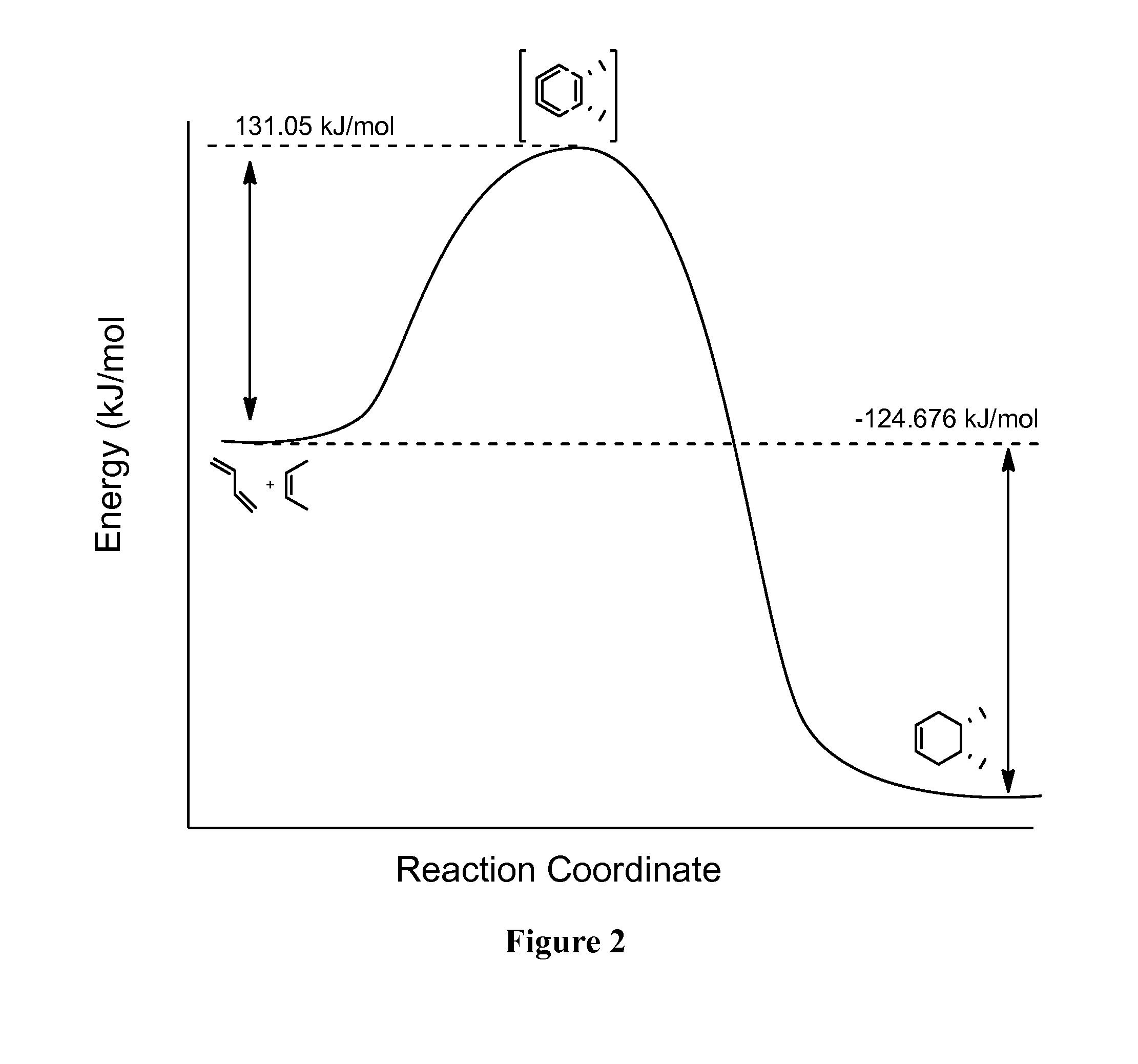
Patents
Literature
79 results about "Activity ratios" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Activity ratios are a category of financial ratios that measure a firm's ability to convert different accounts within its balance sheets into cash or sales. Activity ratios measure the relative efficiency of a firm based on its use of its assets, leverage, or other similar balance sheet items and are important in...
Treatment of female fertility conditions through modulation of the autonomic nervous system
Methods are provided for treating a subject for a fertility condition. In accordance with the subject methods, at least a portion of a subject's autonomic nervous system is modulated to increase the sympathetic activity / parasympathetic activity ratio in a manner that is effective to treat the subject for the condition. Embodiments of the subject invention include modulating a subject's autonomic nervous system using electrical energy and / or one or more pharmacological agents. The subject methods find use in the treatment of a variety of different fertility conditions. Also provided are kits for use in practicing the subject methods.
Owner:PALO ALTO INVESTORS LP
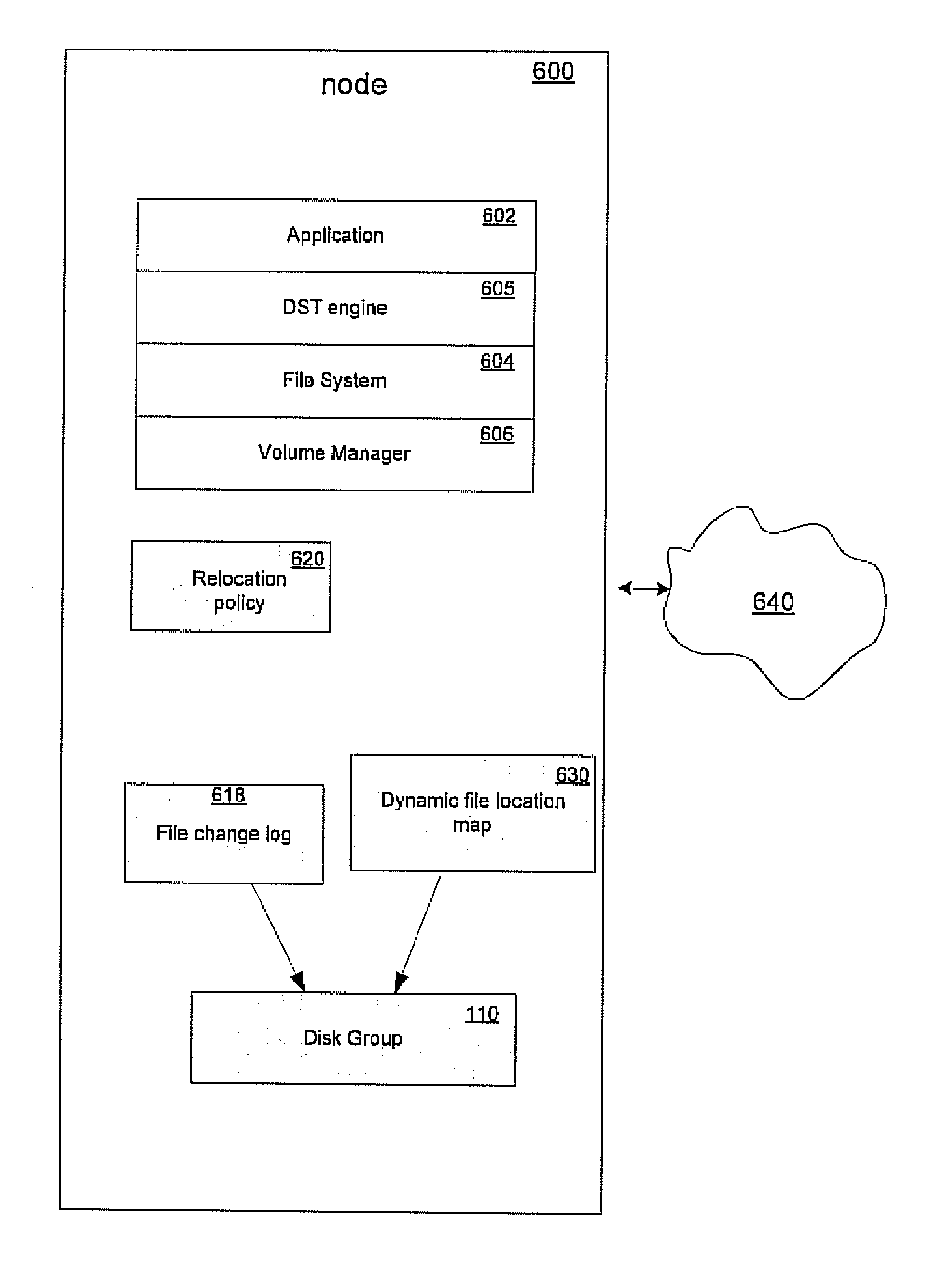
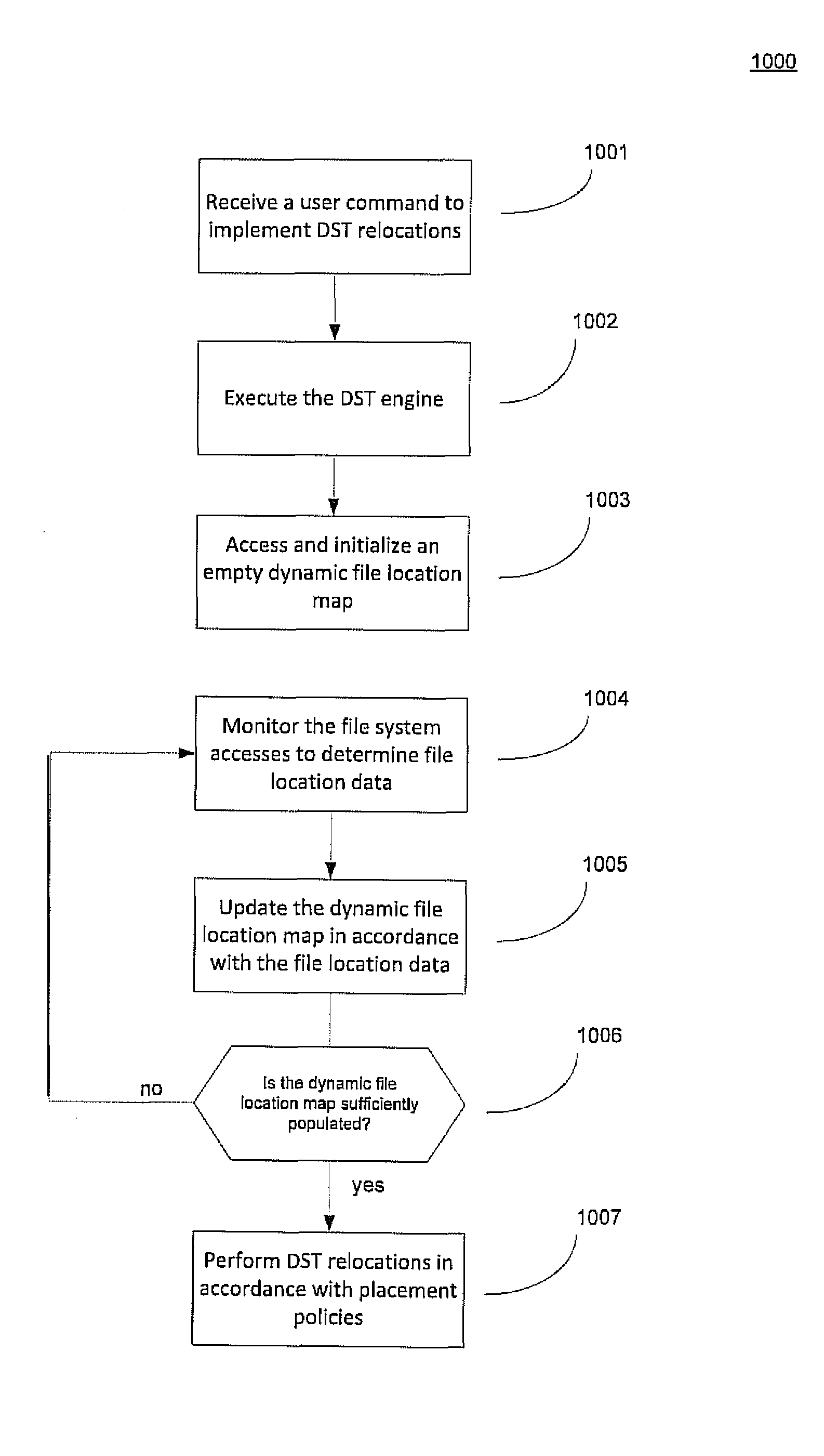
Using a per file activity ratio to optimally relocate data between volumes
ActiveUS20110106863A1Reduce the impactLimited resourceDigital data information retrievalSpecial data processing applicationsFile systemActivity ratios
A method for identifying data for relocation in a multivolume file system. The method includes generating a file location map, the file location map containing a list of the locations of files that occupy space on each of a plurality of volumes of the file system, wherein The file system comprising least a first volume and a second volume. The method further includes updating the file location map in accordance with changes in a file change log for the file system, and identifying data residing on the first volume of the file system by scanning the file location map. Using the identified data, a ratio of per-file activity during a first time period relative to overall file system activity over a second time period is calculated to derive a file activity ratio for each of the files of the identified data. Files are then selected for relocation based on the file activity ratio.
Owner:VERITAS TECH
Complex enzyme preparation for feeding piglets
InactiveCN102119768AImprove metabolic energyReduce chyme viscosityAnimal feeding stuffAccessory food factorsPectinaseDisease
The invention discloses a complex enzyme preparation for feeding piglets. The complex enzyme preparation for feeding piglets comprises the following seven enzymes: acid protease, amylase, xylanase, beta-glucanase, cellulase, pectinase and phytase; and the enzymatic activity ratio of the seven enzymes is 1: 1: (8-10): (3.3-4): 1: (0.5-0.55): (0-0.02). By applying the complex enzyme preparation disclosed by the invention, various anti-nutritional factors in feed can be degraded, the viscosity of chyme in an intestinal tract can be reduced, and the metabolic energy of the feed can be improved; the protein and starch digestibility of the piglets can be improved; excessive reproduction of harmful microorganisms in the intestinal tract can be reduced, damage to the intestinal wall can be reduced, and the microecological balance of the intestinal tract can be regulated; the survival rate and disease resistance of the piglets can be improved, the diarrhea rate can be reduced, and the overall uniformity is improved; and the weight gaining of the piglets can be promoted, and the cultivation cost is reduced.
