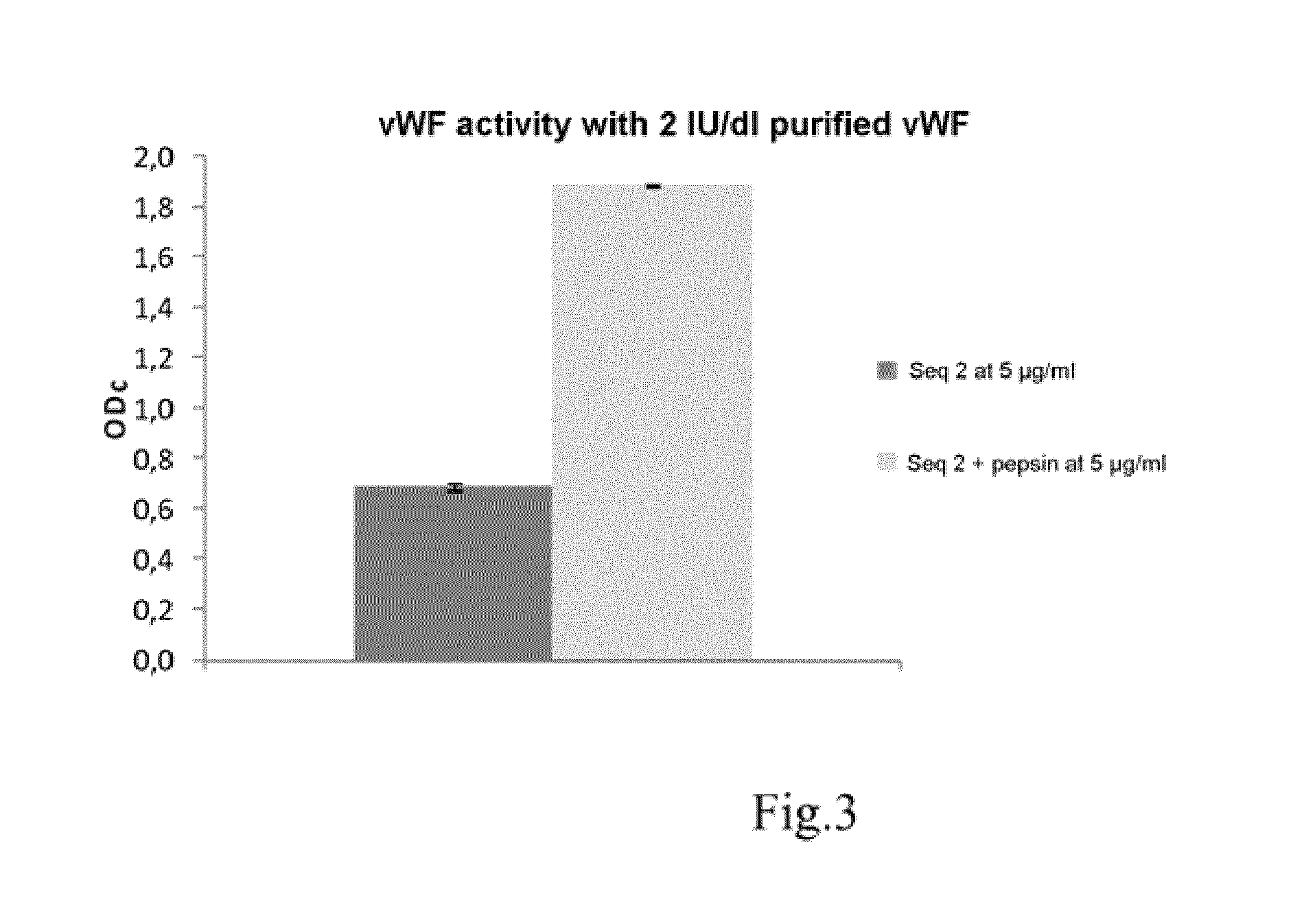
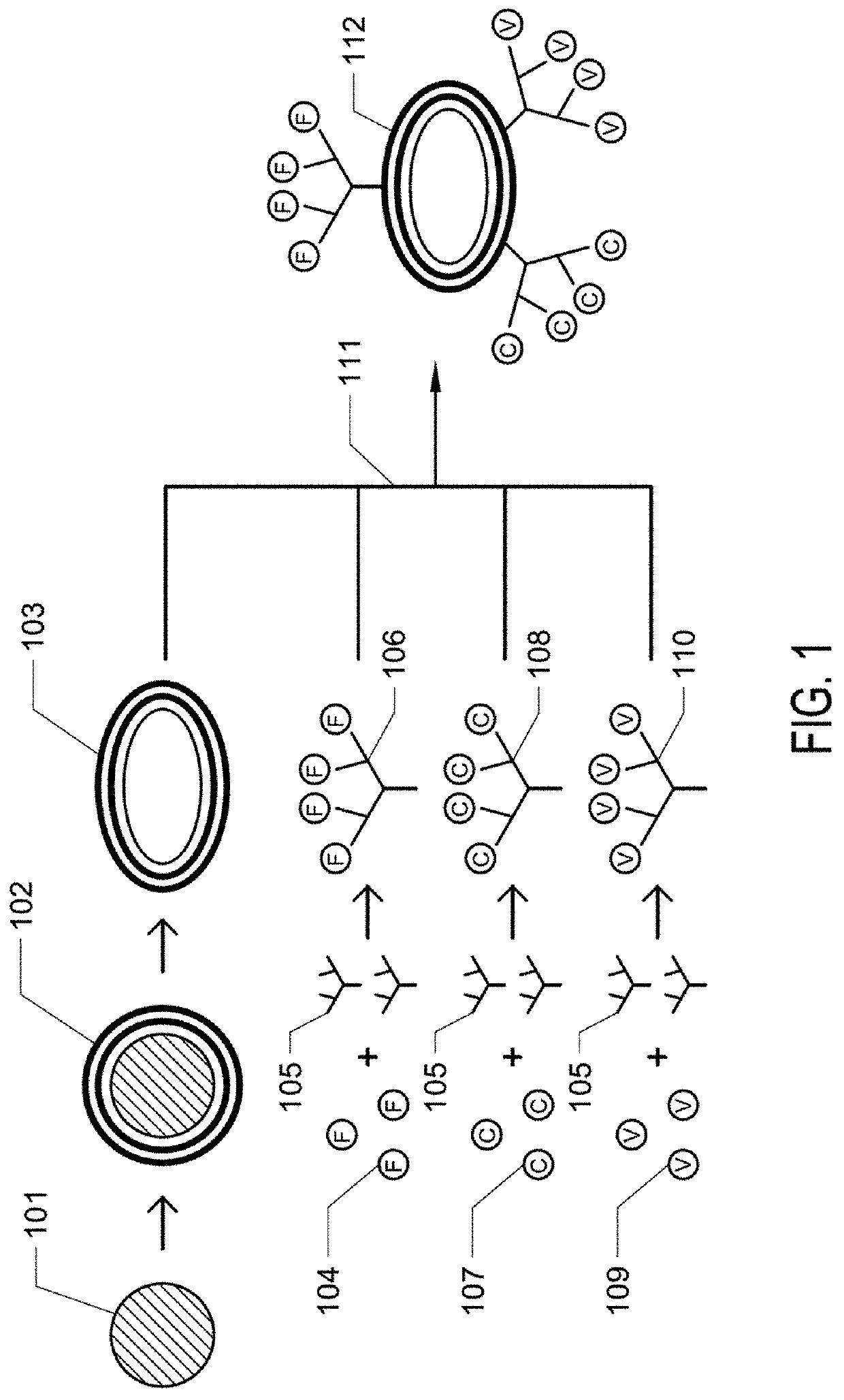
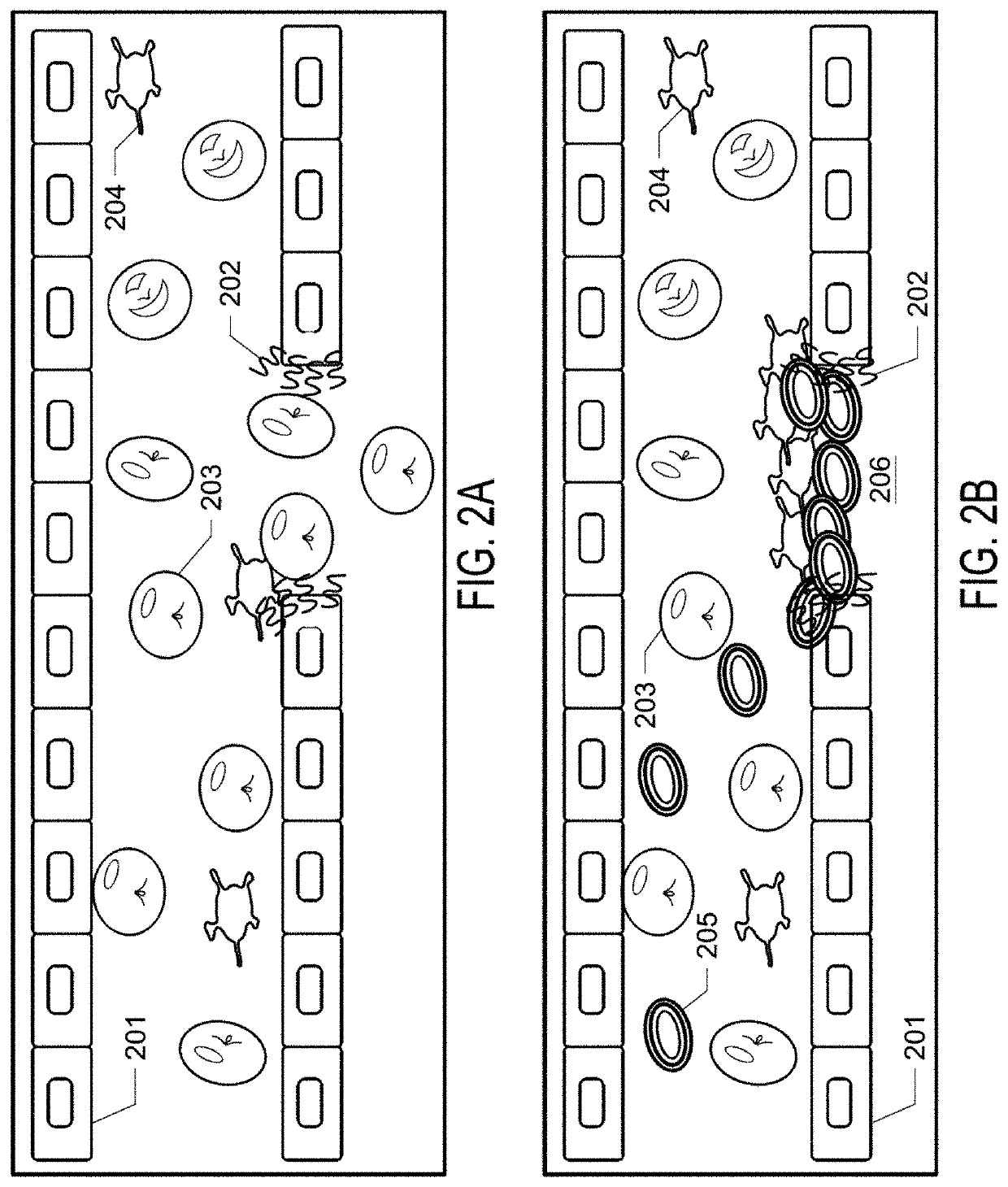
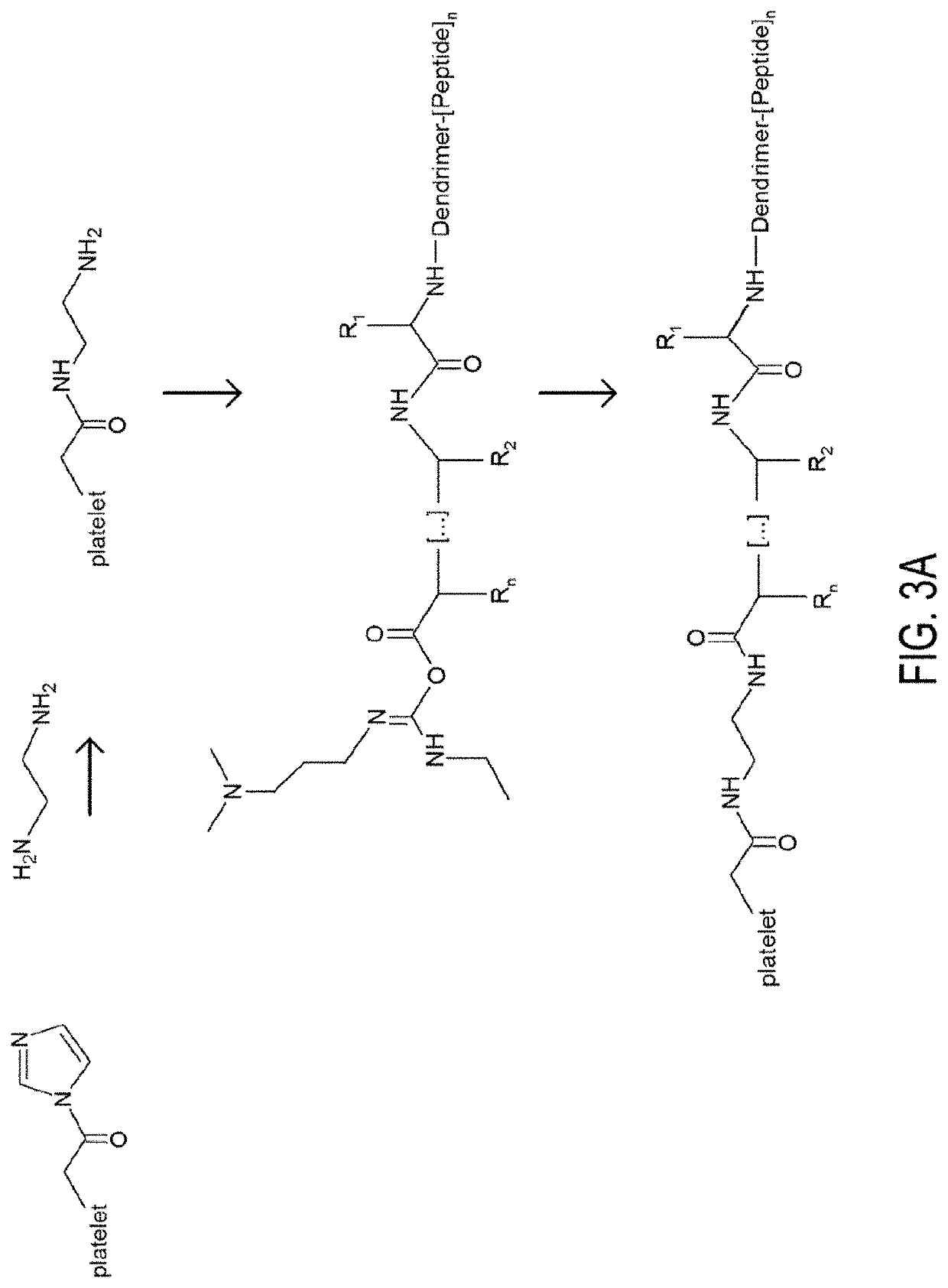
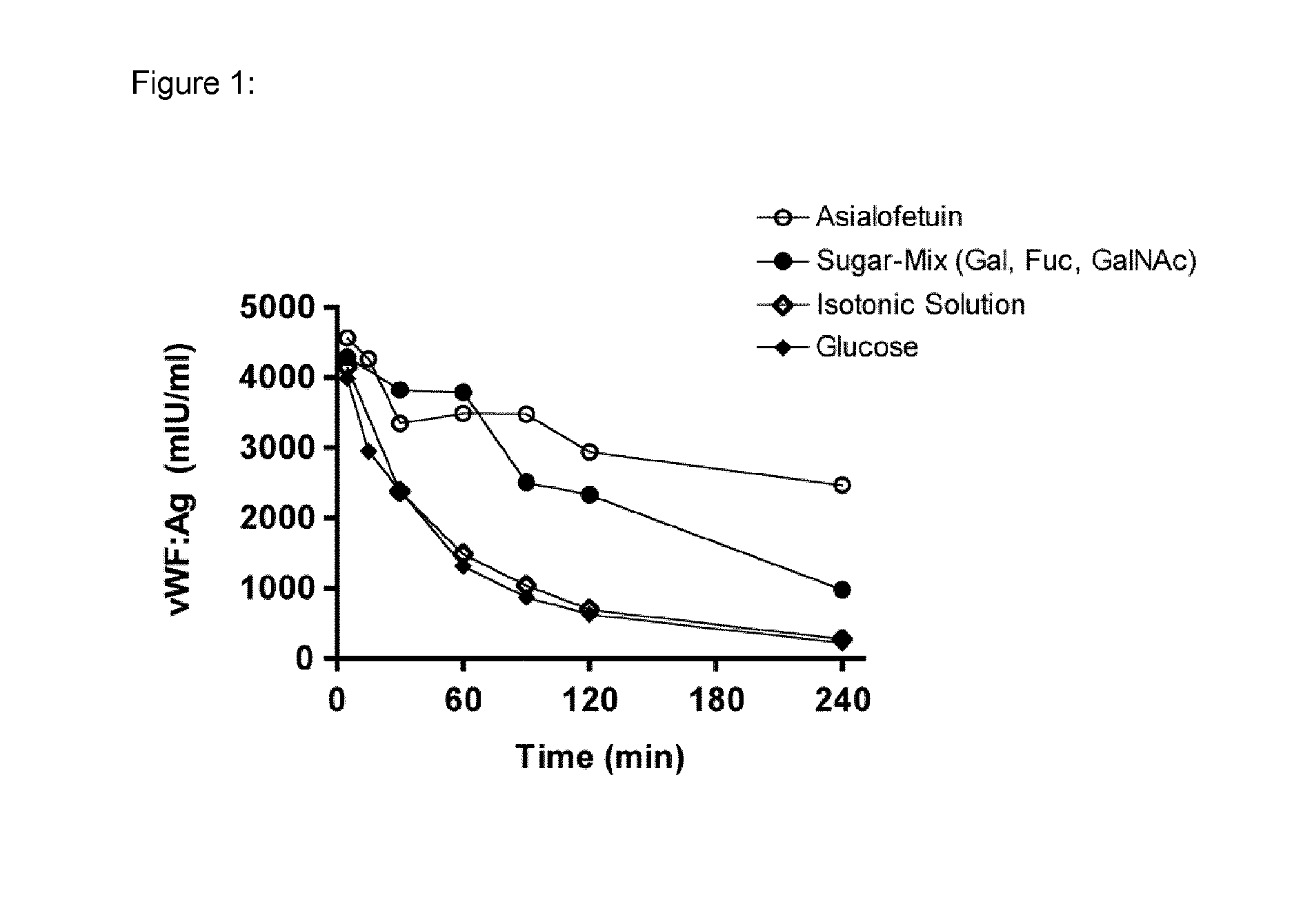
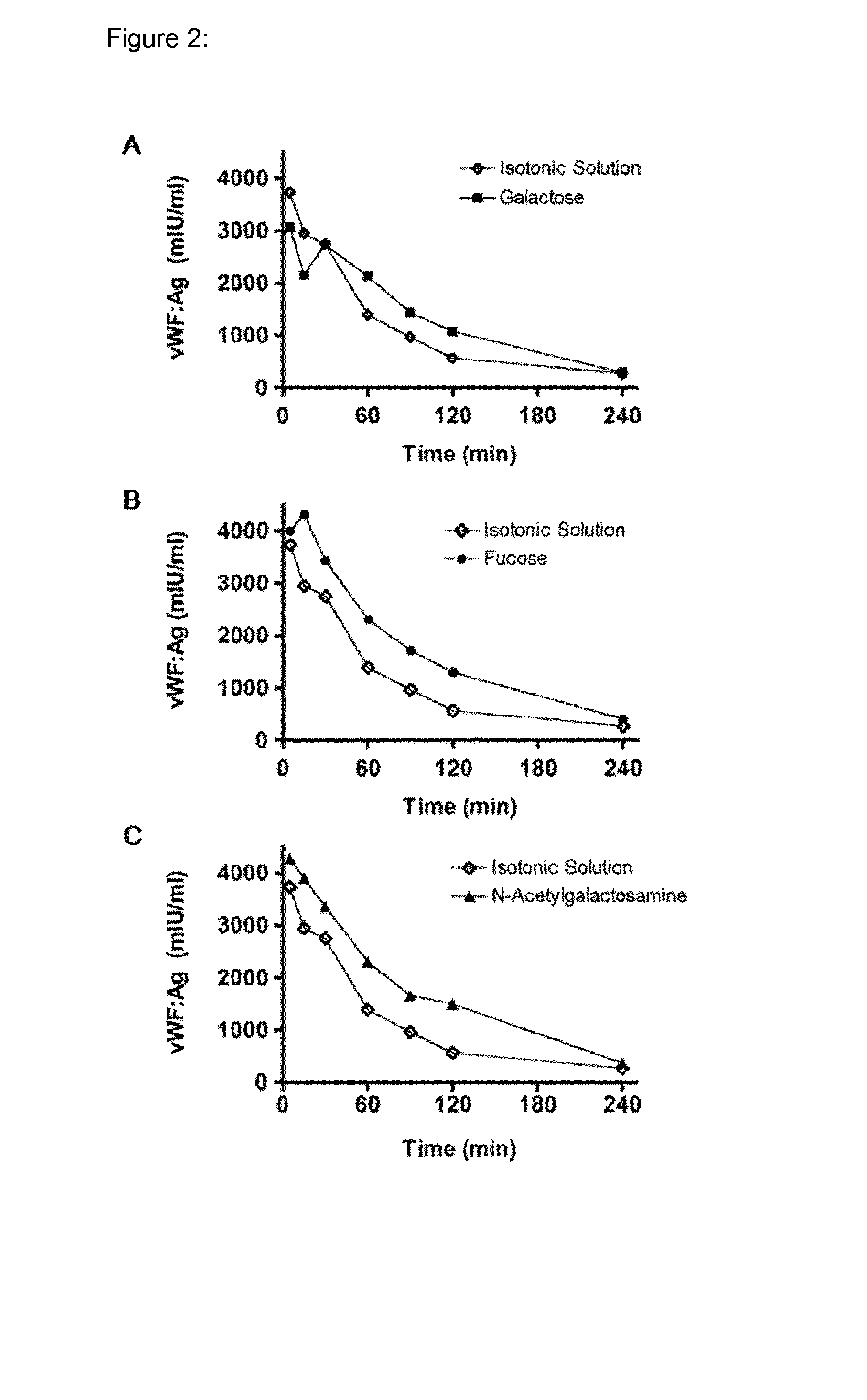
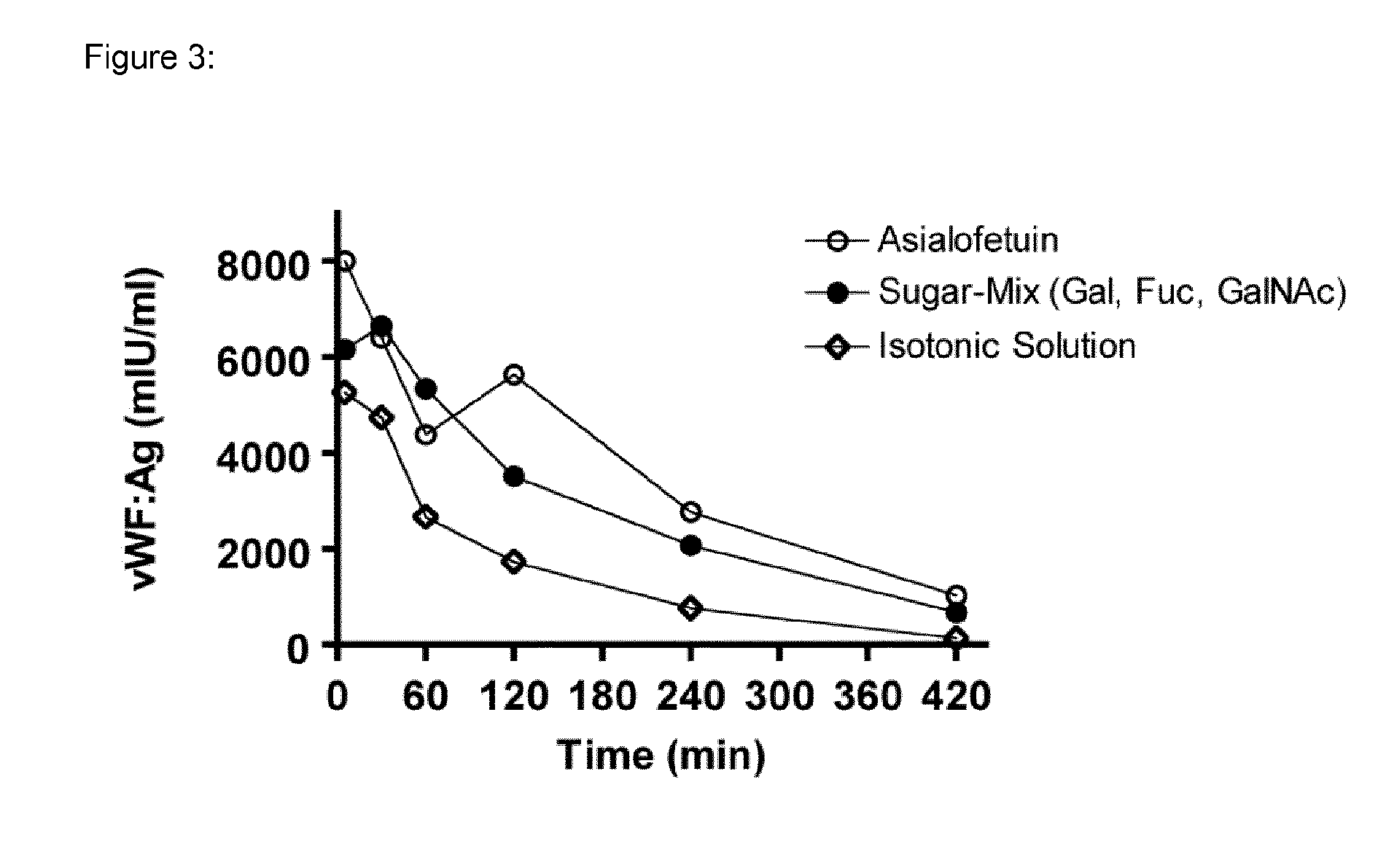
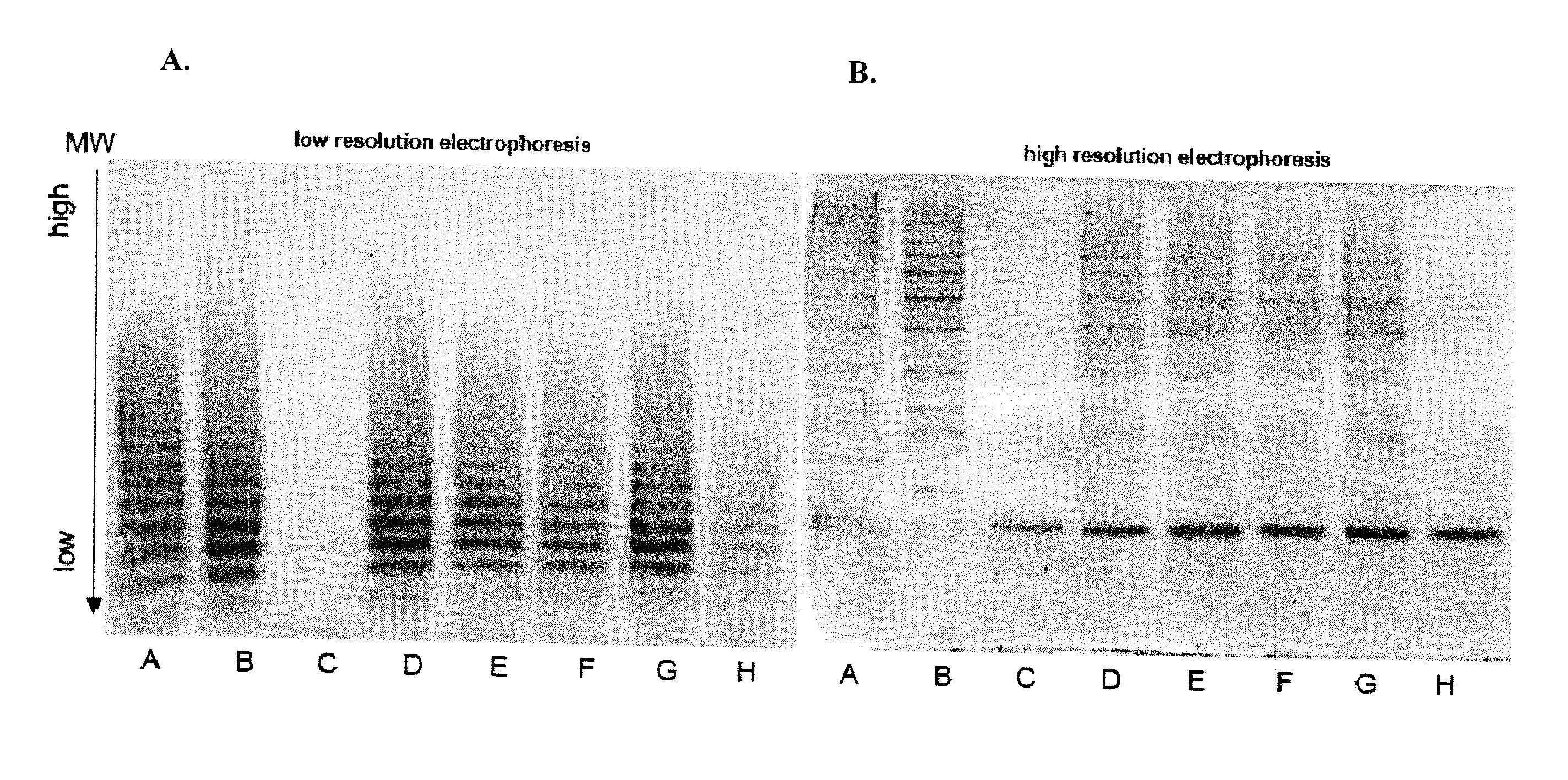
Patents
Literature
37 results about "Von Willebrand disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An inherited bleeding disorder that results from low levels of specific clotting protein in blood.
