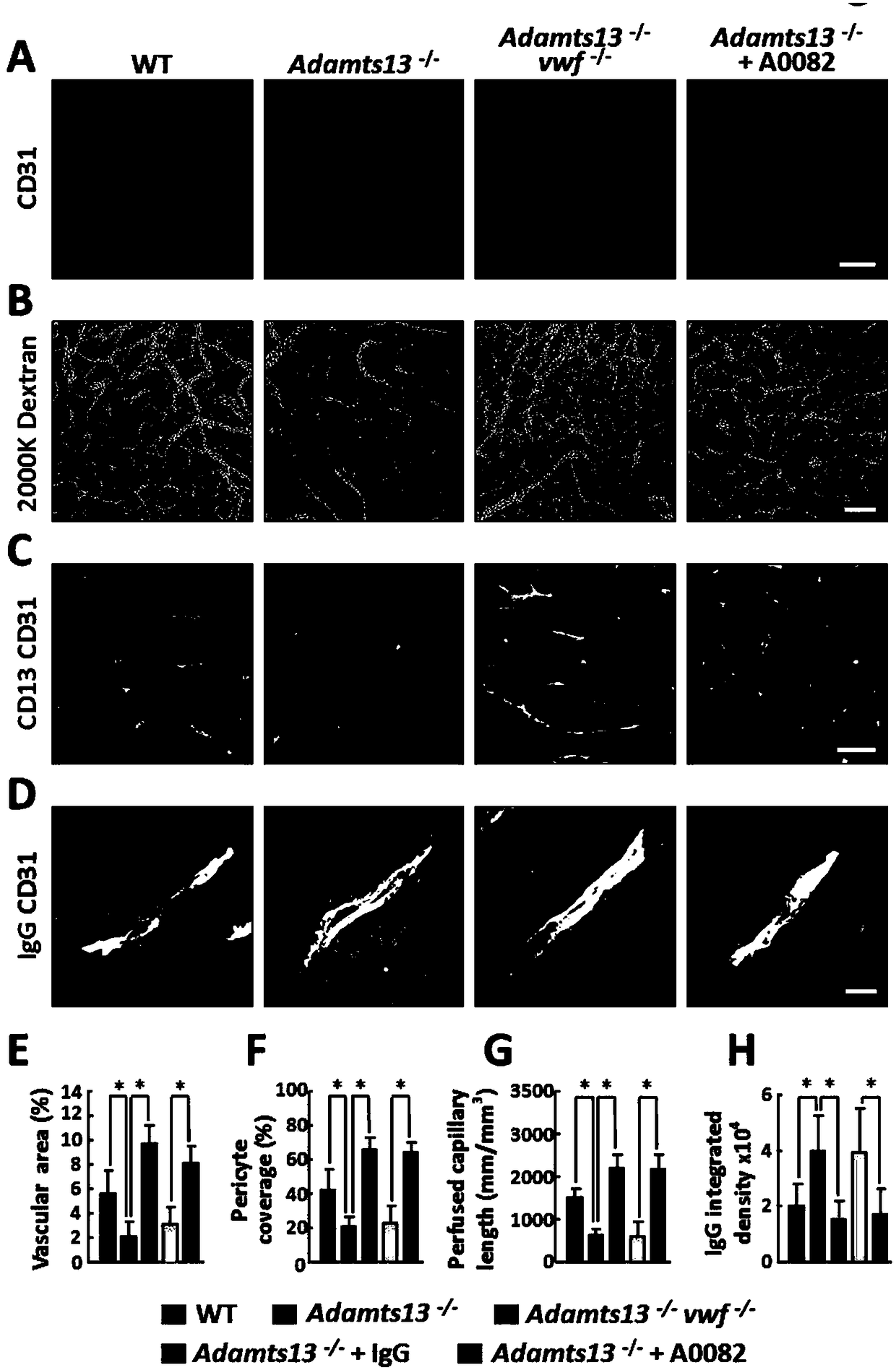
The invention relates to application of ADAMTS13 to preparation of drugs for vascular remodeling, especially application of ADAMTS13 to preparation of drugs used for promoting vascular remodeling after stroke, belonging to the field of biopharmacy. The experimental results of the invention prove that the loss of ADAMTS13 leads to decrease in both revascularization and vascular integrity and aggravation of vascular damage in the peripheral cortex of cerebral ischemia mice; exogenous recombinant ADAMTS13 can significantly increase revascularization, promote the repair of vascular functions and improve the behavioral functions of the cerebral ischemic mice; ADAMTS13 plays an important role in regulating revascularization in the recovery phase of cerebral ischemic; inhibition of revascularization and vascular maturation due to the loss of ADAMTS13 may be related to the opening of vascular permeability; and ADAMTS13 promotes vascular remodeling after cerebral ischemia through the VWF-Ang-2 / Gal-3-pVEGFR-2 pathway.
Owner:FUDAN UNIV
Humanised adamts13 binding antibodies
ActiveUS20200308303A1Improve propertiesAvoid complicationsAntibody ingredientsBlood disorderDiseaseDevice implant
The present invention further relates to specific humanised monoclonal antibodies inhibiting ADAMTS13 function. The present invention relates to methods of treatment by human ADAMTS13 inhibition in circulatory assist device induced haemorrhagic complication such as a bleeding disorder, in particular, bleeding after left ventricular assist device (LVAD) implantation.
Owner:KATHOLIEKE UNIV LEUVEN
Use of recombinant adamts13 in the preparation of intracerebral hemorrhage drugs
ActiveCN103566362BImprove permeabilityAvoid damagePeptide/protein ingredientsCardiovascular disorderPhosphorylationVegf expression
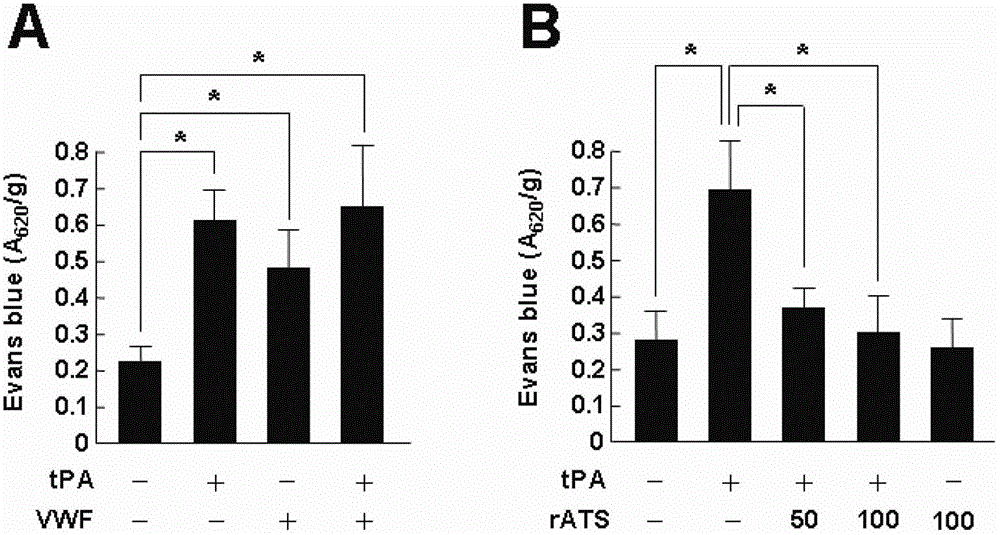
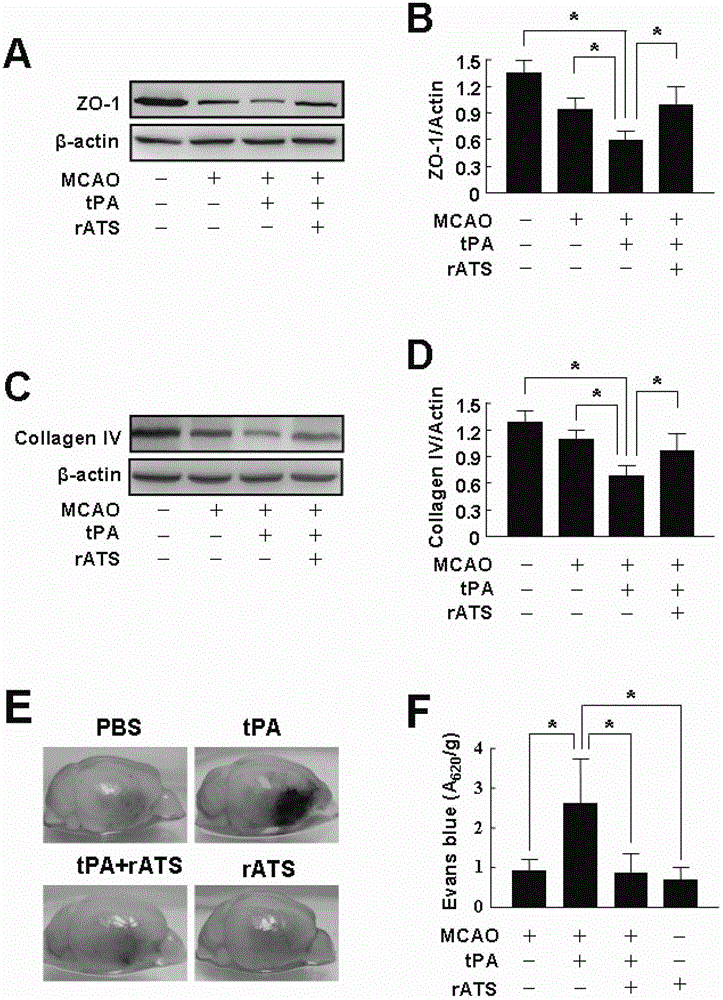
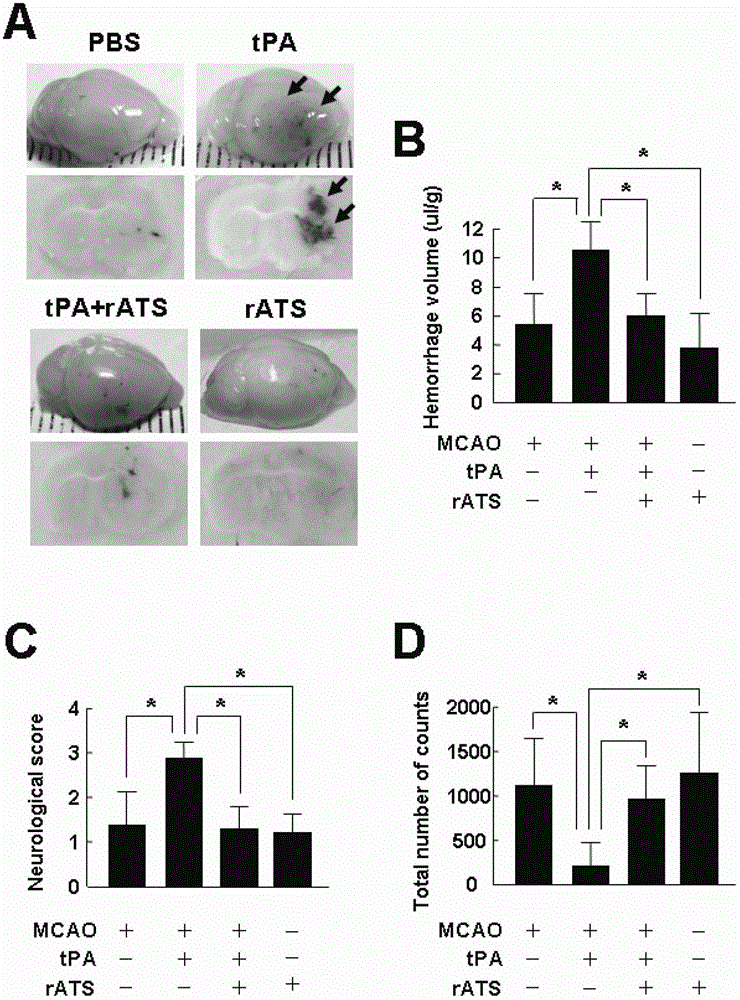
The invention belongs to the field of biological pharmacy, relates to application of recombinant ADAMTS13 to preparation of cerebral hemorrhage medicaments, and especially relates to application of recombinant ADAMTS13 to preparation for medicaments for reducing cerebral hemorrhage caused by tPA thrombolytic therapy on cerebral ischemia. Experiment results show that: VEGF generated by tPA induction at ischemic condition can be blocked by a specific inhibitor of RhoA or Akt, and recombinant ADAMTS13 substantially reduces activation of RhoA and phosphorylation of Akt, which means that recombinant ADAMTS1 is capable of inhibiting tPA-induced VEGF expression through RhoA and Akt pathways. The recombinant ADAMTS13 provided by the invention helps to mitigate damage of tPA to blood cerebral barrier and further to reduce cerebral hemorrhage caused thereby by inhibiting cerebrovascular permeability increase mediated by RhoA and Akt, and therefore combination usage of recombinant ADAMTS13 and tPA is a novel countermeasure and means for increasing thrombolysis security.
