Patents
Literature
51 results about "ADAMTS" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
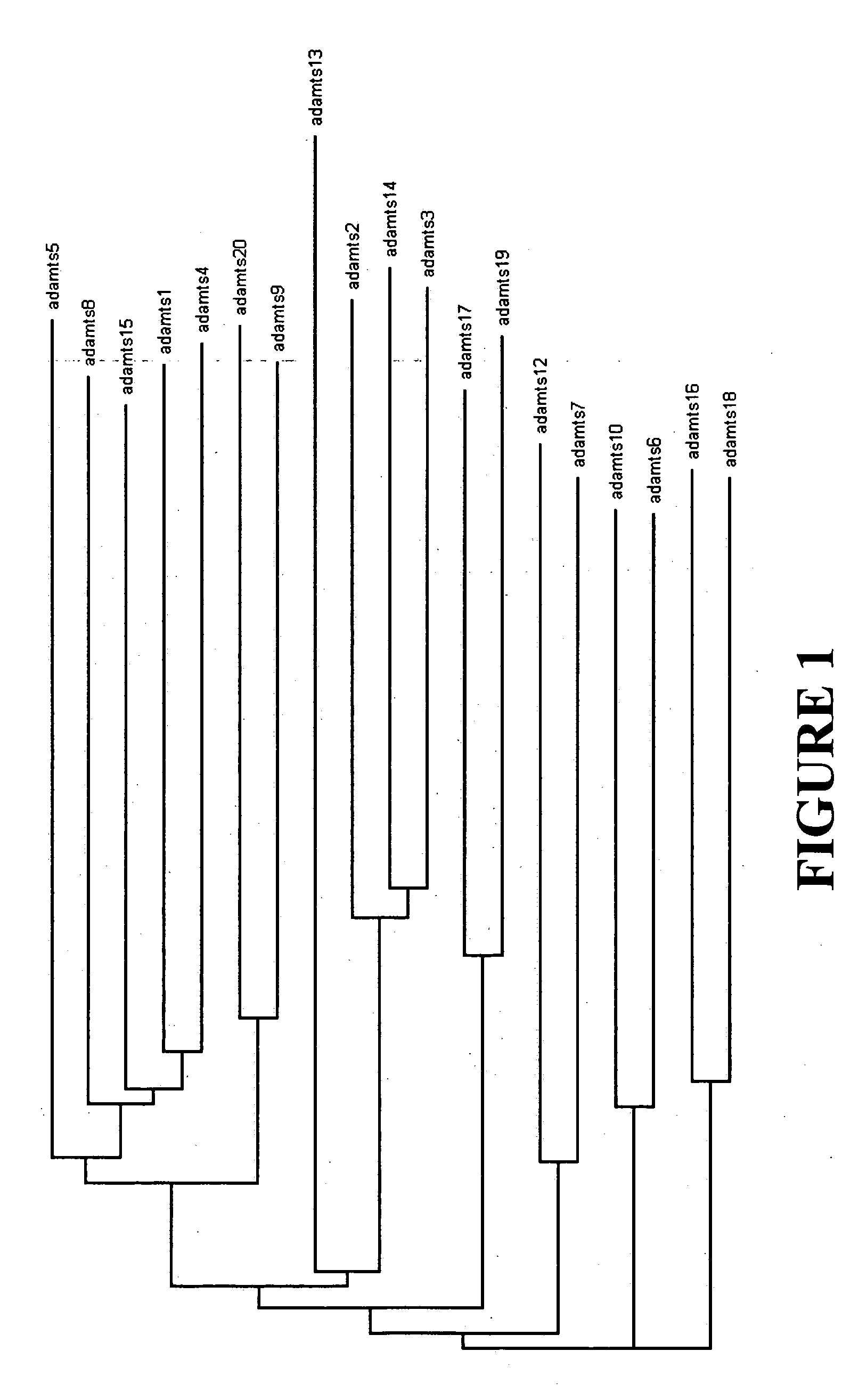
ADAMTS (short for a disintegrin and metalloproteinase with thrombospondin motifs) is a family of multidomain extracellular protease enzymes. 19 members of this family have been identified in humans, the first of which, ADAMTS1, was described in 1997. Known functions of the ADAMTS proteases include processing of procollagens and von Willebrand factor as well as cleavage of aggrecan, versican, brevican and neurocan, making them key remodeling enzymes of the extracellular matrix. They have been demonstrated to have important roles in connective tissue organization, coagulation, inflammation, arthritis, angiogenesis and cell migration. Homologous subfamily of ADAMTSL (ADAMTS-like) proteins, which lack enzymatic activity, has also been described. Most cases of thrombotic thrombocytopenic purpura arise from autoantibody-mediated inhibition of ADAMTS13.
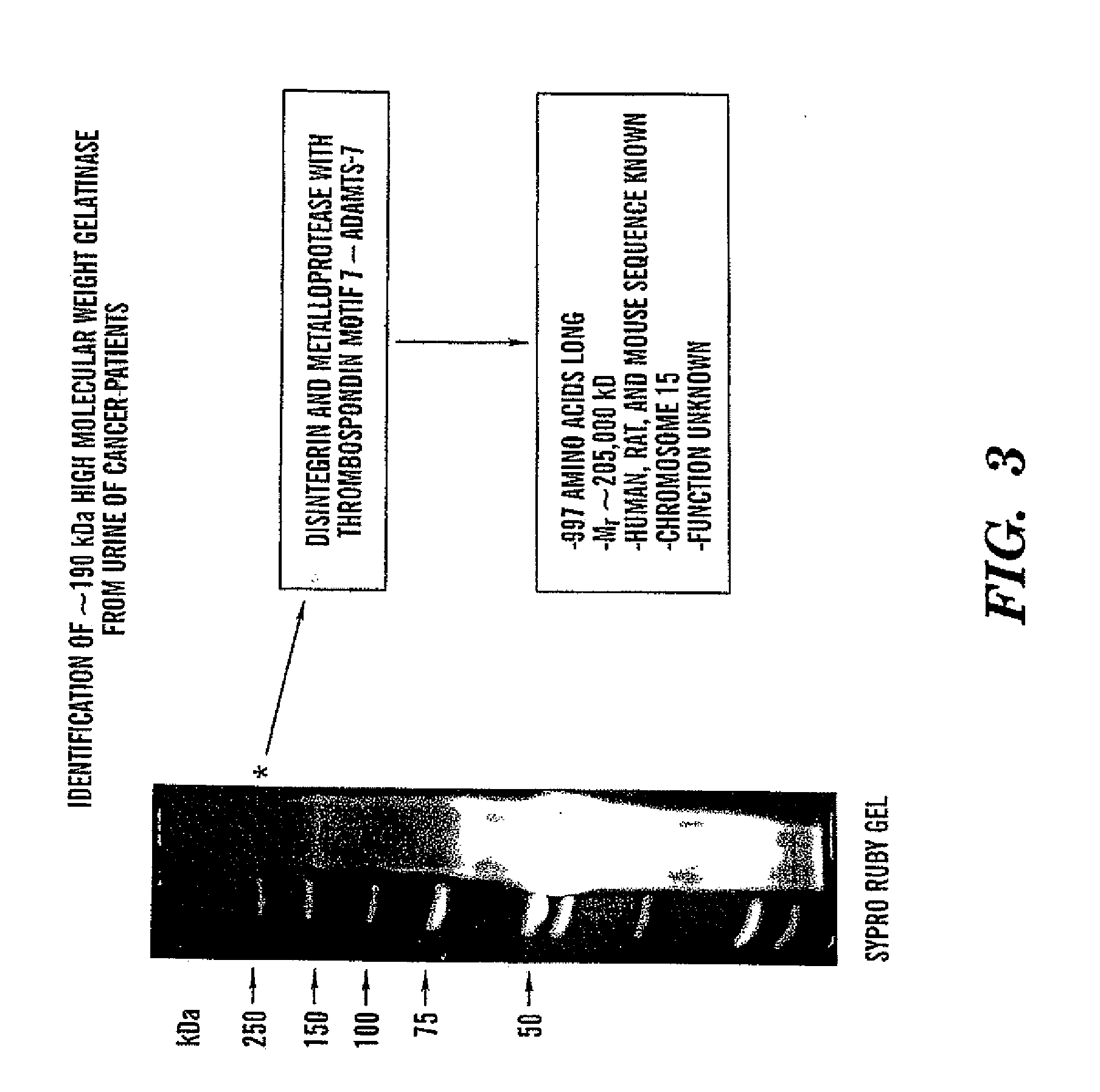
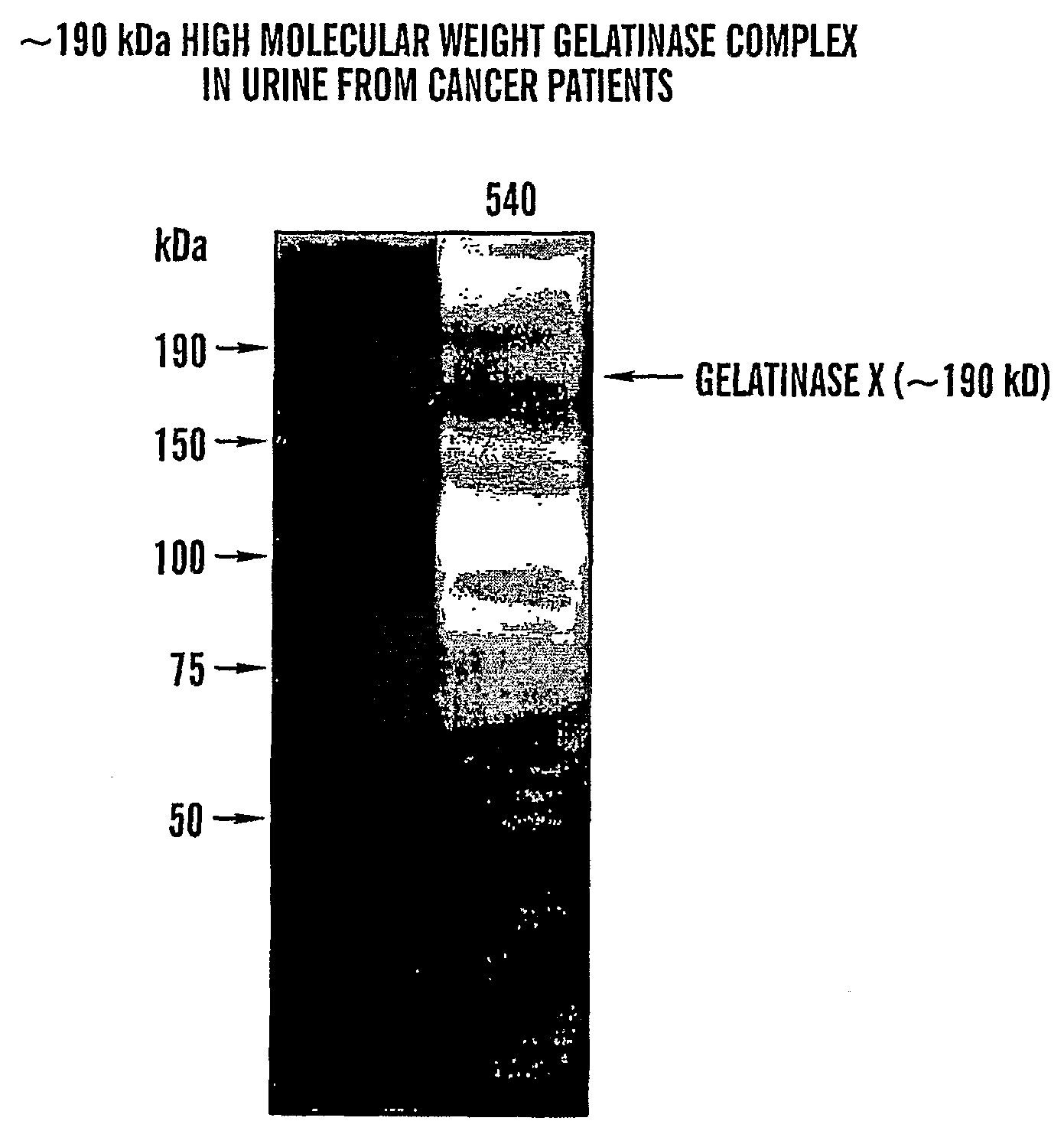
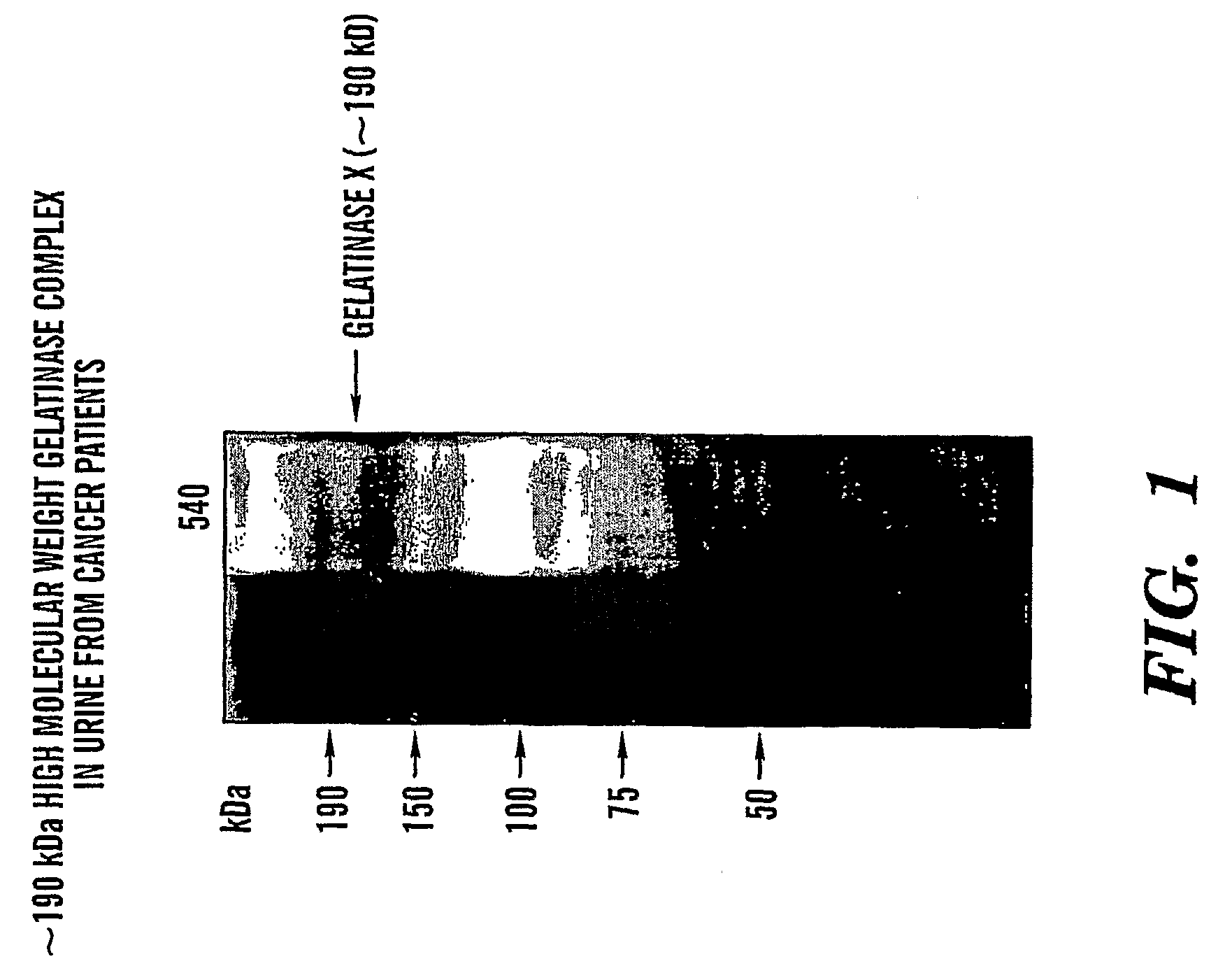
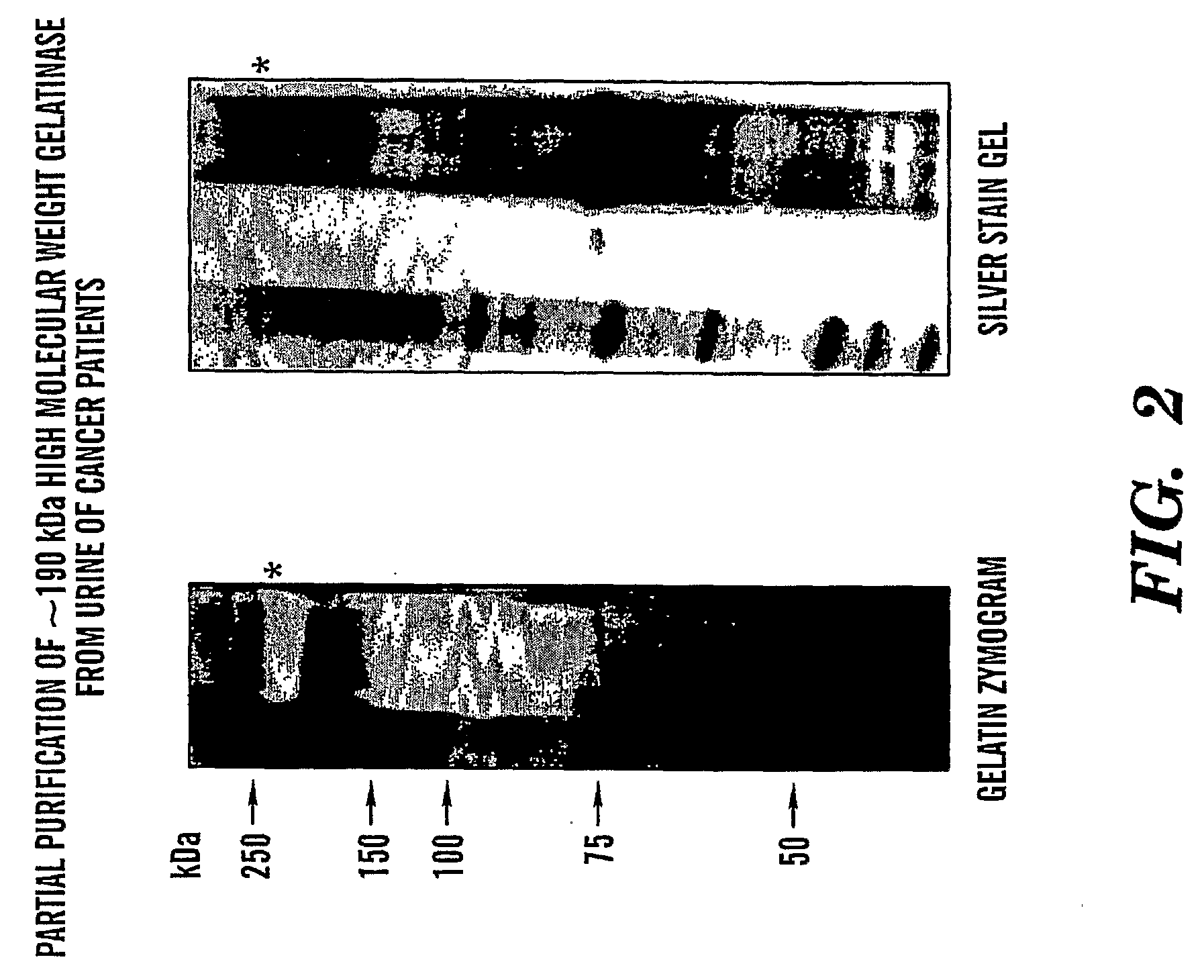
Adamts-7 as a Biomarker for Cancers of Epithelial Origin
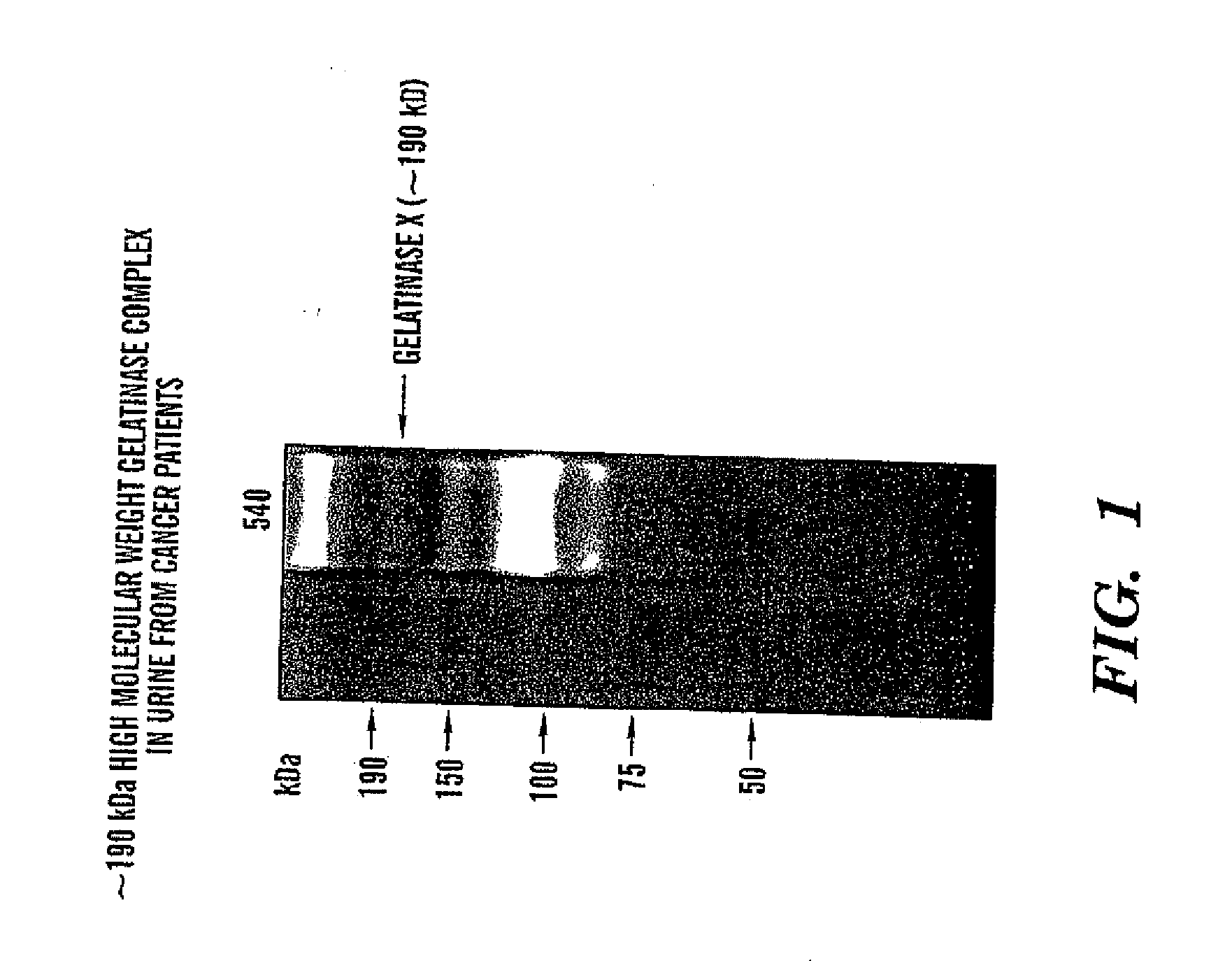
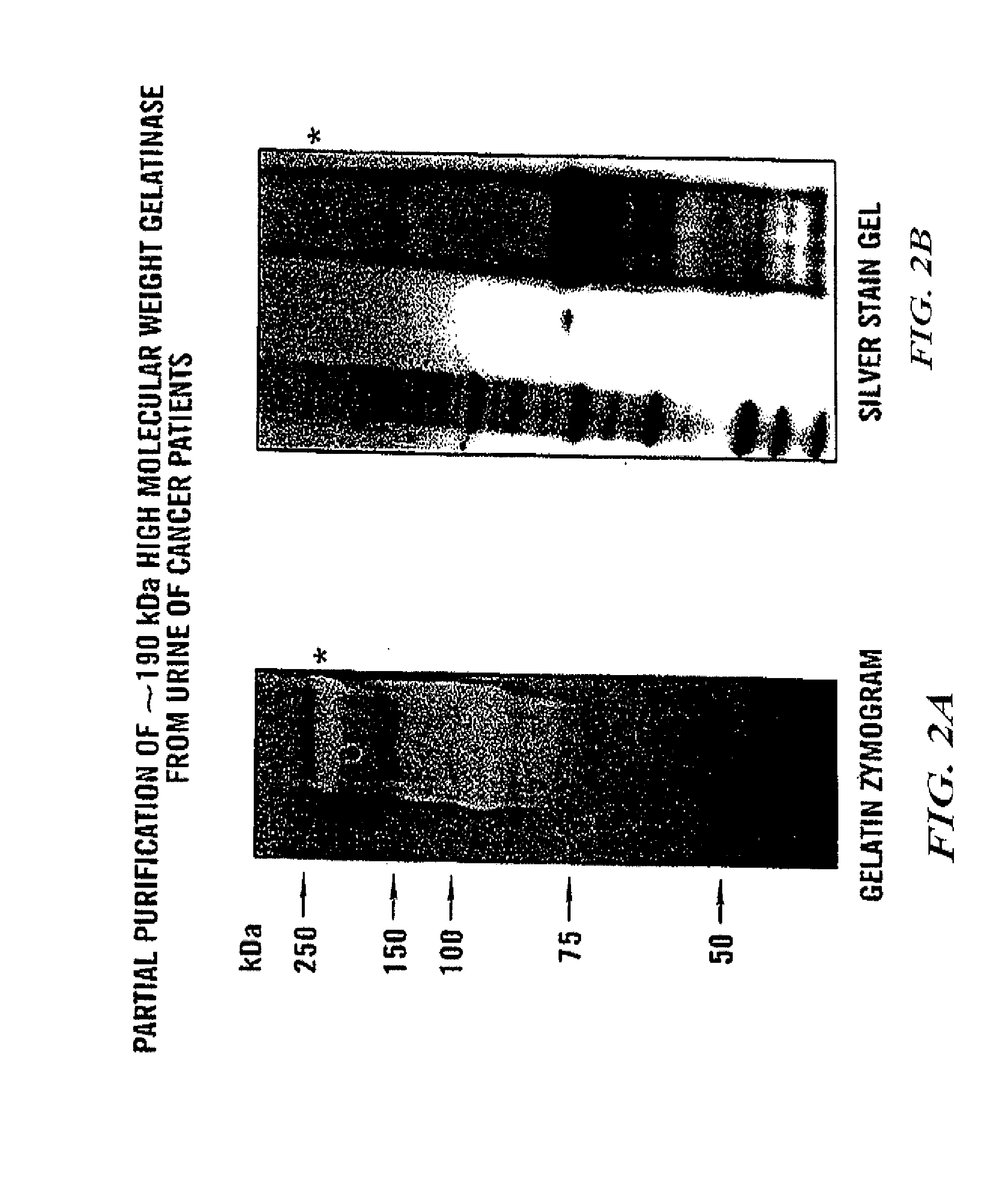
InactiveUS20080268473A1Quick and easy and safeEasy diagnosisBiological material analysisBiological testingBacteriuriaBladder cancer
ADAMTS-7 expression and activity are up regulated in patients that have cancers of epithelial origin. Accordingly, the present invention is directed to methods diagnosis of cancers of epithelial origin (e.g. breast cancer, prostate cancer, bladder cancer, brain cancer and hepatic cancer). In particular, the presence of ADAMTS-7 in a biological sample is indicative of cancer of epithelial origin. Thus, measuring the level of ADAMTS-7 in biological samples (e.g. urine or blood) provides a quick, easy, and safe screen that can be used to diagnose cancer in a patient.
