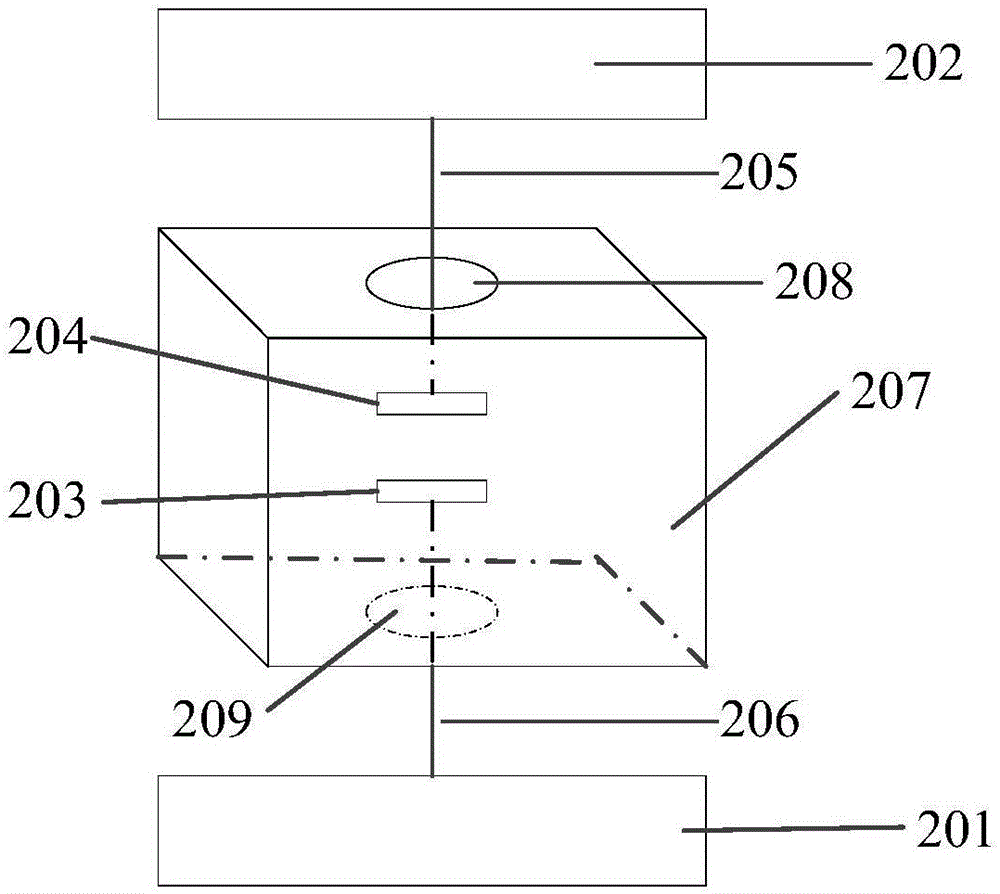
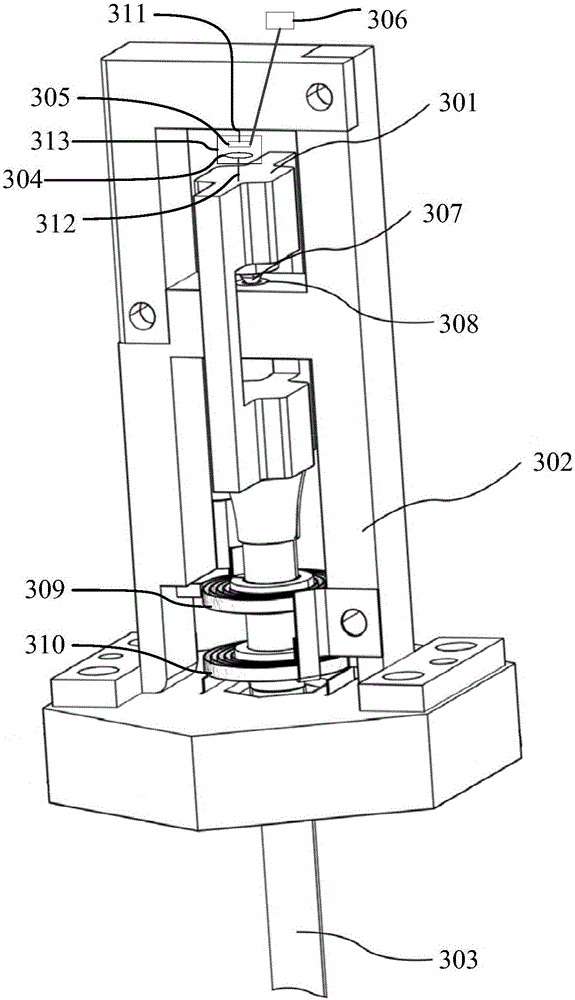
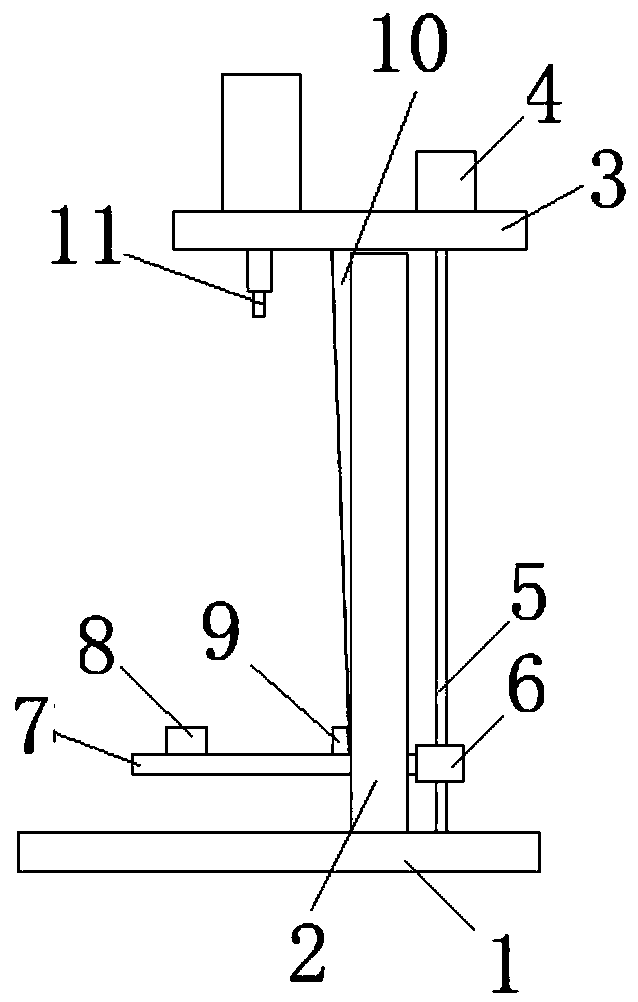
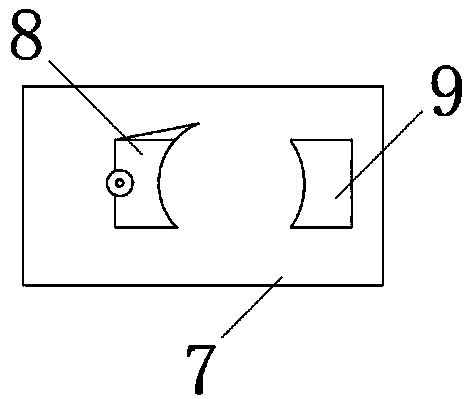
Patents
Literature
58 results about "Thromboelastography" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
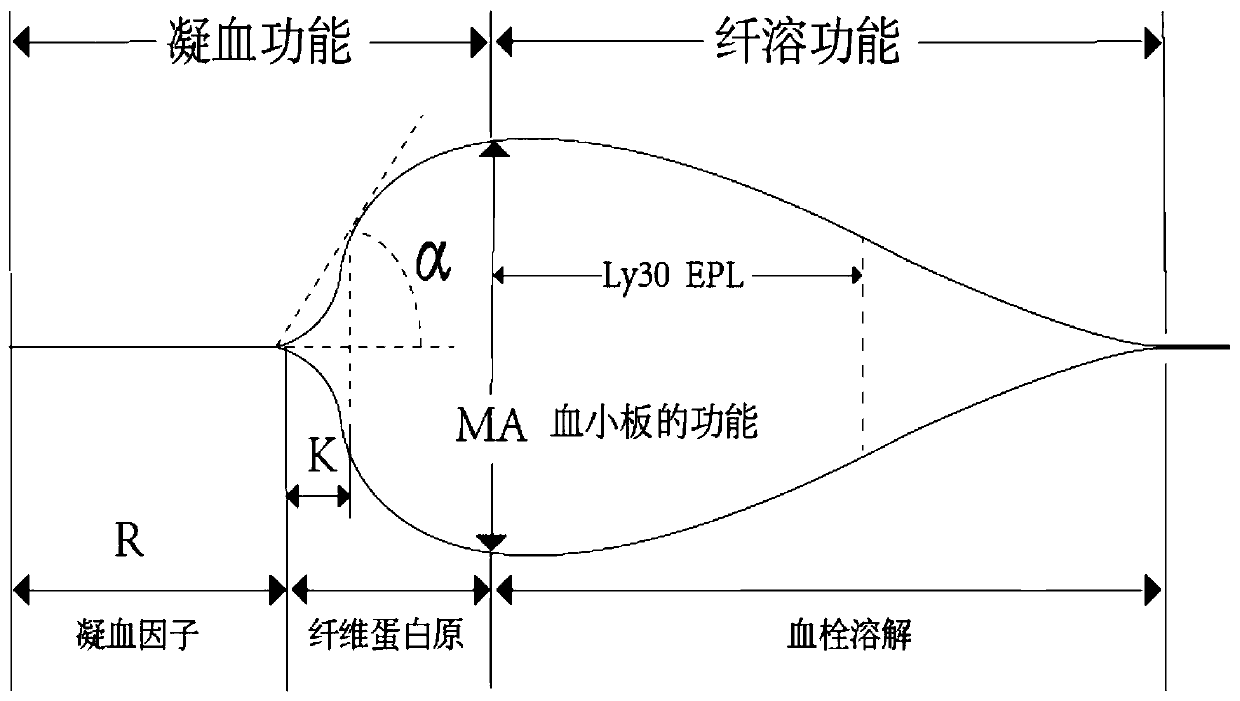
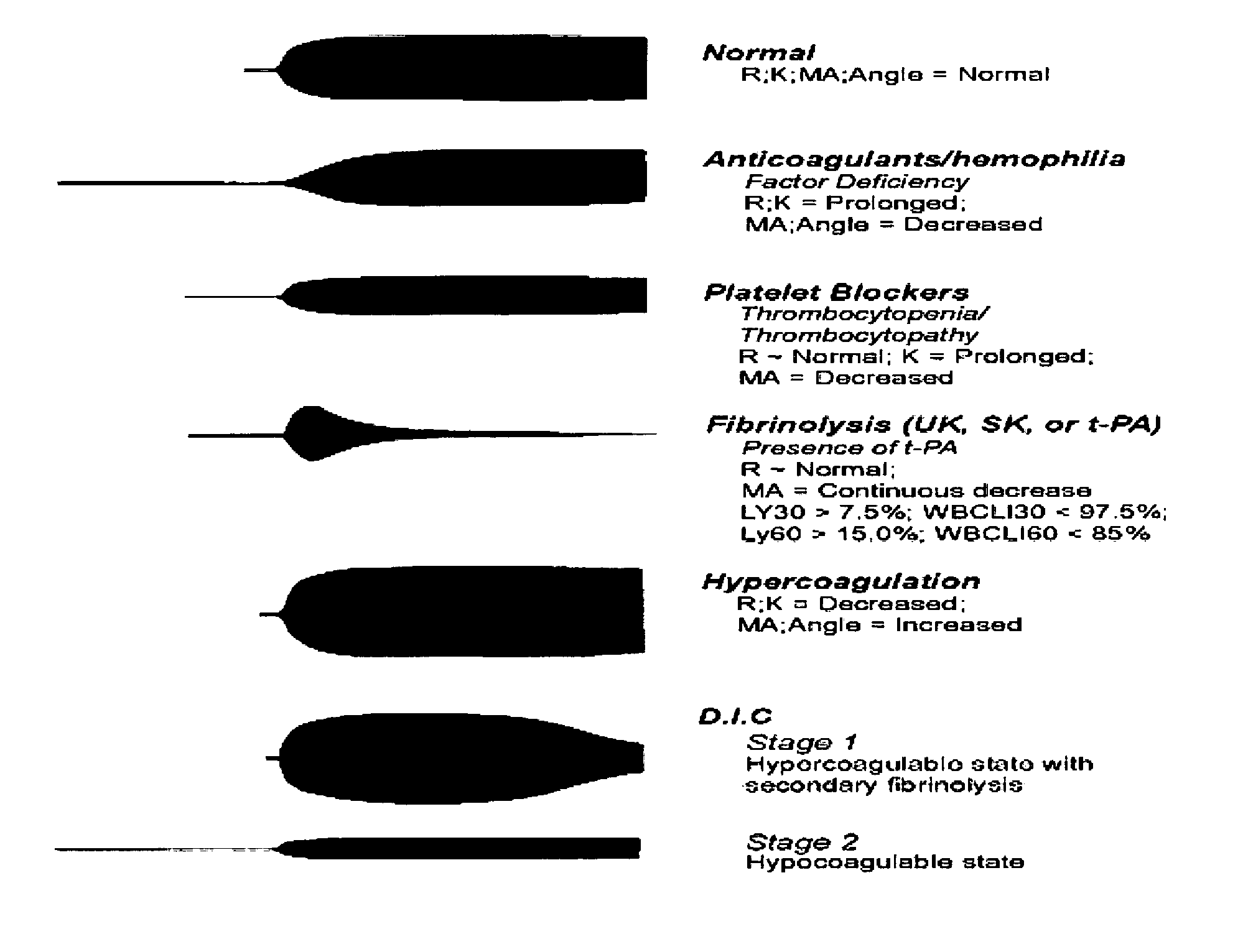
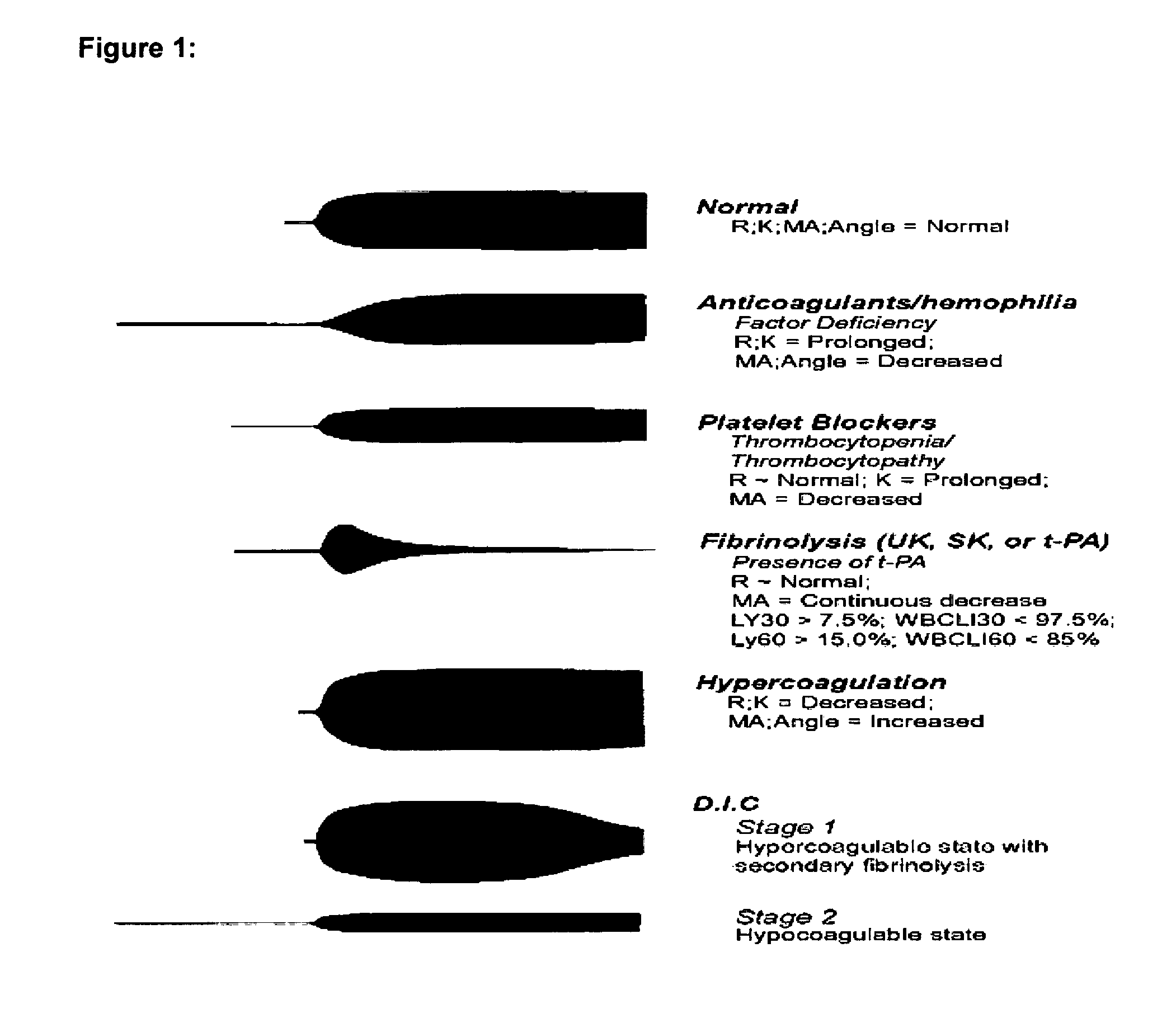
Thromboelastography (TEG) is a method of testing the efficiency of blood coagulation. It is a test mainly used in surgery and anesthesiology, although increasingly used in resuscitations in Emergency Departments, intensive care units, and labor and delivery suites. More common tests of blood coagulation include prothrombin time (PT,INR) and partial thromboplastin time (aPTT) which measure coagulation factor function, but TEG also can assess platelet function, clot strength, and fibrinolysis which these other tests cannot.