Owner:FUDAN UNIV
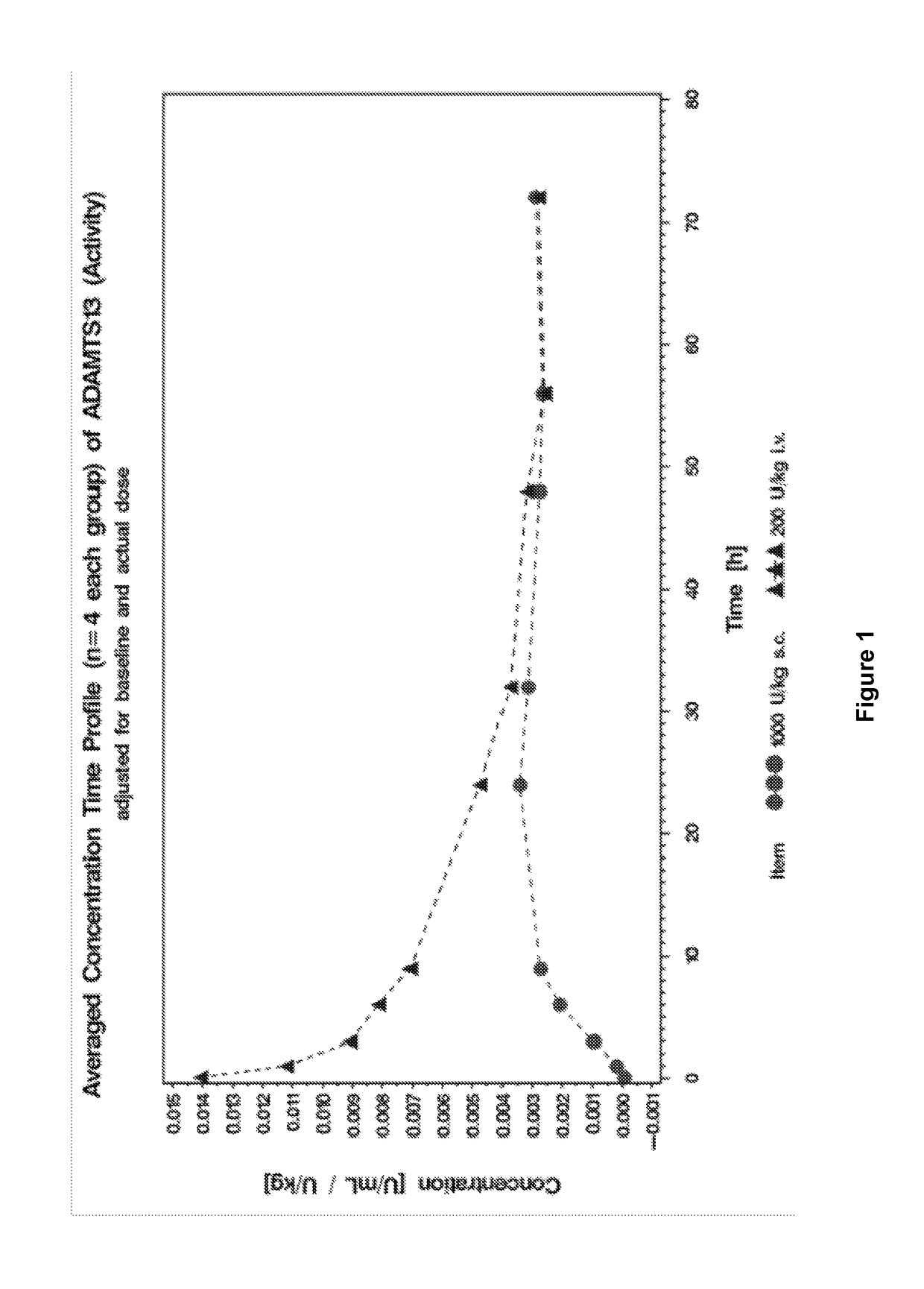
Subcutaneous administration of ADAMTS13
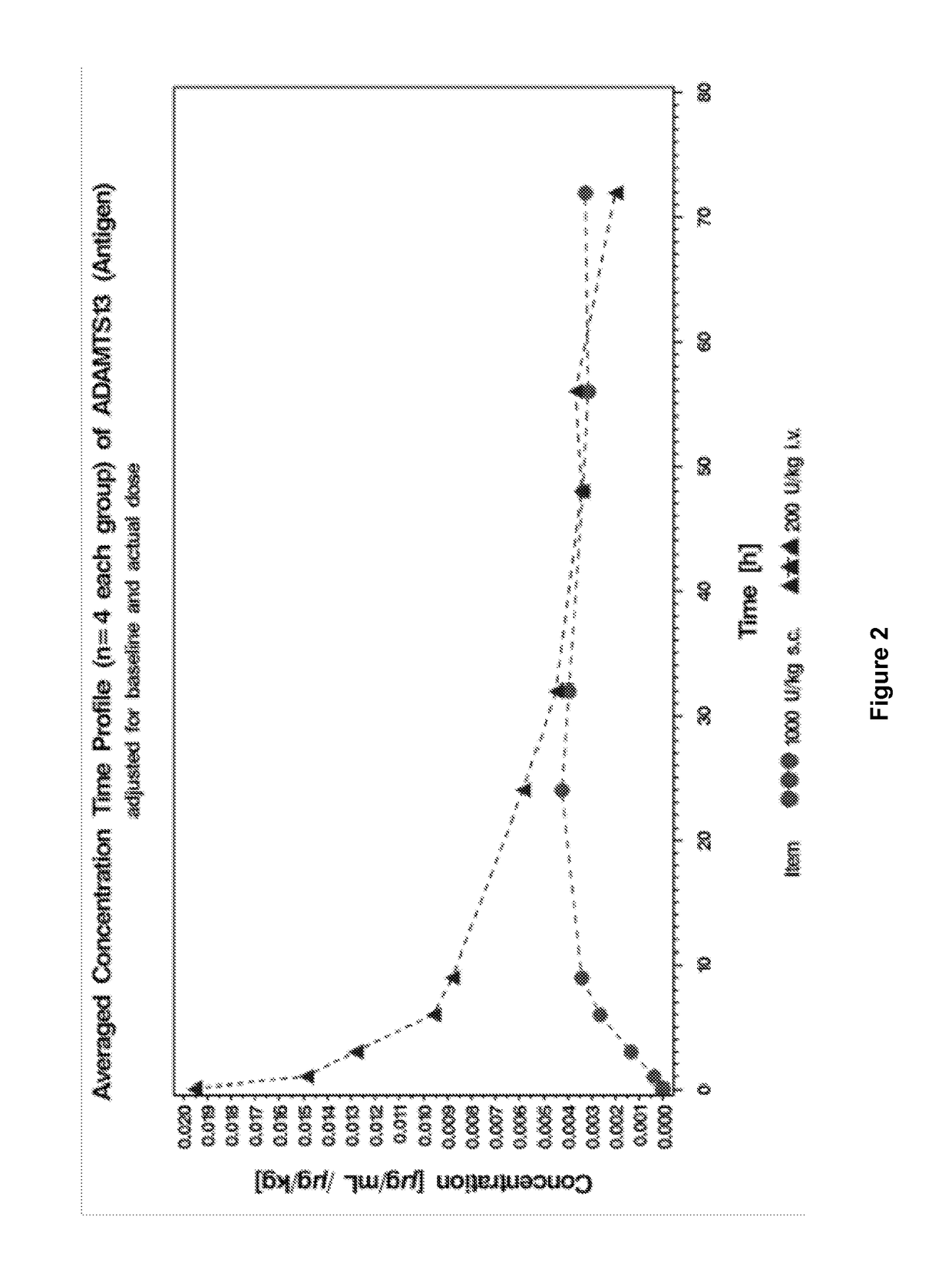
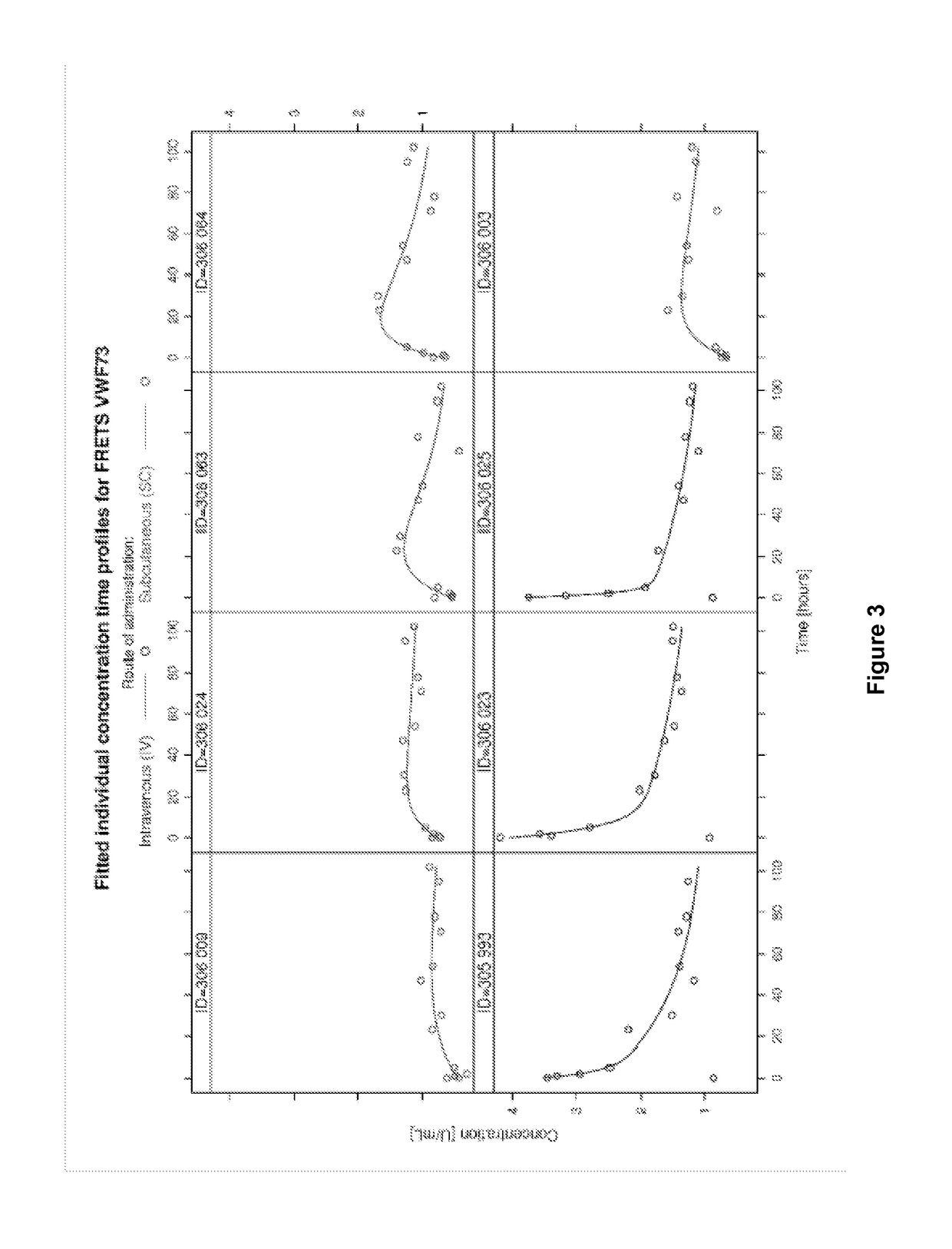
This invention relates to methods of subcutaneous administration of ADAMTS13 formulations to a treat a disease or condition associated with ADAMTS13 and VWF dysfunction. Furthermore, evidence of the unexpectedly high bioavailability of ADAMTS13 formulations administered subcutaneously is provided herein.
