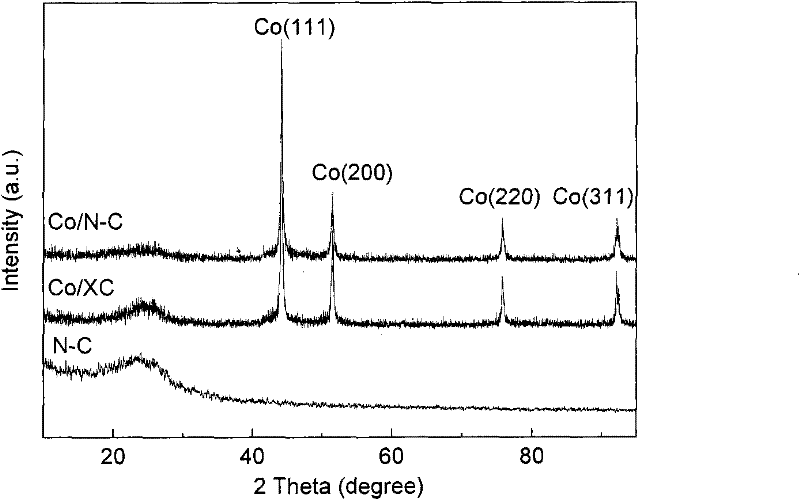
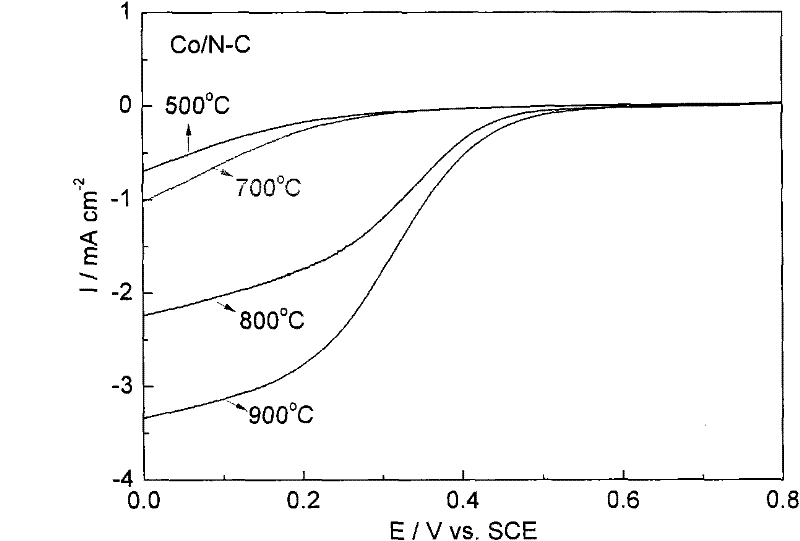
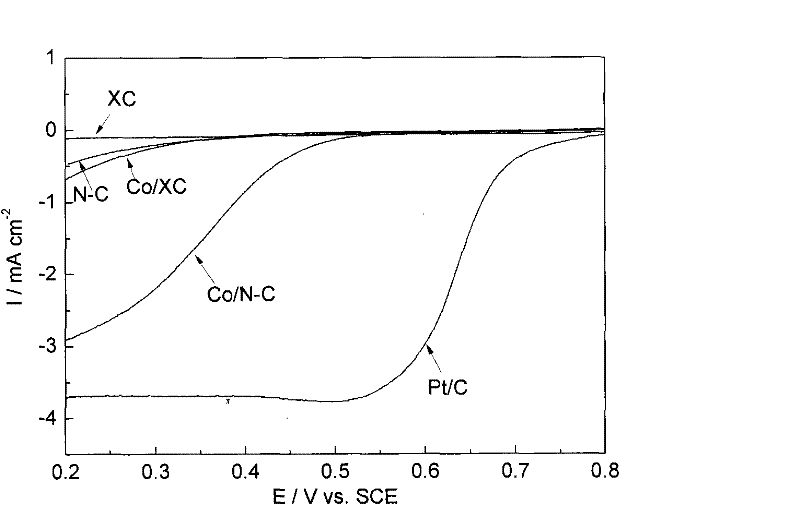
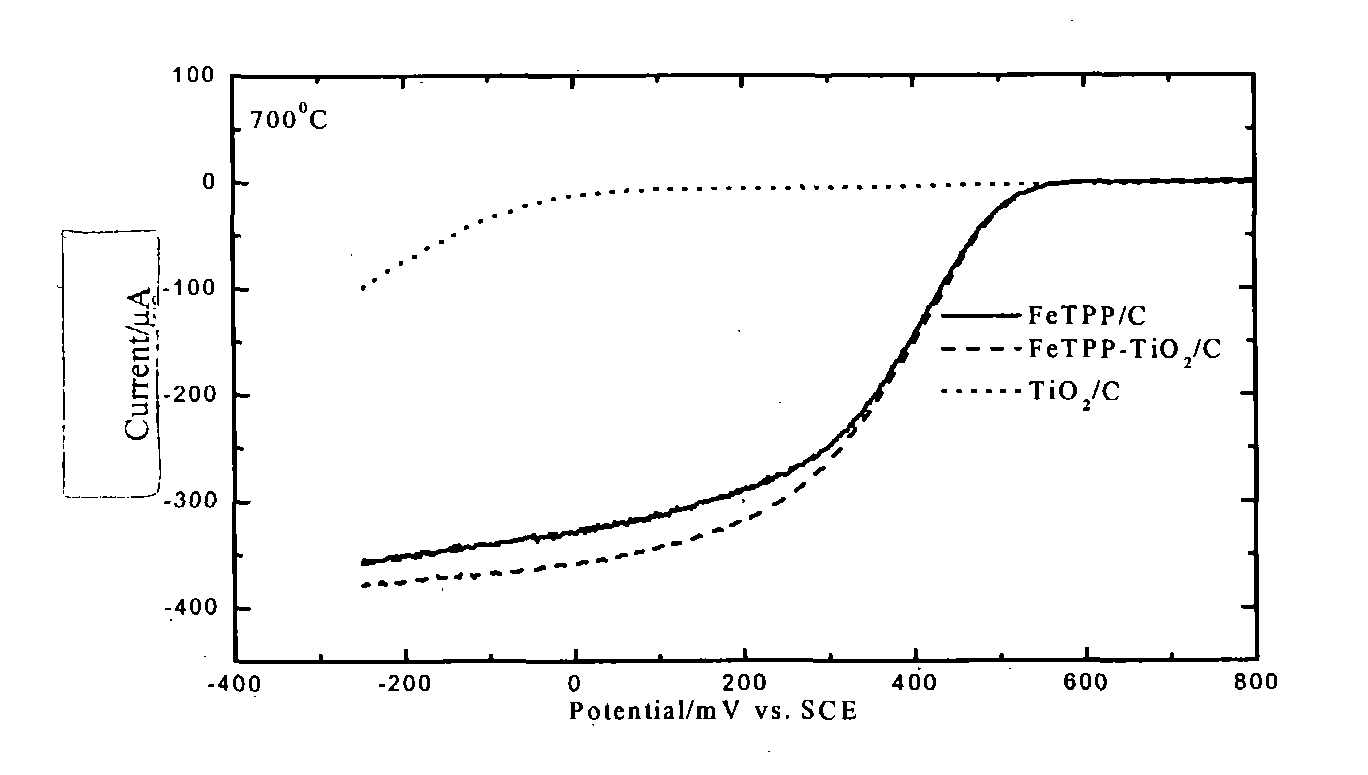
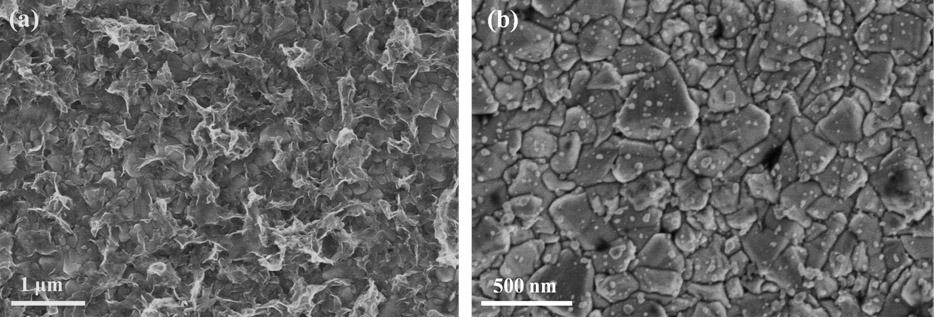
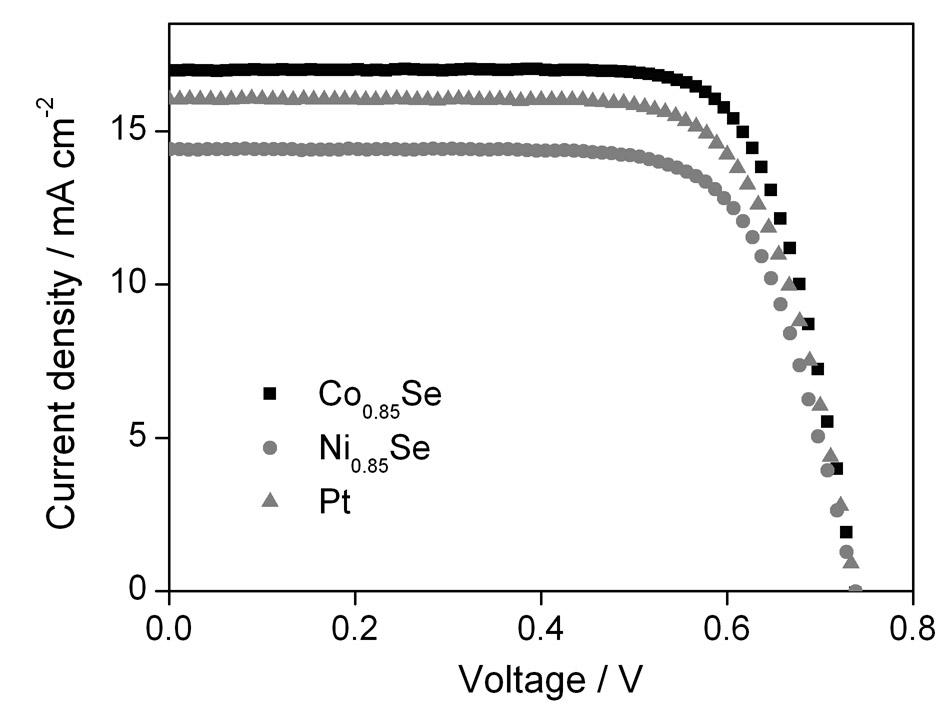
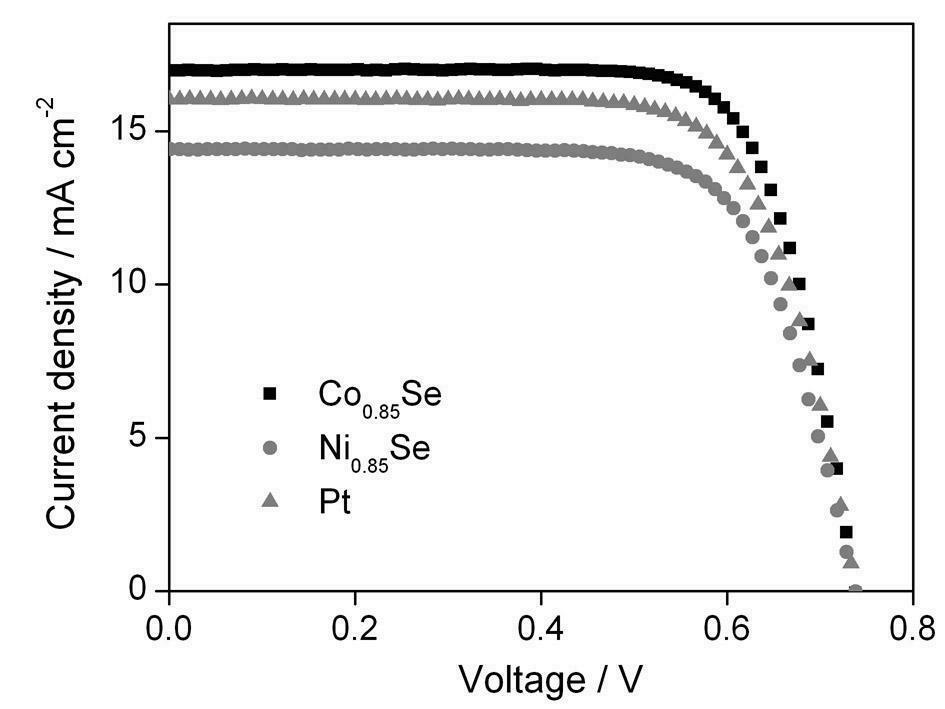
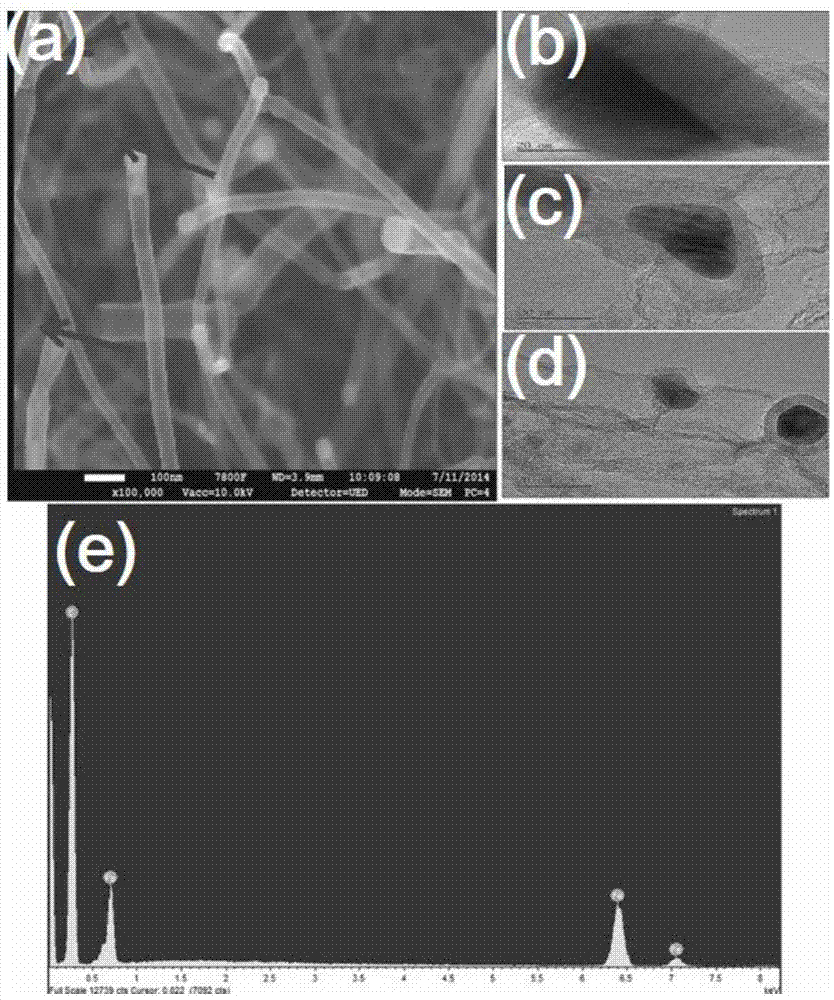
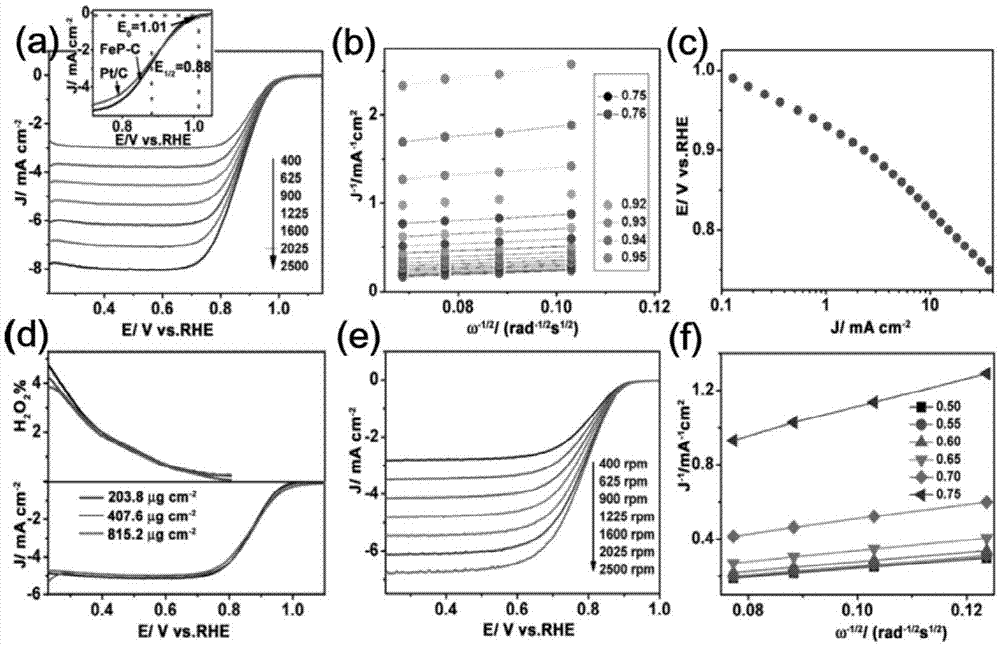
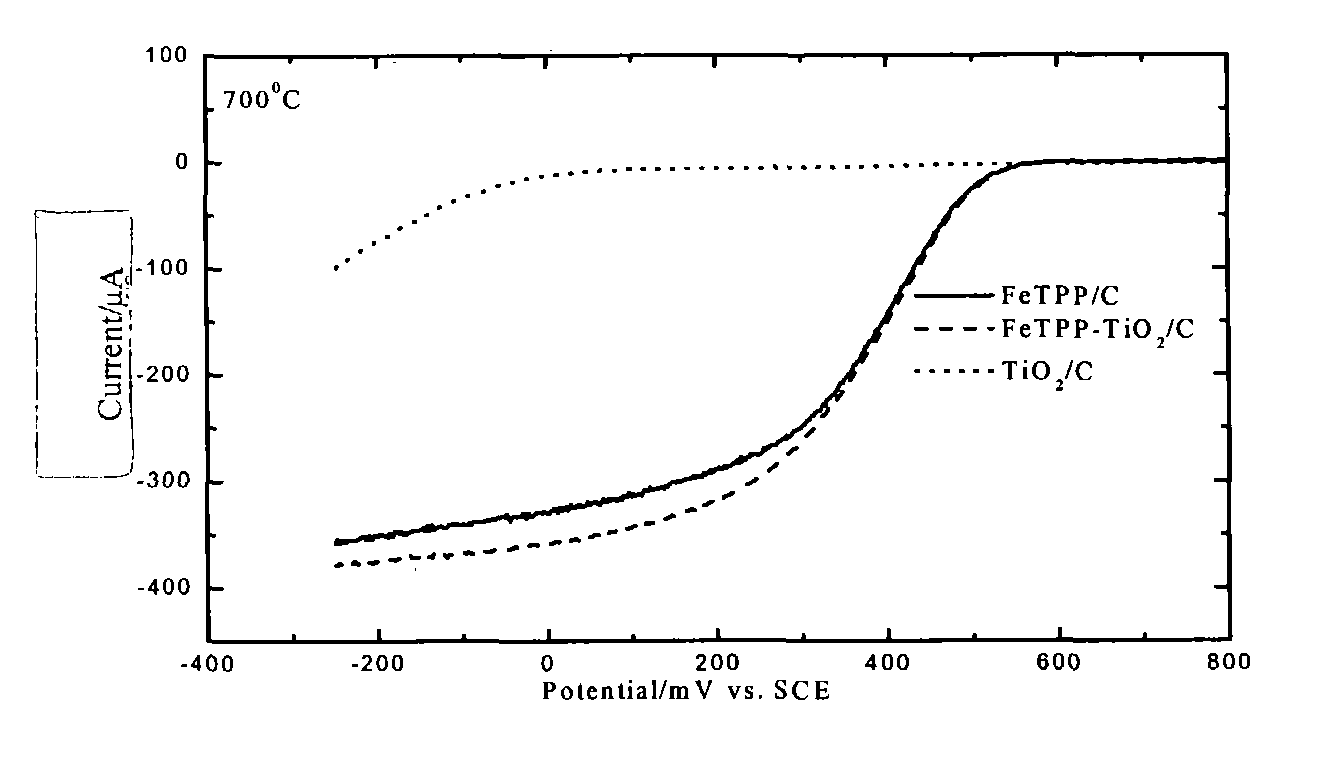
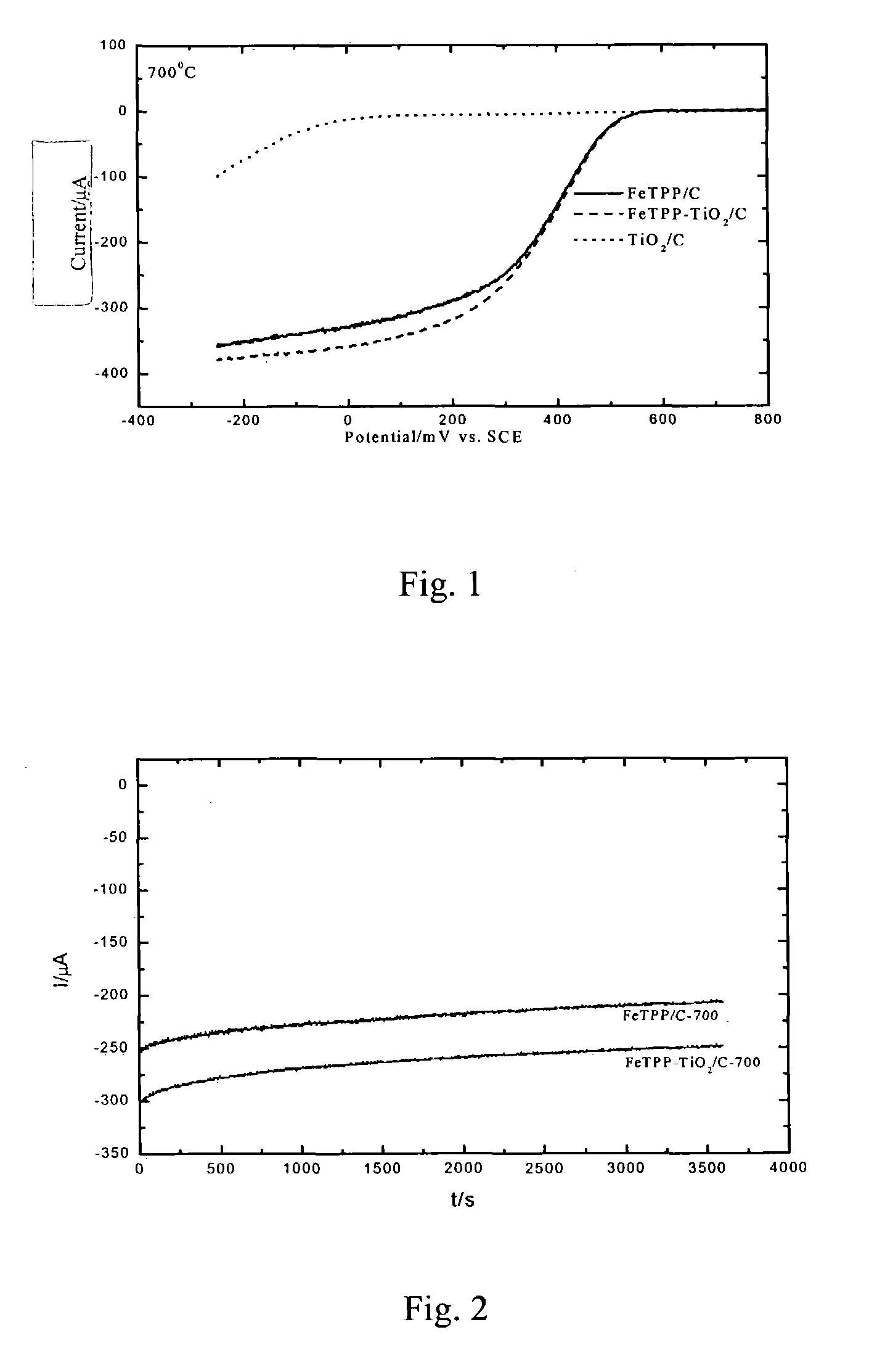
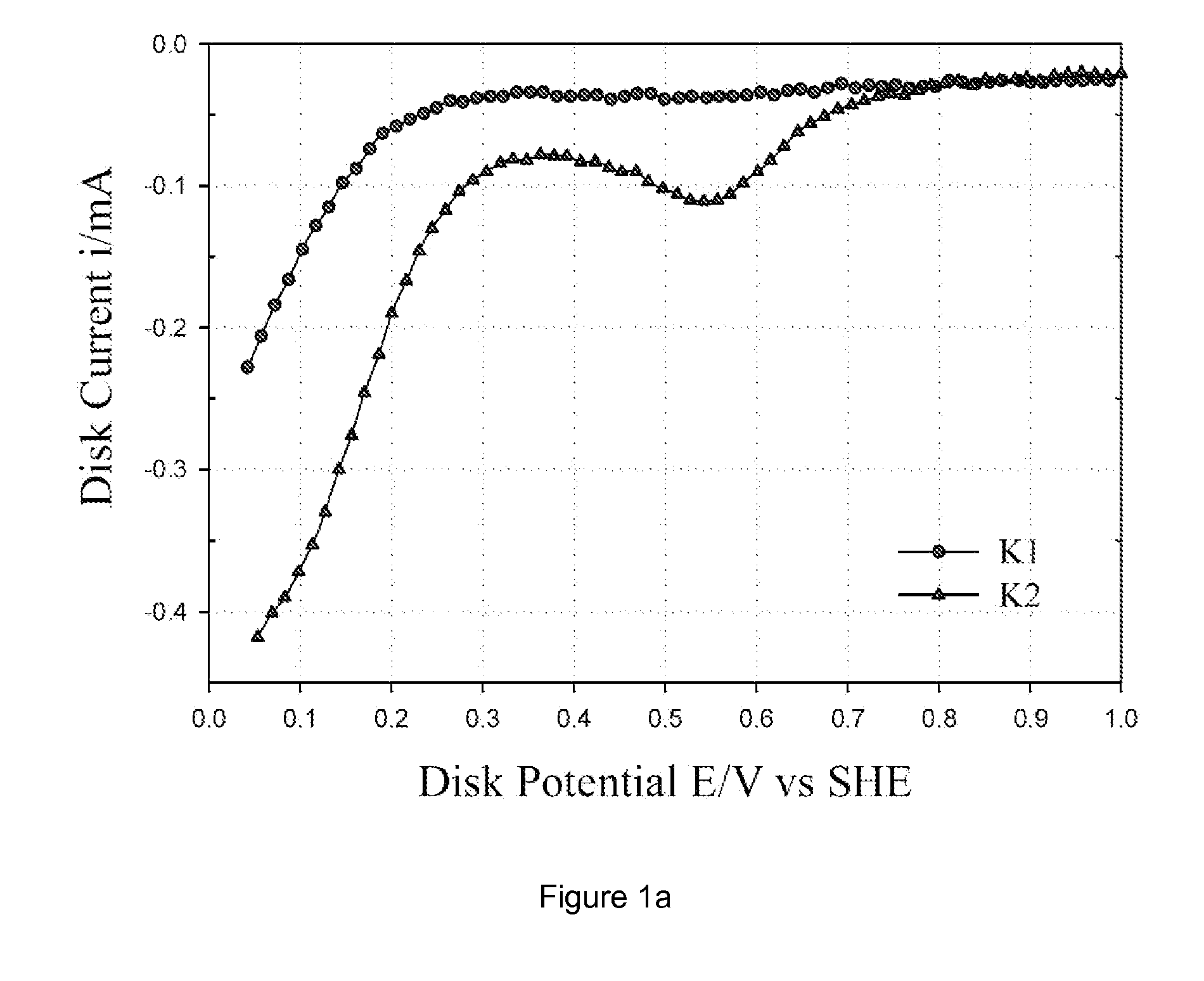
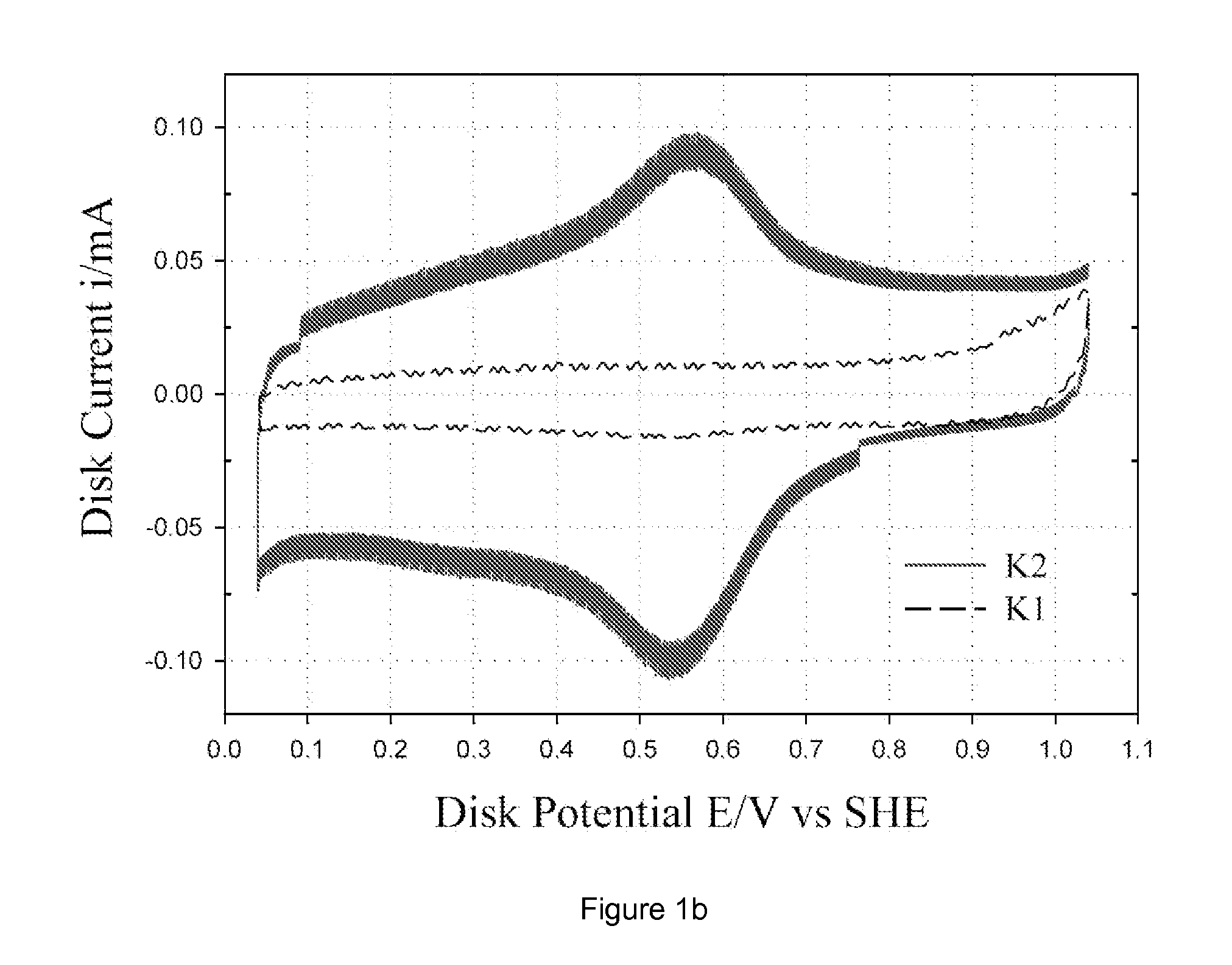
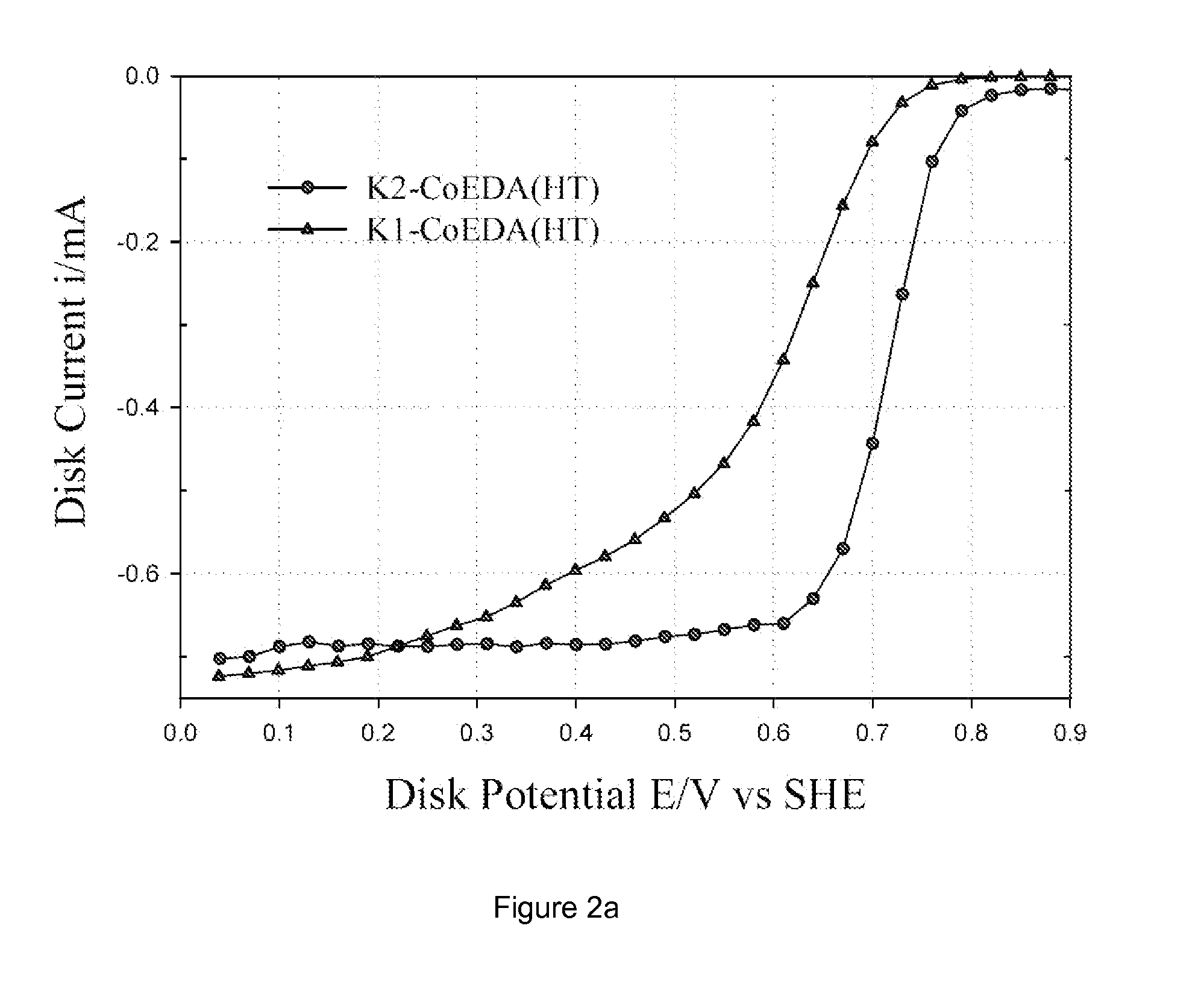
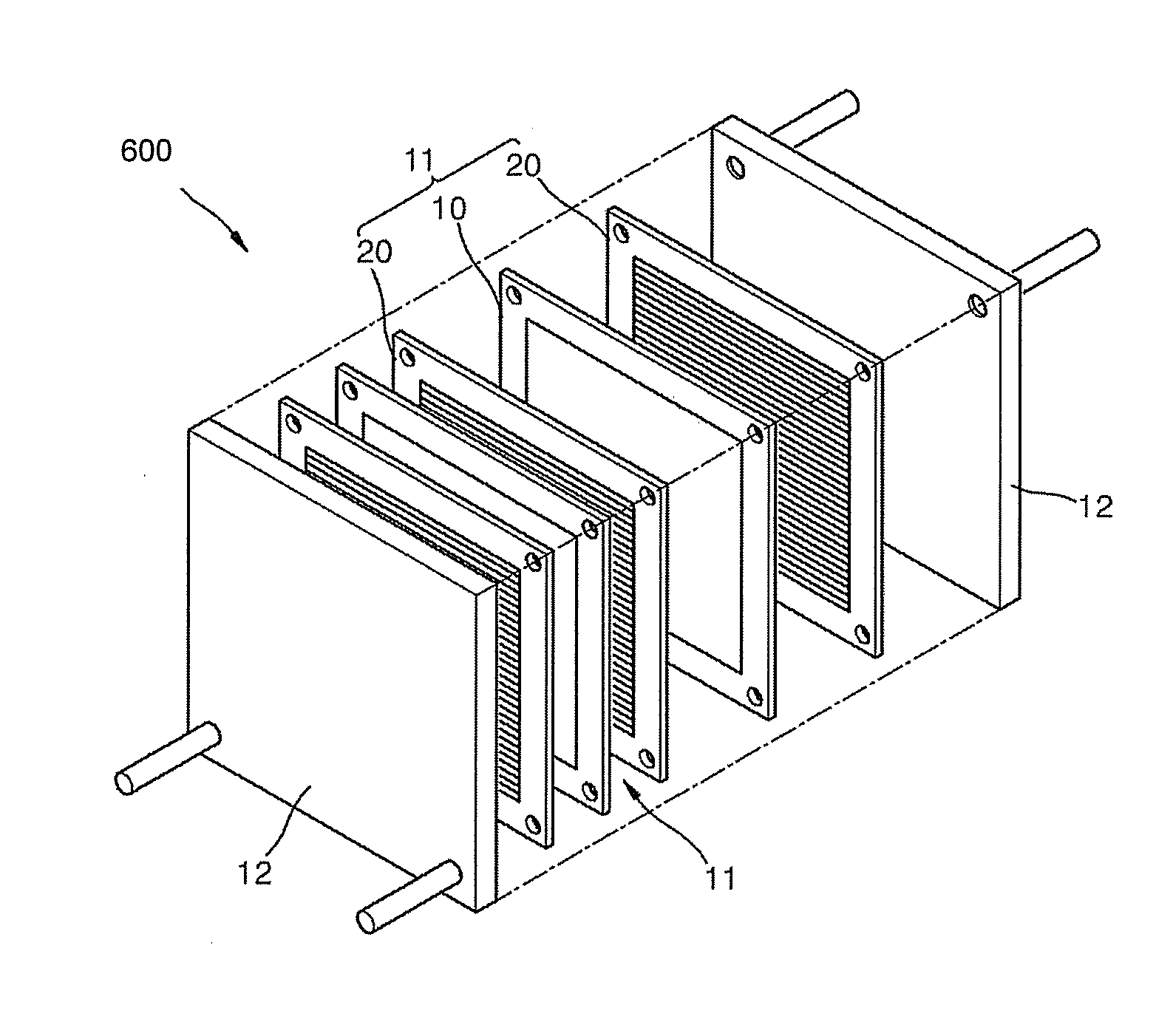
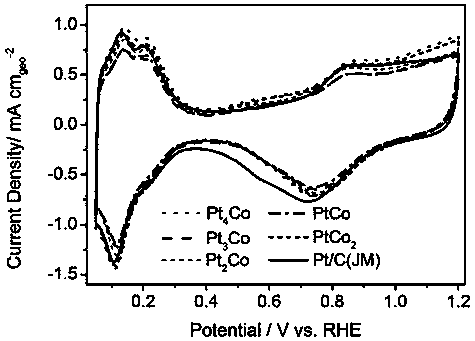
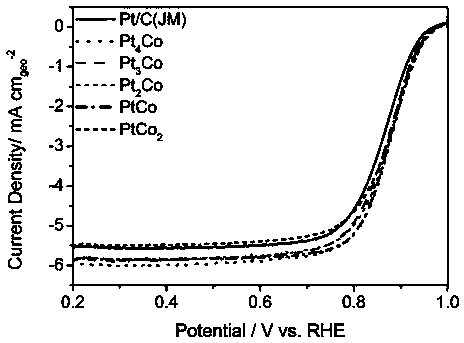
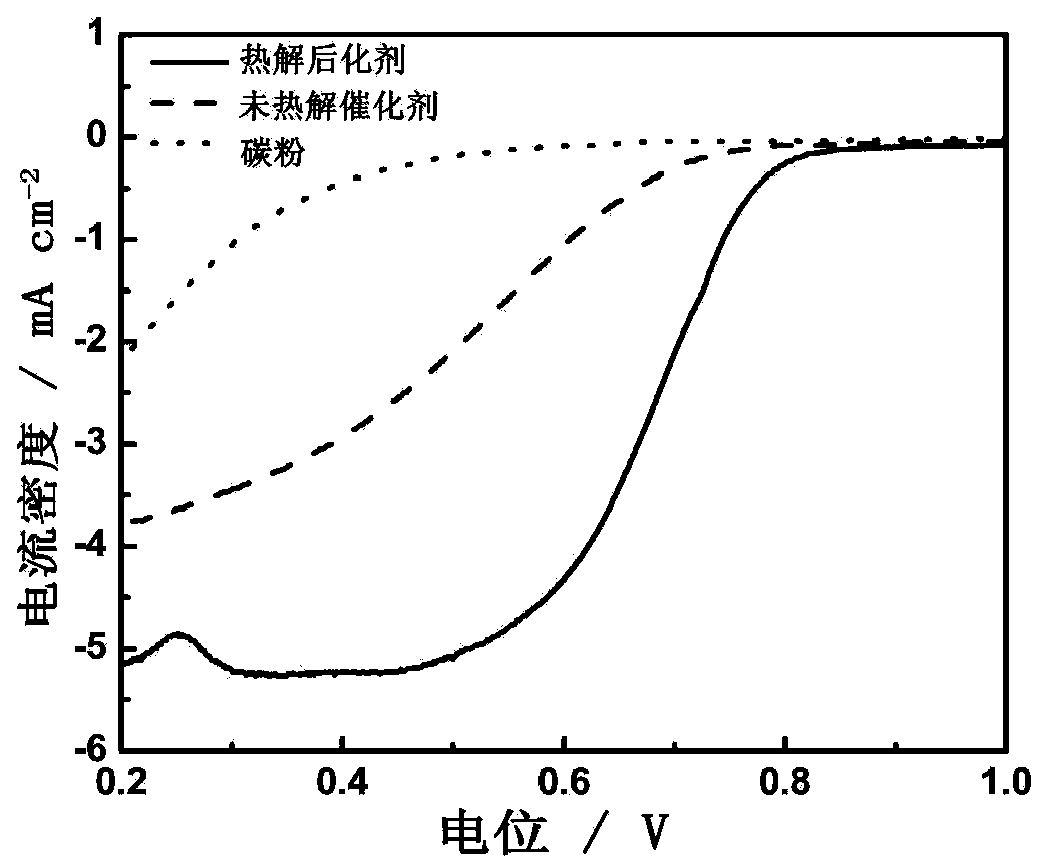
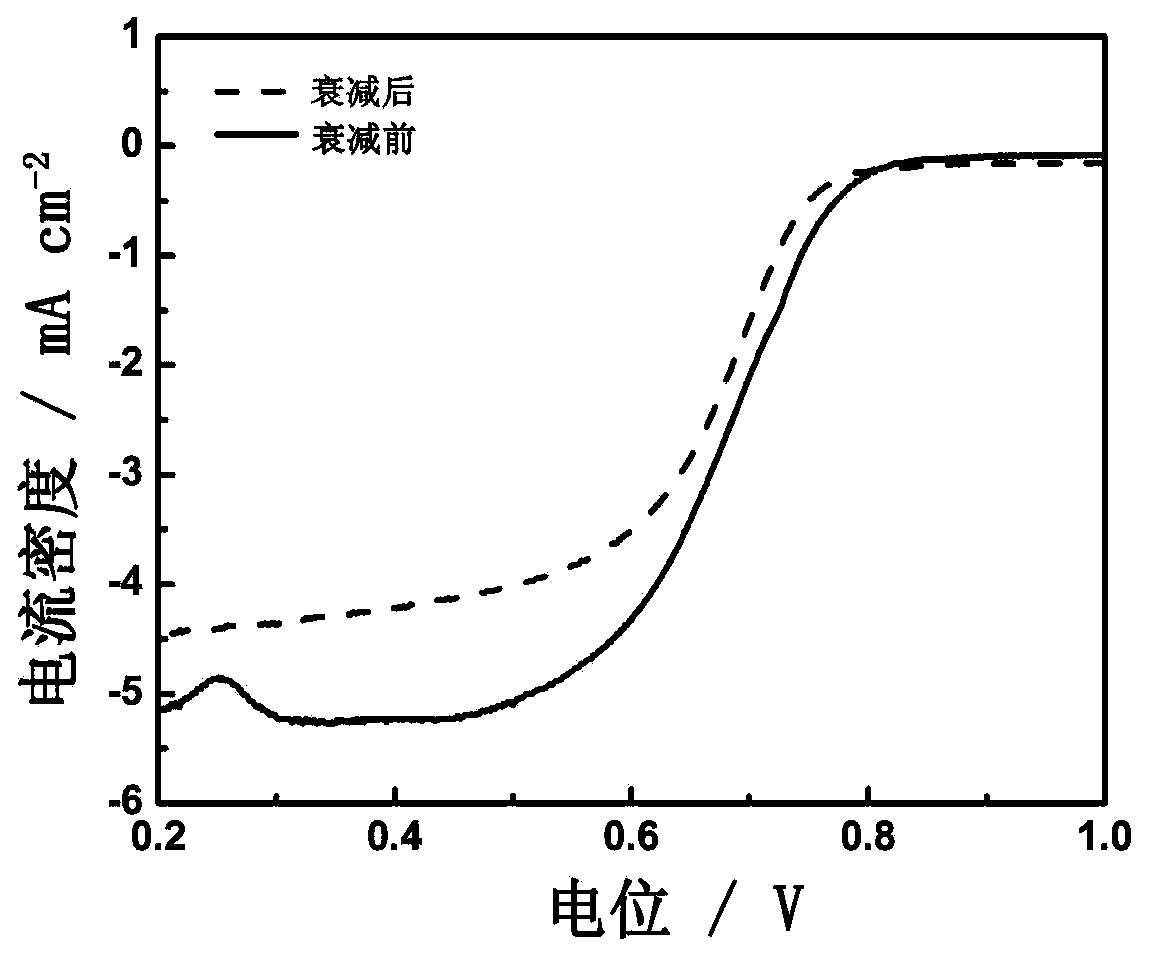
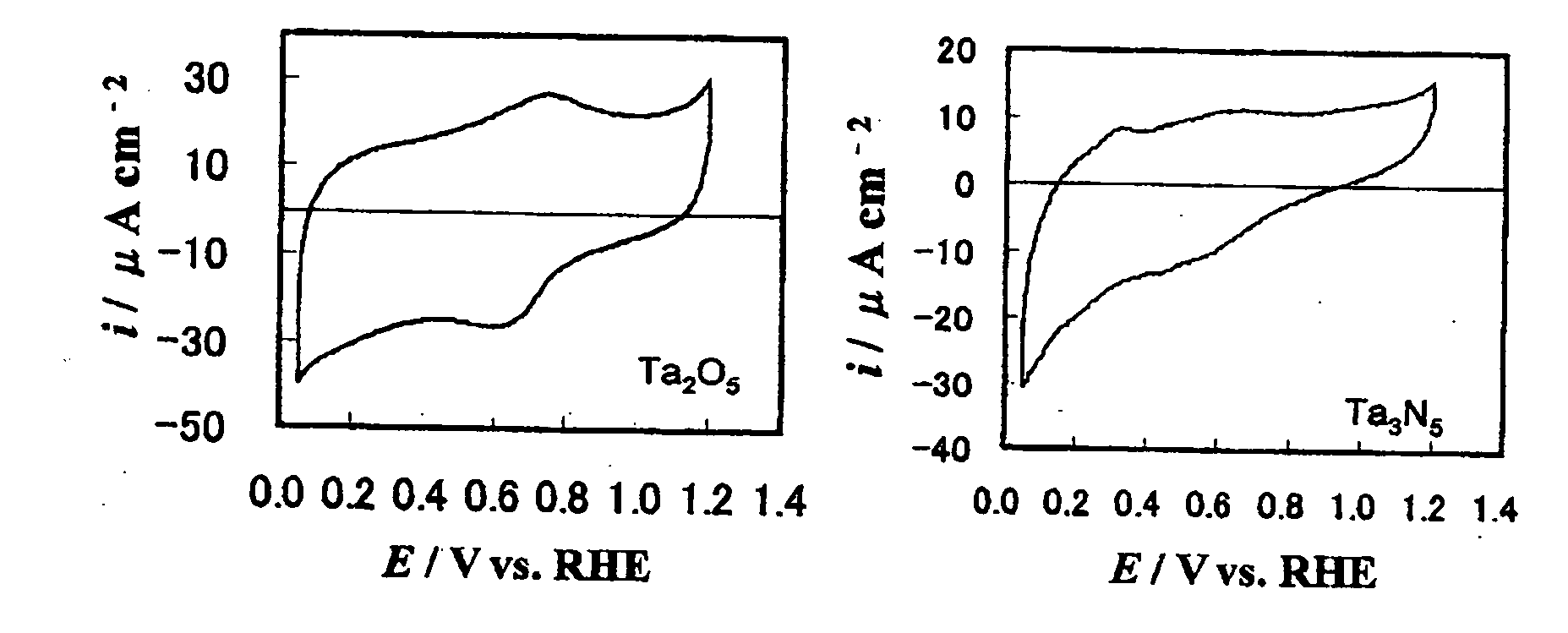
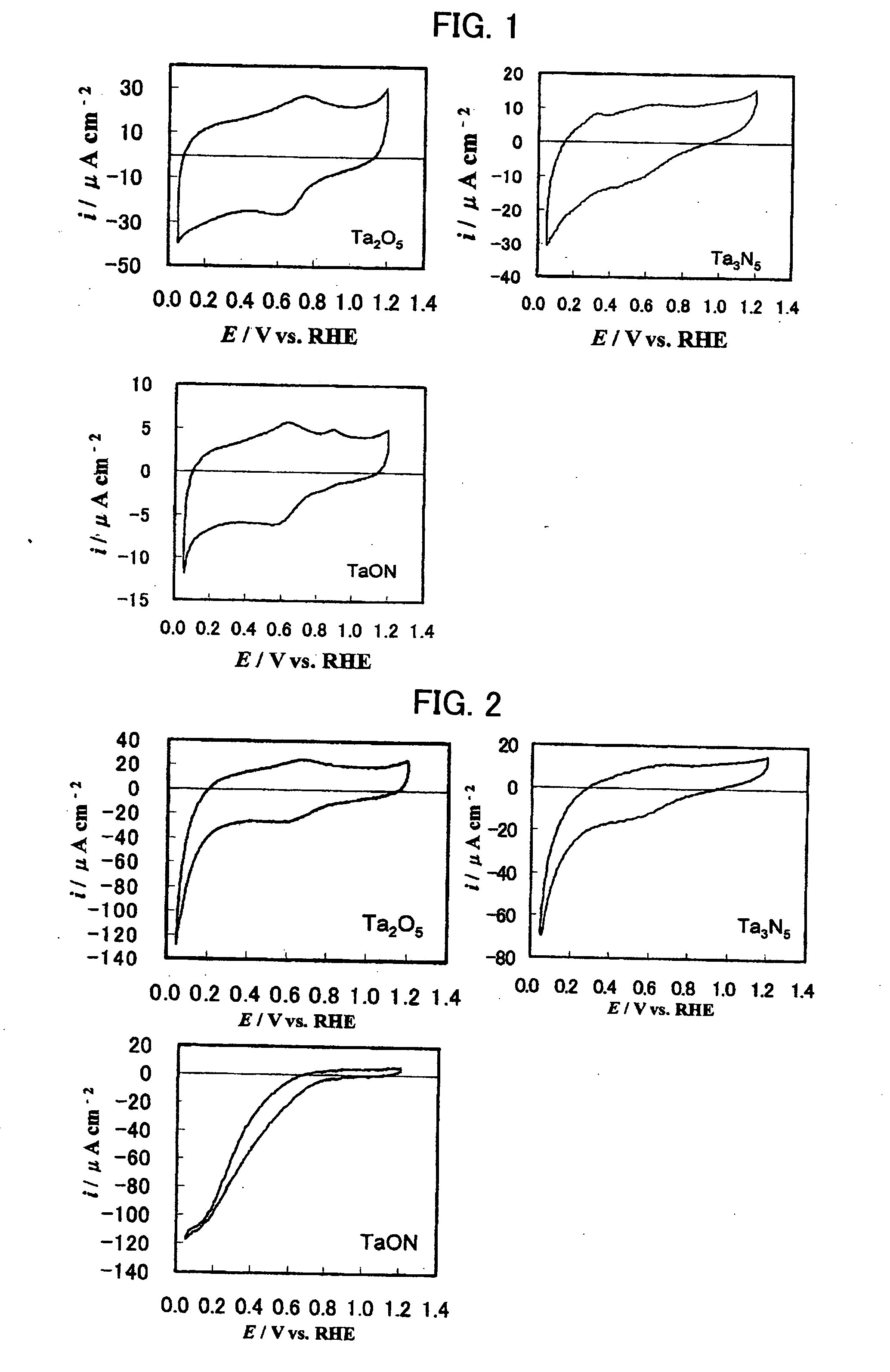
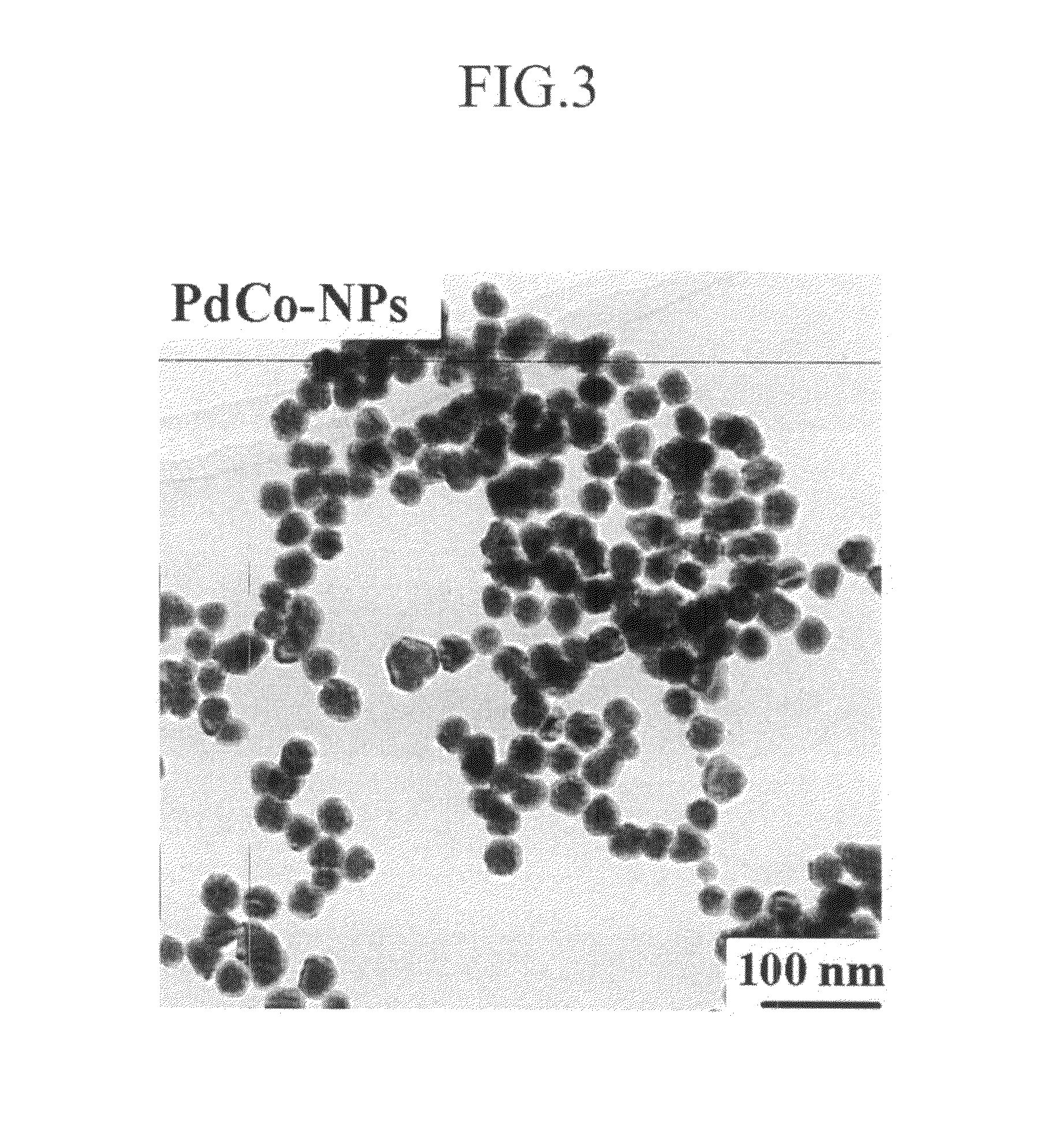
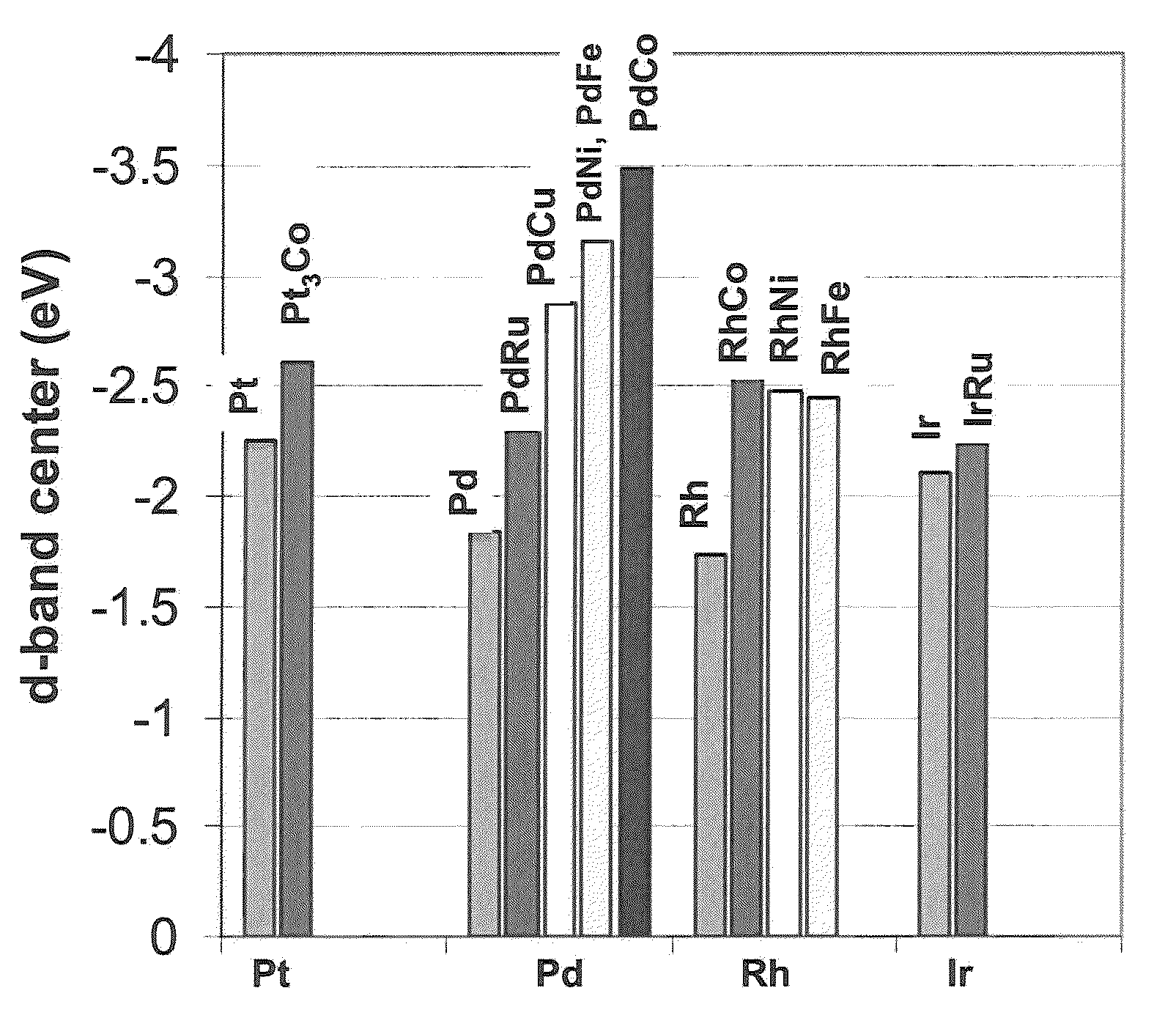
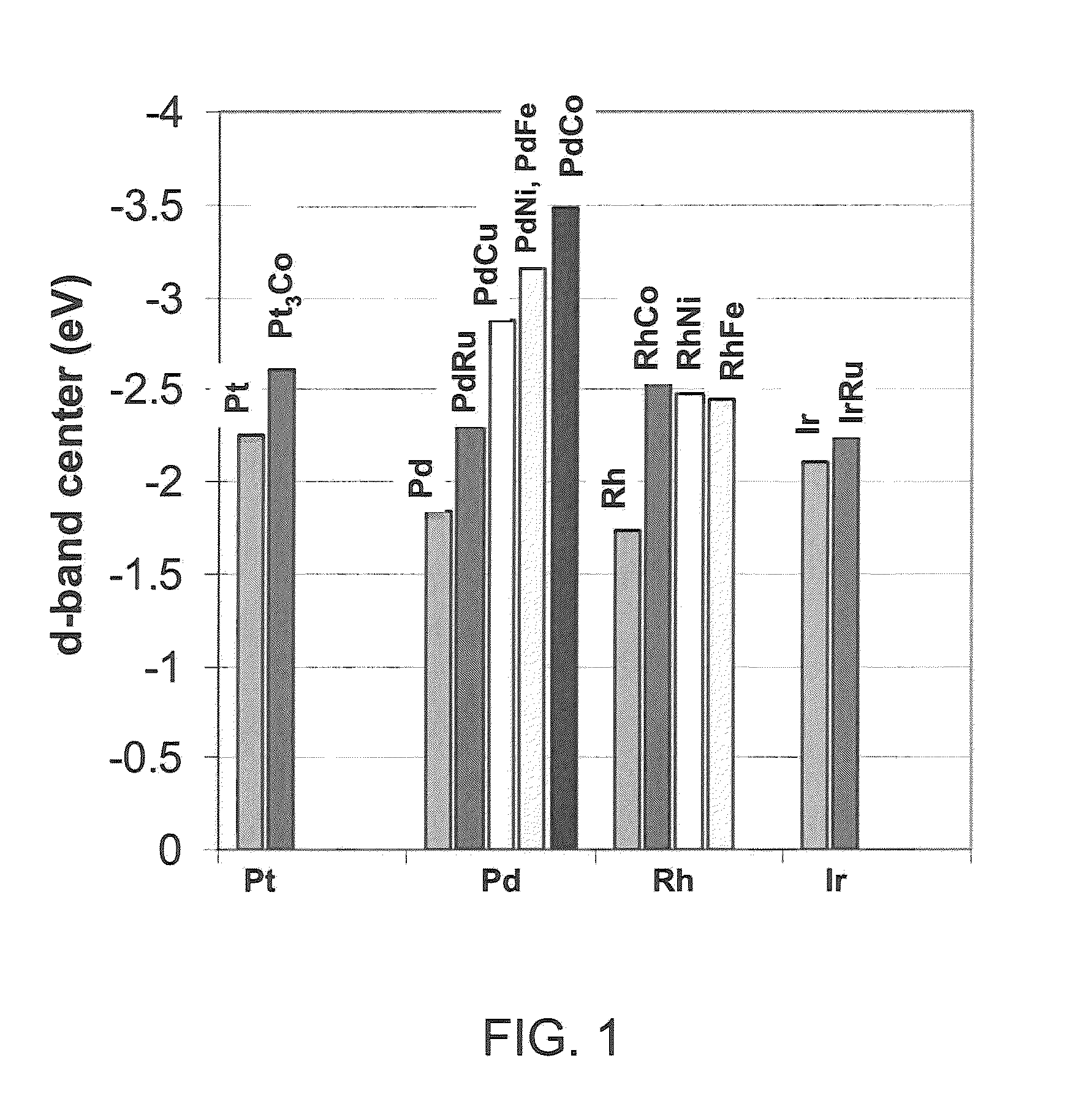
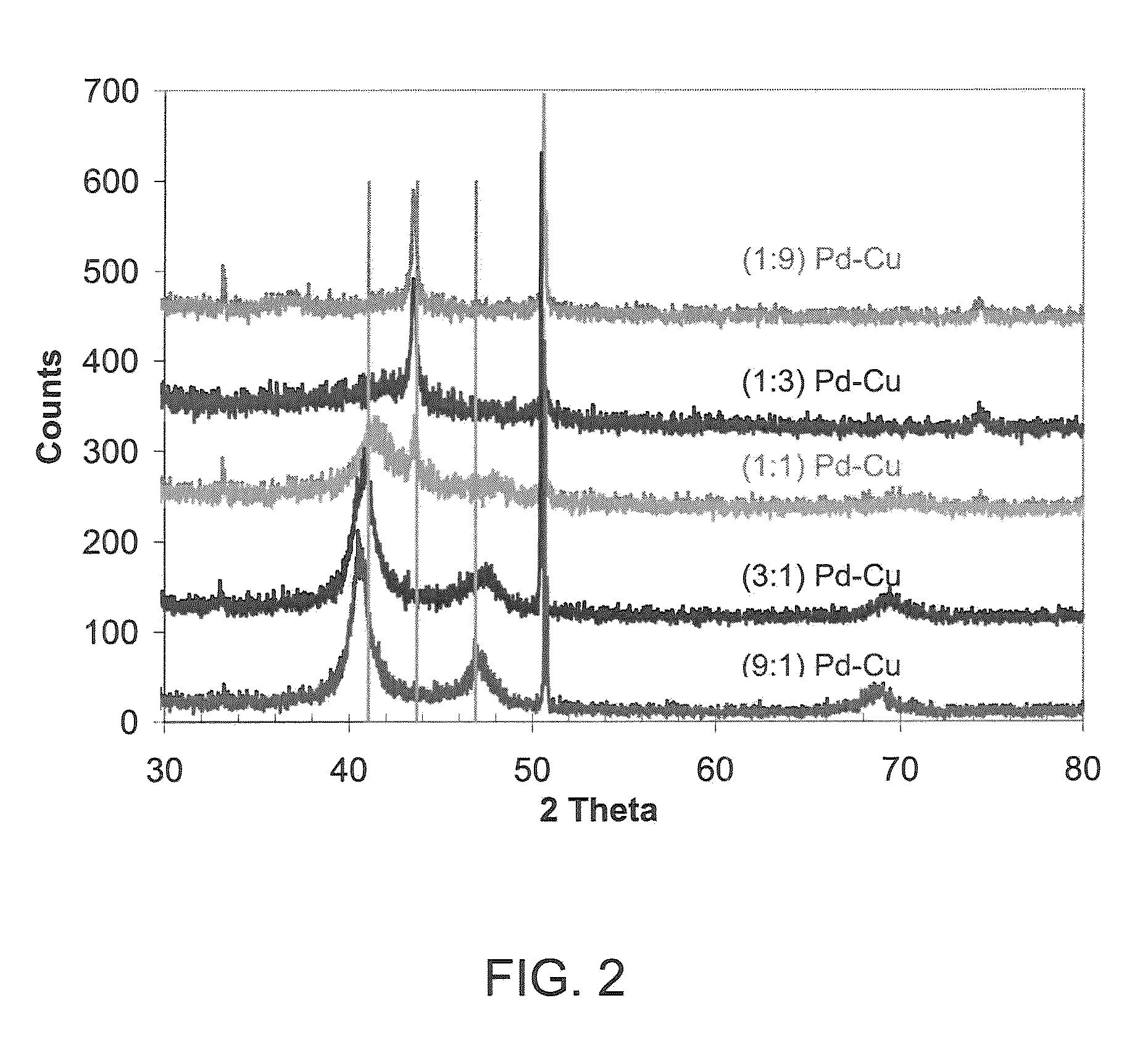
Patents
Literature
100 results about "Non platinum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
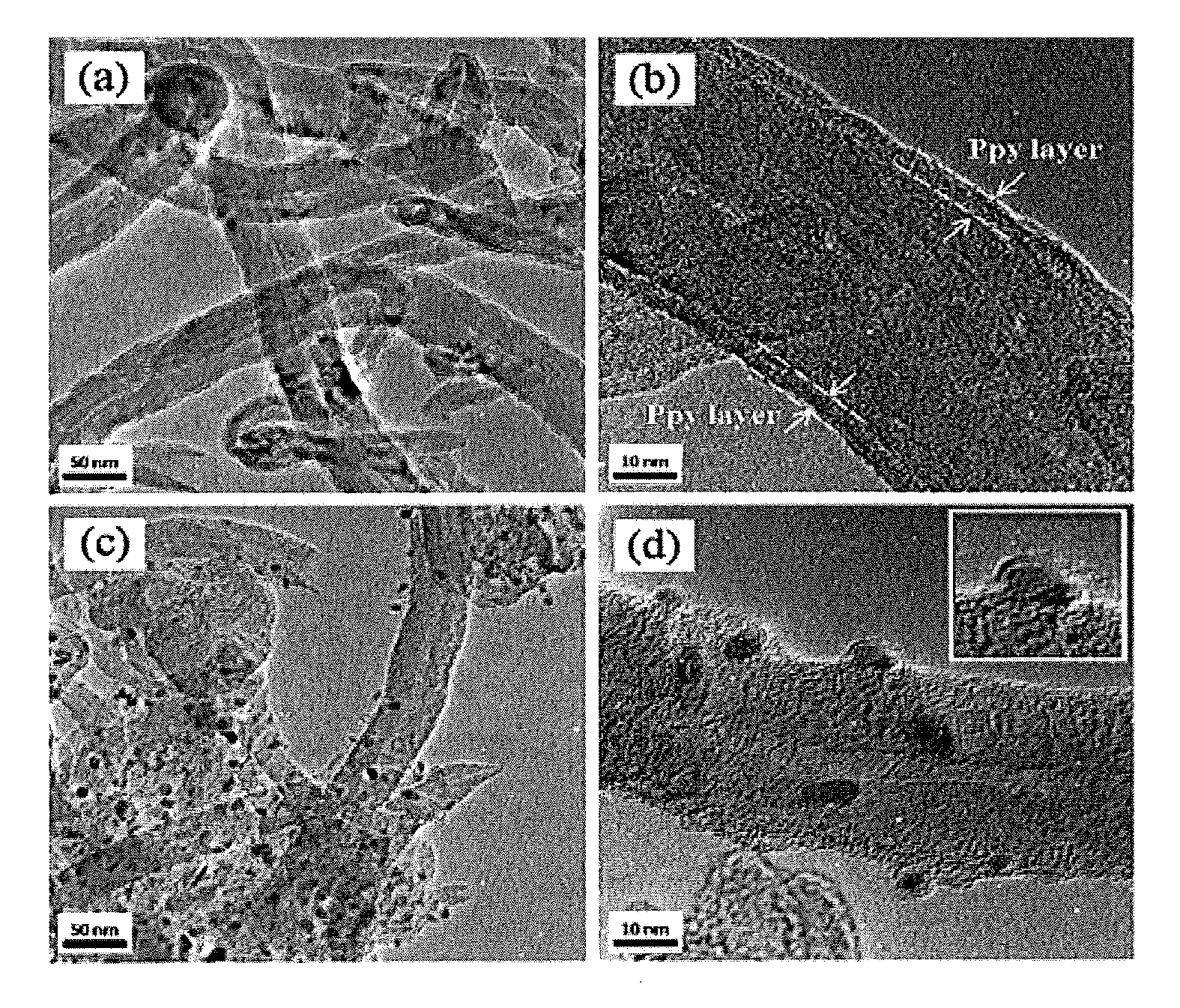
M/N-C catalyst and preparation and application thereof
