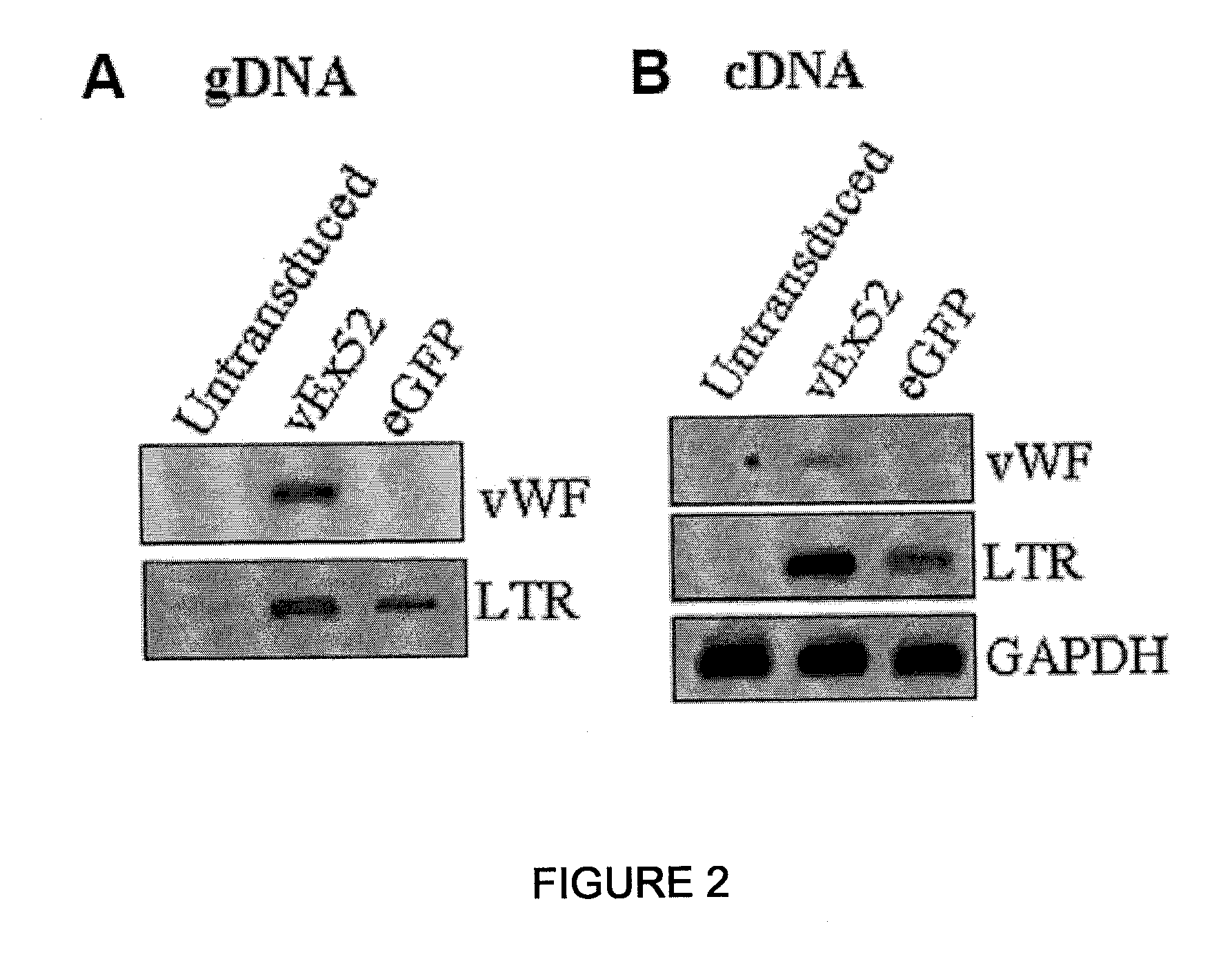
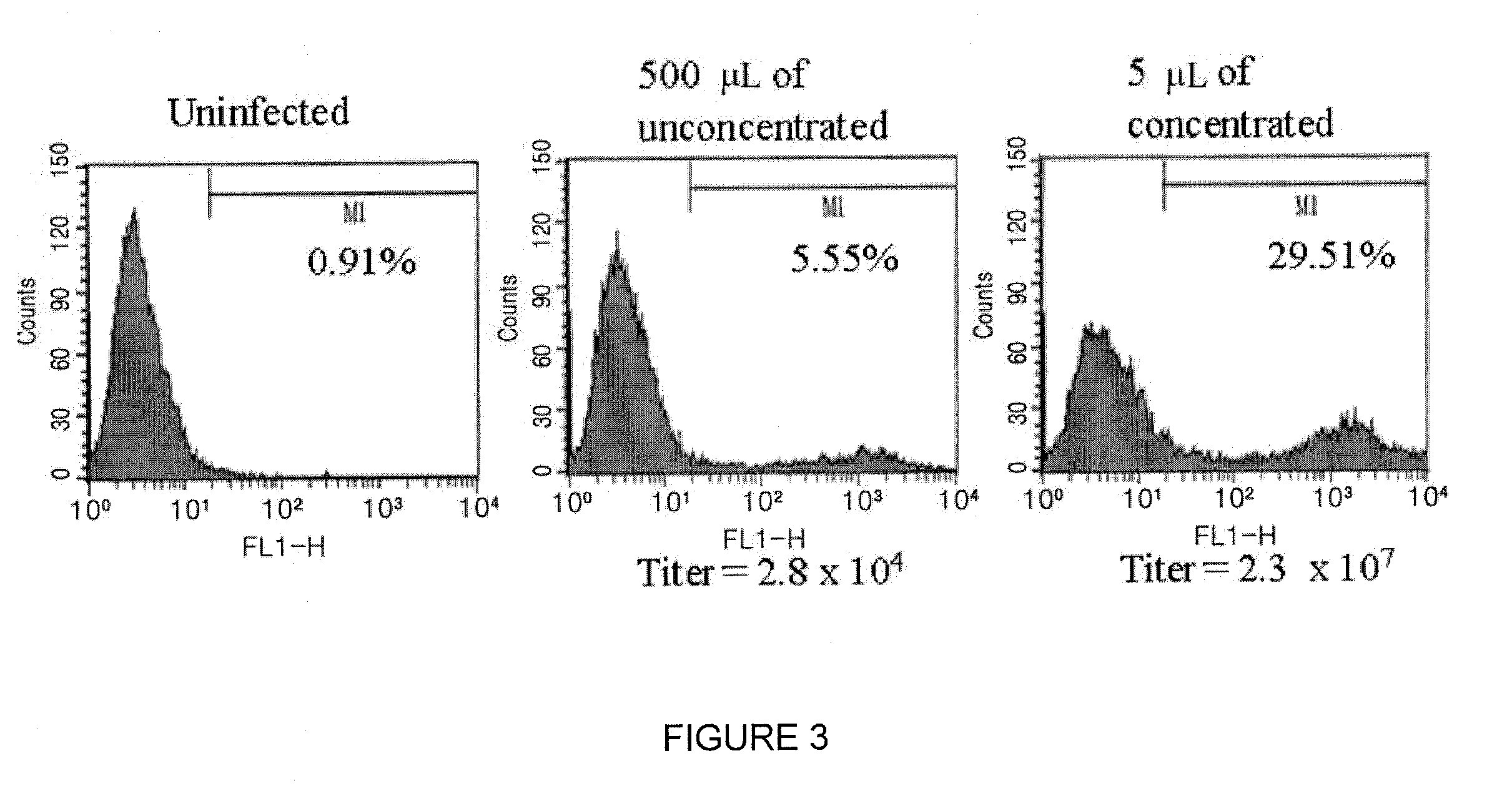
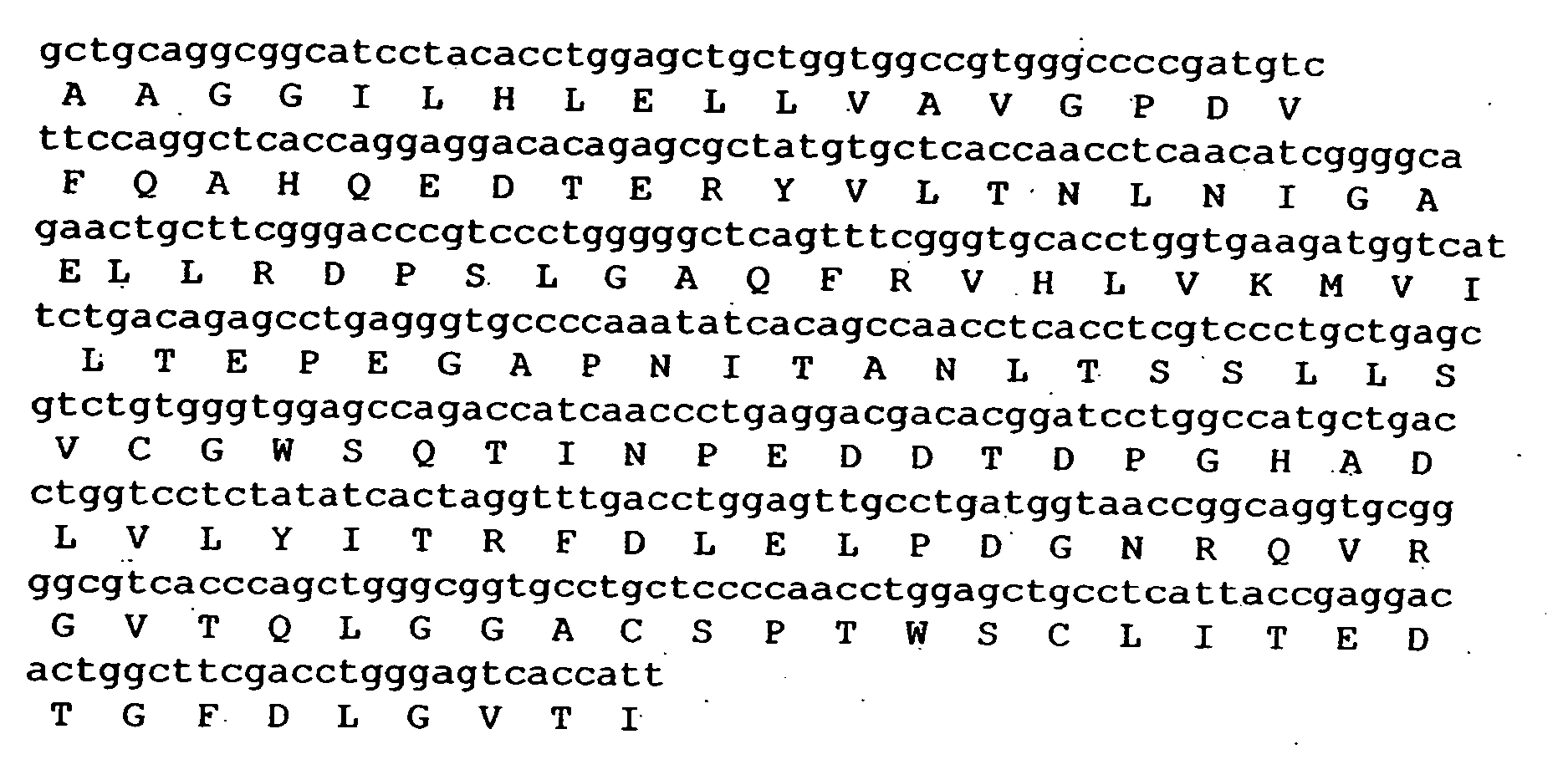Patents
Literature
218 results about "Factor VIII vWF" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
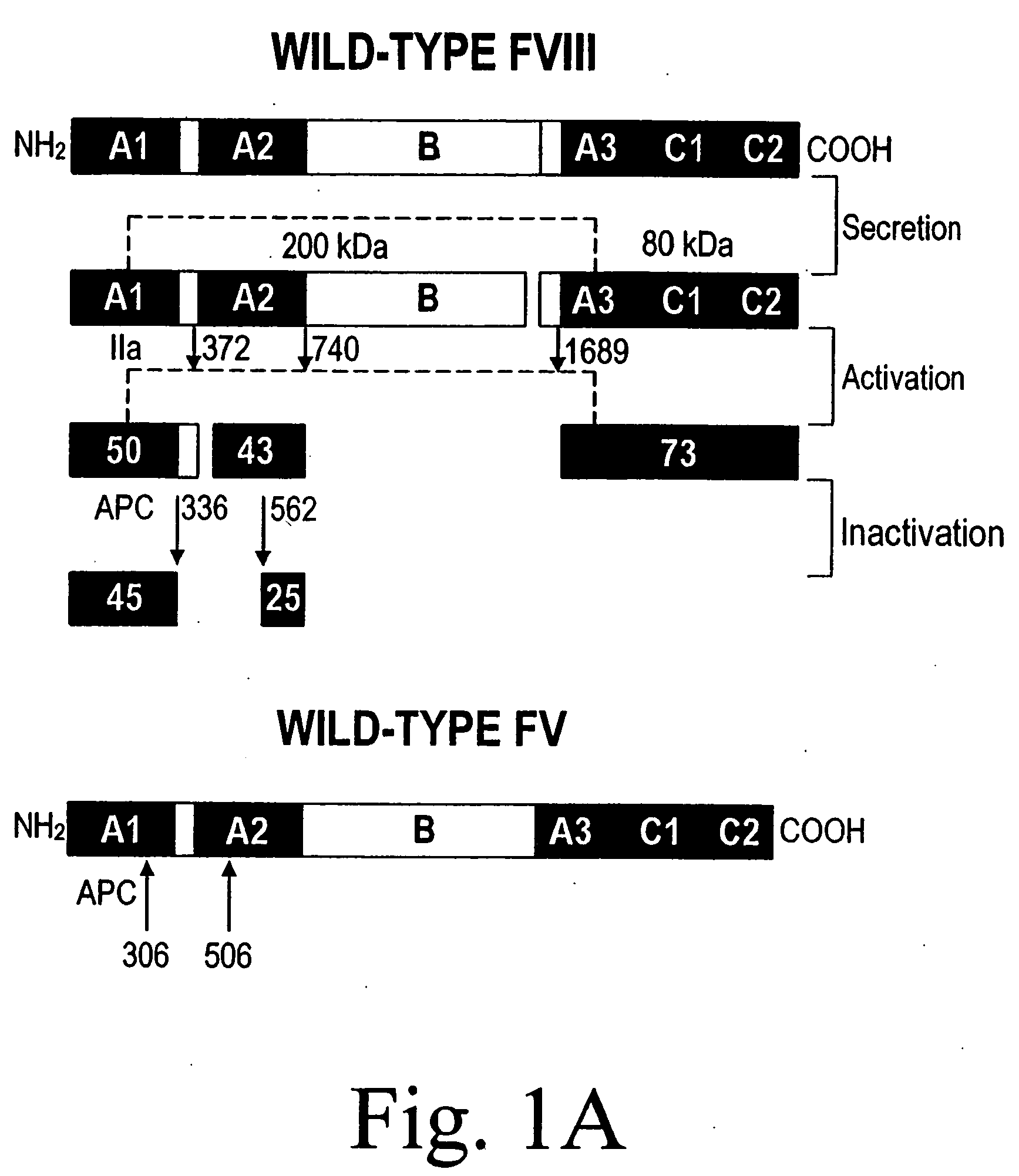
The most important ones are: Factor VIII is bound to vWF while inactive in circulation; factor VIII degrades rapidly when not bound to vWF. Factor VIII is released from vWF by the action of thrombin. vWF binds to collagen, e.g., when it is exposed in endothelial cells due to damage occurring to the blood vessel.
Methods for correcting von willebrand factor point mutations
InactiveUS20150166985A1Nervous disorderFusion with DNA-binding domainHuman DNA sequencingFactor VIII vWF
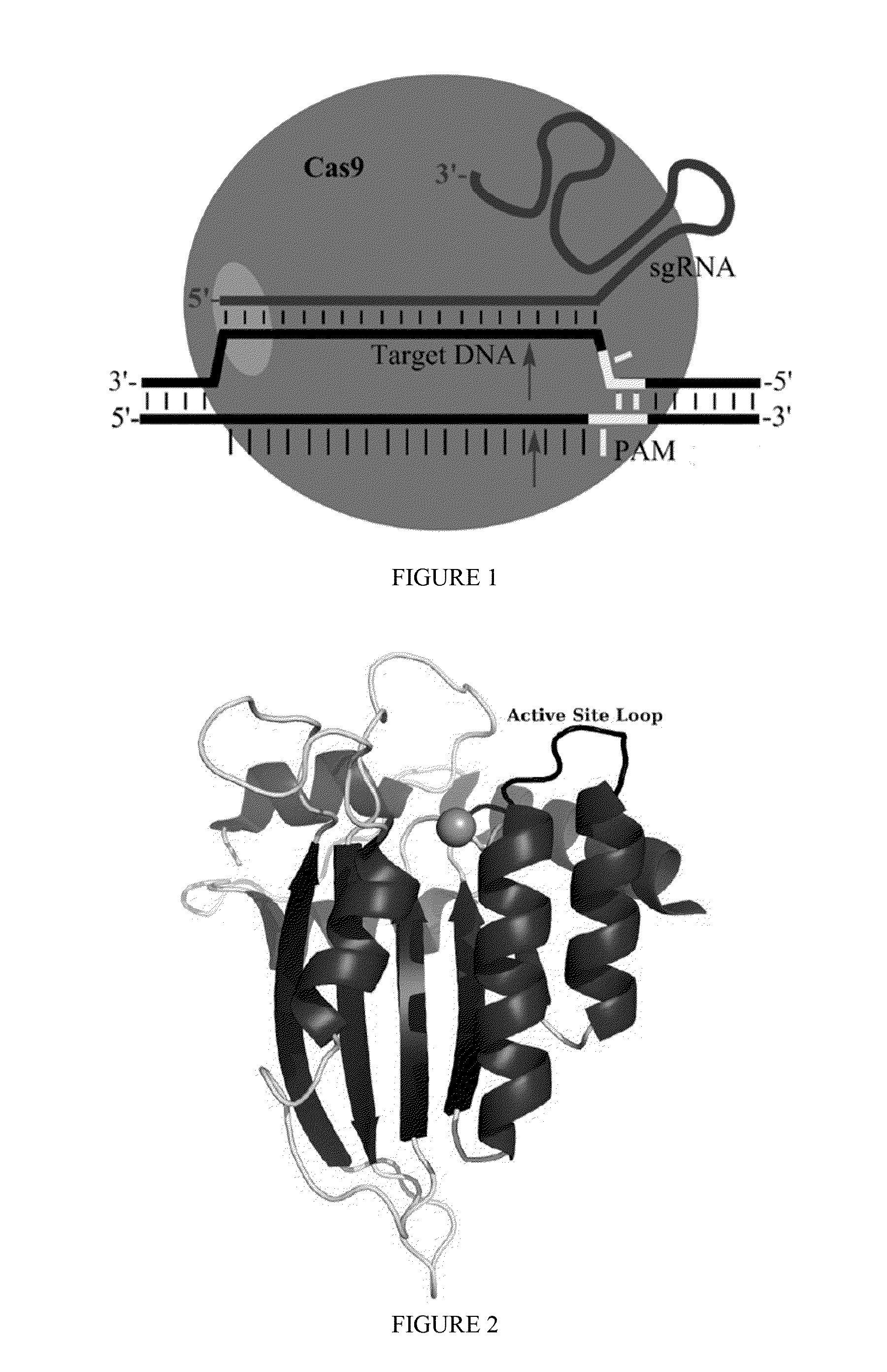
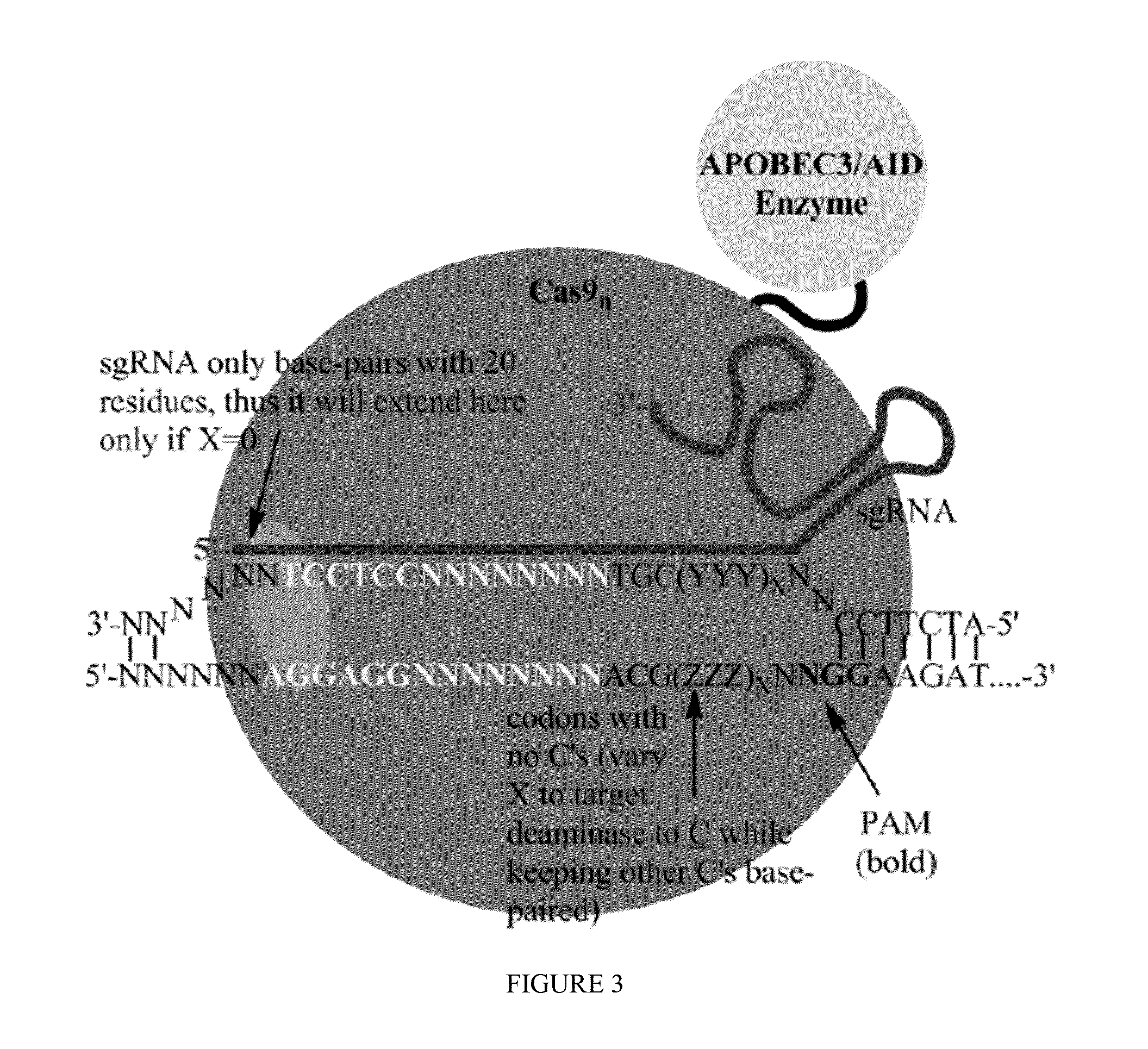
Some aspects of this disclosure provide strategies, systems, reagents, methods, and kits that are useful for the targeted editing of nucleic acids, including editing a nucleic acid encoding a mutant von Willebrand Factor protein to correct a point mutation associated with a disease or disorder, e.g., with von Willebrand disease. The methods provided are useful for correcting a vWF point mutation within the genome of a cell or subject, e.g., within the human genome. In some embodiments, fusion proteins of Cas9 and nucleic acid editing enzymes or enzyme domains, e.g., deaminase domains, are provided. In some embodiments, reagents and kits for the generation of targeted nucleic acid editing proteins, e.g., fusion proteins of Cas9 and nucleic acid editing enzymes or domains, are provided.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Composition exhibiting a von Willebrand factor (vWF) protease activity comprising a polypeptide chain with the amino acid sequence AAGGILHLELLV
InactiveUS20050266528A1Immunoglobulins against blood coagulation factorsFactor VIIFactor VIII vWFProteinase activity
Owner:BAXALTA INC
von Willebrand factor (vWF)-containing preparation, process for preparing vWF-containing preparations, and use of such preparations
A high-purity von Willebrand factor preparation, a process for making it, and use of the preparation and compositions containing it for the treatment of disorders are disclosed.
Owner:GE HEALTHCARE BIOPROCESS R&D +1
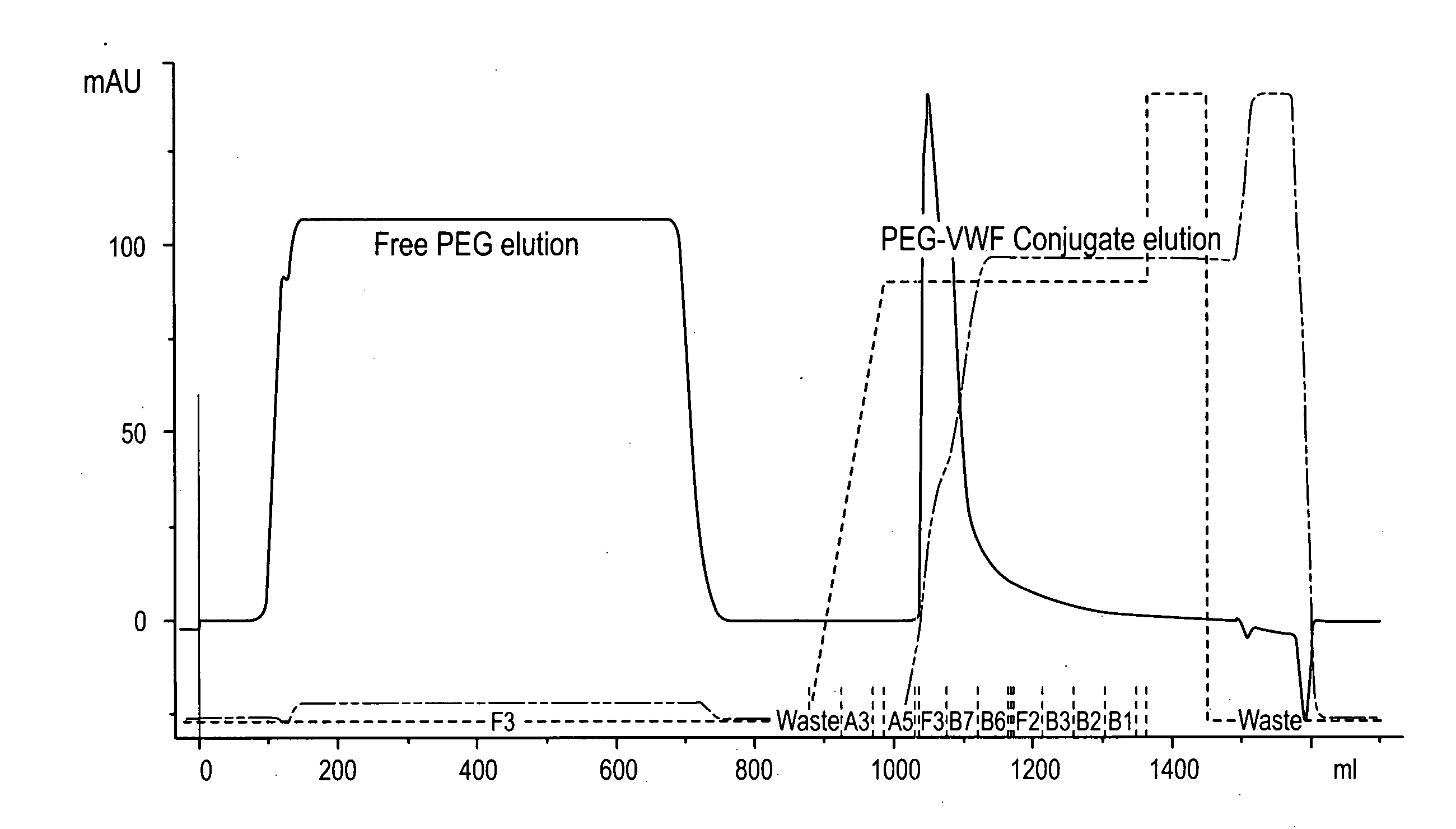
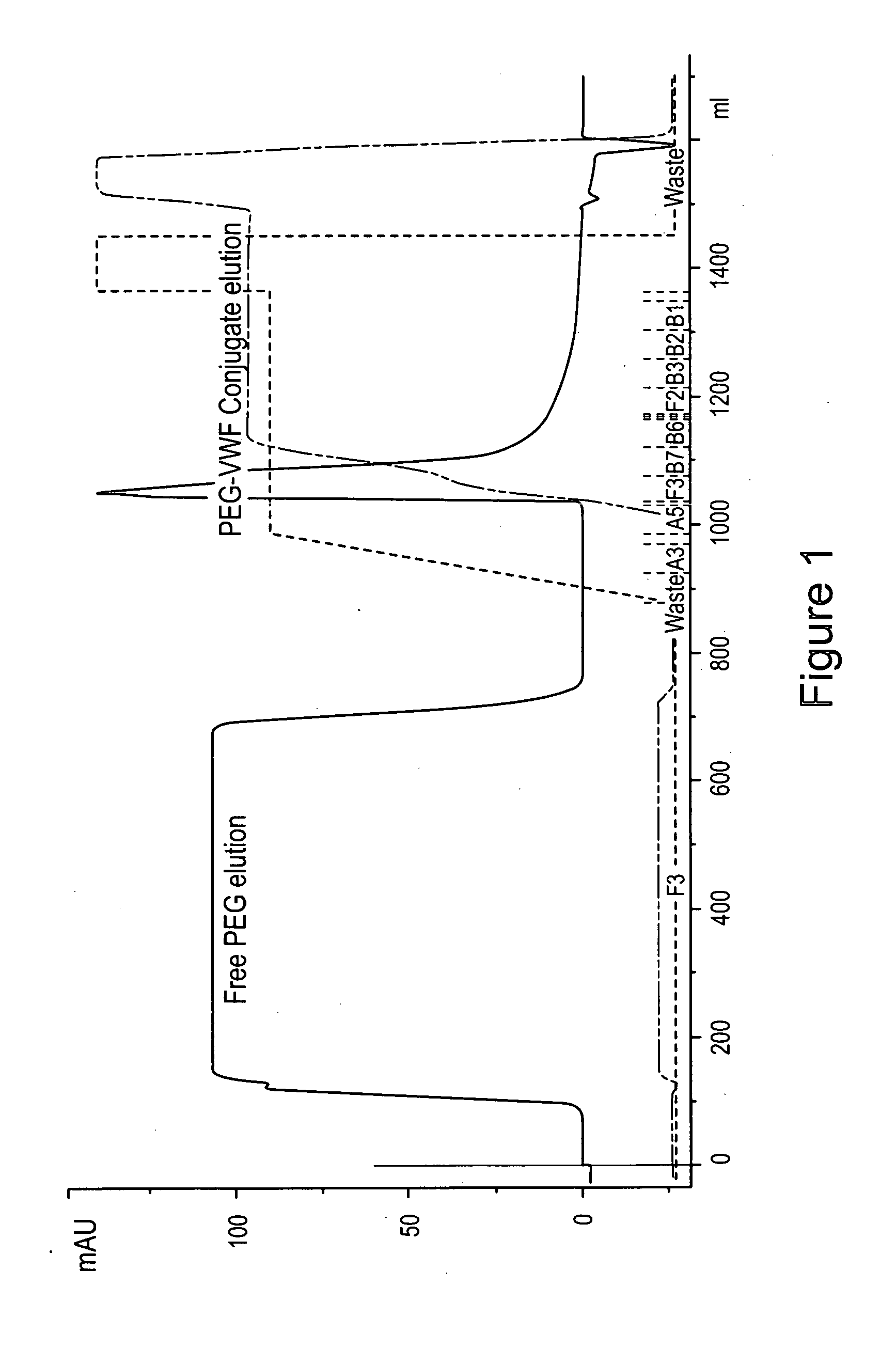
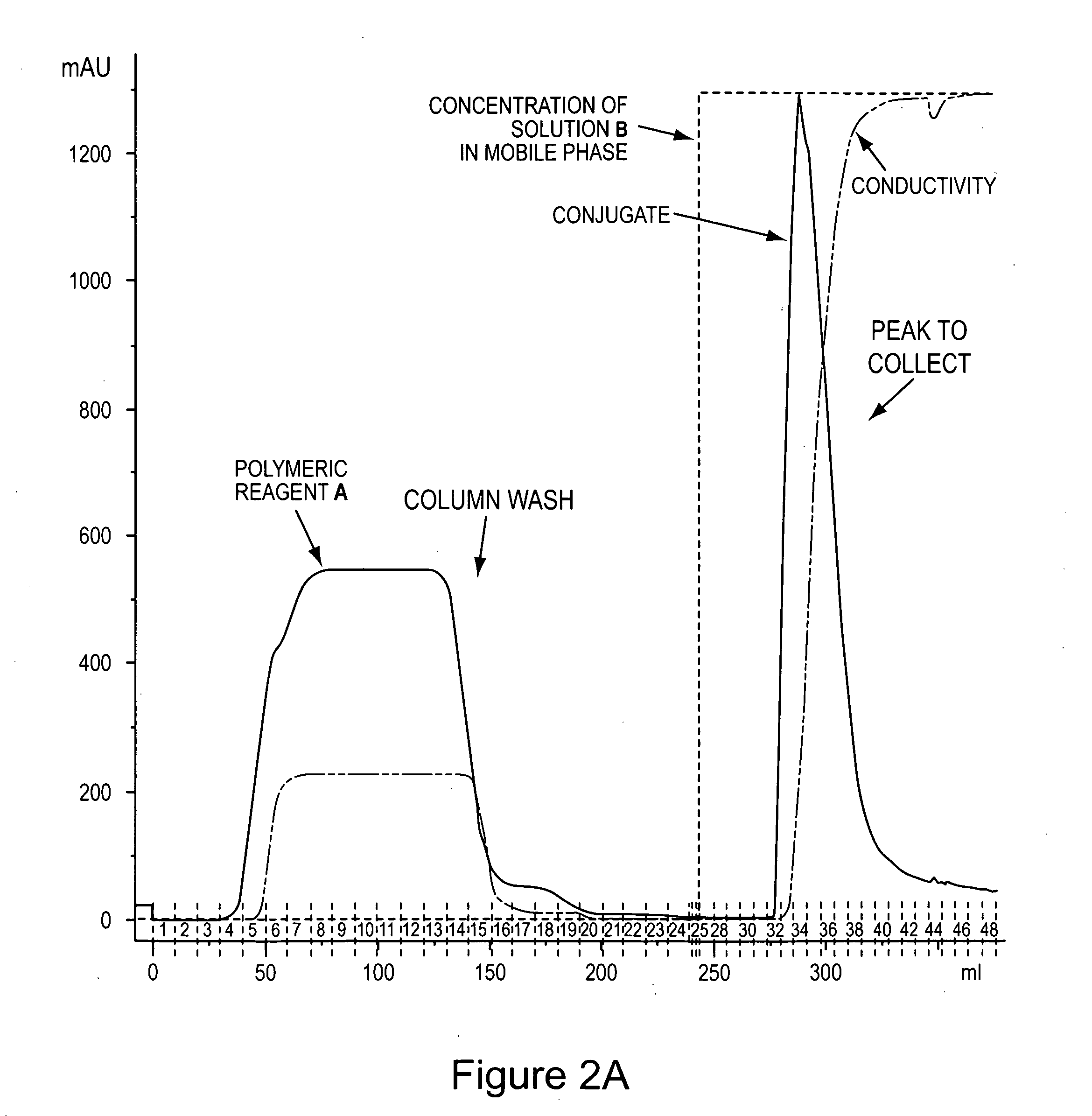
Von Willebrand factor-and factor VIII-polymer conjugates having a releasable linkage
ActiveUS20080234193A1Prolong half-life in vivoFactor VIIPeptide/protein ingredientsFactor VIII vWFFactor ii
The present invention provides von Willebrand Factor-polymer conjugates and Factor VIII-polymer conjugates, each having a releasable linkage. Methods of making conjugates, methods for administering conjugates, are also provided.