Owner:BEIJING CHALLENGE AGRI SCI & TECH CO LTD
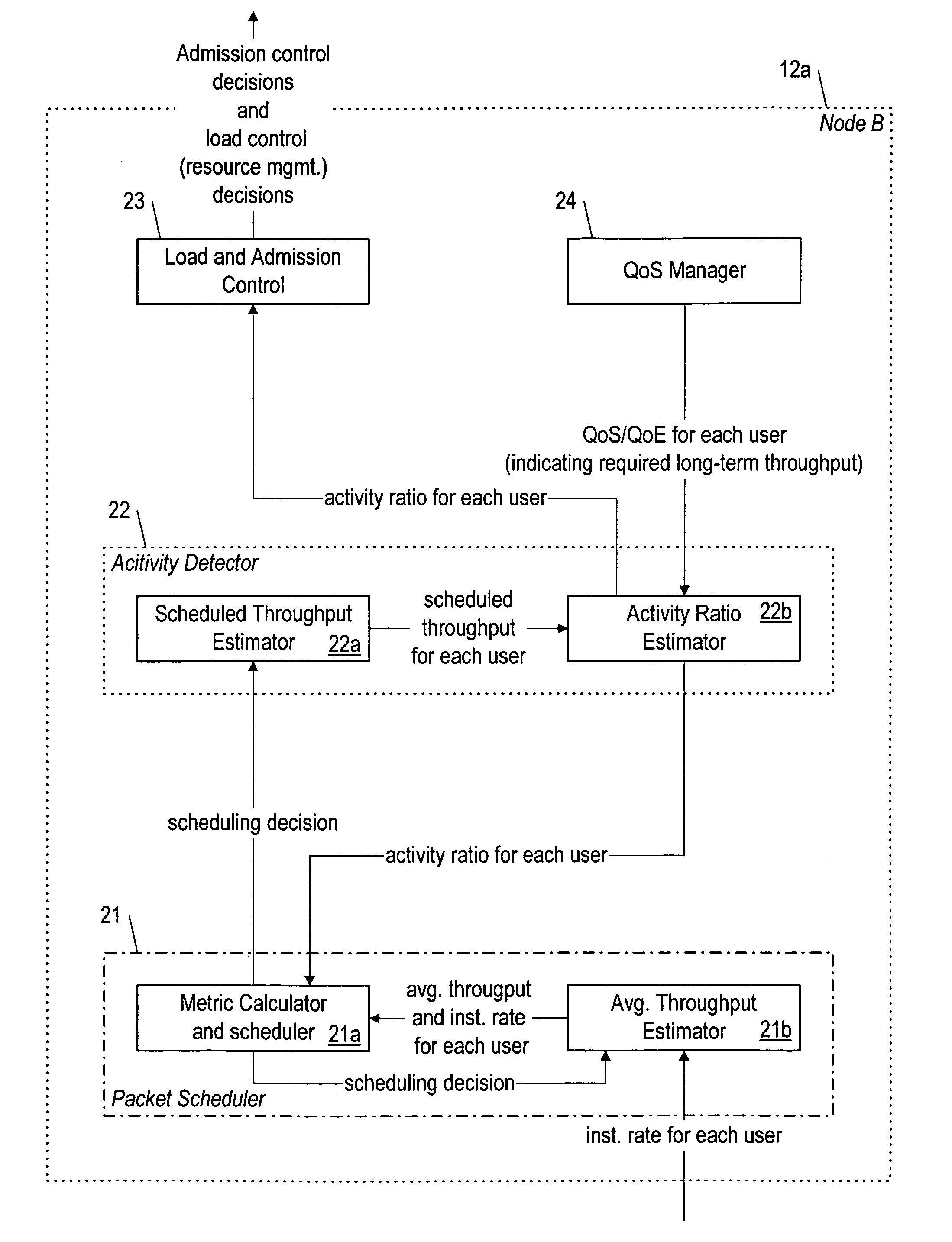
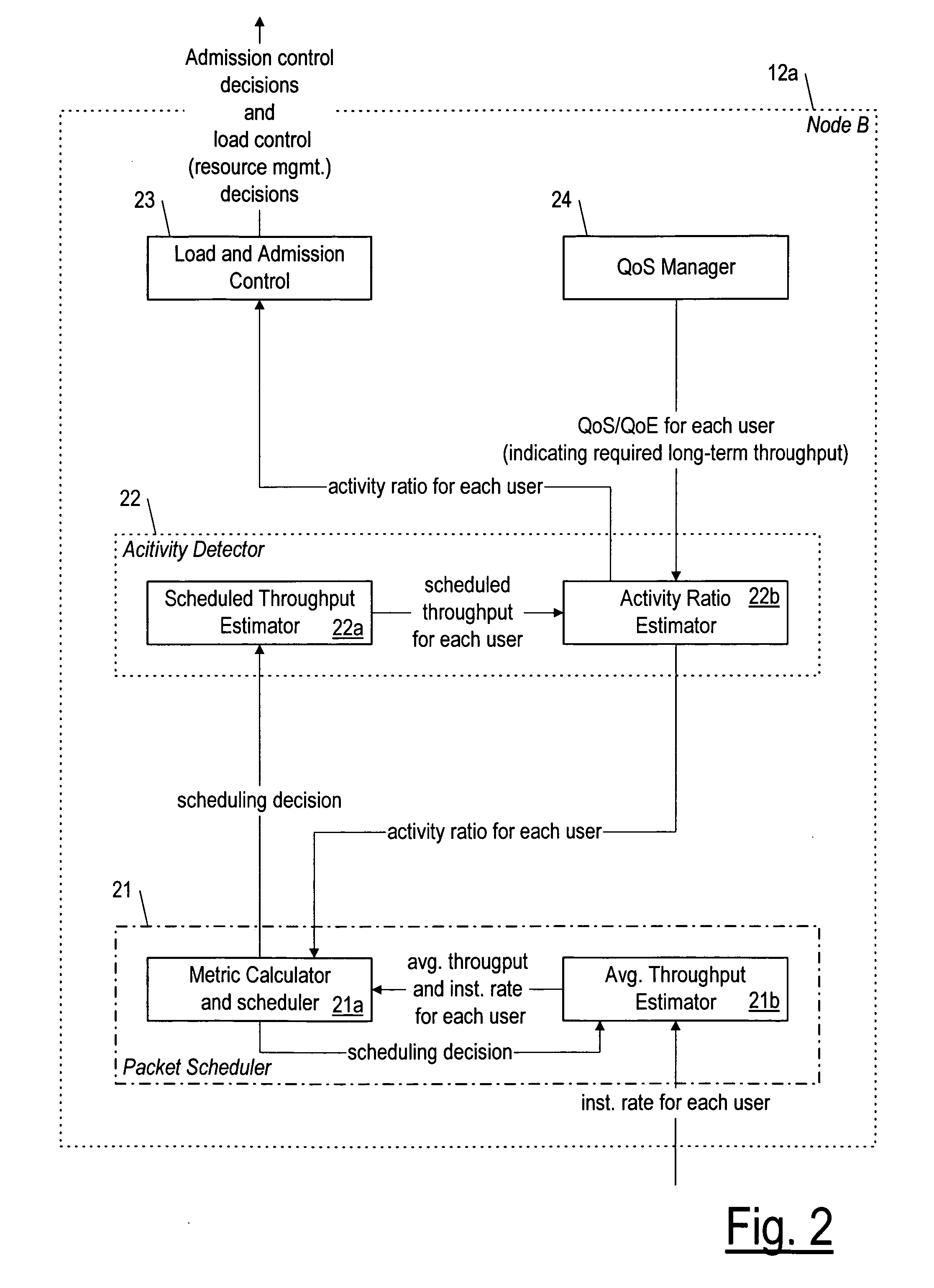
QOS-aware radio resource management (for wireless communication) with activity detection
A packet scheduler (21) that schedules packets for wireless transmission to a UE (16) during a time interval, based on calculating a metric for the UE (16) that takes into account both an activity ratio indicative of the long-term required throughput (if any) for the UE (16) compared to a scheduled throughput. The packet scheduler (21) compares the metric for the UE with that it calculates for other UE's also having packets to be scheduled for delivery during the time interval, and the packets of the UE for which the metric is greatest are scheduled preferentially.
Owner:NOKIA SOLUTIONS & NETWORKS OY
Complex enzyme preparation for growing and fattening pig feed
InactiveCN102106477AImprove metabolic energyReduce chyme viscosityAnimal feeding stuffAccessory food factorsPectinaseAnimal science
The invention discloses a complex enzyme preparation for a growing and fattening pig feed. The complex enzyme preparation for the growing and fattening pig feed comprises the following eight enzymes: acid protease, amylase, beta-mannase, xylanase, beta-dextranase, cellulase, pectinase and phytase; and the enzyme activity ratio of the eight enzymes is sequentially 1: (0.8-14.5): (0.19-0.3): (33.7-35): (25-30): (3.2-3.5): (0.19-2.0): (0-0.025). The complex enzyme preparation can degrade various anti-nutritional factors in the feed, reduce the chime viscosity of the intestinal tract, improve the metabolic energy of the feed, improve the digestion rate of growing and fattening pigs on protein and starch, reduce the nutrient content of the chime entering the intestinal tract, effectively inhibit the reproduction of harmful microbes in the intestinal tract, reconcile the micro-ecological balance of the intestinal tract, promote the health of the pigs, improve the average daily gain of the growing and fattening pigs, reduce the feed-meat ratio, shorten the maintenance period and improve the economic benefit.
Owner:BEIJING CHALLENGE AGRI SCI & TECH CO LTD
Fungal lipolytic enzymes, nucleic acids encoding, and uses thereof
A fungal wild-type lipolytic enzyme having a higher ratio of activity on polar lipids compared with triglycerides, wherein the enzyme preferably has a phospholipid:triglyceride activity ratio of at least 4. Preferably, the lipolytic enzyme according to the present invention has a glycolipid:triglyceride hydrolyzing activity ratio of at least 1.5. In one embodiment, the fungal lipolytic enzyme according to the present invention comprises an amino acid sequence as shown in SEQ ID NO: 1 or SEQ ID No. 2 or SEQ ID No. 4 or SEQ ID No. 6 or an amino acid sequence which has at least 90% identity thereto. The present invention further encompasses a nucleic acid encoding a fungal lipolytic enzyme, which nucleic acid is selected from the group consisting of: (a) a nucleic acid comprising a nucleotide shown in SEQ ID No. 3, SEQ ID No. 5 or SEQ ID No. 7; (b) a nucleic acid which is related to the nucleotide sequence of SEQ ID No. 3, SEQ ID No. 5 or SEQ ID No. 7 by the degeneration of the genetic code; and (c) nucleic acid comprising a nucleotide sequence which has at least 90% identity with the nucleotide sequence shown in SEQ ID No. 3, SEQ ID No. 5 or SEQ ID No. 7.
Owner:DUPONT NUTRITION BIOSCIENCES APS
A non-crystal alloy catalyst as well as its preparing method and purpose
InactiveCN101157034AGood catalyticParticle size controllableOrganic reductionMetal/metal-oxides/metal-hydroxide catalystsNitro compoundReaction rate
The invention discloses amorphous alloy catalyst with controllable grain size being smaller than 50 nm and uniform grain size distribution, and adds a new variety for the prior amorphous alloy catalyst field. The activity ratio surface area of the amorphous alloy catalyst is 10 to 50 m<2> / g, the particle diameter can be controlled in the range of 2 to 50 nm, and the grain size distribution is uniform. The invention realizes the amorphous alloy catalyst with the grain size being smaller than 50 nm and achieves the controllable grain size and the uniform grain size distribution through the preparation method for controlling the reduction reaction rate by oil in water microemulsion. The amorphous alloy catalyst of the invention can be taken as hydrogenation catalyst containing unsaturated functional group compounds such as alkene, alkyne, arene, nitrile, nitro compound, carbonyl compound, etc., not only the catalytic performance is superior to the catalytic performance of the ordinary amorphous alloy catalyst, but also the catalytic performance can be controlled; the life of the catalyst is longer than the life of the ordinary amorphous alloy catalyst, and the catalyst can be recovered and reused time after time.
Owner:SHANGHAI NORMAL UNIVERSITY
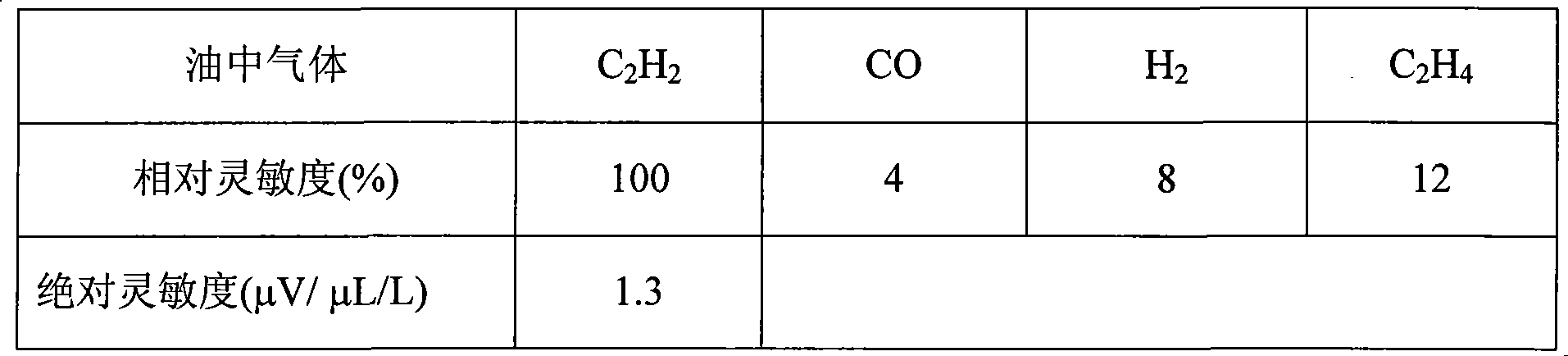
Gas sensor for monitoring gas content in insulating oil
InactiveCN101363813AImproved cross sensitivityReduce concentrationSemi-permeable membranesMaterial electrochemical variablesHigh concentrationAmbient pressure
The invention relates to a gas sensor for monitoring the gas content in insulating oil, in particular to a gas sensor for the online monitoring of the acetylene gas content in insulating oil. The gas sensor comprises a hollow-structure integrated casing, and combined type oil-gas separation membrane and a fuel cell type gas sensing component which are installed inside the casing; wherein a selective electrocatalysis filtering layer for filtering interfering gas is also installed between the combined type oil-gas separation membrane and the fuel cell. Identical gas diffusion electrodes formed by gold alloy (Au) clectrocatalyst are adopted for the anode and the cathode of the selective acetylene fuel cell. The activity of the selective electrocatalysis filtering layer to the interfering gas is higher than the fuel cell itself to the interfering gas; with the increasing of the activity ratio, the filtering effect is also improved. The gas sensor is resistant to various ambient pressure and temperature changes, and the content of low concentration acetylene gas can be correctly and stably monitored in the environment with high concentration interfering gas such as hydrogen gas, carbon monoxide and ethylene gas.
Owner:ASENSOR TECH
Method for simultaneously extracting alliin and garlic enzyme from garlic
InactiveCN102382020AReduce lossesHigh yieldOrganic chemistryOrganic compound preparationFiltration membraneOrganic solvent
The invention relates to a method for simultaneously extracting alliin and garlic enzyme from garlic, which comprises: full ultrasonic cell wall breakage of mixture of peeled garlic and a polar organic solvent with the mass being at least four times of the mass of the garlic (pharmaceutically acceptable) and the volumetric water content less than or equal to 10 percent under the conditions with the pH being 5.5-6.8 and the temperature being less than or equal to 35 DEG C, solid-liquid separation, filtration and separation of supernatant by molecule ultrafiltration membranes with the molecular weight reducing gradually, wherein the molecular weight of the molecule ultrafiltration membrane at the last stage is 10,000 to 3,000, concentration of the filtrate by a nano filtration membrane, crystallization of the concentrated solution, separation to obtain crystals, and drying under conditions with the temperature less than or equal to 35 DEG C. The problem of reaction of the alliin and the garlic enzyme in cell wall breakage of the garlic can be solved effectively, and garlic extracts with the alliin content being 2.2-98 percent and the activity ratio of the garlic enzyme being 80-900 can be extracted and enriched, the losses of effective ingredients can be reduced, the yield and purity can be improved, the cost is low, the production requirements of cleanness and low energy consumption can be satisfied, and the method has a practical value in industrial applications.
Owner:成都菊乐制药有限公司 +1
Cathode non-platinum catalyst of proton exchange membrane fuel cell and preparation method thereof
InactiveCN102916203AIncreased oxygen reduction activityHigh active specific surface areaCell electrodesMetal/metal-oxides/metal-hydroxide catalystsNon platinumActivity ratios
The invention relates to a cathode non-platinum catalyst of a proton exchange membrane fuel cell and a preparation method thereof. The method comprises the following steps: melamine-formaldehyde resin preparation reaction is carried out, and metal salt is added into the melamine formaldehyde resin; complexing reaction occurs between the melamine formaldehyde resin and the metal salt to form a complex; and after solvent of the complex is evaporated, the complex is decomposed by heat treatment to form the cathode non-platinum catalyst with a hollow spherical structure of the proton exchange membrane fuel cell. The non-platinum catalyst has following advantages that firstly the non-platinum catalyst has a large activity ratio surface, so that the oxygen reduction activity of a catalyst is greatly improved; secondly the catalyst has a rich nitrogen source; thirdly the catalyst has an excellent activity of oxygen reduction; and fourthly the preparation method of the non-platinum catalyst is simple, and cheap metals such as Fe, Co and the like are used as the catalyst, so that the synthesis cost is lowered.
