Heparinase III HLGAG fragments and uses thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
DEPC Inactivates Heparinase III
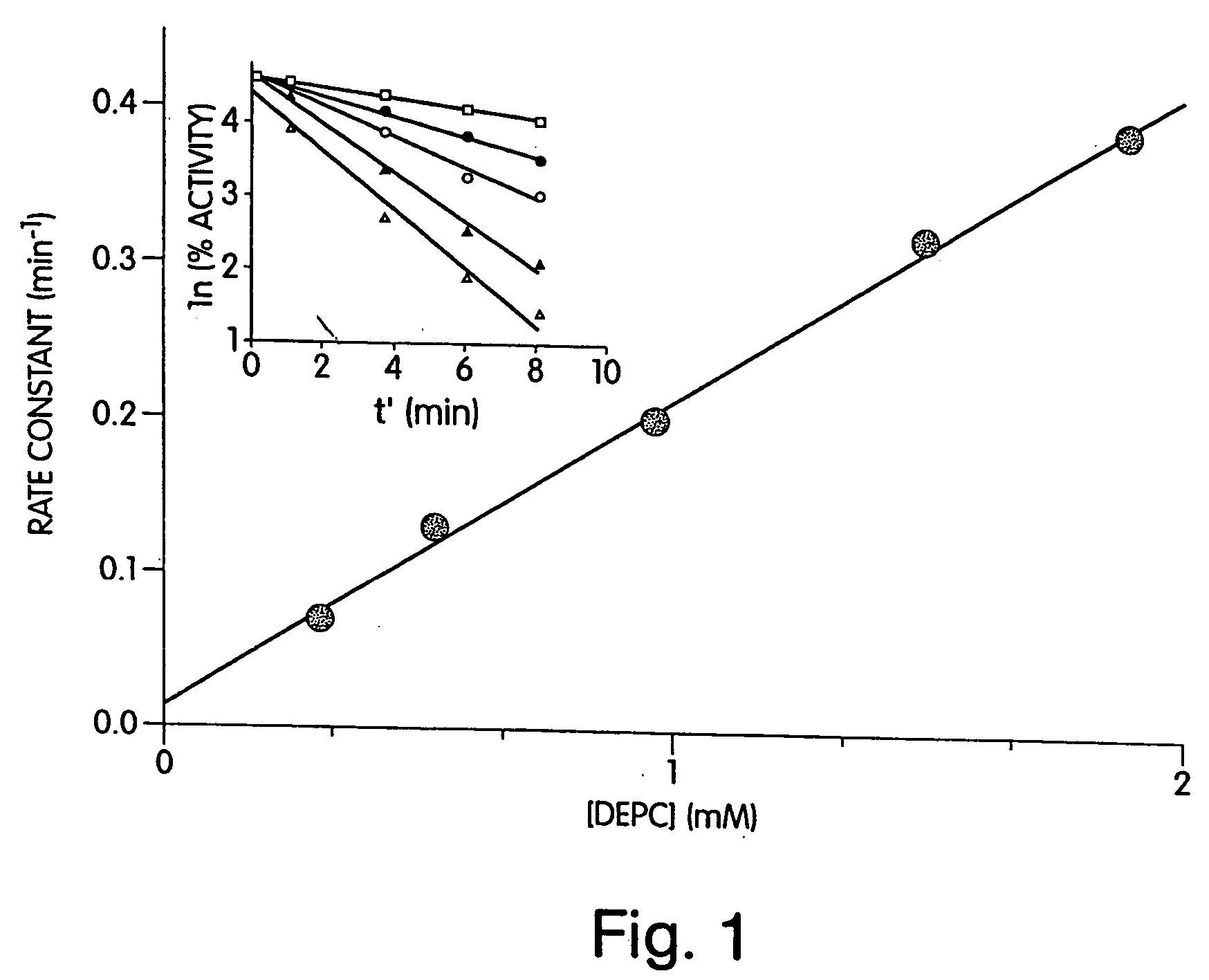
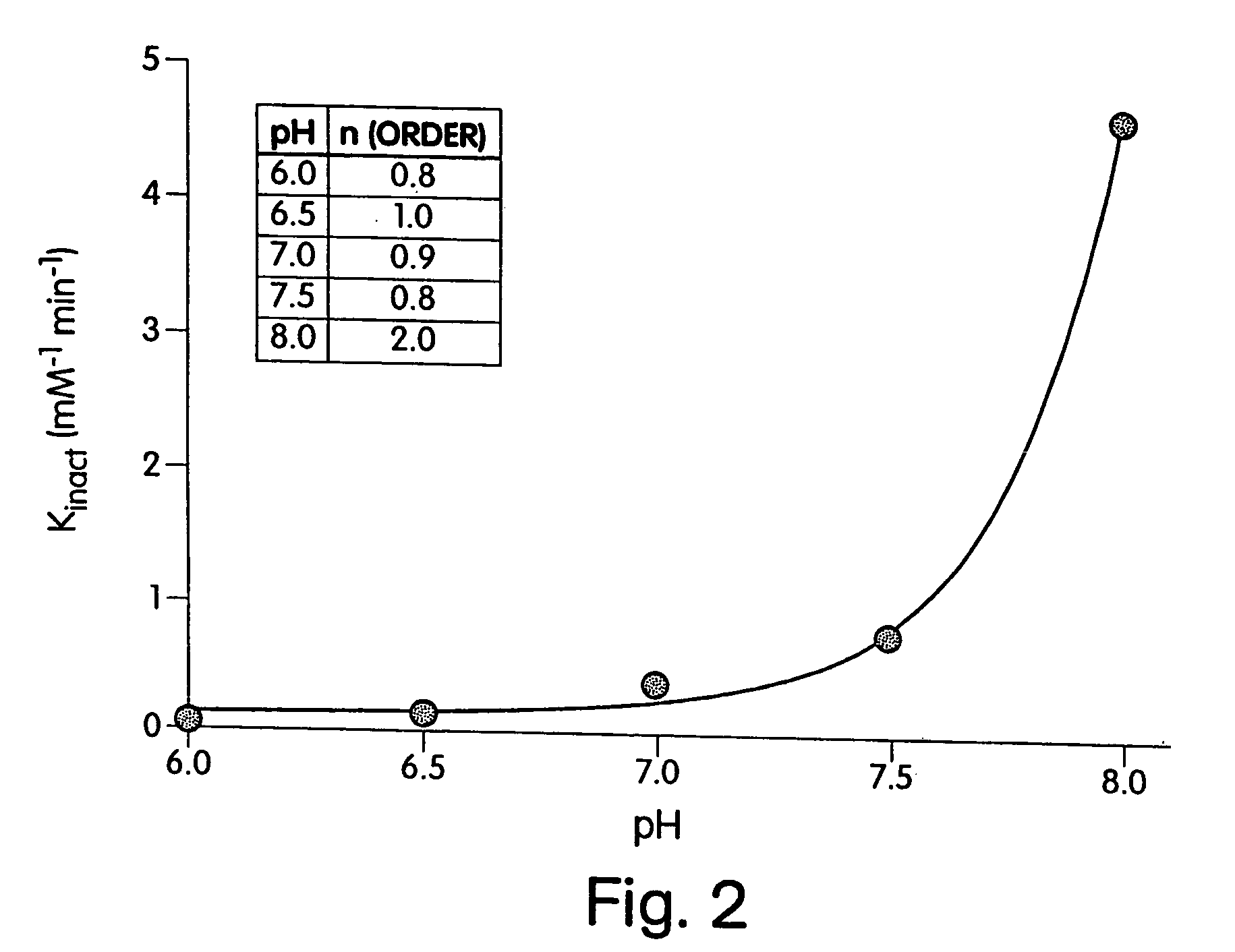
[0190] As a first step towards identifying histidines that are critical for the enzymatic activity of heparinase III, the effect of the modification reagent DEPC on the enzymatic activity of heparinase III was determined. DEPC is a common reagent used for the determination of catalytically critical histidines in enzymes. As stated in early publications (Godavarti, R., Cooney, C. L., Langer, R., and Sasisekharan, R. (1996) Biochemistry 35, 6846-52 and Shriver, Z., Hu, Y., and Sasisekharan, R. (1998) J. Biol. Chem. 273, 10160-67), DEPC is useful for the determination of catalytically critical histidines, however care needs to be taken to ensure that other nucleophilic residues, namely tyrosines, lysines, and cysteines are not modified.
[0191] Heparinase III was incubated with 0.31 (□), 0.54 (●), 0.97 (◯), 1.5 (σ), 1.9 (Δ) mM DEPC at pH 6.5 and at 25° C. (shown in inset of FIG. 1). The natural log of the percent activity remaining was plotted versus an a...