Owner:TAKEDA PHARMA CO LTD
Haemorrhagic disorder due to ventricular assist device
ActiveUS20180362665A1Increase in sizeIncrease the gapAntibody medical ingredientsBlood disorderMedicineLeft ventricular size
The present invention relates to methods of treatment by human ADAMTS13 inhibition in circulatory assist device induced haemorrhagic complication such as a bleeding disorder, in particular, bleeding after left ventricular assist device implantation. The present invention further relates to specific monoclonal antibodies inhibiting ADAMTS13 function.
Owner:KATHOLIEKE UNIV LEUVEN
ADAMTS13 substrate with histidine tags and preparation method and application of ADAMTS13 substrate
InactiveCN110724202ALow costGuaranteed yieldFactor VIIMicrobiological testing/measurementEnzyme digestionElisa kit
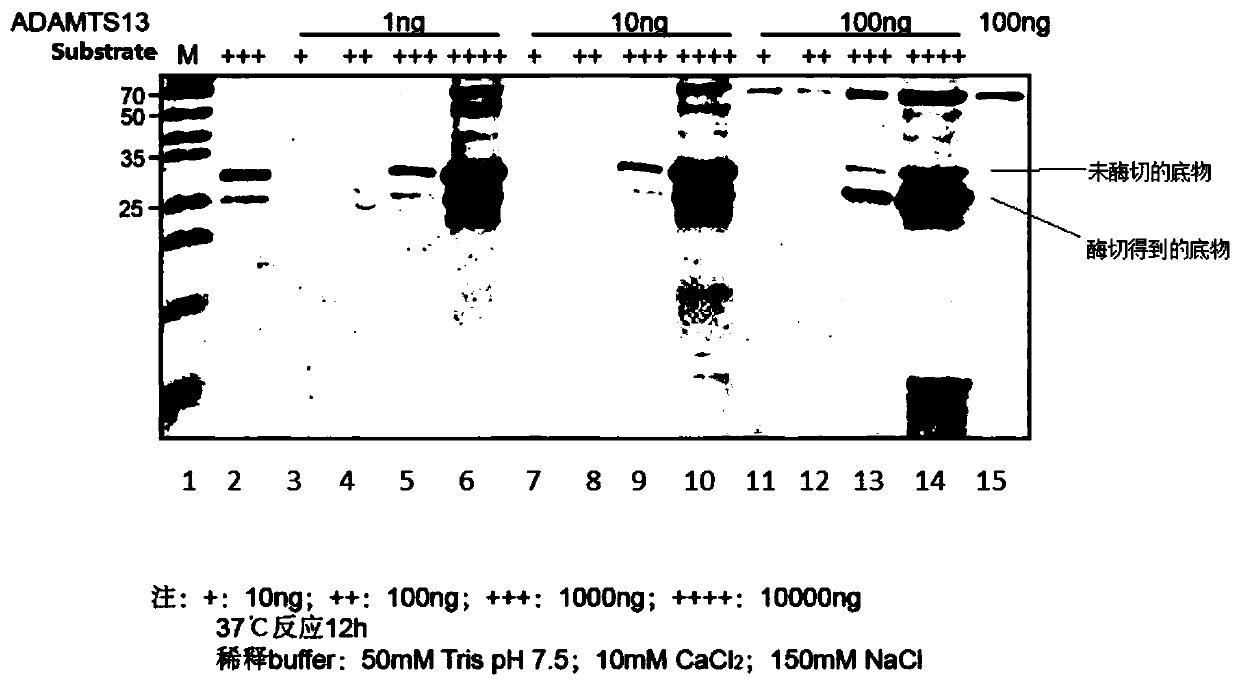
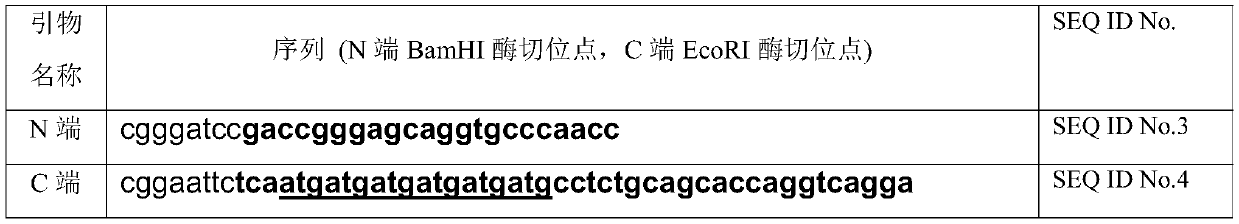
The invention relates to an ADAMTS13 substrate with histidine tags. An amino acid sequence of the substrate is as shown in SEQ ID No.1 and the substrate also has activity of protein enzyme-digested byADAMTS13. The ADAMTS13 enzyme-digested substrate provided by the invention can be efficiently expressed and purified in vitro in a large quantity, does not need to be subjected to fluorescent modification to further reduce cost, and can be widely applied to actual clinic. Besides, the substrate respectively carrying two groups of different tags can be used as a tag tool for protein purification,besides, two products produced after enzyme digestion with the ADAMTS13 respectively have tags with different specificities, and the two products can both be detected by an ELISA kit for the tags. Thenovel ADAMTS13 enzyme-digested substrate provided by the invention can be efficiently enzyme-digested by the ADAMTS13 and can be applied to detection of activity of the ADAMTS13.
Owner:晏才喜
Inhibitory monoclonal antibody resistant to lyase of human von Willebrand factor (VWF) and application of inhibitory monoclonal antibody
ActiveCN106008715AImprove bindingInhibit hydrolysis of VWFAntibody ingredientsBlood disorderFactor VIII vWFLyase
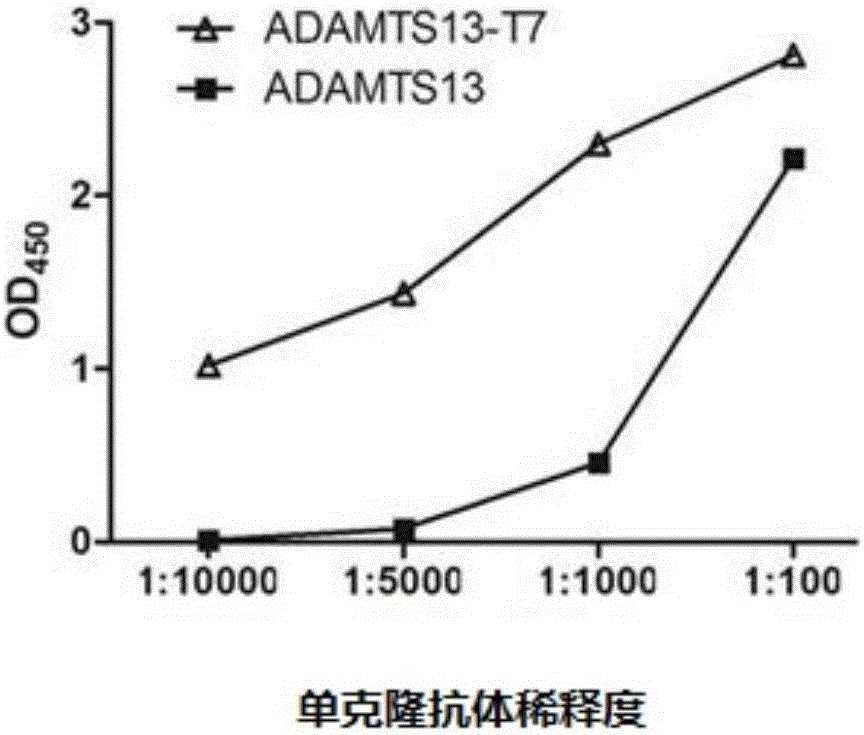
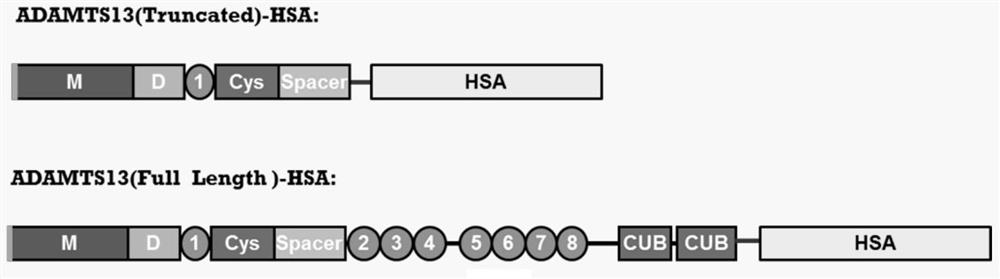
The invention discloses an inhibitory monoclonal antibody resistant to lyase of a human von Willebrand factor (VWF) and application of the inhibitory monoclonal antibody. The monoclonal antibody belongs to an IgG1 subclass antibody and is produced from a hybridoma cell strain SZ-169; the preservation number of the hybridoma cell strain SZ-169 is CCTCC NO: C2016134. Compared with the prior art, the inhibitory monoclonal antibody has the following advantages: (1) the monoclonal antibody can be specifically combined with human overall-length ADAMTS13 and truncated ADAMTS13-T7 proteins, and the bonding capacity with the truncated ADAMTS13-T7 proteins is obviously higher than that with the overall-length ADAMTS13 proteins; (2) under a denaturation condition, the monoclonal antibody can obviously prevent ADAMTS13 from hydrolyzing VWF , and the suppression effect is enhanced with the increase of the concentration of the monoclonal antibody; (3) the monoclonal antibody disclosed by the invention can be effectively applied to medicine preparation, so that the targeting property and the specificity of a medicine are improved.
