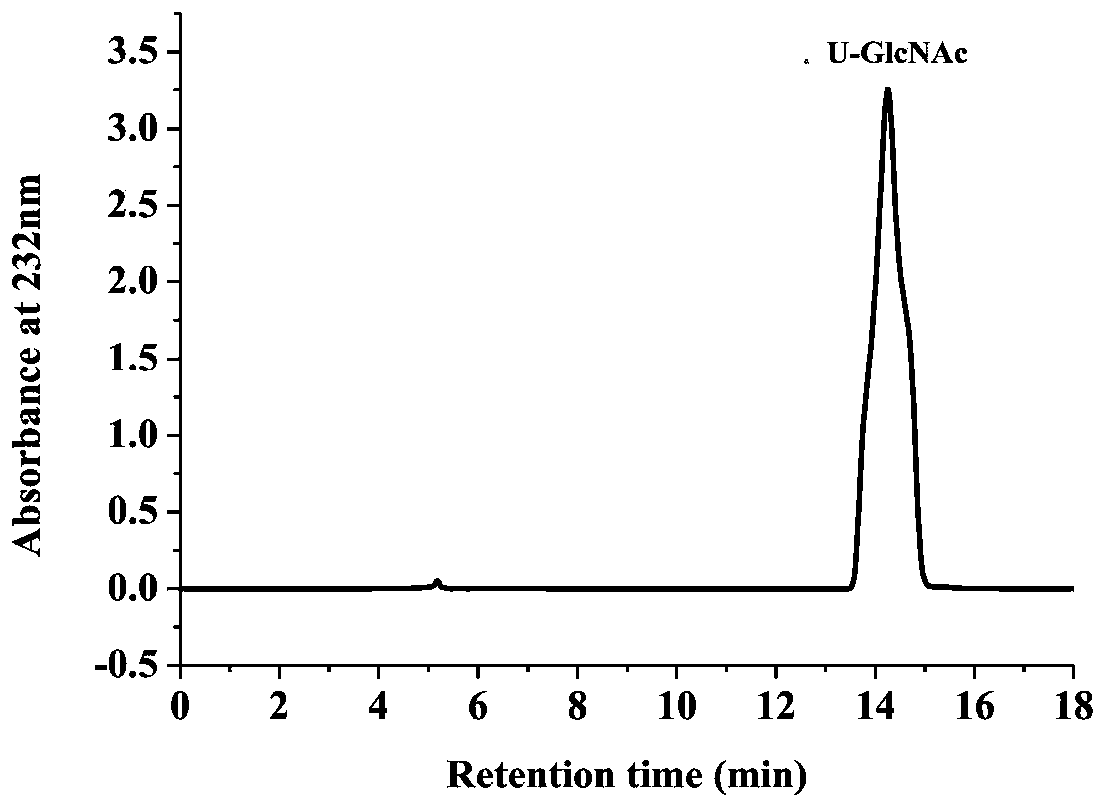
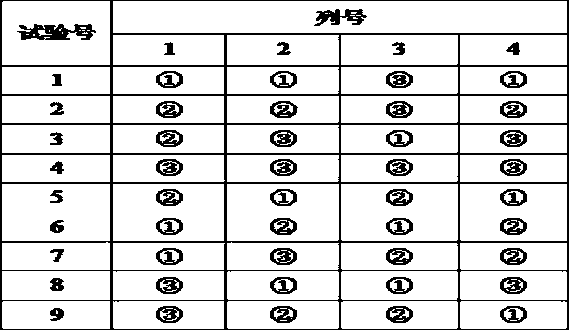
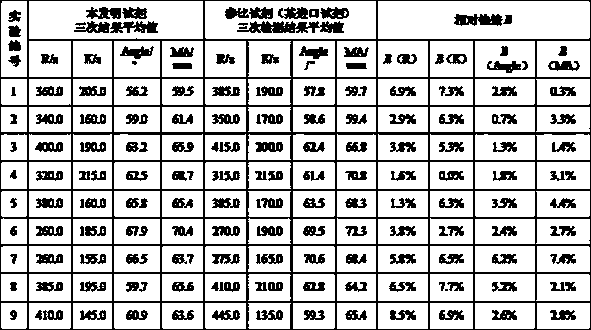
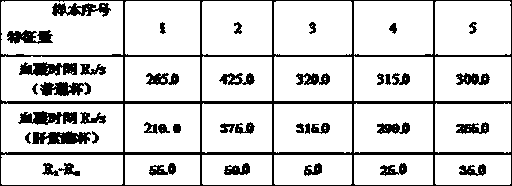
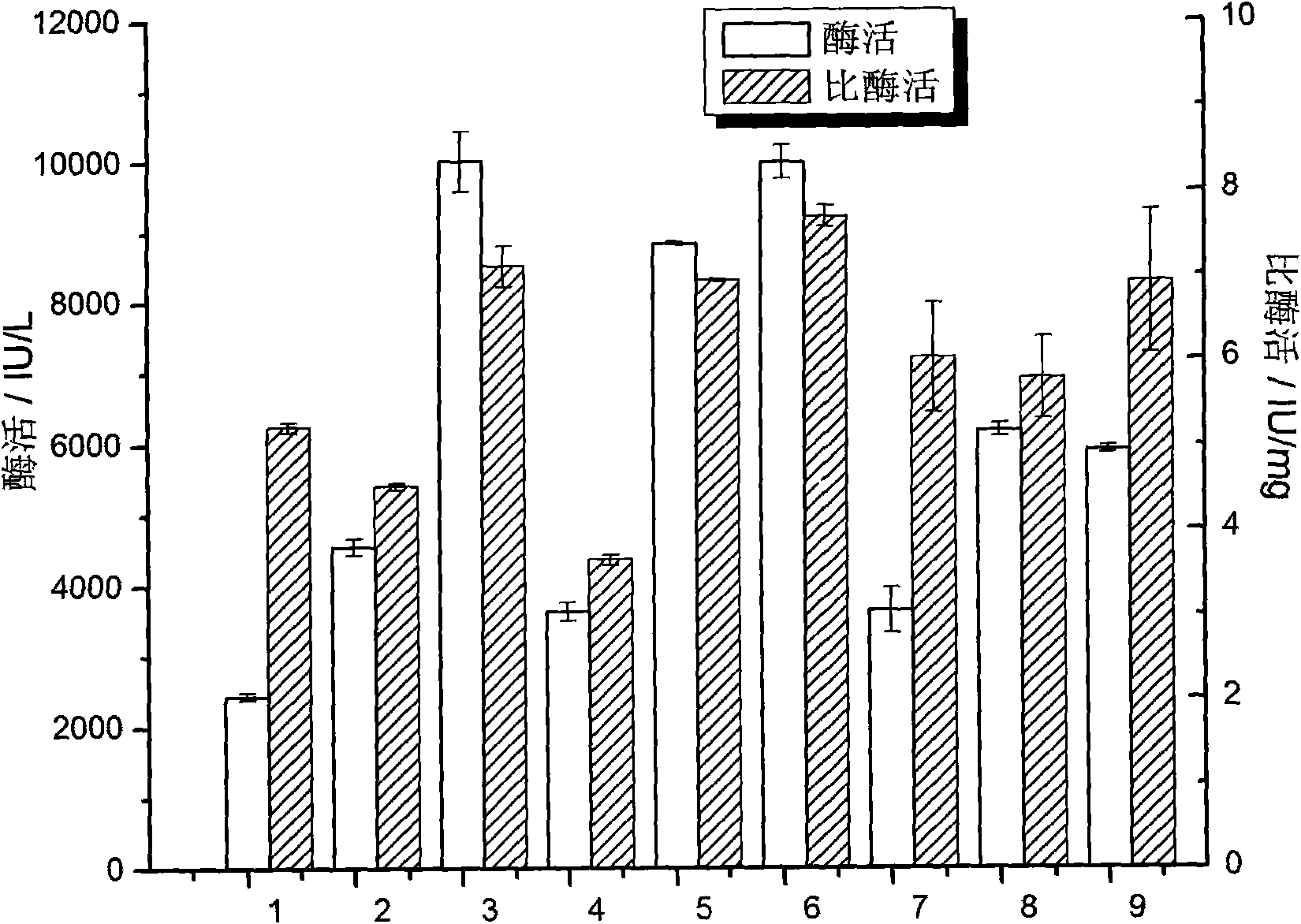
Patents
Literature
80 results about "Heparinase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
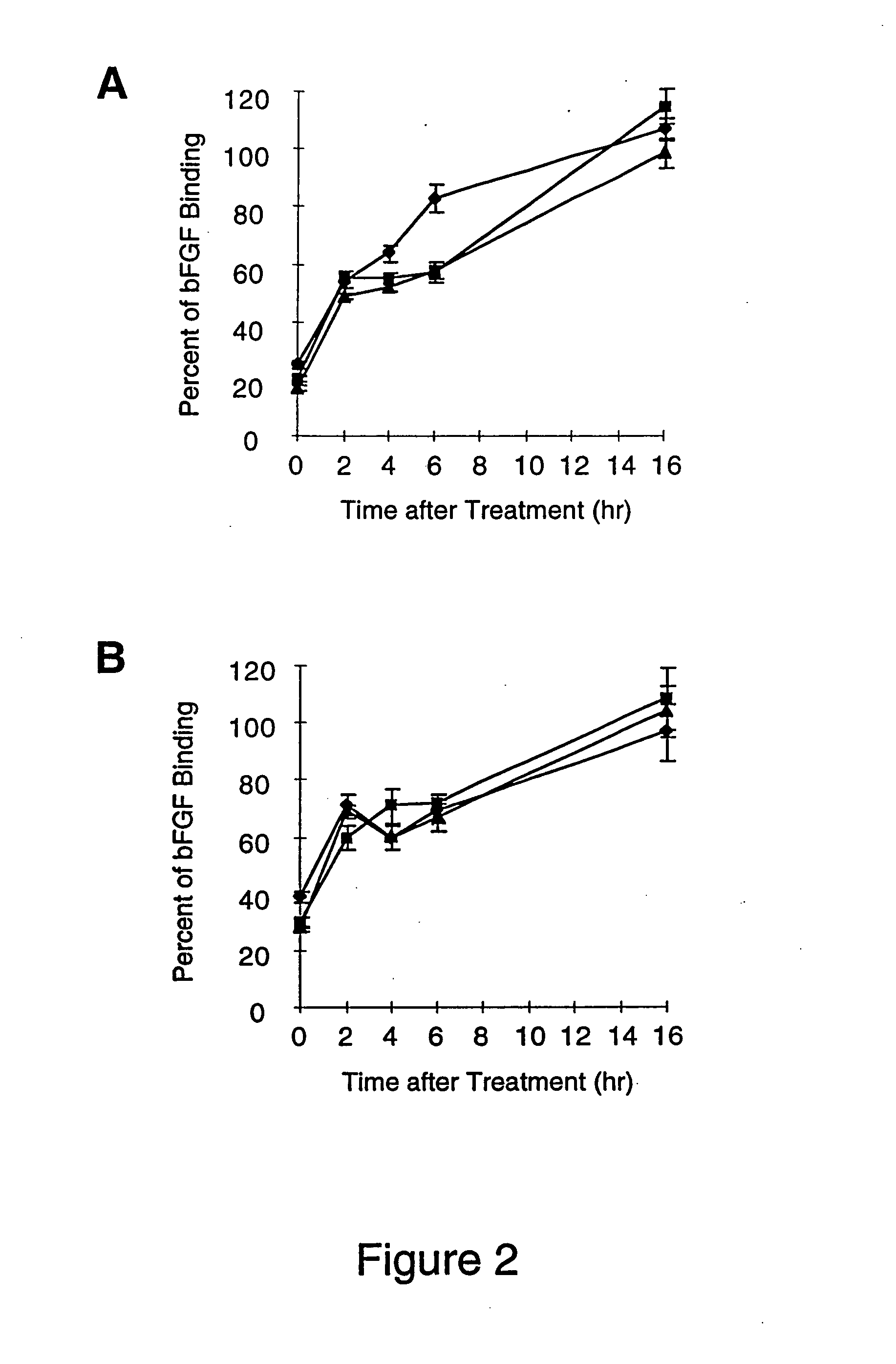
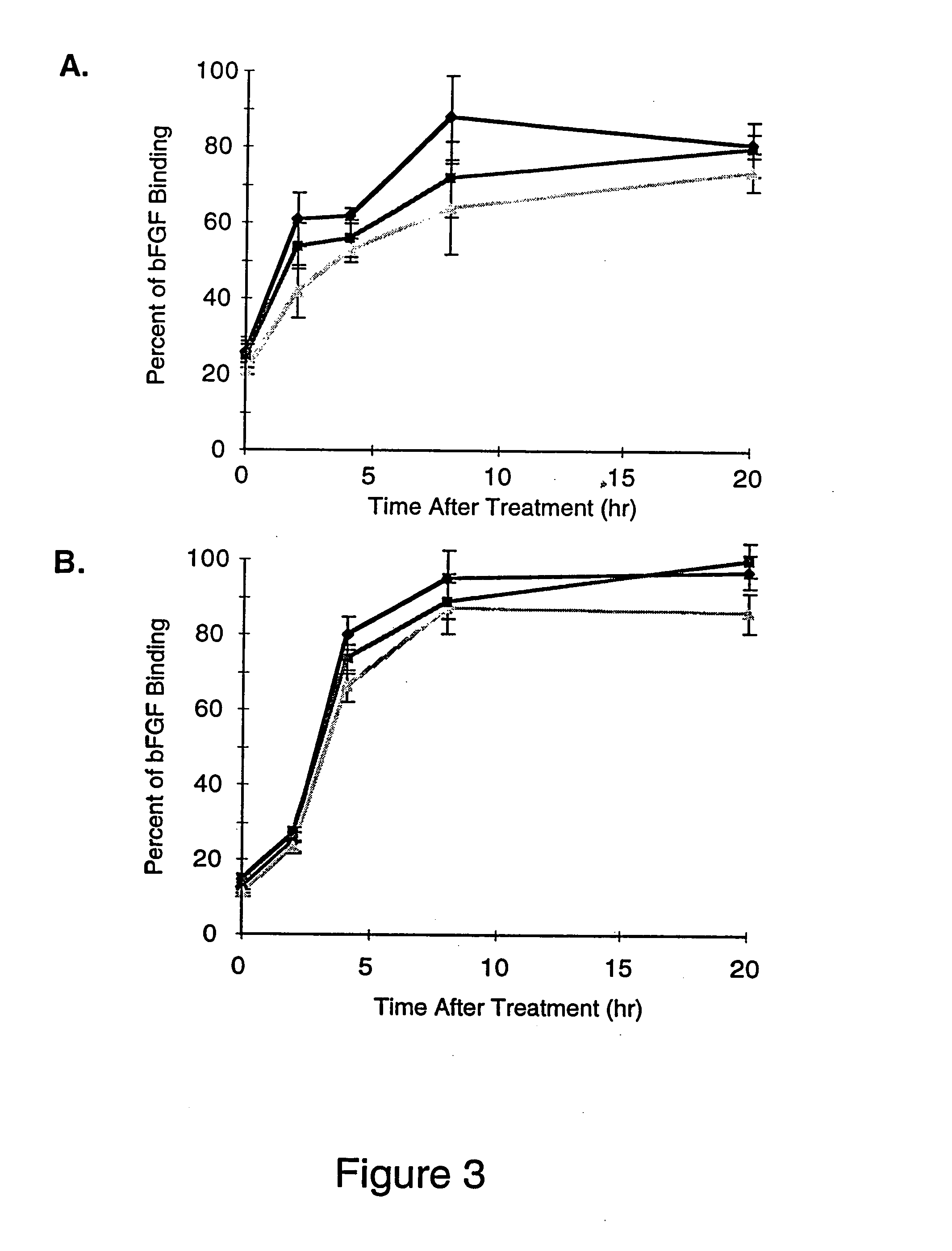
A family of bacterial lyases that catalyze the cleavage of heparin and heparan sulfate glycosaminoglycans.
