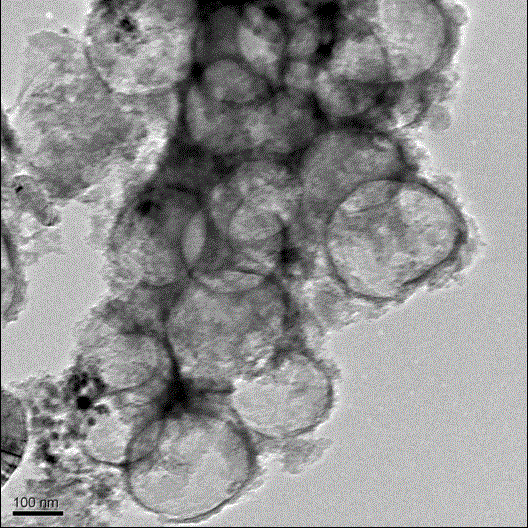
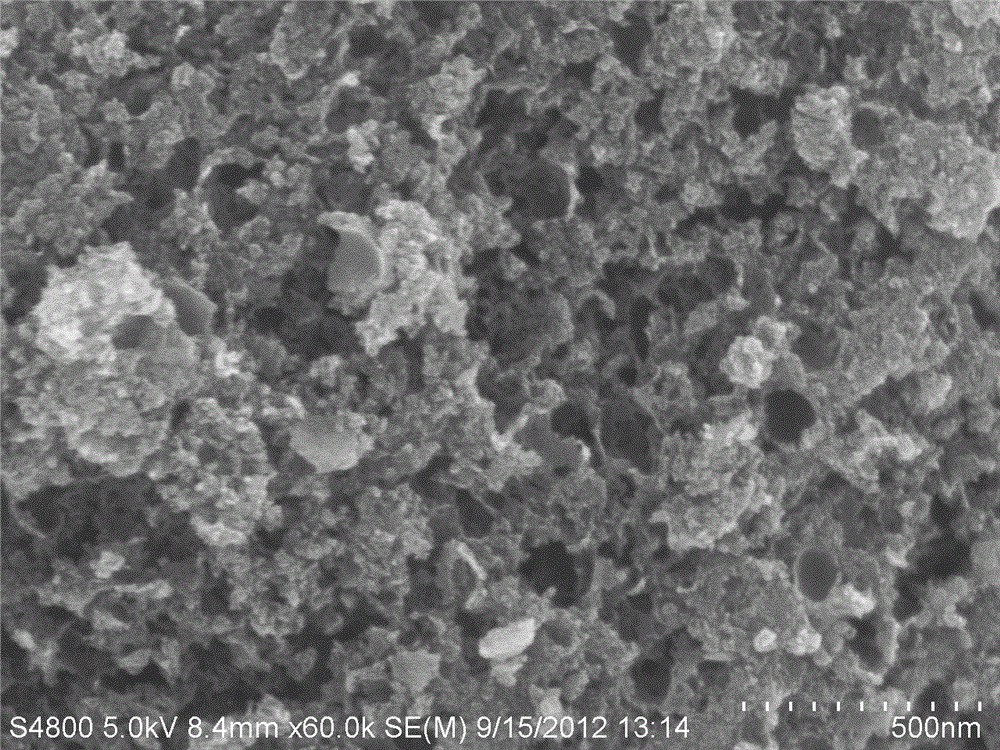
Cathode non-platinum catalyst of proton exchange membrane fuel cell and preparation method thereof
A fuel cell cathode, proton exchange membrane technology, applied in battery electrodes, chemical instruments and methods, physical/chemical process catalysts, etc., can solve unseen problems, achieve large active specific surface area, low synthesis cost, and simple preparation method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1) Measure 37mL formaldehyde (0.5mol) and 40mL isobutanol in a three-necked flask, then add 0.0335g magnesium carbonate while stirring, mix thoroughly, then slowly add 20.2g melamine (0.135mol) while stirring to raise the temperature, the added The molar ratio of melamine to formaldehyde is 1:3.7. When the temperature rises to 60°C, it is kept at about 40 minutes. The feeding time is controlled at about 30 minutes. At 80°C, 0.07g of phthalic anhydride is added to keep it warm for 1.5 hours, and it is slightly cooled and dehydrated under reduced pressure to obtain milky white Gel, that is, melamine formaldehyde resin;
[0042] 2) Weigh 3.2g of triblock polymer F127 (addition polymer of polypropylene glycol and ethylene oxide), 16.0g of ethanol and 2.0g of 0.2M hydrochloric acid solution in a beaker, mix them in a beaker, heat at 40°C and stir for 1 hour; then Add 4.16g of tetraethyl orthosilicate and 2.0g of melamine formaldehyde resin obtained in step 1) and 20wt% ethanol...
Embodiment 2
[0045] 1) Measure 37mL formaldehyde (0.5mol) and 40mL isobutanol in a three-necked flask, then add 0.0170g magnesium carbonate while stirring, mix, then slowly add 20.2g melamine (0.135mol) while stirring and heating up, the added The molar ratio of melamine to formaldehyde is 1:3.7, the time for heating up to 60°C is kept at about 40 minutes, and the feeding time is controlled at about 30 minutes. At 85°C, add 0.07g of phthalic anhydride and keep it warm for 0.5 hours, then cool down and dehydrate under reduced pressure to obtain a milky white gel, which is melamine formaldehyde resin.
[0046] 2) Weigh 1.6g of triblock polymer F127, 10g of ethanol and 1.0g of 0.2M hydrochloric acid solution in a beaker and mix them, heat at 42°C and stir for 50min; then add 3.58g of tetraethyl orthosilicate and the melamine prepared above 30wt% ethanol solution made of formaldehyde resin 3.0g and 7.0g ethanol (add appropriate amount of hydrochloric acid to promote compatibility), then add 2....
Embodiment 3
[0048] 1) Measure 37mL formaldehyde (0.5mol) and 40mL isobutanol in a three-necked flask, then add 0.0935g magnesium carbonate while stirring, mix, then slowly add 20.2g melamine (0.135mol) while stirring to raise the temperature, the added The molar ratio of melamine to formaldehyde is 1:3.7, the time for heating up to 60°C is kept at about 40 minutes, and the feeding time is controlled at about 30 minutes. At 82°C, add 0.07g of phthalic anhydride and keep it warm for 2 hours, then cool down and dehydrate under reduced pressure to obtain a milky white gel, which is melamine formaldehyde resin.
[0049] 2) Weigh 4.8g of triblock polymer F127, 20g of ethanol and 4.0g of 0.2M hydrochloric acid solution in a beaker and mix them, heat at 50°C and stir for 0.5 hours; then add 6.86g of tetraethyl orthosilicate and the above prepared A 10wt% ethanol solution (adding an appropriate amount of hydrochloric acid to promote mutual solubility) made of melamine formaldehyde resin 1.0g and 9...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com