Owner:THE FIRST AFFILIATED HOSPITAL OF SOOCHOW UNIV
Antibodies for the prevention or the treatment of bleeding episodes
ActiveUS10544231B2Extensive treatmentReduce riskImmunoglobulins against blood coagulation factorsAntibody ingredientsHeavy chainVentricular assistance
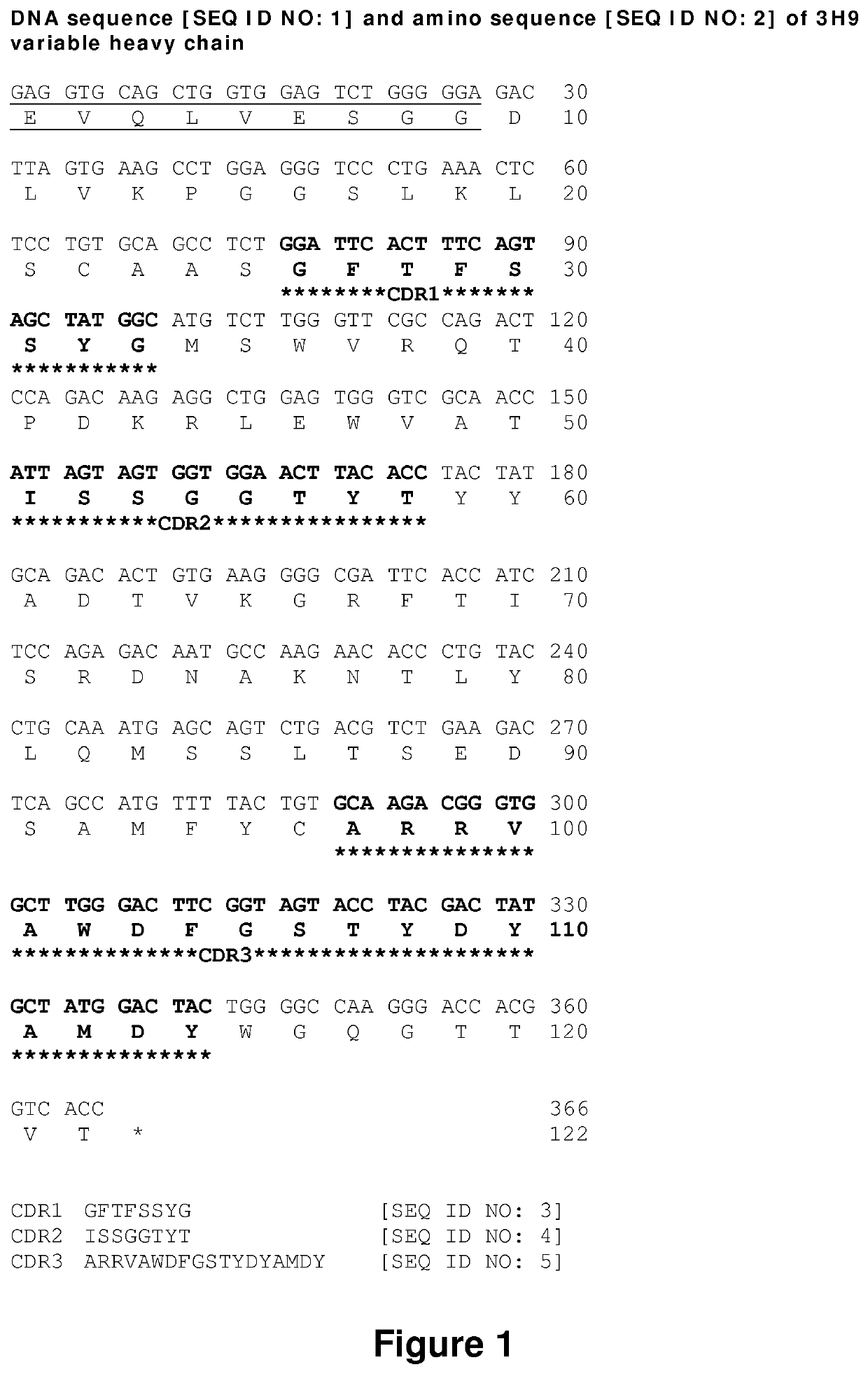
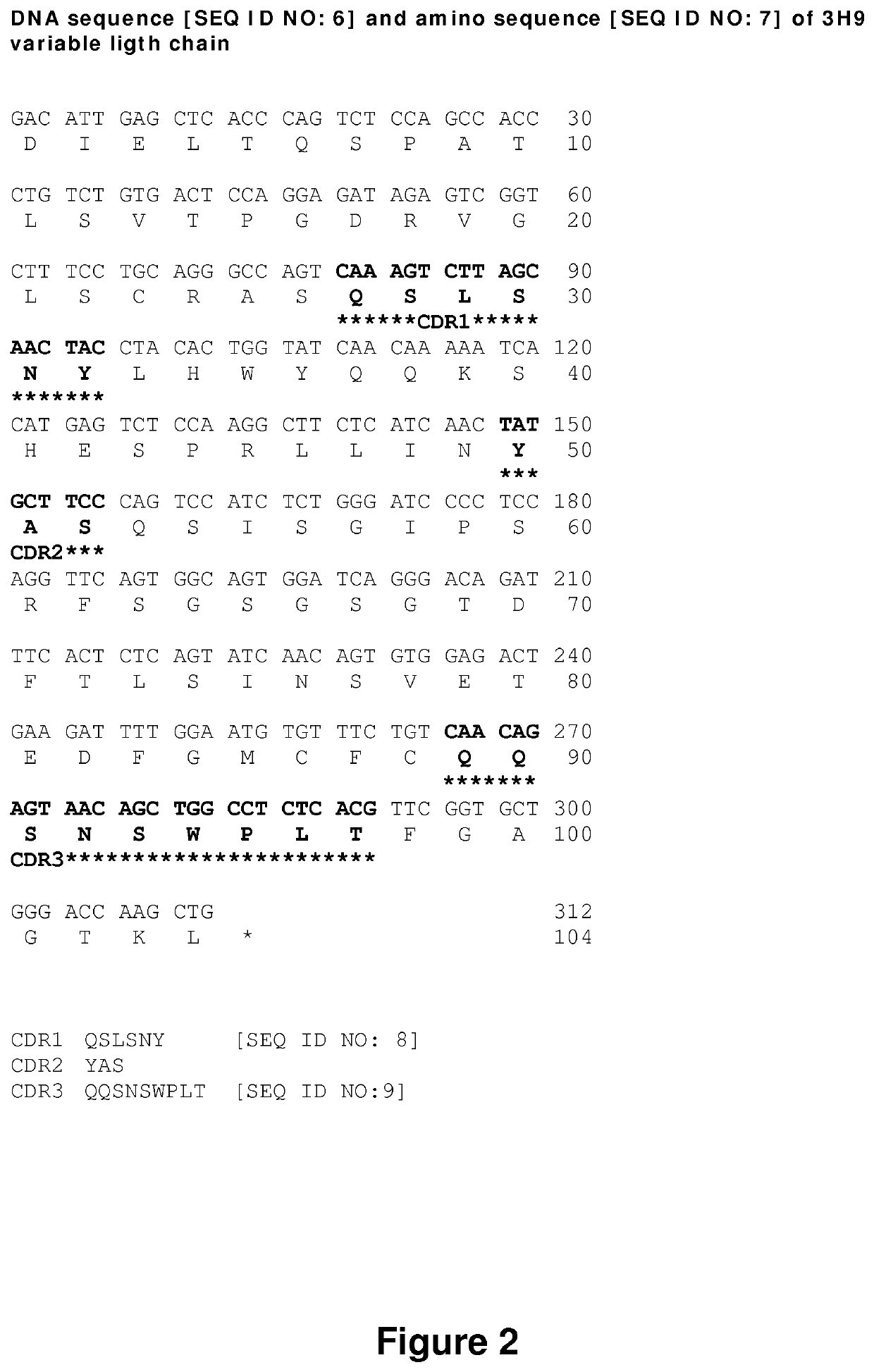
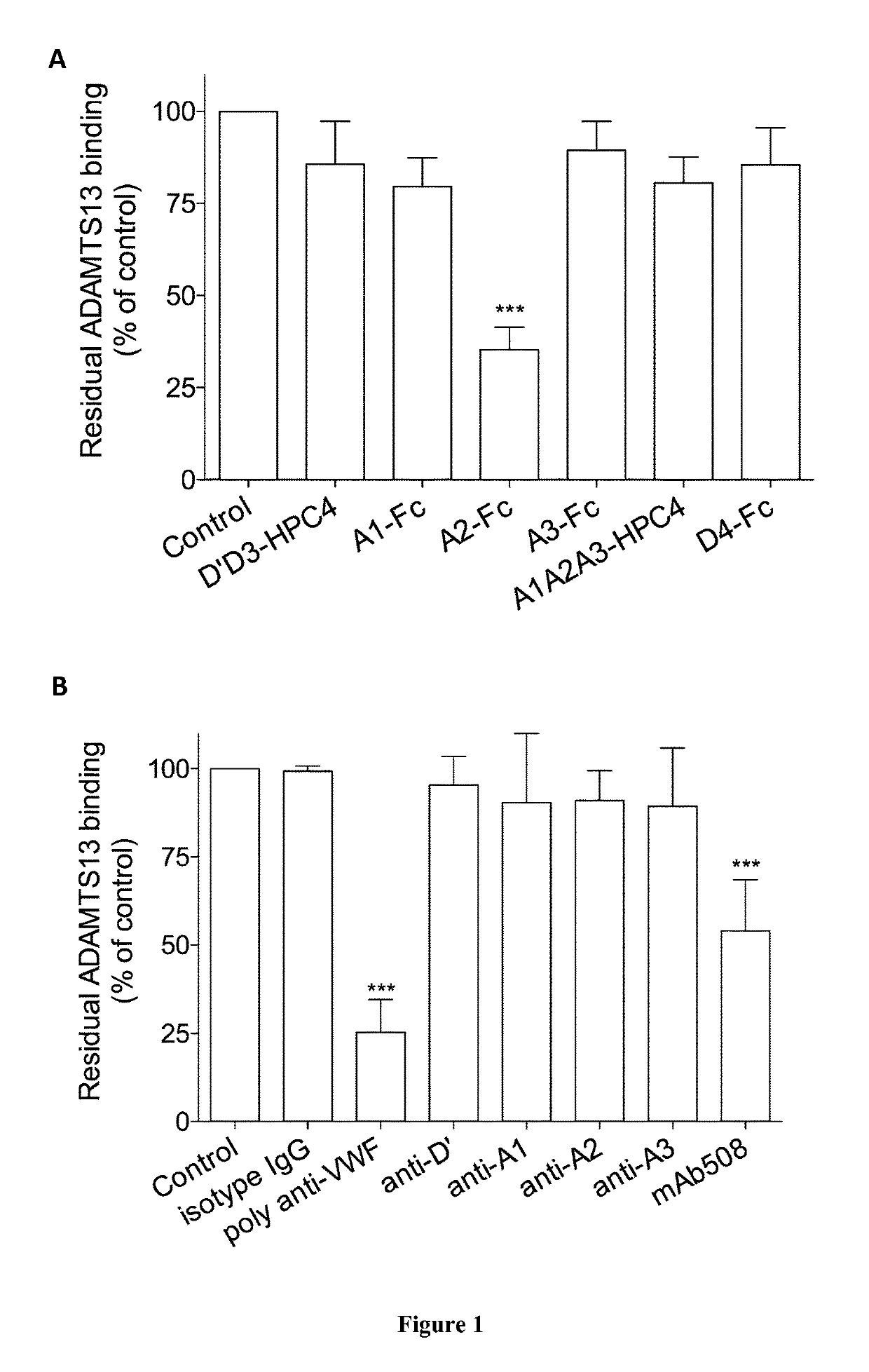
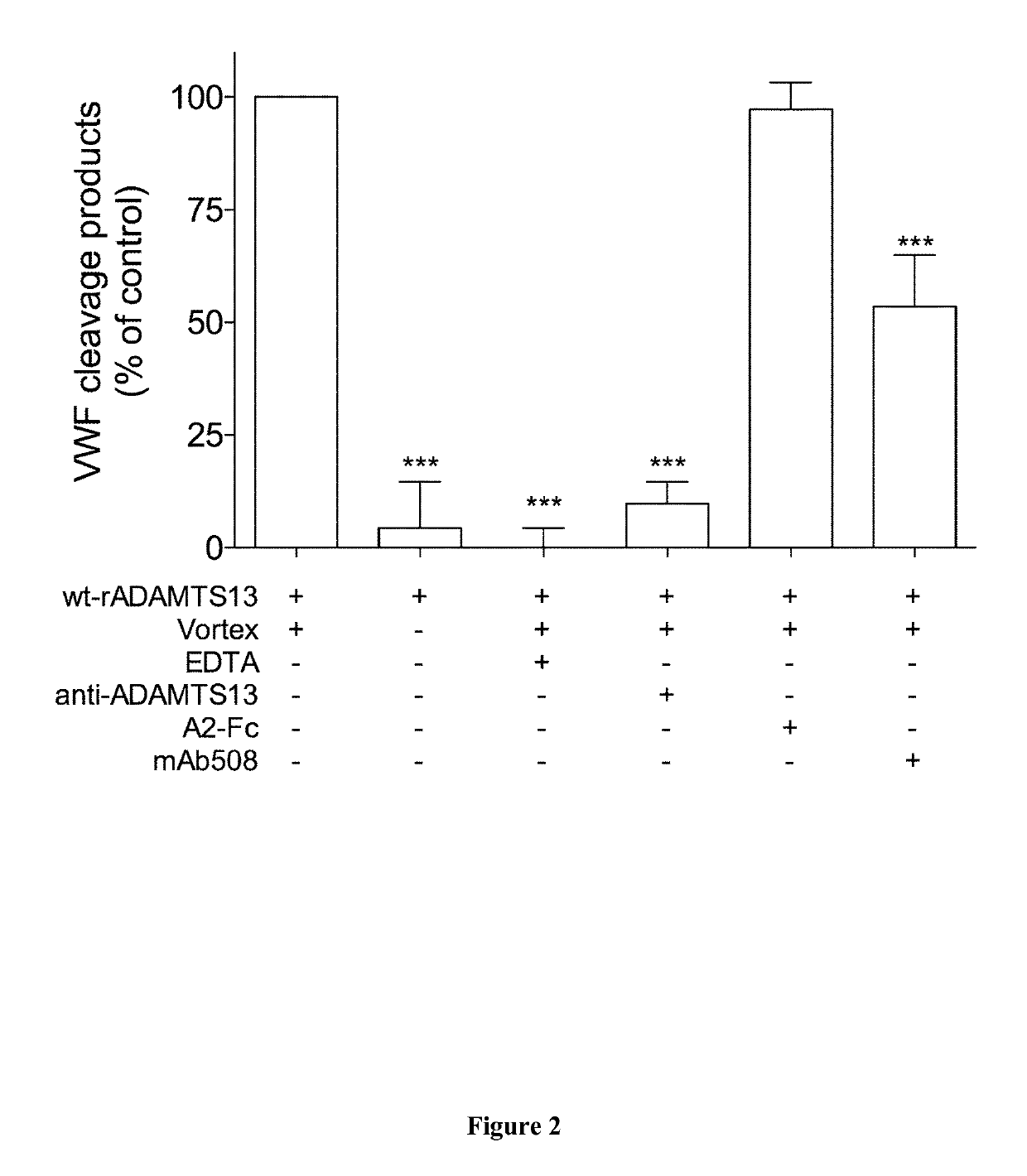
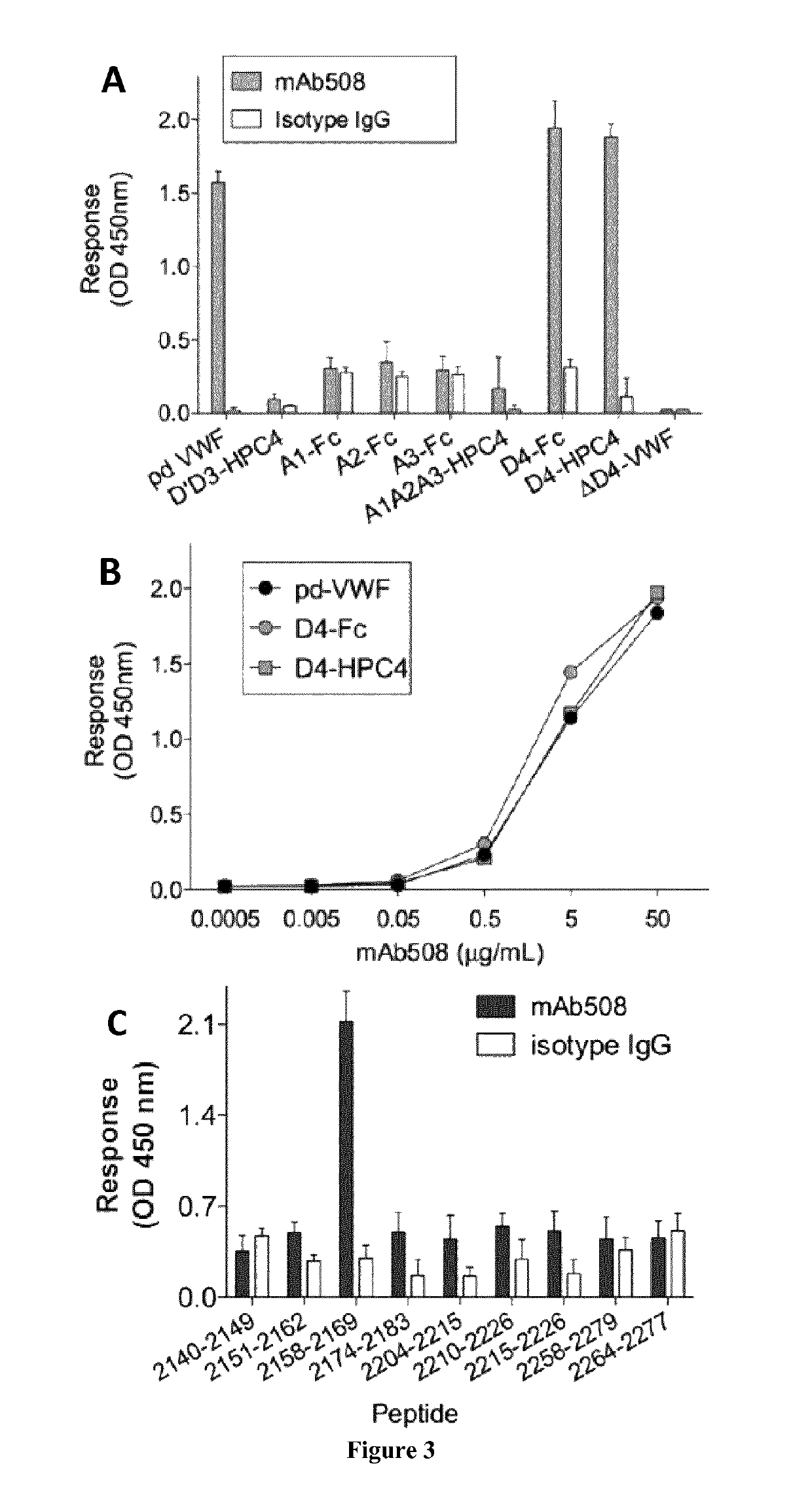
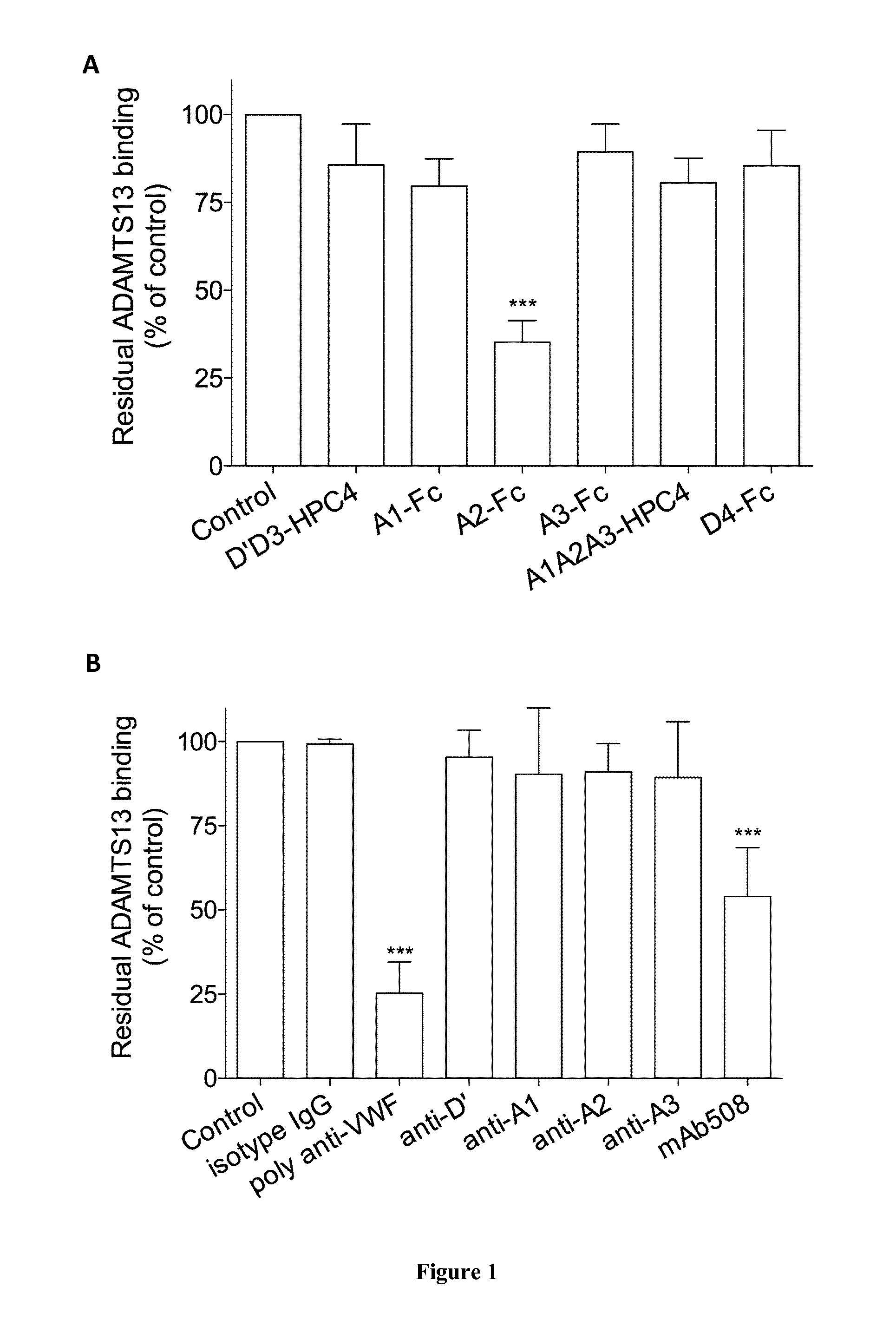
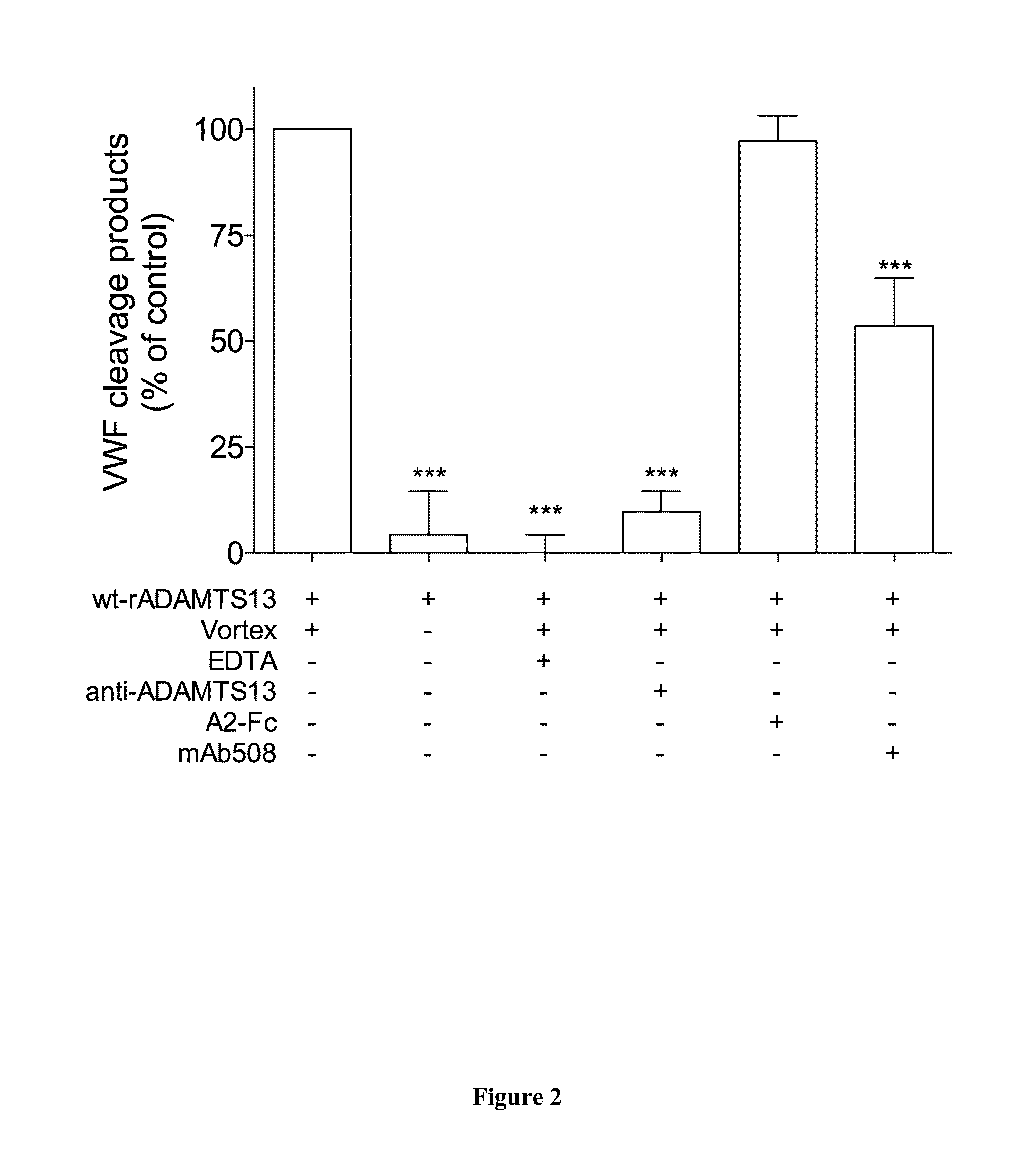
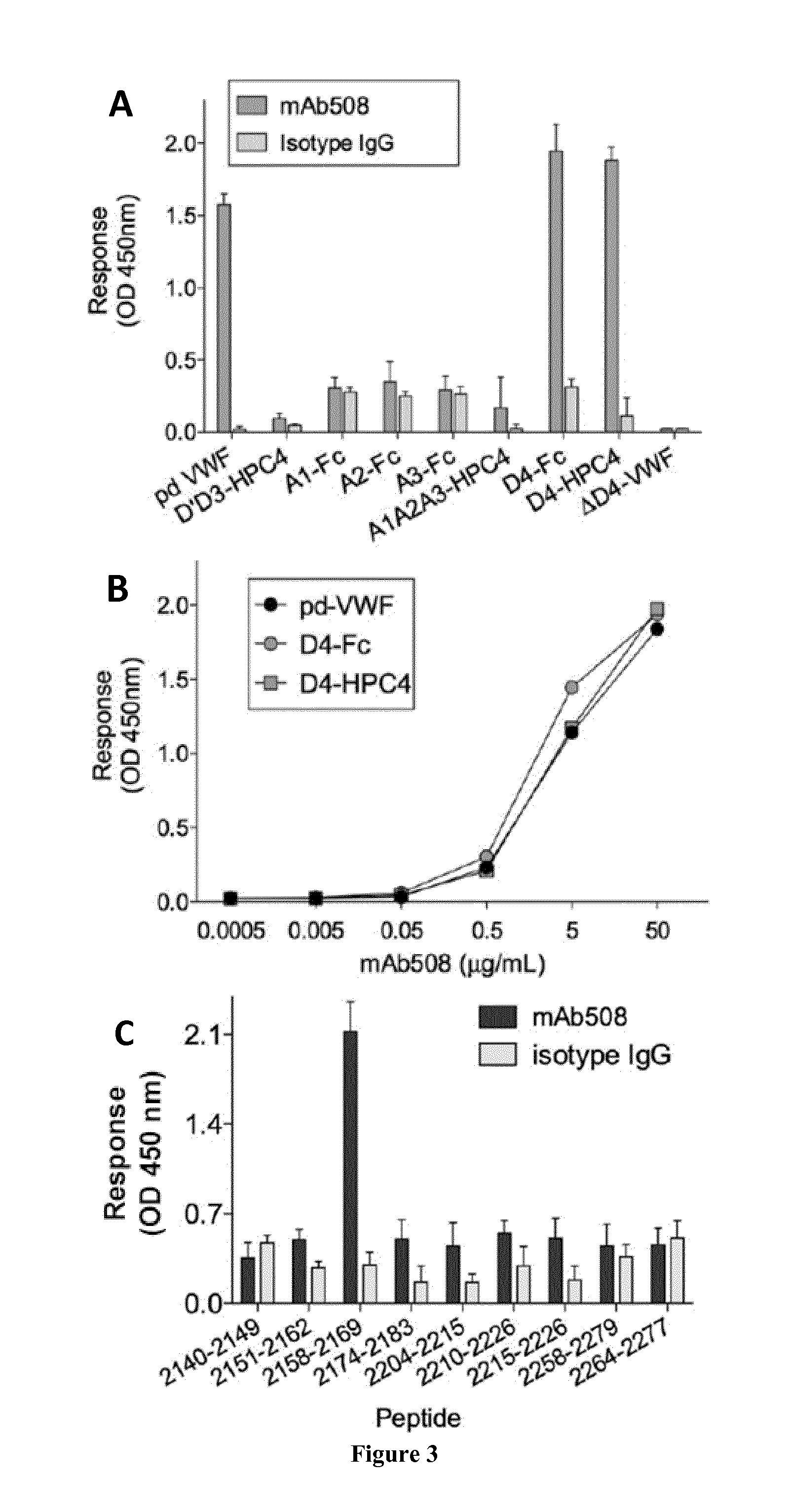