Owner:CHILDRENS MEDICAL CENT CORP
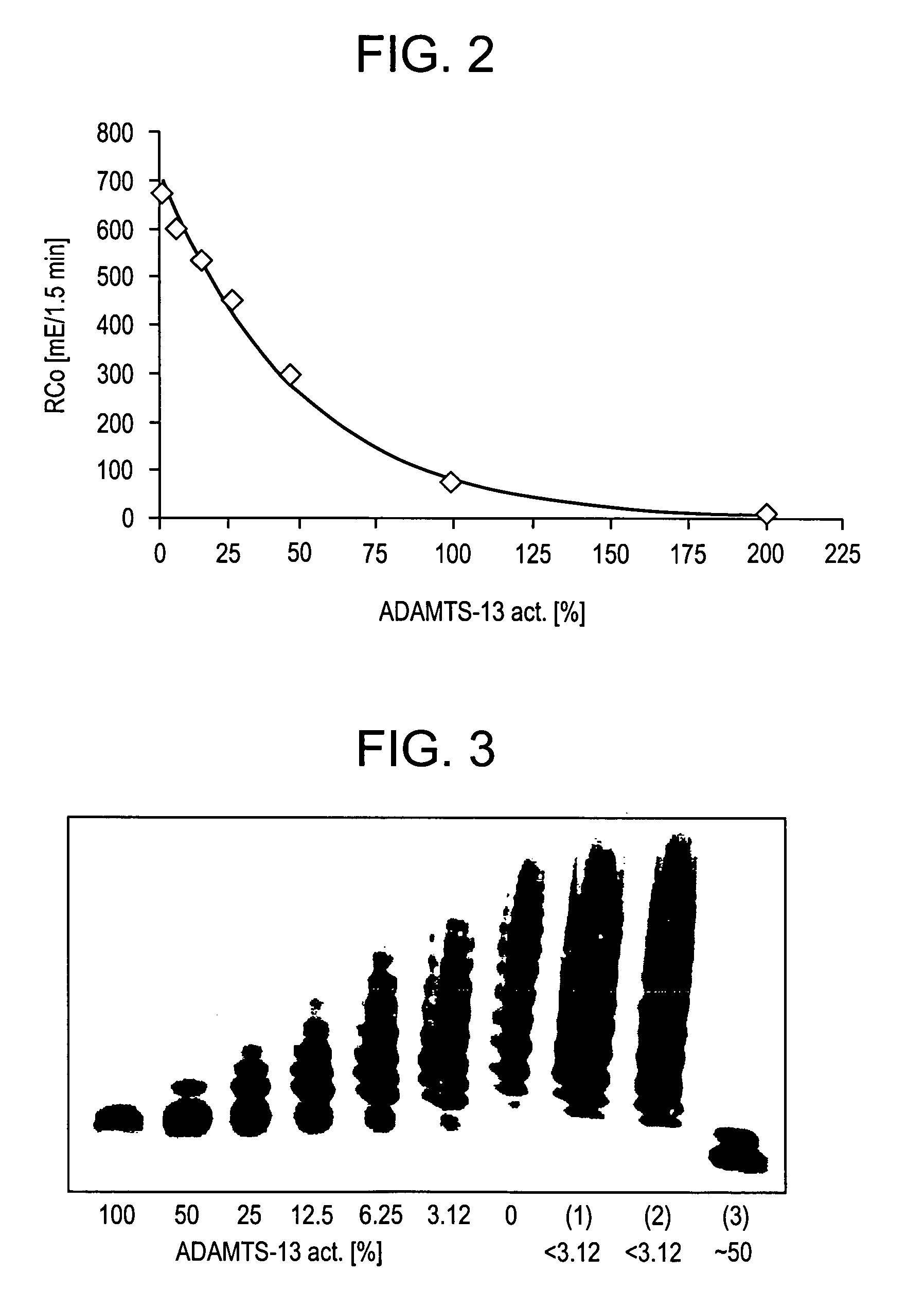
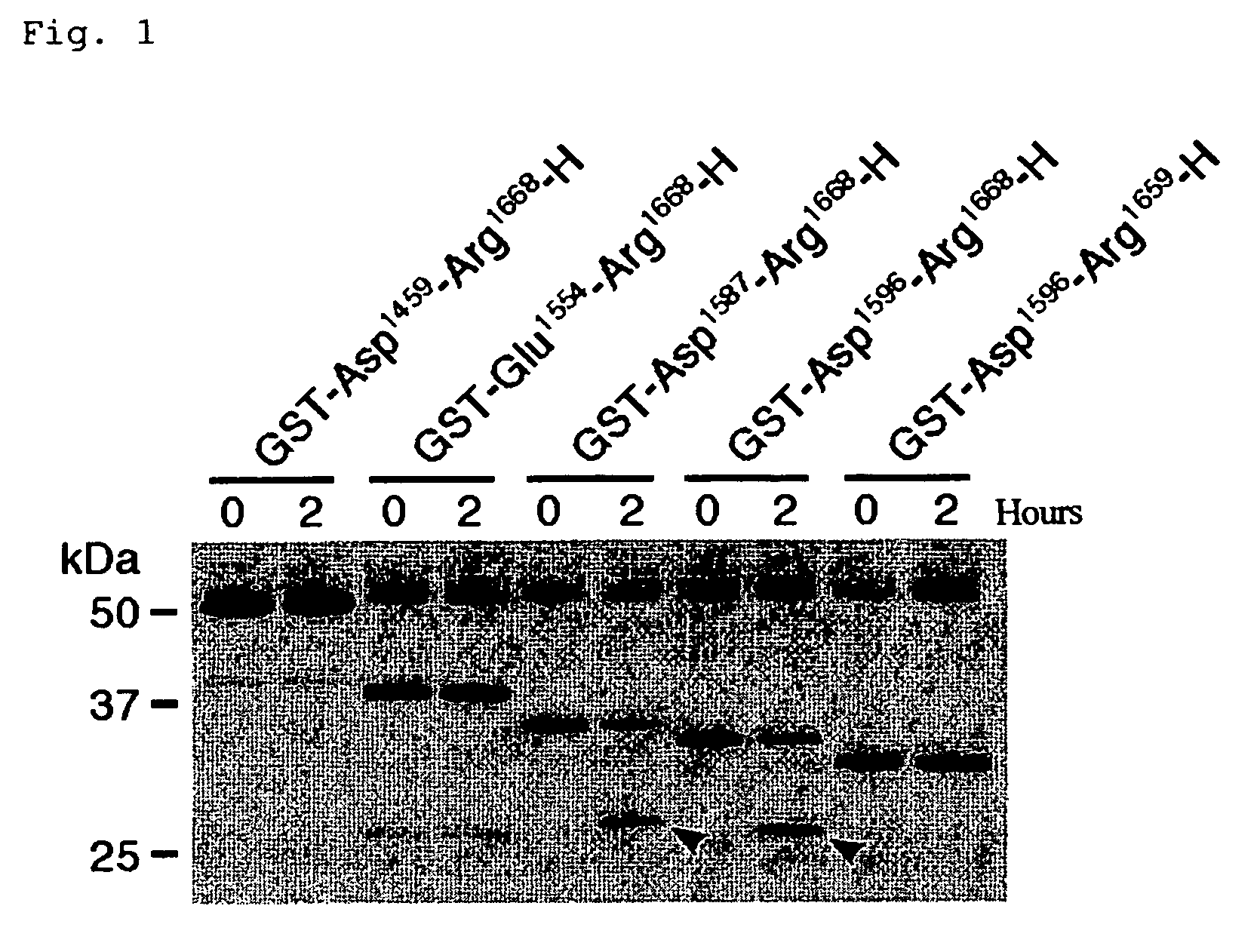
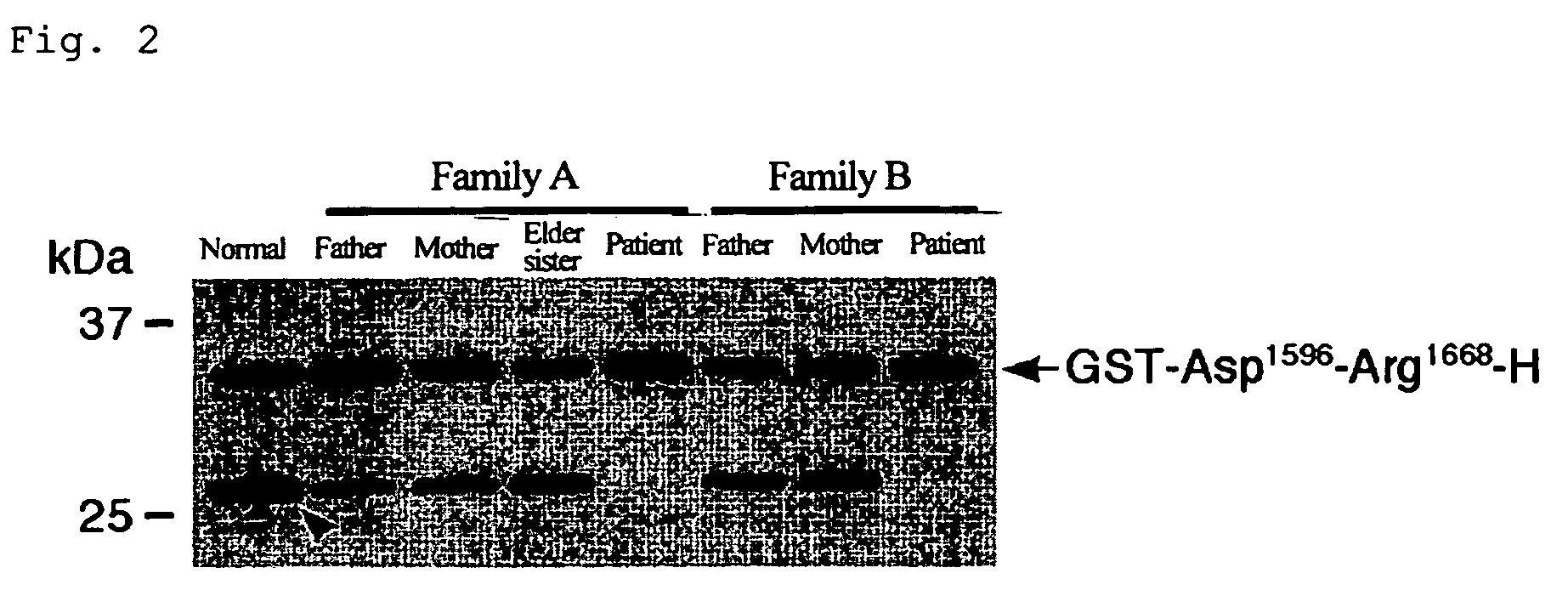
Substrates specific to von willebrand factor cleaving protease and method of assaying the activity
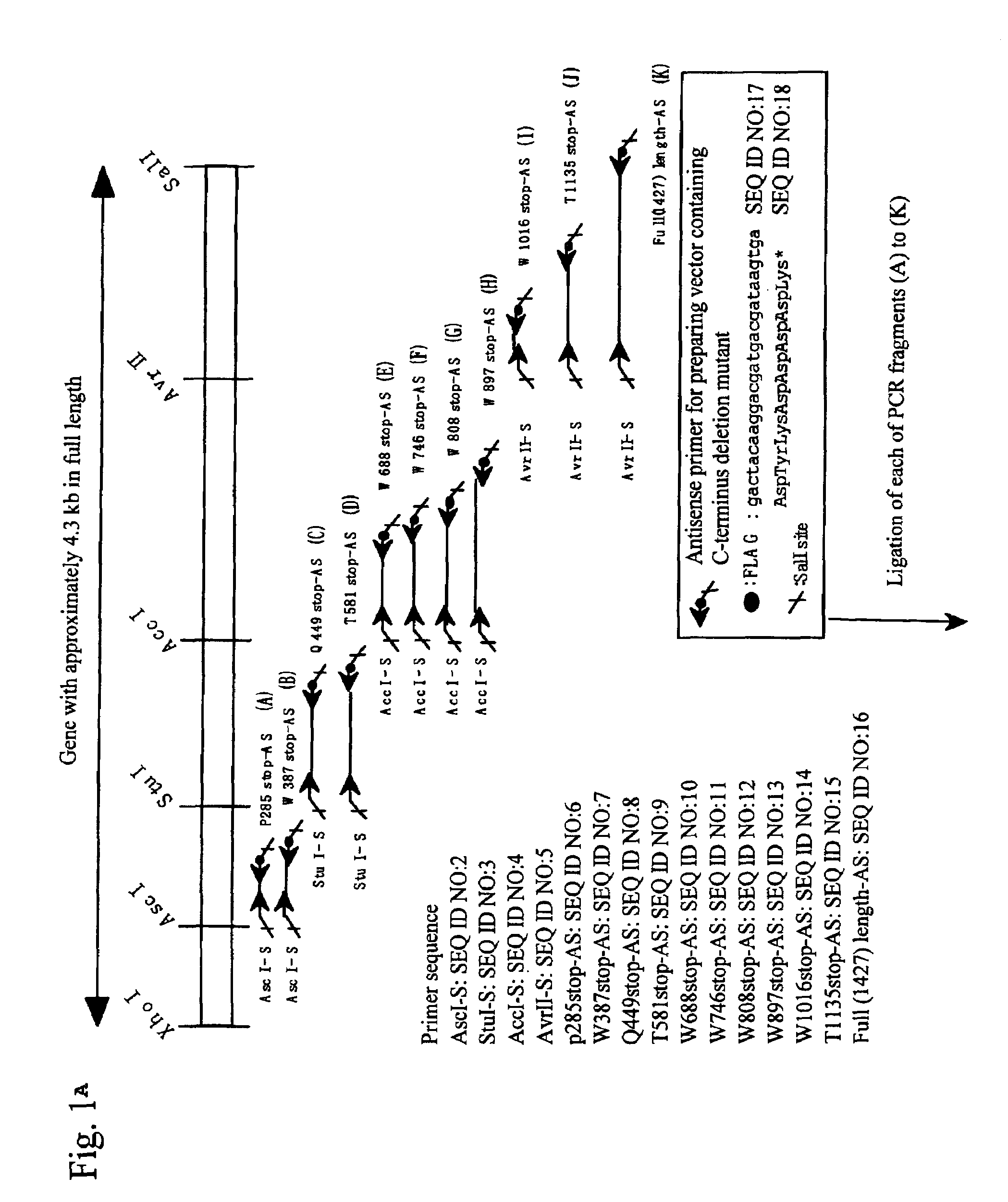
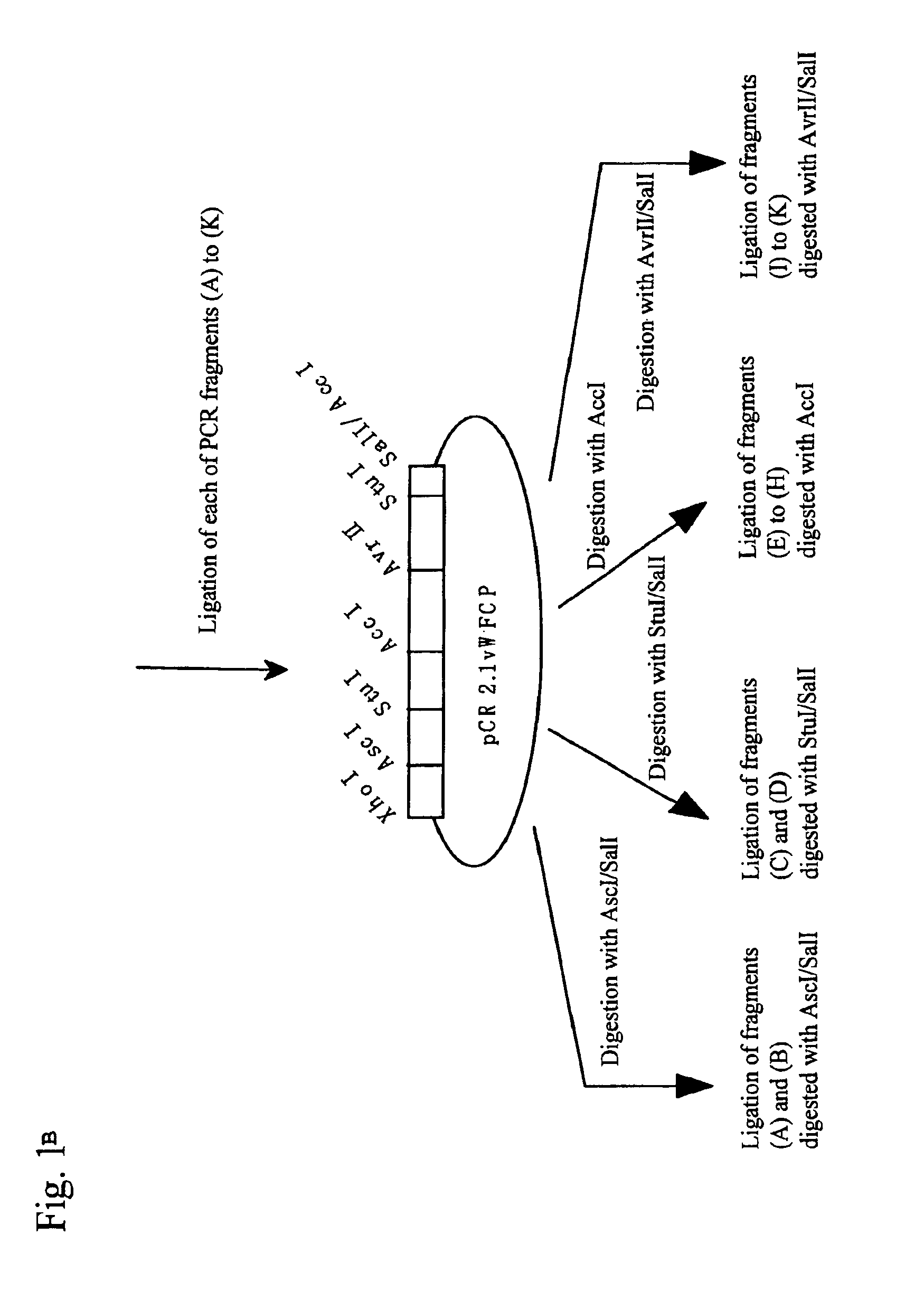
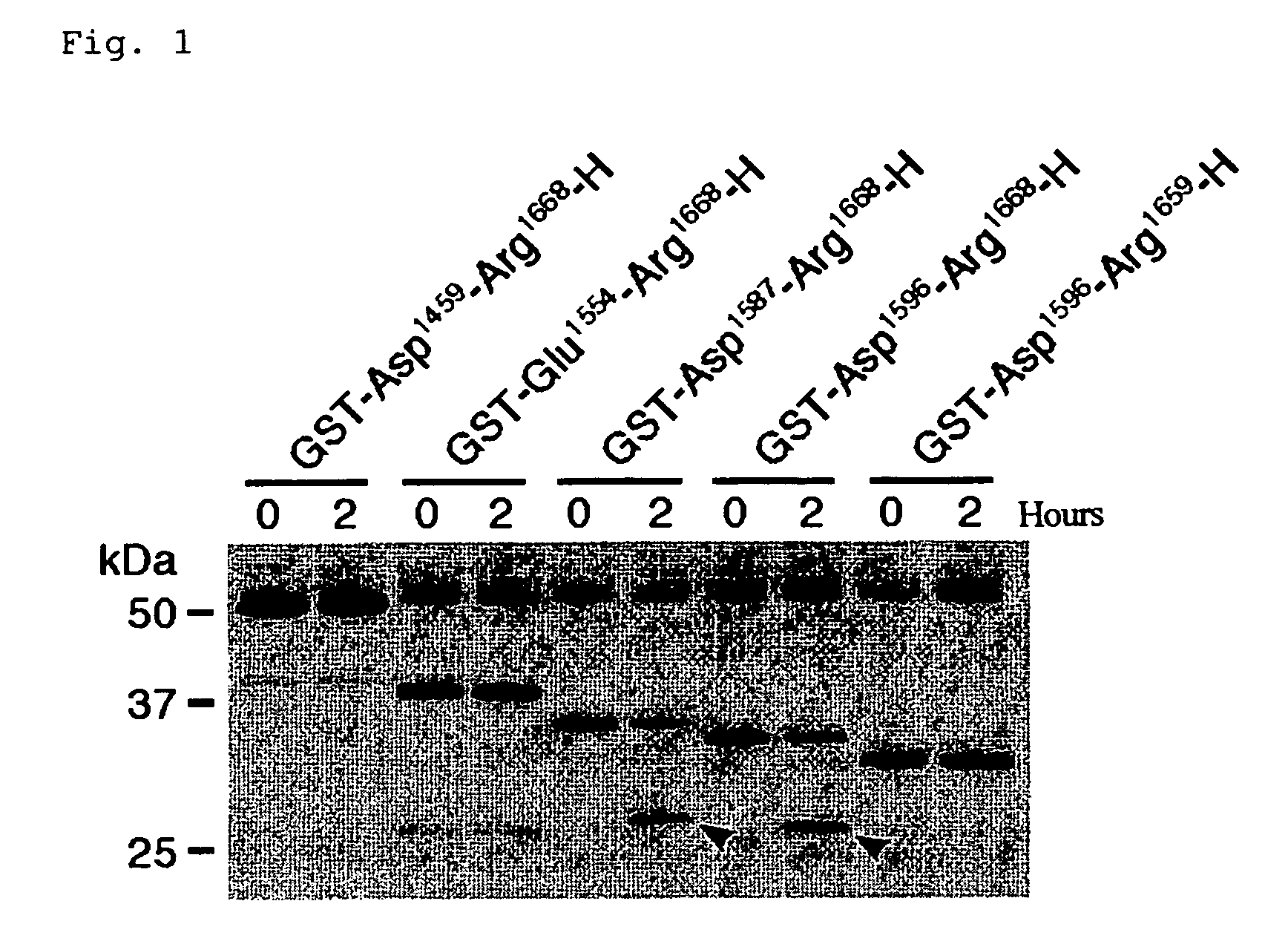
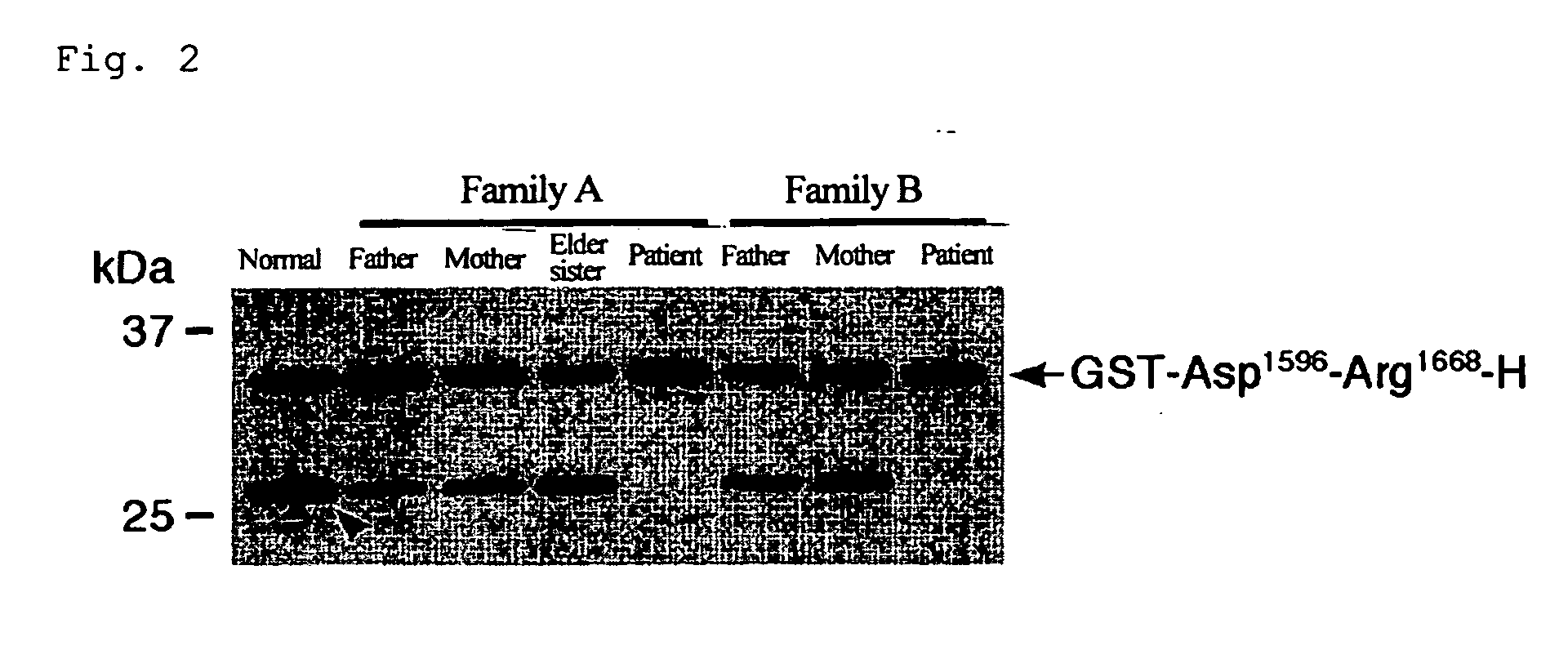
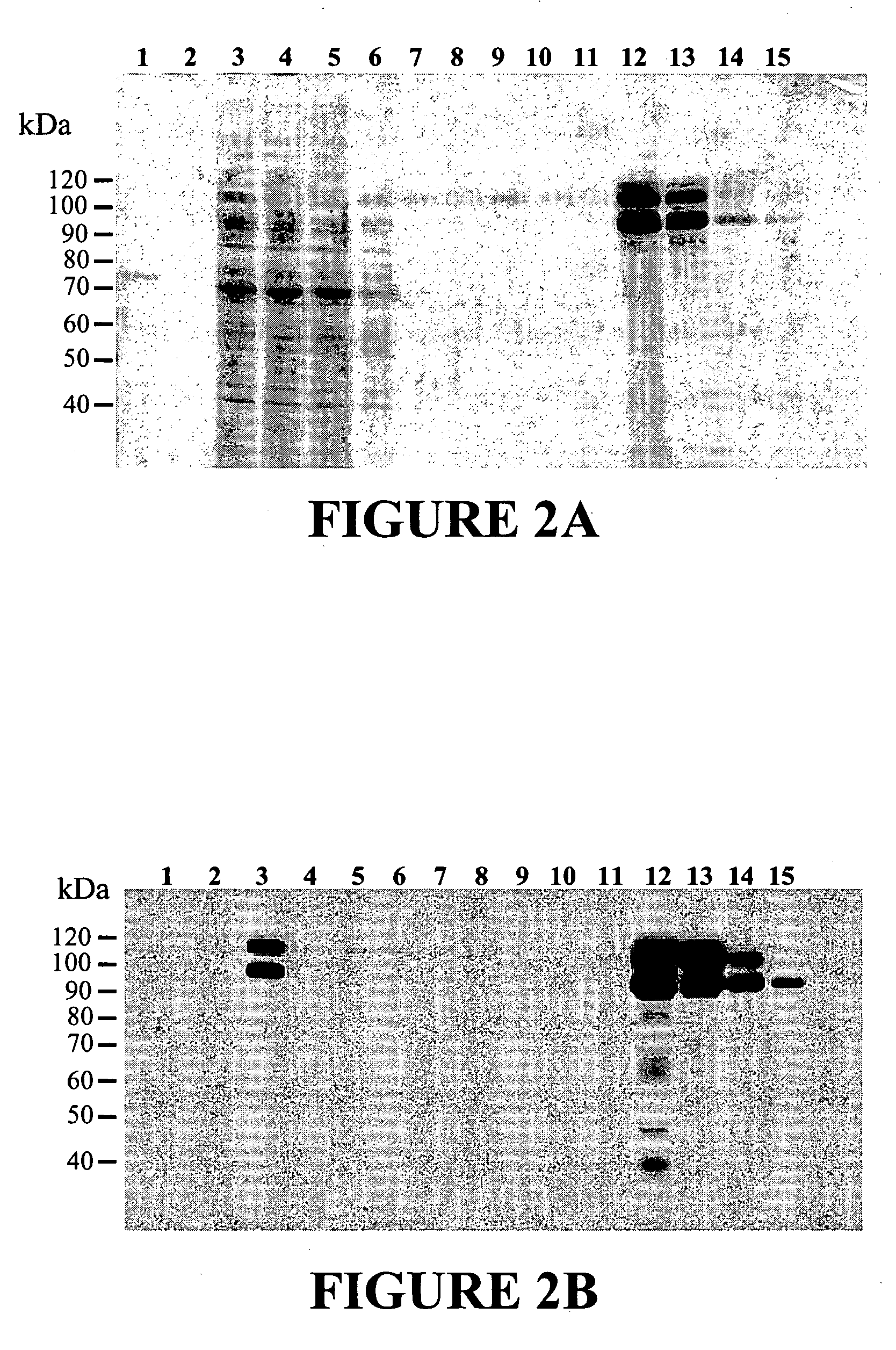
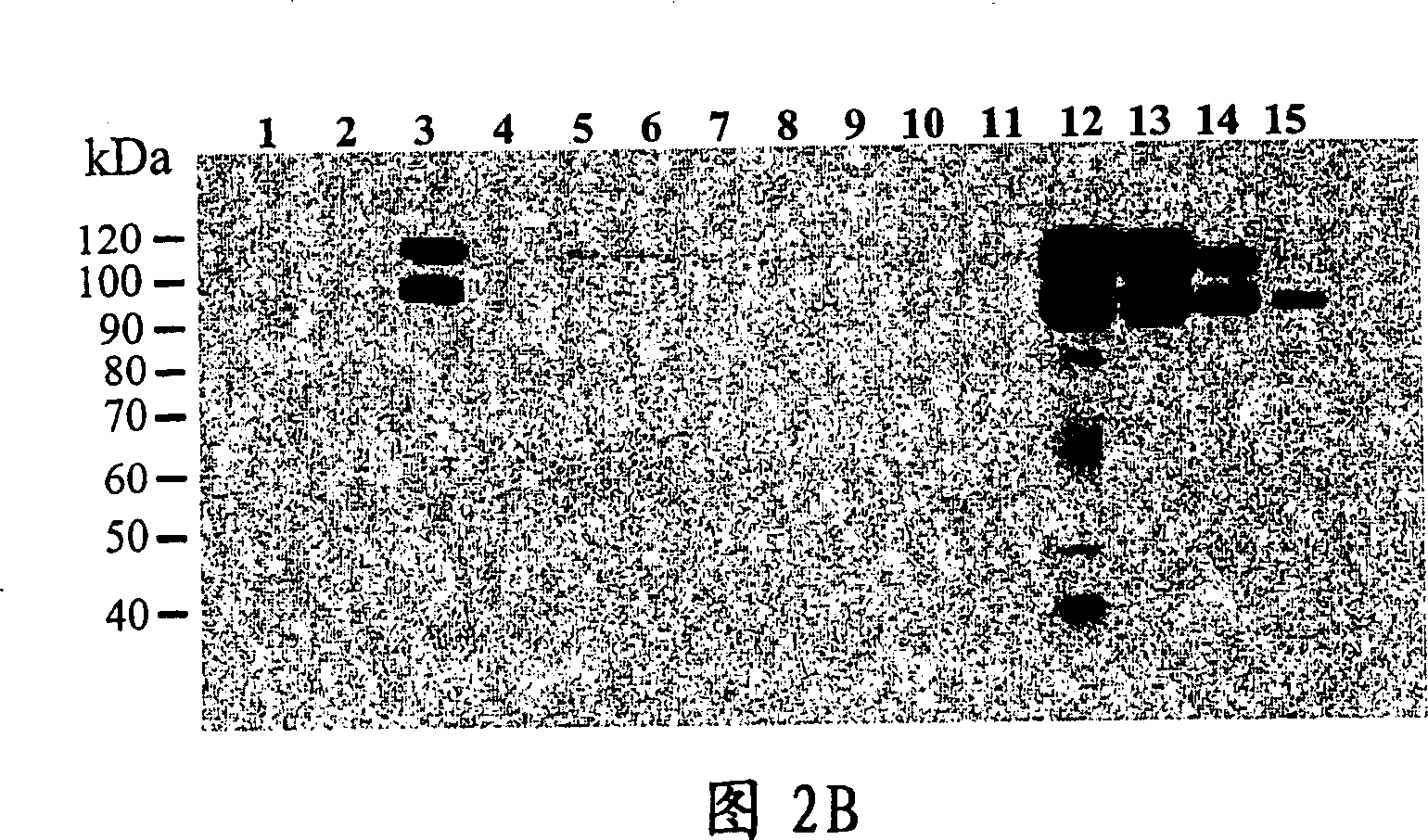
The present invention relates to specific substrates for a von Willebrand factor cleaving enzyme, ADAMTS-13, as well as to diagnosis of ADAMTS-13 deficient patients, diagnostic compositions, and kits employing the substrates. Particularly preferable substrate polypeptides for ADAMTS-13 are the polypeptide which begins at amino acid 1587 and ends at amino acid 1668 of SEQ ID NO: 1 in the Sequence Listing, and the polypeptide which begins at amino acid 1596 and ends at amino acid 1668 of SEQ ID NO: 1 in the Sequence Listing. These substrate polypeptides for ADAMTS-13 have high substrate specificity and also superior quantitativeness, and a suitable size for production by recombinant methods.
Owner:NAT CEREBRAL & CARDIOVASCULAR CENT
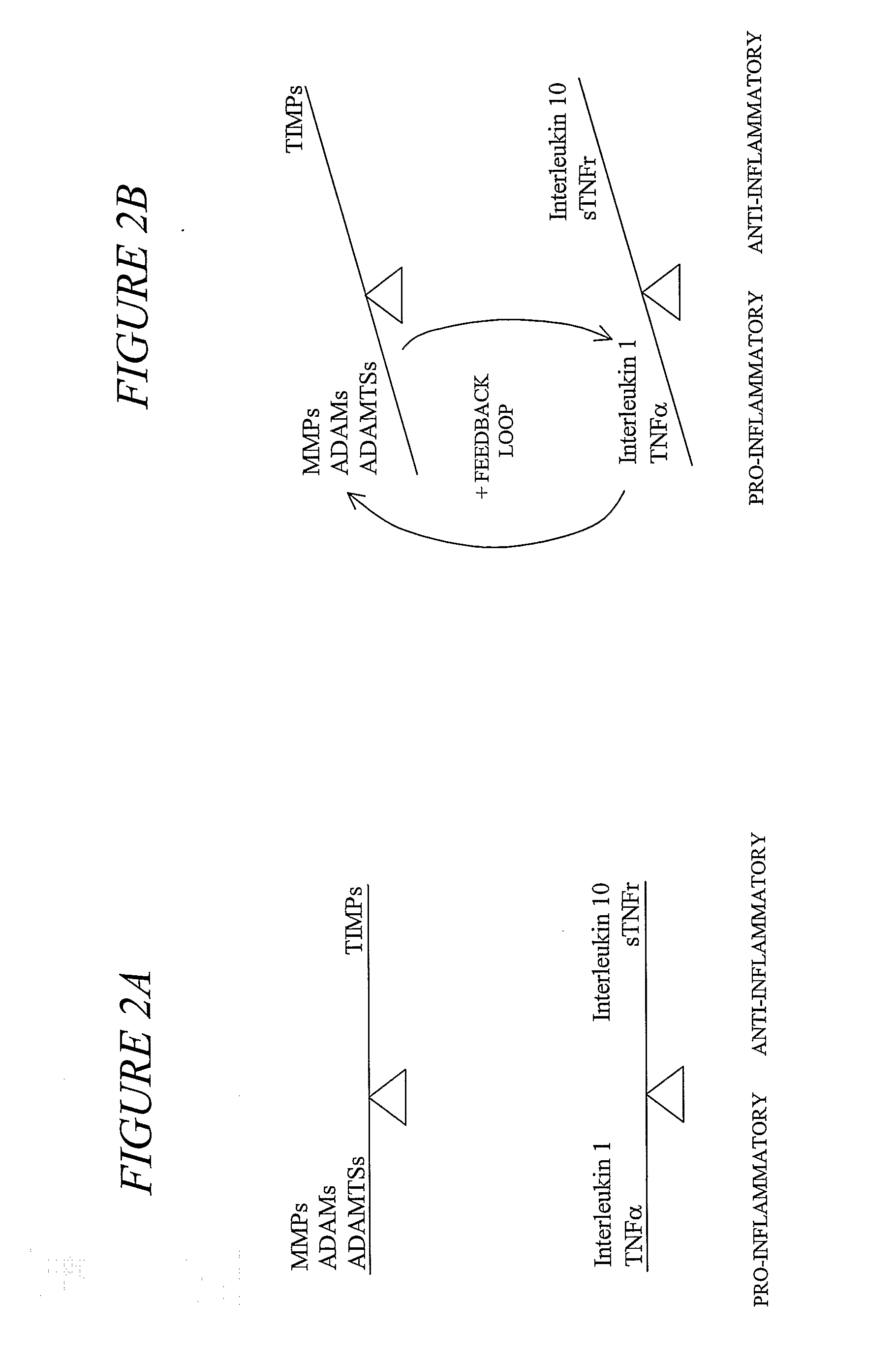
Compositions and Methods for Treating and Preventing Inflammatory and/or Degenerative Processes in Humans and Other Animals
Disclosed are compositions useful for treating Alzheimer's disease, atherosclerosis, arteriosclerosis, osteoarthritis and other degenerative joint diseases, Huntington's chorea, Parkinson's disease, optic atrophy, retinitis pigmentosa, macular degeneration, muscular dystrophy, aging-associated degenerative processes, asthma, dermatitis, laminitis, pemphigoid, pemphigus, reactive airway disease (e.g., COPD, IAD), inflammatory bowel disease (e.g., Crohn's disease, ulcerative colitis), multiple sclerosis, rheumatoid arthritis, periodontal disease, systemic lupus erythematosus, sarcoidosis, psoriasis, type I diabetes, ischemia-reperfusion injury, chronic inflammatory diseases, geriatric wasting, cancer cachexia, cachexia associated with chronic inflammation, sick feeling