Owner:NEKTAR THERAPEUTICS INC +1
Composition exhibiting a von willebrand factor (vWF) protease activity comprising a polypeptide chain with the amino acid sequence AAGGILHLELLV
InactiveUS6926894B2Immunoglobulins against blood coagulation factorsFactor VIIFactor VIII vWFProteinase activity
The invention relates to vWF cleaving entities having a molecular weight of 180 kD, 170 kD, 160 kD, 120 kD or 110 kD and an N-terminal amino acid sequence of AAGGILHLELLV (SEQ ID NO: 1), vWF cleaving complexes and methods for their production.
Owner:BAXALTA INC
Substrates specific to von willebrand factor cleaving protease and method of assaying the activity
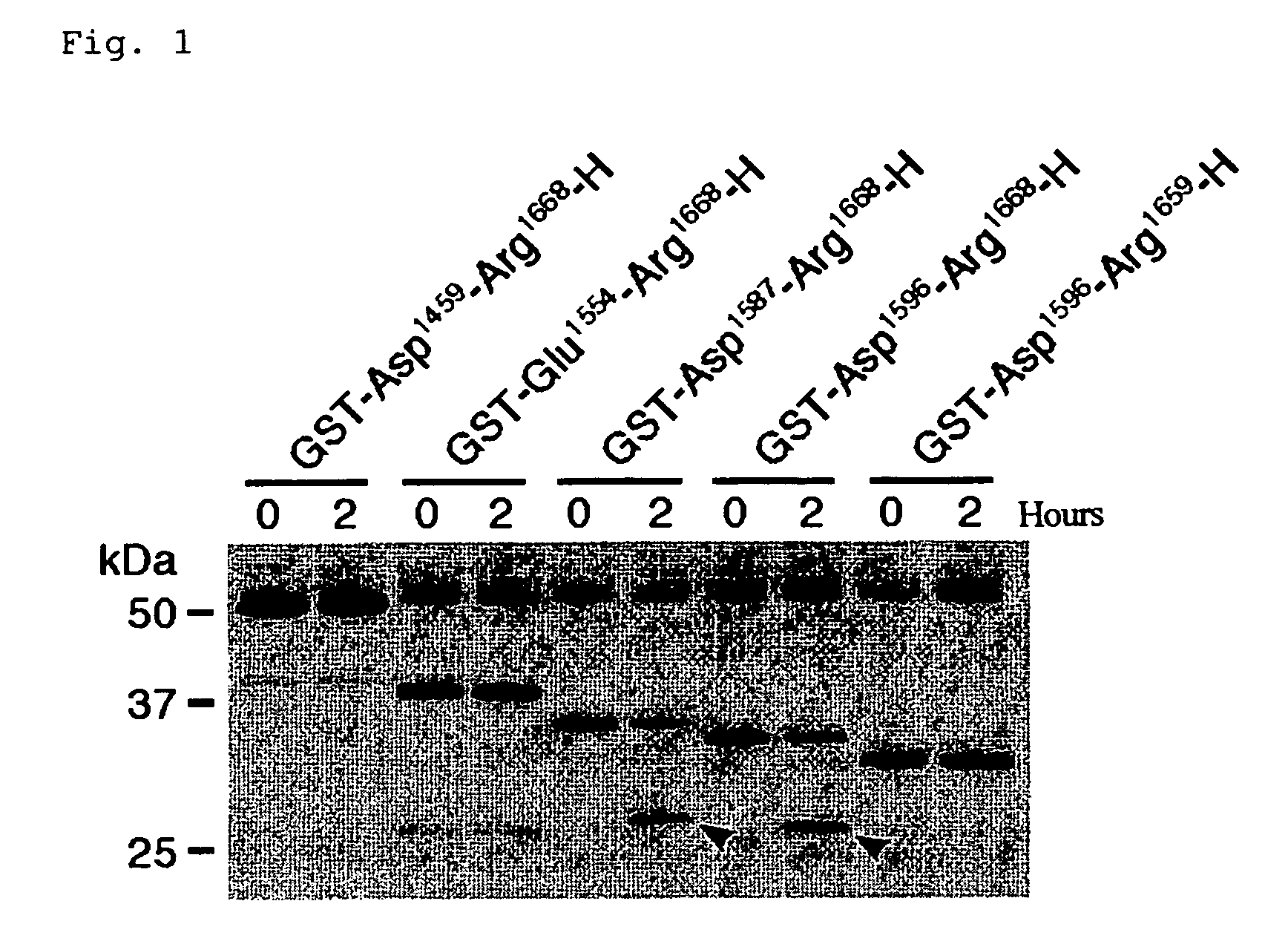
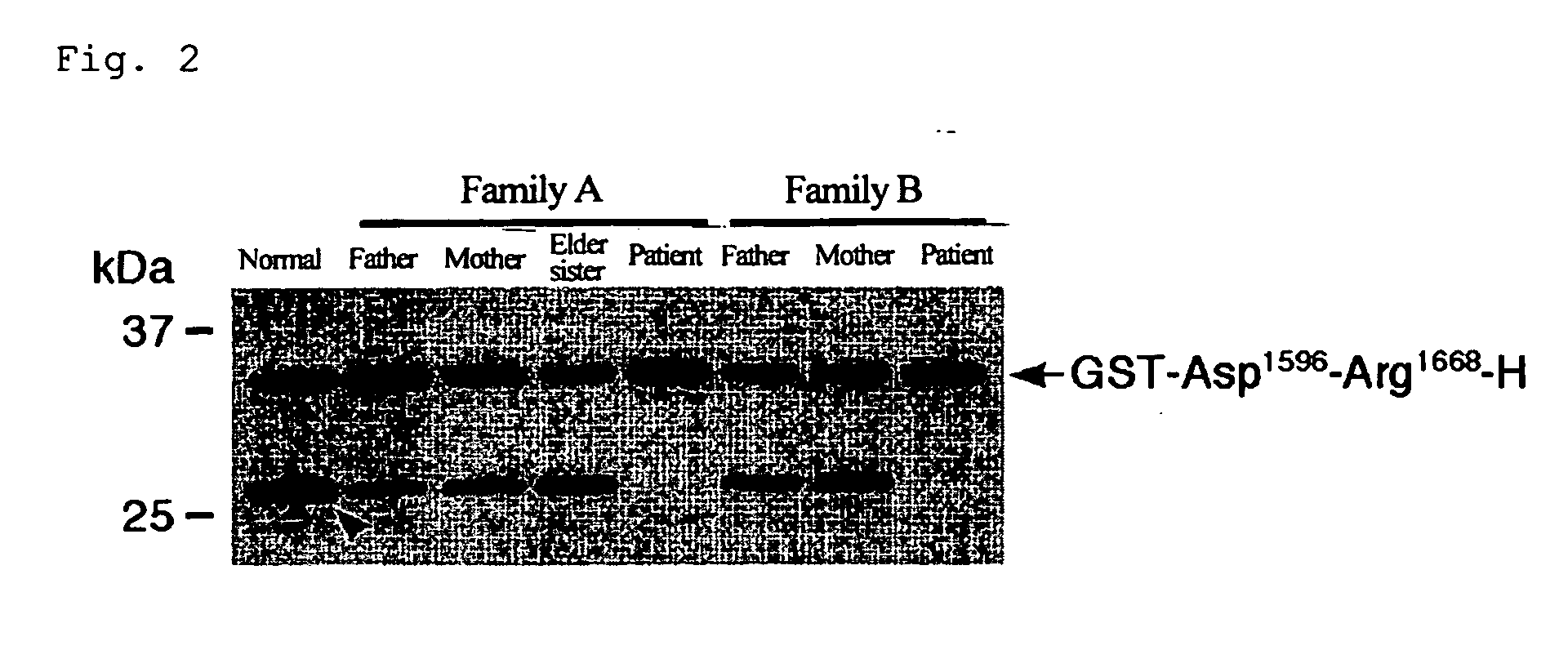
The present invention relates to specific substrates for a von Willebrand factor cleaving enzyme, ADAMTS-13, as well as to diagnosis of ADAMTS-13 deficient patients, diagnostic compositions, and kits employing the substrates. Particularly preferable substrate polypeptides for ADAMTS-13 are the polypeptide which begins at amino acid 1587 and ends at amino acid 1668 of SEQ ID NO: 1 in the Sequence Listing, and the polypeptide which begins at amino acid 1596 and ends at amino acid 1668 of SEQ ID NO: 1 in the Sequence Listing. These substrate polypeptides for ADAMTS-13 have high substrate specificity and also superior quantitativeness, and a suitable size for production by recombinant methods.
Owner:NAT CEREBRAL & CARDIOVASCULAR CENT
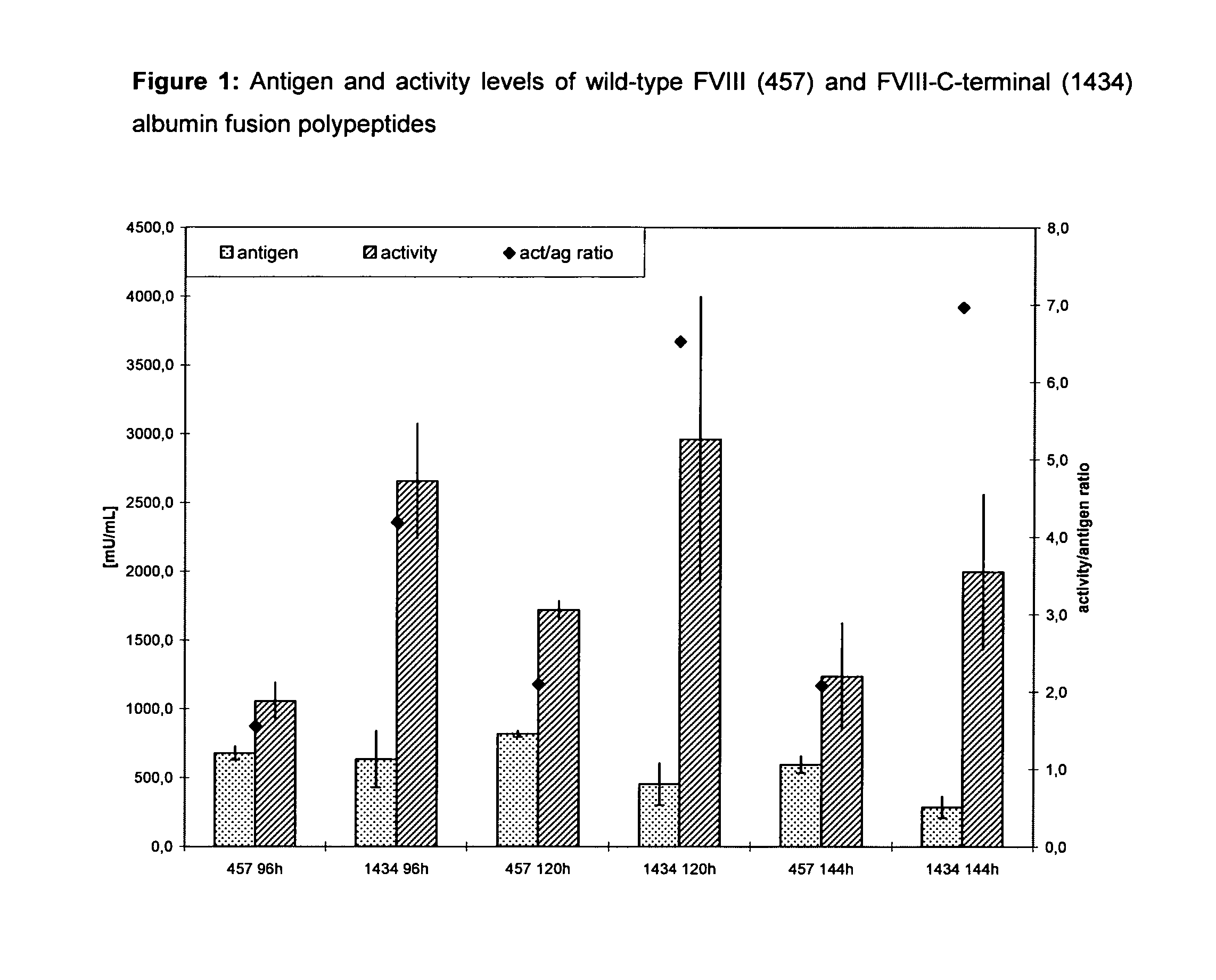
Factor viii, von willebrand factor or complexes thereof with prolonged in vivo half-life
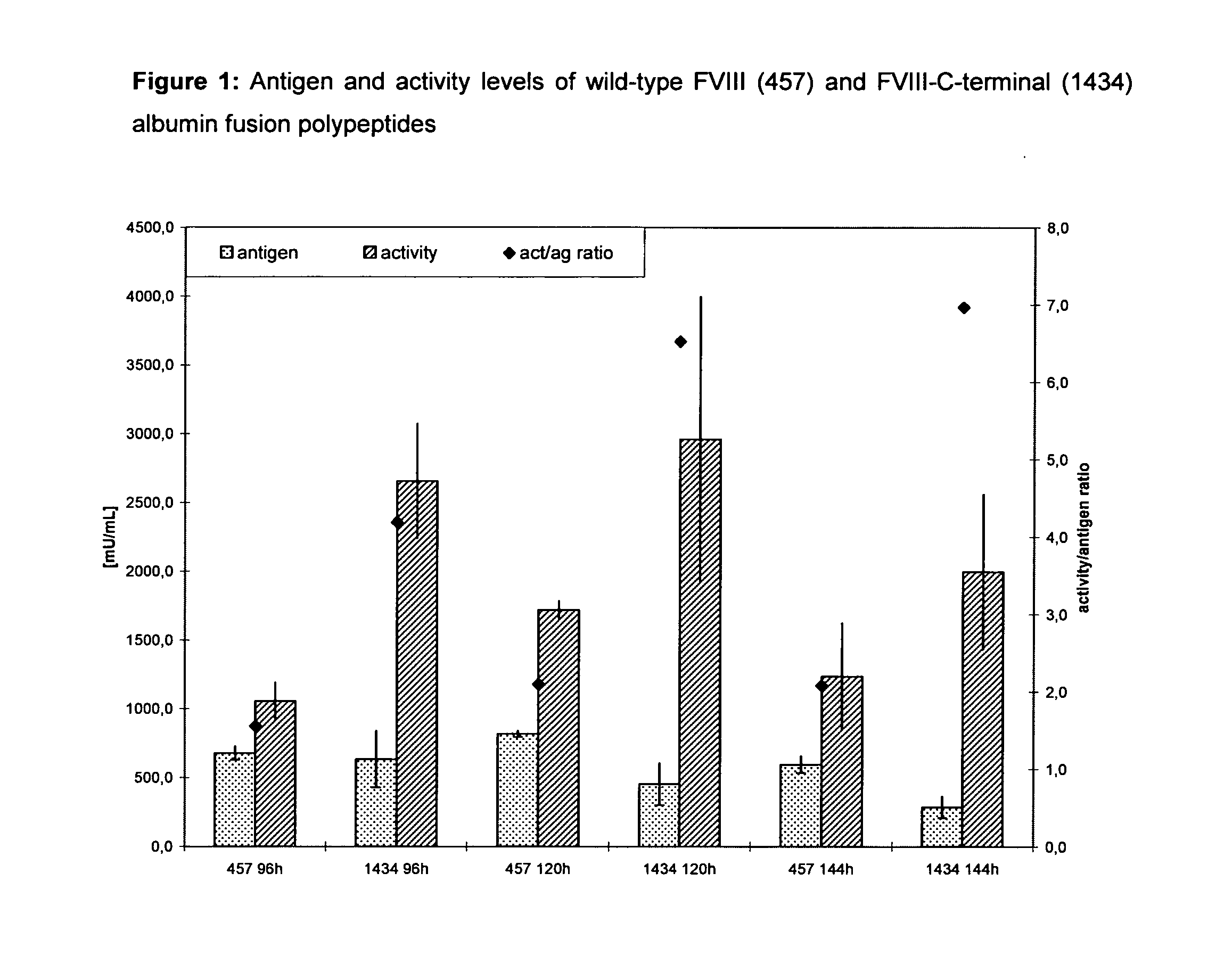
ActiveUS20110183907A1Retain biological activityPromote recoveryOrganic active ingredientsFactor VIIFactor VIII vWFNucleic acid sequencing
The present invention relates to modified nucleic acid sequences coding for coagulation factor VIII (FVIII) and for von Willebrand factor (VWF) as well as complexes thereof and their derivatives, recombinant expression vectors containing such nucleic acid sequences, host cells transformed with such recombinant expression vectors, recombinant polypeptides and derivatives coded for by said nucleic acid sequences which recombinant polypeptides and derivatives do have biological activities together with prolonged in vivo half-life and / or improved in vivo recovery compared to the unmodified wild-type protein. The invention also relates to corresponding FVIII sequences that result in improved expression yield. The present invention further relates to processes for the manufacture of such recombinant proteins and their derivatives. The invention also relates to a transfer vector for use in human gene therapy, which comprises such modified nucleic acid sequences.
