Humanized anti-human von willebrand disease factor monoclonal antibody and application thereof
A technology of hemophilia factor and monoclonal antibody, applied in the direction of anti-coagulation factor immunoglobulin, application, antibody, etc., can solve the problems of unsatisfactory effect and failure of monoclonal antibody, achieve inhibition of adhesion and aggregation, inhibition of Thrombosis, low immunogenicity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Structural analysis of the binding of the murine antibody variable region to the vWF A1 region
[0036] The crystal structure of the vWF A1 and NMC-4Fab complex in the PDB database shows that the fourth α-helix of the A1 region is the main site for binding antibodies, and the arginines at positions 632 and 636 play an important role. The CDR2 and CDR3 of the variable region of the heavy chain of the antibody and the CDR3 of the variable region of the light chain are mainly involved in the combination with A1, so the three CDRs of the heavy chain are kept unchanged during the humanization process, while the CDRs of the light chain have A large degree of trade-offs, in order to achieve a higher degree of humanization.
Embodiment 2
[0037] Example 2: Humanized transformation of murine vWF antibody
[0038] (1) By comparing the amino acid sequences of the variable regions of NMC-4 and the human germline antibody family, it was found that the framework region of the heavy chain variable region has a high homology with the amino acid sequence encoded by the human germline antibody gene 4-59 Sex, and the length of the CDR is also the same, it is speculated that their variable regions have similar spatial