FVIII muteins for treatment of von willebrand disease
A hemophilia, vascular technology, applied in the direction of blood diseases, peptide/protein components, medical raw materials derived from mammals, etc., can solve problems that cannot lead to improved plasma half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1: Mutagenesis
[0089] Substrates for site-directed PEGylation of FVIII can be generated by introducing cysteine codons at sites selected for PEGylation. Using Stratagene cQuickChange TM II site-directed mutagenesis kit to generate all PEG muteins (Stratagene Corporation, La Jolla, CA). use DNA polymerase and temperature cycler for cQuikChange TM Site-directed mutagenesis. use (which does not displace the primers) to extend two complementary oligonucleotide primers containing the desired mutation. dsDNA containing the wild-type FVIII gene was used as template. After multiple extension cycles, the product is digested with DpnI endonuclease, which is specific for methylated DNA. The newly synthesized DNA (containing the mutation) is unmethylated, whereas the parental wild-type DNA is methylated. The digested DNA is then used to transform XL-1Blue supercompetent cells.
[0090] Mutagenesis reactions were performed in pSK207+BDD C2.6 or pSK207+BDD. A...
Embodiment 2
[0094] Example 2: vWF binding ELISA.
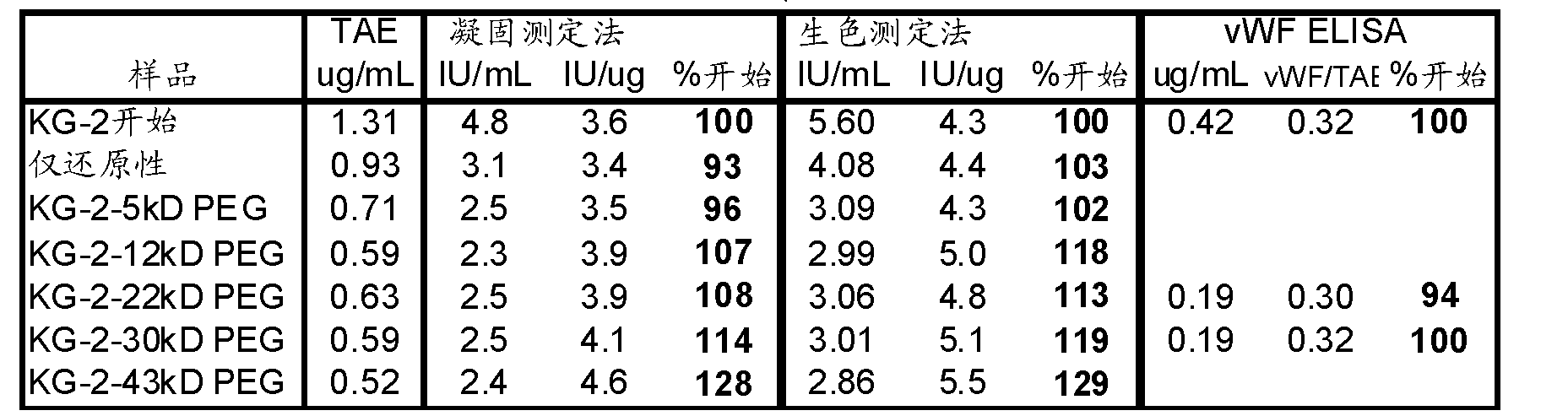
[0095] vWf in severe hemophilia plasma in permissive FVIII binding solution. FVIII-vWf complexes were then captured on microtiter plates that had been coated with vWf-specific monoclonal antibodies. FVIII bound to vWf was detected with FVIII polyclonal antibody and horseradish peroxidase anti-rabbit conjugate. Antibody complexes conjugated to peroxidase produce a color reaction upon addition of substrate. Sample concentrations were interpolated from the standard curve using a four-parameter fit model. FVIII binding results are reported in μg / mL. There was no significant effect on any activity after PEGylation, which would be consistent with PEGylation at the B domain. The results can be seen in Table 2.
