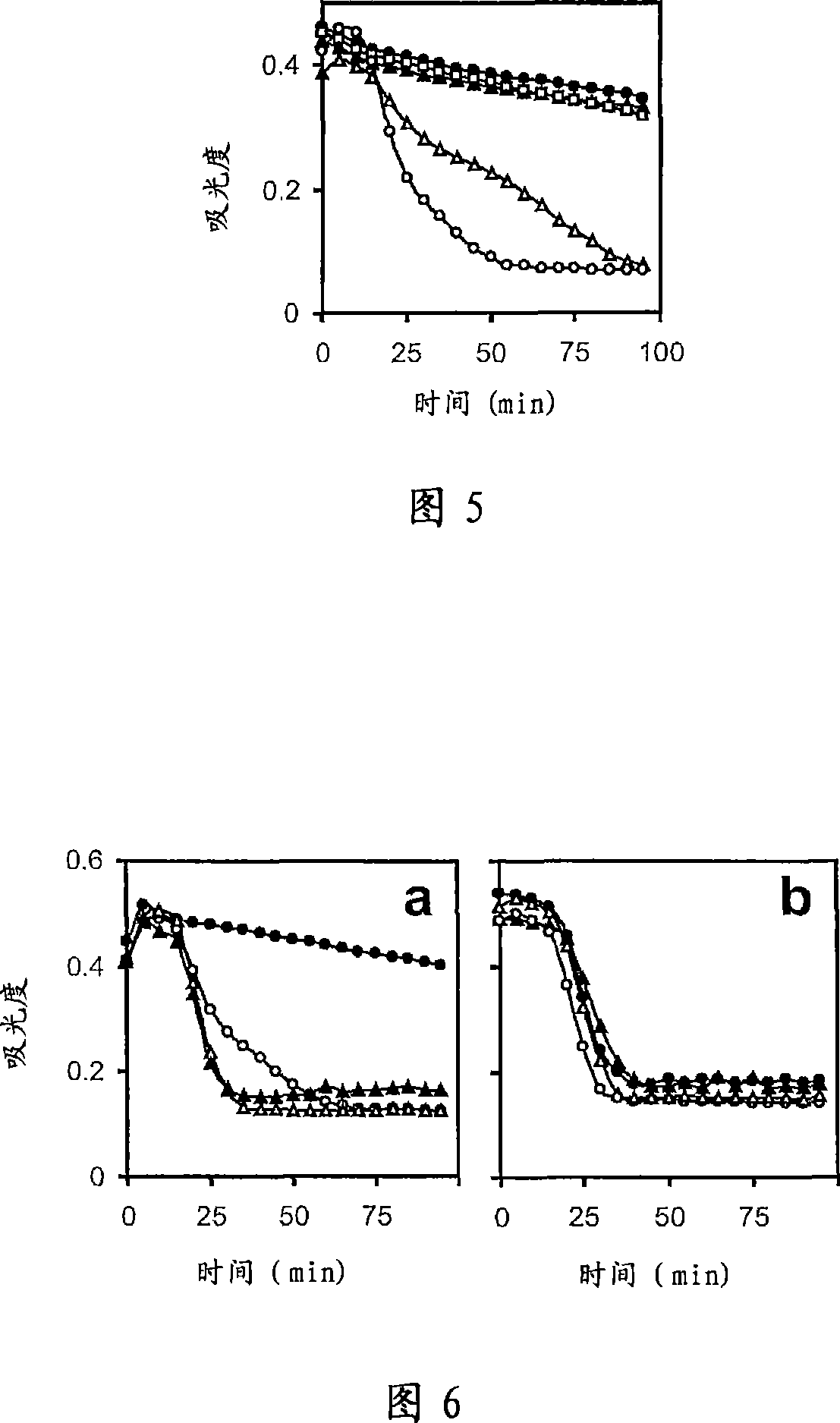
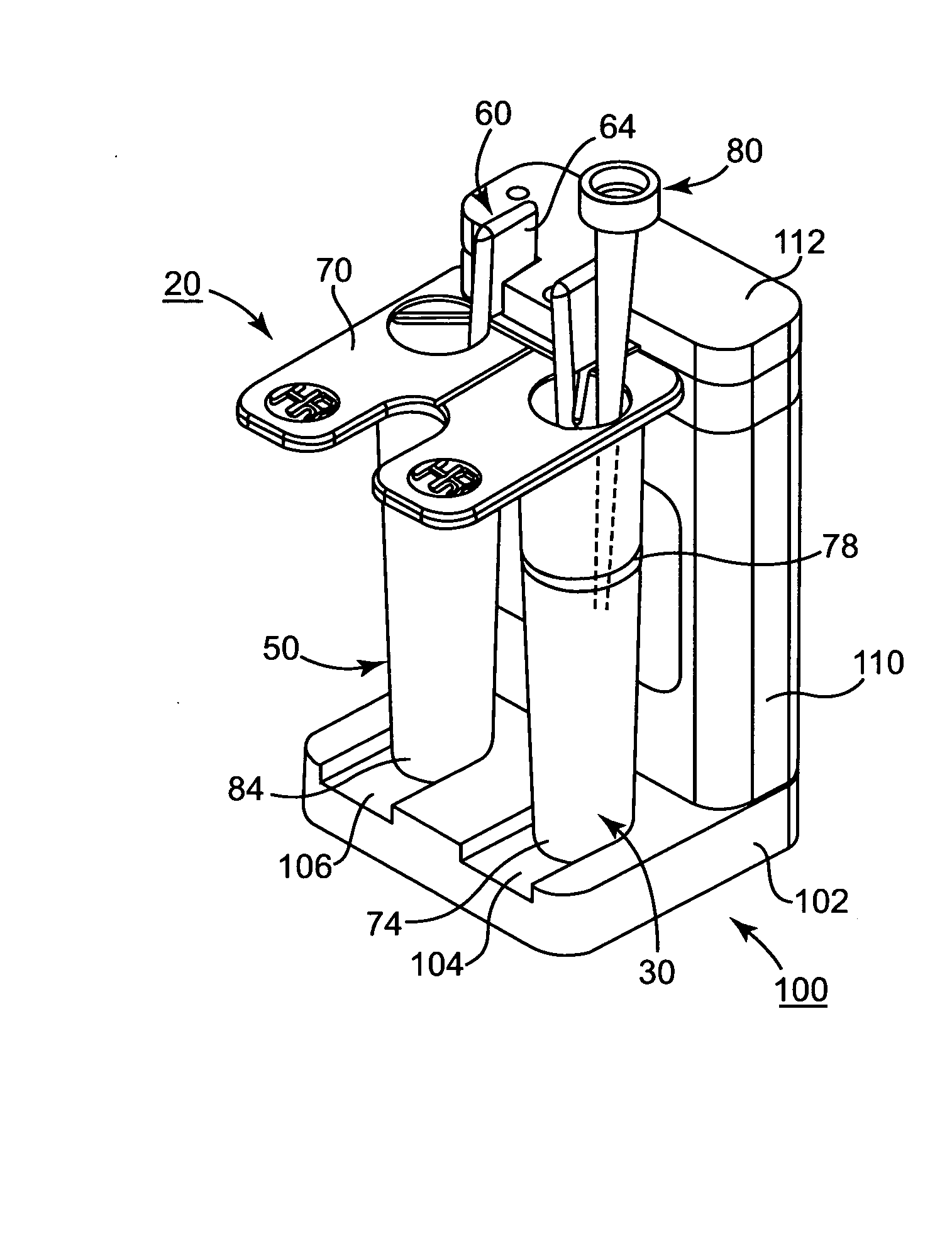
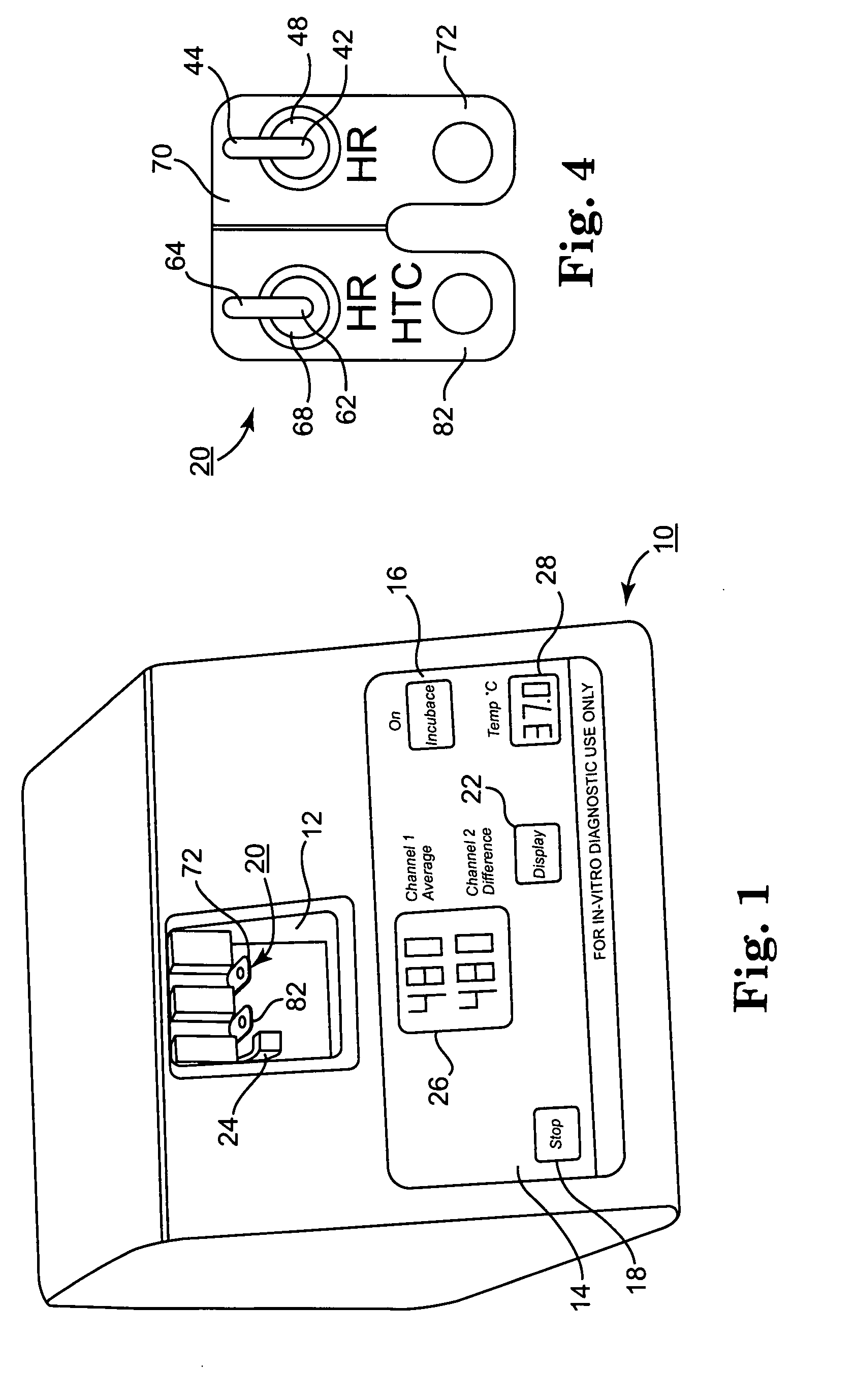
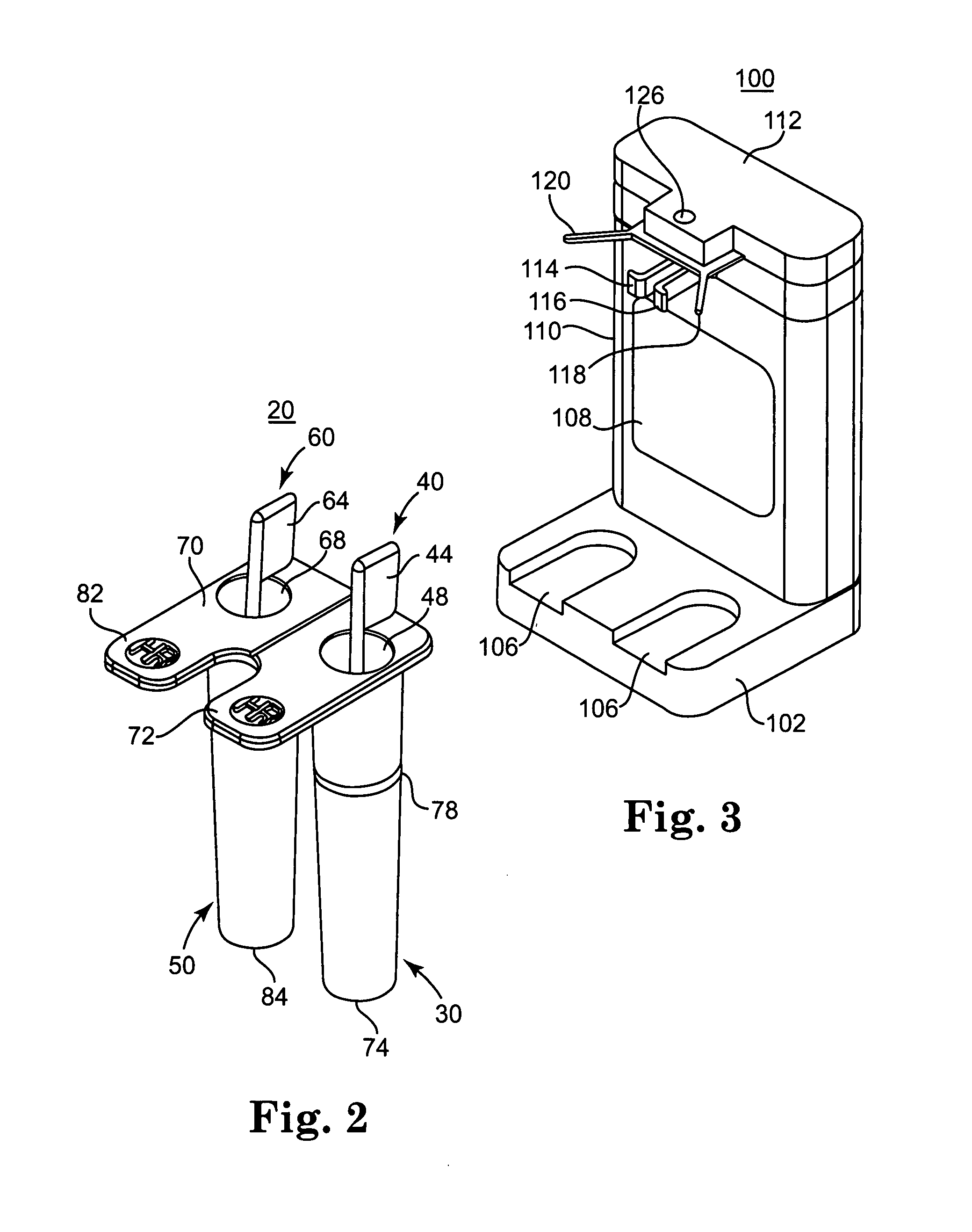
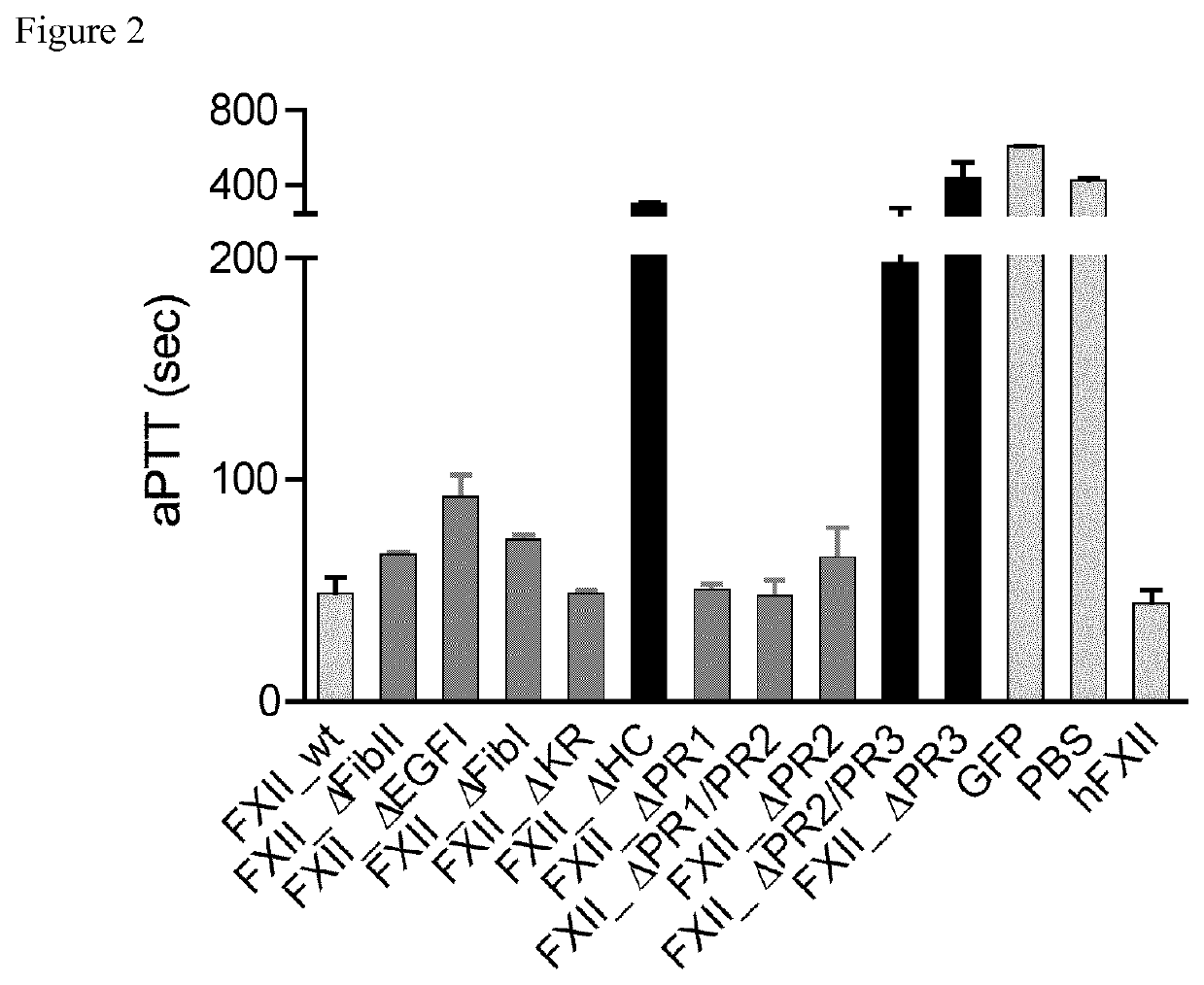
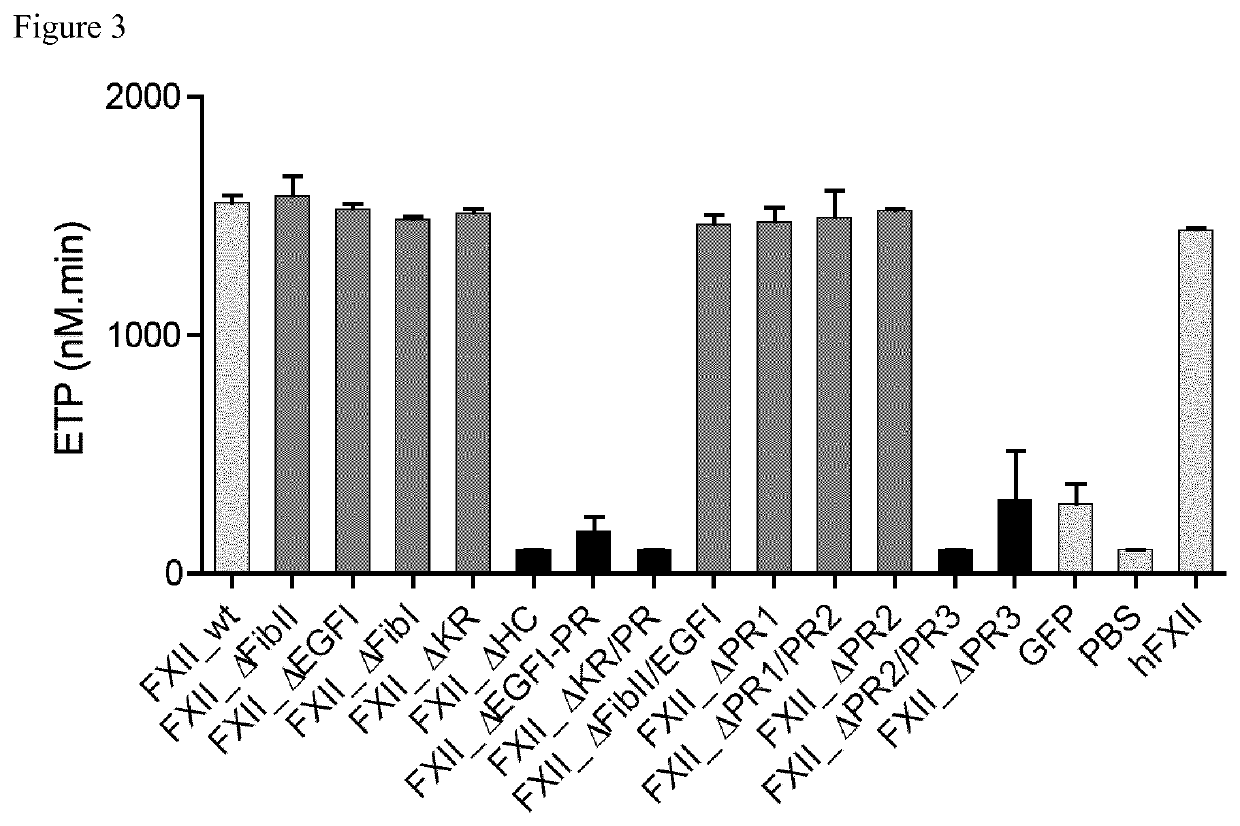
Patents
Literature
37 results about "Blood coagulation test" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Laboratory tests for evaluating the individual's blood clotting mechanisms.