structures, so the framework region of human germline antibody gene 4-59 was selected as the framework region of the humanized antibody heavy chain. Using the same method, it was found that the framework region of the light chain variable region was close to the amino acid sequence encoded by the human germline antibody gene O8, so the framework region of the human germline antibody gene O8 was selected as the framework region of the humanized antibody light chain.
[0039] (2) All the CDRs of the heavy chain...
Embodiment 3
[0048] Example 3: Expression of humanized antibody and detection of antibody activity
[0049] (1) Expression and purification of antibodies
[0050] 293F cells were inoculated into 293SFMII medium (GIBCO) in 8% CO 2 Suspension culture can be subcultured in an incubator, and transient transfection can be performed when the culture density reaches 10E6 / ml. Dilute the humanized antibody plasmid with OPTI-MEMI (GIBCO), dilute PEI (POLYSCIENCE) with the same medium, mix the plasmid and PEI at a mass ratio of 1:2 and let it stand for 5 minutes, then drop it into the suspension cells, and culture After 3-5 days, the cell supernatant was collected, concentrated and purified with a protein A affinity chromatography column.
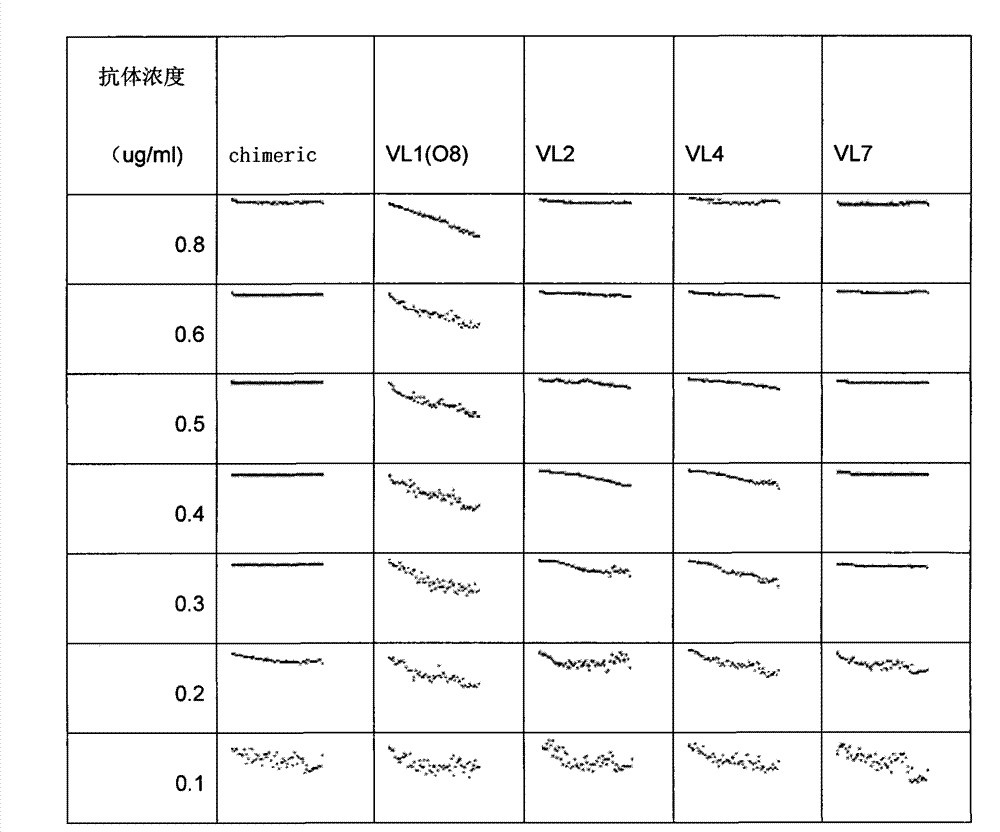
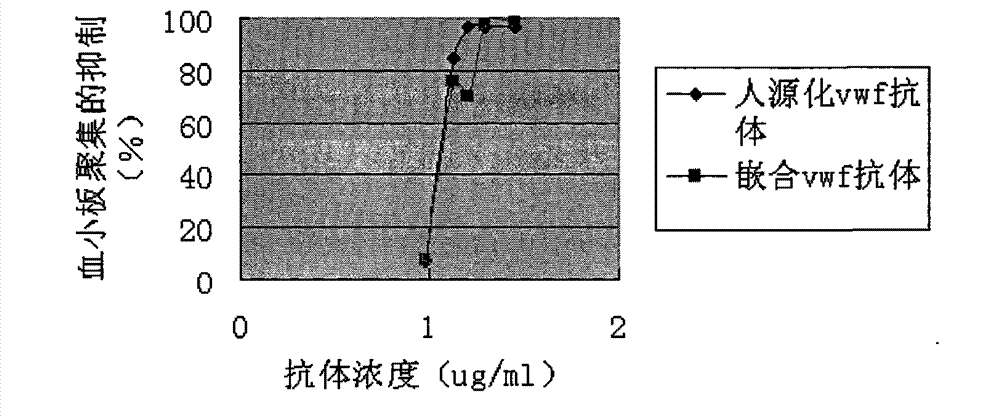
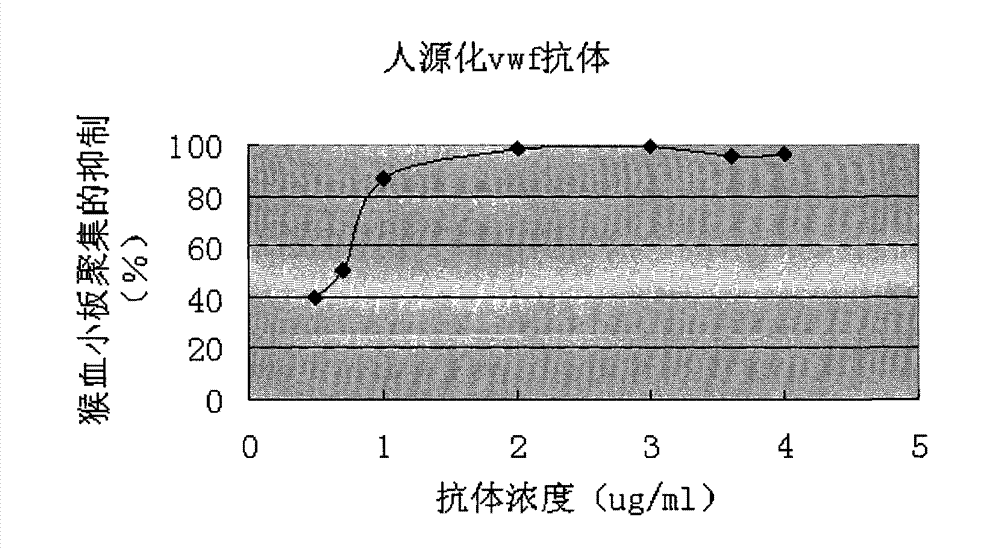
[0051] (2) Platelet aggregation test
[0052] Freeze-dried platelet powder (Biopool) was dissolved in TBS, and distributed into 96-well plates at 60ul per well. Dilute vWF (Calbiochem) to 10ug / ml with PBS, and distribute to 96-well plates at 10ul per well. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com