Methods for correcting von willebrand factor point mutations
InactiveUS20150166985A1Nervous disorderFusion with DNA-binding domainHuman DNA sequencingFactor VIII vWF
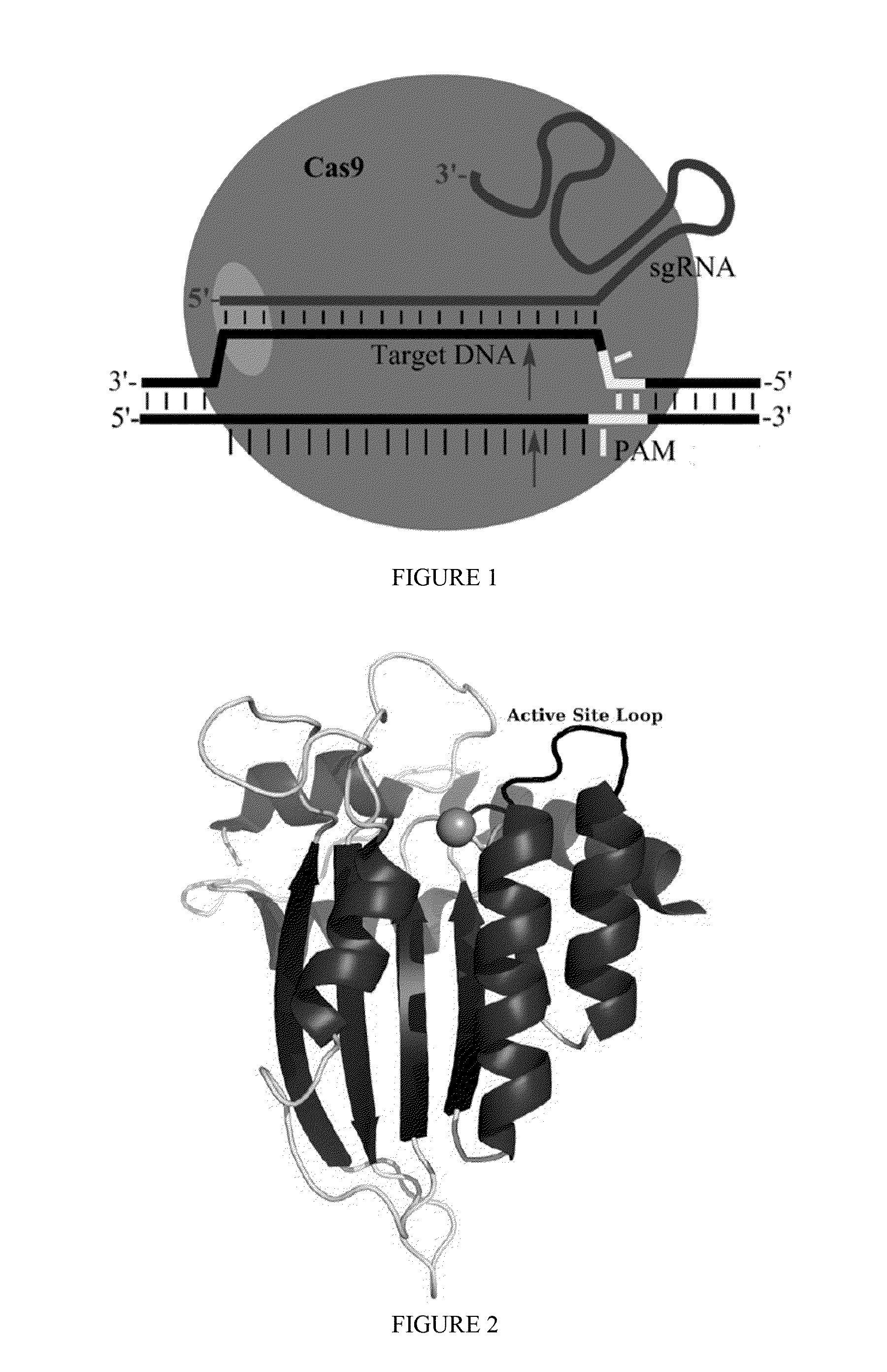
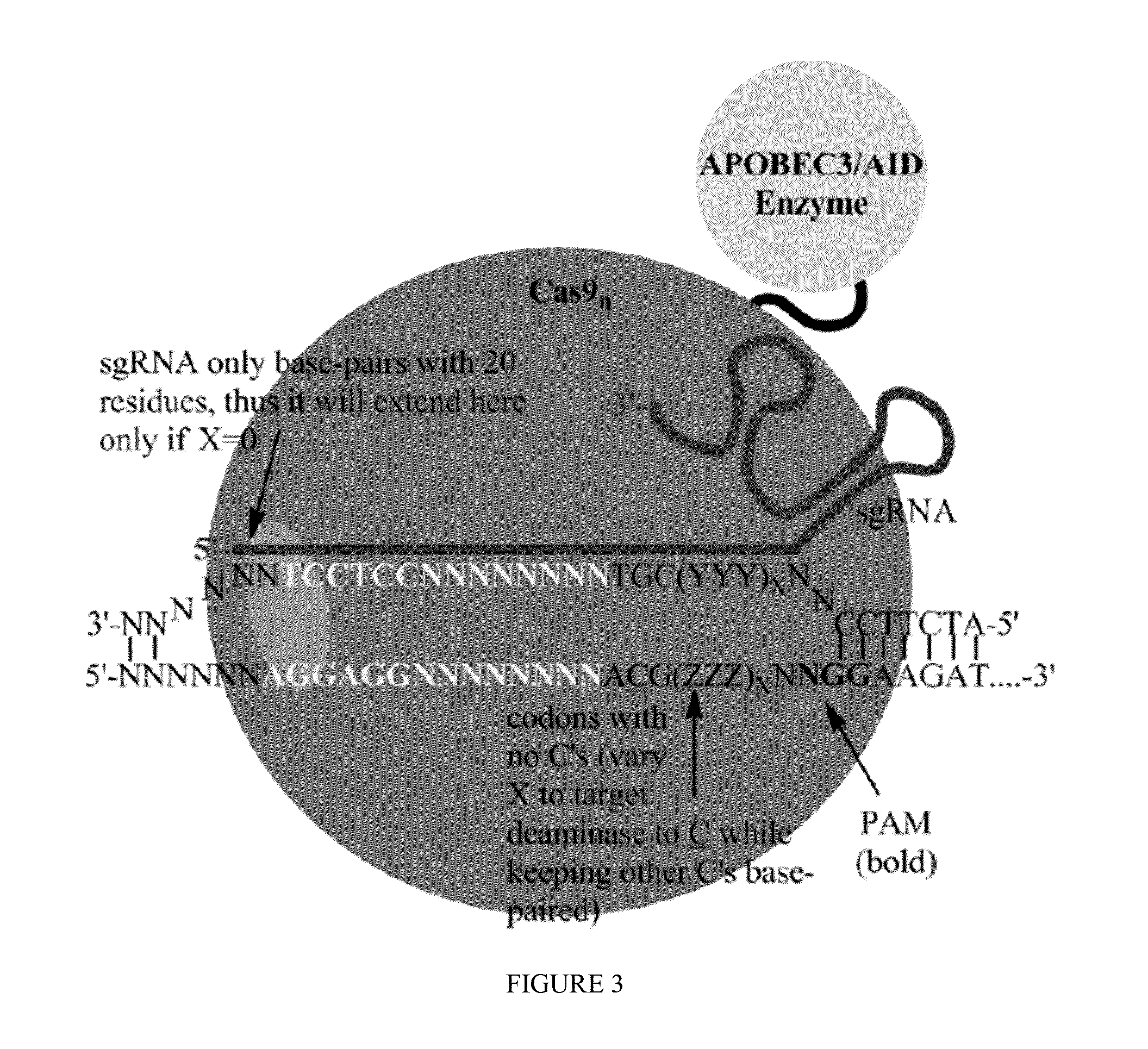
Some aspects of this disclosure provide strategies, systems, reagents, methods, and kits that are useful for the targeted editing of nucleic acids, including editing a nucleic acid encoding a mutant von Willebrand Factor protein to correct a point mutation associated with a disease or disorder, e.g., with von Willebrand disease. The methods provided are useful for correcting a vWF point mutation within the genome of a cell or subject, e.g., within the human genome. In some embodiments, fusion proteins of Cas9 and nucleic acid editing enzymes or enzyme domains, e.g., deaminase domains, are provided. In some embodiments, reagents and kits for the generation of targeted nucleic acid editing proteins, e.g., fusion proteins of Cas9 and nucleic acid editing enzymes or domains, are provided.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Method of providing hemostasis in Anti-coagulated blood
InactiveUS20080254146A1Promote formationBiocideAnimal repellantsBULK ACTIVE INGREDIENTActive ingredient
A method of clotting blood includes the step of administering a therapeutically effective amount of a composition comprising zeolite as the active ingredient to a wound from which the blood emanates. A method of arresting blood flowing from a wound includes the steps of providing a patient being inflicted with a bleeding wound and administering a therapeutically effective amount of a composition comprising zeolite as the active ingredient to the bleeding wound. A method of facilitating the formation of blood clots includes the step of contacting blood with a negatively charged surface wherein upon contacting the blood with the negatively charged surface a clotting mechanism is initiated. In any of the foregoing methods, the blood has a compromised ability to form clots. The blood may be from a person diagnosed with hemophilia or von Willebrand disease.
Owner:TELEFLEX LIFE SCI LTD
Method of providing hemostasis in Anti-coagulated blood
InactiveUS20080254147A1Promote formationBiocideInanimate material medical ingredientsMedicineB hemophilia
In a method of clotting blood in which the blood exhibits a reduced tendency to clot and may be from a person undergoing an anticoagulant therapy or having type A or B hemophilia or von Willebrand disease, a therapeutically effective amount of a composition comprising clay as the active ingredient is administered to a wound from which the blood emanates. Upon contacting the blood, this clay, which may be kaolin, bentonite, or any type of layered clay, causes the blood to clot. In a method of arresting blood flowing from a wound, a therapeutically effective amount of a composition comprising clay as the active ingredient is administered to the bleeding wound. In this method, the blood has a reduced tendency to clot and may be from a person undergoing an anticoagulant therapy or having at least one of hemophilia A or B or von Willebrand disease.
Owner:TELEFLEX LIFE SCI LTD
Inactivation resistant factor VIII
InactiveUS20060293238A1Increase secretionHigh expressionFactor VIIPeptide/protein ingredientsBinding siteENCODE
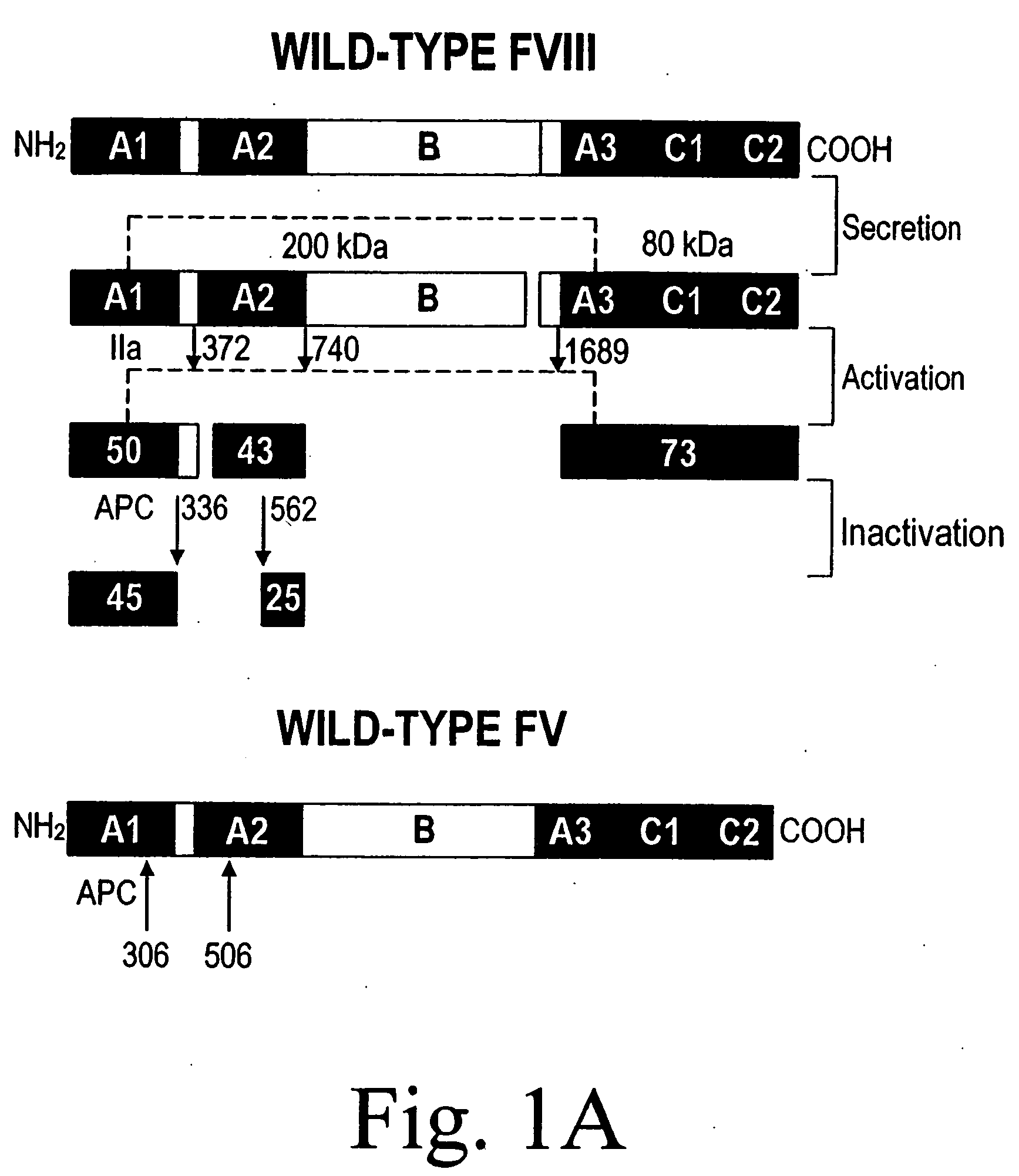
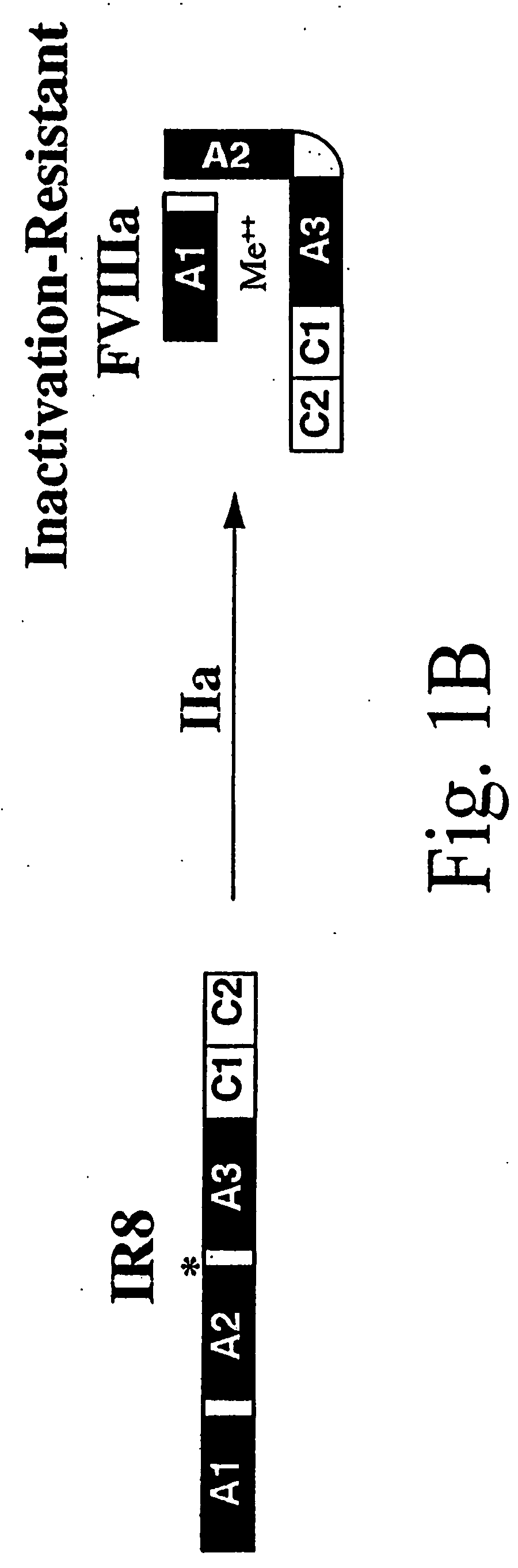
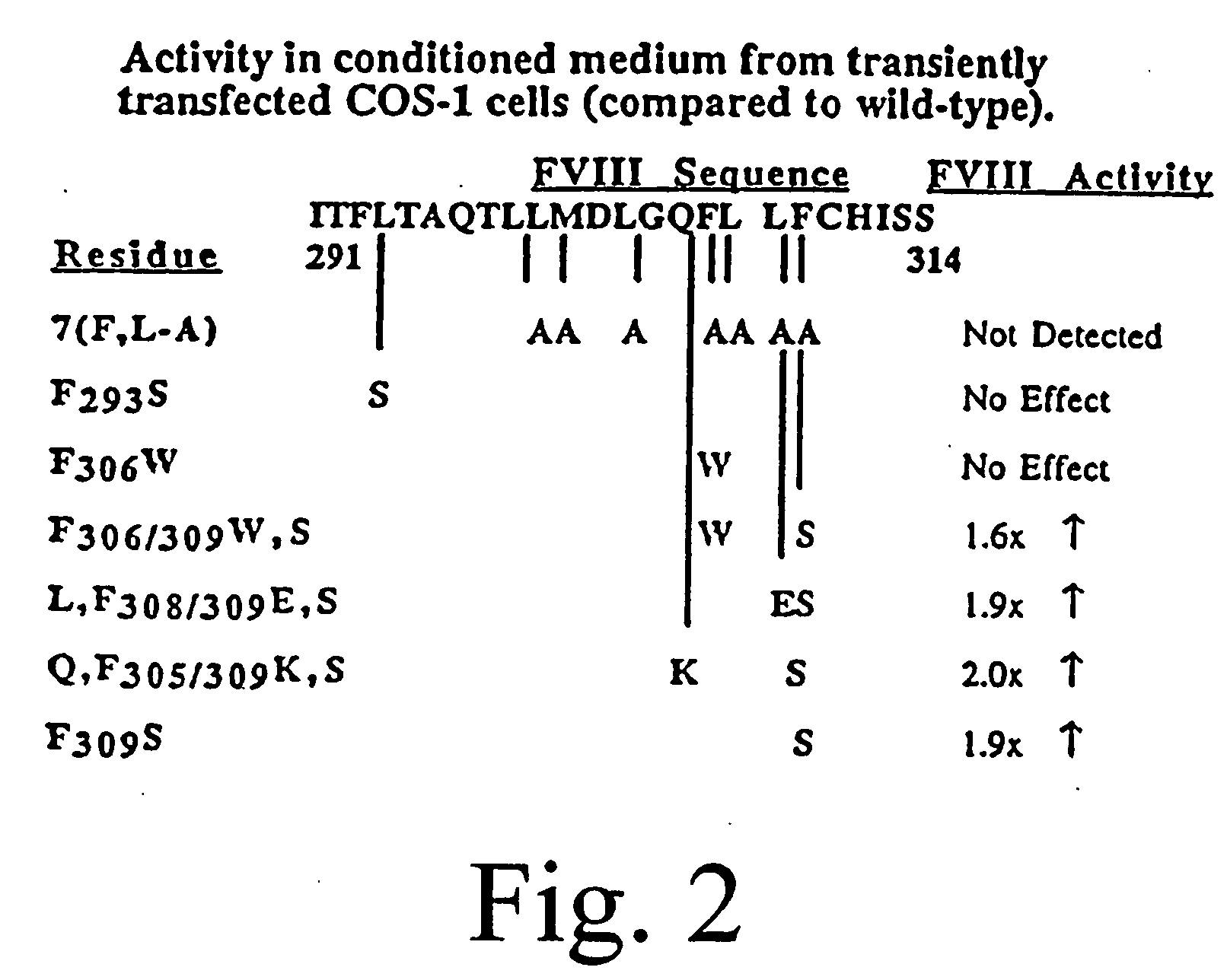
The present invention provides novel purified and isolated nucleic acid sequences encoding procoagulant-active FVIII proteins. The nucleic acid sequences of the present invention encode amino acid sequences corresponding to known human FVIII sequences, wherein residue Phe309 is mutated. The nucleic acid sequences of the present invention also encode amino acid sequences corresponding to known human FVIII sequences, wherein the APC cleavage sites, Arg336 and Ile562, are mutated. The nucleic acid sequences of the present invention further encode amino acid sequences corresponding to known human FVIII sequences, wherein the B-domain is deleted, the von Willebrand factor binding site is deleted, a thrombin cleavage site is mutated, an amino acid sequence spacer is inserted between the A2- and A3-domains. Methods of producing the FVIII proteins of the invention, nucleotide sequences encoding such proteins, pharmaceutical compositions containing the nucleotide sequences or proteins, as well as methods of treating patients suffering from hemophilia, are also provided.