[0096] Table 2
[0097]
Embodiment 3
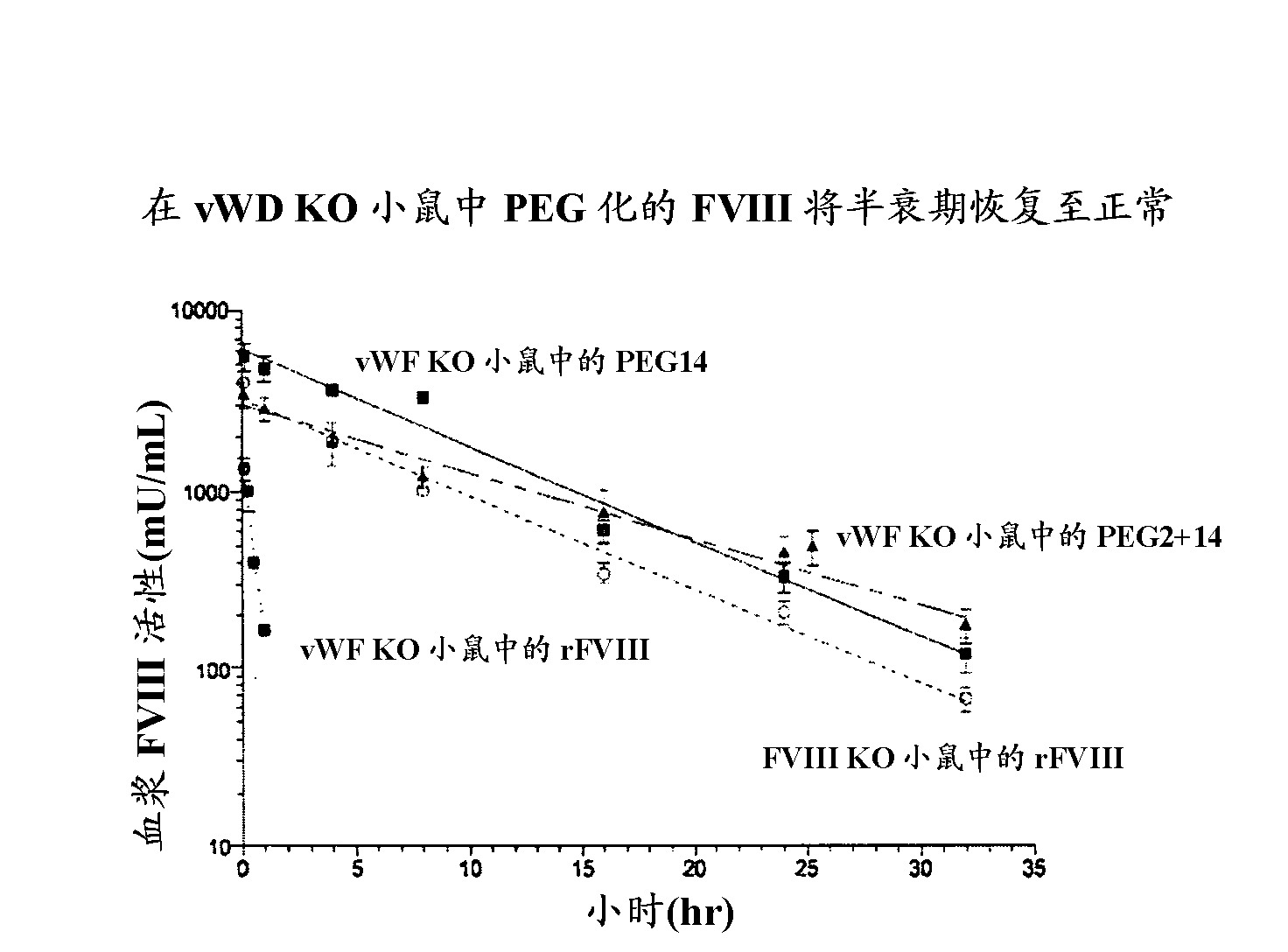
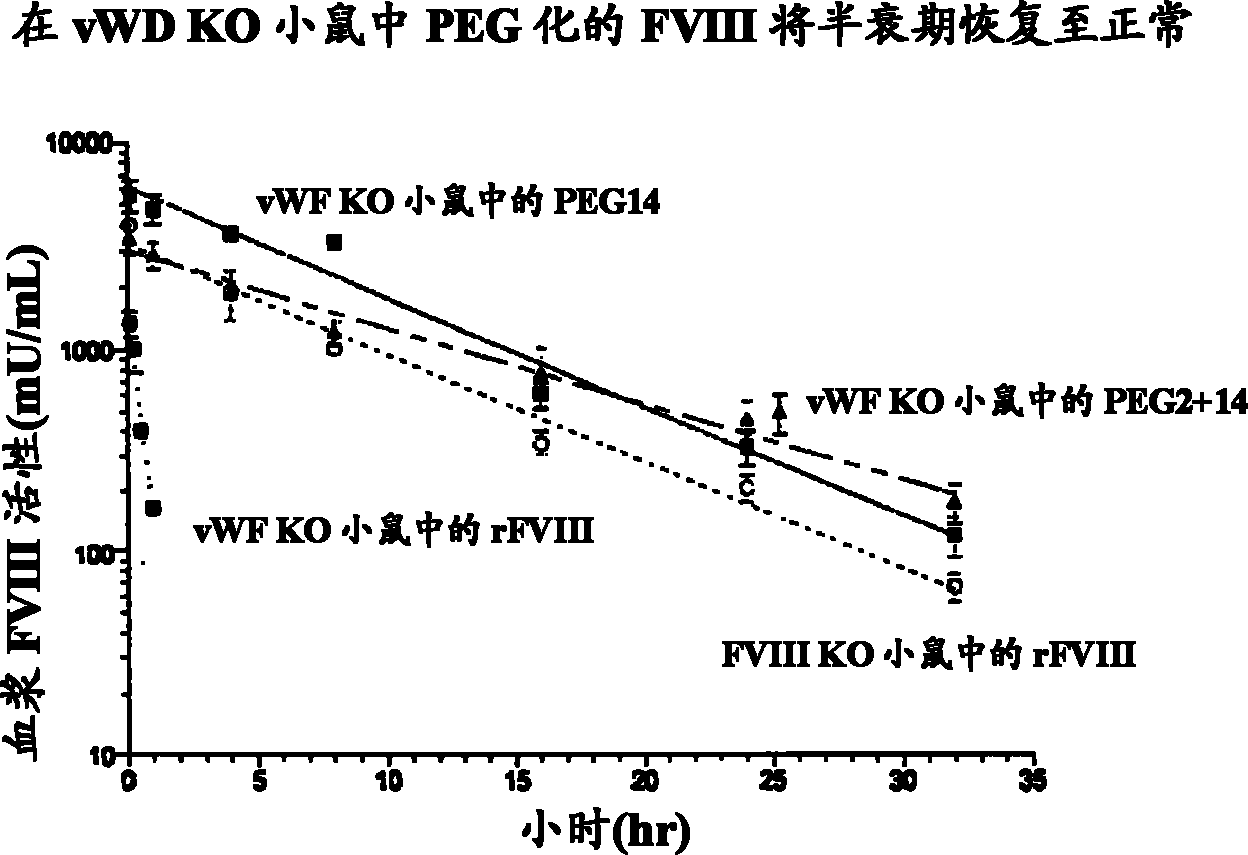
[0098] Example 3: Pharmacokinetic activity
[0099] The PK of PEGylated FVIII and B-domain-deleted FVIII (BDD-FVIII) was determined in FVIII knockout (KO) mice. Mice received 200 IU / kg BDD-FVIII, 108 IU / kg BDD-FVIII conjugated with 64kD PEG (64kD PEG14) at the cysteine mutation introduced at amino acid position 1804, or 194 IU / kg at positions 491 and 1804. Intravenous (i.v) injection of BDD-FVIII conjugated to 64kD PEG (64kD PEG2+14) at each of the cysteine mutations at position 1804. Blood samples were collected from treated mice (5 mice / treatment / time point) at 5 minutes, 4 hours, 8 hours, 16 hours, 24 hours, 32 hours, and 48 hours. Plasma FVIII activity was determined by the Coatest assay. Terminal half-life was determined by noncompartmental modeling of activity versus time curves in WinNonLin. Given the t of BDD-FVIII in FVIII KO mice 1 / 2 is the t of FVIII conjugated with 64kD PEG (64kD PEG14) or 128kD PEG (64kD PEG2+14) at 6 hours 1 / 2 They are 12.43 hours and 12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com