The invention relates to an isolated monoclonal antibody that specifically binds to the D4 domain of VWF, competes for binding to VWF D4 domain with ADAMTS13 and partially inhibits ADAMTS 13-mediated degradation of VWF. More particularly, the invention relates to an isolated monoclonal antibody comprising a heavy chain wherein the variable domain comprises at least one CDR having a sequence selected from the group consisting of SEQ ID NO: 3 for H-CDR1, SEQ ID NO: 4 for H-CDR2 and SEQ ID NO: 5 for H-CDR3 and a light chain wherein the variable domain comprises at least one CDR having a sequence selected from the group consisting of SEQ ID NO: 7 for L-CDR1, SEQ ID NO: 8 for L-CDR2 and SEQ ID NO: 9 for L-CDR3. Antibodies of the invention are presented to be useful in for the prevention or the treatment of bleeding episodes, such as bleeding episodes occurring in patients with aortic stenosis or patients with ventricular assist devices (VAD).
Owner:UNIV PARIS SACLAY +3
Humanised ADAMTS13 binding antibodies
ActiveUS11339226B2Avoid complicationsIncrease shear stressAntibody ingredientsBlood disorderDiseaseAntiendomysial antibodies
The present invention further relates to specific humanised monoclonal antibodies inhibiting ADAMTS13 function. The present invention relates to methods of treatment by human ADAMTS13 inhibition in circulatory assist device induced haemorrhagic complication such as a bleeding disorder, in particular, bleeding after left ventricular assist device (LVAD) implantation.