Owner:RGT UNIV OF MICHIGAN
Modulation platelet adhesion based on the surface-exposed beta-switch loop of platelet glycoprotein IB-alpha
ActiveUS20050192224A1Trend downReduce decreaseFactor VIICell receptors/surface-antigens/surface-determinantsGlycoprotein IbChemistry
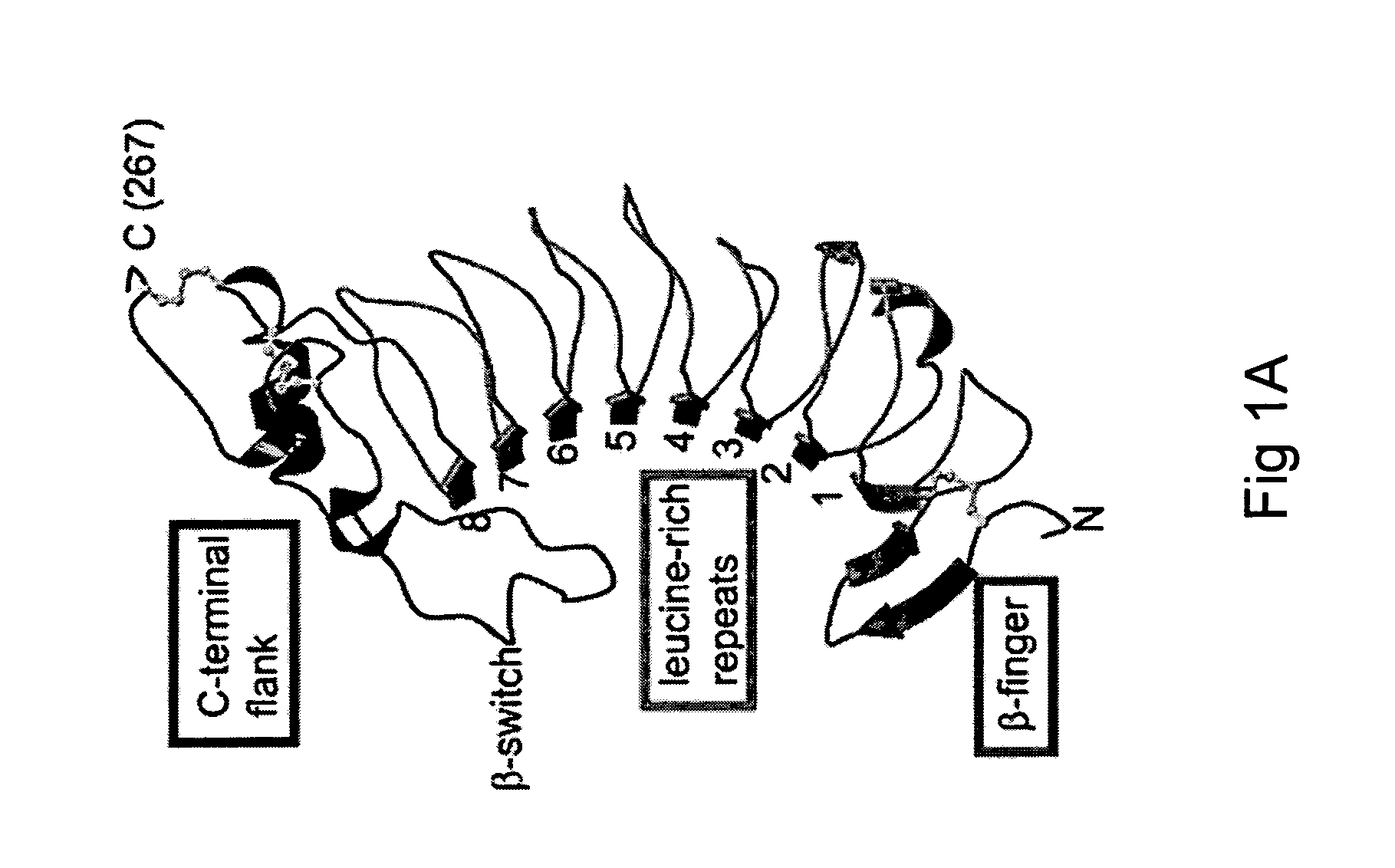
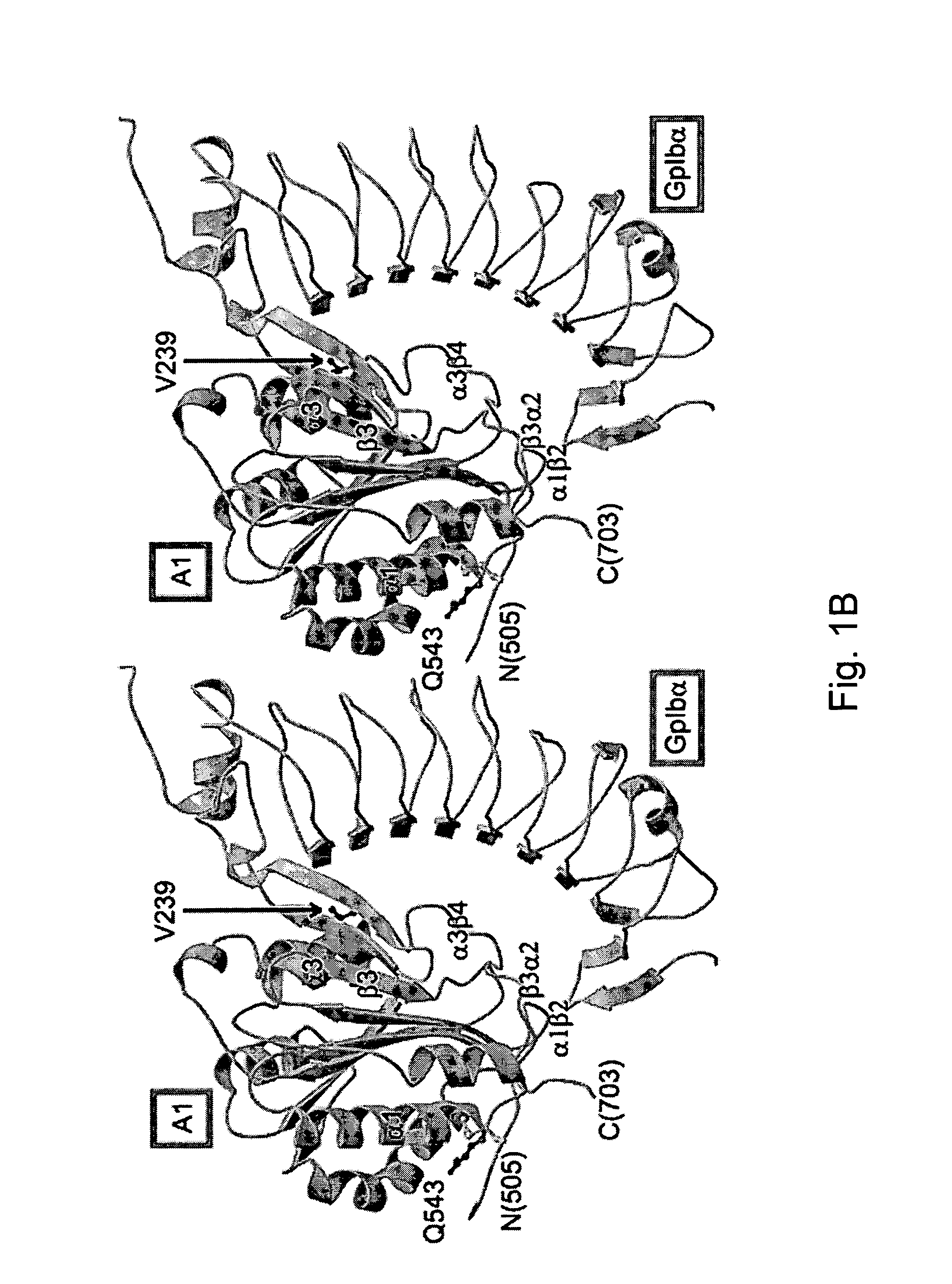
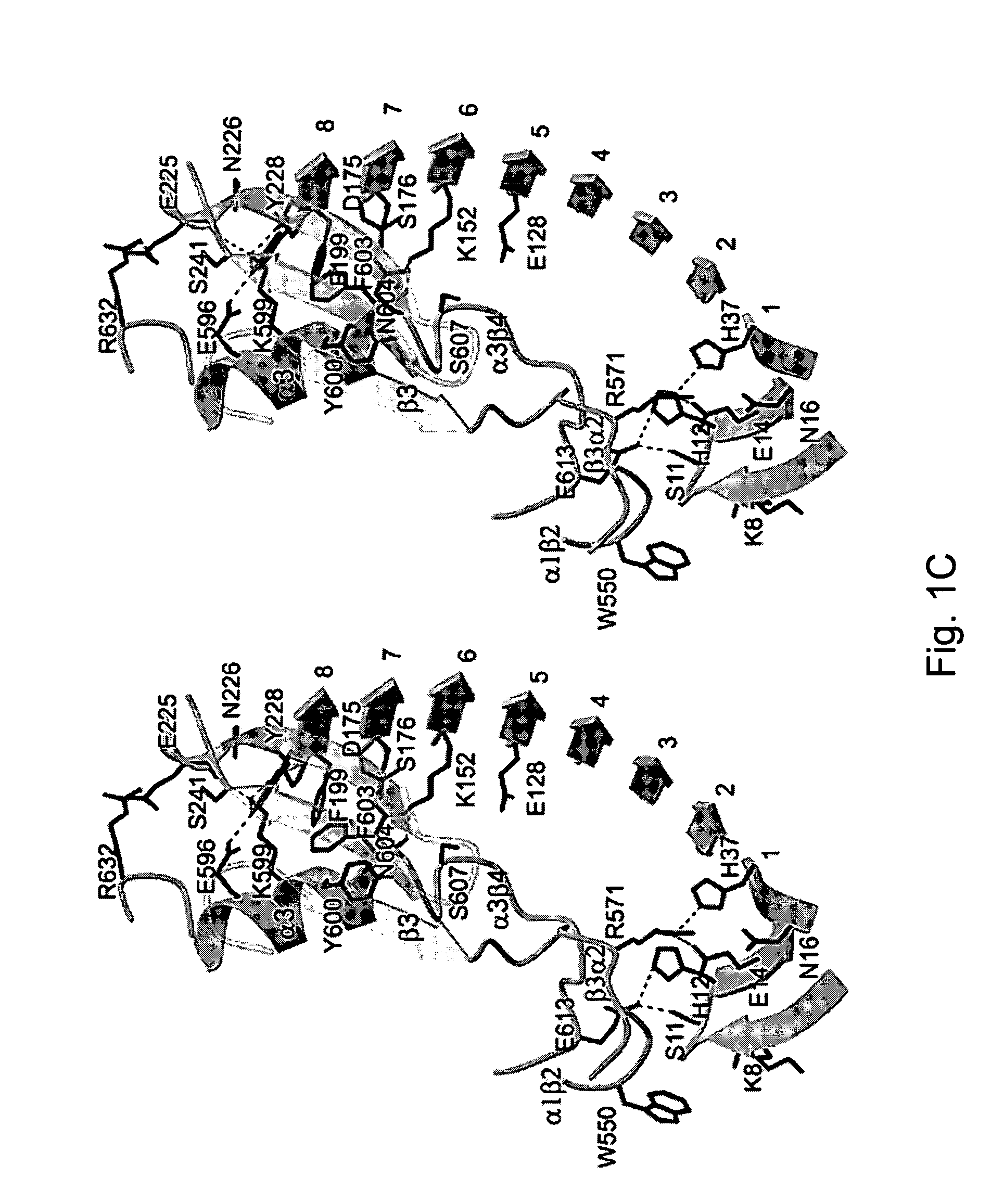
The invention relates to the adhesion of platelet GpIbα to strand β3 of domain A1 of von Willebrand factor (vWF), the strand β3 comprising amino acid residues at amino acid position 560-566 and / or a functional part or equivalent thereof, the platelet GpIbα, the GpIbα region comprising an amino acid sequence corresponding to a beta-switch loop of platelet GpIbα, comprising amino acid residues at amino acid position 227-242 and / or a functional part or equivalent thereof. The invention provides a method of interfering with adhesion of blood platelets to vWF that includes modulating adhesion. The invention further provides proteinaceous compounds, antibodies, medicaments and pharmaceutical compositions to that end. The invention also provides means and methods to increase platelet adhesion by topical application of a compound increasing platelet adhesion.
Owner:ABLYNX NV
Treatment of coagulation disease by administration of recombinant vwf
ActiveUS20120316116A1Extended half-lifeFactor VIIPeptide/protein ingredientsFactor VIII vWFVon willebrand
The present invention provides methods of treating coagulation disease, including hemophilia and von Willebrand disease by administering recombinant von Willebrand Factor alone or in combination with Factor VIII.
Owner:TAKEDA PHARMA CO LTD
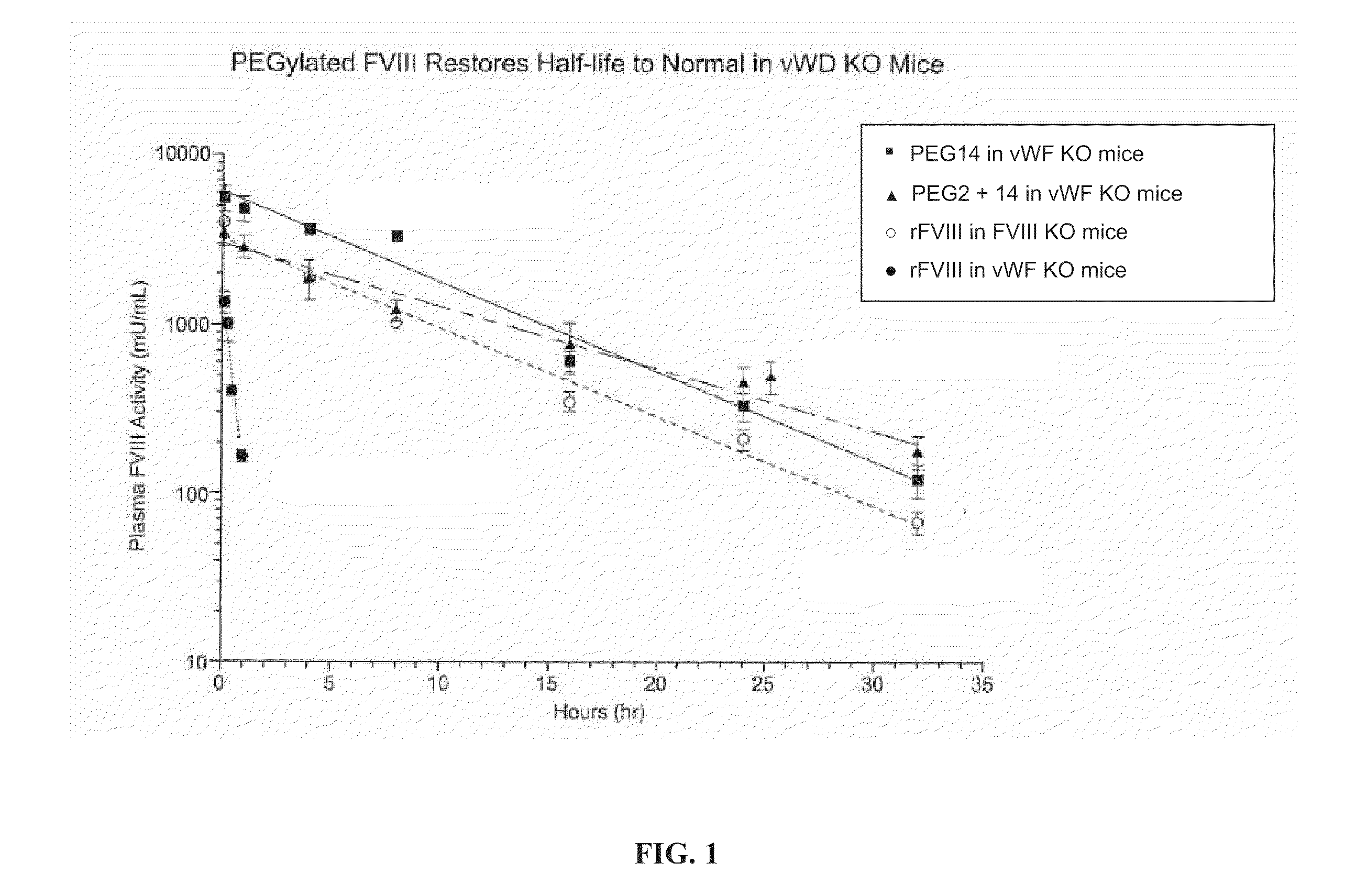
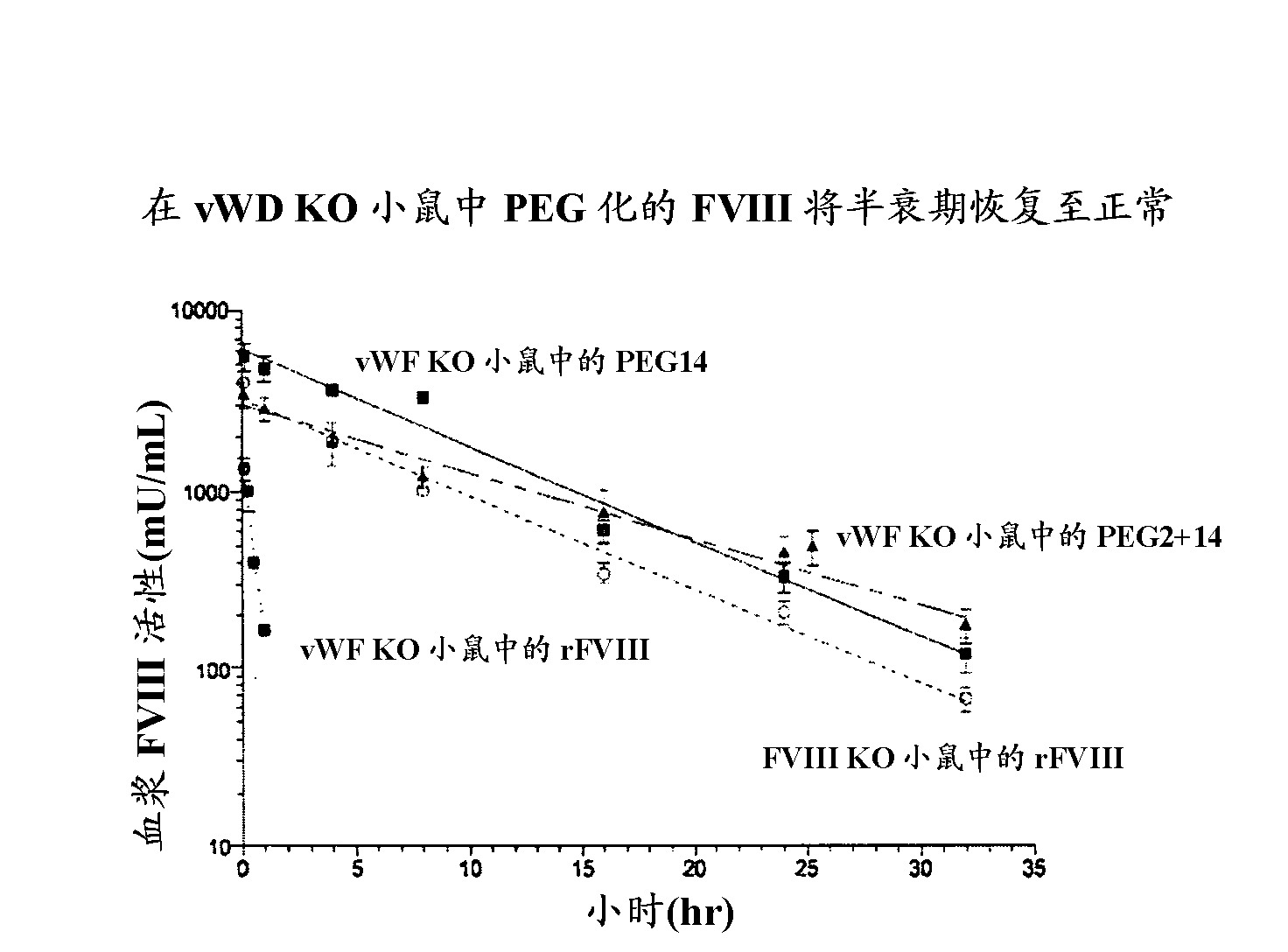
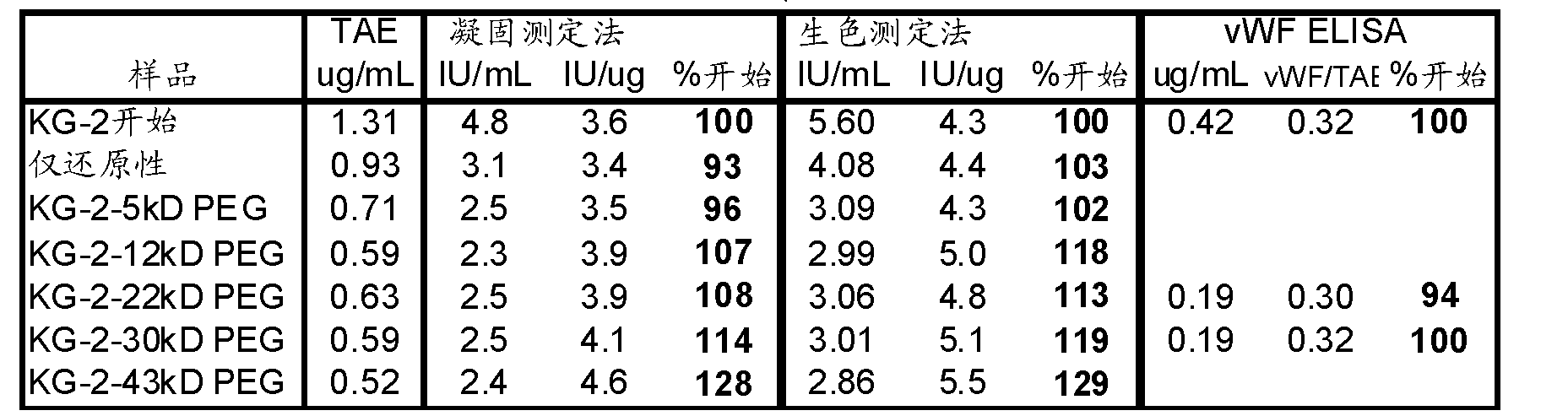
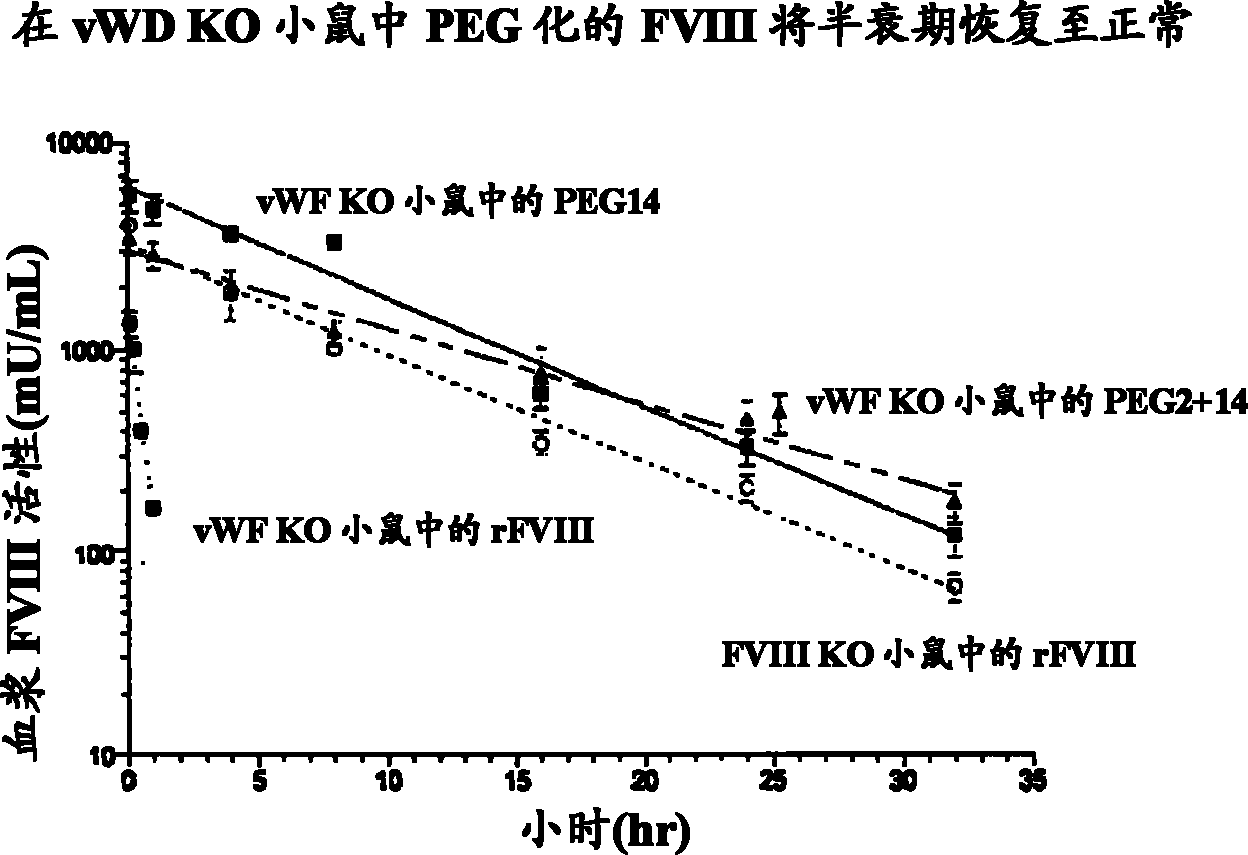
FVIII Muteins for Treatment of Von Willebrand Disease
InactiveUS20110286988A1Improve featuresImproved pharmacokinetic propertiesPeptide/protein ingredientsMammal material medical ingredientsFactor VIII vWFPolyethylene glycol
This invention relates to treatment of von Willebrand Disease by administration of Factor VIII muteins that are covalently bound, at a predefined site that is not an N-terminal amine, to one or more biocompatible polymers such as polyethylene glycol. The mutein conjugates retain FVIII procoagulant activity and have improved pharmacokinetic properties in subjects lacking von Willebrand Factor.
