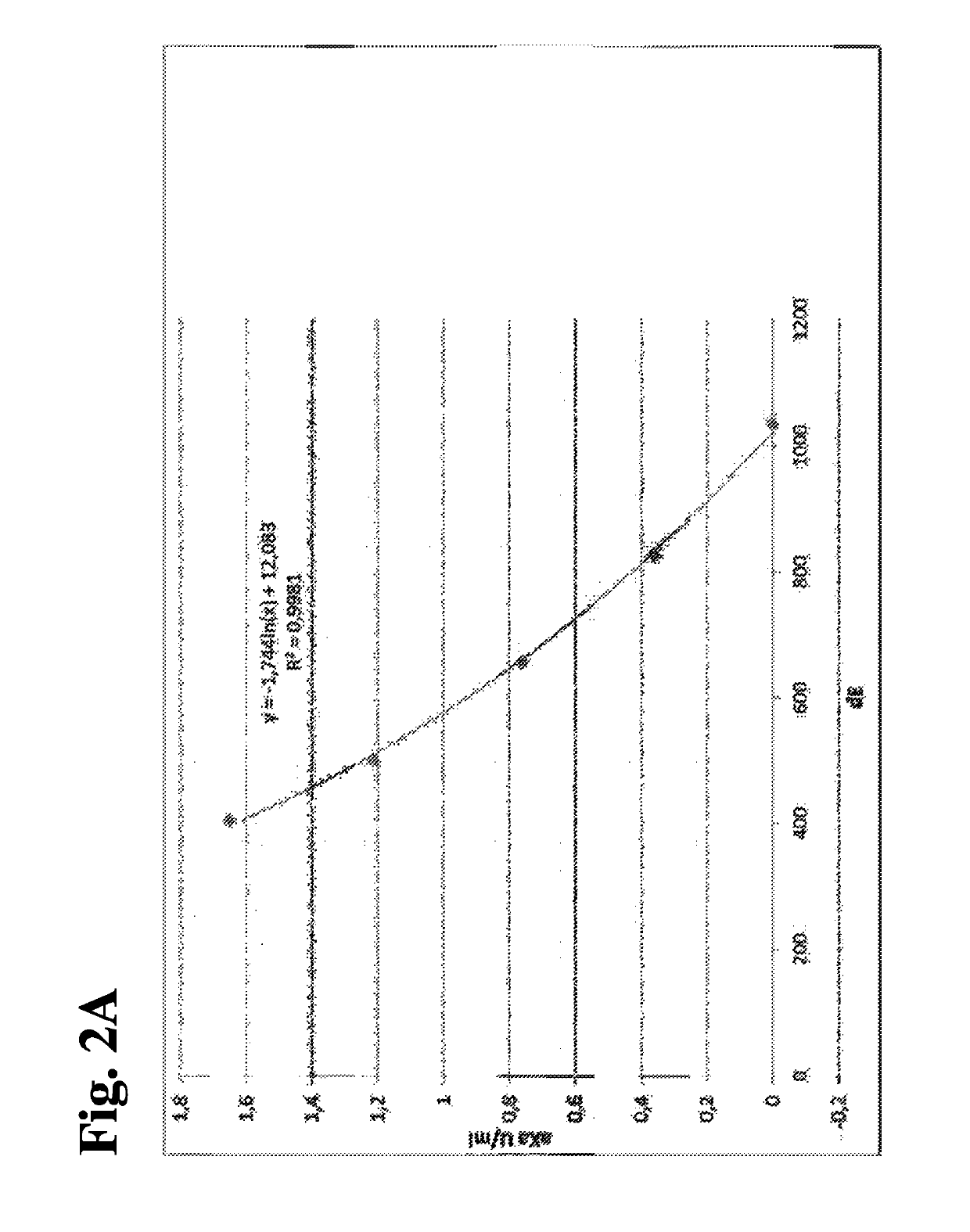
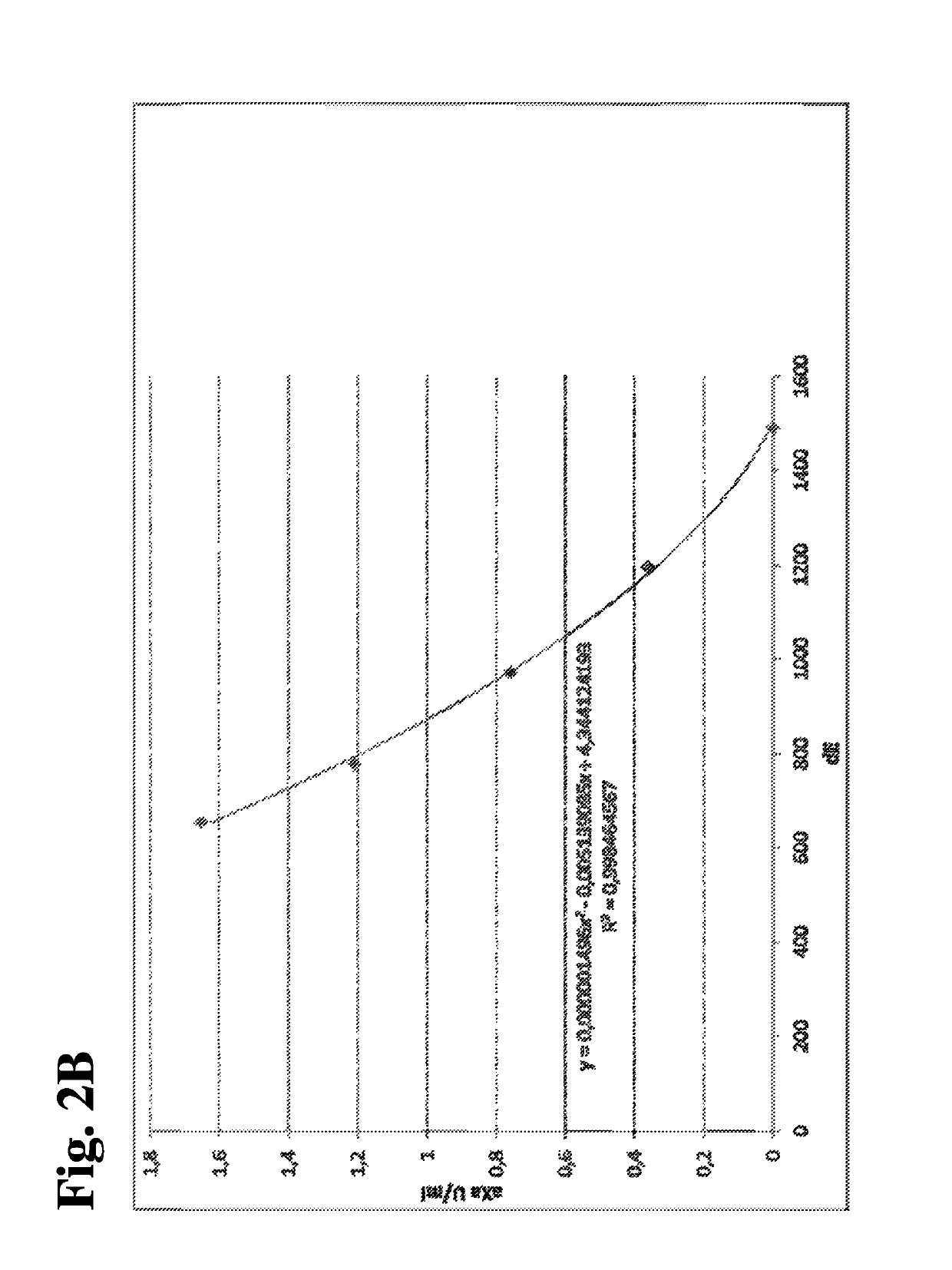
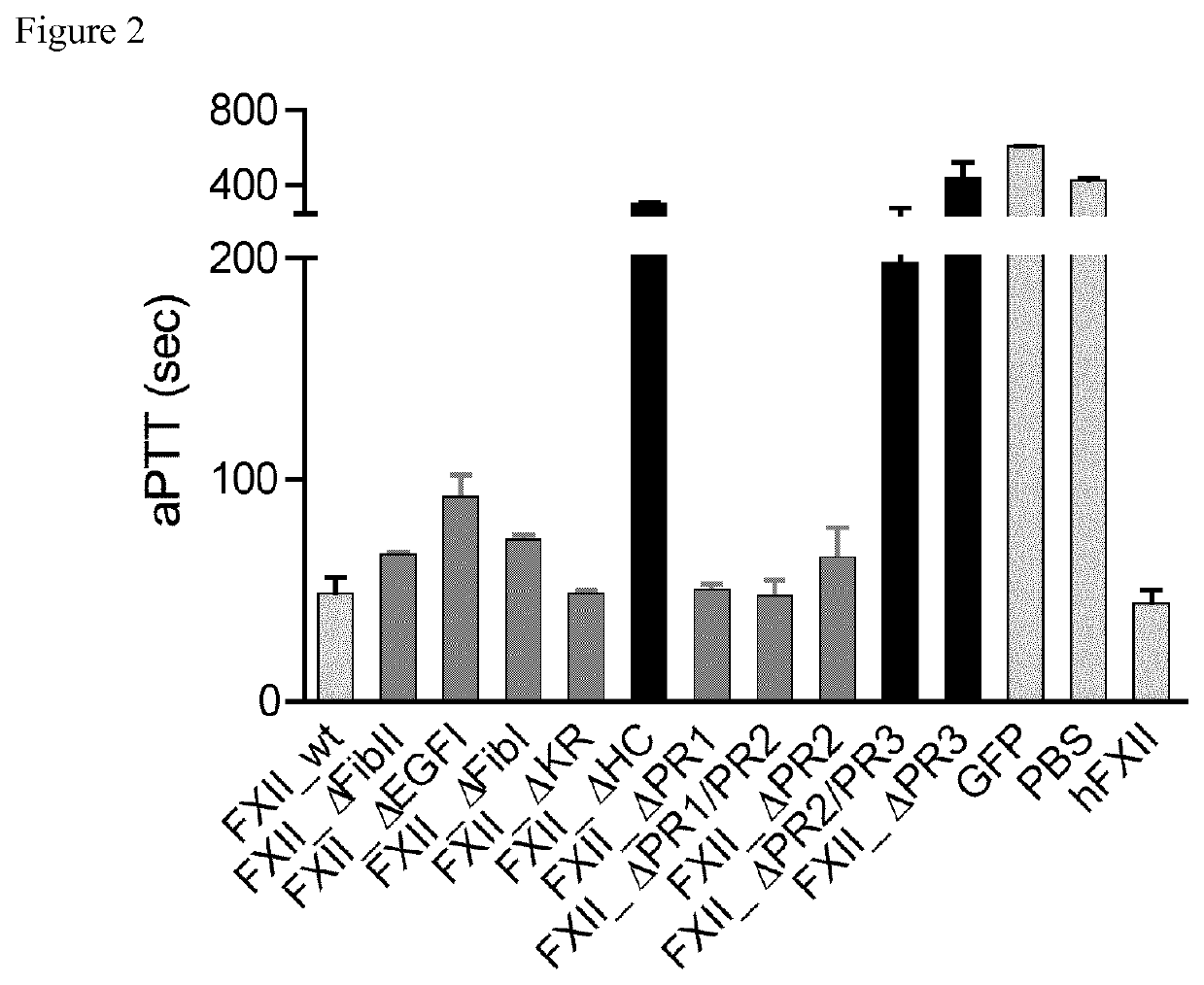
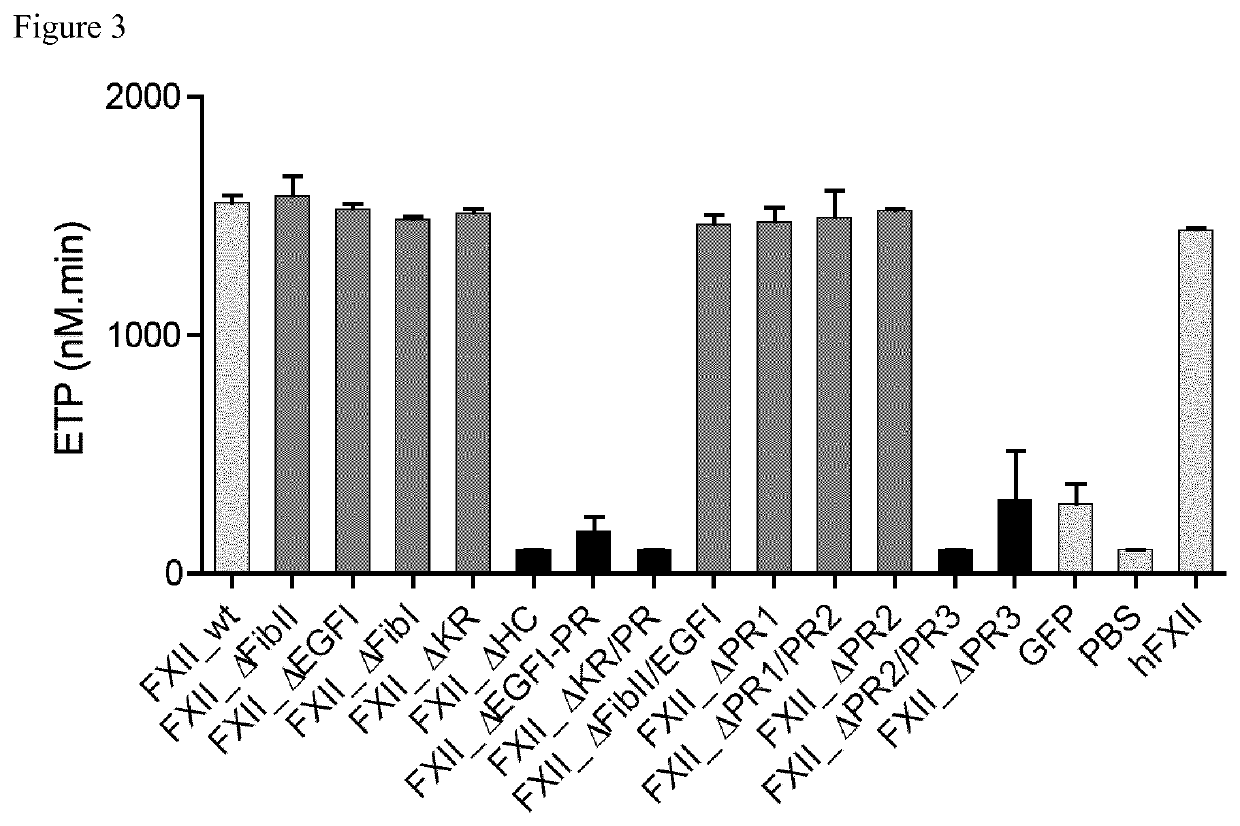
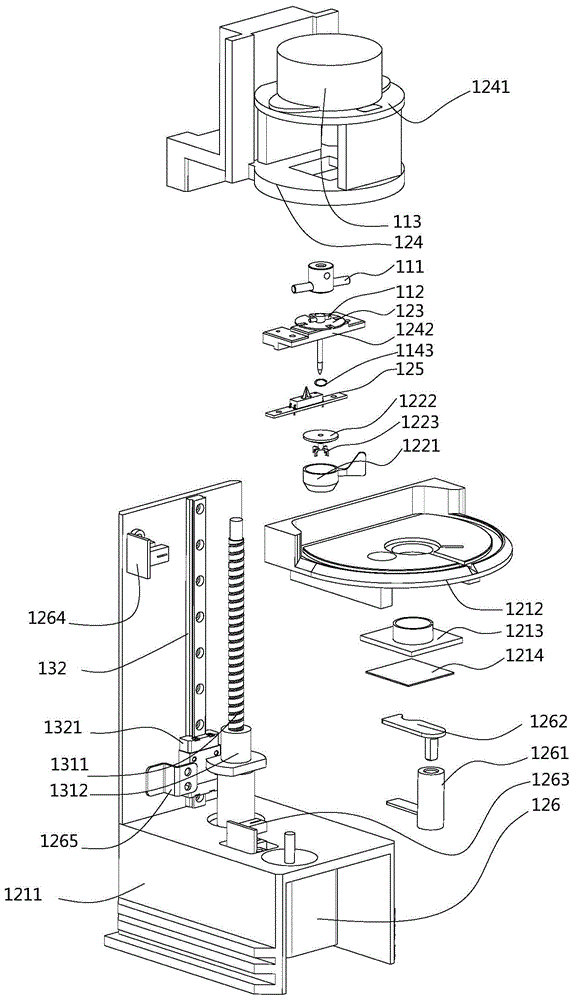
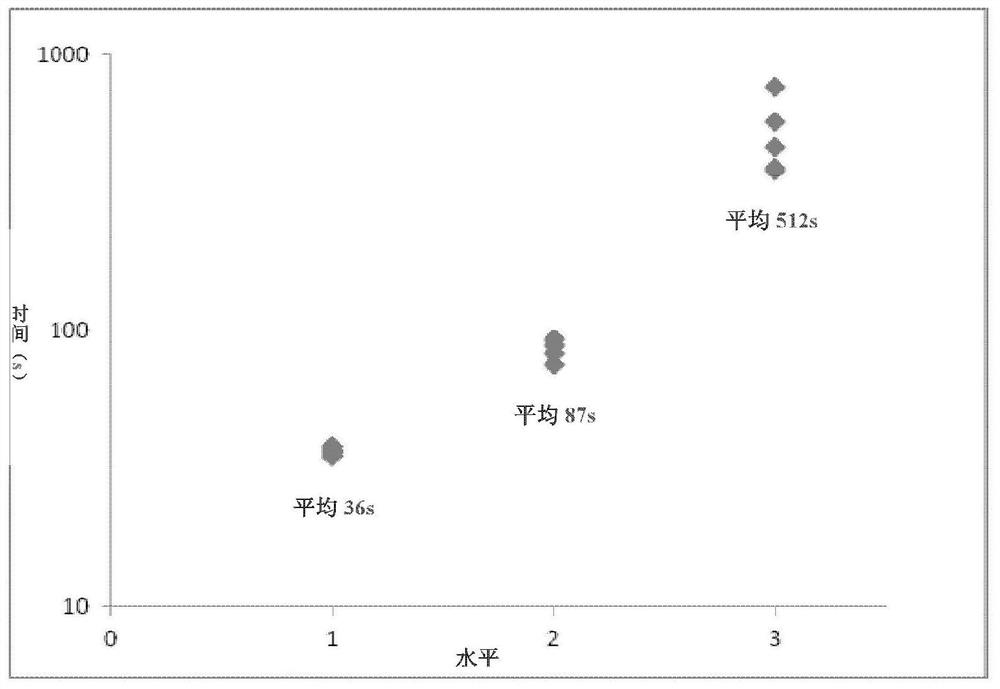
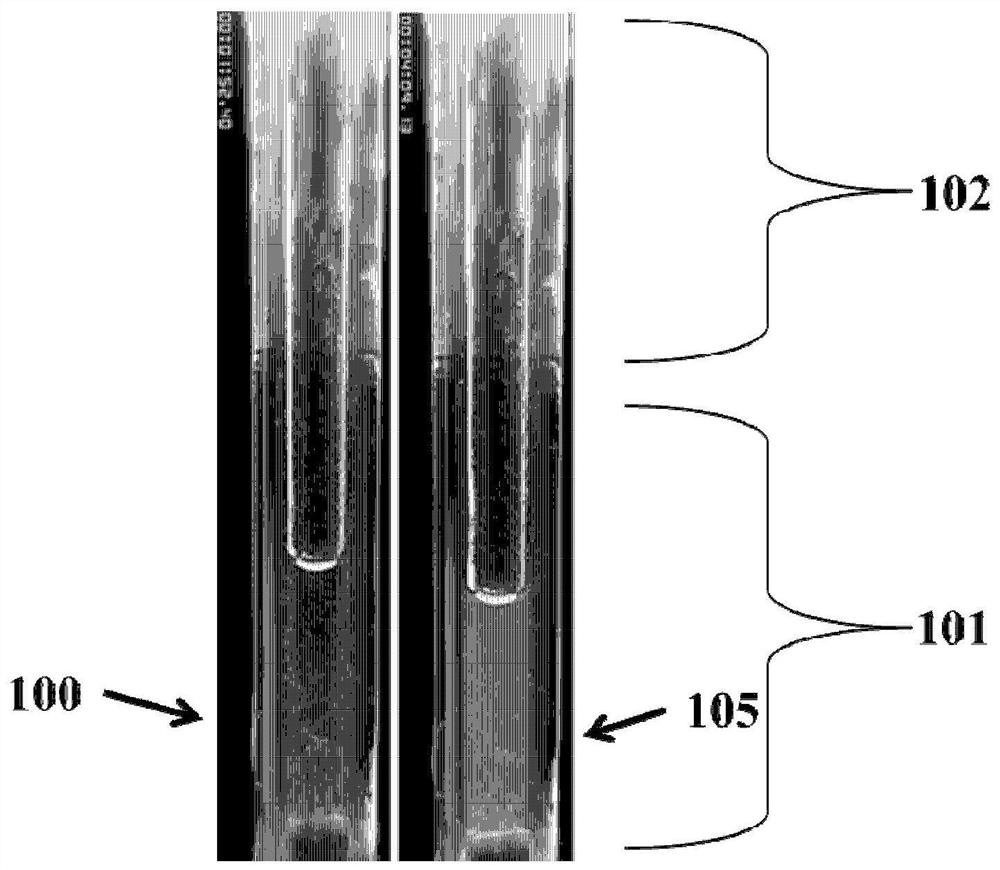
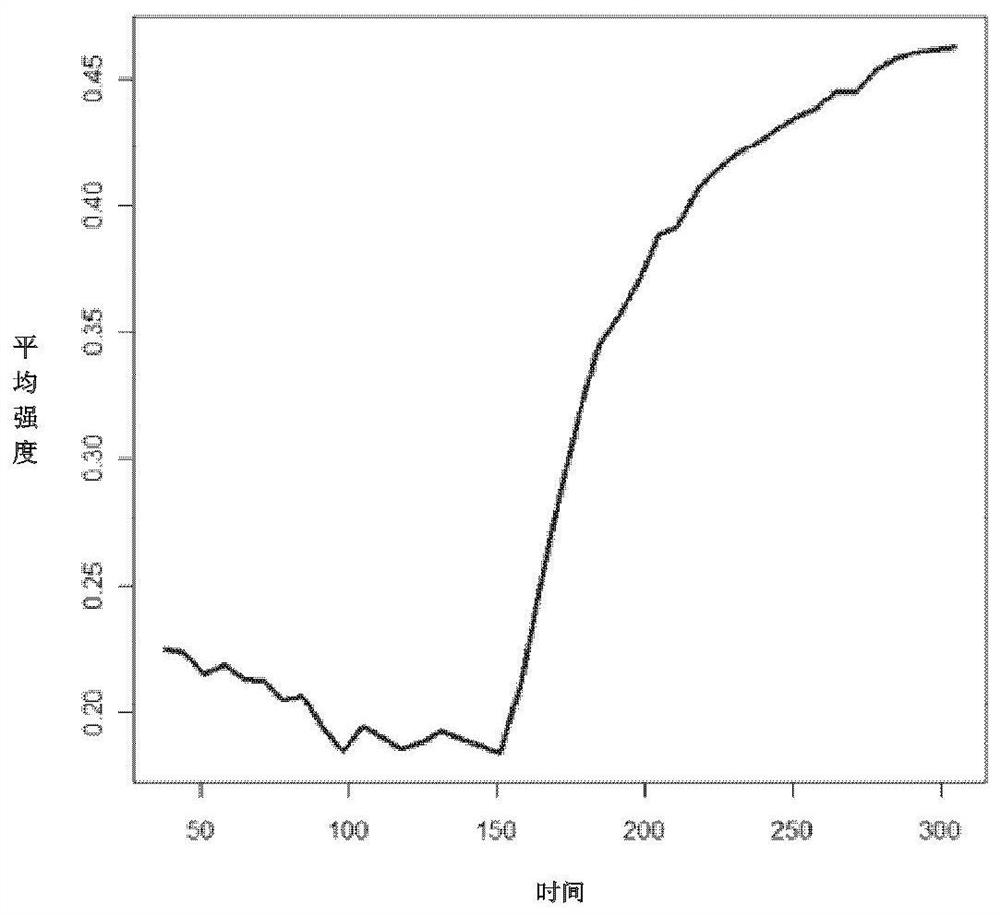
Patents
Literature
35 results about "Blood coagulation testing" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
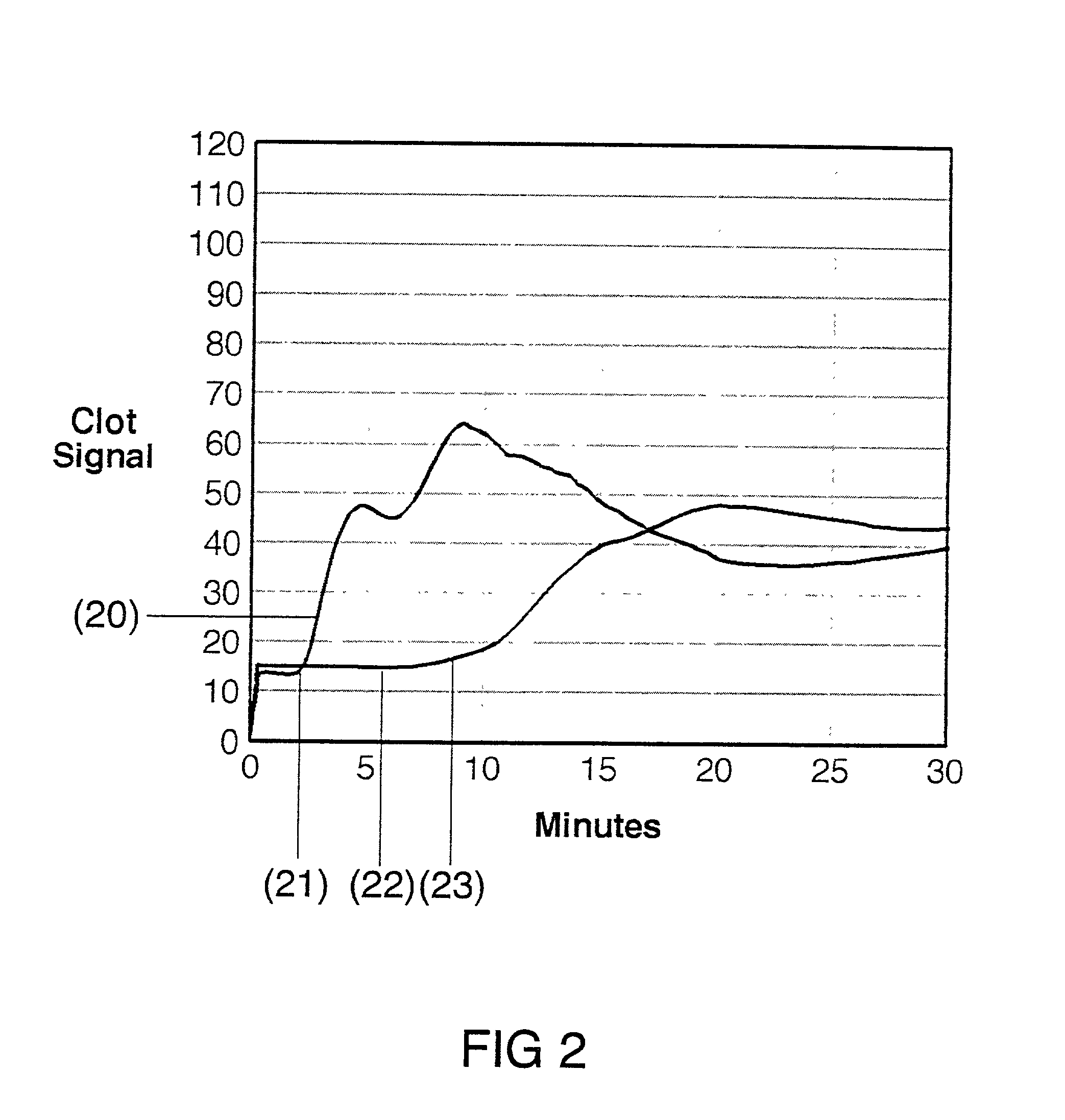
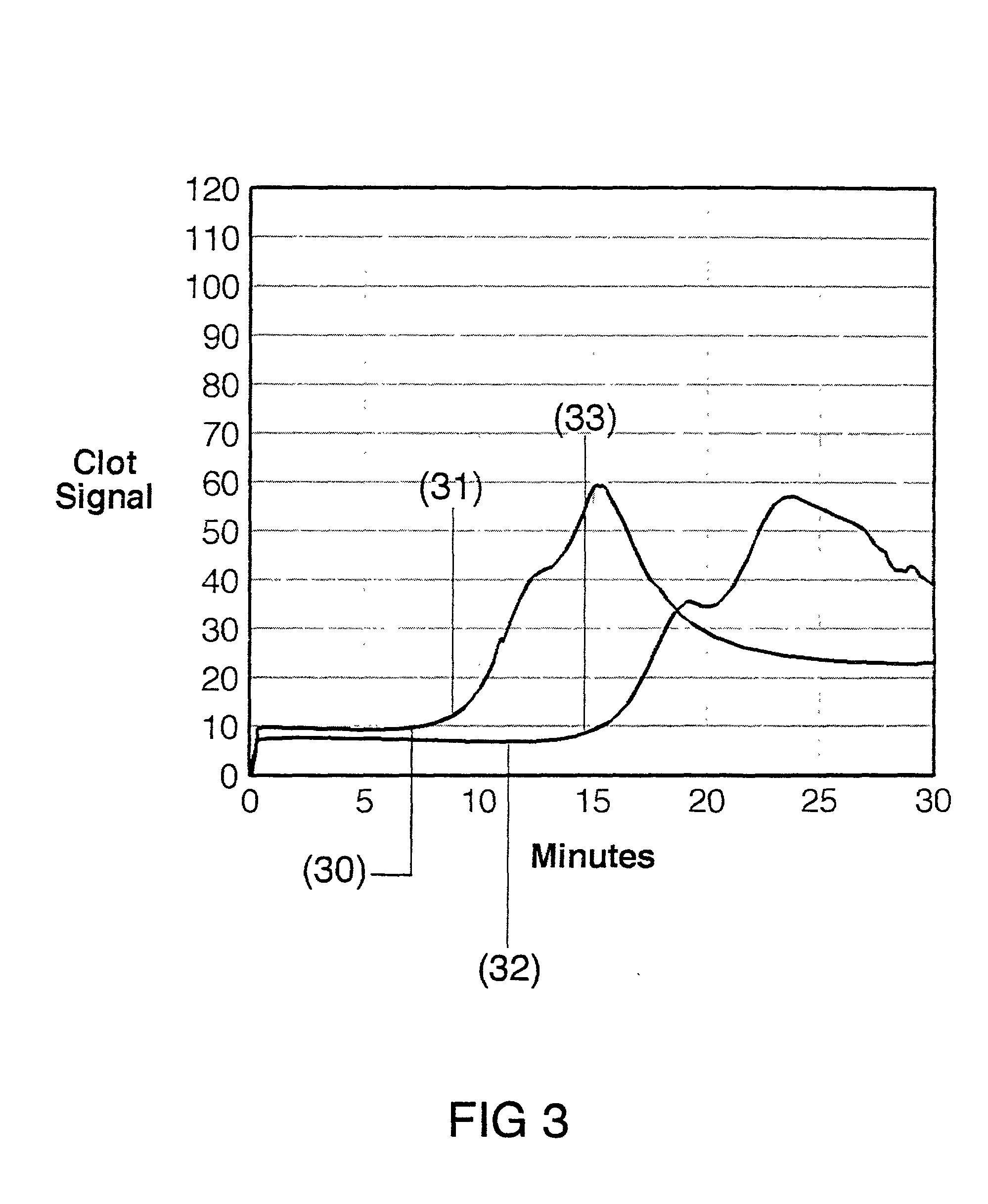
Coagulation testing. Blood clotting tests are the tests used for diagnostics of the hemostasis system. Coagulometer is the medical laboratory analyzer used for testing of the hemostasis system. Modern coagulometers realize different methods of activation and observation of development of blood clots in blood or in blood plasma.