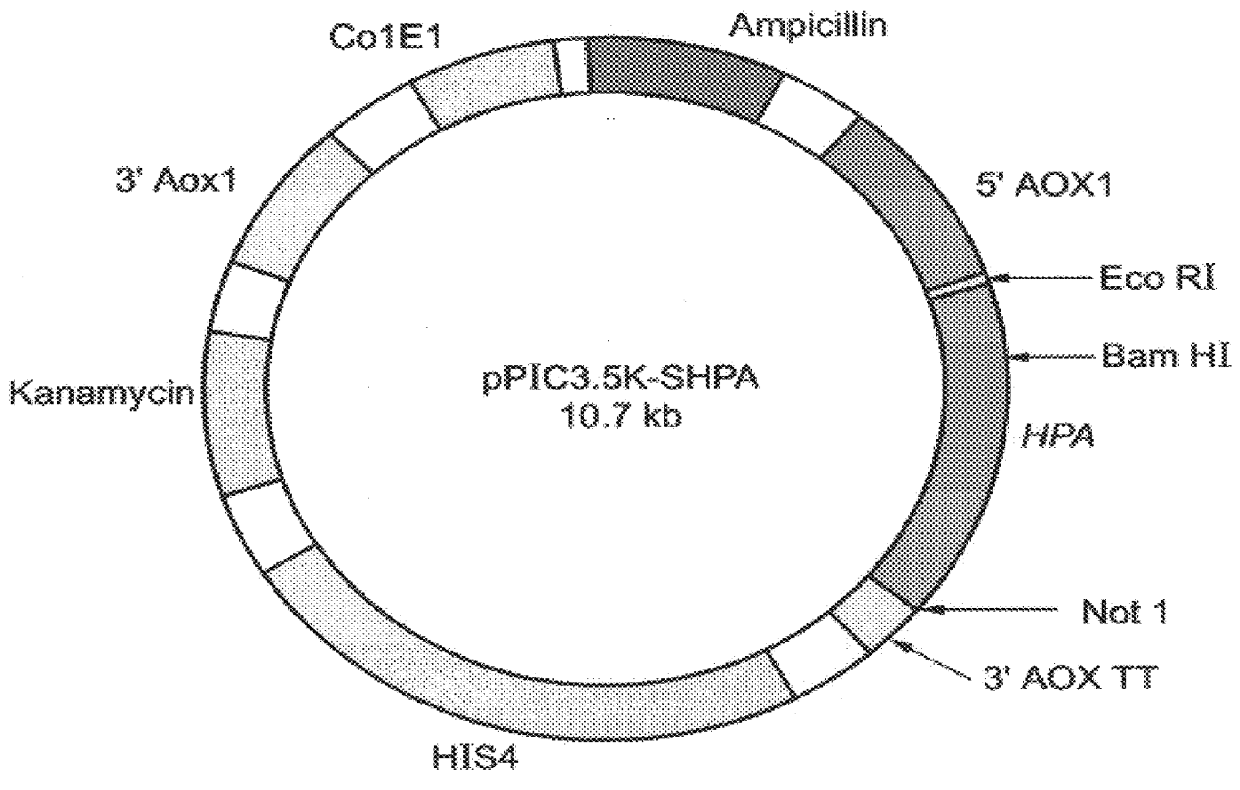
Heparinase III HLGAG fragments and uses thereof
InactiveUS20050233402A1Accelerate tumor growthInhibit primary tumor growthCompound screeningOrganic active ingredientsLymphatic SpreadSulfate
The invention relates to heparinase III and mutants thereof. Modified forms of heparinase III having reduced enzymatic activity which are useful for a variety of purposes, including sequencing of heparin-like glycosaminoglycans (HLGAGs), removing active heparan sulfate from a solution, inhibition of angiogenesis, etc. have been discovered according to the invention. The invention in other aspects relates to methods of treating cancer and inhibiting tumor cell growth and / or metastasis using heparinase III, or products produced by enzymatic cleavage by heparinase III of HLGAGs.
Owner:MASSACHUSETTS INST OF TECH
Novel heparin entities and methods of use
The present invention relates to immobilized biologically active entities that retain a significant biological activity following manipulation. The invention also comprises a medical substrate comprising a heparin entity bound onto a substrate via at least one heparin molecule, wherein said bound heparin entity is heparinase-1 sensitive.
Owner:WL GORE & ASSOC INC
Use of heparinase to decrease inflammatory responses
InactiveUS20010006635A1Inhibiting leukocyte rollingInhibiting chemokine gradient formationOrganic active ingredientsPowder deliveryWhite blood cellDigestion
Heparinase enzymes can be used as a medical treatment to reduce localized inflammatory responses. Treatment of activated endothelium with heparinase inhibits leukocyte rolling, adhesion and extravasation. Most of the heparin and heparan sulfate on endothelial cell surfaces and in basement membranes is degraded by exposure to heparinase. In addition, immobilized chemokines, which are attached to heparin / heparan sulfate on activated endothelium are solubilized by heparinase digestion. Heparinase can be infused into the vascular system to inhibit accumulation of leukocytes in inflamed tissue and decrease damage resulting from localized inflammations. Targeting of heparinase to activated endothelium can be accomplished through localized administration and / or use of genetically engineered heparinase containing endothelium ligand-binding domains.
Owner:BIOMARIN PHARMA INC +1
Method for determining endoglycosidase enzyme activity
ActiveUS20060127945A1Improve throughputMicrobiological testing/measurementLibrary screeningEndorhamnosidaseHeparanase
The invention relates to a method for determining endoglycosidase enzyme activity, and in particular of the heparanase type, in a sample, and also to a method for detecting a compound capable of modulating the activity of an endoglycosidase, and in particular of an endoglycosidase having activity of the heparanase type, by measuring a signal resulting from a close proximity transfer between two compounds attached to a substrate for the enzyme.
Owner:CIS BIO INT
Genetically modified cells and methods for expressing recombinant heparanase and methods of purifying same
Bacterial, yeast and animal cells and methods for overexpressing recombinant heparanase in cellular systems, methods of purifying recombinant heparanase therefrom and modified heparanase species which serve as precursors for generating highly active heparanase by proteolysis.
Owner:INSIGHT BIOPHARMLS
Method of producing heparin oligosaccharide using heparinase
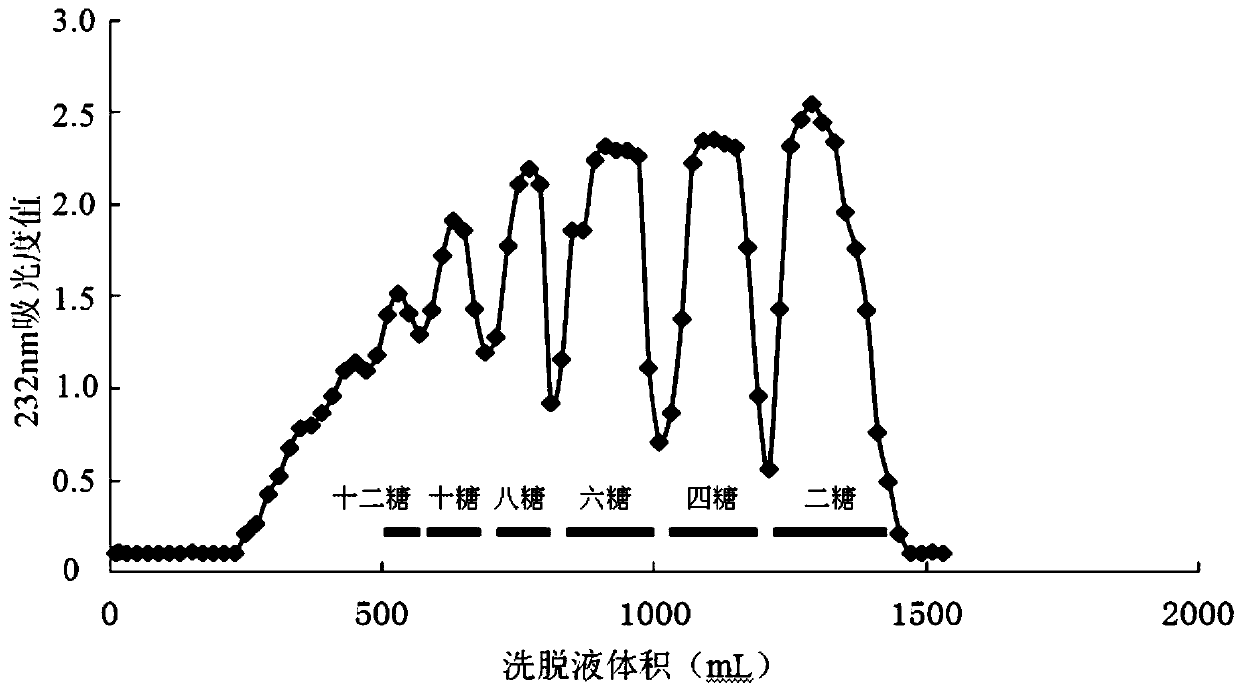
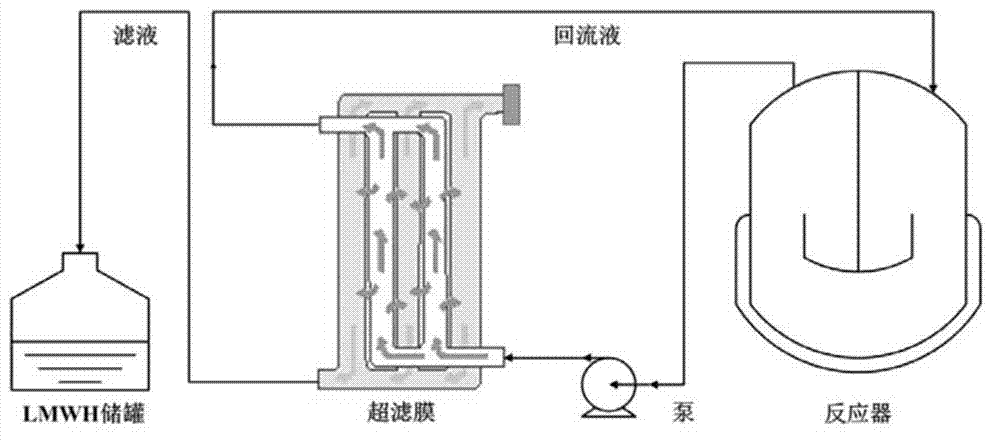
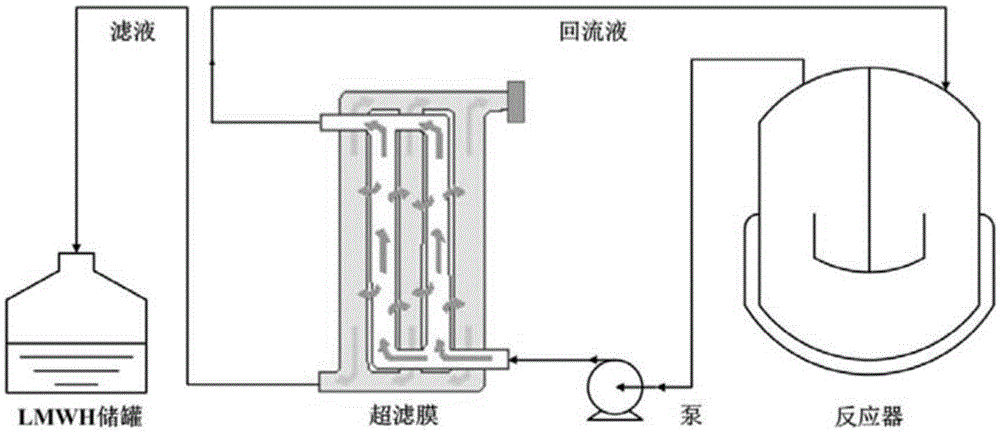
A process for preparing heparin oligose from heparinase includes such steps as culturing sphingobacterium (CGMCC No.0660), preparing non-cell coarse enzyme liquid, extracting and purifying heparinase, degradating heparin by the heparinase at 20-30 deg.C to obtain heparin oligose mixture, ultrafilter, gel filter for fractionation and evaporation concentration. The product has the activity of resisting smooth muscle hyperplasia.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Heparanase activity neutralizing anti-heparanase monoclonal antibody and other anti-heparanase antibodies
InactiveUS20040170631A1Peptide/protein ingredientsBiological material analysisMonoclonal antibodyDrug development
Specific anti-heparanase antibodies which bind specifically to heparanase having sequence homology to human heparanase, which can be used to treat and diagnose conditions associated with heparanase catalytic activity, for purification of heparanase, and for drug development in heparanase associated conditions are disclosed.