Owner:WUHAN UNIV OF TECH
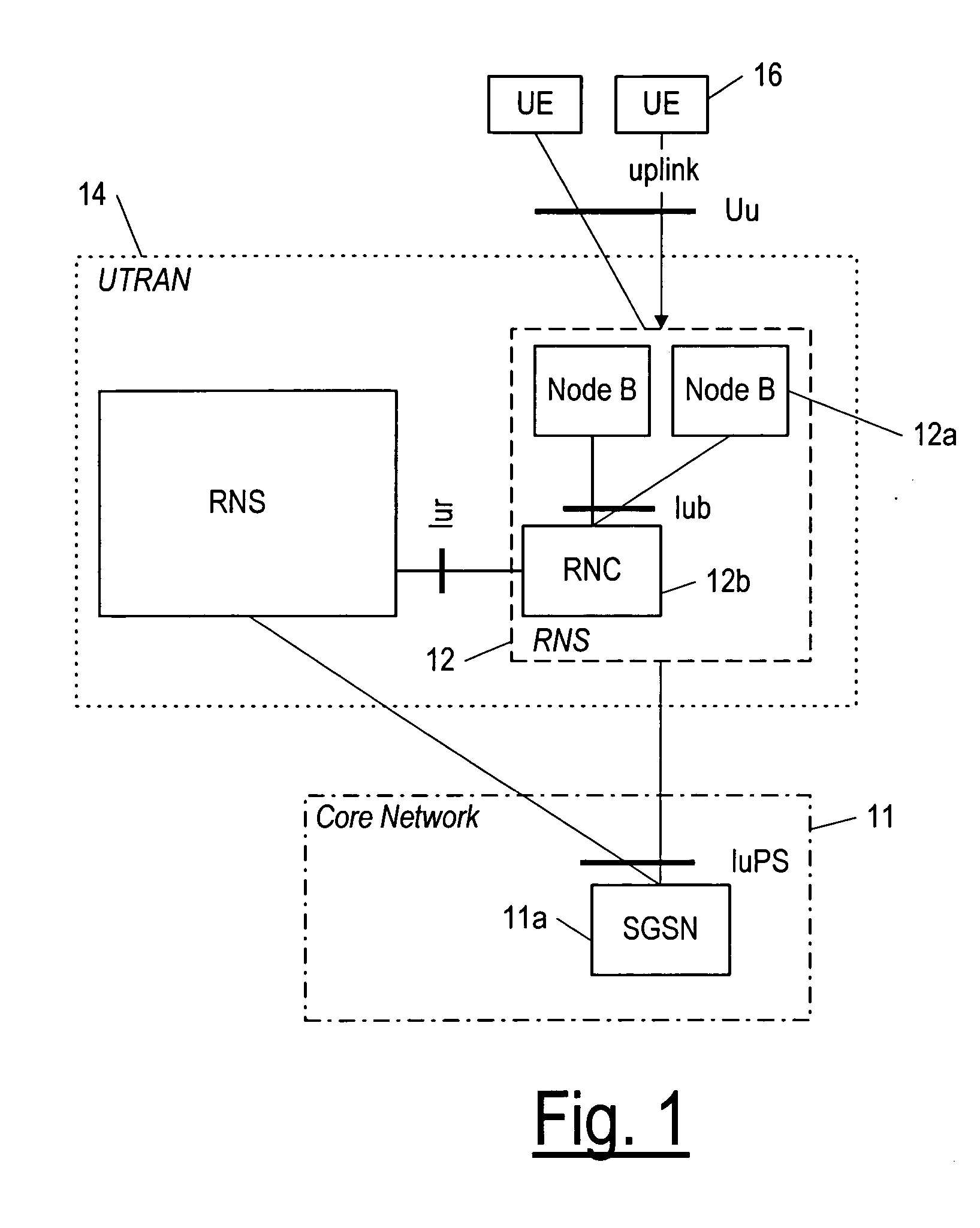
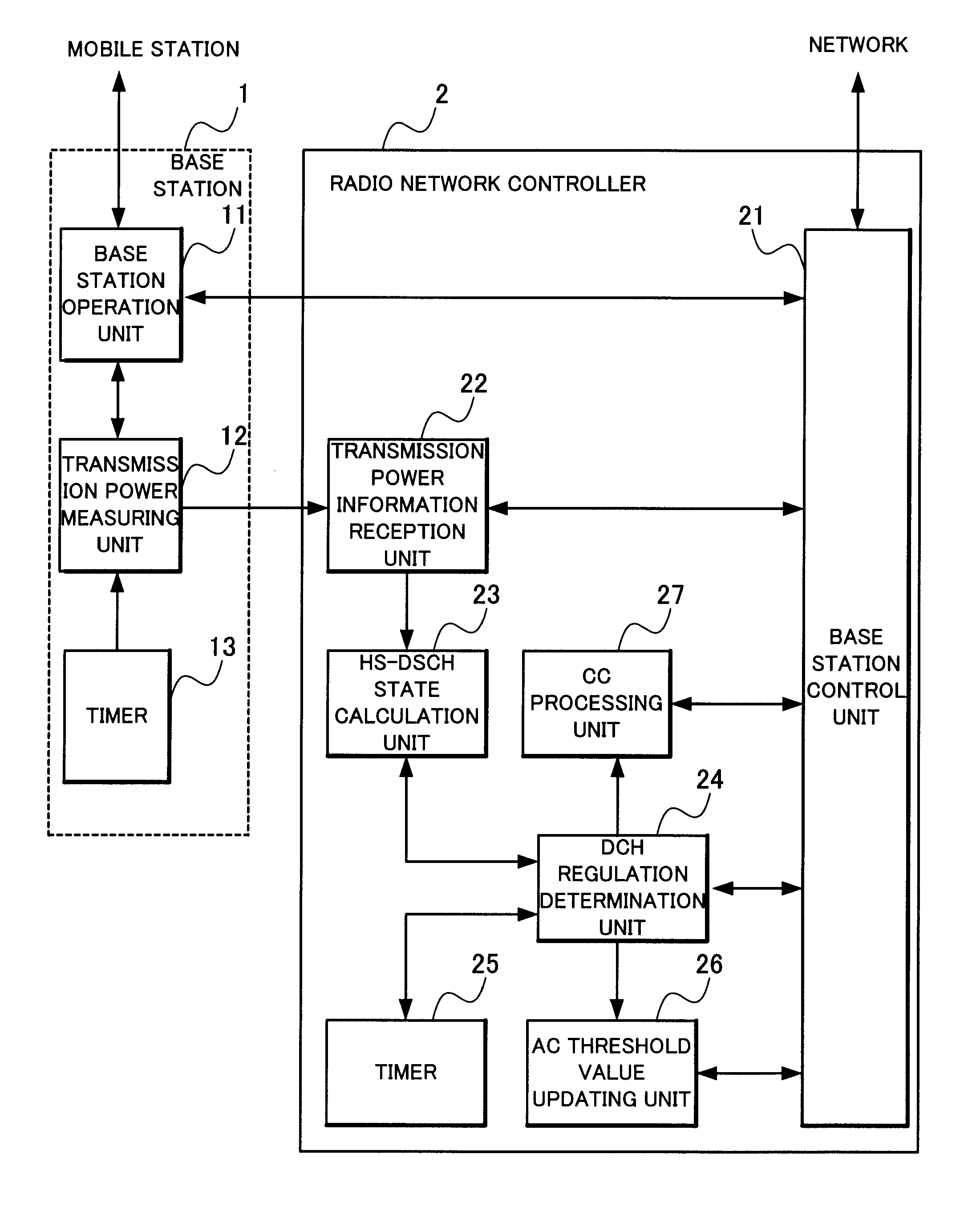
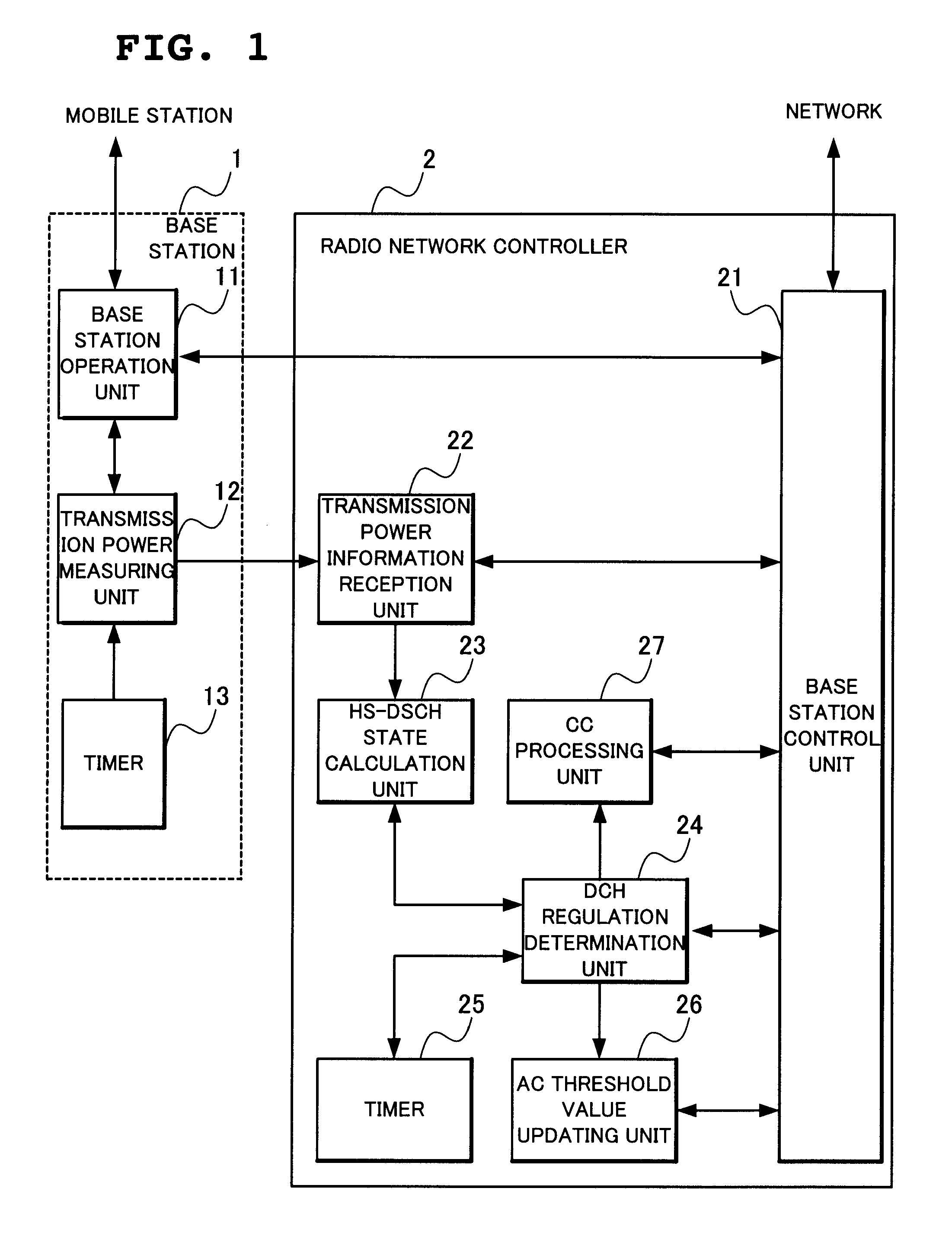
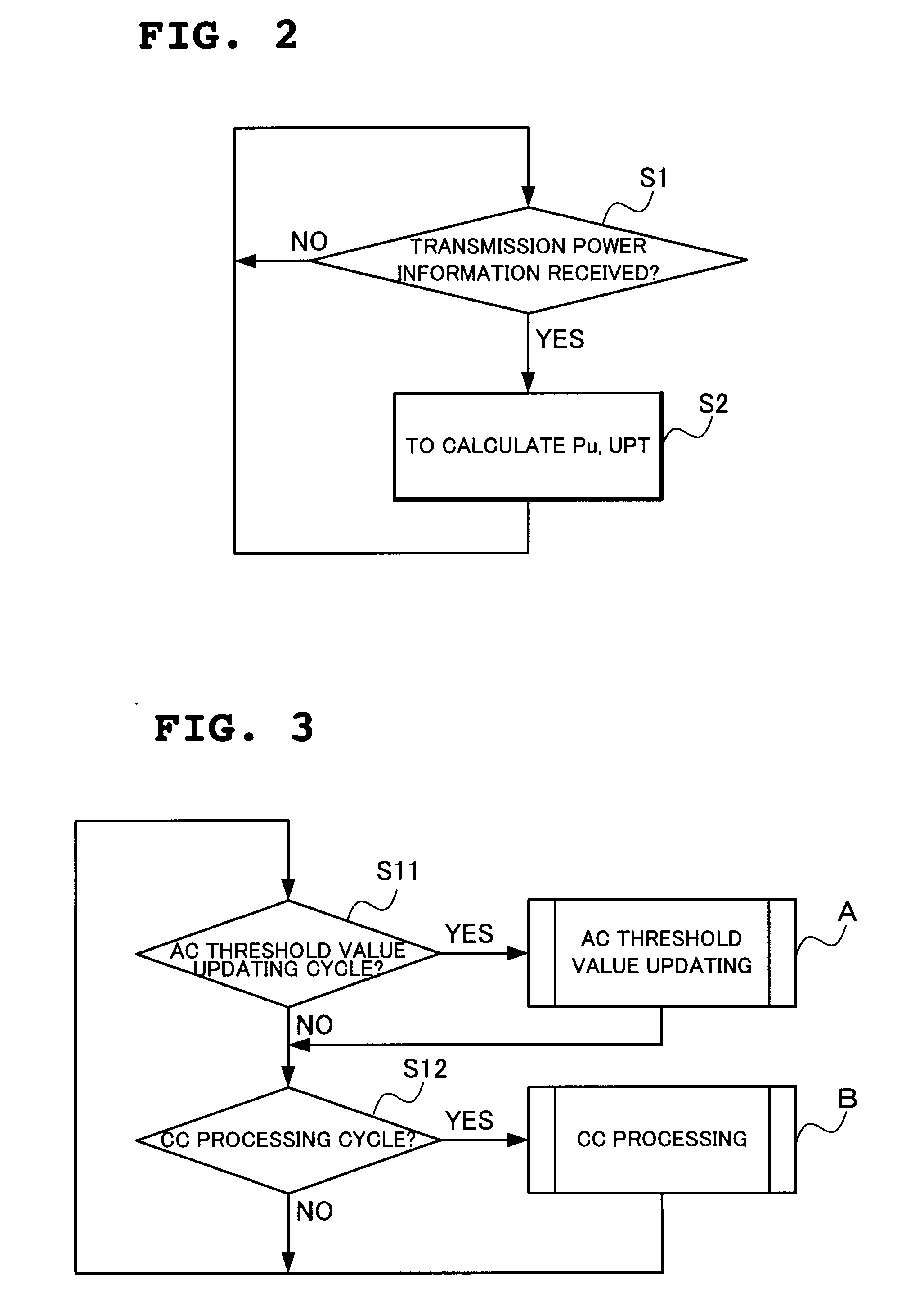
Radio network controlling method, radio communication system and radio network controller
ActiveUS20090154400A1Low transfer ratePower managementNetwork traffic/resource managementCommunications systemRadio networks
When determination is made that a channel for high-speed downlink packet transmission is short in power by the calculation of power assigned to a downlink shared channel and an activity ratio of the power assigned to the downlink shared channel from transmission power of all the channels and transmission power of other channel than a downlink shared channel for high-speed downlink packet transmission, a total sum of transmission power of dedicated channels is reduced by lowering a rate of a connected dedicated channel for data reception, thereby increasing power assigned to the channel for high-speed downlink packet transmission.
Owner:NEC CORP
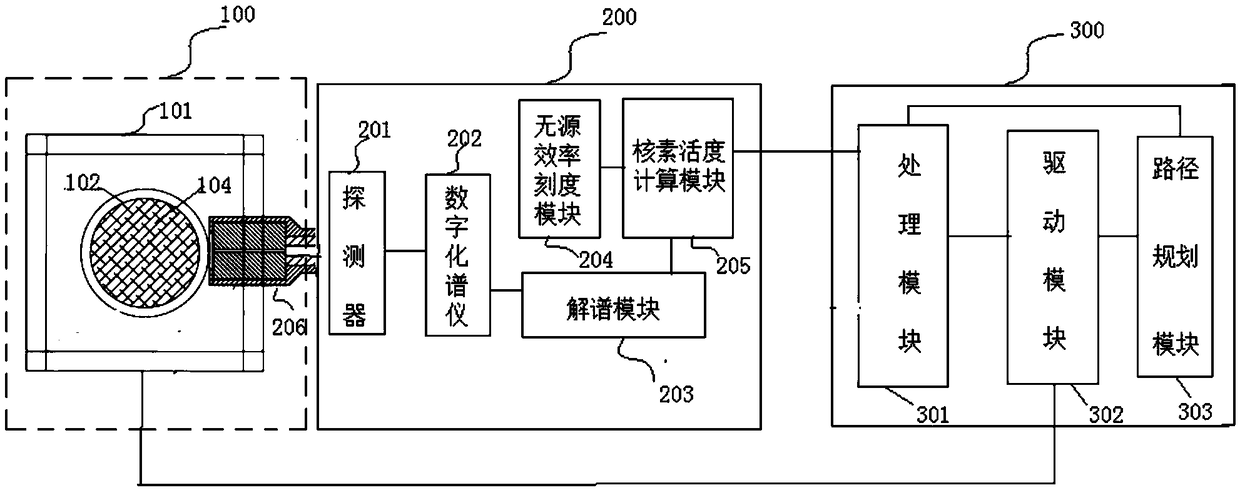
Radioactive water filter waste filter core measuring system and method
ActiveCN109283568AAccurate assessmentSatisfy requirements related to disposalX-ray spectral distribution measurementAutomatic controlWater filter
The invention provides a radioactive water filter waste filter core measuring system and method. The method comprises the following steps that a machinery stand drives a waste filter core to be testedto do three-dimensional movement; a gamma spectrometer and gamma rays released by radionuclides in the waste filter core to be tested act on each other; the distribution of each radionuclide in the waste filter core to be tested, the total activity and the activity ratio are obtained through the total energy peak count rate analysis of each section of the waste filter core to be tested obtained through measurement; and a control unit selects a measuring point, plans a measuring path, automatically controls the movement of a rotating table and visually displays the measuring result. The systemand the method have the advantages that the sectioned multi-point detection on the waste filter core is realized; and a more precise and detailed gamma spectrum type of the waste filter core can be obtained. The guarantee is provided for nuclear power plants to more accurately evaluate the types and the activity of the radionuclides in waste filter core waste barrels by a dose rate reckoning method so that the radiation safety of measuring personnel is ensured and the relevant requirements on national radioactive waste treatment and disposal can be met.