3-level thrombelastogram quality control product and application thereof
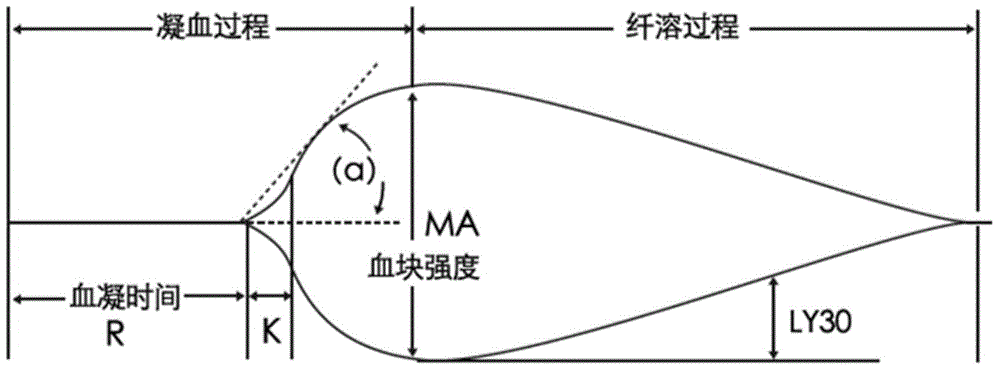
The invention relates to the field of quality control of blood coagulation testing items and provides a 3-level thrombelastogram quality control product. The quality control product is prepared by the following steps: respectively mixing pig blood with 3 different concentrations of sodium citrate, centrifuging, taking blood plasma, respectively adding a coagulation activator and a tissue factor and lyophilizing to prepare a powdery quality control product I, adding a coagulation activator and lyophilizing to prepare a powdery quality control product II, and adding a coagulation activator and human lyophilized platelets and lyophilizing to prepare a powdery quality control product III. The 3-level thrombelastogram quality control product provided by the invention is shaped as a lyophilized powder, can be used to monitor R and MA values simultaneously, is better used for quality control of an thromboelastography instrument and a thrombelastogram detection kit, is fast to detect, has accurate results and is simple to operate.
Owner:北京乐普诊断科技股份有限公司
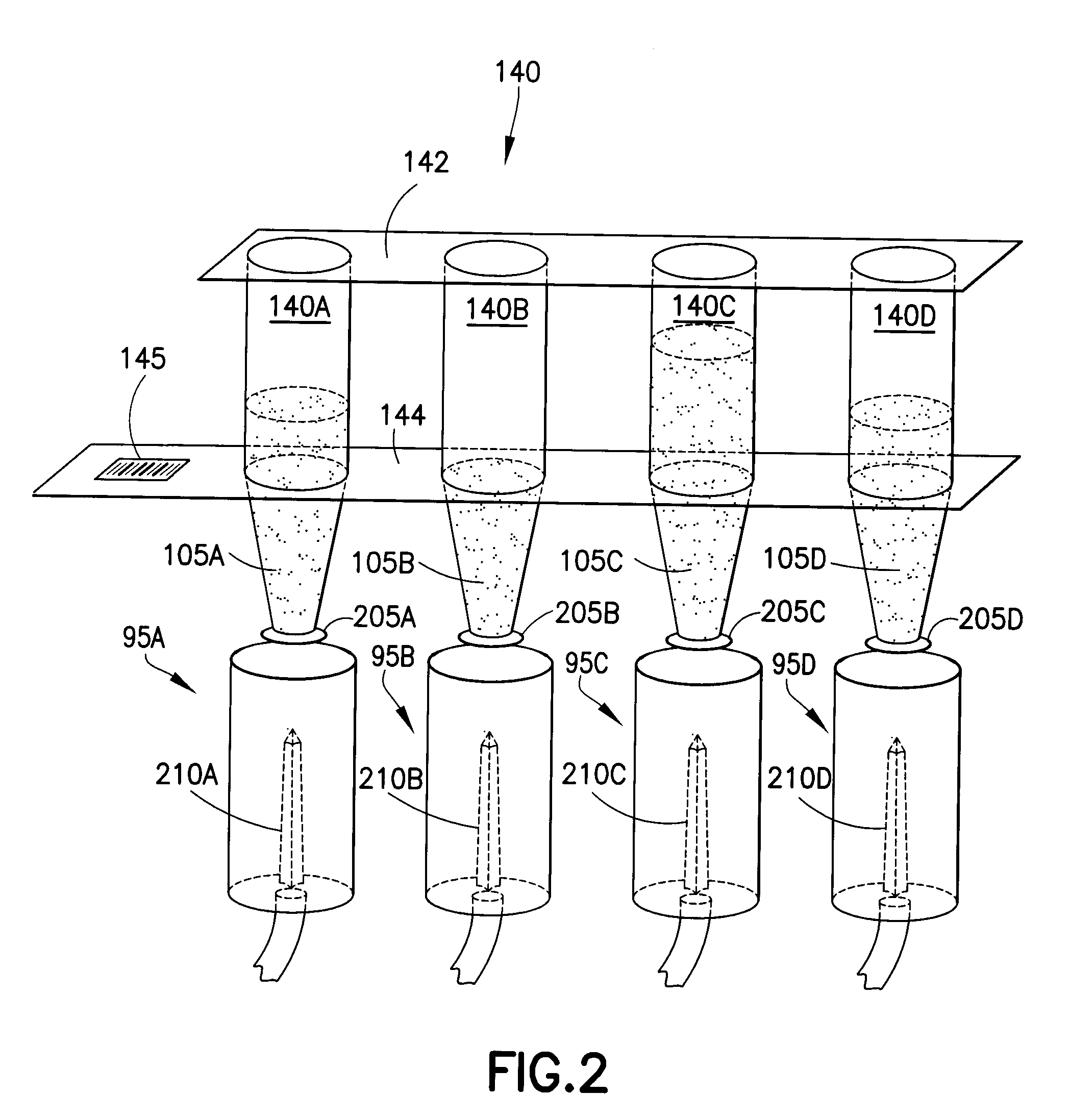
Multiple coagulation test cartridge and method of using same
ActiveUS20090148882A1Satisfies needReduce manufacturing costCompound screeningBioreactor/fermenter combinationsSingle patientGastroenterology
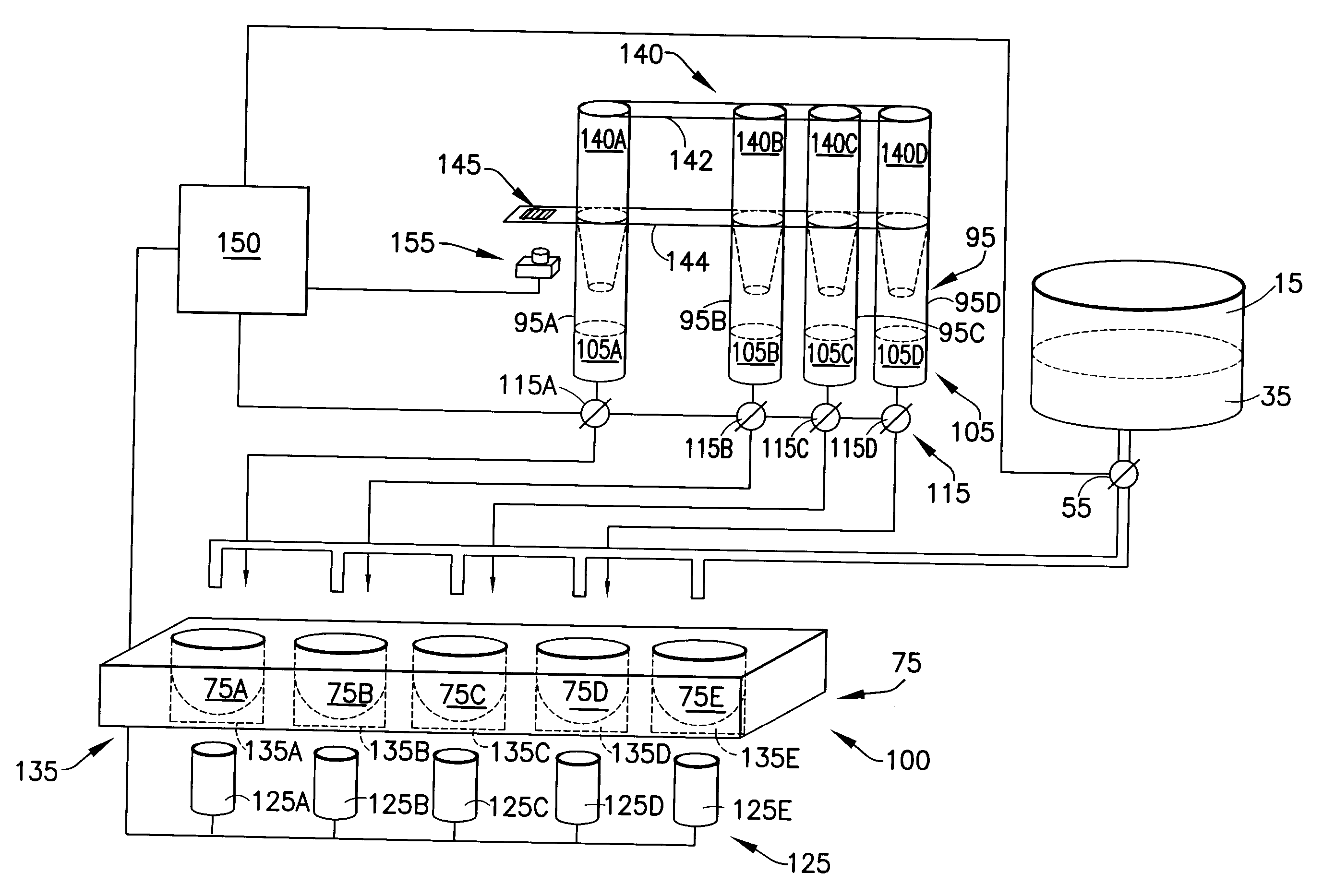
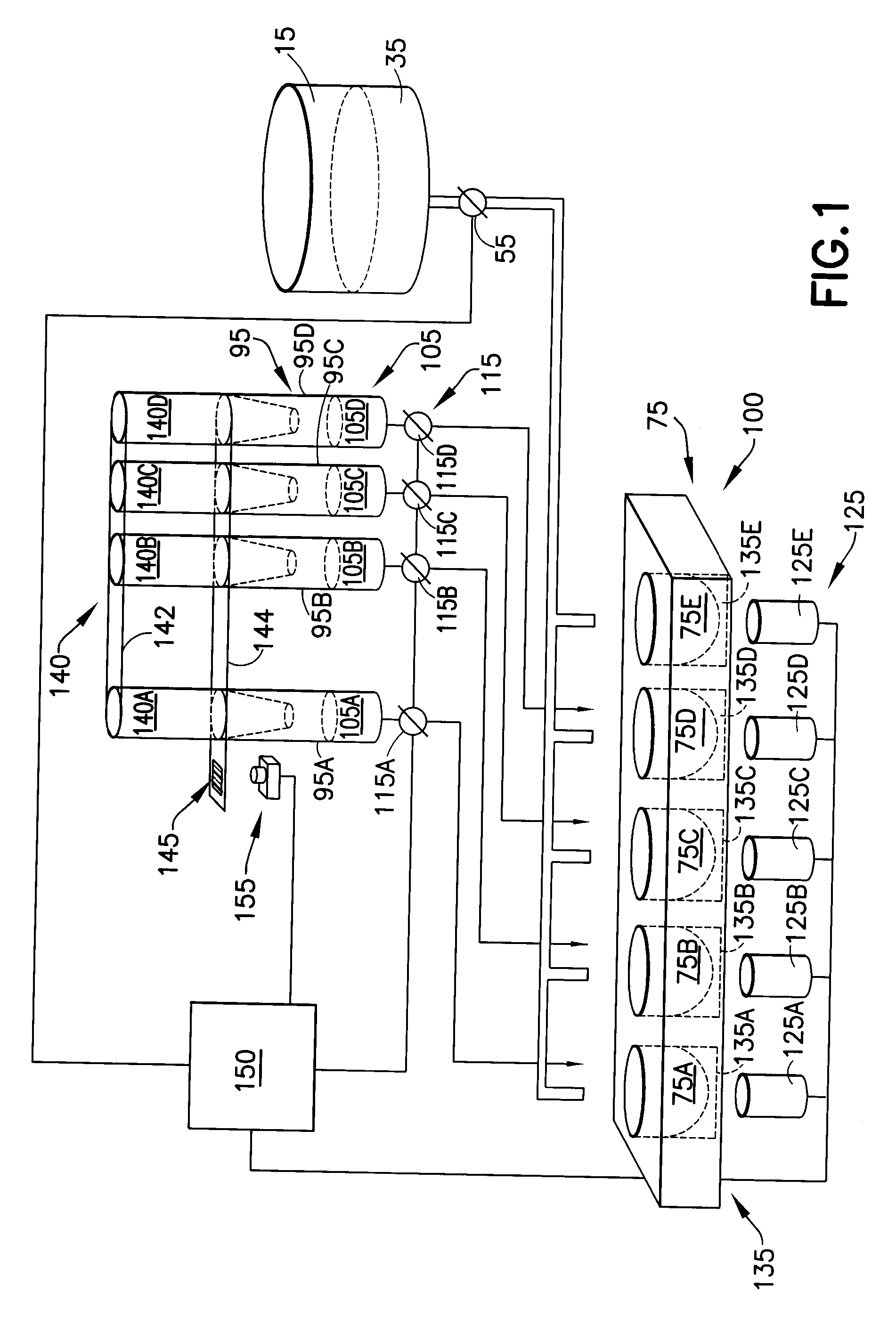
Embodiments of the present invention relate to multiple coagulation test cartridges and methods of using such cartridges. In one embodiment the cartridge is a disposable single-use cartridge for use in evaluating blood clotting. The cartridge includes multiple containers, such as tubes, each of which includes one or more coagulation affecting substances. The containers can be pre-filled with substances in amounts suitable for use with a single patient's blood sample. The cartridge may include one or more containers, each of which has multiple sections, or volumes, each section storing a different coagulation affecting substance. In another embodiment the cartridge is used in a method for determining at least one appropriate coagulation affecting substance for modifying a patient's coagulation status using a multiple coagulation test system.
Owner:COAGULATION SCI LLC
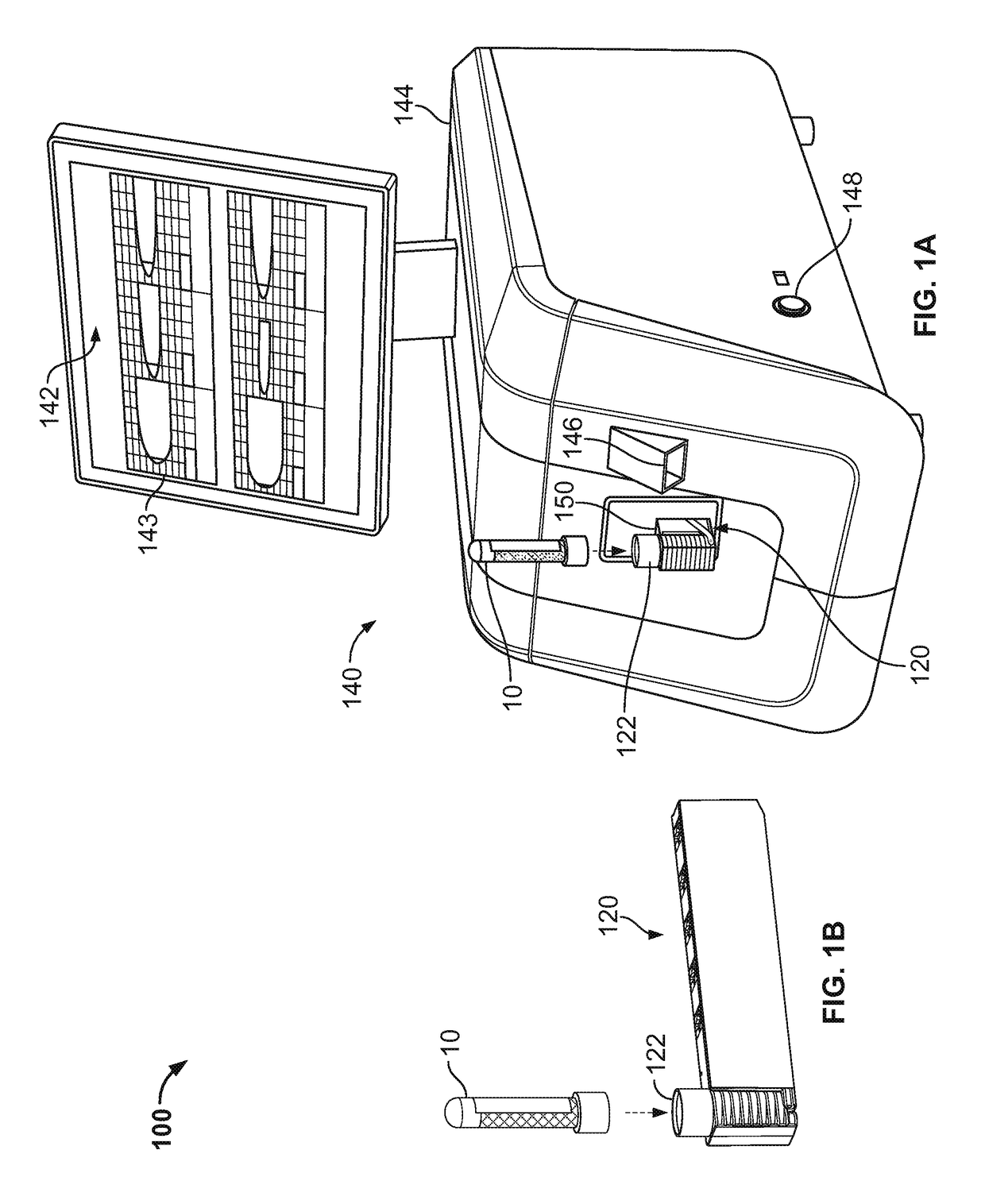
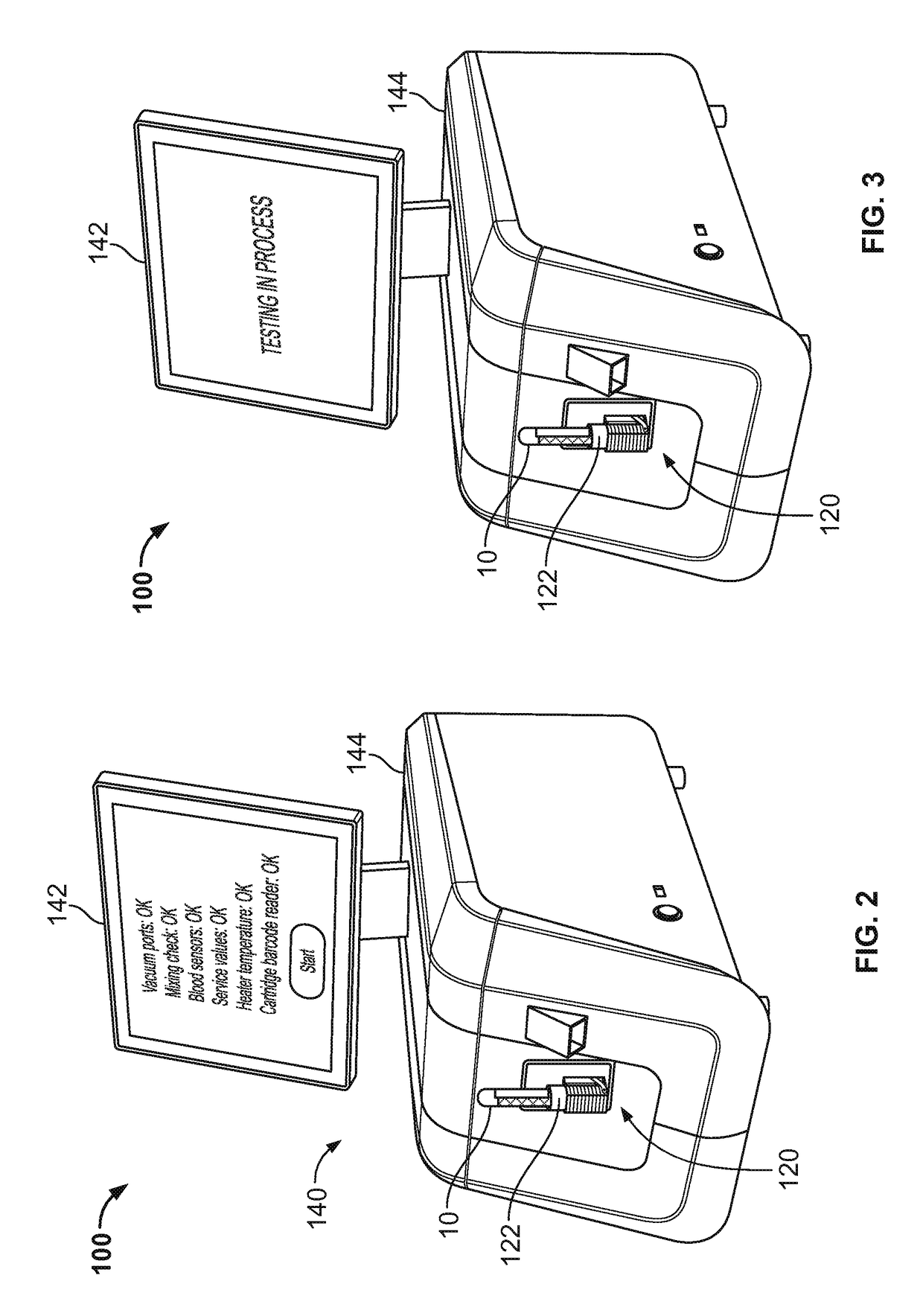
Blood testing system and method
ActiveUS10175225B2Minimizing user interactionReduce chanceFlow propertiesLaboratory glasswaresPoint of careMaximum lysis
Some embodiments of a blood coagulation testing system include an analyzer console device and a single-use cartridge component configured to releasably install into the console device. In some embodiments, the blood coagulation testing system can operate as an automated thromboelastometry system that is particularly useful, for example, at a point-of-care site.
