Carbon-supported CoN fuel-cell catalyst as well as preparation method and application thereof
A fuel cell and catalyst technology, applied in chemical instruments and methods, physical/chemical process catalysts, battery electrodes, etc., can solve the problems of complex operation, non-uniform particle dispersion of metal elements, gaps in activity and stability, etc. The preparation method is simple, the application prospect is good, and the effect of improving the catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
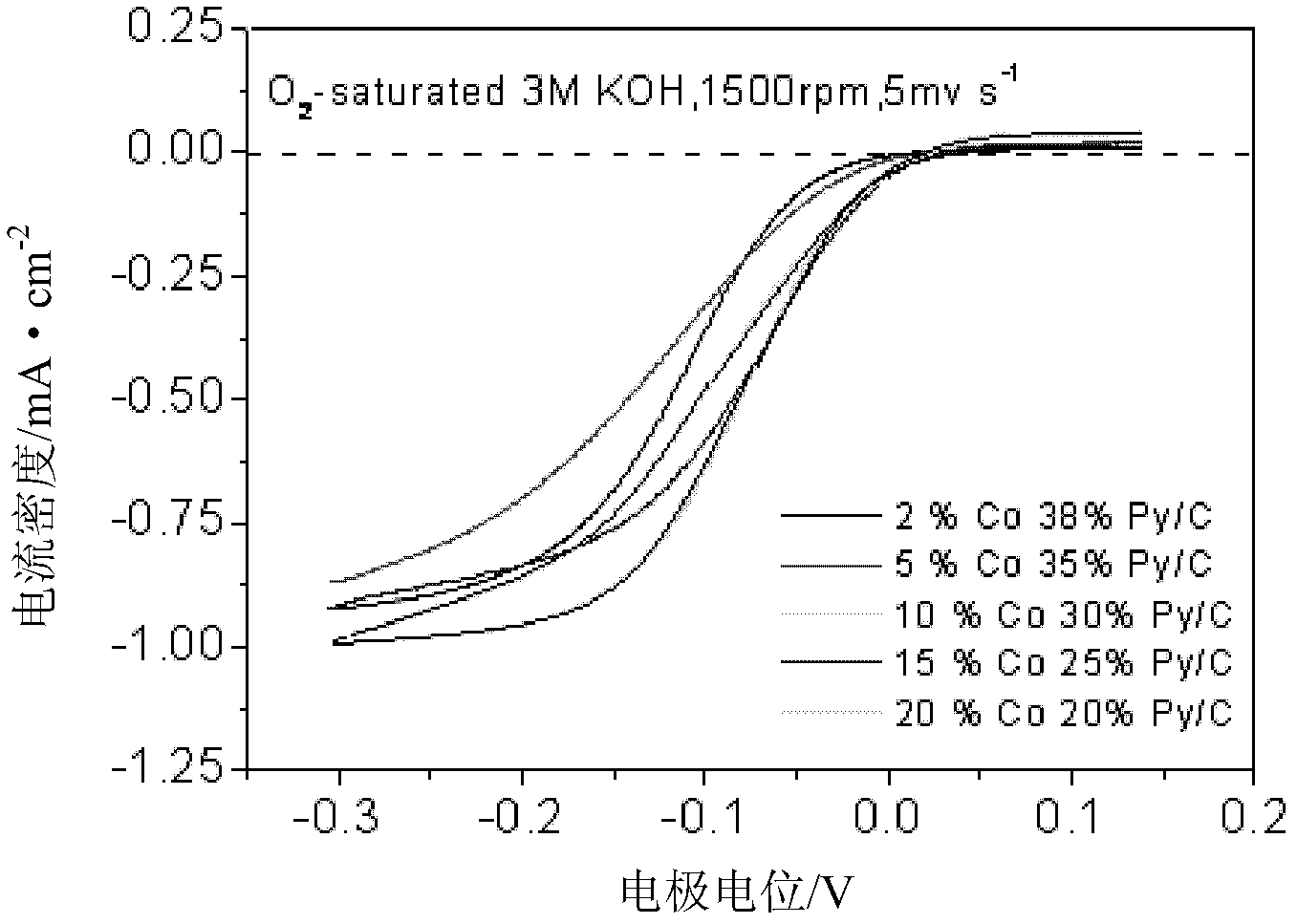
[0028] Preparation of 10%Co30N% / activated carbon catalyst: weigh 0.0477g CoSO 4 ·7H 2 O, 0.0300g pyridine and 0.0600g Vulcan XC-72R carbon powder were placed in an agate mortar. Add 10ml of methanol of analytical grade and grind thoroughly until the methanol is completely volatilized. Put the agate mortar and the mixture into a vacuum oven at 40°C for 1 h in vacuum. Afterwards, the dried mixture was placed in a quartz boat under N 2 Under the protection of the atmosphere, the calcination and reduction treatment was carried out for 2 hours at a heating rate of 20 °C / min to 800 °C to obtain the required CoN / C catalyst.