Owner:YANGJIANG NUCLEAR POWER +1
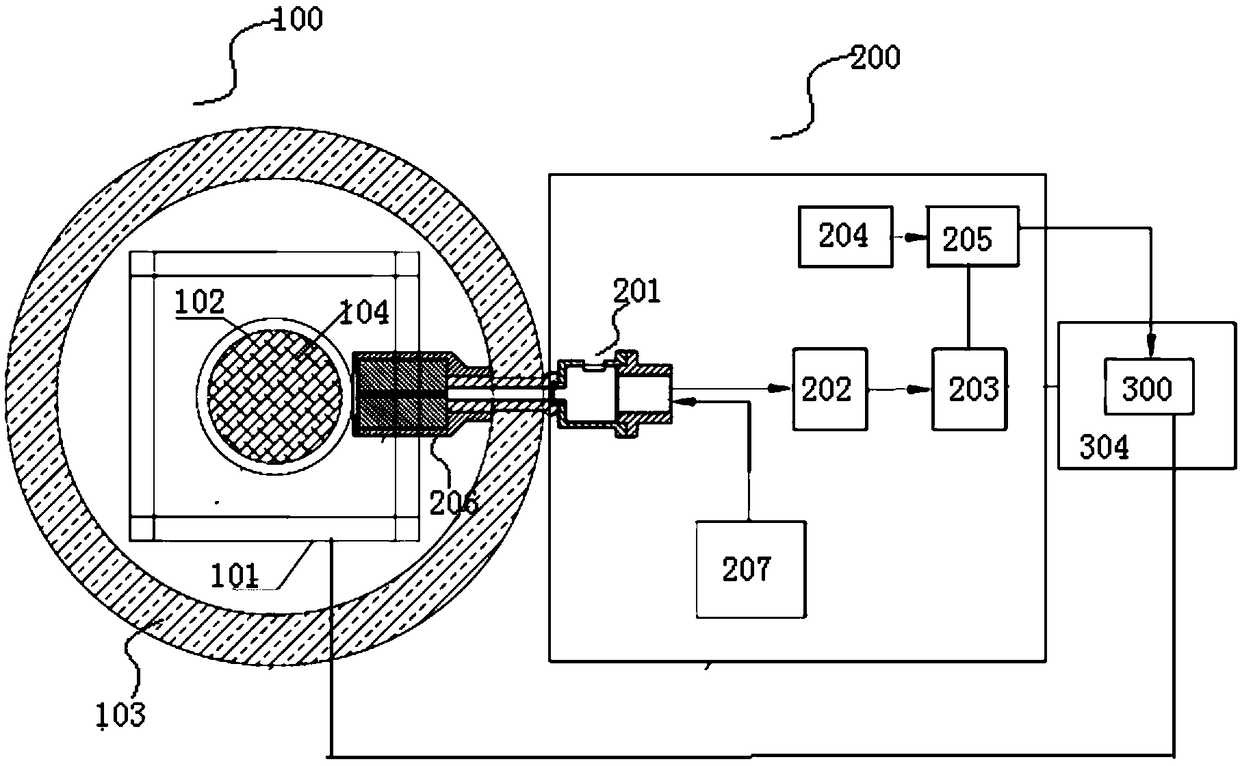
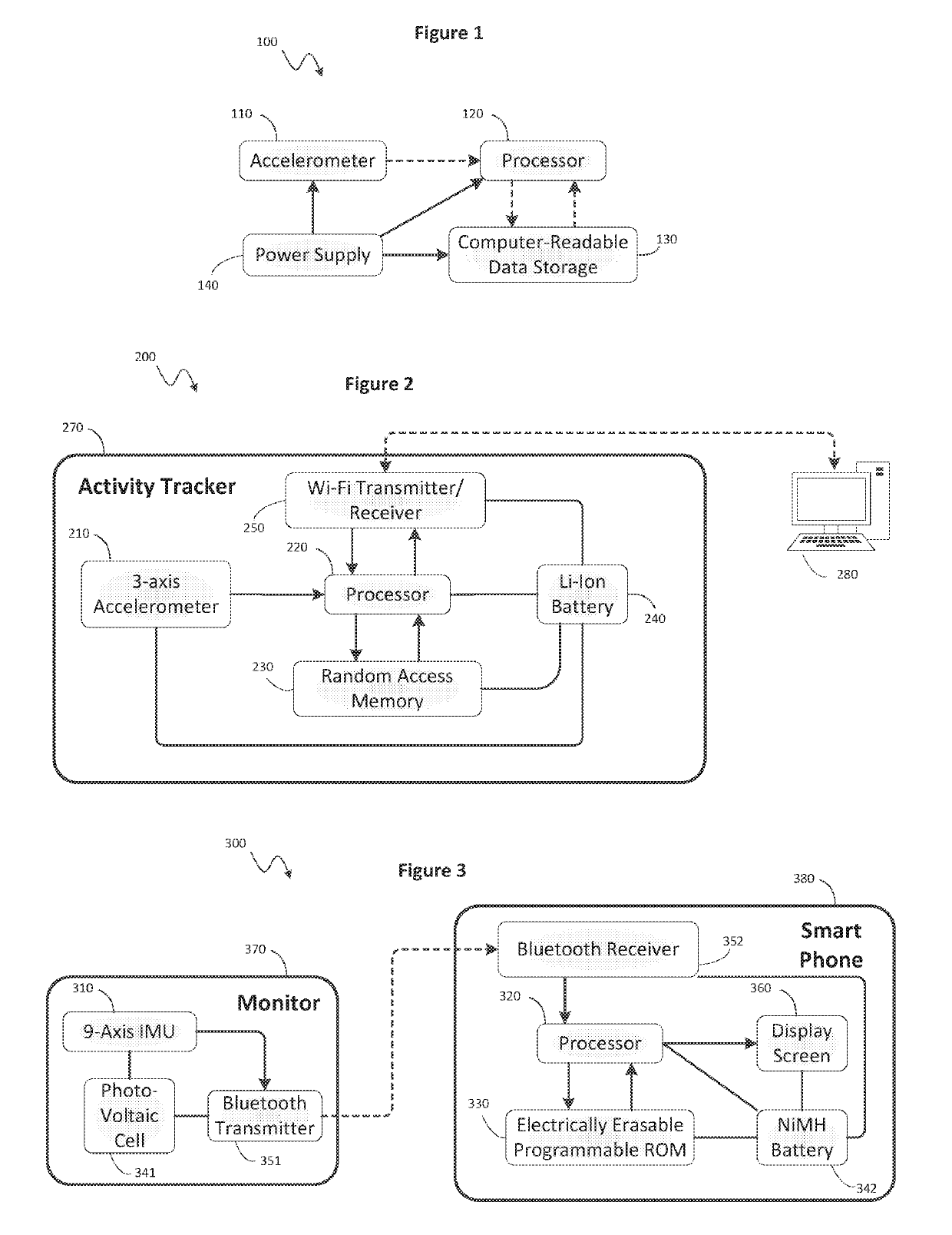
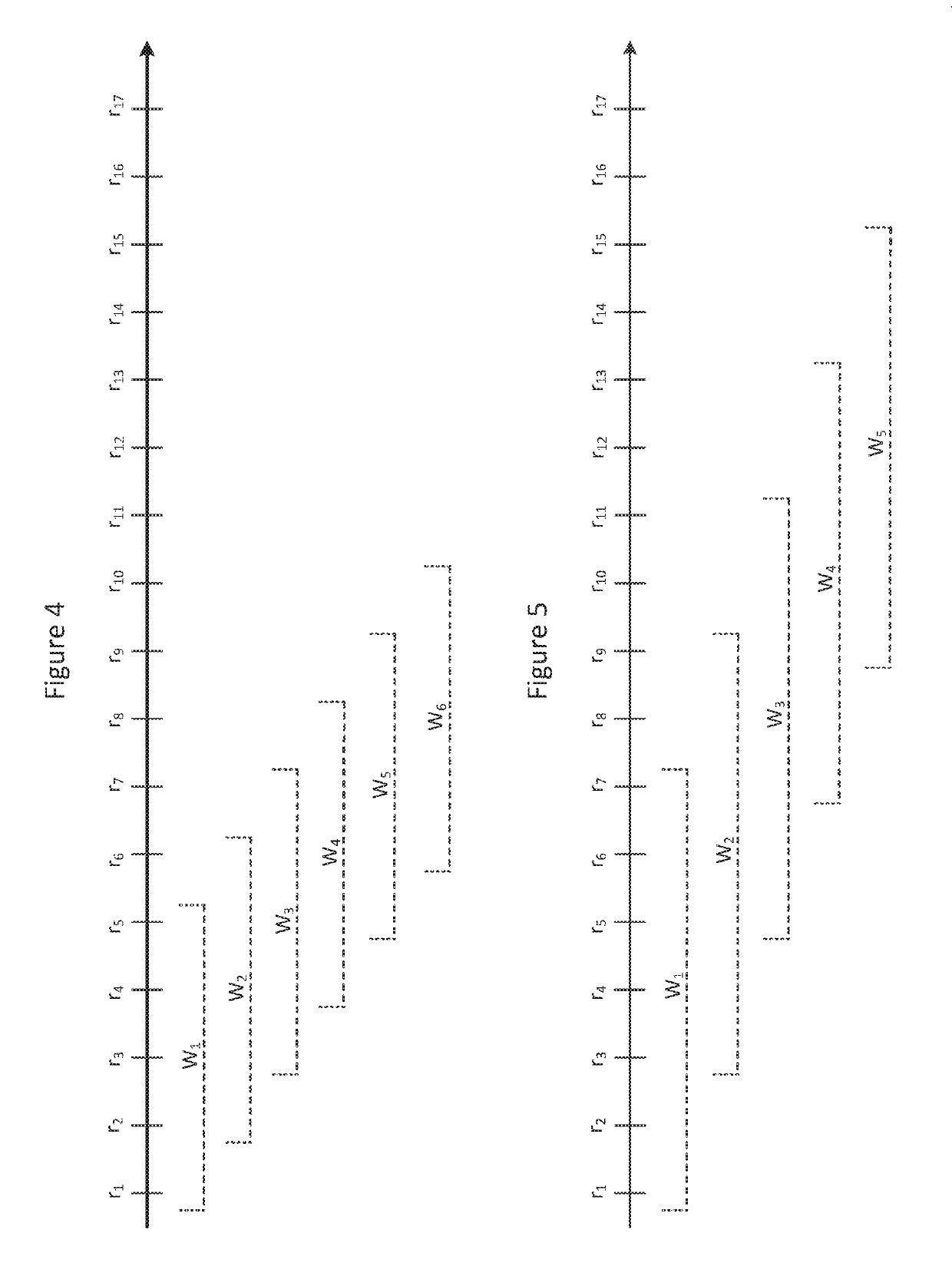
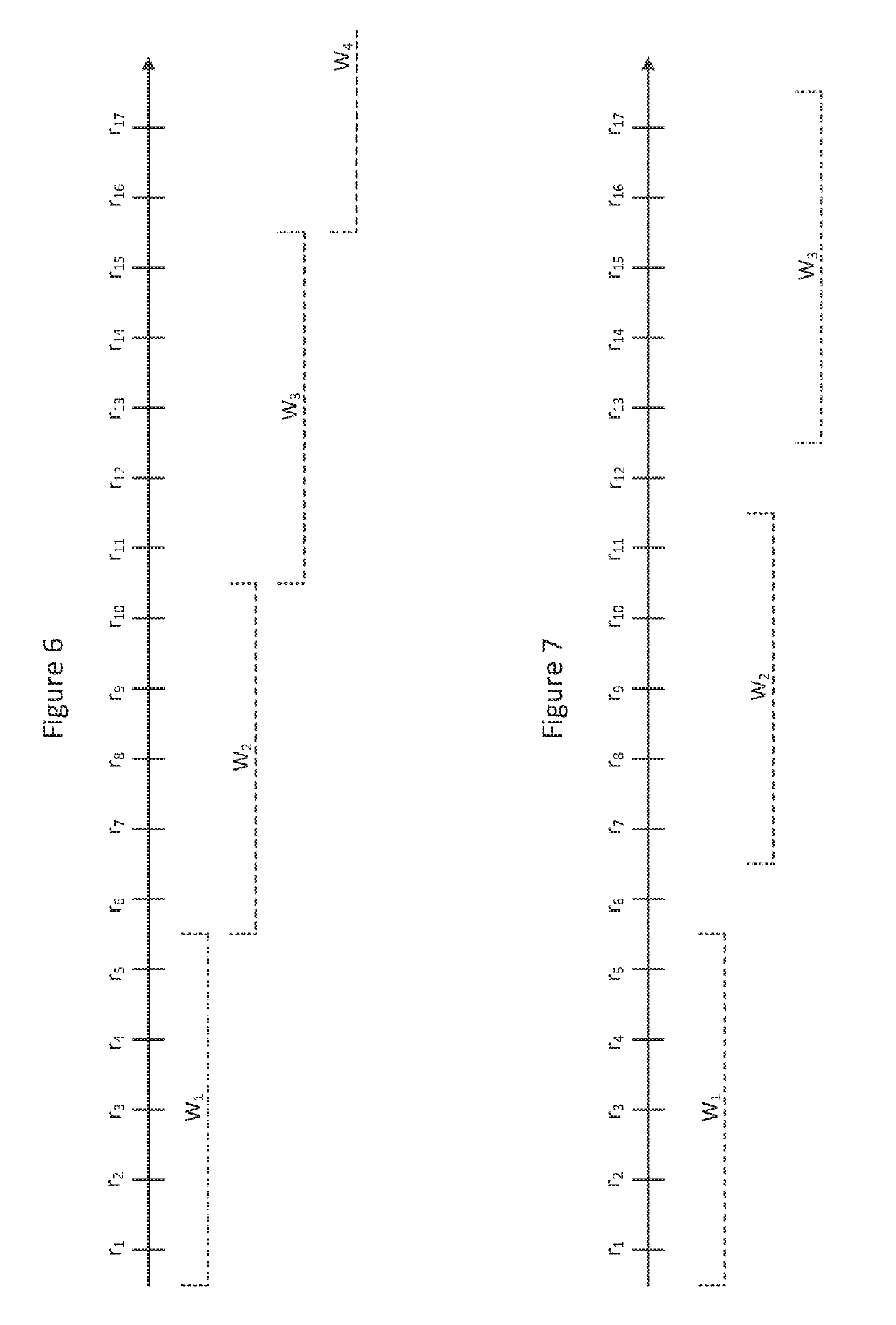
Systems and methods for selecting accelerometer data to store on computer-readable media
Systems and methods select accelerometer data to store on computer-readable media. Systems may comprise an accelerometer, a processor, and a computer-readable medium storing instructions executed by the processor. Accelerometer data from a critical time period are stored on a computer readable medium if an activity ratio for the critical time period exceeds an activity threshold. The numerator of the activity ratio comprises a count of data windows in the critical time period that satisfy one or more count criteria and the denominator comprises the total number of data windows in the critical time period.
Owner:UNITEDHEALTH GROUP
Konjak oligosaccharide buccal tablets
The invention relates to konjak oligosaccharide buccal tablets, which belong to the technical field of food processing. The konjak oligosaccharide buccal tablets are prepared by a method comprising the following steps of: dissolving konjak glucomannan containing 85 percent of glucomannan in water with stirring according to a principle that 10 grams of the konjak glucomannan is dissolved in each 100 mL of water; adding a mixture of mannase, cellulose and hemicellulase into the konjak glucomannan based on a principle that 1 gram of the konjak glucomannan is added with 200 to 300 mu enzymatic activity units, wherein the enzymatic activity ratio in the mixture is 5:1:1; performing thermal-insulation hydrolysis at the temperature of between 30 and 50 DEG C for 4 to 6 hours; heating the hydrolysis solution to boil the hydrolysis solution for 10 minutes; stopping the enzymolysis; subjecting the reaction solution to centrifugation and filtration sequentially; filtering the manna oligosaccharide mixed liquid with a membrane and concentrating the mixed liquid under a reduced pressure to obtain manna oligosaccharide mixed liquid; dissolving 0.05 to 0.08 percent of aspartame, 10 to 15 percent of xylitol, 15 to 20 percent of olive extract powder, 10 to 40 percent of maltodextrin and 10 to 15 percent of starch into the manna oligosaccharide mixed liquid sequentially; drying to the mixture to obtain konjak oligosaccharide powder; and tabletting the konjak oligosaccharide powder to obtain the konjak oligosaccharide buccal tablets. The konjak oligosaccharide buccal tablets have the advantages of uniform taste and color, good mouthfeel, outstanding flavor, edible convenience and good health-care effect. The konjak oligosaccharide buccal tablets are particularly suitable for industrialized production.