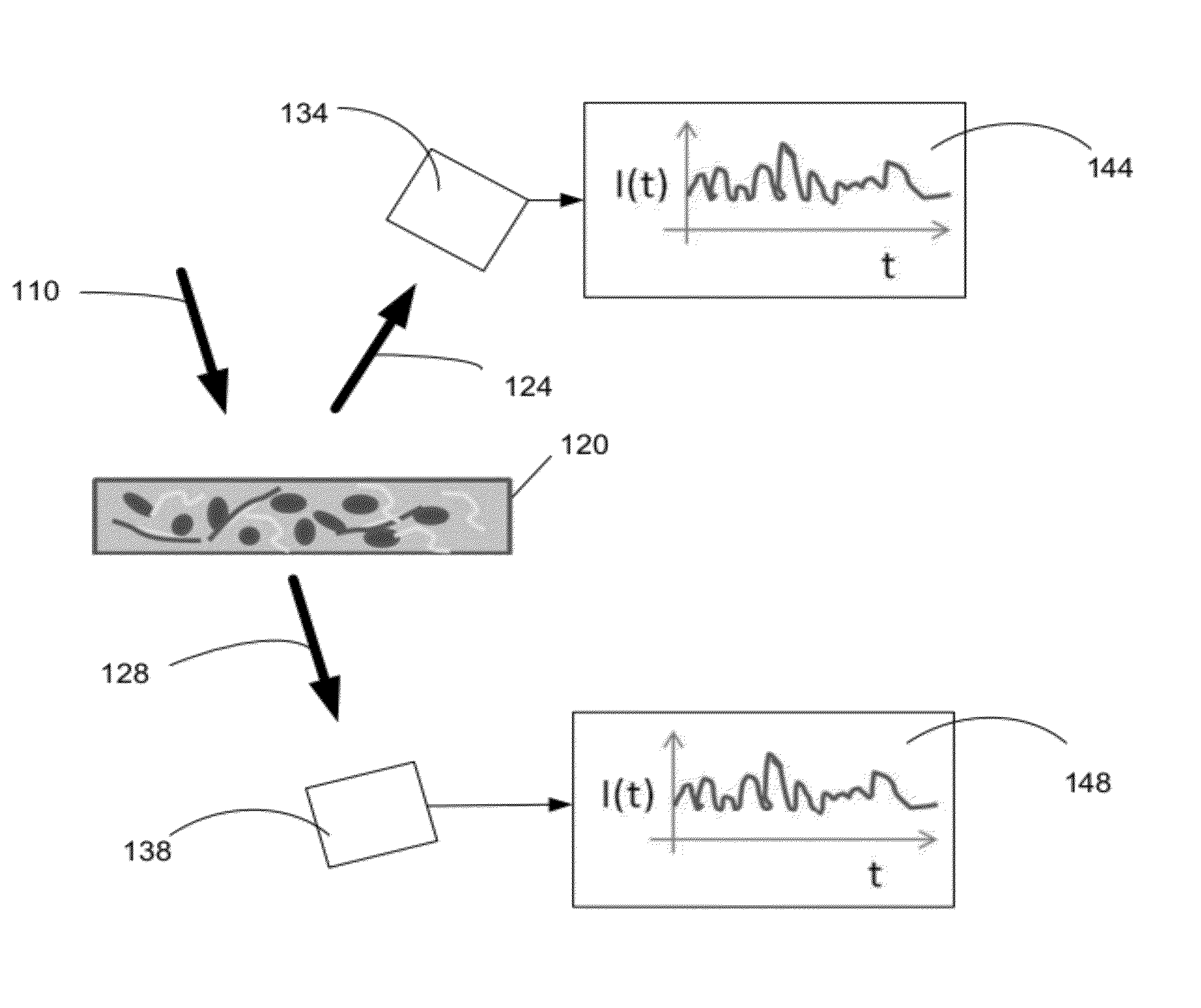
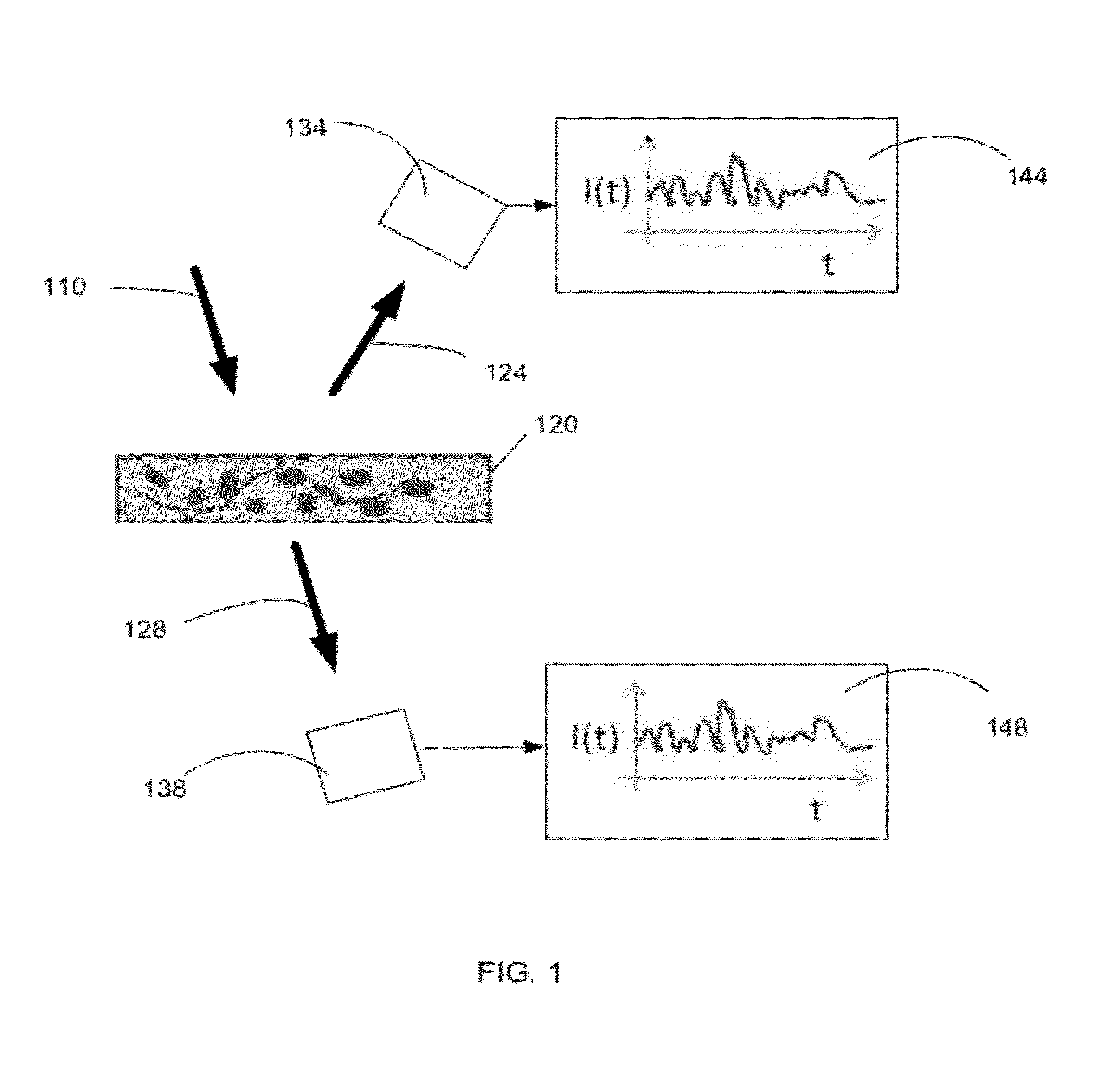
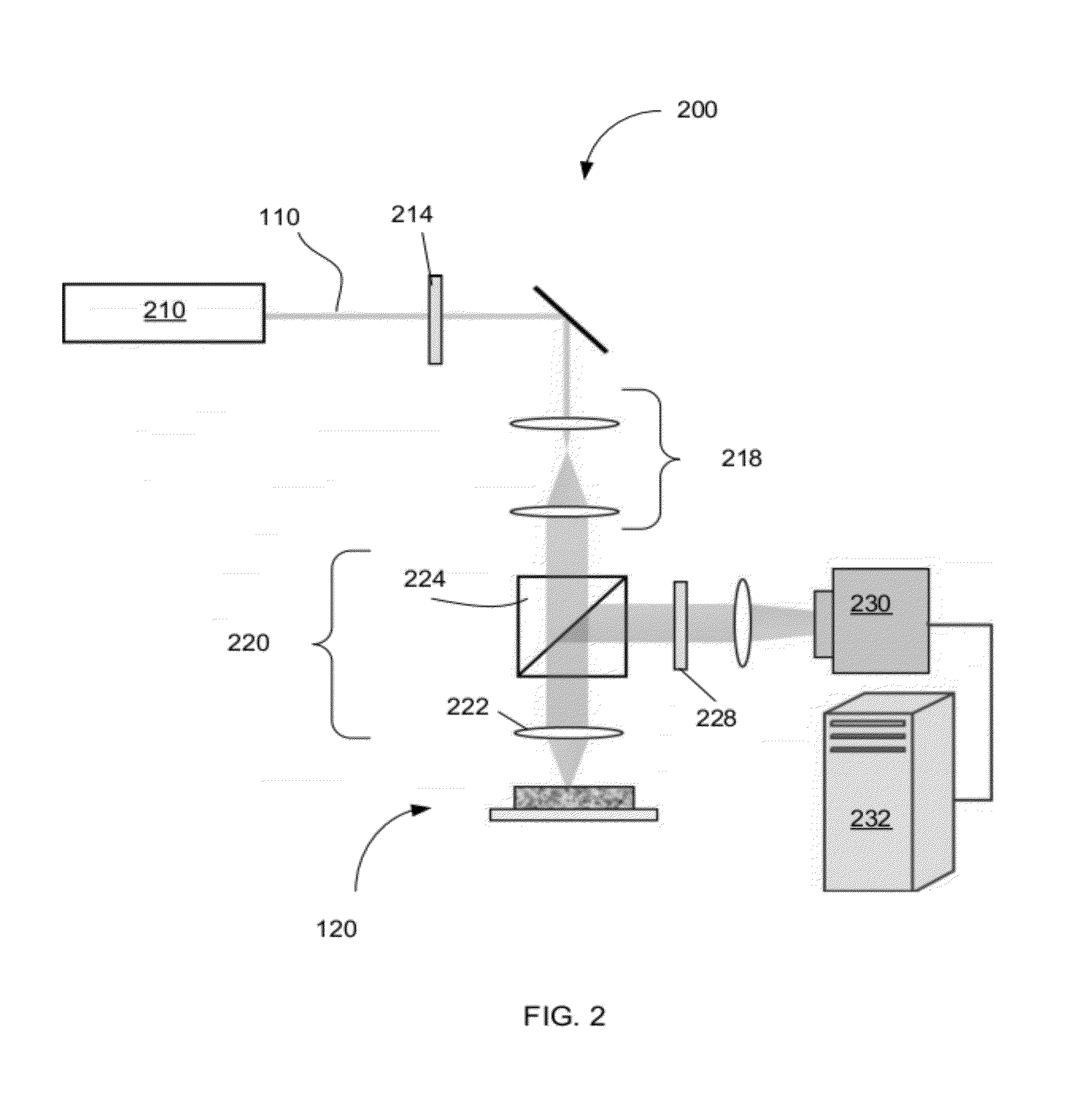
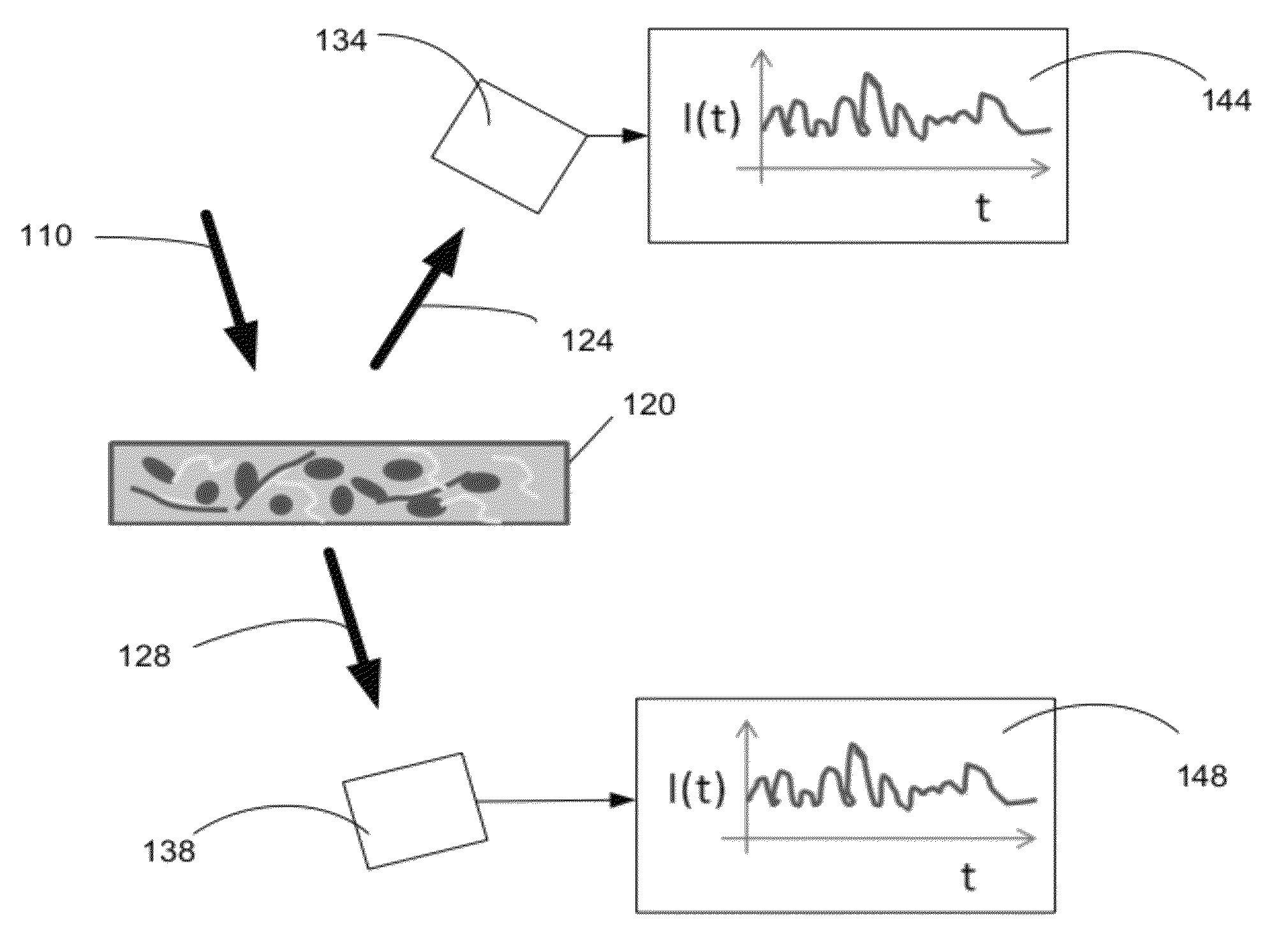
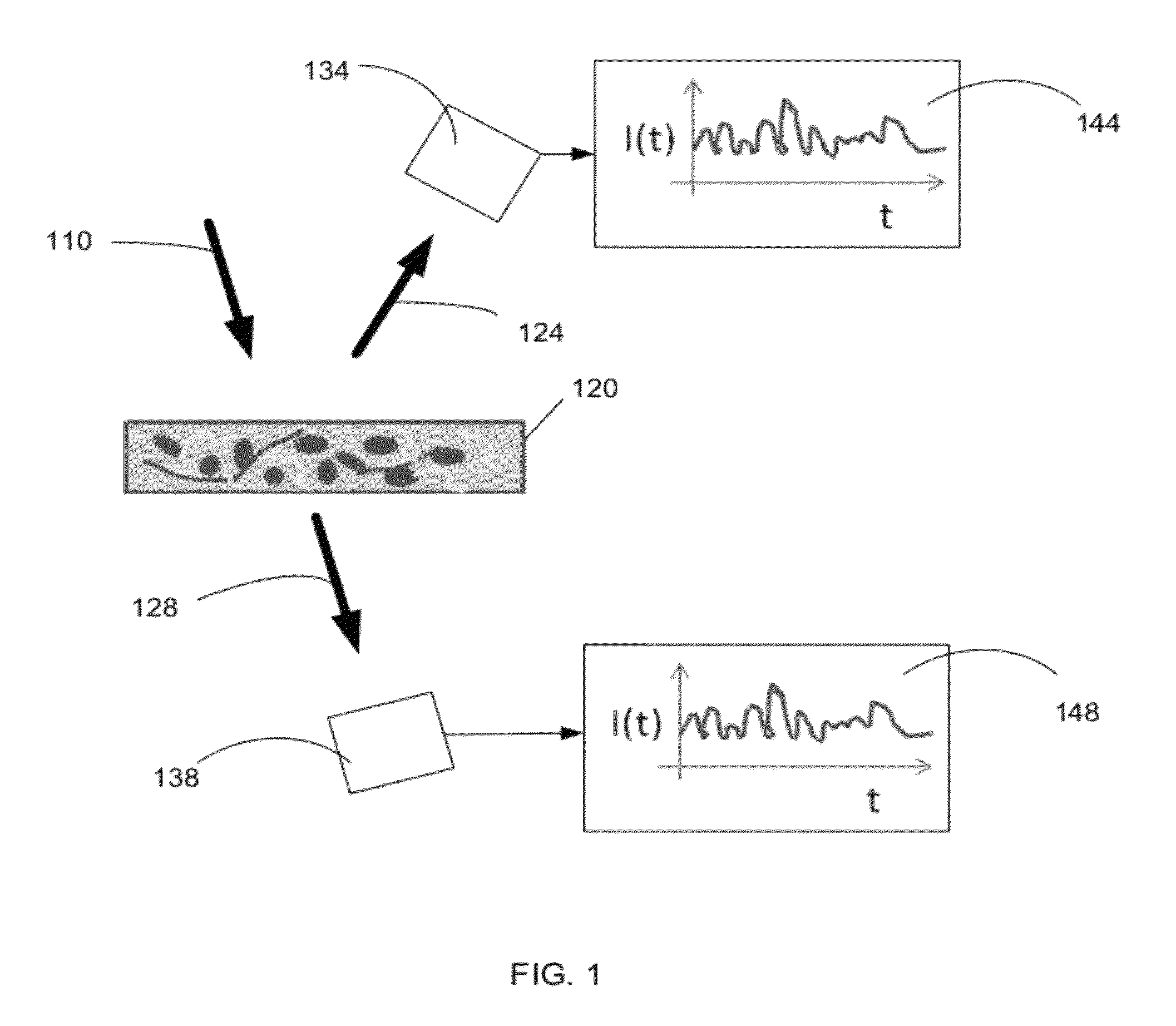
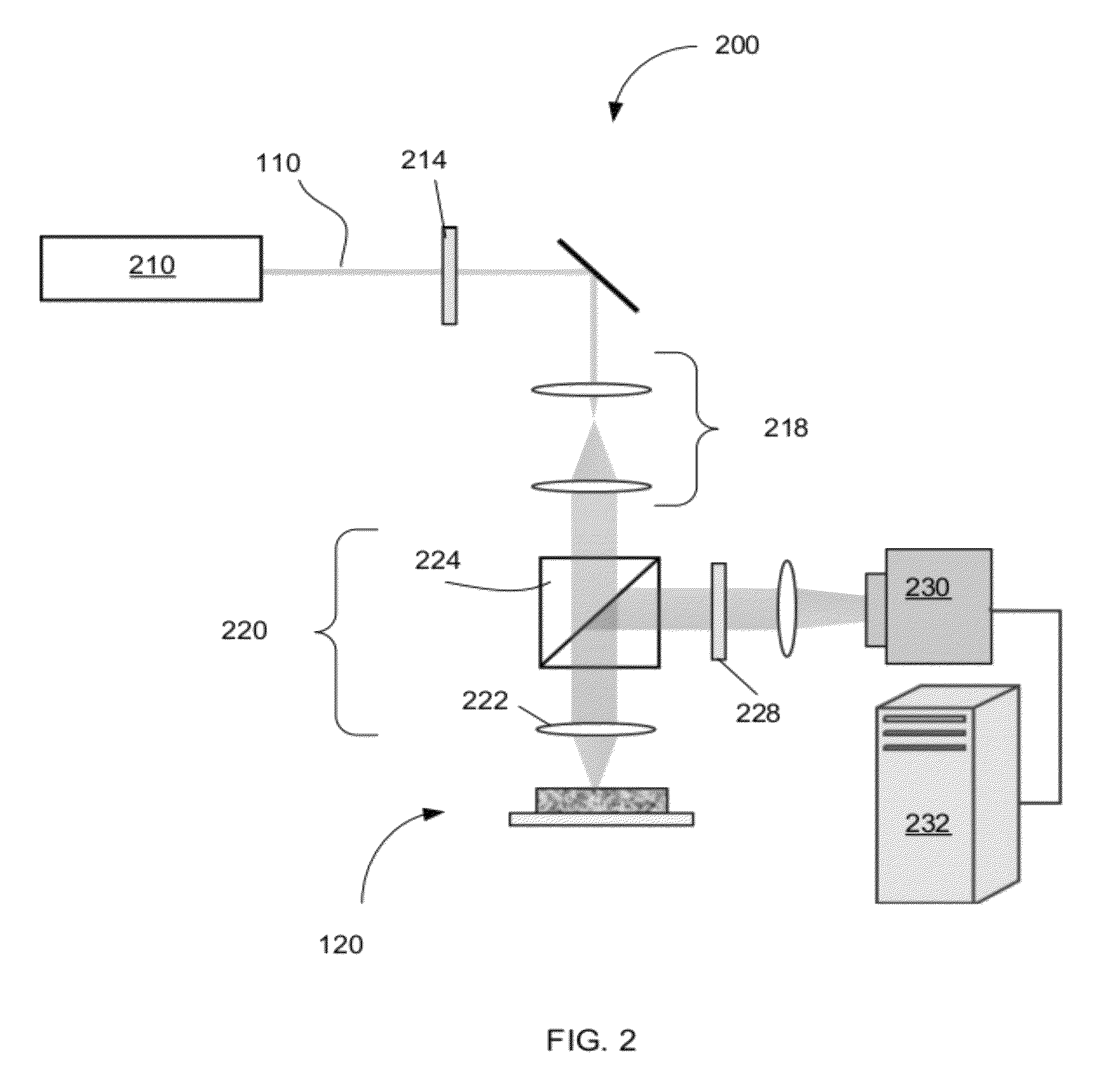
Optical thromboelastography system and method for evaluation of blood coagulation metrics
ActiveUS20120301967A1Scattering properties measurementsDiagnostic recording/measuringLaser lightFibrinolysis
Device, method, and computer program product for determining a material parameter of a blood coagulation cascade based on parameters of light diffused at a biofluid sample. In one example, the biofluid sample includes a blood sample. Laser light scattered by the sample is collected by the optical system in reflection and / or transmission mode. An image of the sample in so collected light is formed, and data representing fluctuations of laser speckle intensity with is processed to derive numerical descriptors associated with blood coagulation and fibrinolysis. In a specific case, such numerical descriptors are derived based on temporal dynamic of a viscoelastic characteristic of the blood sample.
Owner:THE GENERAL HOSPITAL CORP
Optical thromboelastography system and method for evaluation of blood coagulation metrics
ActiveUS8772039B2Scattering properties measurementsAnalysis by subjecting material to chemical reactionLaser lightFibrinolysis
Device, method, and computer program product for determining a material parameter of a blood coagulation cascade based on parameters of light diffused at a biofluid sample. In one example, the biofluid sample includes a blood sample. Laser light scattered by the sample is collected by the optical system in reflection and / or transmission mode. An image of the sample in so collected light is formed, and data representing fluctuations of laser speckle intensity with is processed to derive numerical descriptors associated with blood coagulation and fibrinolysis. In a specific case, such numerical descriptors are derived based on temporal dynamic of a viscoelastic characteristic of the blood sample.
