Multi-level quality control product and preparation method thereof, and application of multi-level quality control product in thrombelastogram detection
A technology of thromboelasticity and quality control products, applied in the direction of biological testing, material inspection products, etc., to achieve the effects of easy industrial production, simple preparation process, and sufficient sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
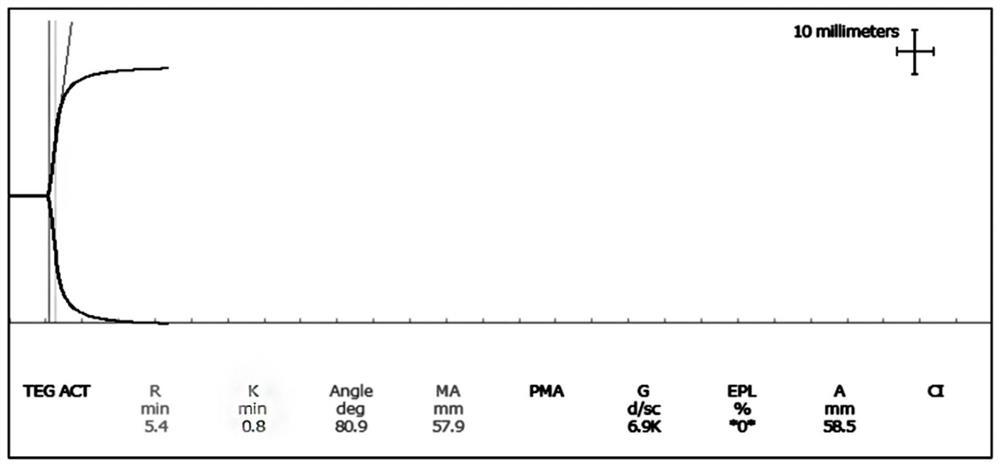
[0039] Embodiment 1: Thromboelastography Quality Control Level 1 (Normal Value Quality Control)
[0040] 1. Preparation of plasma matrix solution and fibrinogen matrix solution
[0041] (1) Collection of anticoagulant pig blood: when pigs are slaughtered, fresh pig whole blood and 3.8% sodium citrate anticoagulant are mixed at a volume ratio of 9:1 to collect anticoagulant pig blood.
[0042] (2) Preparation of porcine plasma: the collected anticoagulated porcine blood was placed at room temperature for 3-6 hours, and then separated into layers. Aspirate the upper liquid, put it into a tube centrifuge for centrifugation, and set the centrifugation parameters: rotation speed 4500rpm, time 15min, temperature 4°C. After centrifugation, the supernatant was carefully sucked out, and sodium azide preservative with a final concentration of 0.02% was added to obtain pig plasma, which was frozen at -20 to -30°C (valid for 3 years). The lower layer is mainly red blood cells, which are...
Embodiment 2
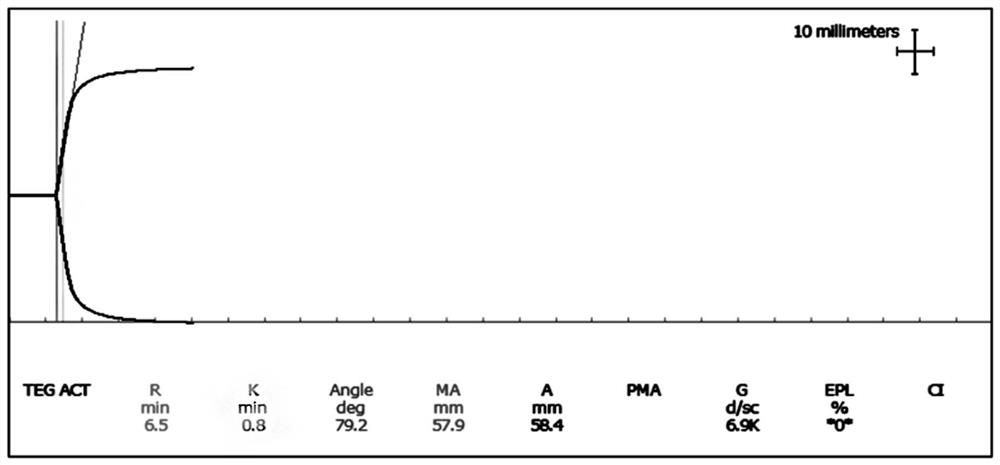
[0069] Embodiment 2: Thromboelastography quality control product level 2 (high coagulation quality control product)
[0070] 1. Preparation of Thromboelastography Quality Control Level 2
[0071] (1) Take out the frozen fibrinogen matrix solution and place it in a 37±2°C water bath to thaw quickly. Add 100.0 mL of thawed fibrinogen matrix solution into a 1000 mL beaker, and then add 900 mL of plasma matrix solution to obtain solution A. In this step, the ratio of fibrinogen matrix solution to plasma matrix solution can be controlled at 5-15:82-94, so that in the subsequent adjustment process, it is easier to adjust the MA value to the range of 60.0-80.0mm .