Owner:YACOBY ZEEVI ORON +9
Heparin entities and methods of use
The present invention relates to immobilized biologically active entities that retain a significant biological activity following manipulation. The invention also comprises a medical substrate comprising a heparin entity bound onto a substrate via at least one heparin molecule, wherein said bound heparin entity is heparinase-1 sensitive.
Owner:WL GORE & ASSOC INC
Transgenic animals expressing heparanase and uses thereof
A transgenic non-human animal expressing heparanase from a transgene, methods for its preparation, compositions-of-matter derived therefrom and uses thereof.
Owner:INSIGHT STRATEGY & MARKETING +1
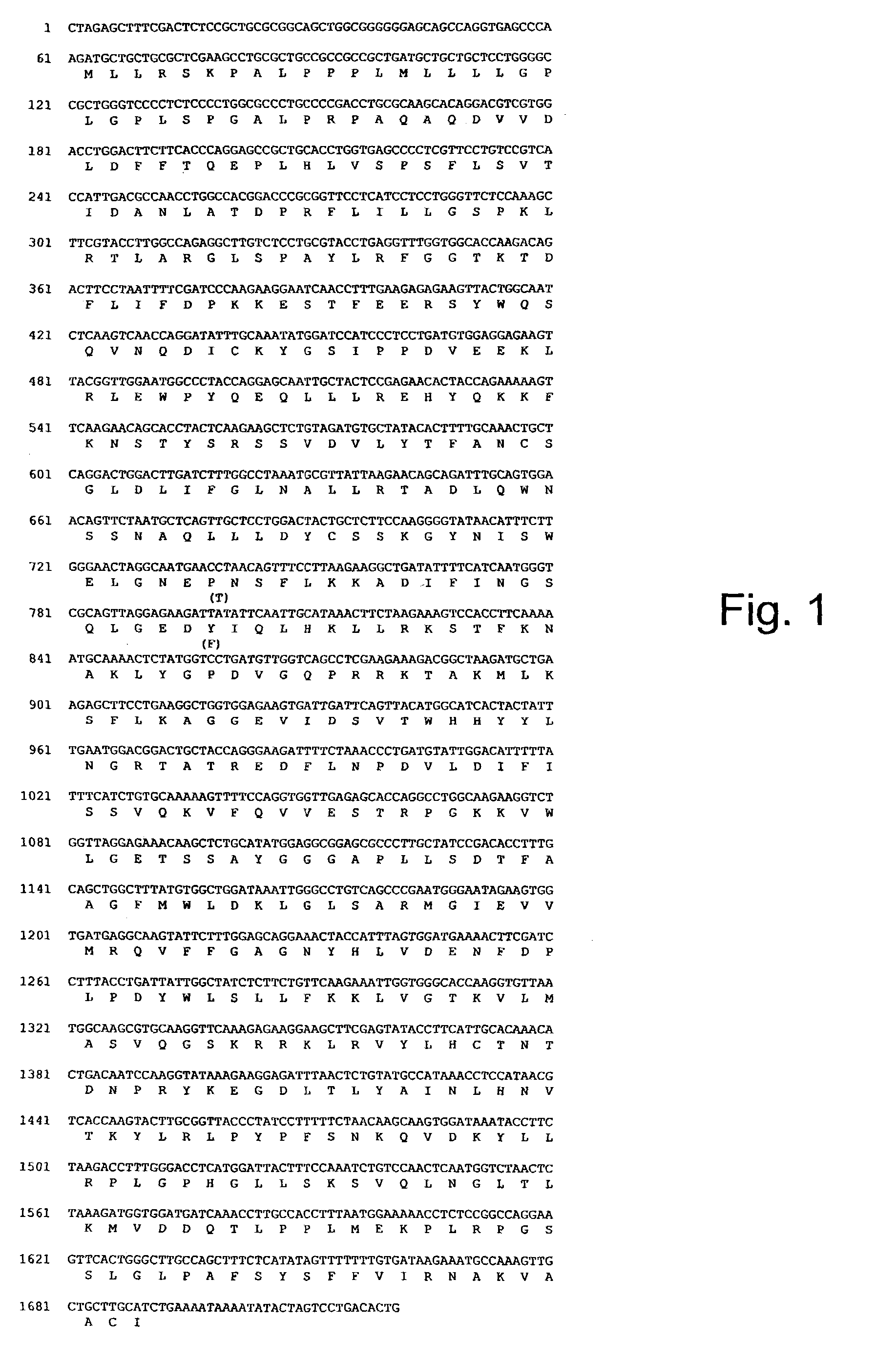
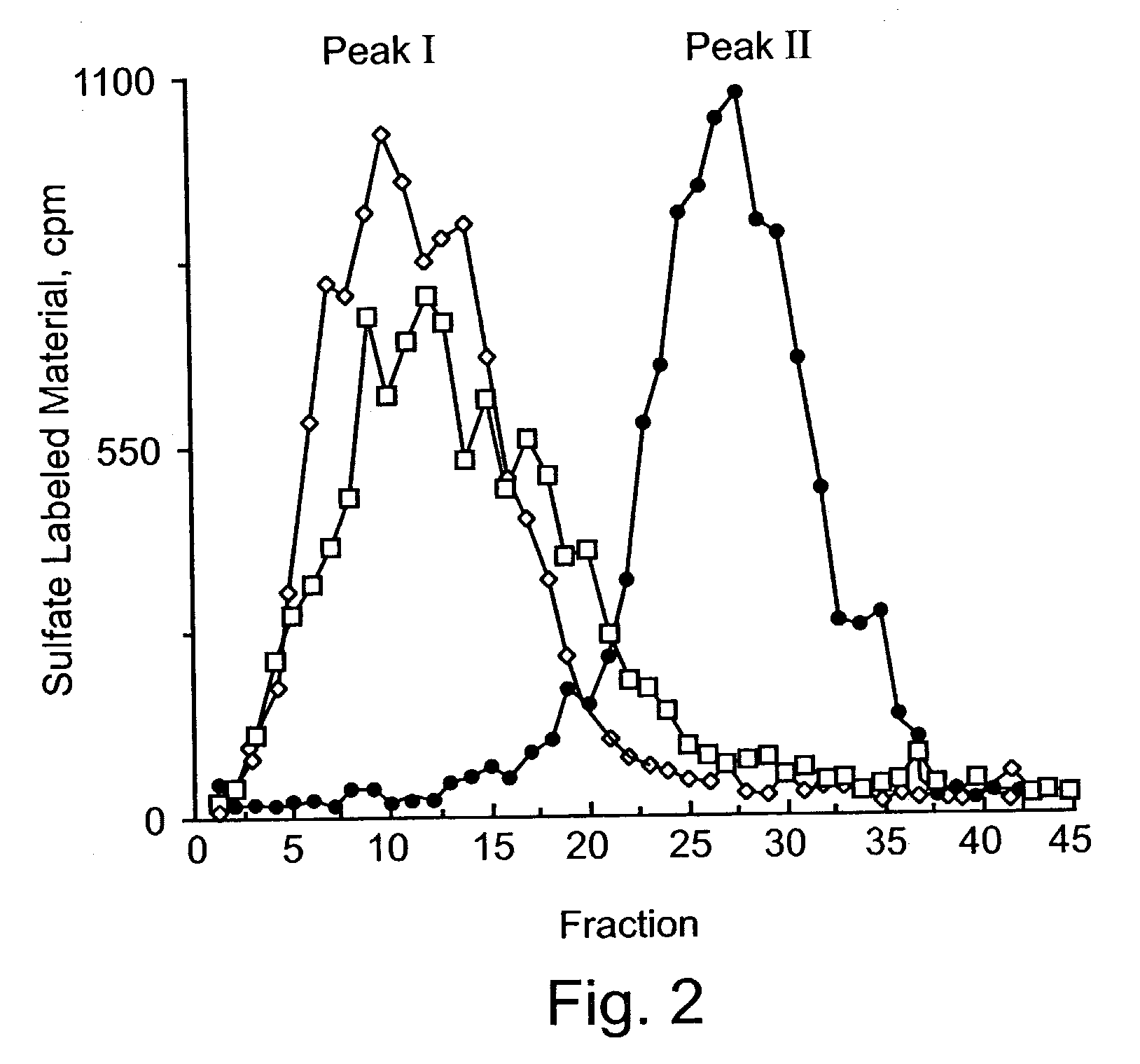
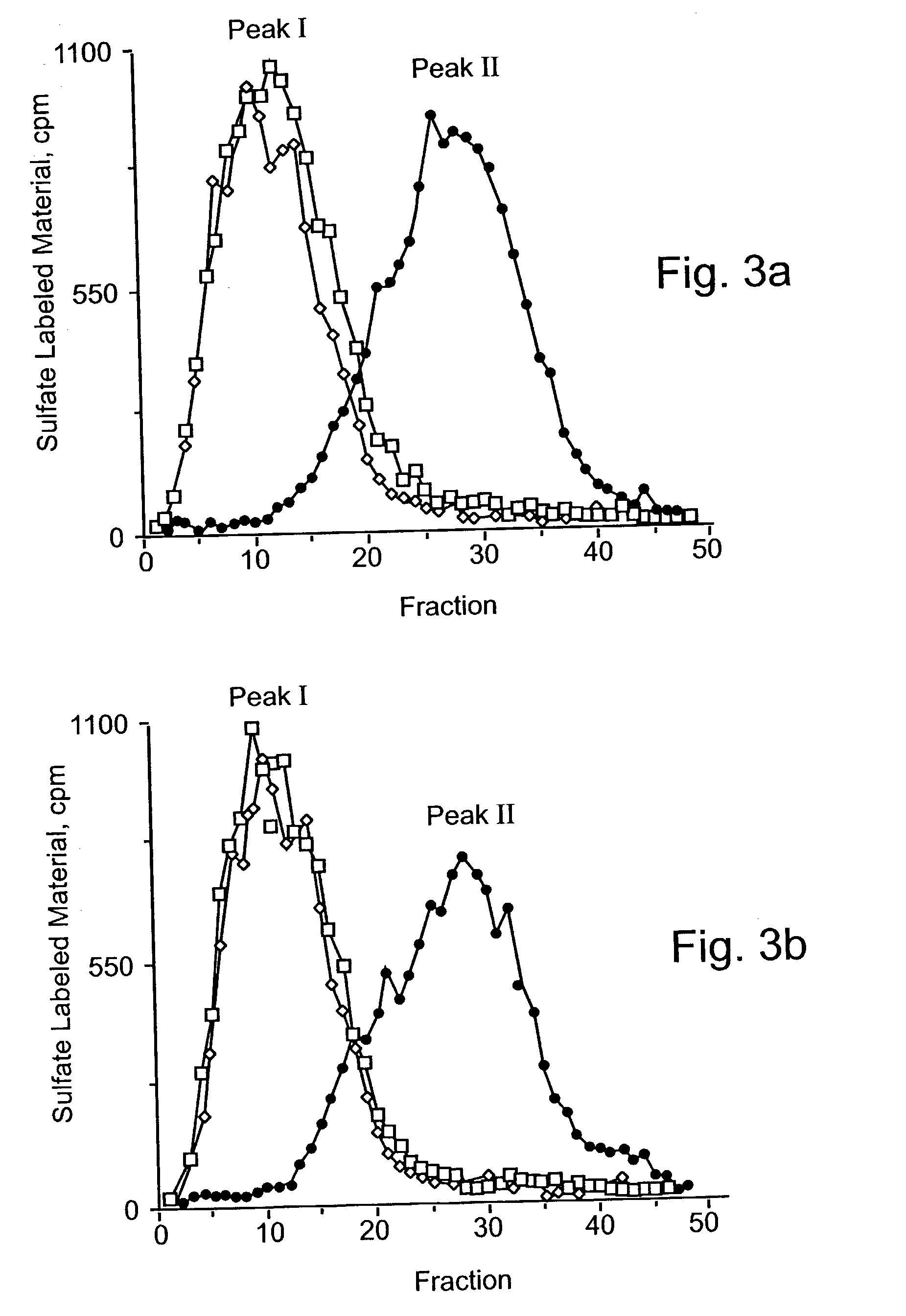
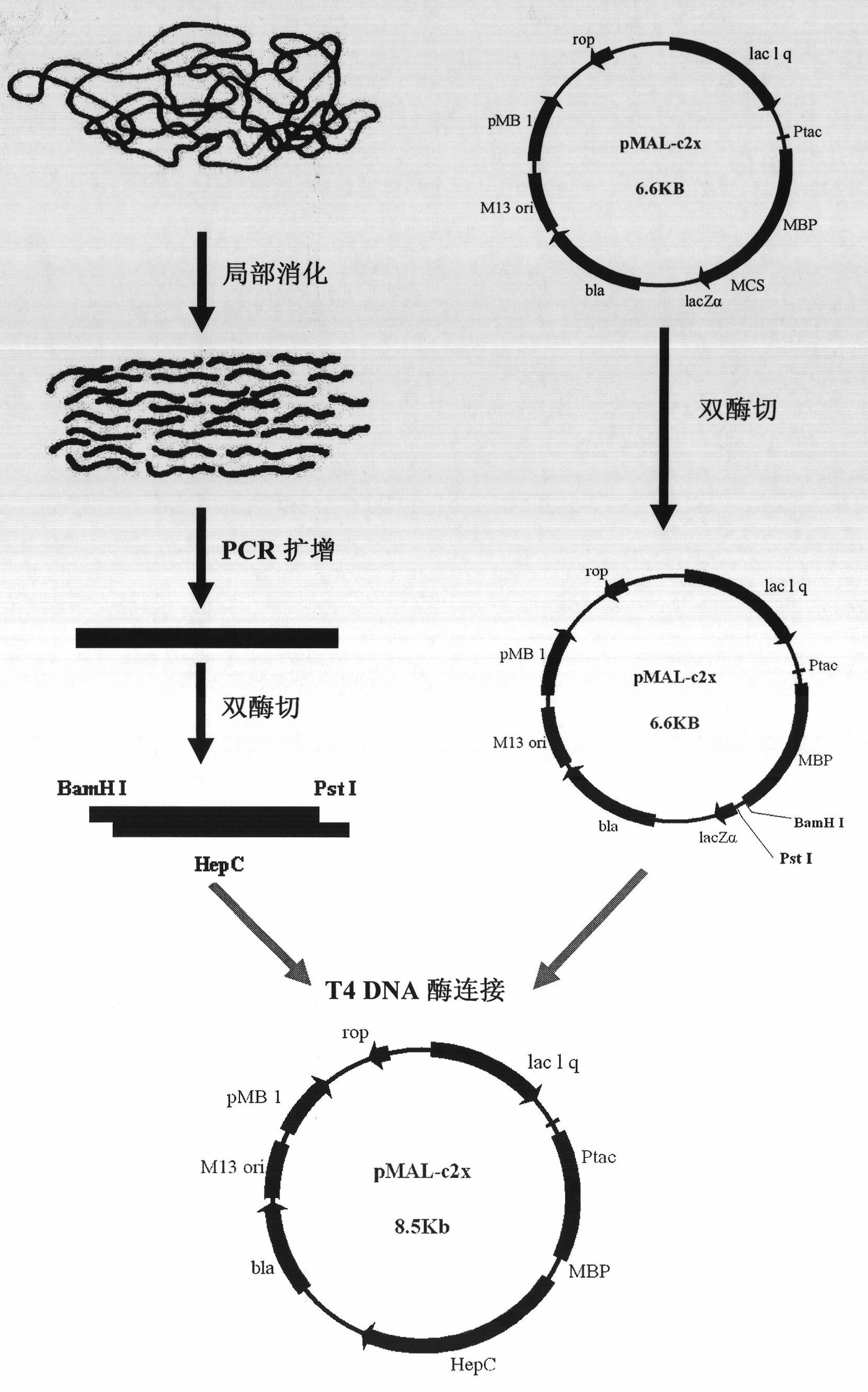
Heparanase III fusion protein and coding gene and expression method thereof
ActiveCN101942025AIncrease productionHigh activityFungiBacteriaEscherichia coliRecombinant escherichia coli
The invention discloses a heparanase III fusion protein and a coding gene and an expression method thereof. The fusion protein of the invention (named as MBP-HepC) is a protein in a) or b): a) a protein consisting of amino acid sequences shown by the 1-1028th site of a sequence 2 in a sequence table; and b) a protein derived from a), provided with heparanase III activity and obtained by substituting and / or deleting and / or adding one or more amino acids in an amino acid sequence of the sequence 2 in the sequence table. The coding gene of the protein is also within the protection range of the invention. By introducing the gene into protein heparanase expressed in recombinant escherichia coli, the enzymatic activity can reach 1,776.3 IU per liter of fermentation liquid, the expression amount can reach 240 mg per liter of fermentation liquid, and the specific enzymatic activity can reach 7.39 IU / mg. The invention also can realize further purification of the fusion protein through affinity separation.
Owner:TSINGHUA UNIV +1
Novel heparin entities and methods of use
InactiveUS20110065085A1Nervous disorderMicrobiological testing/measurementDrug biological activityChemistry
The present invention relates to immobilized biologically active entities that retain a significant biological activity following manipulation. The invention also comprises a medical substrate comprising a heparin entity bound onto a substrate via at least one heparin molecule, wherein said bound heparin entity is heparinase-1 sensitive.
Owner:WL GORE & ASSOC INC
Hemostatic compositions, devices and methods
InactiveUS7094428B2Lower Level RequirementsReduce concentrationPowder deliveryFactor VIIFactor VIIaBiopolymer
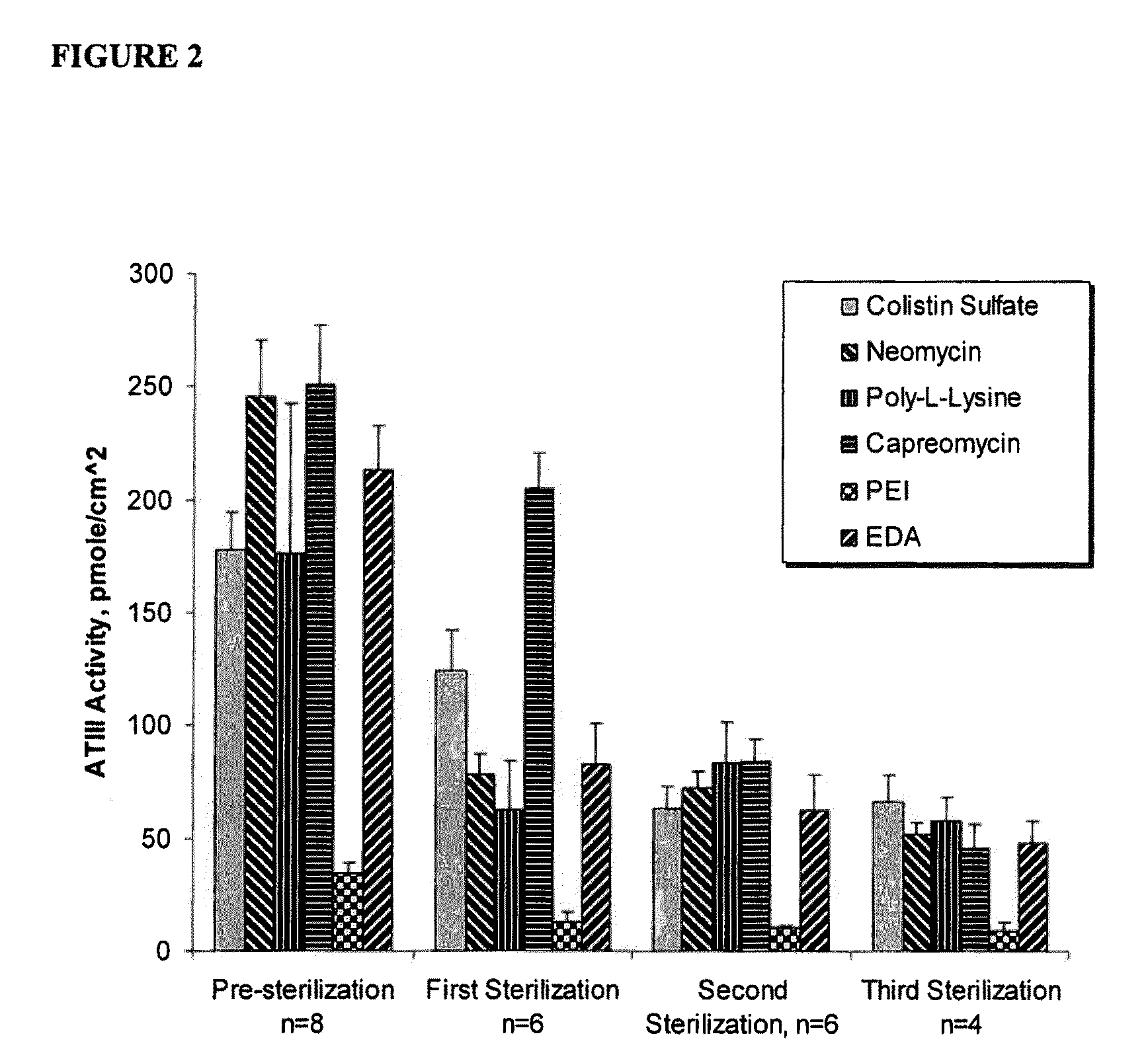