Owner:THE GENERAL HOSPITAL CORP
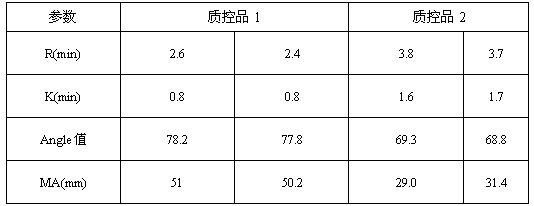
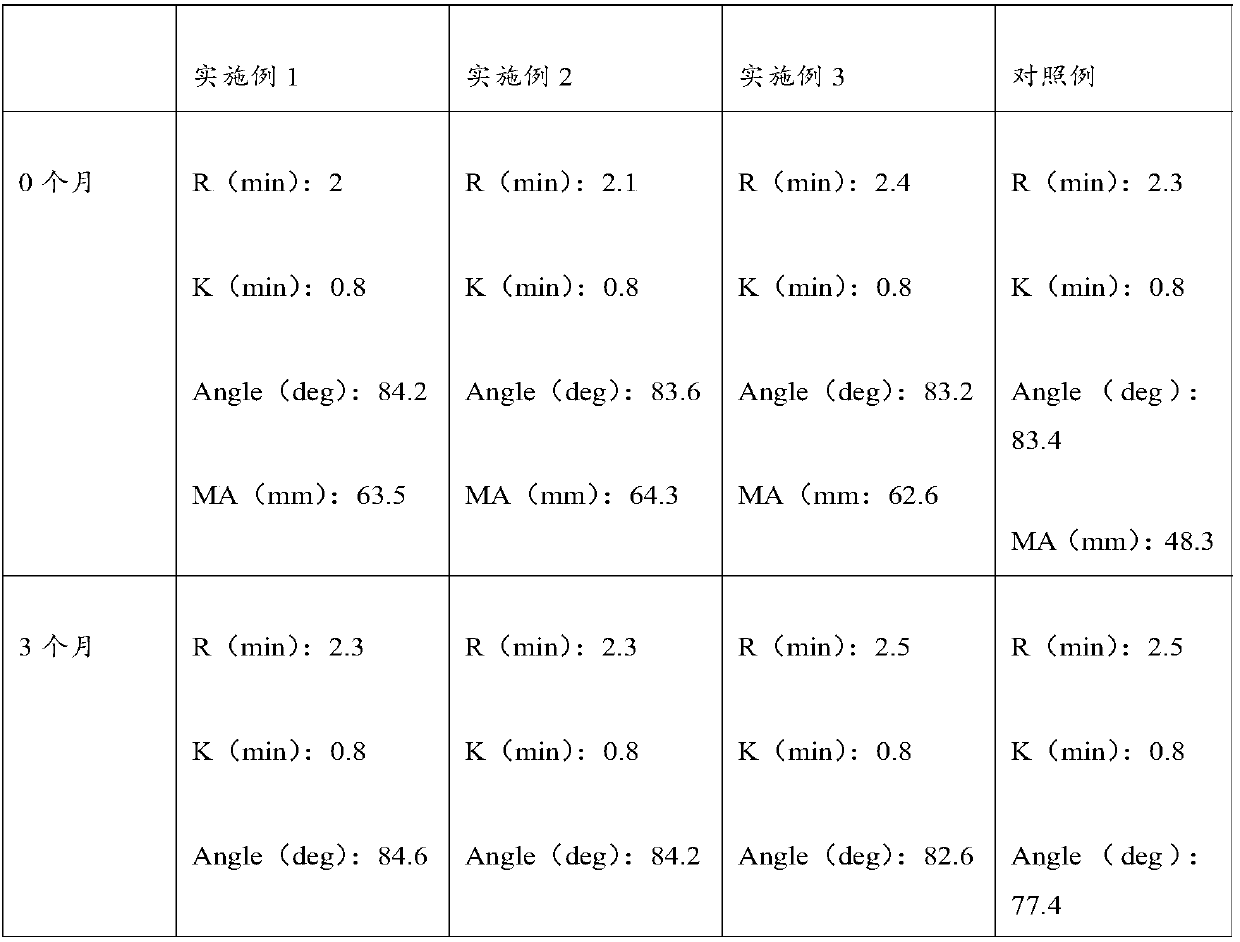
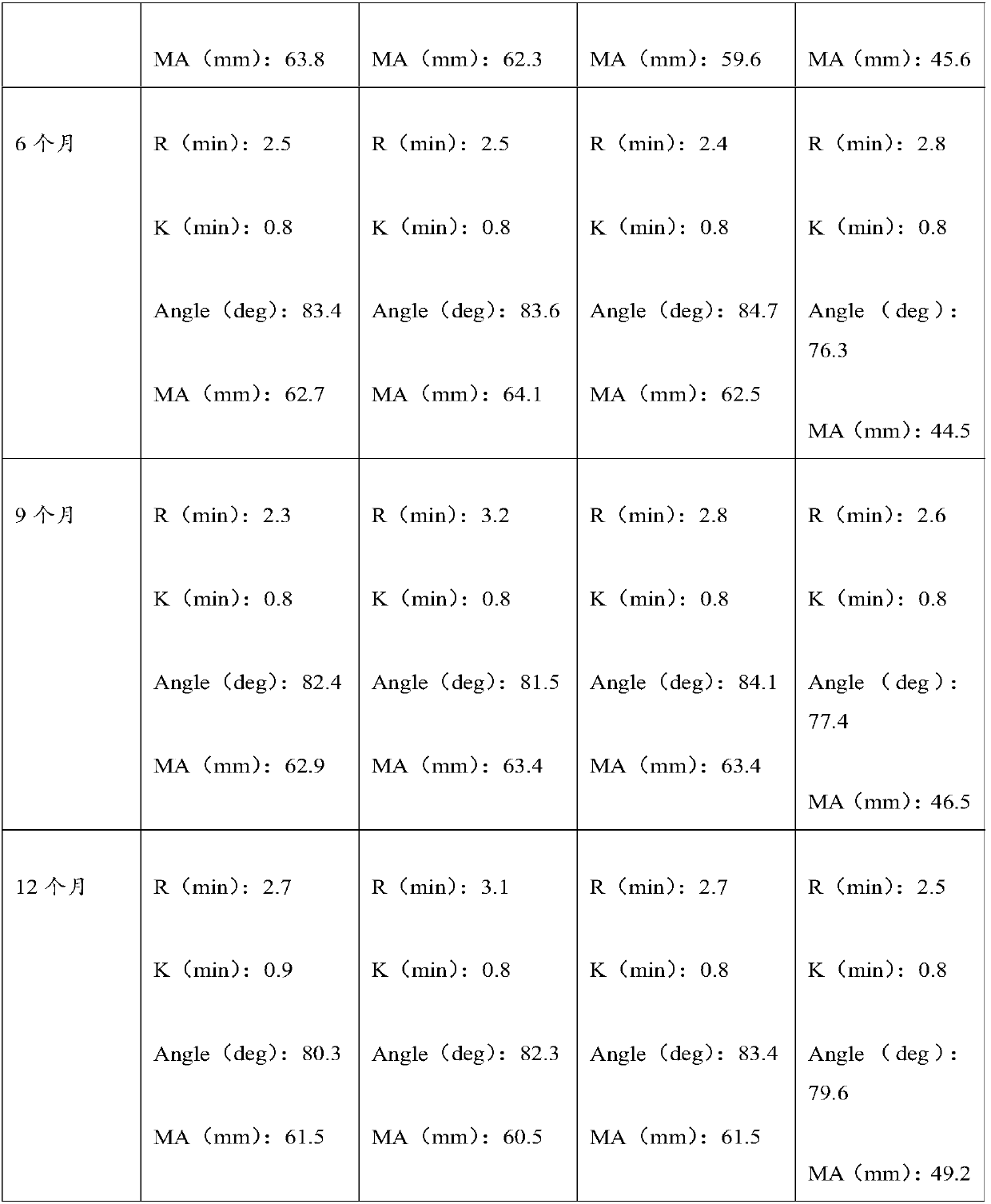
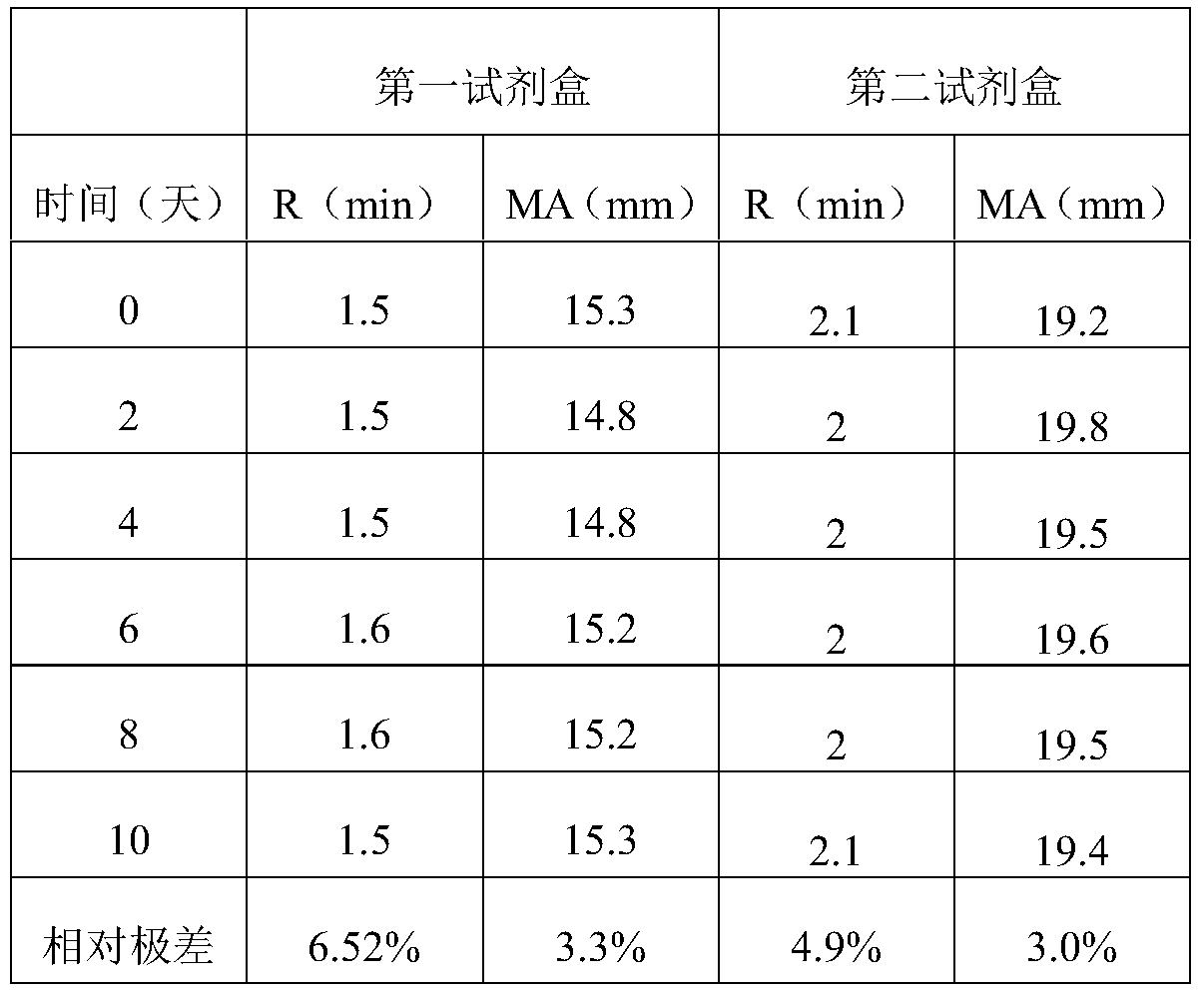
3-level thrombelastogram quality control product and application thereof
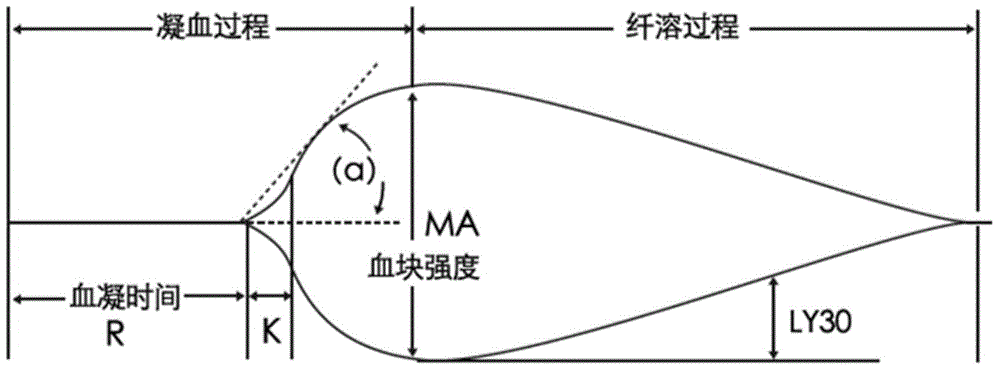
The invention relates to the field of quality control of blood coagulation testing items and provides a 3-level thrombelastogram quality control product. The quality control product is prepared by the following steps: respectively mixing pig blood with 3 different concentrations of sodium citrate, centrifuging, taking blood plasma, respectively adding a coagulation activator and a tissue factor and lyophilizing to prepare a powdery quality control product I, adding a coagulation activator and lyophilizing to prepare a powdery quality control product II, and adding a coagulation activator and human lyophilized platelets and lyophilizing to prepare a powdery quality control product III. The 3-level thrombelastogram quality control product provided by the invention is shaped as a lyophilized powder, can be used to monitor R and MA values simultaneously, is better used for quality control of an thromboelastography instrument and a thrombelastogram detection kit, is fast to detect, has accurate results and is simple to operate.
Owner:北京乐普诊断科技股份有限公司
Method for Differentiation of Factor XIII Deficiency States in Relation to Fibrinogen Deficiency States Using Thrombelastographic Techniques
InactiveUS20080261238A1Stable haemostasisMaterial analysis using sonic/ultrasonic/infrasonic wavesMicrobiological testing/measurementDeficiency stateMedicine
The invention relates to a method for determining a factor XIII deficiency, a method for determining a fibrinogen deficiency, and a method for differentiating between a factor XIII deficiency and a fibrinogen deficiency by means of thrombelastographic techniques. On the basis of the evaluation of the thrombelastographic parameters, a rapid and a selective substitution of factor XIII and / or of fibrinogen in deficiency states is possible.
Owner:KORTE WOLFGANG DR
Device and Method for Performing Blood Thromboelastographic Assays by Magnetic Sensing
ActiveUS20150024473A1Bioreactor/fermenter combinationsBiological substance pretreatmentsMicrocontrollerMotion detector
A magnetic sensor elastometry device (MSED) and a method to perform the whole blood thromboelastography assay. It contains key components, including sample cuvette, detecting head, rotating disc, optical motion detector, and etc; and measures viscoelasticity of whole blood samples. The device optically monitors the physical motion of the magnetically driven rotating disc immersed in the blood sample. The thromboelastograph is recorded by the optical motion detector reading high pulse counts through a gated time window passing through the rotating disc. The device also includes a microcontroller and its embedded firmware to perform the functions of driving the rotating magnetic disc, generating high-frequency pulses, controlling the data pulse time window, as well as handling the user's interface, data analysis, and maintaining communication with an external computer.
Owner:NEOTEK BIOSCI
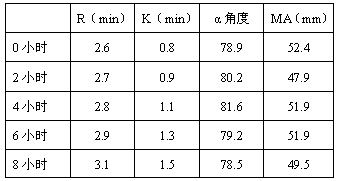
Thromboelastography quality control material and preparation method thereof
ActiveCN105652022ASufficient sourceLow costPreparing sample for investigationBiological testingFreeze-dryingTest room
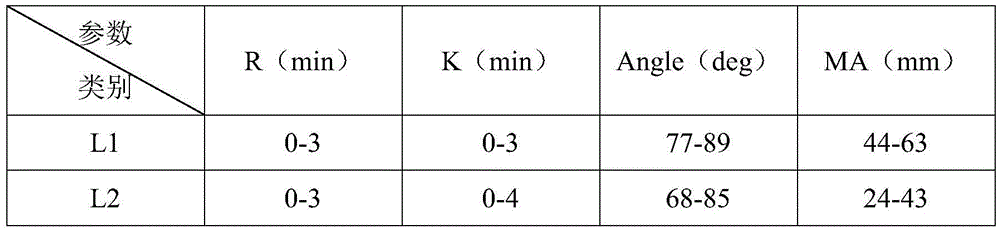
The invention relates to the field of quality control of blood coagulation detection of a clinical thromboelastography, in particular to a thromboelastography quality control material and a preparation method thereof. The preparation method of the thromboelastography quality control material comprises the following steps of step 1, collecting fresh pig whole blood and separating plasma; step 2, dividing the plasma into two parts, wherein the first part is used for extracting fibrinogen and a blood coagulation factor, and the second part is used for properly diluting to obtain diluted plasma; step 3, adding extracted fibrinogen and blood coagulation factor into the diluted plasma to obtain a mother solution, and adjusting an R value, a K value, an Angle value and an MA value to be within the ranges of L1 and L2; step 4, performing subpackage and freeze drying on the solution obtained in the step 3 to obtain a final product. The quality control material prepared by the preparation method disclosed by the invention has two specifications of L1 and L2 and is good in stability; the demand that quality control over normal and abnormal blood samples is simulated in a test room can be met.
Owner:ZHEJIANG SHENGYU MEDICAL TECH CO LTD
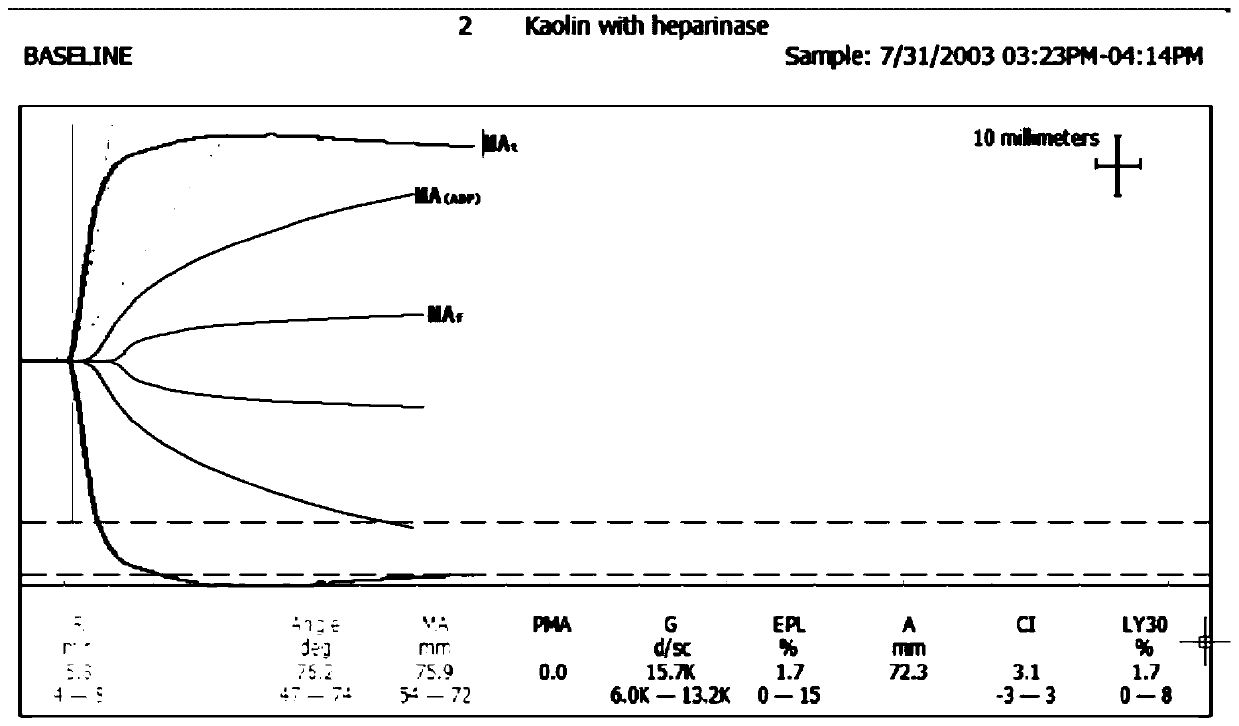
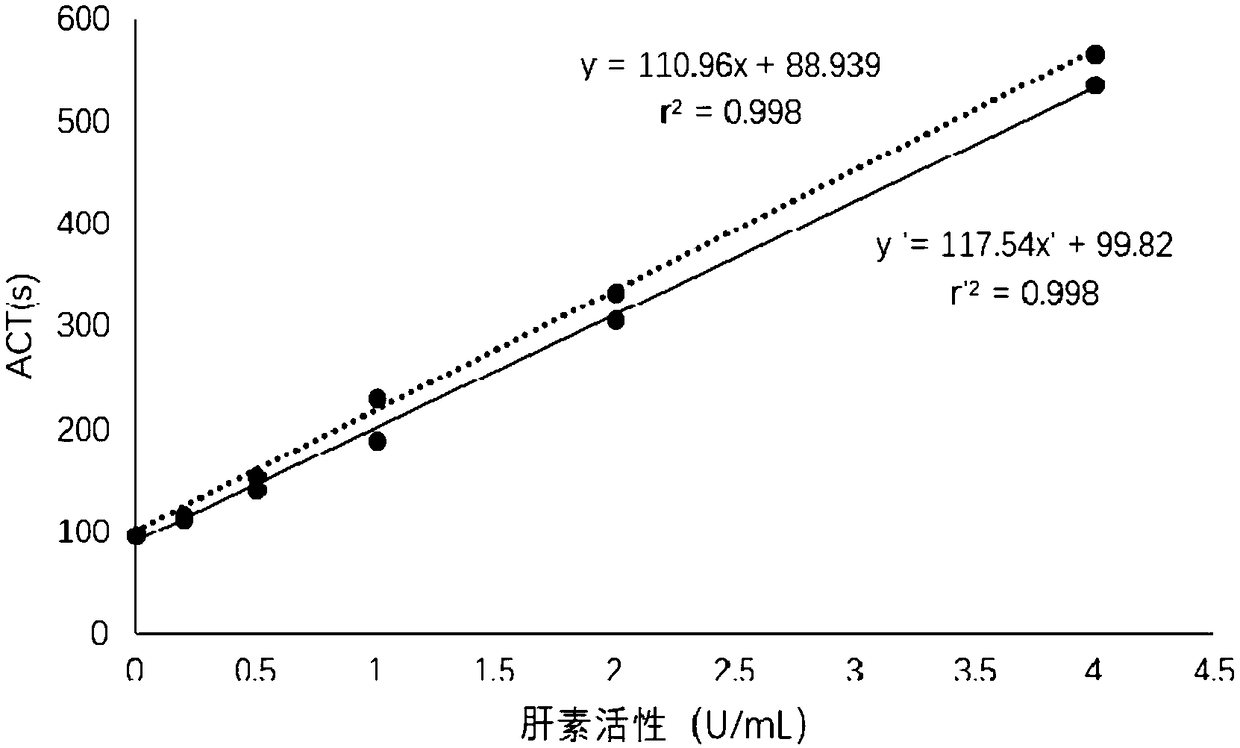
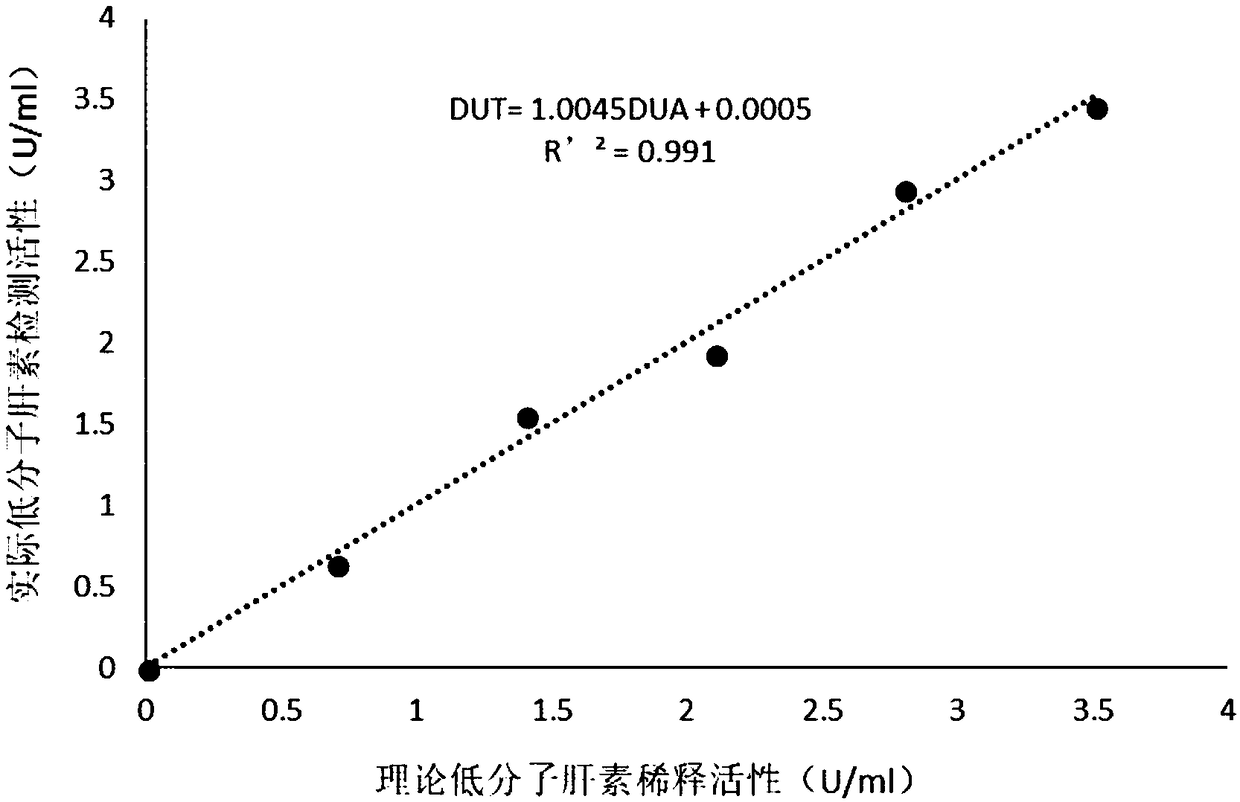
Detection method for evaluating curative effect and residual condition of heparin drugs
The invention relates to the field of blood detection, in particular to a detection method for evaluating the curative effect and the residual condition of heparin drugs. The heparin drugs mainly comprise heparin, low-molecular-weight heparin and some heparinoid drugs. The detection method mainly comprises steps as follows: heparinase, a freeze-drying protective agent and an adhesive are mixed, then a mixed reagent in appropriate proportion is prepared from an obtained mixture by the aid of a buffer solution, the mixed reagent is enveloped in a test-cup and subjected to freezing and vacuum pumping treatment, and a heparinase cup is obtained. The detection method has a plurality of advantages that the operation is simple, the guarantee period is long, the stability is good and the like. Theheparinase cup and a common cup (a blank sample cup) are put in a thrombelastogram, an anticoagulant and a chelating agent are added to the cups respectively, endogenously activated whole blood samples are added to the test-cups, and thromboelastography is started for detection; values R of starting time of blood clot formation in the heparinase cup and the common cup are compared, and whether heparin exists in the blood can be judged; if heparin exists, the value R of the starting time of blood clot formation in the heparinase cup is smaller than that of the starting time of blood clot formation in the common cup. The method is simple to operate, a detection result is good in repeatability, and the accuracy is high.