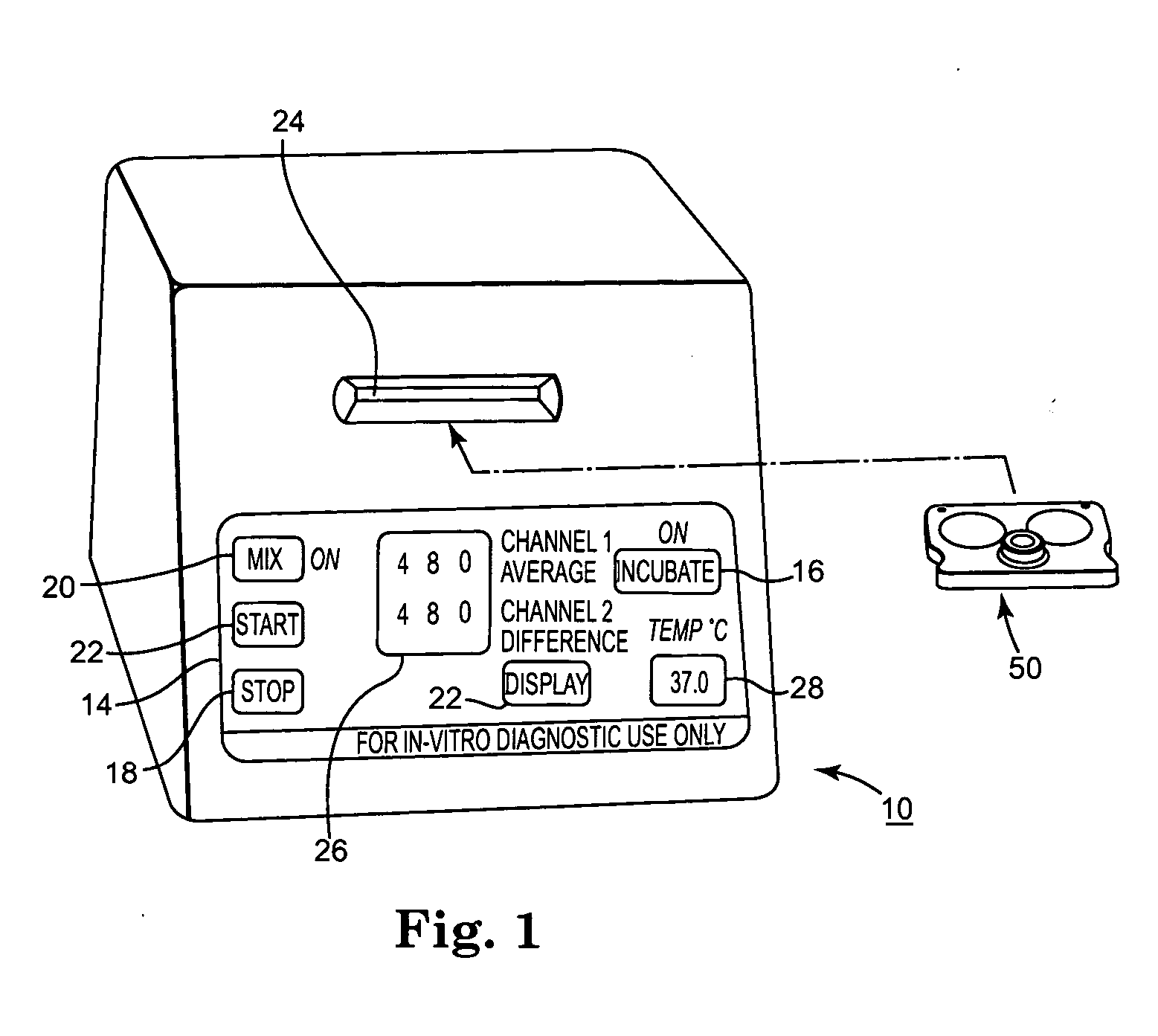
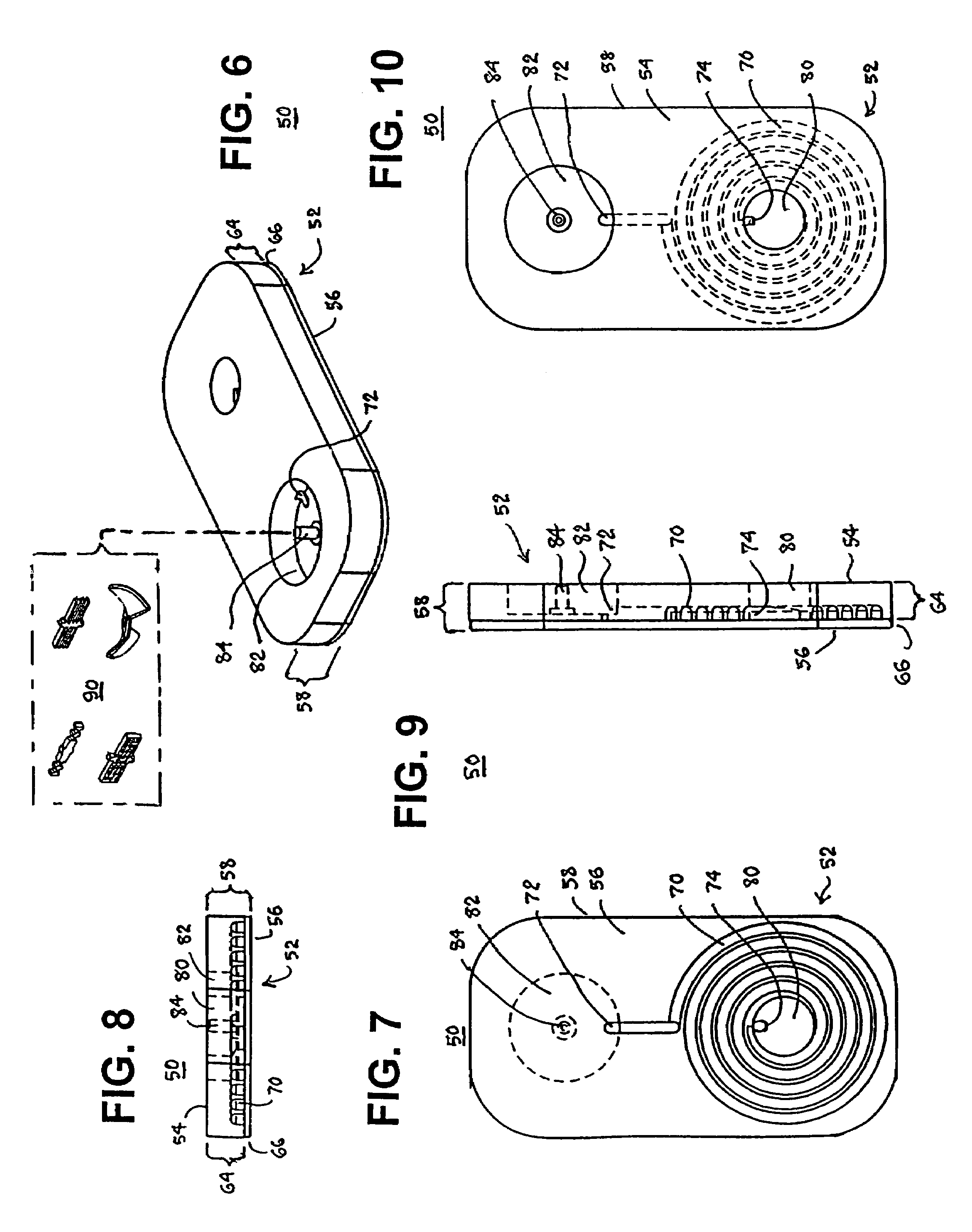
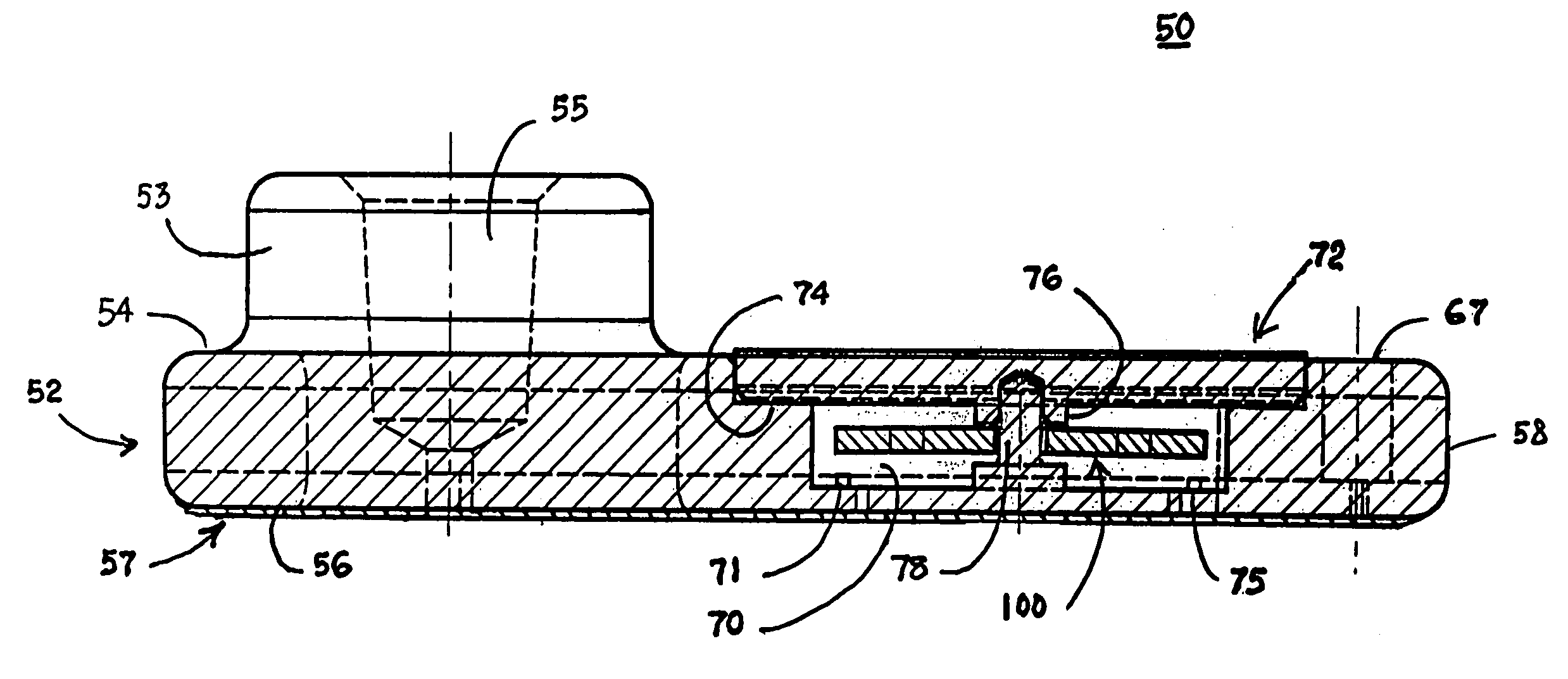
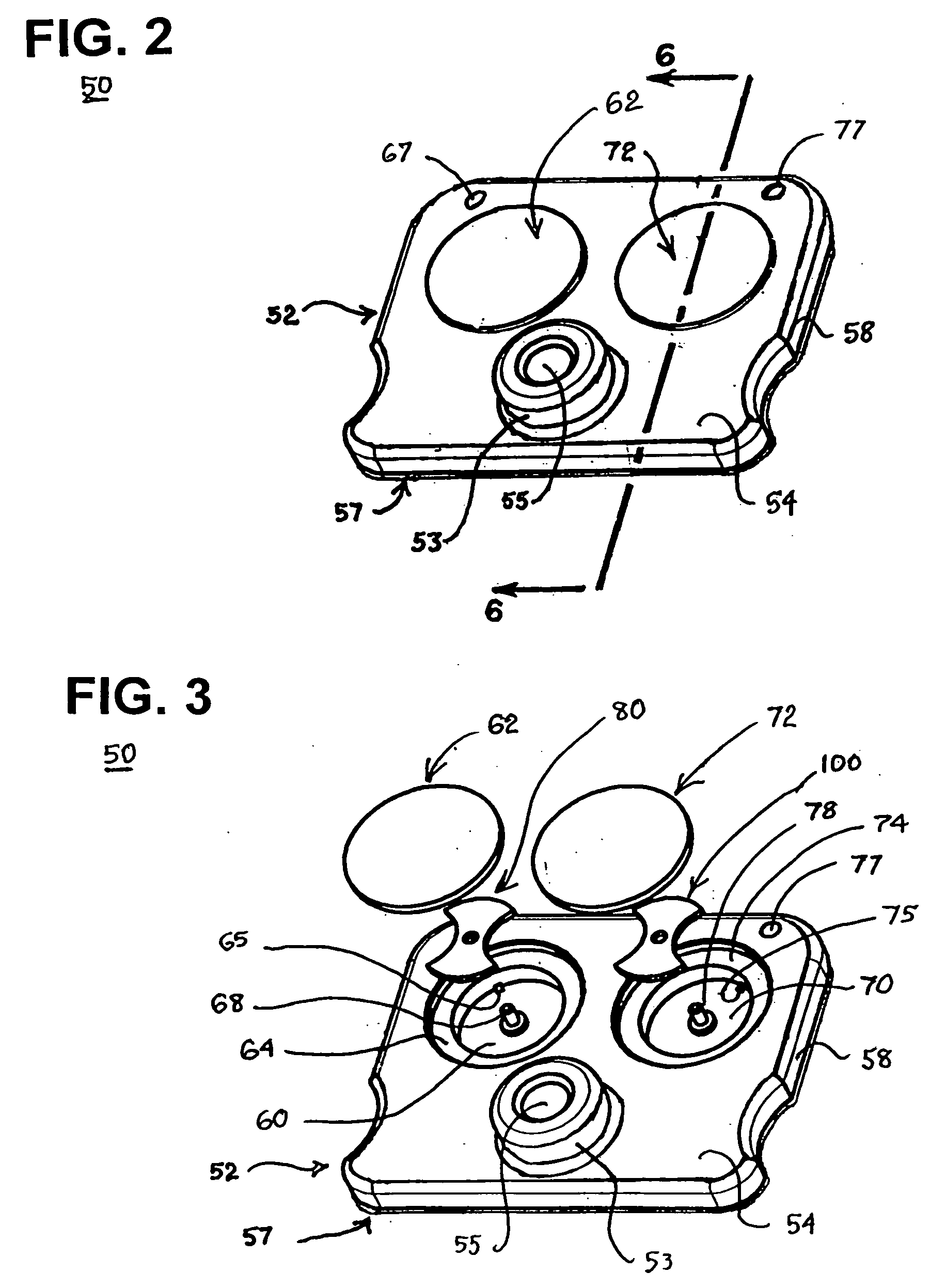
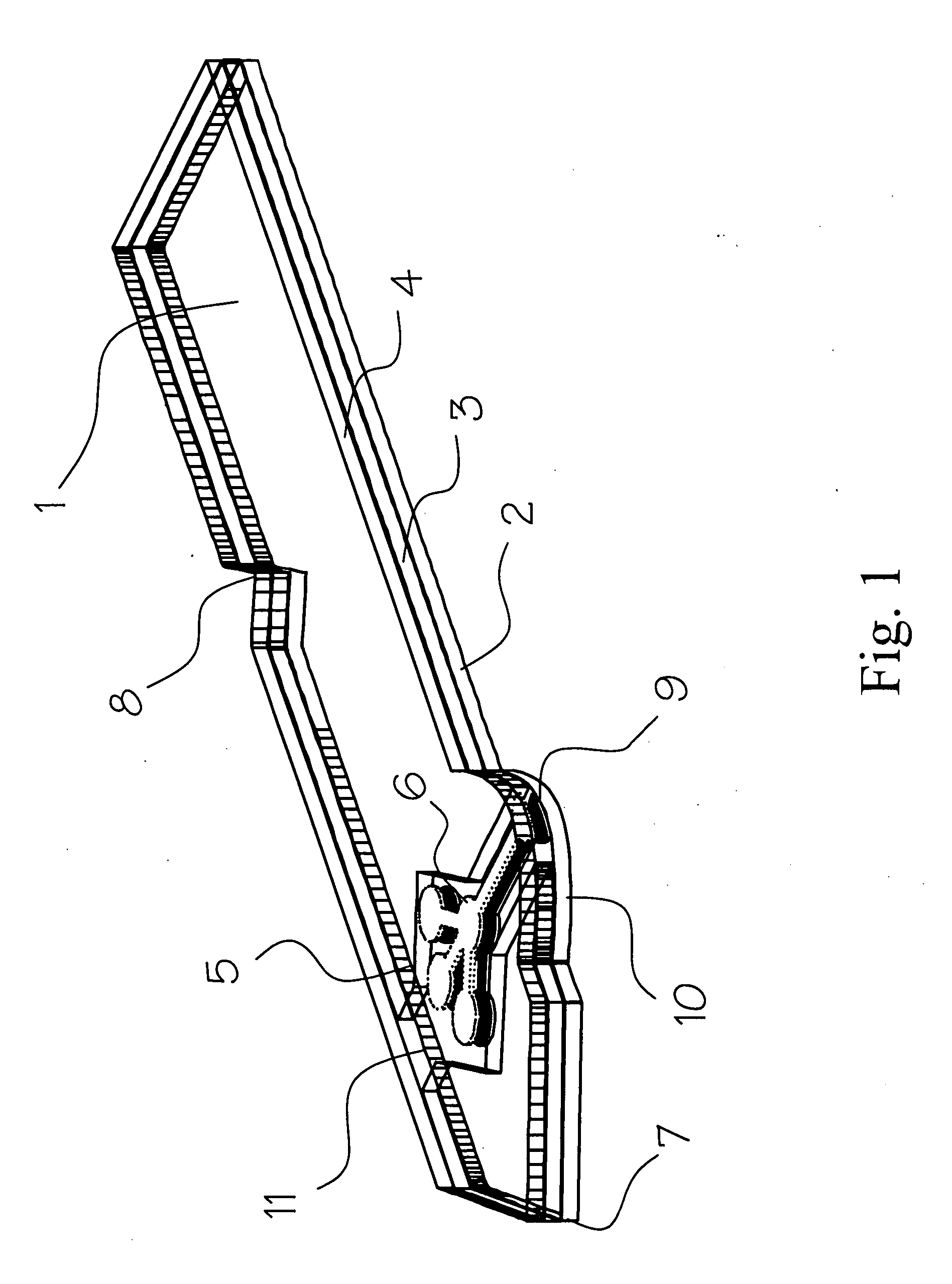
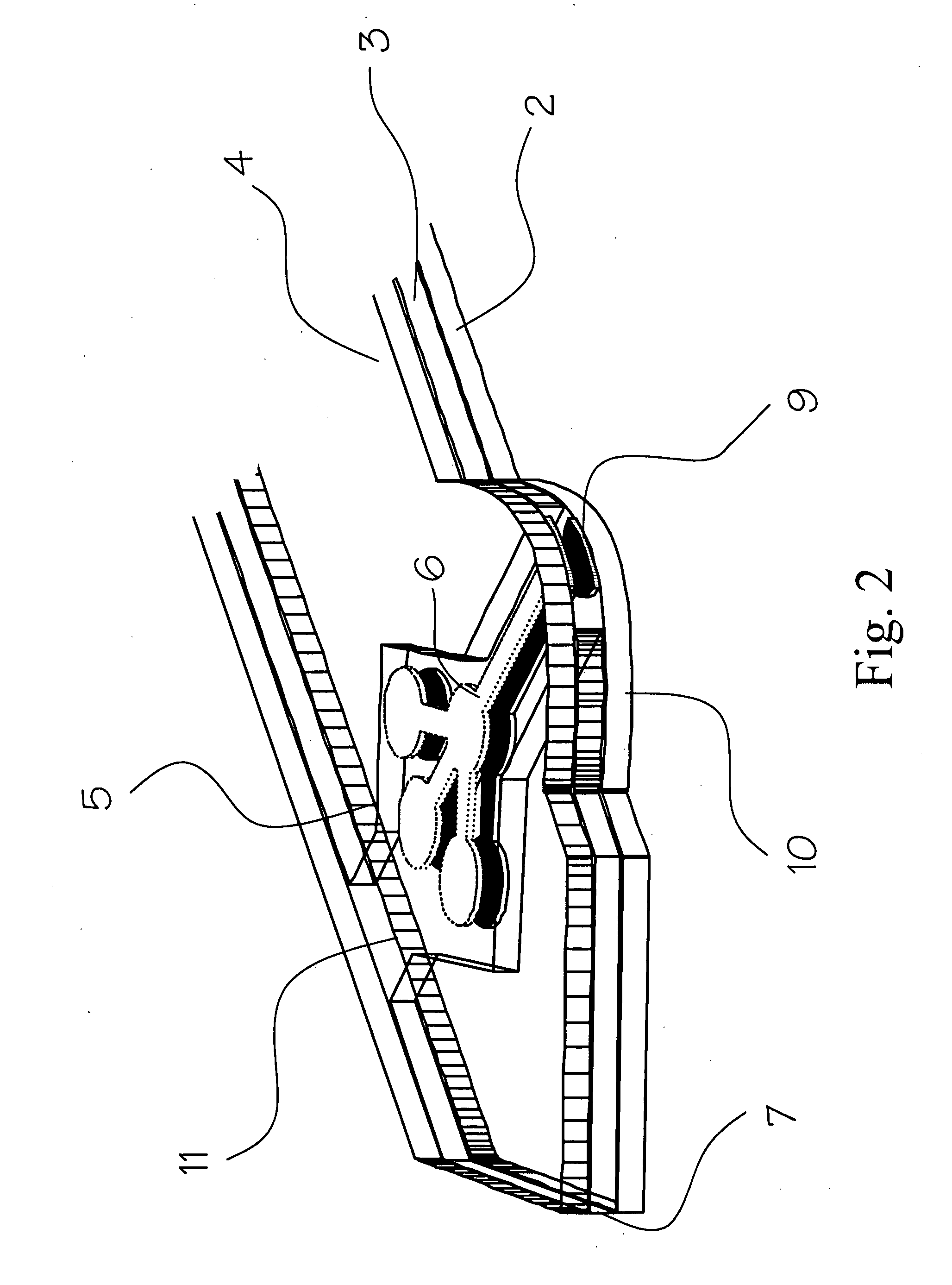
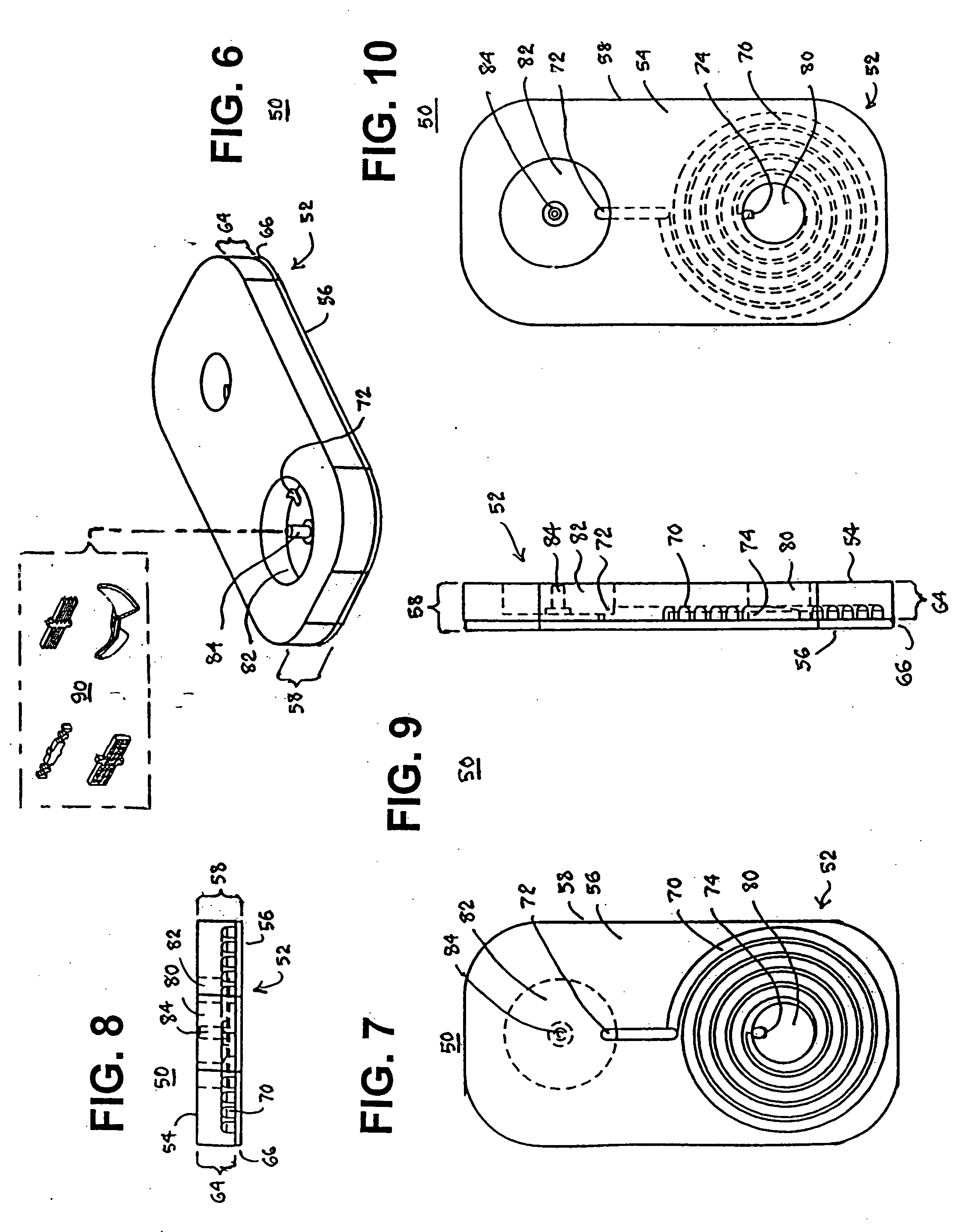
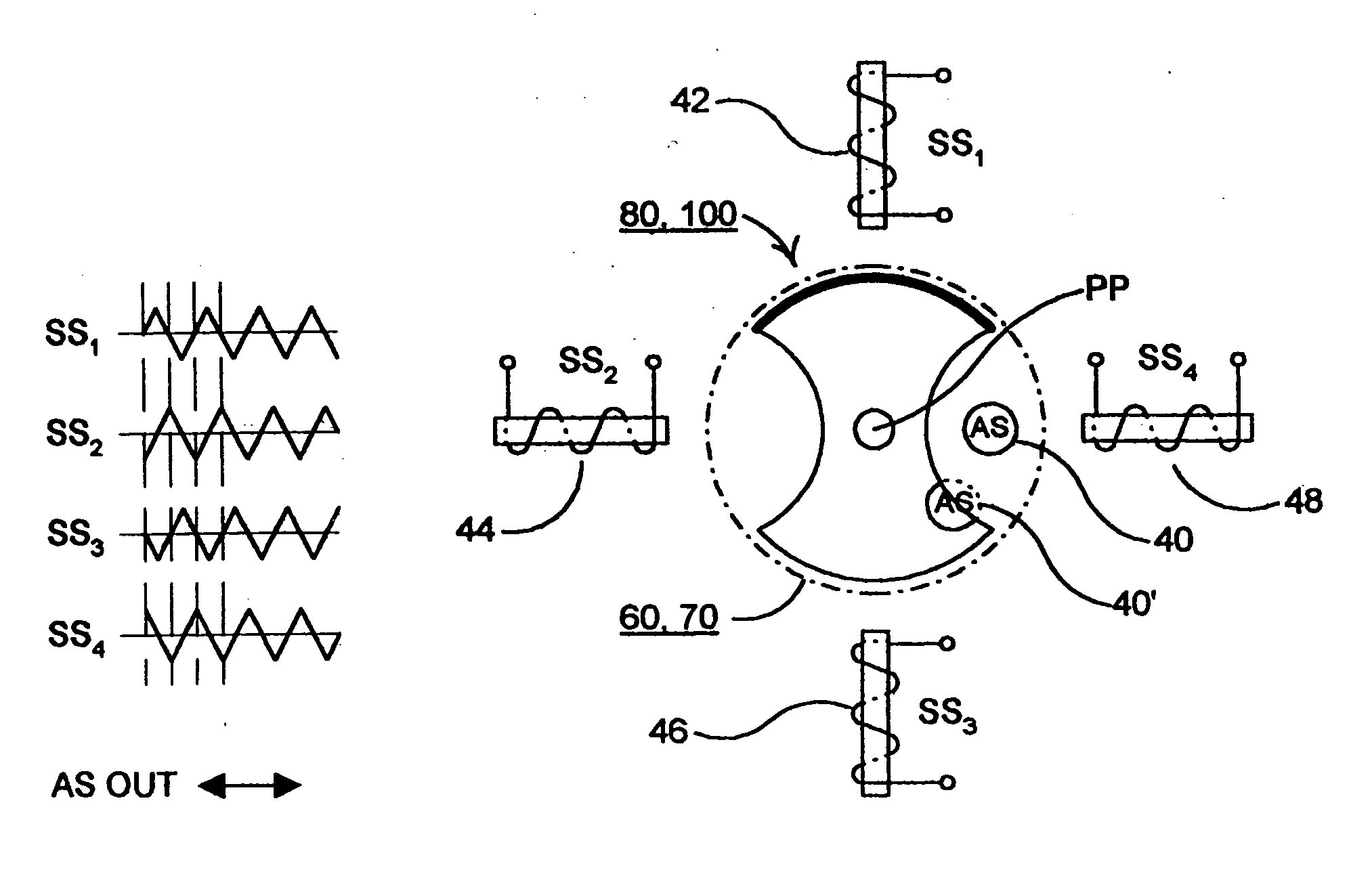
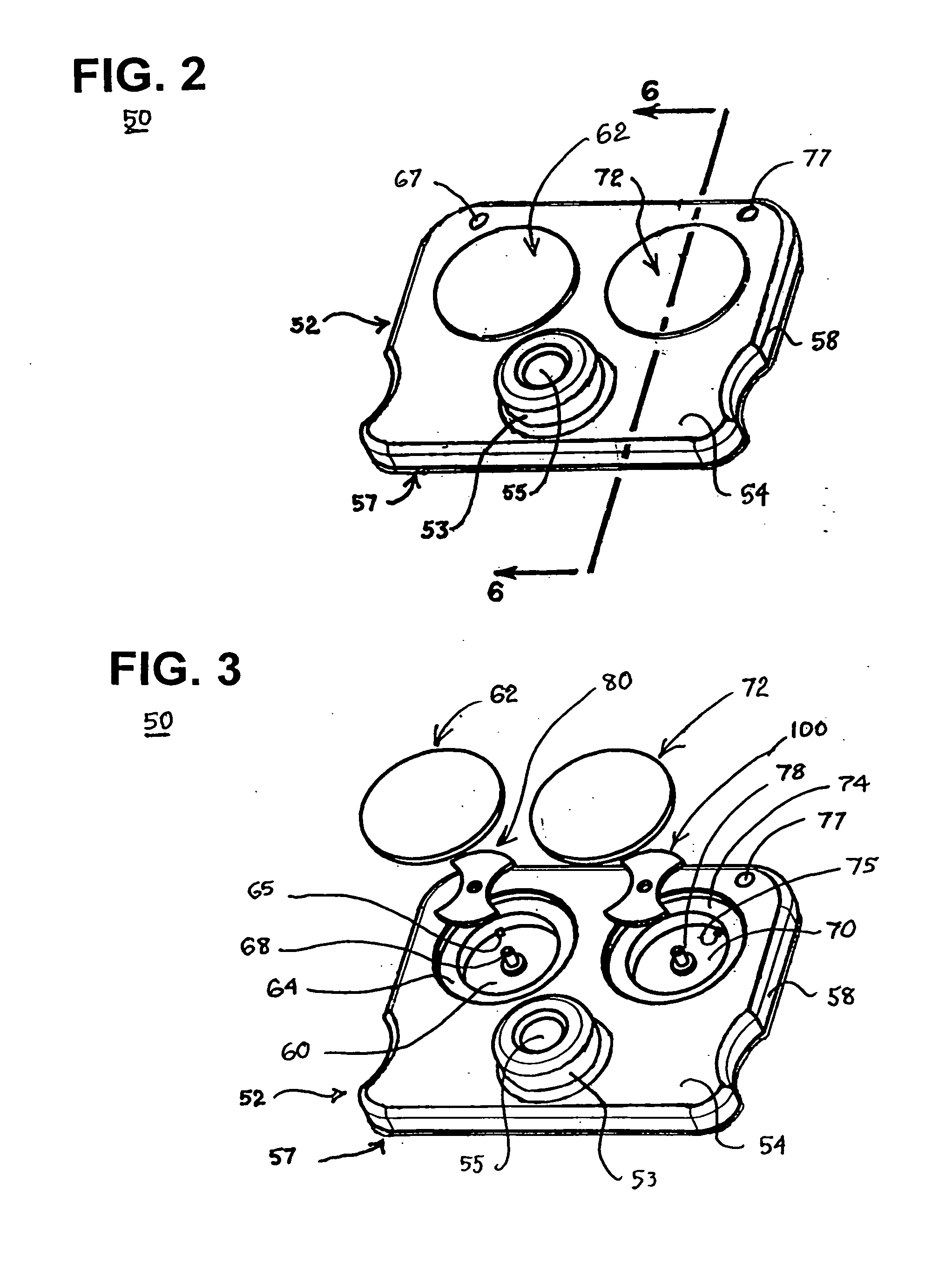
Blood coagulation test cartridge, system, and method
ActiveUS7399637B2Practical and convenientRapid and reliableAnalysis using chemical indicatorsMicrobiological testing/measurementBlood testTest sample
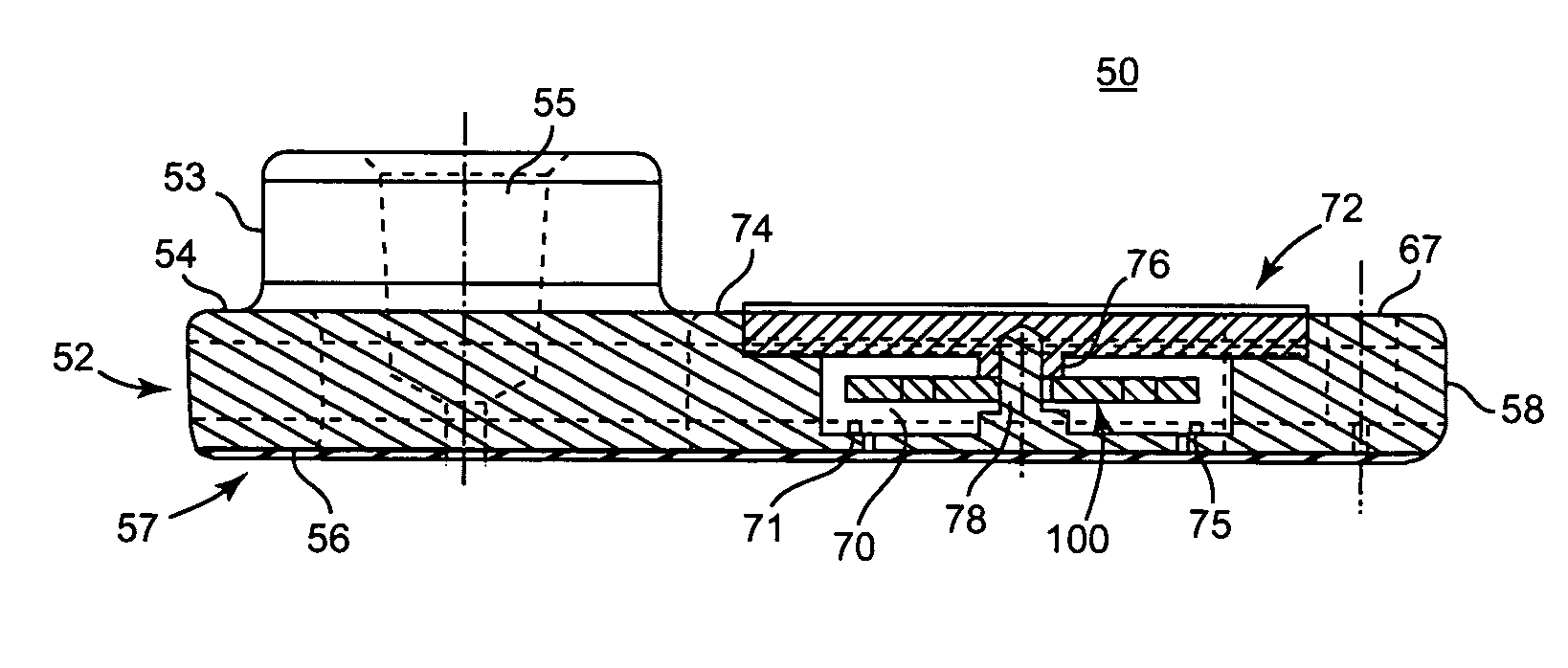
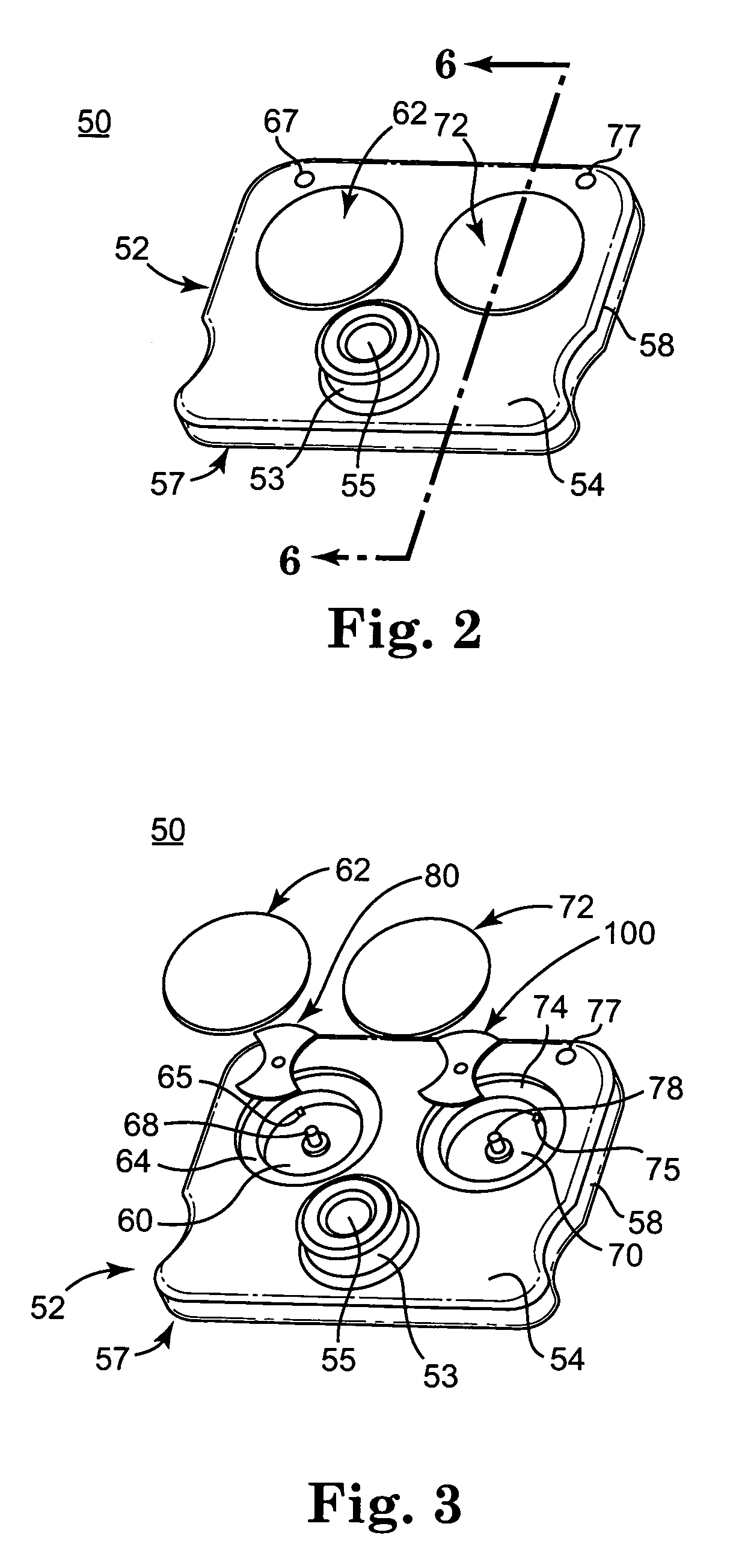
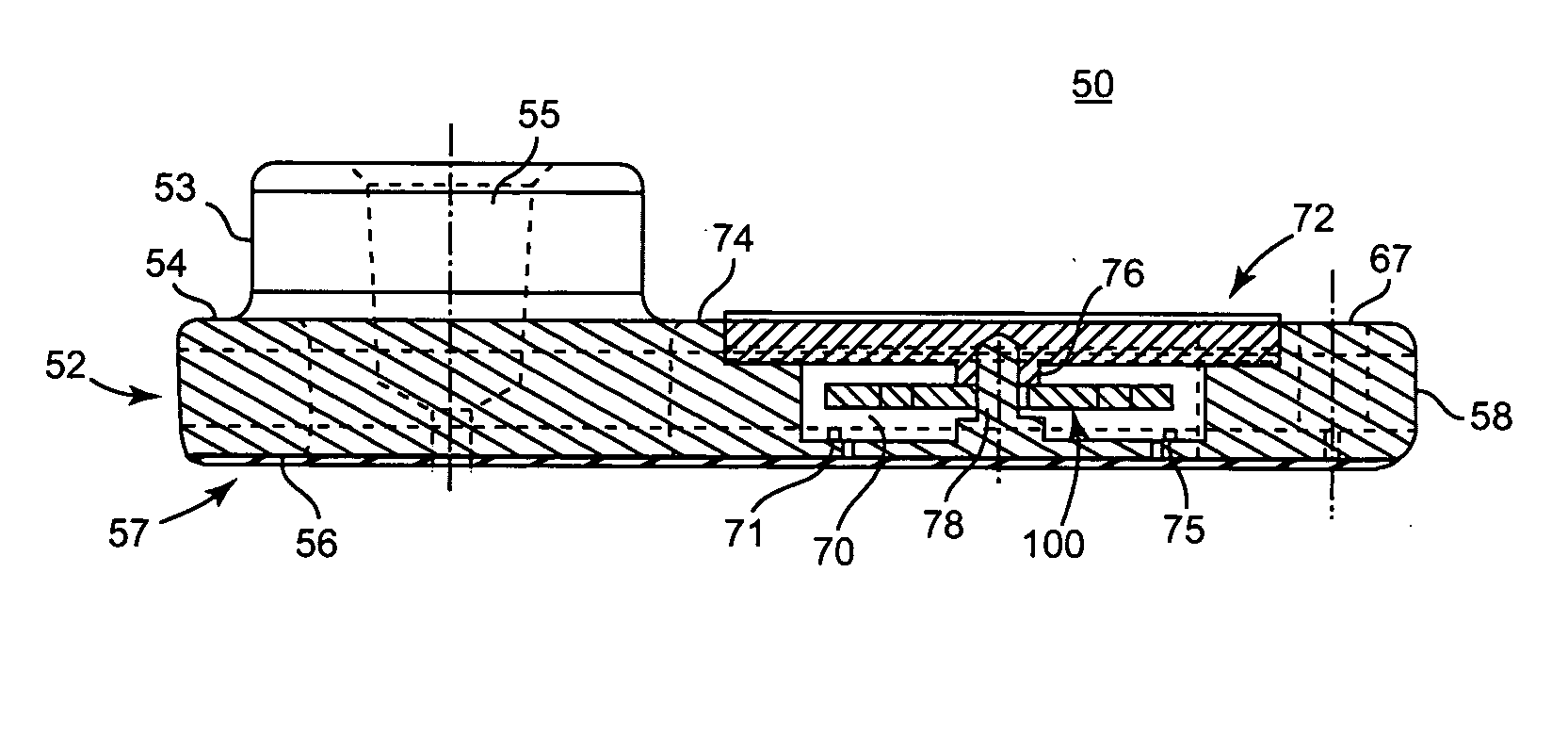
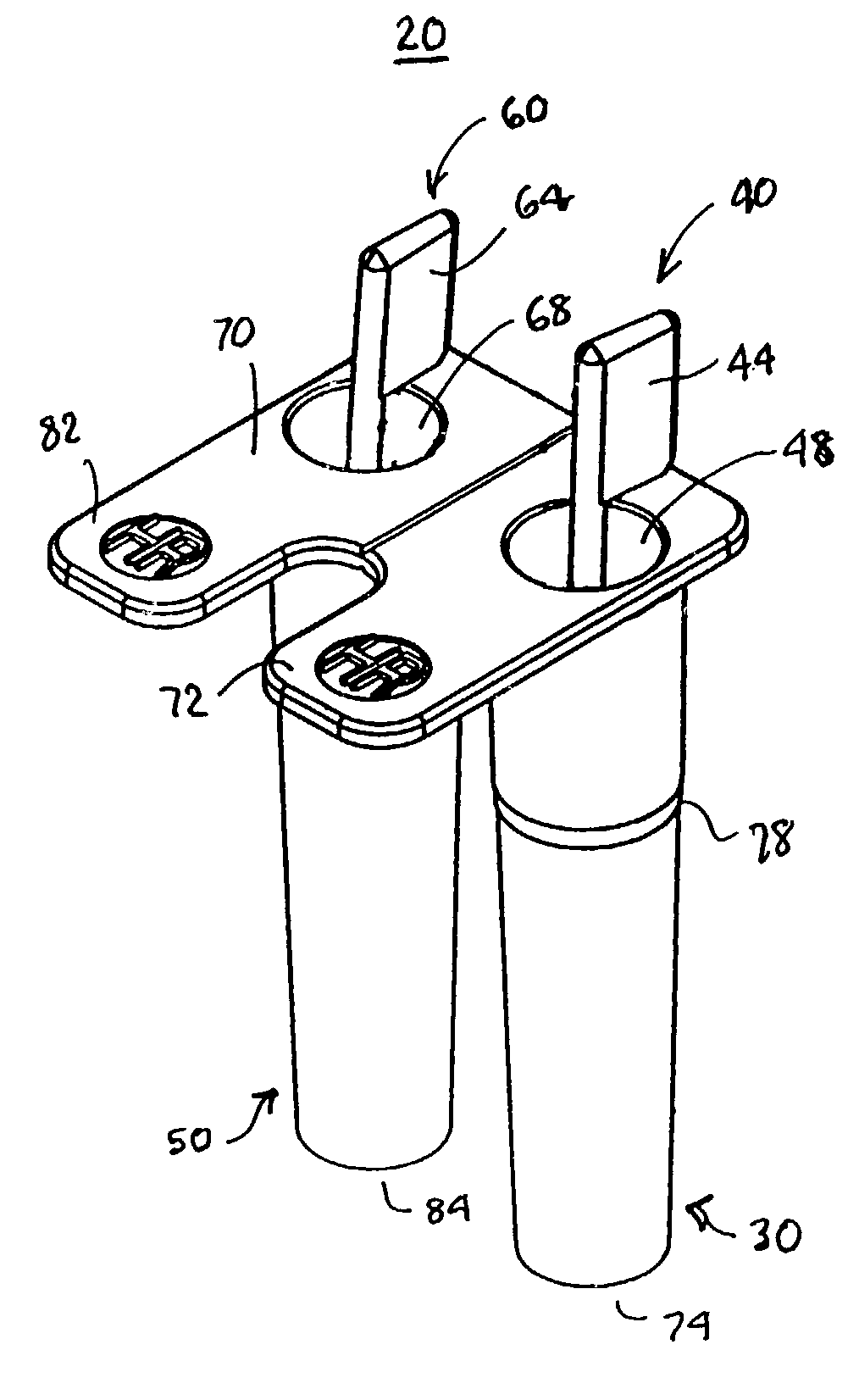
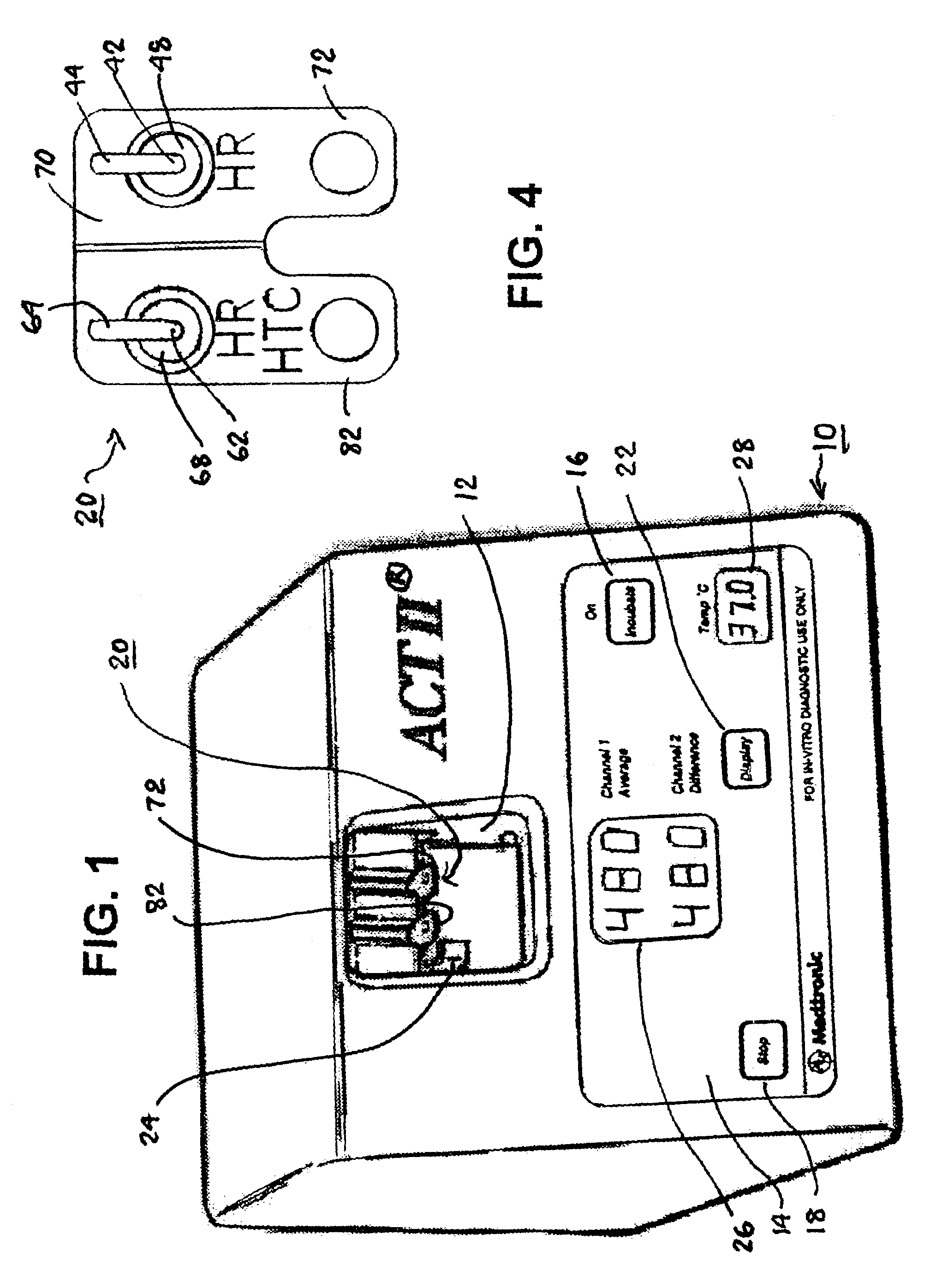
A system and method for determining a coagulation time, e.g., TT, PT, aPTT, and ACT, of a blood test sample deposited in a test cartridge is disclosed. A cartridge housing having upper and lower major sides and a minor sidewall encloses a test chamber having a test chamber pivot element and is provided with a cartridge port for introducing a test sample into the test chamber,. Ferromagnetic agitator vane leaflets extend from an agitator pivot element supported by the test chamber pivot element intermediate the upper and lower major sides for