Owner:C A CASYSO
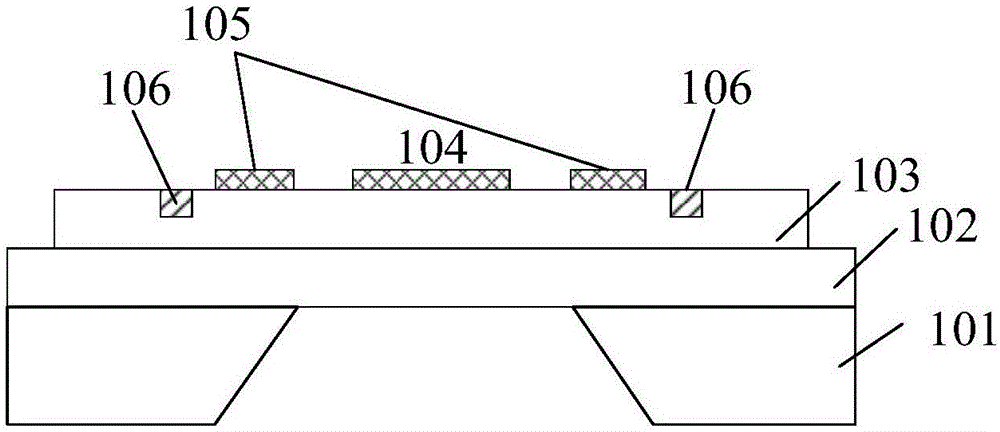
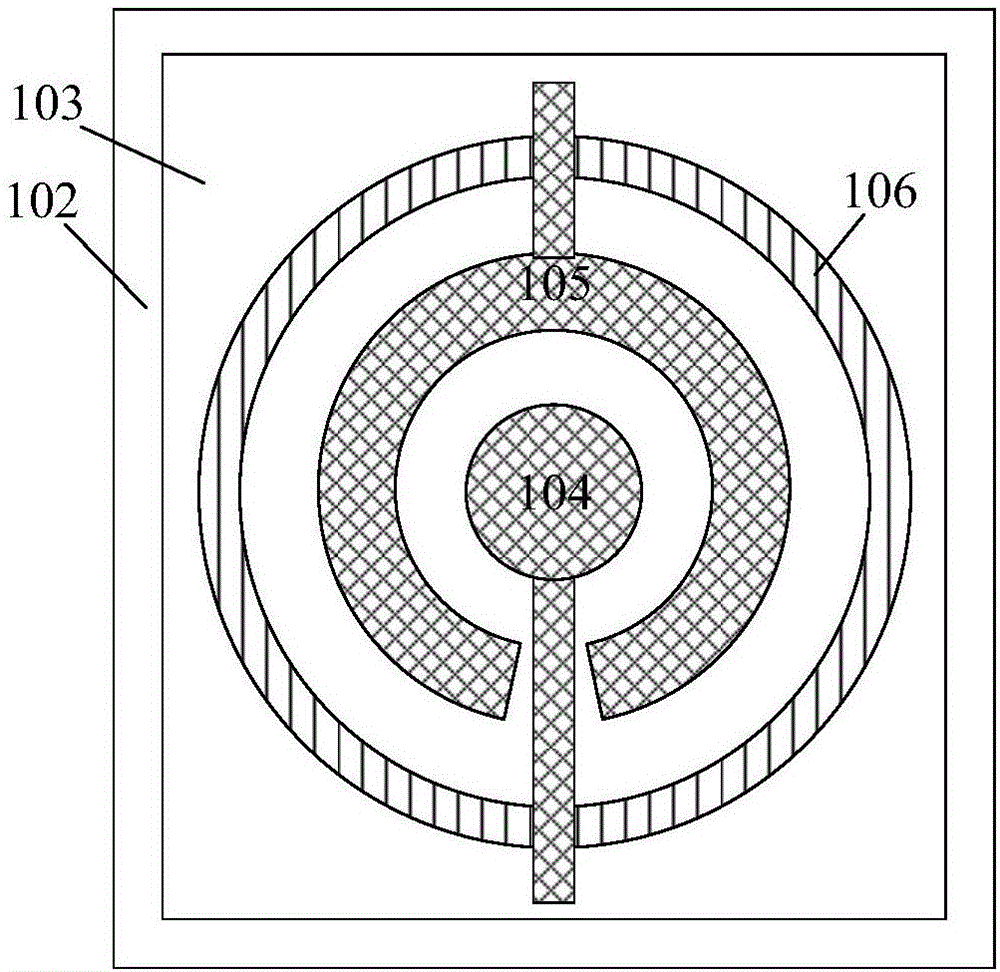
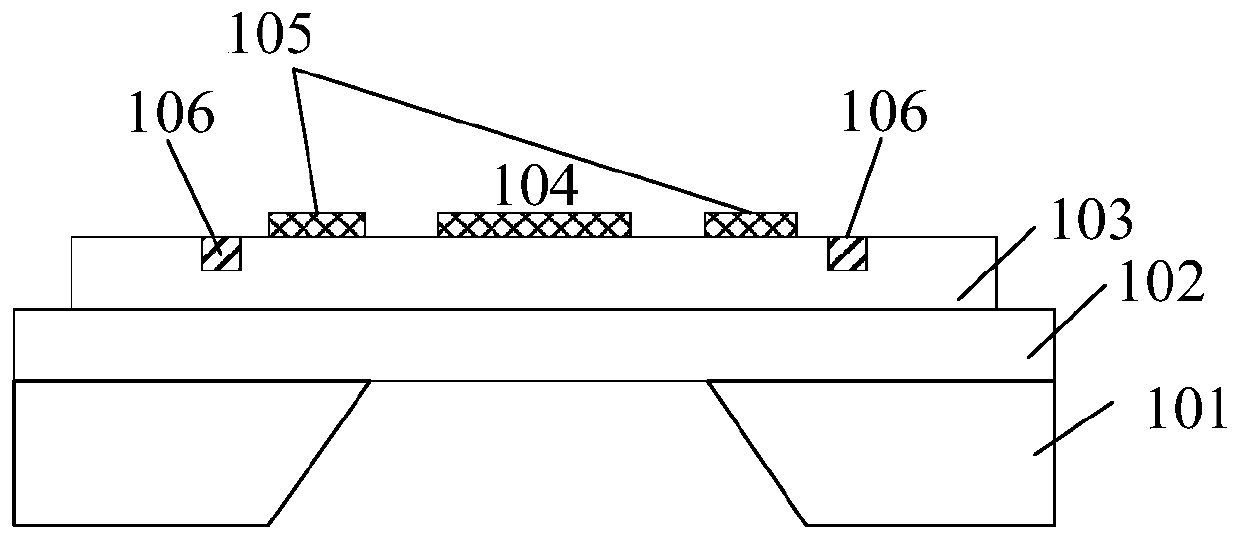
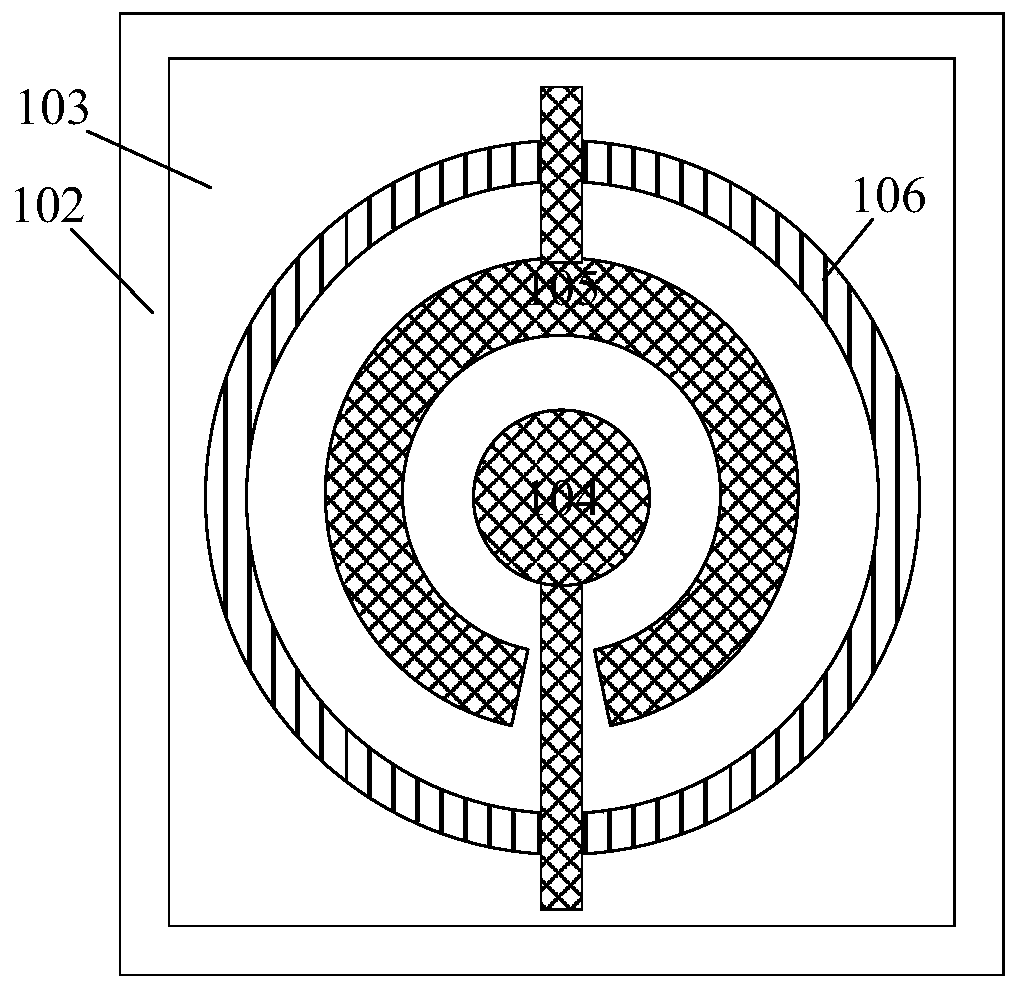
Piezoelectric thin-film resonator, method for manufacturing the same, and method for detecting blood coagulation time
ActiveCN106788317ASimple structureEasy to manufactureImpedence networksMaterial analysis by electric/magnetic meansUltraviolet lightsBlood coagulations
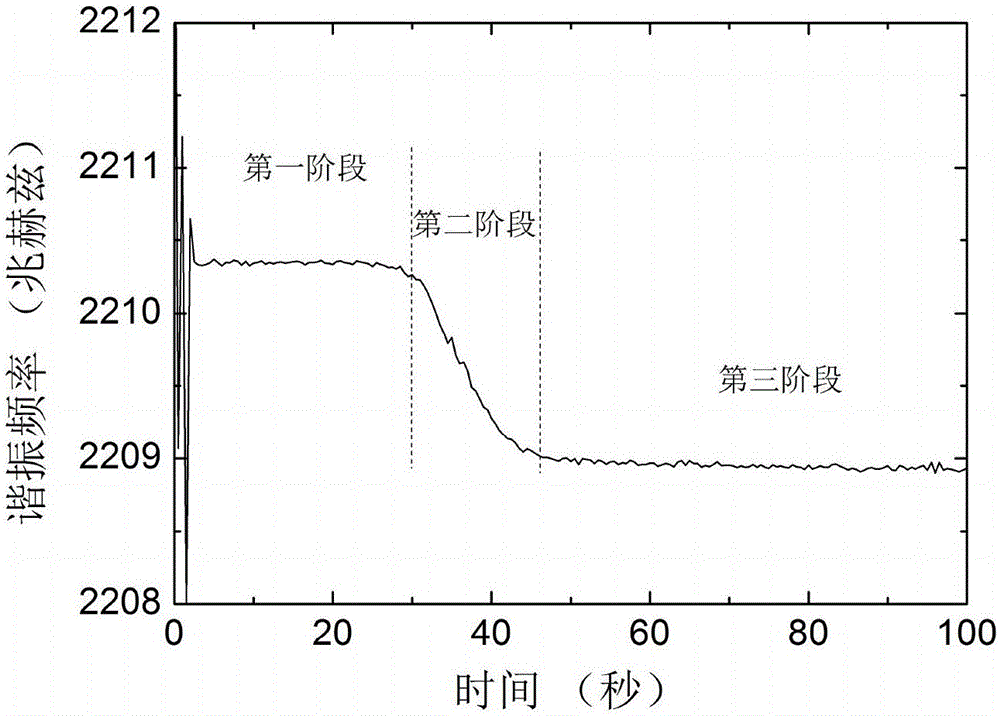
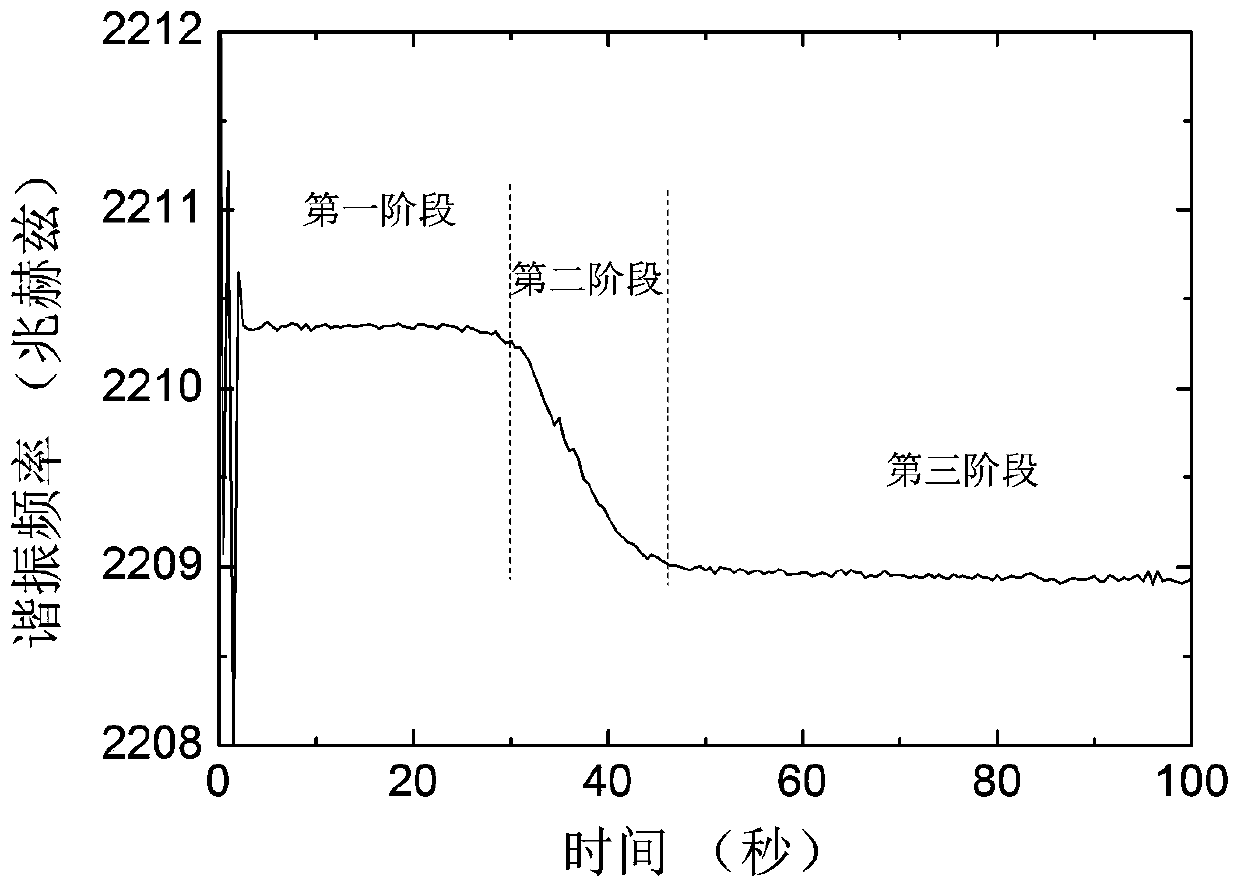
The invention discloses a piezoelectric thin-film resonator for blood coagulation detection. The piezoelectric thin-film resonator comprises a piezoelectric film layer and an annular groove. In addition, the invention also discloses a method for manufacturing the piezoelectric thin-film resonator for blood coagulation detection. The method includes: the inner surface of an annular groove is coated with a hydrophilic polymer material; oxygen plasma treatment or ultraviolet light irradiation is carried out on the inner surface of the annular groove; a piezoelectric thin-film resonator is placed in a sodium carbonate solution and a calcium chloride solution is added into the solution; and the piezoelectric thin-film resonator is baked until the piezoelectric thin-film resonator is dried. Besides, the invention also discloses a method for blood coagulation detection by using a piezoelectric thin-film resonator. The method for blood coagulation detection comprises: dripping mixed liquid having a blood sample and a blood coagulation testing regent into the center of an annular groove; measuring the resonant frequency of a piezoelectric thin film resonator; and according to the recorded stabilized resonant frequency, calculating a second derivative of the resonant frequency to a time change and taking a maximum value of the second derivative as blood coagulation time. The method for blood coagulation detection by using a piezoelectric thin-film resonator is suitable for household application.
Owner:SHANDONG UNIV OF SCI & TECH
Standard/reference/control for blood coagulation testing
ActiveUS7588942B2Control for coagulationSamplingMicrobiological testing/measurementWhite blood cellMedicine
A whole-blood-based substitute composition that is useful in coagulation assays as a standard, reference, control, calibrator, linearity verifier, or training material is prepared by combining a red blood cell lysate that is free of plasma, leukocytes, and platelets with a platelet-free plasma of human origin and an antimicrobial agent.
Owner:BIO RAD LAB INC
Full-automatic coagulation tester
PendingCN107703078ASolving Dispersion ProblemsColor/spectral properties measurementsMaterial magnetic variablesElectricityPeristaltic pump
The invention discloses a full-automatic coagulation tester, and relates to the technical field of coagulation detection. The full-automatic coagulation tester comprises a case as well as a test position, a reagent refrigeration position, an XYZ triaxial linkage system and a peristaltic pump which are arranged in the case, wherein the test position comprises a first base, a first support sliding seat glidingly connected with the first base, a track groove fixedly formed in the first support sliding seat, a test cup disc located on one side of the first support sliding seat, a drive mechanism located on the inlet side of the track groove, a cup withdrawing mechanism located on the outlet side of the track groove, an in-place mechanism fixedly arranged on the cup withdrawing mechanism and acontrol assembly electrically connected with the drive mechanism, the cup withdrawing mechanism and the in-place mechanism; a tape and a test cup of the test cup disc are conveyed into the track groove by the drive mechanism. According to the full-automatic coagulation tester, all independent components are integrated into the same device, and the problem that the components in test positions of existing test cups are bloated and scattered is solved by means of the test position in the tester.
Owner:北京众驰伟业科技发展有限公司
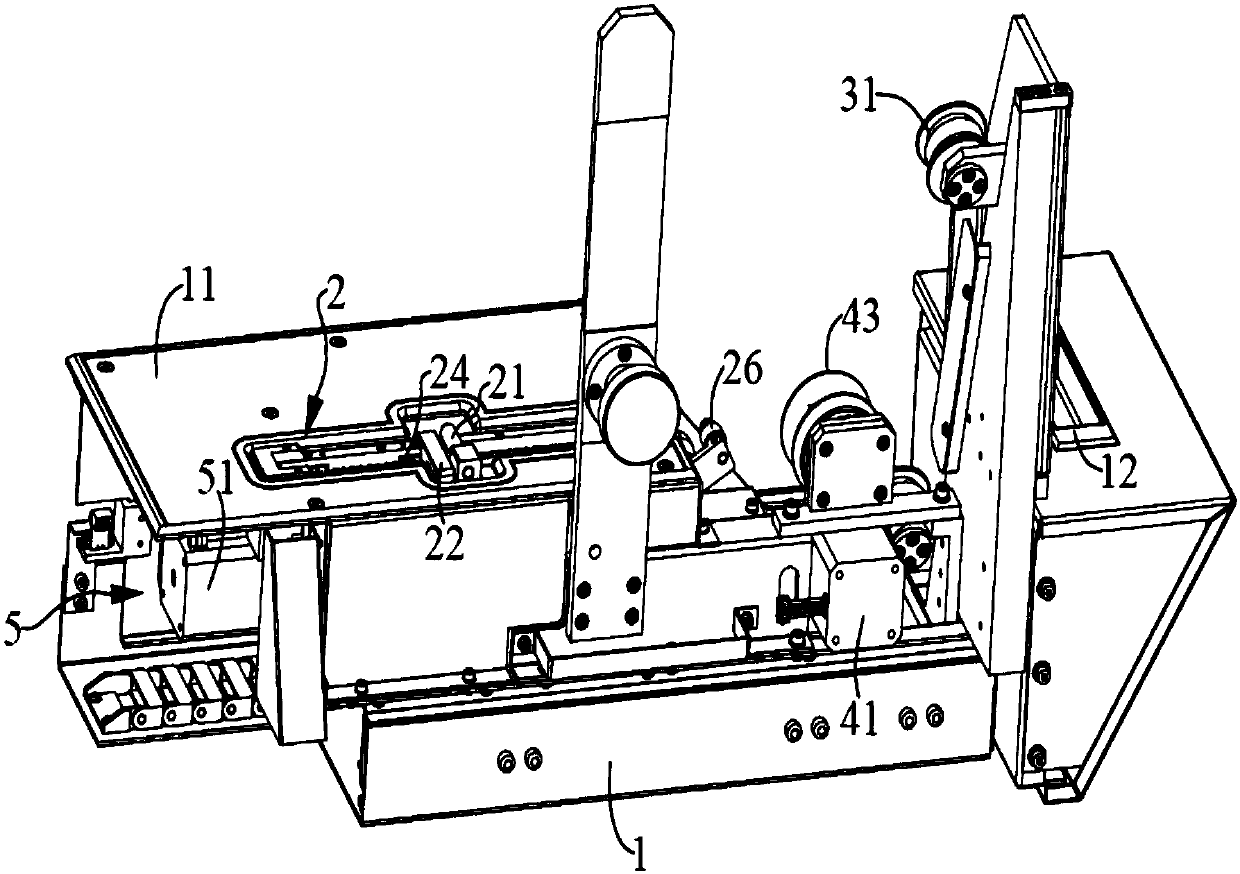
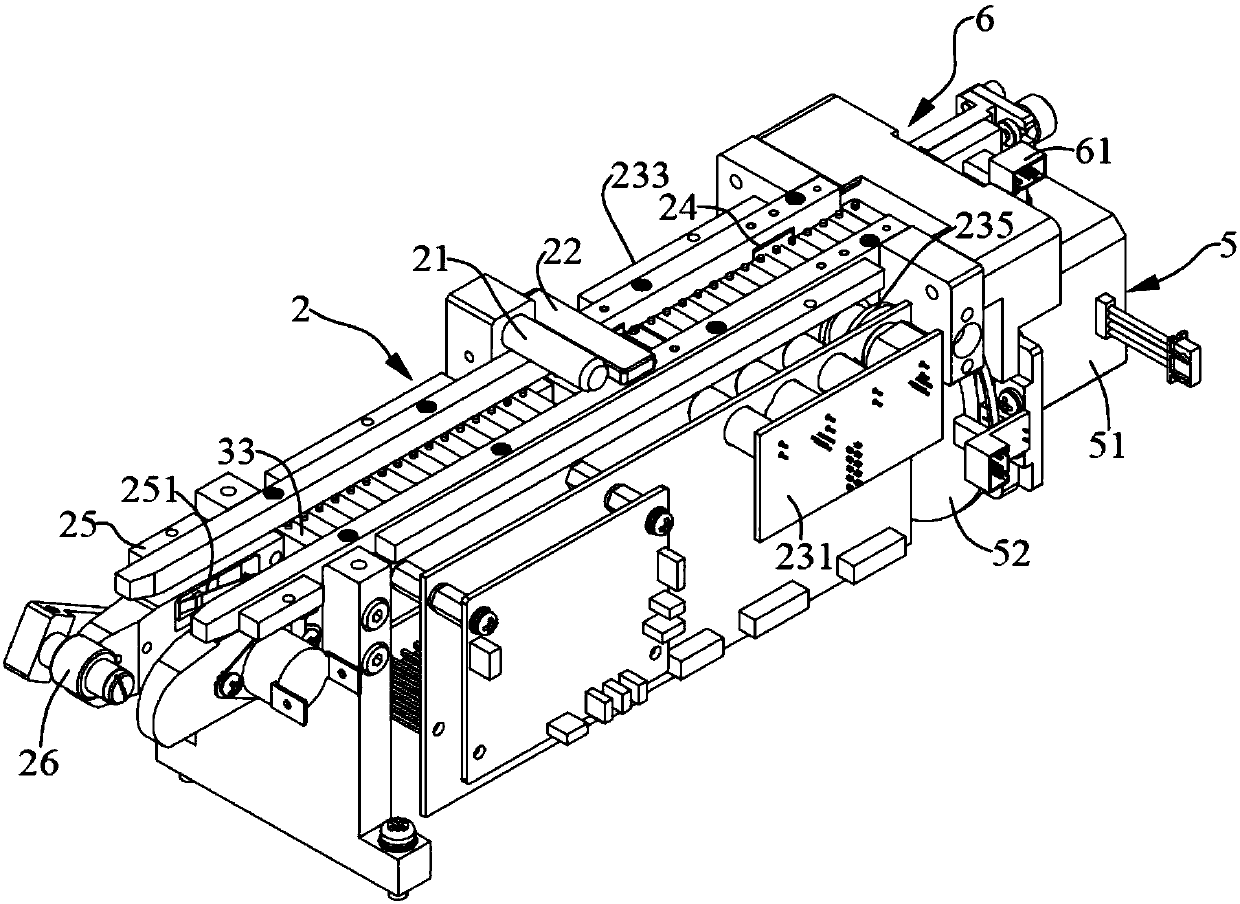
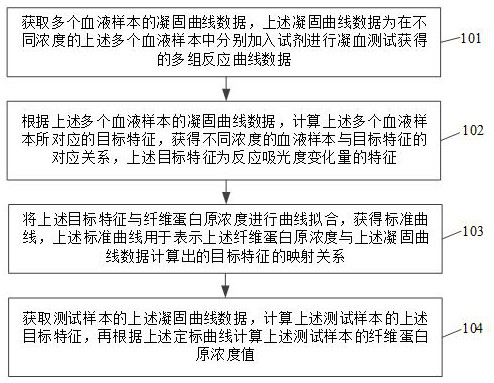
Method and device for determining fibrinogen concentration of sample, blood coagulation analyzer and medium
ActiveCN114324218AEasy accessAccurate acquisitionColor/spectral properties measurementsBiological testingMedicineBlood coagulation analyzer
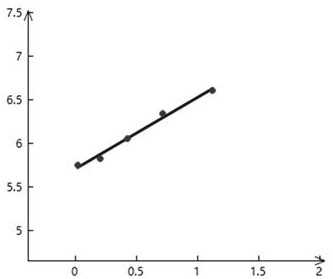
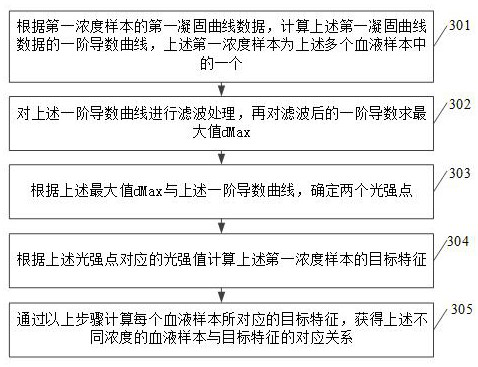
The invention discloses a sample fibrinogen concentration determination method, a sample fibrinogen concentration determination device, a blood coagulation analyzer and a medium. The method comprises the following steps: acquiring coagulation curve data of a plurality of blood samples, wherein the coagulation curve data are multiple groups of reaction curve data obtained by respectively adding reagents into the plurality of blood samples with different concentrations for performing coagulation test; according to the coagulation curve data of the multiple blood samples, target characteristics corresponding to the multiple blood samples are calculated, the corresponding relation between the blood samples of different concentrations and the target characteristics is obtained, and the target characteristics are characteristics reflecting absorbance variation; performing curve fitting on the target characteristics and the fibrinogen concentration to obtain a standard curve which is used for representing a mapping relation between the fibrinogen concentration and the target characteristics calculated by coagulation curve data; and obtaining the solidification curve data of a test sample, calculating the target characteristics of the test sample, and calculating the fibrinogen concentration value of the test sample according to the standard curve.