syndrome, and other inflammatory and / or degenerative diseases, disorders, conditions, and processes in humans and other animals. In one embodiment, the compositions include at least 4 of the following: a MMP1 inhibitor, a MMP2 inhibitor, a MMP3 inhibitor, a MMP7 inhibitor, a MMP9 inhibitor, an ADAMTS-4 inhibitor, a MMP13 inhibitor, and a MMP14 inhibitor. In another embodiment, the compositions include a curcuminoid, a polymethoxylated flavone, a catechin, and a boswellic acid.
Owner:BAKER DONALD J
Method for treating ADAMTS-5-associated disease
The present invention relates to methods of treating ADAMTS-5-associated diseases and particularly osteoarthritis comprising administering an agent capable of modulating ADMATS-5 activity to a subject afflicted with the disease. The agent is preferably a biaryl sulfonamide compound. The invention is based, in part, on the discovery that transgenic animals that do not express functional ADAMTS-5 show a reduction in the degree of osteoarthritis after the induction of osteoarthritis as compared to WT animals. Furthermore, the ADAMTS-5 transgenic animals have reduced aggrecanase activity in articular tissue as compared to WT animals. These animals are good models for ADAMTS-5-associated diseases, and for screening of drugs useful in the treatment and / or prevention of these diseases. There are no other animal models in which the deletion of the activity of a single gene is capable of abrogating the course of osteoarthritis. Accordingly, these animals also show that osteoarthritis can be prevented and / or treated by administering to a subject an ADAMTS-5 inhibitory agent and particularly an agent capable of inhibiting the aggrecanase activity of ADAMTS-5.
Owner:WYETH LLC
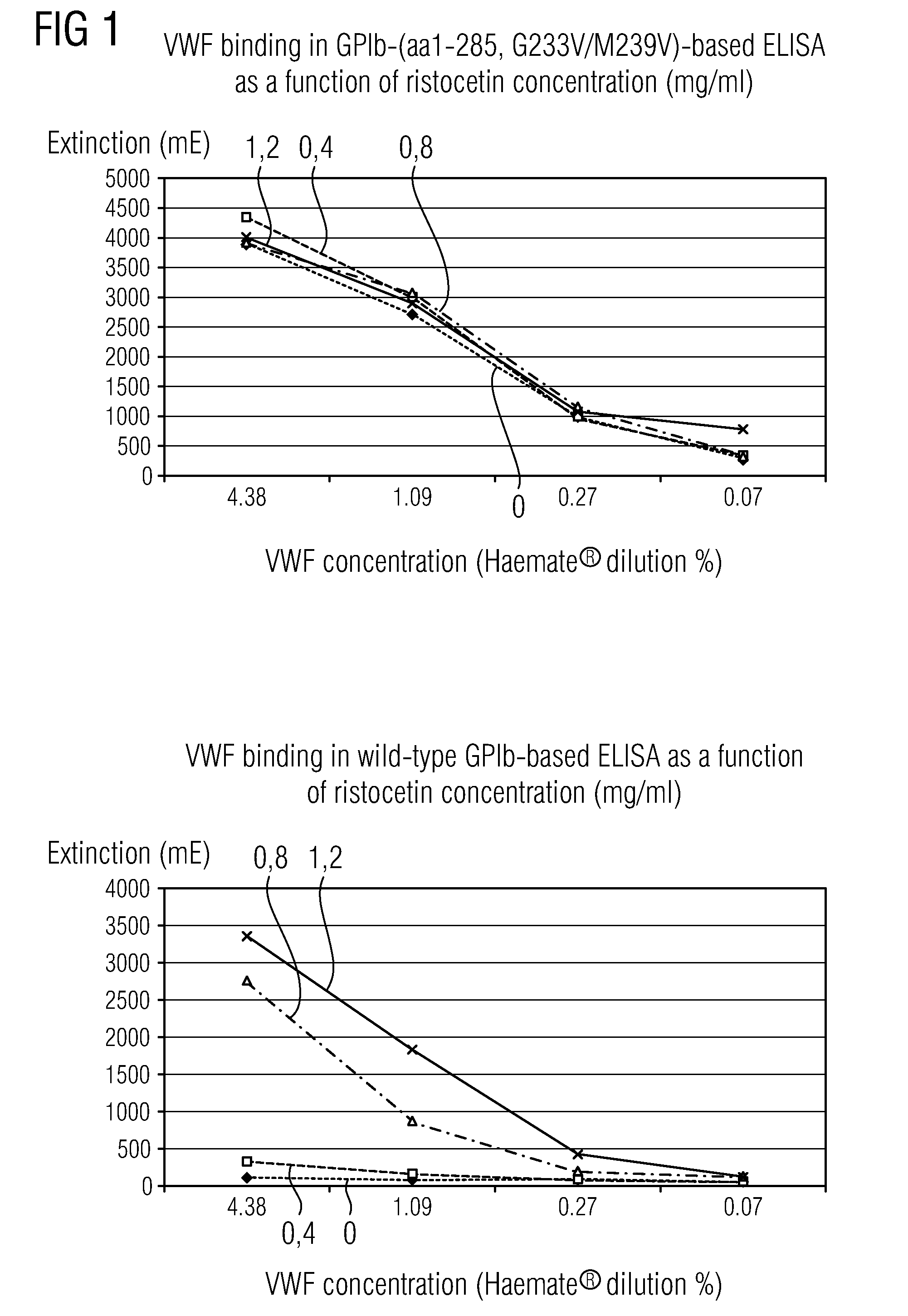
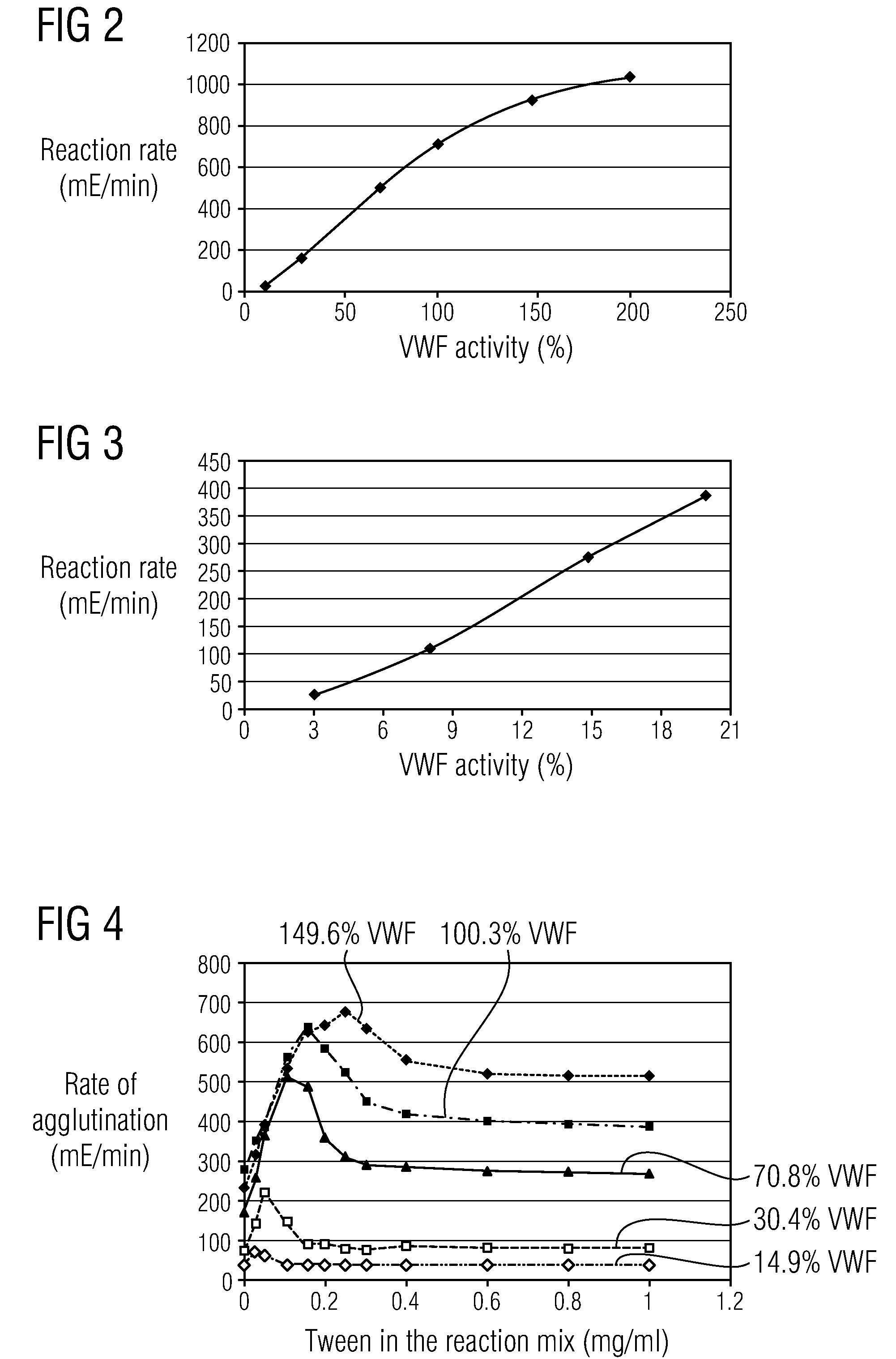
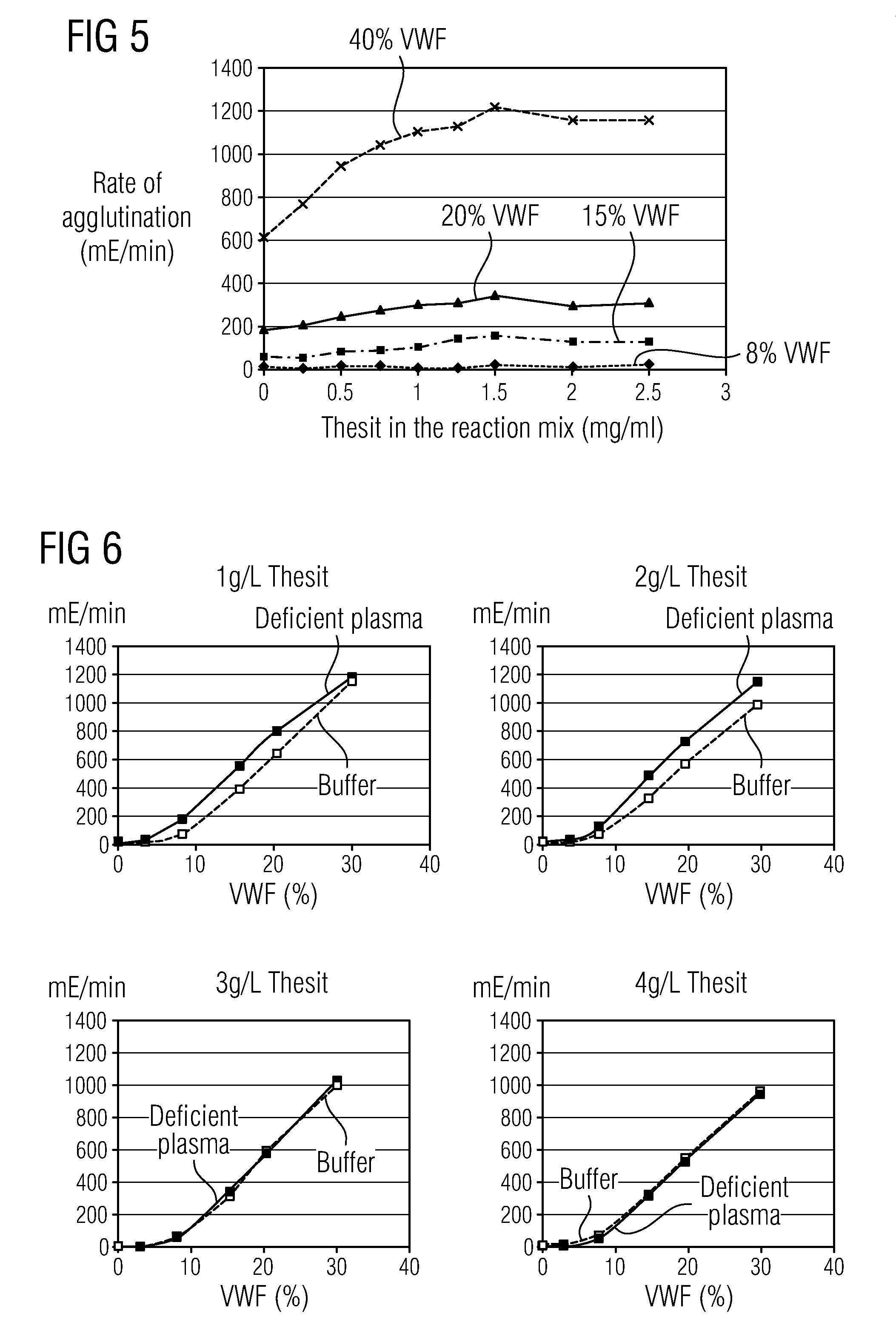
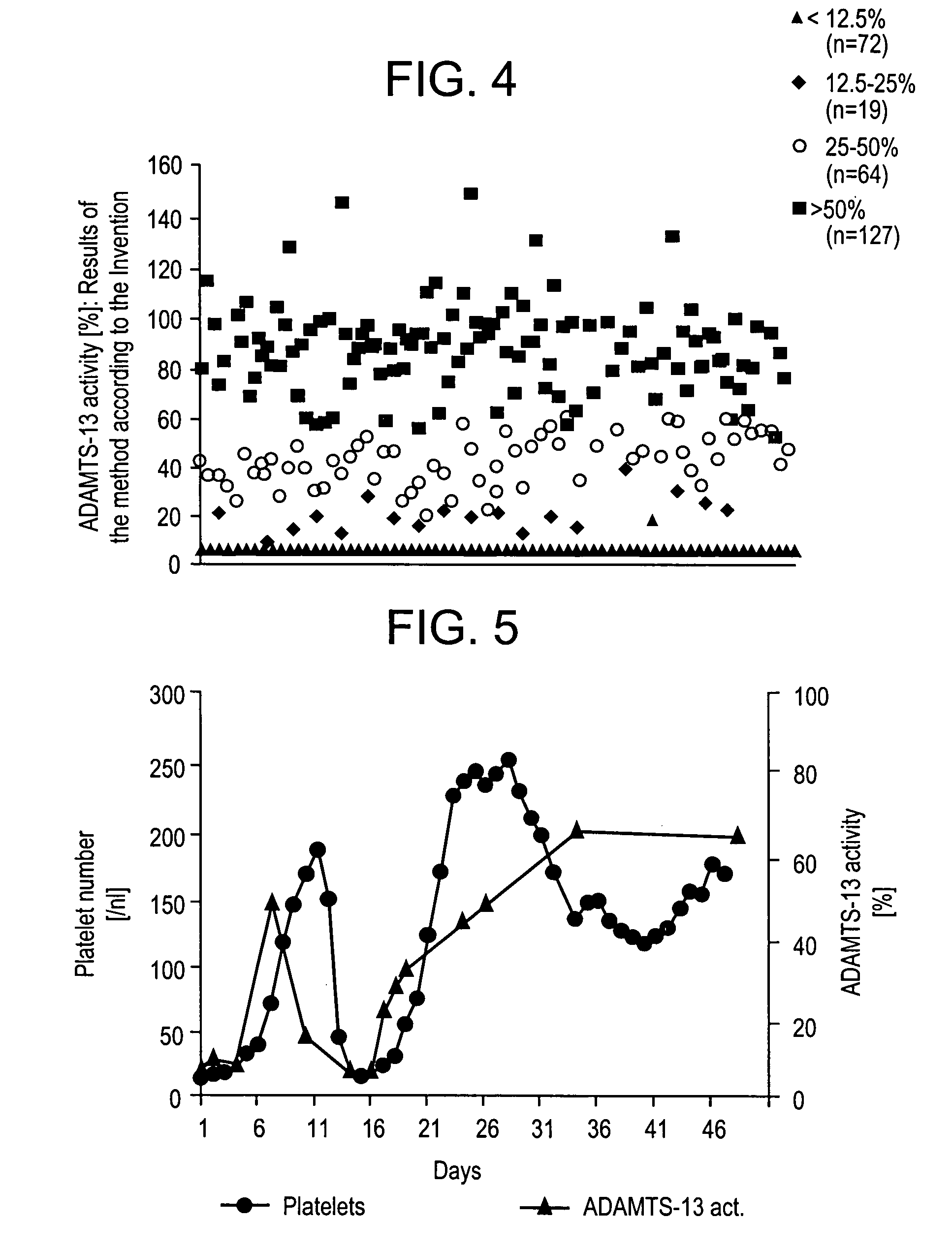
Methods and Kits for Determining von Willebrand Factor Activity in the Absence of Ristocetin and for Determining the Activity of ADAMTS-13 Protease
ActiveUS20100136589A1Microbiological testing/measurementDisease diagnosisProteinase activityFactor VIII vWF