Owner:云南富源金田原农产品开发有限责任公司 +1
Method for preparing Pt-Ru/C catalyst in use for direct methanol fuel cell
InactiveCN1661836AHigh activityIncrease profitCatalyst carriersCell electrodesDecompositionActivity ratios
The method includes following steps: adding processed carbon carrier into mixed liquor of deionized water and isopropyl alcohol and dispersing them evenly; adding predecessor of Pt and Ru compound into evenly dispersed serum containing carbon, drying the said serum through magnetic stirring under constant temperature 50-80 deg.C; grinding the obtained powder of carbon carried predecessor of Pt and Ru compound for ten minutes; deoxidizing the said carbon carried compound in mixed gas of hydrogen and nitrogen gases for 1-3 hours under 350-420 deg.C. The disclosed (NH4)2PtCl6 or (NH4)2RuCl5 is as solid, possessing lower decomposition temperature. Spongy Pt or Ru generated by deoxidizing the said solid forms skeleton structure in porous so as to raise surface area of electrochemical activity ratio and use rate of catalyst.
Owner:HARBIN INST OF TECH
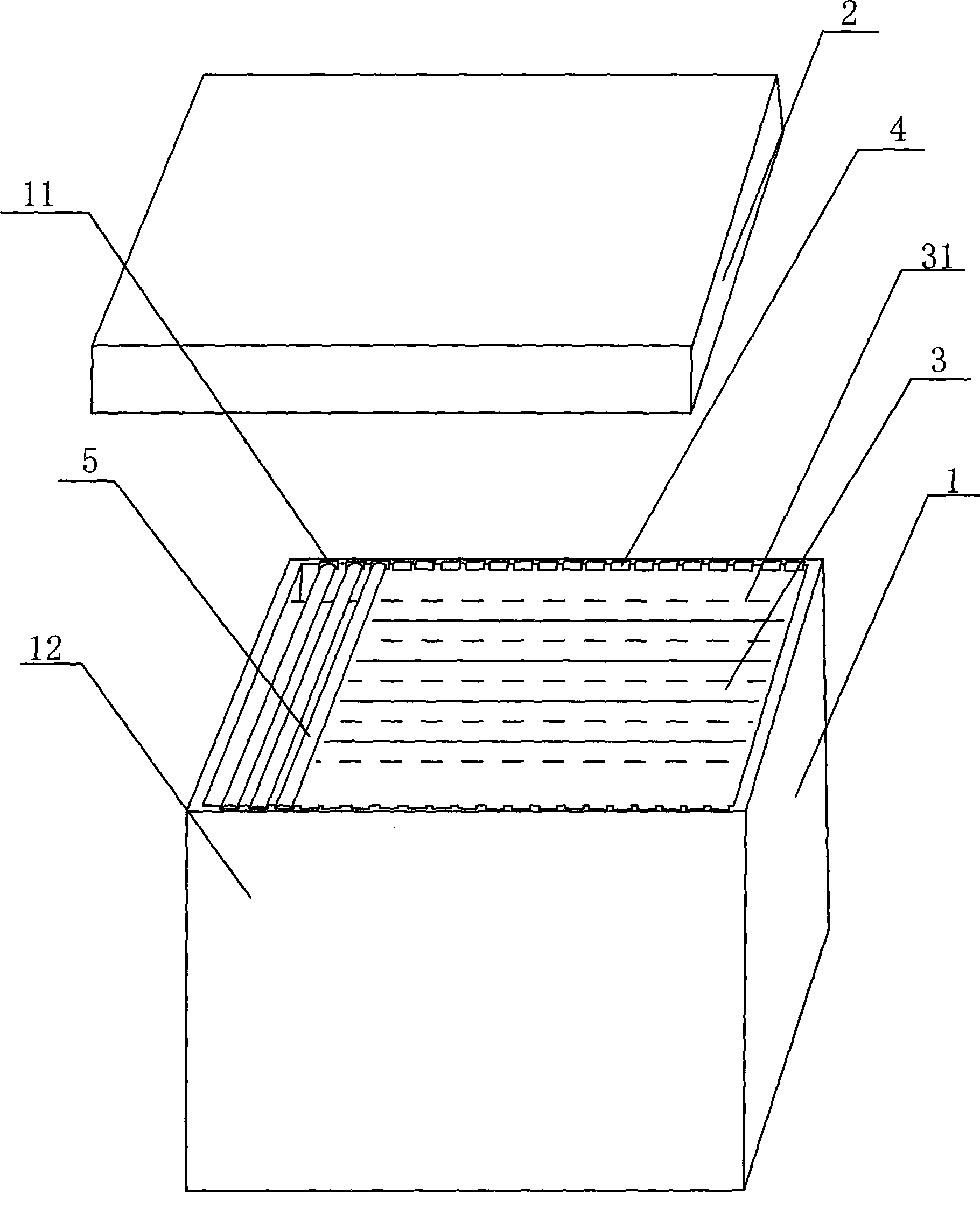
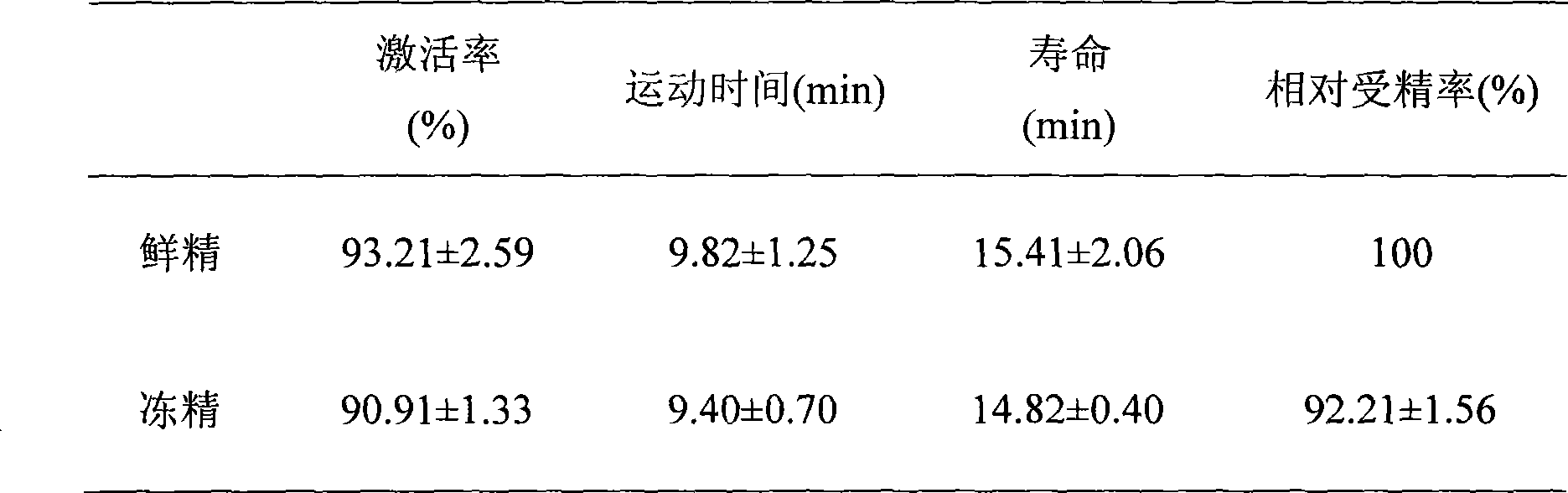
Ultra low temperature cryopreservation method for sperm of large yellow crocker and cryopreservation device
InactiveCN101502256ABest living environmentImprove survival rateDomestic cooling apparatusLighting and heating apparatusCryopreservationActivity ratios
The present invention discloses a method for freezing sperm of large yellow croaker. The method comprises the steps of preparing antifreezing liquid, collecting spectrum, preparing the mixture liquid of antifreezing liquid and spectrum, separately packaging the mixture liquid, freezing the mixture liquid, etc. The method for freezing sperm of large yellow croaker according to the invention has the advantages of convenient operation, high survival rate of sperm, better freezing storing quality of sperm and relatively higher activity ratio of sperm after freezing. The invention also discloses a freezing device of the method, wherein the freezing device comprises a foam box provided with a cover. Liquid oxygen is provided in the foam box. The inner depth of foam box is 18 centimeter and the wall thickness is 2.5 centimeter. Two long walls of foam box are symmetrically provided with grooves which extend downwards from the box top of foam box. The downwards depths of grooves from the box top of foam box is 0.5 centimeter. The distance from the liquid surface of liquid nitrogen to the groove is 2.5-3.5 centimeter. The device of the invention has the advantages of more convenient operation, prevention of the phenomenon of liquid nitrogen vapor enveloping after cover opening, saving to the freezing time, prevention the phenomenon of dropping into the liquid nitrogen caused by the rolling of pulled straws, etc.
Owner:NINGBO UNIV
Low molecular weight heparin and its preparation method
InactiveCN1880344AImprove therapeutic indexReduced anti-factor IIa activityLow activityDecomposition