Owner:SHENZHEN DYMIND BIOTECH
Standard/reference/control for blood coagulation testing
ActiveUS20080032405A1Control for coagulationSamplingMicrobiological testing/measurementWhite blood cellMedicine
A whole-blood-based substitute composition that is useful in coagulation assays as a standard, reference, control, calibrator, linearity verifier, or training material is prepared by combining a red blood cell lysate that is free of plasma, leukocytes, and platelets with a platelet-free plasma of human origin and an antimicrobial agent.
Owner:BIO RAD LAB INC
Coupling type blood coagulation testing system and method
ActiveCN108872615AMaximize coupling efficiencyImprove consistencyColor/spectral properties measurementsBiological testingMeasurement deviceBeam splitting
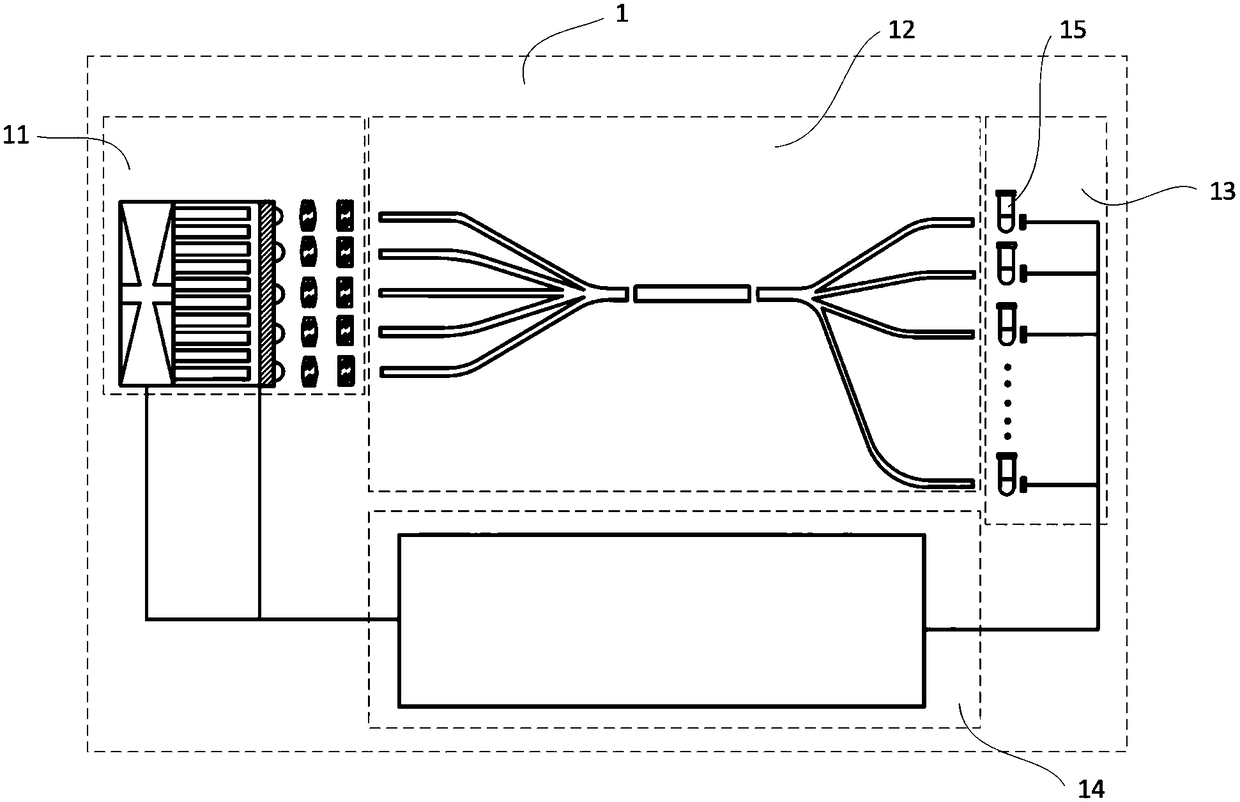
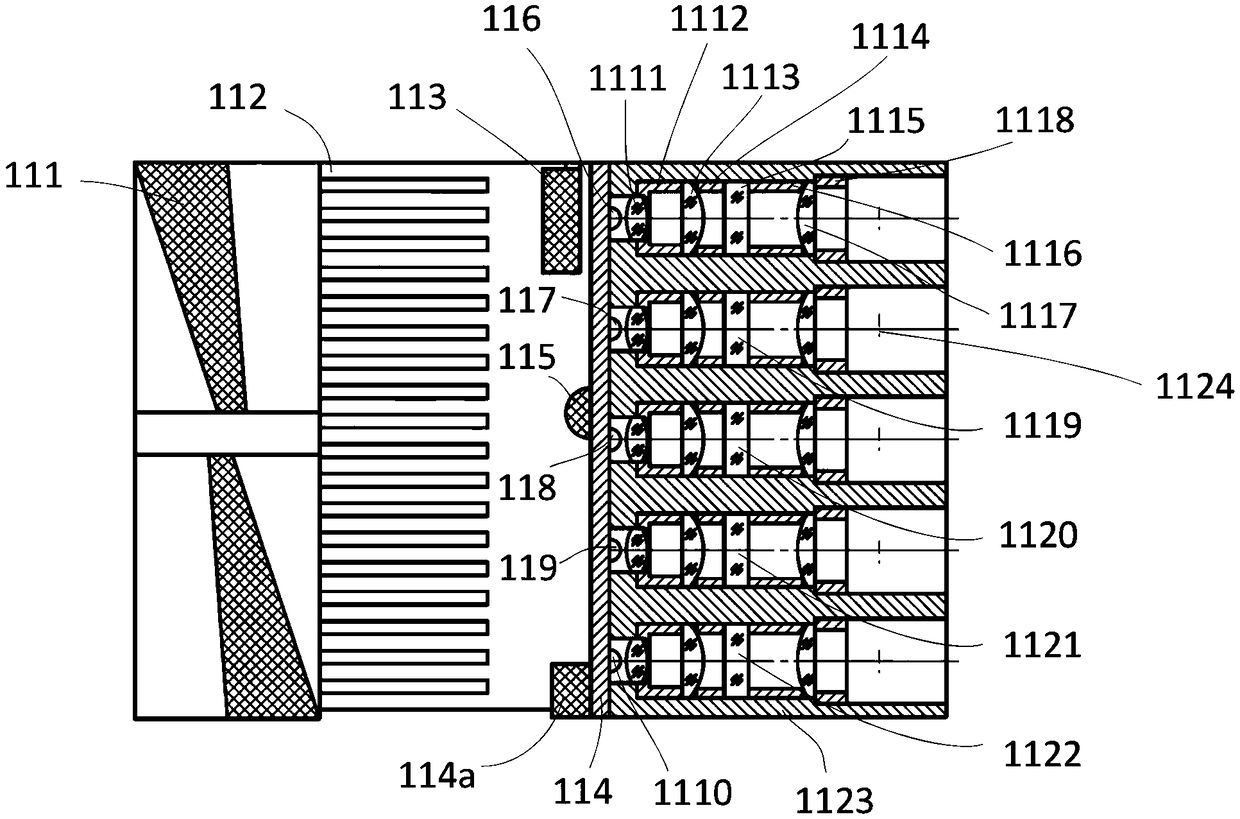
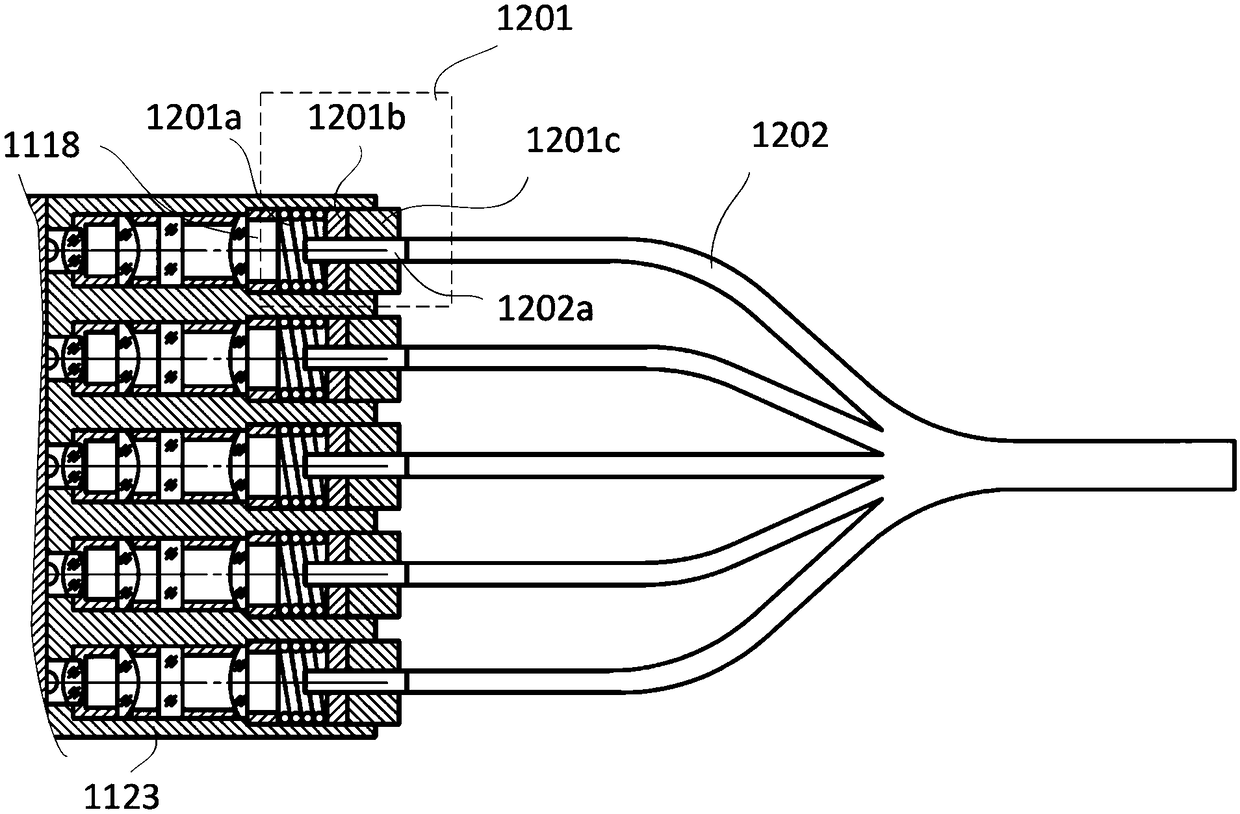
The invention discloses a coupling type blood coagulation testing system and method. The system comprises a light source device, a coupling device used for collecting light to be tested, emitted by the light source device and illuminating an objected to be measured, a measuring device used for measuring an optical signal of the object to be measured and a controller used for controlling the lightsource device and processing the optical signal of the object to be measured; the coupling device comprises an optical fiber coupling regulation component, first beam splitting optical fibers, a dodging device and second beam splitting optical fibers which are connected in sequence; the optical fiber coupling regulation component is connected with the light source device and is used for regulatinga distance between the first beam splitting optical fibers and the light source device; and the second beam splitting optical fibers are connected with the measuring device. As the distance between the first beam splitting optical fibers and the light source device is regulated by the optical fiber coupling regulation component, the optical fiber coupling efficiency is maximized; and dodging is performed on the light to be tested through the dodging device, the consistency of each testing channel is improved.
Owner:DIRUI MEDICAL TECH CO LTD
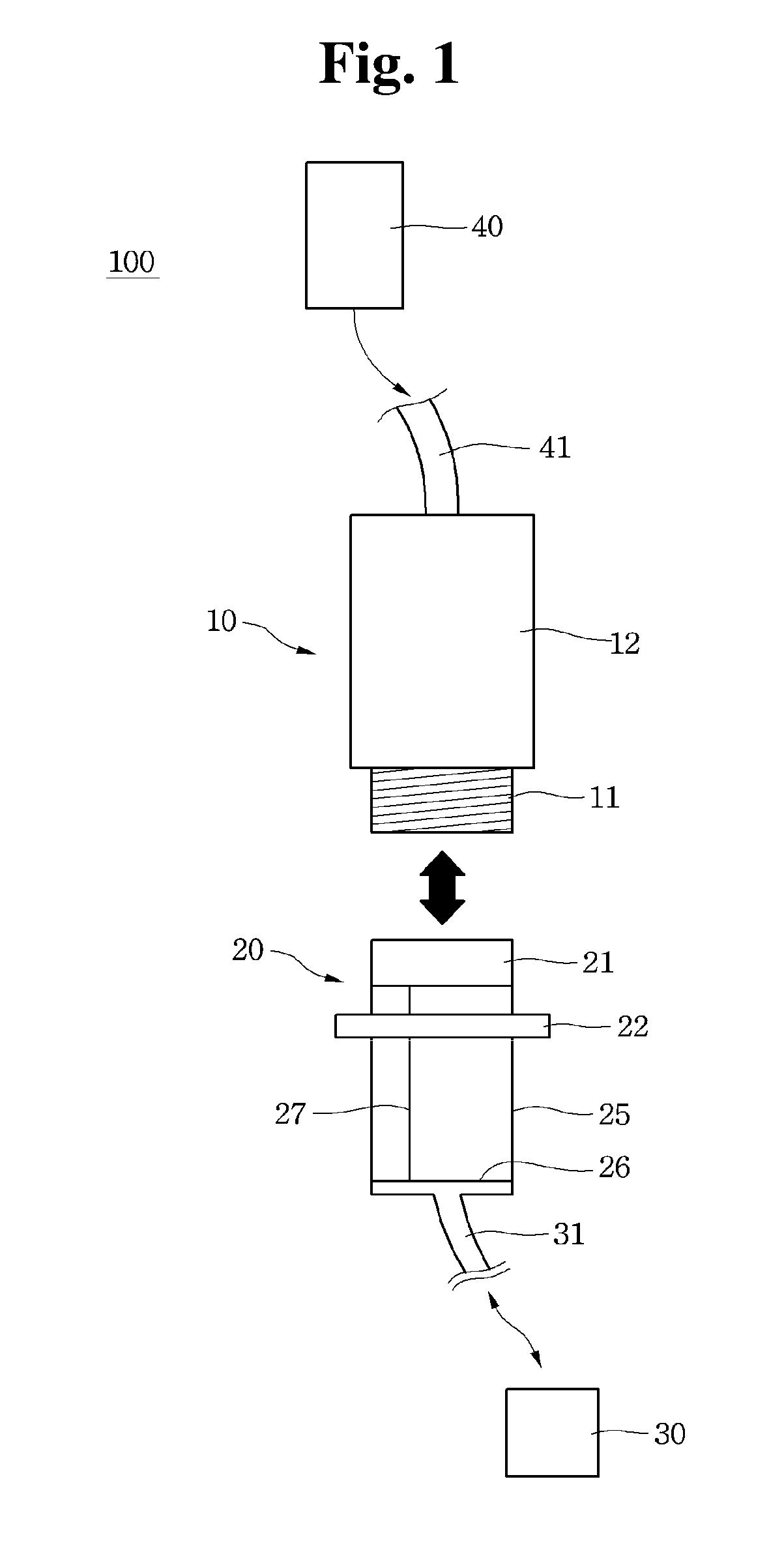
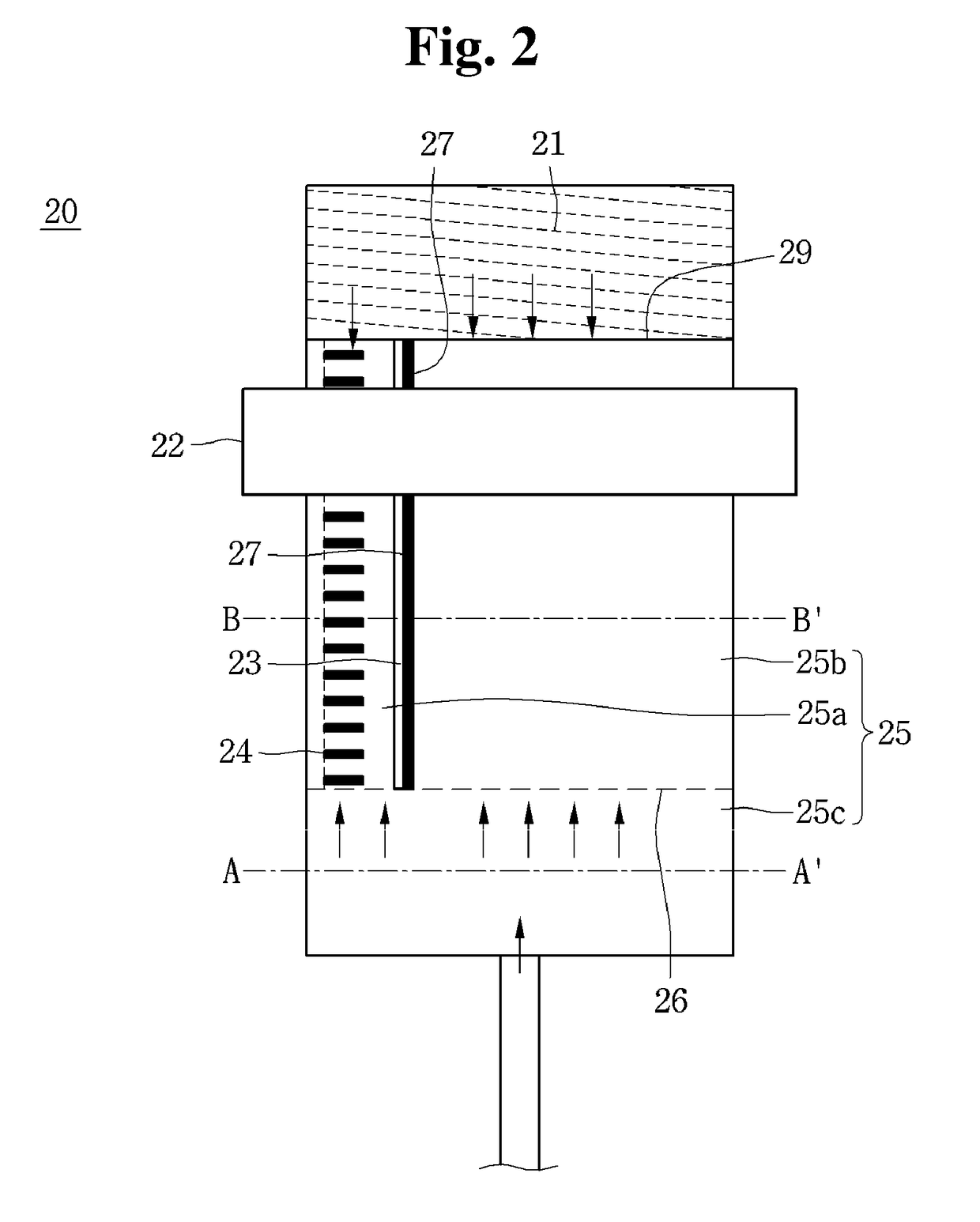
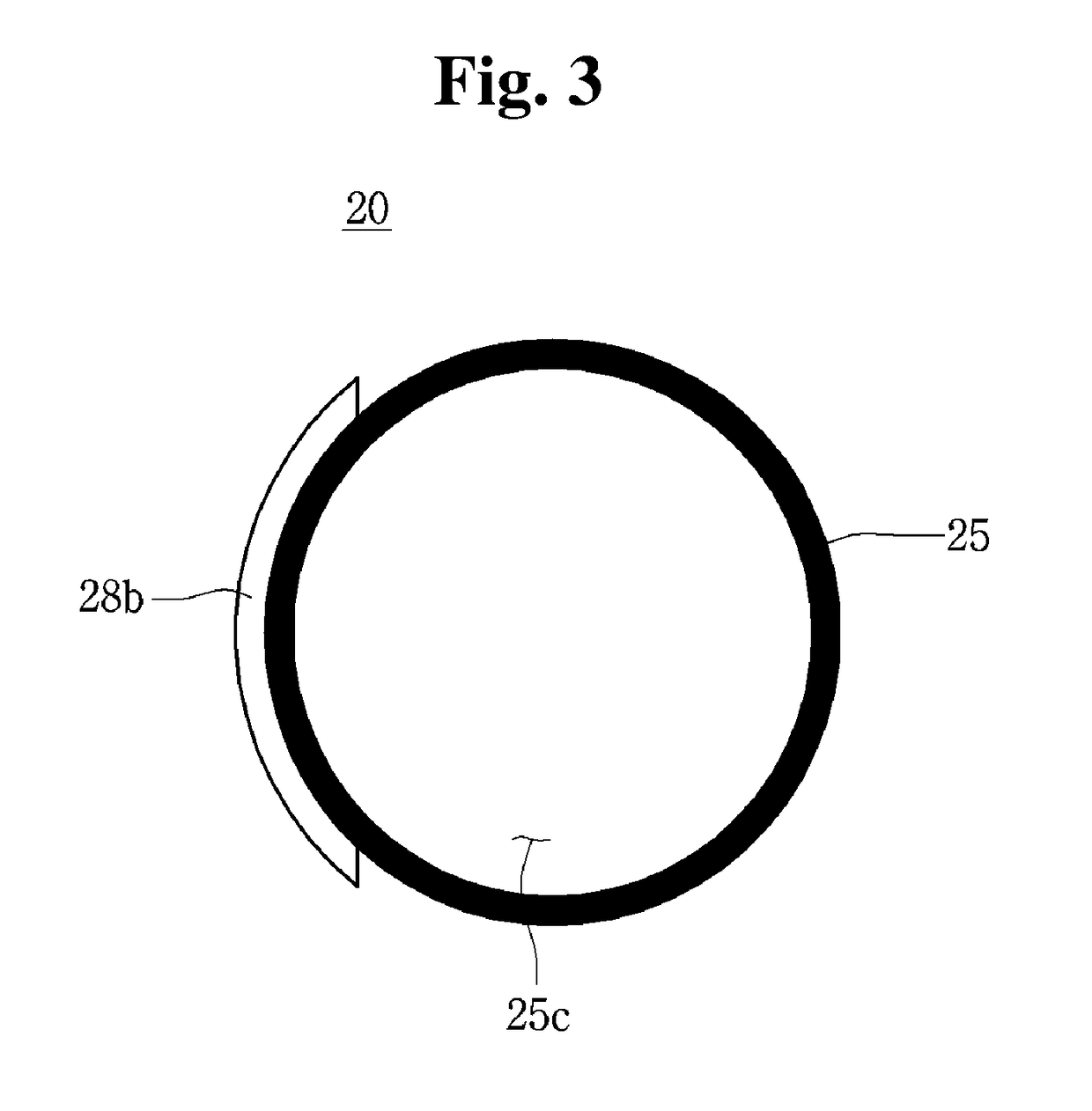
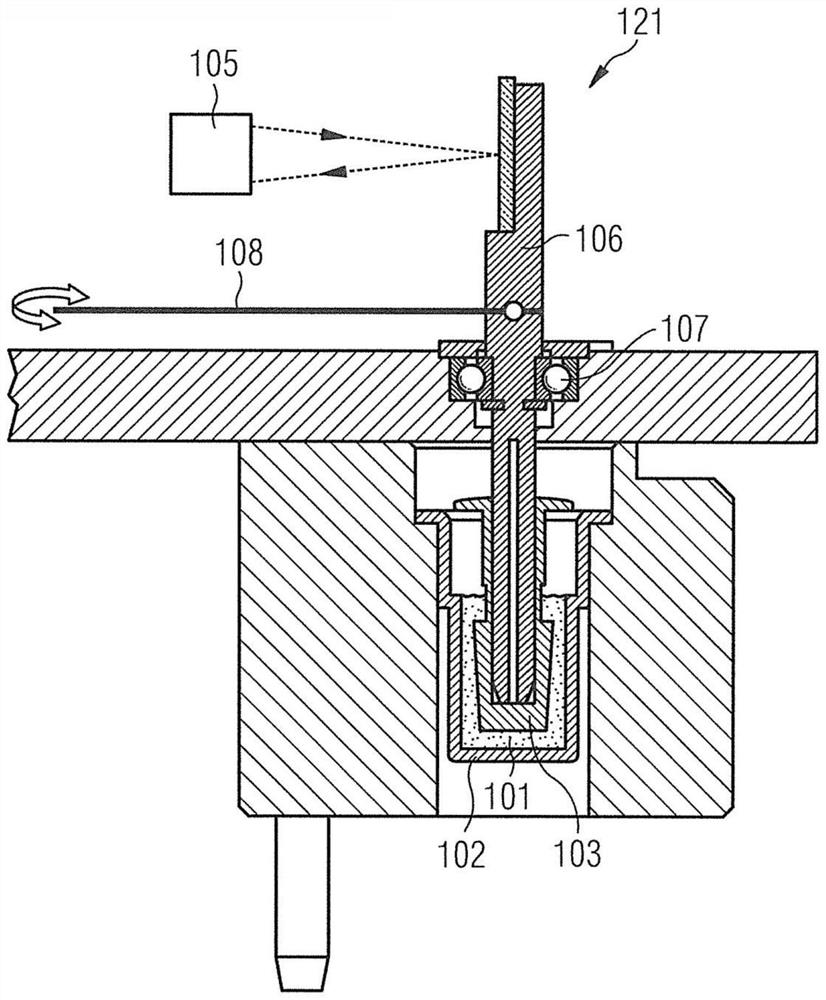
Blood coagulation testing apparatus
InactiveUS20170153222A1Administration of has been hamperedReduce riskBiological testingBlood coagulationsBlood coagulation testing
A blood coagulation testing apparatus for determining compatibility between donor's blood and patient's blood before blood transfusion. The blood coagulation testing apparatus includes: a cylindrical main body connected at one end thereof to a supply tube for delivering donor's blood and connected at the other end thereof to a blood transfer tube for introduction of patient's blood, wherein the main body is divided into two sections by a barrier film extending in a longitudinal direction of the main body such that blood is introduced into the main body through two paths, wherein the donor's blood and the patient's blood are introduced into one of the two sections through one end of the main body and the other end of the main body, respectively, and wherein the barrier film is formed on a surface thereof with a blood coagulation indicator for checking coagulation.
