Dry heat treatment stabilizing agent for human coagulation factor VIII and vWF (von willebrand factor) compound or human coagulation factor VIII preparation
A technology of human coagulation factor and dry heat treatment, applied in the field of medicine, can solve the problems of unsatisfactory protective effect, difficult to meet large-scale production, and difficult to achieve the recovery rate of coagulation factor activity, to prevent protein denaturation and precipitation, stable physical and chemical properties, and improve The effect of recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] The method of the present invention will be described in further detail below in conjunction with specific drawings and embodiments.
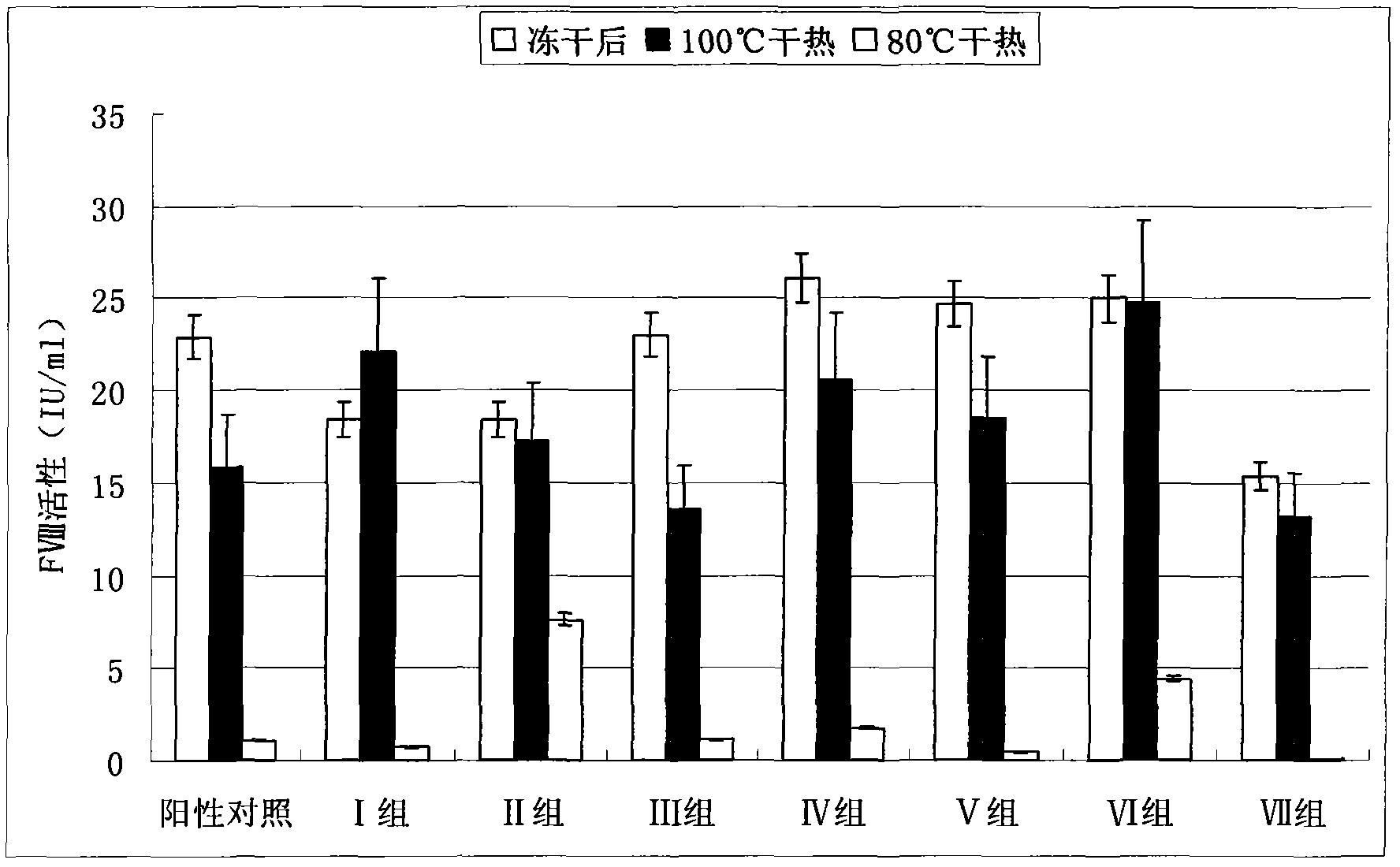
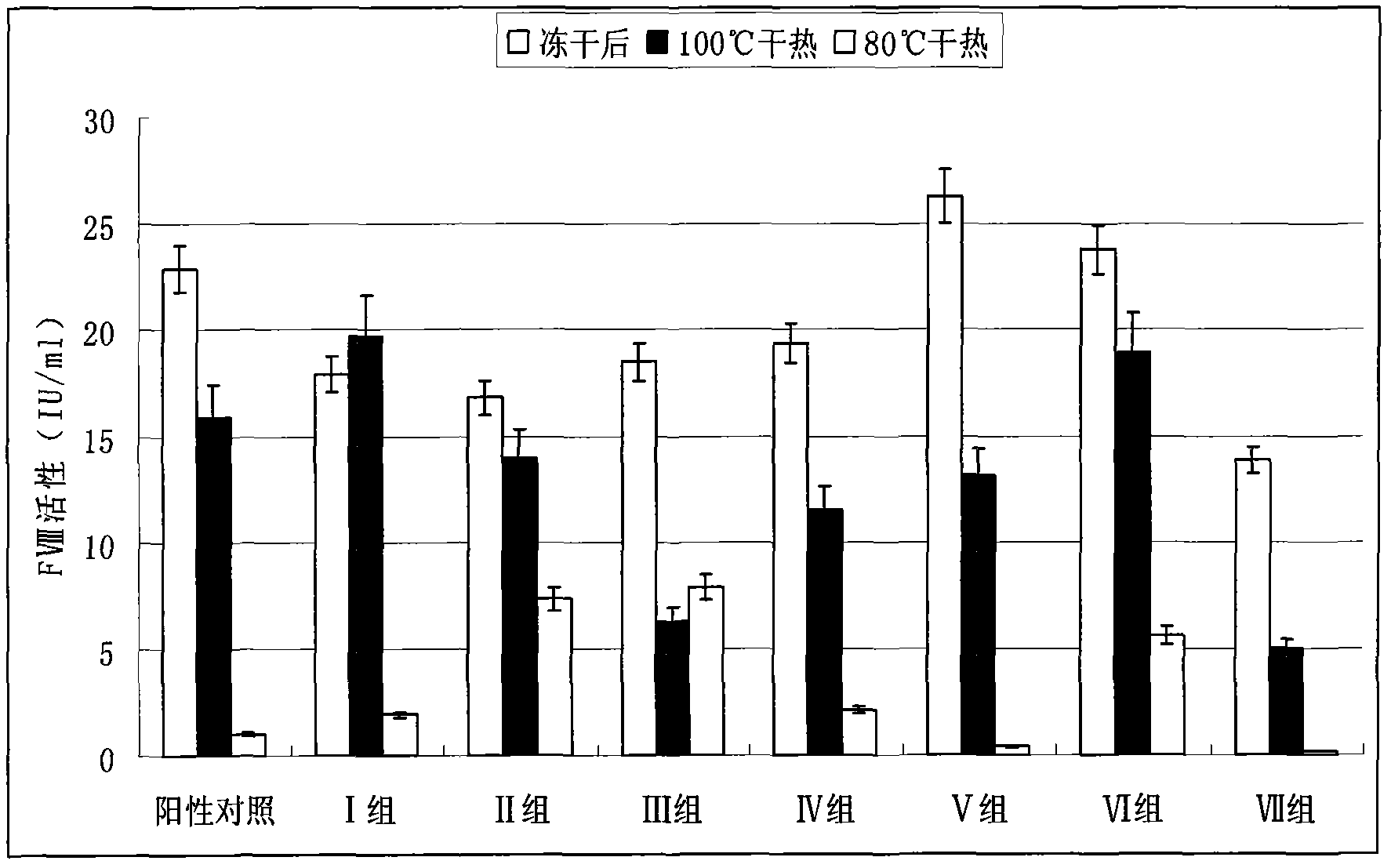
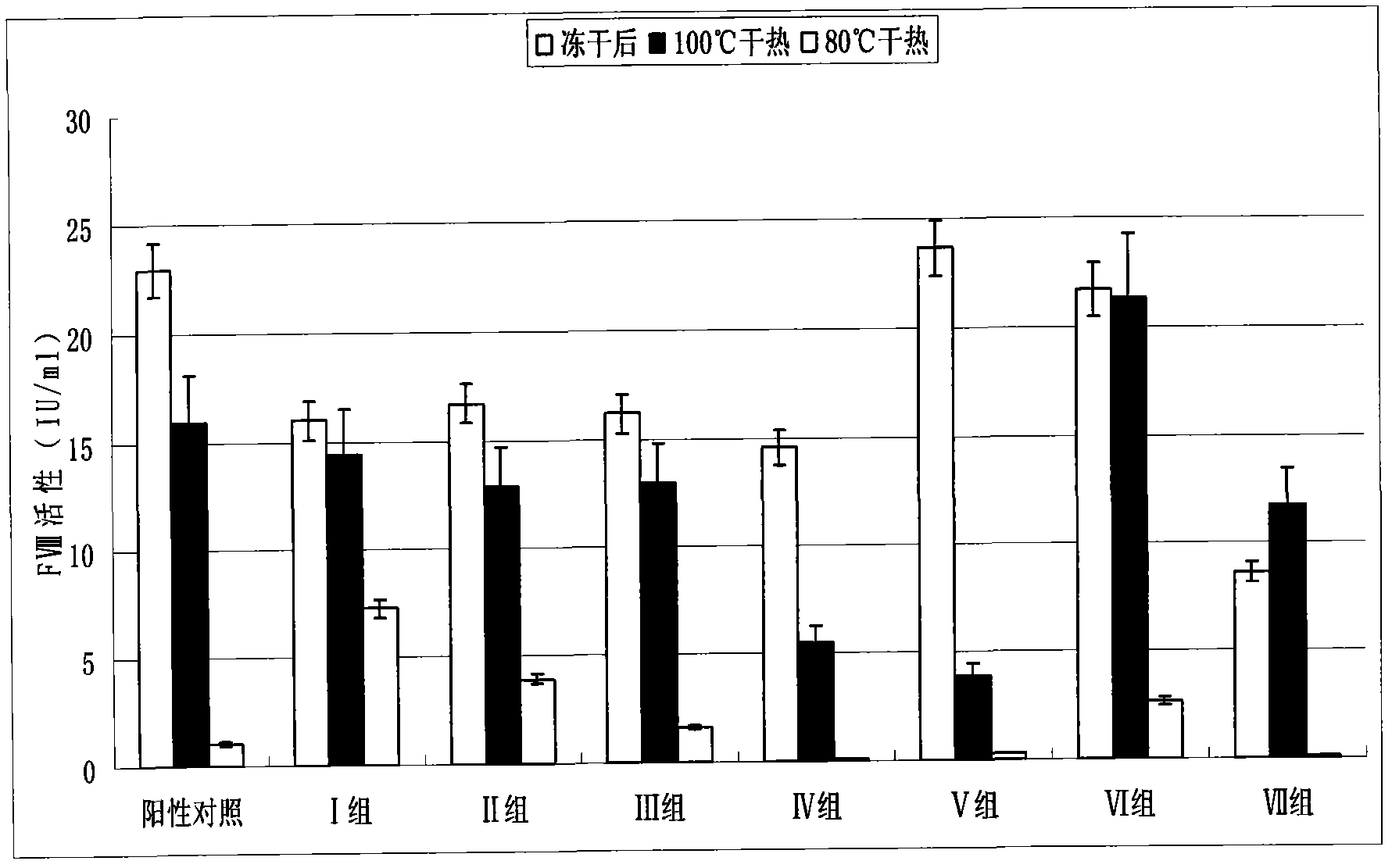
[0019] 1. Preparation of human coagulation factor VIII and vWF complex or human coagulation factor VIII preparation
[0020] According to the conventional method, the cryoprecipitate was dissolved with 4 times the volume of water, and the anticoagulant heparin sodium was added, and after extraction at room temperature for 30 minutes, it was adsorbed with an appropriate amount of 3%-6% aluminum hydroxide gel, centrifuged after adsorption, and the supernatant was The chromatographic column is adsorbed with anionic resin DEAE 650, and after washing the miscellaneous proteins, it is eluted with 0.25M sodium chloride, and the obtained eluate is subjected to ultrafiltration, desalination and concentration to obtain the complex of human blood coagulation factor VIII and vWF or the preparation of human blood coagulation factor VIII Concentrate s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com