This invention discloses a low molecular heparin by decomposing natural heparin with nitrous acid, with average molecular weight between 2100Da and 2700Da, and 90% of the total component between 1500Da and 5400Da. The low molecular heparin of this invention has a low activity of anti-IIa factor, and the activity ratio of anti-Xa factor / anti-IIa factor is over 20, which greatly decreases the bleeding-out possibility. This invention also discloses the method for making the low molecular heparin, comprising procedures of nitrous acid decomposition, sodium borohydride reduction, and ethanol precipitation, with high yield.
Owner:上海复旦张江生物医药股份有限公司
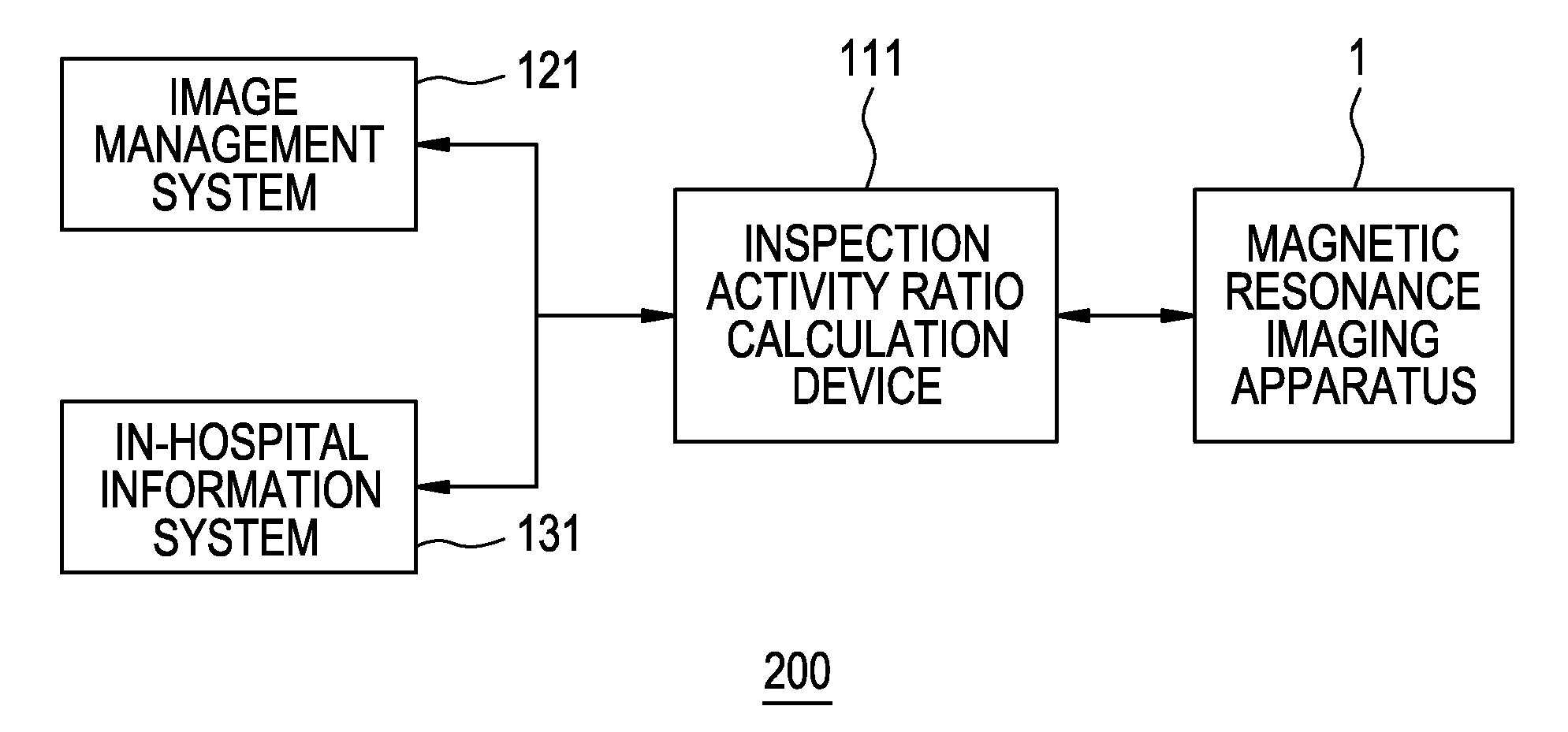
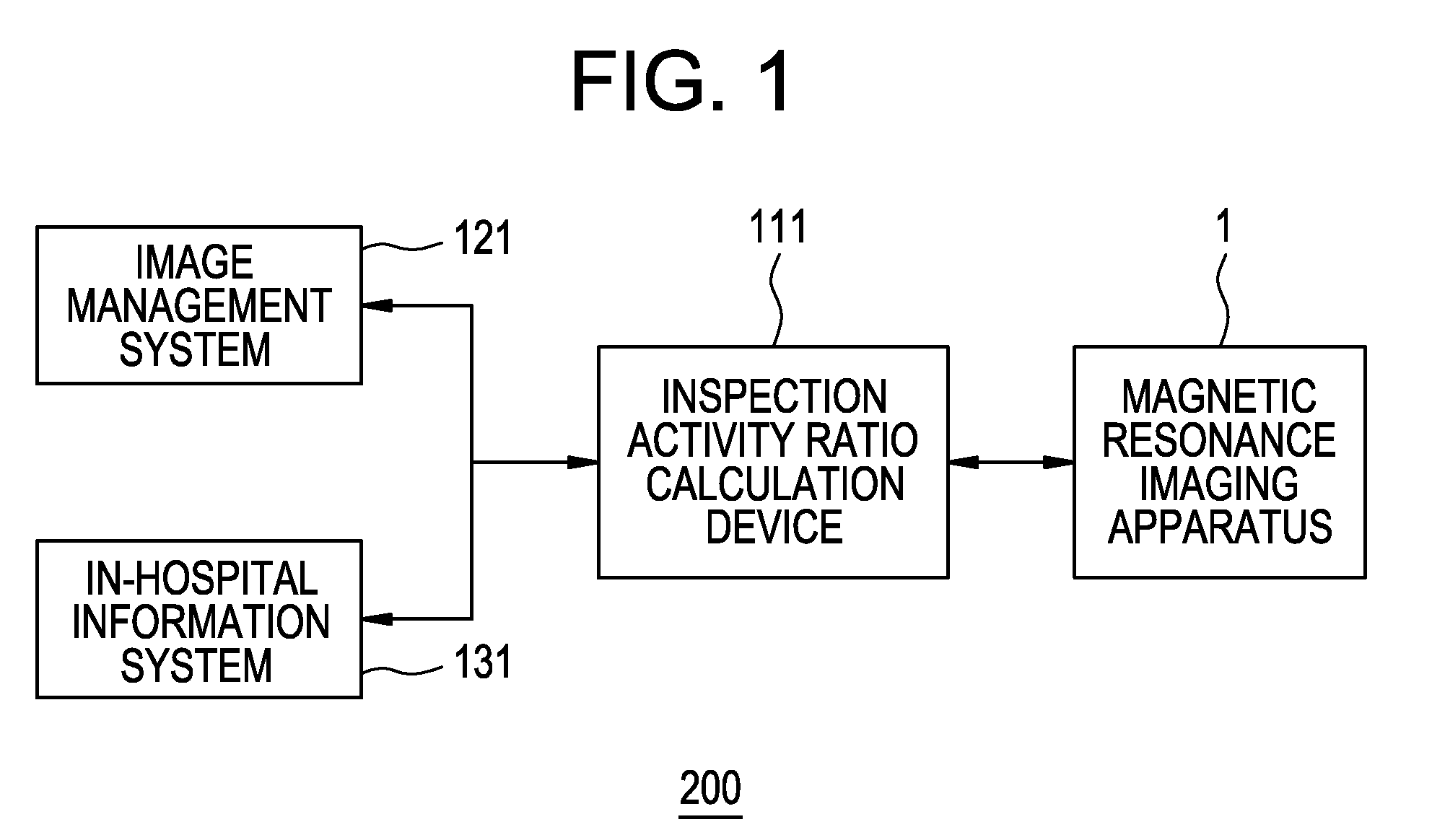
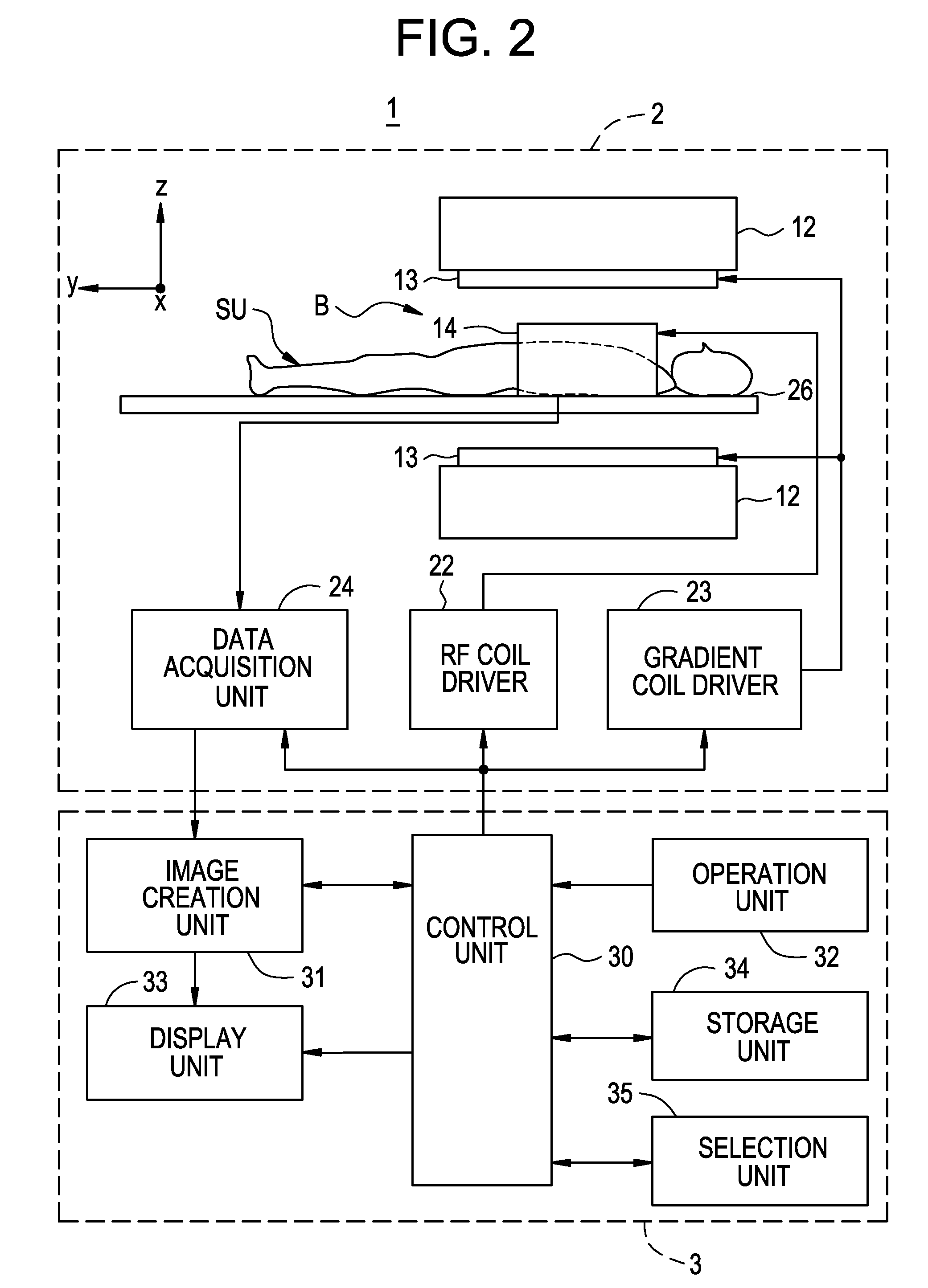
Imaging diagnosis system and its operational apparatus
InactiveUS20080075321A1Easy to operateImprove efficiencyLocal control/monitoringCharacter and pattern recognitionImage diagnosisComputer graphics (images)
The display unit displays a plurality of inspection attributes differently in appearance, depending on the activity ratio of each of the inspection attributes calculated by an inspection activity ratio calculation device. For example, the display unit displays character strings corresponding to the plurality of inspection attributes in different font sizes or different colors, depending on the activity ratio of each inspection attribute calculated by the inspection activity ratio calculation device.
Owner:GE MEDICAL SYST GLOBAL TECH CO LLC
Using a per file activity ratio to optimally relocate data between volumes
ActiveUS8732217B2Reduce impactLimited SSDDigital data information retrievalMemory adressing/allocation/relocationFile systemActivity ratios
Owner:VERITAS TECH
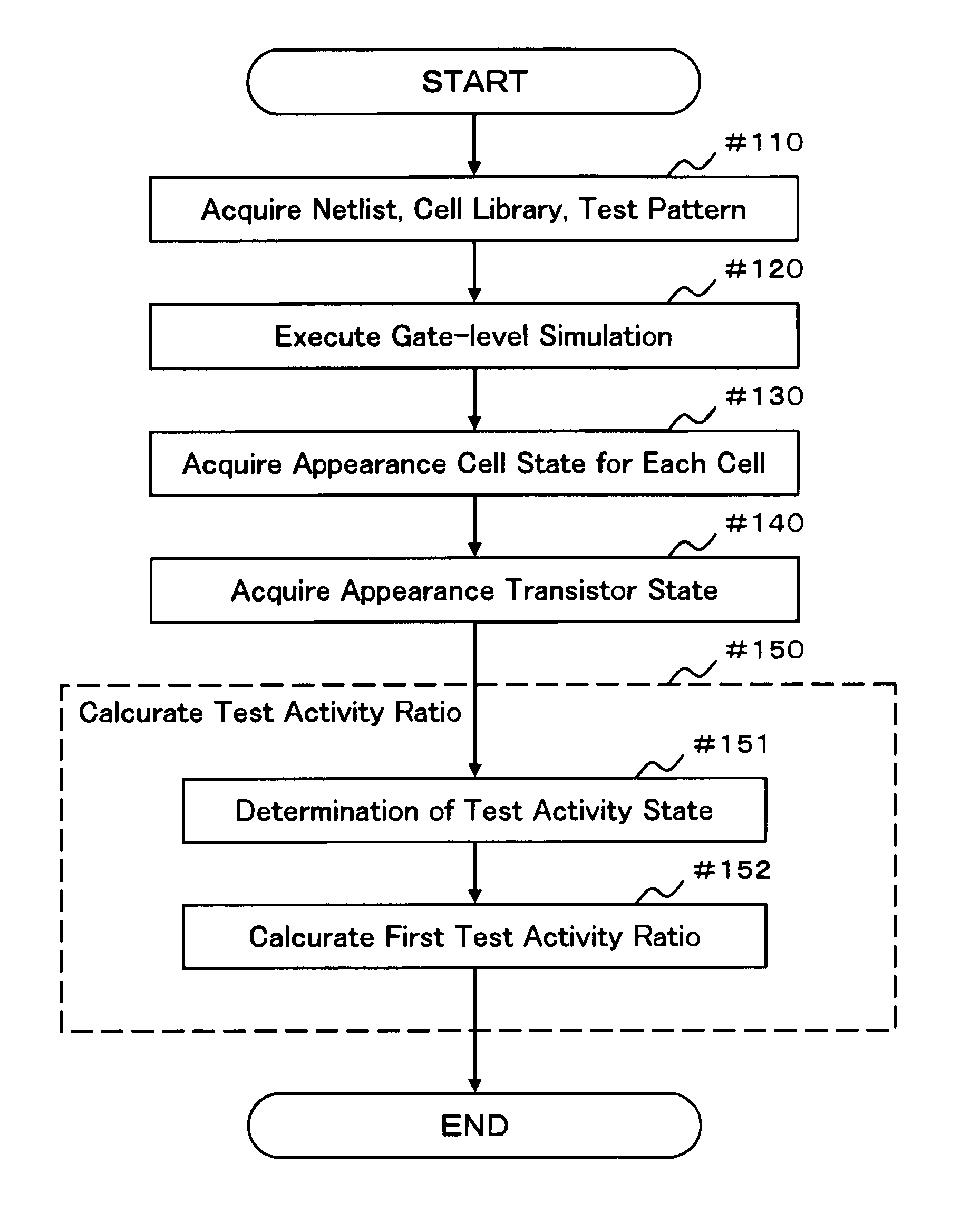
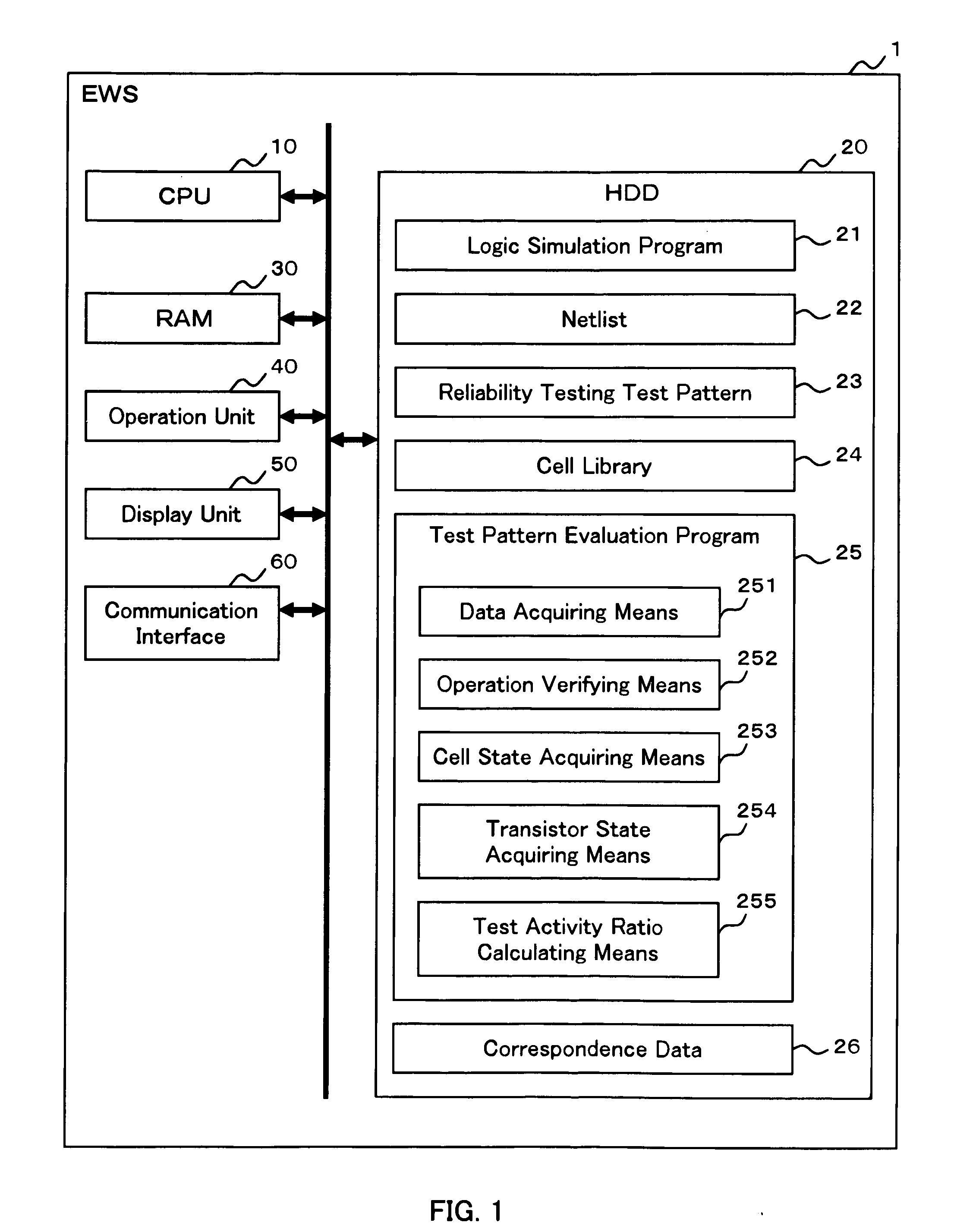
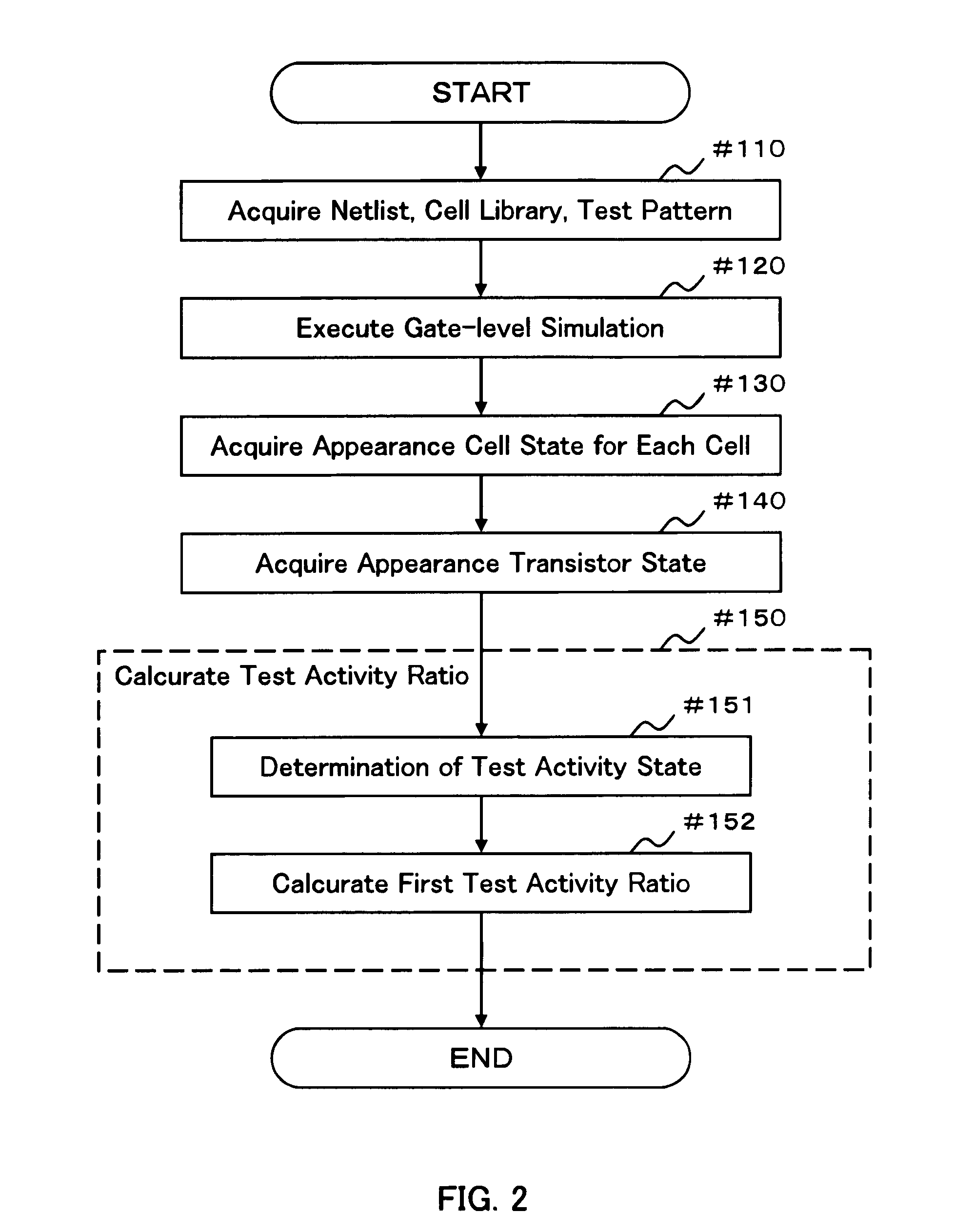
Test pattern evaluation method and test pattern evaluation device
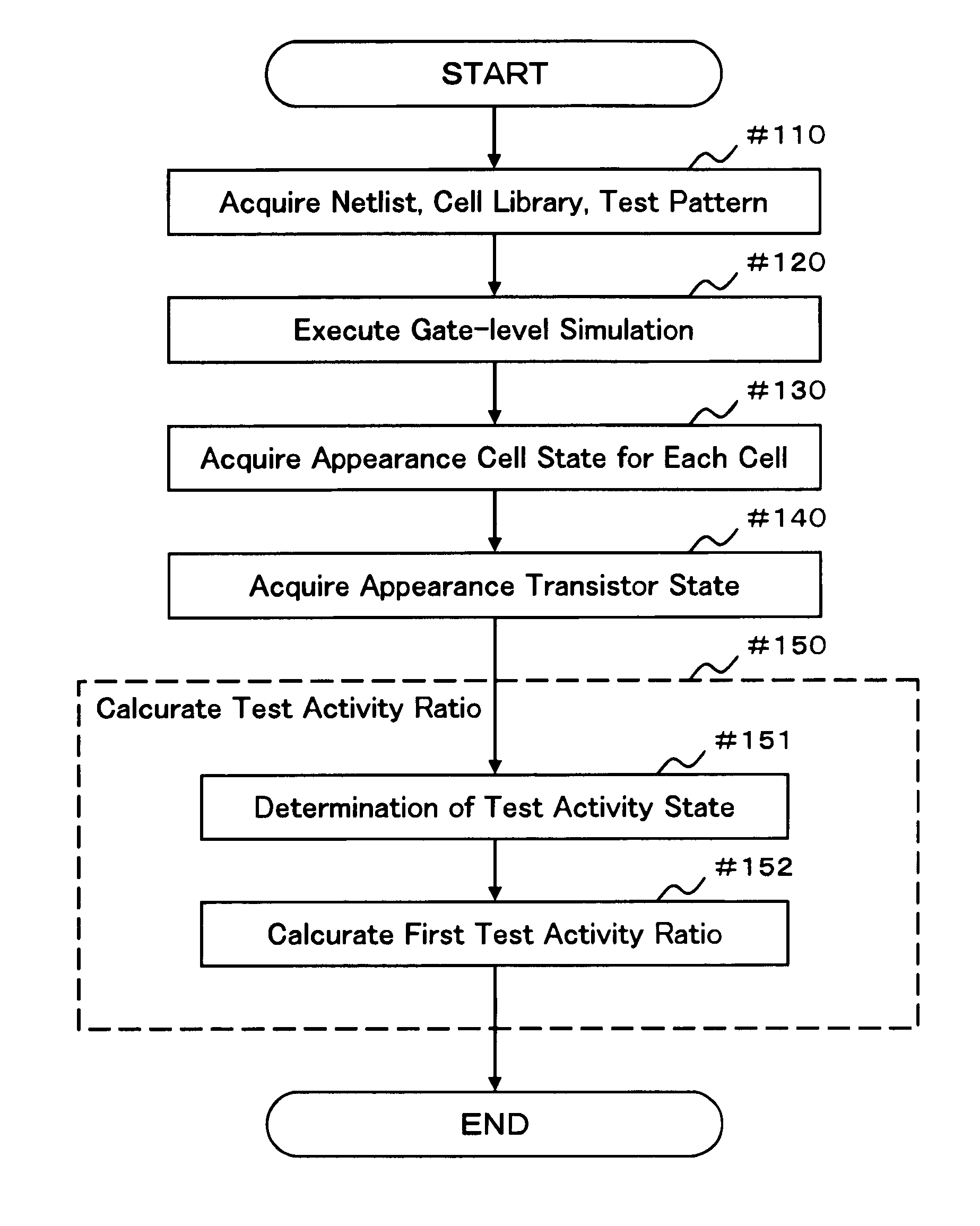
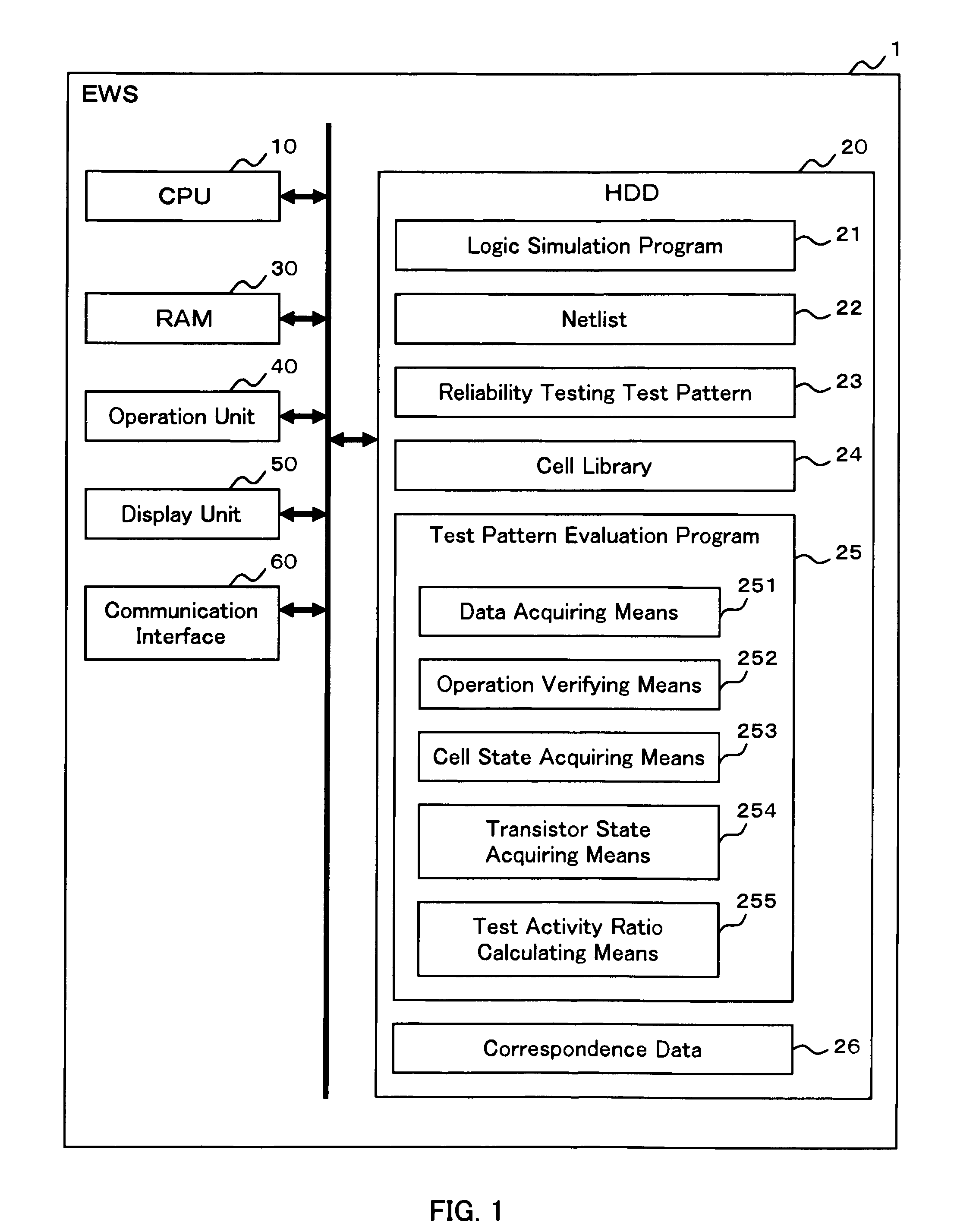
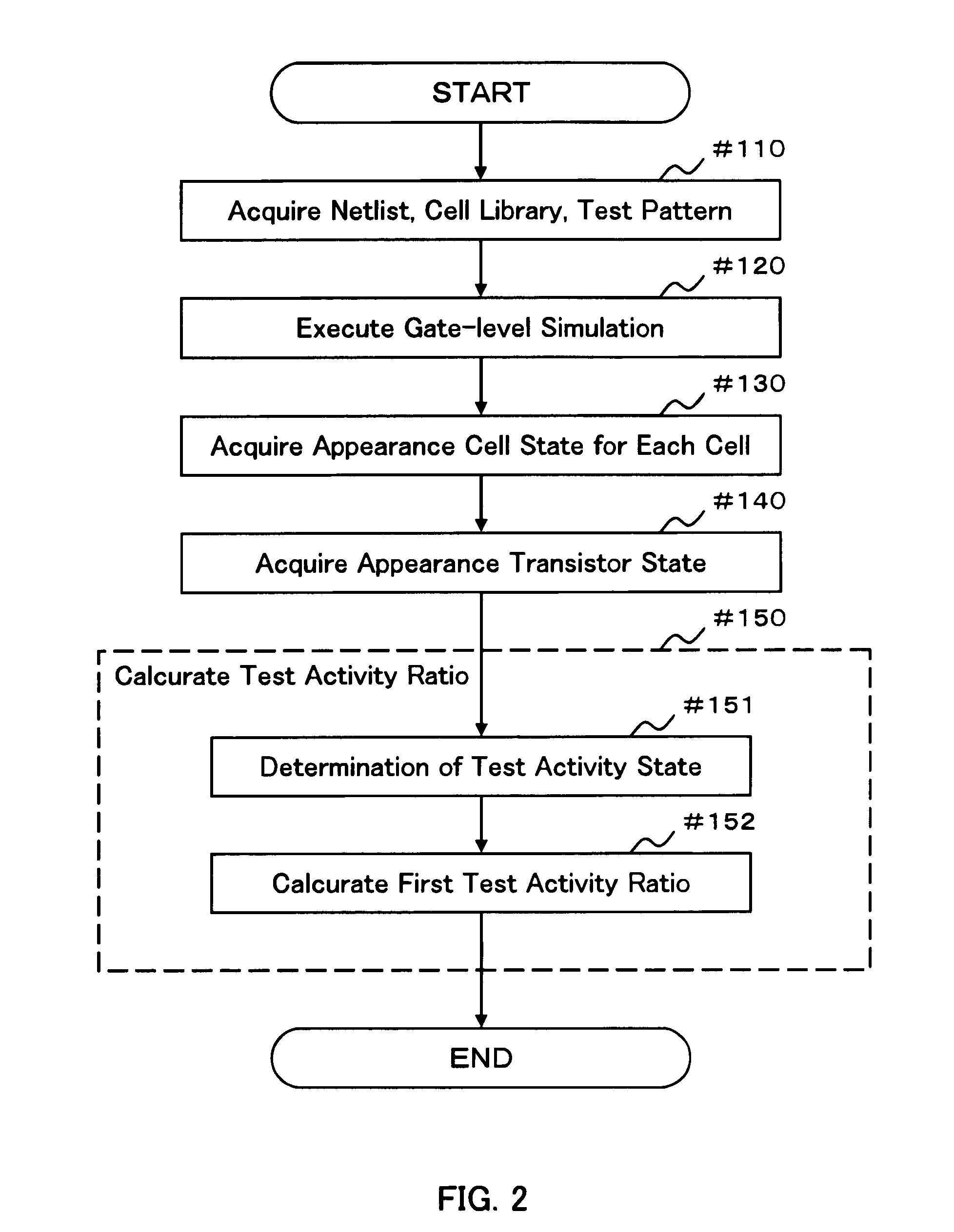
InactiveUS20090094569A1Reduced simulation timeShort simulation timeDetecting faulty computer hardwareCAD circuit designActivity ratiosEngineering
Provided are an evaluation method and device of a test pattern which enable an appropriate evaluation in a reliability test with a simulation time reduced and high accuracy. It is assumed that each possible internal state of a cell determined at least by a logic value or a voltage value of an input terminal is a cell state, and each possible state of a transistor determined by a voltage between terminals of the transistor is a transistor state. The method comprises steps of: verifying operation of a semiconductor integrated circuit at a gate level or higher; acquiring an appearance cell state continuously appearing for a predetermined time or more in the operation verification; acquiring an appearance transistor state using the corresponding appearance cell state in the operation verification for each transistor; and calculating a test activity ratio of the transistor using the corresponding appearance transistor state for each transistor.