Owner:GWANGJU INST OF SCI & TECH
Blood coagulation reagent management method and system
PendingCN112067830ALow costImprove loading efficiencyMaterial analysisEmergency medicineBlood coagulations
Owner:SHENZHEN MINDRAY BIO MEDICAL ELECTRONICS CO LTD +1
Intelligent determining device of cement consistence and coagulation time
InactiveCN104390887AEasy to operateImprove work efficiencySurface/boundary effectMaterial testing goodsGlass sheetIndustrial engineering
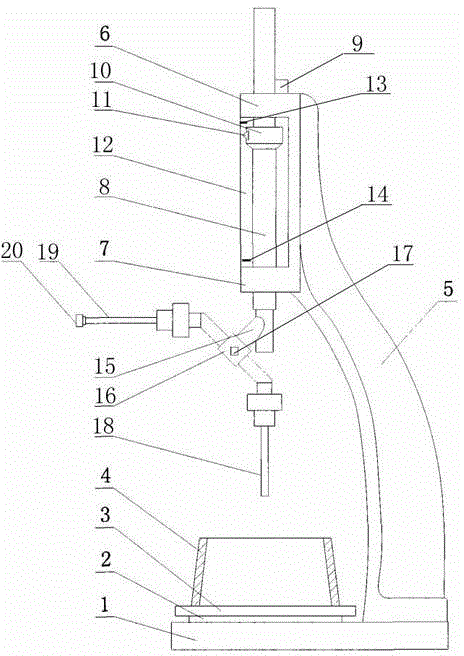
The invention discloses an intelligent determining device of cement consistence and coagulation time. The intelligent determining device comprises a base and a bracket, wherein the bracket is fixed on the base, a base plate, a glass plate and a conical mould are sequentially arranged on the base from bottom to top, an upper sliding sleeve and a lower sliding sleeve which are coaxial are arranged on the bracket vertical to the direction of the conical mould, a sliding rod sleeves between the upper sliding sleeve and the lower sliding sleeve in a penetrating way, a lifting device capable of lifting the sliding rod is arranged on the bracket, the sliding rod between the upper sliding sleeve and the lower sliding sleeve is provided with a fixed ring, a pointer is arranged on the fixed ring, a scale plate corresponding to the pointer is arranged at one side of the sliding rod between the upper sliding sleeve and the lower sliding sleeve, and an upper travel switch and a lower travel switch which correspond to the pointer are arranged at two ends of the scale plate. According to the intelligent determining device, intelligent determination of cement consistence and coagulation time is realized, when the pointer is in contact with the lower travel switch, the lifting device is used for lifting the sliding rod, when the sliding rod is lifted until the pointer is in contact with the upper travel switch, switching of an initial coagulation testing needle and a final coagulation testing needle is realized by virtue of rotation of a converter, so that tedious operation of assembling and dissembling a needle head in a testing process is prevented.
Owner:QIAOJIAN NEW ENERGY TECH SUZHOU
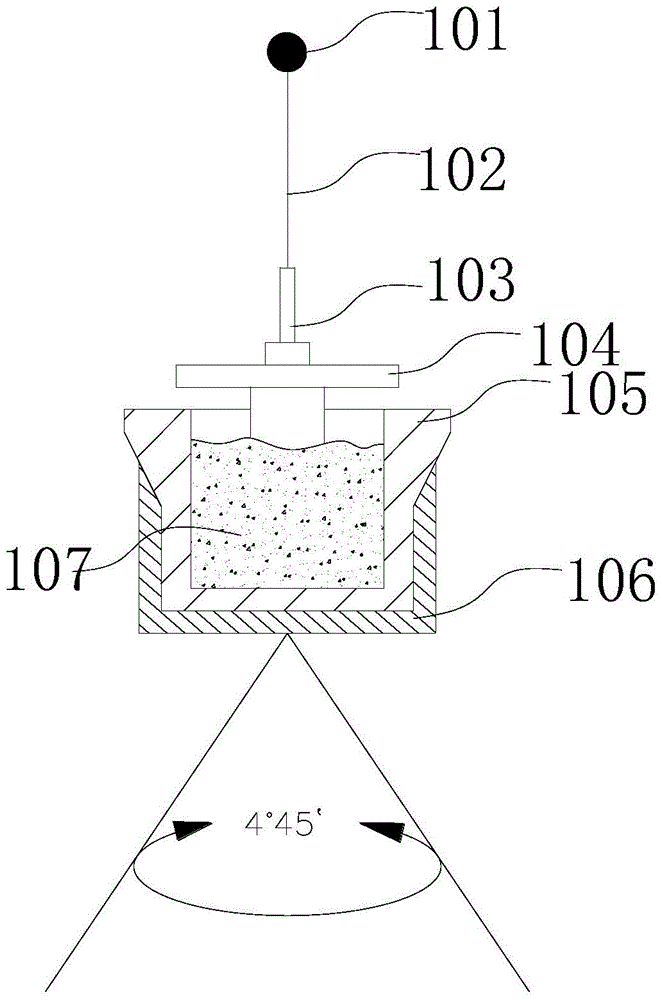
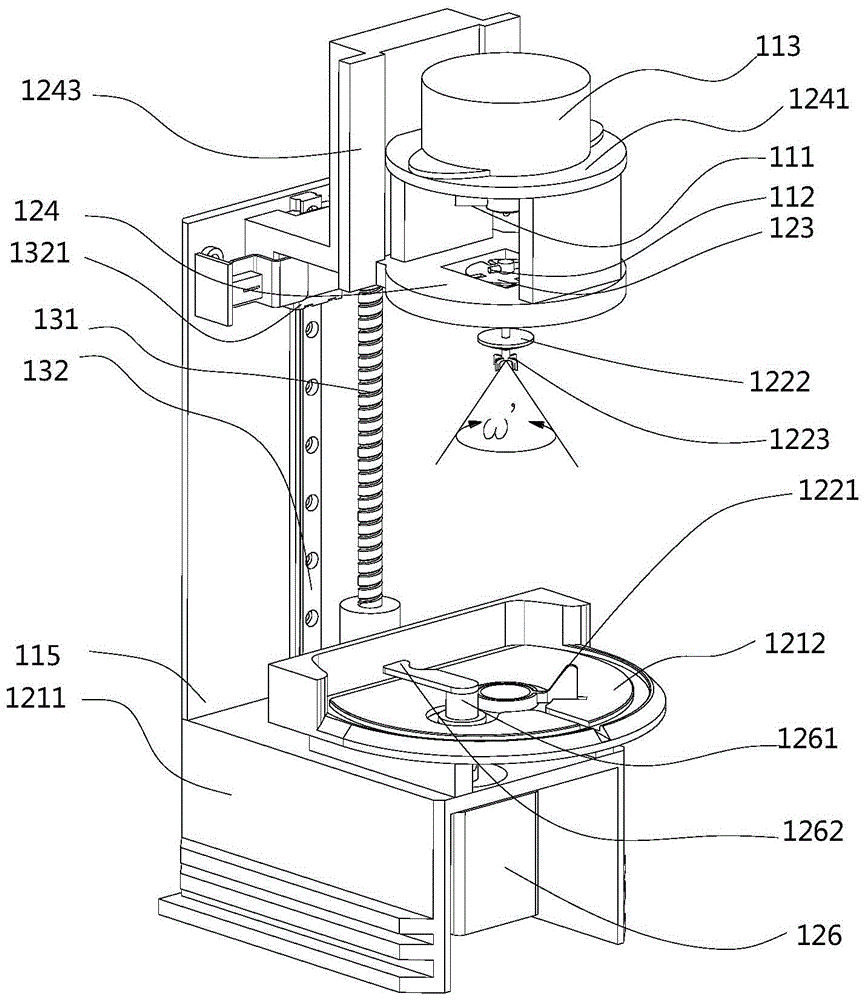
Equipment and method for measuring viscoelasticity changes in sample
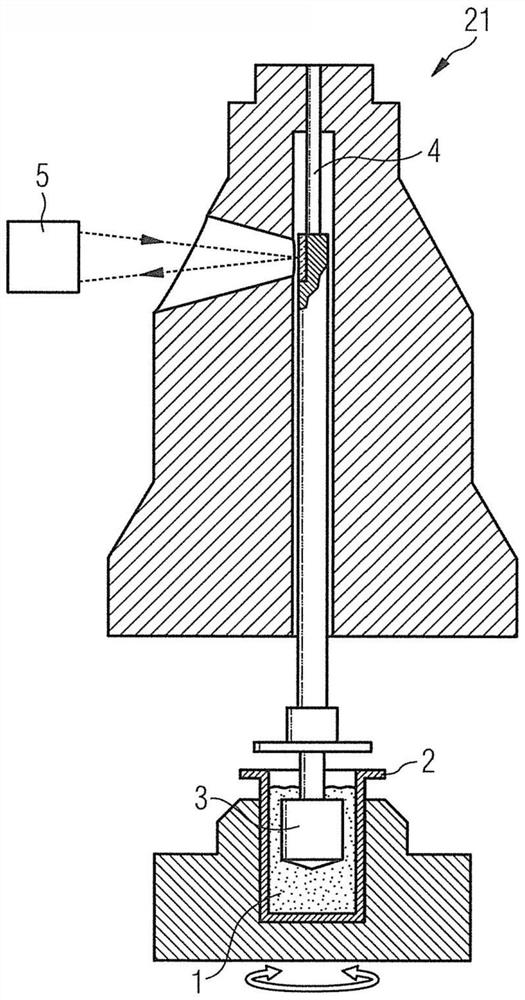
The invention provides an instrument for viscoelasticity analysis, for example, an instrument for coagulation testing of a sample liquid (such as blood and / or a component thereof). In the instrument for viscoelasticity analysis, a rotating device is disposed below a cup, a pin, and a cup accommodating element. The invention further provides a capacitance detection device and temperature control equipment. The capacitance detection device and the temperature control equipment can be used in the instrument for viscoelasticity analysis. The invention further provides a method of performing viscoelastic analysis (e.g., coagulation analysis) on the sample by using the equipment and instrument.
Owner:ENICOR GMBH
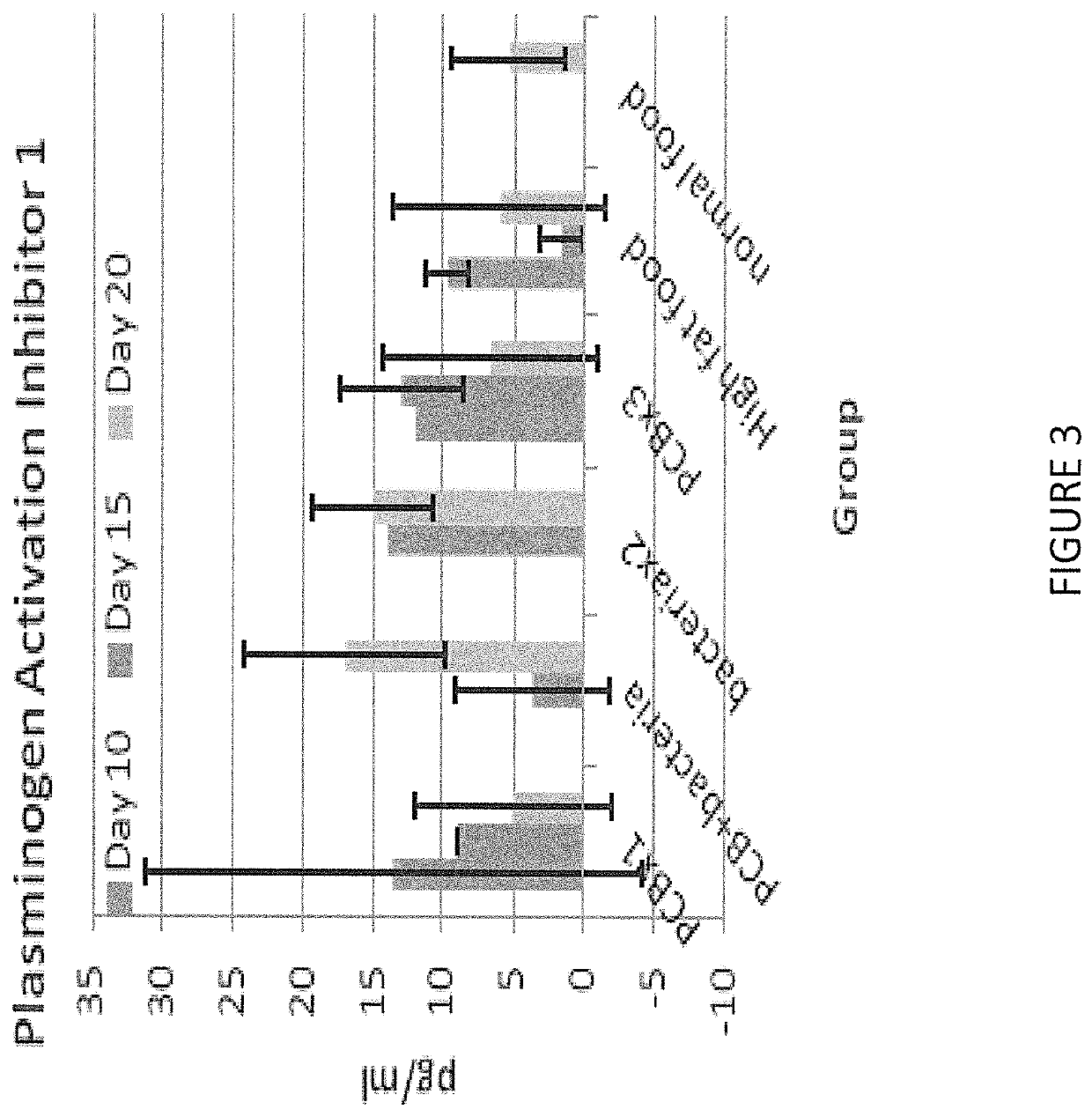
Biomarkers of vascular disease
A biomarker panel including a four-panel test for clotting that detects soluble fibrin (SF), thrombin-antithrombin complex (TAT), antithrombin III (ATIII), and plasminogen activator inhibitor (PAI-1). A biomarker panel including a three-panel test for glycocalyx integrity that detects syndecan-1 (SDC1), heparan sulfate (HS), and hyaluronidase (HAD). A biomarker panel including a test that detects a biomarker chosen from soluble fibrin (SF), thrombin-antithrombin complex (TAT), antithrombin III (ATIII), plasminogen activator inhibitor (PAI-1), syndecan-1 (SDC1), heparan sulfate (HS), hyaluronidase (HAD), and combinations thereof. A kit including a biomarker panel, instructions for use, materials to take and apply samples to the panel, and descriptions of biomarker levels and their meaning. Methods of detecting the presence of vascular disease, determining the stage of vascular disease, monitoring the progress of vascular disease treatments, and monitoring the efficacy of drugs during drug development.