Owner:BAYER HEALTHCARE LLC
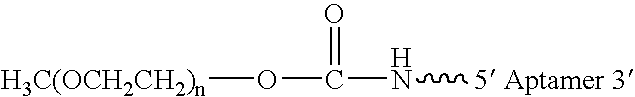
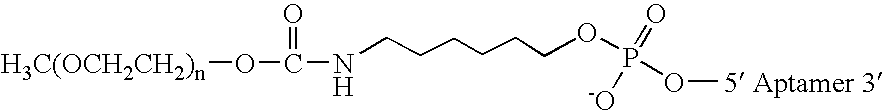
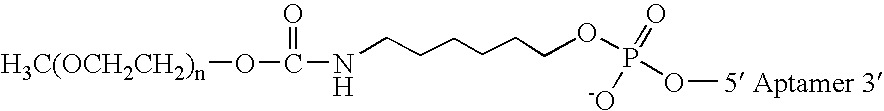
Aptamers to von Willebrand factor and their use as thrombotic disease therapeutics
InactiveUS20060183702A1Shorten bleeding timeInhibits platelet aggregationSugar derivativesGenetic material ingredientsFactor VIII vWFThrombus
The invention relates generally to the field of nucleic acids and more particularly to aptamers capable of binding to von Willebrand Factor useful as therapeutics in and diagnostics of thrombotic diseases and / or other diseases or disorders in which von Willebrand Factor mediated platelet aggregation has been implicated. The invention further relates to materials and methods for the administration of aptamers capable of binding to von Willebrand Factor.
Owner:ARCHEMIX CORP
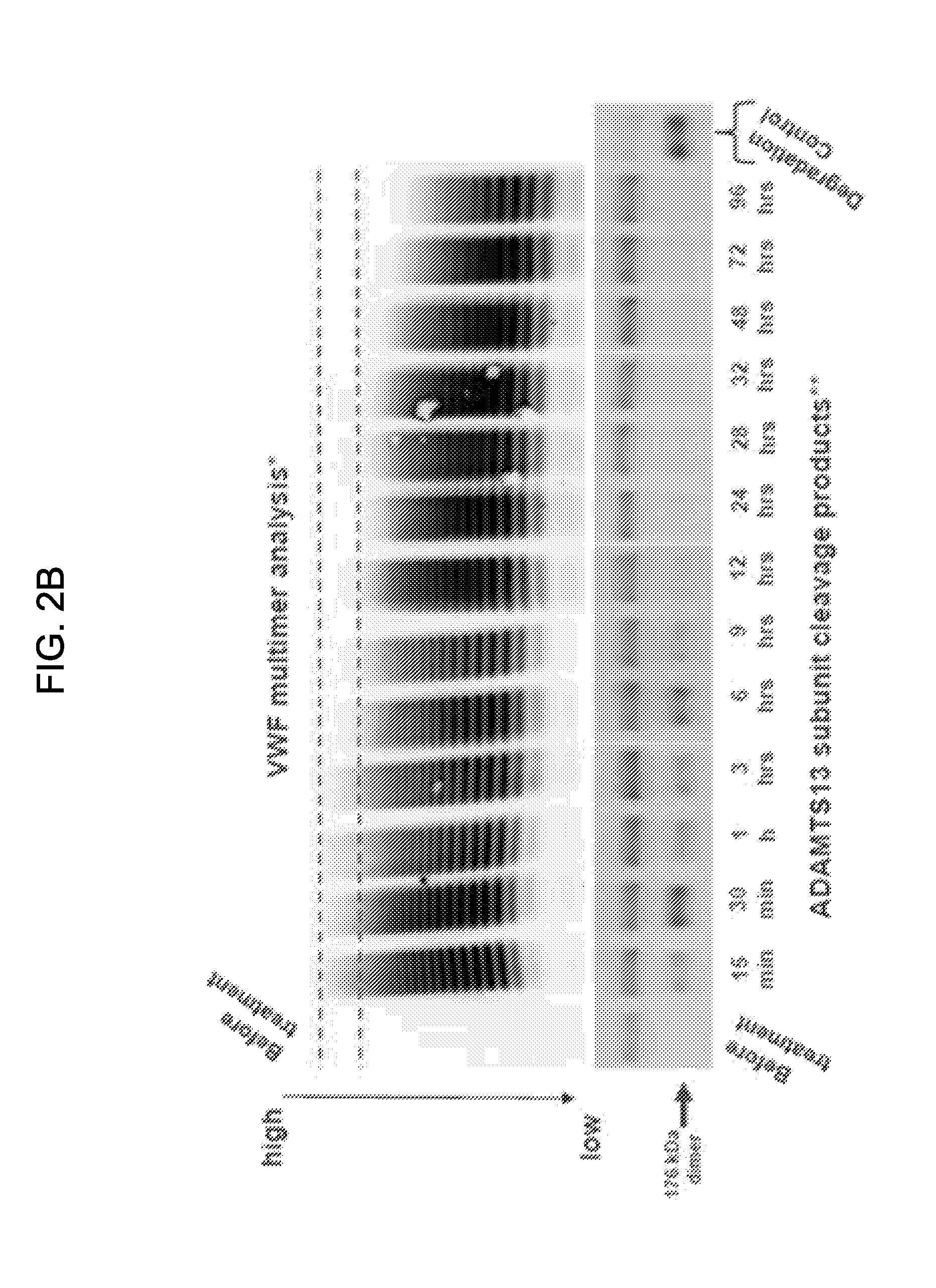
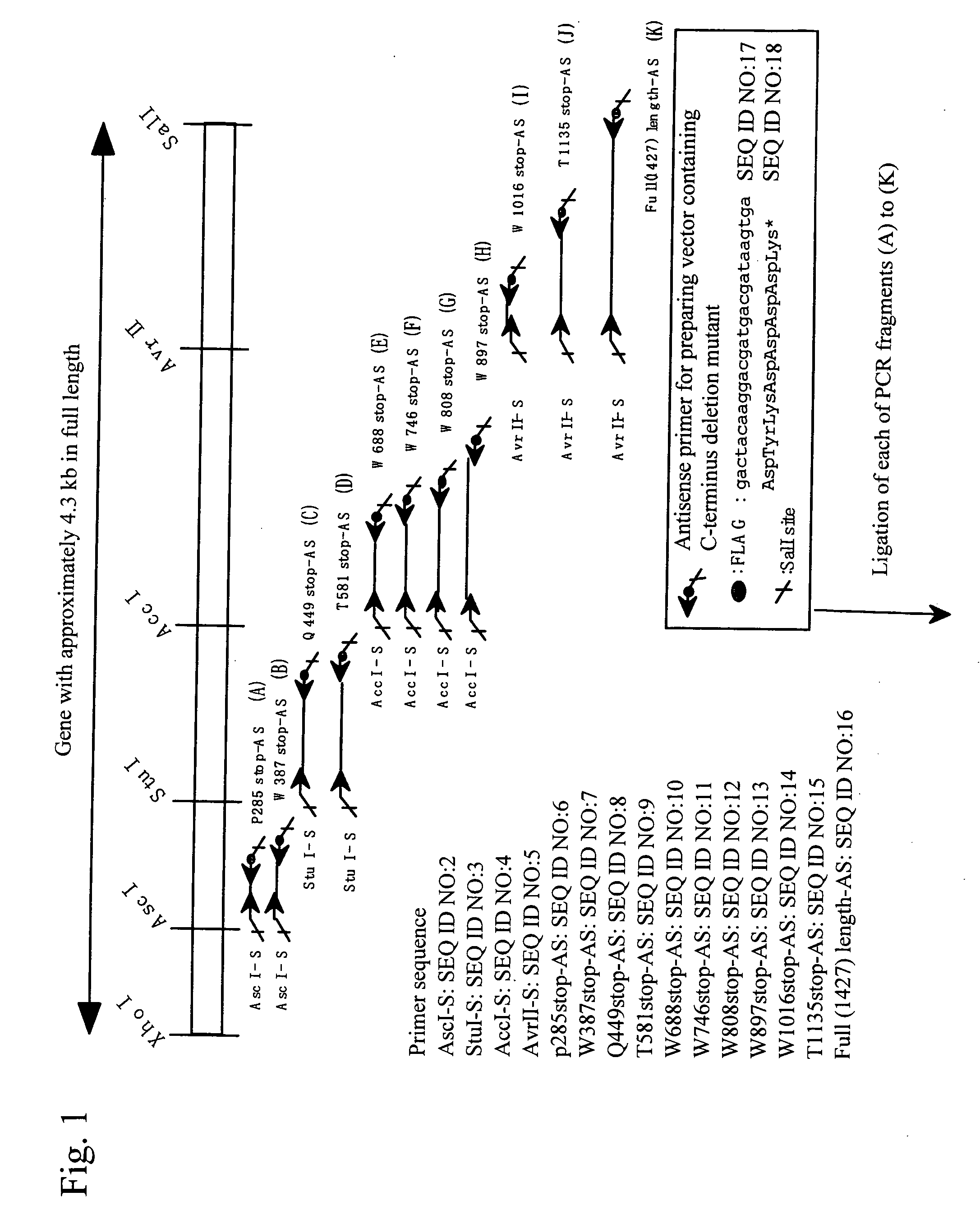
Construct comprising recognition domain of antibody against von willebrand factor-specific cleaving enzyme
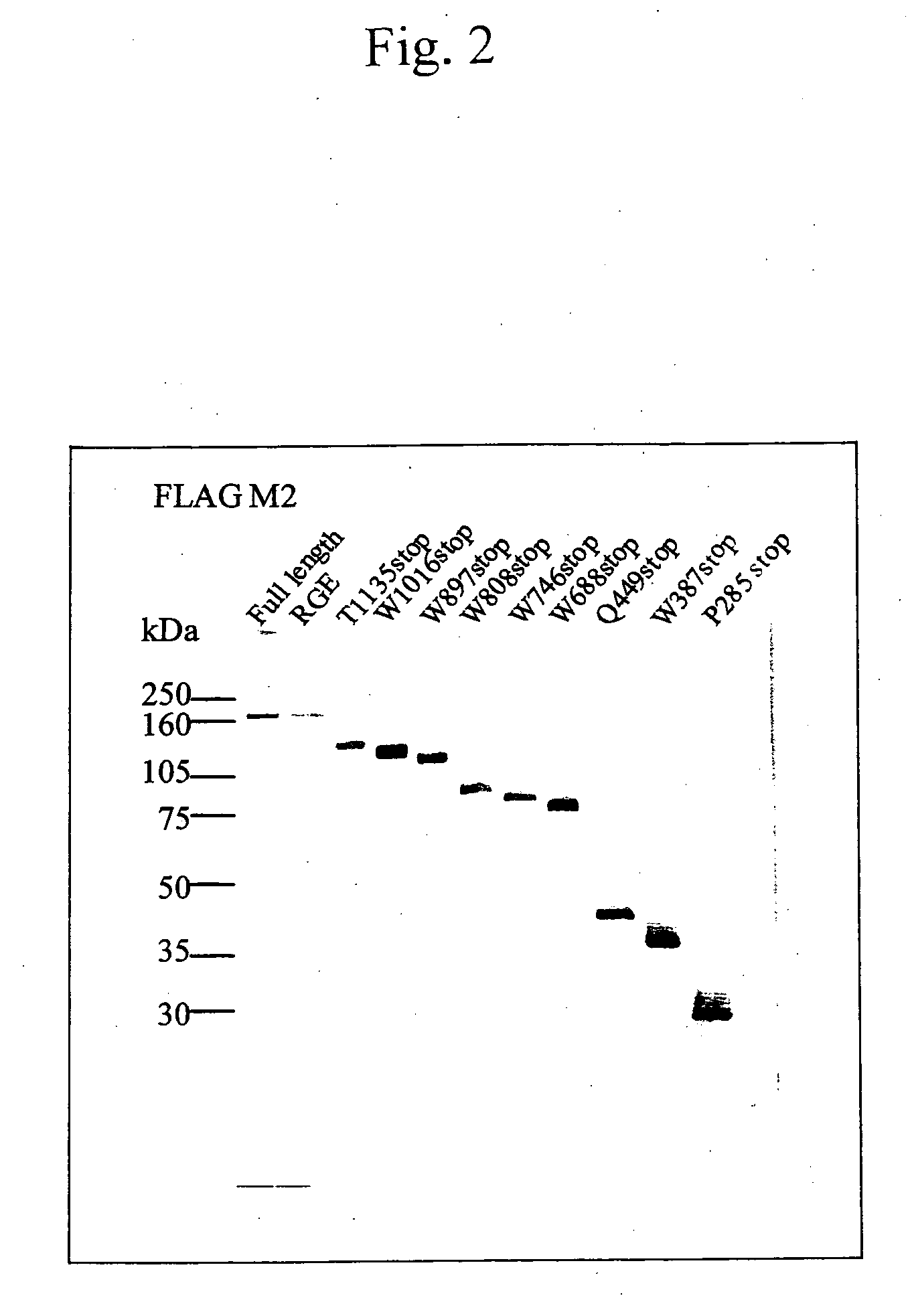
The present invention provides an epitope recognized by an antibody (hereinafter, also referred to as an anti-ADAMTS-13 antibody) against a cleaving protease (hereinafter, also referred to as ADAMTS-13) specific to von Willebrand factor (hereinafter, also referred to as vWF), and a polypeptide comprising the epitope region. The present invention also provides a polypeptide located in a region from position 449 to position 687 in an amino acid sequence composing the ADAMTS-13, which is recognized by the anti-ADAMTS-13 antibody, or a peptide fragment derived from the polypeptide.