Owner:CSL BEHRING GMBH
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
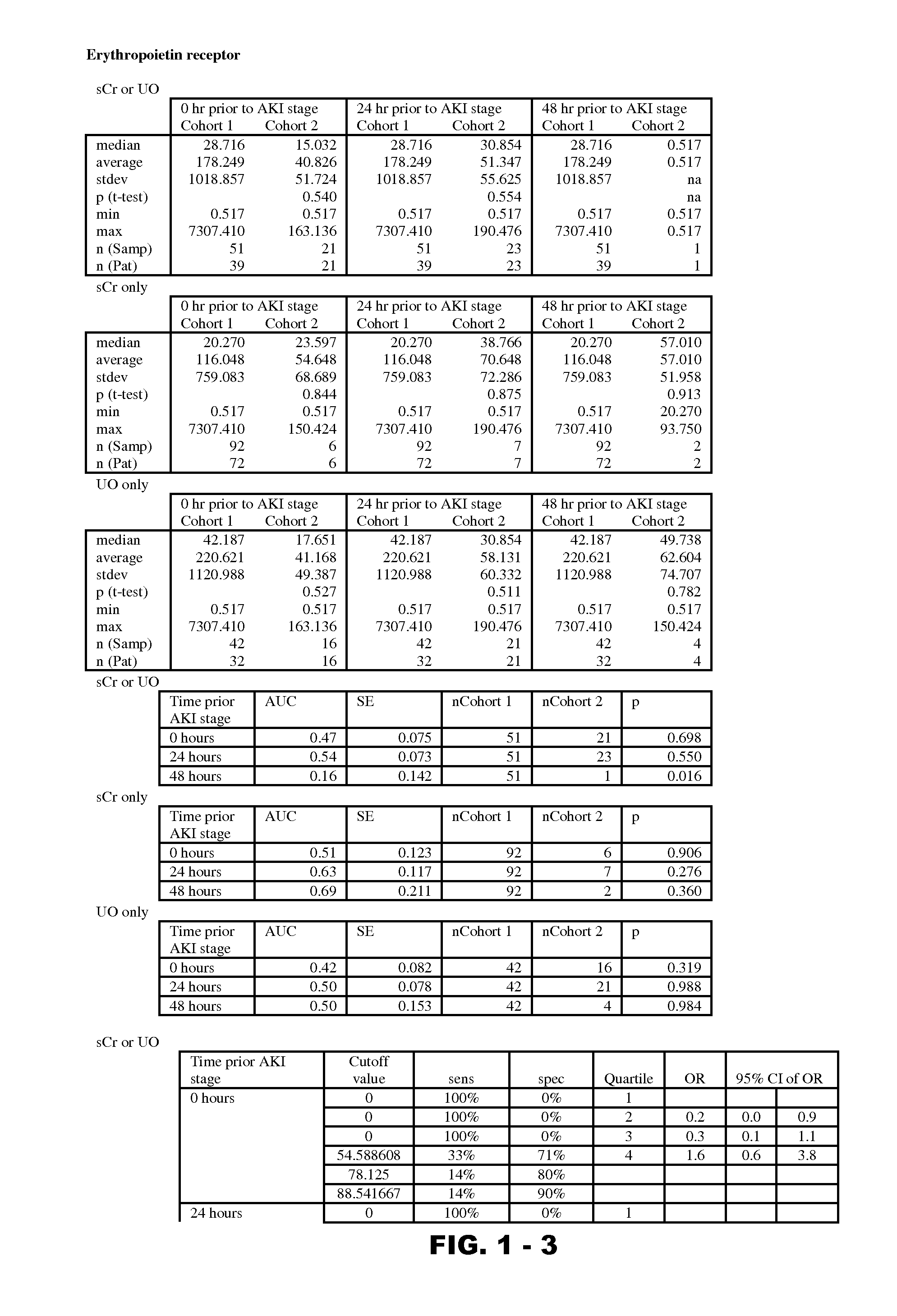
InactiveUS20120190051A1Eliminate needPeptide/protein ingredientsTransferrinsErythropoietin receptorFactor VIII vWF
Disclosed are methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, disclosed are assays that detect one or more markers selected from the group consisting of Prostatic acid phosphatase, Lactotransfenin, Soluble erythropoietin receptor, Von Willebrand factor, Soluble endothelial protein C receptor, and Beta-2-glycoprotein 1 as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
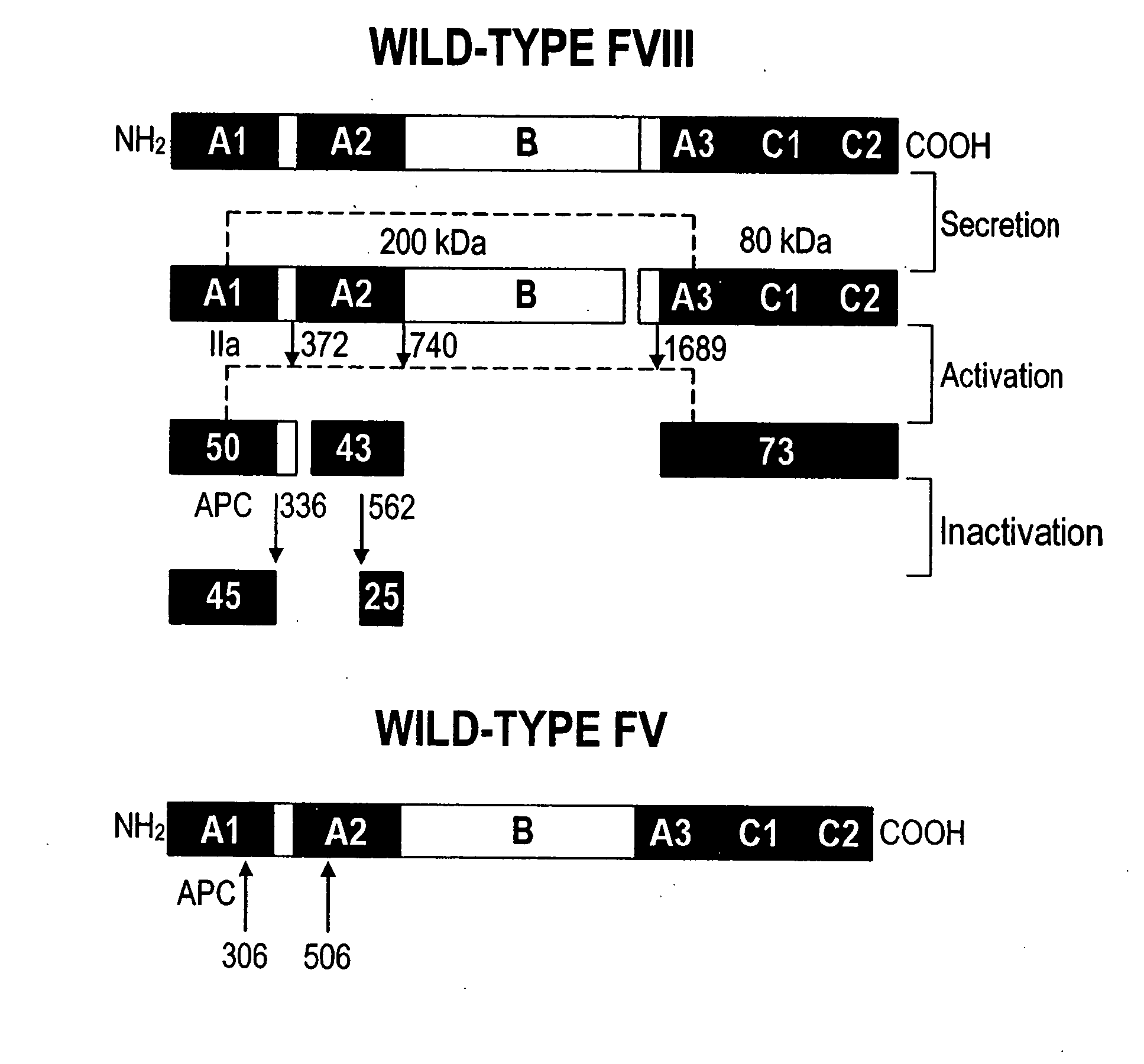
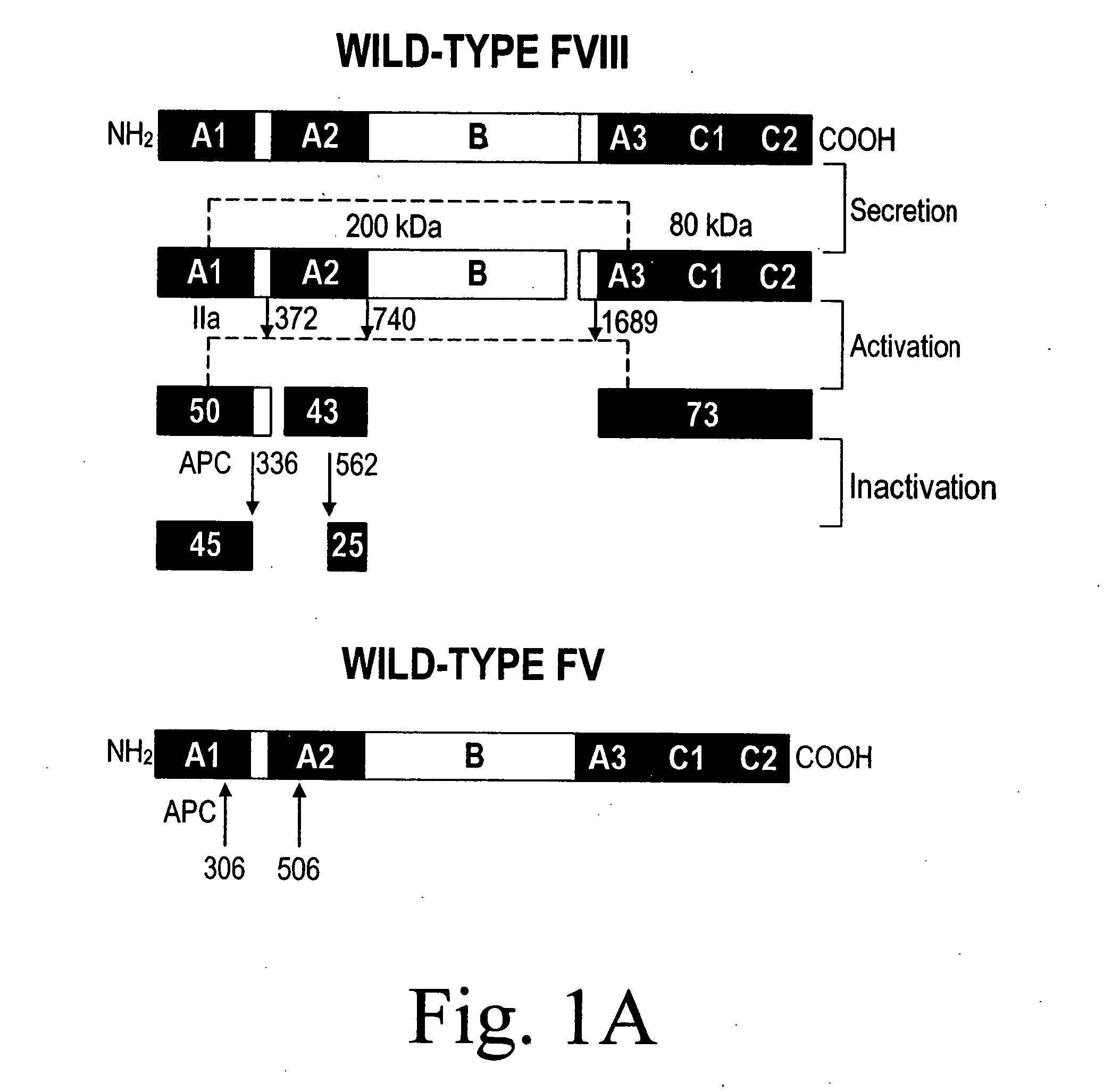
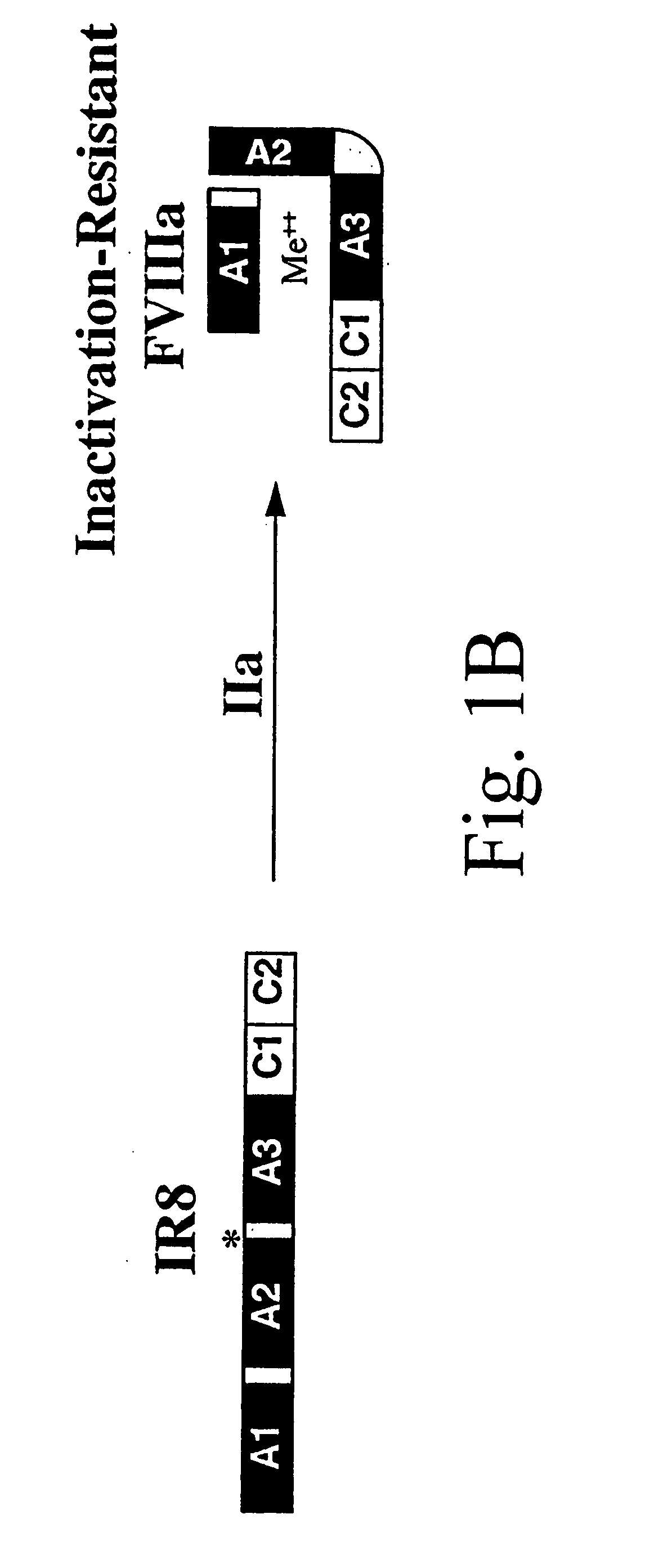
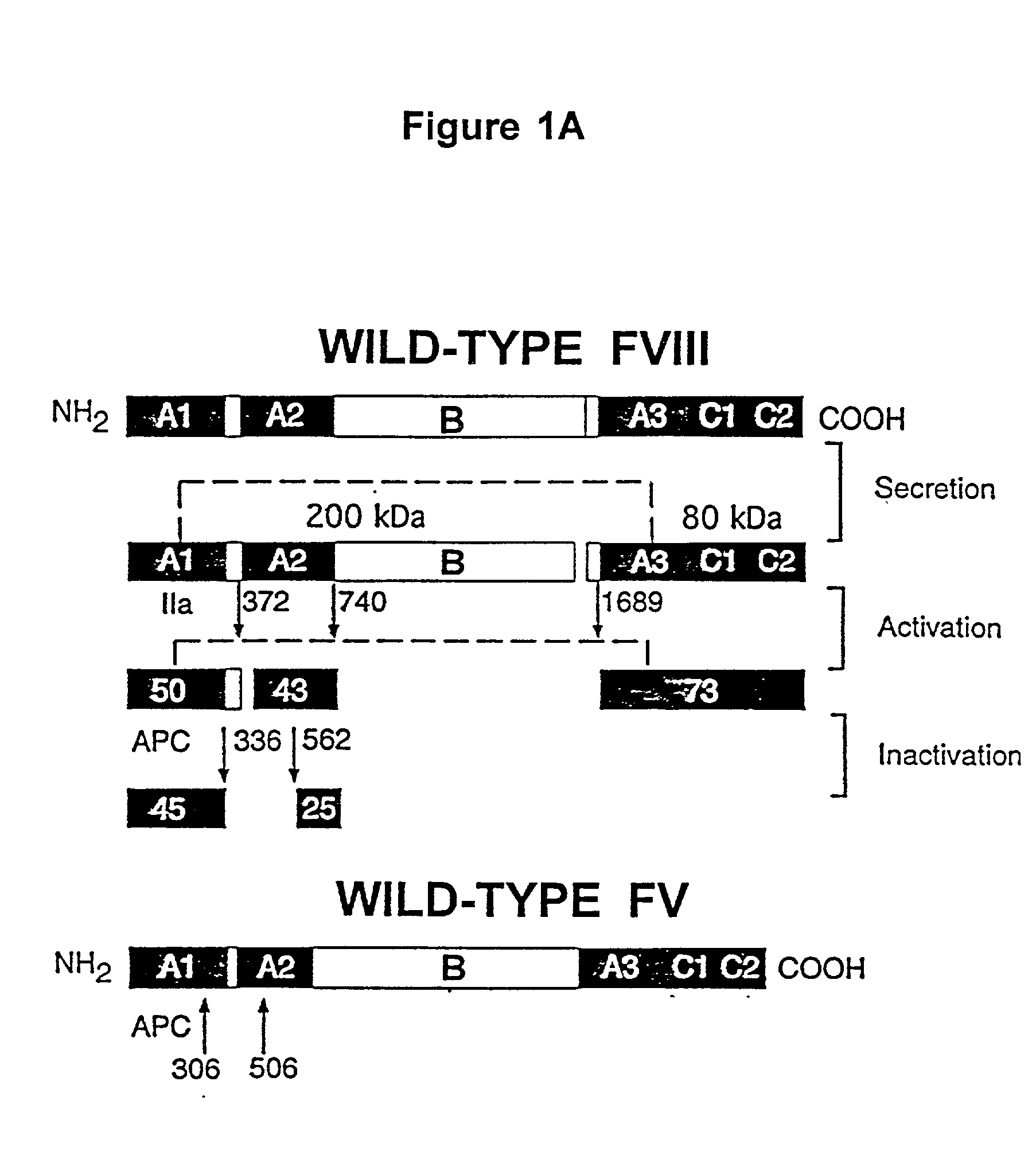
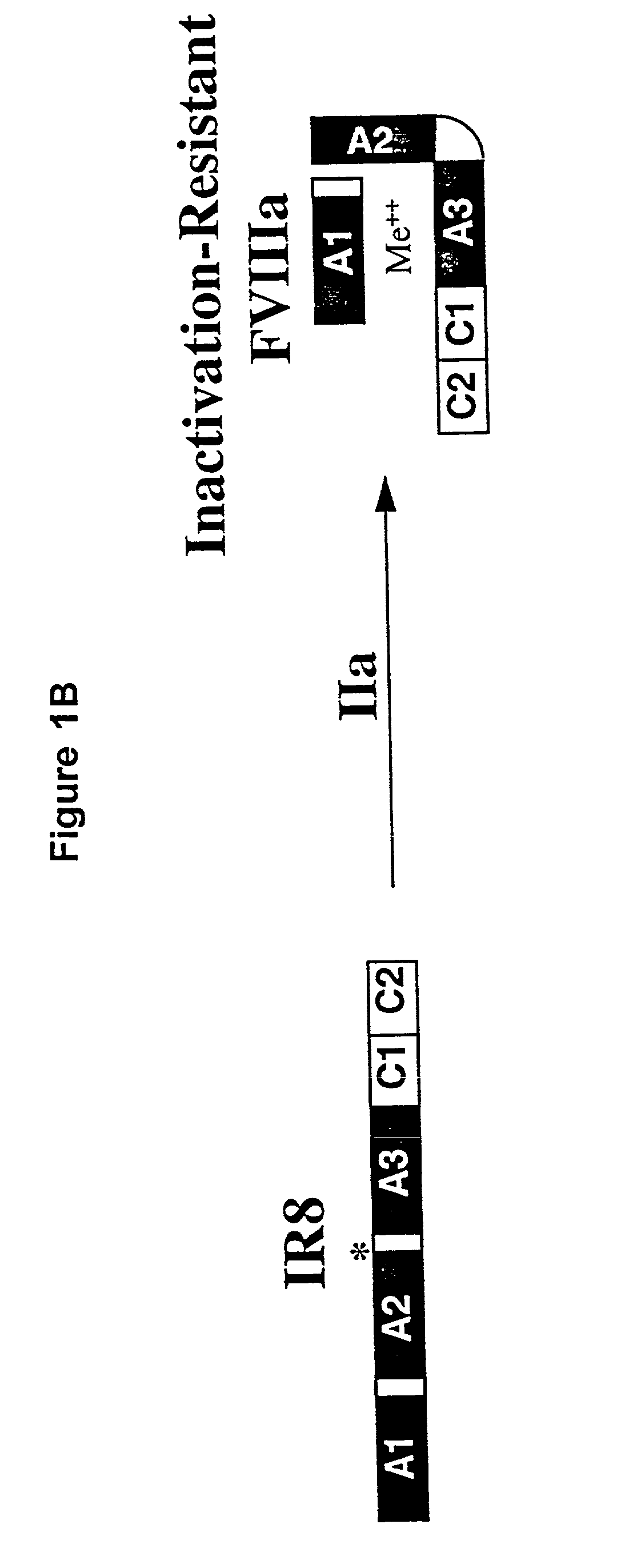
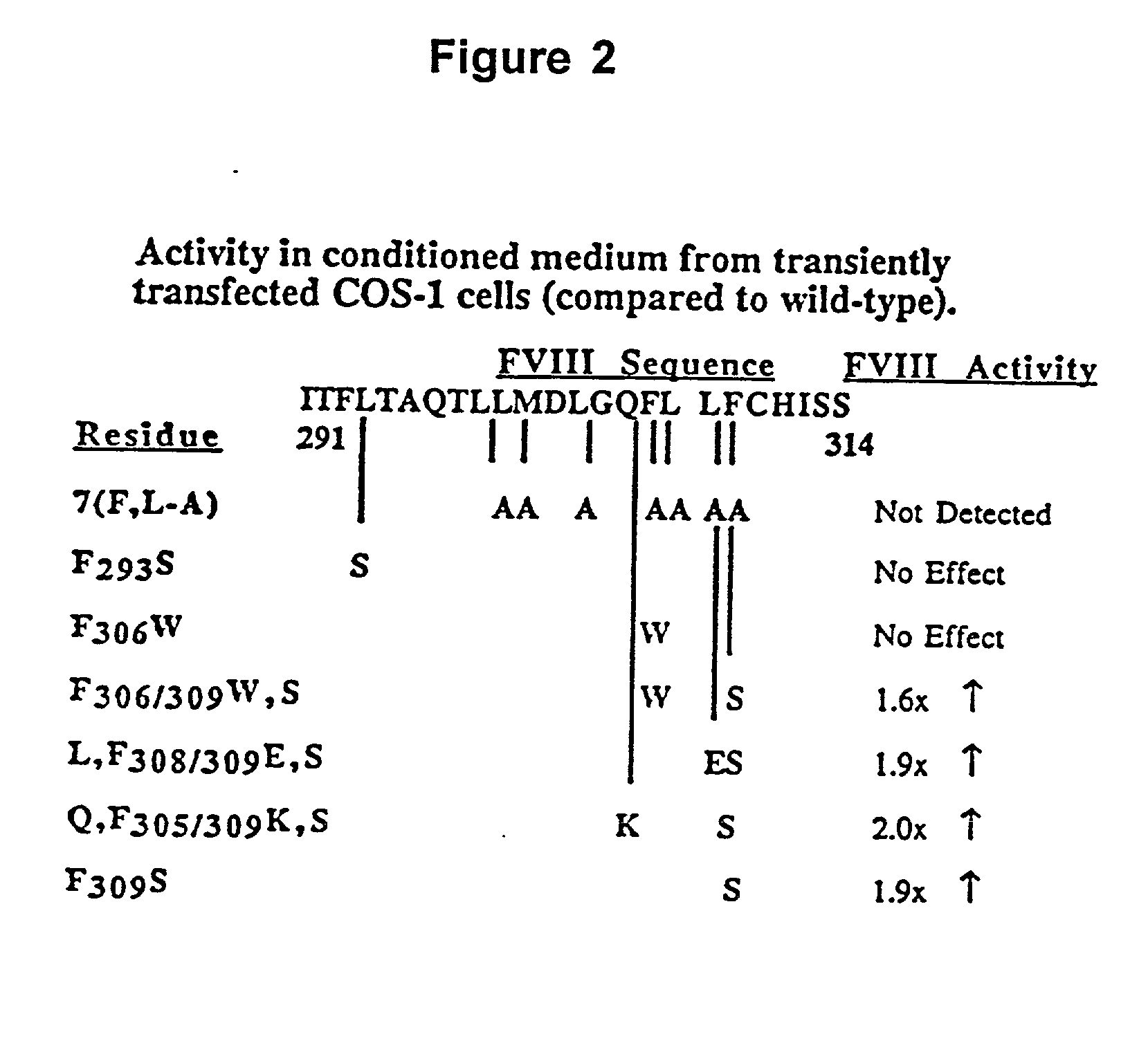
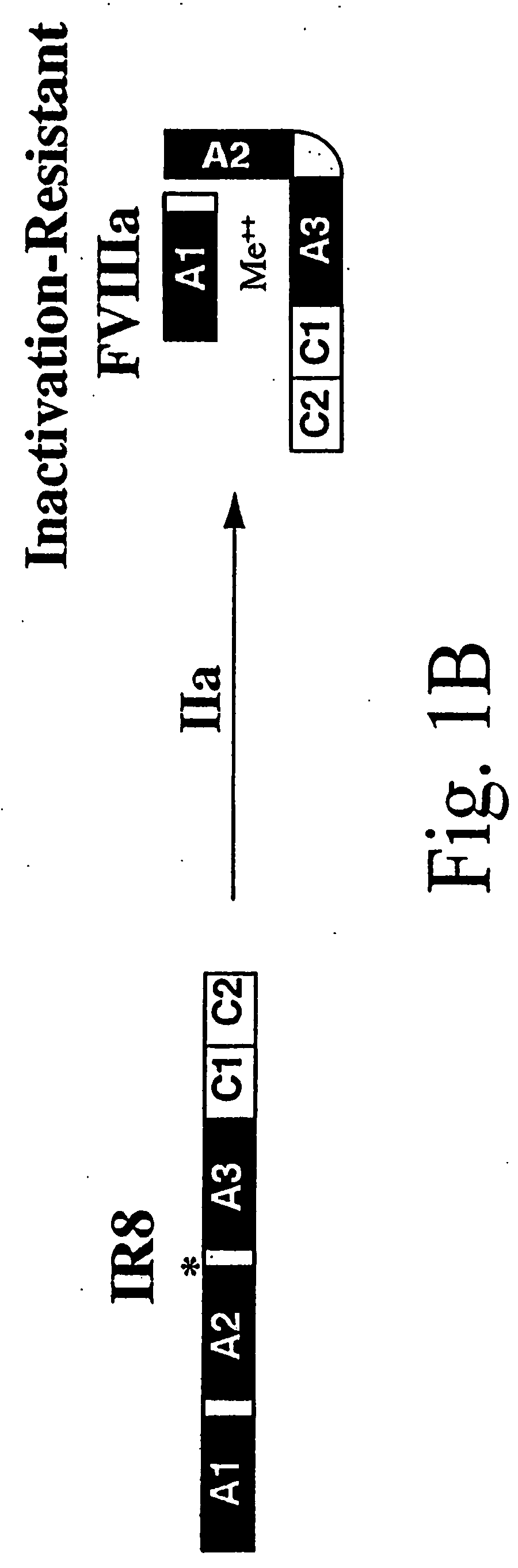
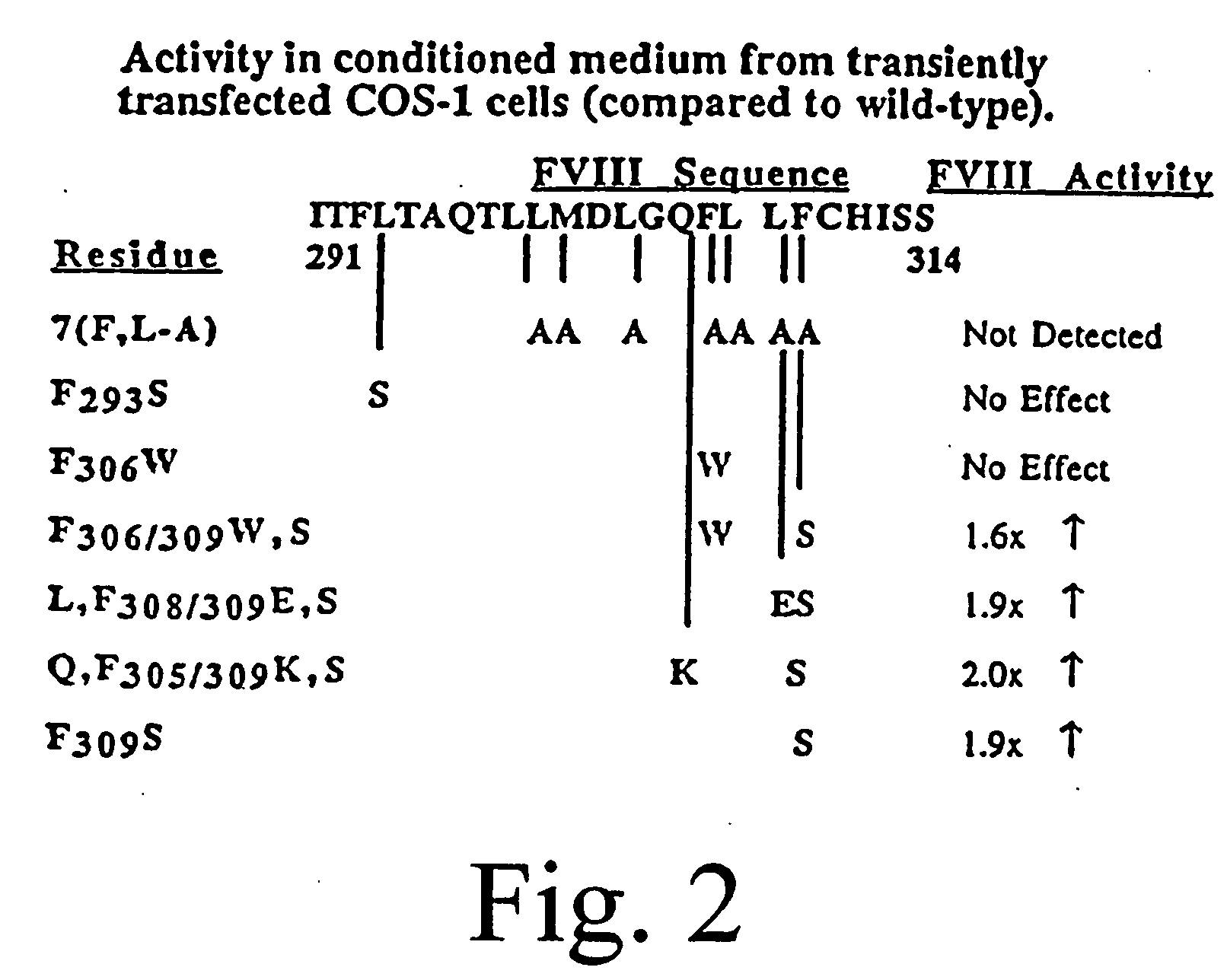
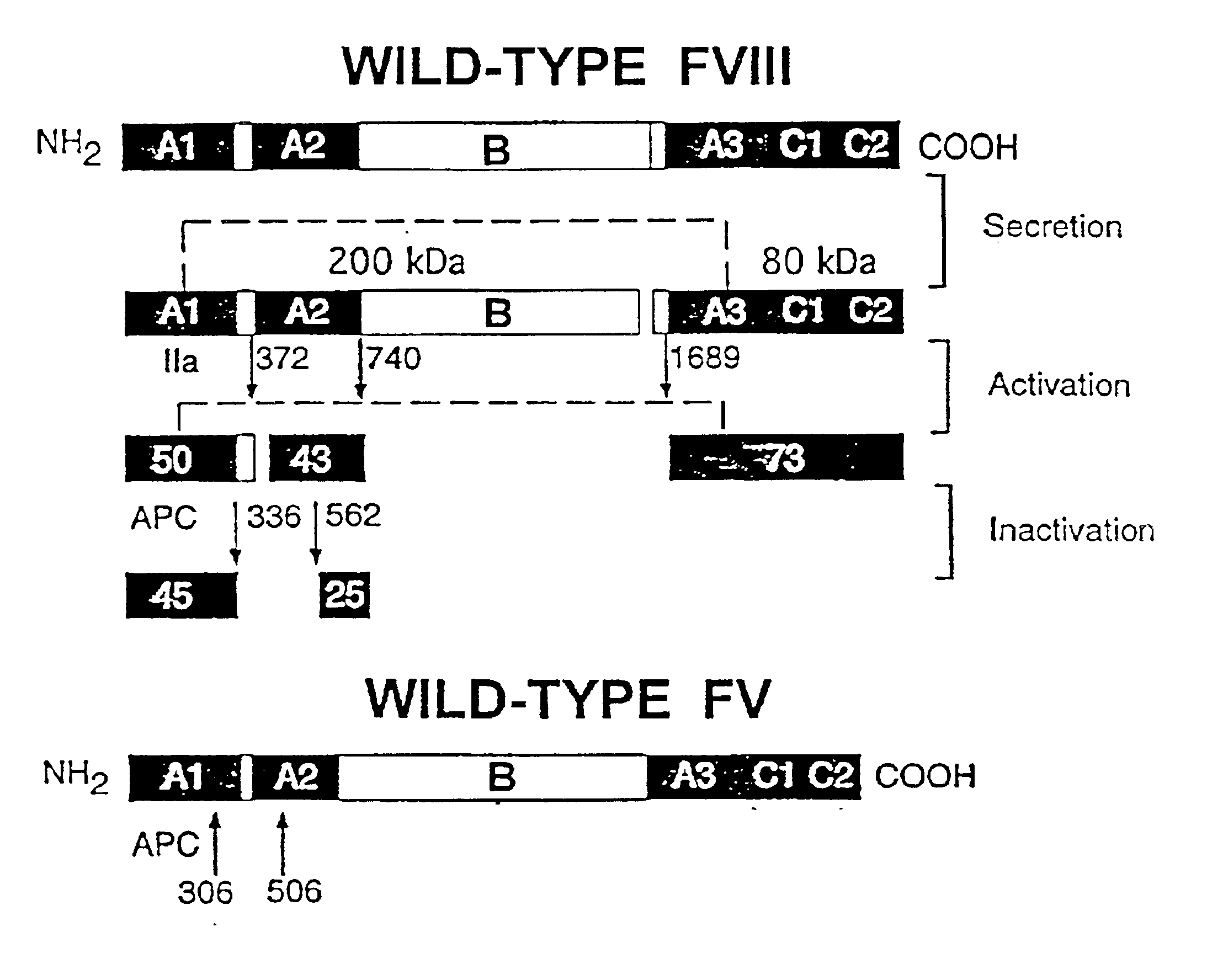
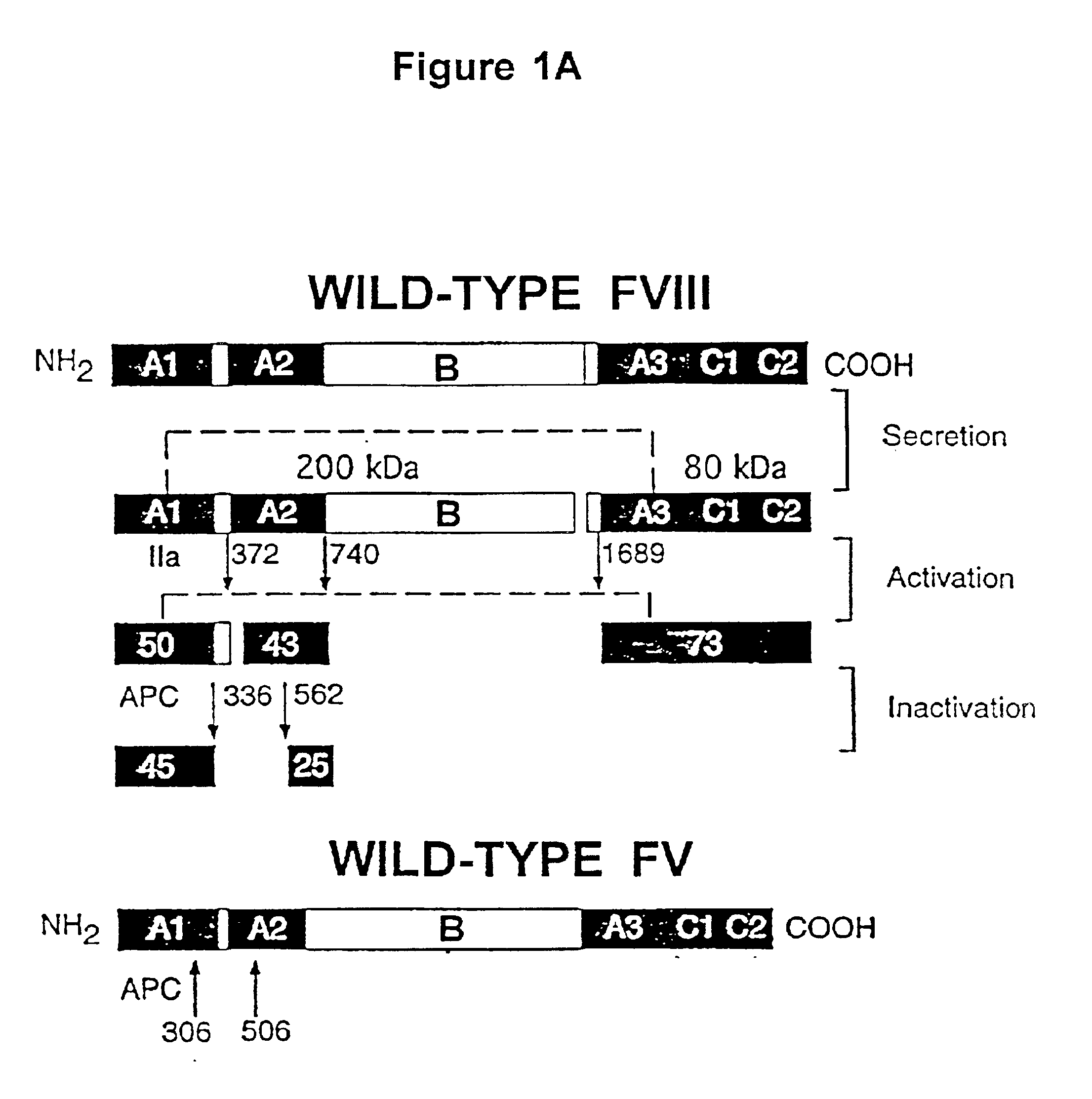
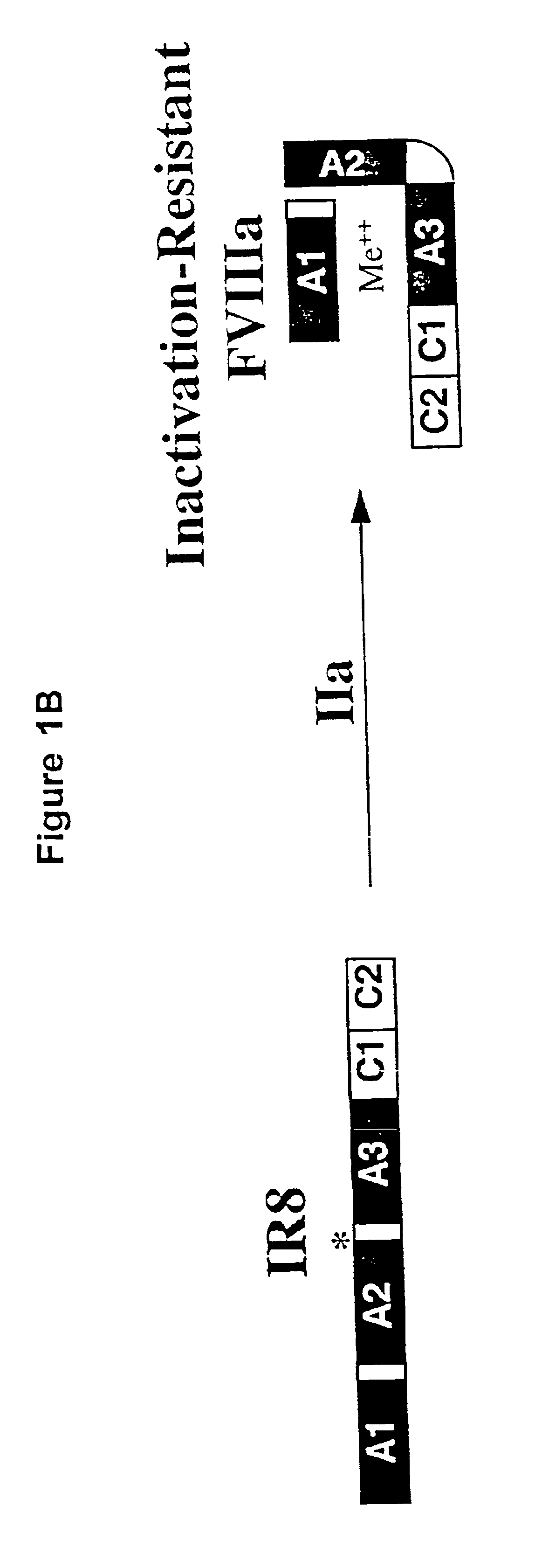
Inactivation resistant factor VIII
InactiveUS20040092442A1Increase secretionIncrease FVIII expressionFactor VIIBacteriaNucleotideBinding site
The present invention provides novel purified and isolated nucleic acid sequences encoding procoagulant-active FVIII proteins. The nucleic acid sequences of the present invention encode amino acid sequences corresponding to known human FVIII sequences, wherein residue Phe3O9 is mutated. The nucleic acid sequences of the present invention also encode amino acid sequences corresponding to known human FVIII sequences, wherein the APC cleavage sites, Arg336 and Ile562, are mutated. The nucleic acid sequences of the present invention further encode amino acid sequences corresponding to known human FVIII sequences, wherein the B-domain is deleted, the von Willebrand factor binding site is deleted, a thrombin cleavage site is mutated, an amino acid sequence spacer is inserted between the A2- and A3-domains. Methods of producing the FVIII proteins of the invention, nucleotide sequences encoding such proteins, pharmaceutical compositions containing the nucleotide sequences or proteins, as well as methods of treating patients suffering from hemophilia, are also provided.