Owner:SHARP KK
Kit for determining mitochondria aspartate aminotransferase
InactiveCN102010890AUndisturbedThe result is accurateMicrobiological testing/measurementLactate dehydrogenaseActivity ratios
The invention discloses a kit for determining mitochondria aspartate aminotransferase, containing a reagent 1 and a reagent 2, wherein the reagent 1 contains aspartic acid protease, alpha- ketoglutaric acid, L-aspartic acid, malate dehydrogenase, lactate dehydrogenase, a preservative and PEG6000. The enzyme activity ratio of the aspartic acid protease to the malate dehydrogenase to the lactate dehydrogenase in the reagent 1 is (0.1-50):(0.1-50):(0.1-10); the ratio of the alpha- ketoglutaric acid to the malate dehydrogenase is (0.1-60)g:(0.1-50)KU; the mass ratio of the alpha- ketoglutaric acid to the L-aspartic acid to the preservative to the PEG6000 is (0.1-60):(5-200):(0.2-10):(1-100); and the mass ratio of NADH (Reduced Form of Nicotinamide-Adenine Dinucleotid) to a preservative to EDTA (Ethylene Diamine Tetraacetic Acid).2Na is (0.5-10):(0.2-10):(1-50). The kit for determining the mitochondria aspartate aminotransferase is used for determining the activity of the determining mitochondria aspartate aminotransferase, is free from the interference of high cell plasma aspartate aminotransferase and obtains accurate result.
Owner:北京迪迈医学诊断技术有限公司
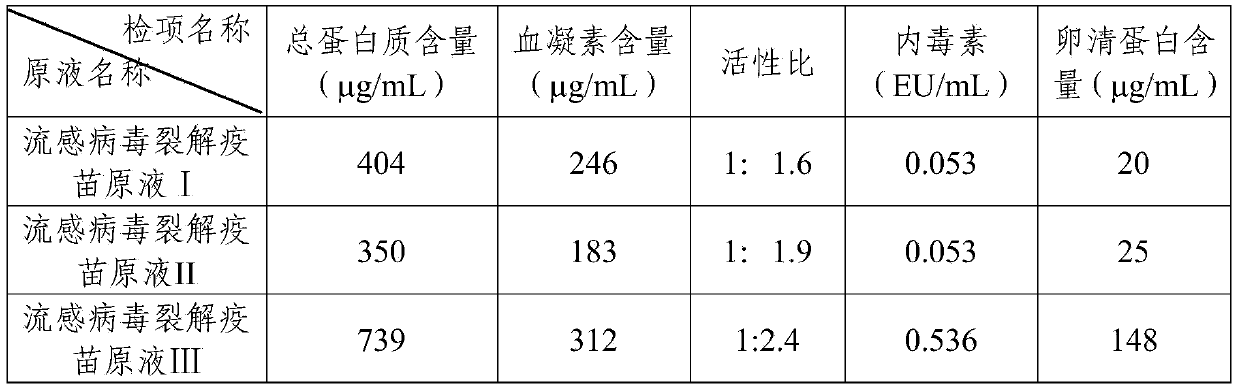
Purification method for split influenza virus vaccine
ActiveCN103721251AGuaranteed uniformityHigh removal rateAntiviralsAntibody medical ingredientsHemagglutininPurification methods
The invention provides a purification method for split influenza virus vaccine. An influenza virus strain is inoculated onto a chick embryo and cultured to obtain a virus solution; the virus solution is sequentially subjected to inactivation and ultrafiltration concentration to obtain an ultrafiltrate; the obtained ultrafiltrate is subjected to cane sugar density gradient centrifugation by using a KII continuous flow centrifuge to obtain an ultra centrifugal solution; the ultra centrifugal solution is subjected to ultrafiltration dialysis for cane sugar removal and molecular sieve gel chromatography to obtain a virus purification solution; the obtained virus purification solution is subjected to virus split by using a split agent TritonX-100 and sodium deoxycholate; after the split is over, the split agent is removed via ultrafiltration dialysis; impure protein is centrifugally removed from the obtained split solution; supernatant is collected, filtered and sterilized so as to obtain the purified influenza virus vaccine primary solution. The purification method is simple and convenient to operate, high in centrifugation capacity and suitable for large-scale production; by adopting a dual-split agent and a centrifugal process, the finished product is greatly improved in activity (hemagglutinin content / protein total content), and approaches the activity ratio of subunit vaccine, as a result, the high-grade split influenza virus vaccine product is obtained.