A hemostatic composition which comprises at least one procoagulant metal ion, such as silver (I) or mercury (II), and at least one procoagulant biopolymer, such as collagen, thrombin, prothrombin, fibrin, fibrinogen, heparinase, Factor VIIa, Factor VIII, Factor IXa, Factor Xa, Factor XII, von Willebrand Factor, a selectin, a procoagulant venom, a plasminogen activator inhibitor, glycoprotein IIb-IIIa, a protease, or plasma. The composition in the form of a paste, dough, glue, liquid, lyophilized powder or foam, may be provided, for application to a wound. A hemostatic device is also described which comprises a hemostatic composition as described above. The device may be in the form of, for example, a plug, bandage, gauze, cloth, tampon, membrane or sponge. Methods are also provided for prophylaxis or treatment of bleeding at a site by application to the site of the composition or device as described.
Owner:RUTGERS THE STATE UNIV
Sulfated heparin oligosaccharide as well as preparation method and application thereof
ActiveCN105504097AShort sugar chainSmall molecular weightOrganic active ingredientsFermentationSulfationLymphatic Spread
The invention provides sulfated heparin oligosaccharide as well as a preparation method and application thereof. The sulfated heparin oligosaccharide is characterized in that a non-reducing end of a sulfated heparin oligosaccharide molecule contains an unsaturated double bond produced through enzymolysis of heparinase as well as uronic acid derivatives and glycosylamine derivatives; the sulfated heparin oligosaccharide has a structure represented by a formula I, wherein R1, R2, R3, R4, R5, R6, R7, R8, R9, Ra, Rb, Rc and Rd are independently SO3<-> or H; Rx', Ry' and Rz' are independently COCH3 or SO3<->, and n is 1-3. The sulfated heparin oligosaccharide with controllable sulfating degree prepared by virtue of the preparation method has very high activity for inhibiting heparanase in vitro, the activity of the sulfated heparin oligosaccharide for inhibiting cell adhesion and migration is 4-5 times higher than that of heparin, and the activity for inhibiting tumor metastasis in mice is 2-3 times higher than that of the heparin; the sulfated heparin oligosaccharide has relatively good tumor metastasis resistance and relatively high specificity.
Owner:SHENZHEN HEPALINK PHARMA GRP CO LTD
Use of heparinase to decrease inflammatory responses
InactiveUS20050191288A1Reduce local concentrationInhibiting chemokine activationOrganic active ingredientsPowder deliveryWhite blood cellDigestion
Heparinase enzymes can be used as a medical treatment to reduce localized inflammatory responses. Treatment of activated endothelium with heparinase inhibits leukocyte rolling, adhesion and extravasation. Most of the heparin and heparan sulfate on endothelial cell surfaces and in basement membranes is degraded by exposure to heparinase. In addition, immobilized chemokines, which are attached to heparin / heparan sulfate on activated endothelium are solubilized by heparinase digestion. Heparinase can be infused into the vascular system to inhibit accumulation of leukocytes in inflamed tissue and decrease damage resulting from localized inflammations. Targeting of heparinase to activated endothelium can be accomplished through localized administration and / or use of genetically engineered heparinase containing endothelium ligand-binding domains.