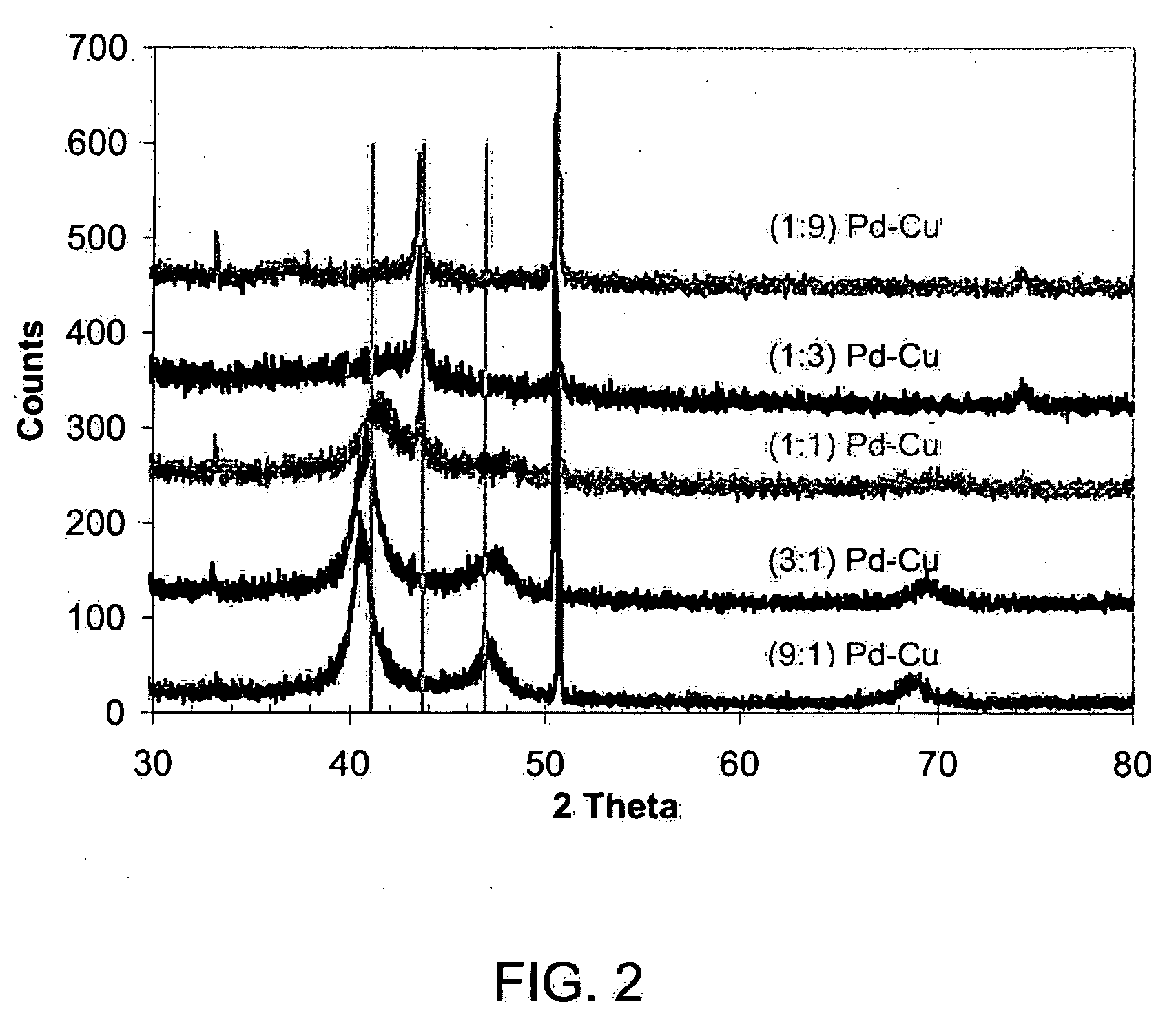
ActiveCN102451727AImprove conductivityImprove stabilityCell electrodesMetal/metal-oxides/metal-hydroxide catalystsPolypyrroleNon platinum
The invention relates to a non-platinum catalyst, in particular to an oxygen reduction catalyst for a proton exchange membrane fuel cell and preparation and application thereof. The catalyst can be prepared by the following steps of: (1) synthesizing polypyrrole (PPy); and (2) preparing an M / N-C catalyst, wherein the M / N-C catalyst can be taken as a cathode oxygen reduction catalyst for the proton exchange membrane fuel cell.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method of preparation of non-platinum composite electrocatalyst for cathode of fuel cell
InactiveUS20040058808A1Improve stabilityImprove the immunityOrganic-compounds/hydrides/coordination-complexes catalystsActive material electrodesOrganic solventFuel cells
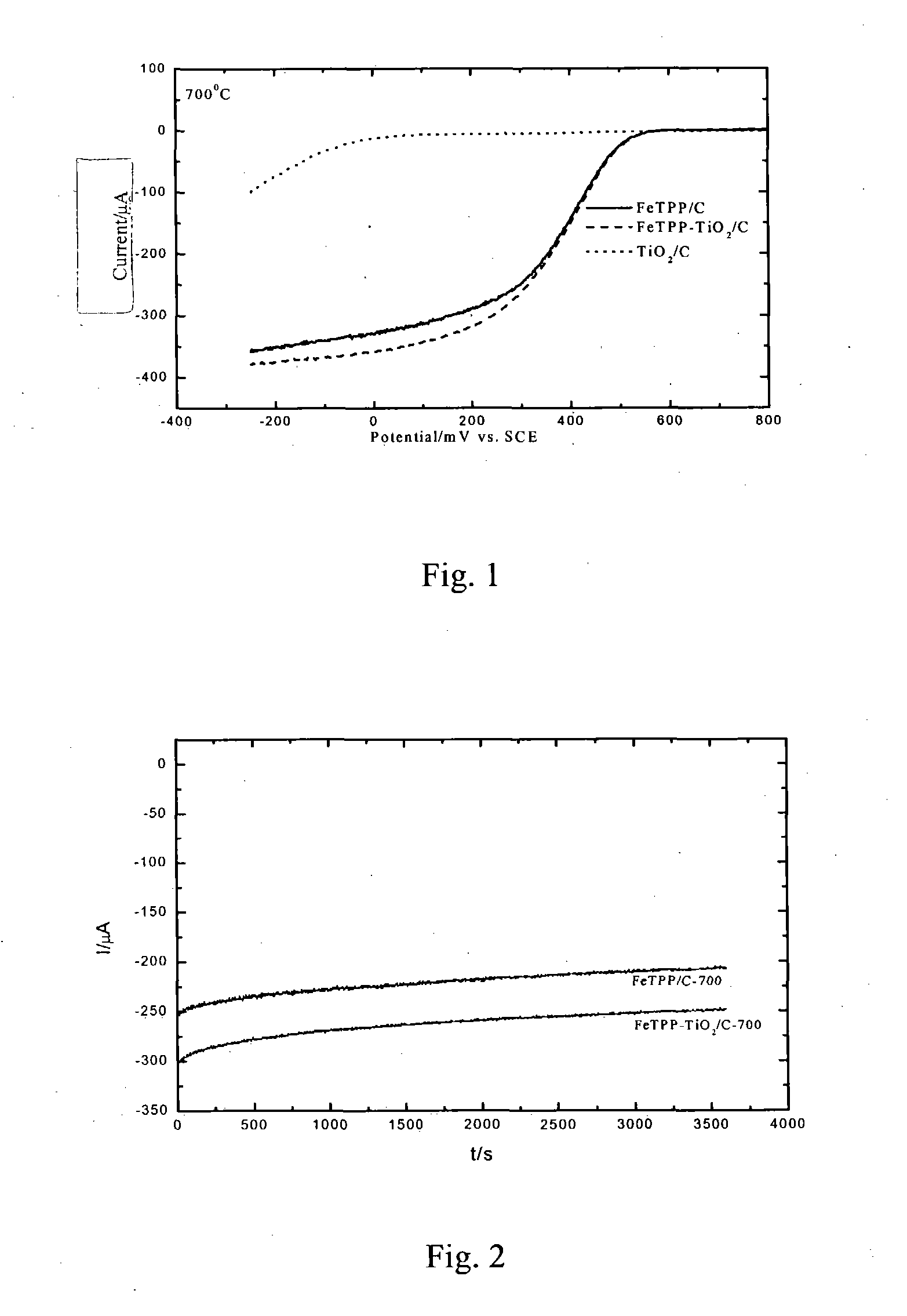
A method of preparing non-platinum composite electrocatalyst for a fuel cell cathode, comprising: (1) preparing a carbon supporting titanium dioxide; (2) compounding the carbon supporting titanium dioxide with a transition metal macrocyclic compound in an organic solvent to produce a