Targeted probe for nuclide labeling and preparation method and application of targeted probe
A nuclide and targeting technology, applied in the field of medical imaging, can solve problems such as enlightenment or teaching, and no design ideas are given, and achieve the effects of increasing radioactivity concentration, improving pharmacokinetic properties, and strong labeling ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
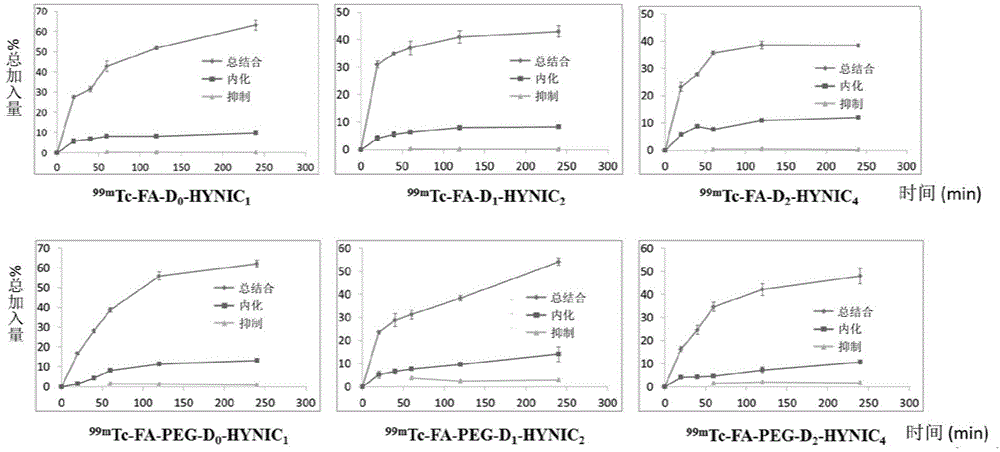
Image
Examples
Embodiment 1
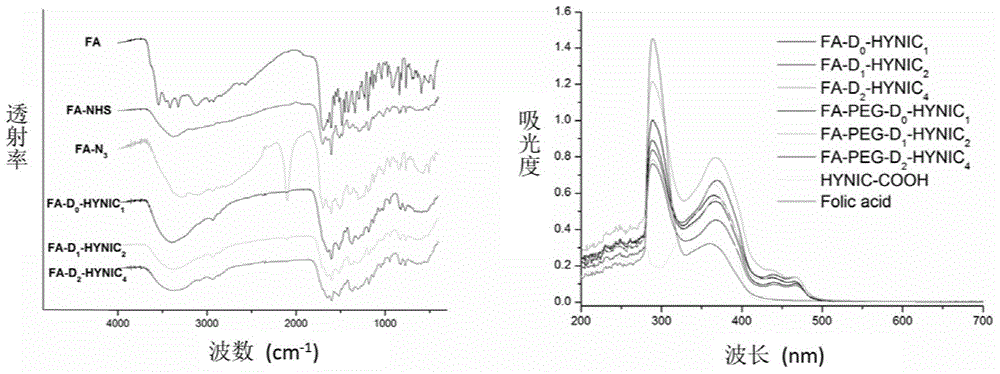
[0055] Example 1 - Synthesis of Folate-Targeted HYNIC Monomers
[0056]
[0057] 1)FA-N 3 Synthesis
[0058] I) Synthesis of FA-NHS: Dissolve folic acid in dimethyl sulfoxide, add N-hydroxysuccinimide (NHS) and dicyclohexylcarbodiimide (DCC), activate in the dark and filter.
[0059] II) FA-N 3 Synthesis of : add 3-azidopropylamine dropwise to the above-mentioned FA-NHS filtrate, add N,N-diisopropylethylamine (DIPEA), after the reaction is complete, drop into diethyl ether to precipitate a precipitate, collect the precipitate and dry it to obtain FA-N 3 .