Owner:BIOMARIN PHARMA INC
Method for controlling production of low-molecular-weight heparin
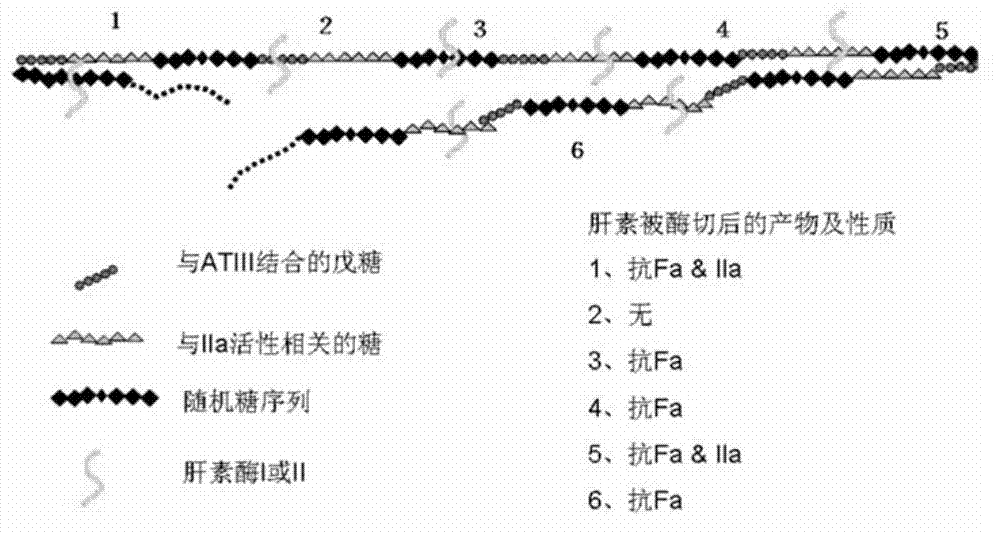
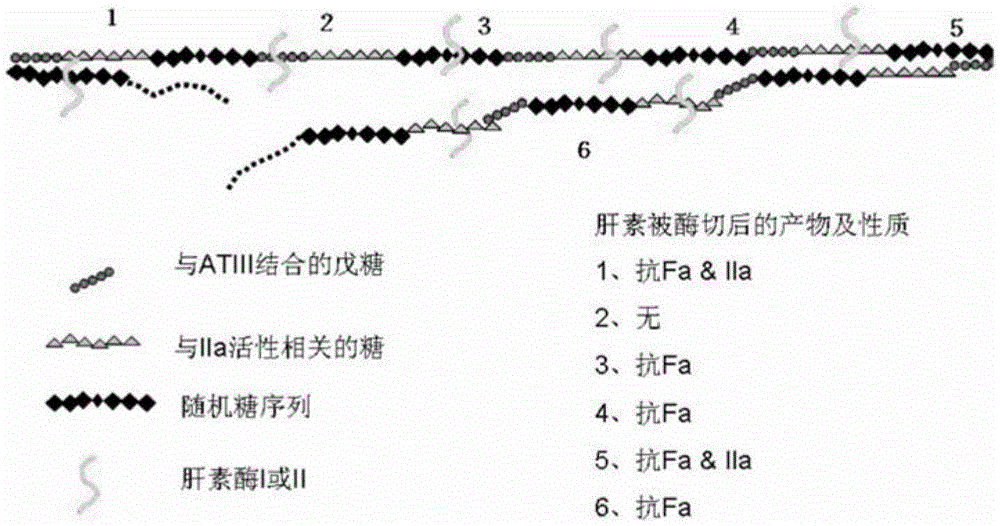
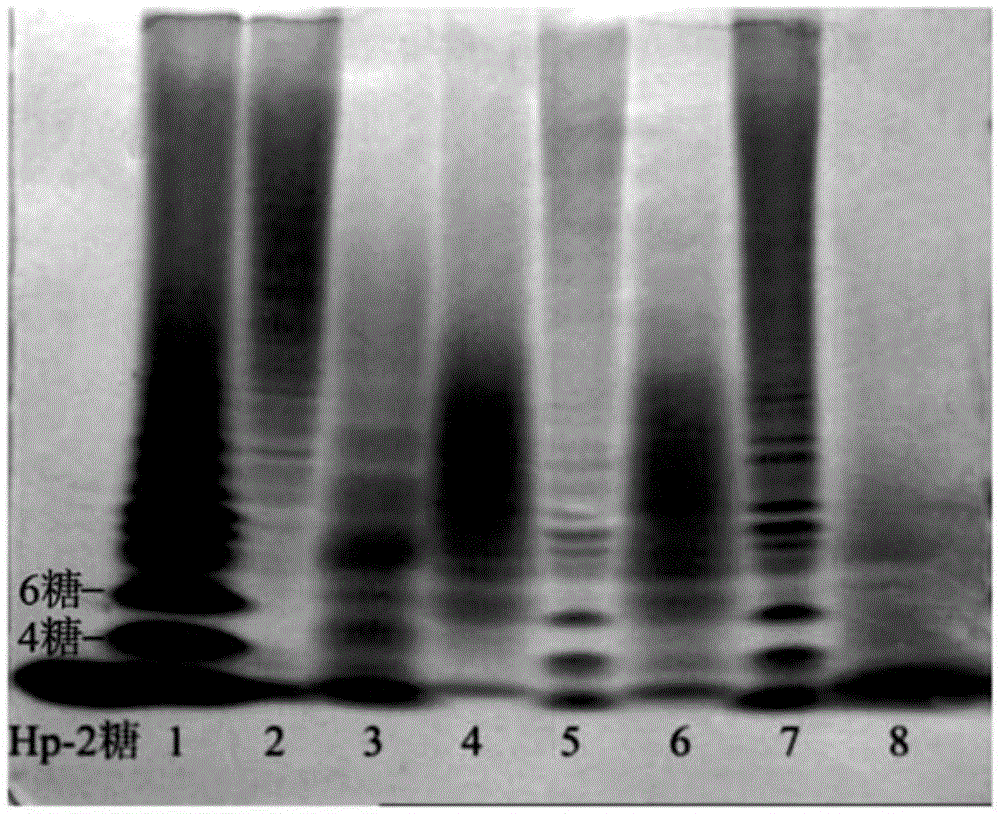
The invention discloses a method for producing low-molecular-weight heparin or ultralow-molecular-weight heparin. According to the method, heparinases selected from more than two of heparinases I, II and III are used for degrading heparin so as to produce the low-molecular-weight heparin or ultralow-molecular-weight heparin.
Owner:TSINGHUA UNIV
Heparanase activity neutralizing anti-heparanase monclonal antibody and other anti-heparanase antibodies
Owner:HADASIT MEDICAL RES SERVICES & DEVMENT +1
Preparation method and use of heparin oligosaccharide of specific length
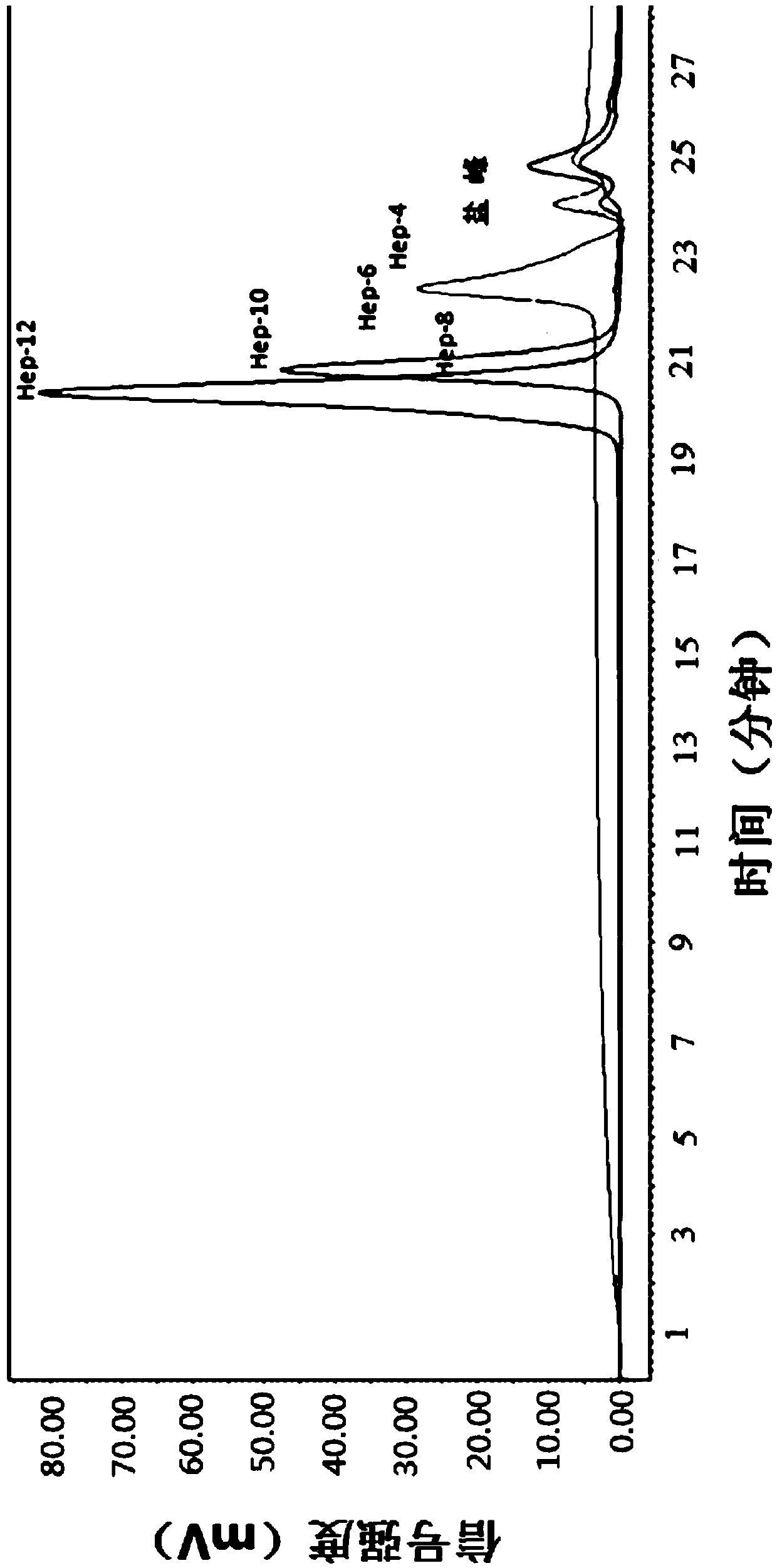
The invention relates to a preparation method and application of heparin oligosaccharides of a specific length. In the invention, heparin is degraded by heparinase, and heparin with a specific sugar chain length is prepared by ultrafiltration, gel chromatography, high performance liquid chromatography and the like. oligosaccharides.