rotational motion. The agitator vane leaflets can be swept, in response to an external magnetic field, through the test sample in the absence of coagulation. A timer is started when the agitator movement is commenced whereupon the agitator moves freely. Resistance to agitator movement due to coagulation is detected, and the coagulation time is measured.
Owner:MEDTRONIC INC
Blood coagulation test cartridge, system, and method
ActiveUS20050233466A1Practical and convenientRapid and reliableAnalysis using chemical indicatorsFlow propertiesBlood testTest sample
A system and method for determining a coagulation time, e.g., TT, PT, aPTT, and ACT, of a blood test sample deposited in a test cartridge is disclosed. A cartridge housing having upper and lower major sides and a minor sidewall encloses a test chamber having a test chamber pivot element and is provided with a cartridge port for introducing a test sample into the test chamber,. Ferromagnetic agitator vane leaflets extend from an agitator pivot element supported by the test chamber pivot element intermediate the upper and lower major sides for rotational motion. The agitator vane leaflets can be swept, in response to an external magnetic field, through the test sample in the absence of coagulation. A timer is started when the agitator movement is commenced whereupon the agitator moves freely. Resistance to agitator movement due to coagulation is detected, and the coagulation time is measured.
Owner:MEDTRONIC INC
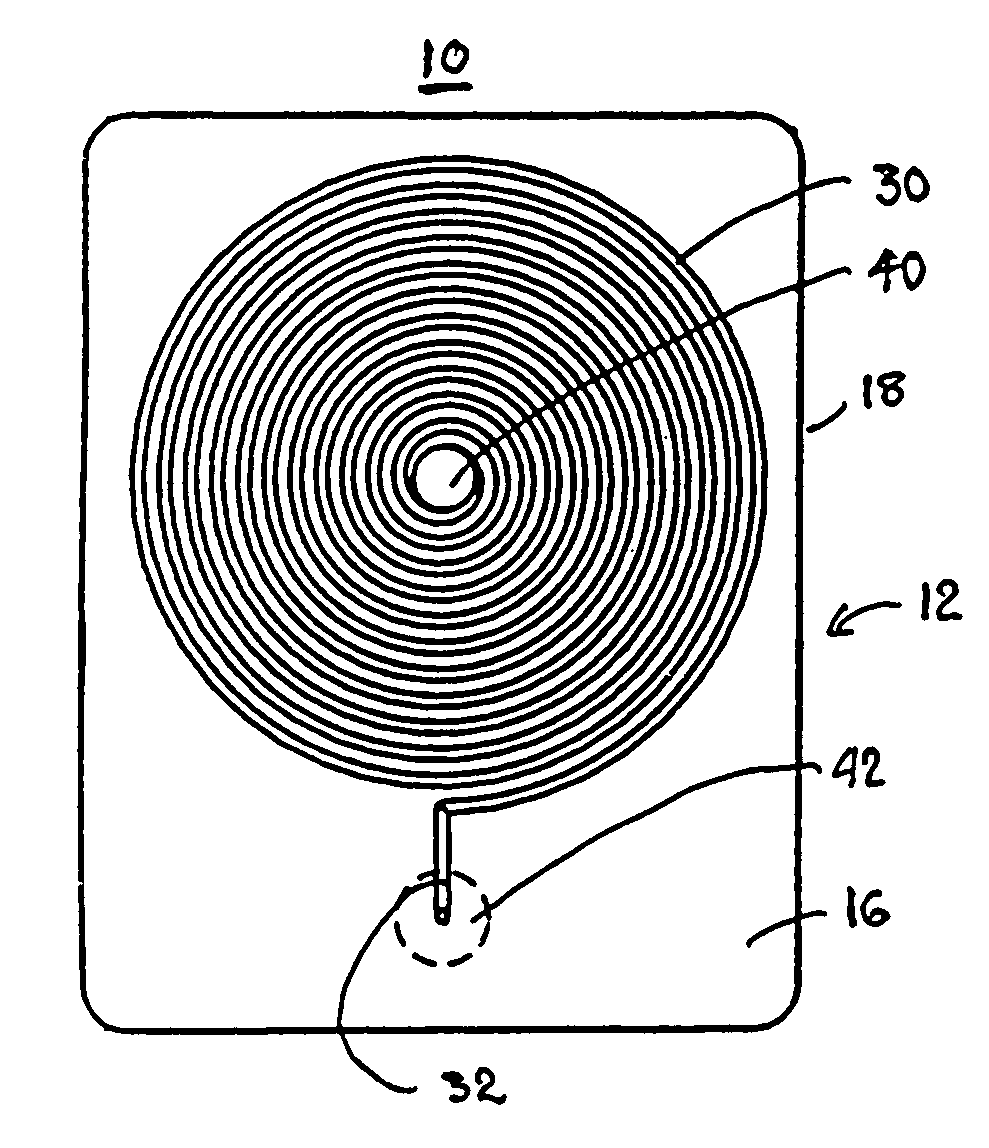
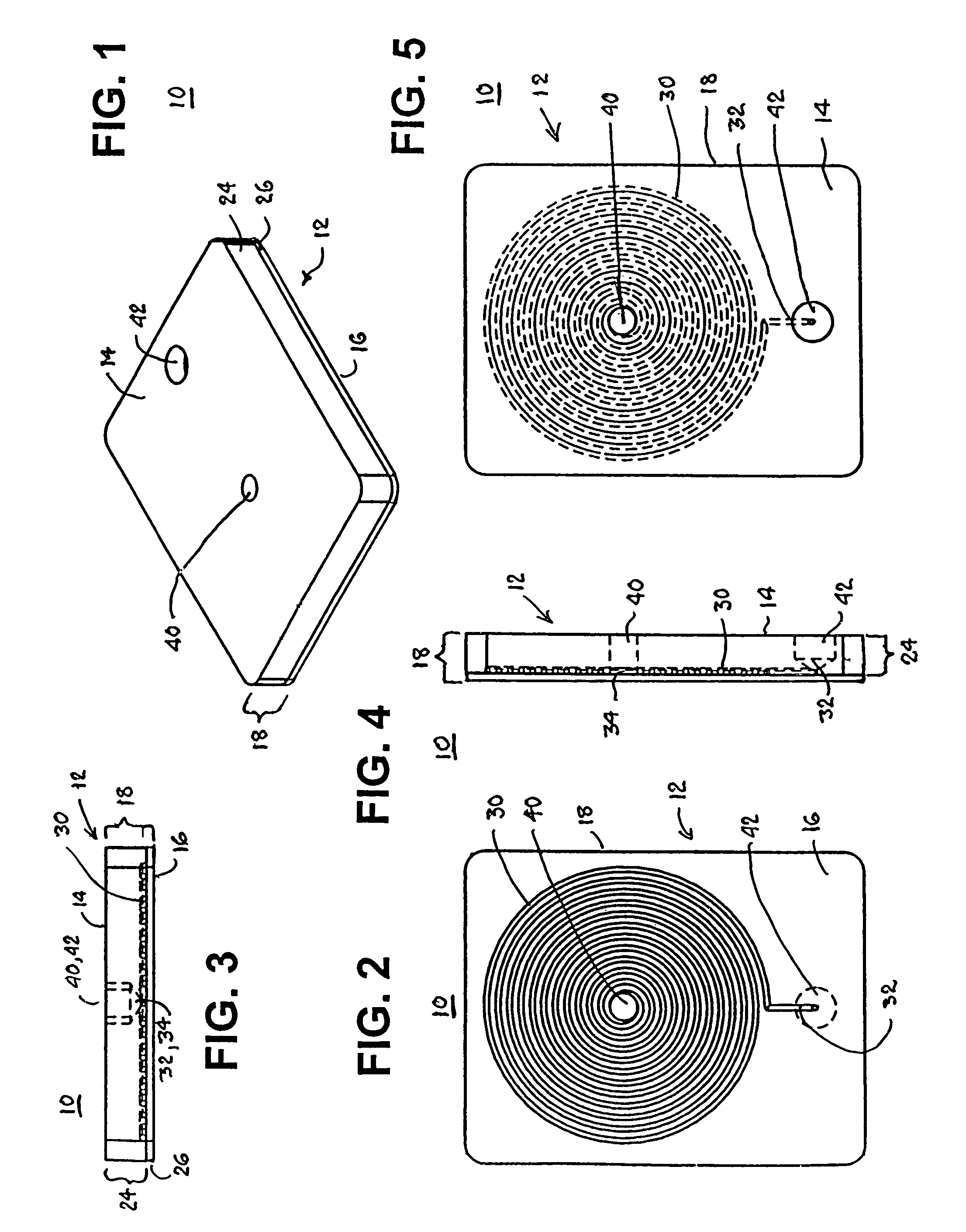
Blood coagulation test cartridge, system, and method
InactiveUS7439069B2Practical and convenientRapid and inexpensiveMicrobiological testing/measurementWithdrawing sample devicesBlood capillaryEngineering
A system and method for determining a coagulation time, e.g., thrombin time, PT, aPTT, and ACT, of a blood sample deposited in a test cartridge is disclosed. The test cartridge includes a blood receptacle that is open to the atmosphere into which a blood sample is to be deposited, a vacuum port that is open to atmosphere, and a spiral capillary within the test cartridge having a capillary length and cross-section area, a first capillary end of the spiral capillary open to the blood receptacle and a second capillary end of the spiral capillary open to the vacuum port, whereby the spiral capillary is closed to atmosphere. When a blood sample is deposited in the blood receptacle, a vacuum is drawn through the vacuum port and the blood is drawn through the spiral capillary until coagulation occurs. A pressure change is detected, and the coagulation time is measured.