example 2
Peptide Mapping of the Histidine Modified by DEPC
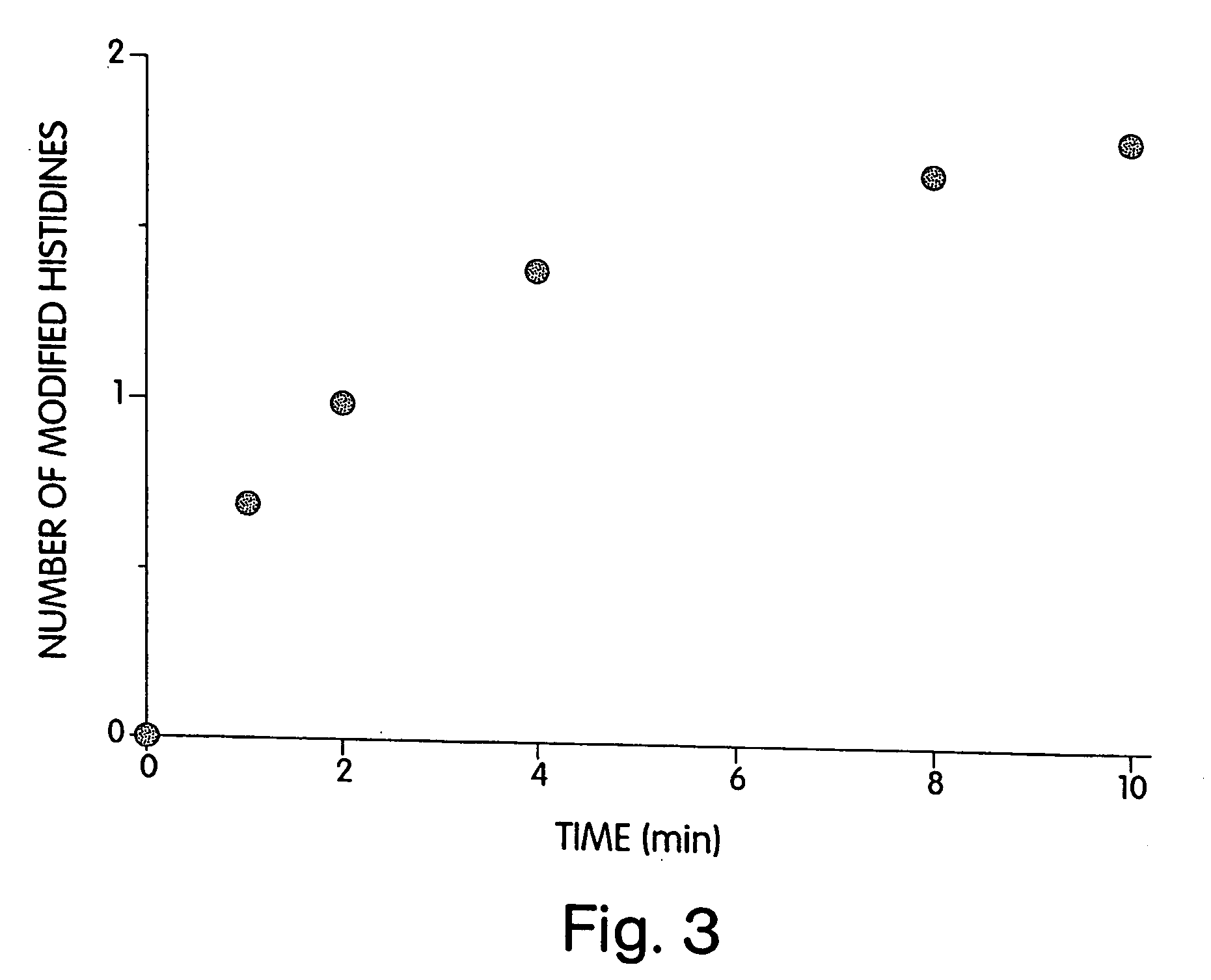
[0200] To identify the histidine(s) modified by DEPC that resulted in the loss of enzymatic activity, DEPC-modified heparinase III was digested with Lys-C. Peptides that had altered retention times and an increased in absorbance at 240 nm as compared to a control digest were collected and sequenced (FIG. 5). Three peptides had altered retention times and increased absorbance at 240 nm were isolated and sequenced. Two of the peptides contained histidine 295 and one contained no modified histidine residues.
[0201] Labeling of the DEPC-reactive histidines was completed by first reacting heparinase III with DEPC, then denaturing the protein in urea. Following an overnight digest with Lys-C, the resultant peptides were separated by using a 1.6%-78.4% acetonitrile gradient over 120 minutes, which included a 5 min isocratic phase (1.6% acetonitrile, 0.1 % trifluoroacetic acid) at the beginning of the run. Lys-C peptides were monitored at 21...
example 3
Site-Directed Mutagenesis of Heparinase III
[0202] In parallel to the mapping studies and to confirm the results of the chemical modification experiments, each of the thirteen histidine residues present in heparinase III was mutated to alanine. The recombinant heparinase III mutant proteins were expressed, purified, and assessed for enzymatic activity towards heparan sulfate (Table 2).
TABLE 2Kinetic Constants for r-heparinase III and the Histidine Mutants.EnzymeKM (uM)akcat (s−1)wild-type8078r-heparinase IIIH36A9886H105ANDbNDbH110A937H139A19168H152A5883H225A8022H234A7523H241A165H295ANDNDH424A5924H469A71100H510ANDNDH539A92132
aCalculated assuming a molecular weight for heparan sulfate of 15 kDa.
bProtein expression levels were too low for heparinase III kinetic assay.
[0203] As a control, the r-heparinase III construct without its putative signal sequence was expressed. The concentration and purity of all recombinant enzyme preparations were determined using SDS-PAGE. The recombinan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Therapeutic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com