carbon supporting titanium dioxide-transition metal macrocyclic compound comprising 0.1-5 g / L of macrocyclic compound; and (3) thermal treating the resulting compound in step (2) at 100-1000° C. to produce a composite catalyst. The composite catalyst prepared with the method according to the present invention also has the advantages of better resistance to methanol and lower cost over the Pt / C catalyst. The said composite catalyst would have better prospects in application.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI +1
Process for preparation of gas perforated electrode catalyst of nucleus-shell structure
InactiveCN101227000AOvercoming the hydrogen evolution problemOvercome hydrolysisCell electrodesCatalyst activation/preparationPlatinum saltsNon platinum
The invention provides a method for preparing a core-shell structure gas porous electrode catalyst, which belongs to the field of fuel cells. First, a homogeneously dispersed non-platinum-group transition metal M core is optionally deposited on a gas porous electrode which is bonded on perfluoro sulfonic acid resin, and then a M@Pt core-shell type catalyst is made through a chemical replacement reaction between the deposited non-platinum-group transition metal M core and a platinum salt solution. The invention has the advantages of simple process and low cost. The M@Pt core-shell type catalyst which is prepared by the invention is capable of effectively reducing the amount of precious metal platinum, improving the utilization ratio of the catalyst, and the catalyst is capable of replacing a prior commercial Pt / C catalyst.
Owner:CHONGQING UNIV
Non-platinum bimetallic polymer electrolyte fuel cell catalysts
ActiveUS20090192030A1Low costPromote formationCell electrodesCatalyst activation/preparationNon platinumAlloy
A polymetallic nanoparticle alloy having enhanced catalytic properties including at least one noble metal and at least one base metal, where the noble metal is preferentially dispersed near the surface of the nanoparticle and the base metal modifies the electronic properties of the surface disposed noble metal. The polymetallic nanoparticles having application as a catalyst when dispersed on a carbon substrate and in particular applications in a fuel cell. In various embodiments a bimetallic noble metal-base metal nanoparticle alloy may be used as an electrocatalyst offering enhanced ORR activity compared to the monometallic electrocatalyst of noble metal.