Owner:MEDTRONIC INC
Blood coagulation test cartridge, system, and method
ActiveUS20050233460A1Practical and convenientRapid and reliableAnalysis using chemical indicatorsMicrobiological testing/measurementTest sampleEngineering
A system and method for determining a coagulation time, e.g., TT, PT, aPTT, and ACT, of a test sample deposited in a test cartridge is disclosed. A cartridge housing having upper and lower major sides and a minor sidewall encloses a test chamber having a test chamber pivot element and is provided with a cartridge port for introducing a test sample into the test chamber,. Ferromagnetic agitator vane leaflets extend from an agitator pivot element supported by the test chamber pivot element intermediate the upper and lower major sides for rotational motion. The agitator vane leaflets can be swept, in response to an external magnetic field, through the test sample in the absence of coagulation. A timer is started when the agitator movement is commenced whereupon the agitator moves freely. Resistance to agitator movement due to coagulation is detected, and the coagulation time is measured.
Owner:MEDTRONIC INC
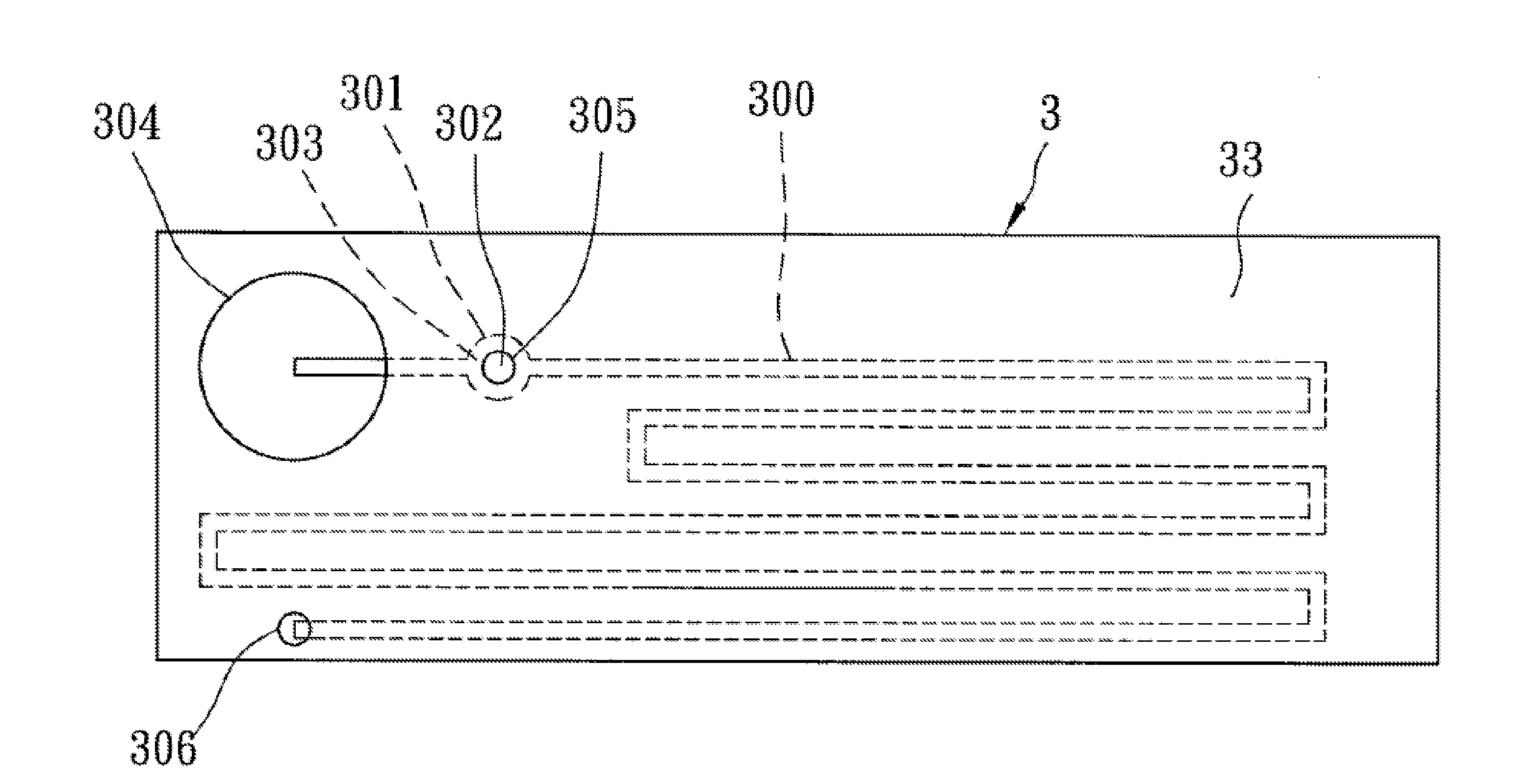
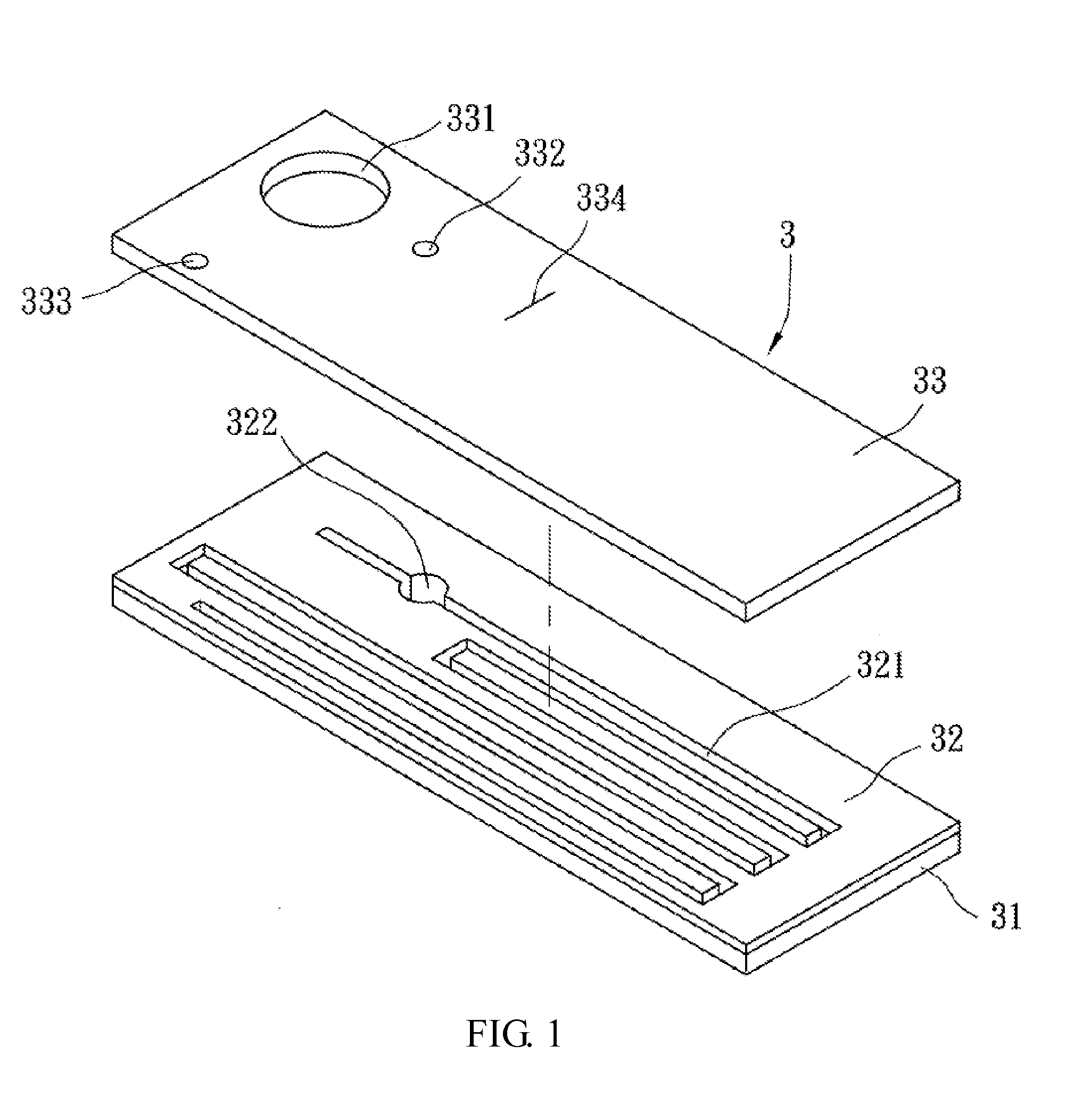
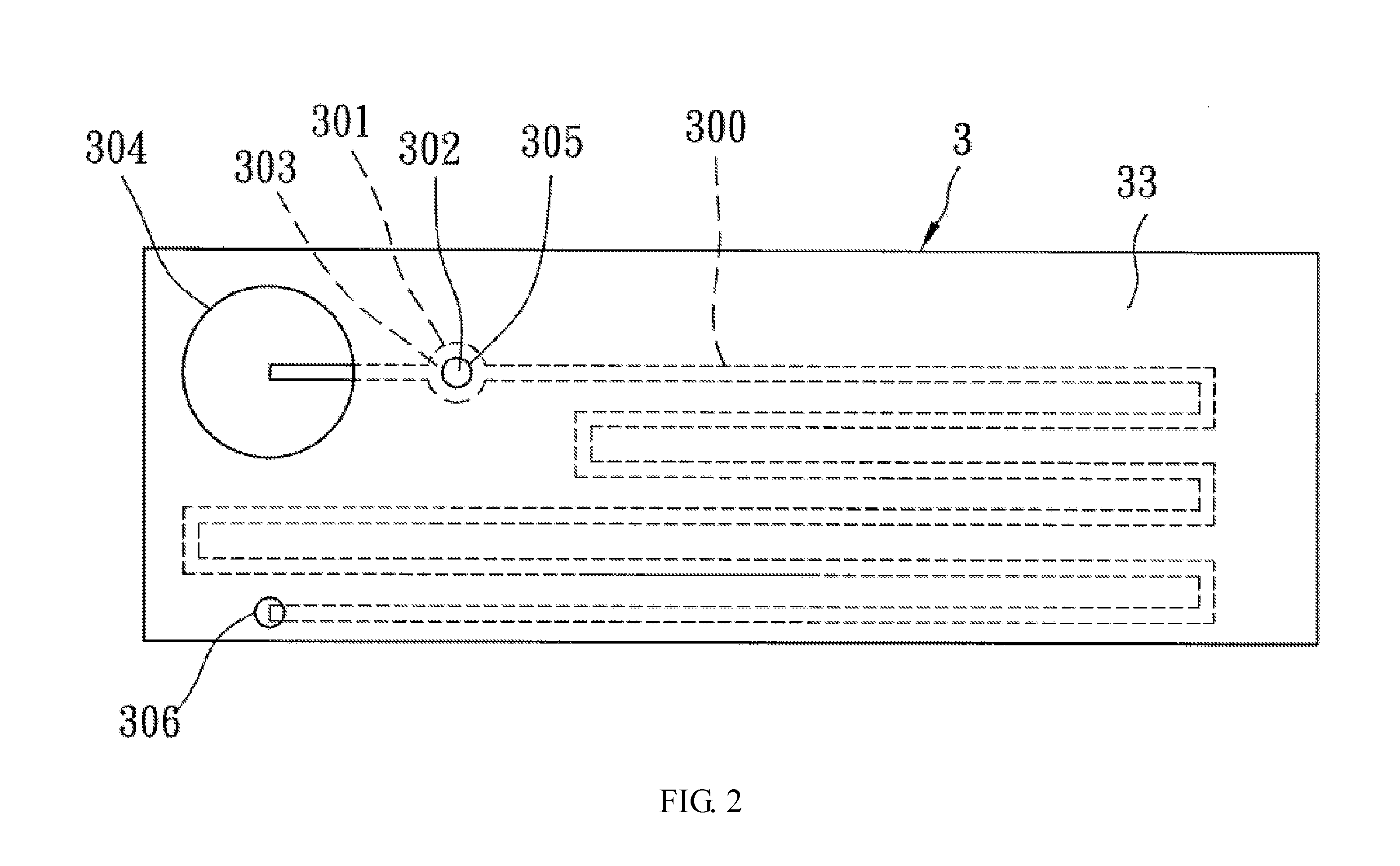
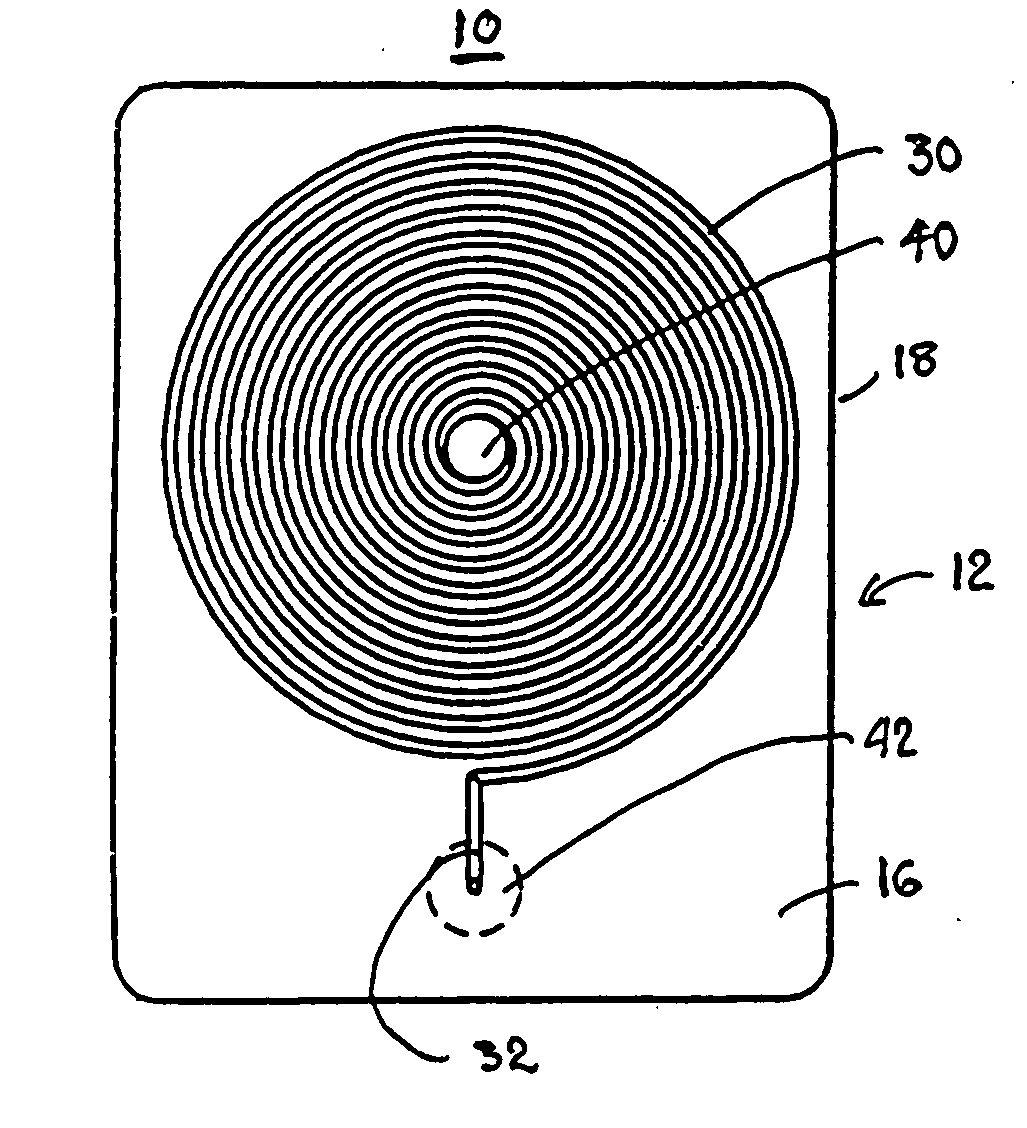
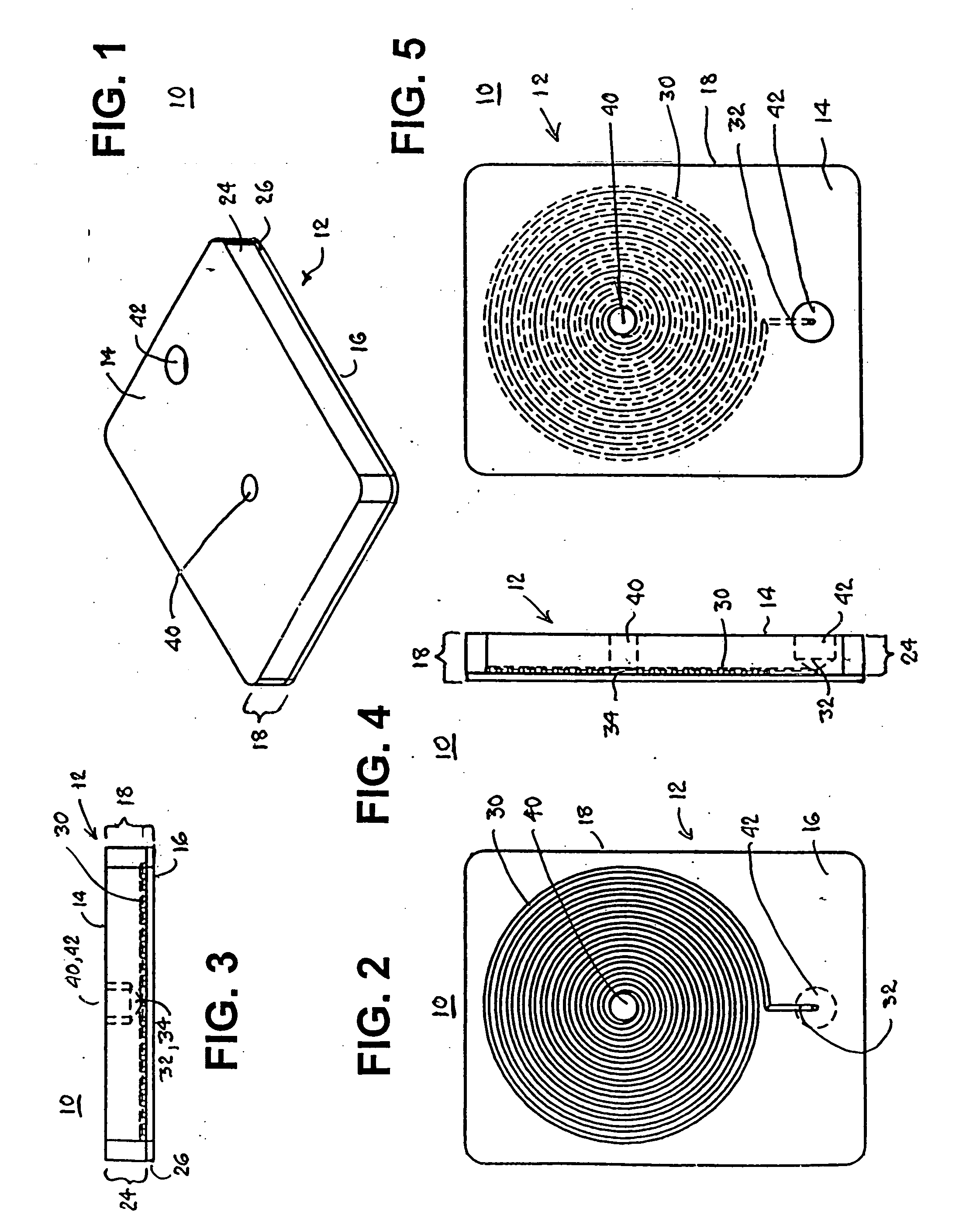
Biomedical chip for blood coagulation test, method of production and use thereof
InactiveUS20110244595A1High viscosityHollow article cleaningCombustion enginesMicrofluidic channelMethods of production
In a biomedical chip for blood coagulation tests and its manufacturing method and use, the biomedical chip comprises a substrate layer, a middle layer, and a cap layer, engaged and stacked with each other to define a microfluidic channel which has a first inlet and an outlet of the microfluidic channel respectively. A mixing interval is expanded outward from the microfluidic channel and interconnected to a second inlet, and has an interconnect portion and a capillary portion disposed between the substrate layer and the cap layer, and more specifically disposed around the periphery of the interconnect portion. With the biomedical chip having the substrate layer and cap layer made of a hydrophilic material, the blood and the reagent can be driven automatically by the capillary force of the microfluidic channel to flow and mix with each other, and the hydrophilic capillary force can be permanently maintained.