Described herein are method(s), kit(s), reagent(s) and the like for determining von Willebrand factor (VWF) activity in a sample in the absence of ristocetin.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS PRODS
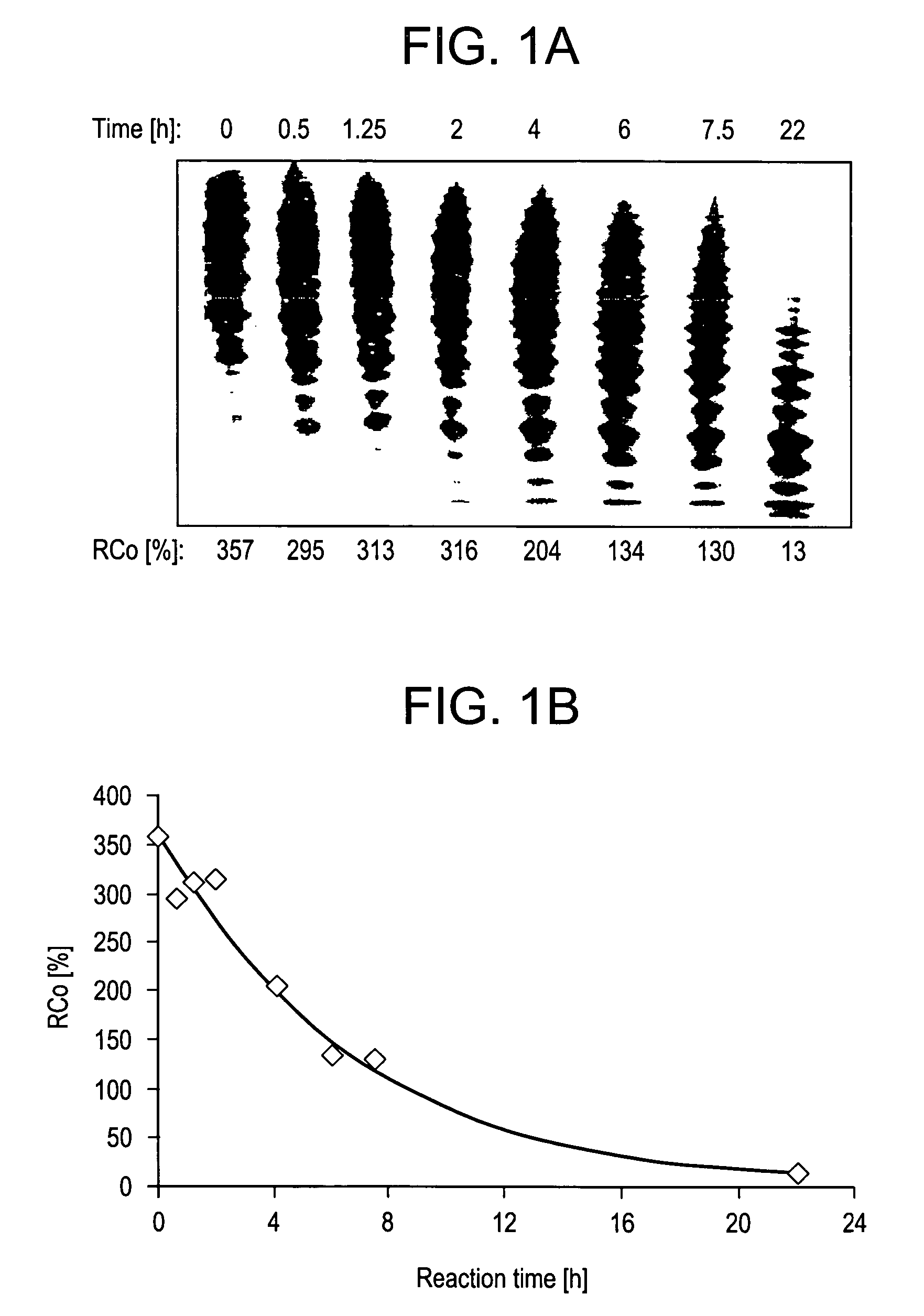
Method for detecting the von Willebrand factor-cleaving protease activity of ADAMTS-13
InactiveUS7291479B2Microbiological testing/measurementBiological testingProteinase activityFactor VIII vWF
The invention relates to a diagnostic method for determining the von Willebrand factor (VWF) cleaving activity of ADAMTS-13 in a test medium during which the test medium is mixed with 0.5 to 5 U / ml of a von Willebrand factor (VWF) that does not contain ADAMTS-13, and after incubation, the ADAMTS-13 activity is determined based on the drop in the VWF-mediated aggregation of thrombocytes.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS PRODS
Antibody against von Willebrand factor cleaving enzyme and assay system using the same
ActiveUS7575872B2Easily isolated and sequencedUseful imageAnimal cellsHydrolasesEpitopeFactor VIII vWF
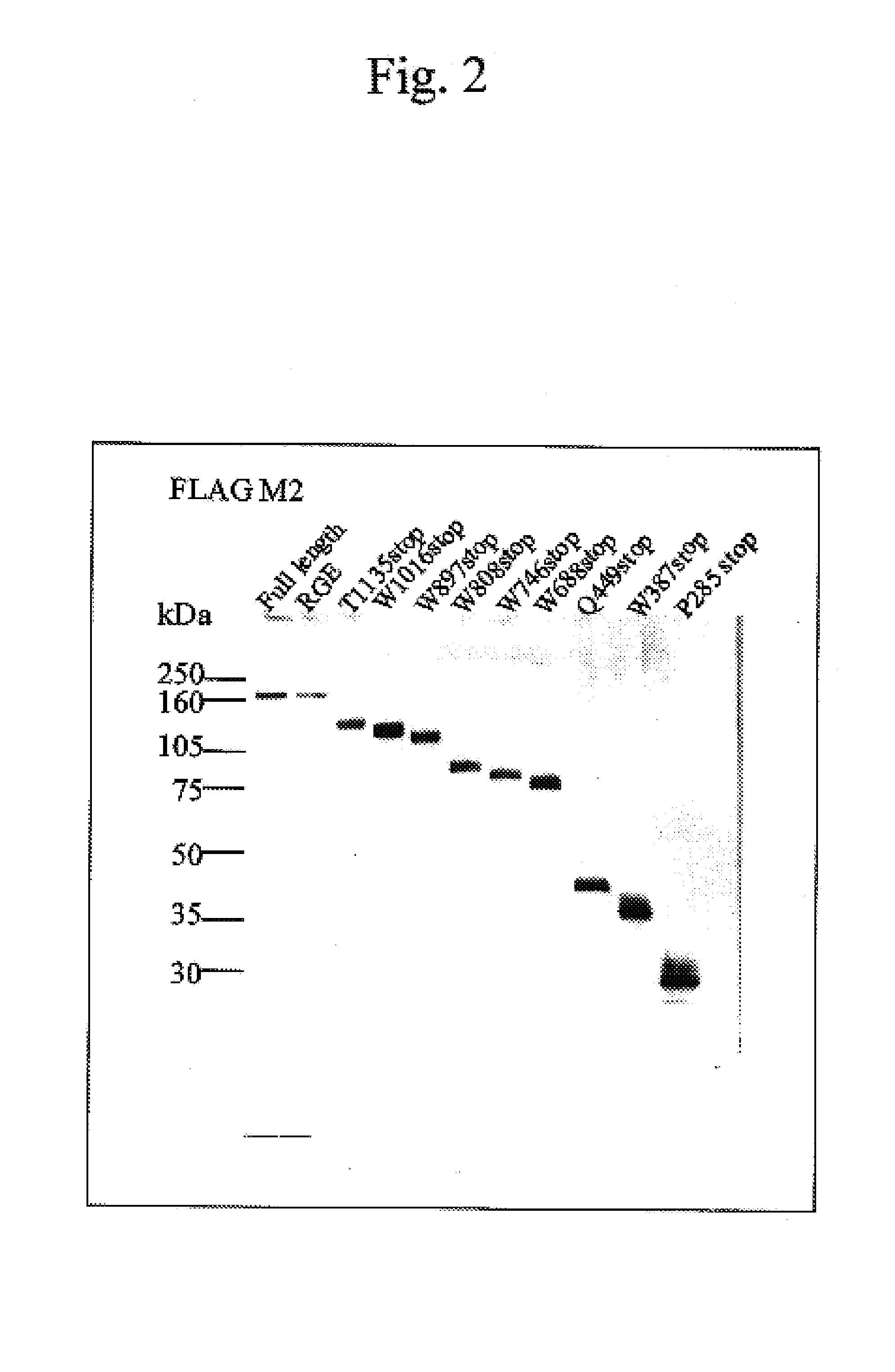
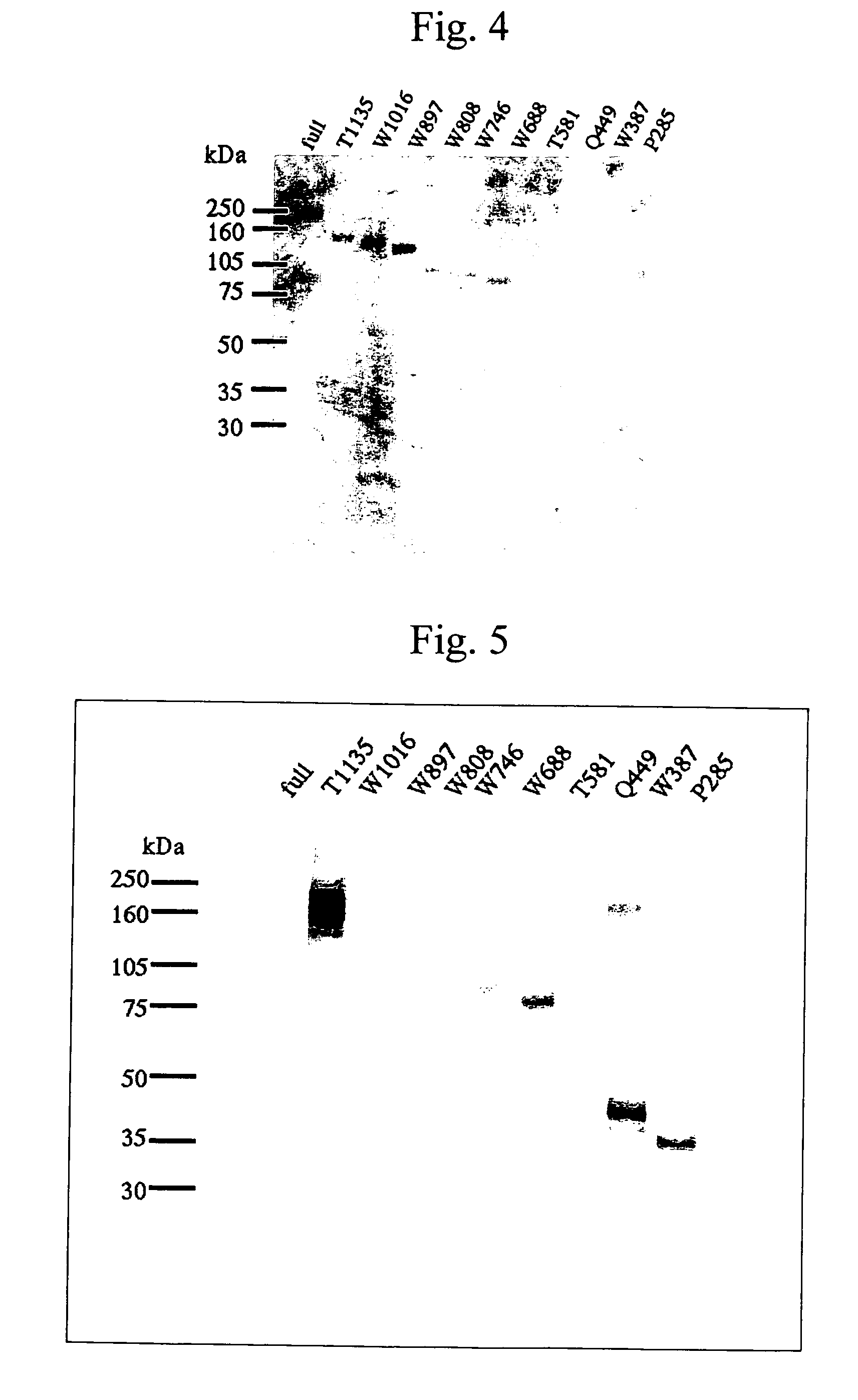
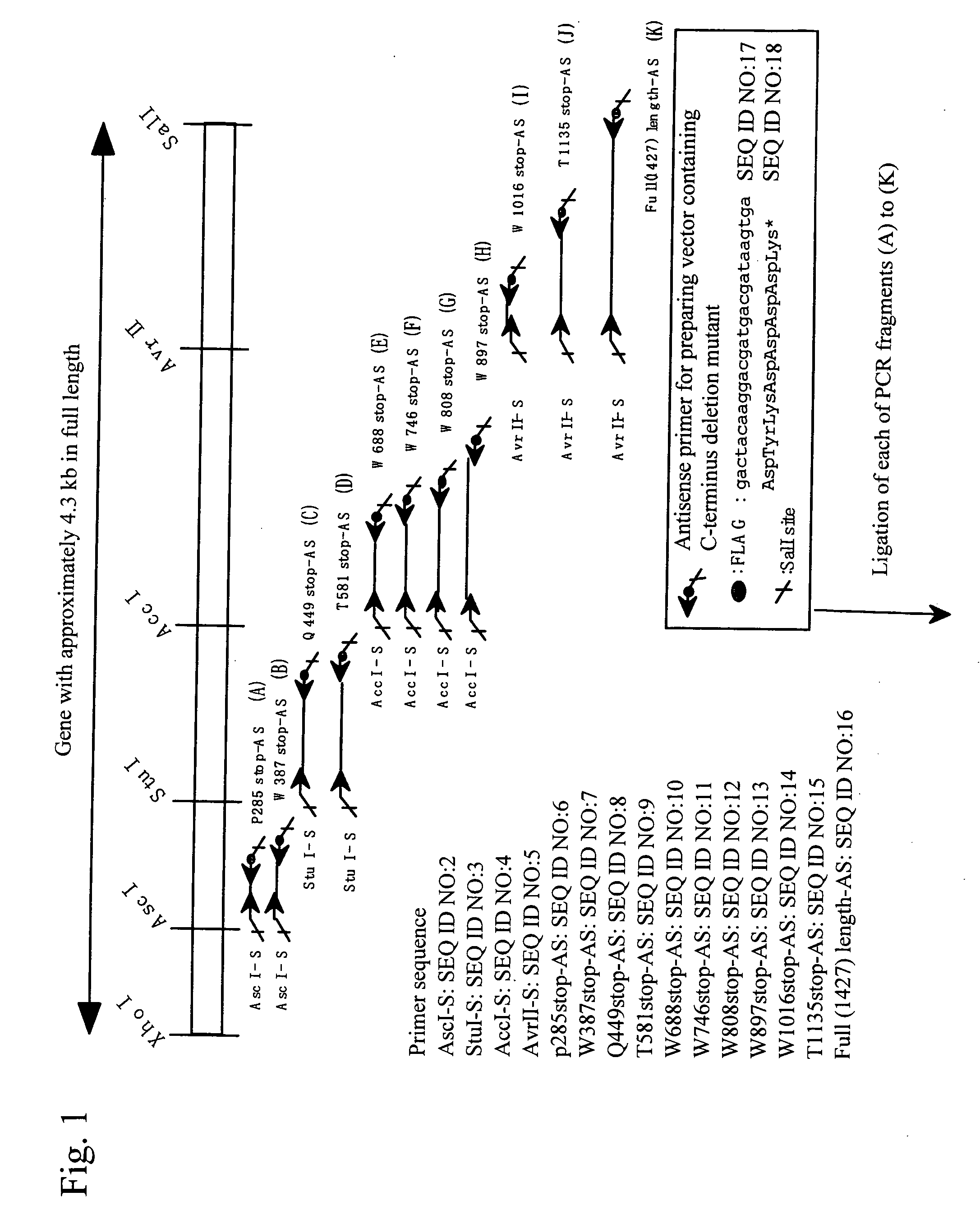
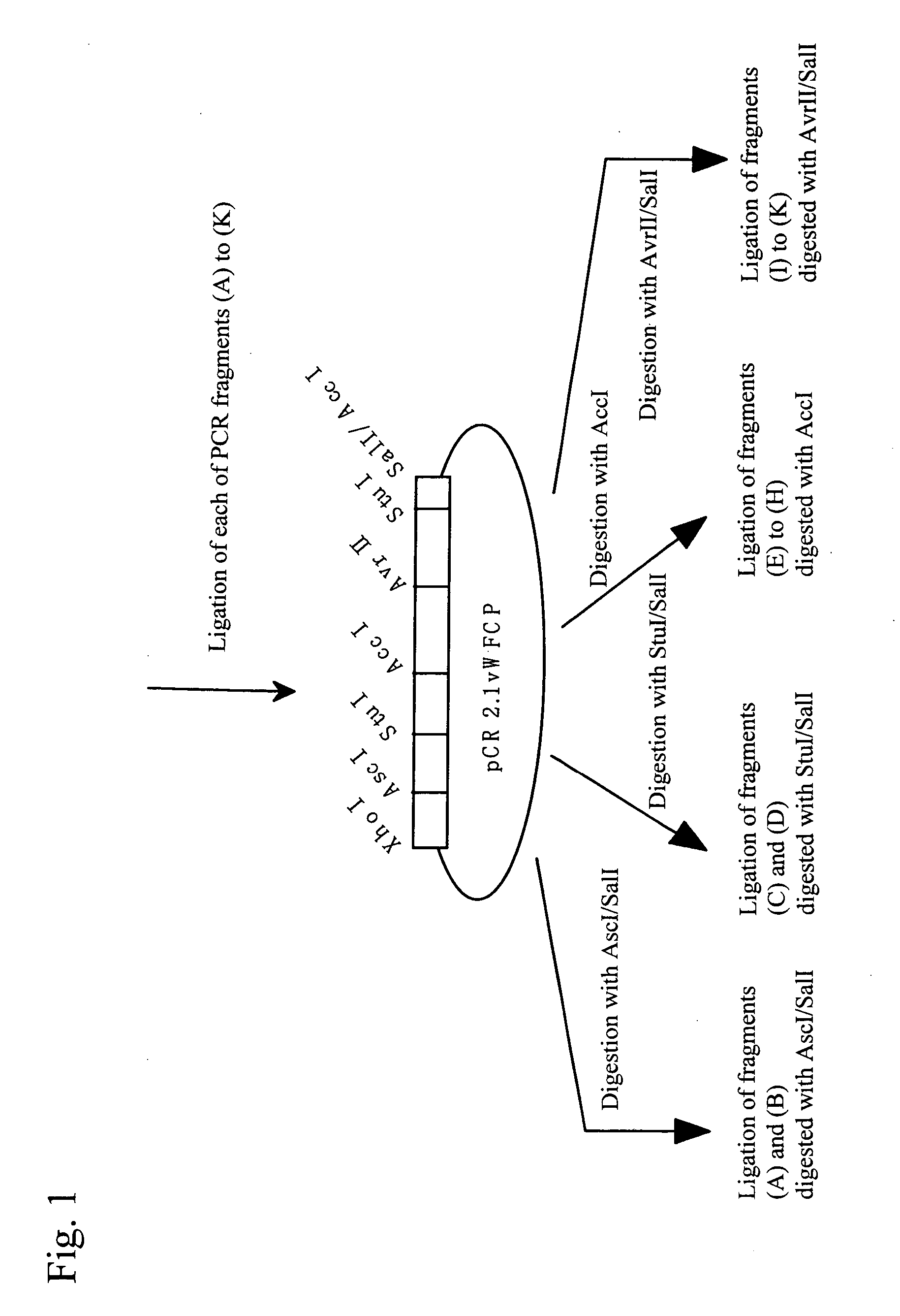
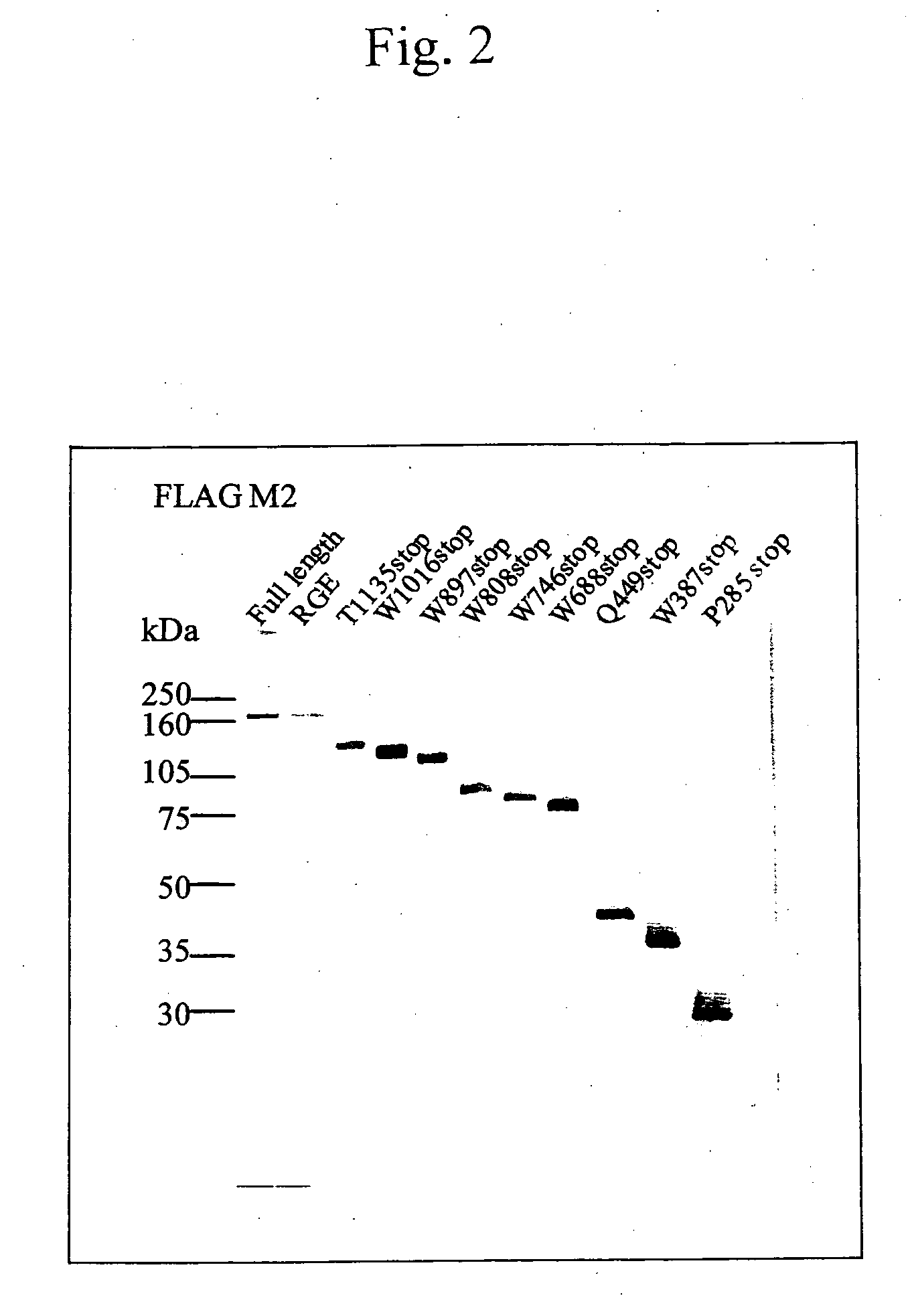
It is intended to provide an antibody showing immunoreactivity selectively to ADAMTS-13 and applications of this antibody in epitope analysis or diagnosis of an ADAMTS-13 autoantibody-positive patient. Alternatively, it is intended to provide a process for producing and use of a modified ADAMTS-13 molecule partially deleted aiming at the application in pharmaceutical products. An antibody specific for ADAMTS-13 which can be obtained from a warm-blooded animal immunized and sensitized with a polypeptide containing a part or the whole of ADAMTS-13 amino acid sequence; a process for producing an antibody comprising a step of immunizing and sensitizing a warm-blooded animal with a polypeptide containing a part or the whole of ADAMTS-13 amino acid sequence; use of the above-described antibody including a method of detecting and purifying ADAMTS-13; and a modified ADAMTS-13 molecule partially deleted are provided.