Owner:UCHICAGO ARGONNE LLC
Carbon-supported CoN fuel-cell catalyst as well as preparation method and application thereof
InactiveCN102324531ALow costHigh catalytic activityPhysical/chemical process catalystsCell electrodesNon platinumSolvent
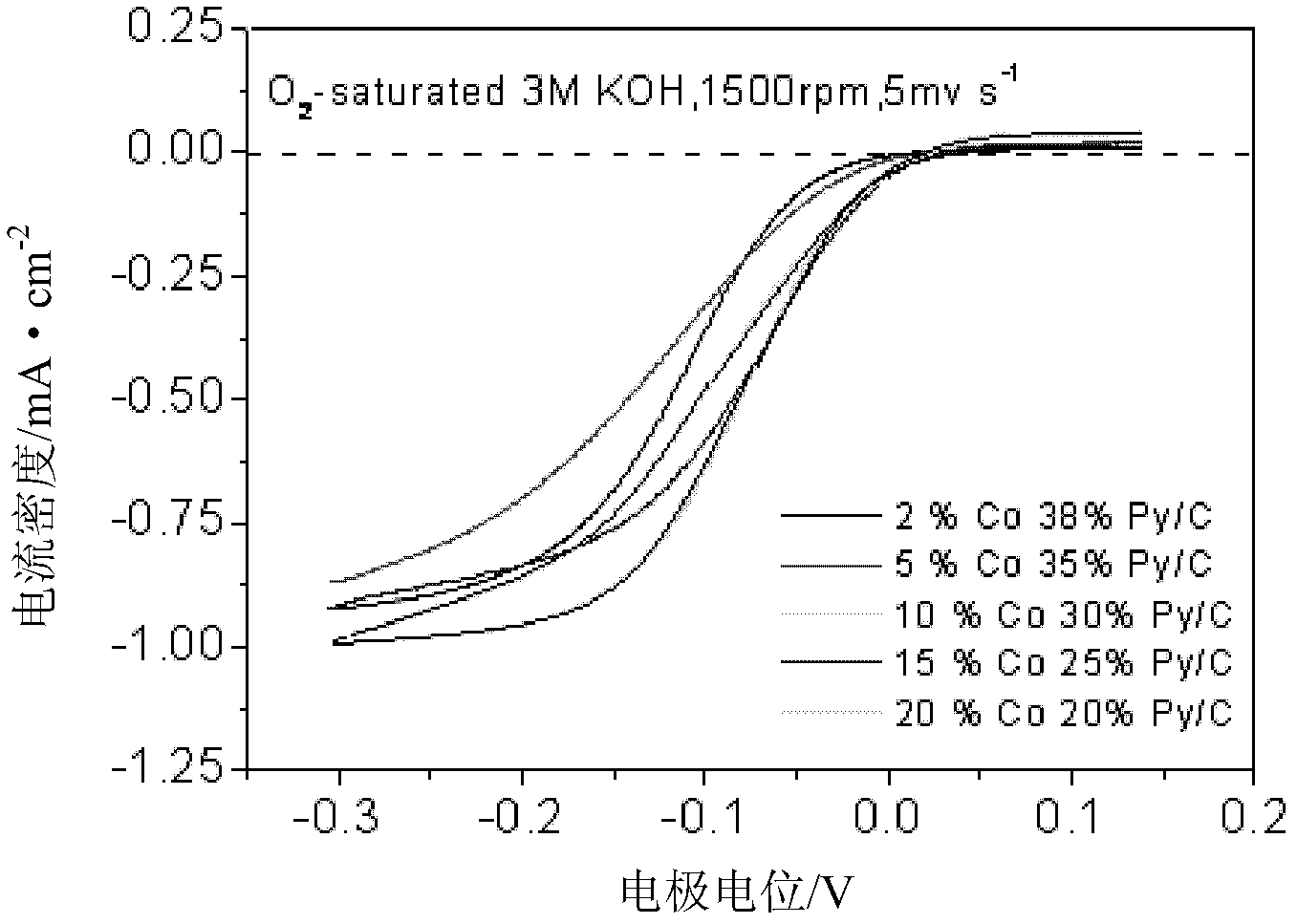
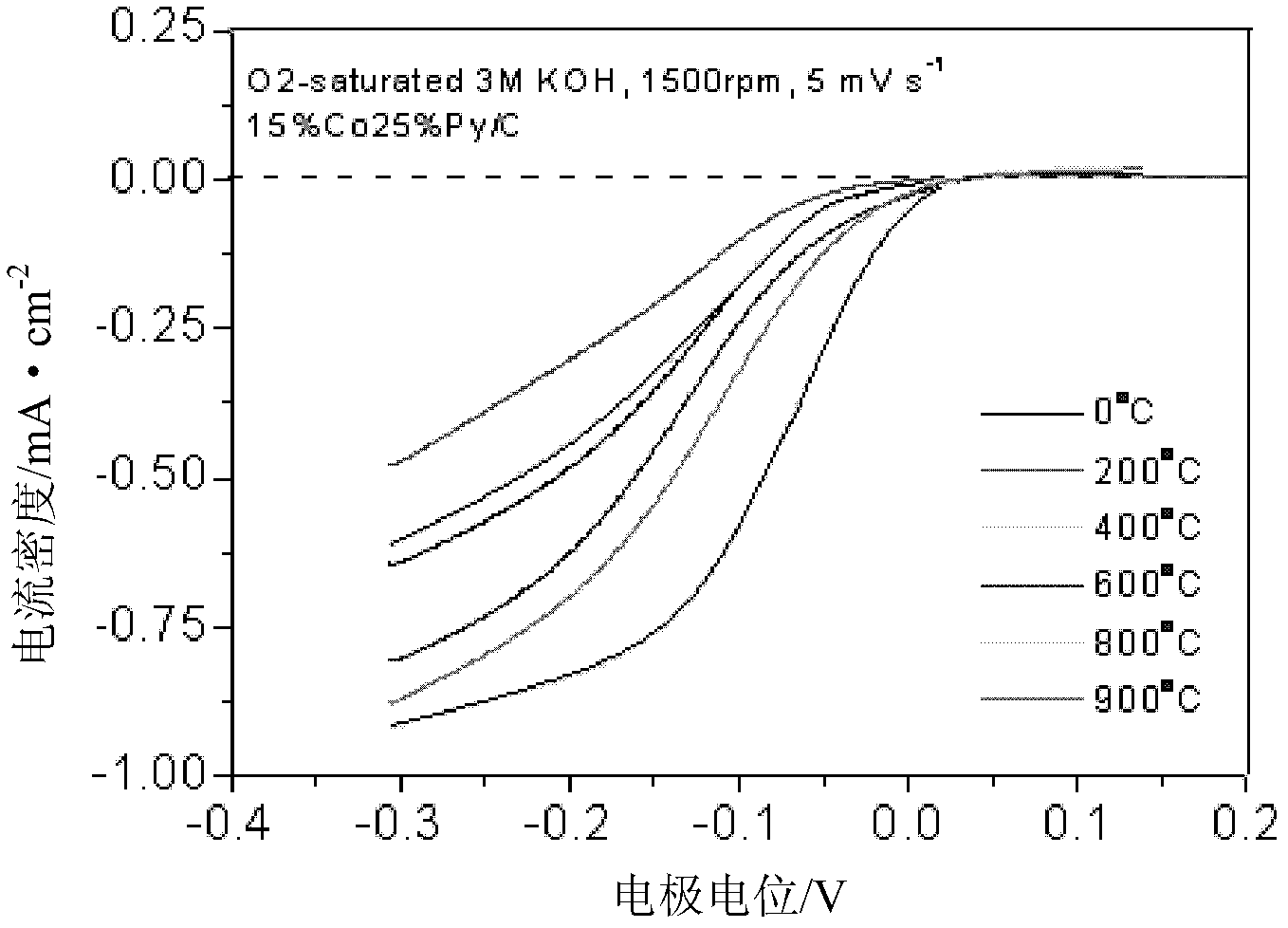
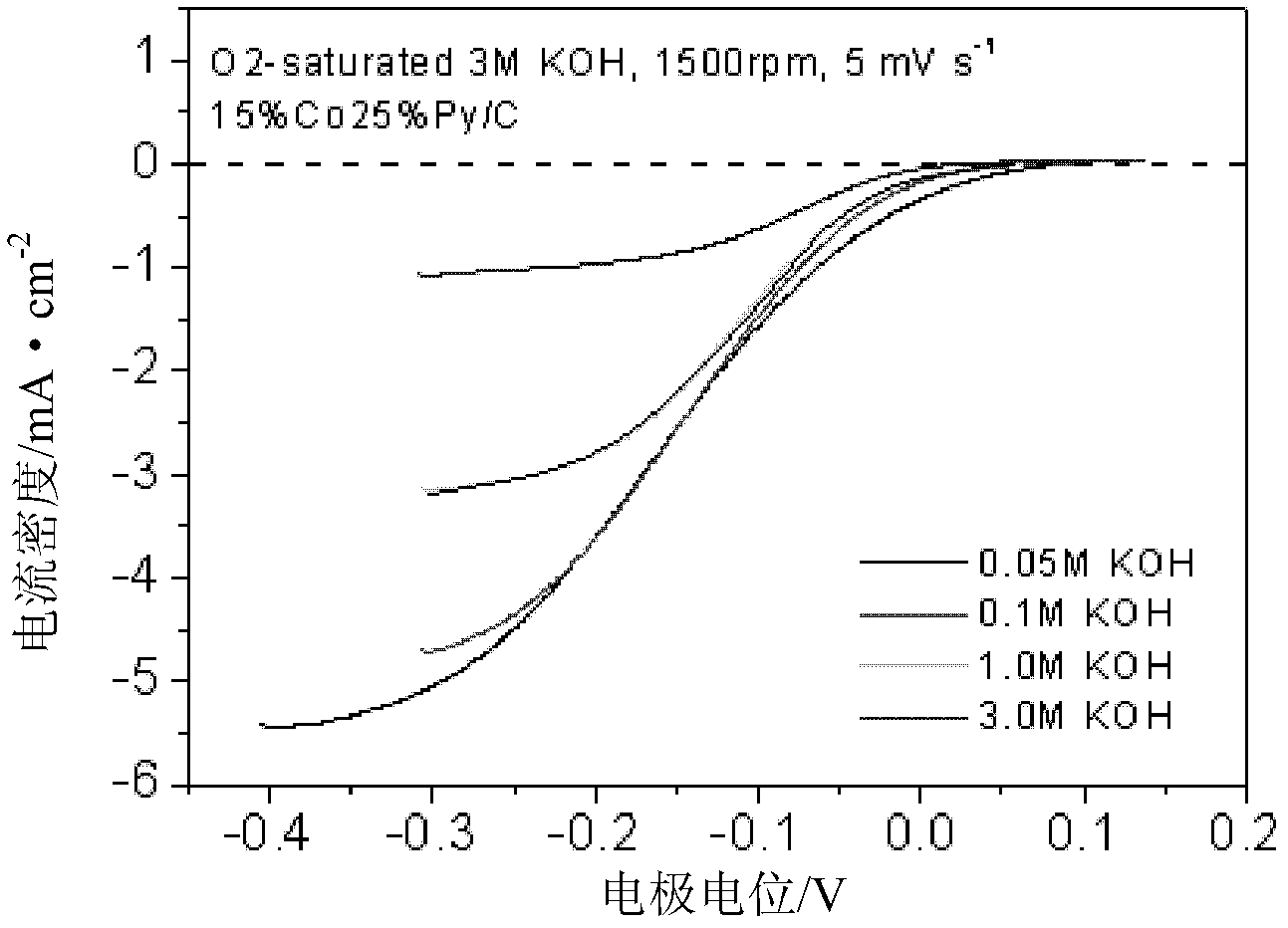
The invention relates to a carbon-supported CoN fuel-cell catalyst as well as a preparation method and application thereof. The catalyst comprises the following components in mass percentage: 40-99 percent of carbon material and 1-60 percent of active component. The catalyst is prepared through the following steps of: dispersing the components into a mortar loaded with a solvent, fully grinding until the solvent is completely volatilized, and obtaining a catalyst precursor after vacuum drying; and roasting for 2-4 hours by increasing the temperature for 200-900 DEG C at the speed of 20 DEG C / minute under the protection of an inert-gas atmosphere. The catalyst is applied to alkaline fuel cells, direct methanol fuel cells, direct ethanol fuel cells or low-temperature fuel cells. The catalyst is a non-platinum catalyst, so that the cost of the fuel cells can be markedly lowered; and the preparation method disclosed by the invention is simple and easy to operate, has low cost, is suitable for industrialized production and has good application prospects.
Owner:DONGHUA UNIV
Metal selenide counter-electrode for dye-sensitized solar cell and preparation method of metal selenide counter-electrode
InactiveCN102610392AReduce manufacturing costSuitable for mass productionLight-sensitive devicesSolid-state devicesNon platinumHydrothermal synthesis
The invention belongs to the technical field of solar cells, and particularly discloses a metal selenide counter-electrode for a dye-sensitized solar cell and a preparation method of the metal selenide counter-electrode. The preparation method includes preparing metal selenide by the aid of a one-step hydrothermal synthesis method; realizing in-situ growth of the metal selenide on a conducting substrate without any other aftertreatment; and directly applying the metal selenide to the dye-sensitized solar cell so that better energy conversion efficiency can be obtained as compared with a pyrolysis platinum counter-electrode. A process is simple, the prepared non-platinum counter-electrode not only is high in catalytic activity, but also is cheap in price, the cost of the counter-electrode is greatly reduced, furthermore, the comprehensive cost of the dye-sensitized solar cell is reduced, and the metal selenide counter-electrode and the preparation method can be applied to large-scale industrial production of dye-sensitized solar cells.
Owner:FUDAN UNIV
Oxygen reduction catalyst as well as preparation method and application thereof
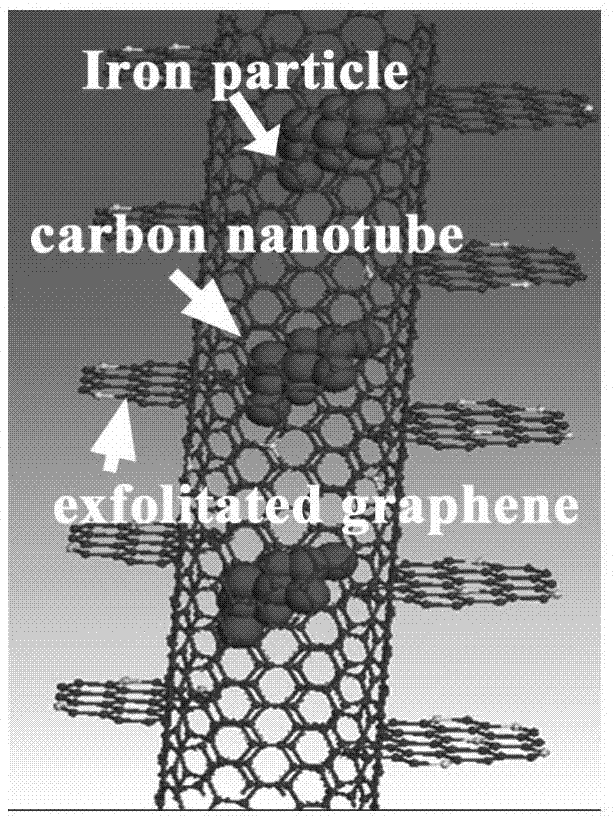
ActiveCN106898786AHigh activityImprove stabilityMaterial nanotechnologyCell electrodesCarbon coatingAlloy
The invention discloses a non-platinum oxygen reduction catalyst and a preparation method thereof. The non-platinum oxygen reduction catalyst is characterized by comprising a three-layer core-shell structure, wherein a core is Fe, Co, Ni metal nanoparticles or alloy of the metal nanoparticles, an intermediate layer is a carbon coating layer, and an outer layer is a peeled graphene nanosheet layer; the size of the metal core of the non-platinum catalyst is 1-50 nanometers; the middle carbon coating layer is of a nanotube structure, and the size of a nanotube is 2-100 nanometers; and the outer layer, namely the peeled graphene nanosheet layer, is bonded with a middle graphite coating layer through covalent bonds, the surface of the peeled graphene nanosheet layer is doped with doping elements such as N and O, and the concentration of the doping elements is 0.5%-20%. The catalyst synthesized by virtue of the preparation method has excellent oxygen reduction catalytic performance and stability.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method of preparation of non-platinum composite electrocatalyst for cathode of fuel cell
InactiveUS7005401B2Improve stabilityImprove the immunityOrganic-compounds/hydrides/coordination-complexes catalystsActive material electrodesOrganic solventFuel cells
A method of preparing non-platinum composite electrocatalyst for a fuel cell cathode, comprising: (1) preparing a carbon supporting titanium dioxide; (2) compounding the carbon supporting titanium dioxide with a transition metal macrocyclic compound in an organic solvent to produce a carbon supporting titanium dioxide-transition metal macrocyclic compound comprising 0.1–5 g / L of macrocyclic compound; and (3) thermal treating the resulting compound in step (2) at 100–1000° C. to produce a composite catalyst. The composite catalyst prepared with the method according to the present invention also has the advantages of better resistance to methanol and lower cost over the Pt / C catalyst. The said composite catalyst would have better prospects in application.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI +1
Composite catalysts supported on modified carbon substrates and methods of making the same
ActiveUS20080113859A1Organic-compounds/hydrides/coordination-complexes catalystsCell electrodesModified carbonNitrogen
A method of producing a composite carbon catalyst is generally disclosed. The method includes oxidizing a carbon precursor (e.g., carbon black). Optionally, nitrogen functional groups can be added to the oxidized carbon precursor. Then, the oxidized carbon precursor is refluxed with a non-platinum transitional metal precursor in a solution. Finally, the solution is pyrolyzed at a temperature of at least about 500° C.
Owner:UNIVERSITY OF SOUTH CAROLINA
Electrode catalyst for fuel cell and fuel cell including electrode having electrode catalyst
InactiveUS20100151296A1High catalytic activityMaterial nanotechnologyActive material electrodesFuel cellsMetal catalyst
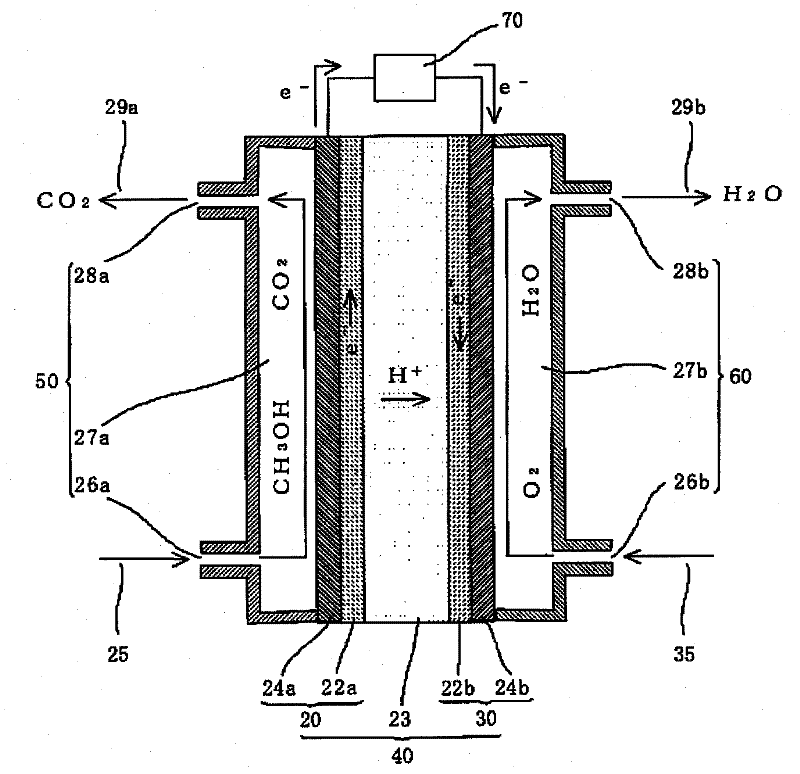
An electrode catalyst for a fuel cell and a fuel cell including an electrode having the electrode catalyst, include a non-platinum (Pt) catalyst, and a cerium (Ce) metal catalyst, both of which are supported on a carbon-based catalyst support having an improved catalytic activity at a decreased cost. The non-Pt catalyst may be at least one selected from the group consisting of Mn, Pd, Ir, Au, Cu, Co, Ni, Fe, Ru, WC, W, Mo, Se, any alloys thereof, and any mixtures thereof, and the Ce metal catalyst may be a Ce oxide.