Owner:北京乐普诊断科技股份有限公司
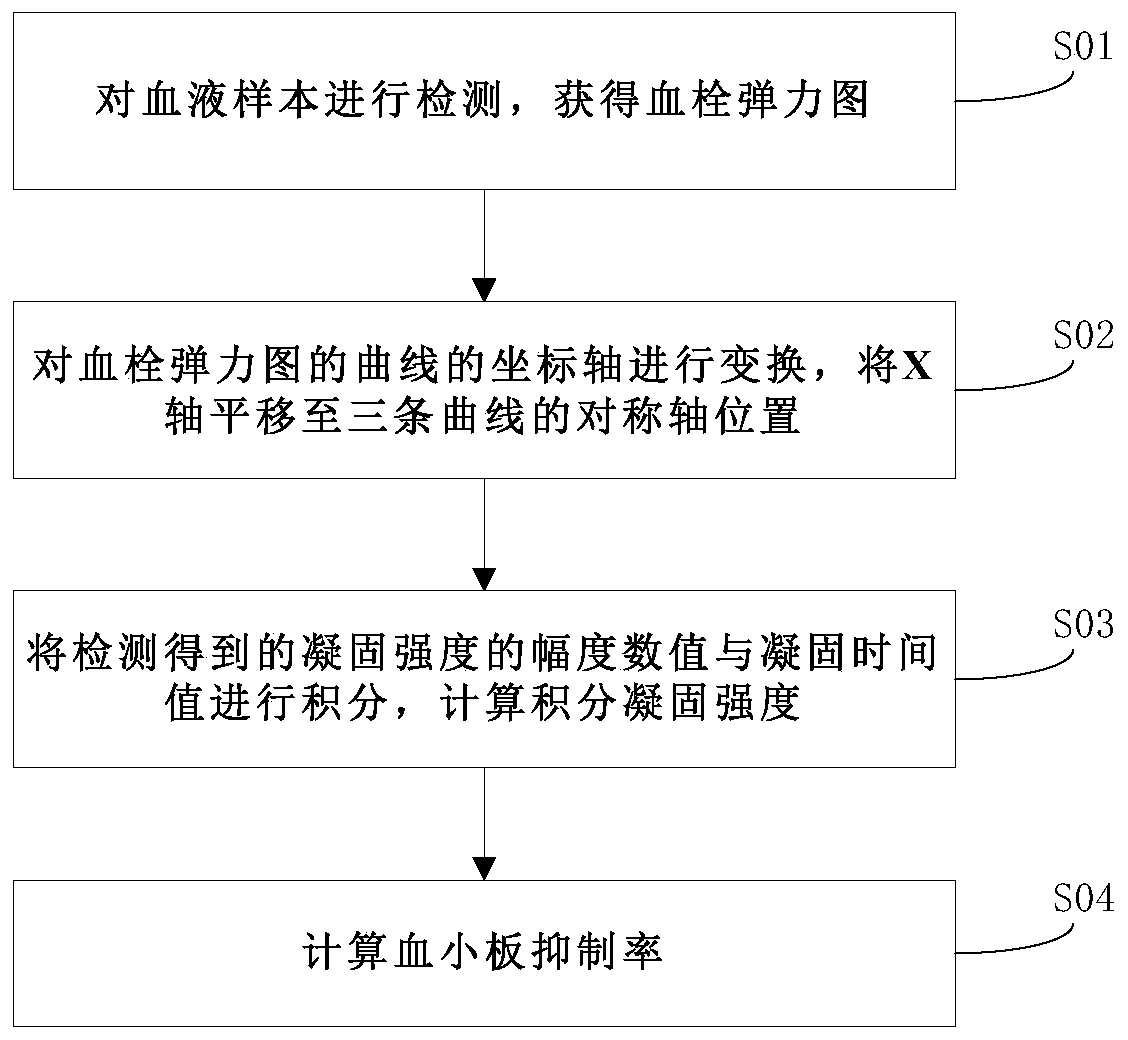
Platelet inhibition rate calculation method based on thromboelastography
ActiveCN110619938AFix bugsMaster the actual medication situationDrug and medicationsBiological testingMedicineEvaluation finding
The invention discloses a platelet inhibition rate calculation method based on thromboelastography. The method comprises the steps that a blood sample is detected to acquire a thromboelastogram, wherein the thromboelastogram comprises the maximum coagulation intensity MAt curve, the minimum coagulation intensity MAf curve and the platelet coagulation curve f(t) of a coagulated blood sample; the coordinate axis of the curves of the thromboelastogram is transformed, the X axis is translated to the position of the symmetry axis of the three curves; the amplitude value of the detected coagulationintensity and the coagulation time value are integrated; the integral coagulation strength DIt of the maximum coagulation intensity MAt curve, the integral coagulation intensity DIf of the minimum coagulation intensity MAf curve and the integral coagulation intensity of the coagulation curve f(t) are calculated respectively; and the platelet inhibition rate BPI=(1-(DI-DIf) / (DIt-DIf))*100% is calculated. The acquired evaluation results are more accurate and can accurately reflect the actual state of platelet inhibition after the use of platelet inhibitory drugs.
Owner:ZIRCON BIOTECH CO LTD
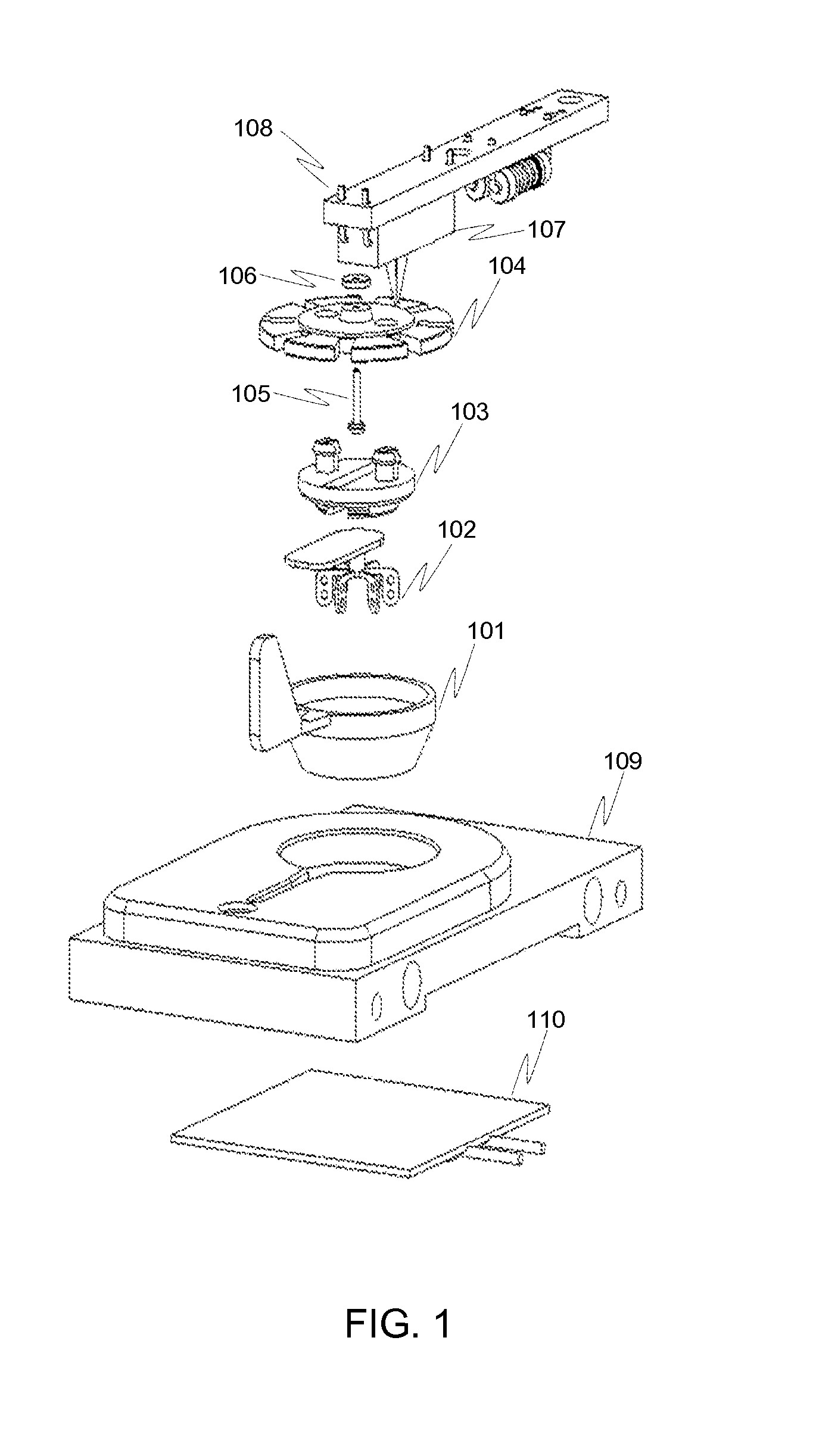
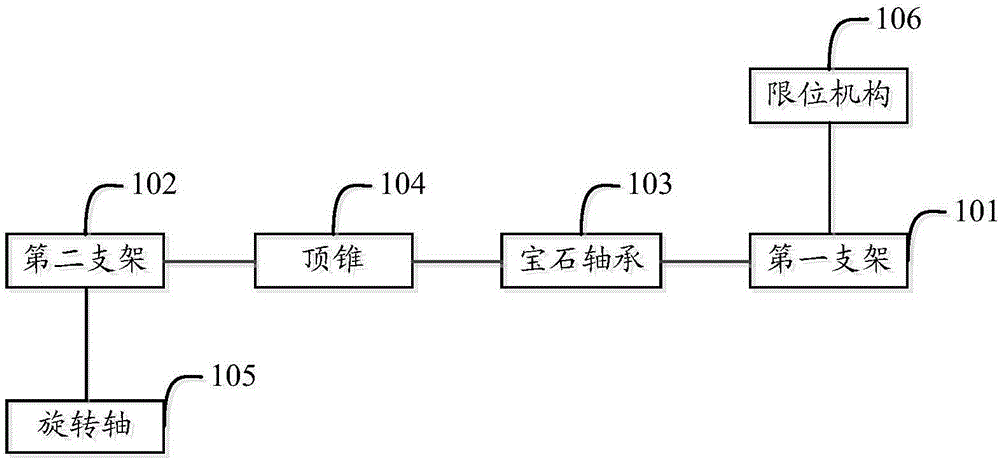
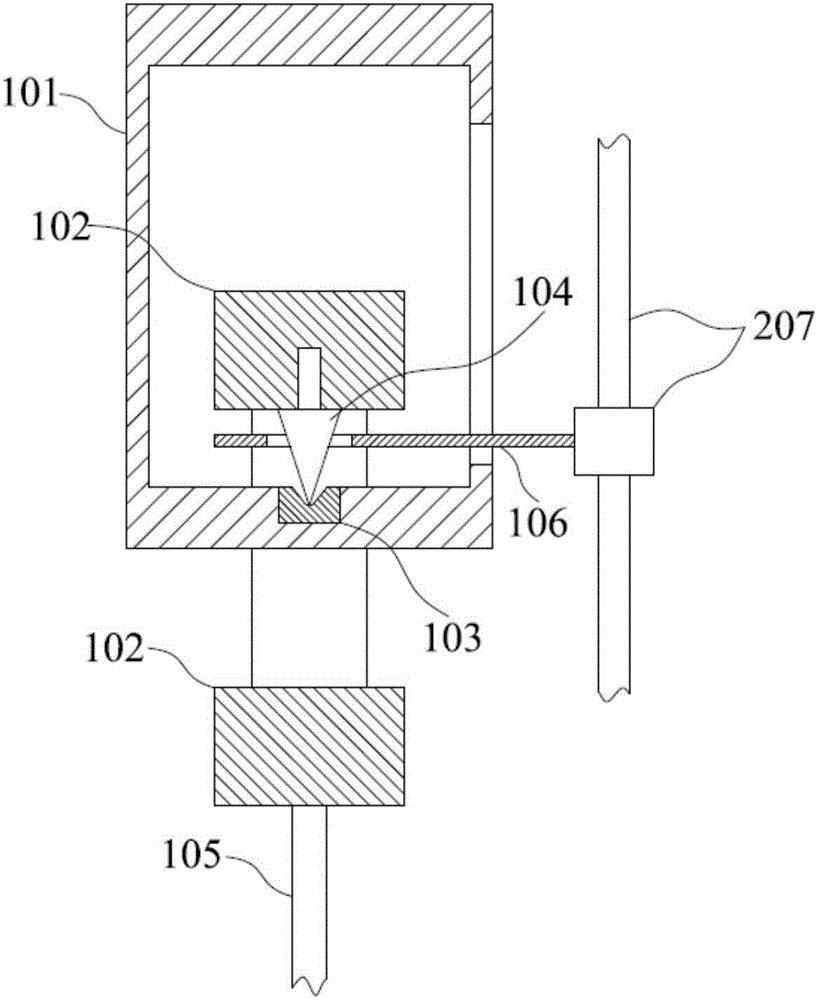
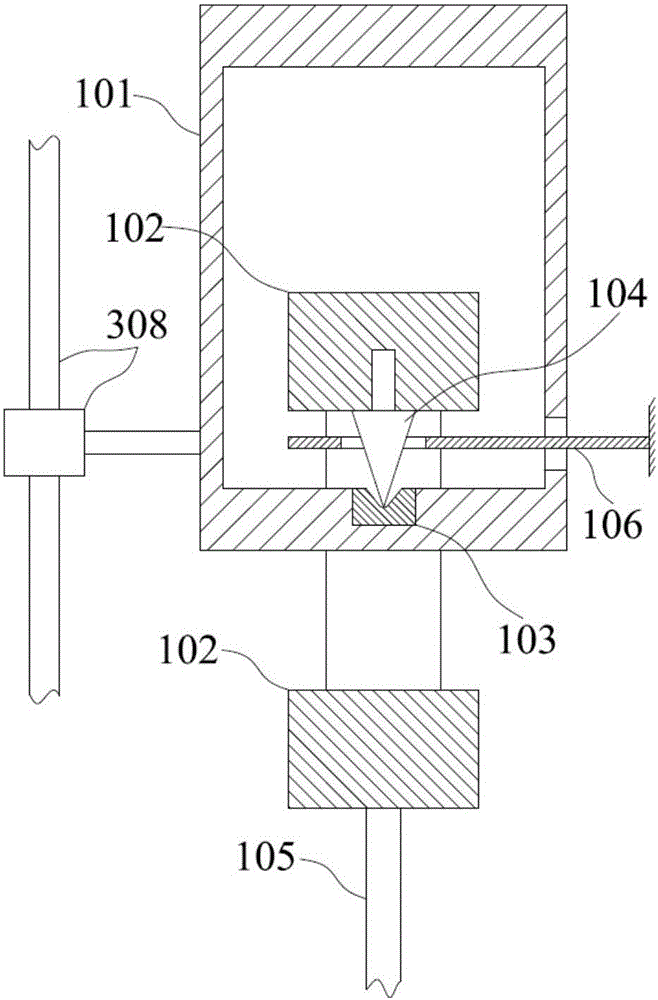
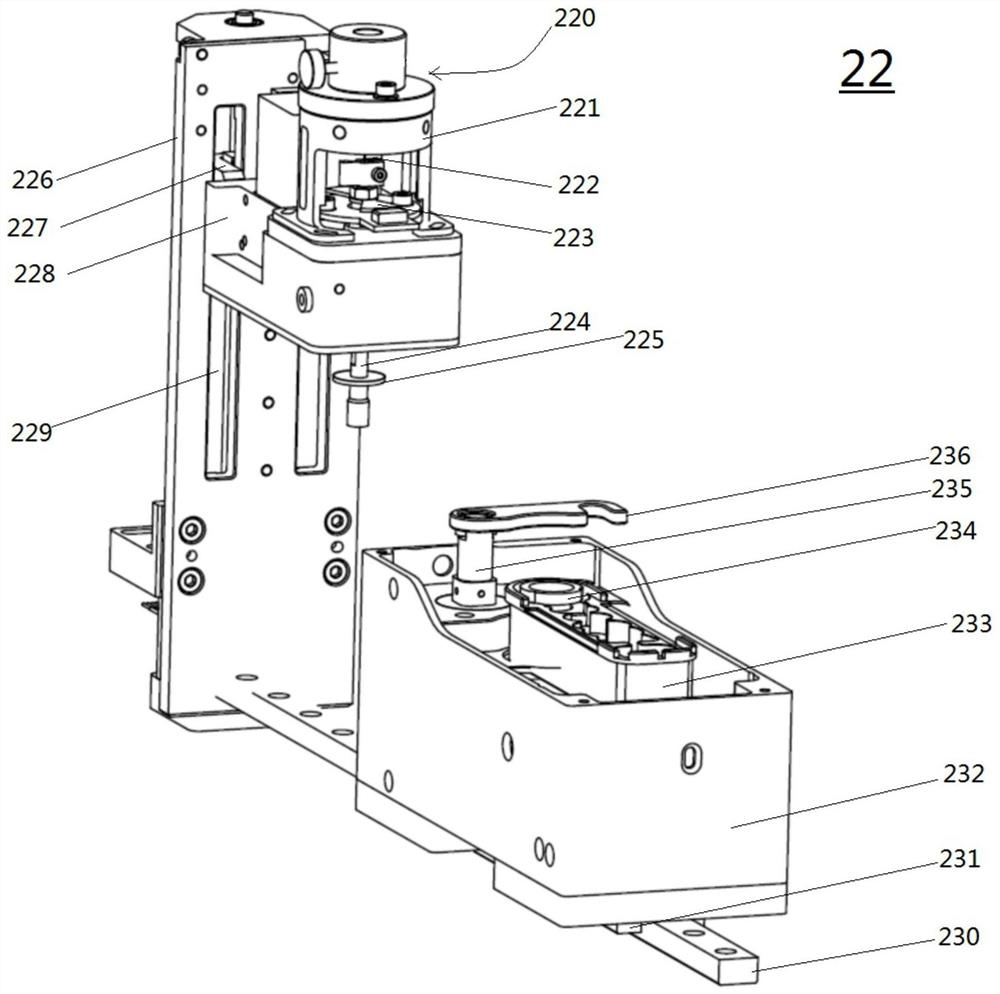
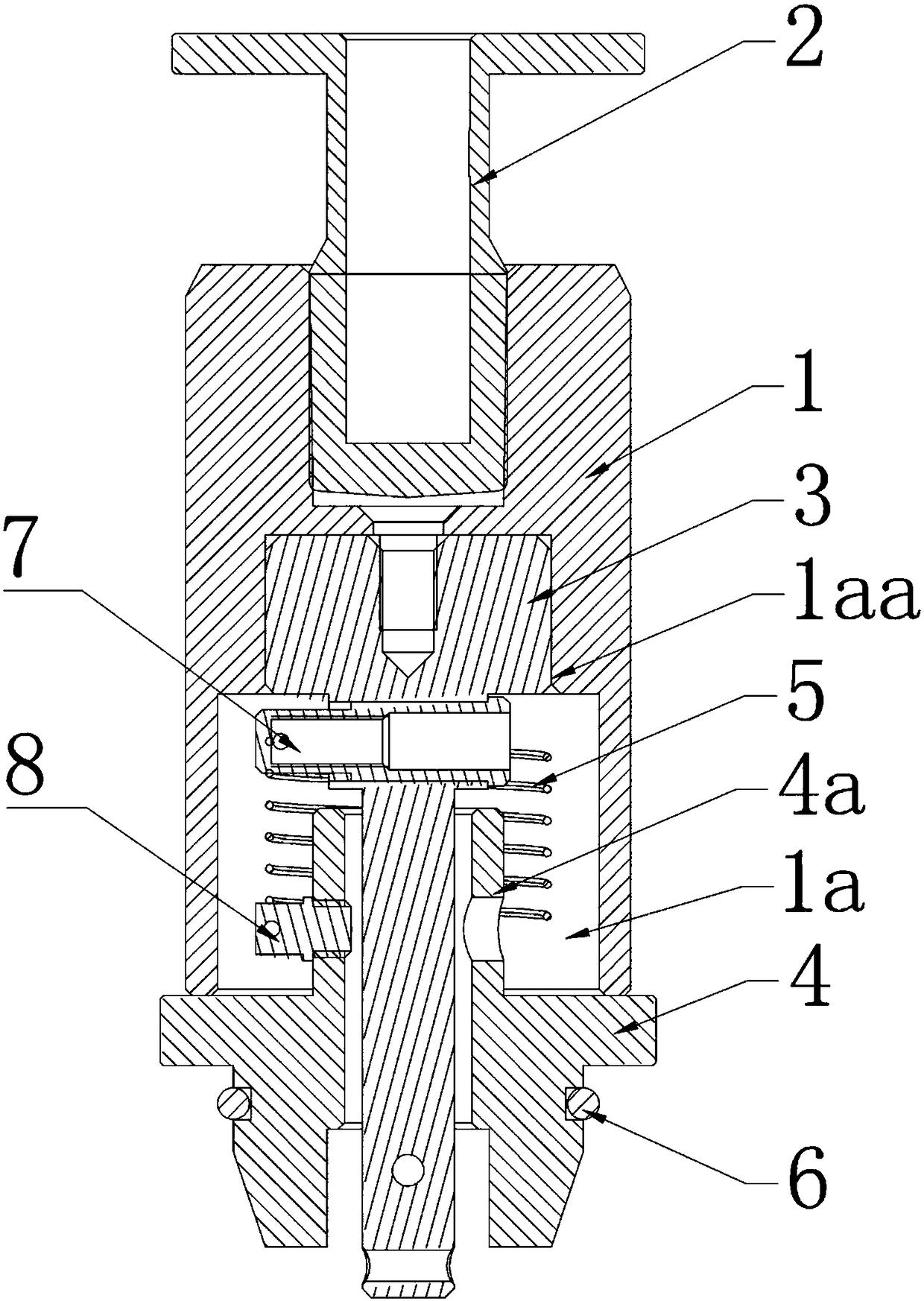
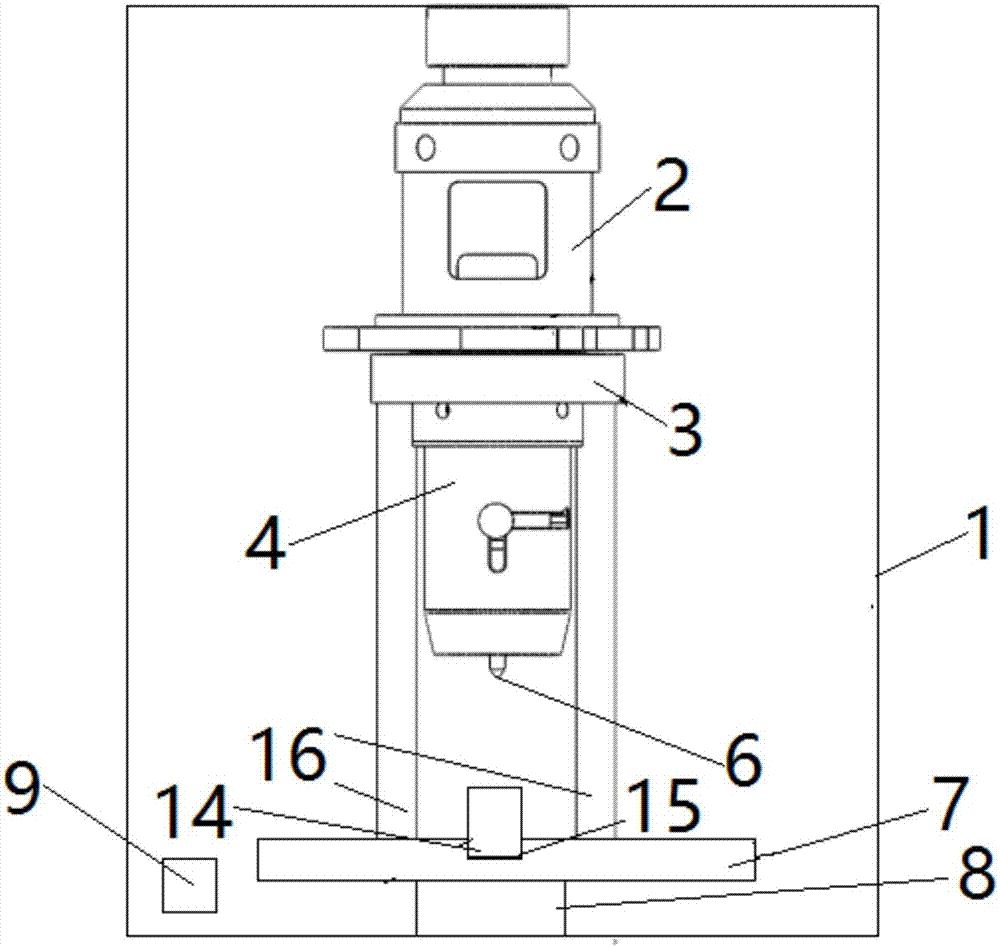
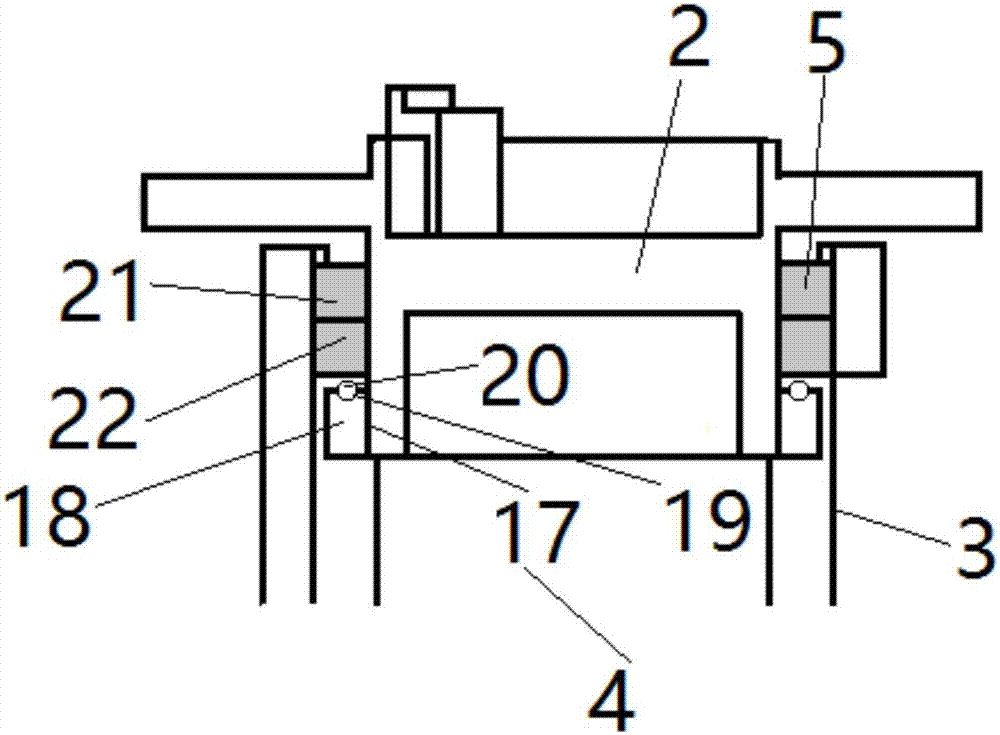
Thromboelastography instrument, and blood containing cup cover assembling and disassembling method
The invention provides a thromboelastography instrument, and a blood containing cup cover assembling and disassembling method. The thromboelastography instrument comprises a first support, a second support, a jewel bearing, an addendum cone and a spacing mechanism; the first support is connected with the jewel bearing, and the second support is respectively connected with the addendum cone and the rotating shaft; the jewel bearing is used for supporting the addendum cone to make the second support and the rotating shaft rotate under the driving of blood to be detected; and the spacing mechanism is used for spacing the positions of the jewel bearing and the addendum cone in the connection or separation process of an external blood containing cup cover from the rotating shaft in order to separate the jewel bearing from the addendum cone. The method comprises the following steps: spacing the positions of the jewel bearing and the addendum cone by the spacing mechanism in order to separate the jewel bearing from the addendum cone; and connecting or separating the blood containing cup cover with / from the rotating shaft. The damage risk of the jewel bearing is reduced in the invention.
Owner:HEMOASSAY SCI & TECH SUZHOU CO LTD
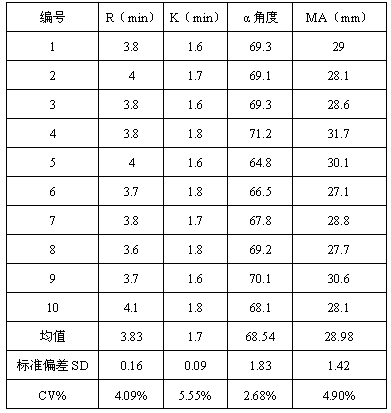
Thromboelastography heparin quantitative detection kit and preparation method thereof
ActiveCN108982865AQuantitative detection is convenient and accurateSimplify the inspection processBiological material analysisBiological testingCoagulation Factor XaCoefficient of variation
The invention relates to a thromboelastography heparin quantitative detection kit, which comprises a buffer agent, a blood coagulation factor Xa, rabbit brain congealed fat, a coagulation factor activator, a supporting agent, and a biological preservative. The preparation method comprises the following steps: preparing a buffer solution, a blood coagulation factor Xa stock solution, a blood coagulation factor activator stock solution, a biological preservative solution, and a rabbit brain congealed fat stock solution; taking the support agent and adding the buffer solution to prepare a supportsolution; adding the blood coagulation factor Xa, the blood coagulation factor activator, and the rabbit brain congealed fat stock solution to the supporting solution according to the requirements ofkit specification, adding the biological preservative solution after uniformly mixing, then adding the buffer solution to the specified amount, subpackaging and lyophilizing after uniformly mixing. The thromboelastography heparin quantitative detection kit provided by the invention can quantitatively detect the heparin in a sample by a thromboelastography, has good correlation in a linear range,meets the heparin detection limit standard and has a coefficient of variation of not more than 10%, and the sample adopts whole blood without extra treatment; the integrity of protein in a clotting cascade is also not required, and the inspection operating process is simplified, thereby facilitating doctors and patients.
Owner:上海原科实业发展有限公司
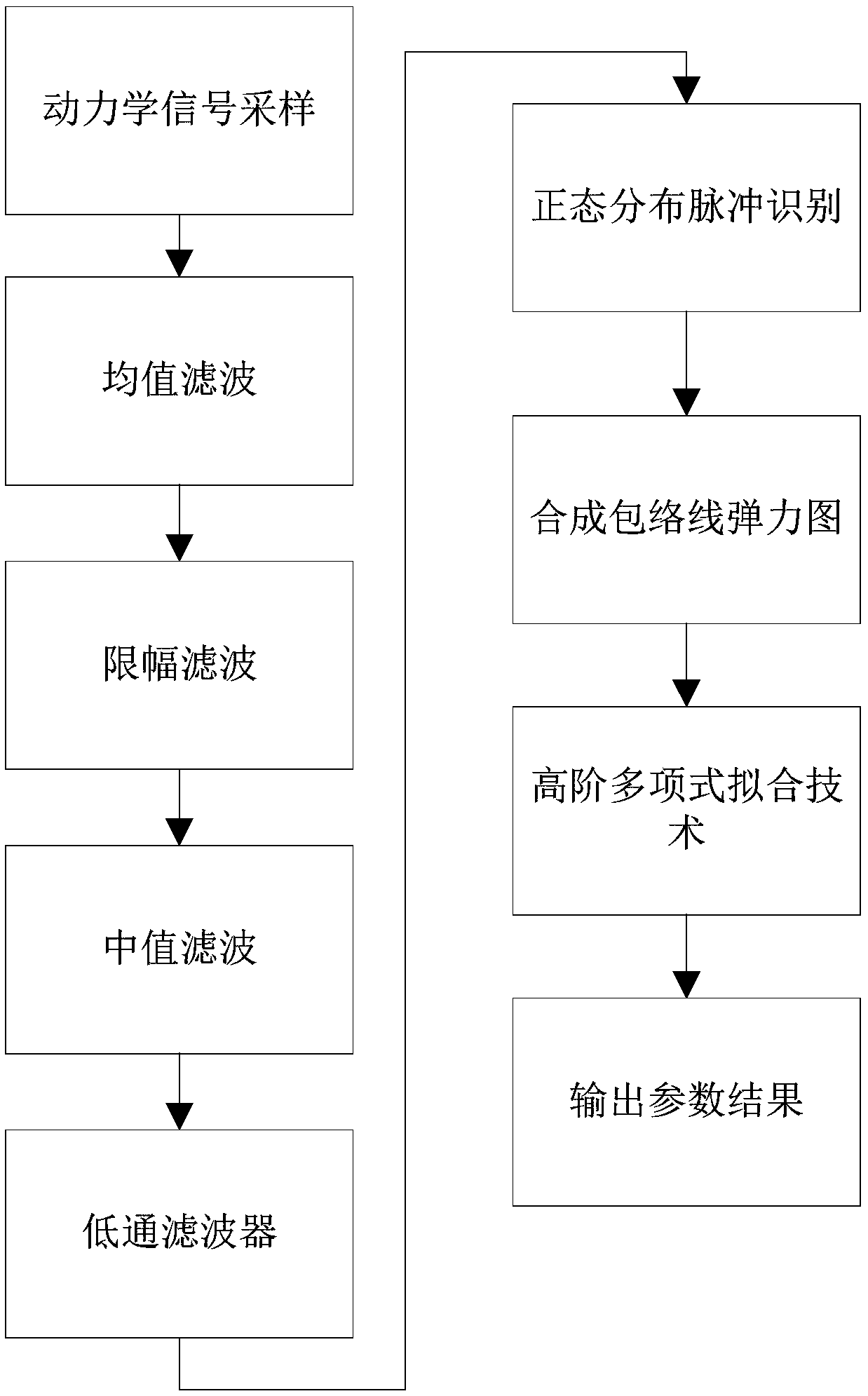
A signal filtering method for a thromboelastography instrument
ActiveCN109684908AEliminate distractionsCancel noiseHealthcare managementCharacter and pattern recognitionCoagulation fibrinolysisHigh frequency vibration
The invention provides a signal filtering method for a thromboelastography instrument. The invention discloses a signal filtering method for a thromboelastography instrument. interference of samplingnoise signals can be eliminated; The method has the advantages that in the whole blood coagulation fibrinolysis period, sharp pulse interference in slow change signals and large-amplitude short-time non-periodic signal interference are achieved, signal oscillation caused by high-frequency vibration of a signal acquisition probe can be shielded, long-period large pulse interference is eliminated, the purpose of data screening is achieved, noise can be well eliminated in the whole blood coagulation fibrinolysis period, and output detection data are more accurate.
Owner:深圳沃德生命科技有限公司
Detection method for functions of platelets
ActiveCN106885895AReliable test resultsReduce testing costsBiological testingTesting medicinal preparationsPlatelet inhibitorsComputer science