Owner:SINOVAC BIOTECH
Method for producing beer
The present invention provides a method for producing beer comprising filtering beer through a porous membrane until such time that the porous membrane is in need of cleaning, contacting the porous membrane with an enzyme selected from the group consisting of cellulases, amylases, and combinations thereof, particularly a cellulase having a crystalline:soluble cellulose activity ratio at 60 minutes of at least about 0.1, to clean the porous membrane, and then reusing the porous membrane to continue filtering beer. The present invention further provides a method for producing beer comprising filtering beer through a porous membrane that progressively clogs during filtration, monitoring the streaming or zeta potential of the porous membrane as a measure of the extent of clogging of the porous membrane, halting filtration of the beer through the porous membrane before the porous membrane becomes fully clogged as determined by the streaming or zeta potential of the porous membrane, cleaning the porous membrane, and then reusing the porous membrane to continue filtering beer.
Owner:PALL CORP
Methods and devices for treating parasympathetic bias mediated conditions
Methods for treating a subject for a parasympathetic bias mediated condition are provided. Aspects of the methods include modulating at least a portion of the subject's autonomic nervous system to increase the sympathetic / parasympathetic activity ratio in a manner effective to treat the subject for the parasympathetic bias mediated condition. In some instances, the subject is known to have parasympathetic bias. Also provided are devices that find use in practicing various embodiments of the methods.
Owner:PALO ALTO INVESTORS LP
Test pattern evaluation method and test pattern evaluation device
InactiveUS7882467B2Short simulation timeReduced simulation timeDetecting faulty computer hardwareCAD circuit designActivity ratiosEngineering
Provided are an evaluation method and device of a test pattern which enable an appropriate evaluation in a reliability test with a simulation time reduced and high accuracy. It is assumed that each possible internal state of a cell determined at least by a logic value or a voltage value of an input terminal is a cell state, and each possible state of a transistor determined by a voltage between terminals of the transistor is a transistor state. The method comprises steps of: verifying operation of a semiconductor integrated circuit at a gate level or higher; acquiring an appearance cell state continuously appearing for a predetermined time or more in the operation verification; acquiring an appearance transistor state using the corresponding appearance cell state in the operation verification for each transistor; and calculating a test activity ratio of the transistor using the corresponding appearance transistor state for each transistor.
Owner:SHARP KK
Methods and Devices for Treating Parasympathetic Bias Mediated Conditions
Methods for treating a subject for a parasympathetic bias mediated condition are provided. Aspects of the methods include modulating at least a portion of the subject's autonomic nervous system to increase the sympathetic / parasympathetic activity ratio in a manner effective to treat the subject for the parasympathetic bias mediated condition. In some instances, the subject is known to have parasympathetic bias. Also provided are devices that find use in practicing various embodiments of the methods.
Owner:PALO ALTO INVESTORS LP
Improved calcium humate and preparation method and application thereof
ActiveCN105694895AHigh carbon contentRaise the pHOther chemical processesOrganic fertilisersActivity ratiosSoil fertility
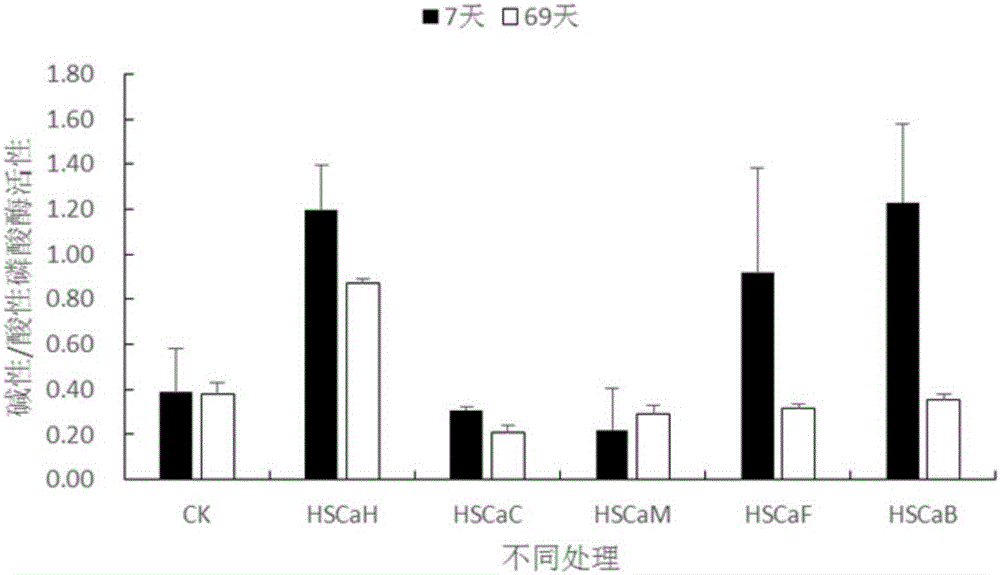
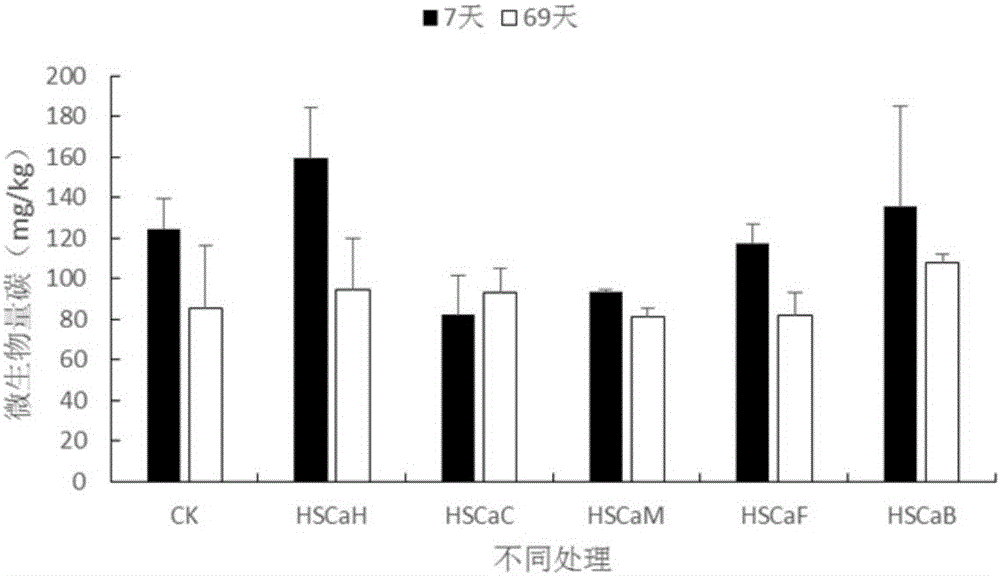
Provided are improved calcium humate and a preparation method and application thereof. The prepared calcium humate product is high in carbon content, and the exchangeable calcium content is higher than that of general commercial calcium humate; when the calcium humate product is applied to acid poor soil, the acid soil aluminum toxicity barrier can be obviously eliminated, the soil pH and base ions are increased, the soil alkaline and acid phosphatase activity ratio is increased, soil microbial biomass is increased, and the oil fertility property and biological function are improved.
Owner:INST OF SOIL SCI CHINESE ACAD OF SCI
Method for preparing rice hull ash active carbon by enzymatic pretreatment of rice hull
The invention discloses a method for preparing rice hull ash active carbon by enzymatic pretreatment of rice hull. The method comprises the steps of: firstly, building a lignin degradation enzyme system with a certain enzyme activity ratio to degrade rice hull, wherein the lignin degradation enzyme system is composed of lignin peroxidase, manganese peroxidase and laccase, then degrading the rice hull by a cellulose degradation enzyme system with a certain enzyme activity ratio, wherein the cellulose degradation enzyme system is composed of endoglucanase, exoglucanase and beta-glucosaccharase, and finally distilling to prepare the rice hull ash. The rice hull is subjected to pretreatment by the lignin degradation enzyme system and the cellulose degradation enzyme system; the surface micro-pore structure of the rich hull active carbon is improved; and the adsorptive property is good.
Owner:HUAIYIN INSTITUTE OF TECHNOLOGY
Methods of preparing para-xylene from biomass
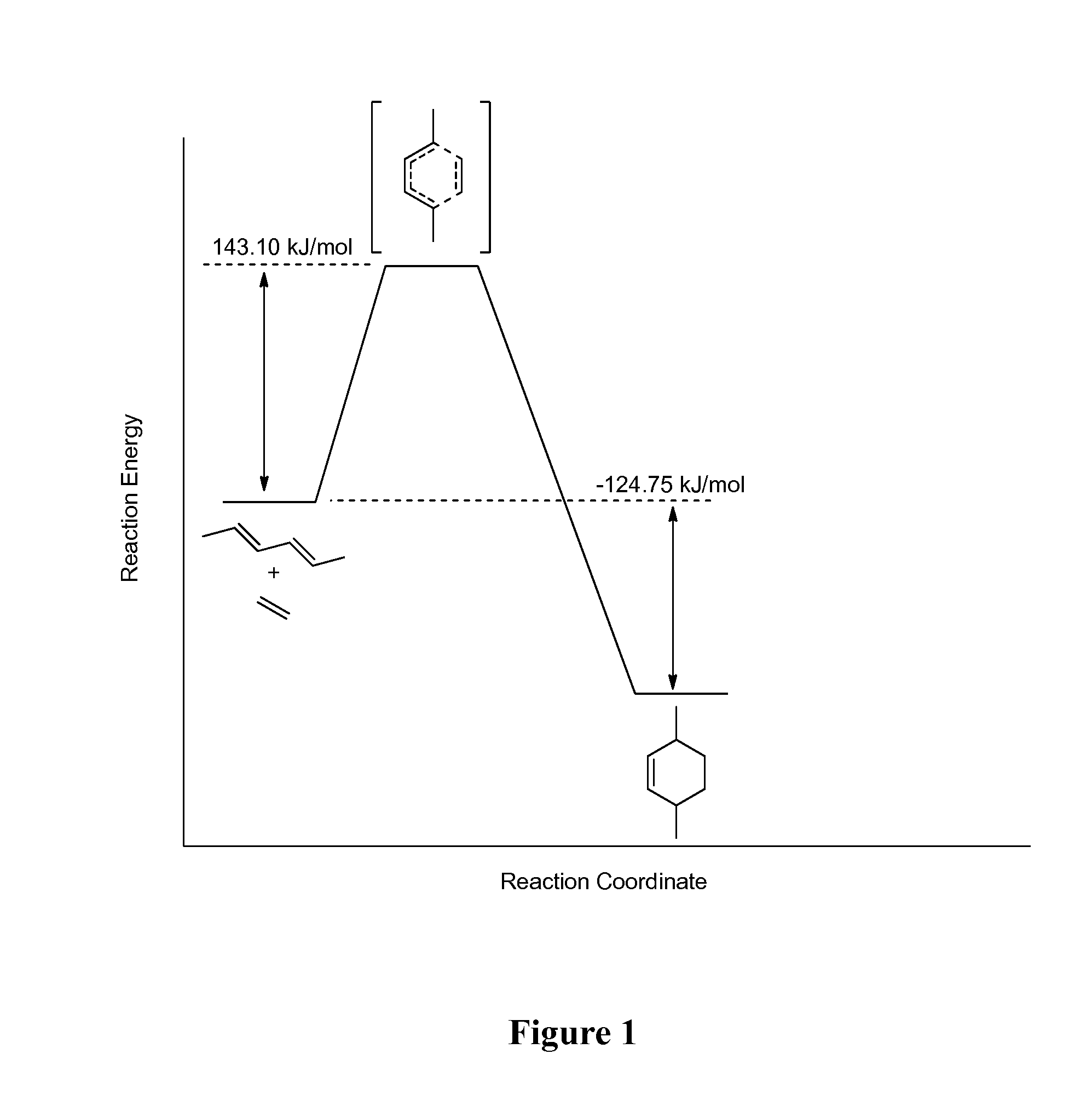
ActiveUS20140017744A1Rate of Diels-AlderImprove reaction speedMolecular sieve catalystOrganic compound preparationCycloadditionActivity ratios
Methods or preparing para-xylene from biomass by carrying out a Diels-Alder cycloaddition at controlled temperatures and activity ratios. Methods of preparing bio-terephthalic acid and bio-poly(ethylene terephthalate (bio-PET) are also disclosed, as well as products formed from bio-PET.
Owner:THE COCA-COLA CO
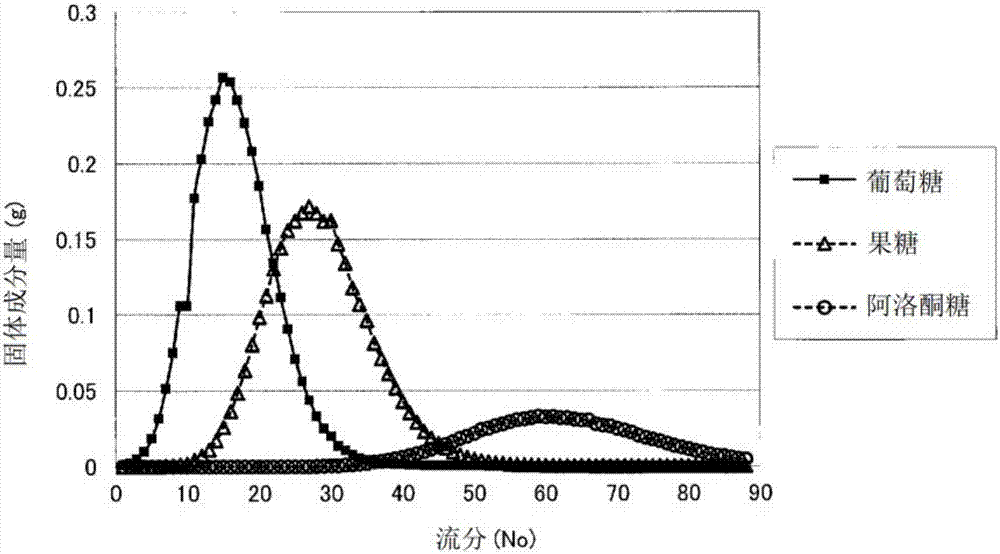
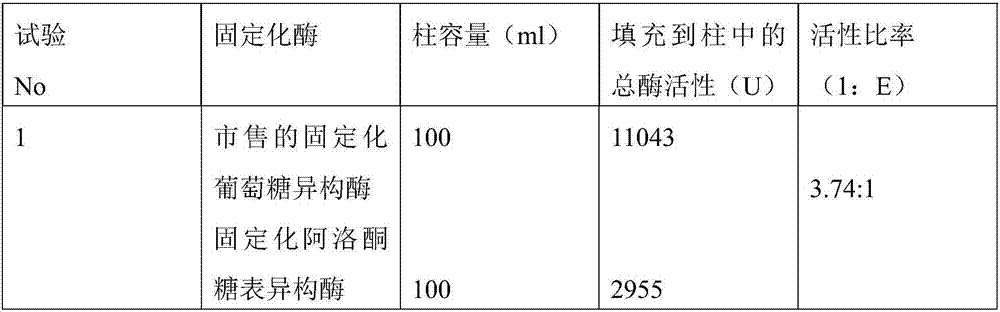
Method for manufacturing allulose-containing sweetener composition
InactiveCN107109448AEfficient manufacturingHigh purityImmobilised enzymesSugar derivativesIsomeraseActivity ratios
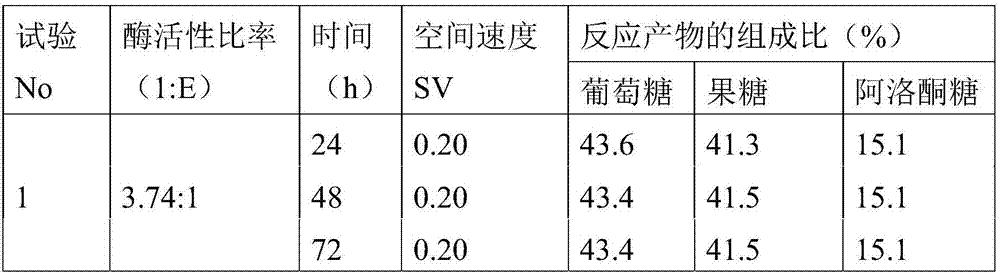
The purpose of the present invention is to provide a technology whereby, in a method for manufacturing a sweetener composition containing glucose, fructose and allulose, said method comprising treating glucose with glucose isomerase and allulose epimerase, the content of allulose in the sweetener composition is increased. A sweetener composition containing glucose, fructose and allulose and having a high allulose content can be continuously manufactured at a high efficiency by immobilizing glucose isomerase and allulose epimerase, packing the same into a column so as to give an activity ratio of the immobilized glucose isomerase to the immobilized allulose epimerase of 1.49:1-5.61:1, and then passing a glucose solution through the column.
Owner:MATSUTANI CHEM INDS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com