Owner:KM BIOLOGICS CO LTD
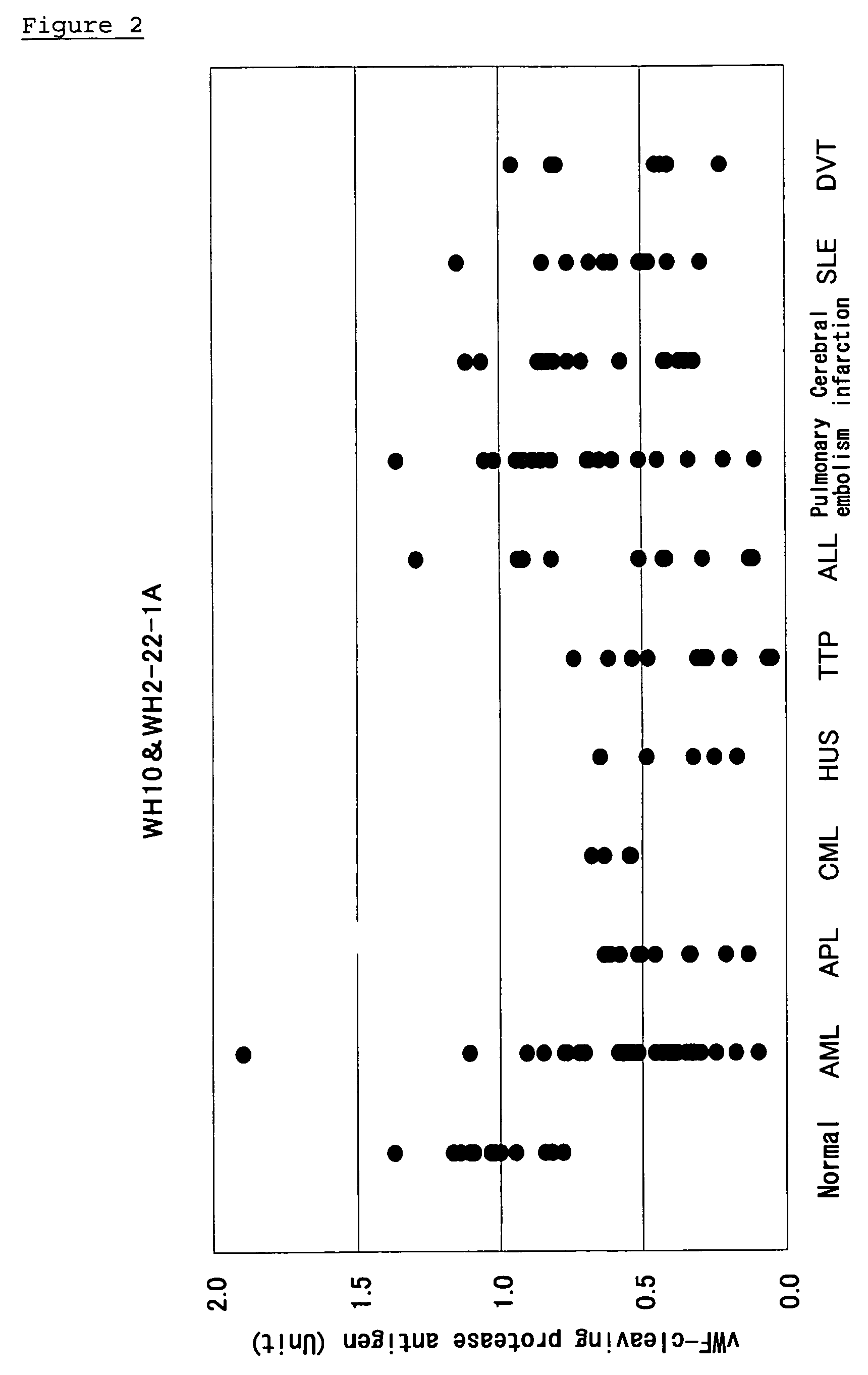
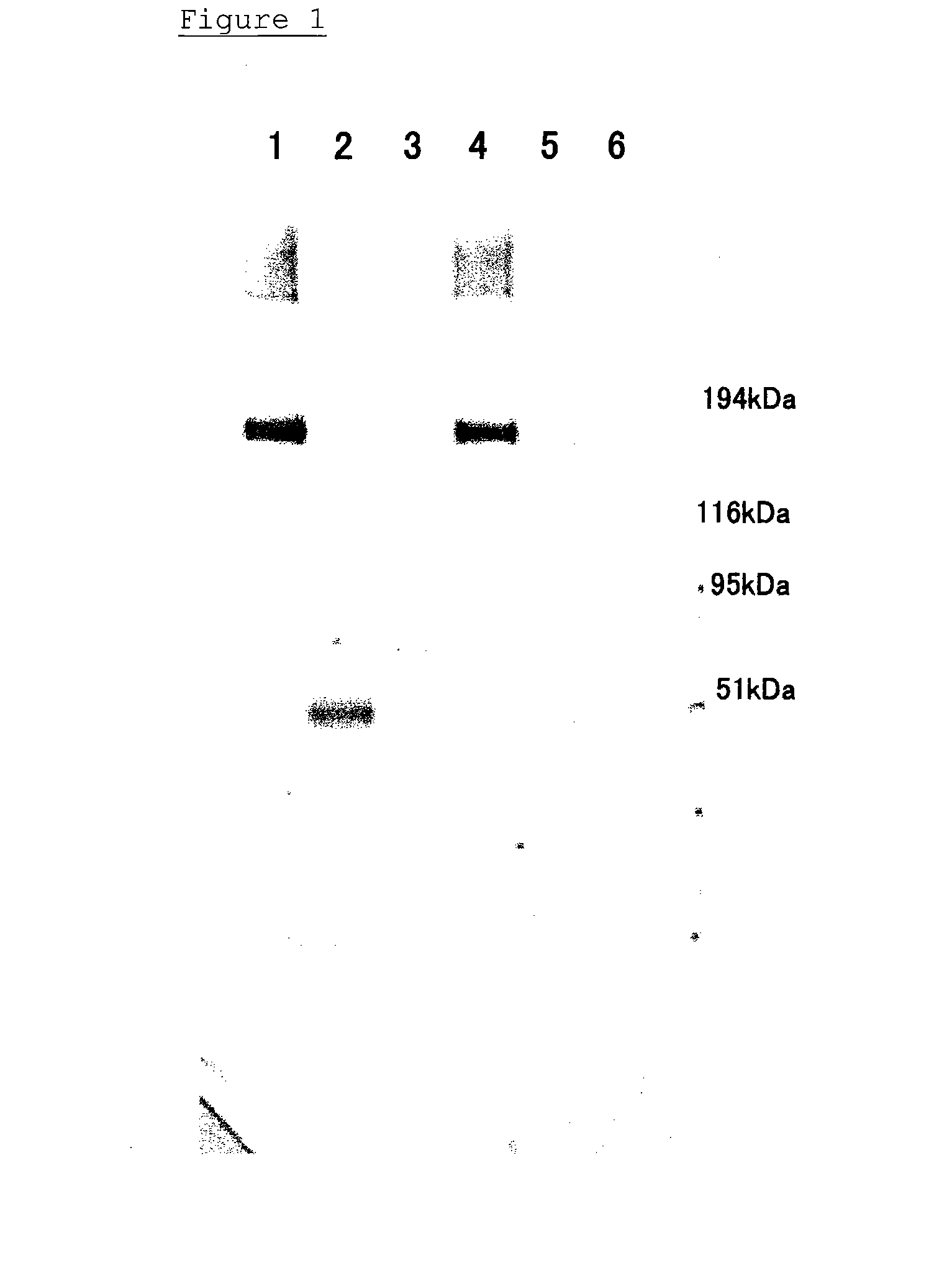
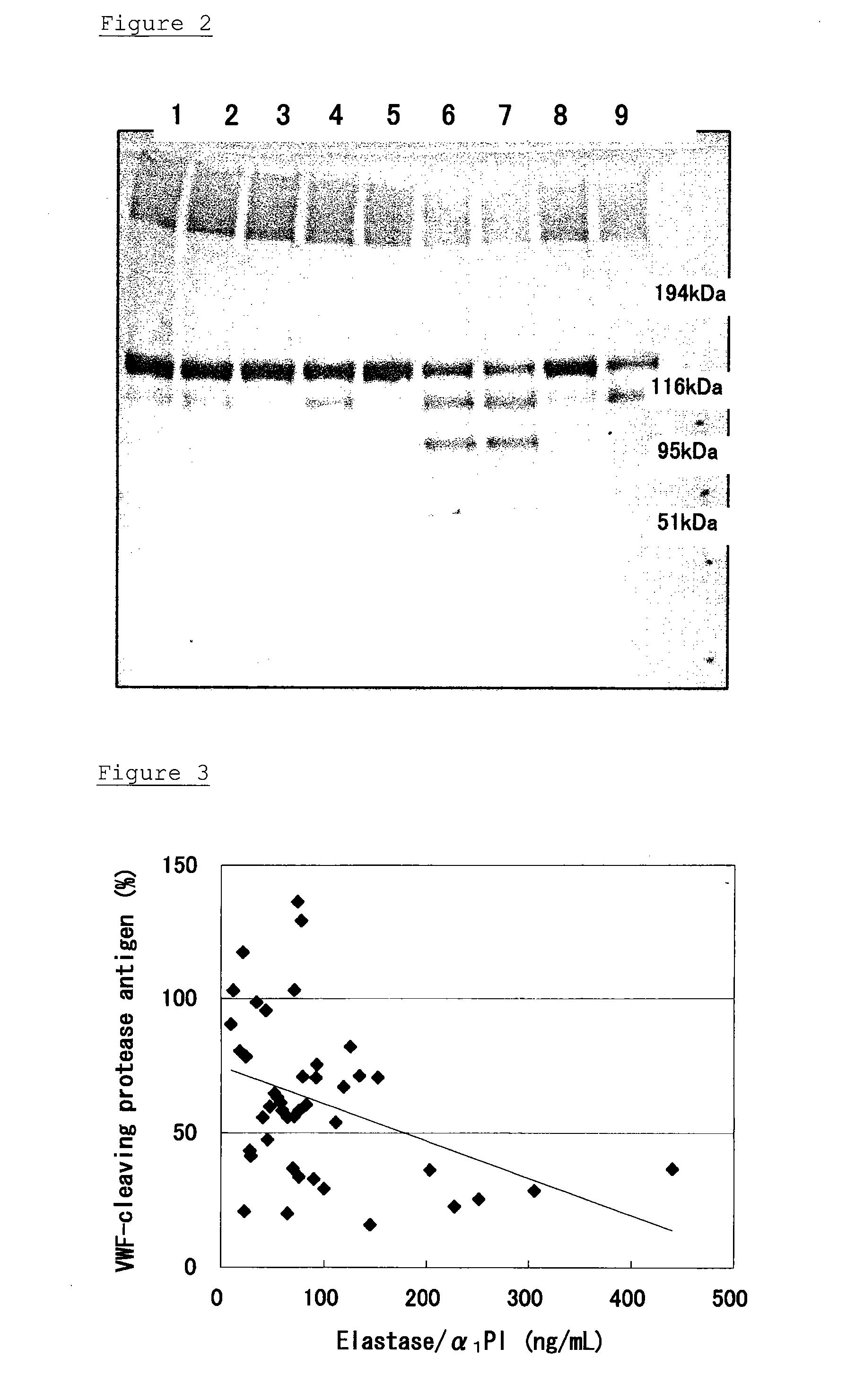
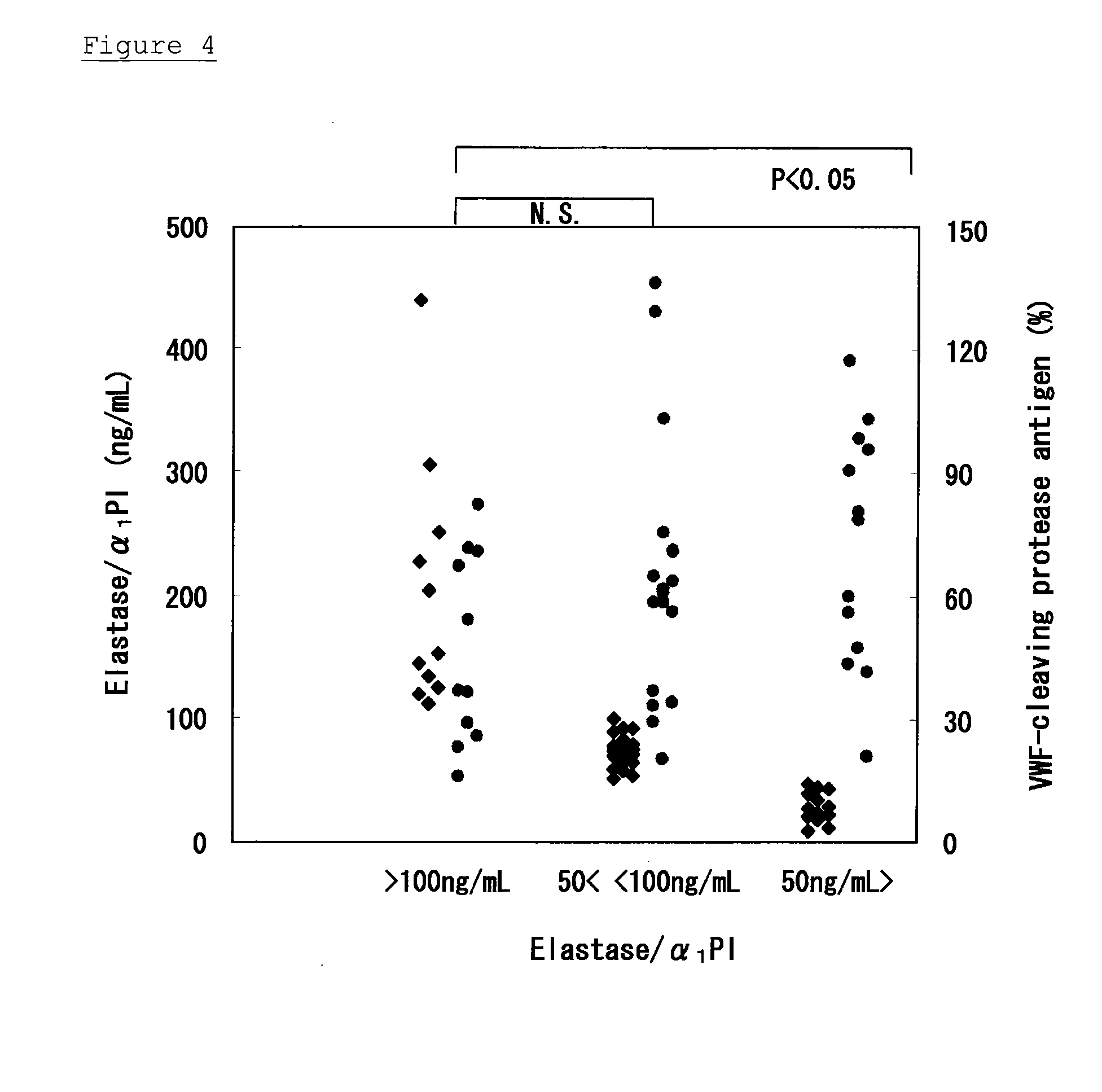
Method of Detecting Thrombosis by Measuring Von Willenbrand Factor-Cleaving Protease
ActiveUS20070275414A1Microbiological testing/measurementDisease diagnosisProteinase activityVon willebrand
A method of detecting thrombosis or the degree of thrombophilia by measuring a von Willebrand factor cleaving protease, and a kit for detecting thrombosis or the degree of thrombophilia, comprising an antibody or a fragment thereof specifically binding to a von Willebrand factor-cleaving protease, are disclosed. The detection method and the detection kit have an excellent convenience, rapidity, and specificity.
Owner:KM BIOLOGICS CO LTD
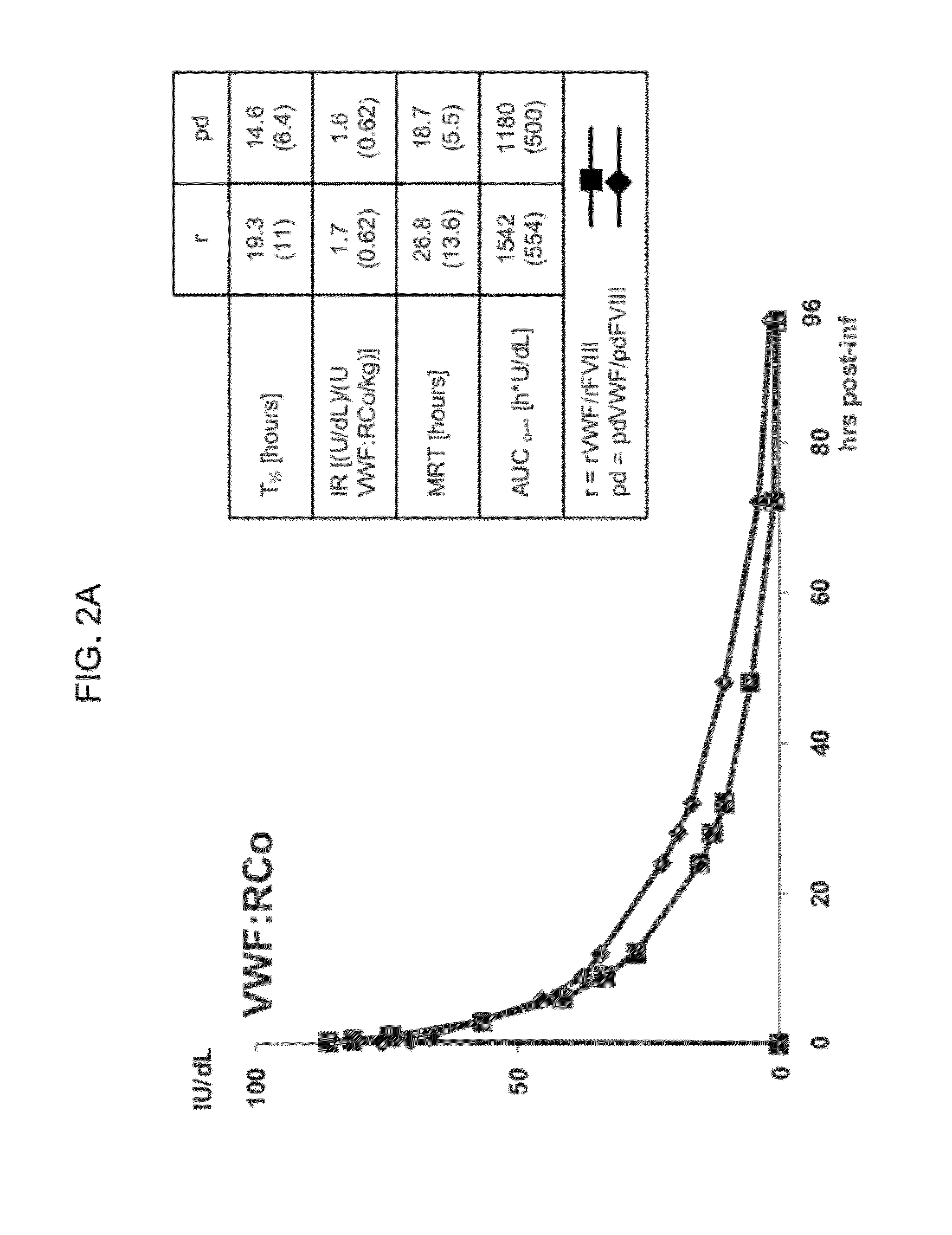
Process for the preparation of a von Willebrand (FvW) factor concentrate by chromatography and a FvW concentrate thus obtainable
InactiveUS20060036081A1Quality improvementHigh specific activityFactor VIIApolipeptidesChromatographic separationVon willebrand
This invention relates to a process for the preparation of a very high purity von Willebrand factor concentrate from a biological fraction containing von Willebrand factor, including a separation by anion exchange chromatography using a vinyl polymer support of weak base type, the said separation comprising the steps of loading of the chromatographic support with the fraction containing von Willebrand factor, previously equilibrated with a suitable buffer, with a predetermined flowrate allowing the retention of the von Willebrand factor, washing of the support with an acidic buffer with a flowrate higher than the flowrate of the step a) until the not-retained proteins and the contaminants are removed, flushing and equilibrating of the chromatographic support with the buffer and using the flowrate of the step a), and elution of the von Willebrand factor by increasing of the ionic strength of the step c). The invention also relates to a von Willebrand factor concentrate for therapeutic use likely to be obtained by implementing of the process wherein the rate of Factor VIII:C / FvW:RCo is less than 0.06%.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
Method Of Detecting Platelet Thrombosis Or Organ Failure
InactiveUS20080096221A1Increase concentrationMicrobiological testing/measurementWithdrawing sample devicesCleavage factorWhole body
Owner:MITSUBISHI CHEM MEDIENCE
Liquid, Aqueous Pharmaceutical Composition of Factor VII Polypeptides
InactiveUS20100166730A1Improve stabilityHeavy metal active ingredientsPeptide/protein ingredientsClotting factor deficiencyOxidation state
The present invention is directed to liquid, aqueous pharmaceutical compositions containing Factor VII polypeptides, and methods for preparing and using such compositions, as well as vials containing such compositions, and the use of such compositions in the treatment of a Factor VII-responsive syndrome, e.g., bleeding disorders, including those caused by clotting Factor deficiencies (e.g. haemophilia A, haemophilia B, coagulation Factor VII deficiency); by thrombocytopenia or von Willebrand's disease, or by clotting Factor inhibitors, and intra cerebral haemorrhage, or excessive bleeding from any cause. The preparations may also be administered to patients in association with surgery or other trauma or to patients receiving anticoagulant therapy. More particularly, the invention relates to liquid compositions stabilised against chemical and / or physical degradation. The main embodiment is represented by a liquid, aqueous pharmaceutical composition comprising a Factor VII polypeptide (i); a buffering agent (ii) suitable for keeping pH in the range of from about 4.0 to about 9.0; at least one metal-containing agent (iii), wherein said metal is selected from the group consisting of first transition series metals of oxidation state +II, except zinc, such as chromium, manganese, iron, cobalt, nickel, and copper; and a non-ionic surfactant (iv).
Owner:NOVO NORDISK HEALTH CARE AG
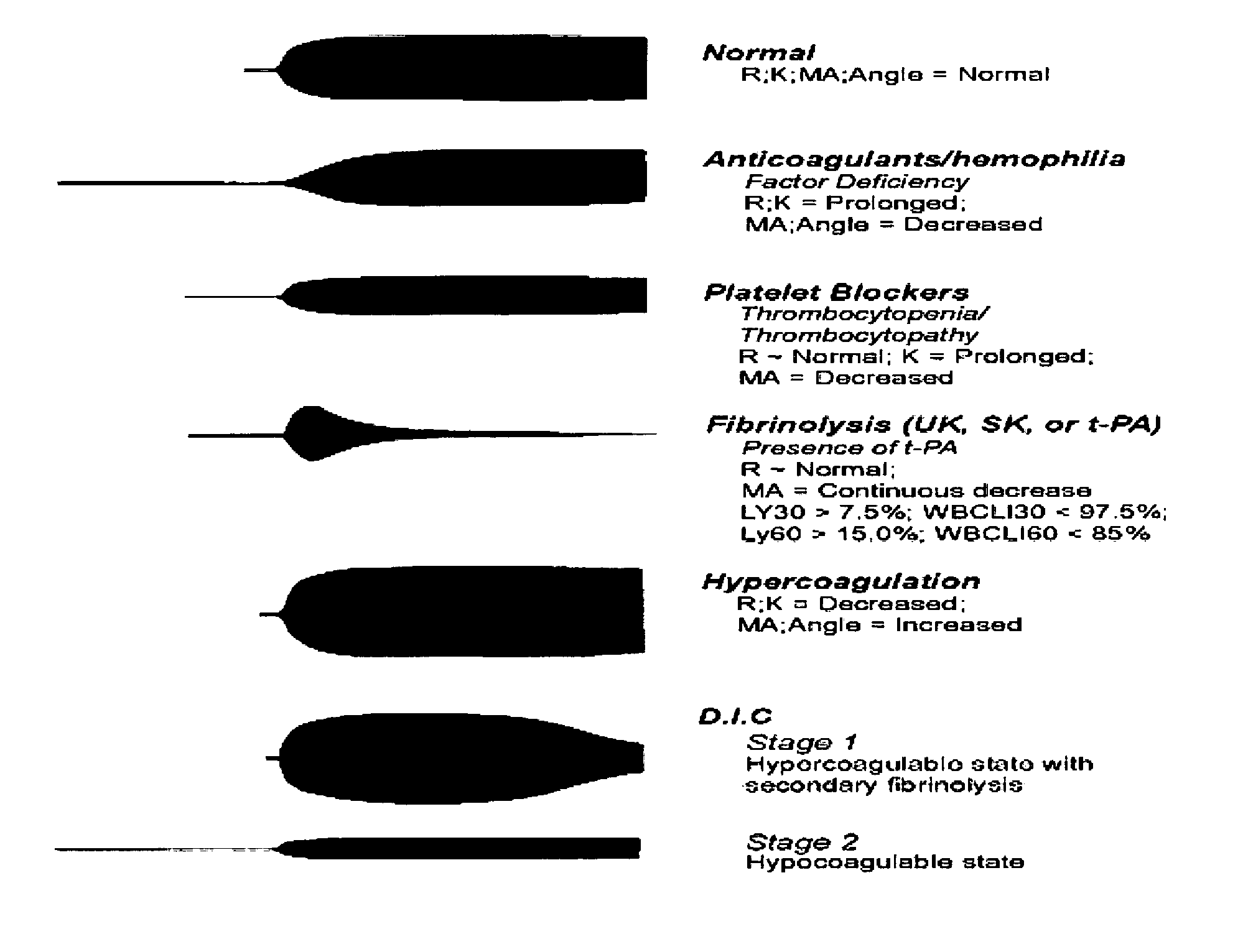
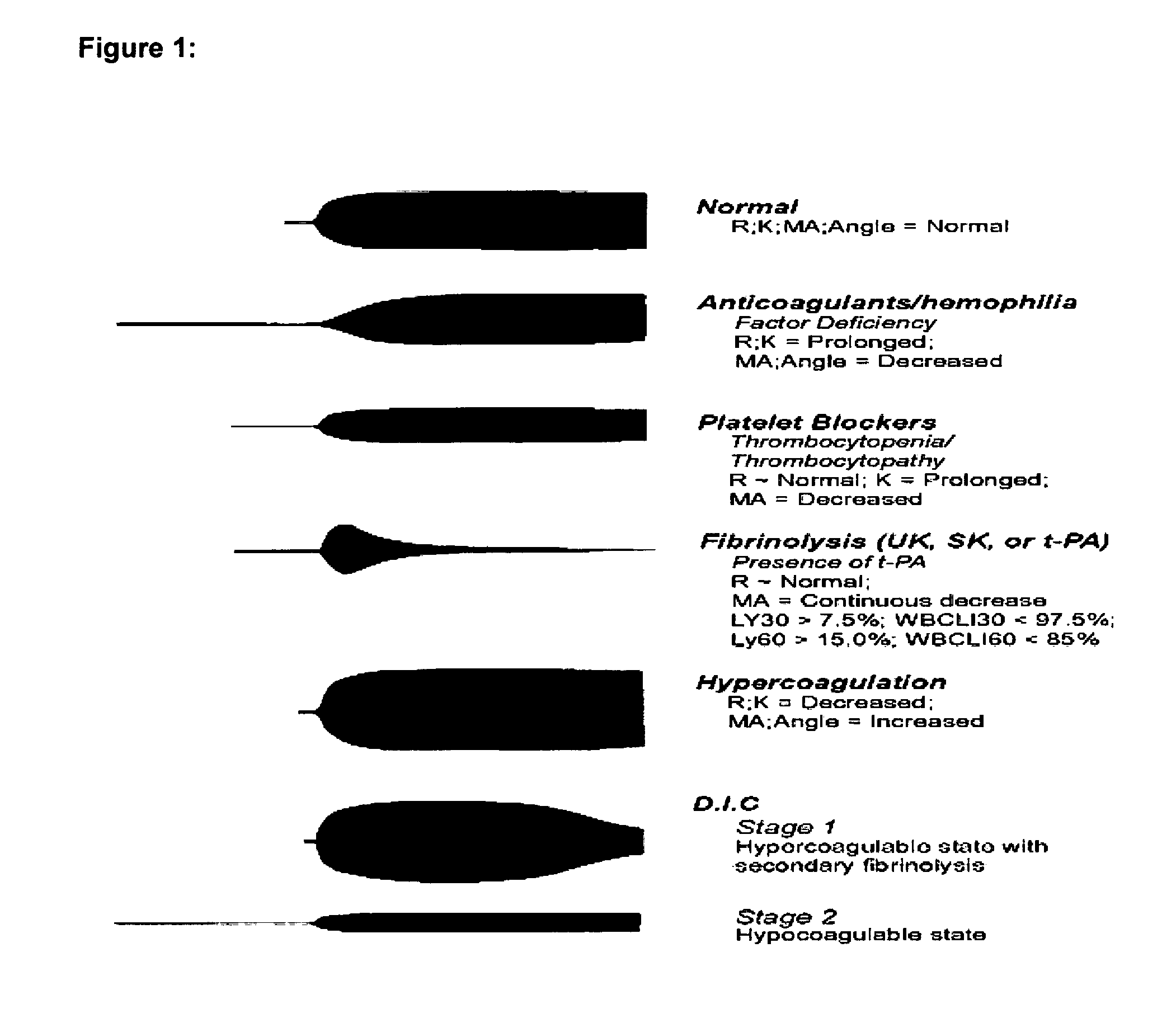
Diagnostic in vitro method for assessing von willebrand disease and increased bleeding risk associated with von willebrand disease and acquired or congenital disorders of platelet function
InactiveUS20100273206A1Superior in predicting bleeding riskMicrobiological testing/measurementDisease diagnosisPoint of careFactor ii
The invention relates to an in-vitro method for diagnosing Von Willebrand Disease (VWD) and an increased bleeding risk associated with Von Willebrand Disease and / or acquired or congenital platelet function defects that reduce the interactions of Von Willebrand Factor (VWF) with platelets. The in-vitro method of the invention may also be used to diagnose further bleeding risks. The test is suitable for use as a screening test based on whole blood and has the additional benefit of being suitable as a point of care test. The method involves the incubation of a sample containing platelets and hemostasis factors with an activator of platelet aggregation and the measurement of the viscoelastic change after inducing coagulation, e.g., by means of thromboelastography (TEG).