Owner:UNIV OF MICHIGAN THE
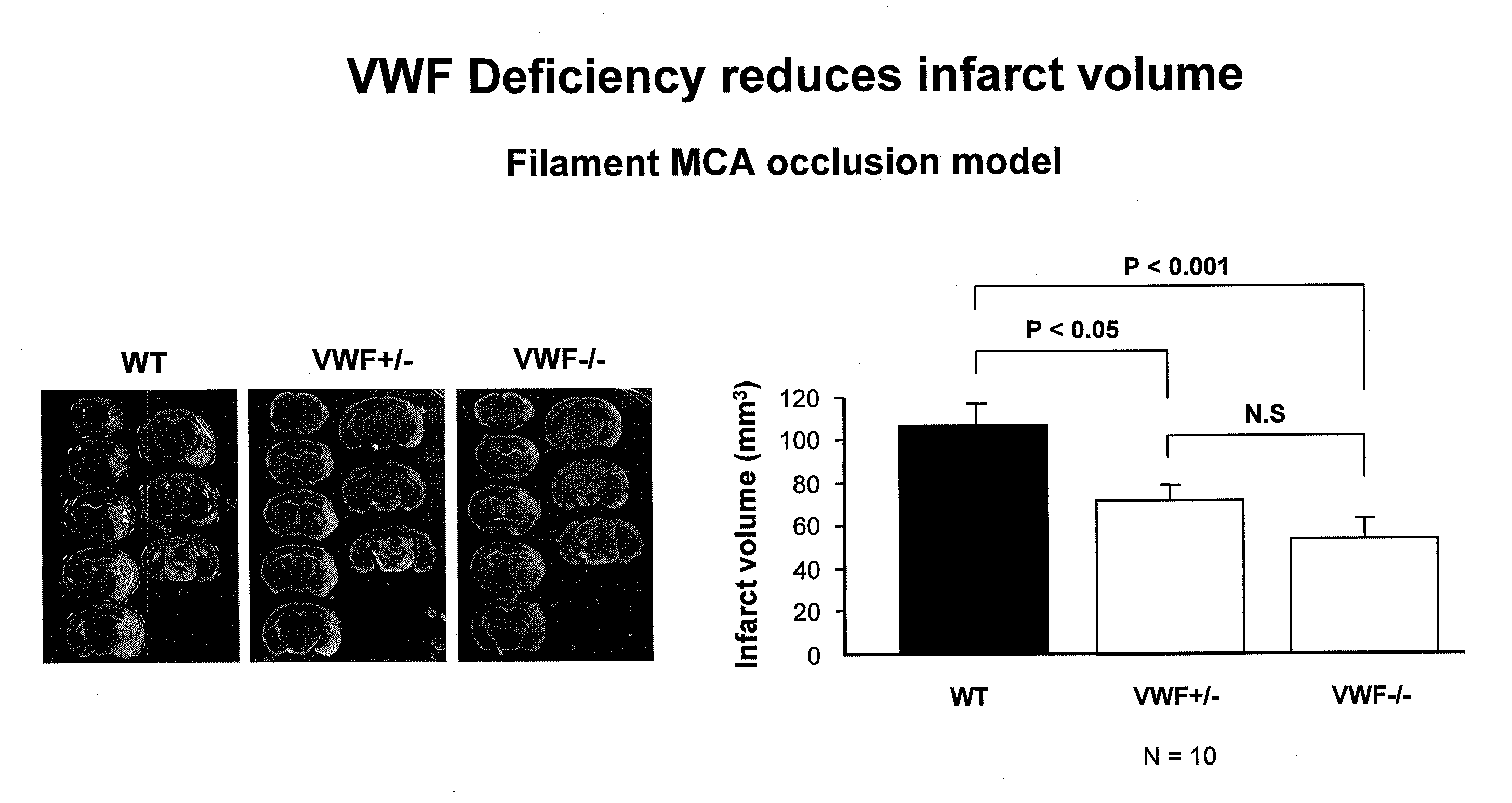
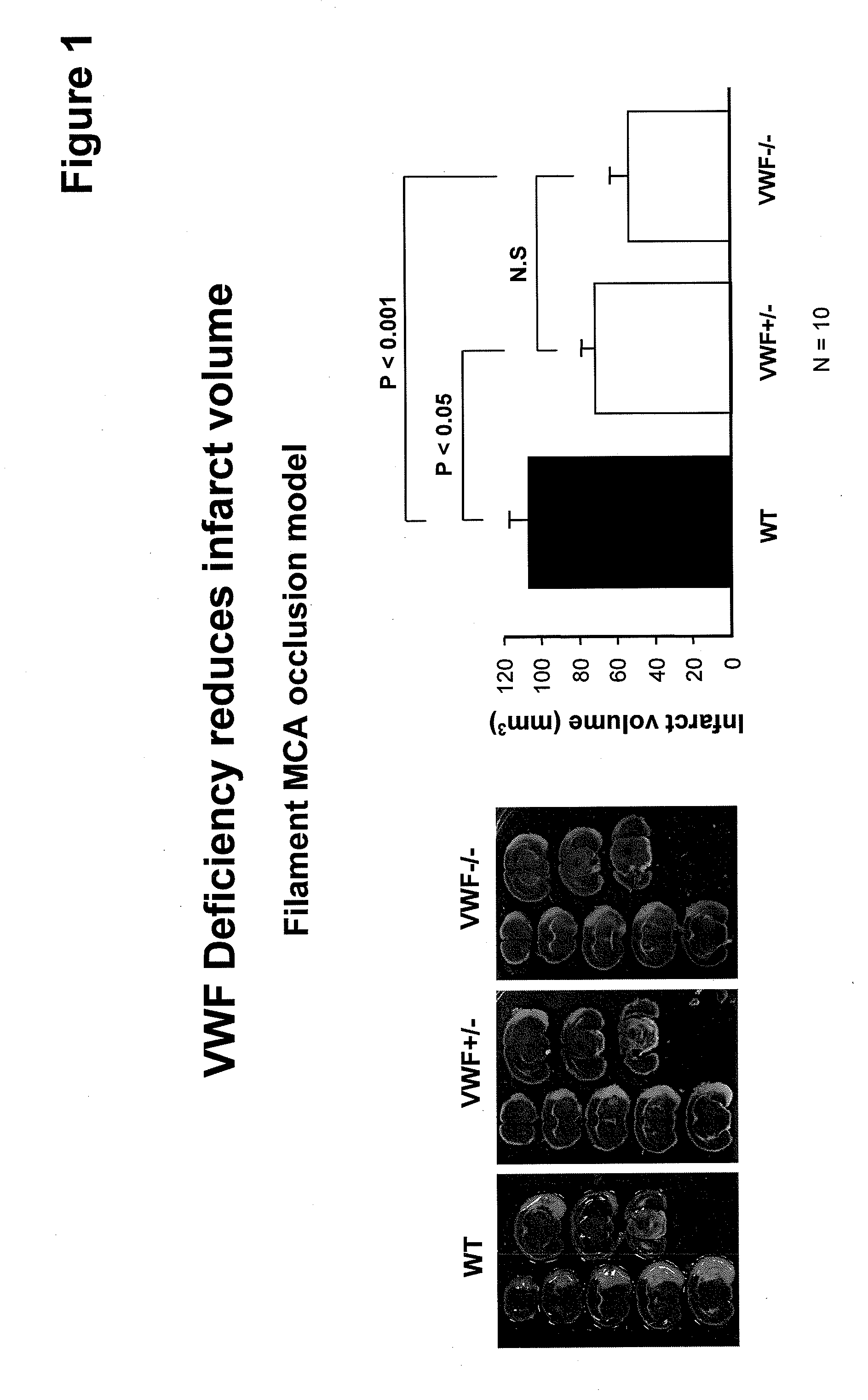
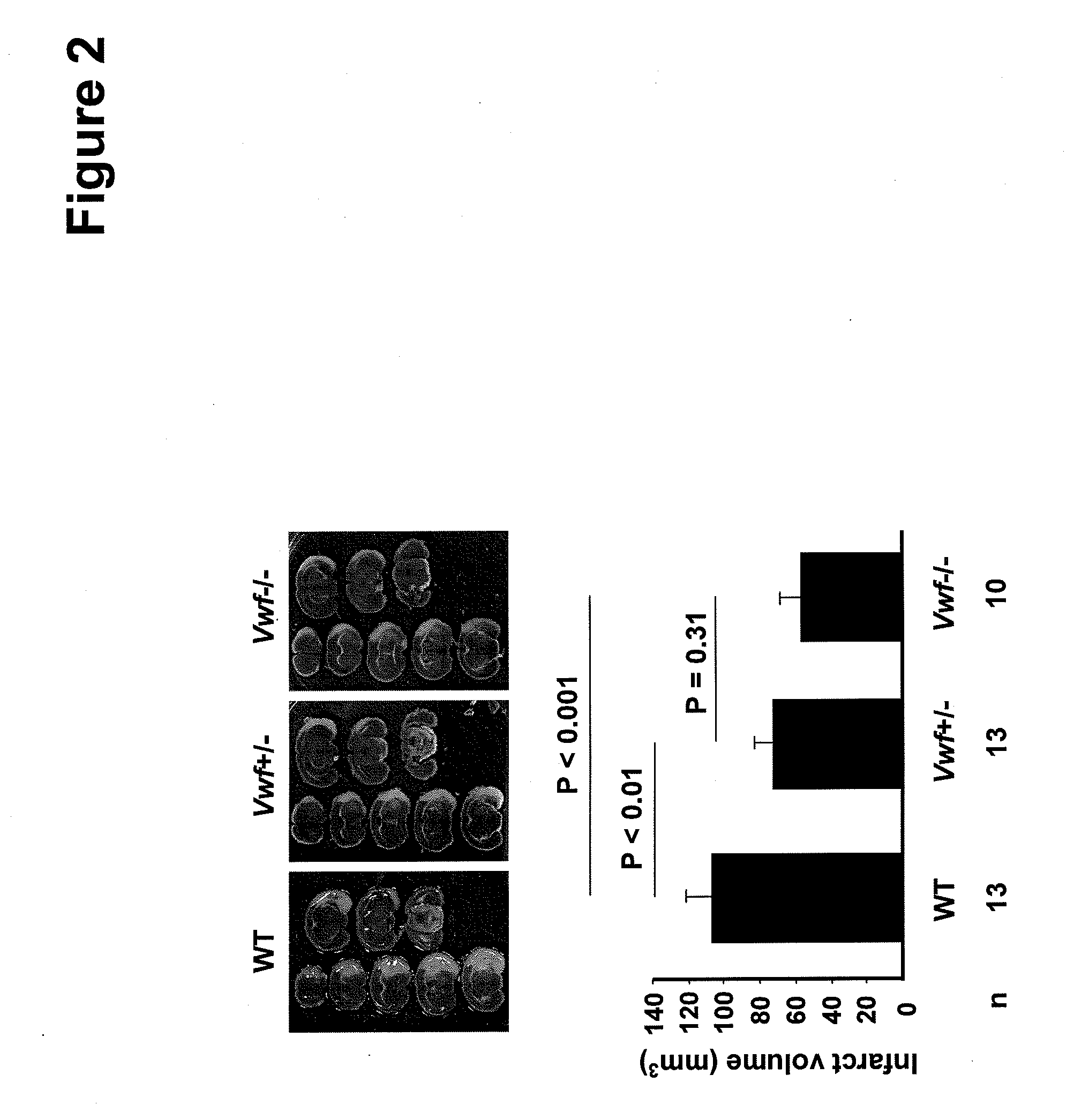
Von willebrand factor (VWF) inhibitors for treatment or prevention of infarction
InactiveUS20090317375A1Reduce capacitySuppress activityPeptide/protein ingredientsCardiovascular disorderFactor VIII vWFFactor ii
This invention relates to methods for treating or preventing an infarction by administering to a patient in need thereof a compound capable of suppressing the expression or activity of the von Willebrand Factor (VWF). Thus, the invention relates to the use of a pharmaceutically effective amount of a VWF inhibitor, such as ADAMTS13, for the preparation of a medicament for treating conditions known to involve infarction to reduce or eliminate the symptoms and effect of an infarction.
Owner:CHILDRENS MEDICAL CENT CORP
Factor VIII, von willebrand factor or complexes thereof with prolonged in vivo half-life
ActiveUS8575104B2Prolong half-life in vivoFactor VIIPeptide/protein ingredientsFactor VIII vWFHalf-life
The present invention relates to modified nucleic acid sequences coding for coagulation factor VIII (FVIII) and for von Willebrand factor (VWF) as well as complexes thereof and their derivatives, recombinant expression vectors containing such nucleic acid sequences, host cells transformed with such recombinant expression vectors, recombinant polypeptides and derivatives coded for by said nucleic acid sequences which recombinant polypeptides and derivatives do have biological activities together with prolonged in vivo half-life and / or improved in vivo recovery compared to the unmodified wild-type protein. The invention also relates to corresponding FVIII sequences that result in improved expression yield. The present invention further relates to processes for the manufacture of such recombinant proteins and their derivatives. The invention also relates to a transfer vector for use in human gene therapy, which comprises such modified nucleic acid sequences.
Owner:CSL BEHRING GMBH
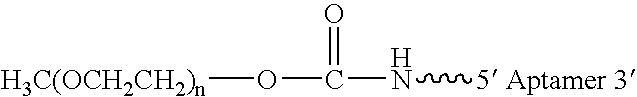
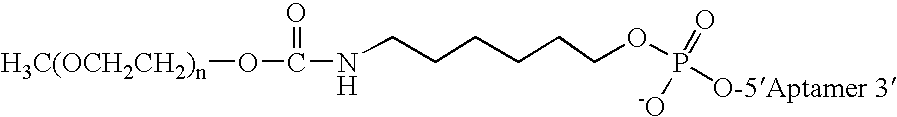
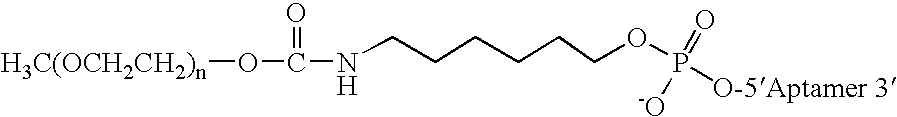
Aptamers to von Willebrand Factor and their use as thrombotic disease therapeutics
InactiveUS7566701B2Shorten bleeding timeInhibits platelet aggregationBiocideSugar derivativesFactor VIII vWFThrombus
The invention relates generally to the field of nucleic acids and more particularly to aptamers capable of binding to von Willebrand Factor useful as therapeutics in and diagnostics of thrombotic diseases and / or other diseases or disorders in which von Willebrand Factor mediated platelet aggregation has been implicated. The invention further relates to materials and methods for the administration of aptamers capable of binding to von Willebrand Factor.
Owner:ARCHEMIX CORP
Inactivation resistant factor VIII related applications
InactiveUS20020132306A1Reduce the possibilityLow costOrganic active ingredientsBiocideBinding siteENCODE
The present invention provides novel purified and isolated nucleic acid sequences encoding procoagulant-active FVIII proteins. The nucleic acid sequences of the present invention encode amino acid sequences corresponding to known human FVIII sequences, wherein residue Phe3O9 is mutated. The nucleic acid sequences of the present invention also encode amino acid sequences corresponding to known human FVIII sequences, wherein the APC cleavage sites, Arg336 and Ile562, are mutated. The nucleic acid sequences of the present invention further encode amino acid sequences corresponding to known human FVIII sequences, wherein the B-domain is deleted, the von Willebrand factor binding site is deleted, a thrombin cleavage site is mutated and an amino acid sequence spacer is inserted between the A2- and A3-domains. Methods of producing the FVIII proteins of the invention, nucleotide sequences encoding such proteins, pharmaceutical compositions containing the nucleotide sequences or proteins, as well as methods of treating patients suffering from hemophilia, are also provided.
Owner:RGT UNIV OF MICHIGAN
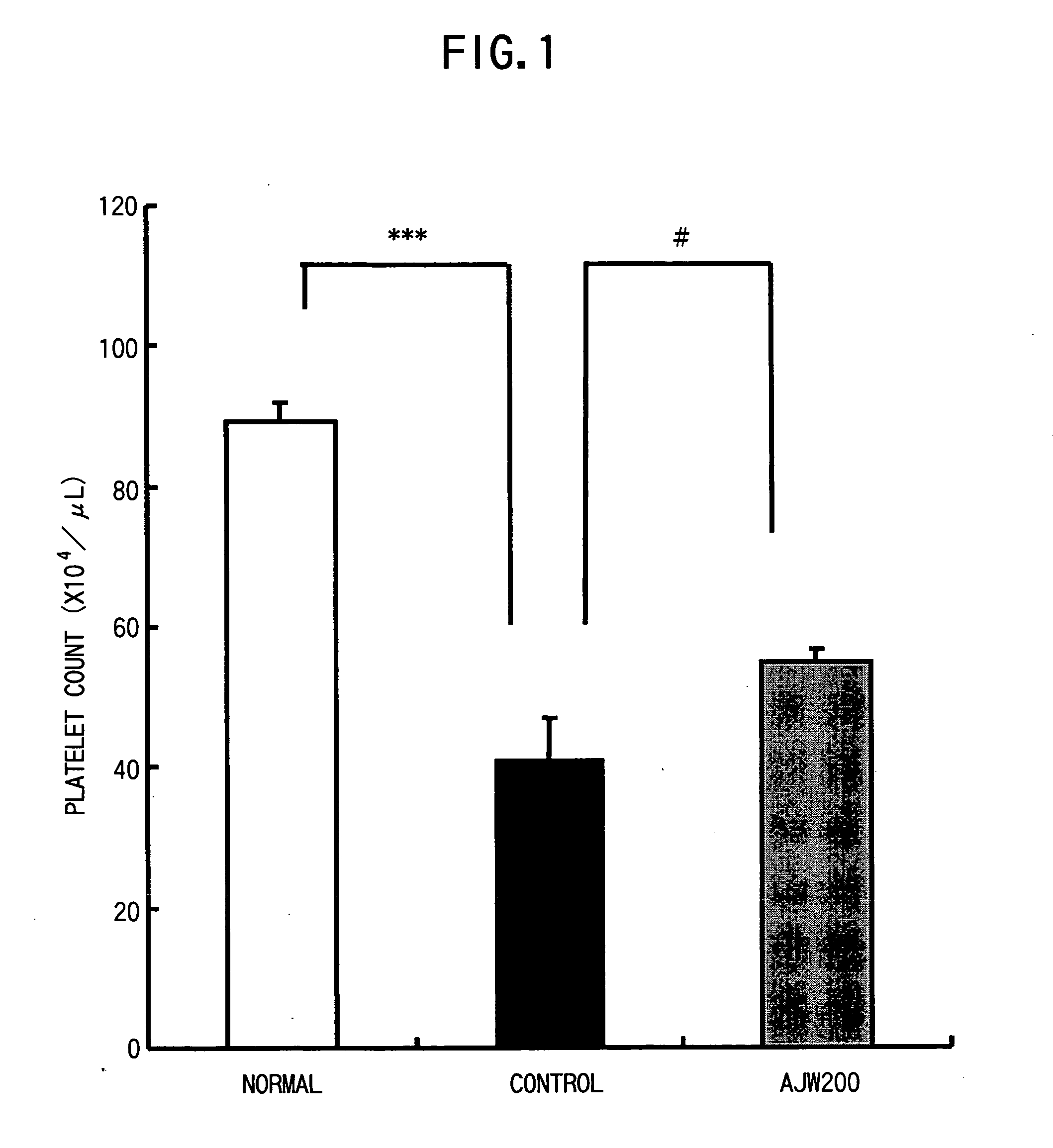
Pharmaceutical composition for the treatment of thrombocytopenia
InactiveUS20050136056A1Shorten the counting processImprove bindingImmunoglobulins against blood coagulation factorsImmunoglobulins against cell receptors/antigens/surface-determinantsGlycoprotein IbFactor VIII vWF
The present invention provides a drug for the treatment of thrombocytopenia caused by hepatic failure, preferably a drug with few adverse drug reactions. A substance that inhibits binding between glycoprotein Ib (GPIb) and von Willebrand factor (vWF), for example, anti-GPIb antibody or anti-vWF antibody that inhibits binding between GPIb and vWF is an active ingredient of the drug for the treatment of thrombocytopenia.
Owner:AJINOMOTO CO INC
Aptamers to von Willebrand Factor and their use as thrombotic disease therapeutics
InactiveUS20060264369A1Shorten bleeding timeInhibits platelet aggregationPeptide/protein ingredientsGenetic material ingredientsAptamerDisease
The invention relates generally to the field of nucleic acids and more particularly to aptamers capable of binding to von Willebrand Factor useful as therapeutics in and diagnostics of thrombotic diseases and / or other diseases or disorders in which von Willebrand Factor mediated platelet aggregation has been implicated. The invention further relates to materials and methods for the administration of aptamers capable of binding to von Willebrand Factor.
Owner:ARCHEMIX CORP
Inactivation resistant factor VIII
InactiveUS20060293238A1Increase secretionHigh expressionFactor VIIPeptide/protein ingredientsBinding siteENCODE
The present invention provides novel purified and isolated nucleic acid sequences encoding procoagulant-active FVIII proteins. The nucleic acid sequences of the present invention encode amino acid sequences corresponding to known human FVIII sequences, wherein residue Phe309 is mutated. The nucleic acid sequences of the present invention also encode amino acid sequences corresponding to known human FVIII sequences, wherein the APC cleavage sites, Arg336 and Ile562, are mutated. The nucleic acid sequences of the present invention further encode amino acid sequences corresponding to known human FVIII sequences, wherein the B-domain is deleted, the von Willebrand factor binding site is deleted, a thrombin cleavage site is mutated, an amino acid sequence spacer is inserted between the A2- and A3-domains. Methods of producing the FVIII proteins of the invention, nucleotide sequences encoding such proteins, pharmaceutical compositions containing the nucleotide sequences or proteins, as well as methods of treating patients suffering from hemophilia, are also provided.
Owner:RGT UNIV OF MICHIGAN
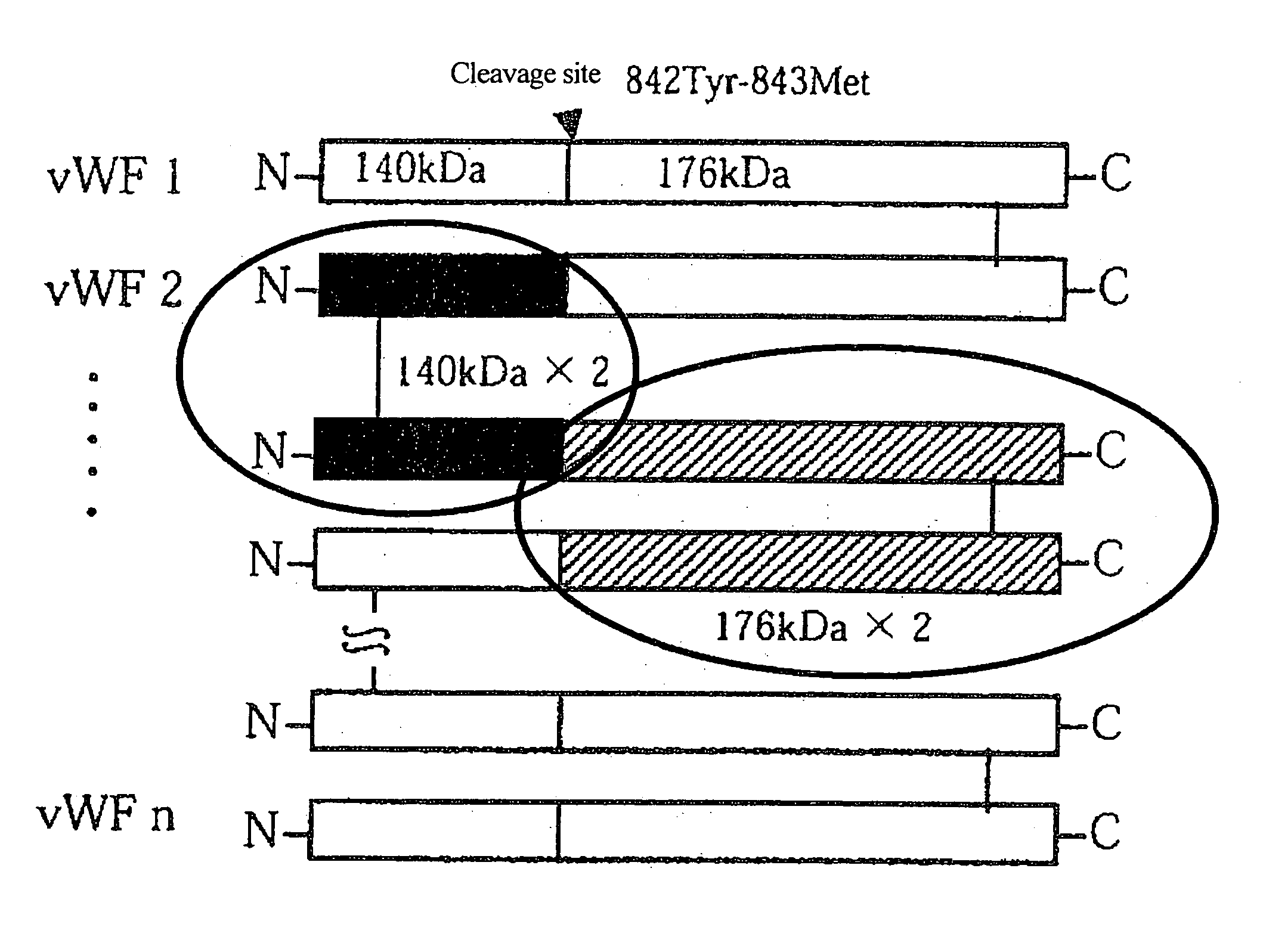
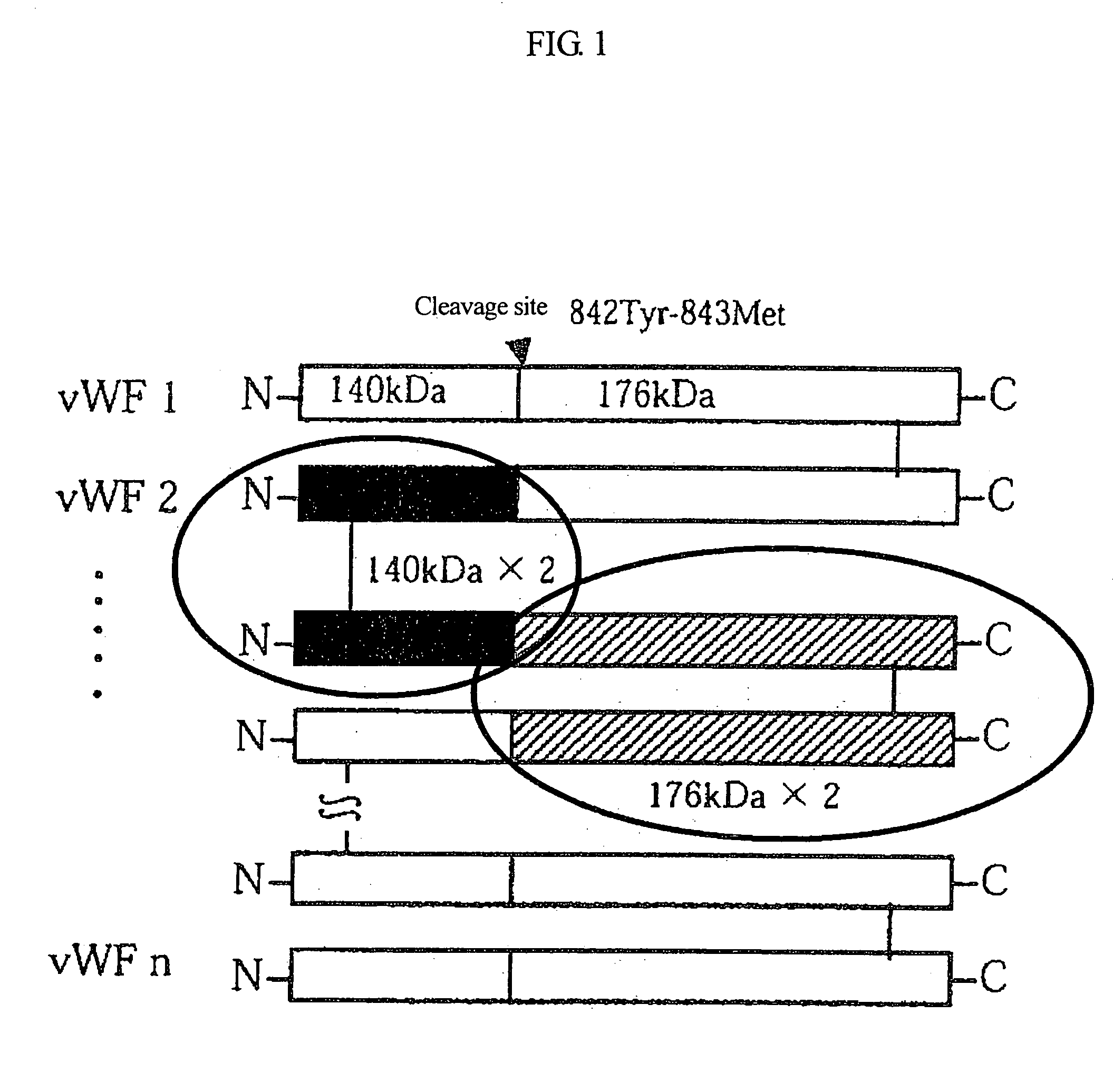
Von Willebrand Factor (Vwf)-cleaving protease
This invention is intended to isolate and identify a vWF-specific cleaving protease.The vWF-specific cleaving protease cleaves a bond between residues Tyr 842 and Met 843 of vWF and comprises a polypeptide chain having Leu-Leu-Val-Ala-Val as a partial sequence, and more preferably comprises a polypeptide chain having the partial N-terminal amino acid sequence of a mature protein, Ala-Ala-Gly-Gly-Ile-Leu-His-Leu-Glu-Leu-Leu-Val-Ala-Val, and having a molecular weight of 105 to 160 kDa in SDS-PAGE under reducing or non-reducing conditions. Isolation and identification of this vWF-specific cleaving protease have led to the possibility of replacement therapy for patients having diseases resulting from a deficiency of the protease, such as thrombotic thrombocytopenic purpura.