The invention discloses a detection method for functions of platelets. The detection method is used for analyzing and evaluating the functions of the platelets and comprises platelet detection as follows: (1) carrying out platelet activated detection; (2) carrying out blood clotting factor activated detection; (3) carrying out platelet inhibited detection. The detection method is applicable to the platelet function determination of a thromboelastography by a clotting method, and a platelet function detection result is obtained through separately adding obtained platelet detection values in a detected blood sample and carrying out further calculation. According to the method, by adopting a platelet inhibitor, a detection value serves as a background control parameter in platelet function analysis, and thus, the detection result is more reliable. Compared with the former detection methods, the method has the advantages that expensive hemocoagulase is not required to be used, so that the detection cost is reduced, and the method is applicable to clinical popularization.
Owner:ZIRCON BIOTECH CO LTD
Thromboelastography instrument quality control product and preparation method thereof
ActiveCN107588998AReduce use costSimple preparation stepsPreparing sample for investigationBiological testingBiochemical engineeringQuality control
The invention discloses a quality control product which comprises a quality control product 1 and a quality control product 2, wherein the quality control product 1 comprises the following components:2-6 parts by weight of factor-rich goat plasma, 2-5 parts by weight of sheep serum and 1-4 parts by weight of fibrinogen mother liquor; the quality control product 2 comprises the following components: 1 to 4 parts by weight of factor-deficient sheep plasma, 5 to 10 parts by weight of sheep serum and 0. 3 to 2 parts by weight of the fibrinogen mother liquor, the fibrinogen mother liquor is prepared by dissolving fibrinogen in the sheep serum, wherein the mass percentage of the fibrinogen is 10%; clotting factor extraction is not needed in the preparation process of the thromboelastography instrument quality control product, preparation steps are simplified, operation is easy, cost is low, results are accurate, and stability is good; test results of the thromboelastography instrument quality control product basically meet that of a quality control product produced by already listed US Haemoscope company, the thromboelastography instrument quality control product can replace the qualitycontrol product produced by the already listed US Haemoscope company, and customer use cost is reduced.
Owner:上海原科实业发展有限公司
Diagnostic in vitro method for assessing von willebrand disease and increased bleeding risk associated with von willebrand disease and acquired or congenital disorders of platelet function
InactiveUS20100273206A1Superior in predicting bleeding riskMicrobiological testing/measurementDisease diagnosisPoint of careFactor ii
The invention relates to an in-vitro method for diagnosing Von Willebrand Disease (VWD) and an increased bleeding risk associated with Von Willebrand Disease and / or acquired or congenital platelet function defects that reduce the interactions of Von Willebrand Factor (VWF) with platelets. The in-vitro method of the invention may also be used to diagnose further bleeding risks. The test is suitable for use as a screening test based on whole blood and has the additional benefit of being suitable as a point of care test. The method involves the incubation of a sample containing platelets and hemostasis factors with an activator of platelet aggregation and the measurement of the viscoelastic change after inducing coagulation, e.g., by means of thromboelastography (TEG).
Owner:CSL BEHRING GMBH
Integrated module structure and equipment for thrombus elasticity and blood coagulation analysis
PendingCN113219188AImprove detection efficiencyImprove convenienceMaterial analysisTest sampleThrombus
The invention belongs to the technical field of blood sample detection, and discloses an integrated module structure and equipment for thrombus elasticity and blood coagulation analysis. The integrated module structure a carrier, a blood coagulation analysis module for performing conventional blood coagulation detection on a first to-be-detected sample, a thromboelastography module for performing thromboelastography detection on a second to-be-detected sample, and a sample transfer module, the sample transfer module is used for respectively transmitting the first to-be-detected sample and the second to-be-detected sample to the blood coagulation analysis module and the thrombus elasticity module for detection. According to the invention, double-item detection is realized, and the detection efficiency and convenience are improved; the transfer efficiency of the to-be-detected sample in the double detection modules and the test tube is improved, and the detection efficiency is correspondingly improved; a second to-be-tested sample needed by the thrombus elastic module and a first to-be-tested sample needed by the blood coagulation analysis module can be provided at the same time, and the situation that two tubes of blood samples are extracted from apatient is avoided.
Owner:深圳沃德生命科技有限公司
Kaolin reagent quality control product and preparation method and application thereof
ActiveCN109541242AMeet quality control requirementsIncreased sensitivityPreparing sample for investigationBiological testingQuality controlBlood plasma
The invention discloses a kaolin reagent quality control product. The quality control product is lyophilized formulation, and the raw material of the lyophilized formulation includes platelet-deficient animal plasma. The R value of the quality control product is 6-8 min, the K value is 2-5 min, the Angle value is 60 DEG-80 DEG, and the MA value is 23-45 mm. The kaolin reagent quality control product is sensitive to the kaolin reagent reaction, so the accuracy of the quality detection is high, and the quality control requirements for the kaolin preparation in thromboelastography test are met. The quality control product has good reconstitution stability and can be stored for a long time under low temperature. The quality control product uses animal plasma as raw material, thus reducing thecost of the quality control and improving the safety of the quality control operations. The invention also discloses a preparation method of a kaolin reagent quality control product, which can preparethe kaolin reagent quality control product with accurate detection results and high stability. The preparation method is stable and reliable, the preparation process is simple and easy to operate, and the preparation method is suitable for the production and application of kaolin reagent quality control products.