[0072] (2) Take solution A, use thromboelastography instrument (DRNX-Ⅲ, DRNX-Ⅳ, DRNX-Ⅴ, TEG 5000, etc.) and supporting reagents (thromboelastography test (activated coagulation) reagent (coagulation method), etc ) detection, the MA value is required to be in the range of 60.0-80.0mm; when the MA value is 80.0mm, add an...
Embodiment 3
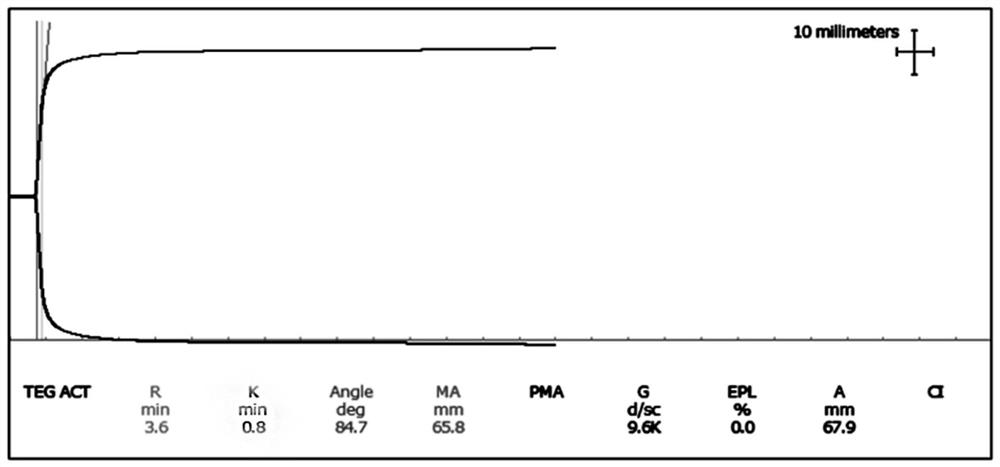
[0096] Embodiment 3: Thromboelastography quality control product level 3 (low coagulation quality control product)
[0097] 1. Preparation of Thromboelastography Quality Control Level 3
[0098] (1) Take out the frozen fibrinogen matrix solution and place it in a 37±2°C water bath to thaw quickly. Take 70.0mL of thawed fibrinogen matrix solution and add it to a 1000mL beaker, then add 200mL of plasma matrix solution and 730mL of 6% hydroxyethyl starch 130 / 0.4 saline solution (6% hydroxyethyl starch saline solution, that is, artificial plasma ), to obtain solution A'. In this operation step, the volume ratio of fibrinogen matrix solution, plasma matrix solution and 6% hydroxyethyl starch 130 / 0.4 saline solution is controlled at 2-10:18-22:67-74, all of which can be prepared to meet the requirements quality control products.
[0099] (2) Take the solution A' and test it with a thromboelastograph (such as DRNX-Ⅲ type) and supporting reagents (thromboelastography test (activate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com