Owner:KM BIOLOGICS CO LTD
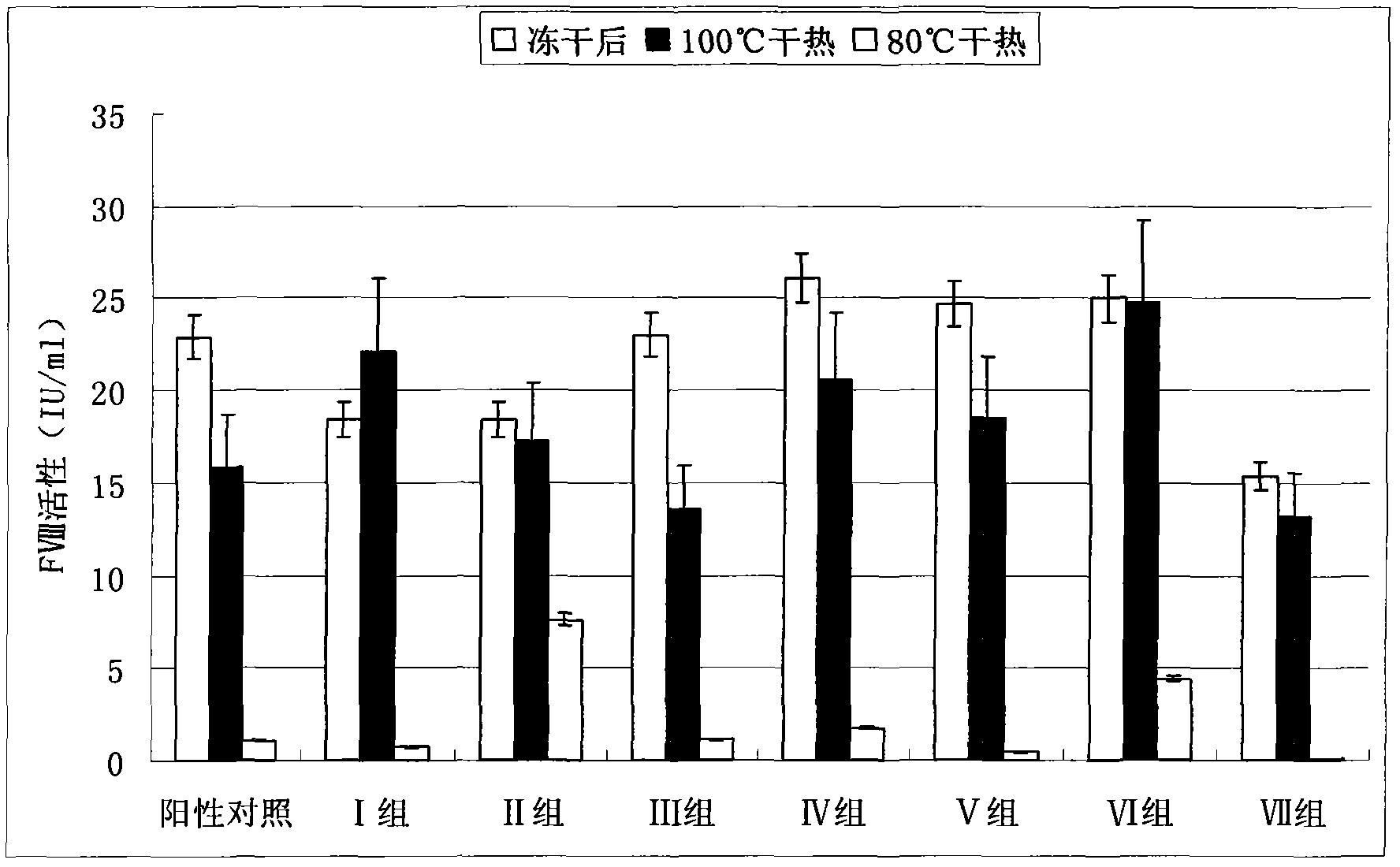
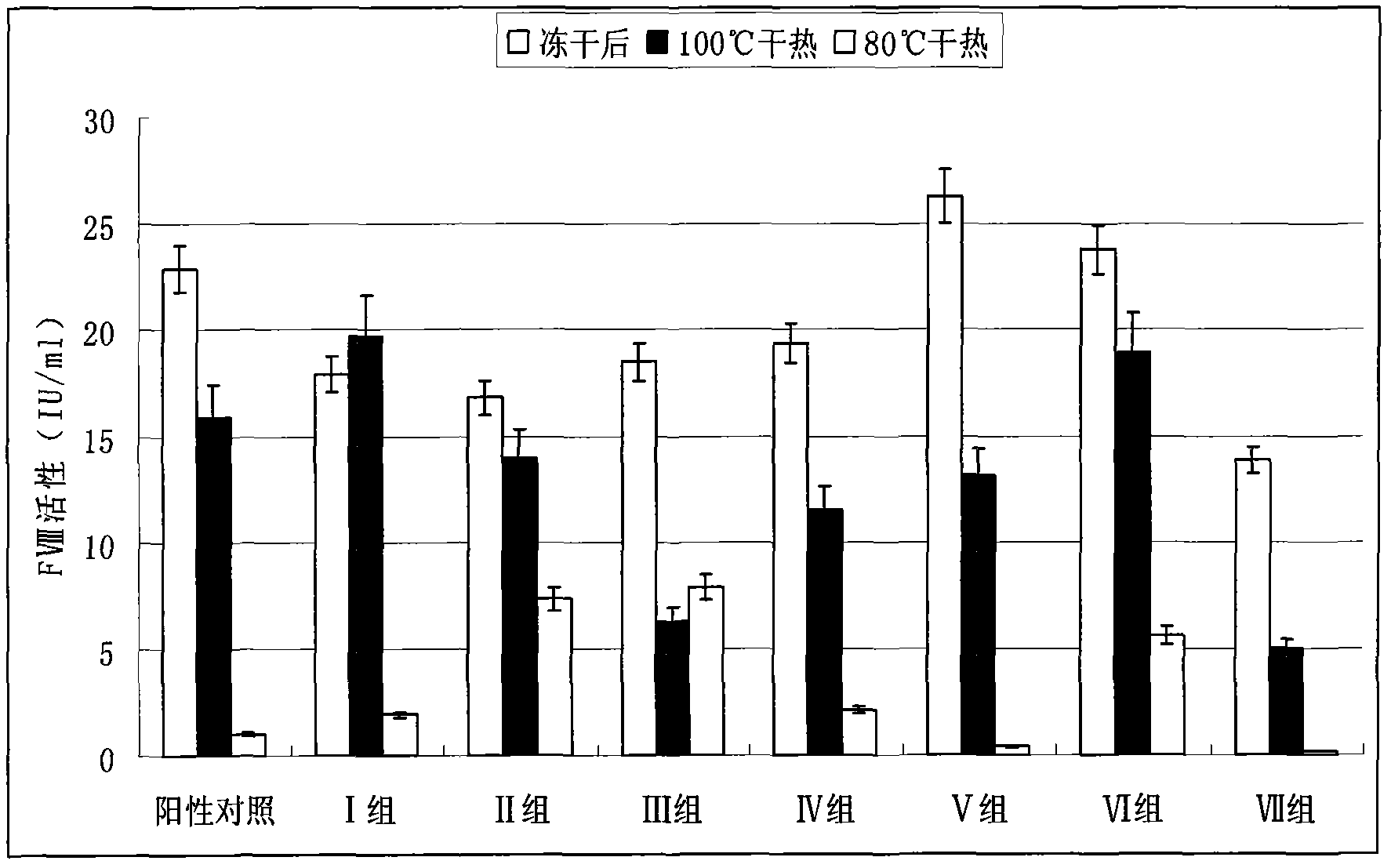
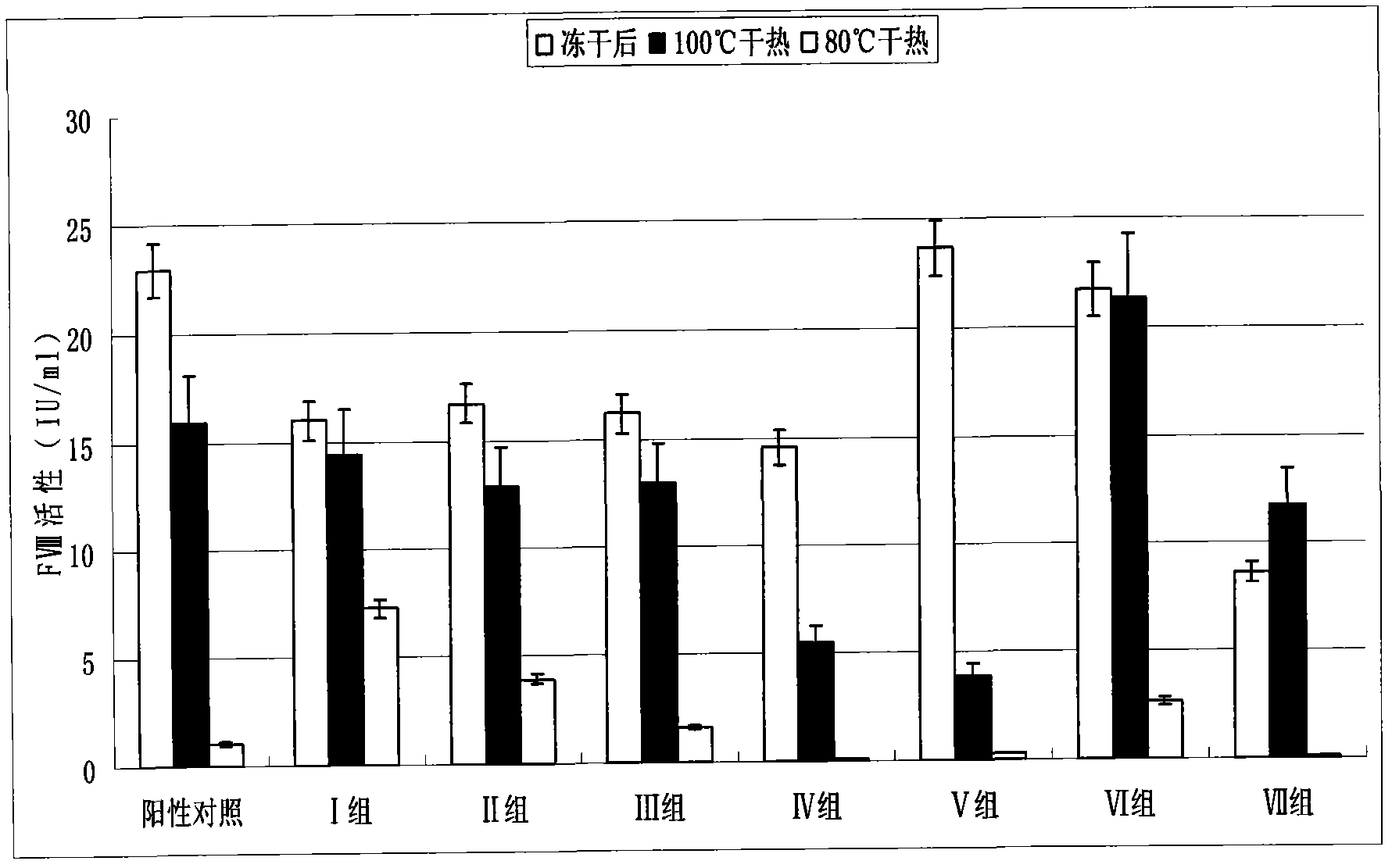
Dry heat treatment stabilizing agent for human coagulation factor VIII and vWF (von willebrand factor) compound or human coagulation factor VIII preparation
ActiveCN102580062ALittle loss of activityIncreased loss of activityPeptide/protein ingredientsPharmaceutical non-active ingredientsSucroseFreeze-drying
The invention discloses a dry heat treatment stabilizing agent for a human coagulation factor VIII and vWF compound or a human coagulation factor VIII preparation. The stabilizing agent of the invention comprises histidine or its salt, arginine or its salt, lysine or its salt, mannitol, mycose, and sucrose, and also can comprise one or several of common glycine, sucrose, common salt, calcium chloride, sodium citrate, and heparins. Experiments prove that the human coagulation factor VIII and vWF compound or the human coagulation factor VIII preparation contains 0.1-10% of histidine or its salt, 0.1-10% of arginine or its salt, and one or several of 0.1-10% of lysine or its salt, 0.1-10% of glycine, 0.1-10% of mannitol, 0.1-10% of sucrose, and 0.1-10% of mycose, so the human coagulation factor VIII and vWF compound or the human coagulation factor VIII preparation can effectively inactivate viruses under a 80-100DEG C dry heat environment, can effectively protect the activity of the human coagulation factor VIII, and has a qualified freeze-drying appearance and a redissolving appearance. So the stabilizing agent of the invention can be used as the dry heat treatment stabilizing agent for the human coagulation factor VIII and vWF (von willebrand factor) compound or the human coagulation factor VIII preparation.
Owner:BLOOD TRASFUSION INST CHINESE ACAD OF MEDICAL SCI
Recombinant vwf formulations
InactiveUS20090192076A1Improve stabilityUseful in therapyPeptide/protein ingredientsInorganic non-active ingredientsFactor VIII vWFPharmaceutical formulation
Owner:BAXALTA GMBH
Recombinant expression vector system for variants of coagulation factor VIII and von Willebrand factor
ActiveUS20100183556A1Good coagulationReduce the possibilityOrganic active ingredientsBiocideFactor iiExon
Disclosed is an expression vector system for variants of coagulation factor VIII (FVIII) and von Willebrand factor (vWF). In detail, mutant vWF the size of which is significantly reduced by deleting exons but which has remarkably increased FVIII stabilizing and activating efficiency, and an expression vector system useful for the treatment of hemophilia which is capable of expressing the same along with FVIII are disclosed. Use of the mutant vWF with a reduced size enables effective expression of FVIII in a viral vector and significantly enhanced FVIII activity. Further, the viral vector may be effectively used to treat hemophilia through gene therapy.
Owner:KOREA UNIV IND & ACADEMIC CALLABORATION FOUND
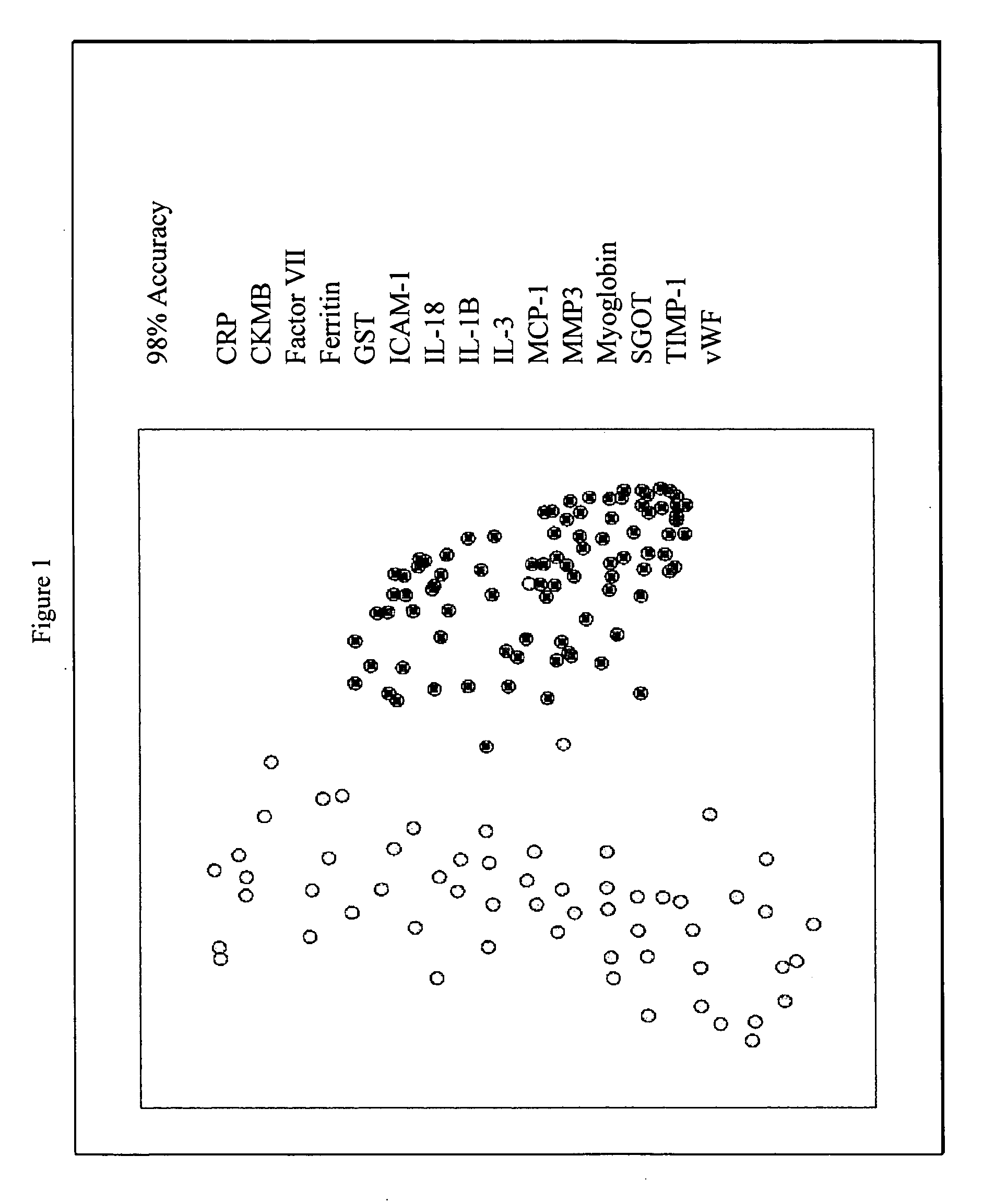
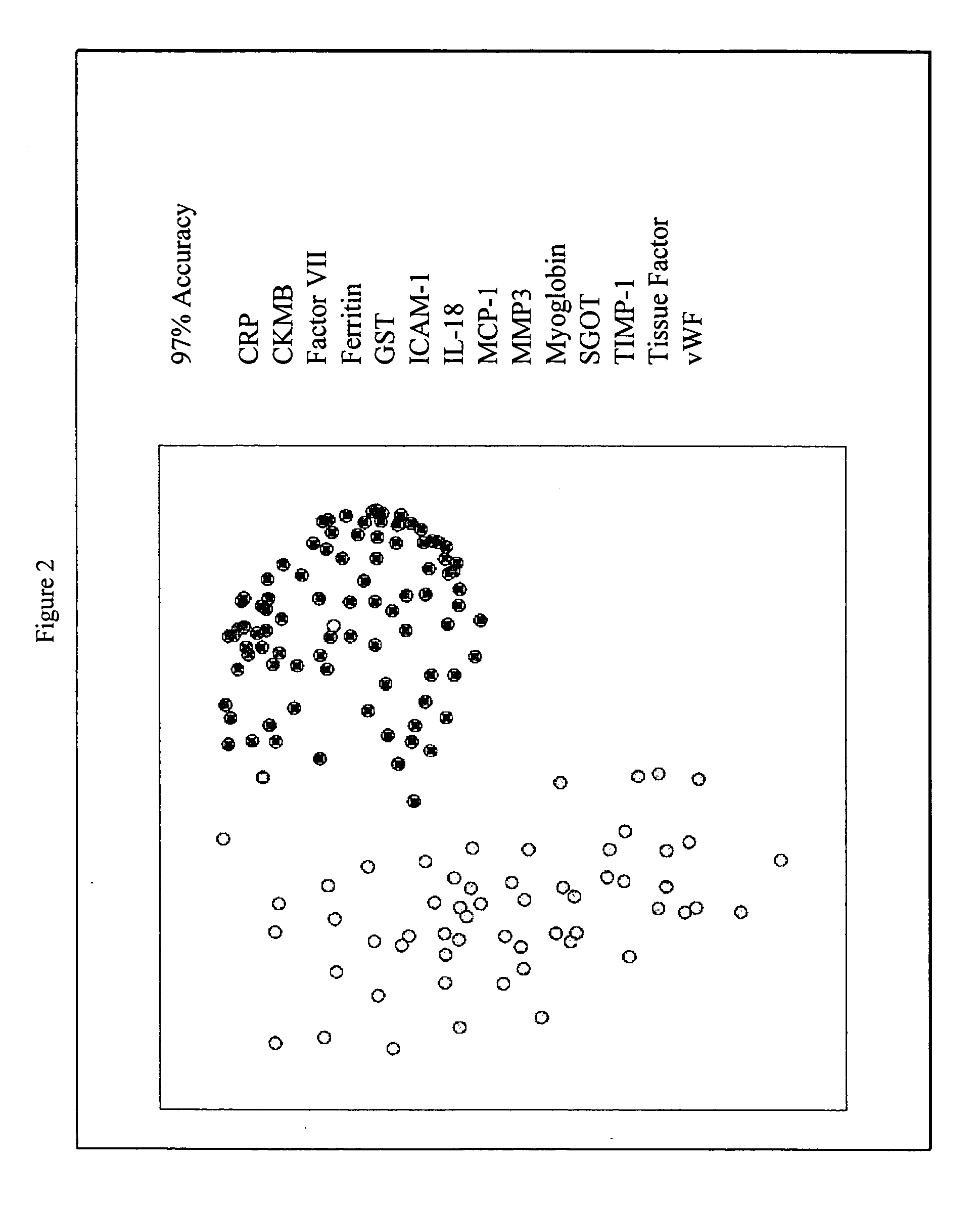
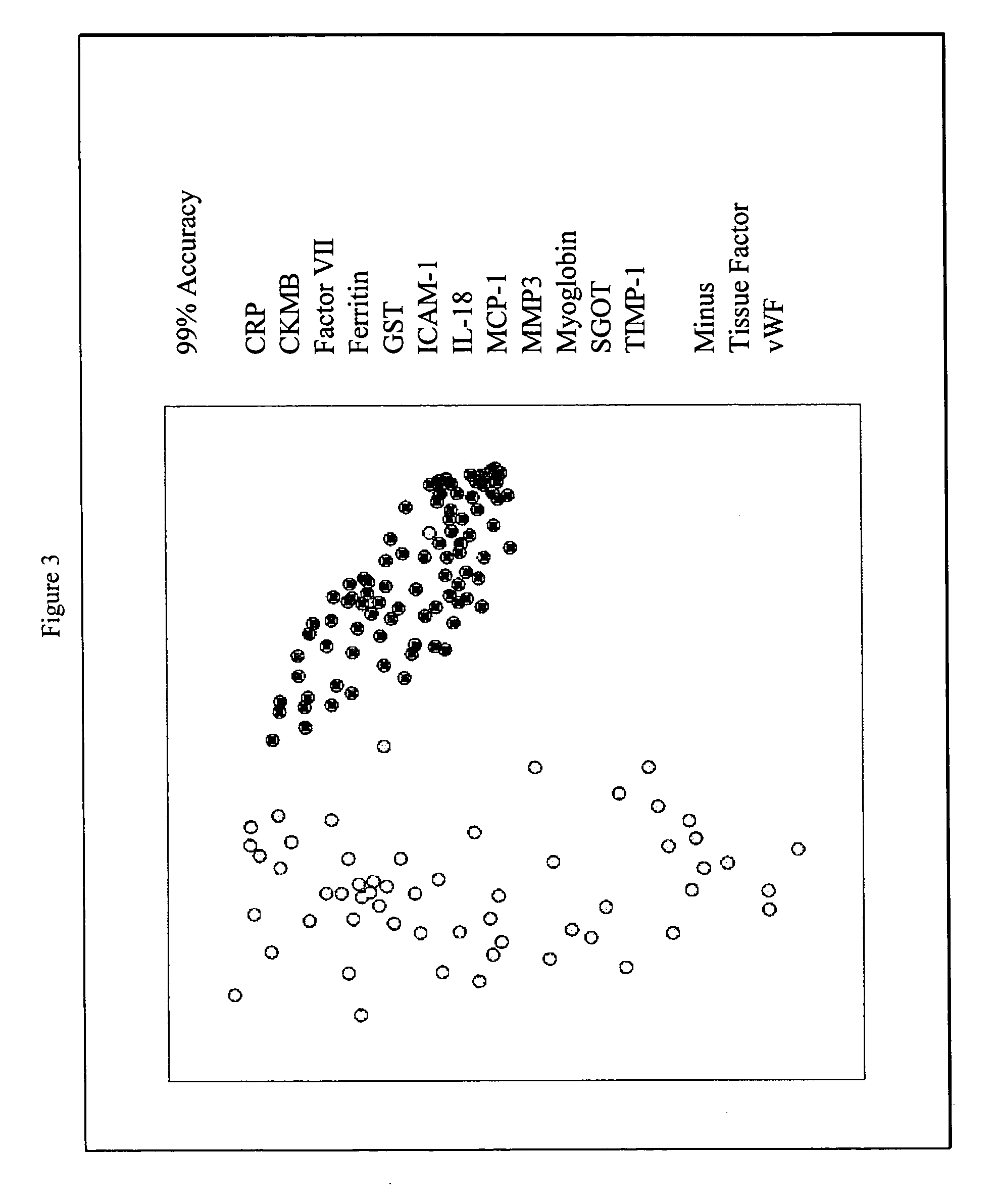
Methods and kits for the diagnosis of acute coronary syndrome
InactiveUS20070003981A1Quick checkAccurate diagnosisMicrobiological testing/measurementDisease diagnosisComplement 3Factor VII
Provided are methods for the detection and diagnosis of acute coronary syndrome or ACS. The methods are based on the discovery that abnormal levels of selected analytes in sample fluid, typically blood samples, of patients who are at risk are supportive of a diagnosis of ACS. At least two new biomarkers for ACS are thus disclosed, MMP-3 and SGOT. Altogether the concentrations of twelve analytes provide a sensitive and selective picture of the patient's condition, namely, whether the patient is suffering a heart attack. Other important biomarkers for ACS are described, including but not limited to IL-18, Factor VII, ICAM-1, Creatine Kinase-MB, MCP-1, Myoglobin, C Reactive Protein, von Willebrand Factor, TIMP-1, Ferritin, Glutathione S-Transferase, Prostate Specific Antigen (free), IL-3, Tissue Factor, alpha-Fetoprotein, Prostatic Acid Phosphatase, Stem Cell Factor, MIP-1-beta, Carcinoembryonic Antigen, IL-13, TNF-alpha, IgE, Fatty Acid Binding Protein, ENA-78, IL-1-beta, Brain-Derived Nerotrophic Factor, Apolipoprotein A1, Serum Amyloid P, Growth Hormone, Beta-2 microglobulin, Lipoprotein (a), MMP-9, Thyroid Stimulating hormone, alpha-2 Macroglobulin, Complement 3, IL-7, Leptin, and IL-6. Kits containing reagents to assist in the analysis of fluid samples are also described.