Owner:ARTEREZ INC
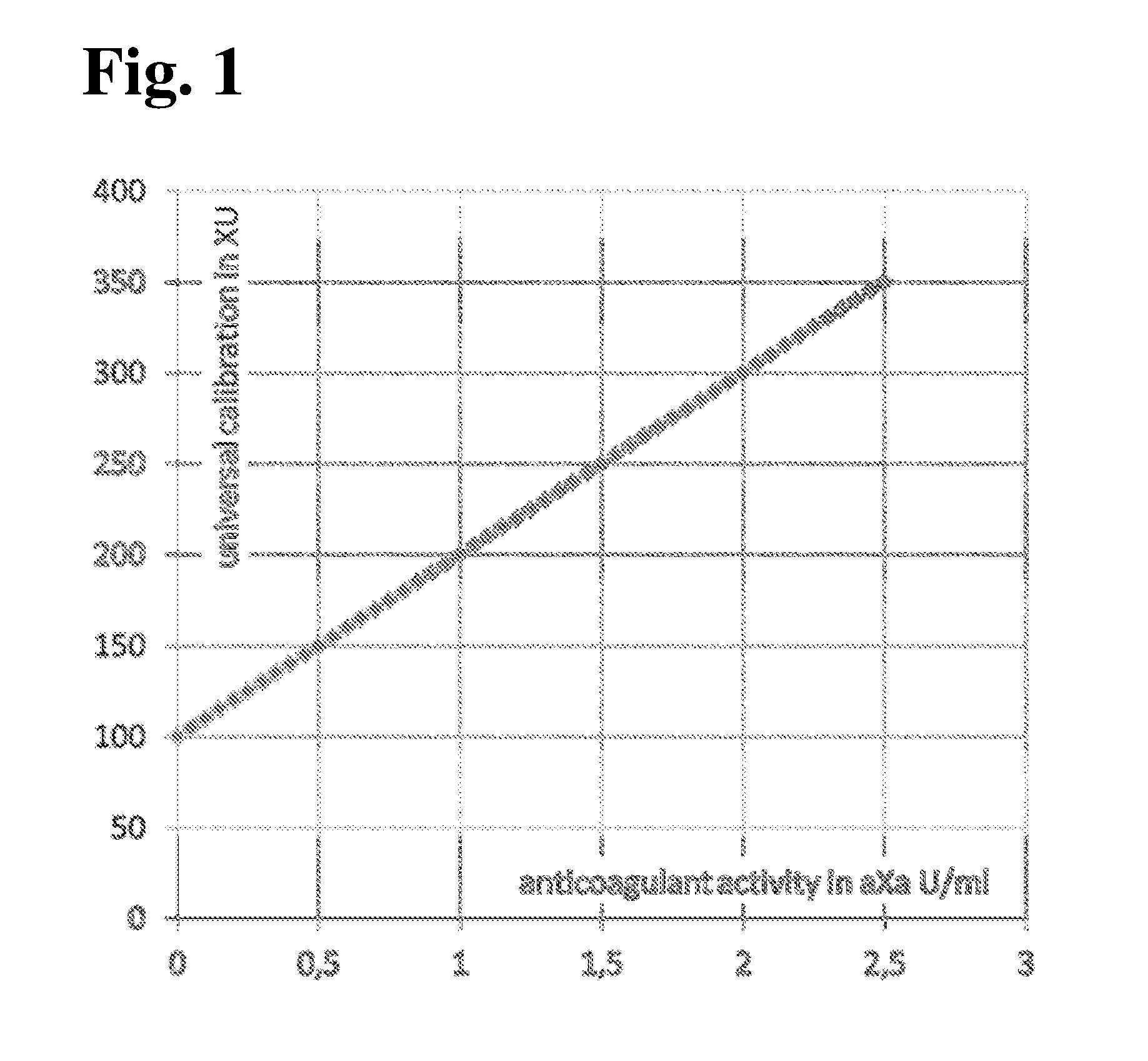
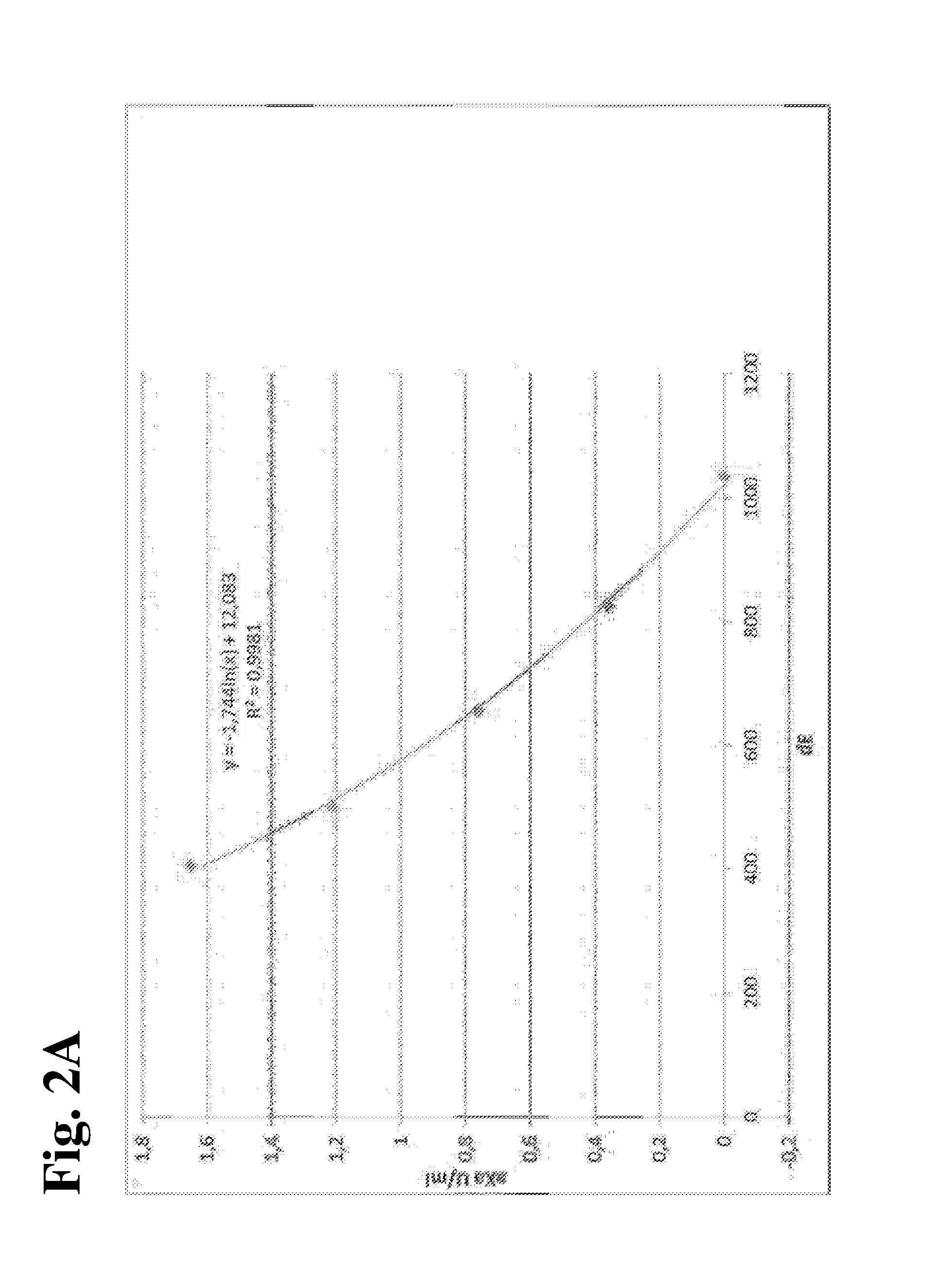
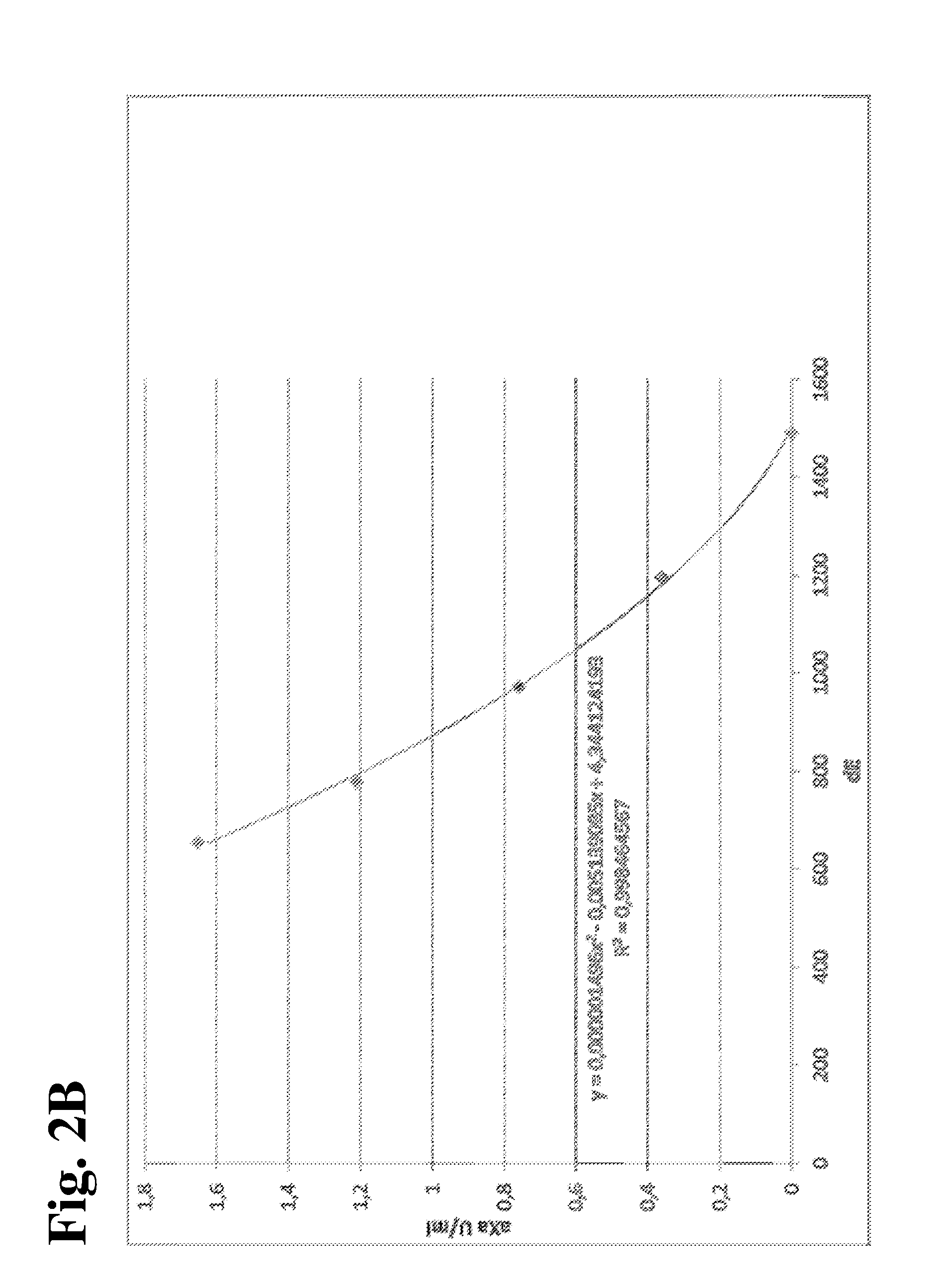
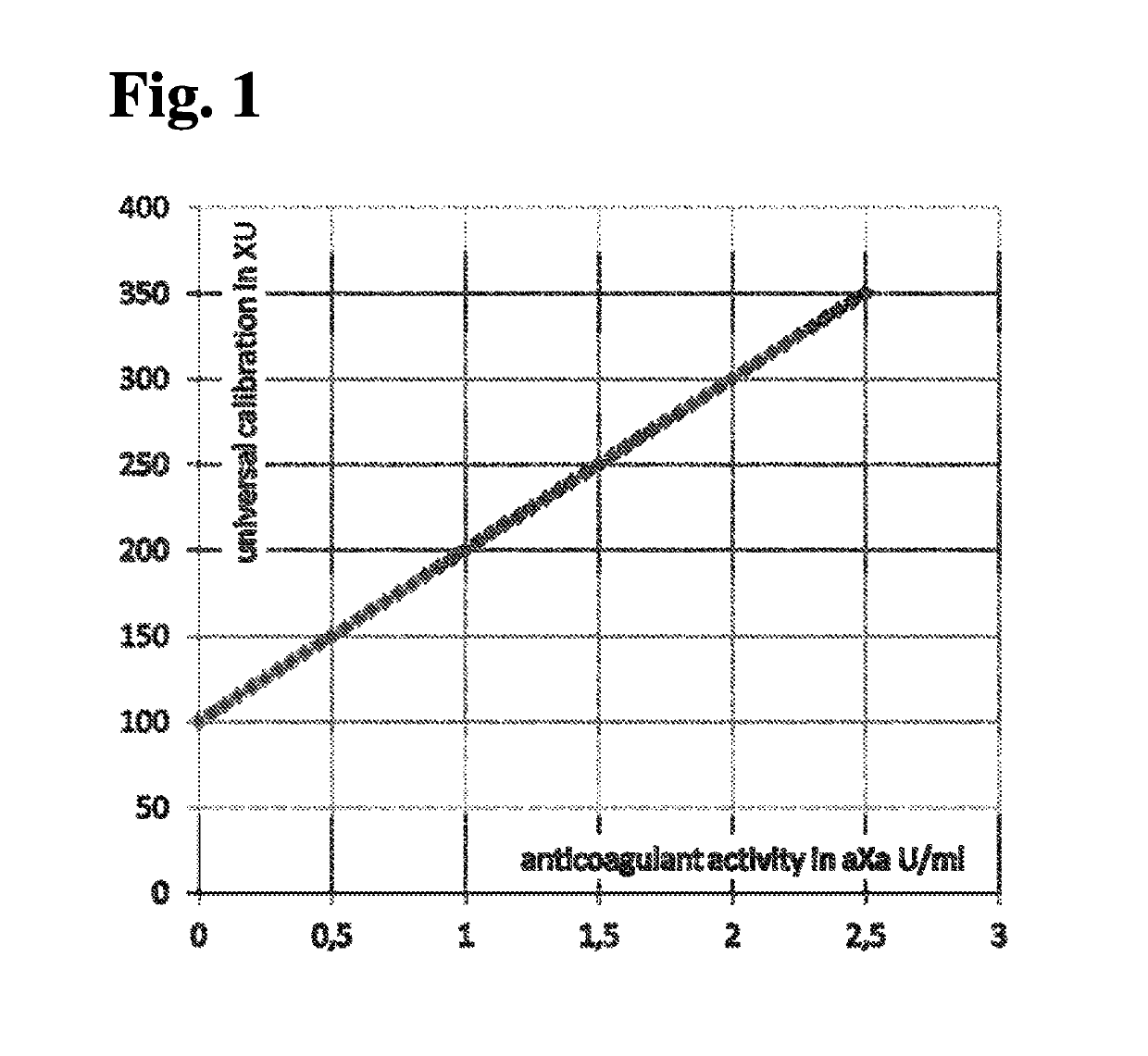
MEANS AND METHODS FOR UNIVERSAL CALIBRATION OF ANTI-FACTOR Xa TESTS
The present invention concerns diagnostic methods for coagulation testing involving determining anticoagulant activity elicited by a first anticoagulant in a sample comprising measuring a first Factor Xa activity in a body fluid test sample of said subject, measuring a second Factor Xa activity in at least one calibrator sample comprising a predefined anticoagulation activity for a second anticoagulant, calculating an universal parameter for the anticoagulation activity comprised in the test sample based on the first and the second measured Factor Xa activities and comparing the said parameter for the anticoagulation activity with predefined ranges of expected anticoagulation activity for at least three anticoagulants. Further provided is a computer program code assisting the method as well as a system for carrying out the said method as well as a kit.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Blood testing system and method
InactiveCN108495682AMinimize interactionEfficient use ofFlow propertiesLaboratory glasswaresBlood testThrombus
Some embodiments of a blood coagulation testing system include an analyzer console device and a single-use cartridge component configured to releasably install into the console device, in some embodiments, the blood coagulation testing system can operate as an automated thrornboelastometry system that is particularly useful, for example, at a point-of-care site.
Owner:CA卡希索有限公司
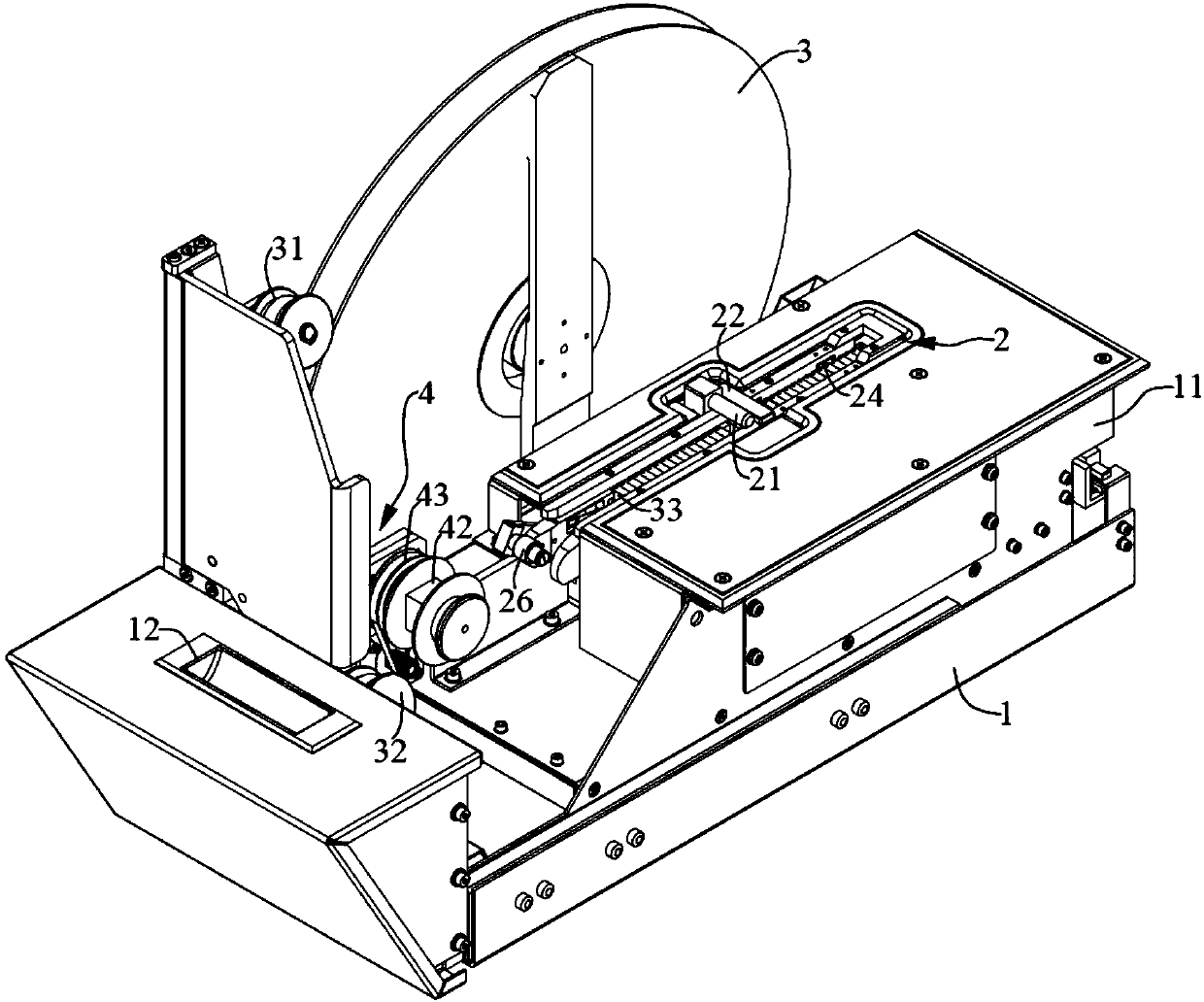
Full-automatic blood coagulation tester
PendingCN111323609ACompact structureThe detection process is fastMaterial analysisTester deviceBlood coagulations
The invention relates to the technical field of blood coagulation detection, and discloses a full-automatic blood coagulation tester. The full-automatic blood coagulation tester comprises a test platform, a sample adding arm unit and a test cup conveying unit, wherein the test platform includes a bath temperature position used for loading a test cup and heating, a first reagent position used for storing an R1 reagent, a sample position used for storing a sample, a test position used for a cup conveying test and a second reagent position used for storing an R2 reagent; the sample adding arm unit is used for adding the reagent R1 and the sample into the test cup at the bath temperature position and adding the reagent R2 into the test cup at the test position; and the test cup conveying unitis used for conveying the test cup at the bath temperature position to the test position and discarding the tested test cup to a specified position. The full-automatic blood coagulation tester provided by the invention is compact in structure, excellent in performance and high in reliability.
Owner:北京众驰伟业科技发展有限公司
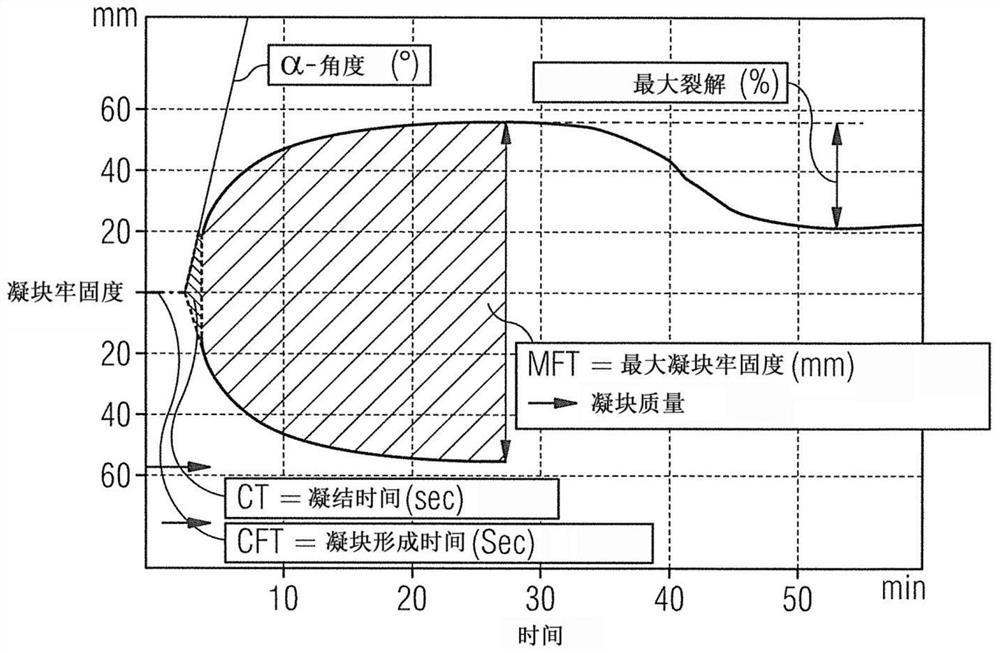
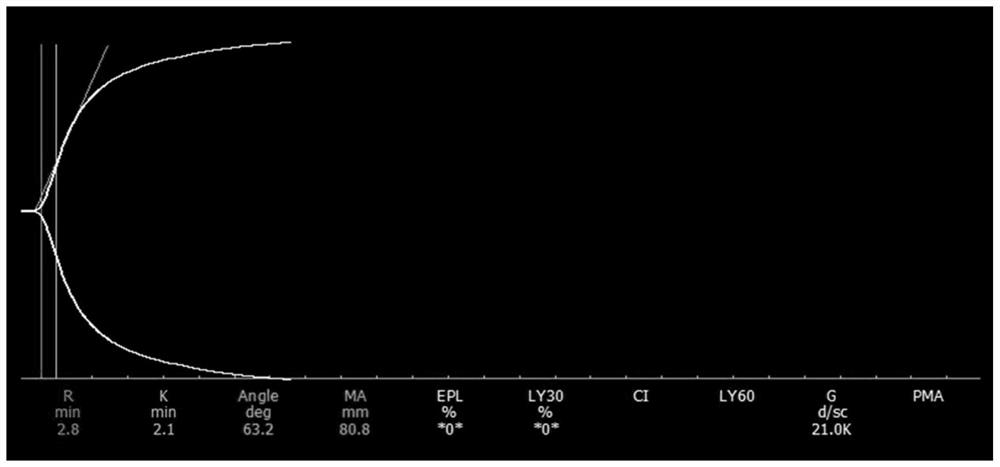
Physical quality control product as well as preparation method and application thereof
PendingCN114544296ASimple preparation processLow costPreparing sample for investigationBiological testingThrombusQuality control
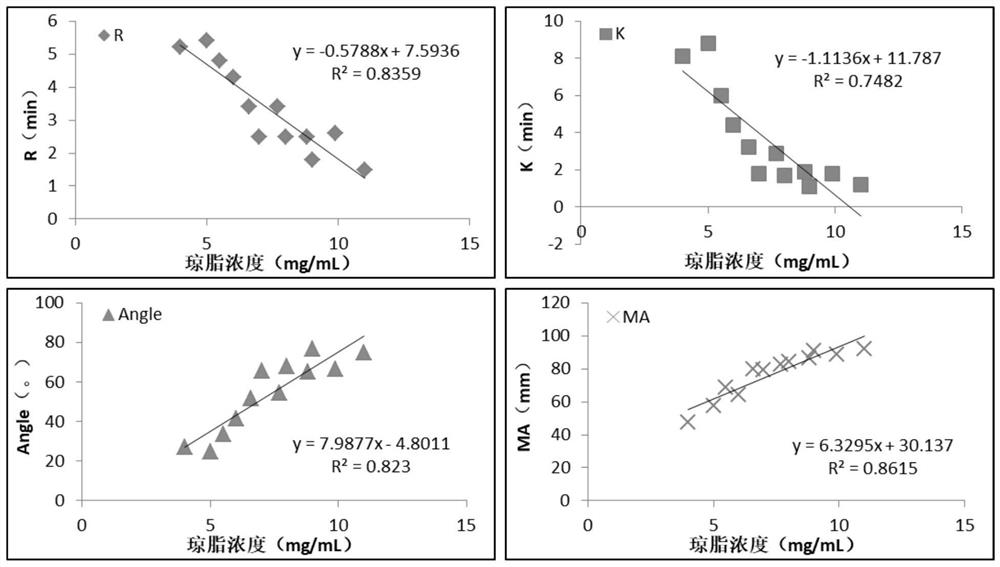
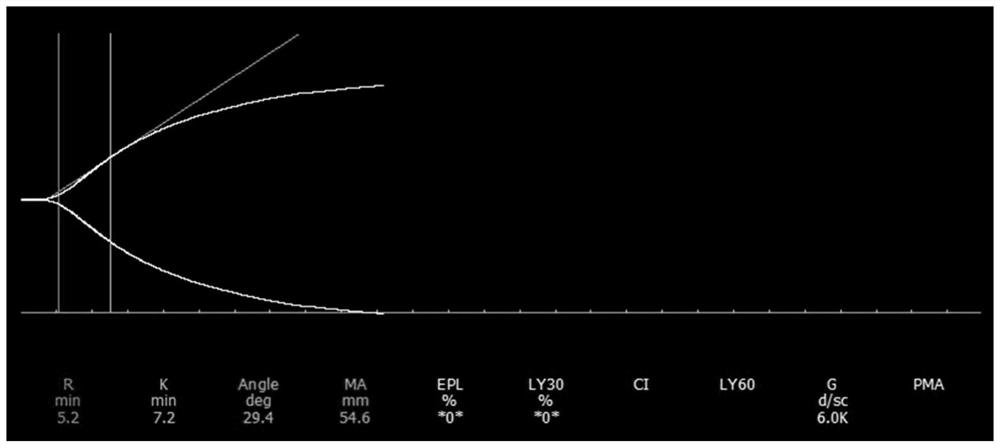
The invention relates to the technical field of clinical blood coagulation testing, and discloses a physical quality control product as well as a preparation method and application thereof. Physical quality control products in the scheme comprise agar and water, the agar and the water are separately stored, and the quality control products are divided into a quality control product I, a quality control product II and a quality control product III according to agar solutions with different concentrations. When in use, agar and water are uniformly mixed and then heated and melted to prepare a colorless, transparent and uniformly dispersed quality control product in a solution state; the quality control product gradually forms a clot along with the cooling of the solution, the clot is described by a thrombelastogram instrument to obtain various parameters (R, K, Angle, MA and the like), and the quality of the thrombelastogram instrument is accurately evaluated by comparing the assignment range of the quality control product. The quality control product has the advantages that the raw materials are convenient to store and transport, the cost is low, the stability is good, the manufacturing process is simple, the detection result is stable and the like, different working states of the thrombelastogram can be accurately evaluated through agar solutions with different concentrations, and the quality control product is very suitable for evaluating and detecting the thrombelastogram in clinic.