Owner:中科精瓒(武汉)医疗技术有限公司
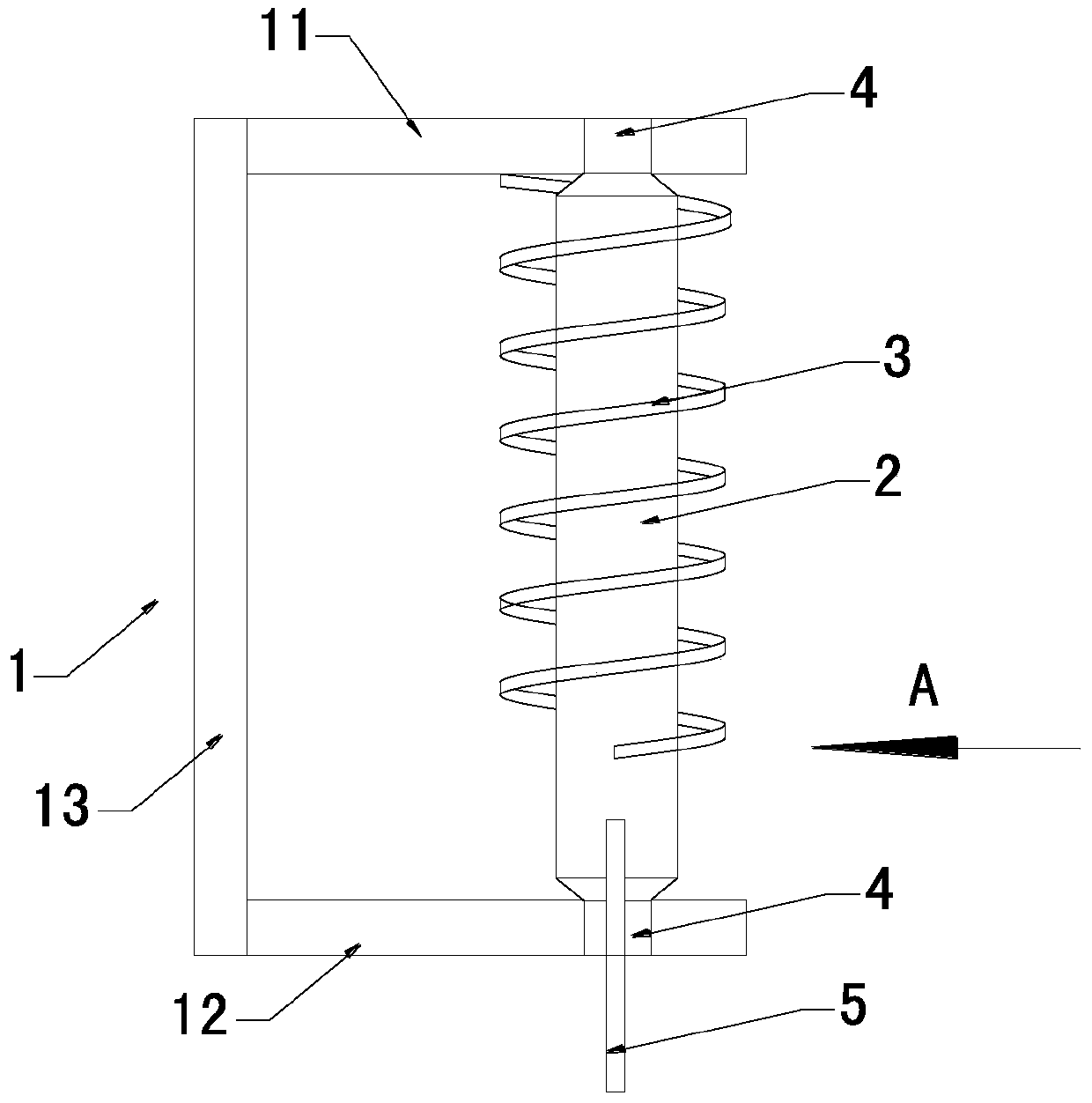
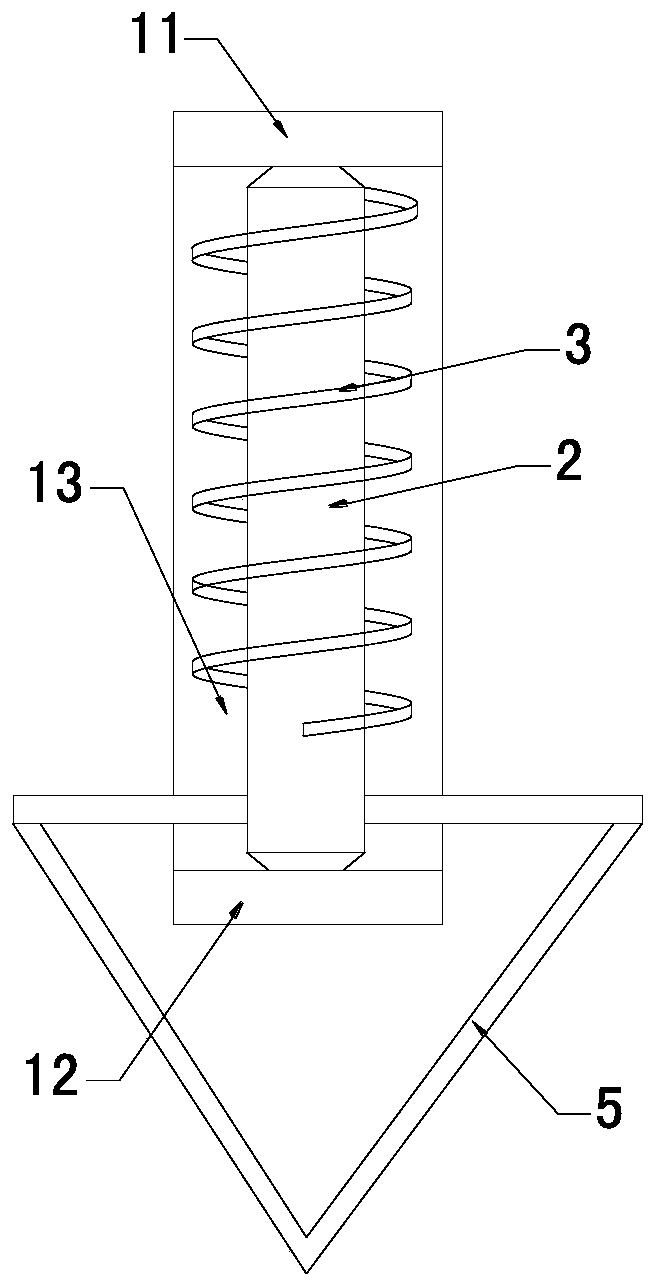
Torsion sensing assembly suitable for thromboelastography instrument
PendingCN110987257ASimple structureEasy to manufactureWork measurementBiological testingMedical equipmentMechanical engineering
The invention relates to the technical field of medical equipment, in particular to a torsion sensing assembly suitable for a thromboelastography instrument. The torsion sensing assembly suitable comprises a U-shaped bracket, a rotating shaft and a spring, wherein the rotating shaft is vertically and rotatably arranged on the U-shaped bracket; the spring sleeves the rotating shaft; one end of thespring is fixed on the rotating shaft; and the other end of the spring is fixed on the U-shaped bracket. A traditional overhang wire structure is replaced by arranging the rotating shaft and the spring, the rotating shaft and the spring are convenient to manufacture and simple in structure, the problems of great torsion wire manufacturing difficulty and low production efficiency are solved, and the induction device of the thromboelastography instrument can be rapidly manufactured and is high in yield and low in cost.
Owner:CHONGQING NANFANG NUMERICAL CONTROL EQUIP
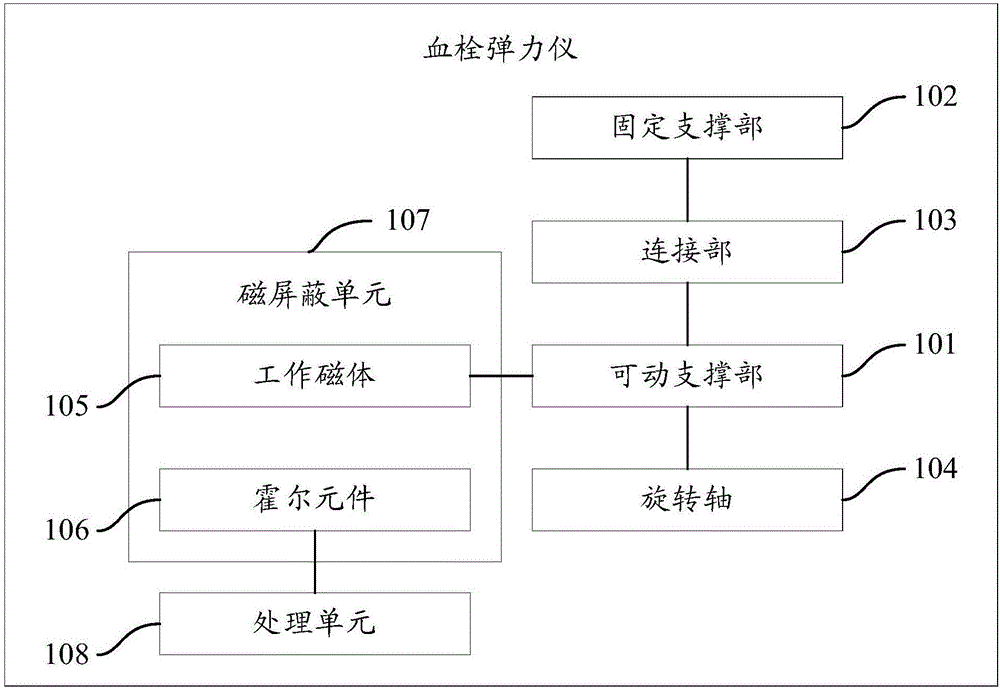
Thromboelastography instrument and use method thereof
InactiveCN106404888AImprove accuracyAccurately reflectBiological testingMaterial magnetic variablesHall elementEngineering
The invention provides a thromboelastography instrument and a use method thereof. The thromboelastography instrument comprises a movable supporting portion, a fixed supporting portion, a connecting portion, a rotating shaft, a working magnet, a Hall element, a magnetic shielding unit and a processing unit; one end of the movable supporting portion is fixedly connected with the rotating shaft, and the other end of the movable supporting portion is connected with the fixed supporting portion through the connecting portion; the movable supporting portion is fixedly connected with the working magnet; the rotating shaft rotates relative to the fixed supporting portion under the driving action of detected blood; the rotating shaft drives the movable supporting portion to rotate; the movable supporting portion drives the working magnet to move; the Hall element is connected with the processing unit; the magnetic shielding unit shields influences of external magnetic field on the Hall element and the working magnet; the Hall element outputs a detection electric signal according to the magnetic field change of the working magnet; and the processing unit determines the blood clotting data of the detected blood according to the detection electric signal. The detection accuracy of the detected blood is improved in the invention.
Owner:NEOTEK BIOSCI
Thromboelastography
InactiveCN108896748AReasonable structural designSimple structureBiological testingEngineeringBlock structure
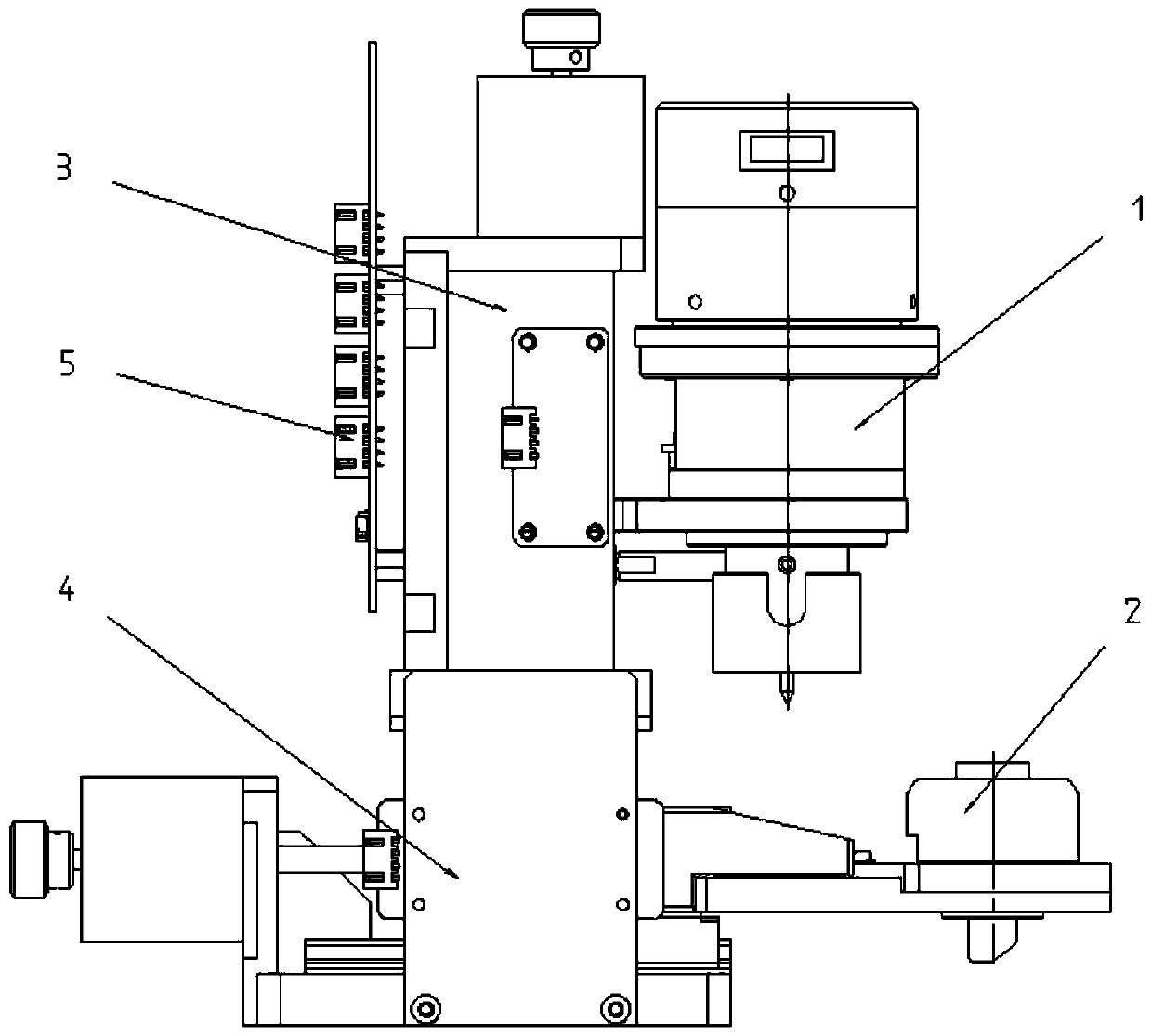
The invention discloses a thromboelastography. The thromboelastography comprises a bottom plate, a detection head and a detection cup supporting plate, wherein the detection cup supporting plate is arranged corresponding to the detection head and used for containing a detection reaction cup, a top plate is arranged above the bottom plate, the top plate is provided with a motor, a lifting lead screw is connected to the lower portion of the motor, the lifting lead screw is provided with a lifting block, the detection cup supporting plate is connected with the lifting block, and the detection cupsupporting plate is provided with a positioning block structure used for positioning the detection cup. The thromboelastography has the advantages that the structure is designed reasonably, the loading and unloading operations of the detection reaction cup are simple and convenient, positioning is reliable, the structure is simple, positioning does not need manual auxiliary operation, and the detection efficiency and accuracy are greatly improved.
Owner:安徽健朗医疗器械有限公司
Thromboelastography TEG quality control and preparation method thereof
PendingCN109596845AImprove quality inspectionImprove detection qualityBiological testingMedicineFibrinogen
The invention discloses a thromboelastography TEG quality control and a preparation method thereof. The preparation method comprises the following steps: taking animal whole blood as a raw material, and protecting blood coagulation factor, fibrinogen, blood platelet and like blood coagulation components by adopting a natural settling process, thereby preventing the components from being lost or activated, and improving the quality of the thromboelastography TEG.
Owner:GUIZHOU JINJIU BIOTECH
Calibration instrument for calibrating detection channel of thromboelastography
PendingCN108562731ANovel ideaReasonable designBiological testingApparatus for force/torque/work measurementRubber ringWhole body
The invention discloses a calibration instrument for calibrating a detection channel of a thromboelastography. The calibration instrument comprises a measurement outer barrel, wherein a suspension part is arranged at the upper part of the measurement outer barrel; a positioning rod is fixedly arranged in the measurement outer barrel; the positioning rod downwards extends out of the measurement outer barrel and penetrates through the center of a testing head; the testing head is suspended on the positioning rod through a spring; a rubber ring is arranged at the periphery of the testing head. The calibration instrument disclosed by the invention has the advantages of novel conception, reasonable design and convenience for operation; a torque value of a plurality of detection channel torsionwires in the multi-channel thromboelastography can be initially calibrated so that the detection precision of a whole body is improved.