Owner:黄欣
Detection method for evaluating curative effect and residual condition of heparin drugs
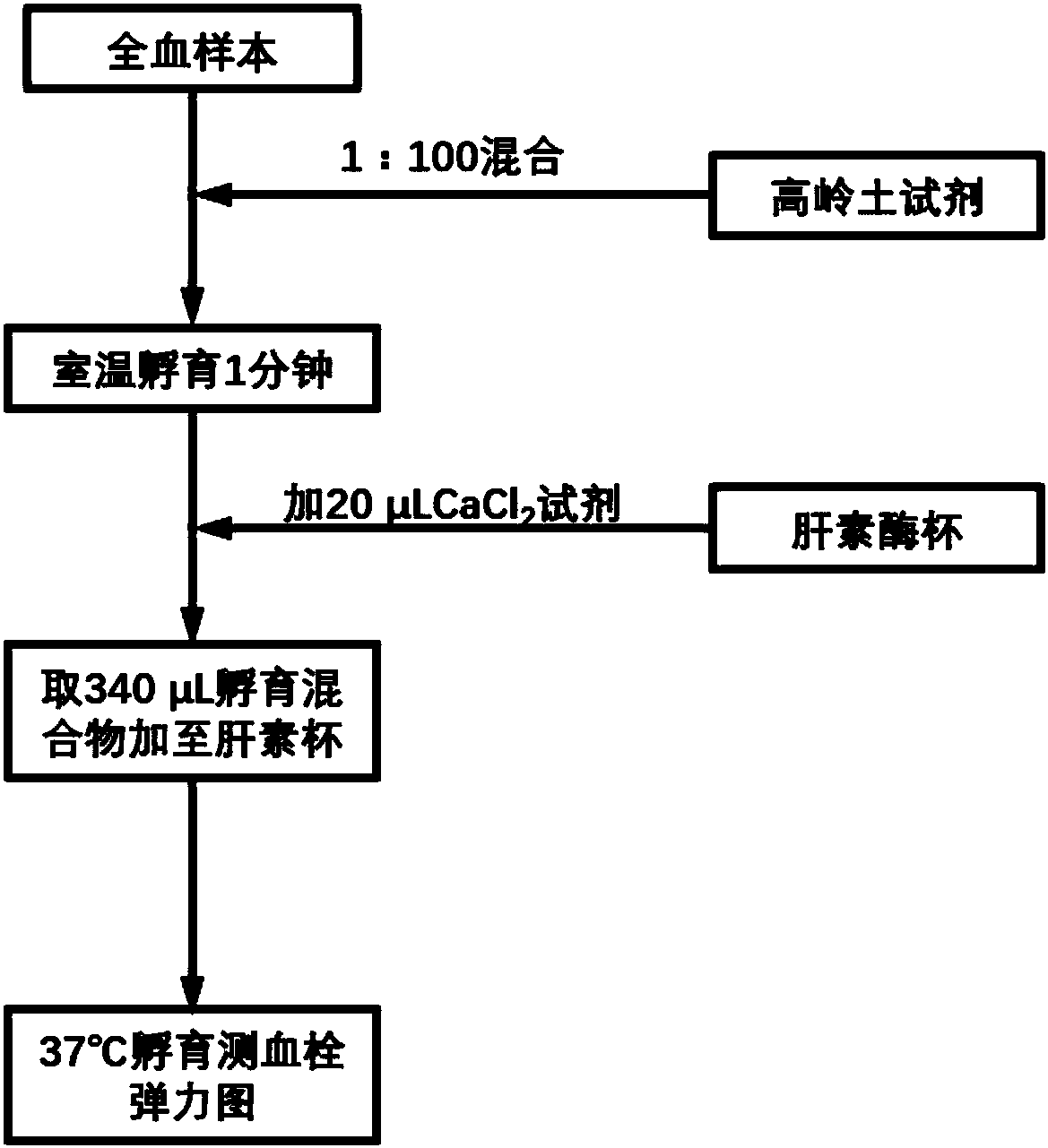
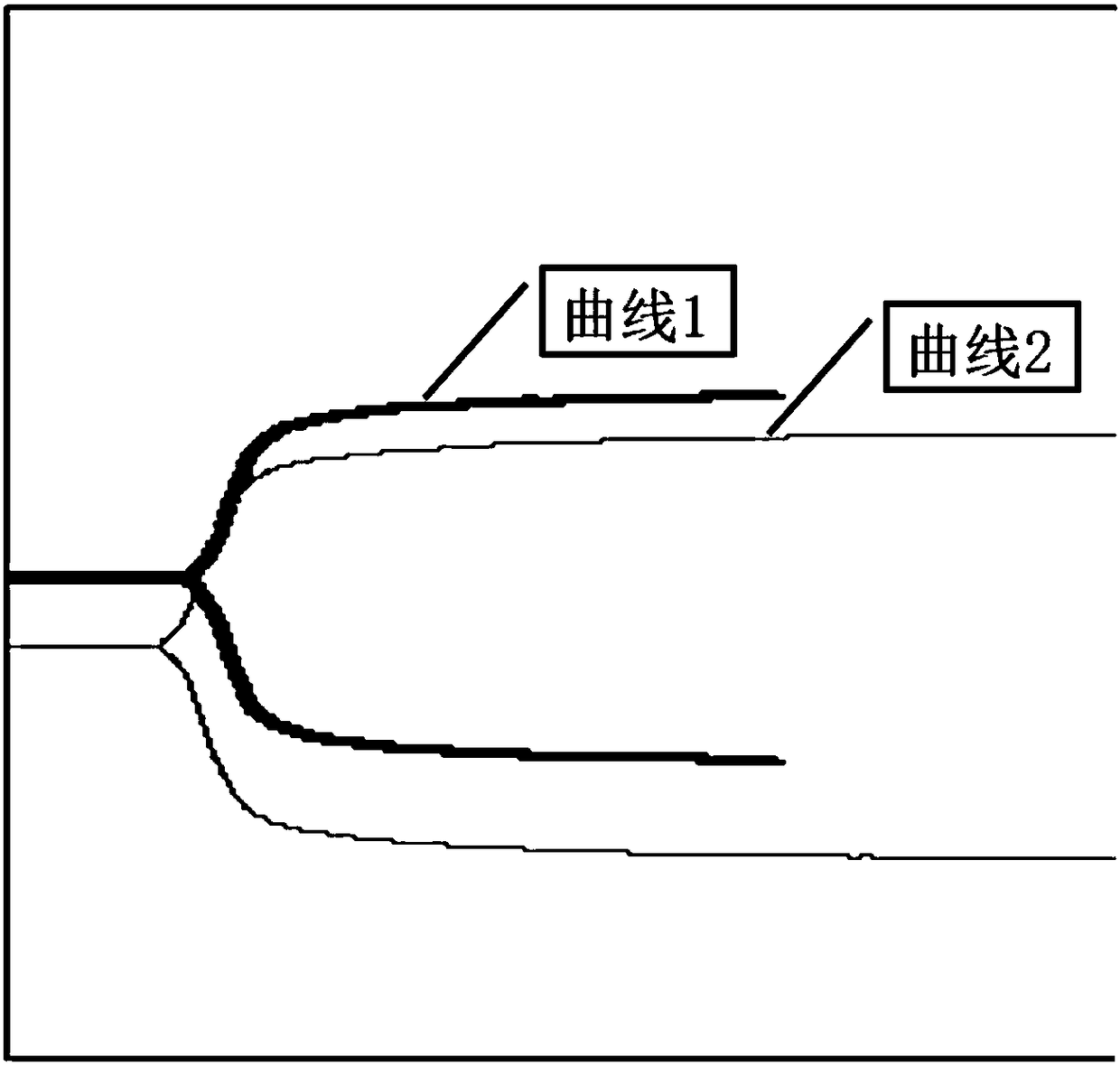
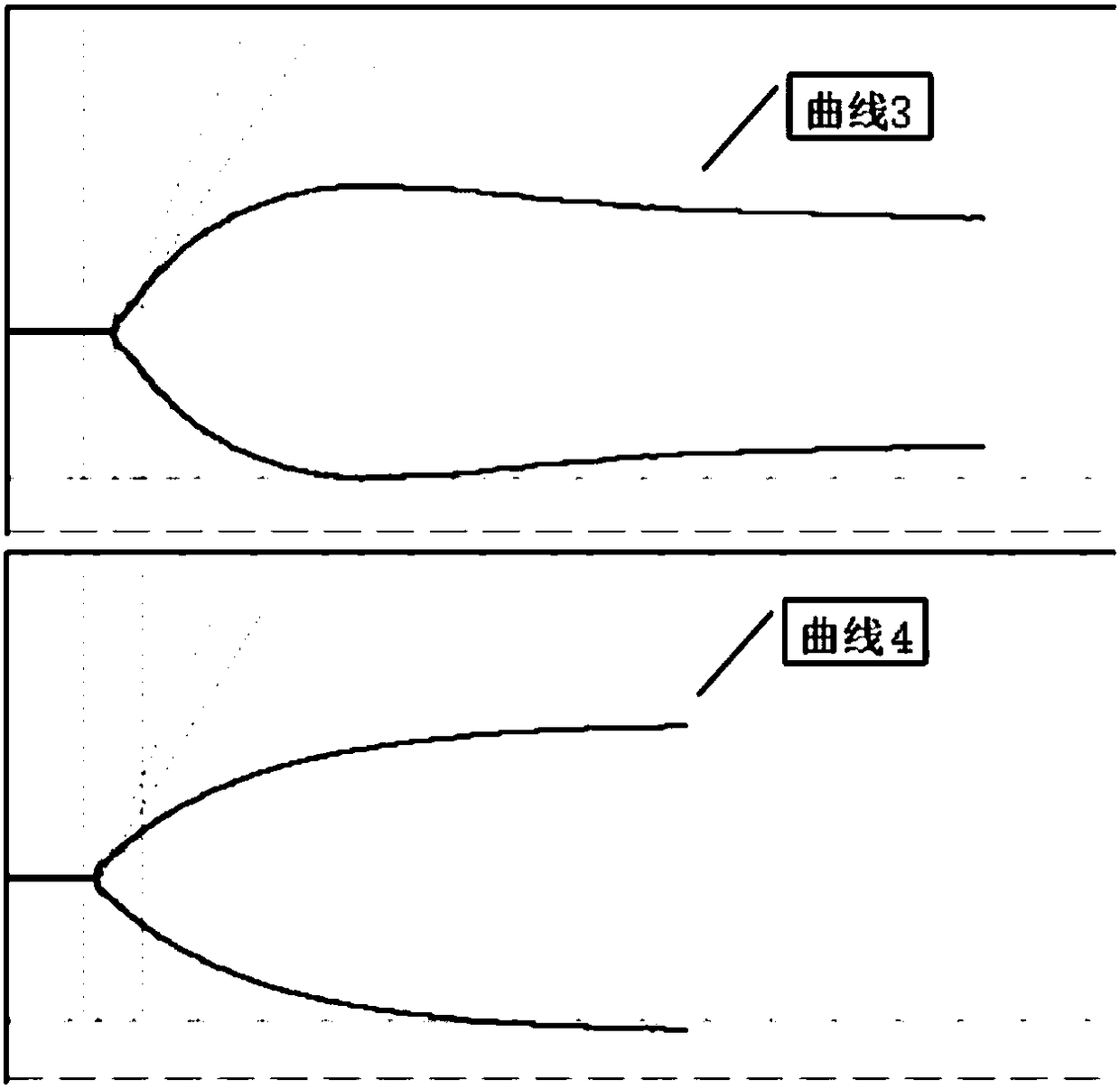
The invention relates to the field of blood detection, in particular to a detection method for evaluating the curative effect and the residual condition of heparin drugs. The heparin drugs mainly comprise heparin, low-molecular-weight heparin and some heparinoid drugs. The detection method mainly comprises steps as follows: heparinase, a freeze-drying protective agent and an adhesive are mixed, then a mixed reagent in appropriate proportion is prepared from an obtained mixture by the aid of a buffer solution, the mixed reagent is enveloped in a test-cup and subjected to freezing and vacuum pumping treatment, and a heparinase cup is obtained. The detection method has a plurality of advantages that the operation is simple, the guarantee period is long, the stability is good and the like. Theheparinase cup and a common cup (a blank sample cup) are put in a thrombelastogram, an anticoagulant and a chelating agent are added to the cups respectively, endogenously activated whole blood samples are added to the test-cups, and thromboelastography is started for detection; values R of starting time of blood clot formation in the heparinase cup and the common cup are compared, and whether heparin exists in the blood can be judged; if heparin exists, the value R of the starting time of blood clot formation in the heparinase cup is smaller than that of the starting time of blood clot formation in the common cup. The method is simple to operate, a detection result is good in repeatability, and the accuracy is high.
Owner:北京乐普诊断科技股份有限公司
Method for preparing protamine-deoxycholic acid conjugate with heparin transfer function
InactiveCN103623421AInhibition of growth and proliferationImprove reprinting efficiencyPowder deliveryOrganic active ingredientsCross-linkNano composites
The invention belongs to the technical field of biological medicine, and particularly relates to a method for preparing a protamine-deoxycholic acid conjugate with a heparin transfer function. The amidogen of protamine and the carboxyl of deoxycholic acid form an amide bond through teh effect of a cross-linking agent to prepare an amphipathic conjugate, and then the amphipathic conjugate and heparin form a self-assembled aggregation in order to conveniently release the heparin in cells. The hydrophobic modification of the conjugate can enhance the self-aggregation stability of nano-composites; the cationic performance of the conjugate entraps the heparin, so that the distribution capability of the heparin in the cells is increased, and the heparin is prevented from, degradation caused by the effect of heparinase and further the transfer of the heparin into cancer cells and the release and biological effect of the heparin in the cells are realized.
Owner:JIANGNAN UNIV
Preparation method of mediated-heparin intracellular delivery crosslinking nanometer carrier
InactiveCN104524591AAvoid degradationEfficient releaseOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityNano composites
The invention belongs to the technical field of biological medicines, and particularly relates to a preparation method of a mediated-heparin intracellular delivery crosslinking nanometer carrier. PEG-modified PEI is adopted; and heparin is entrapped by using cation performance of PET, so that the cell endocytosis ability is increased; the water solubility, the stability and the blood metabolic capability of a carrier and nano composites are improved by PEG molecules; removal of combination of composites and protein is avoided; meanwhile, degradation of the heparin caused by heparin enzyme action is avoided; in addition, effective release of the heparin in cells is achieved; and a disulfide bond is introduced through a crosslinking agent on the basis, so that nanoparticles are degraded to release the heparin, thus the heparin plays a biological effect in the cells.
Owner:JIANGNAN UNIV
Method for controlling the production of low molecular weight heparin
This article discloses a production method of low molecular weight heparin or ultra-low molecular weight heparin. This method utilizes two or more heparinase enzymes selected from heparinase I, II and III to degrade heparin to produce low molecular weight heparin or ultra-low molecular weight heparin.
Owner:TSINGHUA UNIV
Lyophilized heparanase preparation, heparanase cup as well as preparation method and application of lyophilized heparanase preparation and heparanase cup
InactiveCN108148889AImprove stabilityImprove viabilityMicrobiological testing/measurementBiological material analysisPreservativeAqueous solution
The invention relates to the technical field of in vitro diagnostic reagents, and particularly relates to a lyophilized heparanase preparation, a heparanase cup as well as a preparation method and application of the lyophilized heparanase preparation and the heparanase cup. A redissolved enzyme activity yield of the lyophilized heparanase preparation provided by the invention is at least 75 percent. The heparanase cup provided by the invention comprises a cup body and the lyophilized heparanase preparation, wherein an accommodating space is defined in the cup body; the lyophilized heparanase preparation is arranged in the accommodating space. The invention also provides a method for preparing the lyophilized heparanase preparation. The method comprises the following steps: sequentially performing pre-freezing treatment, sublimation treatment and drying treatment on aqueous solutions prepared from heparinase, a protective agent, buffer salt and a preservative in a predetermined proportion so as to obtain the lyophilized heparanase preparation. The lyophilized heparanase preparation and the heparanase cup are used in a detection process of blood samples, and the detection sensitivityis high; moreover, the activity and the stability of the heparinase in a lyophilization process can be obviously improved.