Owner:KATHOLIEKE UNIV LEUVEN
Red blood cells expressing von willebrand factor protease and methods of use thereof
PendingUS20220213462A1Immunoglobulins against blood coagulation factorsFactor VIIFactor VIII vWFRed blood cell
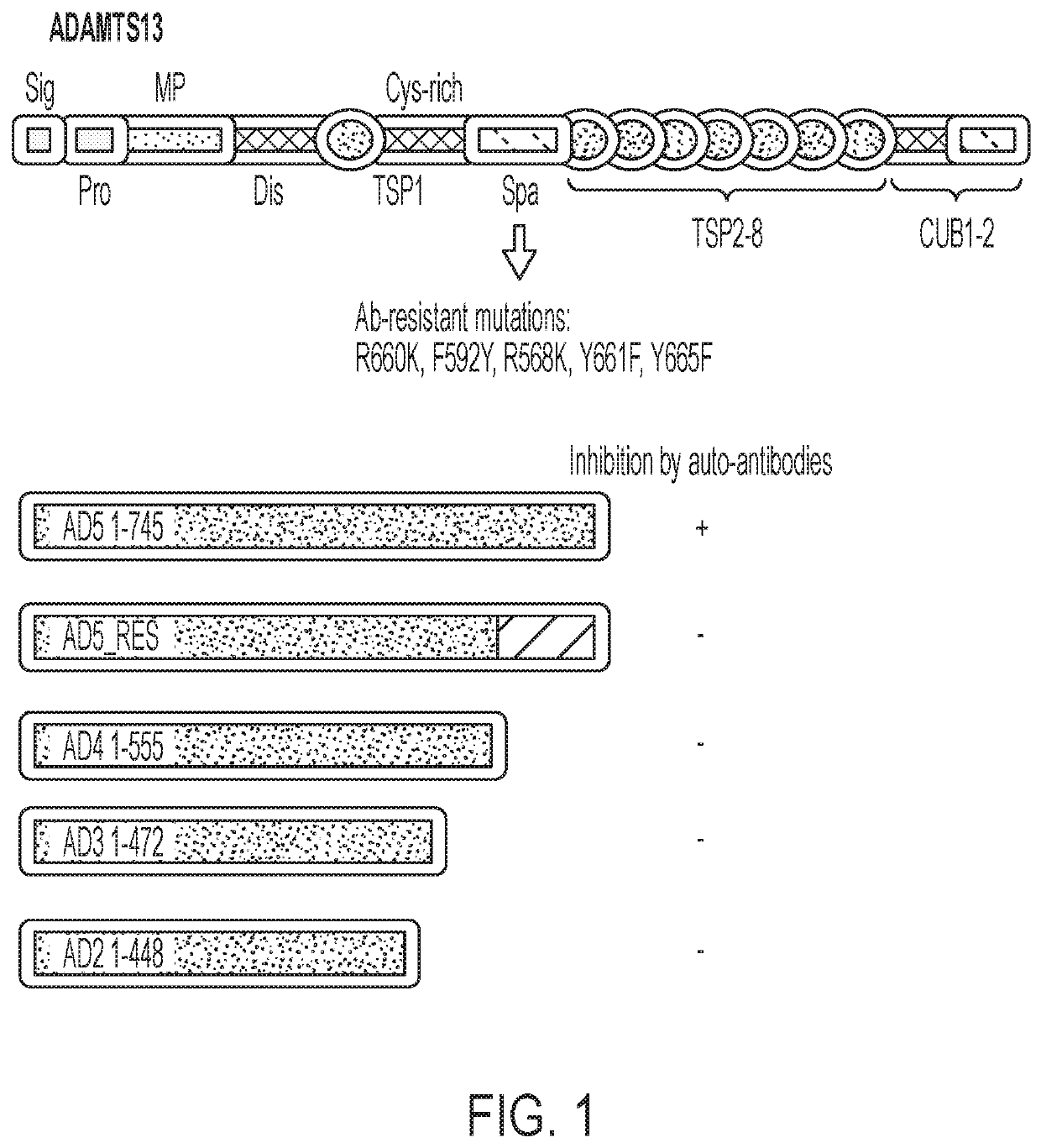
This disclosure provides methods and compositions for treating TTP based on transfusion of a relatively small number of genetically modified red blood cells. The genetically modified red blood cells express a fusion protein including a fragment of ADAMTS13 that is enzymatically active against von Willebrand factor (VWF). The fragments of ADAMTS13 can be resistant to the inhibitors, e.g., the auto-immune antibodies, which are responsible for the acquired form of TTP.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
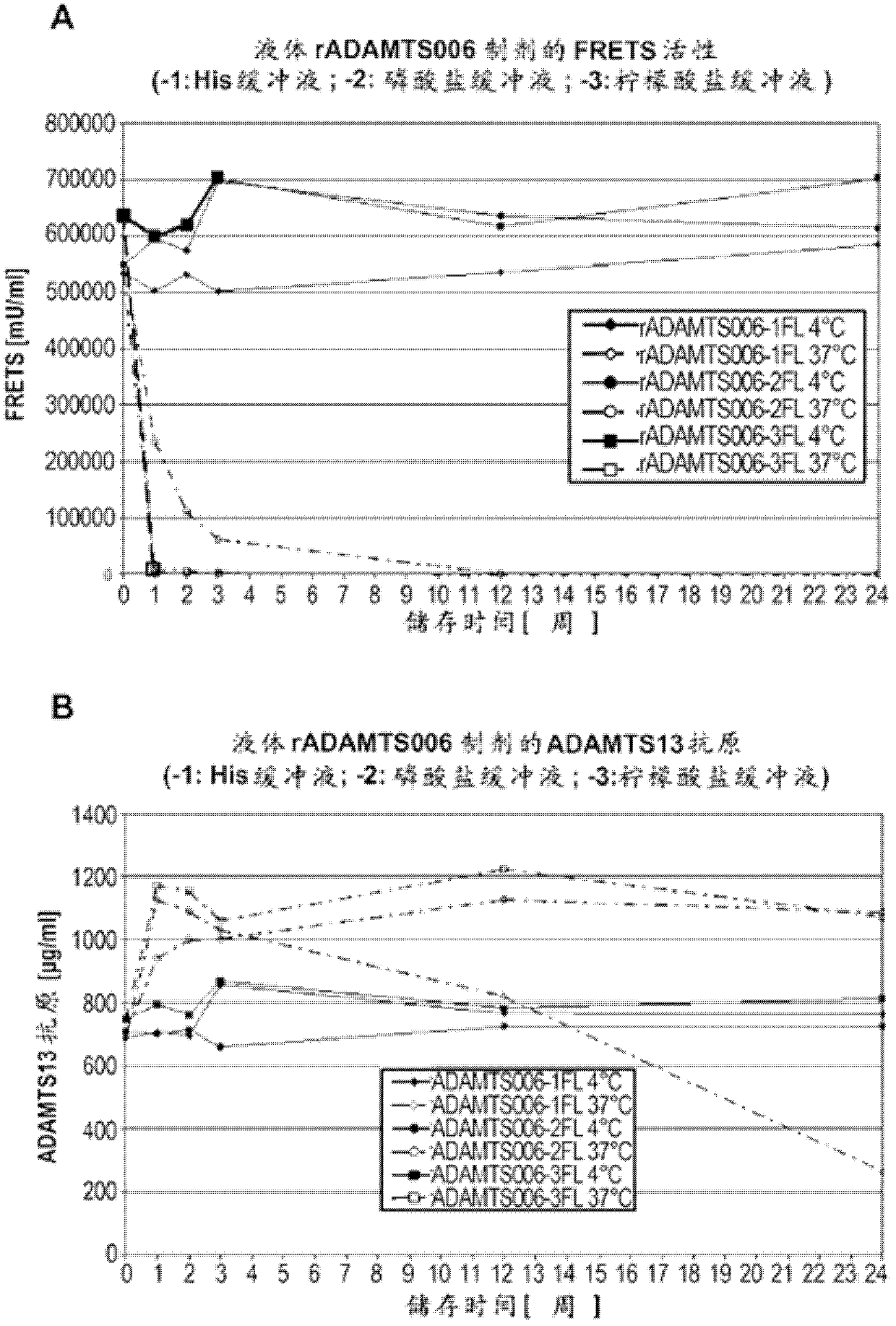
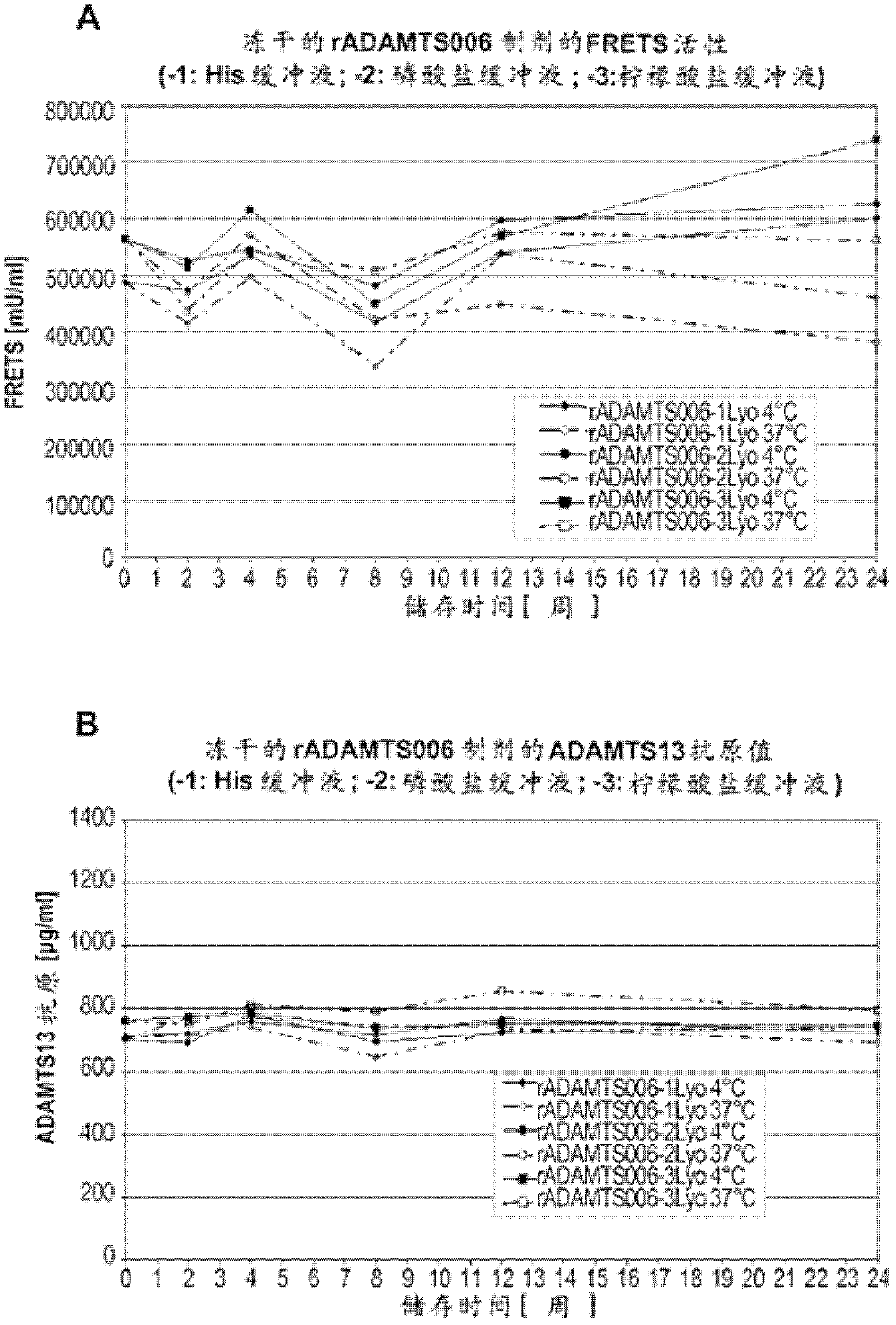
Stabilized liquid and lyophilized formulations of adamts13
ActiveCN102573792BHigh specific activityDelayed formationPeptide/protein ingredientsInorganic non-active ingredientsDiseaseFunctional disturbance
The present invention relates to formulations of ADAMTS13 with improved or desirable properties. Accordingly, the present invention provides liquid and lyophilized formulations of ADAMTS13 suitable for pharmaceutical administration. Among other aspects, the present invention provides methods of treating various diseases and conditions associated with VWF and / or ADAMTS13 dysfunction in a subject. Also provided herein are kits comprising formulations of ADAMTS13 useful in the treatment of various diseases and conditions.