Owner:重庆康巨全弘生物科技有限公司
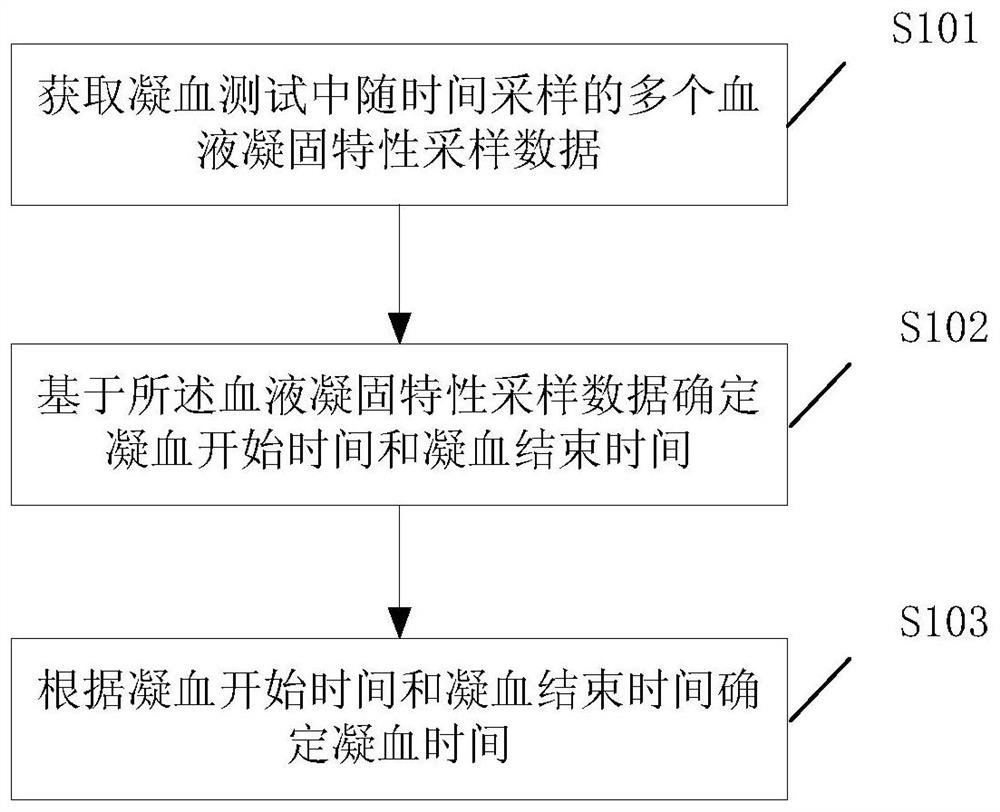
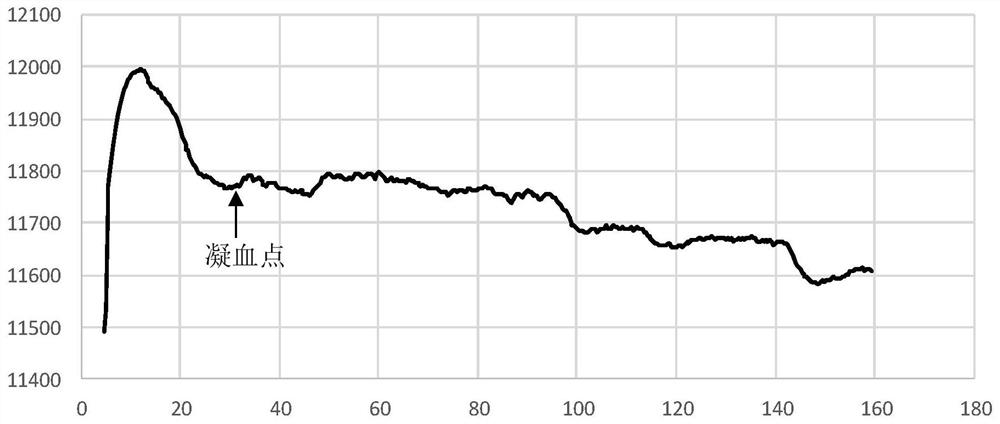
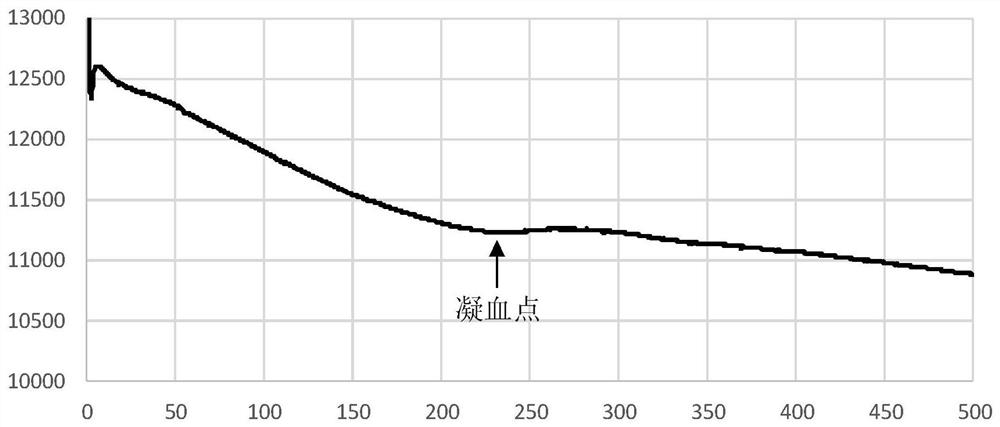
Clotting time determination method, electronic equipment and storage medium
ActiveCN108761105BImprove judgment efficiencyBiological testingHematological testIntensive care medicine
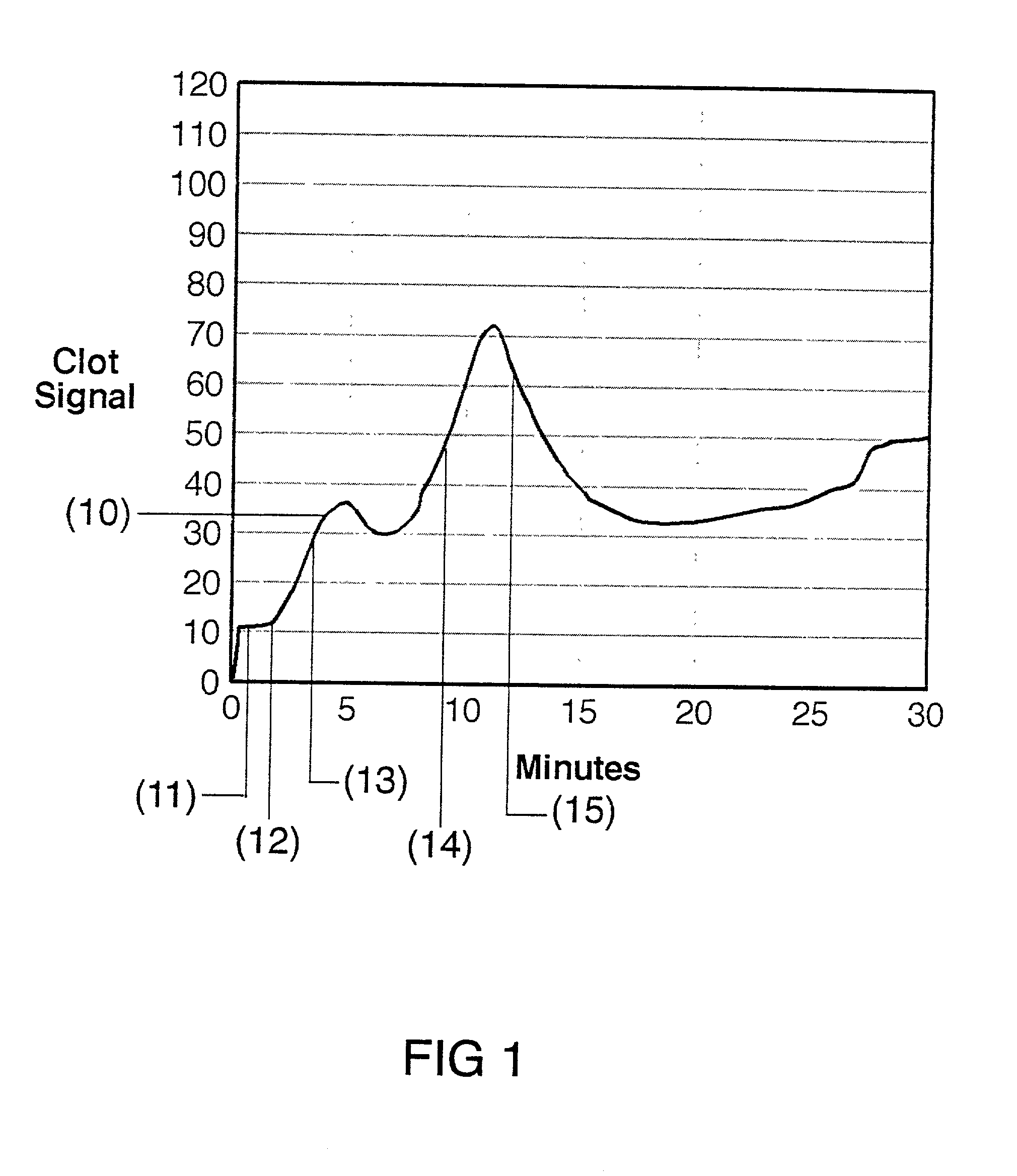
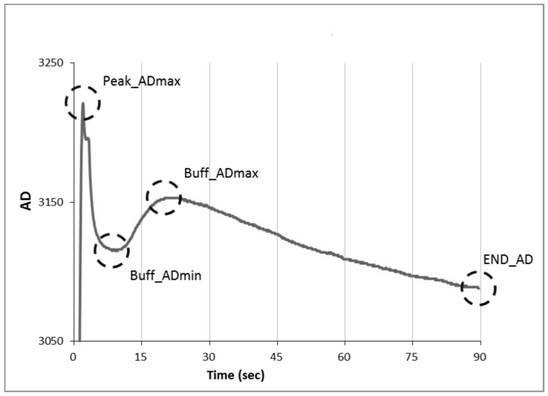
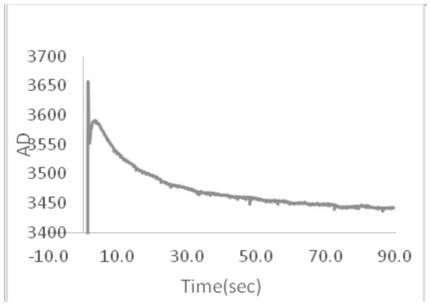
A method for determining blood coagulation time, an electronic apparatus, and a storage medium. The method comprises: acquiring multiple pieces of blood coagulation feature sampling data sampled over time during a blood coagulation test; determining a blood coagulation starting time and a blood coagulation ending time on the basis of the blood coagulation feature sampling data; and determining a blood coagulation time according to the blood coagulation starting time and the blood coagulation ending time. The method determines blood coagulation time by means of data analysis, thereby improving the efficiency of determining a blood coagulation time.
Owner:GUANGZHOU WONDFO BIOTECH
Piezoelectric thin film resonator, its manufacturing method and method for detecting coagulation time
ActiveCN106788317BSimple structureEasy to manufactureImpedence networksMaterial analysis by electric/magnetic meansUltraviolet lightsBlood coagulations
The invention discloses a piezoelectric film resonator used for coagulation detection, which comprises a piezoelectric film layer and an annular groove. The invention also discloses a manufacturing method of a piezoelectric thin film resonator for blood coagulation detection, which includes coating the inner surface of the annular groove with a hydrophilic polymer material; performing oxygen plasma on the inner surface of the annular groove treatment or ultraviolet light irradiation; placing the piezoelectric thin film resonator in sodium carbonate solution, and then adding calcium chloride solution in the solution; baking the piezoelectric thin film resonator until dry. The invention also discloses a method for detecting coagulation time by using a piezoelectric thin film resonator. Calculate the second derivative of the resonance frequency with respect to the time change of the stabilized resonance frequency, and take the maximum value of the second derivative as the coagulation time. Using the piezoelectric thin film resonator to detect blood coagulation is suitable for home use.
Owner:SHANDONG UNIV OF SCI & TECH
Methods for universal determination of anticoagulant activity
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Binding Molecule Activating FXII
PendingUS20200182890A1Immunoglobulins against blood coagulation factorsBiological material analysisAntiendomysial antibodiesBlood coagulations
The present invention relates to a binding molecule, in particular an antibody or binding fragment thereof, capable of activating FXII, which binds to the proline rich domain of FXII. In particular, the invention is directed to FXII activating antibodies or binding fragments thereof which binds to the proline rich domain of FXII. The invention also encompasses the use of the binding molecule directed to the proline rich domain of FXII as blood coagulation activator, e.g. in diagnostic blood coagulation tests. Corresponding methods and blood coagulation test are also encompassed.
Owner:UNIVERSITAETSKLINIKUM HAMBURG EPPENDORF
Thrombus coagulation testing device and testing method
InactiveCN113777289AMaintain fluidityControl flow rateBiological testingPeristaltic pumpBlood coagulation analyzer
The invention relates to a thrombus coagulation testing device and testing method, and belongs to the technical field of thrombus testing equipment. According to the influence of material surface characteristics on thrombus coagulation, the testing device for testing thrombus coagulation caused by different material surface structures is provided, modular design is adopted, blood is pumped into a pipeline through a constant-temperature peristaltic pump box aiming at the blood compatibility problem of a medical in-vivo material, the blood enters the blood coagulation analyzer after flowing through a material sleeve, and the blood coagulation routine in the blood is tested through the tester. The testing device can be used for performing thrombus coagulation testing on two materials in a double-pipeline mode, so that testers can conveniently compare the advantages and disadvantages of the two materials. The invention provides a more efficient and accurate method for thrombus coagulation test of the surface structure of the in-vivo material.
Owner:长春中医药大学附属第三临床医院
Method for performing activated clotting time test with reduced sensitivity to the presence of aprotinin and for assessing aprotinin sensitivity
InactiveUS20020127730A1Prolongs ACT resultImprove anticoagulant safetyMicrobiological testing/measurementBiological material analysisActivated Coagulation TimeSodium Bentonite
A coagulation test for determining the activated clotting time (ACT) of blood in the presence of heparin that produces test results that are substantially insensitive to the drug aprotinin. The activator is formulated to be a combination of celite and bentonite. The ACT results obtained with this formulation are similar to celite ACT tests on heparinized blood while simultaneously being unaffected by aprotinin. Additionally, a method for quantifying the aprotinin effect of different ACT formulations is disclosed.
Owner:SIENCO
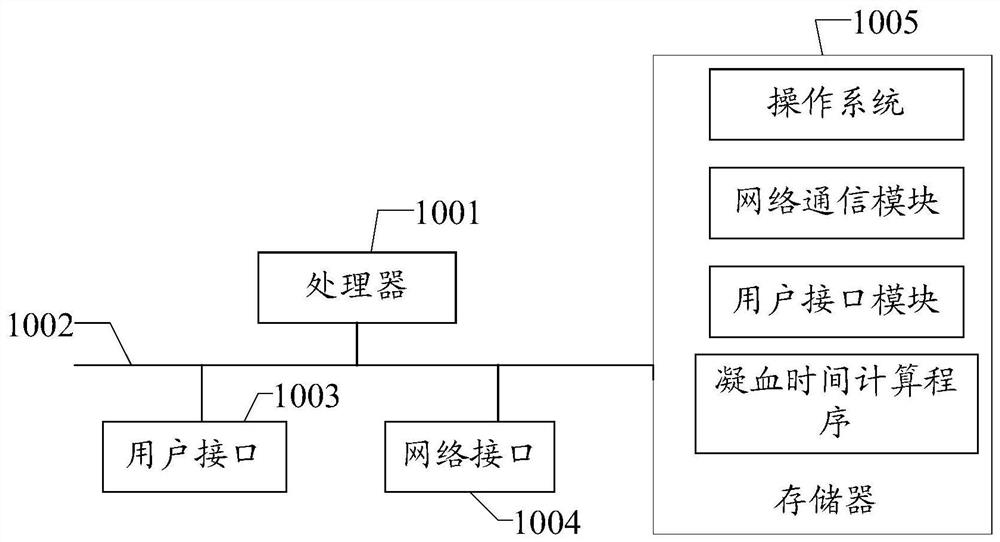
A blood coagulation detection instrument
Owner:GUANGZHOU IMPROVE MEDICAL TECH CO LTD
Coagulation testing for low sample volumes
Some components and methods for the determination of coagulation parameters using trace amounts of blood are provided. Advantageously, the methods described herein can be assayed in a single drop of blood (approximately 20 [mu]L), while generally reserving a sufficient amount of sample for performing other assays, optionally in a multiplex assay format. The method and device do not require an experienced operator and can be performed in a designated service location, which is very important for the management and treatment of coagulation abnormalities.
Owner:THERANOS IP CO LLC
Blood coagulation time calculation method, device and system and readable storage medium
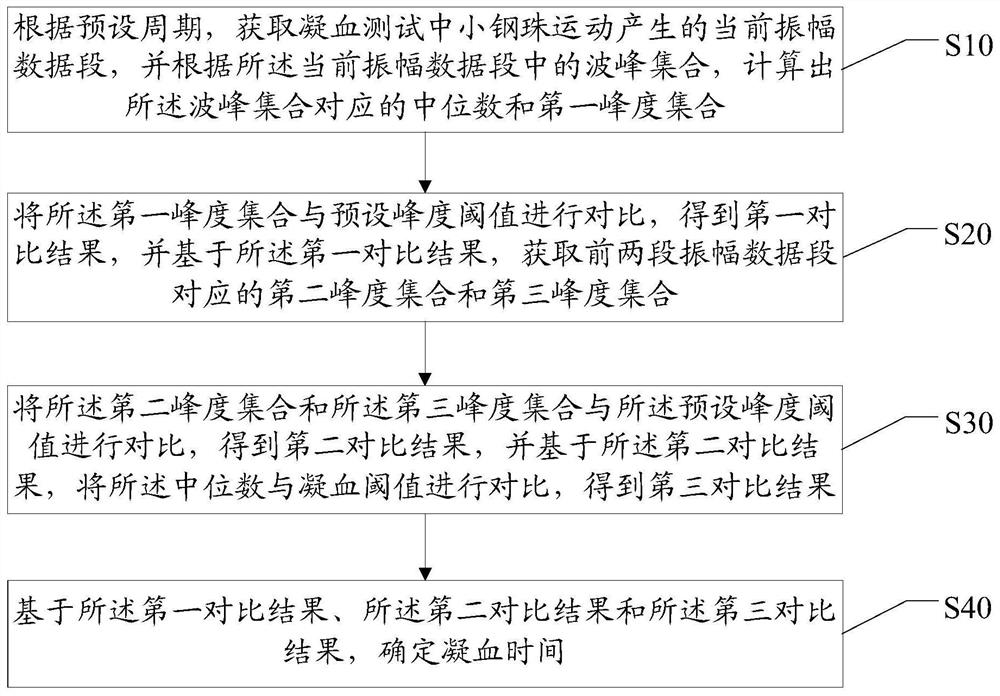
ActiveCN113962252AImprove accuracyCharacter and pattern recognitionBiological testingBlood coagulationsMechanics
The invention discloses a blood coagulation time calculation method, device and system and a readable storage medium. The method comprises the following steps: obtaining a current amplitude data segment generated by the movement of a small steel ball in a blood coagulation test according to a preset period, and calculating a median and a first kurtosis set corresponding to a wave crest set according to the wave crest set in the current amplitude data segment; comparing the first kurtosis set with a preset kurtosis threshold to obtain a first comparison result, and based on the first comparison result, obtaining a second kurtosis set and a third kurtosis set corresponding to the first two amplitude data segments; comparing the second kurtosis set and the third kurtosis set with a preset kurtosis threshold to obtain a second comparison result, and comparing the median with a blood coagulation threshold based on the second comparison result to obtain a third comparison result; based on the first comparison result, the second comparison result and the third comparison result, blood coagulation time is determined; the blood coagulation time is determined according to the three continuous amplitude data segments, and the accuracy of determining the blood coagulation time is improved.
Owner:SHENZHEN GOLDSITE DIAGNOSTICS
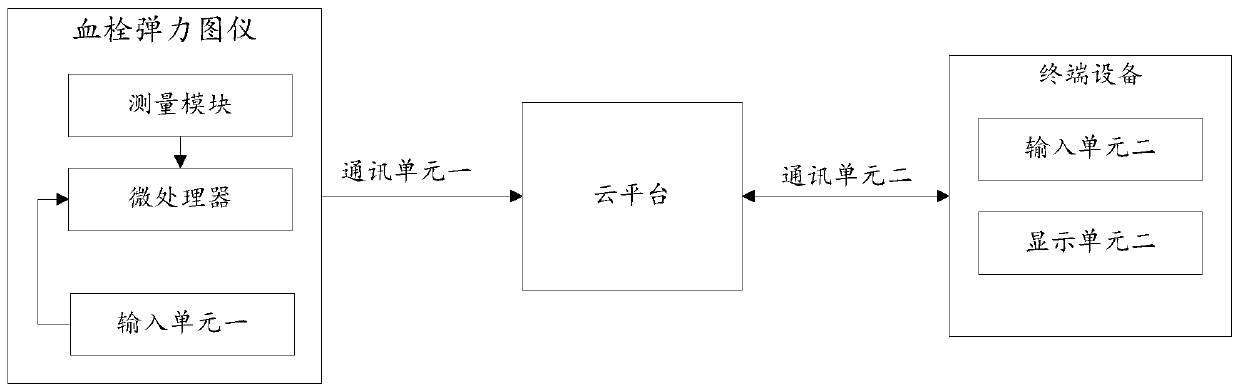
Remote monitoring system for thrombus and method thereof
InactiveCN110189820AAchieve sharingRich data supportTransmissionMedical equipmentThrombusMonitoring system
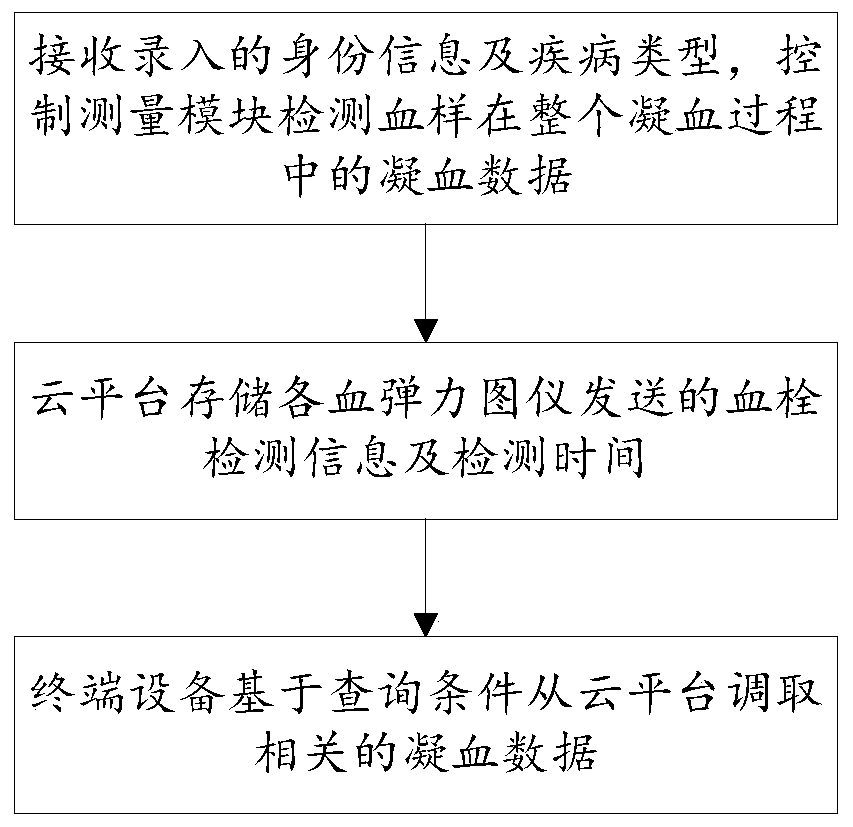
The invention is suitable for the field of thrombus detecting technology, and provides a remote monitoring system for thrombus and a method thereof. The method comprises the following steps of receiving recorded identity information and disease type, controlling a measuring module detect coagulation data of a blood sample in a whole coagulation process; storing thrombus detecting information and detecting time by a cloud platform, wherein the thrombus detecting information and the detecting time are transmitted from each thromboelastography instrument; and calling related coagulation data by terminal equipment based on a searching condition from a cloud platform. The remote monitoring system realizes big data sharing based on the cloud platform. Medical personnel can call the current coagulation testing data, historical coagulation testing data of the patient and the coagulation data of other patient with the same disease type, thereby supplying more abundant data support for clinicalpredicting and realizing high accuracy in clinical predicting.
Owner:安徽协同创新设计研究院有限公司
A method and device for blood coagulation detection
ActiveCN110954687BEnhanced coagulation eigenvalue signalBiological testingMaterial impedanceEngineeringErythrocyte volume
The purpose of this application is to provide a method and device for blood coagulation detection. Compared with the prior art, the present application loads at least two combinations of voltage frequency and amplitude on the blood coagulation detection card during the blood coagulation detection process, and according to the combination of the voltage frequency and amplitude, the blood coagulation detection card Determine the coagulation characteristic value of the blood sample in the blood coagulation test card according to the impedance change of the blood sample, and then determine the coagulation detection index corresponding to the coagulation characteristic value according to the coagulation characteristic value. The present application uses variable voltage frequency and amplitude to drive the detection of the coagulation process, which can enhance the coagulation characteristic value signal and significantly increase the application range of the patient's blood sample HCT (hematocrit) level, thereby serving more patients.