Owner:CSL BEHRING GMBH
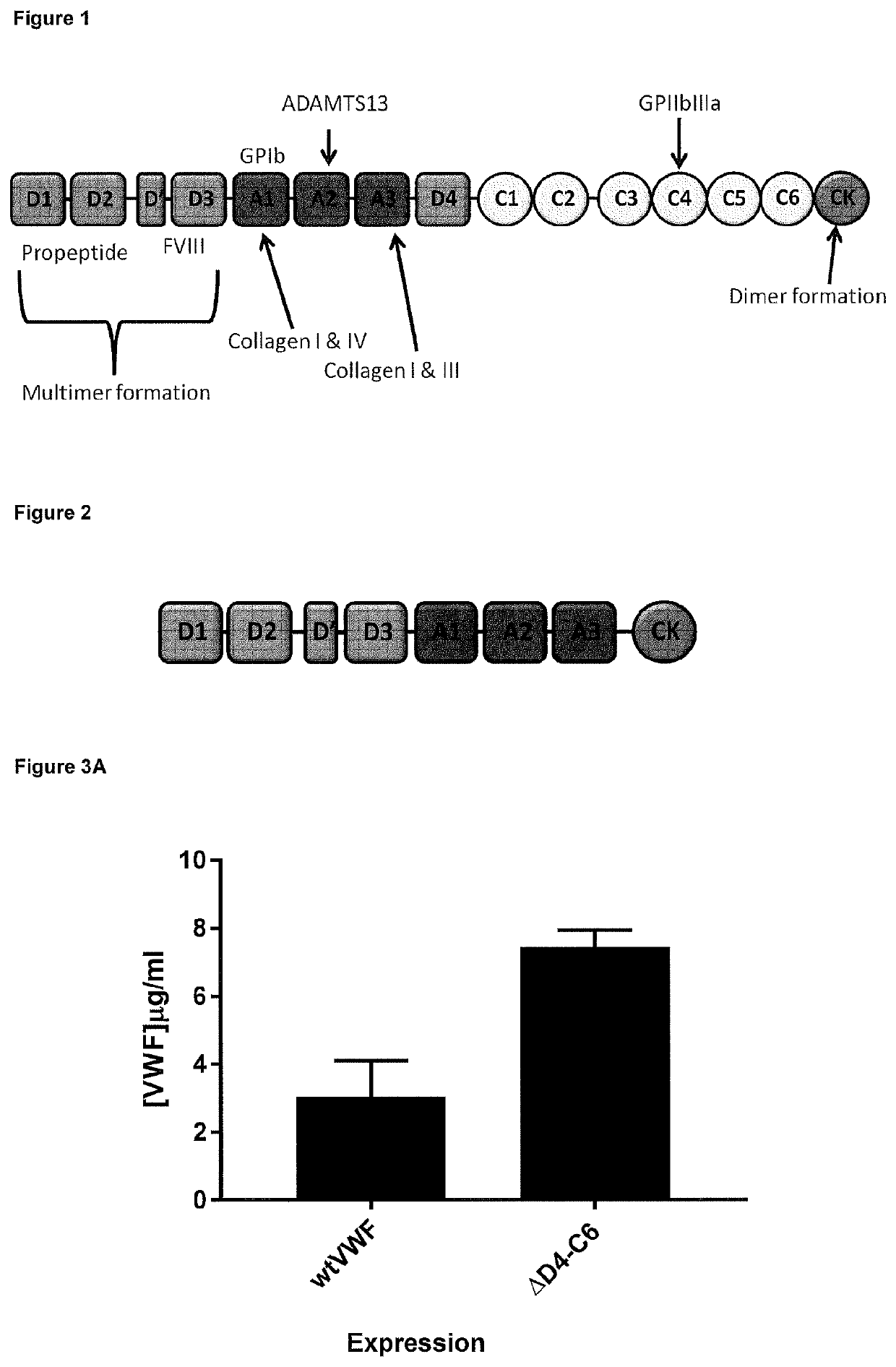
Truncated vwf
ActiveUS20200157186A1Improve stabilityEasy to transportFactor VIIOrganic active ingredientsFactor VIII vWFEndocrinology
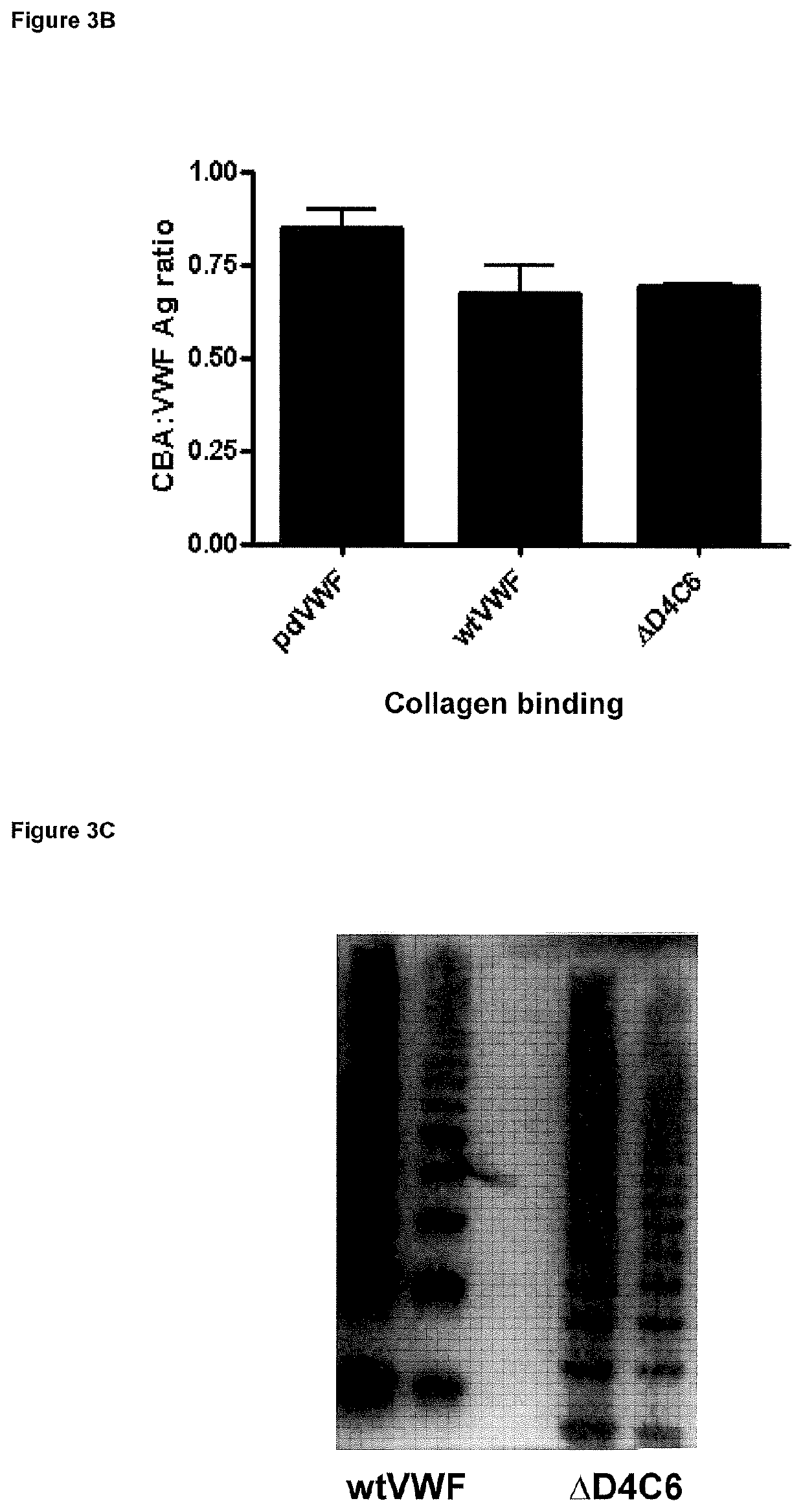
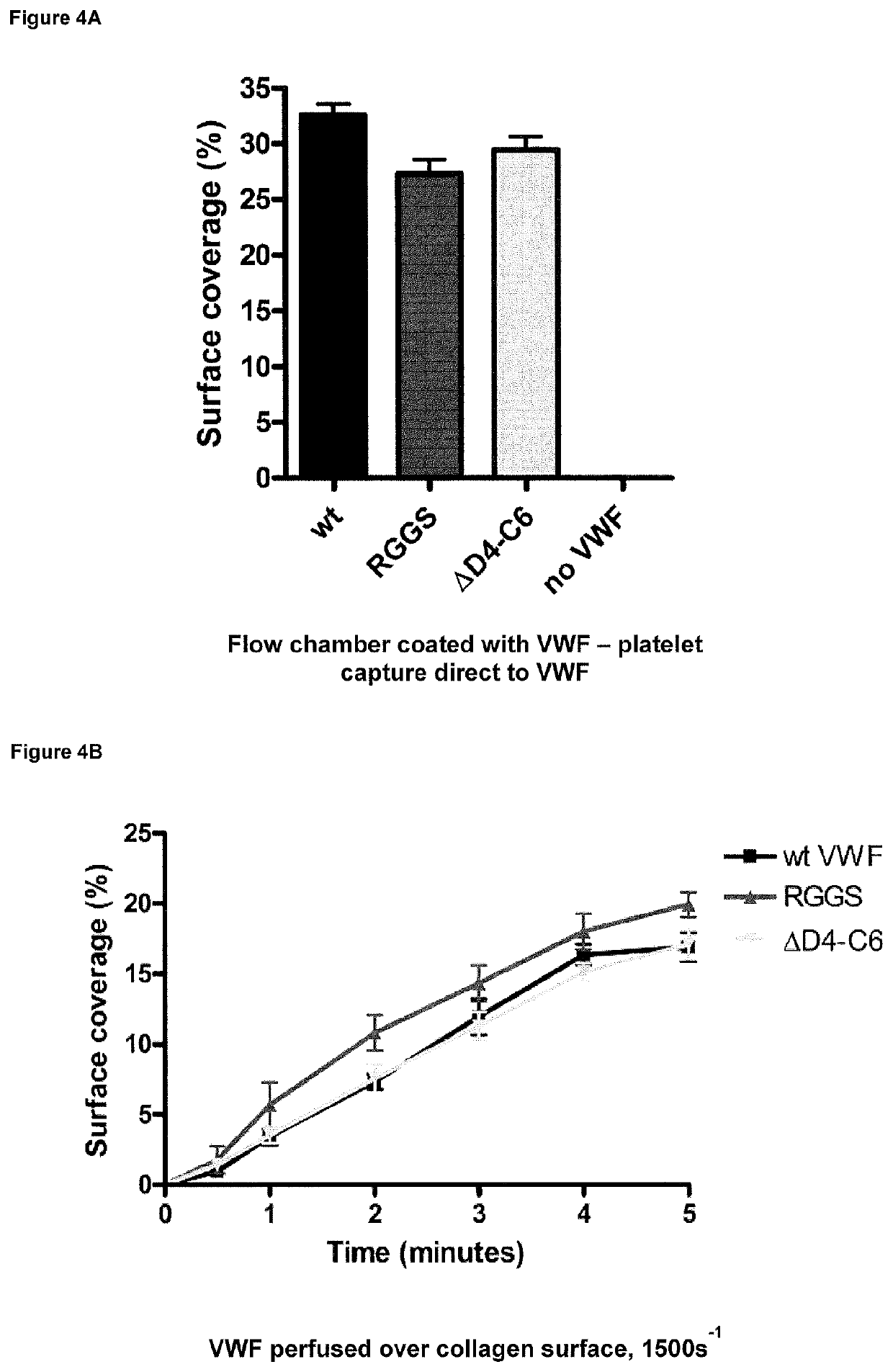
The present invention relates to novel truncated fragments of von Willebrand factor (VWF) and the use of such fragments and nucleic acids encoding such fragments in the treatment of von Willebrand disease (VWD) and haemophilia.
Owner:IMPERIAL INNOVATIONS LTD
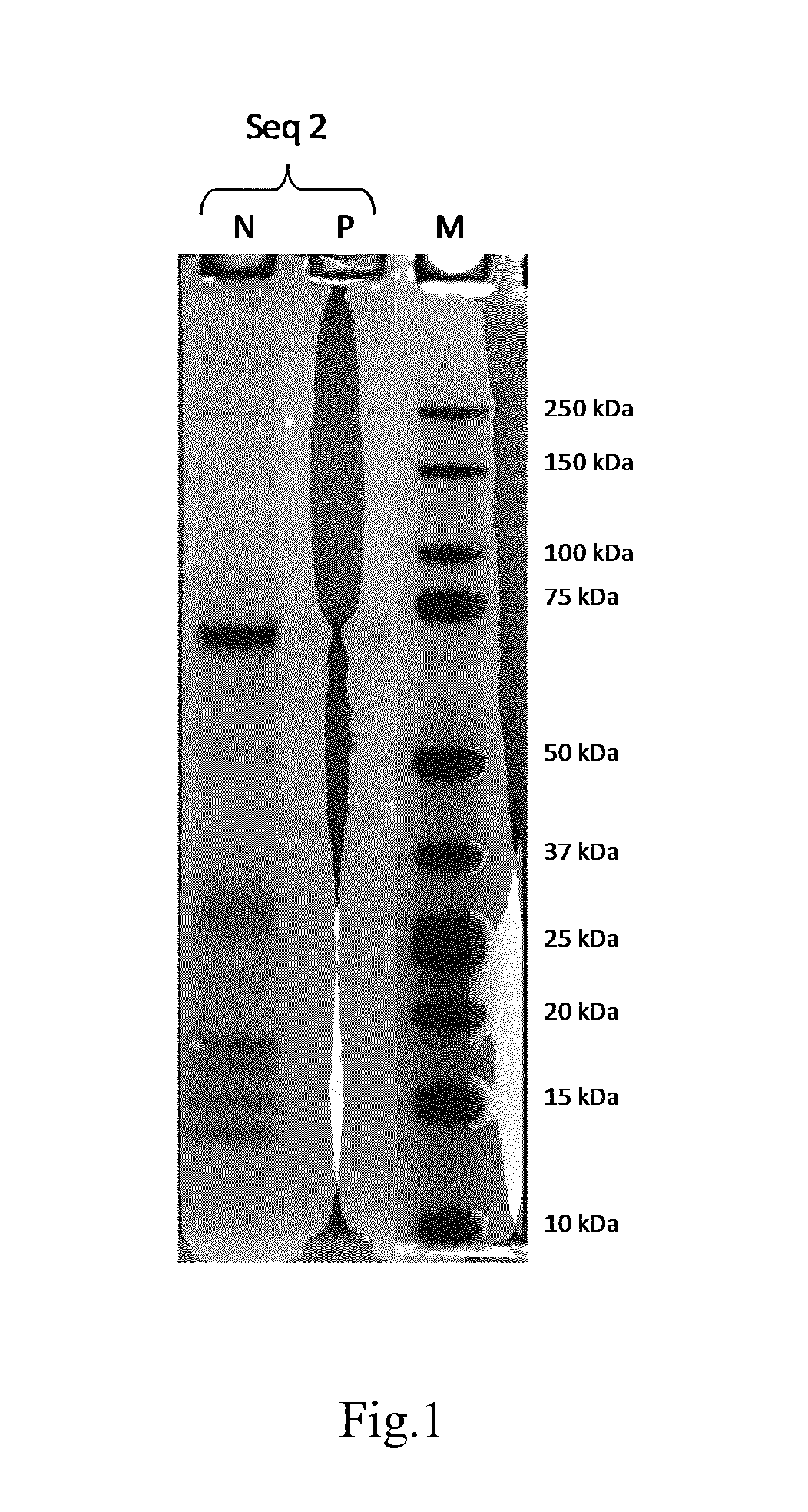
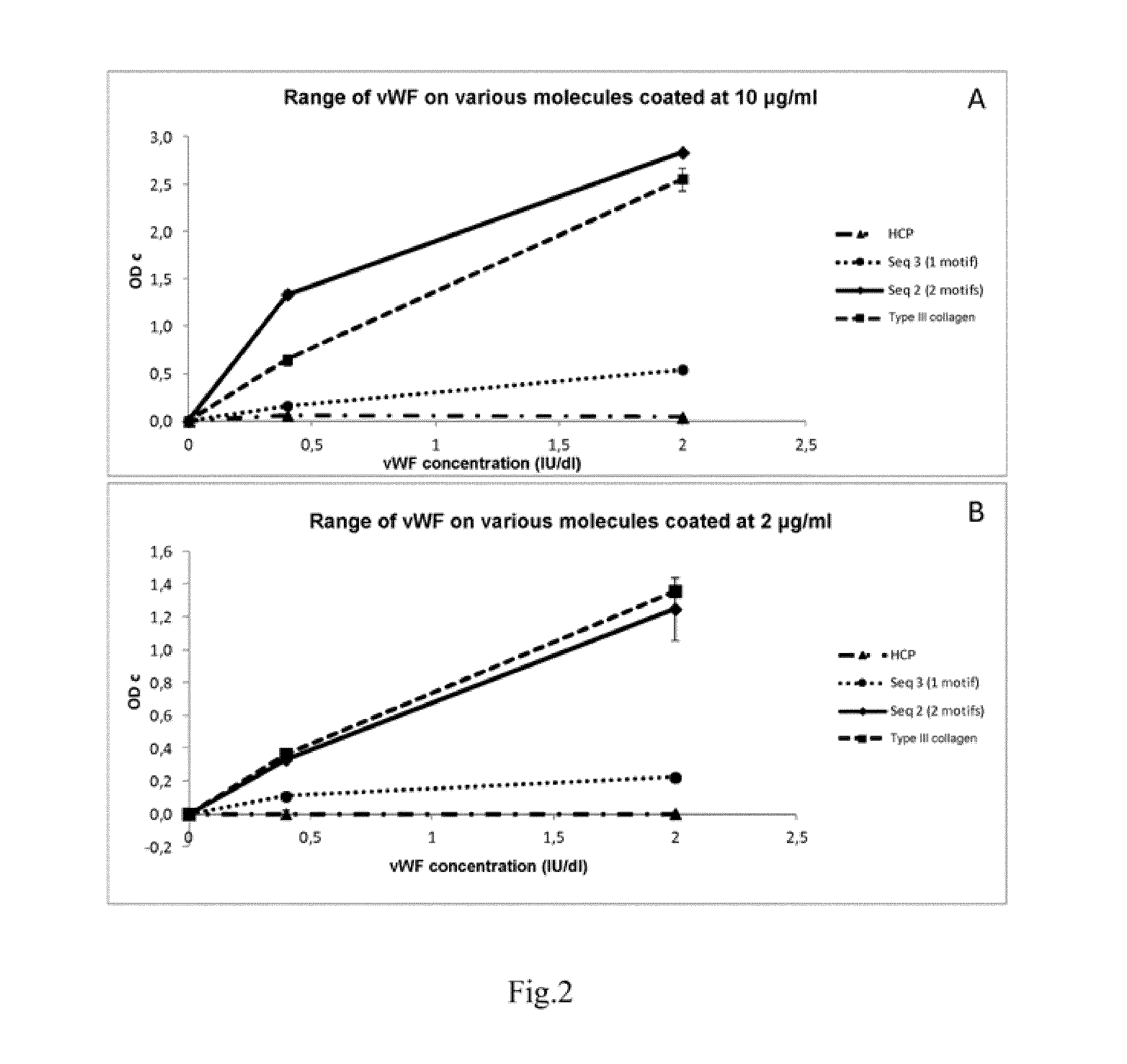
Collagen-Derived Recombinant Proteins with Von Willebrand Factor-Binding Activity
ActiveUS20150276762A1Not easy to produceDifficult to purifyPolypeptide with localisation/targeting motifConnective tissue peptidesFactor VIII vWFProtein C
A method for diagnosing von Willebrand disease and novel polypeptides which bind to von Willebrand factor.
