Treatment of patients with severe von willebrand disease undergoing elective surgery by administration of recombinant vwf
a technology of recombinant vwf and treatment of patients with severe von willebrand disease, which is applied in the direction of drug composition, extracellular fluid disorder, peptide/protein ingredient, etc., and can solve the problem of 1/10,000 patients actually needing treatmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
c Efficacy and Safety of rVWF
[0228]This study evaluated the hemostatic efficacy and safety of rVWF with or without ADVATE (antihemophilic factor [recombinant]), Baxalta US Inc., Westlake Village, Calif. (rFVIII) in patients with severe VWD undergoing elective surgery.
Methods
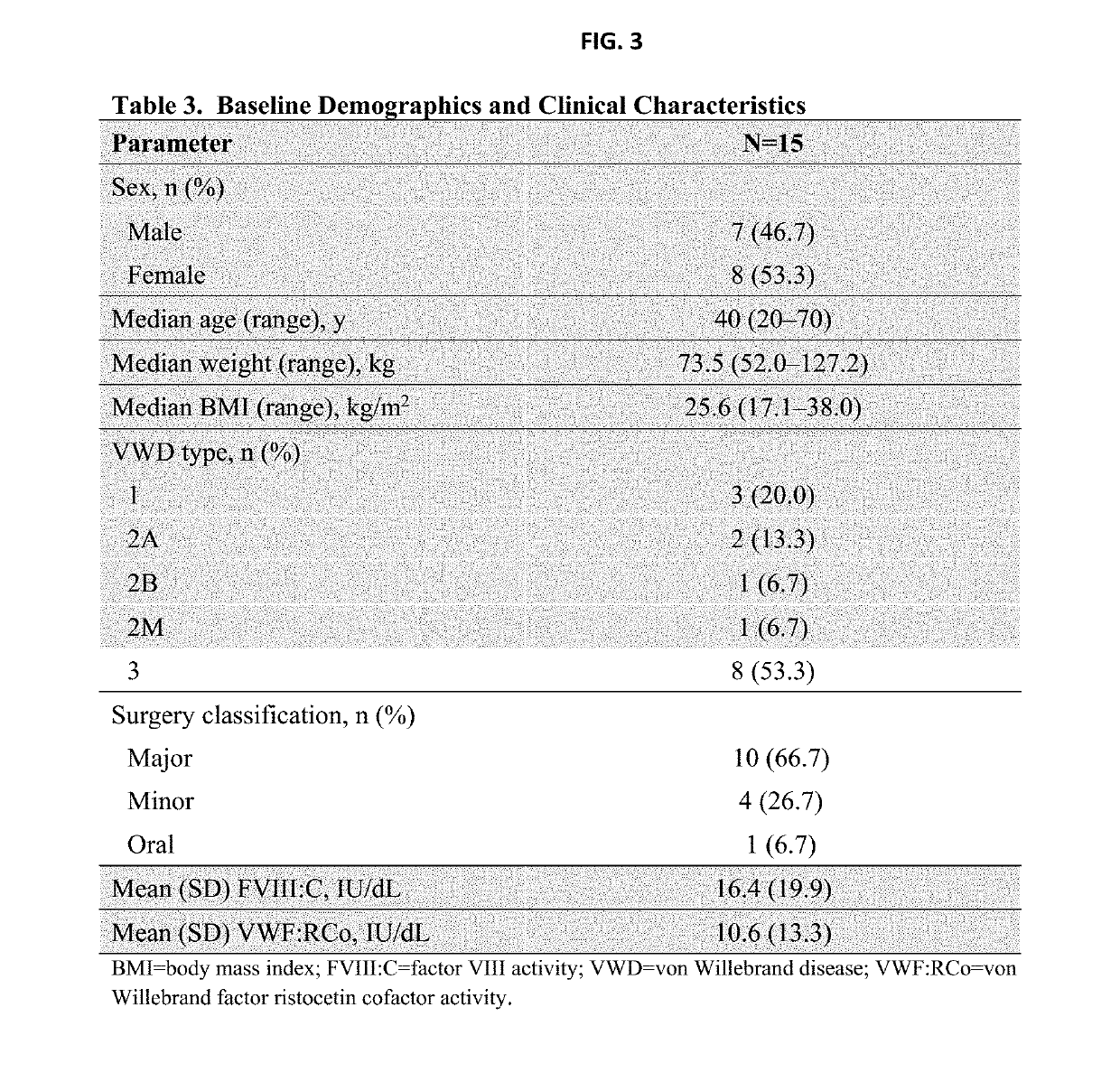
[0229]Phase 3, open-label, uncontrolled, nonrandomized study at 14 sites in 10 countries (NCT02283268) in patients ≥18 y of age who had severe VWD and were scheduled to undergo elective surgery. Patients were monitored for 14 d after surgery.