Owner:NAT CHENG KUNG UNIV
Coagulation test system
InactiveUS20090221011A1Microbiological testing/measurementPharmaceutical containersSmall sampleQuality control system
A test element for the determination of coagulation in a plasma or whole blood sample having a first surface (2a) and a second surface (4a) in a predetermined distance opposite from each other, said both surfaces being provided with two substantially equivalent patterns forming areas of high and low surface energy which are aligned mostly congruent, whereby the areas of high surface energy create a sample distribution system (6) with at least one detection area (6a), wherein the detection area(s) (6a, 6′a) of the first and second surfaces (2a, 4a) is / are provided with at least one coagulation stimulation reagent. The coagulation test element is provided with an integrated quality control system suitable for dry reagent test strip format with a very small sample volume of about 0.5 μL. The production of the inventive coagulation test element involves only a small number of uncomplicated production steps enabling an inexpensive production of the element.
Owner:EGOMEDICAL TECH
Blood coagulation test cartridge, system, and method
InactiveUS20050255601A1Practical and convenientRapid and inexpensiveMicrobiological testing/measurementWithdrawing sample devicesBlood capillaryEngineering
A system and method for determining a coagulation time, e.g., thrombin time, PT, aPTT, and ACT, of a blood sample deposited in a test cartridge is disclosed. The test cartridge comprises a blood receptacle that is open to the atmosphere into which a blood sample is to be deposited, a vacuum port that is open to atmosphere, and a spiral capillary within the test cartridge having a capillary length and cross-section area, a first capillary end of the spiral capillary open to the blood receptacle and a second capillary end of the spiral capillary open to the vacuum port, whereby the spiral capillary is closed to atmosphere. When a blood sample is deposited in the blood receptacle, a vacuum is drawn through the vacuum port and the blood is drawn through the spiral capillary until coagulation occurs. A pressure change is detected, and the coagulation time is measured.
Owner:MEDTRONIC INC
Blood coagulation test cartridge, system, and method
ActiveUS20080206880A9Practical and convenientRapid and reliableAnalysis using chemical indicatorsFlow propertiesTest sampleEngineering
Owner:MEDTRONIC INC
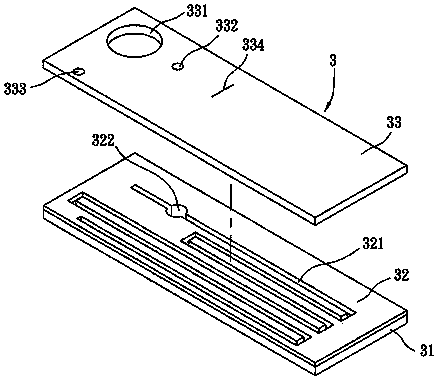
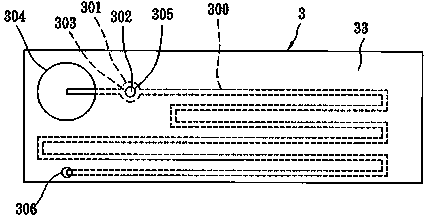
Portable blood coagulation test card
The invention discloses a portable blood coagulation test card. The portable blood coagulation test card comprises a bottom layer with a printed detection electrode, a middle layer through which a blood sample flows, and an upper layer for blood siphon, wherein the upper layer, the middle layer and the bottom layer are stuck in sequence, the upper layer is provided with a sample add area for adding a blood sample, the middle layer is provided with at least two test channels, one end of the test channel is communicated with the sample add area, the other end of the test channel is provided witha test area, one test channel is a delay channel, an obstacle is arranged in the middle in the delay channel, the end part of the test area is provided with an air hole communicated with the upper layer, the inner side wall of the bottom layer is provided with an electrode conduction area connected with the test area, and the end part of the electrode conduction area is inserted into a test hostto be detected. The portable blood coagulation test card can deliver a detection result within one or two minutes through a drop of fingertip blood or venous whole blood only, thereby being convenientto test, being accurate and sensitive, being portable, and being convenient and flexible to use.
Owner:BEIJING LEADMAN BIOCHEM
Full-automatic Newcastle disease detector
InactiveCN105974145AFully automatedImprove sampling efficiencyMaterial analysisMedical equipmentNewcastle disease virus NDV
The invention discloses a full-automatic Newcastle disease detector and relates to the technical field of animal medical equipment. The full-automatic Newcastle disease detector comprises a red blood cell supply unit, a virus liquid supply unit, a reagent supply unit, a reaction plate production unit, a vision interpretation unit, an interpretation region motion unit, a suction head supply unit, a sample feeding region motion unit and a single-shaft double-sliding block motion unit, wherein all the units are mounted on a base. According to the full-automatic Newcastle disease detector, Newcastle disease blood coagulation test and Newcastle disease blood coagulation inhibition test can be simultaneously carried out on Newcastle disease virus samples. The full-automatic Newcastle disease detector is simple and compact in structure and is suitable for batch, rapid and effective tests.
Owner:SHANDONG DOLANG TECH EQUIP
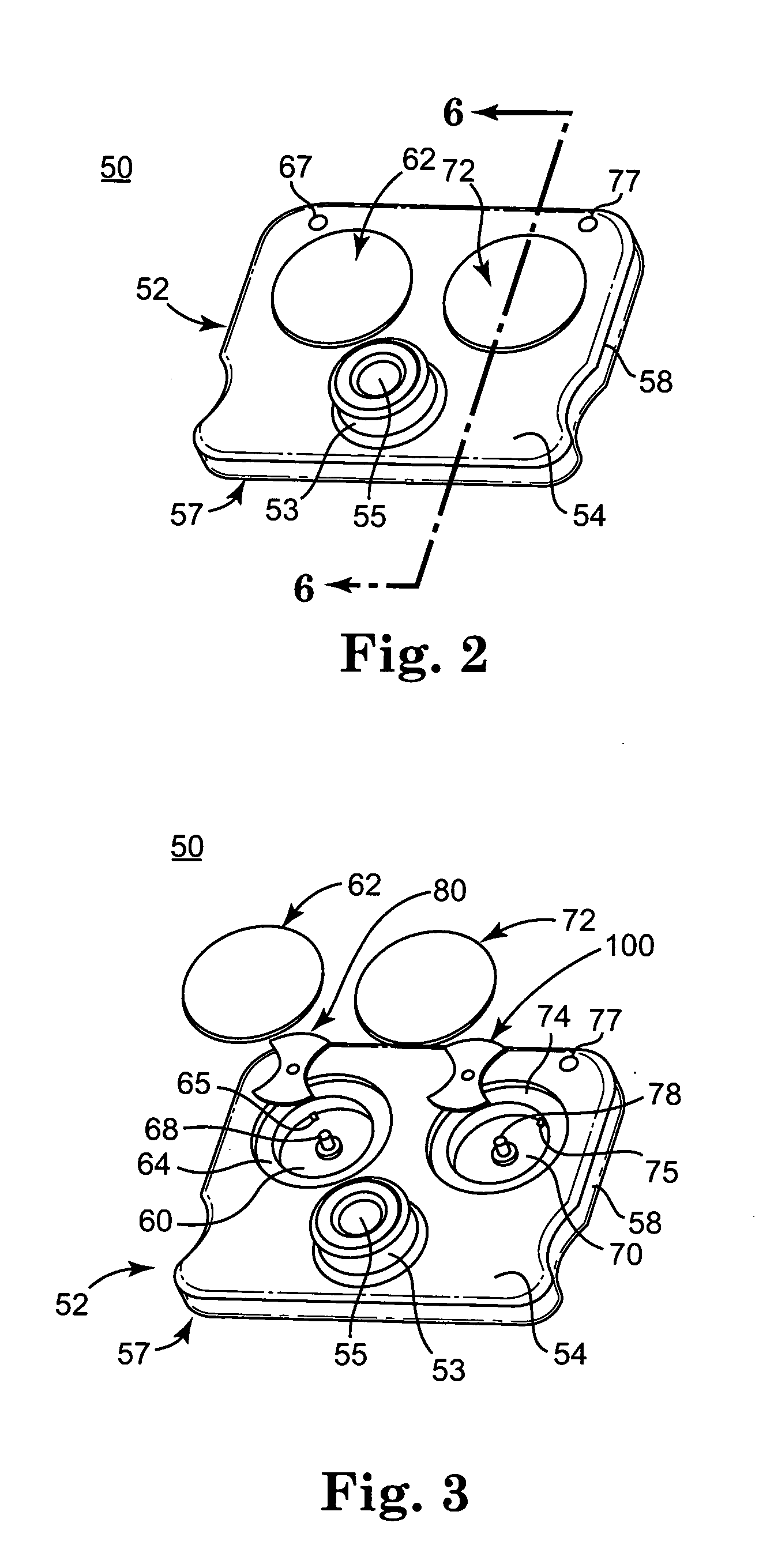
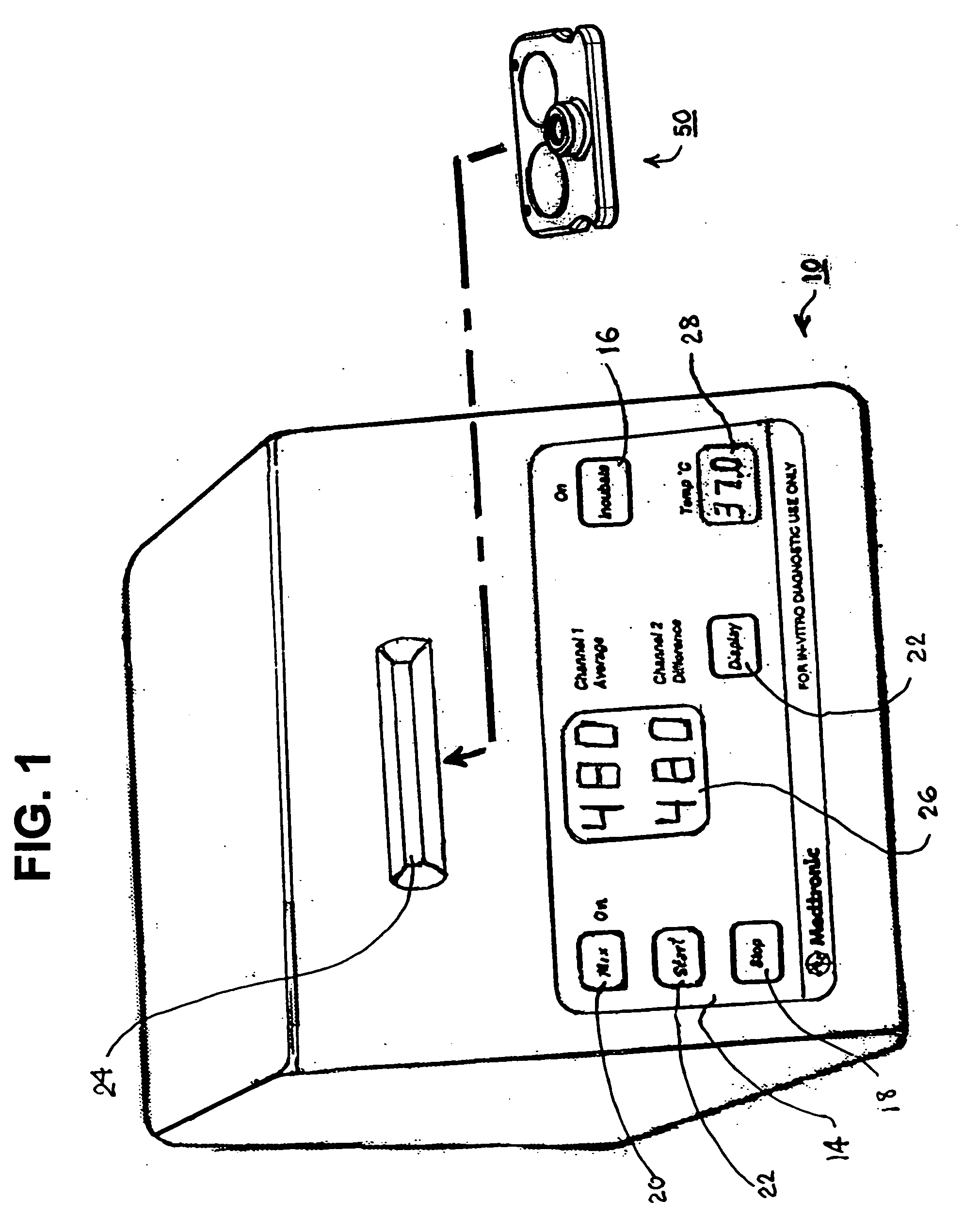
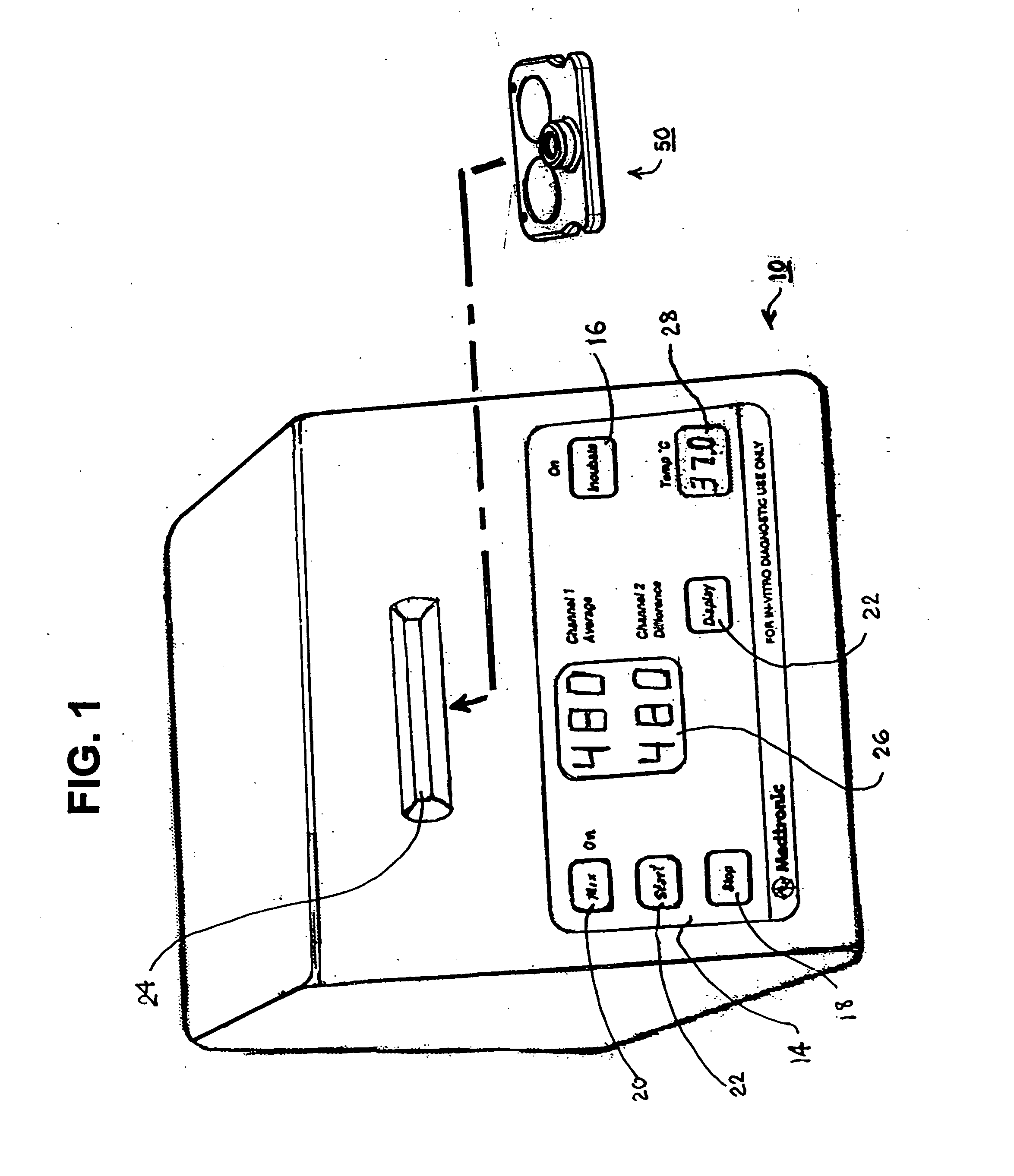
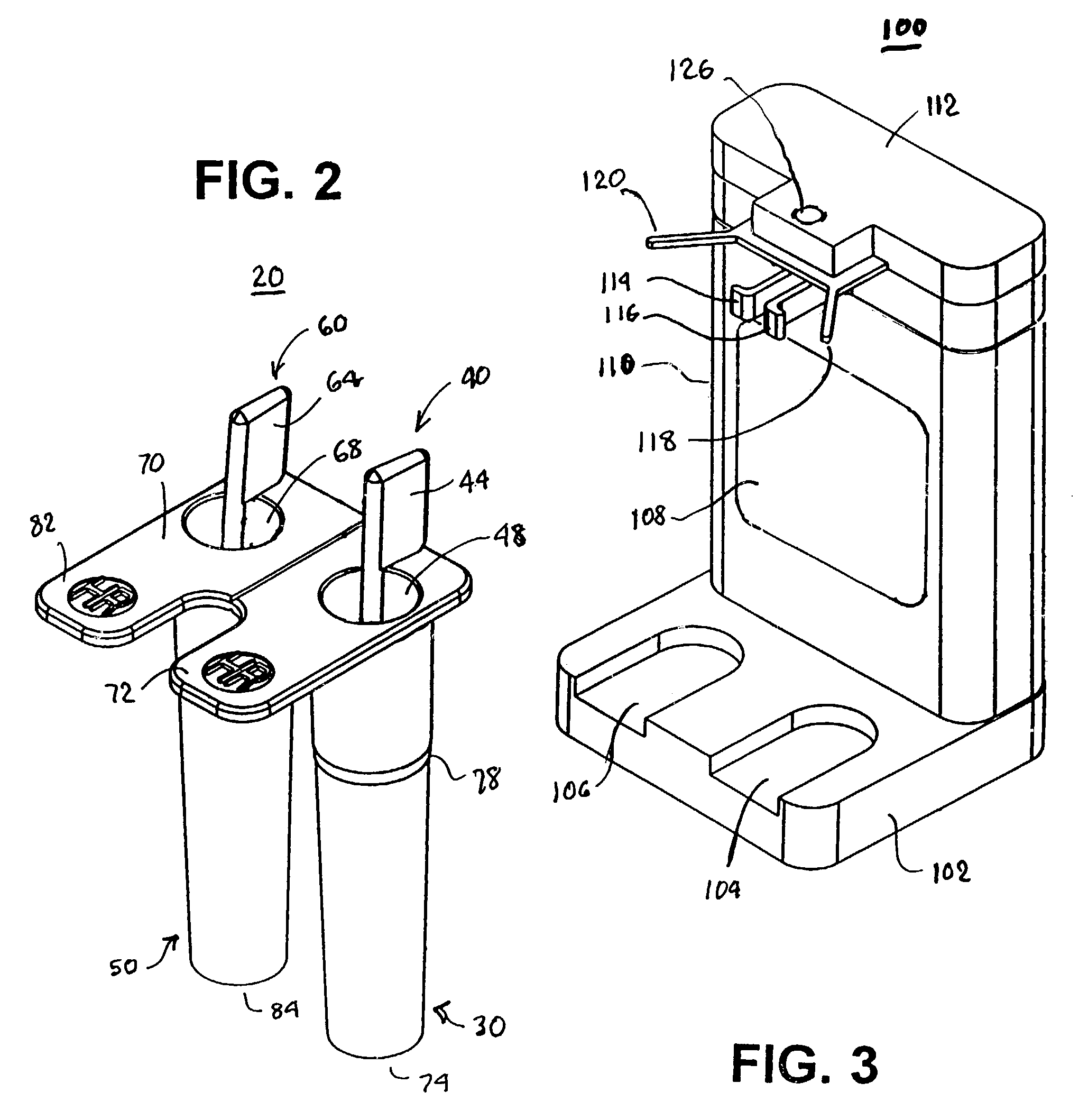
Test cartridge holder for blood samples
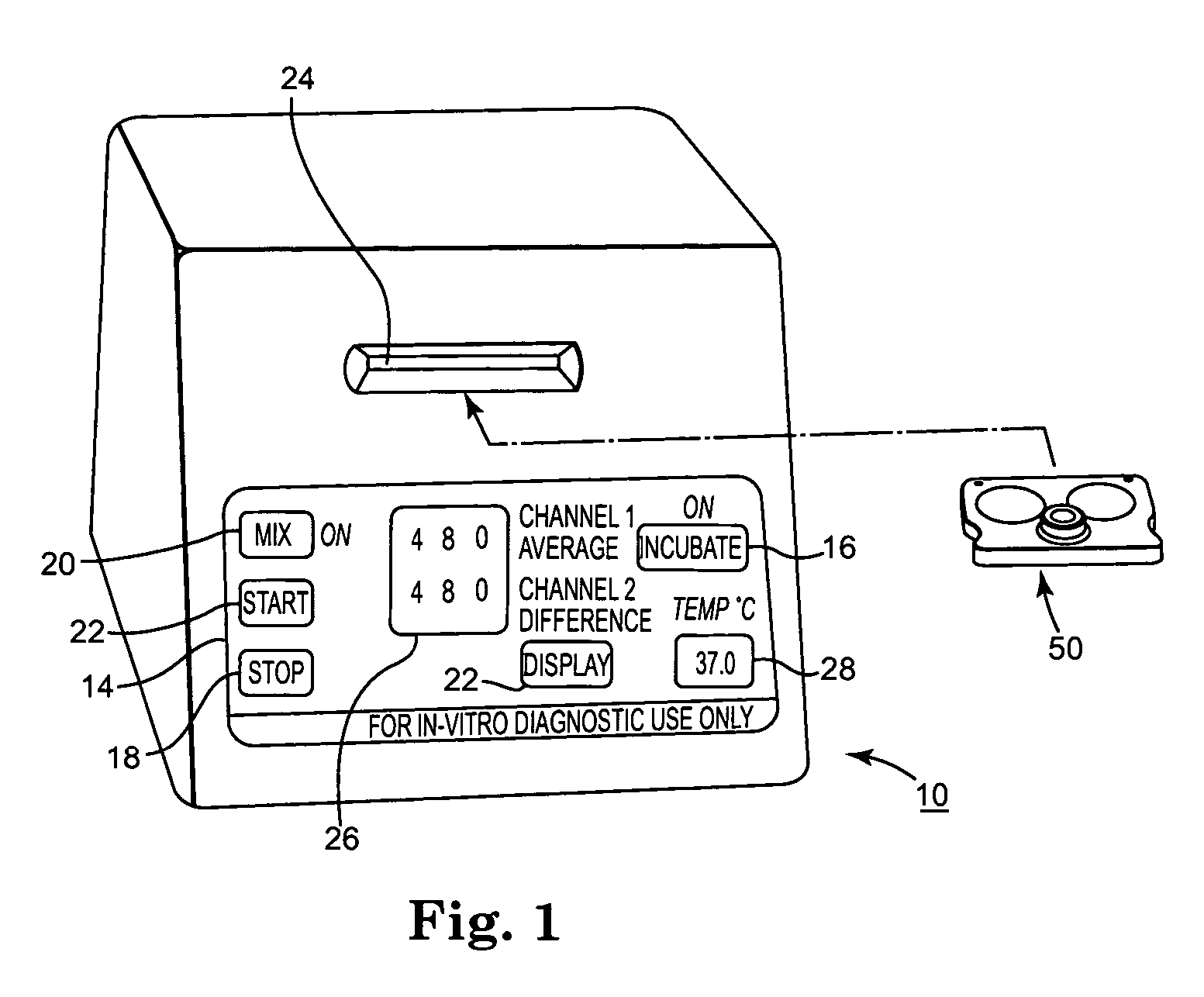
ActiveUS7294312B2More accurateReduce riskJet injection syringesWithdrawing sample devicesManual insertionMagnification
Improved methods and apparatus that make more accurate and reduces risk of filling reaction chambers of cartridge cells with blood samples to conduct blood coagulation tests of the type employing the plunger technique are disclosed. A cartridge holder is provided that secures a test cartridge in a fixed upright position and deflects the plunger flag of each cartridge cell to enable manual insertion of a blood dispenser deeply into the reaction chamber to fill the reaction chamber and avoid contamination of surfaces of the cartridge outside the reaction chamber. Preferably, the cartridge holder provides illumination of the reaction chamber during filling, so that the user can judge when the reaction chamber is properly filled with blood dispensed from the blood dispenser. The cartridge holder may incorporate image magnification to facilitate viewing of the reaction chamber as it is filled.