Owner:NVH MEDICINAL +1
Synthetic platelets
Provided herein are particles comprising a polymer substrate comprising one or more hyaluronic acid chains; and two or more peptide moieties bound directly to each hyaluronic acid chain. In some embodiments, the two or more peptide moieties comprising collagen-binding peptide (CBP) and von Willebrand binding peptide (VBP). The particles can be utilized in, e.g., methods of hemostatic treatment.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
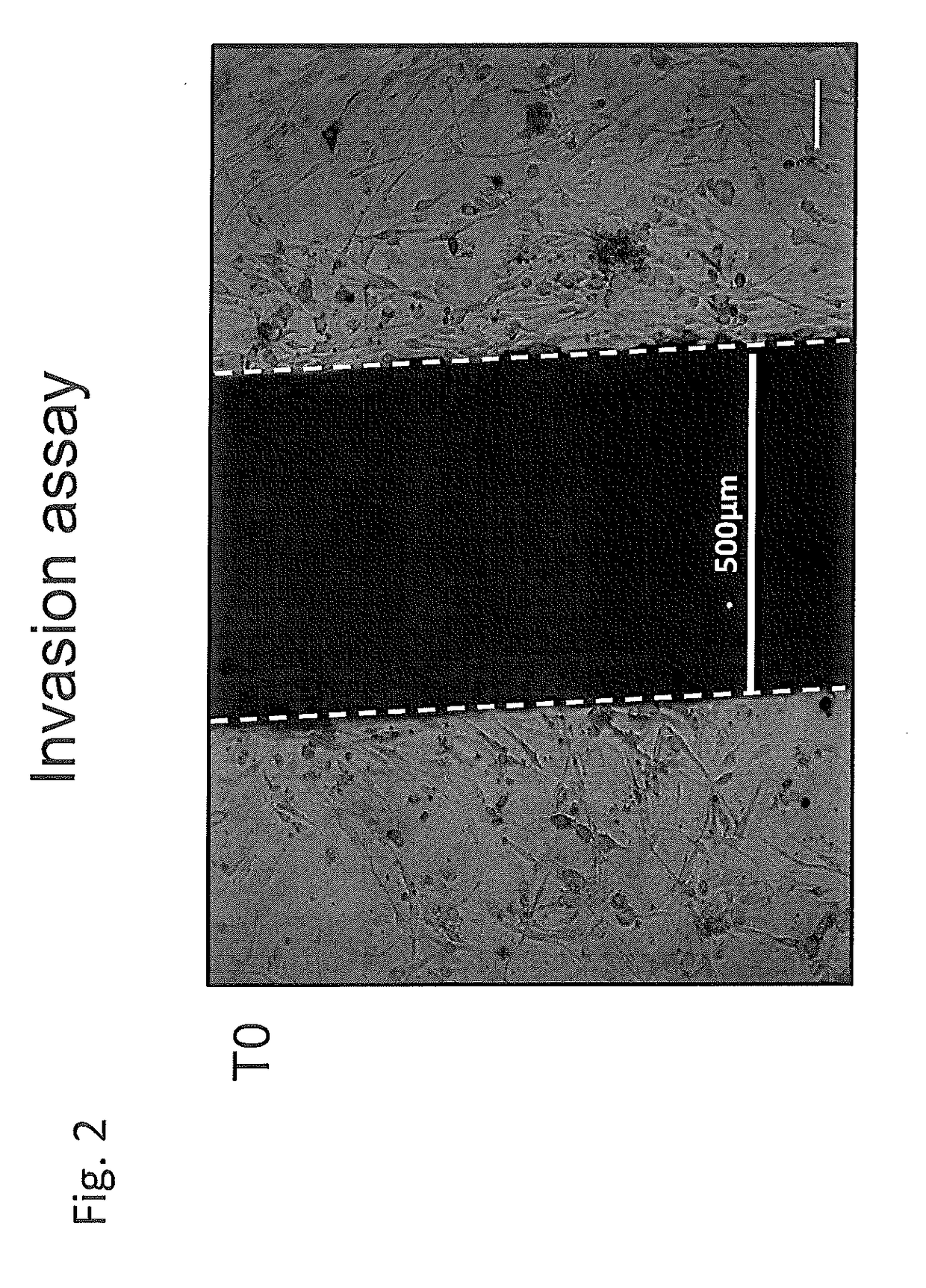
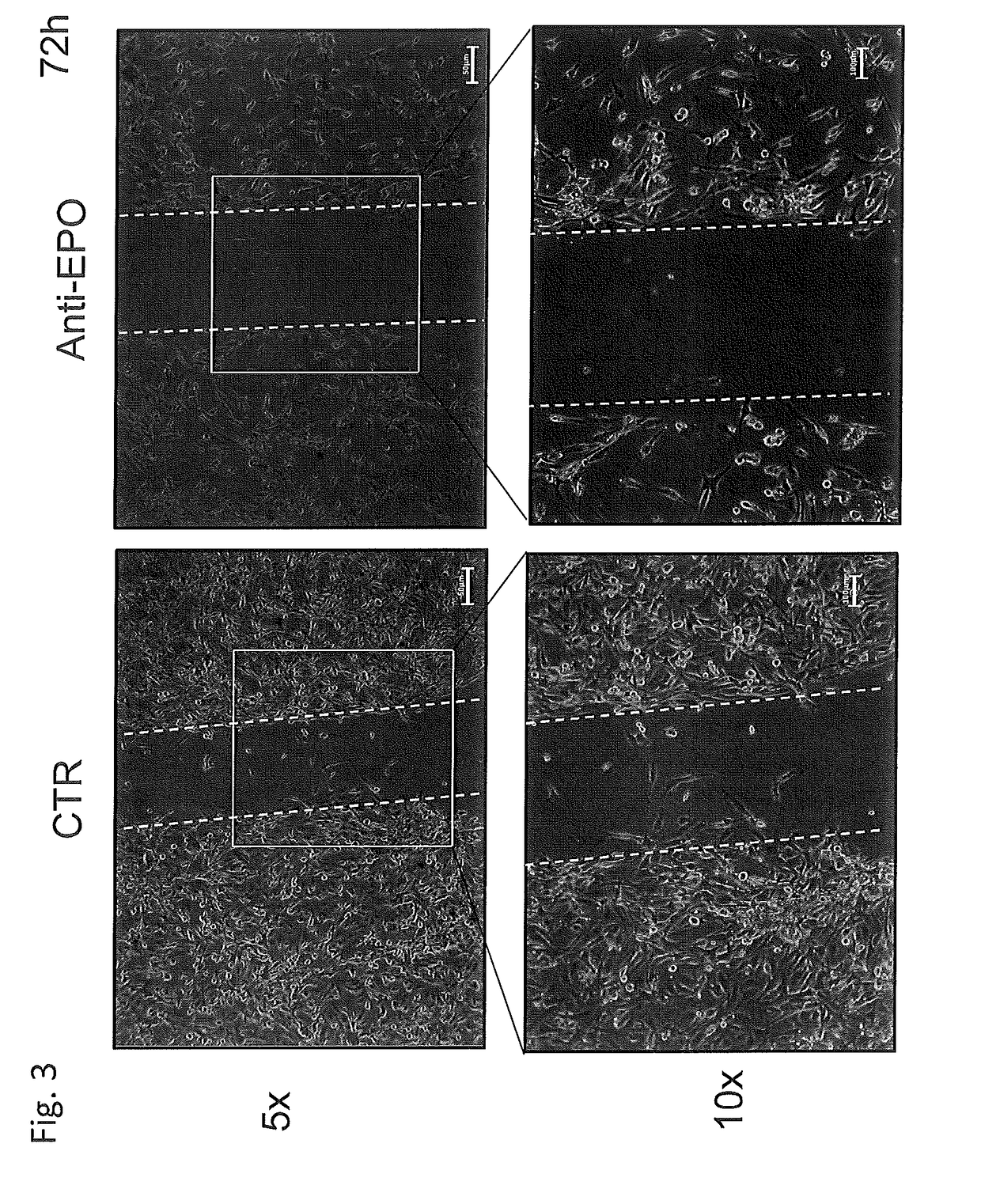
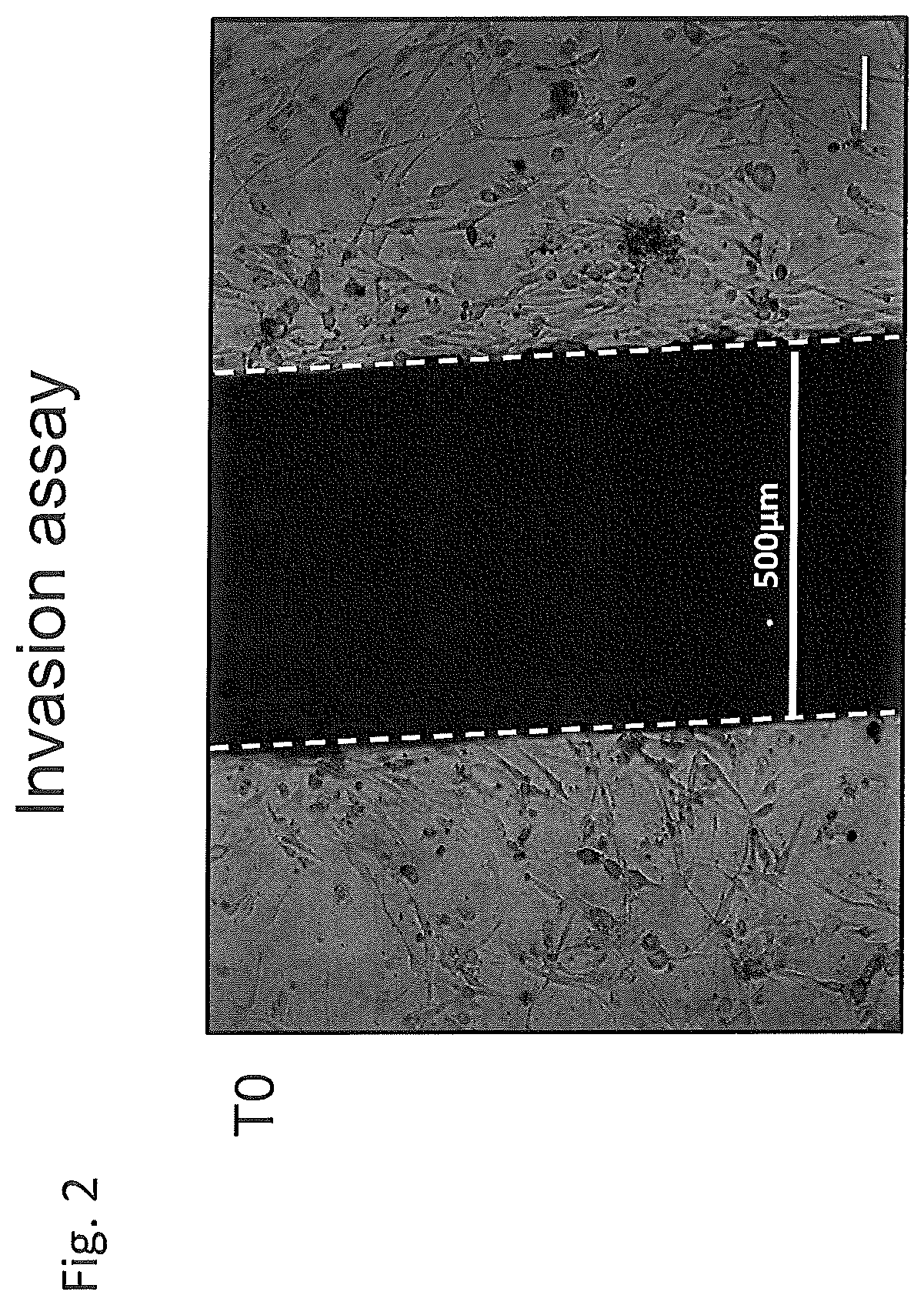
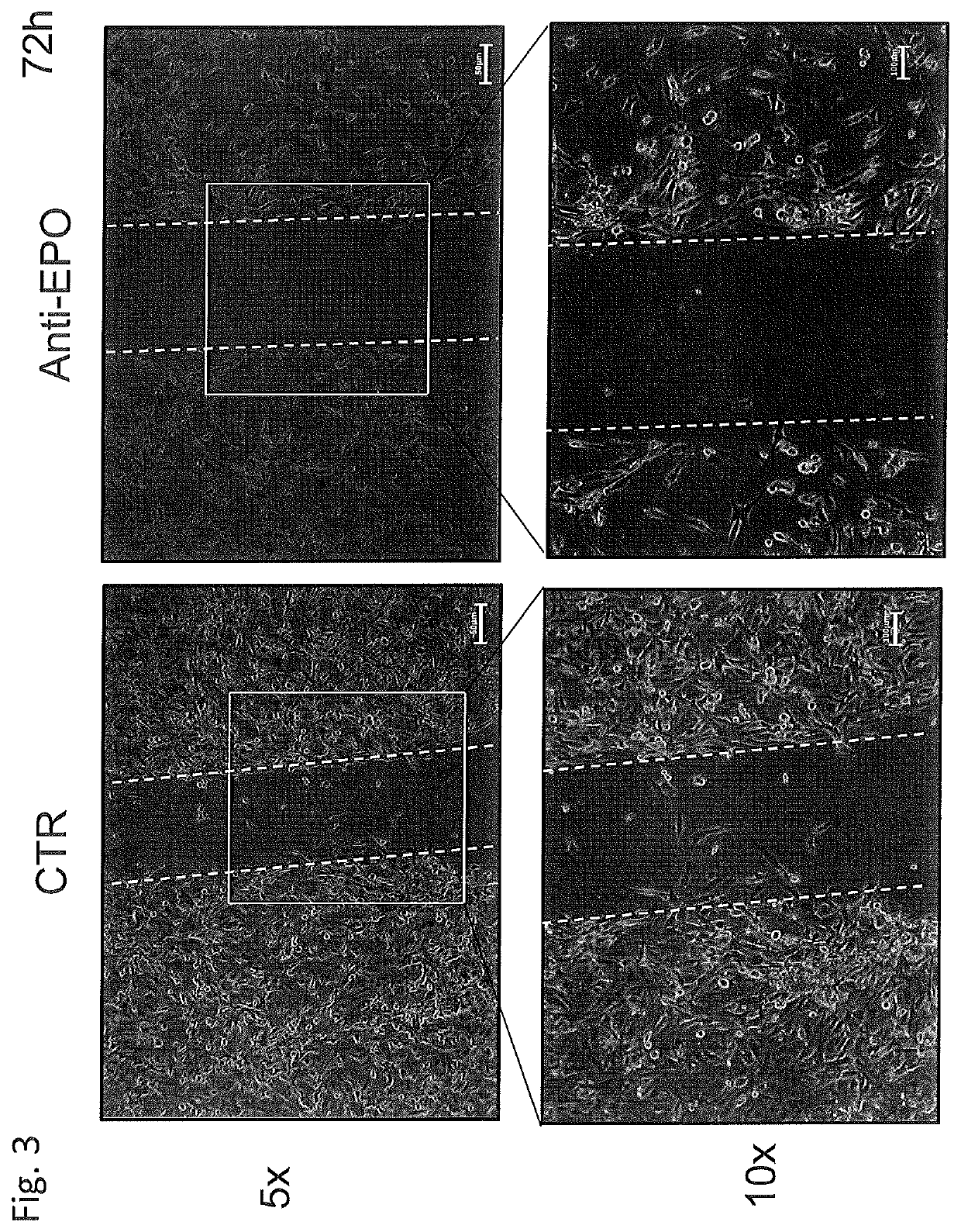
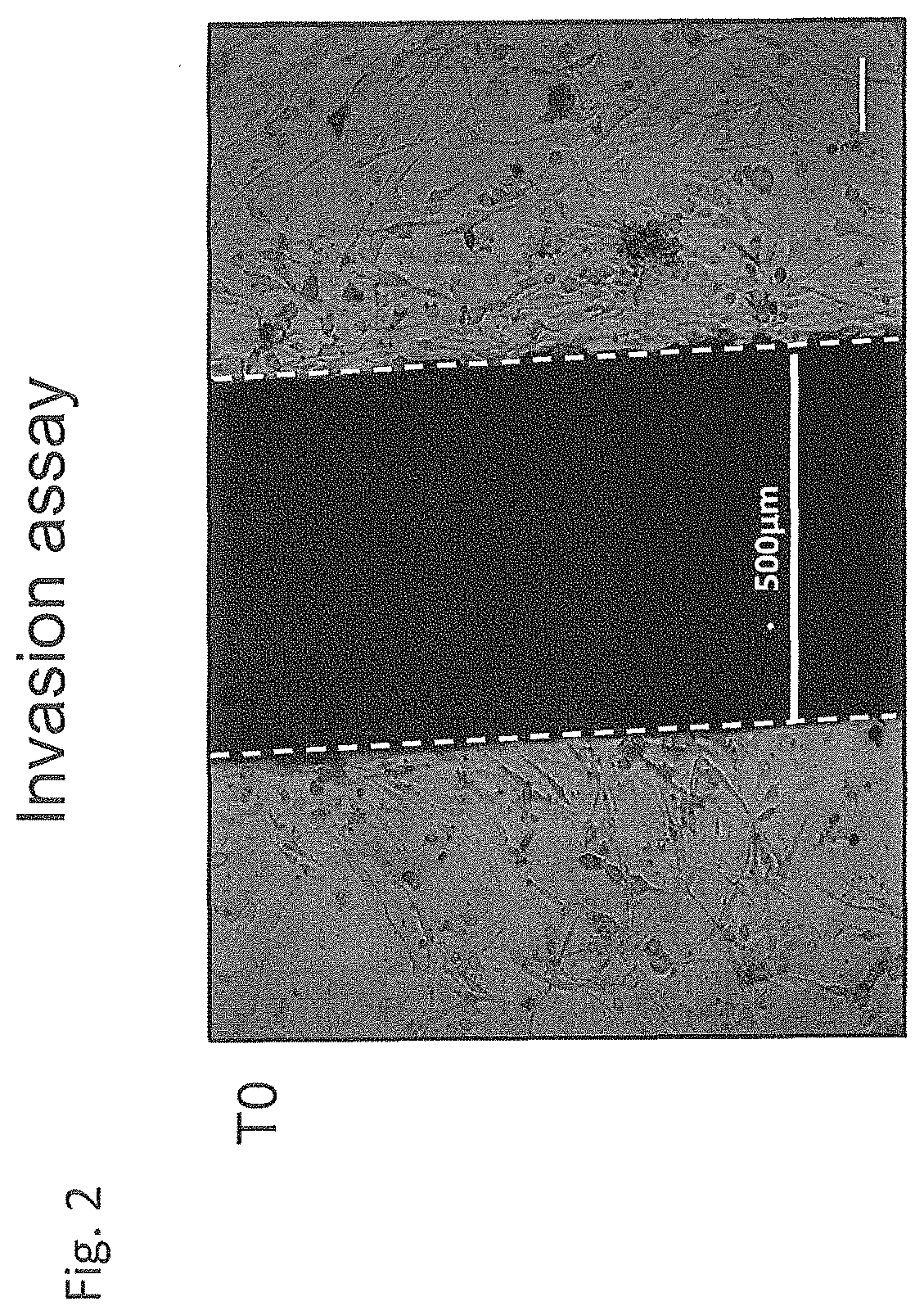
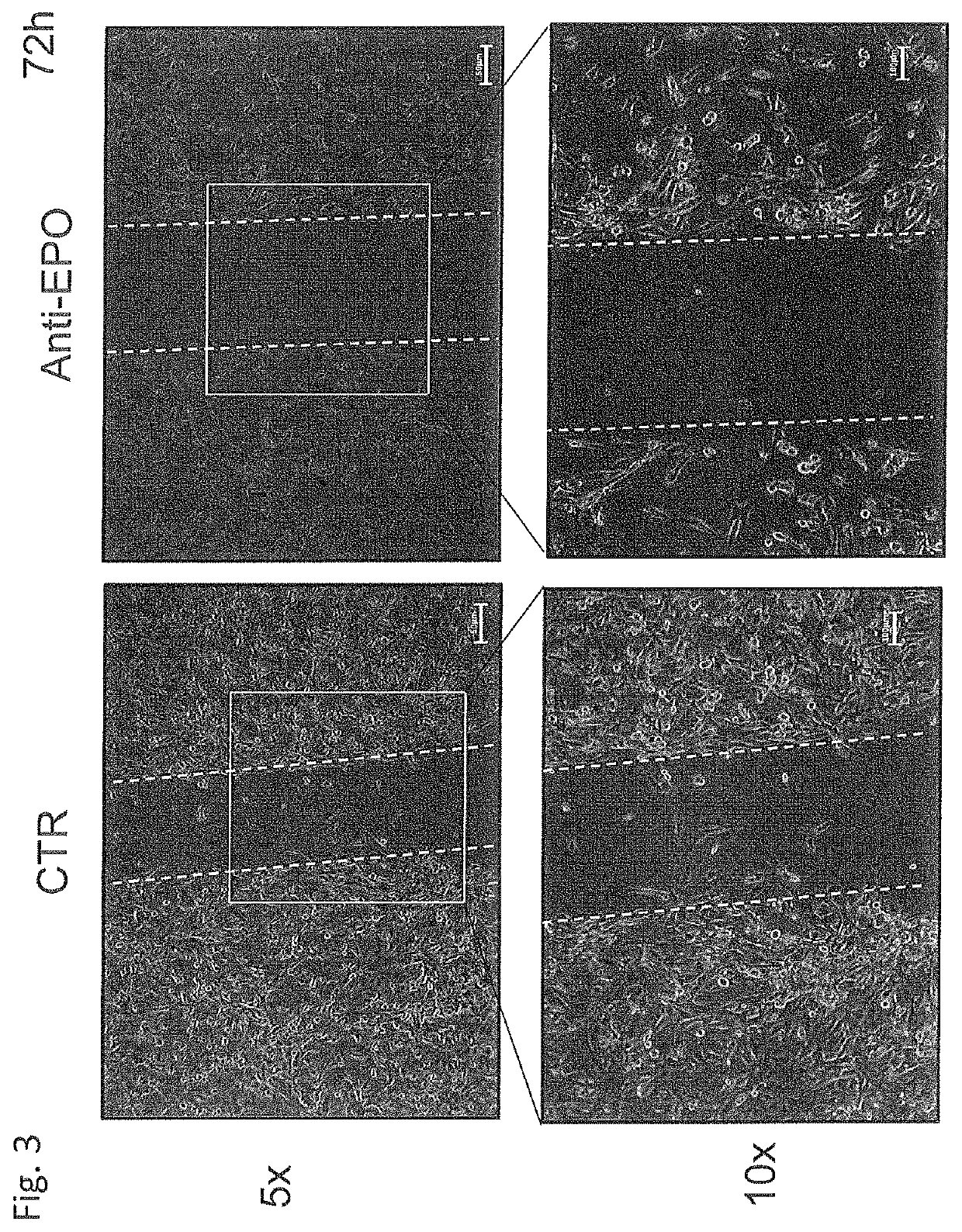
Use of negative functional modulators of erythropoietin for therapy
ActiveUS20170114132A1Lower Level RequirementsImprove the level ofCompound screeningOrganic active ingredientsAutoimmune responsesAntagomir
The invention relates to negative functional modulators of erythropoietin (EPO) for use in the treatment of cancers, in the therapy of autoimmune -based and non-autoimmune based chronic inflammatory diseases, and in the treatment of patients under-going organ or tissue transplant, or for the treatment of hemophilic arthropathy, hemophilia A and B, von Willebrand disease, angiodysplasia, proliferative disorders and neurological diseases characterized in their pathogenesis by primary neuroinflammation and / or neuroinflammation secondary to other causes. Such modulators are anti-EPO antibodies and their derivatives: anti-EPO receptor antibodies (EPOR), antisense oligonucleotides, decoy DNA, decoy RNA, ribozyme, antagomir, shRNA, LNA and / or siRNAs that inhibit the expression of the gene encoding EPO or EPOR.
Owner:ANDREMACON
FVIII muteins for treatment of von willebrand disease
InactiveCN102112623AAttenuated episodic bleedingPeptide/protein ingredientsMammal material medical ingredientsFactor VIII vWFPolyethylene glycol
This invention relates to treatment of von Willebrand Disease by administration of Factor VIII muteins that are covalently bound, at a predefined site that is not an N-terminal amine, to one or more biocompatible polymers such as polyethylene glycol. The mutein conjugates retain FVIII procoagulant activity and have improved pharmacokinetic properties in subjects lacking von Willebrand Factor.