Owner:深圳优迪生物技术有限公司
Method for preparing recombinant heparinase III by utilizing SUMO fusion expression system and SUMO_heparinase III fusion protein prepared by method
PendingCN113025598AImprove stabilityImprove solubilityFusion with degradation motifFermentationEscherichia coliInclusion bodies
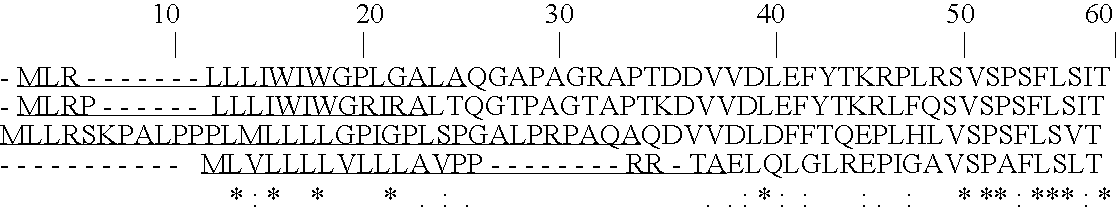
The invention discloses a method for preparing recombinant heparinase III by utilizing an SUMO fusion expression system and the heparinase III prepared by the method, the preparation method comprises the following steps: selecting a heparinase III sequence from Pedobacter heparinus, wherein the amino acid sequence of the heparinase III is shown as SEQ ID No.1, and the total number is 659aa; removing a signal peptide sequence to obtain a DNA sequence of the heparinase III, such as SEQ ID No.2; inserting the DNA sequence of the heparinase III into a pSMART vector plasmid with an N-terminal SUMO protein tag; transforming the correct plasmids into BL21 (DE3) escherichia coli competent cells, selecting monoclone, and obtaining fusion protein through fermentation and purification; and cutting the SUMO tag protein from the fusion protein by using the SUMO protease to obtain the heparinase III. The method has the advantages that the solubility of the target protein is improved, correct folding of the target protein is promoted, and inclusion bodies are prevented from being formed; the purification cost is reduced and the purification efficiency is improved; the molecular weight of the SUMO protein tag is small, the influence on the enzymatic activity of the heparinase III is small, and the purified heparinase III is obtained.
Owner:上海宝维医药技术有限公司
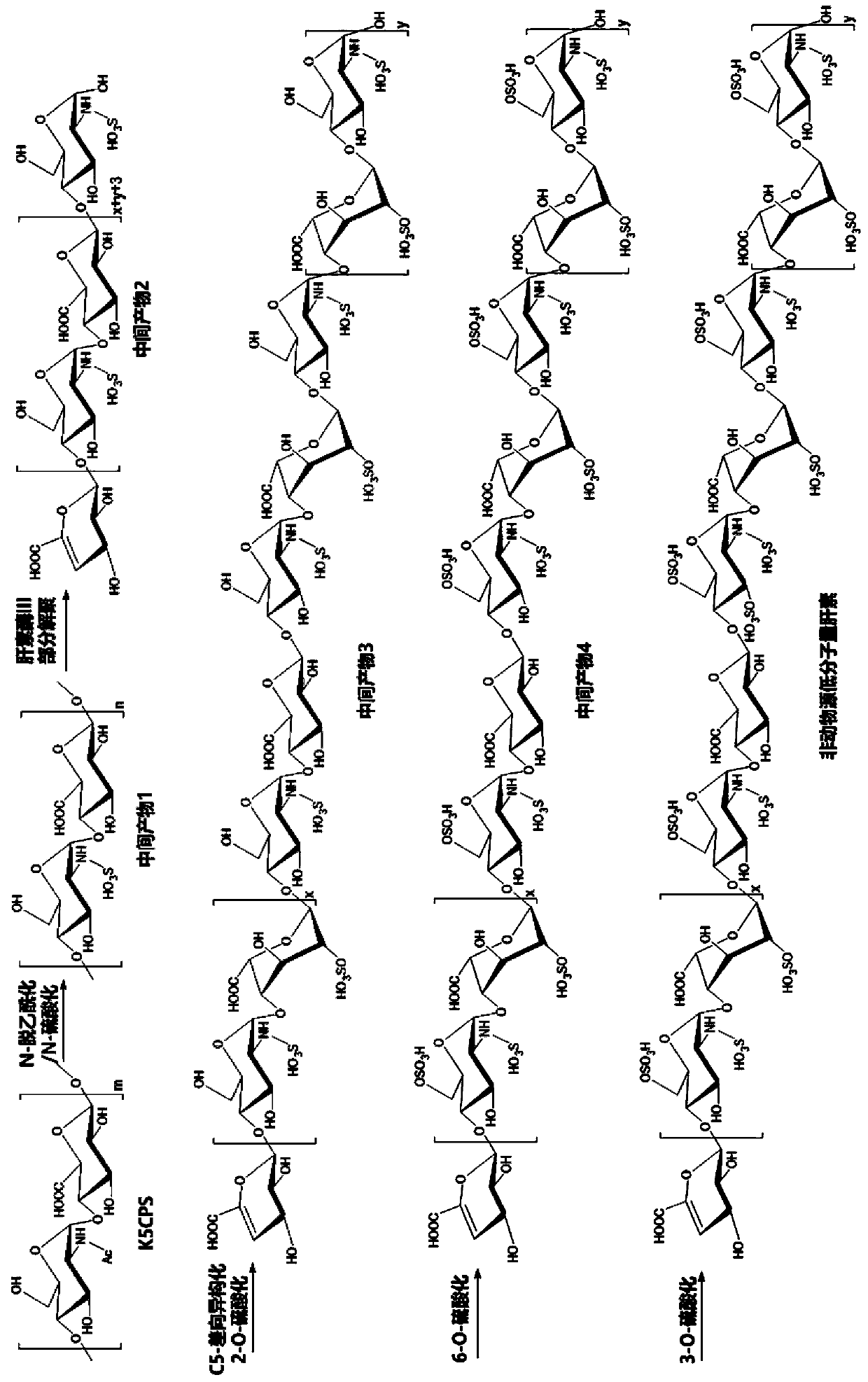
Non-animal-derived LMW (low molecular weight) heparin, preparation method therefor and application of non-animal-derived LMW heparin
ActiveCN111154819AEasy to separateReduce the risk of contaminationOrganic active ingredientsFermentationBiotechnologyAnimal science
The invention relates to non-animal-derived LMW (low molecular weight) heparin, a preparation method therefor and application of the non-animal-derived LMW heparin. The preparation method disclosed bythe invention comprises the steps of performing a one-step chemical method and a variety of enzyme catalysis methods in order: extracting an exopolysaccharide K5CPS, subjecting the exopolysaccharideK5CPS to chemical-method N-deacetylation / N-sulfation modification, and then, carrying out partial depolymerization in a buffer solution through heparinase III; and subjecting obtained LMW products toenzyme-method C-5 epimerization / 2-O-sulfation modification, 6-O-sulfation modification and 3-O-sulfation modification sequentially, thereby obtaining a product with anti-FXa and anti-FIIa activity. The invention provides a novel method for preparing the LMW product with typical structure characteristics of the heparin and remarkable anticoagulation activity. According to the method, raw materialsare non-animal-derived, the quality is controllable, the risk of contamination is low, adopted reaction conditions are mild and efficient, the obtained product is easy to separate, and large-scale preparation of the LMW heparin with good structural homogeneity and high anticoagulation activity can be achieved.
Owner:SHANDONG UNIV
Preparation method of low-molecular-weight heparin
ActiveCN103060404AIncreased thrombin activityAvoid infectionFermentationSpectrometerHigh-performance liquid chromatography
The invention provides a method for preparing low-molecular-weight heparin by using non-mammal fish resources. Specific steps comprise: ungraded heparin component is obtained by rough extraction from fish tissues; and the ungraded heparin is degraded by using heparanase, such that low-molecular-weight heparin lyophilized pure product is obtained. The content is calculated with a carbazole colorimetric method; molecular weight and purity are analyzed with a high-performance liquid chromatography-mass spectrometer and a polyacrylamide gel electrophoresis method; and activity evaluation is carried out by determining anti-IIa titer with a sheep plasma method and by determining anti-Xa titer with a chromogenic substrate method. According to the invention, non-mammal fish resources are utilized, such that adverse influences caused by inter-generic cross virus and prion infection existing among different species of mammals are overcome. Also, an enzyme extraction method is improved, such that the high-thrombin-activity low-molecular-weight heparin is finally obtained.
Owner:青岛亚博生物科技有限公司
Heparanase composition capable of complete specific enzymolysis of enoxaparin sodium and application of heparanase composition
InactiveCN104792896AReduce the introductionSolve the problem of 1,6-anhydride disaccharide analysisComponent separationStrength propertiesActivity ratiosImpurity
The invention provides a heparanase composition capable of complete specific enzymolysis of enoxaparin sodium. The heparanase composition is a mixture of heparanase II and heparanase III, wherein the enzyme activity ratio of heparanase II to heparanase III is 3: 1. According to the invention, the heparanase composition can realize the complete specific enzymolysis of enoxaparin sodium and is reduced to obtain 1, 6-anhydride derivatives, and the contents are in an acceptable range; the heparanase composition is free from heparanase I to reduce the introduction of enzyme impurities, so that the method is more reliable, is an important supplement for several pharmacopoeia methods, has high practical use value and significance.
Owner:SUZHOU RONGXI BIOTECH CO LTD
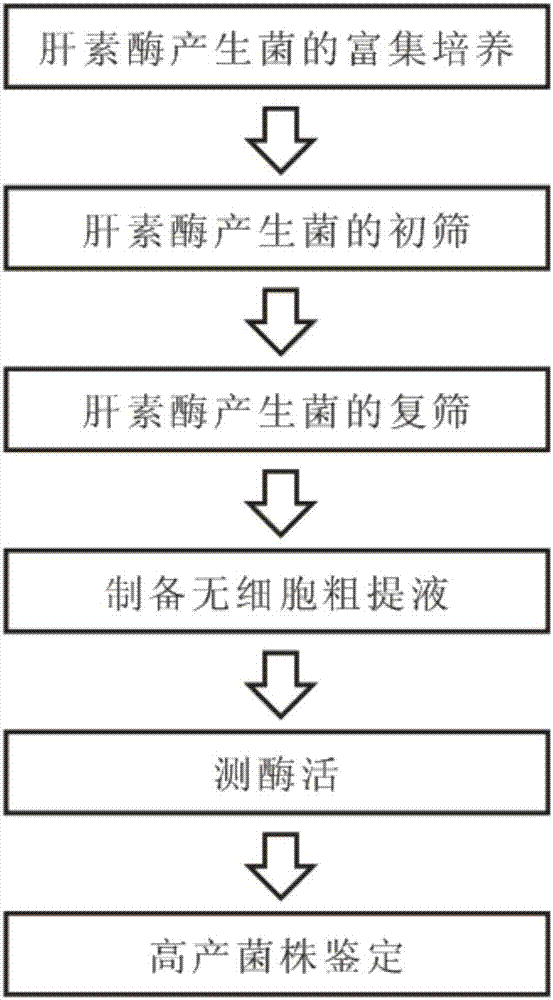
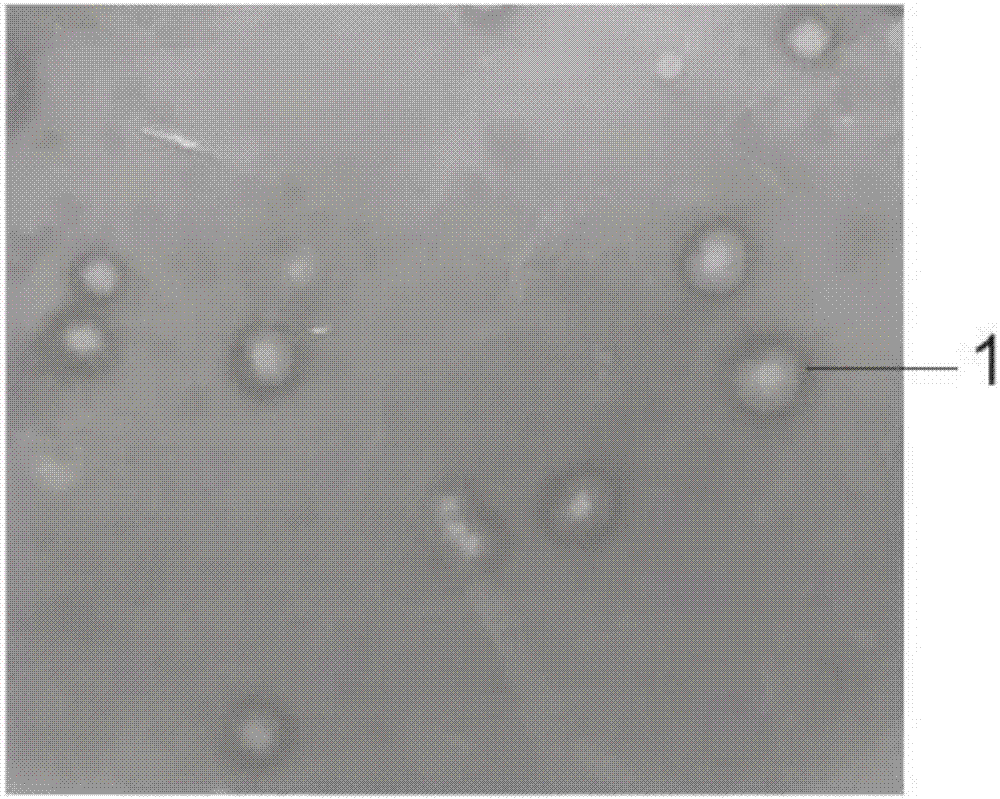
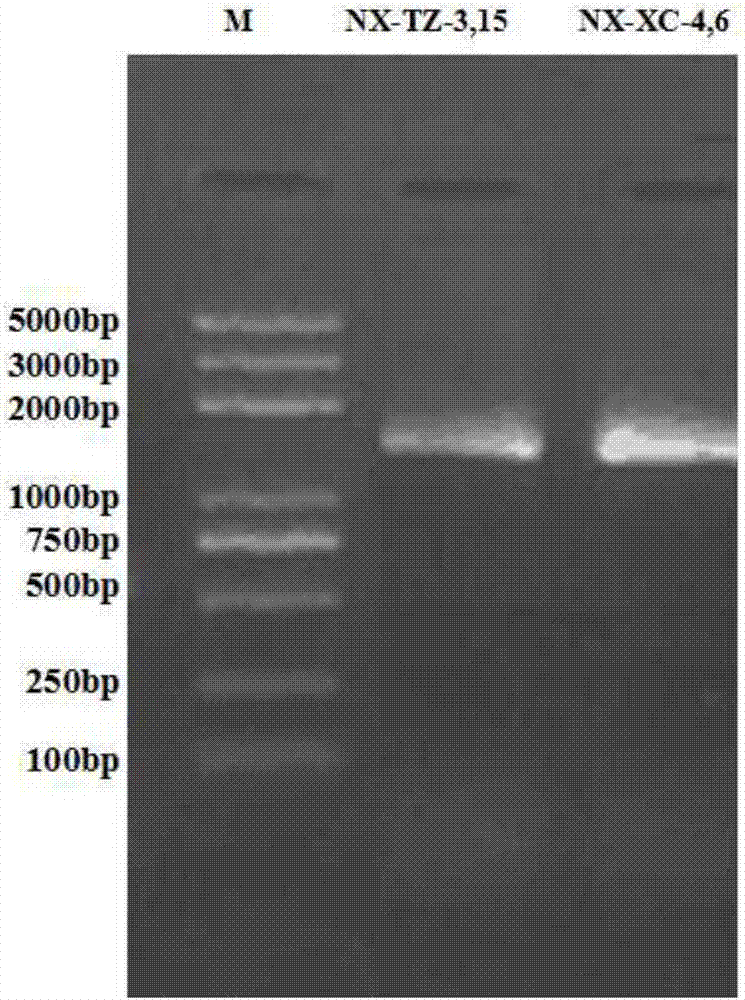
Heparinase high-yielding strain and breeding method thereof
ActiveCN107189956AIncrease productionTime consuming to solveBacteriaMicrobiological testing/measurementMicroorganismIntellectual property
The invention provides a heparanase high-yielding strain. The strain is Raoultella SP.NX-TZ-3,15. The strain is already preserved in a preserve department specified by the State Intellectual Property Office, the preserve date is March 6 th, 2017, the preserve department is the China Common Microorganism Strain Preserve and Management center, and the preserve serial number is CGMCC No.13723. In breeding of the heparanase high-yielding strain, precipitation generated through the reaction of protamine and heparin is utilized as an indicator, heparanase generated through microorganisms are subjected to a reaction of cracking heparin to make the precipitation disappear, transparent rings are generated around thalli, further an effect of directly indicating heparanase source microorganism strains is achieved, through secondary screening and a 16S r DNA method, the heparanase yielding strain SP.NX-TZ-3,15 is identified. In the heparanase high-yielding strain, the disadvantages that in an original screening method of heparanase source microorganisms, time, labor and materials are consumed is solved, the precipitation generated through the reaction of protamine and heparin is utilized as the indicator, and thus the labor, materials and time are sharply saved.