Owner:TAKEDA PHARMA CO LTD
Antibodies for the prevention or the treatment of bleeding episodes
ActiveUS20170037148A1Avoid degradationImmunoglobulins against blood coagulation factorsBlood disorderMonoclonal antibodyVariable domain
The invention relates to an isolated monoclonal antibody that specifically binds to the D4 domain of VWF, competes for binding to VWF D4 domain with ADAMTS13 and partially inhibits ADAMTS 13 -mediated degradation of VWF. More particularly, the invention relates to an isolated monoclonal antibody comprising a heavy chain wherein the variable domain comprises at least one CDR having a sequence selected from the group consisting of SEQ ID NO: 3 for H-CDR1, SEQ ID NO: 4 for H-CDR2 and SEQ ID NO: 5 for H-CDR3 and a light chain wherein the variable domain comprises at least one CDR having a sequence selected from the group consisting of SEQ ID NO: 7 for L-CDR1, SEQ ID NO: 8 for L-CDR2 and SEQ ID NO: 9 for L-CDR3. Antibodies of the invention are presented to be useful in for the prevention or the treatment of bleeding episodes, such as bleeding episodes occurring in patients with aortic stenosis or patients with ventricular assist devices (VAD).
Owner:UNIV PARIS SACLAY +3
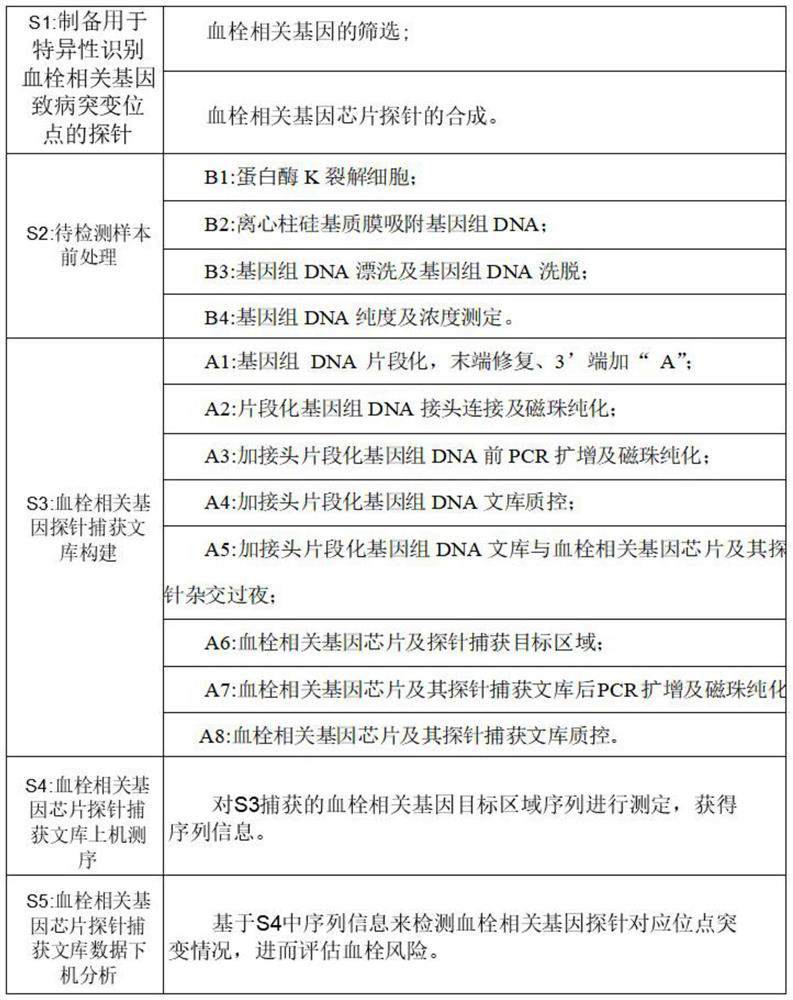
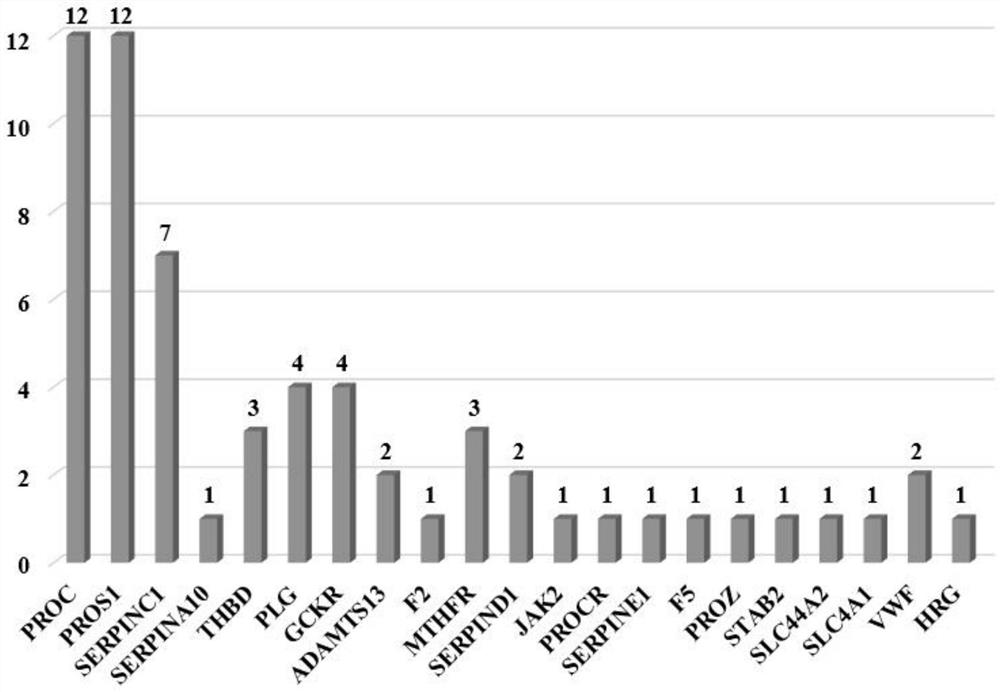
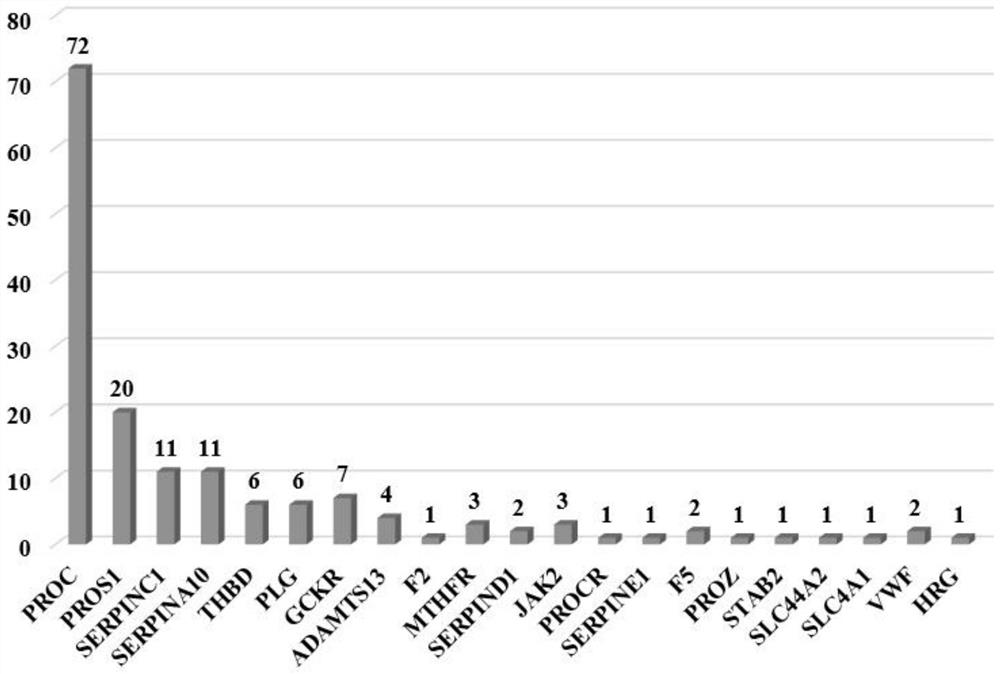
Detection method of thrombus-related gene chip
PendingCN114807336AImprove accuracyDetection target is clearMicrobiological testing/measurementGenes mutationBiological studies
The invention provides a detection method of a thrombus-related gene chip, relates to the field of biological detection, and particularly discloses corresponding 62 pathogenic mutation sites in the thrombus-related gene chip, the detection method of the thrombus-related gene chip comprises probes for the following 21 thrombus-related genes: PROC, PROS1, SERPINC1, SERPINA10, THBD, PLG, GCKR, ADAMTS13, F2, MTHFR, SERPIND1, JAK2, PROCR, SERPINE1, F5, PROZ, STAB2, HRG, SLC44A2, SLC4A1 and VWF. According to the detection method of the thrombus-related gene chip, the probes are used for capturing the 21 thrombus-related genes; 62 pathogenic mutation sites of the gene can be specifically captured; a probe capture method next-generation sequencing technology is adopted, mutation of all exons and regulatory regions of the genes can be comprehensively and systematically detected at a time, and the invention aims to be applied to molecular diagnosis and biological research of thrombus caused by gene mutation. The method has the advantages of clear detection target, good coverage, high accuracy, stable detection, short period, low cost and the like.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
Method for determining treatment of disseminated intravascular coagulation
ActiveUS20110143369A1Difficult to accuratelyImprove survival rateMicrobiological testing/measurementDisease diagnosisFactor VIII vWFDisseminated coagulopathy
Owner:KM BIOLOGICS CO LTD
Adamts13-mdtcs fusion protein with extended half-life in vivo and its application
ActiveCN104926946BExtended half-lifeNo biological activityPeptide/protein ingredientsSerum albuminLyaseIn vivo
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com