Treatment
[0230]12-24 h before surgery, rVWF 40-60 IU / kg rVWF:RCo was given intravenously to allow endogenous FVIII:C levels to increase to ≥30 IU / dL (minor / oral surgery) or ≥60 IU / dL (major surgery). FVIII:C levels were assessed within 3 h of initiation of surgery. If target FVIII:C levels were achieved, rVWF alone was administered 1 h before surgery to achieve the peak levels described in Table 2. If target FVIII:C levels were achieved, rVWF alone was administered 1 h before...
example 2
nt Von Willebrand Factor in Subjects with Severe Von Willebrand Disease Undergoing Surgery
[0245]This example provides the study results from a study examining treatment of subjects with severe von Willebrand Disease (VWD) undergoing surgery.
Primary Outcome Measures:
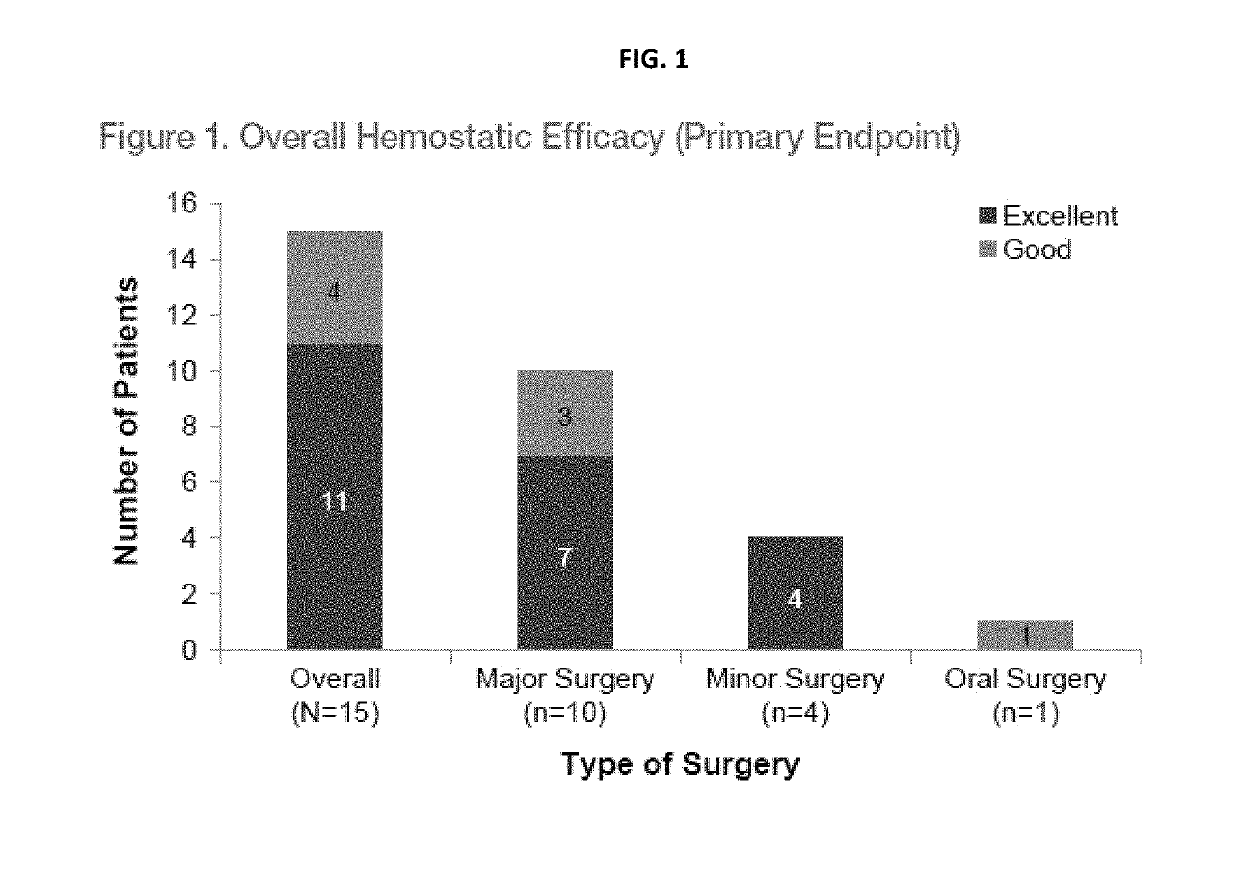
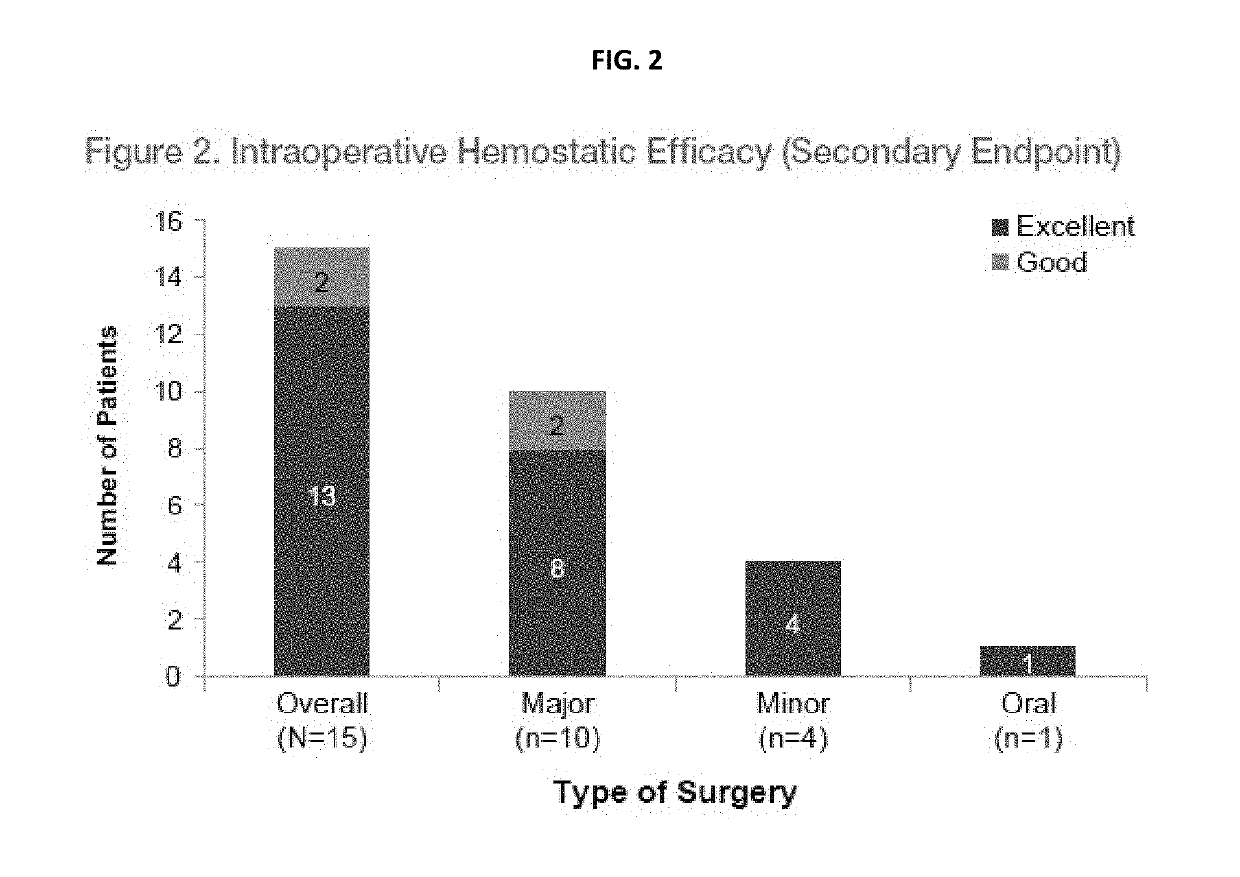
[0246]Overall Hemostatic Efficacy as Assessed by the Investigator (Hemophilia Physician) [Time Frame: 24 hours after last pen-operative infusion or at completion of Day 14 (±2 days) visit, whichever occurs earlier]. Hemostatic efficacy was rated on a scale of excellent—good—moderate—none.[0247]Excellent: Intra-, and postoperative hemostasis achieved with rVWF with or without ADVATE was as good or better than that expected for the type of surgical procedure performed in a hemostatically normal subject.[0248]Good: Intra-, and postoperative hemostasis achieved with rVWF with or without ADVATE was probably as good as that expected for the type of surgical procedure performed in a hemostatically normal subject...
PUM
| Property | Measurement | Unit |
|---|---|---|
| average molecular weight | aaaaa | aaaaa |
| average molecular weight | aaaaa | aaaaa |
| average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com