Owner:SHENZHEN HEPALINK PHARMA GRP CO LTD
Unhairing enzyme preparation and unhairing method using same
The invention discloses an unhairing enzyme preparation and an unhairing method using the same, and the unhairing enzyme preparation is prepared by compounding heparinase, lipase and WB 600 / KerT keratinase according to an enzyme activity ratio of 3: 3: 5. The unhairing enzyme preparation has the following technical effects: the unhairing enzyme preparation can effectively solve the pollution caused by traditional sulfide unhairing, effectively acts on original fur hair through heparinase, keratinase and lipase, greatly reduces the dosage of sodium sulfide in the unhairing process, and reducesthe treatment cost of wastewater, and reduces sulfide emissions, thereby reducing environmental pollution and further realizing cleaner production in depilation process.
Owner:QILU UNIV OF TECH
Heparin-containing blood sample detection kit and preparation method thereof
The invention provides a heparin-containing blood sample detection kit and a preparation method thereof. The kit comprises a reagent 1, a reagent 2, a common cup and a heparinase cup. The reagent 1 contains kaolin, sodium chloride and purified water; the reagent 2 contains calcium chloride, sodium chloride and purified water; the heparinase cup is coated with heparinase and a freeze-drying protective agent; and the common cup is an empty test cup. According to the invention, different reactivity of the diagnostic reagent to heparin-containing drug blood is fully considered, and the kit has good sensitivity to heparin drugs commonly used clinically at present, including common heparin, dalteparin, enoxaparin, nadroparin, paheparin, paheparin and the like. The heparin-containing blood sampledetection kit is used for heparin-containing blood detection, the detection result accuracy is good, and the intra-batch precision and the inter-batch precision are high.
Owner:郑州普湾医疗技术有限公司
Culture medium used for culturing microbial expression heparanase
InactiveCN101591633AReduce manufacturing costIncrease enzyme activityBacteriaHydrolasesCulture mediumsYeast extract
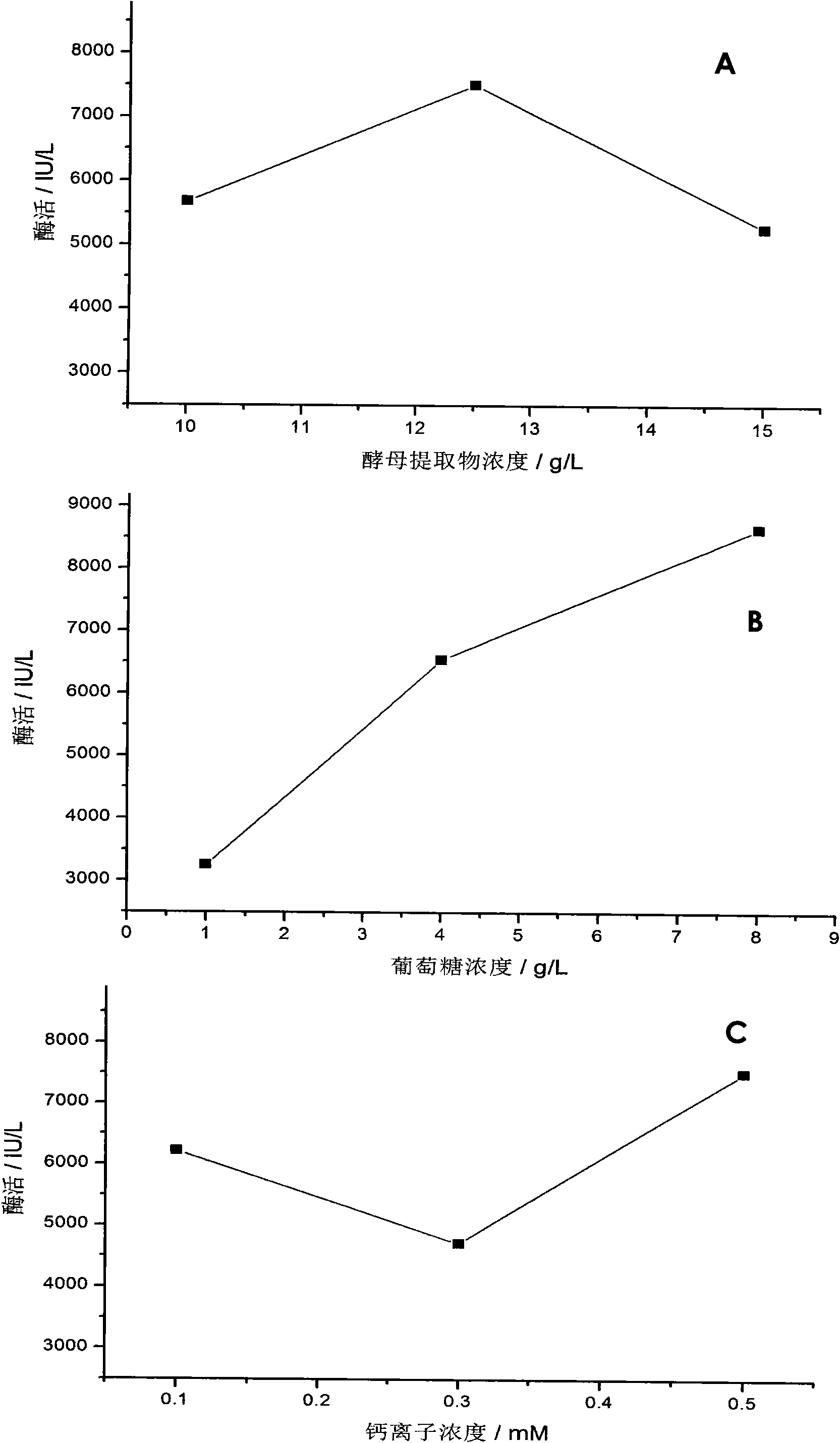
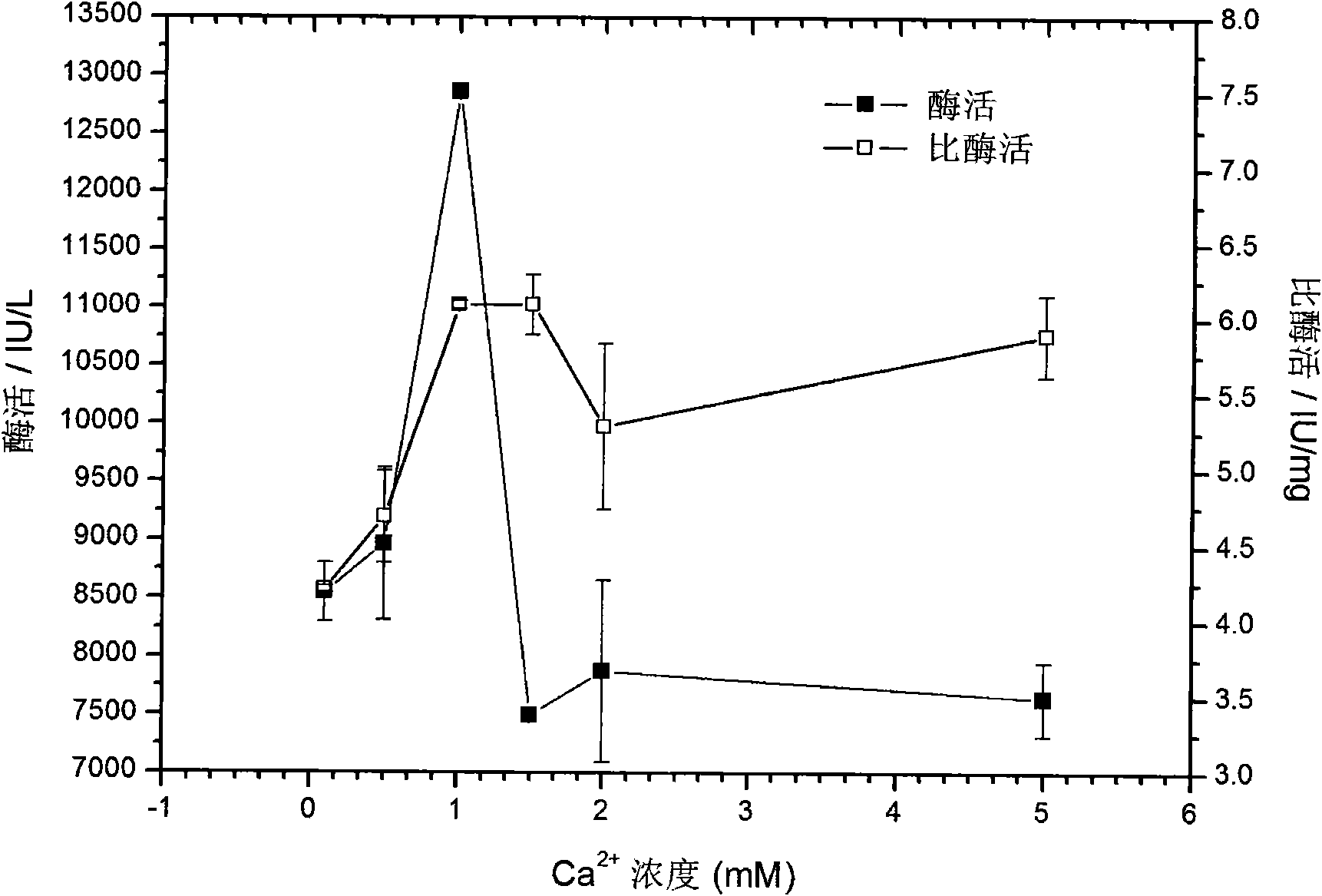
The invention discloses a culture medium used for culturing microbial expression heparanase. The culture medium contains 5 to 20g / L of yeast extract, 0.5 to 20g / L of glucose and 0.05mM to 5.0mM of calcium ion. The culture medium also contains 10.0 to 25.0g / L of Na2HPO.12H2O, 1.0 to 5.0g / L of KH2PO4, 0.1 to 1.0g / L of NaC1 and 0.5 to 5.0g / L of NH4C1. Recombinant heparanase can be expressed in a high yield manner in the culture medium, and the maximal enzyme activity of the heparanase reaches 20652.63IU / L of fermentation liquid and is far higher than the expression effect of a common LB culture medium. The culture medium greatly reduces the production cost of the heparanase and has a very obvious economic meaning for the application of the heparanase, in particular to heparin which is prepared by an enzymatic method and has low molecular weight.
Owner:TSINGHUA UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com