[0060] 3)D 0 -Synthesis of HYNIC
[0061] I) Synthesis of HYNIC-NHS: Add 6-chloro-nicotine acid to an appropriate amount of 85% hydrazine hydrate, heat at high temperature to make it completely react, then cool and concentrate to obtain a light yellow solid. Dissolve the solid in water, adjust the pH to be weakly acidic with acid, filter after precipitation, wash the obtained solid with 95% ethanol and ether, and ...
Embodiment 2
[0065] Example 2 - Synthesis of PEG-modified folic acid targeting HYNIC monomer
[0066]
[0067] 1) FA-PEG-N 3 Synthesis
[0068] I) Synthesis of FA-NHS: Refer to Example 1 for the synthesis of FA-NHS.
[0069] II) Synthesis of FA-PEG: slowly drop the filtrate of FA-NHS into DMSO of 2,2'-(ethylene dioxy)bis(ethylamine), add N,N-diisopropylethylamine (DIPEA ), reaction 2d at room temperature. Add the reaction solution dropwise to a large amount of dichloromethane to produce a large amount of yellow precipitate, centrifuge or filter with a sand core funnel to obtain a solid, wash with dichloromethane and ether, and dry in a vacuum oven to obtain FA-PEG as a yellow powder solid.
[0070] III) Synthesis of FA-PEG-COOH: Dissolve 1 eq. FA-PEG in DMSO, add 2 eq. succinic anhydride and 0.5 eq. 4-dimethylaminopyridine (DMAP), react at room temperature for 2 d. Add the reaction solution dropwise to a large amount of dichloromethane to produce a large amount of yellow precipitate...
Embodiment 3
[0077] Example 3 - Synthesis of folic acid-targeted HYNIC polymers
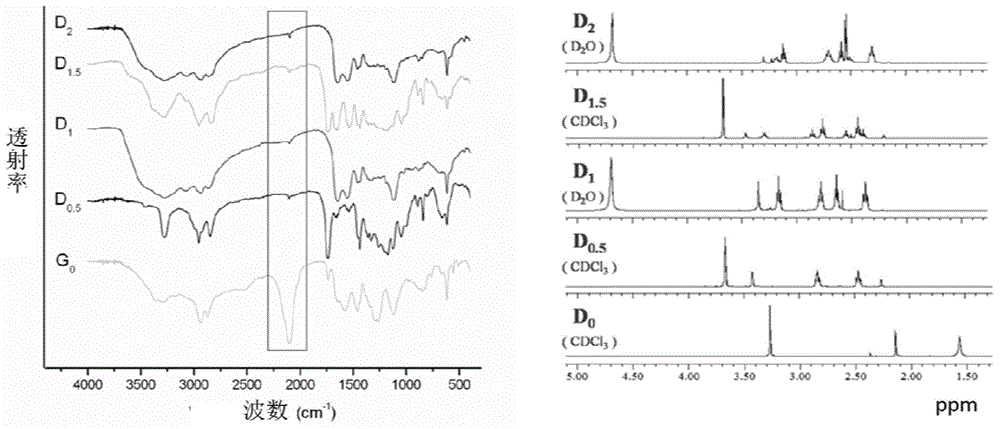
[0078] 1) Molecular skeleton D n Synthesis
[0079]
[0080] I)D 0.5 The synthesis of: in ice-water bath, will be dissolved with propargylamine (D 0 ,) methanol solution was slowly added dropwise to excess methyl acrylate methanol solution, reacted at room temperature for 3d under nitrogen protection, concentrated under reduced pressure to obtain a yellow liquid (D 0.5 ).
[0081] II) D 1 Synthesis: In an ice-water bath, the D 0.5 Dissolved in methanol, slowly added dropwise to the methanol solution containing excess ethylenediamine under the protection of nitrogen, then stirred at room temperature for 3 days, concentrated to obtain light yellow liquid D 1 .
[0082] III) D 1.5 Synthesis: In an ice-water bath, D will be dissolved 1 Slowly added dropwise the methanol solution of excess methyl acrylate in the methanol solution, reacted at room temperature under nitrogen protection for 4d, and concentr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com