Owner:KM BIOLOGICS CO LTD
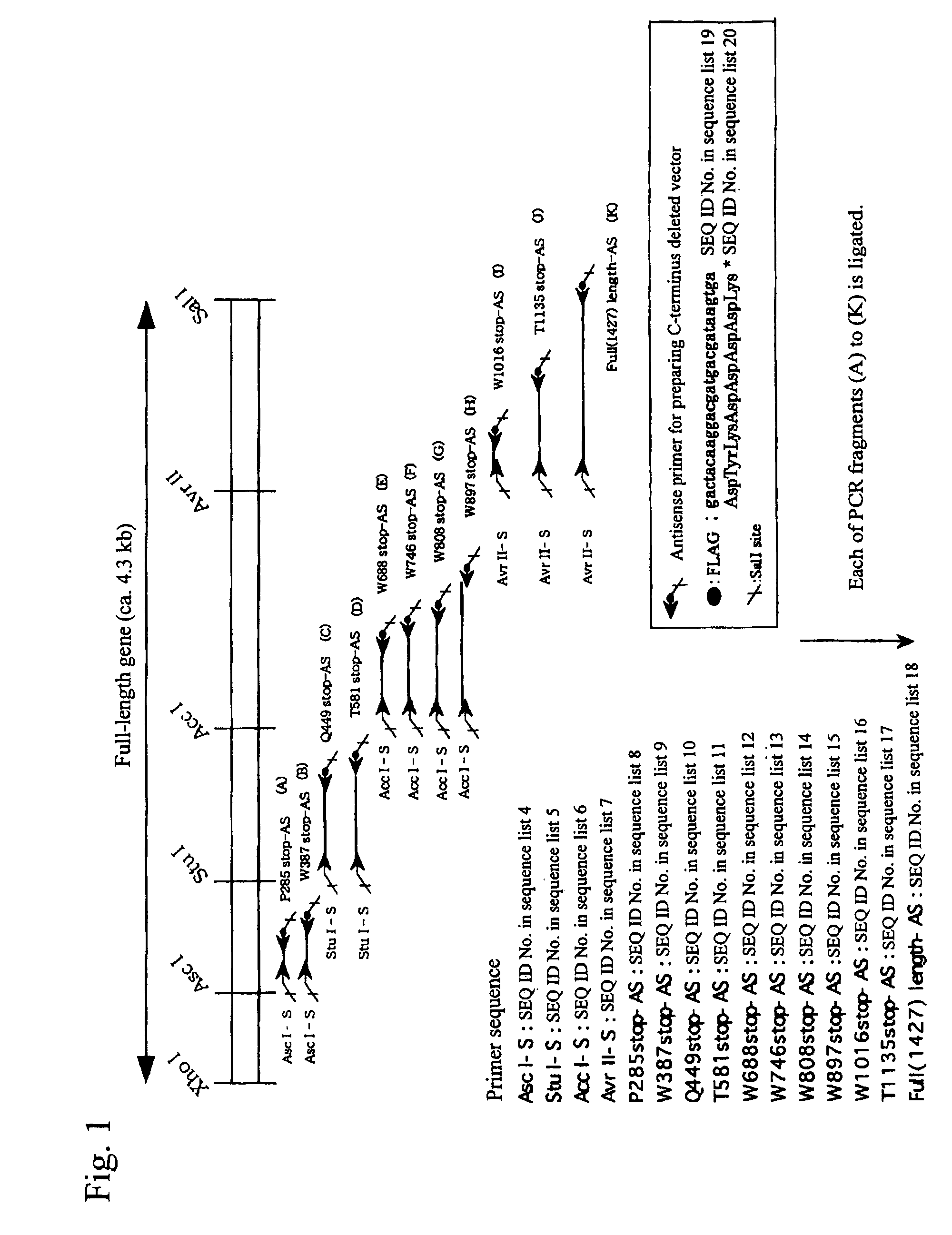
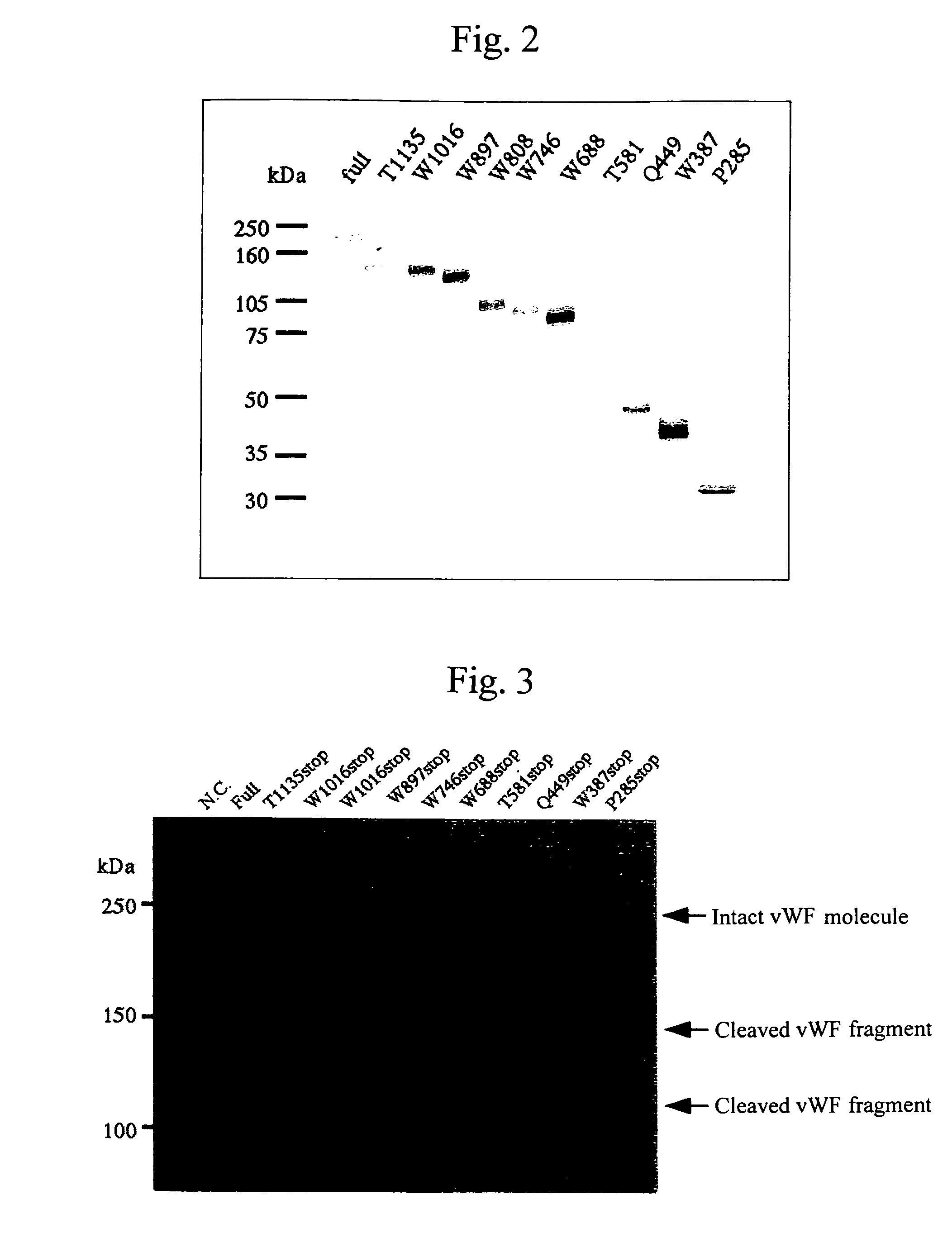
Construct comprising recognition domain of antibody against von willebrand factor-specific cleaving enzyme
The present invention provides an epitope recognized by an antibody (hereinafter, also referred to as an anti-ADAMTS-13 antibody) against a cleaving protease (hereinafter, also referred to as ADAMTS-13) specific to von Willebrand factor (hereinafter, also referred to as vWF), and a polypeptide comprising the epitope region. The present invention also provides a polypeptide located in a region from position 449 to position 687 in an amino acid sequence composing the ADAMTS-13, which is recognized by the anti-ADAMTS-13 antibody, or a peptide fragment derived from the polypeptide.
Owner:KM BIOLOGICS CO LTD
Methods and kits for detecting and measuring ADAMTS13/FXI complexes
InactiveUS20080138837A1Inhibit and enhance formationMicrobiological testing/measurementDisease diagnosisHemostatic DisordersPeptide substrate
Provided are assays and kits to detect ADAMTS13 activity using peptide substrates and ADAMTS13 / Factor XI complexes using ELISA. These assays and kits can be used for diagnostic applications and to evaluate treatment of thrombotic or hemostatic disorders, for example, thrombotic thrombocytopenic purpura (TTP). Also provided is a novel form of ADAMTS13 found on platelets, and anti-ADAMTS13 antibodies.
Owner:AMERICAN DIAGNOSTICA
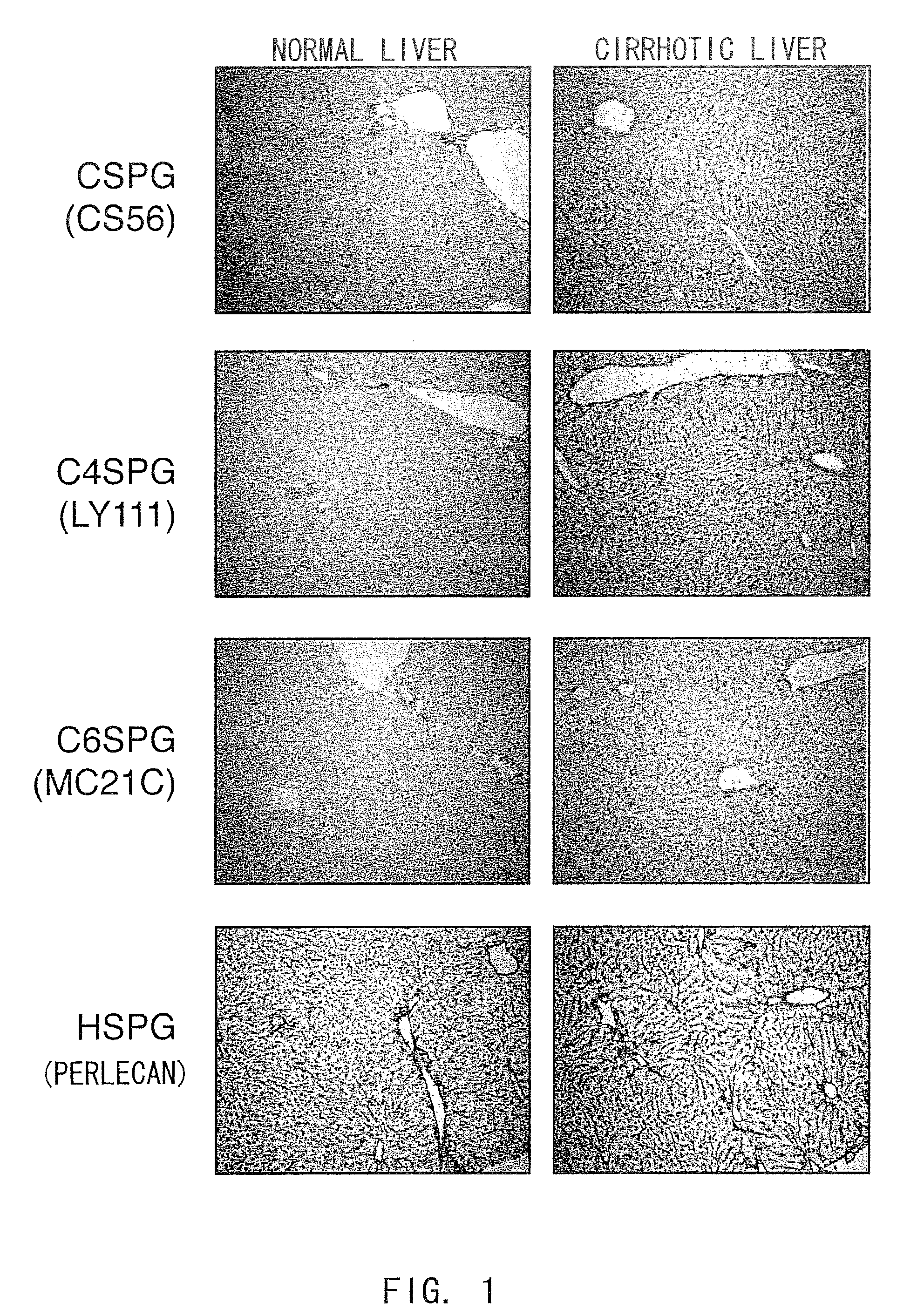
Agents for suppressing hepatic fibrosis
InactiveUS20090060892A1Enhance liver tissue fibrosisEfficient degradationPeptide/protein ingredientsMetabolism disorderLiver tissueMedicine
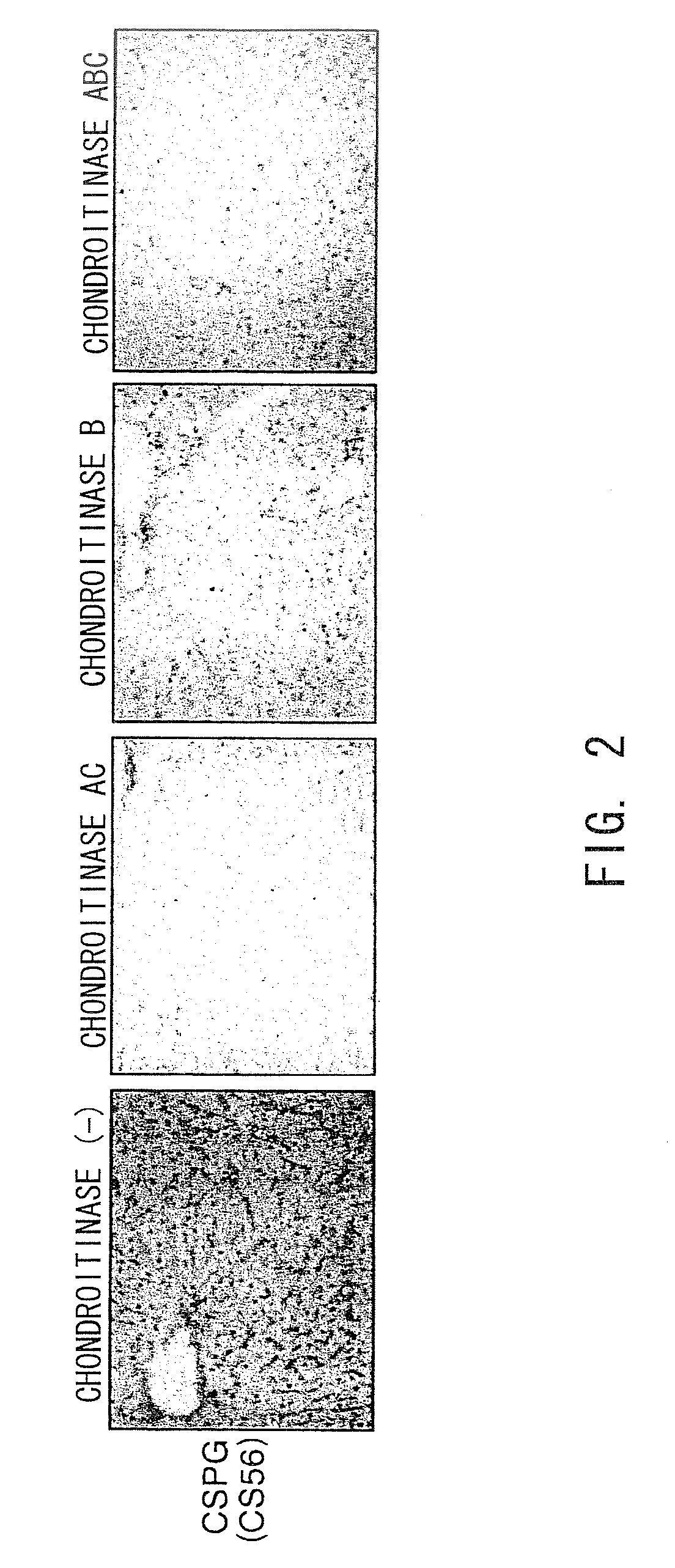
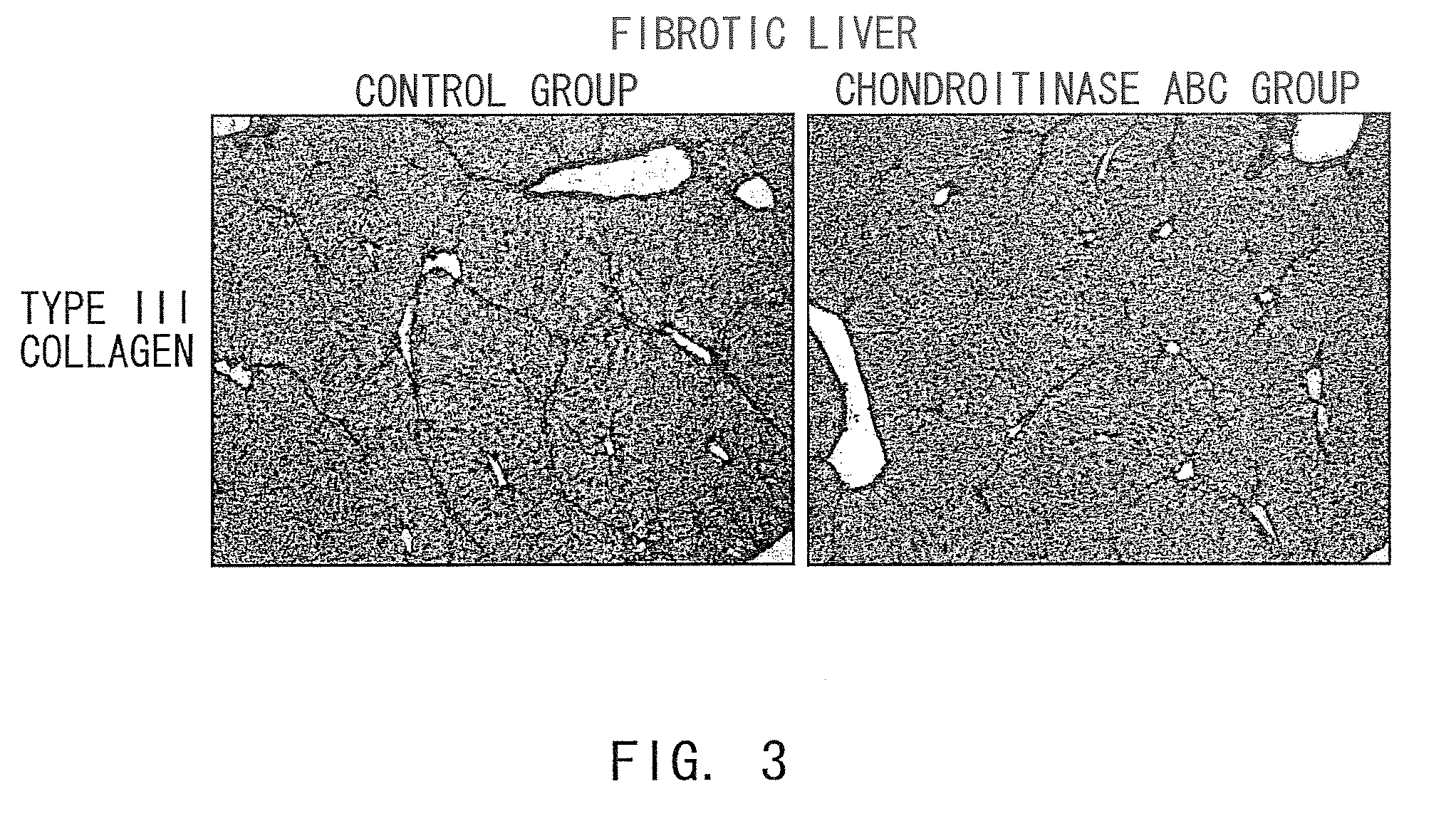
The present invention relates to hepatic fibrosis-suppressing agents that are suitable for treating or preventing fibrotic liver diseases such as cirrhosis, which comprise as an active ingredient a substance that inhibits the production or accumulation of chondroitin sulfate proteoglycans including chondroitinase ABC and ADAMTS-4; and methods of screening for the agents.The present inventors discovered for the first time that hepatic fibrosis could be efficiently suppressed by suppressing the production or accumulation of chondroitin sulfate proteoglycans. Specifically, fibrosis of liver tissues can be suppressed by administering chondroitinase ABC, a chondroitin sulfate proteoglycan-degrading enzyme, or by using siRNA to suppress the expression of C4ST-1, C6ST-1, or C6ST-2, a sulfotransferase for chondroitin sulfate proteoglycans. Compounds such as nucleic acids that are used as siRNA can be used as effective agents for suppressing hepatic fibrosis. Furthermore, hepatic fibrosis-suppressing agents can be found by screening for compounds that suppress the production or accumulation of chondroitin sulfate proteoglycans.
