High-specific activity L-glutamate oxidase gene multisite mutant, and preparation method and application thereof
A technology of glutamate oxidase and multi-site mutation, which is applied in the field of microorganisms and can solve problems such as application limitations and expensive L-glutamate oxidase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 DNA molecular rearrangement (DNA Shuffling) of L-glutamate oxidase (SDLDOX) gene derived from amylase Streptomyces chromogenes
[0049] 1.1 Gene synthesis of L-glutamic acid oxidase (SDLDOX) derived from amylase Streptomyces chromogenes
[0050] By gene synthesis method (Nucleic Acids Research, 2004, 32, e98) synthetic amylase L-glutamic acid oxidase gene (SDLDOX) of Streptomyces chromogenes, used primer is as follows:
[0051] SDLG1: GGATCCATGACTGAAACTCCACGTGATAATTCTGCAACTCGTGCACGTTGGCAAACTTGT (shown in SEQ ID NO.1)
[0052] SDLG2: CATCAGGACCAACAAGAAGCAGTTCACGTGCCAGCTT GAGACAAGTTTGCCAACGTGCAC (shown in SEQ ID NO.2)
[0053] SDLG3: GCTTCTTGTTGGTCCTGATGACAAGGATCTGAAACTGT CCTATCTGCATACTCTGATTGA (shown in SEQ ID NO.3)
[0054] SDLG4: CTTCTTACGTGGATGATGAGTTGGACCCAG ACGACCAG TATCAATCAGAGTATGCAGATA (shown in SEQ ID NO.4)
[0055] SDLG5: CTCATCATCCACGTAAGAAGATCCTGGTCATTGGTGCT GGTATCACTGGTCTGGTTGCTG (shown in SEQ ID NO.5)
[0056] SDLG6: ATGATAGTGACATCGTAACCAGCATC...
Embodiment 2
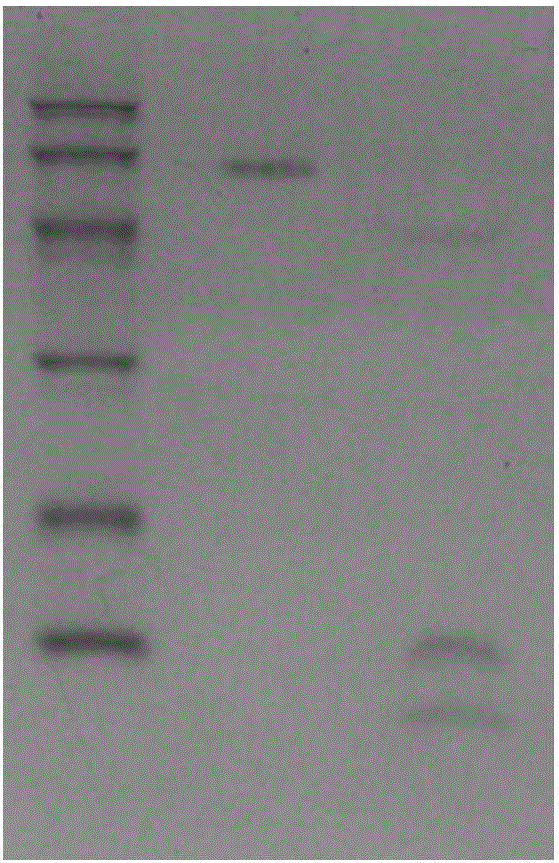
[0110] Example 2 High specific activity L-glutamic acid oxidase (SDLDOX) gene screening
[0111] The rearranged L-glutamic acid oxidase gene fragment recovered in Example 1 was constructed between the prokaryotic expression vector pG251 (CN1338515) promoter and the t1t2 terminator after BamH I and SacI double digestion. Ampicillin resistance gene. Transform Escherichia coli strain DH5α by electroporation to obtain mutant expression library with a capacity of 10 8 , and then use a large number of plasmid extraction kits (Omega, USA) for plasmid extraction.
[0112] Take 1 μl of a large amount of extracted plasmid and transfer it into Escherichia coli DH5α, spread it on the antibiotic medium containing 100mg / L ampicillin, and cultivate it at 37°C for 16 hours, and select resistant transformants for L-glutamic acid oxidase activity screening. The specific library screening method is as follows: transformants are picked into a 40-well bacterial culture plate, 50 μl of pH 8.0 pho...
Embodiment 3
[0115] Example 3 Acquisition of High Specific Activity L-Glutamic Acid Oxidase Gene Mutant
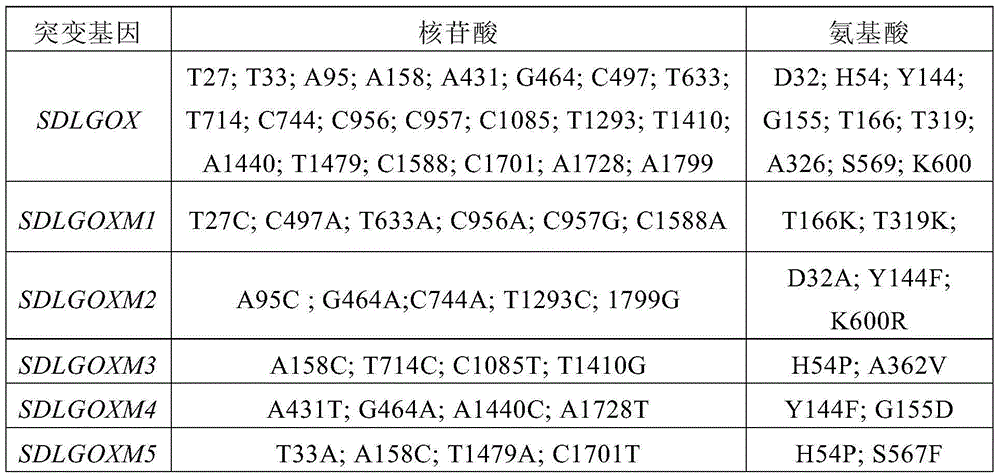
[0116]Using the step-by-step sequencing method to carry out DNA sequencing on the full sequence of the five high specific activity L-glutamic acid oxidase genes screened in Example 2, it was shown that there were changes in 21 nucleotide sites T27C, T33A, A95C , A158C, A431T, G464A, C497A, T633A, T714C, C744A, C956A, C957G, C1085T, T1293C, T1410G, A1440C, T1479A, C1588A, C1701T, A1728T, A15F42A, H( G155D, T166K, T319K, A362V, S567F, K600R), the specific results are shown in Table 1.
[0117] Table 1 Nucleotide and amino acid changes of L-glutamate oxidase gene mutants
[0118]
[0119] Using the L-glutamic acid oxidase (SDLDOX) gene SDLGOXM2 with mutated 3 sites as a template, all the other 6 screened mutation sites were mutated to complete 9 site mutations. Primers used for mutation are as follows.
[0120] Primers for the H54P mutation:
[0121] 54Z: GCCGCCTCGGCCCCACCCCCCA (sh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com