Owner:RULES BASED MEDICINE
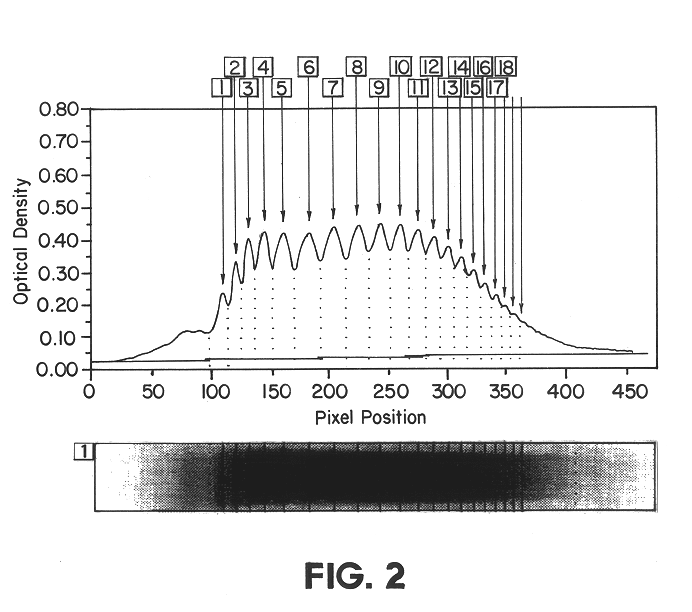
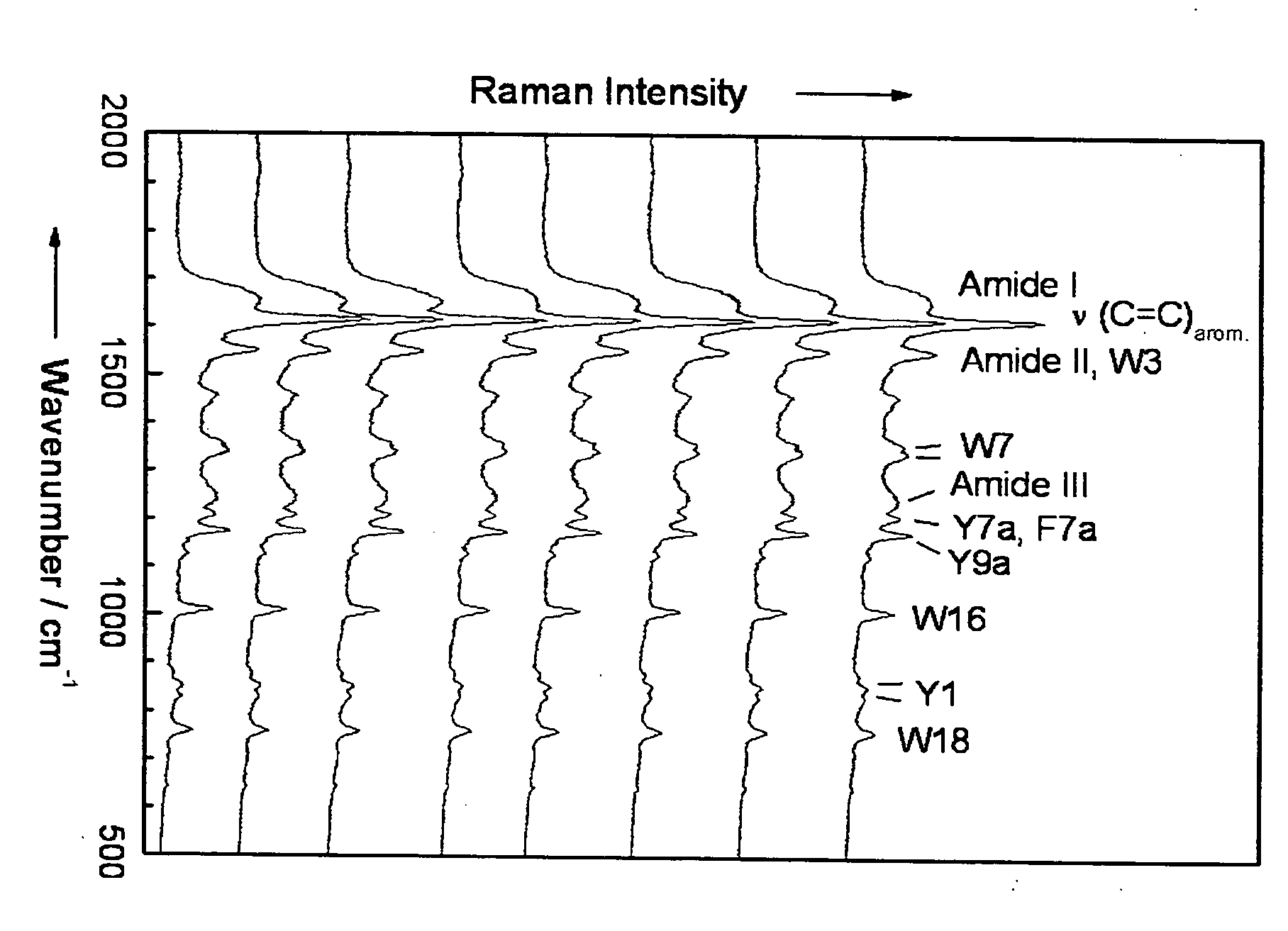
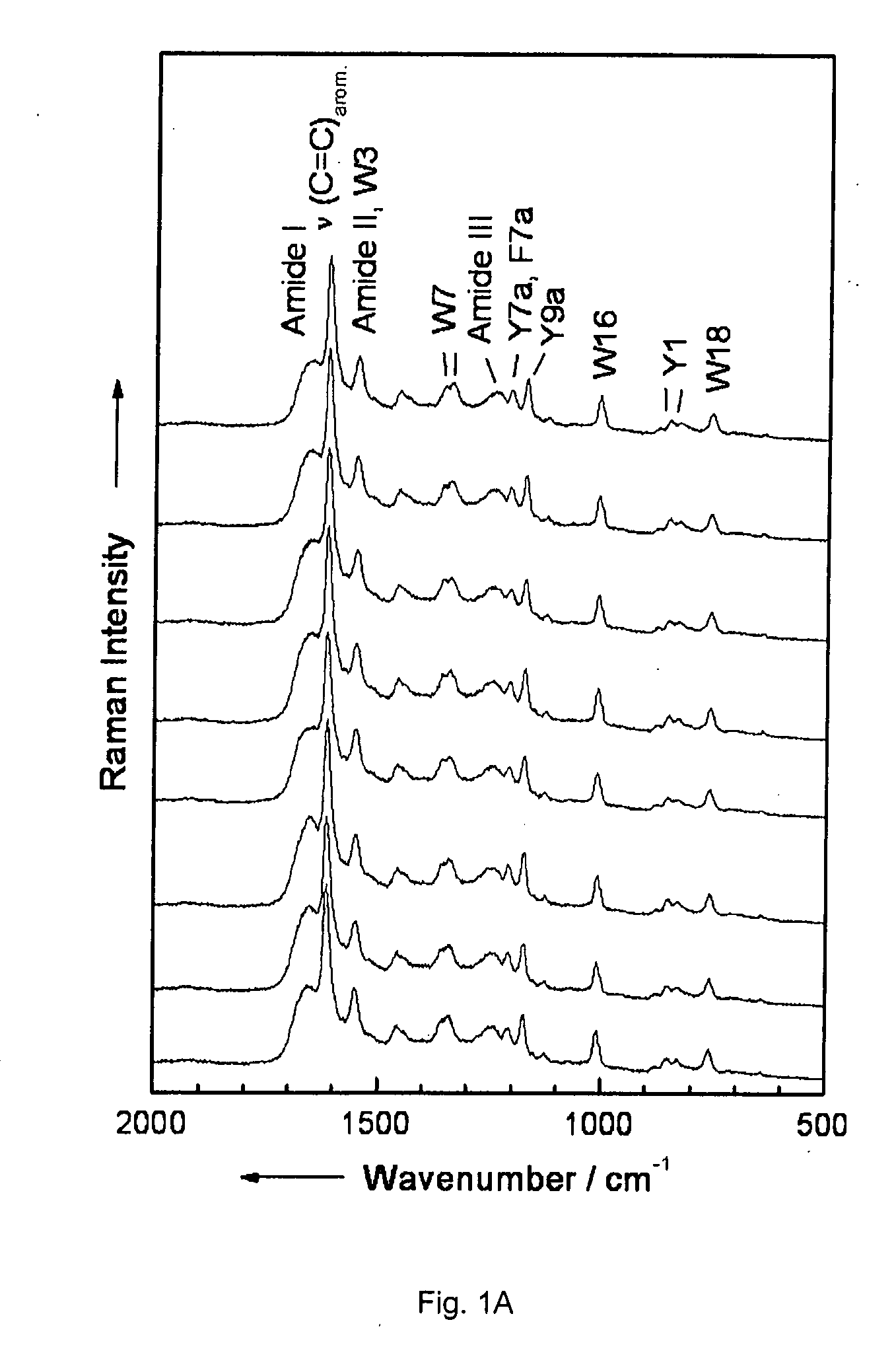
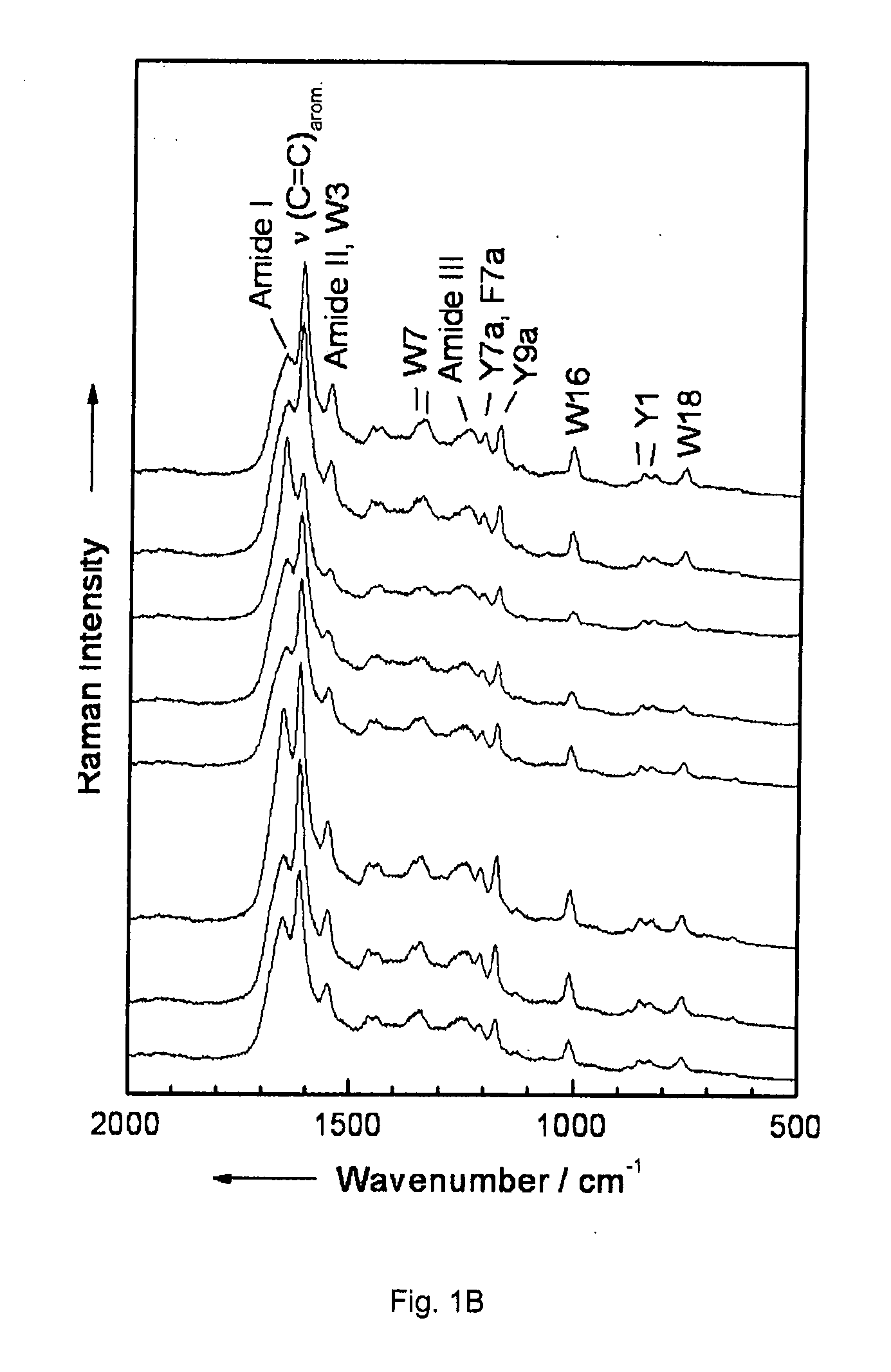
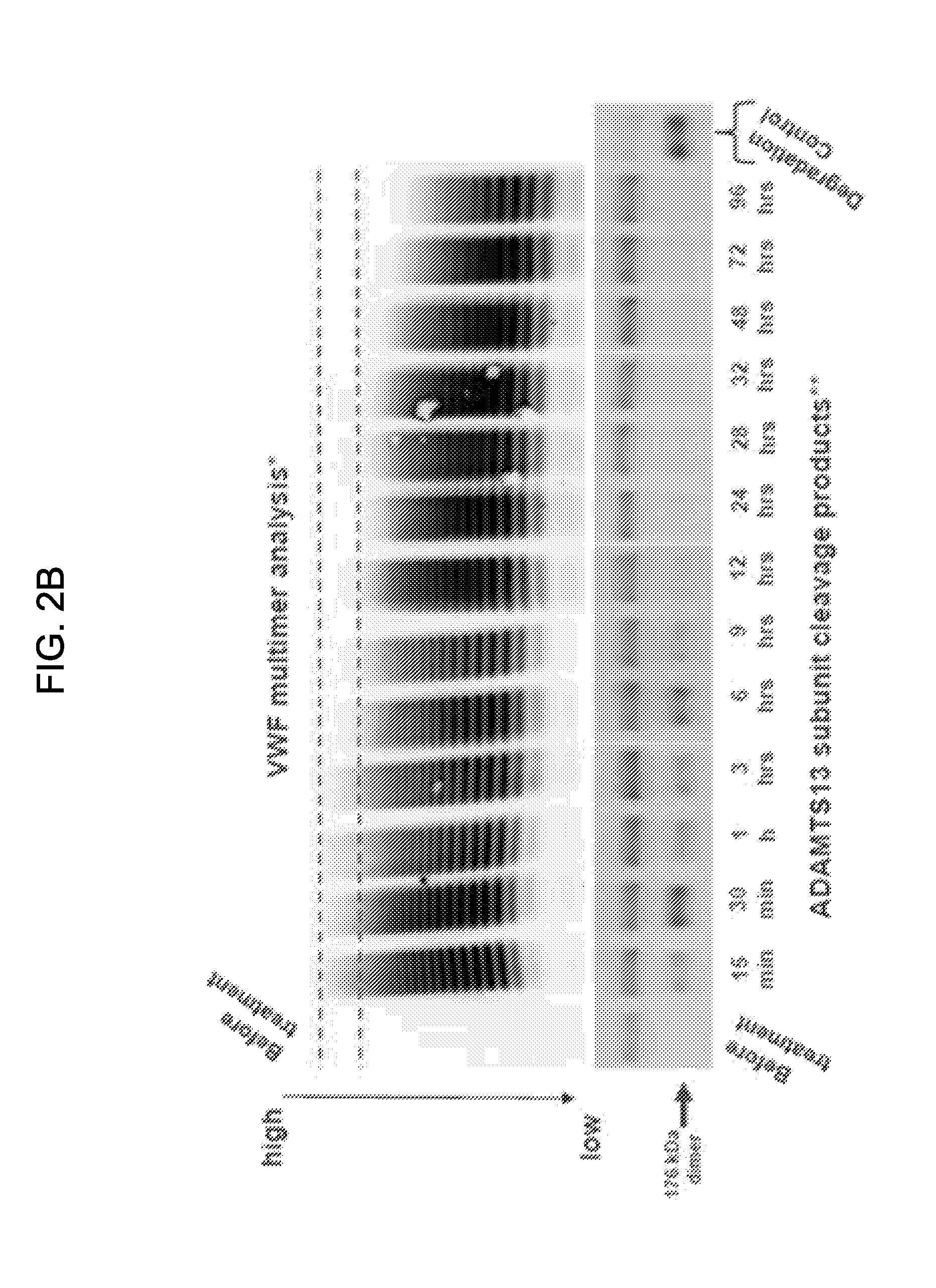
Diagnostic tool detecting the degradation status of Von Willebrand Factor multimers
InactiveUS20080306346A1Quick checkQuick identificationDiagnostics using lightDrug and medicationsDiseaseHuman body
A method in which the cleavage profile and size distribution of von Willebrand factor (VWF) multimers is analyzed, includes: providing a sample medium of human body fluids comprising a plurality of VWF multimers of different size; enrichment or purification of the VWF multimers by cryoprecipitation or chromatography to obtain a separated preparation of the VWF multimers from said sample medium; exposing the separated preparation of VWF multimers to a light source to produce signals obtained by vibrational spectroscopy; detecting said signals; transformation by mathematical alogrithms; generation of patterns based on computing of data of original resonance spectra and determining the cleavage profile and the size distribution of said separated VWF multimers by chemometrics; and acquisition of a databank obtained from healthy individuals for identifying subjects at risk of developing at least one of the following diseases: sepsis, coagulopathy, thrombotic disease, infection, and inflammation.
Owner:BOCKMEYER CLEMENS +2
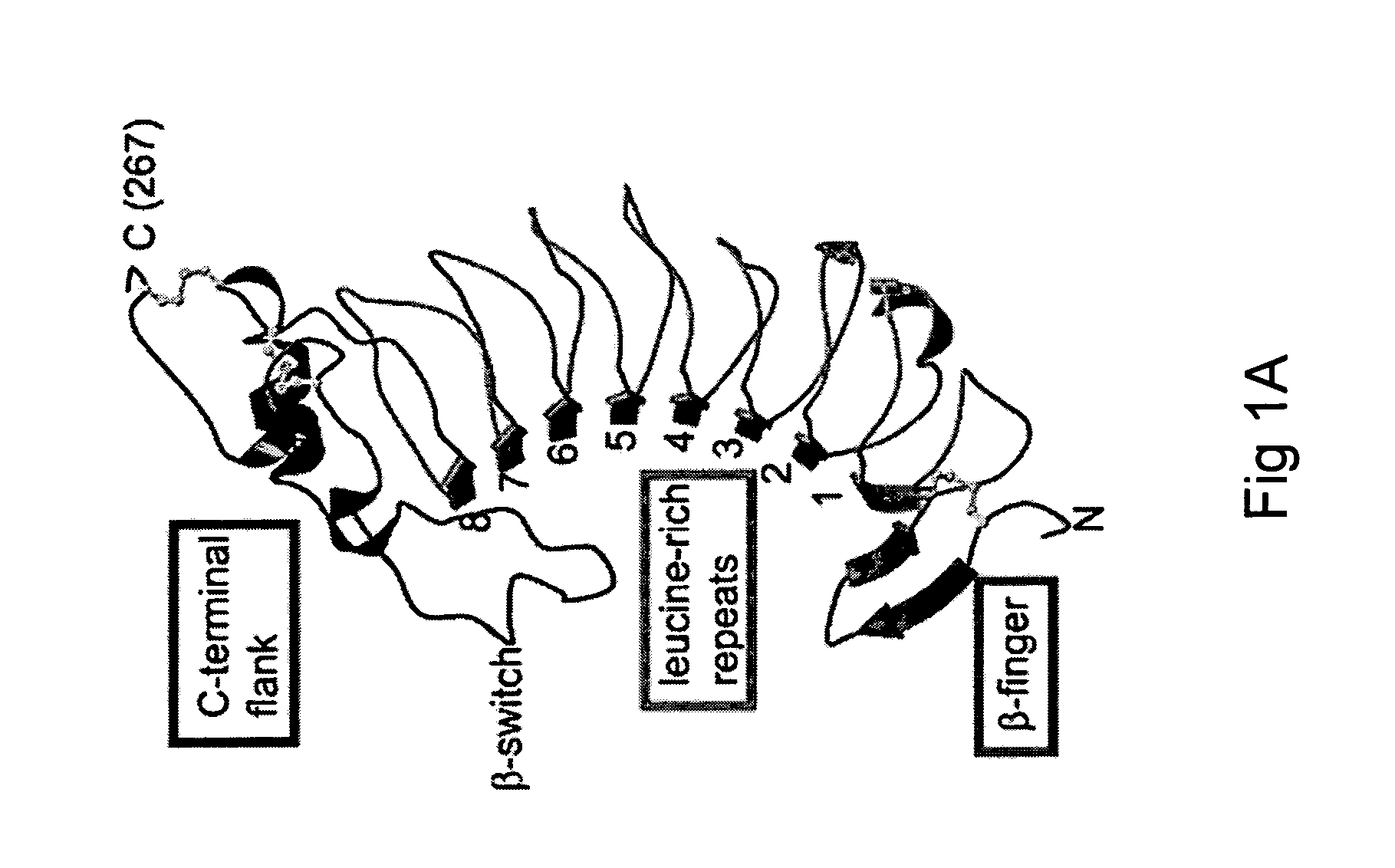
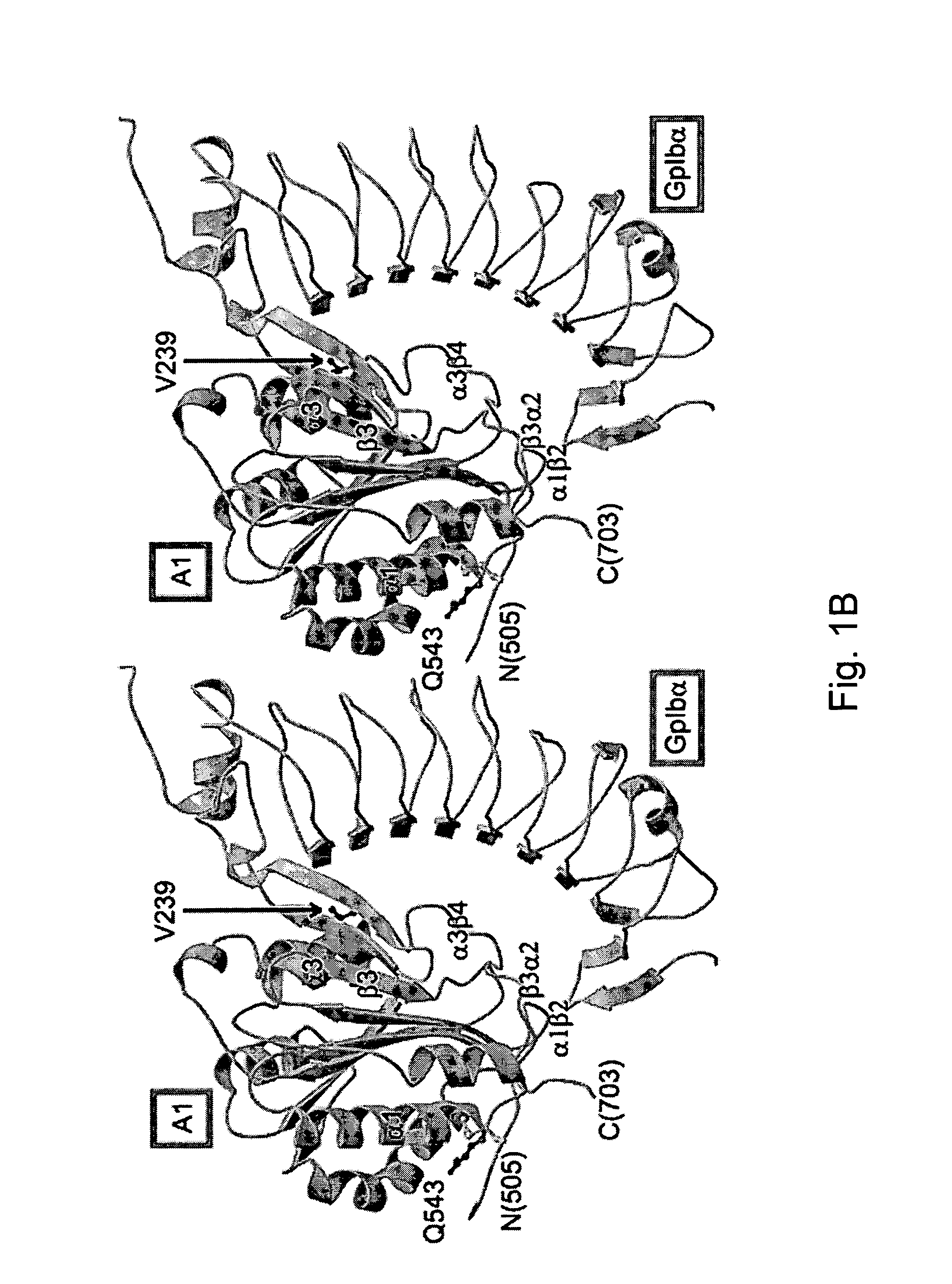
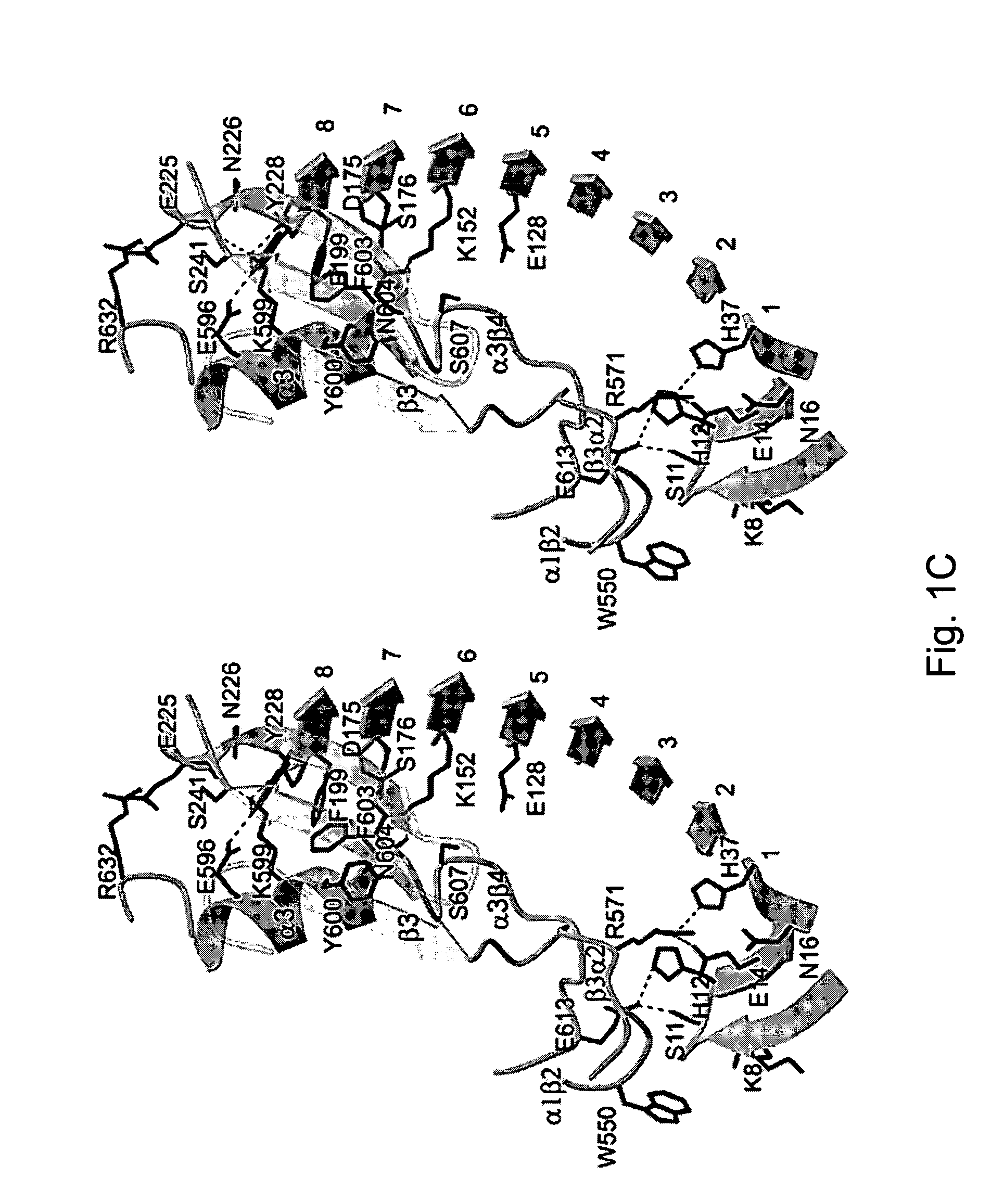
Modulation platelet adhesion based on the surface-exposed beta-switch loop of platelet glycoprotein IB-alpha
ActiveUS20050192224A1Trend downReduce decreaseFactor VIICell receptors/surface-antigens/surface-determinantsGlycoprotein IbChemistry
The invention relates to the adhesion of platelet GpIbα to strand β3 of domain A1 of von Willebrand factor (vWF), the strand β3 comprising amino acid residues at amino acid position 560-566 and / or a functional part or equivalent thereof, the platelet GpIbα, the GpIbα region comprising an amino acid sequence corresponding to a beta-switch loop of platelet GpIbα, comprising amino acid residues at amino acid position 227-242 and / or a functional part or equivalent thereof. The invention provides a method of interfering with adhesion of blood platelets to vWF that includes modulating adhesion. The invention further provides proteinaceous compounds, antibodies, medicaments and pharmaceutical compositions to that end. The invention also provides means and methods to increase platelet adhesion by topical application of a compound increasing platelet adhesion.