Owner:BAYER HEALTHCARE LLC
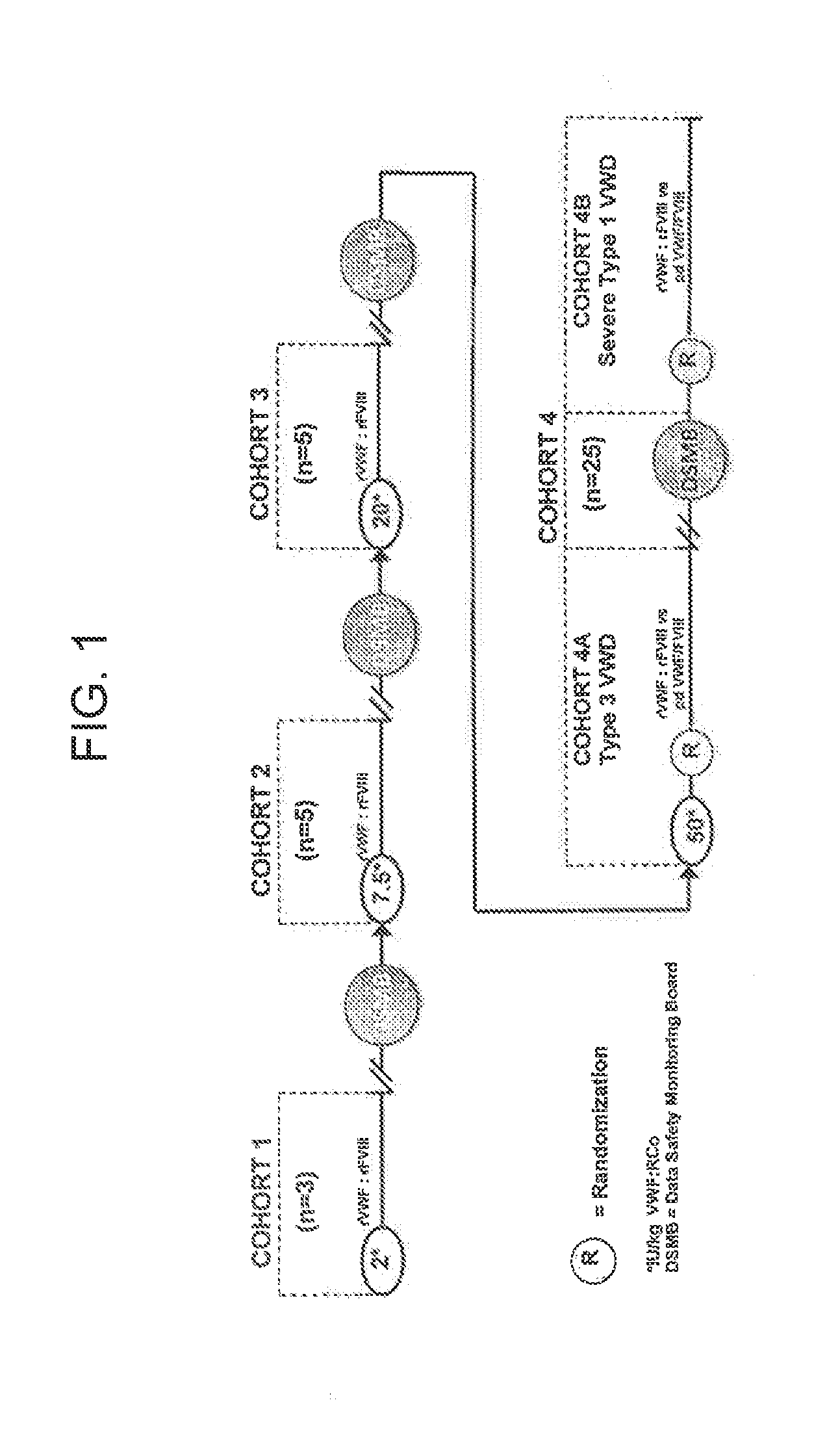
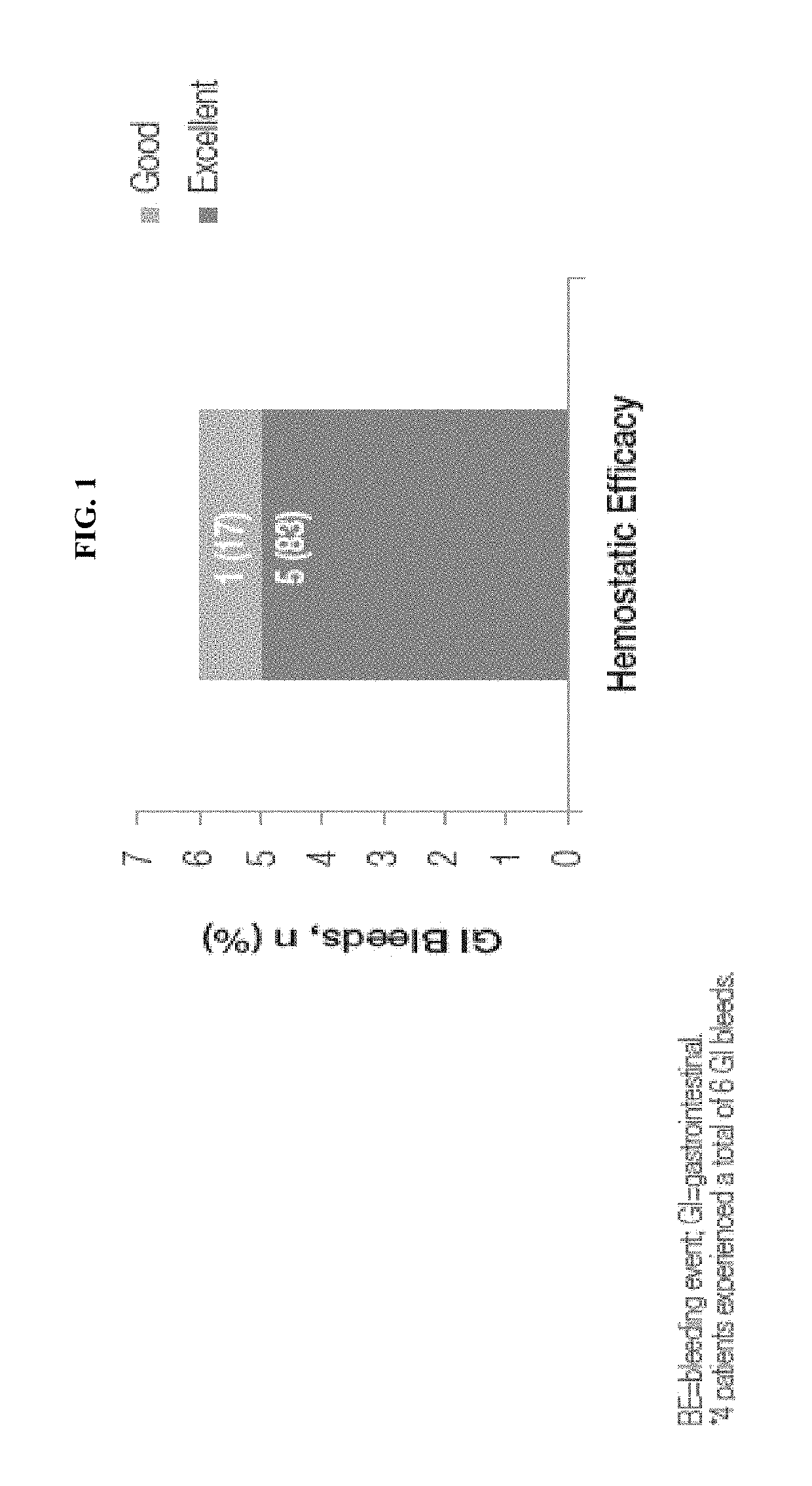
Treatment of gastrointestinal bleeding in patients with severe von willebrand disease by administration of recombinant vwf
ActiveUS20190091299A1High specific activityPeptide/protein ingredientsBlood disorderFactor VIII vWFVon willebrand
The present invention relates to a method for treating gastrointestinal bleeding in a subject with severe von Willebrand Disease comprising administering to the subject at least one dose of recombinant von Willebrand Factor (rVWF) ranging from about 40 IU / kg to about 100 IU / kg, wherein the first dose further comprises recombinant Factor VIII (rFVIII).
Owner:TAKEDA PHARMA CO LTD
Use of negative functional modulators of erythropoietin for therapy
ActiveUS11078270B2Low toxicitySafety managementCompound screeningOrganic active ingredientsAngiodysplasiaAntiendomysial antibodies
The invention relates to negative functional modulators of erythropoietin (EPO) for use in the treatment of cancers, in the therapy of autoimmune -based and non-autoimmune based chronic inflammatory diseases, and in the treatment of patients under-going organ or tissue transplant, or for the treatment of hemophilic arthropathy, hemophilia A and B, von Willebrand disease, angiodysplasia, proliferative disorders and neurological diseases characterized in their pathogenesis by primary neuroinflammation and / or neuroinflammation secondary to other causes. Such modulators are anti-EPO antibodies and their derivatives: anti-EPO receptor antibodies (EPOR), antisense oligonucleotides, decoy DNA, decoy RNA, ribozyme, antagomir, shRNA, LNA and / or siRNAs that inhibit the expression of the gene encoding EPO or EPOR.
Owner:ANDREMACON
Preparation for treating haemophilia caused by Qi and blood deficiency and von Willebrand disease and method
InactiveCN107693630APromote absorptionHigh dissolution rateUnknown materialsBlood disorderSide effectVon willebrand
A preparation for treating haemophilia caused by Qi and blood deficiency and von Willebrand disease and a method. The preparation is produced from, by weight, 18-48 parts of raw astragalus membranaceus, 6-15 parts of codonopsis pilosula, 9-24 parts of raw atractylodes macrocephala, 9-24 parts of poria cocos, 6-15 parts of pericarpium citri reticulatae, 6-15 parts of angelica sinensis, 6-15 parts of colla corii asini, 6-15 parts of Chinese wolfberries, 6-15 parts of fructus psoraleae, 6-15 parts of pomegranate peel, 9-24 parts of Chinese dates, and 4-10 parts of honey-fried licorice roots. In the invention, active components in the raw material medicines are extracted in a manner of water boiling, wherein the activities are maintained, so that in the preparation, the dissolution rates of the active components are greatly increased, and the medicines are easy to absorb, thus increasing the bioavailability of the preparation; in addition, the preparation is a pure-traditional Chinese medicine compound preparation, so that no toxic and side effect is generated after long time use by a patient, and the preparation is good in safety. The preparation method is simple and is convenient tocarry out.
Owner:天津市善济宏兴科技发展有限公司
Methods of treating thrombotic diseases with von willebrand factor specific antibodies
InactiveUS20050197494A1Immunoglobulins against blood coagulation factorsAntibody mimetics/scaffoldsFactor VIII vWFThrombus
Owner:PDL BIOPHARMA INCORPORATED
Use of negative functional modulators of erythropoietin for therapy
PendingUS20210340241A1Lower Level RequirementsImprove the level ofOrganic active ingredientsImmunoglobulins against growth factorsAngiodysplasiaAntiendomysial antibodies
The invention relates to negative functional modulators of erythropoietin (EPO) for use in the treatment of cancers, in the therapy of autoimmune-based and non-autoimmune based chronic inflammatory diseases, and in the treatment of patients undergoing organ or tissue transplant, or for the treatment of hemophilic arthropathy, hemophilia A and B, von Willebrand disease, angiodysplasia, proliferative disorders and neurological diseases characterized in their pathogenesis by primary neuroinflammation and / or neuroinflammation secondary to other causes. Such modulators are anti-EPO antibodies and their derivatives: anti-EPO receptor antibodies (EPOR), antisense oligonucleotides, decoy DNA, decoy RNA, ribozyme, antagomir, shRNA, LNA and / or siRNAs that inhibit the expression of the gene encoding EPO or EPOR.
Owner:ANDREMACON
Transgenic Mouse Lacking Endogenous FVIII and VWF - A Model of Hemophilia A
InactiveUS20110072524A1Improvement in clinical readoutFactor VIIPeptide/protein ingredientsFactor VIII vWFEndogenous Factors
The present invention relates, generally, to a transgenic non-human animal model of hemophilia A, wherein the transgenic animal is deficient in endogenous Factor VIII and endogenous von Willebrand Factor, and methods to treat hereditary or acquired hemophilia A or von Willebrand Disease (VWD) by administration of exogenous human VWF.
Owner:BAXTER INT INC +1
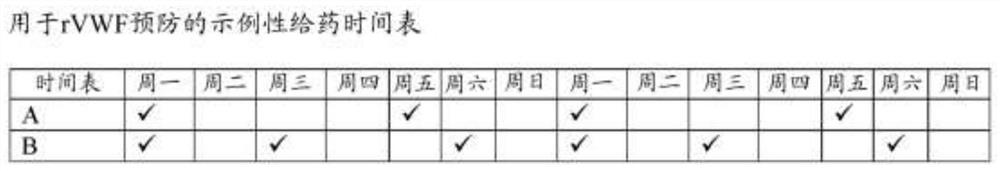
Methods of prophylactic treatment using recombinant vwf (RVWF)
Owner:TAKEDA PHARMA CO LTD
Preparation for treating hemophilia and von willebrand disease caused by heat stagnation blood aspect and method
InactiveCN107669921APromote absorptionHigh dissolution rateConiferophyta medical ingredientsBlood disorderNepetaSide effect
The invention relates to a preparation for treating hemophilia and von willebrand disease caused by heat stagnation blood aspect and a method. The preparation is made of, by weight ratio, 6-15% of stir-fried cape jasmine fruit, 6-15% of tree peony bark, 6-15% of raw rhubarb, 18-48% of lalang grass rhizome, 6-15% of polygonum aviculare, 6-15% of Japanese thistle herb or root, 6-15% of common cephalanoplos herb, 9-24% of radix rubiae, 18-48% of hairyvein agrimonia herb and bud, 6-15 parts of Chinese arborvitae twig, 6-15% of carbonized catnip and 4-10% of radix glycyrrhizae. According to the preparation, a boiling extraction method is adopted to extract active ingredients in the raw medicines and keep activities thereof, so that not only can the dissolution rate of the active ingredients inthe medicines be improved, but also the absorption to the medicines can be facilitated, thereby improving the biological utilization degree thereof. In addition, the medicine is a pure traditional Chinese medicine compound preparation, so a patient does not produce toxic and side effects after long-term use, and the safety is good. In addition, the preparation method has a simple process and is convenient to operate.
Owner:天津市善济宏兴科技发展有限公司
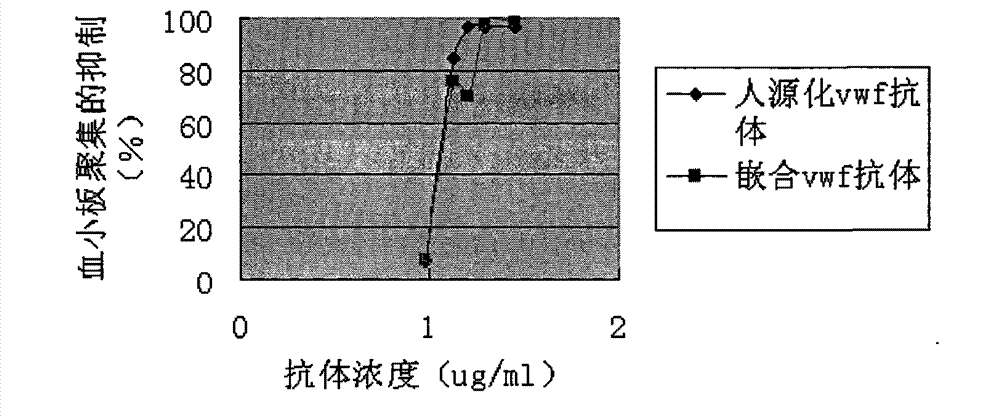
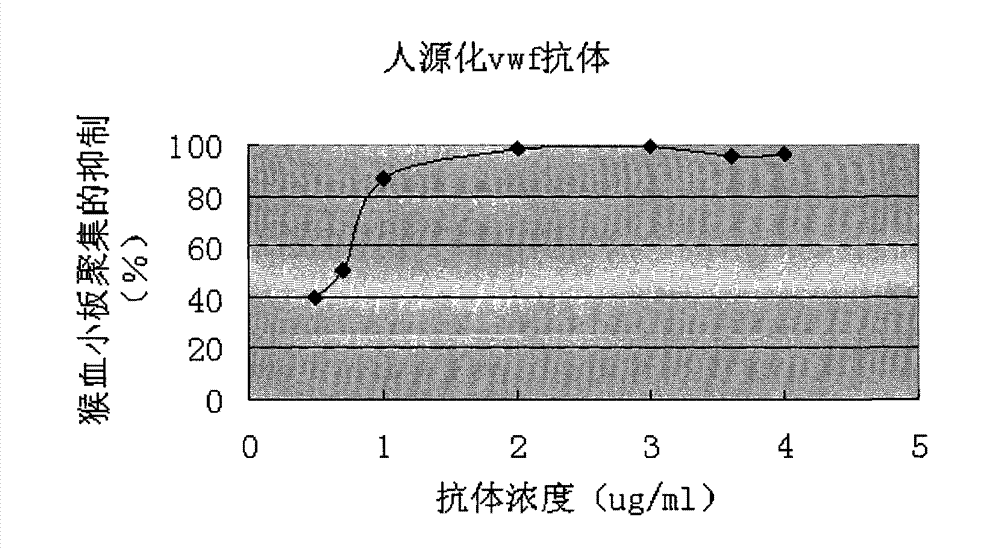
Humanized anti-human von willebrand disease factor monoclonal antibody and application thereof
InactiveCN101891820BInhibition formationAvoid stickingImmunoglobulins against blood coagulation factorsAntibody ingredientsDiseaseThrombus
The invention discloses a humanized anti-human von willebrand disease factor monoclonal antibody and an application thereof. The antibody consists of a heavy chain variable region of mouse antibody NMC-4 and a light chain variable region of mouse antibody NMC-4, wherein amino acid sequences of CDR1, CDR2 and CDR3 contained in the heavy chain variable region are shown as serial numbers 1, 2 and 3 in sequence; and the light chain variable region comprises CDR1, CDR2 and CDR3 or CDR2 and CDR3 or CDR3, and the amino acid sequences of CDR1, CDR2 and CDR3 are shown as serial numbers 4, 5 and 6 in sequence. The monoclonal antibody is characterized by having vWF bonding capability, inhibiting thrombopoiesis from formation, having low immunogenicity and the like, can be used for preparing drugs for preventing and curing various thrombus diseases, and provides a possibility for preventing and curing human thrombus diseases, particularly thrombus of artery.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
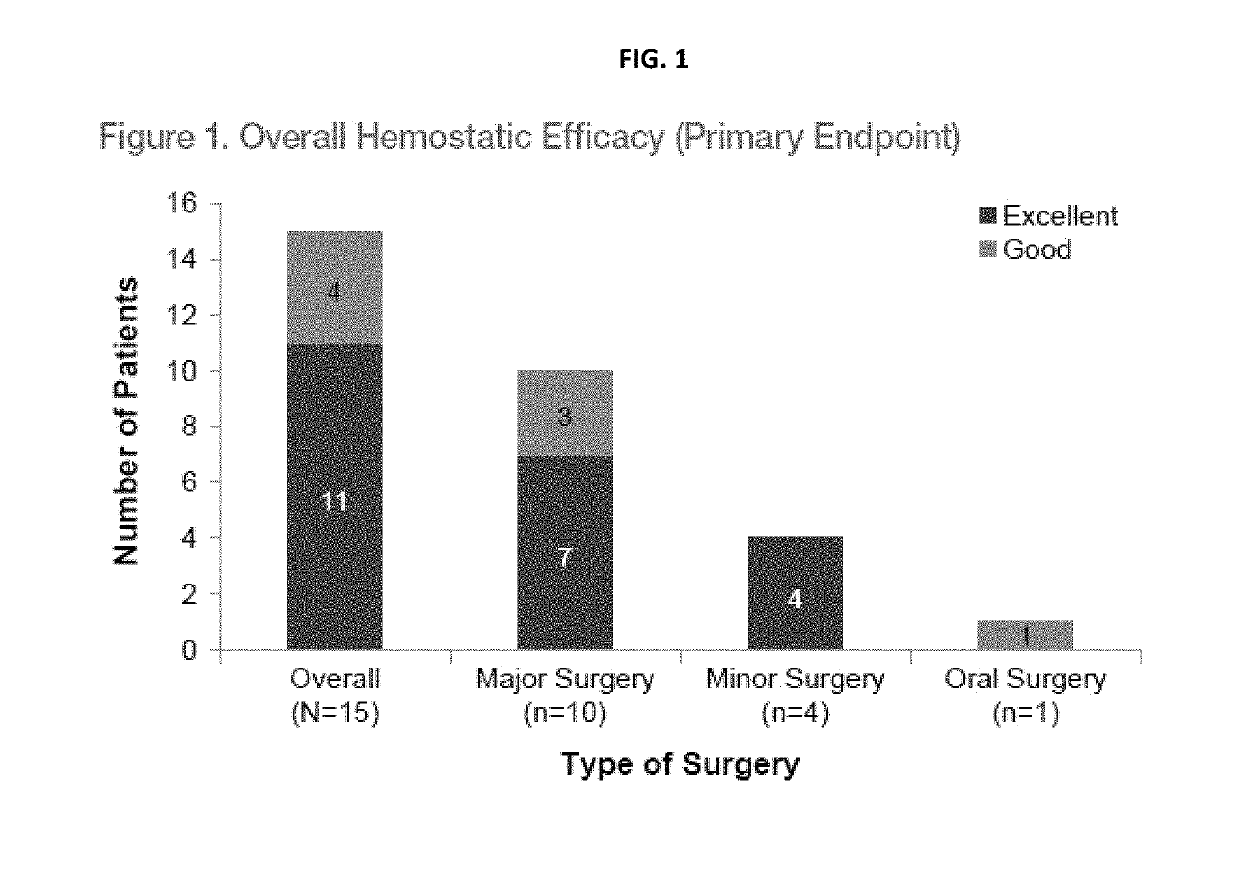
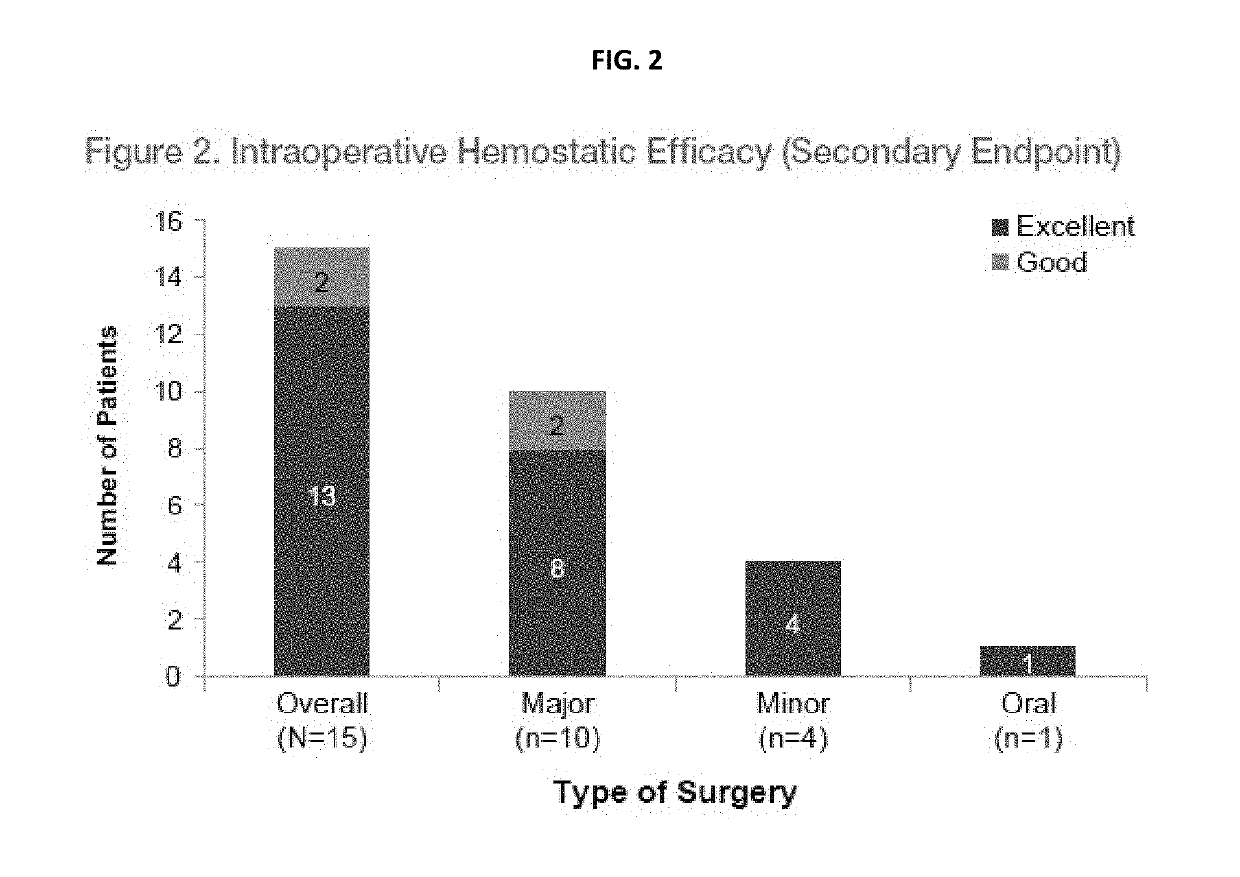
Treatment of patients with severe von willebrand disease undergoing elective surgery by administration of recombinant vwf
InactiveUS20190091298A1High specific activityPeptide/protein ingredientsBlood disorderFactor iiPre treatment
The present invention relates to method for pretreating a subject with severe von Willebrand disease prior to a surgical procedure comprising administering to the subject a dose ranging from about 20 IU / kg to about 60 IU / kg rVWF between about 12 hours and about 24 hours prior to the surgical procedure, and wherein Factor VIII is not administered with the rVWF prior to the surgical procedure.
Owner:TAKEDA PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com