Owner:CHONGQING NANFANG NUMERICAL CONTROL EQUIP
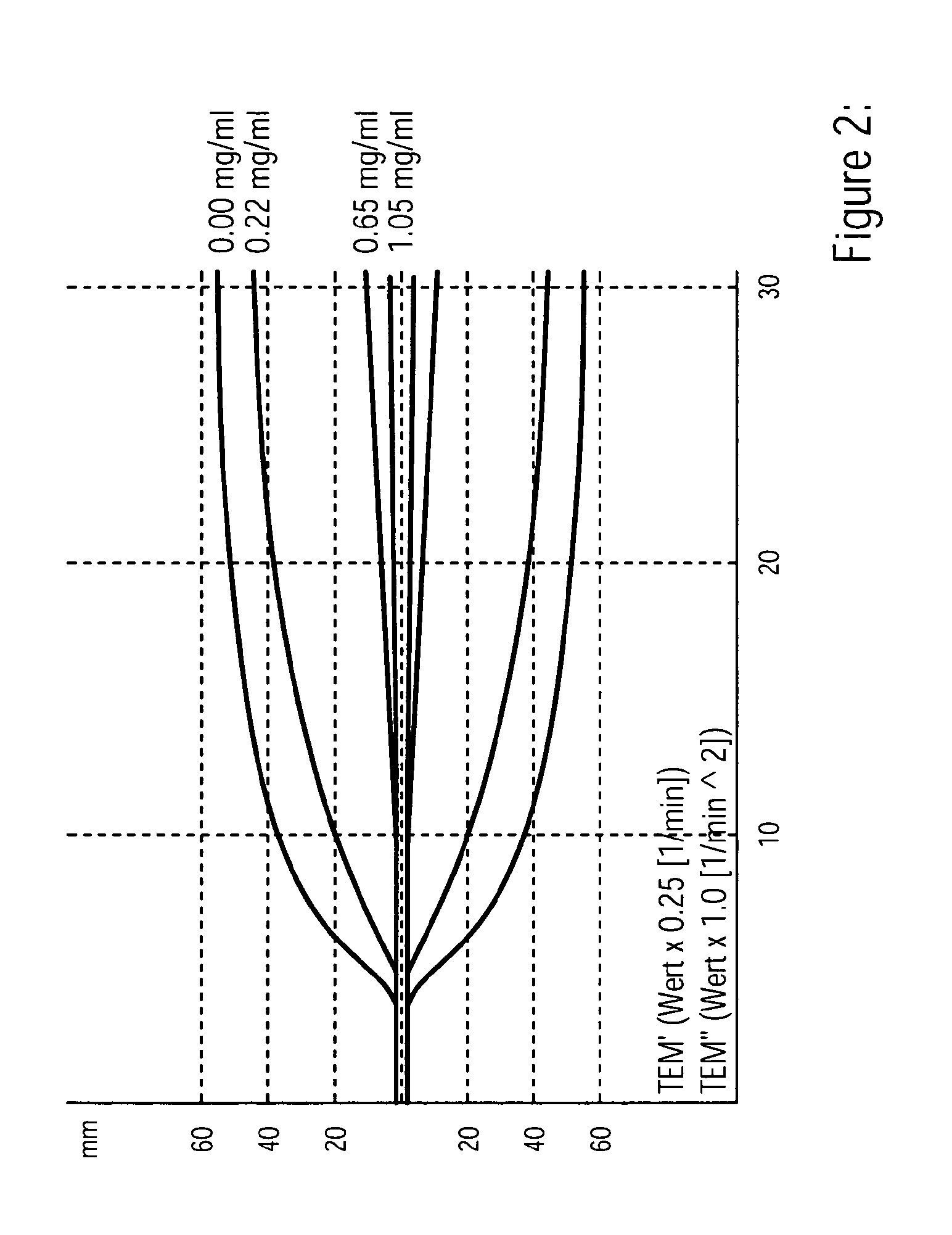
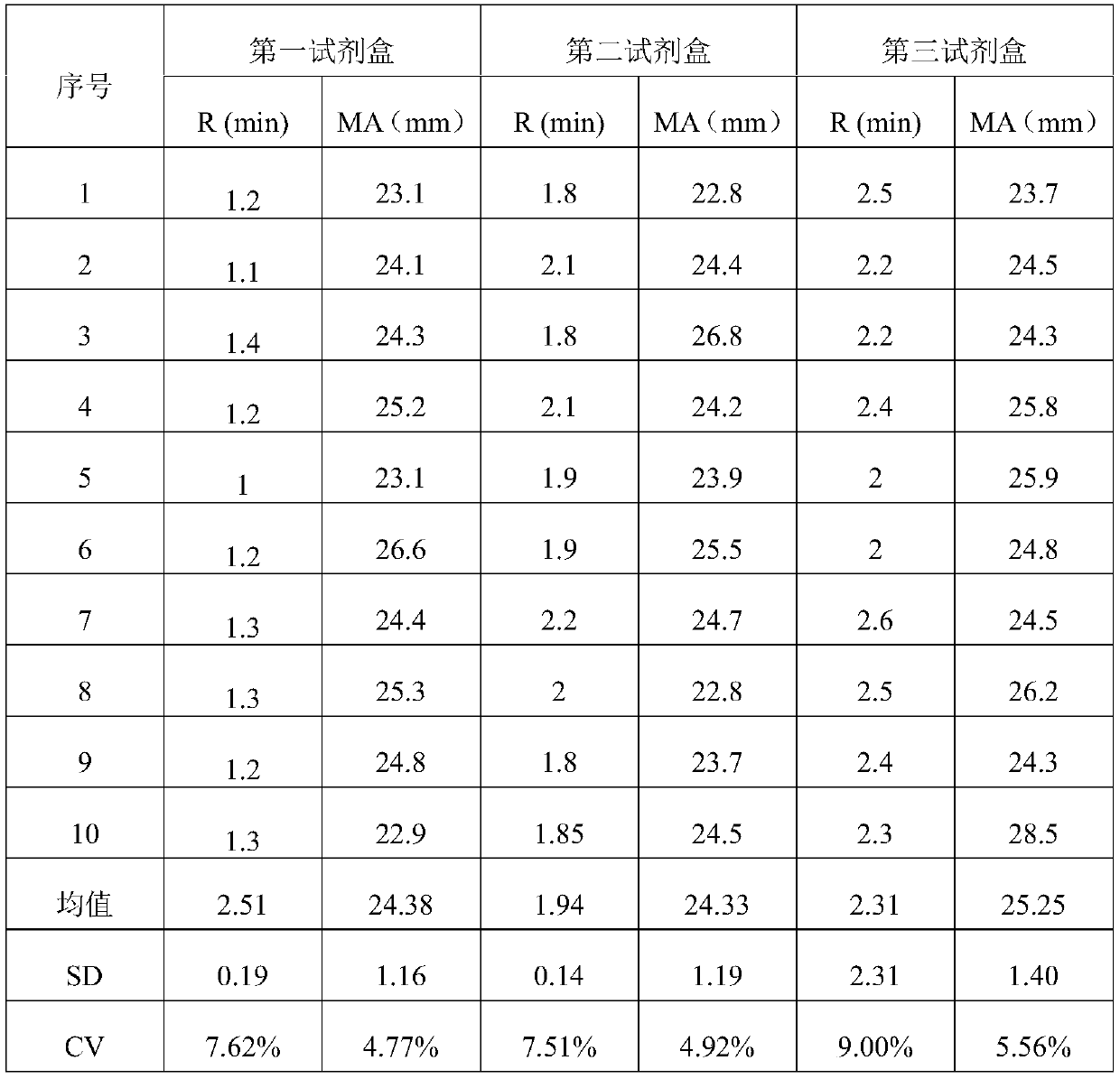
Calibration method for thromboelastography instruments
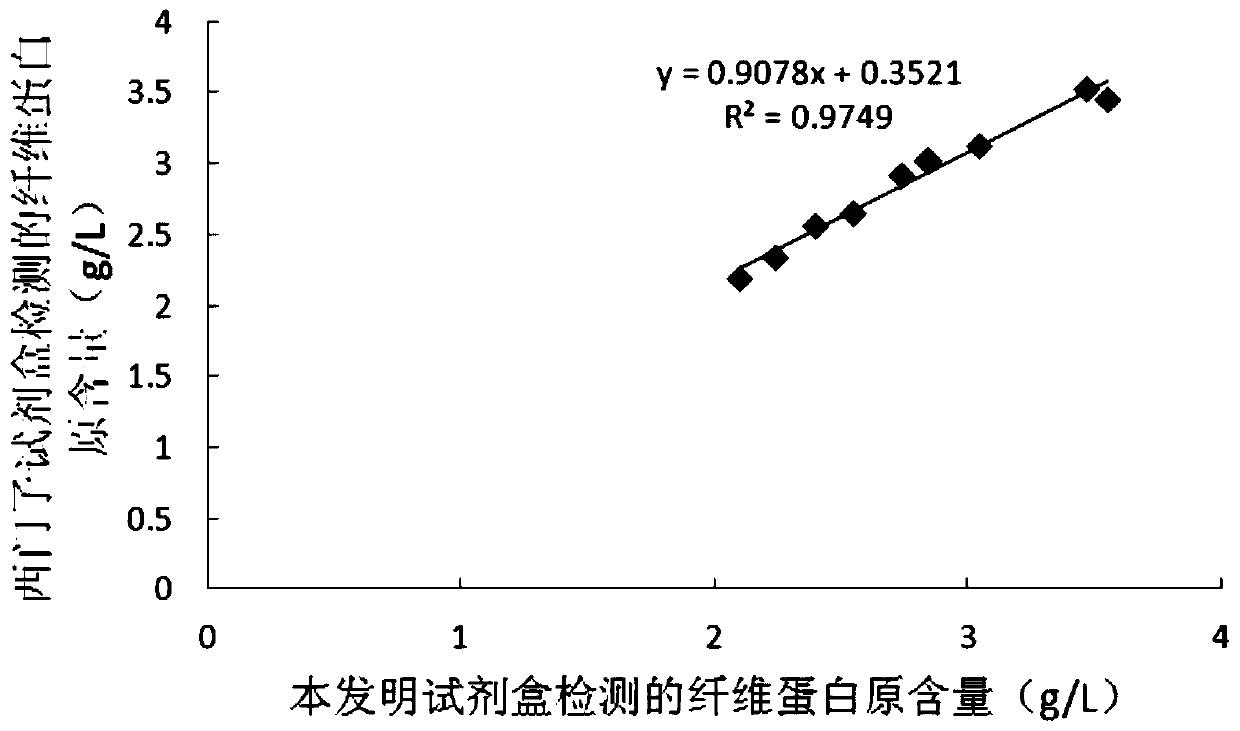
ActiveCN107102125APerfect calibration processImprove calibration accuracyBiological testingFibrinogenBuffer solution
The invention discloses a calibration method of thromboelastography instruments. The method comprises the steps of preparing a buffer solution, namely preparing a mixed solution comprising Tris, NaCl and CaCl2 and adding hydrochloric acid to the mixed solution to adjust the pH to be 7.0-8.0; preparing thrombin and fibrinogens of different concentration according to the buffer solution; testing standard MA values and calibrated MA values, namely successively adding the thrombin and the fibrinogens to a standard thromboelastography instrument and a to-be-calibrated thromboelastography instrument separately and testing the standard MA values and the calibrated MA values of the fibrinogens of different concentration; and calculating a collaboration coefficient for correcting real-time measurement of the calibrated thromboelastography instrument according to the calibrated MA values and the standard MA values. According to the calibration method for thromboelastography instruments, the property that the fibrinogens are solidified under the action of the thrombin is utilized and the fibrinogens are taken as a calibration product, so that the calibratin process is complete and the calibration accuracy is high.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
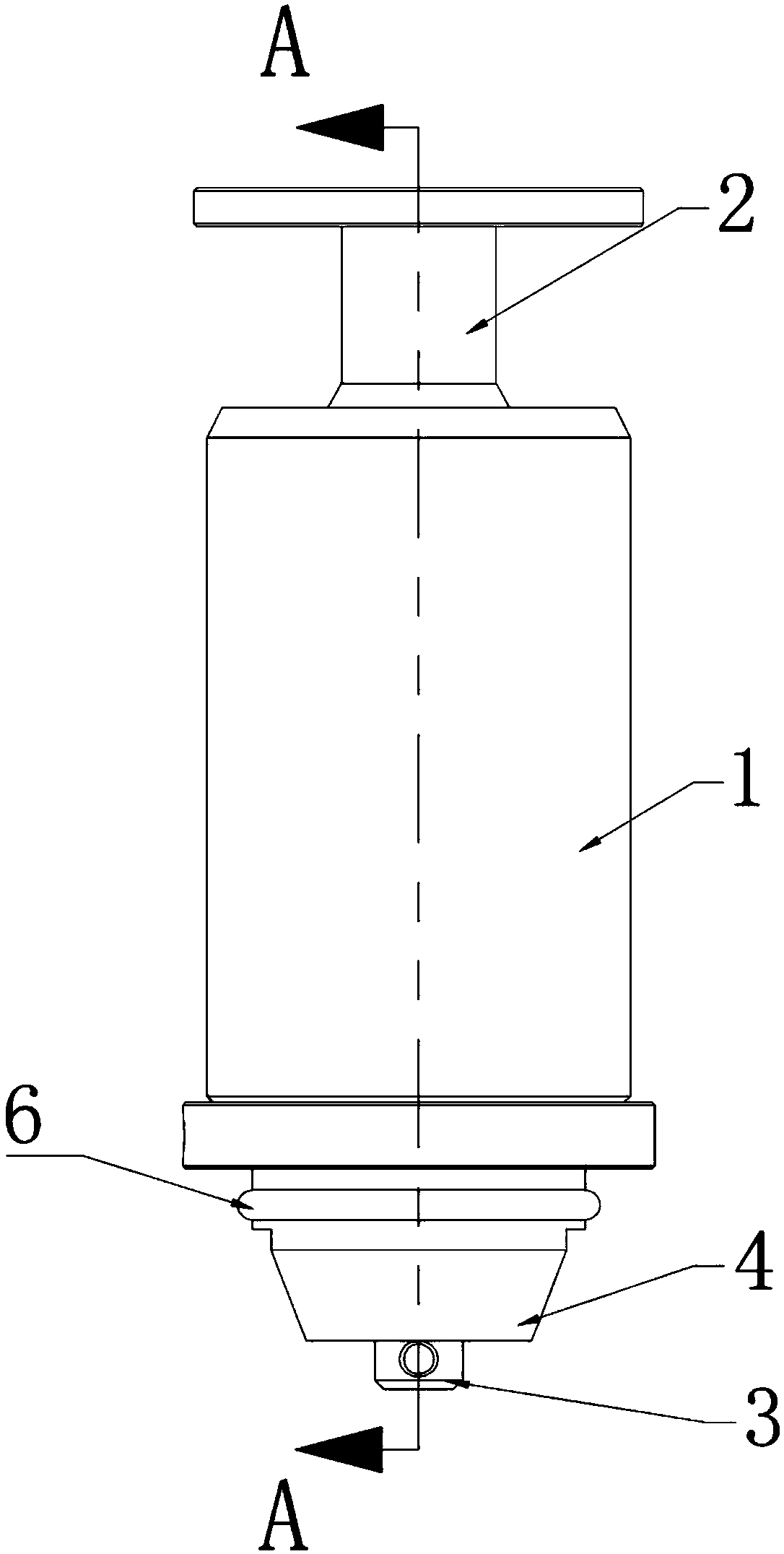
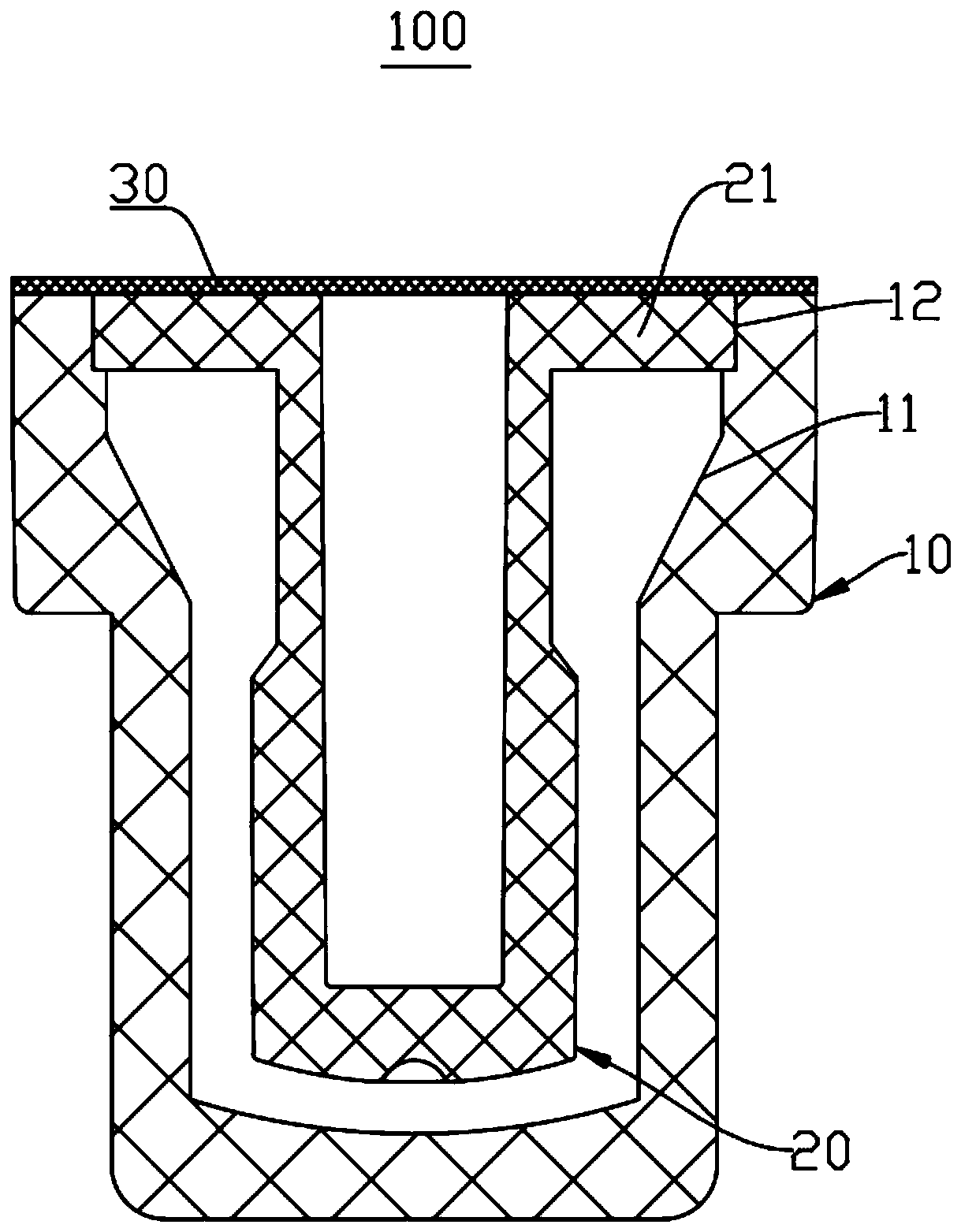
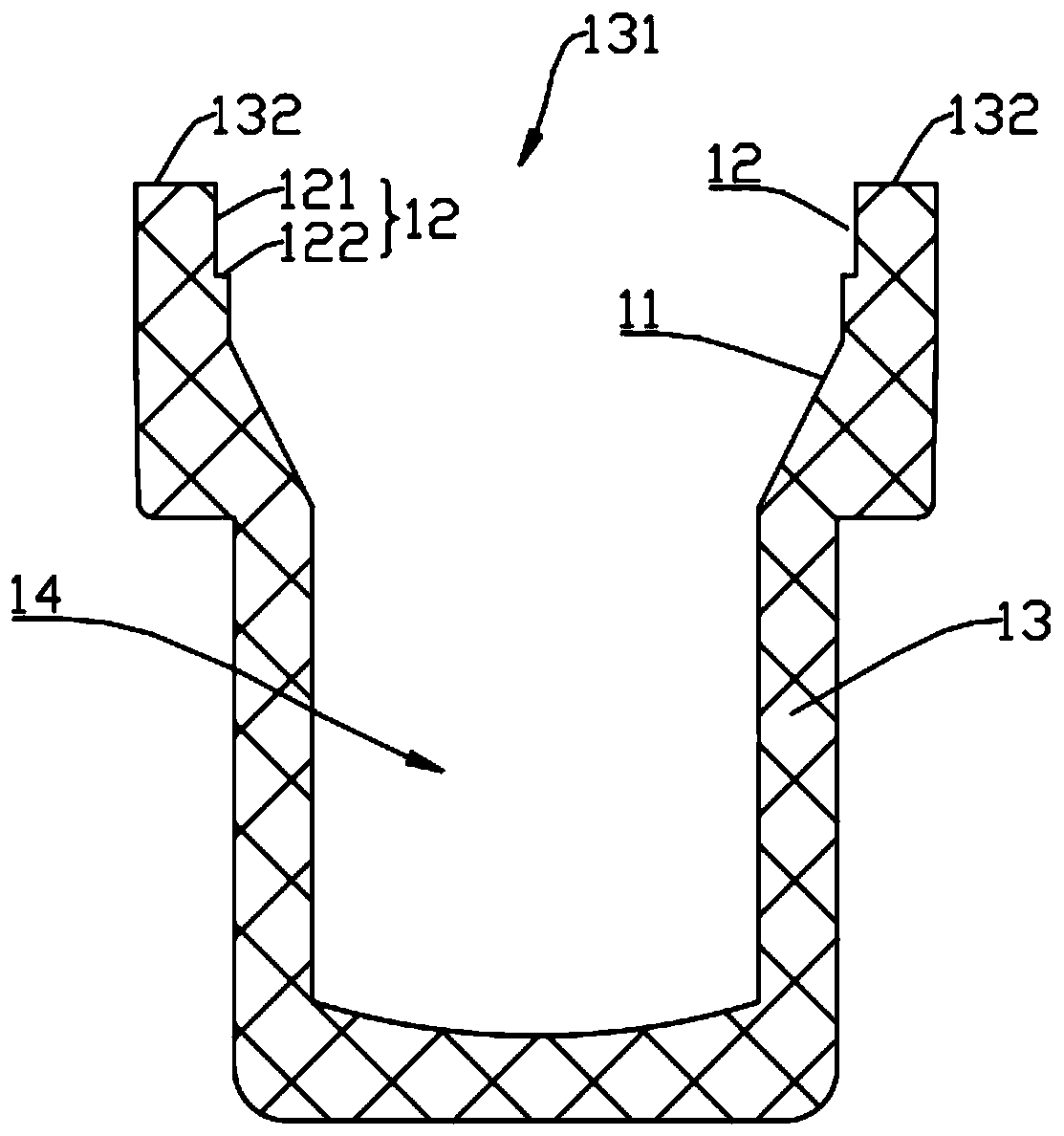
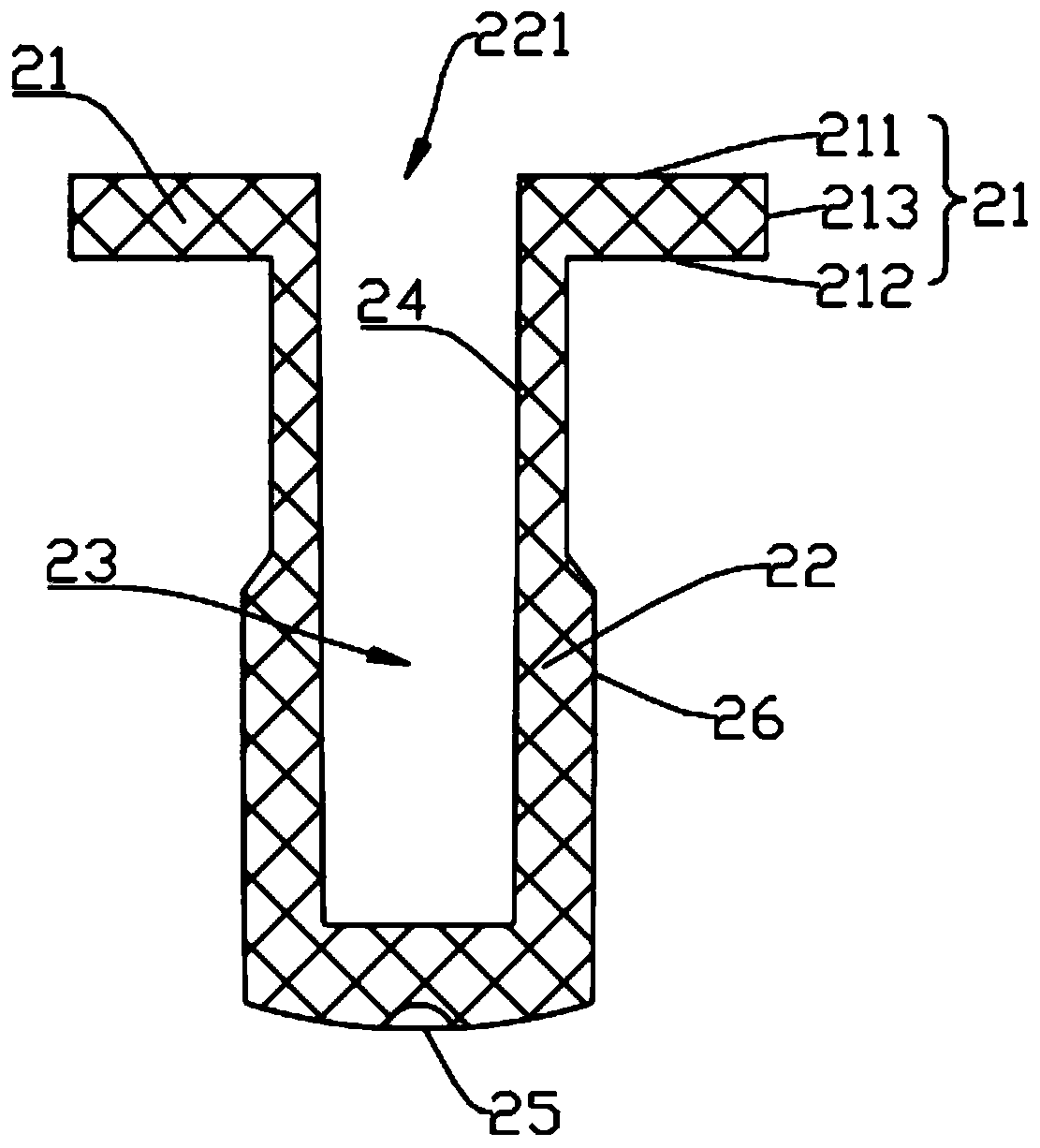
Reaction vessel
The invention provides a reaction vessel. The reaction vessel comprises a sample container and a detection container, wherein a concave part is formed in the inner side wall of the sample container; the detection container comprises a convex part; and the convex part is used for clamping and fixing the detection container in the concave part when the detection container is put in the sample container. The reaction vessel provided by the invention has the advantages that the shaking of the reaction vessel is avoided when the reaction vessel is mounted on a thromboelastography, thereby guaranteeing the accuracy of the later sample detection.
Owner:MEDCAPTAIN MEDICAL TECH
Checking device for detection head of thromboelastography
PendingCN108562730AEasy to debug standardNovel ideaUsing optical meansBiological testingEngineeringThromboelastography
The invention discloses a checking device for a detection head of a thromboelastography. The checking device comprises a lower supporting plate, wherein an upper supporting plate is supported on the lower supporting plate through a supporting rod; the upper supporting plate is provided with a through hole for the detection head to penetrate; a swinging device for swinging the detection head back and forth is arranged on the upper surface of the upper supporting plate; the swinging device comprises a circular ring; an inner hole of the circular ring directly faces the through hole; an adjustingdevice is arranged on the lower supporting plate; the adjusting device faces the position of the through hole; the adjusting device is provided with a camera for observing a centering situation of atorsion wire in the detection head. The checking device disclosed by the invention has the advantages of novel concept, reasonable design and convenience for operation and is used for checking the detection head of the thromboelastography; each type of detection head is conveniently debugged to be standard and is assembled on the thromboelastography in a qualified manner, so that the precision ofthrombus detection in an application process is ensured.
Owner:CHONGQING NANFANG NUMERICAL CONTROL EQUIP
Shifting block mechanism and blood detection device using same
The invention relates to a shifting block mechanism and a blood detection device using the same. The shifting block mechanism comprises a shifting block bottom plate, a sliding groove is formed in the middle of the shifting block bottom plate, notches on two sides of the sliding groove inwards form shifting block flanges, a shifting block is arranged in the sliding groove, the shifting block can move in the sliding groove, and a shifting block groove is formed in the shifting block. The blood detection device comprises a thromboelastography instrument, a test head and a test base are arranged on the thromboelastography instrument, test connecting rods penetrate through the two sides of the test base respectively, the test base can move up and down along the test connecting rods on the two sides of the test base, a button is arranged at the bottom of the test base, and the shifting block is positioned under the test base and is matched with the button. Compared with the prior art, the device is provided with the shifting block mechanism, the shifting block mechanism is located under the button and provided with the shifting block groove matched with the button, and when the test base is moved downwards, the button can enter the shifting block groove and can also be extruded, and thus a more convenient and faster effect is achieved.