Owner:MEDTRONIC INC
Method of measuring blood coagulation time to detect lupus anticoagulants
ActiveCN103688177AEasy and Sensitive DetectionMicrobiological testing/measurementDisease diagnosisLiver functionFactor ii
Provided is a method of measuring blood coagulation time which makes it possible to detect lupus anticoagulants more easily and with a higher degree of sensitivity in comparison to the method recommended by the International Society on Thrombosis and Haemostasis (ISTH), and which is not affected by blood-coagulation-factor deficiencies, even in blood samples from patients on warfarin, patients with vitamin K deficiencies and patients with hepatic insufficiency. The method for measuring blood coagulation time to detect lupus anticoagulants is characterized in that a buffering solution composition containing blood coagulation factors is added to a blood sample both before and during the measurement of the blood coagulation time, and the blood coagulation time is measured.
Owner:SCHOOL JURIDICAL PERSON HIGASHI NIPPON GAKUEN +1
coagulation detection device
ActiveCN106153439BRealize multi-dimensional measurementHigh measurement accuracyStrength propertiesCapacitanceEngineering
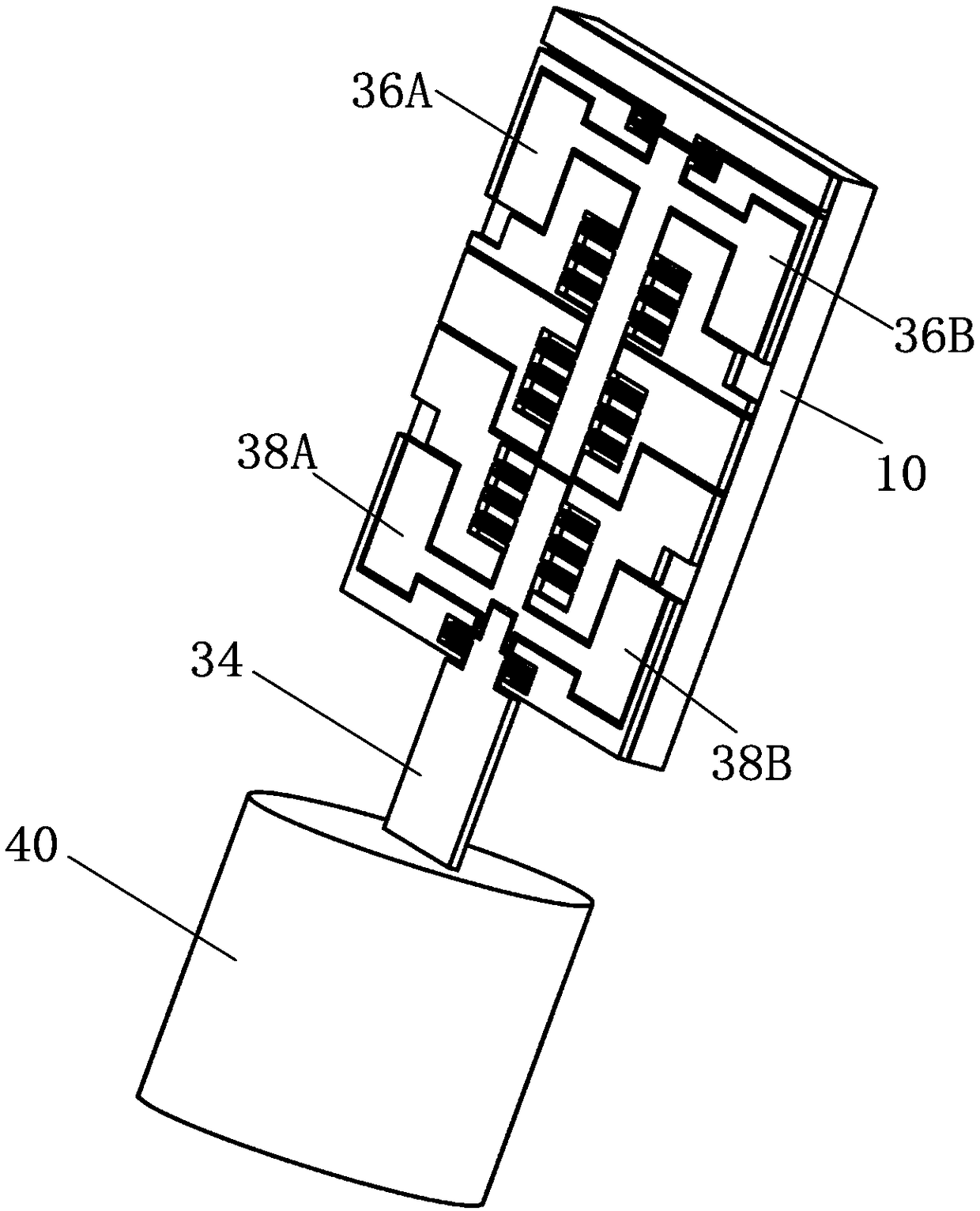
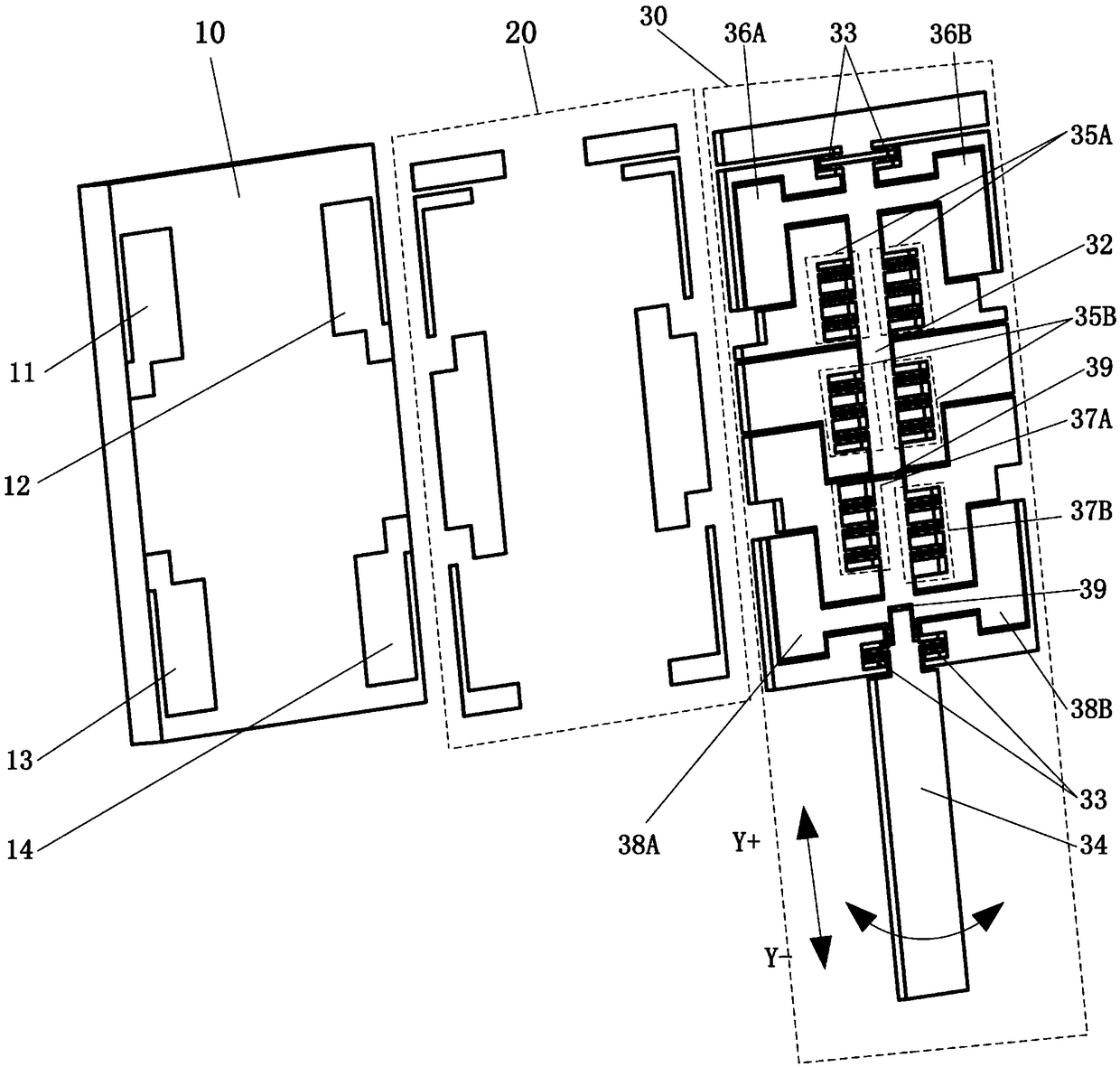
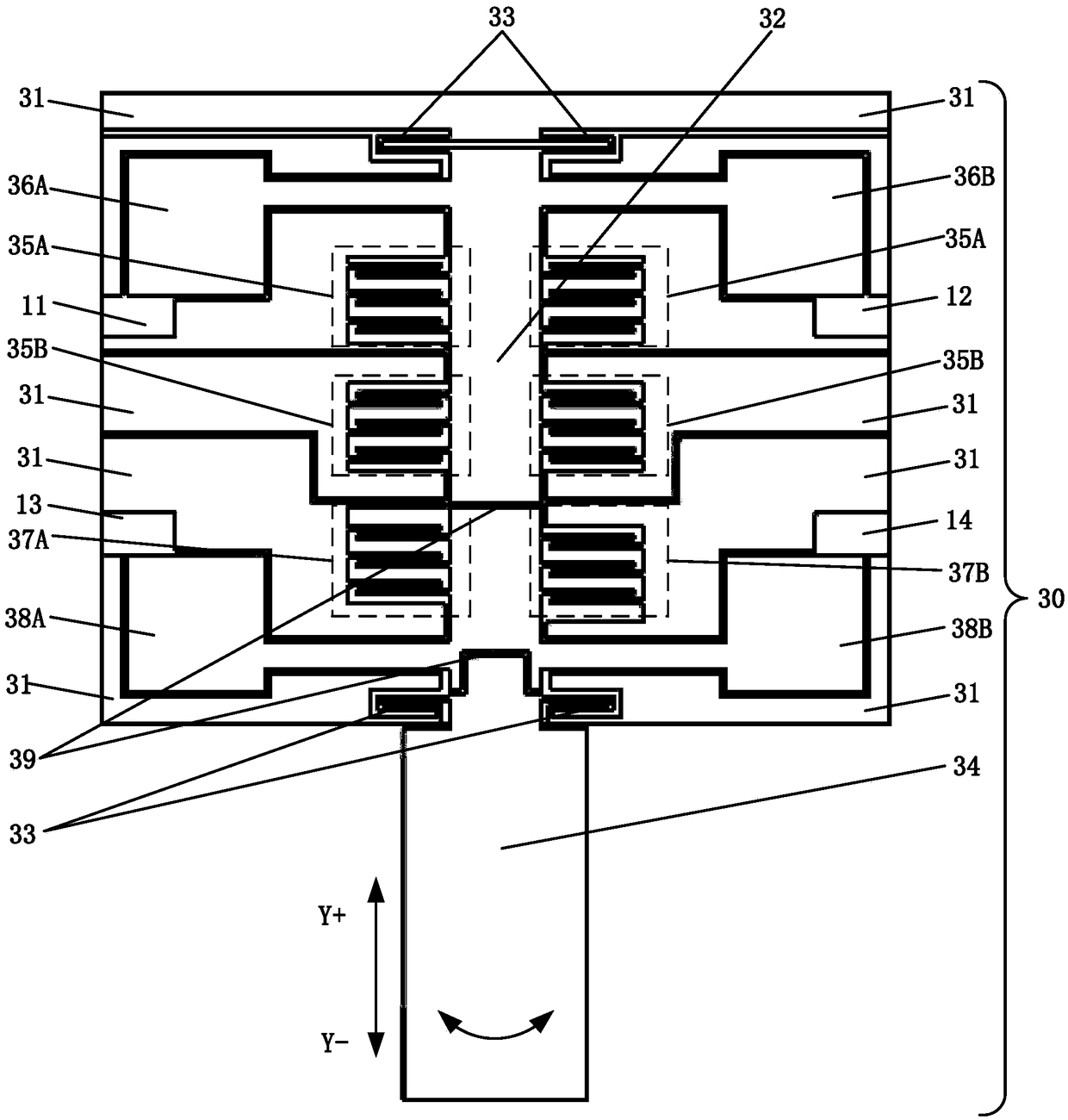
The invention discloses a blood coagulation detector which comprises a base layer of a lamellar structure, a bonding layer of a lamellar structure and a device layer, wherein the device layer comprises a fixed unit, a movable unit and an elastic member; the fixed unit is connected with the base layer through the bonding layer; the movable unit is connected with the fixed unit through the elastic member so that the movable unit is suspended above the base layer; the device layer also comprises a drive capacitor capable of driving the movable unit and a to-be-detected object to do relative motion, a measuring capacitor and a probe; one end of the probe is fixedly connected with the movable unit, and the other end enters the to-be-detected object to do relative motion under the drive of the drive capacitor; the relative motion means that the movable unit reciprocates along a direction and rotates around a direction relative to the to-be-detected object; and the real-time capacitance error of the measuring capacitor is used for calculating the stress of the movable unit reciprocating along a direction and the torque rotating around a direction. The blood coagulation detector disclosed by the invention integrates drive and measurement, has the advantages of high measurement precision, good integration level and low cost and is suitable for batch production.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
Blood coagulation test device and blood coagulation test method
ActiveCN109642909AGet resolutionFlow propertiesBiological testingHematological testBiomedical engineering
The blood coagulation test device according to the present invention is provided with: a container into which test-object blood is placed; a stirring part for stirring the test-object blood in the container; an elastic body capable of deforming in response to a force received through stirring of the test-object blood from the stirring part, the elastic body being connected to the stirring part; acontrol part for transmitting a predetermined rotary motion to the stirring part and causing the stirring part to rotate in reciprocating fashion in a circumferential direction by rotating the elasticpart in reciprocating fashion about an axis of the stirring part as a rotational axis and controlling the reciprocating rotation in a position separated a predetermined diameter from the rotational axis; and a measurement part for measuring the rotation angle pertaining to the reciprocating rotation of the stirring part.