Owner:STELIC INST OF REGENERATIVE MEDICINE
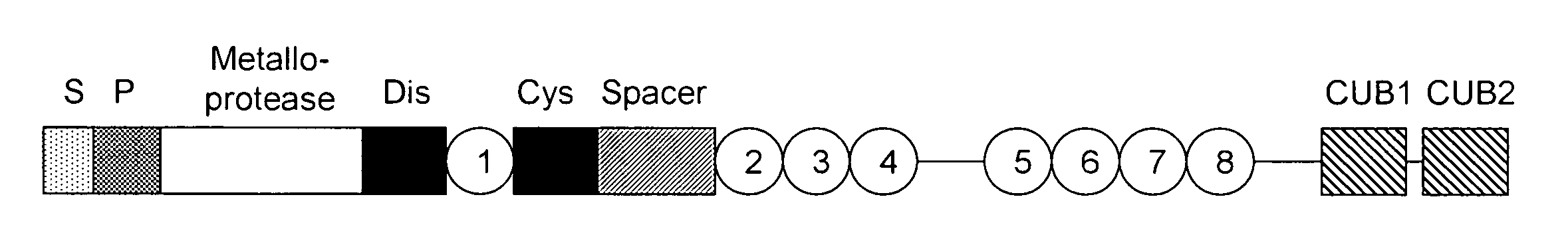
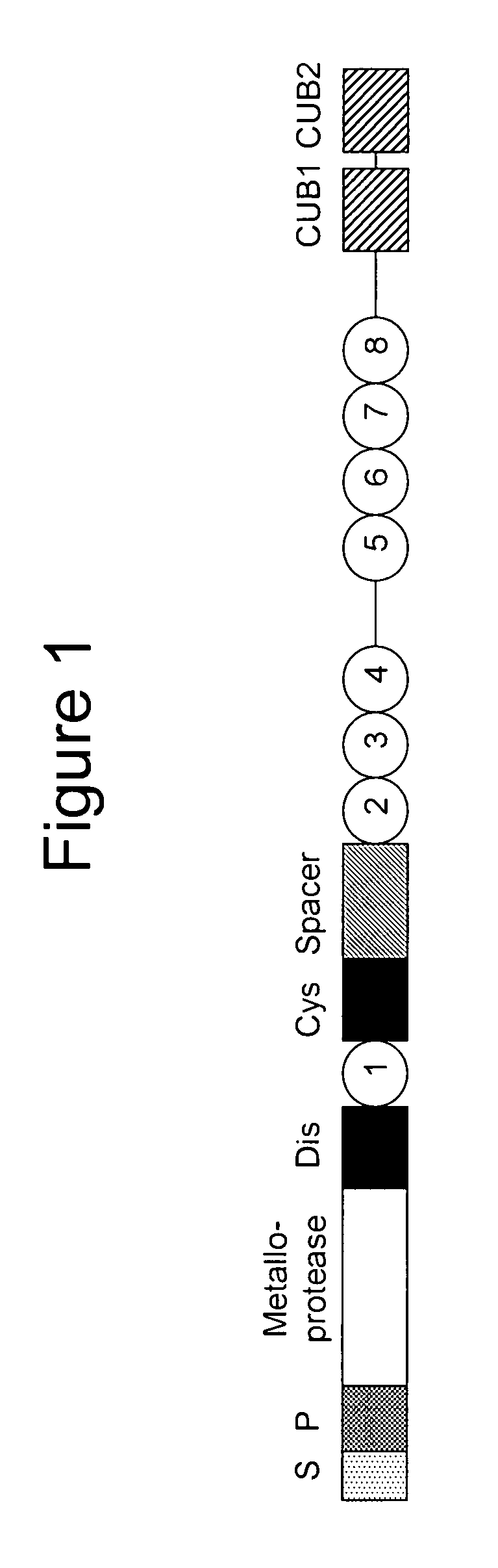
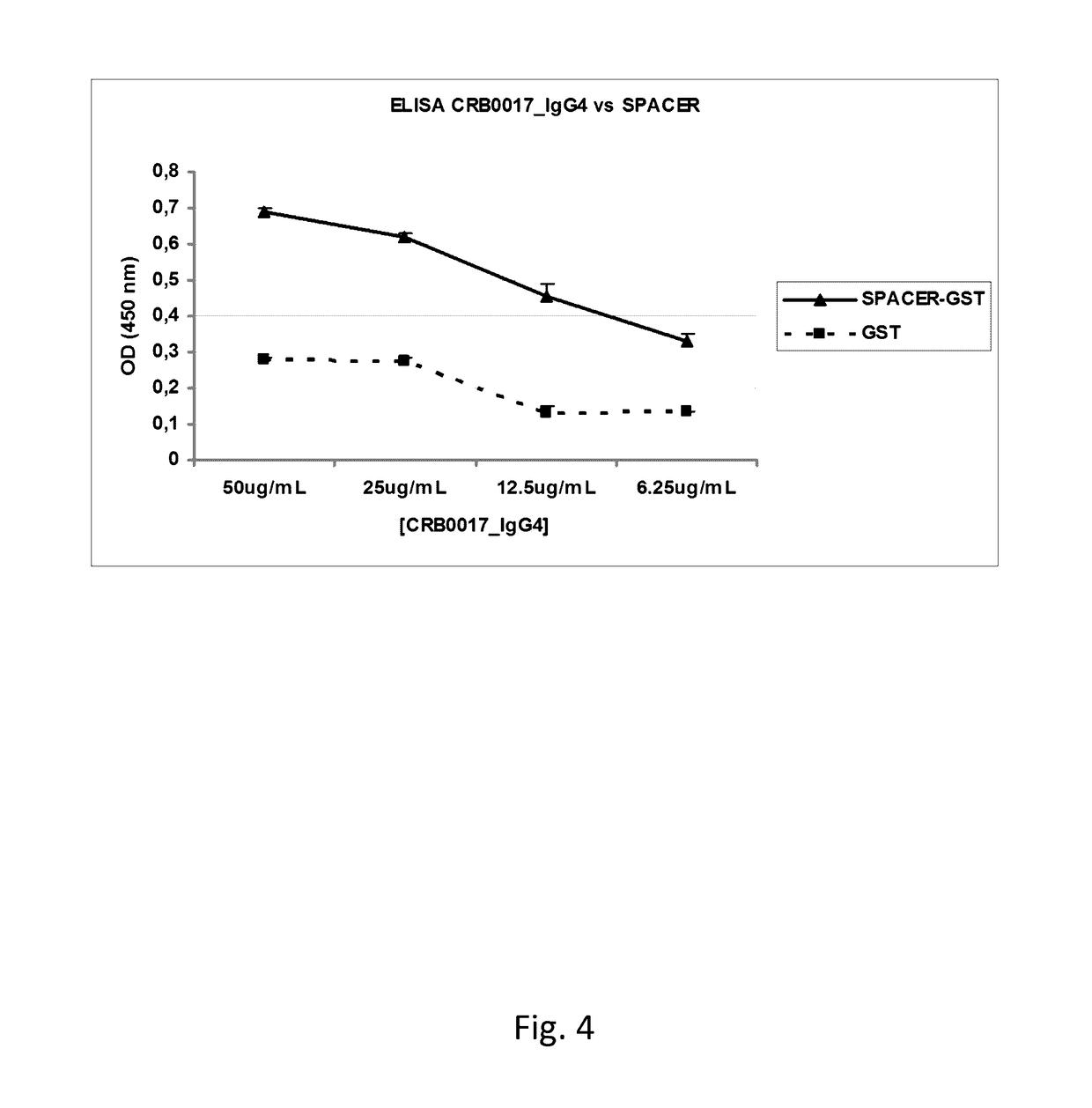
Anti-ADAMTS-5 antibody, derivatives and uses thereof
InactiveUS9631029B2Risk minimizationIncrease synthesisPeptide/protein ingredientsImmunoglobulins against animals/humansSynthetic antigenVirology
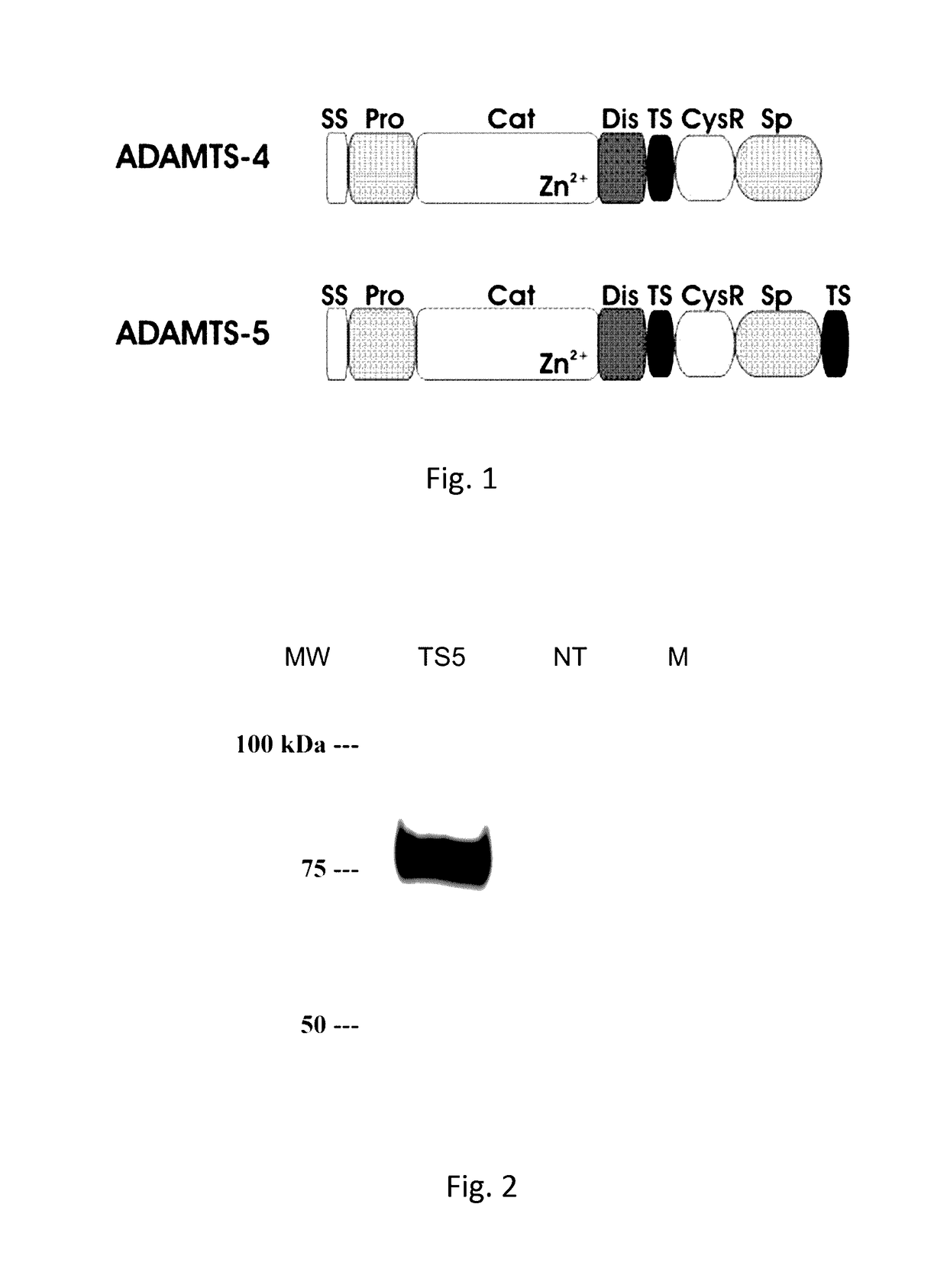
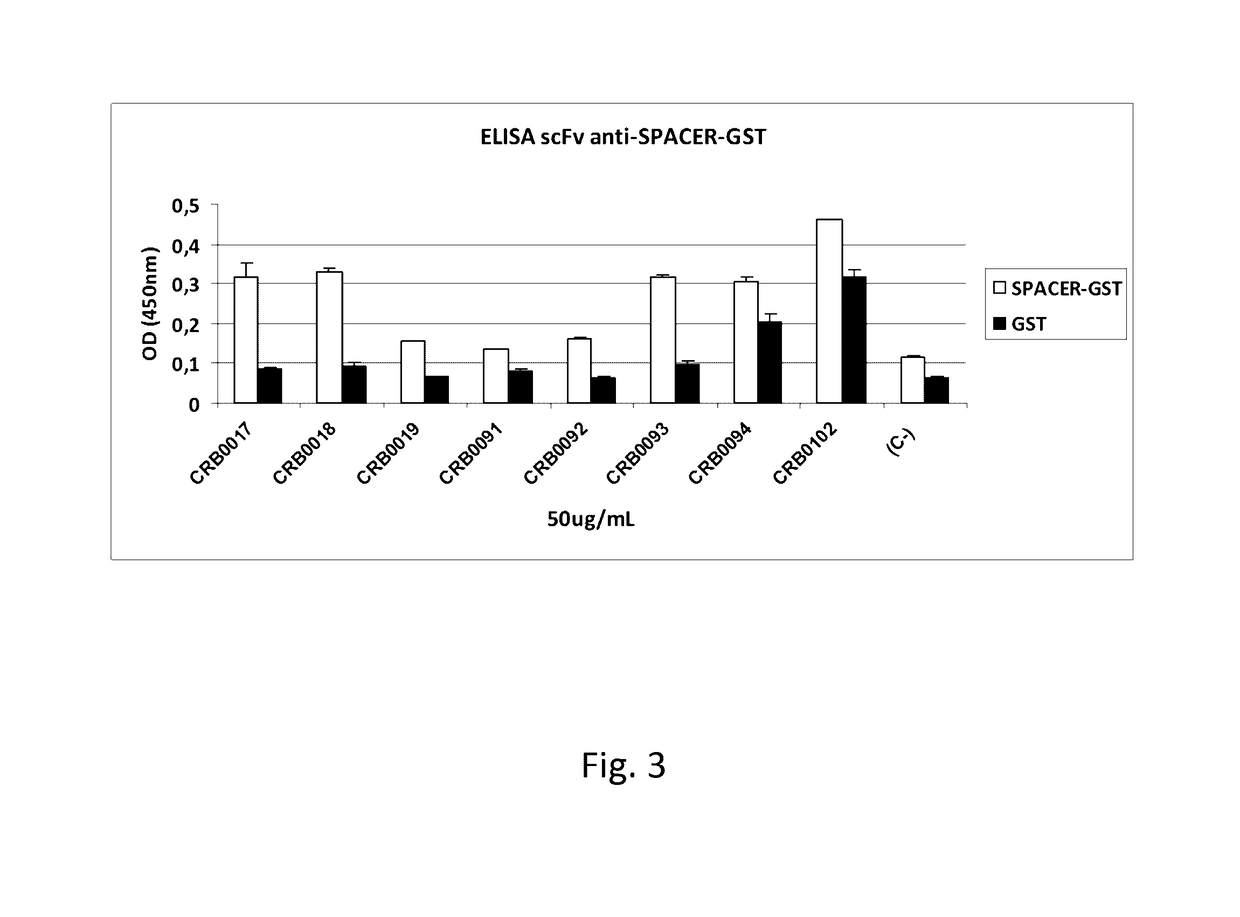
The present invention relates to an antibody, recombinant or synthetic antigen-binding fragments thereof able to recognize and bind an epitope comprised in the spacer domain of ADAMTS-5, nucleic acid and expression vector encoding the same, method of production and uses thereof.
Owner:ROTTAPHARM BIOTECH SRL
Substrate polyeptides for von Willebrand factor cleaving protease ADAMTS-13
The present invention relates to specific substrates for a von Willebrand factor cleaving enzyme, ADAMTS-13, as well as to diagnosis of ADAMTS-13 deficient patients, diagnostic compositions, and kits employing the substrates. Particularly preferable substrate polypeptides for ADAMTS-13 are the polypeptide which begins at amino acid 1587 and ends at amino acid 1668 of SEQ ID NO: 1 in the Sequence Listing, and the polypeptide which begins at amino acid 1596 and ends at amino acid 1668 of SEQ ID NO: 1 in the Sequence Listing. These substrate polypeptides for ADAMTS-13 have high substrate specificity and also superior quantitativeness, and a suitable size for production by recombinant methods.
Owner:NAT CEREBRAL & CARDIOVASCULAR CENT
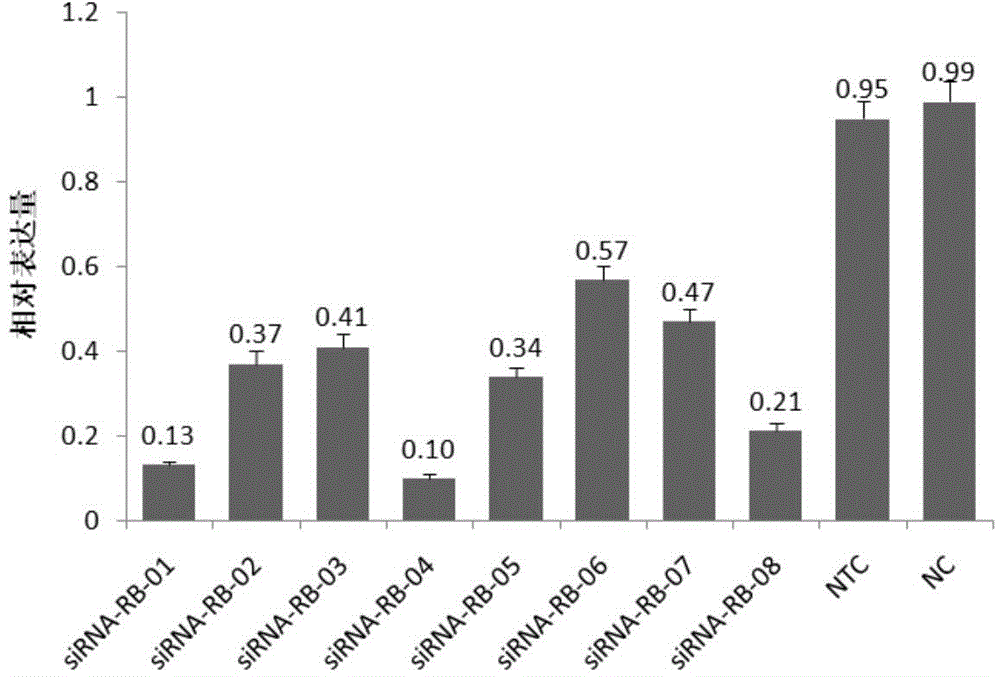
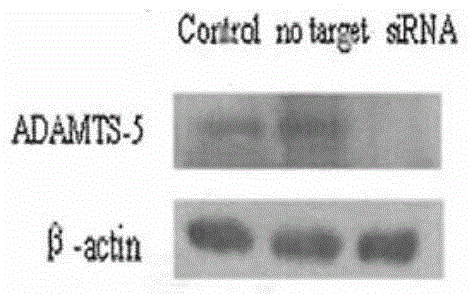
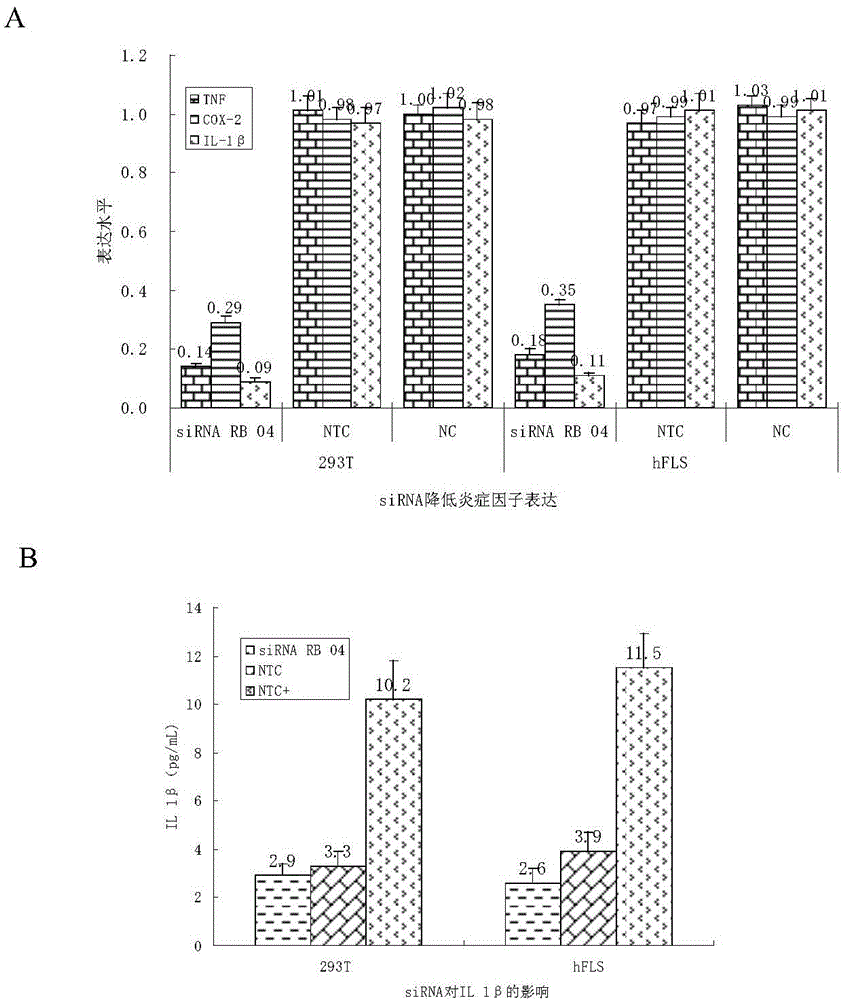
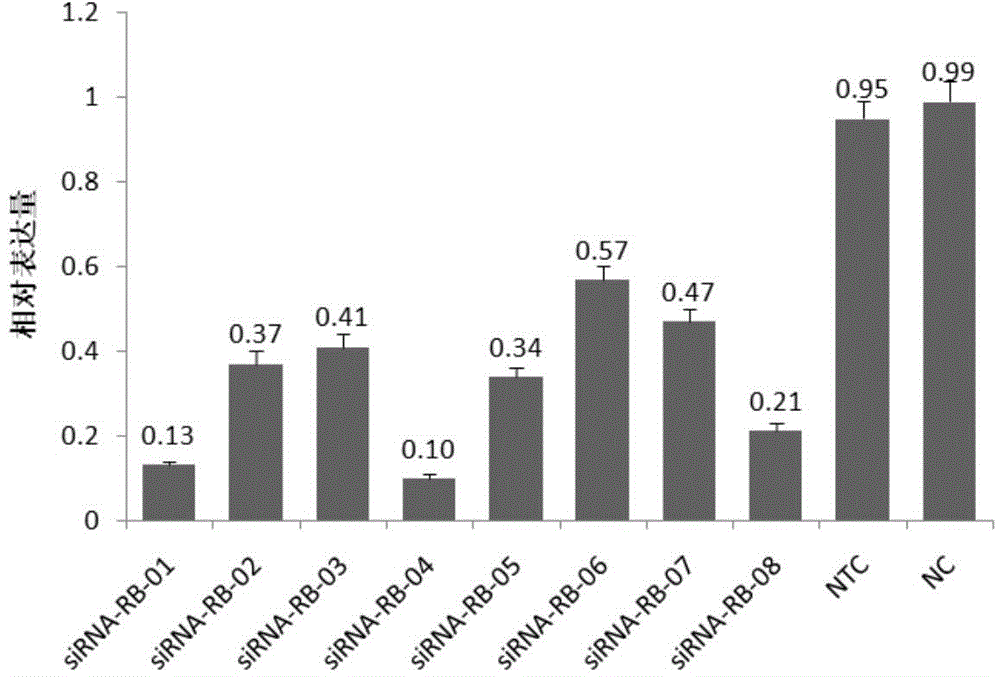
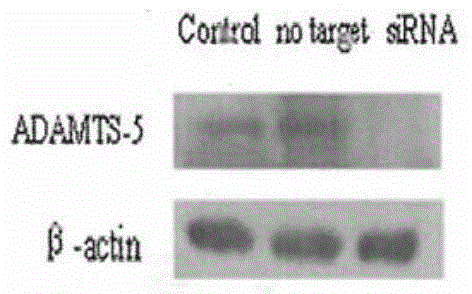
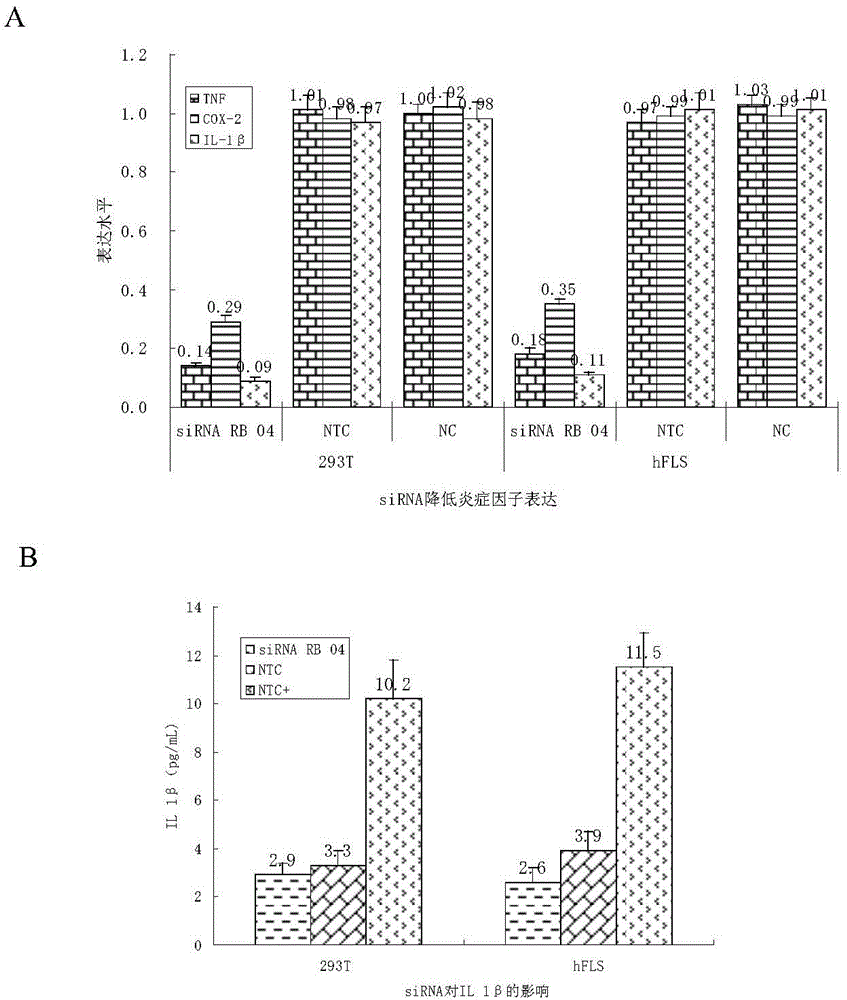
SiRNA (small interfering ribonucleic acid) composition inhibiting ADAMTS-5 (a disintegrin-like and metalloproteinase with thrombospondin type 1motifs-5) and ADAM17 (a disintegrin-like and metalloproteinase with thrombospondin type 1motifs 17) genes and application of siRNA composition
ActiveCN104560997AAchieve healingAntipyreticGenetic material ingredientsInflammatory factorsMolecular composition
The invention discloses an siRNA (small interfering ribonucleic acid) composition inhibiting an ADAMTS-5 (a disintegrin-like and metalloproteinase with thrombospondin type 1motifs-5) gene and an ADAM17 (a disintegrin-like and metalloproteinase with thrombospondin type 1motifs 17) gene and an application of the siRNA composition. The invention discloses the chemically modified double-chain siRNA molecular composition which comprises at least one of double-chain siRNA molecules as shown as (1) and at least one double-chain siRNA molecules as shown as (2). The siRNA composition can serve as a treatment medicine of arthritis and relevant inflammation, and the siRNA composition and a preparation of the siRNA composition can be injected locally through a bone articular cavity to inhibit inflammatory factor expression to achieve treatment of the joint inflammation.