Owner:SAMSUNG ELECTRONICS CO LTD
Platinum-containing catalyst, process for producing the platinum-containing catalyst, electrode, and electrochemical device
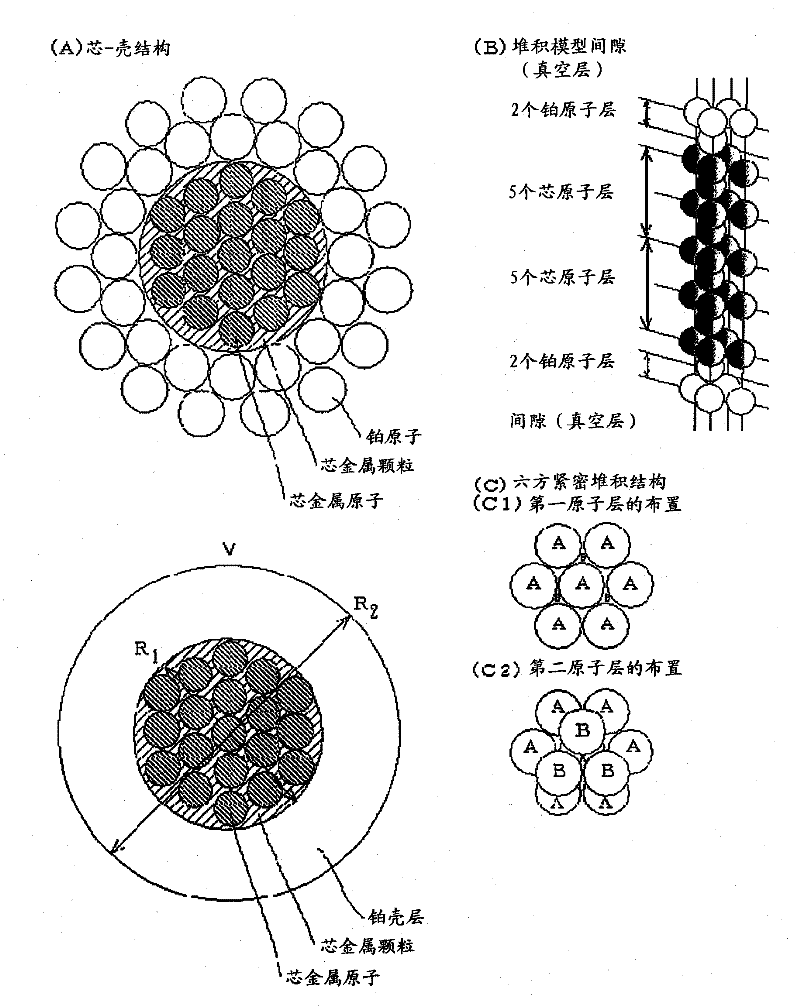
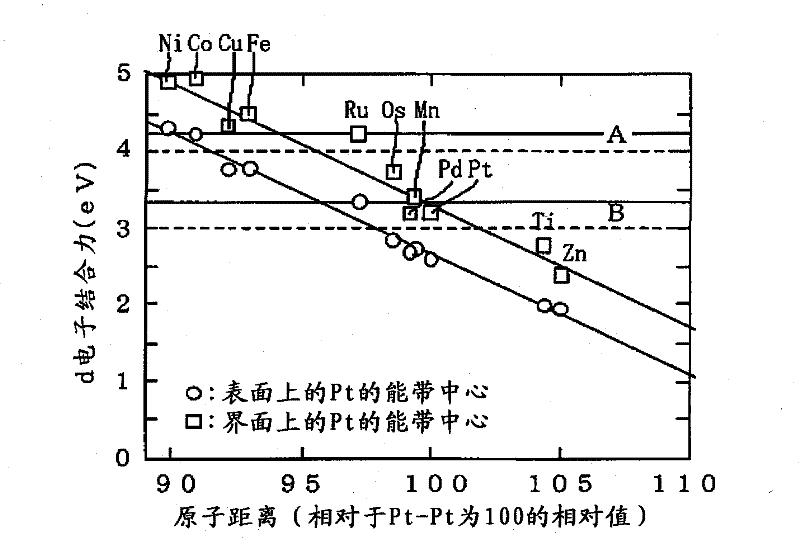
InactiveCN102196863AHigh output maintenanceIncreased durabilityCell electrodesCatalyst activation/preparationBinding energyPower flow
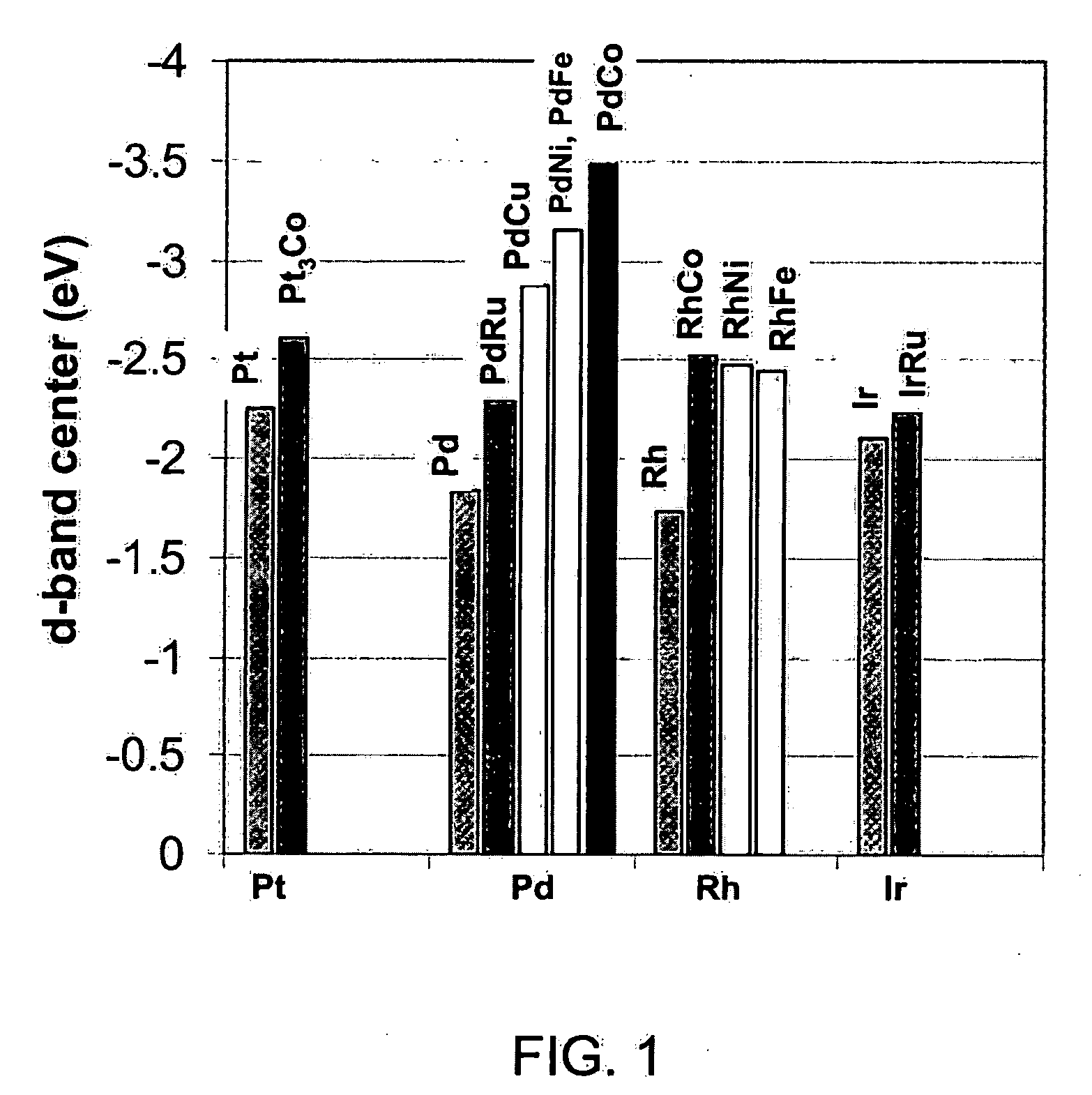
Disclosed is a core-shell-type platinum-containing catalyst that can reduce the amount of platinum used and has high catalytic activity and stability. Also disclosed are a process for producing the core-shell-type platinum-containing catalyst, an electrode, and an electrochemical device. The core-shell-type platinum-containing catalyst comprises particles each comprising a core particle (average particle diameter: R1) formed of a non-platinum element and a platinum shell layer (average thickness: ts) and satisfies the following relationships: 1.4 nm = R1 = 3.5 nm; and 0.25 nm = ts = 0.9 nm. The core particle contains an element satisfying Eout = 3.0 eV wherein Eout represents the average bound energy, based on Fermi level, of 5d orbit electrons of platinum present on the outermost surface of the platinum shell. A fuel cell comprising a platinum-containing catalyst as an anode catalyst, the platinum-containing catalyst comprising a Ru particle as a core particle, has an output density of not less than 70 mW / cm2 at a current density of 300 mA / cm2 and an output retention of not less than about 90%.
Owner:SONY CORP
Preparing method for supported core-shell-structure catalyst for low-temperature fuel cell
InactiveCN103157519ASmall particle sizeUniform particle size distributionCell electrodesCatalyst activation/preparationDispersityNano catalyst
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Catalyst for generating hydrogen by visible light photocatalytic reduction of water, and preparation method thereof
InactiveCN102039131AHigh hydrogen production activityAchieve non-platinizationHydrogen productionMetal/metal-oxides/metal-hydroxide catalystsCarbon nanotubeCopper oxide
The invention provides a catalyst for generating hydrogen by visible light photocatalytic reduction of water, and a preparation method thereof, wherein each 100mg of the catalysts contain 10-60mg of eosin-Y, 35-85mg of carbon nano tubes and 1-10mg of oxides. With the technical scheme of the invention, the eosin is used as a sensitizing agent, the carbon nano tubes are used as carriers and photogenerated electron channels, the oxides, such as copper oxide, are used as co-catalysts, so that hydrogen generation by visible light photocatalytic reduction of water is implemented. The catalyst has higher hydrogen generation activity under irradiation of the visible light, and can be used for generating hydrogen by visible light photocatalytic reduction of water. And the oxides substitute Pt to be used as the co-catalyst, thereby implementing non-platinum property of the catalyst.
Owner:EAST CHINA UNIV OF SCI & TECH
Cathode non-platinum catalyst of proton exchange membrane fuel cell and preparation method thereof
InactiveCN102916203AIncreased oxygen reduction activityHigh active specific surface areaCell electrodesMetal/metal-oxides/metal-hydroxide catalystsNon platinumActivity ratios
The invention relates to a cathode non-platinum catalyst of a proton exchange membrane fuel cell and a preparation method thereof. The method comprises the following steps: melamine-formaldehyde resin preparation reaction is carried out, and metal salt is added into the melamine formaldehyde resin; complexing reaction occurs between the melamine formaldehyde resin and the metal salt to form a complex; and after solvent of the complex is evaporated, the complex is decomposed by heat treatment to form the cathode non-platinum catalyst with a hollow spherical structure of the proton exchange membrane fuel cell. The non-platinum catalyst has following advantages that firstly the non-platinum catalyst has a large activity ratio surface, so that the oxygen reduction activity of a catalyst is greatly improved; secondly the catalyst has a rich nitrogen source; thirdly the catalyst has an excellent activity of oxygen reduction; and fourthly the preparation method of the non-platinum catalyst is simple, and cheap metals such as Fe, Co and the like are used as the catalyst, so that the synthesis cost is lowered.
Owner:WUHAN UNIV OF TECH
Preparation method of supported platinum-based alloy catalyst for low temperature fuel cell
InactiveCN109935847ALarge electrochemical active areaImprove electrochemical activityMaterial nanotechnologyCell electrodesHydrazine compoundUnit mass
The invention provides a preparation method of a supported platinum-based alloy catalyst. The catalyst can be used as a low temperature fuel cell catalyst. The preparation method comprises the steps of taking ethylene glycol as a solvent and stabilizer, dispersing a carrier in the solvent, and reducing platinum and non-platinum precursors by strong reducing agents such as borohydride, hydrazine hydrate and tetrabutyl borohydride to obtain supported platinum-based alloy nanoparticles. The preparation method adopted by the invention is simple, effective and free of a problem of difficult removalof the stabilizer. The prepared alloy catalyst has a small particle size and uniform particle size distribution, and has good dispersion on the carrier at the same time. In addition, the catalyst canshow high area specific activity and unit mass Pt specific activity for oxygen reduction reaction, can effectively reduce the platinum consumption and has potential application prospects in the low temperature fuel cell.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
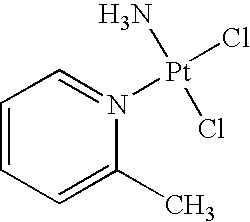
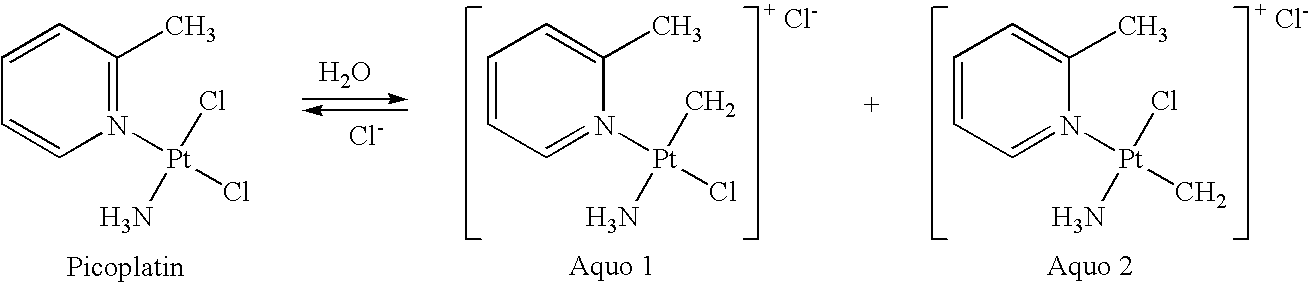
Stabilized picoplatin dosage form
Methods for stabilizing aqueous solutions of picoplatin are provided. Such stable, preferably aseptic solutions are particularly useful for preparing unit dosages of picoplatin for oral or intravenous administration, preferably in combination with at least one additional non-platinum anti-cancer agent.
Owner:PONIARD PHARMA INC
Use of polymerized phthalocyanine compound as cell cathode catalyst with direct alcohols as fuel
InactiveCN1634919AHigh catalytic activityHigh selectivityOrganic chemistryAqueous electrolyte fuel cellsFuel cellsElectrical conductor
Polymerized phthalocyanine compound with following general formulas can be used as cell cathode catalyst with direct alcohols as fuel. Polymerized phthalocyanine compound has more atoms which can form big ªð bonds, high catalytic activity and high selectivity, and large probability of 4e reduction by catalyzing oxygen for releasing oxygen completely. The compound belongs to non-platinums and alcohol-resistant catalyst.
Owner:SHANDONG UNIV OF TECH
Electrode catalyst for fuel cell, method of preparing electrode catalyst, and fuel cell using electrode catalyst
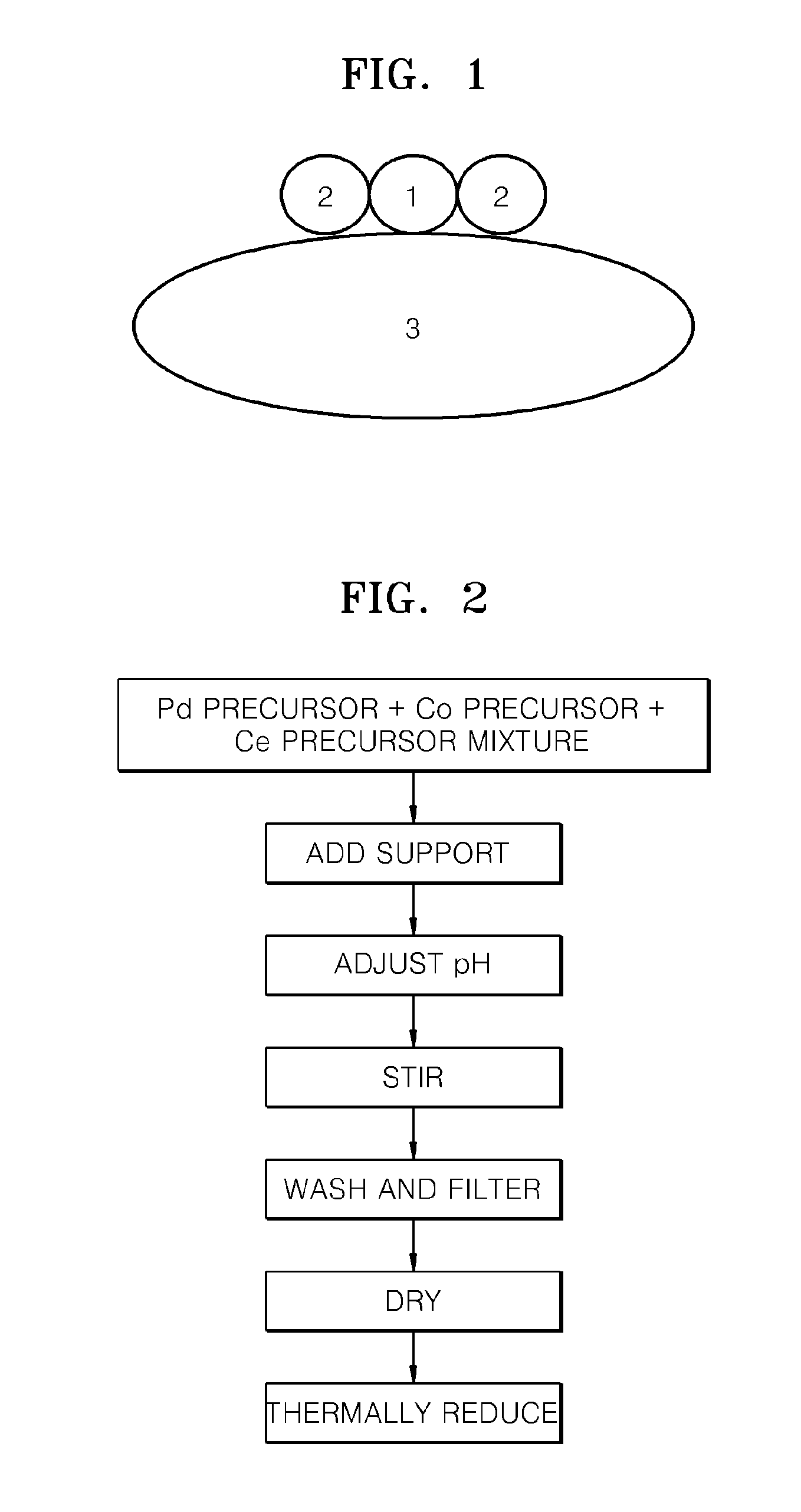
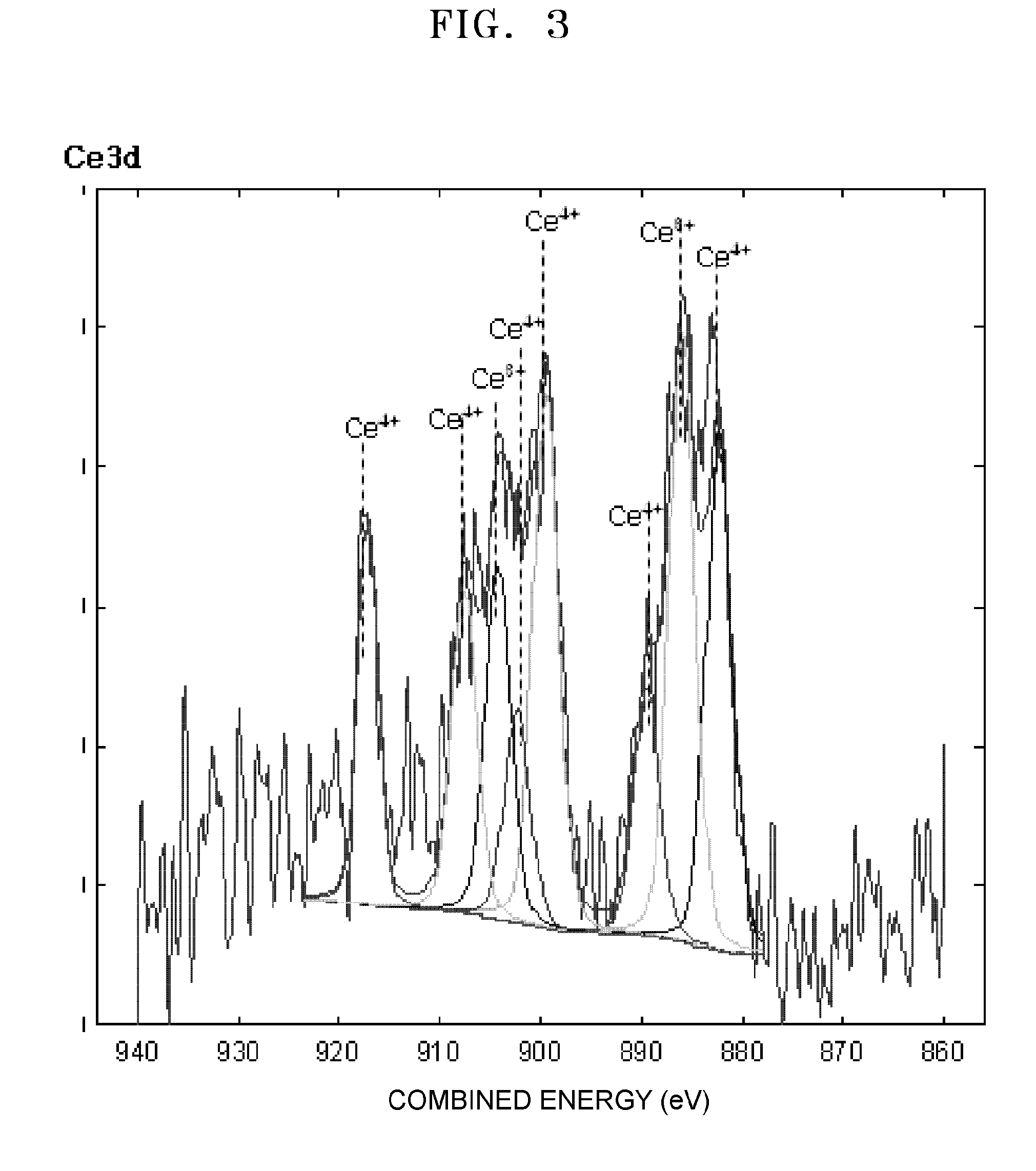
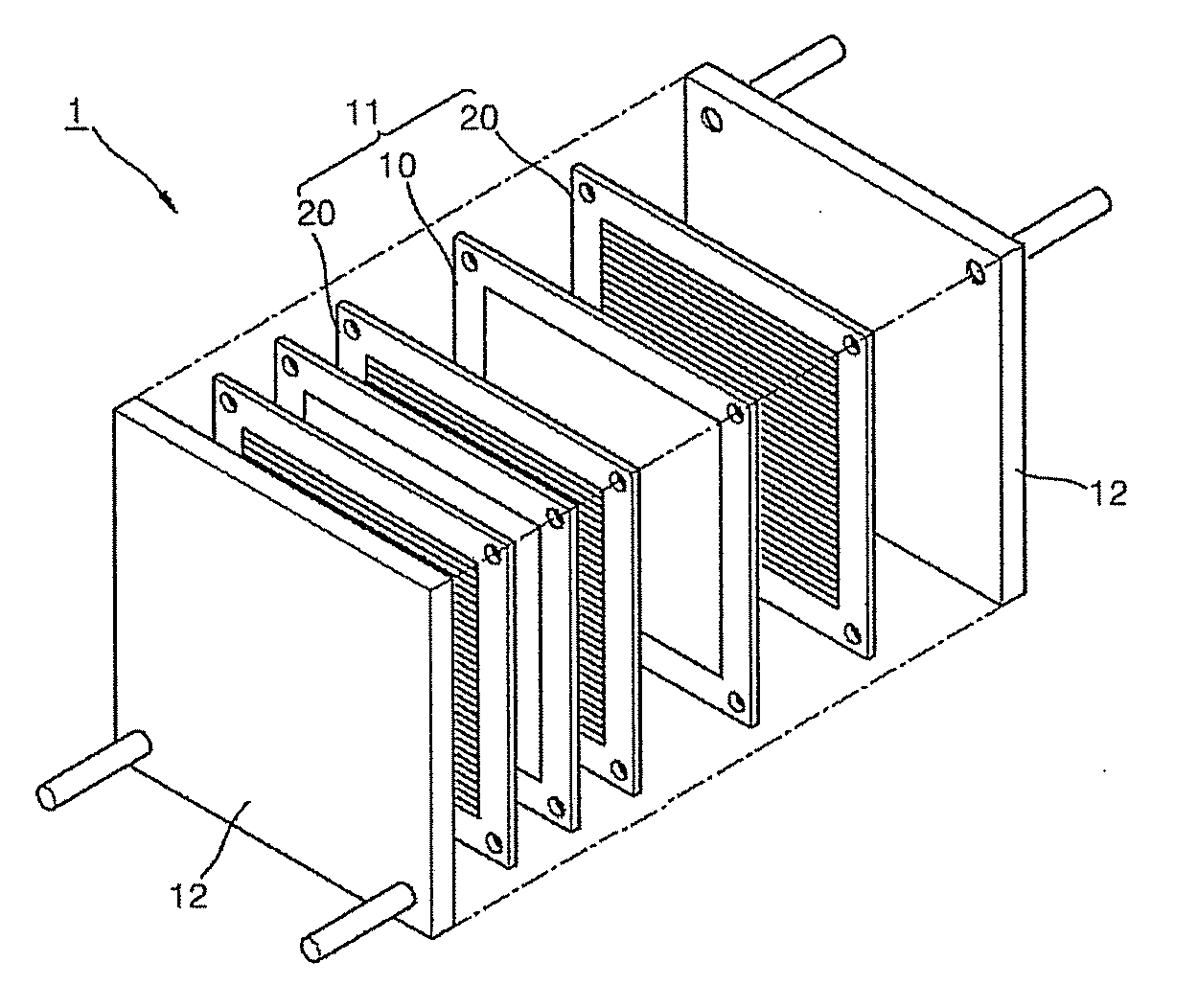
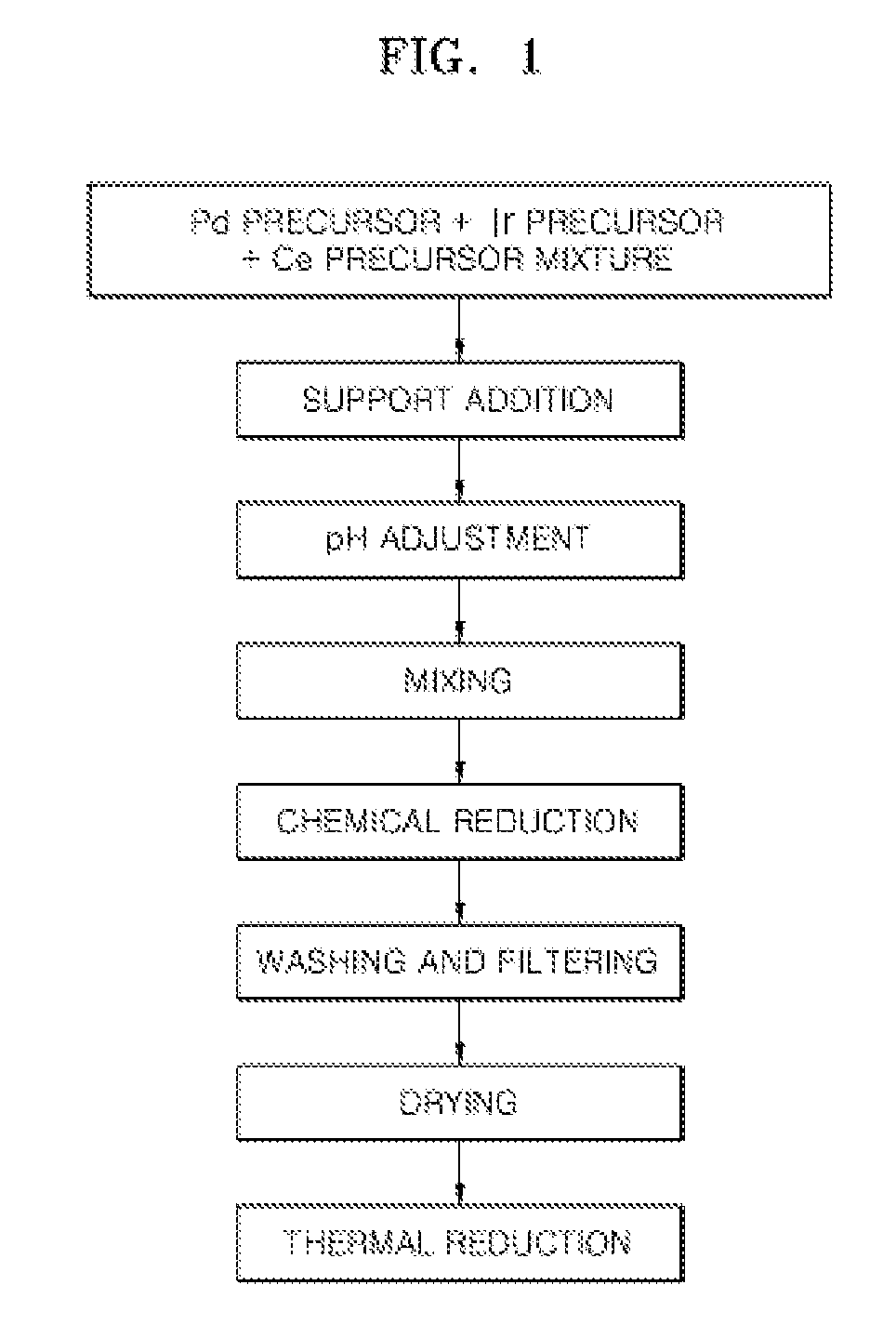
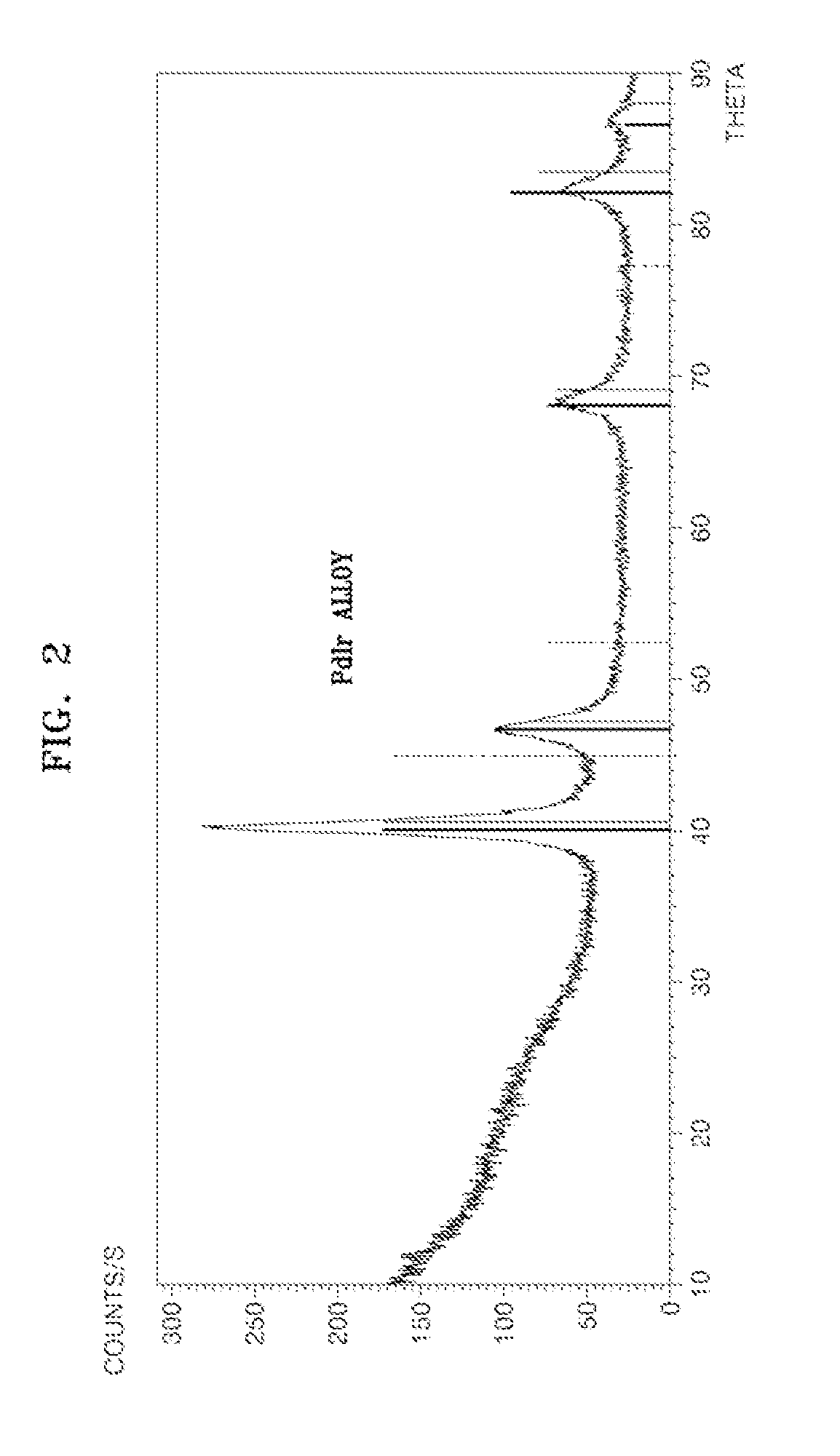
ActiveUS20110081599A1Reducing pH-adjusted mixtureFinal product manufactureActive material electrodesIridiumFuel cells
Non-platinum (Pt) electrode catalysts for fuel cells, methods of manufacturing the same, and fuel cells including the non-Pt electrode catalysts. Each of the non-Pt electrode catalysts for fuel cells includes at least palladium (Pd) and iridium (Ir), and further includes a metal, oxide of the metal, or mixture thereof for compensating for the activity of Pd and Ir.