Owner:ABLYNX NV
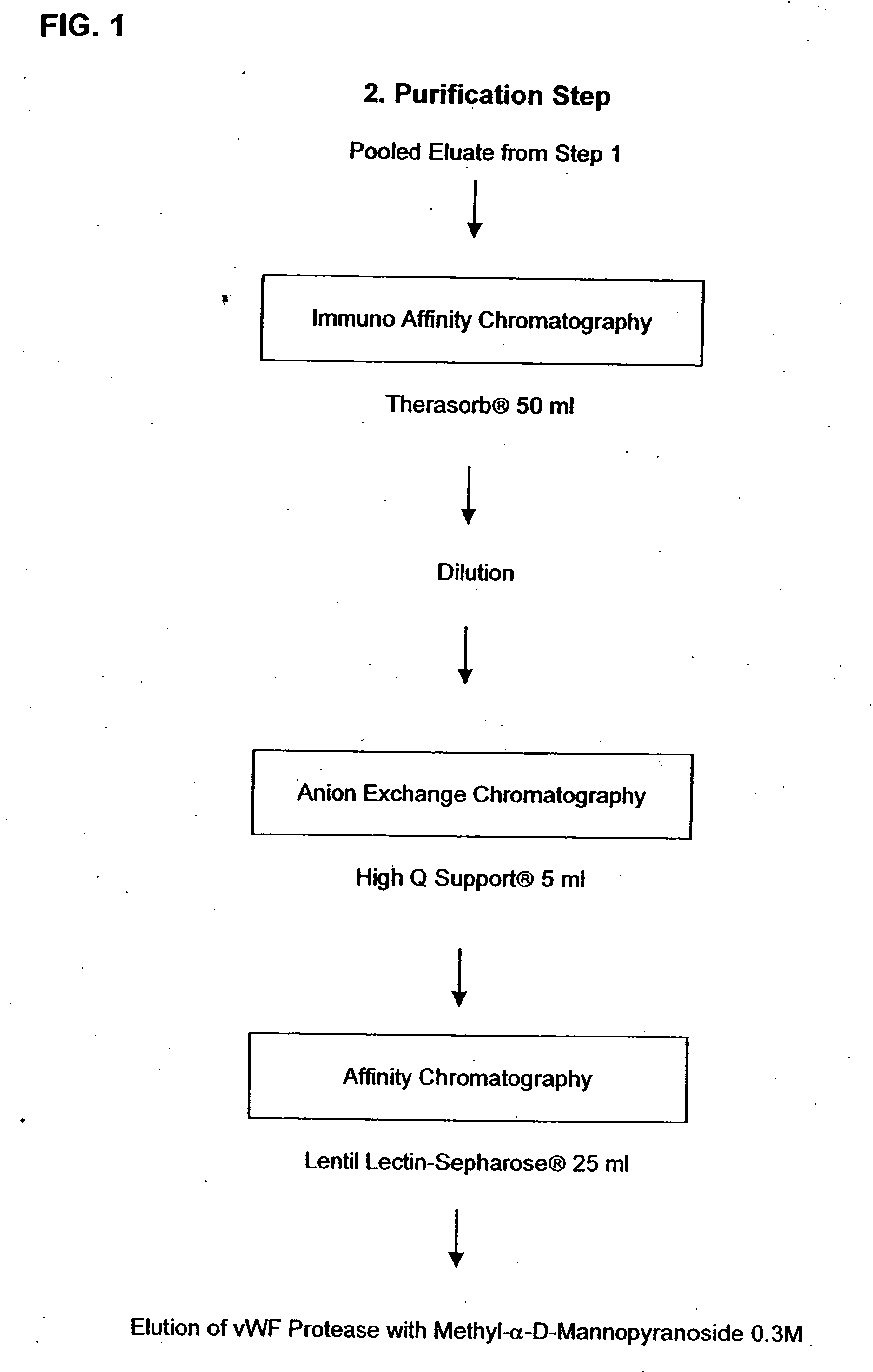
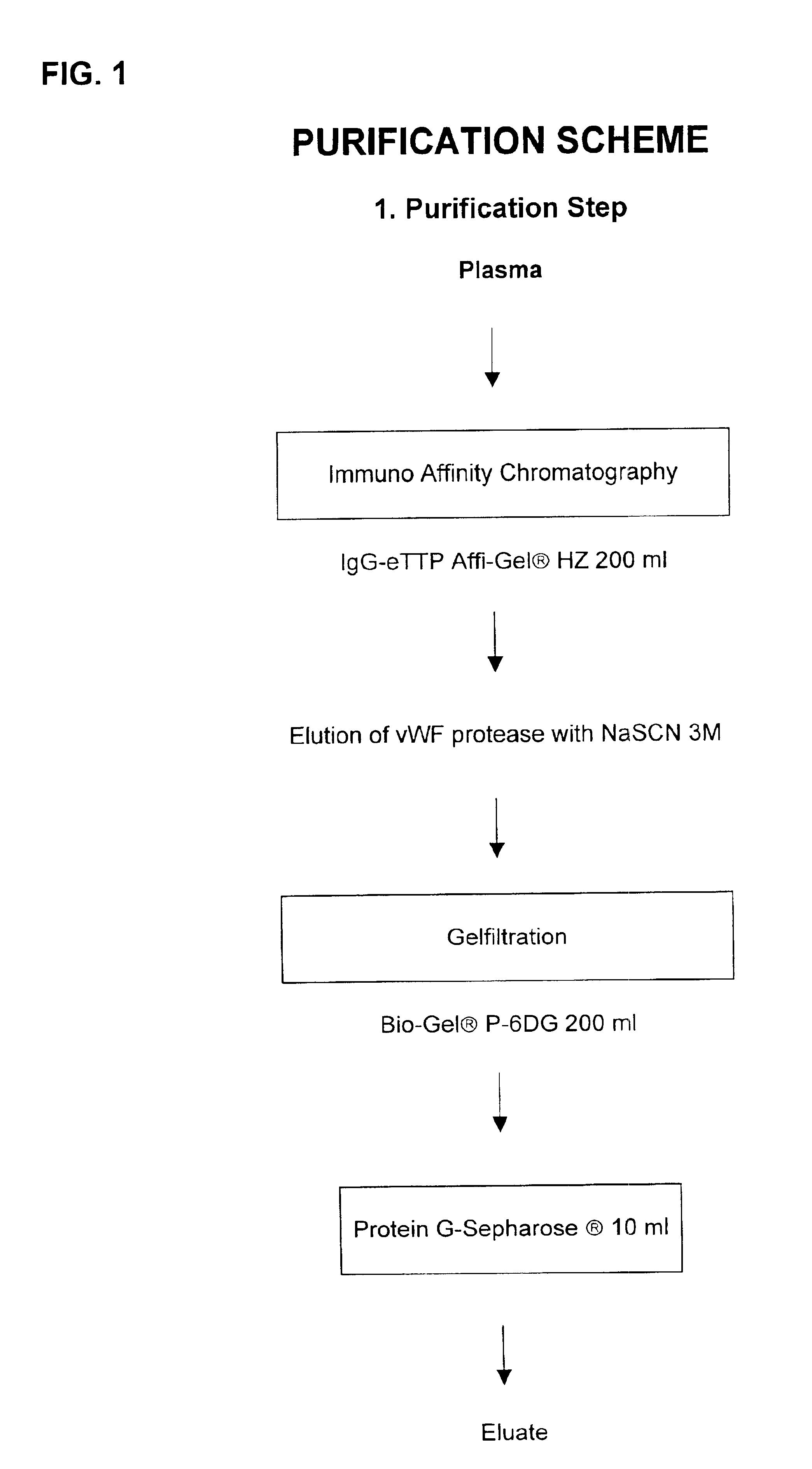
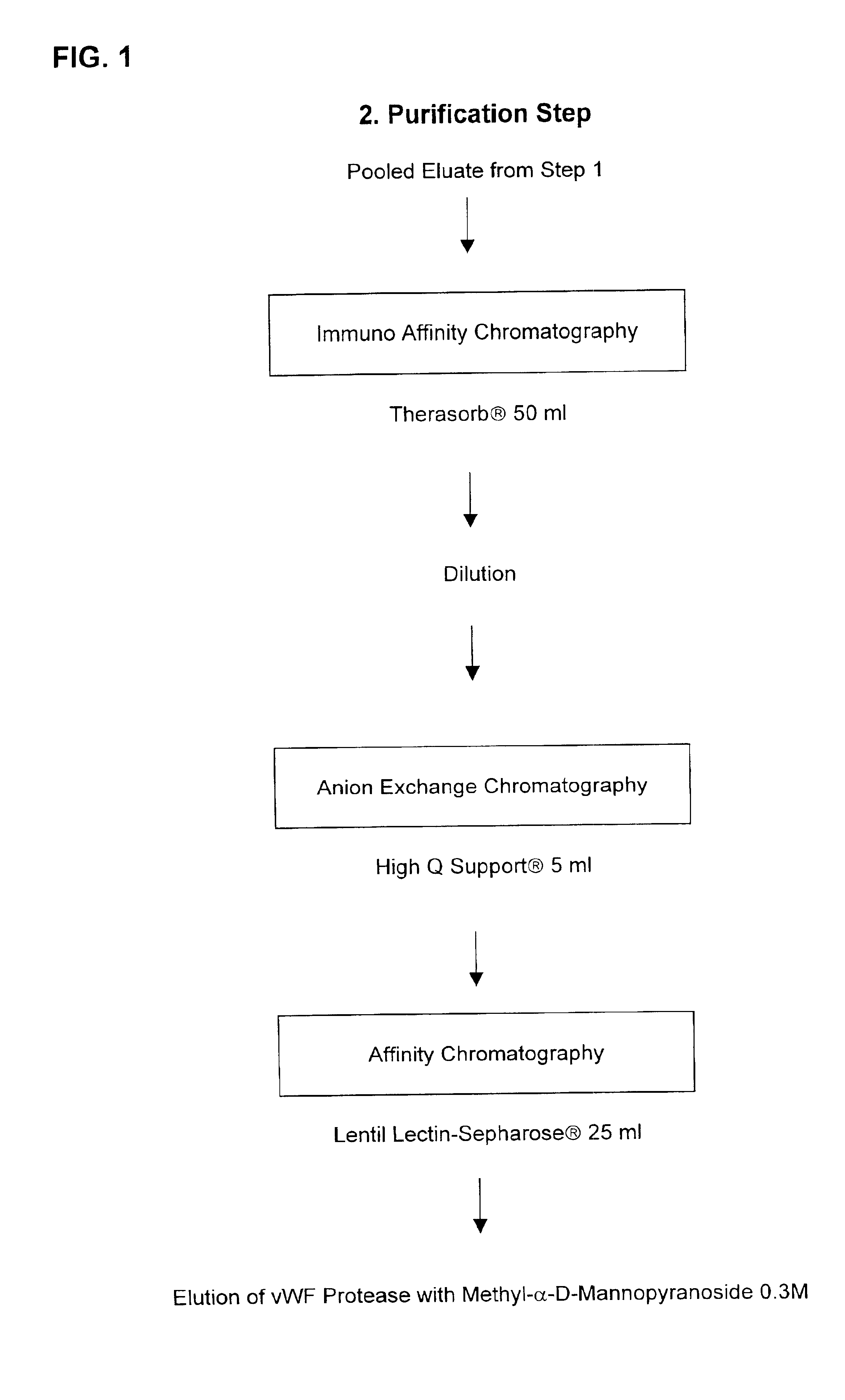
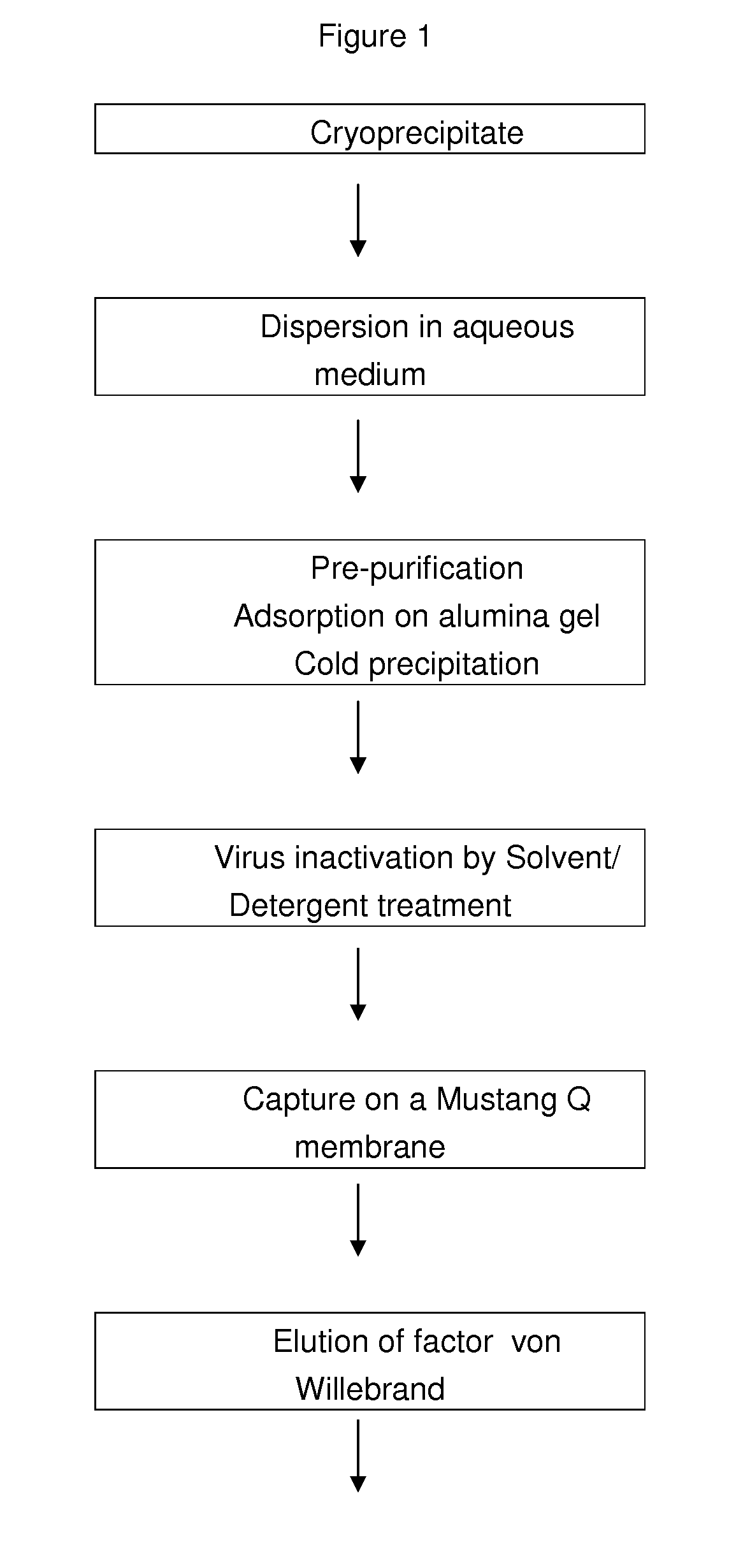
Method for purifying factor viii and von willebrand factor
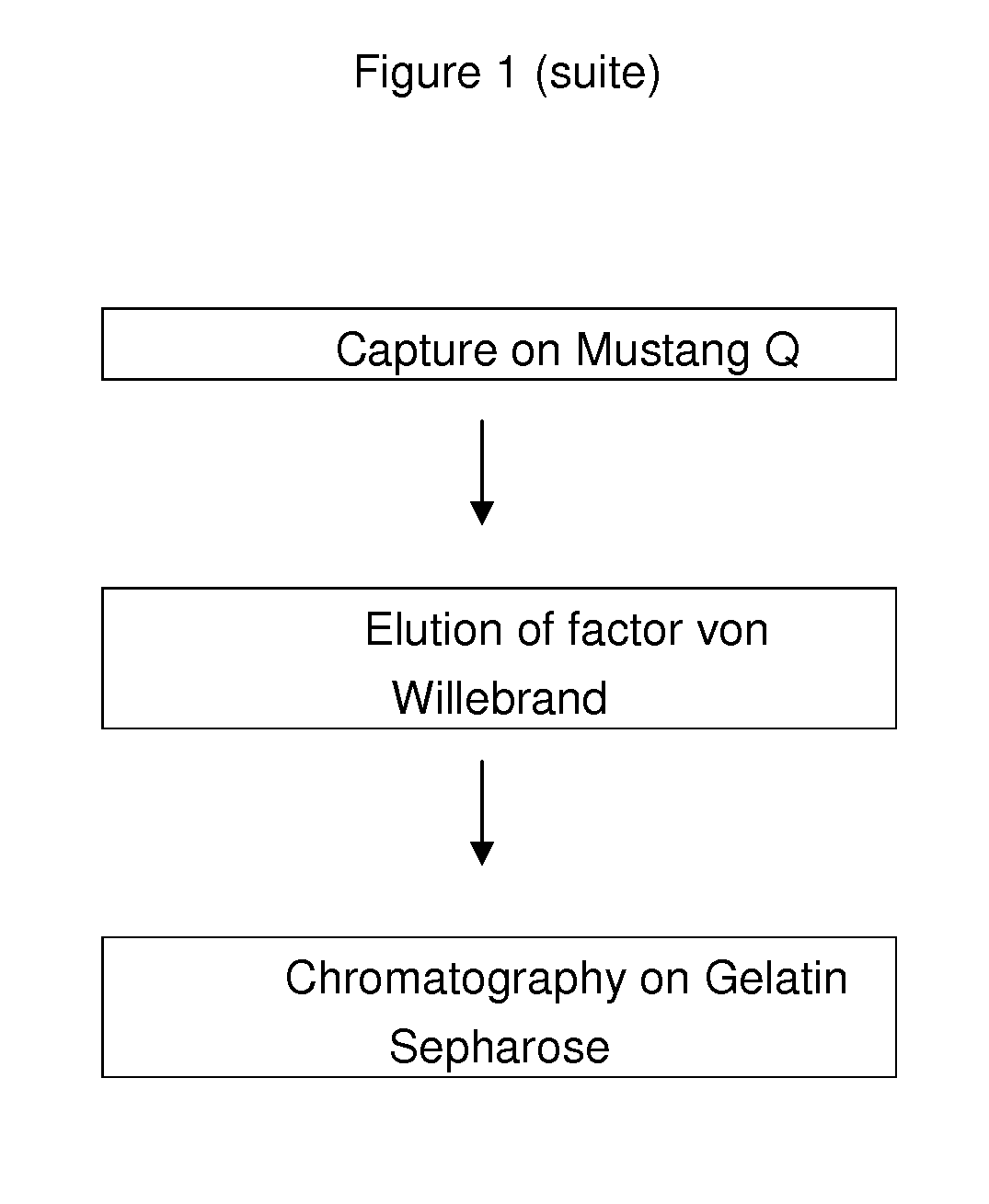
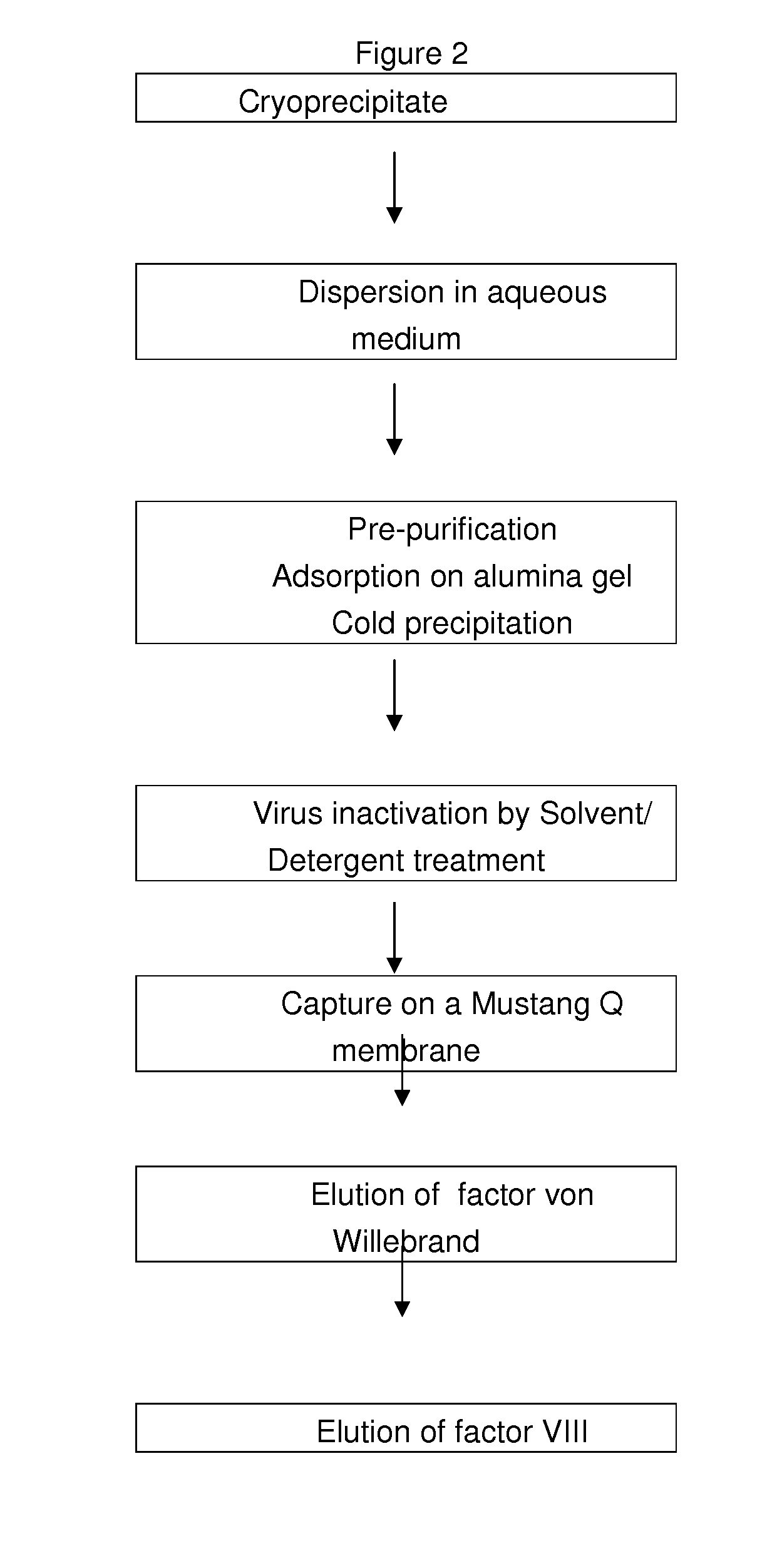
InactiveUS20100305305A1Improve health and safetyIncrease flow rateFactor VIIMammal material medical ingredientsHuman animalFactor VIII vWF
The purification method includes, starting from a solution selected from (i) a solution containing a mixture of FVIII and FvW, (ii) a solution containing FvW, (iii) a solution derived from a secretion of a non-human animal and (iv) a solution derived from a FVIII-containing plant extract, a step of absorption of FVIII or FvW on an ion-exchange chromatography filter-type membrane.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
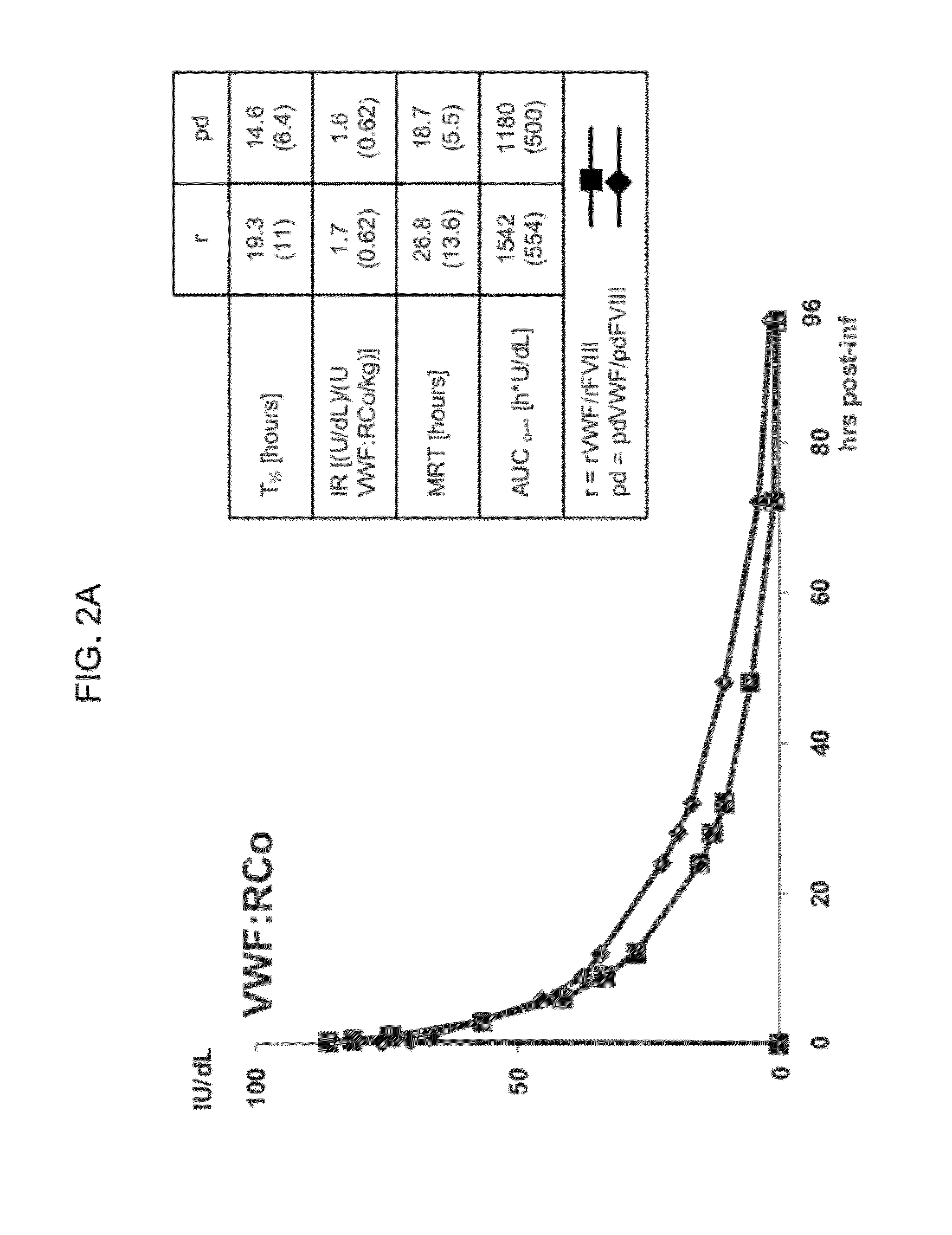
Treatment of coagulation disease by administration of recombinant vwf
ActiveUS20120316116A1Extended half-lifeFactor VIIPeptide/protein ingredientsFactor VIII vWFVon willebrand
The present invention provides methods of treating coagulation disease, including hemophilia and von Willebrand disease by administering recombinant von Willebrand Factor alone or in combination with Factor VIII.
Owner:TAKEDA PHARMA CO LTD
Inactivation resistant factor VIII
The present invention provides novel purified and isolated nucleic acid sequences encoding procoagulant-active FVIII proteins. The nucleic acid sequences of the present invention encode amino acid sequences corresponding to known human FVIII sequences, wherein residue Phe3O9 is mutated. The nucleic acid sequences of the present invention also encode amino acid sequences corresponding to known human FVIII sequences, wherein the APC cleavage sites, Arg336 and Ile562, are mutated. The nucleic acid sequences of the present invention further encode amino acid sequences corresponding to known human FVIII sequences, wherein the B-domain is deleted, the von Willebrand factor binding site is deleted, a thrombin cleavage site is mutated and an amino acid sequence spacer is inserted between the A2- and A3-domains. Methods of producing the FVIII proteins of the invention, nucleotide sequences encoding such proteins, pharmaceutical compositions containing the nucleotide sequences or proteins, as well as methods of treating patients suffering from hemophilia, are also provided.