Owner:ARGORNA PHARM CO LTD
siRNA for inhibiting gene ADAMTS-5 and application of siRNA
ActiveCN104560999AAchieve healingOrganic active ingredientsAntipyreticInflammatory factorsBones joints
The invention discloses siRNA for inhibiting a gene ADAMTS-5 and application of the siRNA. The invention discloses a chemically modified double-chain siRNA molecule which is formed in the way that any chemical modification from (1) to (13) in the Specification is performed on at least one of the chains in the siRNA molecule in A (in the Specification), and the complementation is performed. The siRNA disclosed by the invention can be used as a medicine for treating arthritis and related inflammations, and the siRNA and a preparation thereof can be injected into local parts of bone joint cavities to inhibit the expression of inflammatory factors and further realize treatment of joint inflammation.
Owner:ARGORNA PHARM LTD
Antibody against enzyme specifically cleaving von villebrand factor and assay system using the same
It is intended to provide an antibody showing immunoreactivity selectively to ADAMTS-13 and applications of this antibody in epitope analysis or diagnosis of an ADAMTS-13 autoantibody-positive patient. Alternatively, it is intended to provide a process for producing and use of a modified ADAMTS-13 molecule partially deleted aiming at the application in pharmaceutical products. An antibody specific for ADAMTS-13 which can be obtained from a warm-blooded animal immunized and sensitized with a polypeptide containing a part or the whole of ADAMTS-13 amino acid sequence; a process for producing an antibody comprising a step of immunizing and sensitizing a warm-blooded animal with a polypeptide containing a part or the whole of ADAMTS-13 amino acid sequence; use of the above-described antibody including a method of detecting and purifying ADAMTS-13; and a modified ADAMTS-13 molecule partially deleted are provided.
Owner:KM BIOLOGICS CO LTD
Proteases and uses thereof
InactiveUS20050260733A1Altering cleavage activityCleavage activity can be modulatedCompound screeningApoptosis detectionProteinase activityProteolysis
The present invention features methods of using ADAMTS-8 proteins or their functional derivatives to cleave aggrecan or other proteoglycan molecules. The present invention also features methods for identifying ADAMTS-8 modulators that are capable of inhibiting or enhancing ADAMTS-8 proteolytic activities. In addition, the present invention features pharmaceutical compositions comprising ADAMTS-8 proteins or their derivatives or modulators. These pharmaceutical compositions can be used to treat diseases that are characterized by deficiencies or abnormalities in proteoglycan cleavage or metabolism.
Owner:WYETH LLC
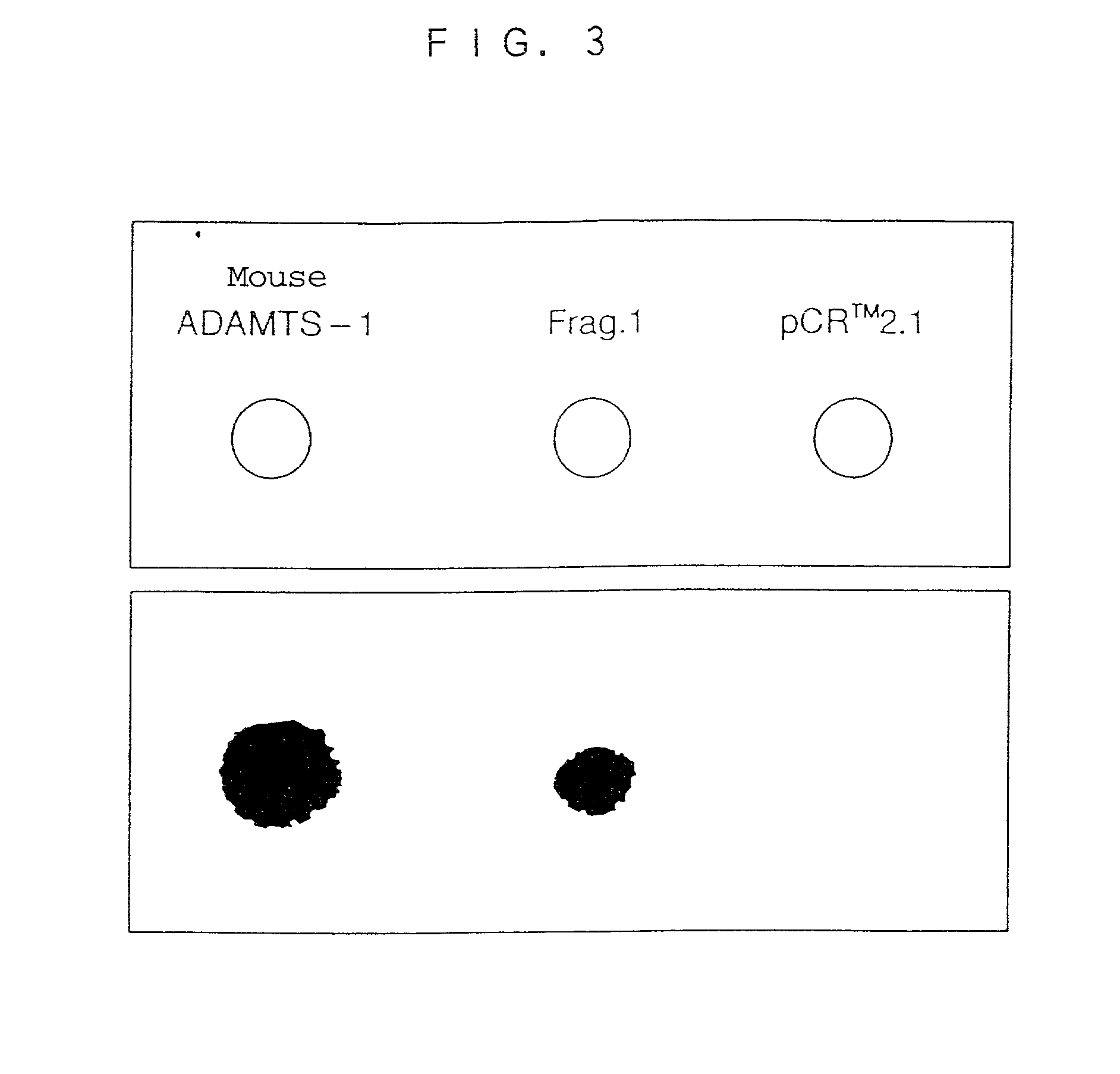
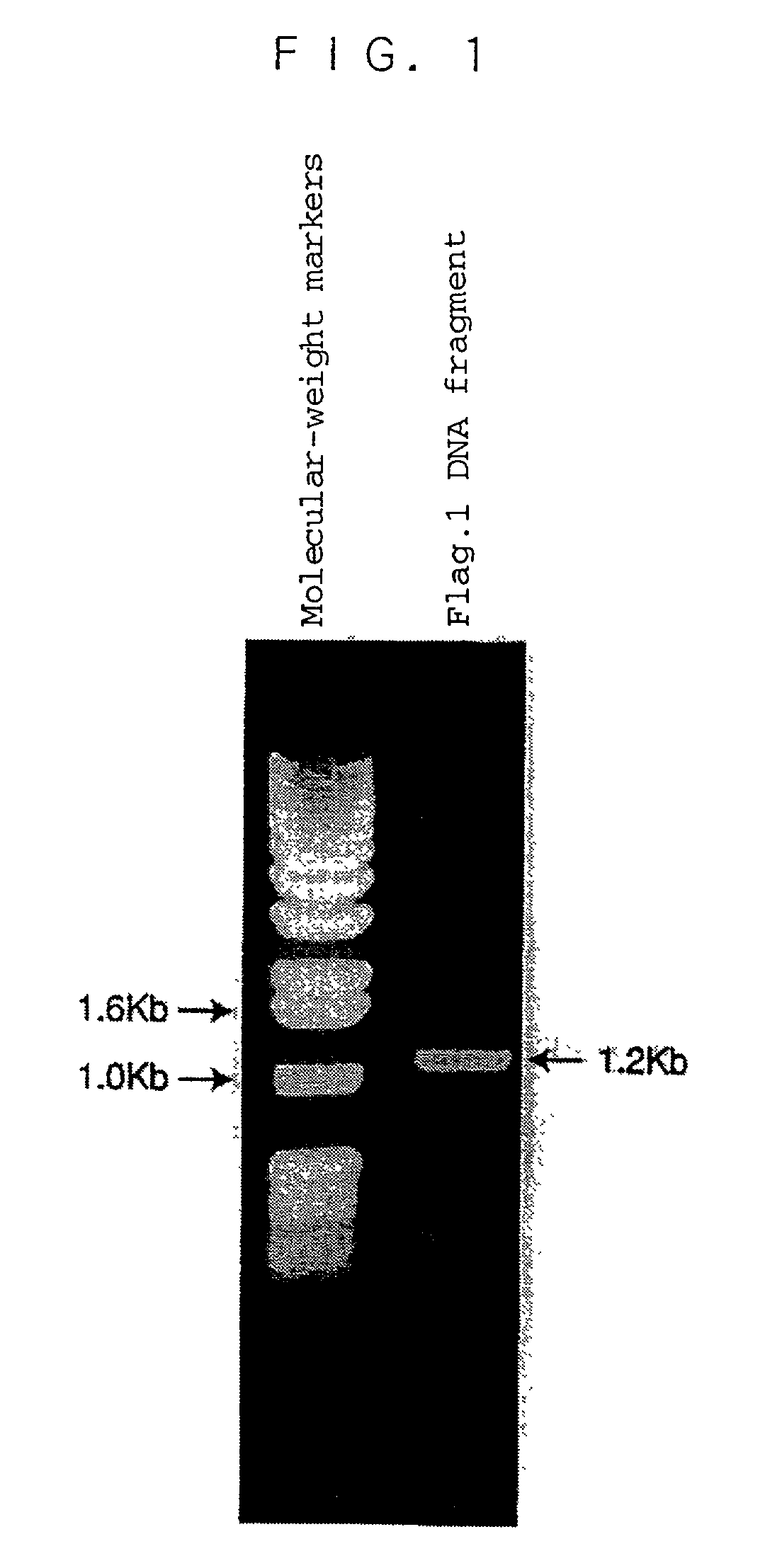
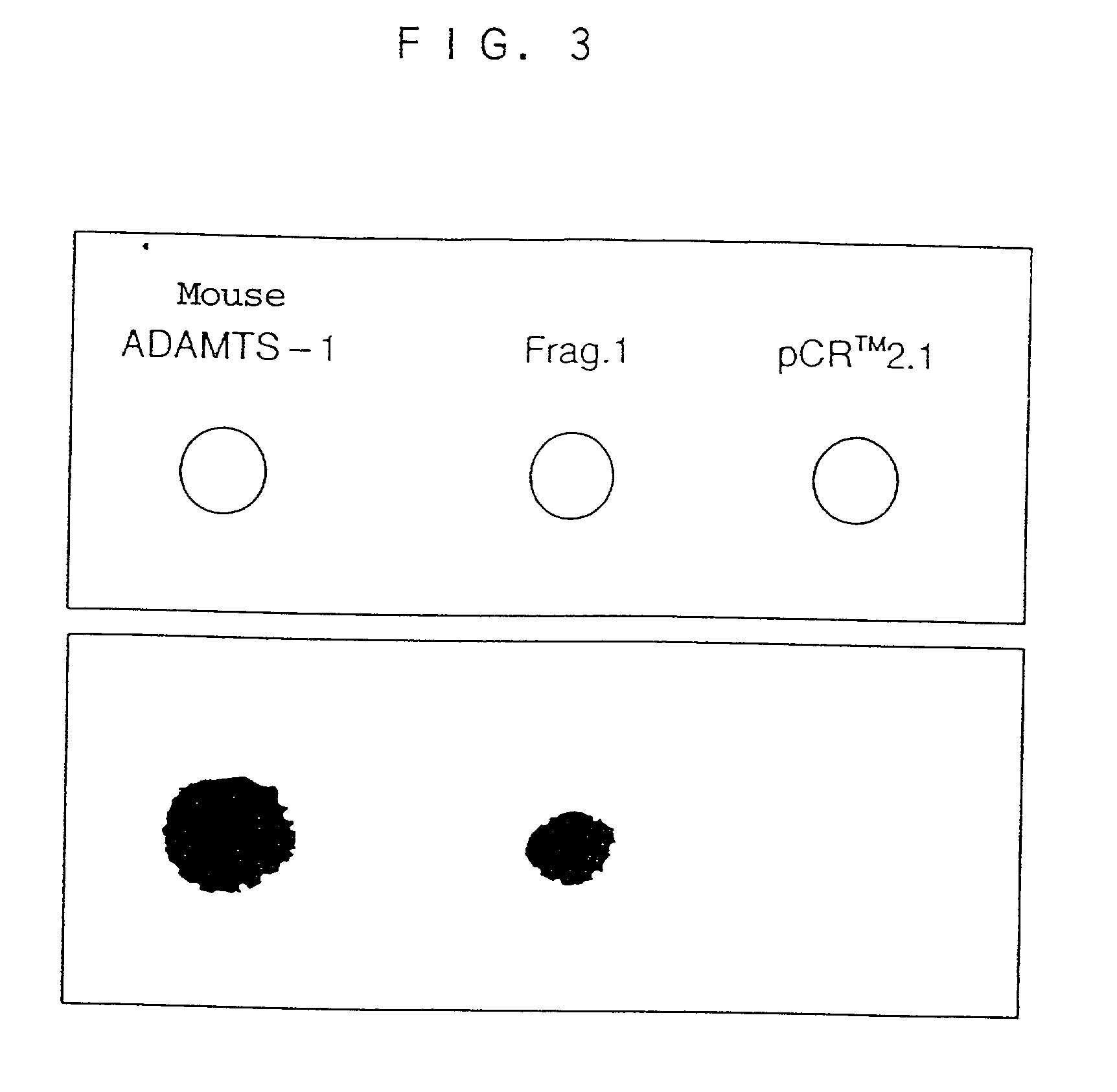
Human ADAMTS-1 protein, gene encoding the same, pharmaceutical composition, and method for immunologically analyzing human ADAMTS-1 protein
InactiveUS20030022352A1Improve isolationEasy to purifySugar derivativesHydrolasesWhite blood cellRed blood cell
A human ADAMTS-1 protein, a gene encoding the same, a pharmaceutical composition containing the protein as an active ingredient, and a method for immunologically analyzing the human ADAMTS-1 protein are disclosed. The protein can decrease the number of leukocytes and platelets, and at the same time, increase the number of erythrocytes.
Owner:HIROSE KUNITAKA +6
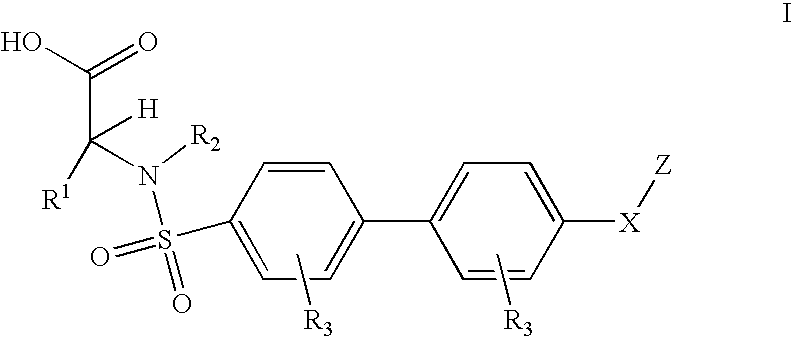
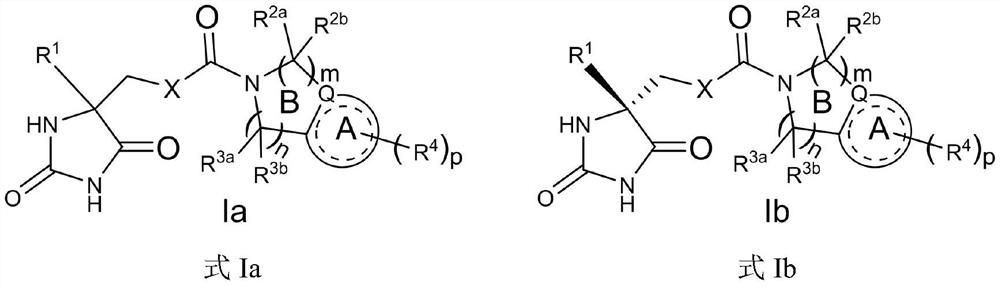
5-[(piperazin-1-yl)-3-oxo-propyl]-imidazolidine-2,4-dione derivatives as ADAMTS inhibitors for the treatment of osteoarthritis
ActiveUS10487060B2Reduced activityHigh drug safetyOrganic chemistrySkeletal disorderDiseaseImidazolidine
The present invention discloses compounds according to Formula I:Wherein R1, R2, R3a, R3b, and Cy are as defined herein.The present invention relates to compounds inhibiting ADAMTS, methods for their production, pharmaceutical compositions comprising the same, and methods of treatment using the same, for the prophylaxis and / or treatment of inflammatory conditions, and / or diseases involving degradation of cartilage and / or disruption of cartilage homeostasis by administering a compound of the invention.
Owner:GALAPAGOS NV
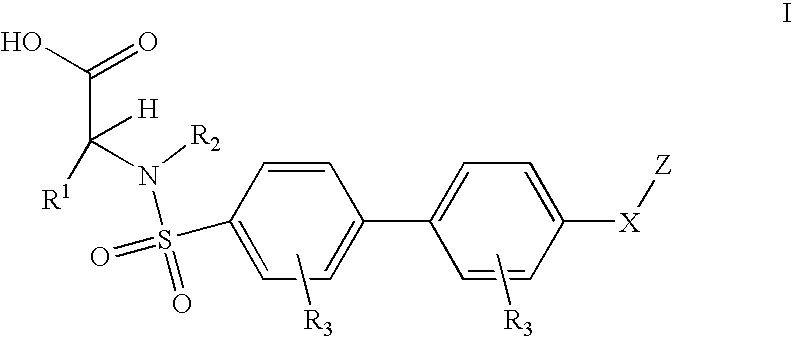
5-[3-[piperazin-1-yl]-3-oxo-propyl]-imidazolidine-2,4-dione derivatives as adamts 4 and 5 inhibitors for treating e.g. osteoarthritis
ActiveUS20190300503A1Reduced activityHigh drug safetyOrganic chemistrySkeletal disorderDiseaseCartilage homeostasis
The present invention discloses compounds according to Formula I:Wherein R1, R2, R3a, R3b, R6, Cy, and the subscript n are as defined herein.The present invention relates to compounds inhibiting ADAMTS, methods for their production, pharmaceutical compositions comprising the same, and methods of treatment using the same, for the prophylaxis and / or treatment of inflammatory diseases, and / or diseases involving degradation of cartilage and / or disruption of cartilage homeostasis by administering the compound of the invention.
Owner:GALAPAGOS NV
Aconite extract injection for treating osteoarthritis as well as preparation method and application of aconite extract injection
InactiveCN107485651ASignificant reliefSignificant analgesic effectAntipyreticAnalgesicsArthritisHigh pressure
The invention discloses an aconite extract injection for treating osteoarthritis as well as a preparation method and application of the aconite extract injection, and belongs to the technical field of traditional Chinese medicine production. The preparation method comprises the steps: weighing radix aconiti and radix aconiti kusnezoffii as raw materials, adding uniformly mixed medicine powder into an alcohol solution, performing sealed soaking, transferring into a high-pressure reaction kettle, performing pressurized extraction, performing depressurization, performing filtering, separating medicine liquid from a filter residue, adding 8-10 volume times of water into the filter residue, performing uniform mixing, transferring into the high-pressure reaction kettle, performing pressurized extraction, concentrating the two medicine liquids until no alcohol odor exists, performing filtering, adding absolute alcohol to allow the alcohol content to reach 75%, performing standing overnight, performing filtering, concentrating the medicine liquid until no alcohol odor exists, performing dilution with injection water to be 10 times, and performing encapsulation and sterilization to obtain the aconite extract injection. The aconite extract injection has the significant influence on MMP-3, ADAMTS, IL-1 beta, IL-18, TNF-alpha and the like, and is confirmed to have a protective effect on chondrocyte damage caused by arthritis.
Owner:HEILONGJIANG ACAD OF TCM
Human ADAMTS-1 protein, gene encoding the same, pharmaceutical composition, and method for immunologically analyzing human ADAMTS-1 protein
InactiveUS20030032168A1Improve isolationEasy to purifySugar derivativesHydrolasesWhite blood cellPlatelet
A human ADAMTS-1 protein, a gene encoding the same, a pharmaceutical composition containing the protein as an active ingredient, and a method for immunologically analyzing the human ADAMTS-1 protein are disclosed. The protein can decrease the number of leukocytes and platelets, and at the same time, increase the number of erythrocytes.
Owner:HIROSE KUNITAKA +6
ADAMTS-8 proteases and uses thereof
The present invention features methods of using ADAMTS-8 proteins or their functional derivatives to cleave aggrecan or other proteoglycan molecules. The present invention also features methods for identifying ADAMTS-8 modulators that are capable of inhibiting or enhancing ADAMTS-8 proteolytic activities. In addition, the present invention features pharmaceutical compositions comprising ADAMTS-8 proteins or their derivatives or modulators. These pharmaceutical compositions can be used to treat diseases that are characterized by deficiencies or abnormalities in proteoglycan cleavage or metabolism.
Owner:WYETH LLC
Cell culture processes
Culturing heterologous protein-secreting mammalian cells, such as CHO or BHK cells, at 35.1-36.5° C. and / or at pH 7.15-7.20 and / or at a dissolved CO2 concentration of 10% or lower. Preferred heterologous proteins are Factor VIII, ADAMTS-13, furin or Factor VII.
Owner:TAKEDA PHARMA CO LTD
Condensed ring compound and preparation method and application thereof
The present invention provides a condensed ring compound and a preparation method and application thereof, the compound has a good inhibitory effect on ADAMTS-5 and / or ADAMTS-4, particularly, the compound exhibits excellent selectivity and bioavailability.