Owner:SAMSUNG ELECTRONICS CO LTD
Graphene/iron-nickel sulfo-spinel composite catalyst, preparation method therefor, and method for preparing dye-sensitized solar cell
ActiveCN105448526AStable structureLower resistanceLight-sensitive devicesFinal product manufactureManufacturing technologyNon platinum
The invention discloses a graphene / iron-nickel sulfo-spinel composite catalyst, and the composite catalyst comprises graphene oxide serving as a substrate, and iron-nickel sulfide which is in in-situ growth on graphene and is in a spinel structure. The atom ratio of iron, nickel and sulfur in the iron-nickel sulfide is x: (3-x): 4, wherein x is greater than zero and less than three. The invention also provides a preparation method for the composite catalyst, and a method for preparing a dye-sensitized solar cell. The composite catalyst is ingenious in design, is simple in manufacturing technology, well solves problems that a non-platinum system catalyst is low in catalytic efficiency and a platinum catalyst is high in cost, and can meet the demands of the industrial production well. Therefore, the composite catalyst is wide in application range, and is very suitable for large-scale production.
Owner:MATERIAL INST OF CHINA ACADEMY OF ENG PHYSICS
Novel Non-Platinum Metal Catalyst Material
InactiveUS20160149229A1Little activityPhysical/chemical process catalystsMultiple component coatingsFuel cellsMetal catalyst
The present invention relates to a novel non-platinum metal catalyst material for use in low temperature fuel cells and electrolysers and to fuel cells and electrolysers comprising the novel non-platinum metal catalyst material. The present invention also relates to a novel method for synthesizing the novel non-platinum metal catalyst material.
Owner:DANMARKS TEKNISKE UNIV
Non-noble metal catalytic electrode, film electrode and preparation method of non-noble metal catalytic electrode
InactiveCN109346728AExcellent gasExcellent electronicsFinal product manufactureCell electrodesNon platinumNitrogen source
The invention discloses a non-noble metal catalytic electrode, a film electrode and a preparation method of the non-noble metal catalytic electrode, wherein a catalytic layer on the surface of an electrode diffusion layer of the non-noble metal catalytic electrode is formed by thermally treating a precursor mixture of a catalytic layer material at the high temperature and growing or loading a catalytic ingredient on the electrode diffusion layer, the precursor mixture of the catalytic layer material comprises a transition metal precursor, a carbon source material and a nitrogen source material, the catalytic layer is porous ordered large-specific surface area non-platinum high-nitrogen carbon based catalytic ingredient containing transition metal, the catalytic activity is mainly from a multi-component system comprising carbon, nitrogen and the transition metal, a catalytic region is porous and highly ordered, has the characteristic of high hydrophobicity and the excellent performanceof conduction of gas and electrons, the integrally direct forming preparation method directly loads or grows a catalytic activity component on the surface of the electrode diffusion surface at one time, the time is saved, the production steps of the electrode are simplified, the waste of a catalyst is avoided, and the preparation method has the advantages of simple preparation, low cost and excellent performances.
Owner:CHINA-SINGAPORE INT JOINT RES INST
Fuel-cell catalyst with non-platinum core-shell structure and preparation method of fuel-cell catalyst
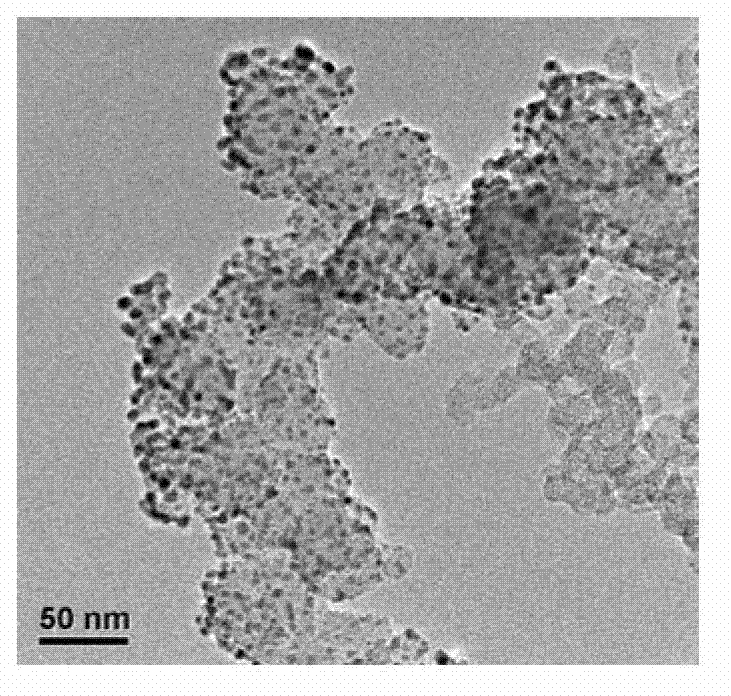
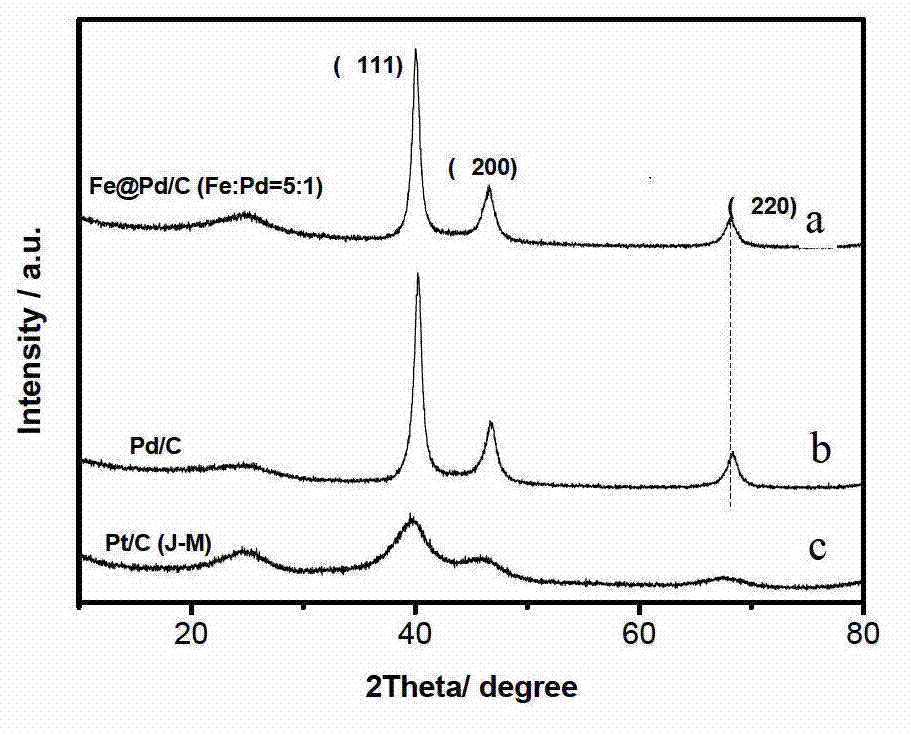
InactiveCN102903939ALow costSimple manufacturing methodCell electrodesMetal/metal-oxides/metal-hydroxide catalystsShielding gasNon platinum
The invention provides a fuel-cell catalyst with a non-platinum core-shell structure and a preparation method of the fuel-cell catalyst, and relates to a fuel-cell catalyst. A carrier of the catalyst is activated carbon, the active ingredient of the carrier is Fe-coated Pd, the molar ratio of nanocrystalline iron of the active ingredient Fe-coated Pd to palladium of the active ingredient Fe-coated Pd is 5:1, and the active ingredient Fe-coated Pd accounts for 40%-50% of the total mass of the fuel-cell catalyst with the non-platinum core-shell structure. The preparation method comprises the followings steps of: dissolving the activated carbon into water; performing ultrasonic treatment on the mixture; leading shielding gas in the mixture; adding reducing agents in the mixture; then adding a ferric salt aqueous solution in the mixture; reacting and then adding a palladium chloride aqueous solution in the mixture to perform replacement reaction; and filtering, washing and drying the mixture after the replacement reaction so as to obtain a product. The electrocatalytic activity of the catalyst is 10.63 times that of the traditional carbon load palladium nanocrystal Pd / C catalyst, and is 23.05 times that of a commercial Pt / C catalyst. The cost is low, the preparation method is simple and practicable, the particle size of the catalyst is easy to control, and the fuel-cell catalyst is suitable for large-scale industrial production.
Owner:XIAMEN UNIV
Non-noble metal catalyst for fuel cells, and its application
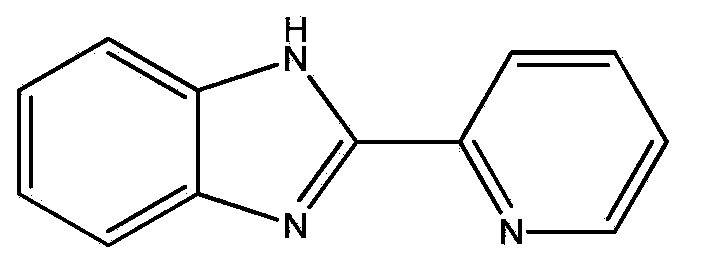
ActiveCN104138759ALow priceOxygen reduction activityCell electrodesMetal/metal-oxides/metal-hydroxide catalystsNon platinumOxygen
The invention relates to a fuel cell catalyst, and concretely relates to an application of a novel non-noble metal catalyst in fuel cells. The catalyst comprises resorcinol, formaldehyde, a metal salt and 2-(2-pyridyl)-benzimidazole (C12H4N3). A molar ratio of resorcinol to formaldehyde is 1:5-5:1, and a molar ratio of the metal salt (by metal) to 2-(2-pyridyl)benzimidazole is 1:10-10:1. A highly active non-platinum catalyst is prepared through the steps of carbonizing an organogel in 500-1200DEG C inert gas environment to prepare a carbon gel, dipping a metal organic complex (prepared through coordinating the metal salt with 2-(2-pyridyl)benzimidazole), and pyrolyzing in 500-1200DEG C nitridation atmosphere environment. The non-noble metal catalyst has good redox activity and electrochemical stability in a flue cell cathode catalyst as a nonmetal catalyst.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Metal oxynitride electrode catalyst
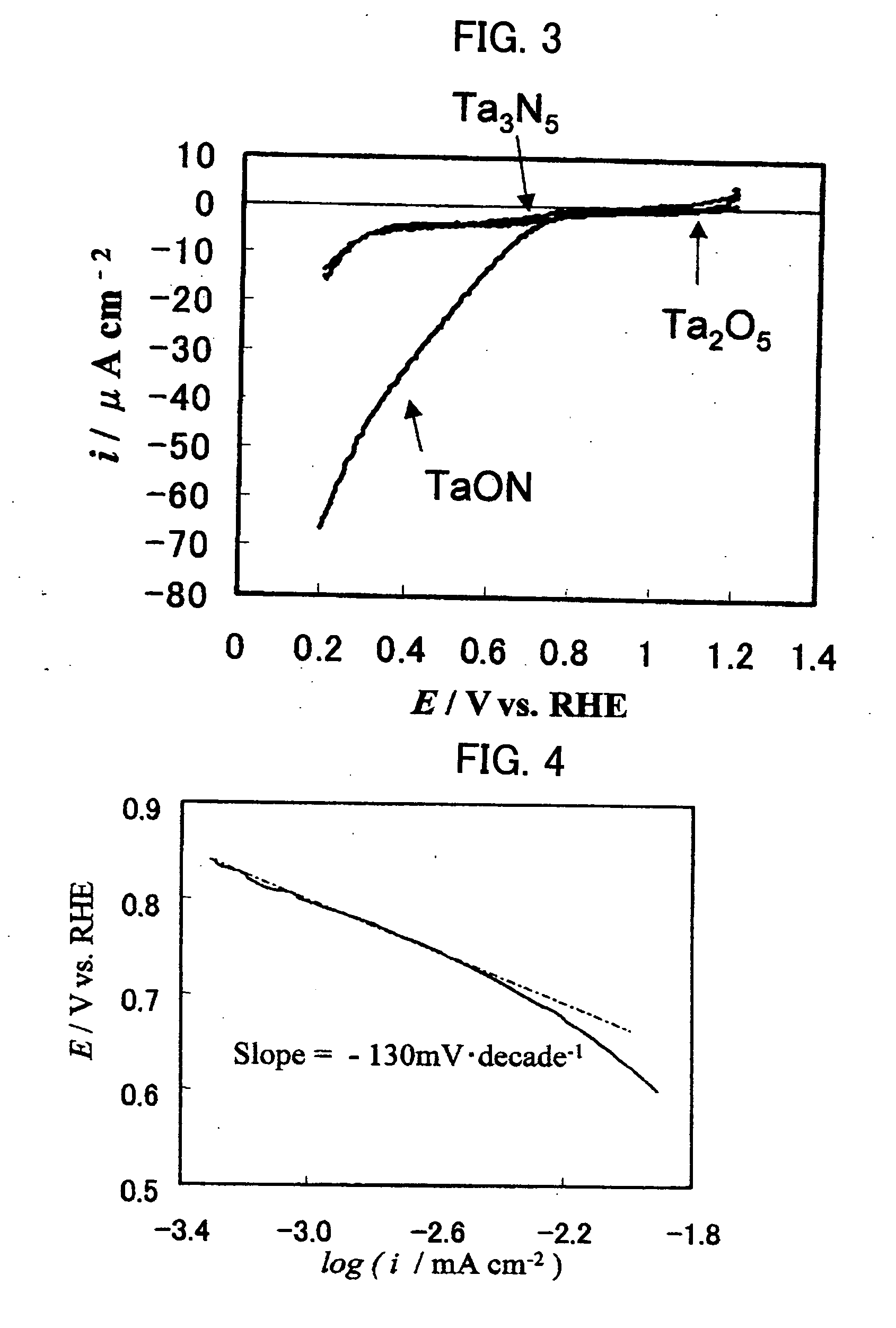
InactiveUS20070128884A1Cell electrodesSemiconductor/solid-state device manufacturingElectrode potentialElectrolysis
[Problems] Carbides and many other non-platinum-based compounds are activated and dissolved and cannot be stably present in an acidic electrolyte under conditions of an electrode potential as high as 0.4 V or above, and thus, the application range of these compounds as an electrode catalyst is limited to low electrode potentials. There has been need for development of an electrode catalyst that maintains catalytic activity under these conditions and exhibits improved stability. [Means for Solving Problems] To provide a metal oxynitride electrode catalyst composed of an oxynitride containing at least one transition metal element selected from the group consisting of La, Ta, Nb, Ti, and Zr, the metal oxynitride electrode catalyst being used at a potential of 0.4 V or higher relative to the reversible hydrogen electrode potential in an acidic electrolyte. The metal oxynitride electrode catalyst is useful as an electrode catalyst for electrochemical systems used in acidic electrolytes in the fields of water electrolysis, organic electrolysis, fuel cells, etc.