Owner:FUJIMORI KOGYO CO LTD
Coagulation and fibrinolytic cascades modulator
InactiveCN101184775APeptide/protein ingredientsMicrobiological testing/measurementFactor iiPolyphosphate
The invention provides a thromboplastin reagent comprising: tissue factor, a phospholipid and a polyphosphate that acts as a blocker of tissue factor pathway inhibitor (TFPI). Also provided are a composition for promoting clotting comprising polyphosphate, and a reagent for a clotting assay also comprising polyphosphate together with an activator of clotting. Methods for stopping or slowing wound bleeding and fibrinolysis using compositions comprising polyphosphate are also disclosed.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Test Cartridge Holder for Blood Samples
ActiveUS20080009073A1More accurateReduce riskAnalysis using chemical indicatorsWithdrawing sample devicesManual insertionEngineering
Owner:MEDTRONIC INC
A blood coagulation test system driven by sound waves
ActiveCN109765269BRapid responseShorten production timeFlow propertiesMaterial analysis by electric/magnetic meansHematological testSound wave
The invention discloses a blood coagulation test system driven by sound waves. The blood coagulation test system driven by sound waves comprises a hollow glass substrate, an interdigital transducer, apiezoelectric plate, a blood movement reaction tank, a detection electrode and an image acquisition and analysis system, and an interdigital electrode is evaporated on the substrate; the piezoelectric plate is placed on the interdigital electrode; an annular blood reaction tank obtained through cutter cutting is in bonding with the piezoelectric plate through surface treatment; the detection electrode is fixed to the reaction tank through a fixing spring; and by adjusting the position of a bracket, it is ensured that the image acquisition system is located at a suitable position, and the movement condition of blood can be recorded in real time. The blood coagulation test system driven by sound waves is simple and convenient in equipment, the integration level is high, required samples areless, energy consumption is lower, the reaction efficiency is high, open type reaction is easy to operate and maintain, and detection and analysis of various parameters in the blood coagulation process can be realized.
Owner:XI AN JIAOTONG UNIV
Blood coagulation reagent management method and system
PendingCN112067830ALow costImprove loading efficiencyMaterial analysisEmergency medicineBlood coagulations
Owner:SHENZHEN MINDRAY BIO MEDICAL ELECTRONICS CO LTD +1
Biomedical chip used for blood coagulation tests, its manufacturing method and application
ActiveCN102692515ALong-lasting hydrophilicityDecorative surface effectsBiological testingInjection portBlood coagulations
The invention relates to a biomedical chip used for blood coagulation tests, its manufacturing method and application. The biomedical chip used for blood coagulation tests contains a substrate layer, an intermediate layer and a top cover layer in superposed connection, and the substrate layer, the intermediate layer and the top cover layer cooperate to define a microchannel, and a first injection port, an outlet and a second injection port that are respectively connected to the microchannel. An outward expanded mixing zone of the microchannel has connecting portion communicated with the second injection port, and a capillary portion disposed between the substrate layer and the top cover layer, set around and communicated with the connecting portion. With the biomedical chip composed of the substrate layer and the top cover layer prepared from a hydrophilic material, the intrinsic capillary force of the microchannel can drive the flow and mixing of blood and a reagent, and a hydrophilic capillary force can be maintained permanently.
Owner:NAT CHENG KUNG UNIV
Biomedical chip used for blood coagulation tests, its manufacturing method and application
ActiveCN102692515BLong-lasting hydrophilicityDecorative surface effectsBiological testingInjection portBlood coagulations
The invention relates to a biomedical chip used for blood coagulation tests, its manufacturing method and application. The biomedical chip used for blood coagulation tests contains a substrate layer, an intermediate layer and a top cover layer in superposed connection, and the substrate layer, the intermediate layer and the top cover layer cooperate to define a microchannel, and a first injection port, an outlet and a second injection port that are respectively connected to the microchannel. An outward expanded mixing zone of the microchannel has connecting portion communicated with the second injection port, and a capillary portion disposed between the substrate layer and the top cover layer, set around and communicated with the connecting portion. With the biomedical chip composed of the substrate layer and the top cover layer prepared from a hydrophilic material, the intrinsic capillary force of the microchannel can drive the flow and mixing of blood and a reagent, and a hydrophilic capillary force can be maintained permanently.
Owner:NAT CHENG KUNG UNIV
Coagulation test device, system, and method of use
PendingCN113906295ASolve key problemsFlow propertiesLaboratory glasswaresEtiologyPhysical medicine and rehabilitation
A coagulation test device for measuring clotting time and clot characteristics of a whole blood sample under different hemostatic conditions. Results of the test are used as an aid in management of patients with coagulopathy of unknown etiology in order to help the physician determine appropriate clinical action to arrest bleeding in a patient.
Owner:COAGULATION SCI LLC
Multi-level quality control product and preparation method thereof, and application of multi-level quality control product in thrombelastogram detection
PendingCN113325185AQuality is easy to controlEasy to detectBiological testingThrombusQuality control
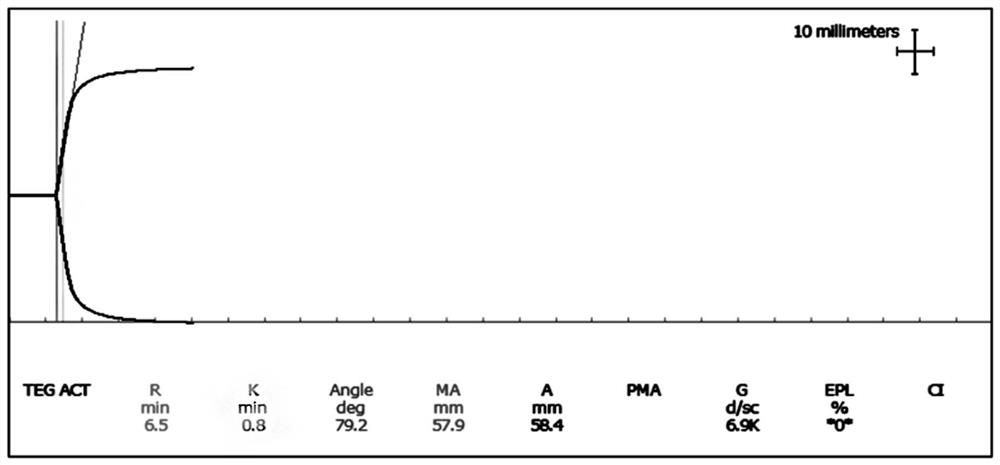
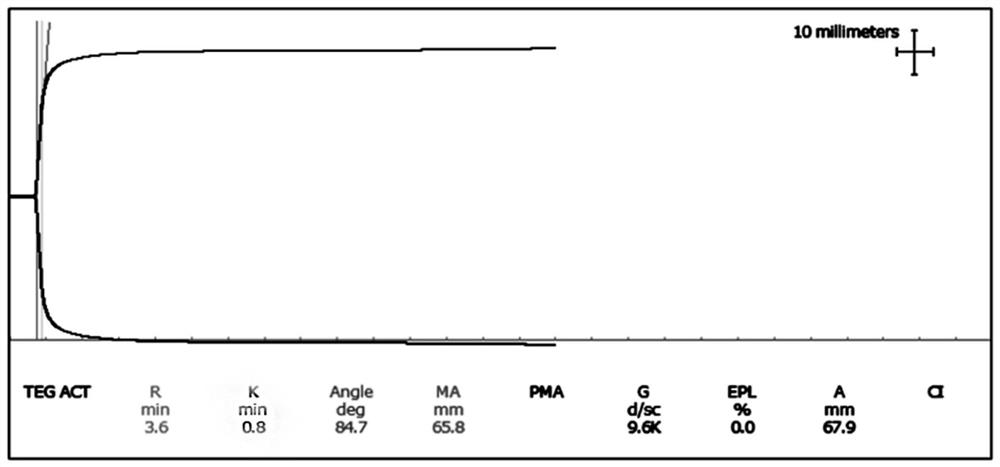
The invention relates to the technical field of quality control of blood coagulation test items, in particular to a multi-level quality control product and a preparation method thereof and application of the multi-level quality control product to thromboelastogram detection. The thrombelastogram quality control product provided by the invention has a level 1 (normal value), a level 2 (high coagulation) and a level 3 (low coagulation), covers various states such as clinical normal coagulation, high coagulation, low coagulation, small Angle and the like, simulates a clinical actual detection environment, is combined with a thrombelastogram instrument, a matched reagent and a calcium chloride solution for use, and can carry out quality control or detection system evaluation on a thromboelastogram detection system. The thrombelastogram quality control product can be applied to quality control practice operation of a thrombelastogram detection system composed of equipment and matched reagents, and the reliability of clinical sample detection results is guaranteed.
Owner:重庆鼎润医疗器械有限责任公司
Blood coagulation test system driven by sound waves
ActiveCN109765269ARapid responseShorten production timeFlow propertiesMaterial analysis by electric/magnetic meansElectricityEngineering
The invention discloses a blood coagulation test system driven by sound waves. The blood coagulation test system driven by sound waves comprises a hollow glass substrate, an interdigital transducer, apiezoelectric plate, a blood movement reaction tank, a detection electrode and an image acquisition and analysis system, and an interdigital electrode is evaporated on the substrate; the piezoelectric plate is placed on the interdigital electrode; an annular blood reaction tank obtained through cutter cutting is in bonding with the piezoelectric plate through surface treatment; the detection electrode is fixed to the reaction tank through a fixing spring; and by adjusting the position of a bracket, it is ensured that the image acquisition system is located at a suitable position, and the movement condition of blood can be recorded in real time. The blood coagulation test system driven by sound waves is simple and convenient in equipment, the integration level is high, required samples areless, energy consumption is lower, the reaction efficiency is high, open type reaction is easy to operate and maintain, and detection and analysis of various parameters in the blood coagulation process can be realized.
Owner:XI AN JIAOTONG UNIV
Blood coagulation testing device and blood coagulation testing method
The present invention provides a blood coagulation testing device and a blood coagulation testing method. The blood coagulation testing device comprises: a container, which is filled with the blood of the test object; a stirring part, which stirs the blood of the test target in the container; The received force deforms correspondingly; the control part makes the elastic body reciprocate with the shaft of the stirring part as the rotating shaft, and controls the reciprocating rotation at a position separated from the rotating shaft by a predetermined diameter, thereby transmitting a predetermined rotational motion to the stirring part, The stirring part is reciprocated in the circumferential direction; the measuring part measures the rotation angle involved in the reciprocating rotation of the stirring part.
Owner:FUJIMORI KOGYO CO LTD
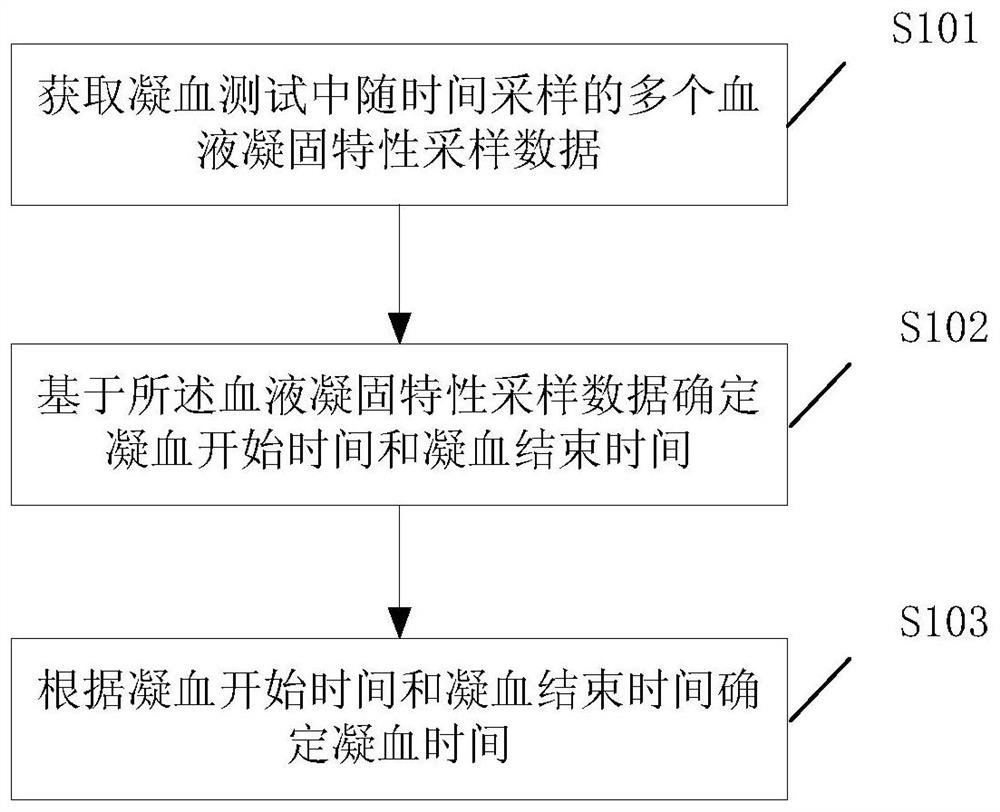
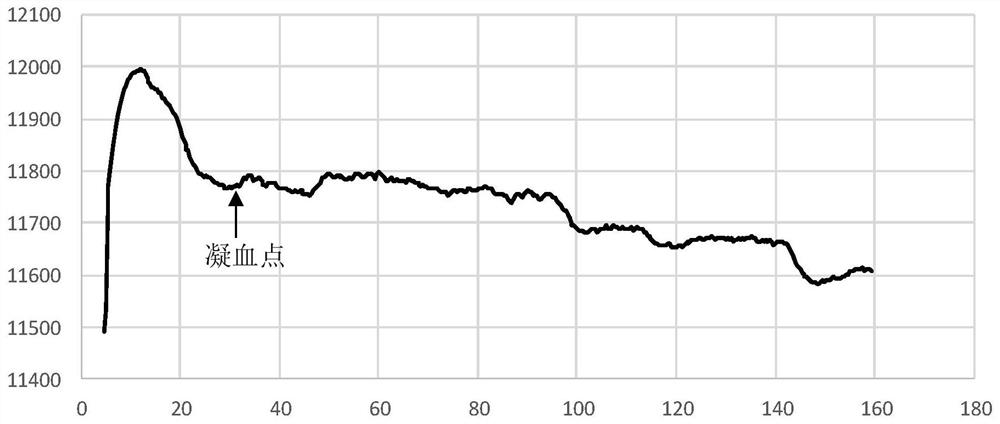
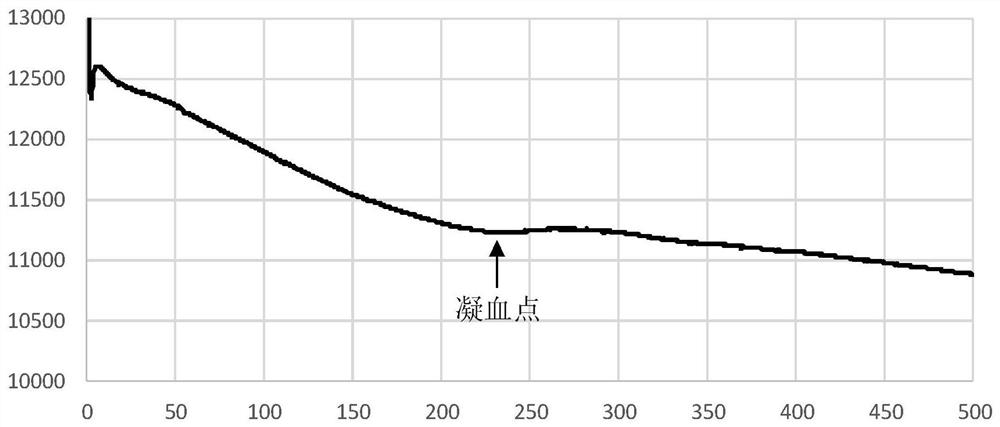
Clotting time determination method, electronic equipment and storage medium
ActiveCN108761105BImprove judgment efficiencyBiological testingHematological testIntensive care medicine
A method for determining blood coagulation time, an electronic apparatus, and a storage medium. The method comprises: acquiring multiple pieces of blood coagulation feature sampling data sampled over time during a blood coagulation test; determining a blood coagulation starting time and a blood coagulation ending time on the basis of the blood coagulation feature sampling data; and determining a blood coagulation time according to the blood coagulation starting time and the blood coagulation ending time. The method determines blood coagulation time by means of data analysis, thereby improving the efficiency of determining a blood coagulation time.
Owner:GUANGZHOU WONDFO BIOTECH
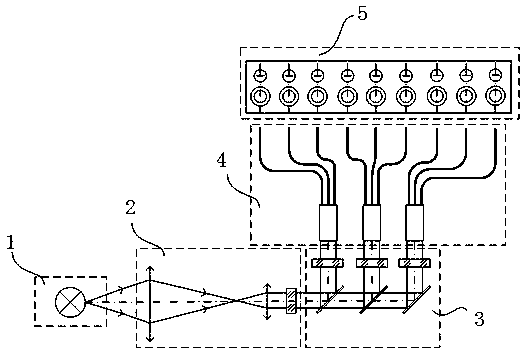
Two-way light splitting blood coagulation test device
InactiveCN109709345AWork reliablyLow costTransmissivity measurementsBiological testingBlood coagulation analyzerLight guide
The invention discloses a two-way light splitting blood coagulation test device. The device comprises a base plate and a first light source device arranged on the base plate. The base plate is successively connected to a light collecting device for collecting polychromatic light emitted by the first light source device, a light splitting device for dividing the polychromatic light into monochromatic light with different wavelengths, a light guiding device for conducting the monochromatic light to a test area and a first measuring device for measuring the optical signal of an object to be tested. The light splitting device comprises a two-way light splitting component so as to realize the multi-project detection of a sample. The polychromatic light is converted into the monochromatic lightwith different wavelengths to be detected through the light splitting device and the light guiding device, and a motor and a driver which filter rotation wheel movement in a traditional blood coagulation analyzer depends on are eliminated. Working is reliable and cost is low.
Owner:DIRUI MEDICAL TECH CO LTD
Blood testing system and method
PendingCN114113563AMinimize interactionEfficient use ofFlow propertiesLaboratory glasswaresBlood testThrombus
Some embodiments of a blood coagulation test system include an analyzer console device and a single-use cartridge component configured to be releasably mounted into the console device. In some embodiments, the blood coagulation test system may operate as an automatic thrombus elasticity measurement system that is particularly useful, for example, in the location of point-of-care testing.
Owner:CA卡希索有限公司
Binding Molecule Activating FXII
PendingUS20200182890A1Immunoglobulins against blood coagulation factorsBiological material analysisAntiendomysial antibodiesBlood coagulations
The present invention relates to a binding molecule, in particular an antibody or binding fragment thereof, capable of activating FXII, which binds to the proline rich domain of FXII. In particular, the invention is directed to FXII activating antibodies or binding fragments thereof which binds to the proline rich domain of FXII. The invention also encompasses the use of the binding molecule directed to the proline rich domain of FXII as blood coagulation activator, e.g. in diagnostic blood coagulation tests. Corresponding methods and blood coagulation test are also encompassed.
Owner:UNIVERSITAETSKLINIKUM HAMBURG EPPENDORF
Portable coagulation test card
The invention discloses a portable blood coagulation test card. The portable blood coagulation test card comprises a bottom layer with a printed detection electrode, a middle layer through which a blood sample flows, and an upper layer for blood siphon, wherein the upper layer, the middle layer and the bottom layer are stuck in sequence, the upper layer is provided with a sample add area for adding a blood sample, the middle layer is provided with at least two test channels, one end of the test channel is communicated with the sample add area, the other end of the test channel is provided witha test area, one test channel is a delay channel, an obstacle is arranged in the middle in the delay channel, the end part of the test area is provided with an air hole communicated with the upper layer, the inner side wall of the bottom layer is provided with an electrode conduction area connected with the test area, and the end part of the electrode conduction area is inserted into a test hostto be detected. The portable blood coagulation test card can deliver a detection result within one or two minutes through a drop of fingertip blood or venous whole blood only, thereby being convenientto test, being accurate and sensitive, being portable, and being convenient and flexible to use.
Owner:BEIJING LEADMAN BIOCHEM
Automatic Newcastle Disease Detector
InactiveCN105974145BSimple structureCompact structureMaterial analysisNewcastle disease virus NDVRed Cell
The invention discloses a full-automatic Newcastle disease detector and relates to the technical field of animal medical equipment. The full-automatic Newcastle disease detector comprises a red blood cell supply unit, a virus liquid supply unit, a reagent supply unit, a reaction plate production unit, a vision interpretation unit, an interpretation region motion unit, a suction head supply unit, a sample feeding region motion unit and a single-shaft double-sliding block motion unit, wherein all the units are mounted on a base. According to the full-automatic Newcastle disease detector, Newcastle disease blood coagulation test and Newcastle disease blood coagulation inhibition test can be simultaneously carried out on Newcastle disease virus samples. The full-automatic Newcastle disease detector is simple and compact in structure and is suitable for batch, rapid and effective tests.
Owner:SHANDONG DOLANG TECH EQUIP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com