Owner:昕迪精密机械(东莞)有限公司
Thromboelastography TEG
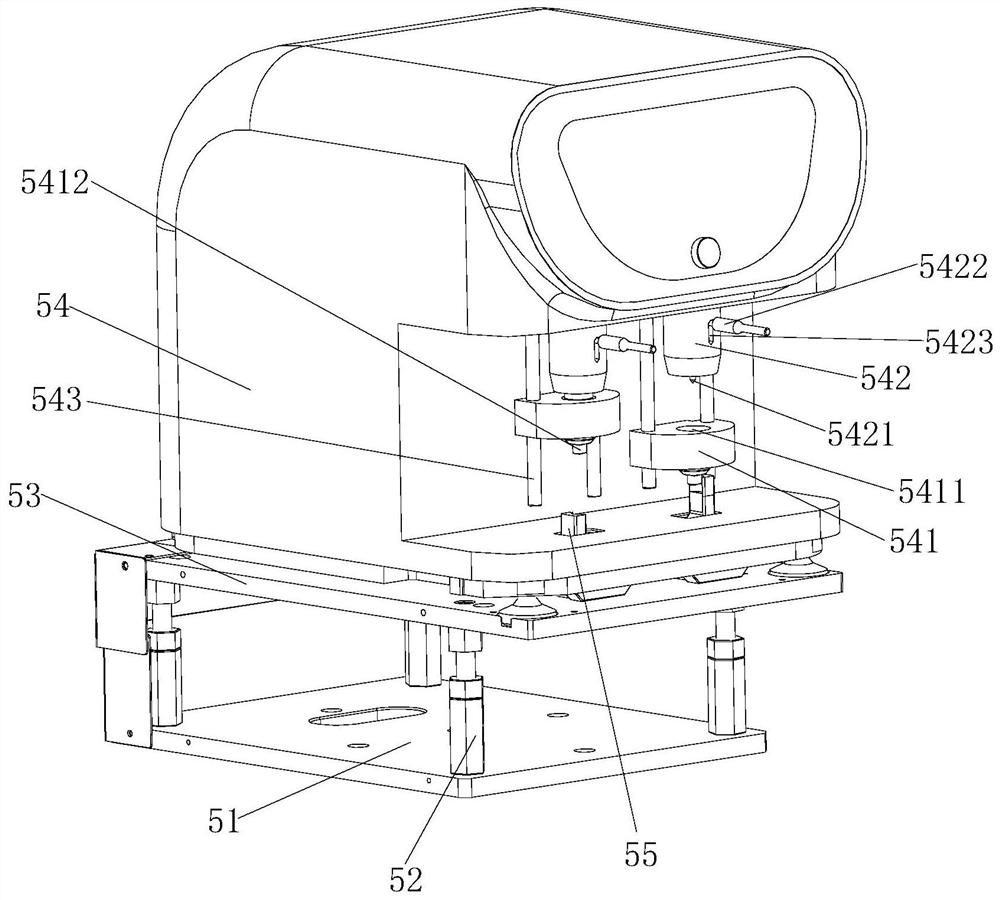
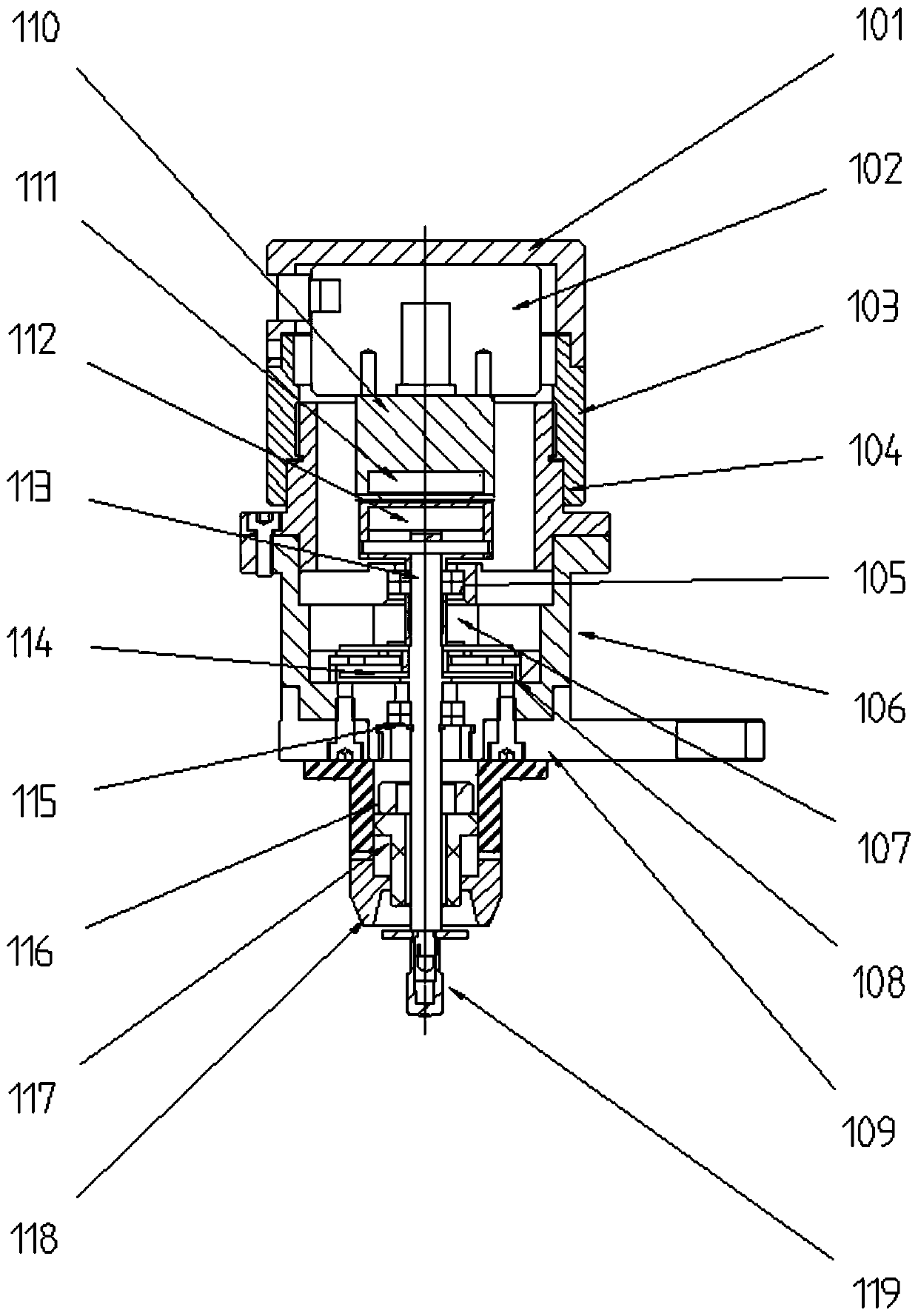
The invention belongs to the field of medical coagulation detection equipment, and relates to a thromboelastography TEG comprising a channel coagulation detection module, a blood sample heating and placing module, an upper and lower cups and lid removing movement module, a cup entering and cup body removing module, and a main control circuit board, wherein the body of a sample cup carries the blood sample, and is placed in the blood sample heating and placing module and driven by the cup entering and cup body removing module to move to the position; the channel coagulation detection module isdriven by the upper and lower cups and lid removing movement module to lift, its rotating spindle extends into the body of the sample cup to rotate, and the sensor detects the change in the angle of the rotating main axis to obtain the blood coagulation change of the blood sample. The thromboelastography TEG provided by the invention can reduce the dependence of the thrombus detection equipment onhuman operation and the requirements on the external use environment, thereby improving the stability of the detection result and improving the detection efficiency.
Owner:重庆鼎润医疗器械有限责任公司
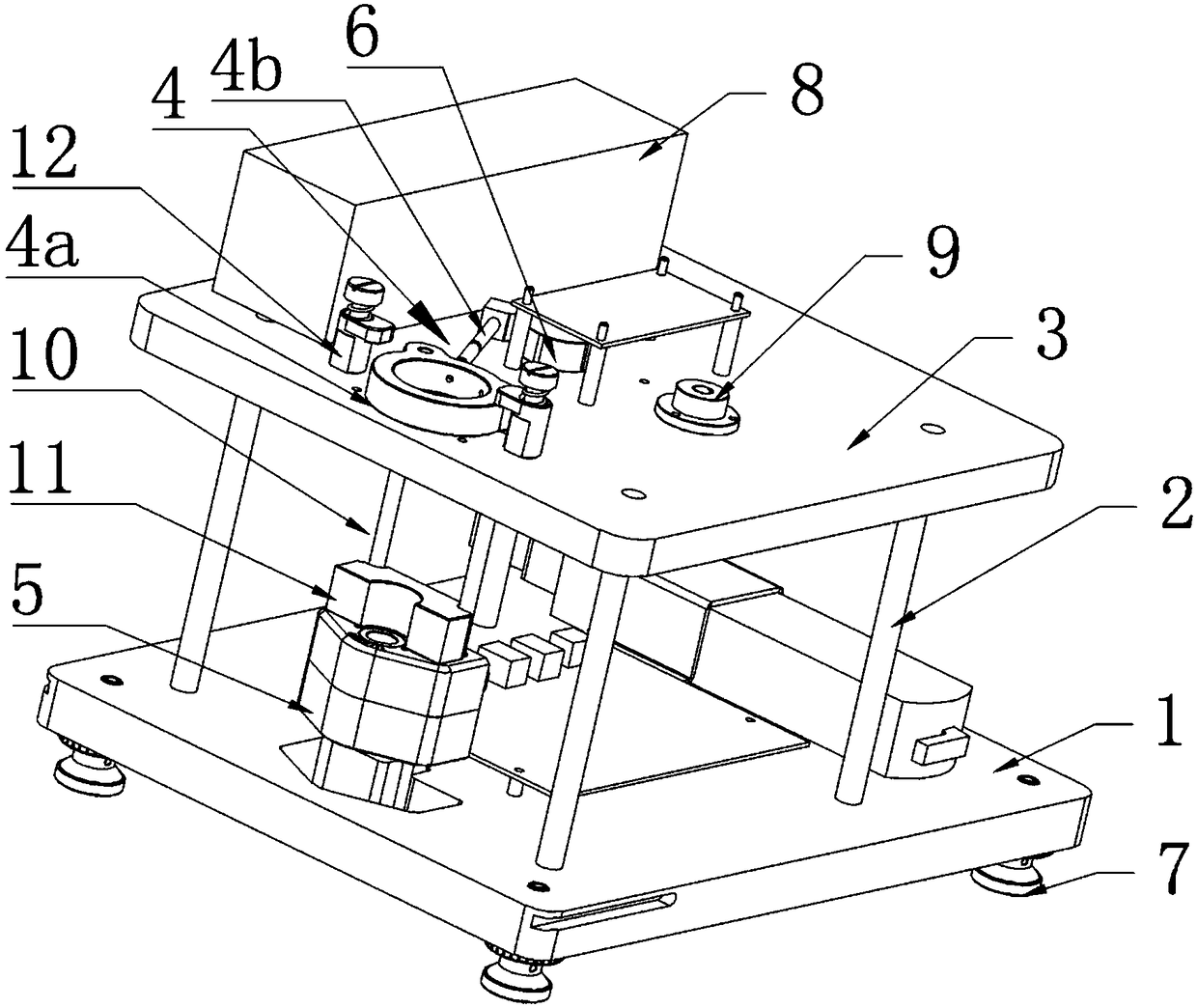
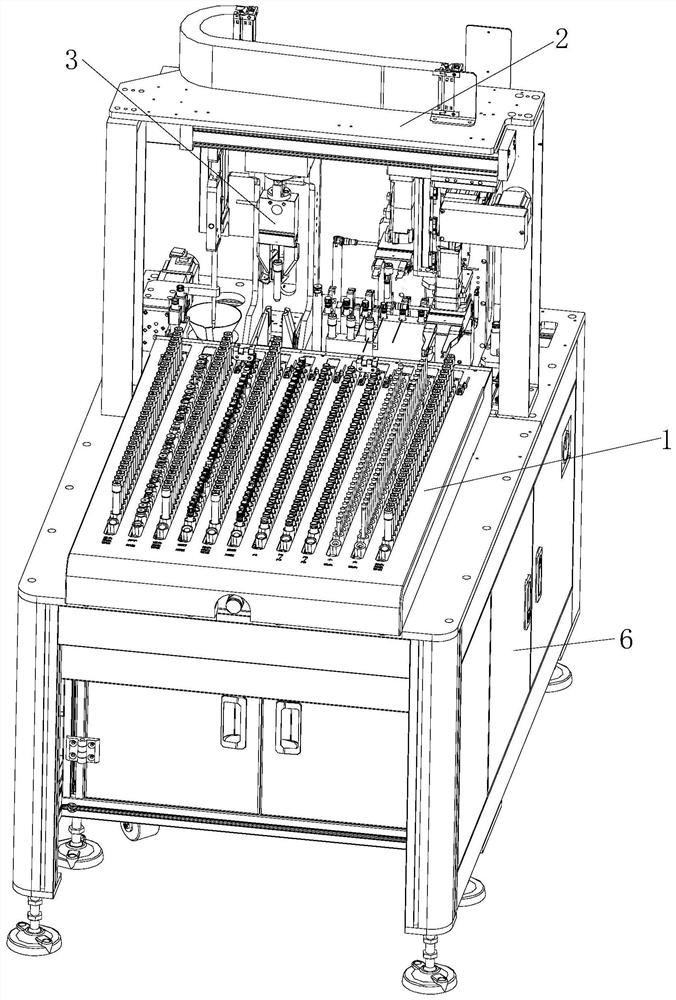
Full-automatic detection type thromboelastography and application method thereof
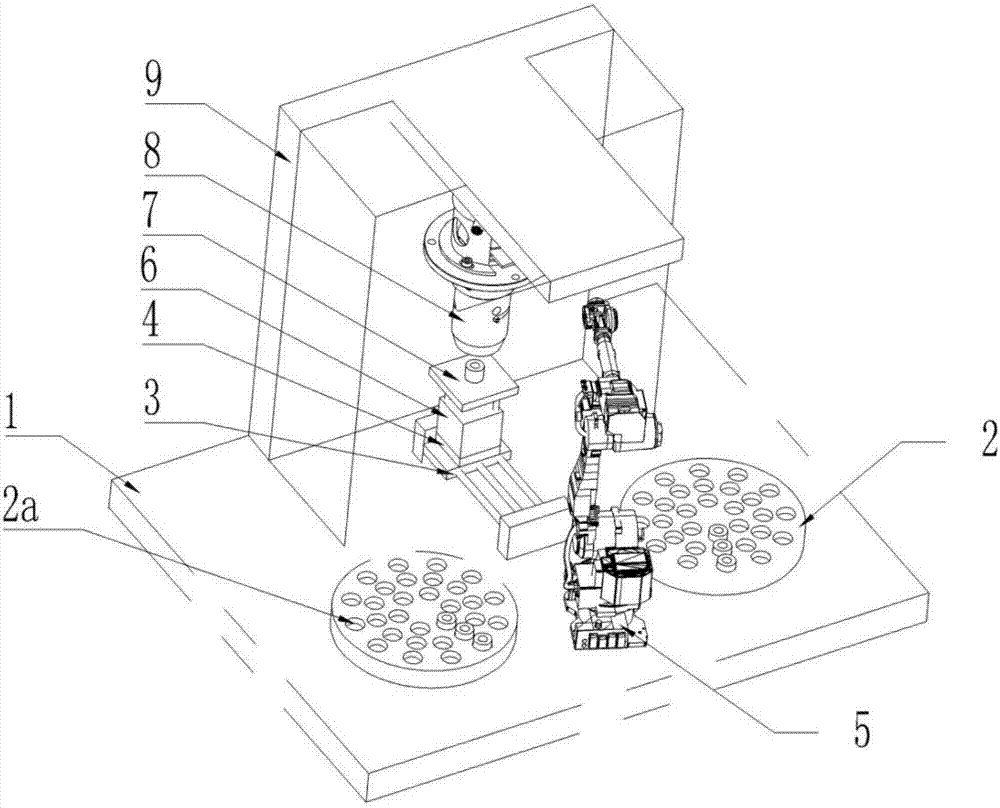
The invention discloses a full-automatic detection type thromboelastography and an application method thereof. Two test cup boxes are arranged on a base frame side by side, multiple test cup holes are formed in each test cup box, a joint robot used for grabbing a test cup is arranged in front of a middle position between the two test cup boxes, a second mounting table is arranged at the upper end of a second cylinder, a first cylinder and the second cylinder are respectively arranged in a chassis, the chassis is arranged on the base frame, and a detection device which can be opposite to the second mounting table in position is fixedly arranged on a top plate in the chassis. The application method disclosed by the invention comprises the following steps: sequentially controlling the first cylinder, the joint robot and the second cylinder, taking out a detection cup with blood from one test cup box and then moving into another detection cup box. The full-automatic detection type thromboelastography disclosed by the invention is novel in conception, reasonable in design and easy to produce and operate, by virtue of the joint robot and the cylinders, the whole process is automatically completed, labor cost and material resource cost are greatly reduced, and for a large number of to-be-detected blood samples, efficiency is greatly improved.
Owner:CHONGQING NANFANG NUMERICAL CONTROL EQUIP
Functional fibrinogen activator detection, and application thereof
The invention puts forward a functional fibrinogen activator, which comprises extrinsic coagulation activator, platelet inhibitor, buffer salt and protective agent. The functional fibrinogen activatordisclosed by the invention simultaneously contains the extrinsic coagulation activator and the platelet inhibitor, the aggregation of platelets can be inhibited while an extrinsic coagulation mechanism is activated, and in addition, salts and protective agent are simultaneously added into the activator to perform a function of guaranteeing coagulation. When the activator is adopted to carry out thromboelastography activated coagulation detection, the contents of fibrinogen in a blood sample can be accurately measured, and therefore, the functional fibrinogen activator has a wide application prospect.
Owner:深圳优迪生物技术有限公司
Thromblastography TEG
InactiveCN106950359AReduce thicknessEliminate gapsBiological testingMechanical engineeringThin walled
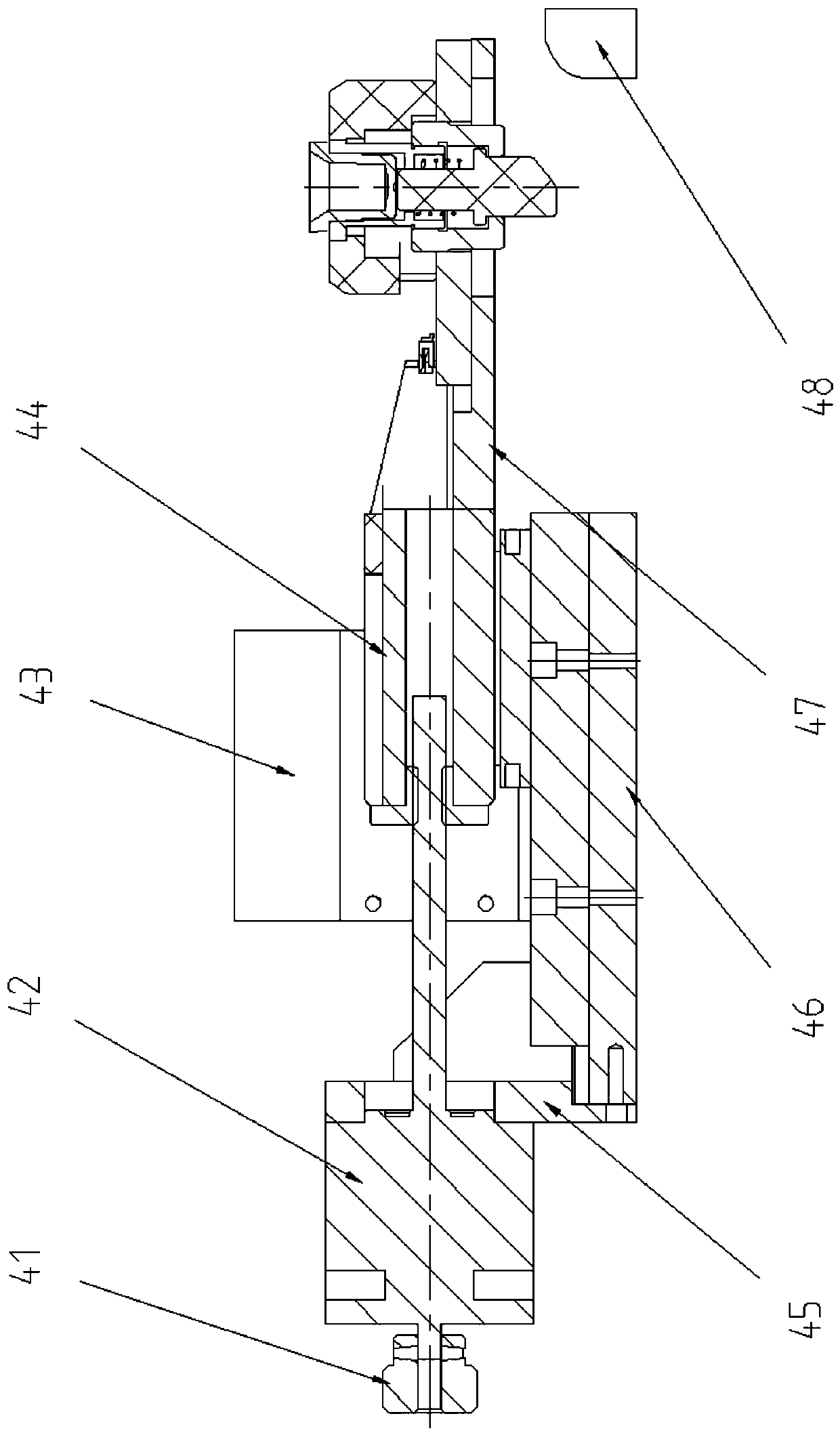
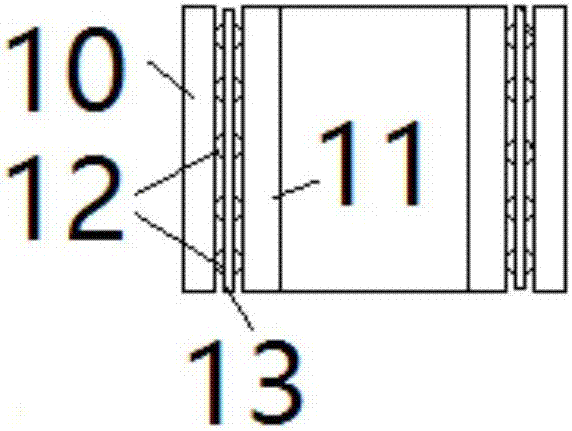
The present invention provides a thromboelastography instrument applied in the technical field of thromboelastogram drawing equipment, wherein the rotating part (3) of the thromboelastography instrument is movably connected with the protruding part (4) of the fixing part (2), A thin-walled bearing (5) is arranged between the rotating part (3) and the protruding part (4), a probe (6) is arranged at the lower end of the protruding part (4), and a tray (7) is arranged below the protruding part (4), The lower end of the tray (7) is connected with the motor (8), the motor (8) is installed on the body part (1), and the motor (8) is connected with the control part (9) capable of controlling the start-stop and rotating speed of the motor (8). The thrombelastograph has a simple structure, and can ensure flexible and stable connection between the rotating part and the fixed part of the thrombelastograph when the thrombelastograph is used for drawing the thrombelastograph, and the gap between the rotating part and the fixed part can be effectively obtained. Eliminate, ensure reliable and accurate test results, improve service life and test accuracy.
Owner:凌中鑫
Serum-free quality control material for thromboelastography and its use
The invention relates to a serum-free quality control material for a thromboelastography (TEG) and use of the serum-free quality control material. The serum-free quality control material is prepared from the following components by weight percent: 3.5-25% of acrylamide and methylene diacrylamide mother liquor (24 to 1), 0.1-1% of tetramethyl ethylenediamine (TEMED), 1-15% of persulfate and 0.01% of stabilizer; water is used as a solvent, and inorganic polymers with definite chemical compositions are used; the serum-free quality control is simple in production process and low in cost, can be stored at the room temperature, and is good in stability; by controlling the concentration and amount of a catalyst, the kinetic parameters of the blood can be well simulated in three states of hypocoagulability, hypercoagulability and normal blood coagulation; a user can use very low cost to achieve the test or correction of operating states of the instrument in the three states of hypocoagulability, hypercoagulability and normal blood coagulation every day, thus guaranteeing the stability and reliability of test data.
Owner:重庆鼎润医疗器械有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com