Owner:CHENGDU KANGHONG PHARMA GRP
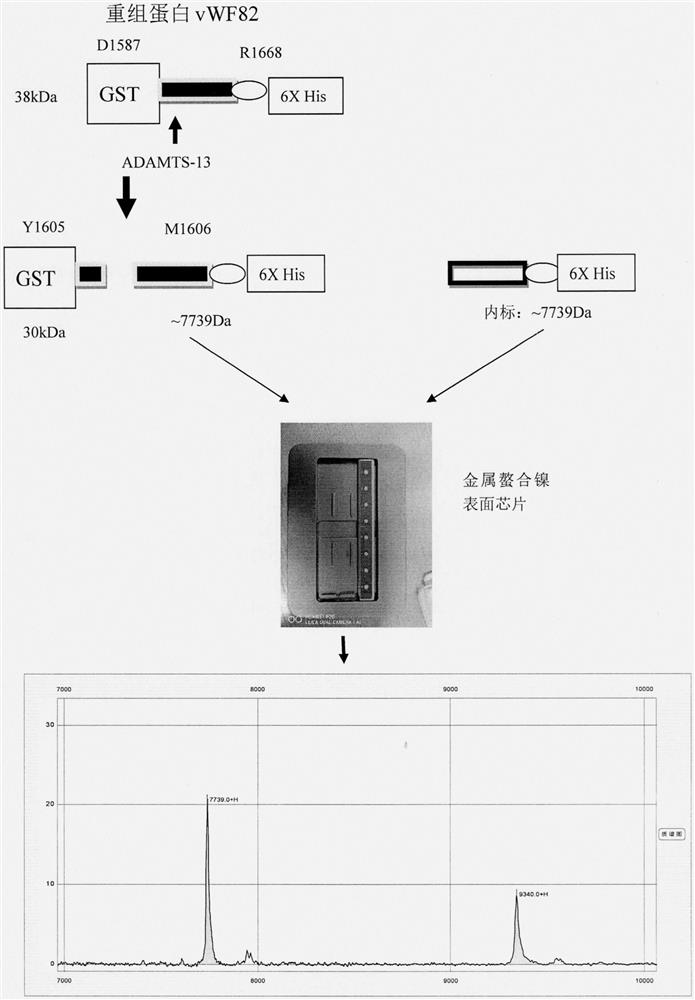
Substrate, method and kit capable of detecting enzymatic activity of metalloproteinase ADAMTS-13 by utilizing MALDI-TOF-MS
PendingCN111983003AThe detection method is fast and effectiveAccurate detectionMaterial analysis by electric/magnetic meansDisease diagnosisEnzyme digestionSerum samples
The invention belongs to the technical field of medical molecular biology and protein polypeptide detection, and particularly relates to a substrate, a method and a kit capable of detecting the enzymatic activity of metalloproteinase ADAMTS-13 by utilizing MALDI-TOF-MS. The method comprises the following steps: (1) performing expression and purification of an ADAMTS-13 enzyme digestion substrate GST-vWF82-6XHis recombinant protein; (2) performing enzyme digestion reaction; and (3) enriching the ADAMTS-13 enzyme digestion product by a metal chelating chip, identifying and detecting the ADAMTS-13 enzyme digestion product by MALDI-TOF-MS, and obtaining the enzyme digestion activity of ADAMTS-13 in the serum sample by analysis software. The substrate, the method and the kit for in-vitro quantitative determination of ADAMTS-13 enzyme activity are good in sensitivity and accuracy, simple and convenient to operate, low in price and suitable for wide patient screening.
Owner:刘学博 +8
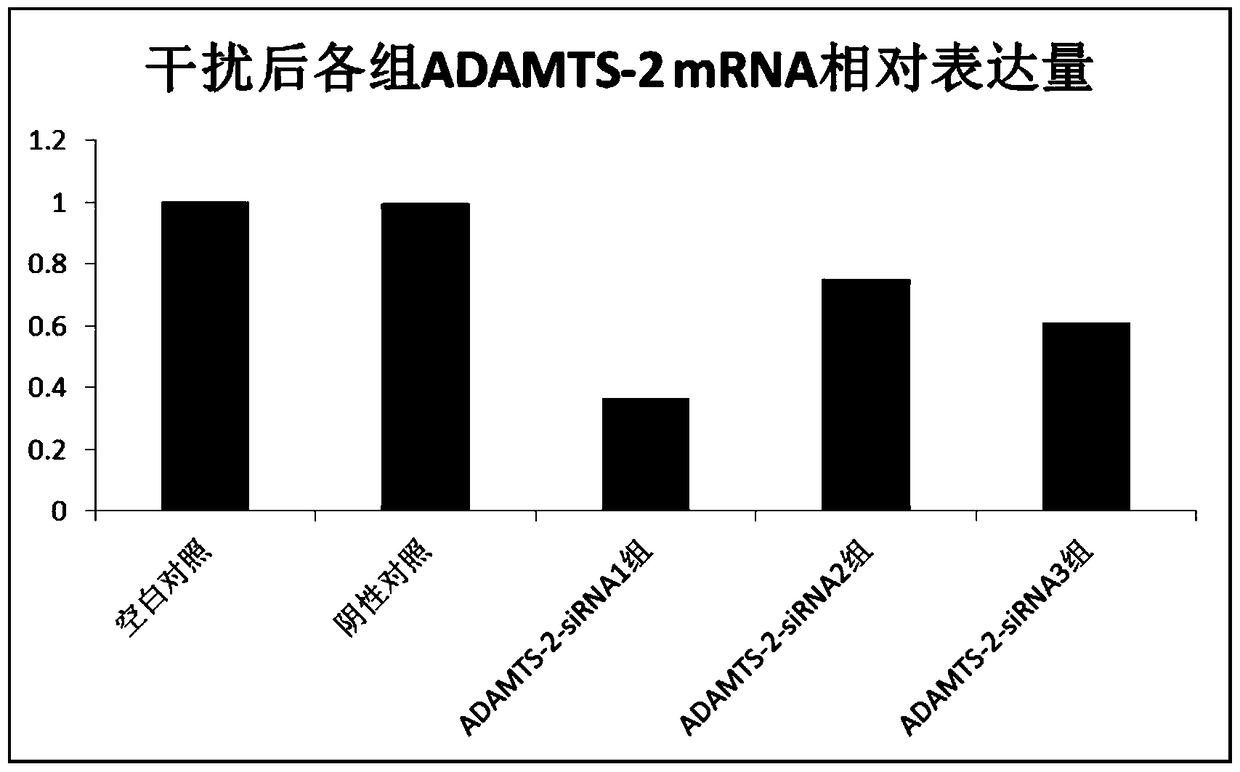
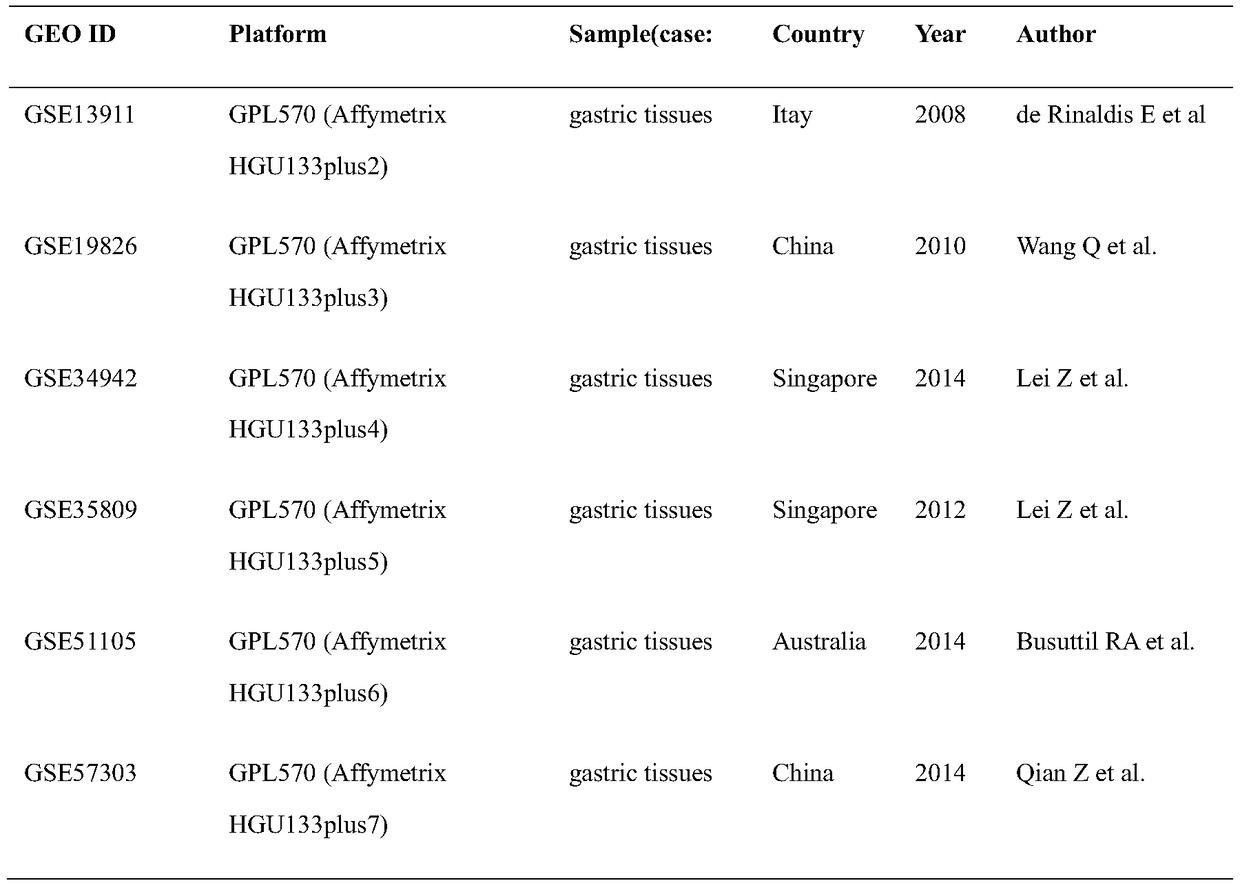
Application of adamts-2 gene and its expression product in diagnosis and treatment of gastric cancer
InactiveCN105420366BOrganic active ingredientsMicrobiological testing/measurementInformation analysisAnalysis tools
The invention relates to an ADAMTS-2 gene and application of an expression product of the ADAMTS-2 gene in the diagnosis and treatment of stomach cancer. An inventor integrates the existing gene expression microarray data, six sets of stomach cancer expression data are obtained by retrieving and screening a GEO database, data are processed and analyzed again by utilizing a biological information analysis tool, the ADAMTS-2 gene is screened out, and validation of molecular biology is further performed, an RT-PCR result shows that high expression of the ADAMTS-2 gene is performed in gastric cancer tissues, an interference test indicates that ADAMTS-2-siRNA1 can effectively reduce the expression of the ADAMTS-2 gene. The invention provides a new target spot of diagnosis and treatment of stomach cancer, and has important clinical application value.
Owner:张军利 +3
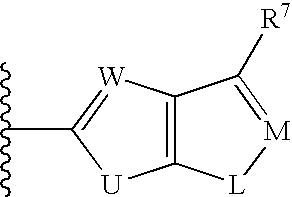
5-[3-[piperazin-1-yl]-3-oxo-propyl]-imidazolidine-2,4-dione derivatives as ADAMTS 4 and 5 inhibitors for treating e.g. osteoarthritis
ActiveUS10829478B2Reduced activityHigh drug safetyOrganic chemistrySkeletal disorderDiseaseCartilage homeostasis
The present invention discloses compounds according to Formula I:Wherein R1, R2, R3a, R3b, R6a, R6b, the subscript n and Cy are as defined herein.The present invention relates to compounds inhibiting ADAMTS, methods for their production, pharmaceutical compositions comprising the same, and methods of treatment using the same, for the prophylaxis and / or treatment of inflammatory diseases, and / or diseases involving degradation of cartilage and / or disruption of cartilage homeostasis by administering the compound of the invention.
Owner:GALAPAGOS NV
Construct comprising recognition domain of antibody against von Willebrand factor-specific cleaving enzyme
Owner:KM BIOLOGICS CO LTD
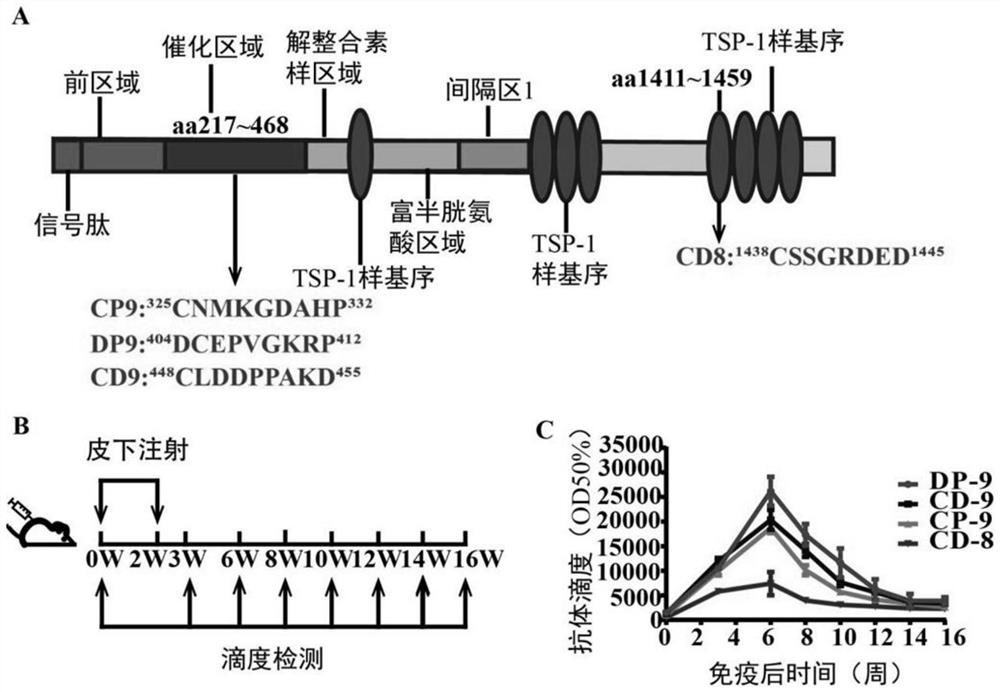
Immunogenic peptides of metalloprotease adamts-7 and its application in anti-atherosclerosis and related diseases
ActiveCN112553184BBlocking activityRestenosis prevention or treatmentPeptide/protein ingredientsVertebrate antigen ingredientsDiseaseImmunogenic peptide
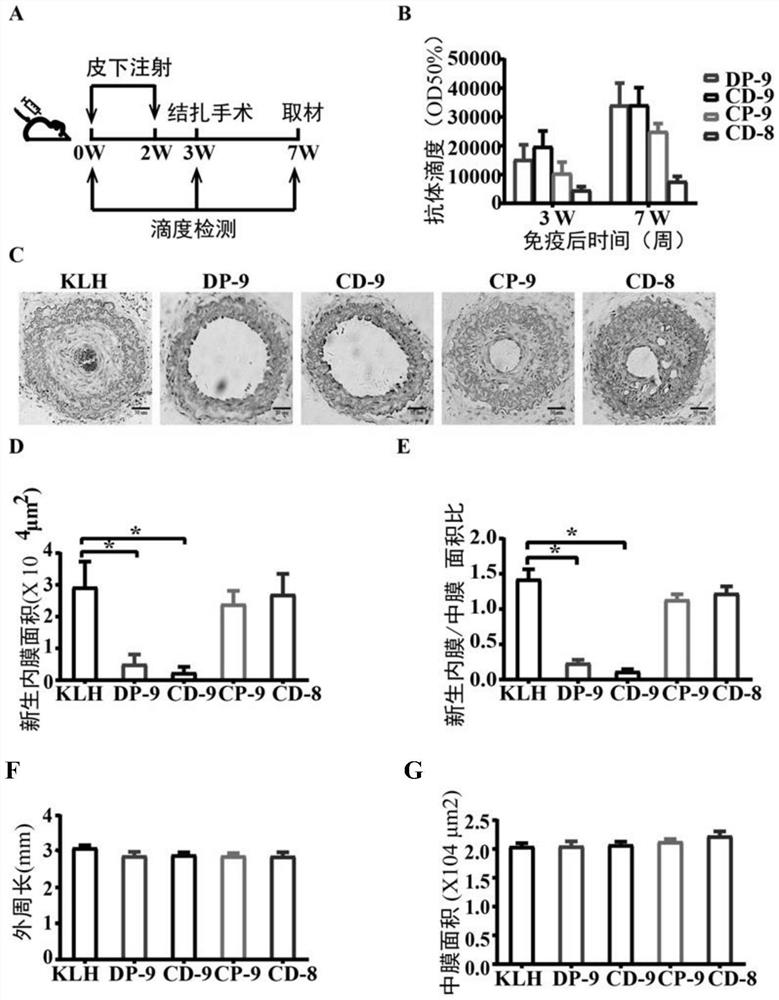
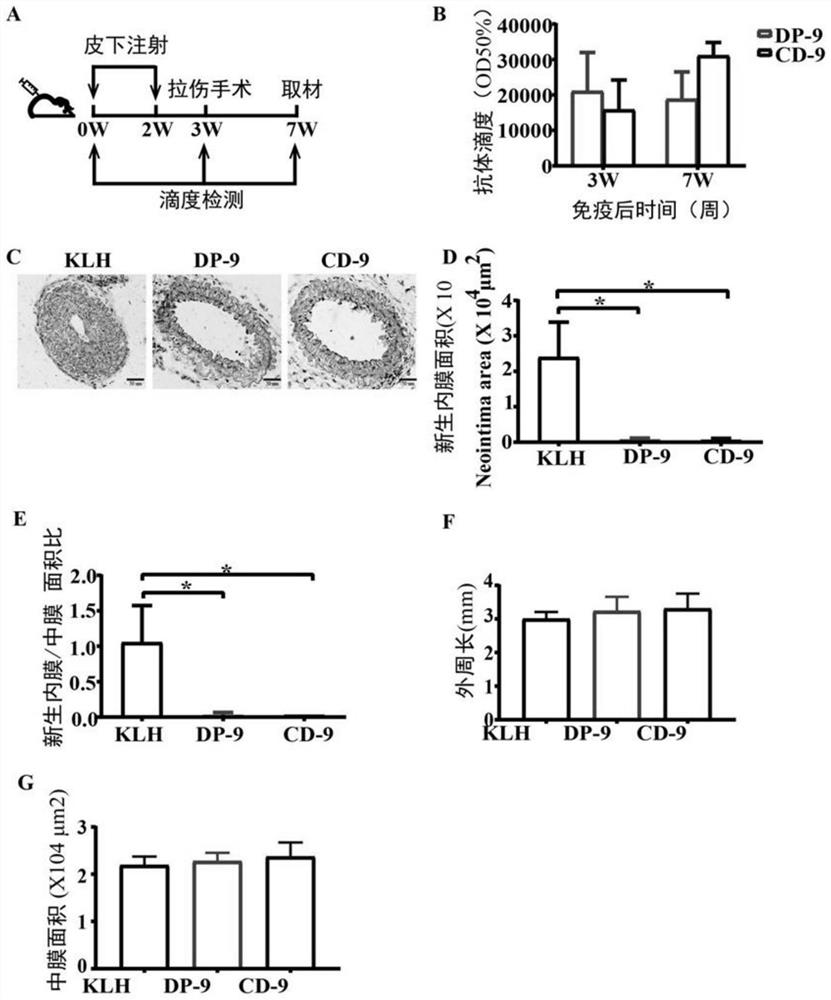
The present invention discloses a metalloprotease ADAMTS-7 immunogenic peptide and its product and application; the present invention first discloses a short peptide, including any of the following: the amino acid sequence is shown in Sequence 1 in the sequence table Short peptide; the amino acid sequence is the short peptide shown in sequence 2 in the sequence listing; the amino acid sequence is the short peptide shown in sequence 3 in the sequence listing; the amino acid sequence is the short peptide shown in sequence 4 in the sequence listing. The invention further discloses a conjugate comprising the above short peptide, a vaccine comprising the conjugate and applications thereof. The vaccine containing the short peptide of the present invention can significantly inhibit neointimal neointimal in mouse models of vascular restenosis and atherosclerosis in mice fed with high fat, and can be used for preventing or treating atherosclerosis and / or vascular restenosis.
Owner:北京经纬康泰生物科技有限公司
Adamts-7 as a biomarker for cancers of epithelial origin
InactiveUS20100209944A1Quick and easy and safeEasy diagnosisAnalysis using chemical indicatorsBiological material analysisBladder cancerProstate cancer
ADAMTS-7 expression and activity are up regulated in patients that have cancers of epithelial origin. Accordingly, the present invention is directed to methods diagnosis of cancers of epithelial origin (e.g. breast cancer, prostate cancer, bladder cancer, brain cancer and hepatic cancer). In particular, the presence of ADAMTS-7 in a biological sample is indicative of cancer of epithelial origin. Thus, measuring the level of ADAMTS-7 in biological samples (e.g. urine or blood) provides a quick, easy, and safe screen that can be used to diagnose cancer in a patient.
Owner:MOSES MARSHA A +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![5-[(piperazin-1-yl)-3-oxo-propyl]-imidazolidine-2,4-dione derivatives as ADAMTS inhibitors for the treatment of osteoarthritis 5-[(piperazin-1-yl)-3-oxo-propyl]-imidazolidine-2,4-dione derivatives as ADAMTS inhibitors for the treatment of osteoarthritis](https://images-eureka.patsnap.com/patent_img/ef92b52f-3add-451e-835a-4d0c579beca2/US10487060-C00001.png)
![5-[(piperazin-1-yl)-3-oxo-propyl]-imidazolidine-2,4-dione derivatives as ADAMTS inhibitors for the treatment of osteoarthritis 5-[(piperazin-1-yl)-3-oxo-propyl]-imidazolidine-2,4-dione derivatives as ADAMTS inhibitors for the treatment of osteoarthritis](https://images-eureka.patsnap.com/patent_img/ef92b52f-3add-451e-835a-4d0c579beca2/US10487060-C00002.png)
![5-[(piperazin-1-yl)-3-oxo-propyl]-imidazolidine-2,4-dione derivatives as ADAMTS inhibitors for the treatment of osteoarthritis 5-[(piperazin-1-yl)-3-oxo-propyl]-imidazolidine-2,4-dione derivatives as ADAMTS inhibitors for the treatment of osteoarthritis](https://images-eureka.patsnap.com/patent_img/ef92b52f-3add-451e-835a-4d0c579beca2/US10487060-C00003.png)
![5-[3-[piperazin-1-yl]-3-oxo-propyl]-imidazolidine-2,4-dione derivatives as adamts 4 and 5 inhibitors for treating e.g. osteoarthritis 5-[3-[piperazin-1-yl]-3-oxo-propyl]-imidazolidine-2,4-dione derivatives as adamts 4 and 5 inhibitors for treating e.g. osteoarthritis](https://images-eureka.patsnap.com/patent_img/2b37a3e9-1f8c-4378-93c4-e018c44e4aa2/US20190300503A1-C00001.png)
![5-[3-[piperazin-1-yl]-3-oxo-propyl]-imidazolidine-2,4-dione derivatives as adamts 4 and 5 inhibitors for treating e.g. osteoarthritis 5-[3-[piperazin-1-yl]-3-oxo-propyl]-imidazolidine-2,4-dione derivatives as adamts 4 and 5 inhibitors for treating e.g. osteoarthritis](https://images-eureka.patsnap.com/patent_img/2b37a3e9-1f8c-4378-93c4-e018c44e4aa2/US20190300503A1-C00002.png)
![5-[3-[piperazin-1-yl]-3-oxo-propyl]-imidazolidine-2,4-dione derivatives as adamts 4 and 5 inhibitors for treating e.g. osteoarthritis 5-[3-[piperazin-1-yl]-3-oxo-propyl]-imidazolidine-2,4-dione derivatives as adamts 4 and 5 inhibitors for treating e.g. osteoarthritis](https://images-eureka.patsnap.com/patent_img/2b37a3e9-1f8c-4378-93c4-e018c44e4aa2/US20190300503A1-C00003.png)



![5-[3-[piperazin-1-yl]-3-oxo-propyl]-imidazolidine-2,4-dione derivatives as ADAMTS 4 and 5 inhibitors for treating e.g. osteoarthritis 5-[3-[piperazin-1-yl]-3-oxo-propyl]-imidazolidine-2,4-dione derivatives as ADAMTS 4 and 5 inhibitors for treating e.g. osteoarthritis](https://images-eureka.patsnap.com/patent_img/07f71154-de0d-433a-bd14-f4dfec7b2e2a/US10829478-C00001.png)
![5-[3-[piperazin-1-yl]-3-oxo-propyl]-imidazolidine-2,4-dione derivatives as ADAMTS 4 and 5 inhibitors for treating e.g. osteoarthritis 5-[3-[piperazin-1-yl]-3-oxo-propyl]-imidazolidine-2,4-dione derivatives as ADAMTS 4 and 5 inhibitors for treating e.g. osteoarthritis](https://images-eureka.patsnap.com/patent_img/07f71154-de0d-433a-bd14-f4dfec7b2e2a/US10829478-C00002.png)
![5-[3-[piperazin-1-yl]-3-oxo-propyl]-imidazolidine-2,4-dione derivatives as ADAMTS 4 and 5 inhibitors for treating e.g. osteoarthritis 5-[3-[piperazin-1-yl]-3-oxo-propyl]-imidazolidine-2,4-dione derivatives as ADAMTS 4 and 5 inhibitors for treating e.g. osteoarthritis](https://images-eureka.patsnap.com/patent_img/07f71154-de0d-433a-bd14-f4dfec7b2e2a/US10829478-C00003.png)