Embodiment 2
[0030] Preparation of 2%Co38%N / graphene catalyst: Weigh 0.0095g of cobalt nitrate, 0.0380g of triethylamine, 0.009g of ammonium vanadate and 0.0600g of graphene, and place them in an agate mortar. Add 8ml of analytical grade ethanol, and grind thoroughly until the ethanol evaporates completely. Subsequently, the agate mortar and the mixture were placed in a vacuum oven at 40° C. for 1 h in vacuum. Afterwards, the dried mixture was placed in a quartz boat under N 2 Calcination treatment at 200° C. for 2 h to obtain the desired CoN / C catalyst.
Embodiment 3
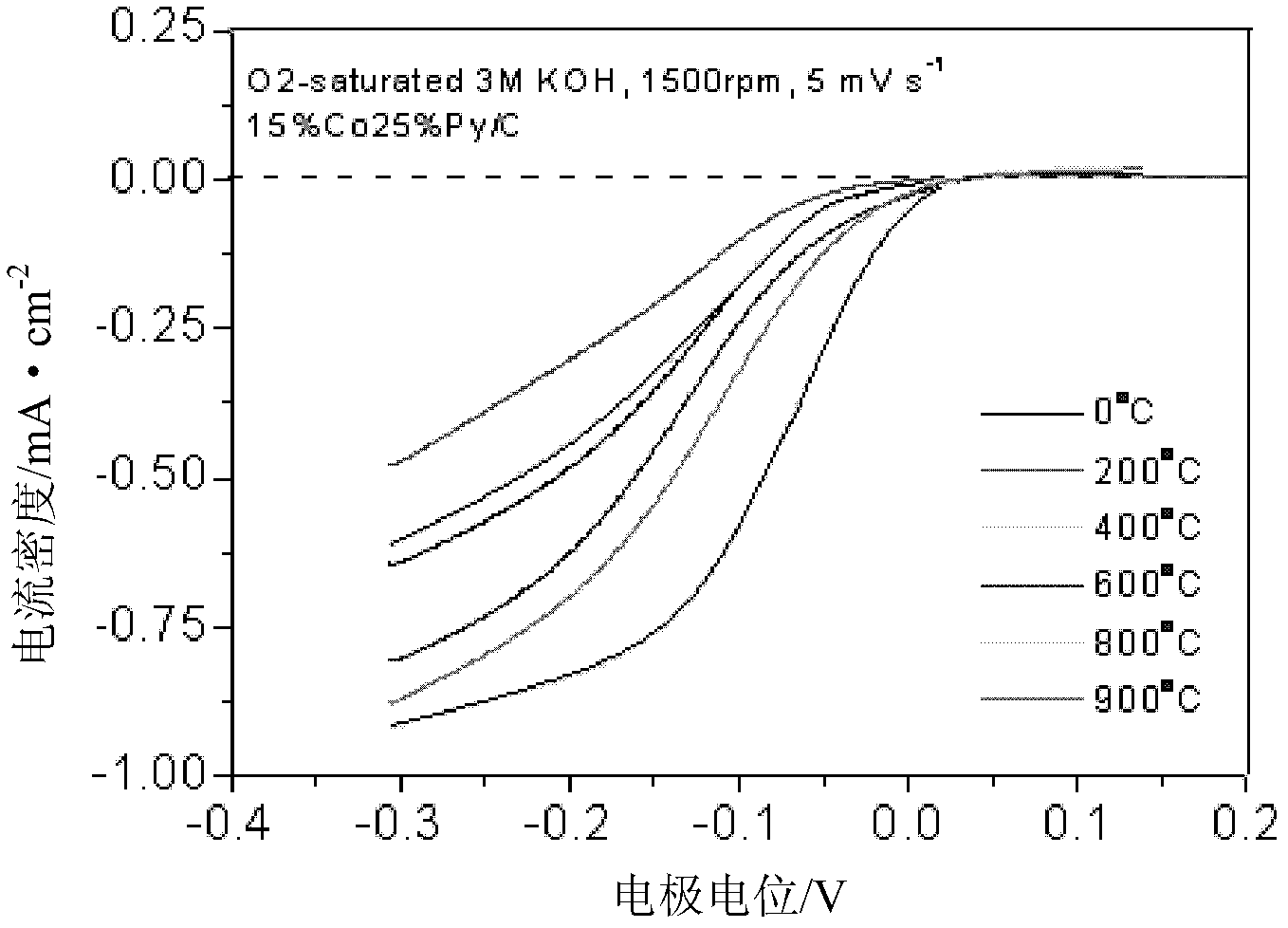
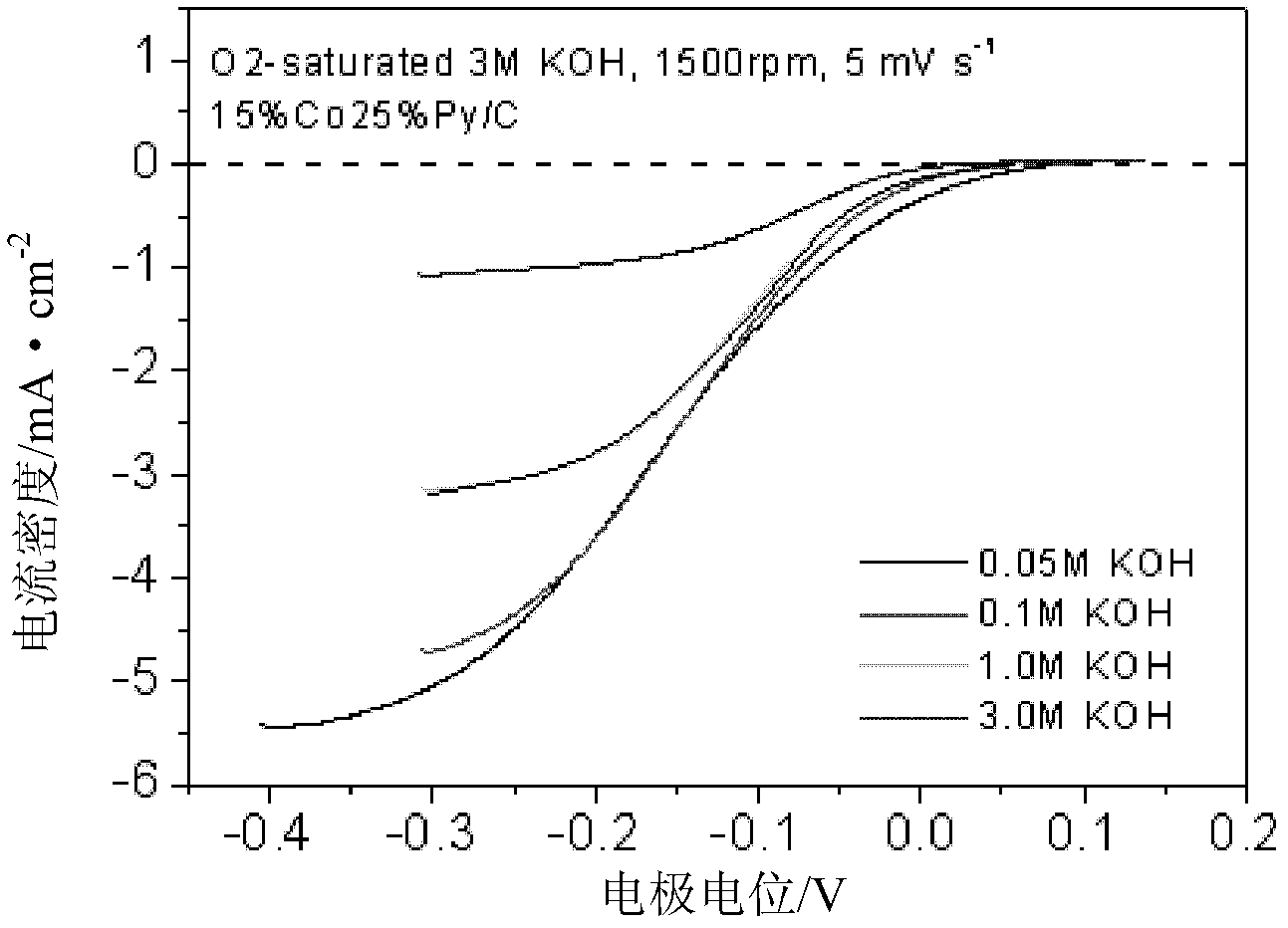
[0032] Preparation of 15%Co25%N / carbon nanotube catalyst: Weigh 0.0716g ethyl acetate cobalt, 0.0250g N, N-dimethylacetamide, 0.007g sodium tungstate and 0.0600g nano-carbon fiber and place them in an agate mortar . Add 8ml of water and grind until the water evaporates completely. Put the agate mortar and the mixture into a vacuum oven at 40°C for 1 h in vacuum. Afterwards, the dried mixture was placed in a quartz boat, and under the protection of an argon atmosphere, the temperature was raised to 900 °C at a rate of 20 °C / min for 2 h to obtain the desired CoN / C catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com