Owner:MICROPOINT BIOTECHNOLOGIES CO LTD
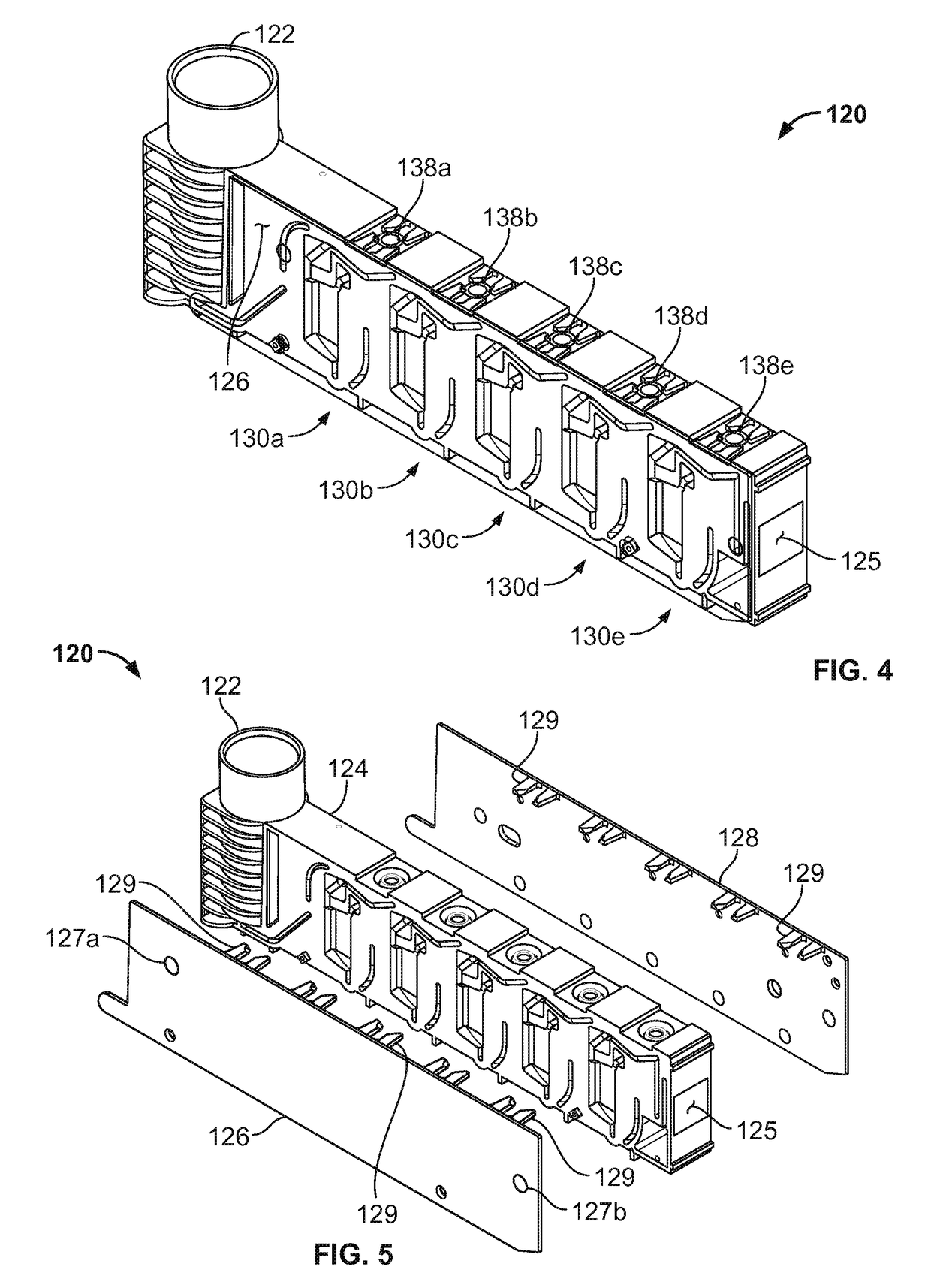
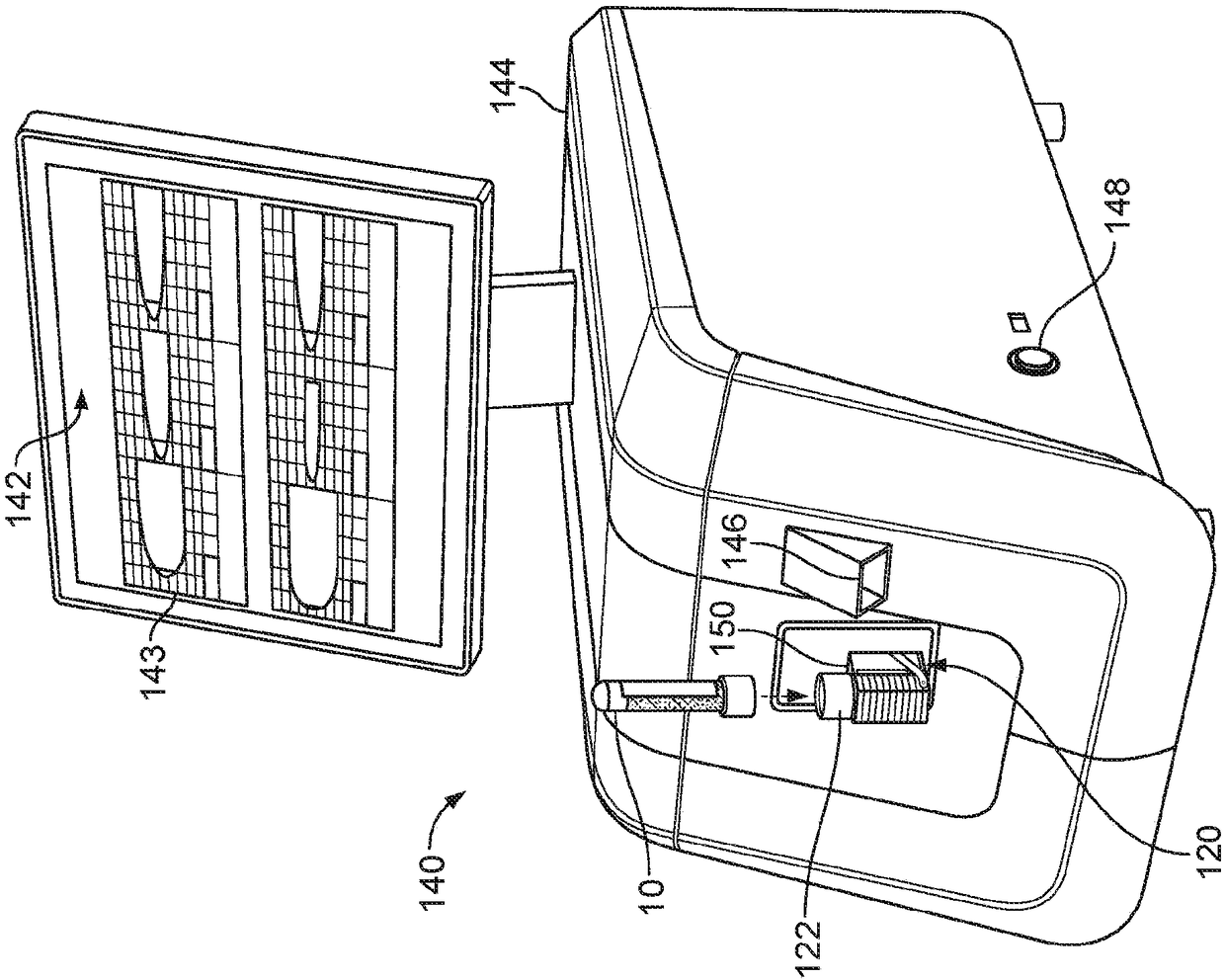
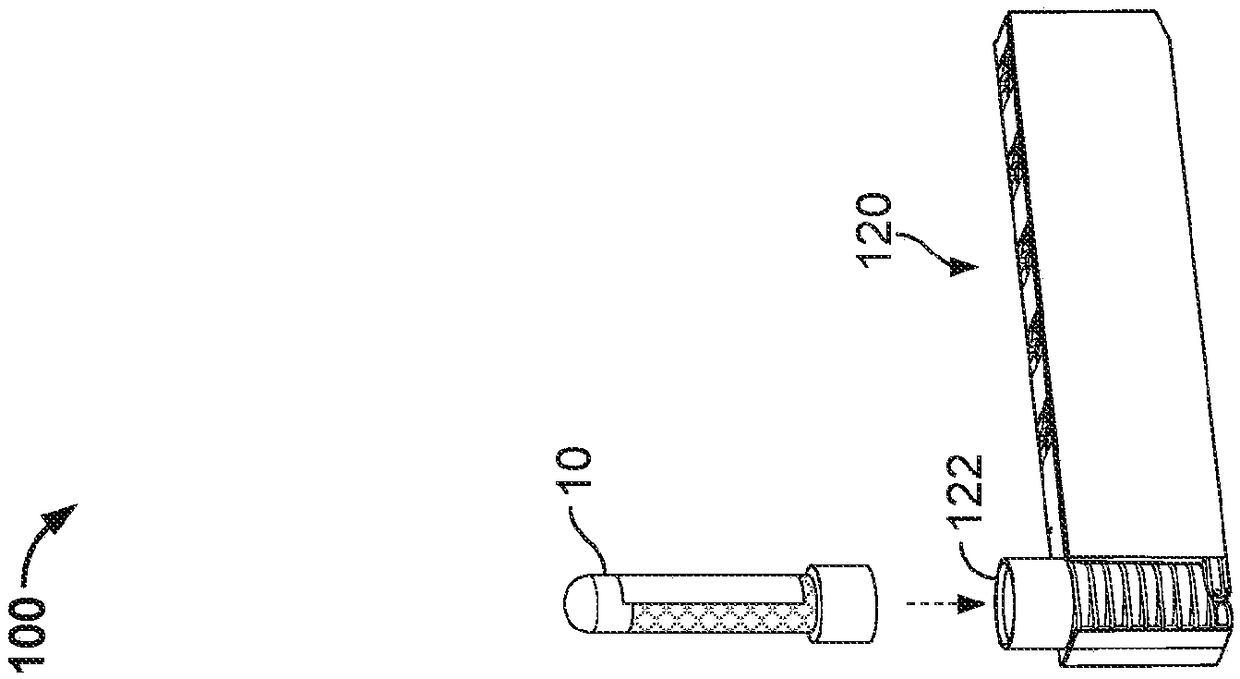
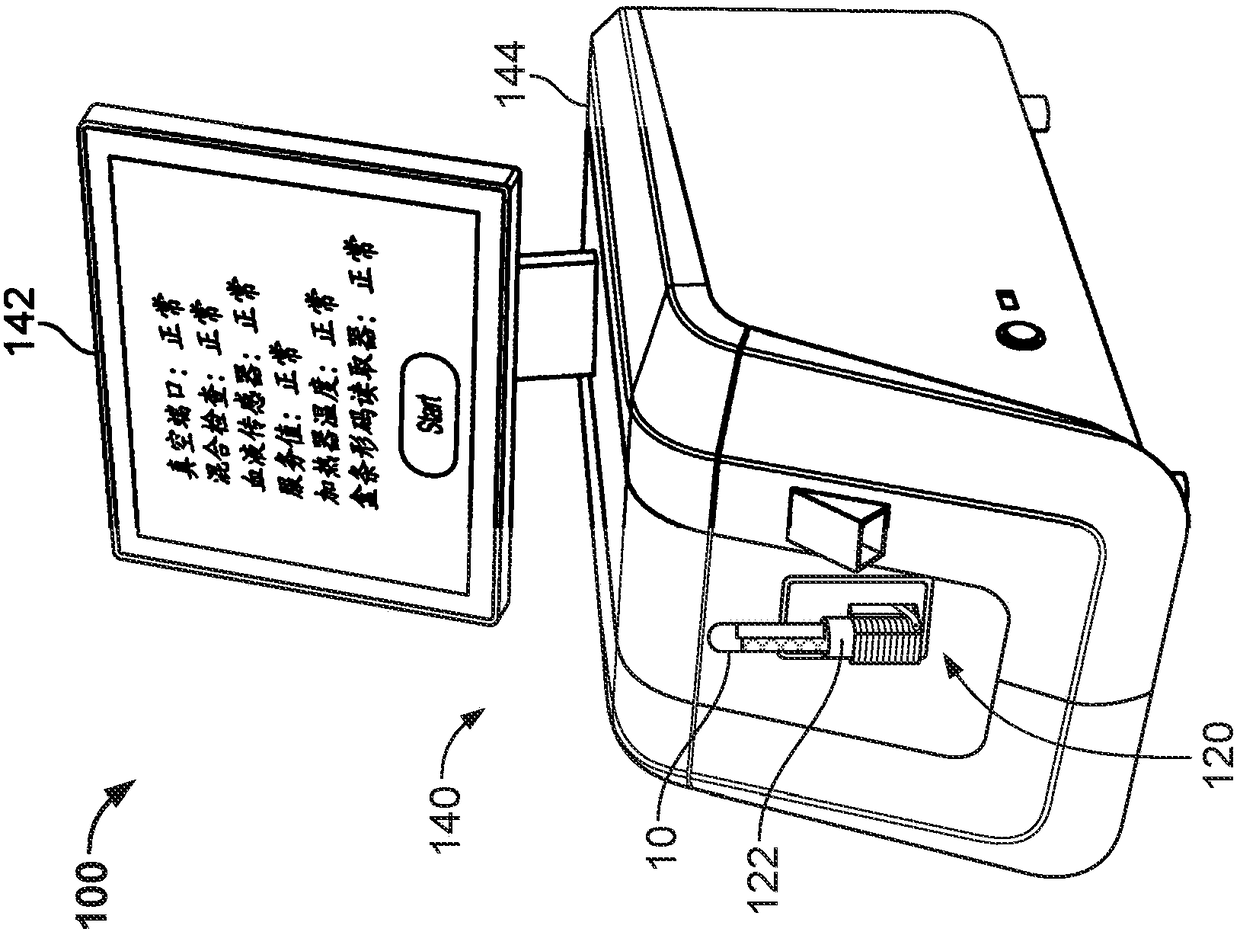
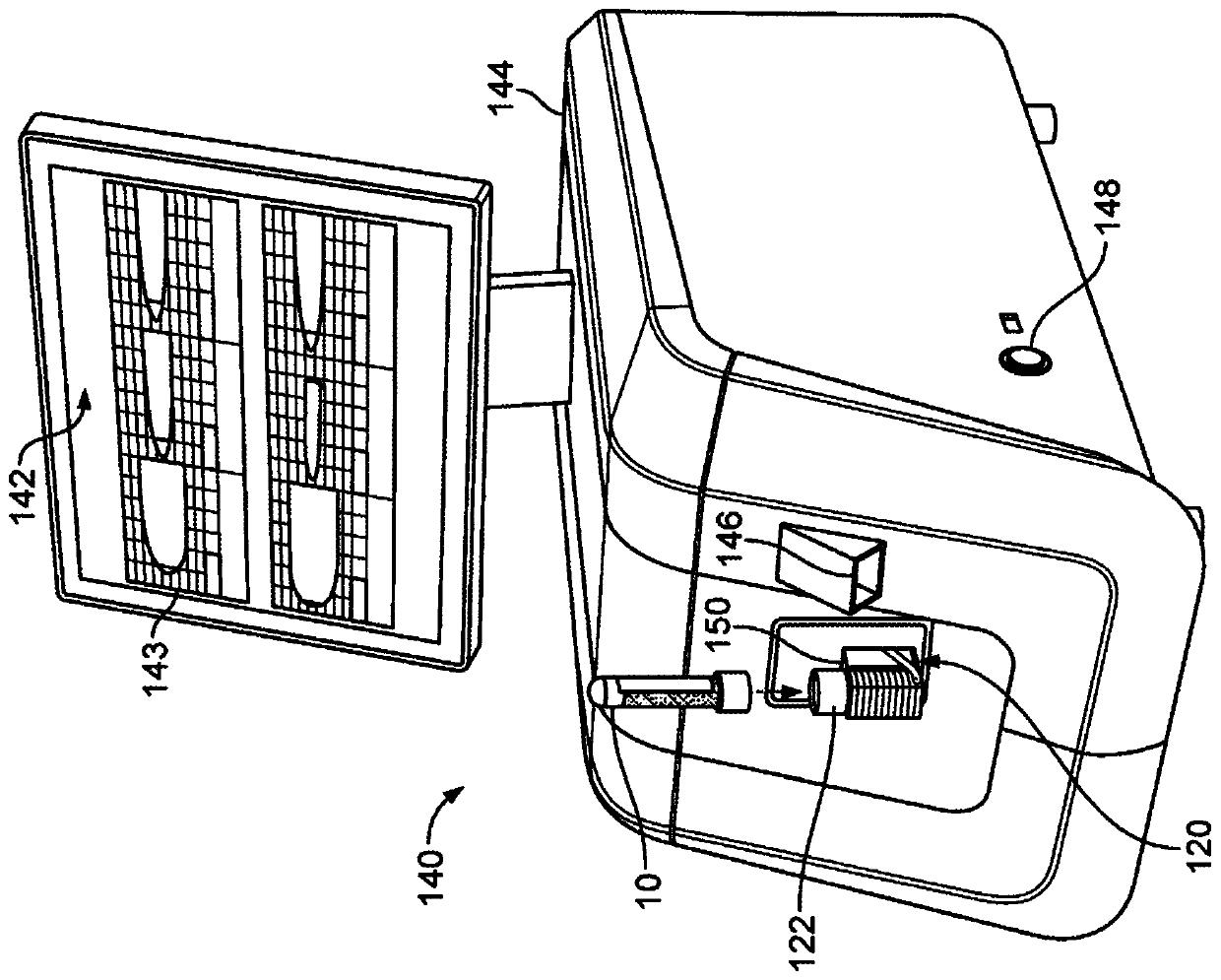
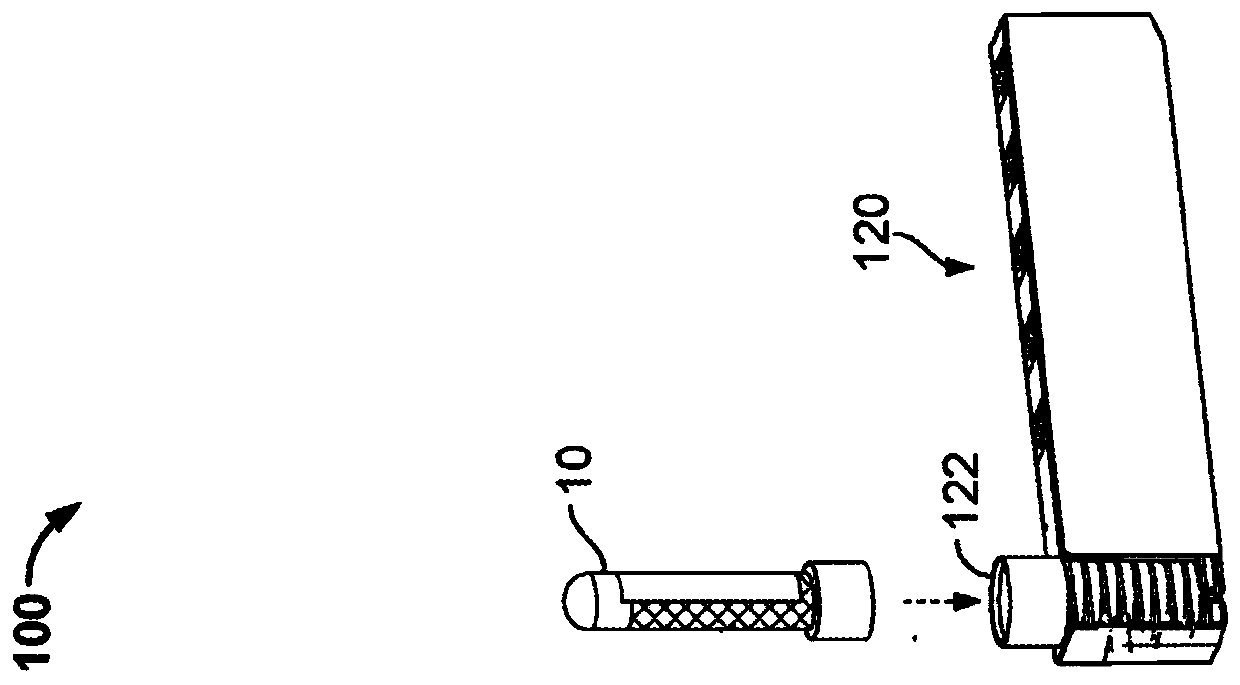
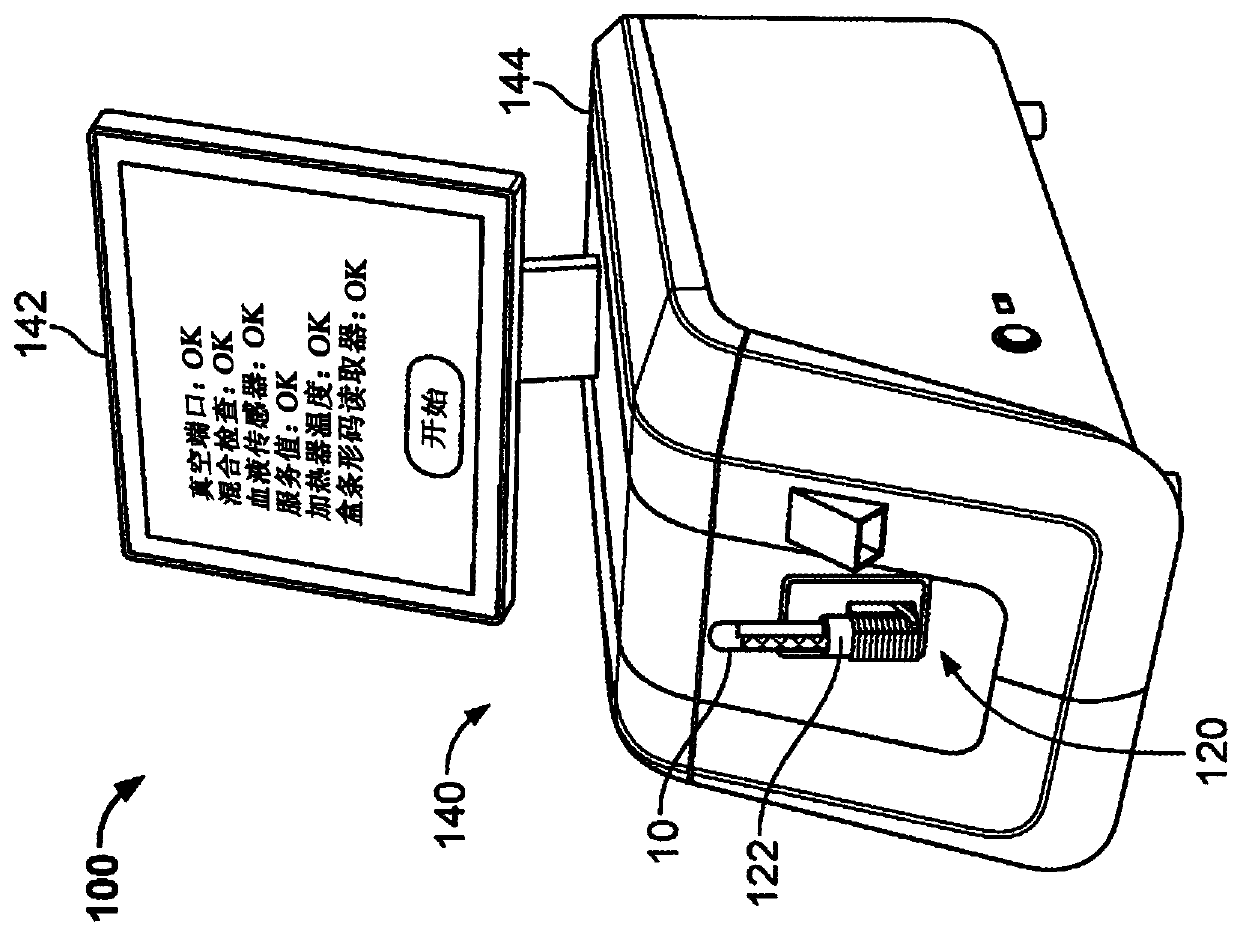
Controlled blood delivery to mixing chamber of a blood testing cartridge
ActiveCN110114144AMinimize interactionEfficient use ofTransportation and packagingPreparing sample for investigationPoint of careThrombus
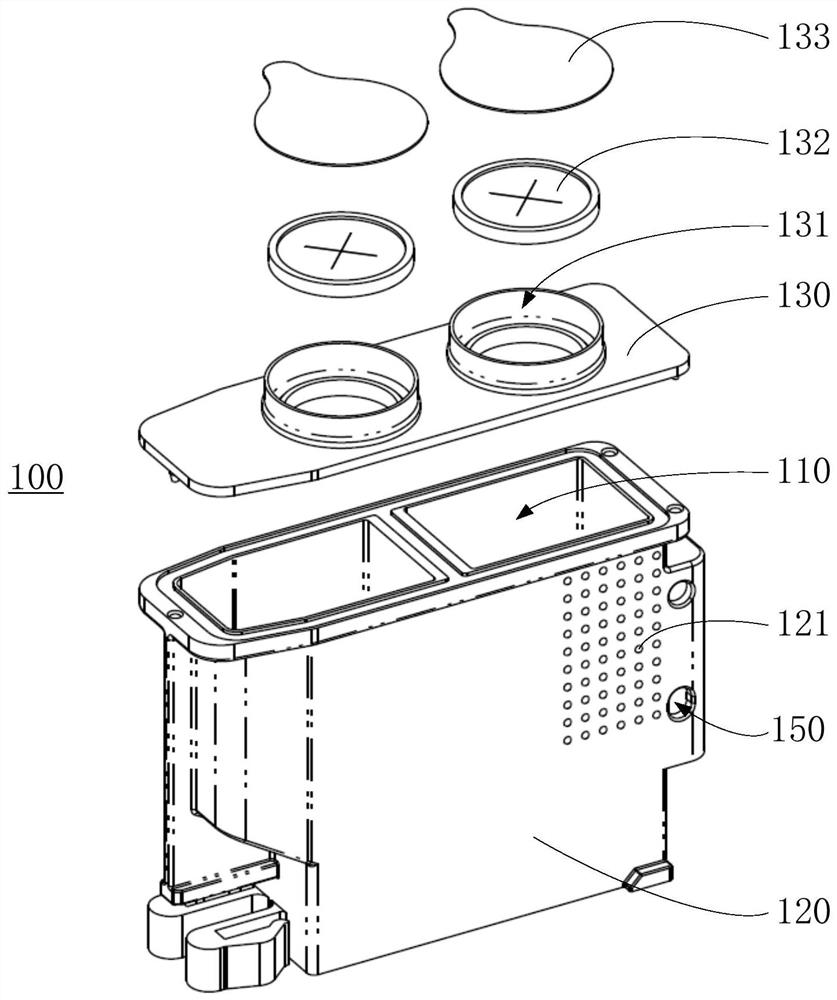
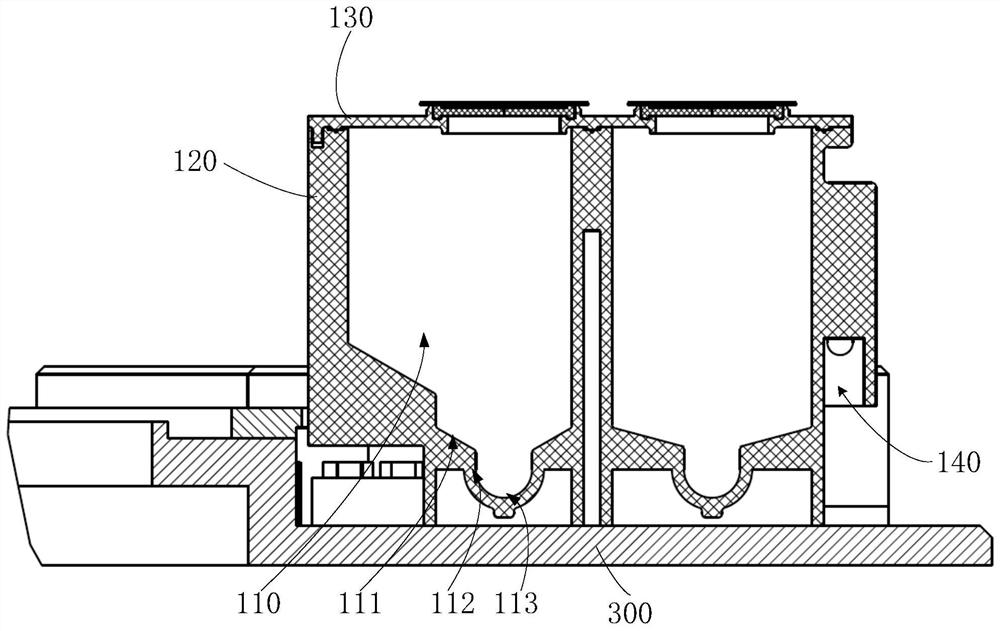
Embodiments of a blood coagulation testing system can operate as an automated thromboelastometry system that is particularly useful, for example, at a point-of-care site. In some embodiments, the blood coagulation testing system includes a single-use cartridge component configured to measure and mix reagents with blood received from a blood sample reservoir. A mixing chamber in the single-use cartridge includes different reagent beads that, when exposed to a pre-determined volume of blood, dissolve and mix specific reagents with the blood. The assembled blood cartridge further includes configurations that are designed to prevent blood from prematurely mixing with reagent beads in the mixing chamber and to guide blood flow in the mixing chamber to dissolve reagent beads in a desired order.Thus, the mixture obtained from the mixing chamber can be readily utilized to generate results for the blood coagulation testing system.
Owner:CA卡西索有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com