Owner:RGT UNIV OF MICHIGAN
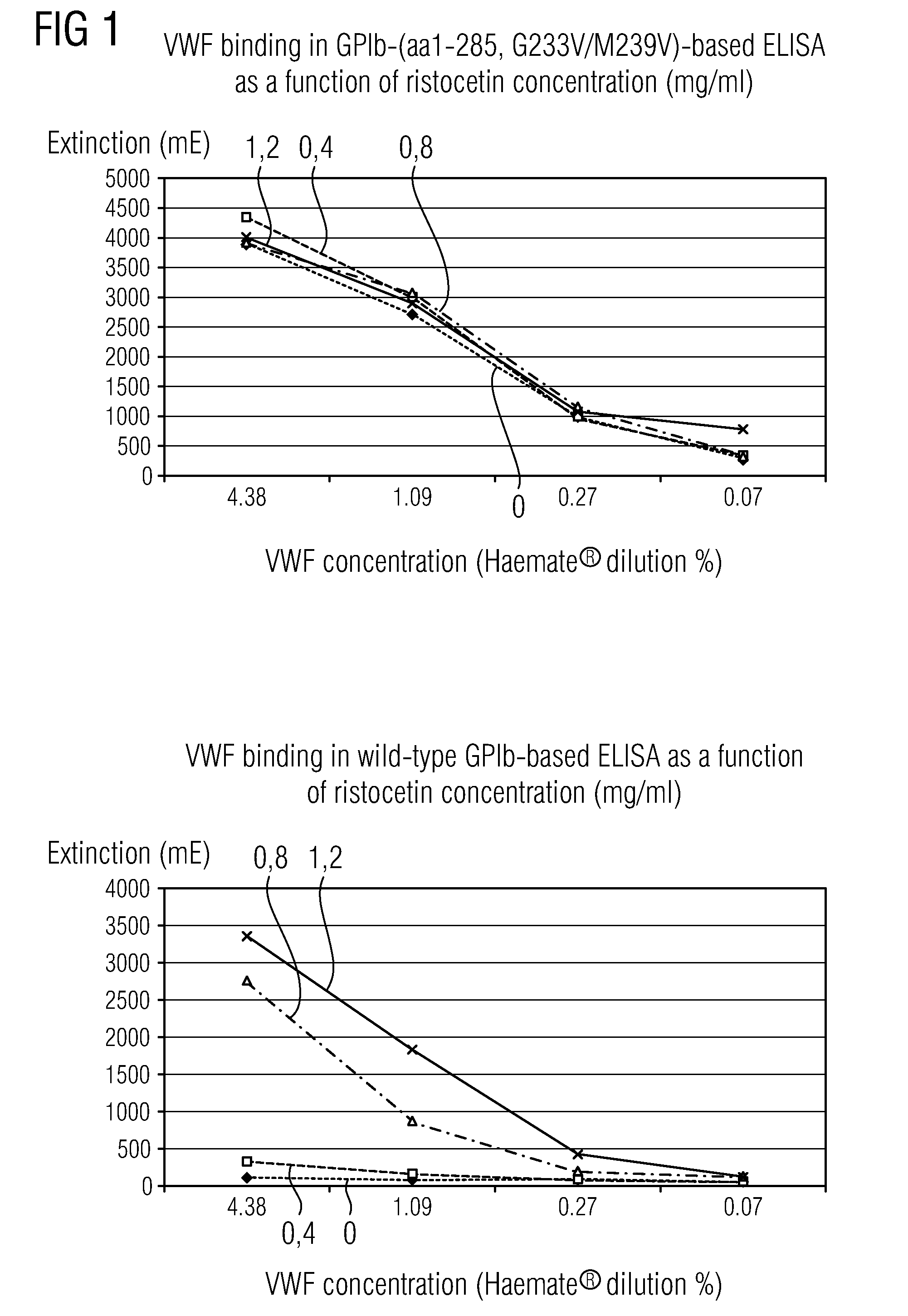
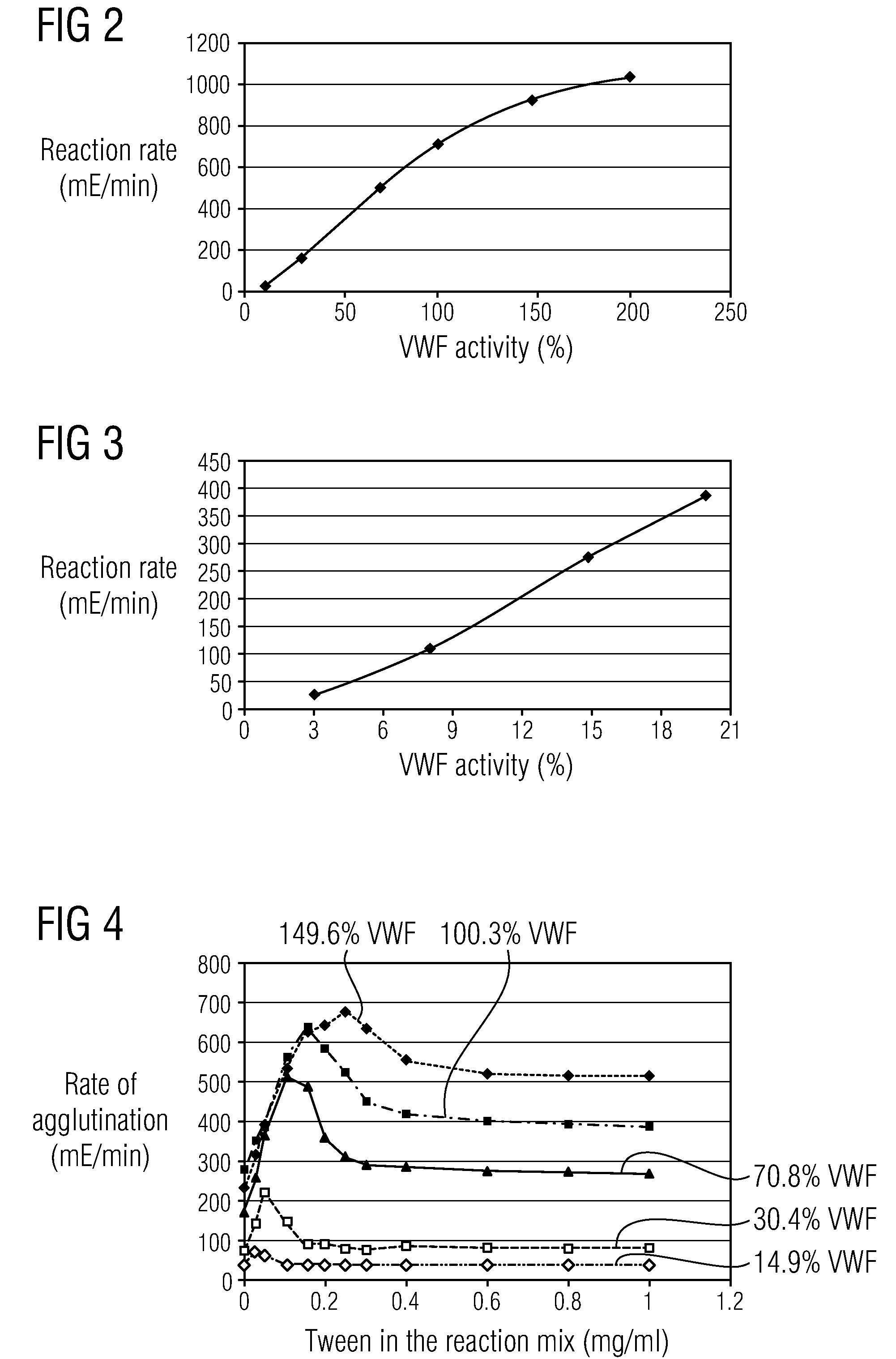
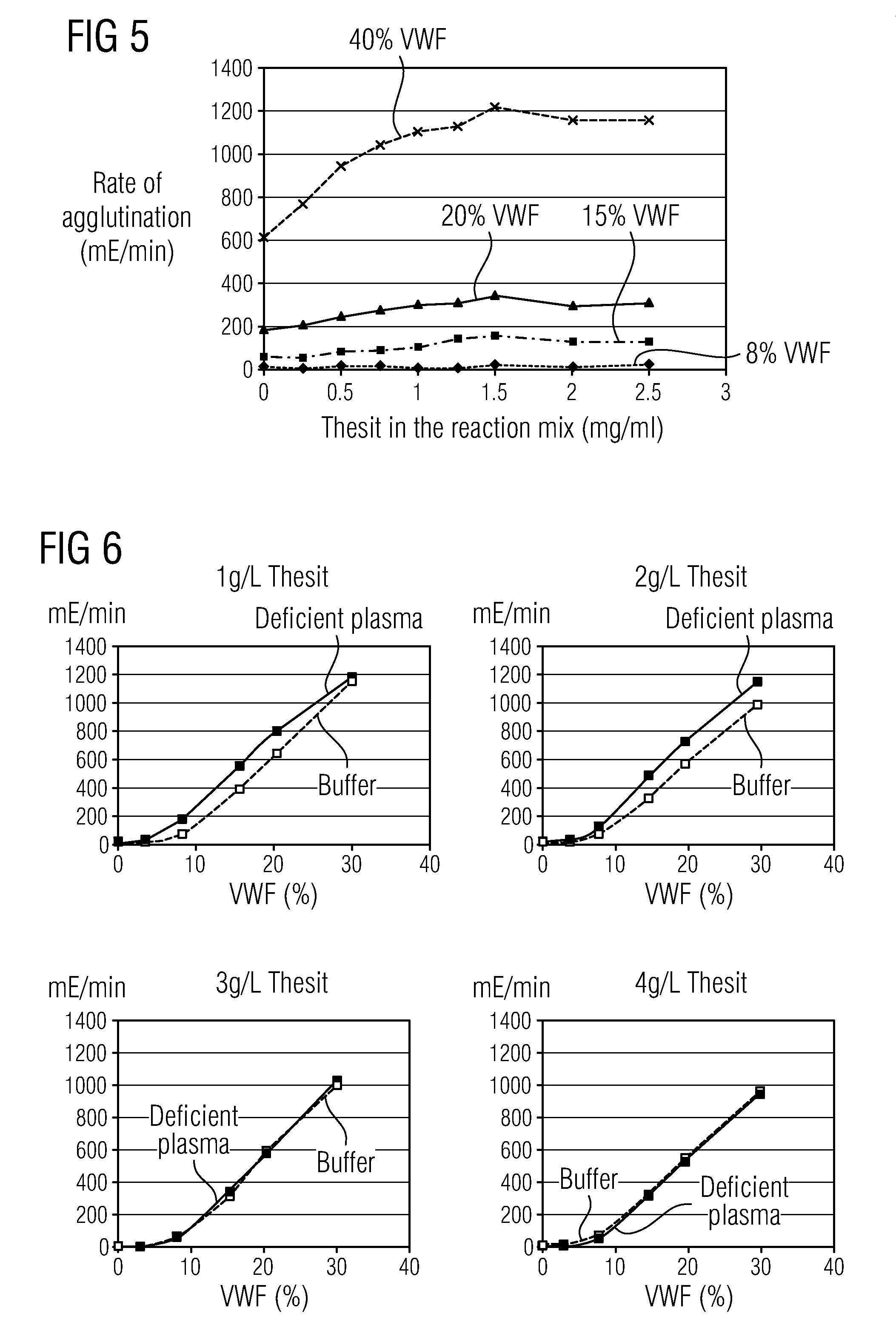
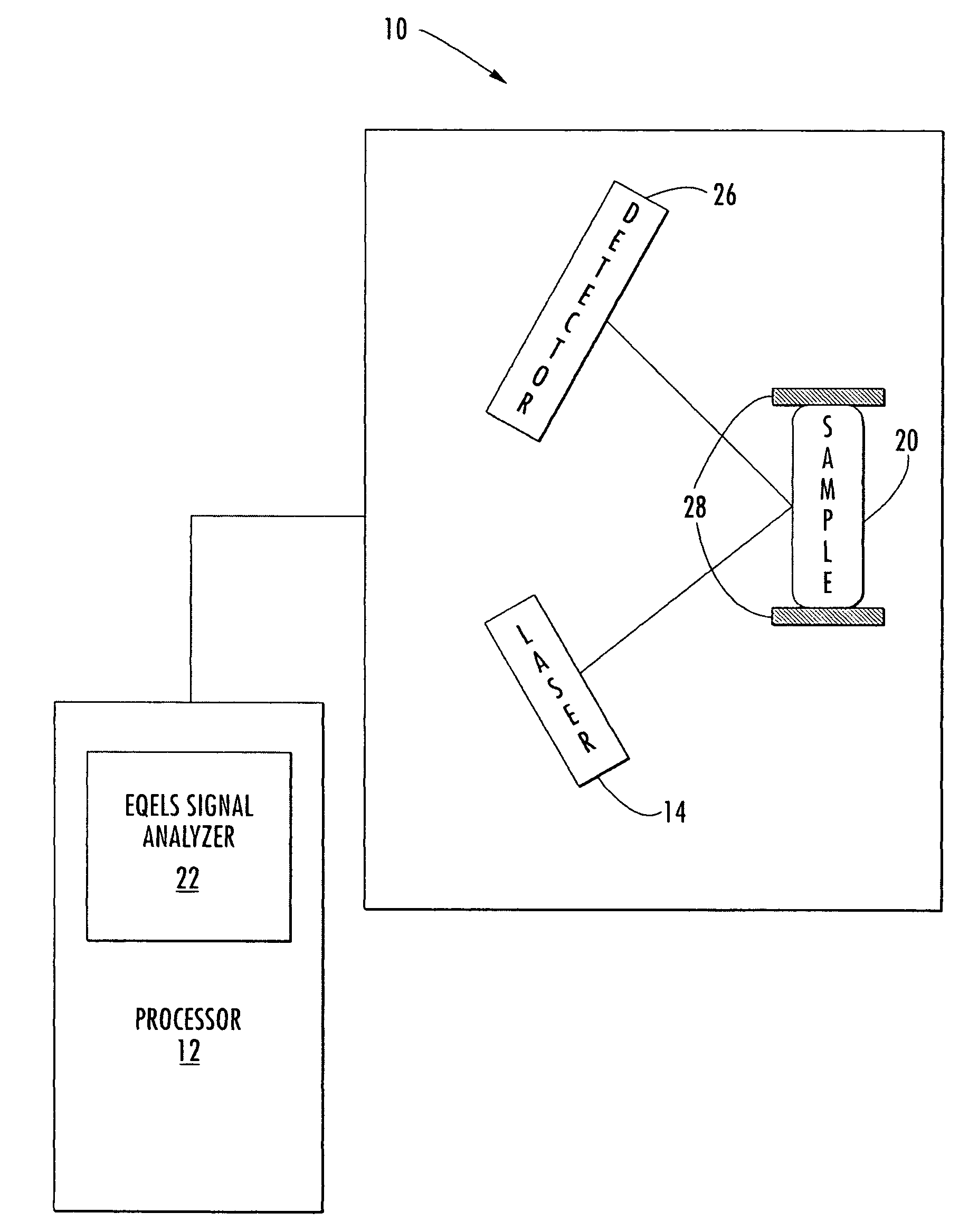
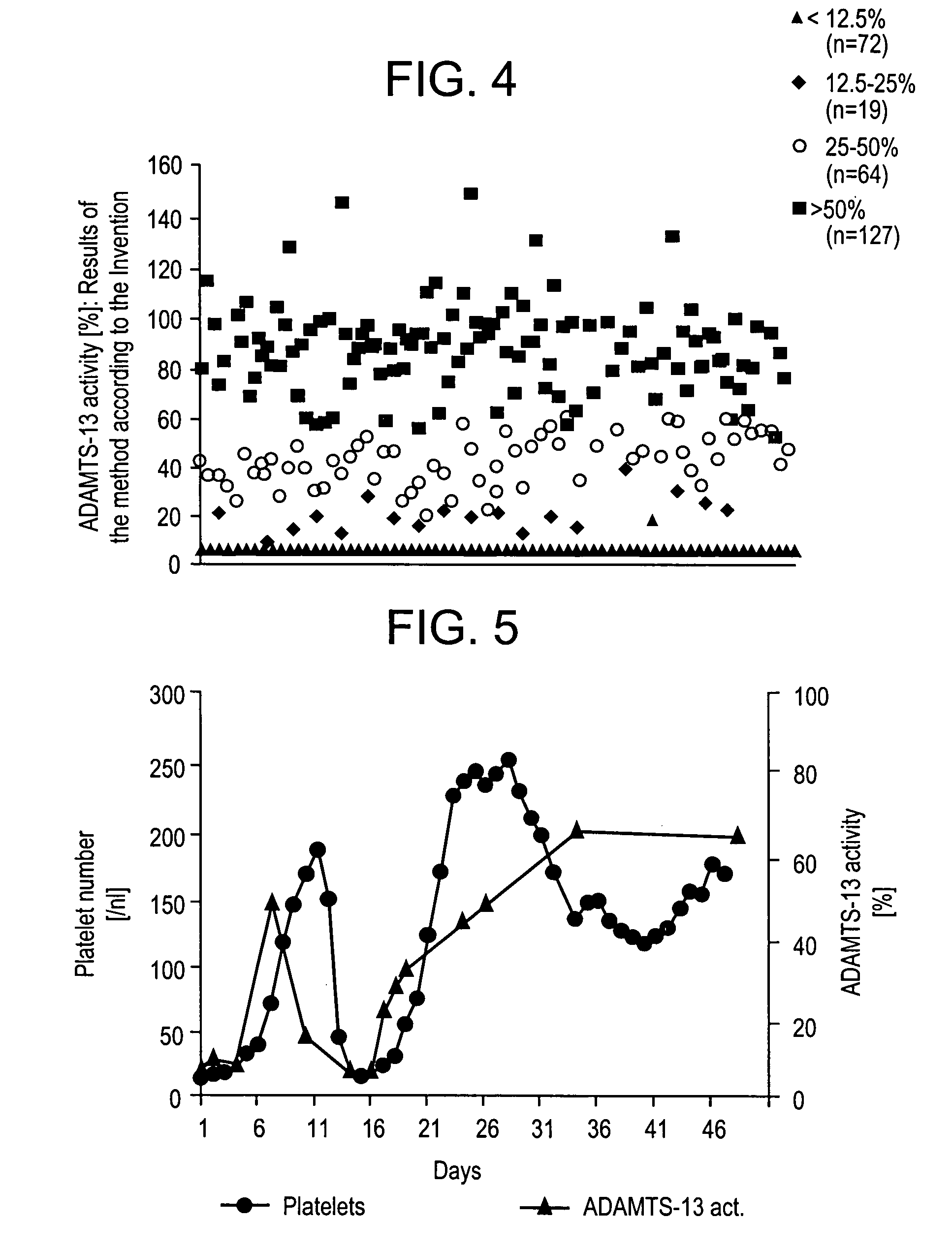
Methods and Kits for Determining von Willebrand Factor Activity in the Absence of Ristocetin and for Determining the Activity of ADAMTS-13 Protease
ActiveUS20100136589A1Microbiological testing/measurementDisease diagnosisProteinase activityFactor VIII vWF
Described herein are method(s), kit(s), reagent(s) and the like for determining von Willebrand factor (VWF) activity in a sample in the absence of ristocetin.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS PRODS
Assays for detection of von willebrand factor (vWF) multimers and for degradation of vWF by agents such as ADAMTS13 and related methods
Owner:NORTH CAROLINA AT CHAPEL HILL THE UNIV OF
Method for detecting the von Willebrand factor-cleaving protease activity of ADAMTS-13
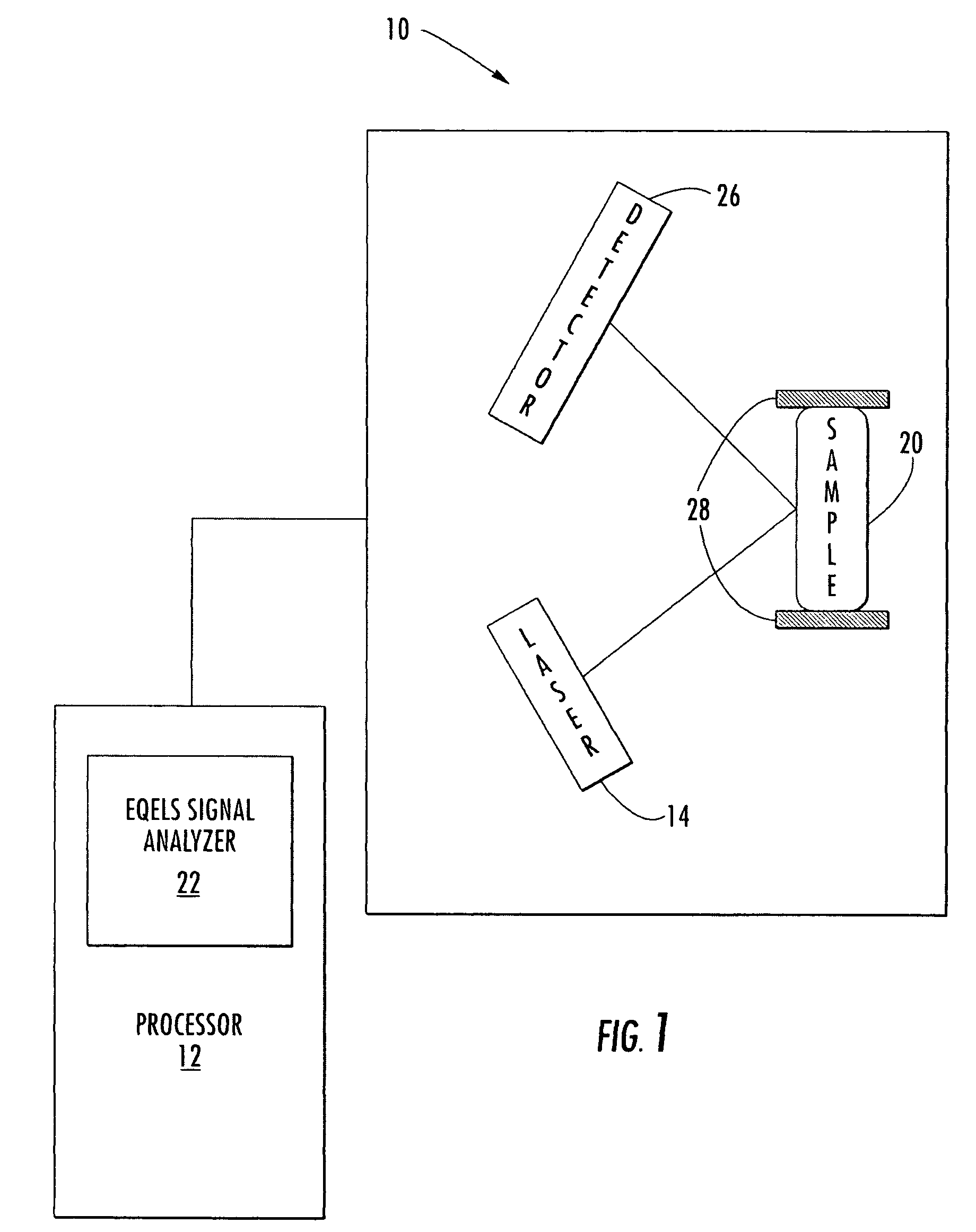
InactiveUS7291479B2Microbiological testing/measurementBiological testingProteinase activityFactor VIII vWF
The invention relates to a diagnostic method for determining the von Willebrand factor (VWF) cleaving activity of ADAMTS-13 in a test medium during which the test medium is mixed with 0.5 to 5 U / ml of a von Willebrand factor (VWF) that does not contain ADAMTS-13, and after incubation, the ADAMTS-13 activity is determined based on the drop in the VWF-mediated aggregation of thrombocytes.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS PRODS
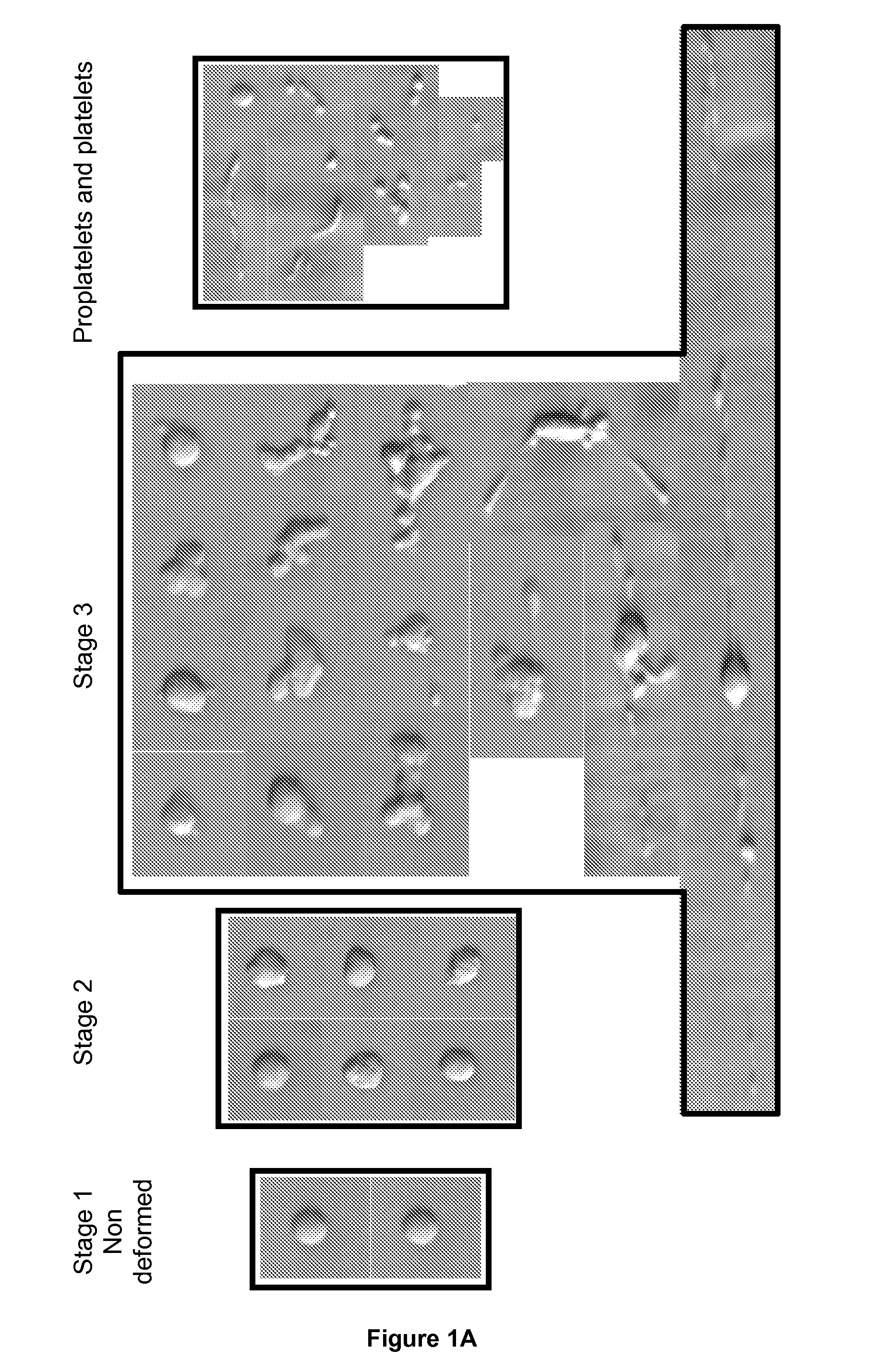
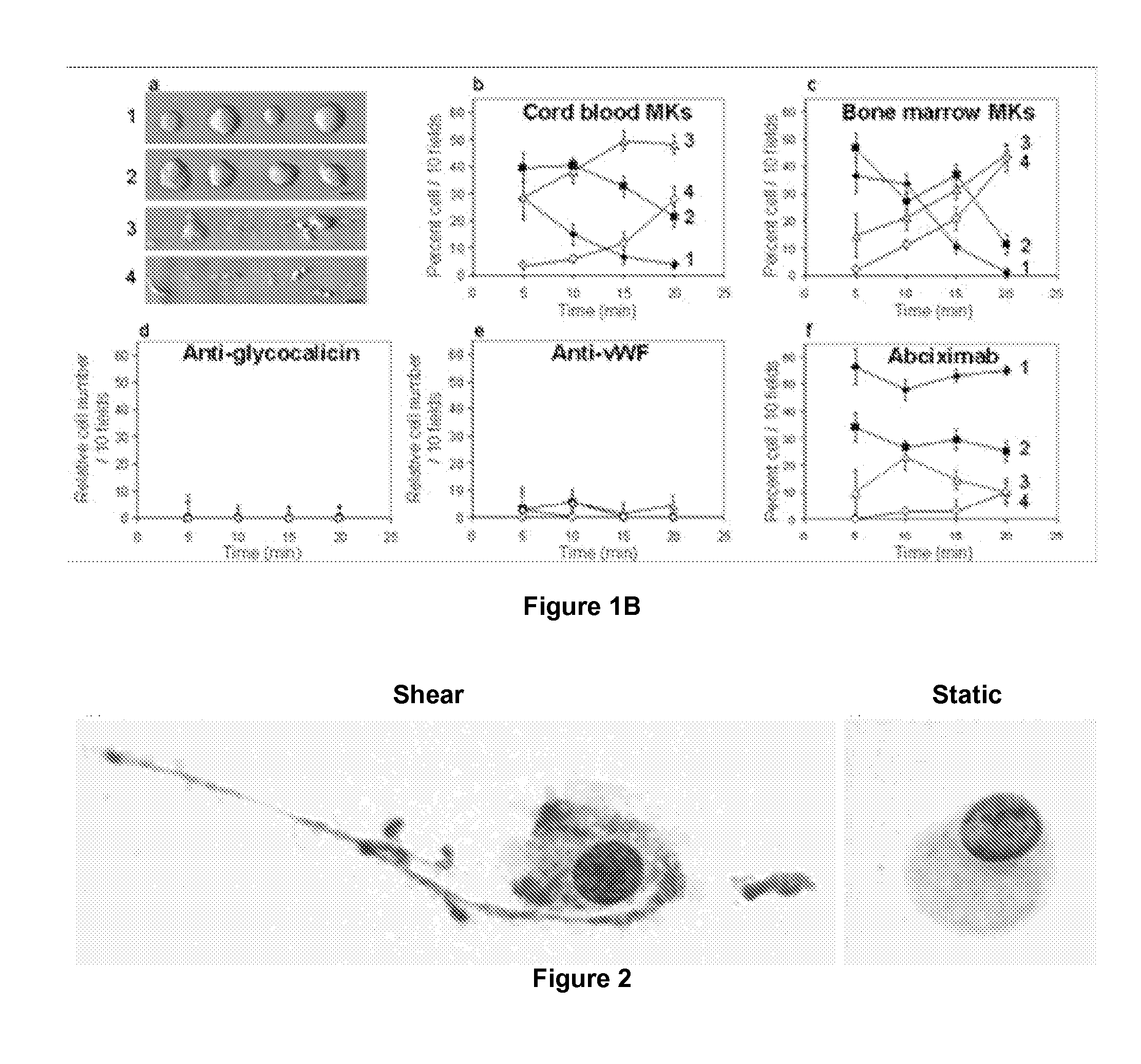
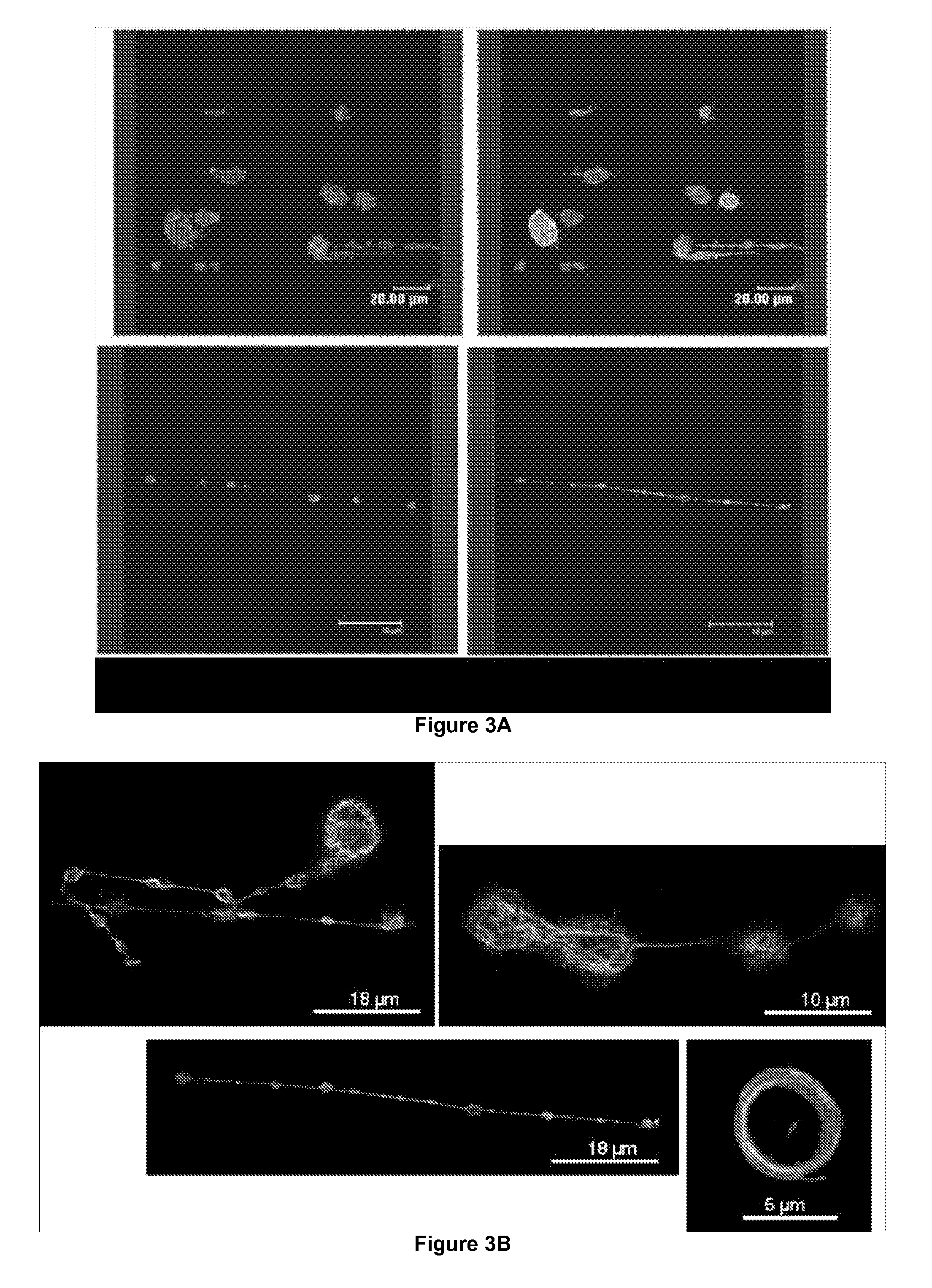
Method for Producing Platelets
ActiveUS20120014933A1Easy to produceBioreactor/fermenter combinationsBiocideFactor VIII vWFShear rate
The present invention relates to a method for producing platelets from mature megakaryocytes. More particularly, the invention relates to an ex vivo method for producing platelets, from mature megakaryocytes, said method comprising a step of subjecting a suspension of mature megakaryocytes to a flow having a minimal shear rate of 600 s−1 on a solid phase coated with Von Willebrand factor.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +3
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com