Owner:JAPAN SCI & TECH CORP
Fuel cell membrane electrode employing ruthenium telluride as cathode and preparation method
InactiveCN108448128ALow costImprove performanceMaterial nanotechnologyCell electrodesNano catalystTellurium compounds
The invention provides a fuel cell membrane electrode employing ruthenium telluride as a cathode and a preparation method. The membrane electrode comprises a proton exchange membrane, a cathode catalyst layer and an anode catalyst layer, wherein a carrier load of the cathode catalyst layer is a ruthenium-tellurium compound catalyst formed by ruthenium and tellurium or a ruthenium-based alloy nano-catalyst. The preparation method of the fuel cell membrane electrode comprises the steps of preparing paste for an anode, preparing the anode catalyst layer, preparing paste for a cathode and preparing the cathode catalyst layer, thereby finally completing preparation of the whole membrane electrode. The fuel cell membrane electrode has the beneficial effects that the fuel cell membrane electrodeis prepared by adopting the ruthenium telluride as the cathode catalyst layer instead of a traditional platinum-based catalyst layer, so that the preparation process is simple and the obtained membrane electrode is stable in performance and high in yield; and a non-platinum catalyst is utilized as the catalyst layer by the cathode, so that the cost of the fuel cell membrane electrode is greatly reduced, the poisoning resistance and the methanol oxidation resistance of the cathode are obviously improved and the fuel cell membrane electrode has a wide application prospect in a methanol fuel celland a small portable hydrogen fuel cell.
Owner:福建水利电力职业技术学院
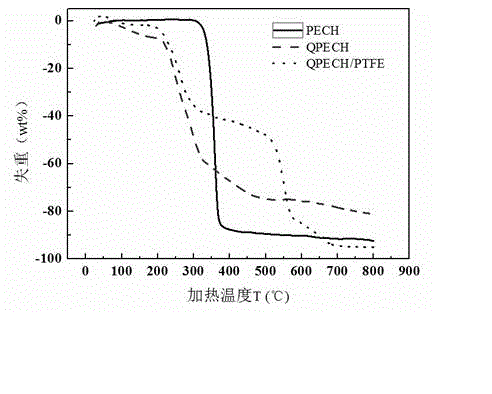
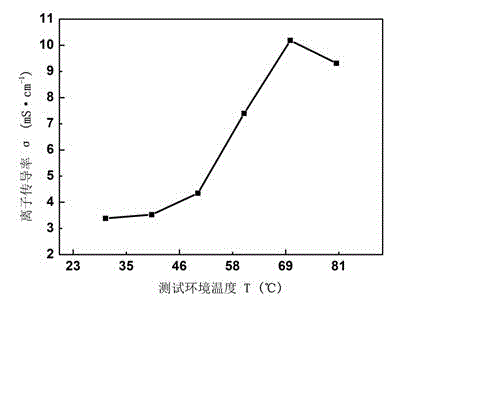
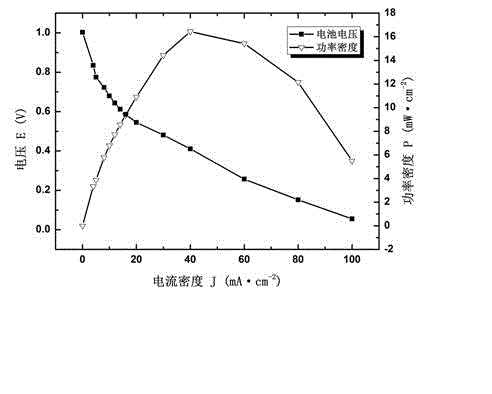
Composite polyepoxy chloropropane alkaline polymer membrane electrode and preparation method thereof
InactiveCN102945968AEasy to prepareLittle toxic pollutionCell electrodesHydrophobic polymerSynthesis methods
The invention discloses a composite polyepoxy chloropropane alkaline polymer membrane electrode and a preparation method thereof. The membrane electrode comprises an alkaline anion-exchange membrane and an electrode layer, wherein the alkaline anion-exchange membrane is prepared by the following steps: carrying out nucleophilic substitution reaction on halogen atom and tertiary amine to obtain quaternized polyepoxy chloropropane, and compounding with a porous polytetrafluoroethylene membrane; the electrode layer comprises a hydrogen electrode and an oxygen electrode, the hydrogen electrode comprises a gas diffusion layer and a hydrogen electrode catalytic layer sprayed on the gas diffusion layer, and the oxygen electrode comprises a gas diffusion layer and an oxygen electrode catalytic layer sprayed on the gas diffusion layer; the membrane electrode is integrally formed according to the combination sequence of the hydrogen electrode, the alkaline anion-exchange membrane and the oxygen electrode; and the gas diffusion layer is formed by adding hydrophobic polymer to the matrix carbon material. The alkaline membrane electrode disclosed by the invention has the advantages of simple synthesis method of the alkaline anion-exchange membrane, and high ionic conductivity, can adopt a low-cost non-platinum-based catalyst, and is suitable for alkaline anion-exchange membrane fuel batteries.
Owner:SHANGHAI INST OF SPACE POWER SOURCES
Non-platinum oxygen reduction catalysts for polymer electrolyte membrane fuel cell and method for preparing the same
InactiveUS20120028790A1Good oxygen reduction activityIncreased durabilityMaterial nanotechnologyCell electrodesPolymer electrolytesFuel cells
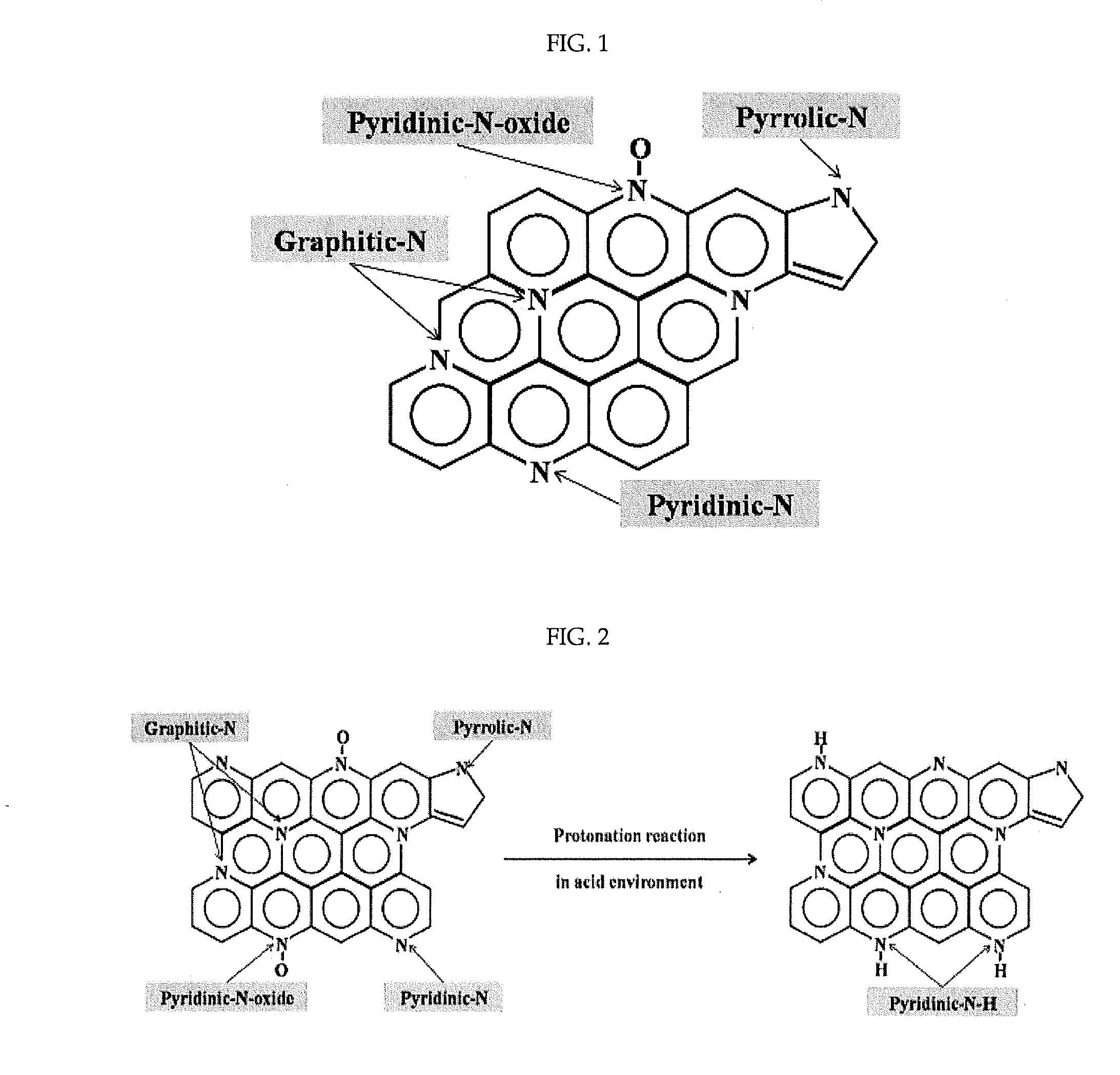
Provided are a non-platinum oxygen reduction catalyst for a polymer electrolyte membrane fuel cell and a method for preparing the same. More particularly, an oxygen reduction catalyst in which chelated cobalt is impregnated on a conductive polymer coated on a carbon support and a method for preparing the oxygen reduction catalyst by coating a conductive polymer on a carbon support, impregnating chelated cobalt on the conductive polymer and then treating the same with heat and an acid are disclosed. The platinum-free oxygen reduction catalyst of the present invention has superior oxygen reduction activity and durability with high proportion of pyridinic and graphitic nitrogens on the catalyst surface, and thus can be usefully applied for a polymer electrolyte membrane fuel cell.
Owner:HYUNDAI MOTOR CO LTD +1
Catalyst for cathode of fuel cell, preparing method and fixing method thereof, and fuel cell including same
InactiveUS20080102350A1Increased durabilityIncrease concentrationFinal product manufactureActive material electrodesHigh concentrationFuel cells
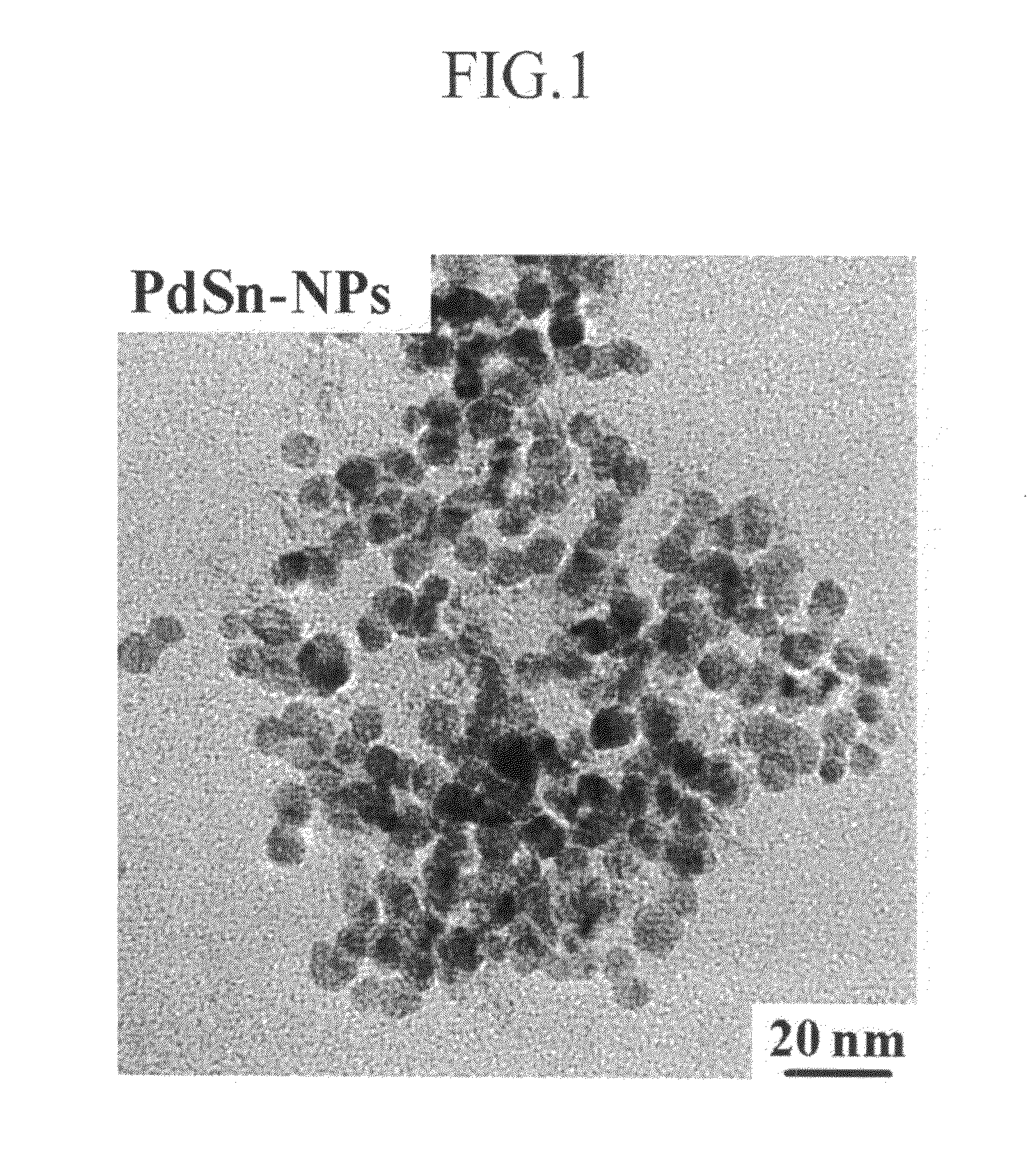
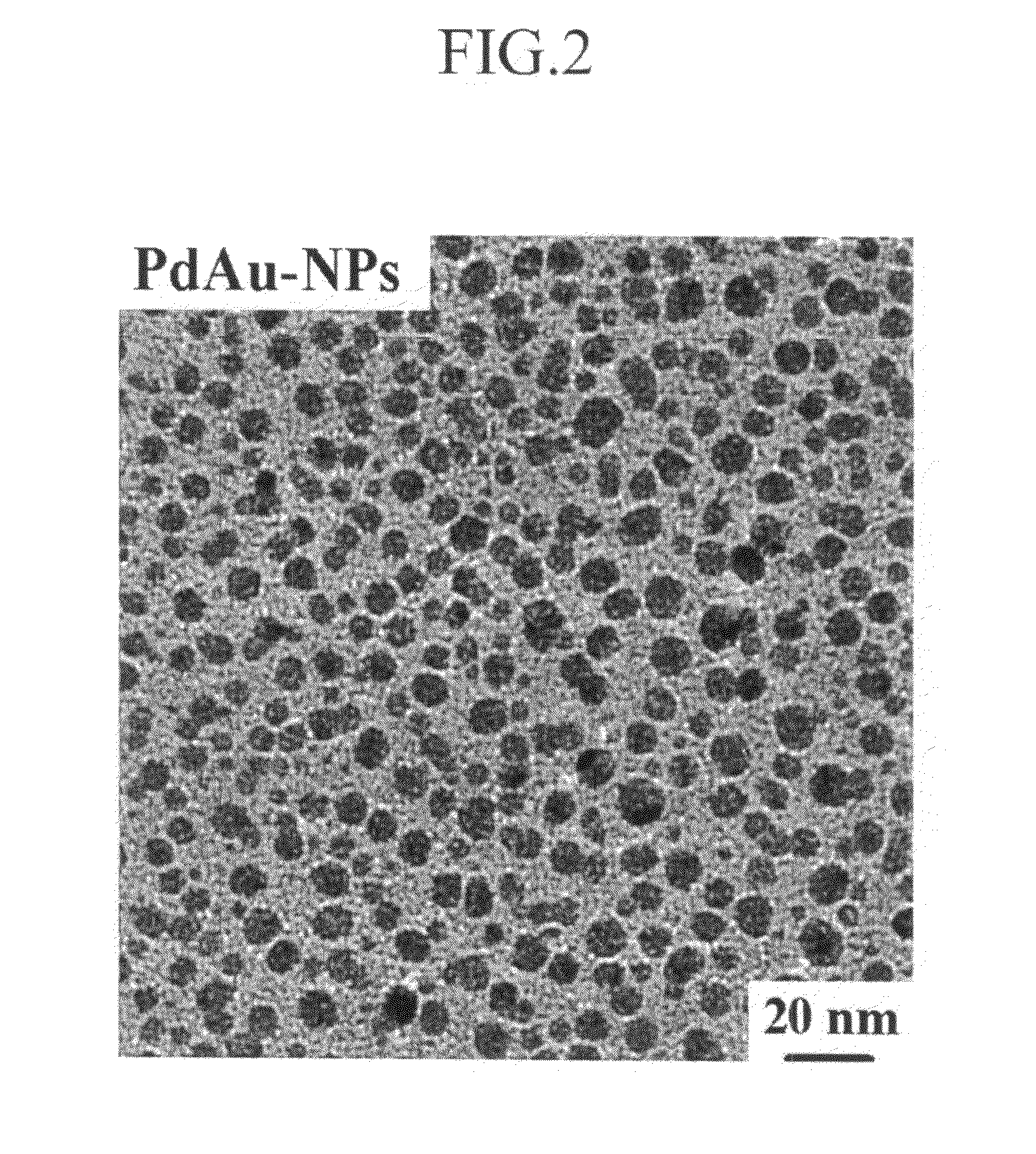
A cathode catalyst for a fuel cell is inexpensive and has high durability against methanol. A method of manufacturing and fixing the cathode catalyst, and a fuel cell including it, are disclosed. The cathode catalyst includes a compound selected from the group consisting of PdSn, PdAu, PdCo, PdWO3, and mixtures thereof. The present invention can provide a non-platinum-based cathode catalyst as a substitute for a platinum catalyst, the cathode catalyst having a low cost and improved catalyst activity, thereby contributing to popular use of a fuel cell. In addition, since the cathode catalyst of the present invention has high durability against methanol and can thereby be used with a fuel in a high concentration, it can increase the energy density of a direct methanol fuel cell (DMFC).
Owner:WASEDA UNIV +1
Non-platinum bimetallic polymer electrolyte fuel cell catalysts
ActiveUS8129306B2Low costPromote formationCell electrodesCatalyst activation/preparationNon platinumAlloy
A polymetallic nanoparticle alloy having enhanced catalytic properties including at least one noble metal and at least one base metal, where the noble metal is preferentially dispersed near the surface of the nanoparticle and the base metal modifies the electronic properties of the surface disposed noble metal. The polymetallic nanoparticles having application as a catalyst when dispersed on a carbon substrate and in particular applications in a fuel cell. In various embodiments a bimetallic noble metal-base metal nanoparticle alloy may be used as an electrocatalyst offering enhanced ORR activity compared to the monometallic electrocatalyst of noble metal.
Owner:UCHICAGO ARGONNE LLC
Treatment method for optically transmissive body
InactiveUS20090000700A1Good optical performanceVacuum evaporation coatingSputtering coatingZinc selenideGibbs free energy
A method of treating a transmissive body of zinc sulfide or zinc selenide includes placing a non-platinum metal layer, such as a layer of cobalt, silver, or iron on a surface of the transmissive body, and improving the optical properties of the transmissive body by subjecting the body and the layer to an elevated temperature and elevated pressure. The zinc sulfide or zinc selenide may be chemical vapor deposited material. The non-platinum metal of the layer may be such that a Gibbs free energy of formation of a most stable sulfide (or selenide) of the non-platinum metal is more negative than a Gibbs free energy of formation of a most stable zinc sulfide (or zinc selenide) configuration that is thermodynamically capable of reacting with the non-platinum metal. With this condition the non-platinum metal preferentially chemically bonds with free sulfur (or free selenium) in preference to zinc sulfide (or zinc selenium).
Owner:RAYTHEON CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com