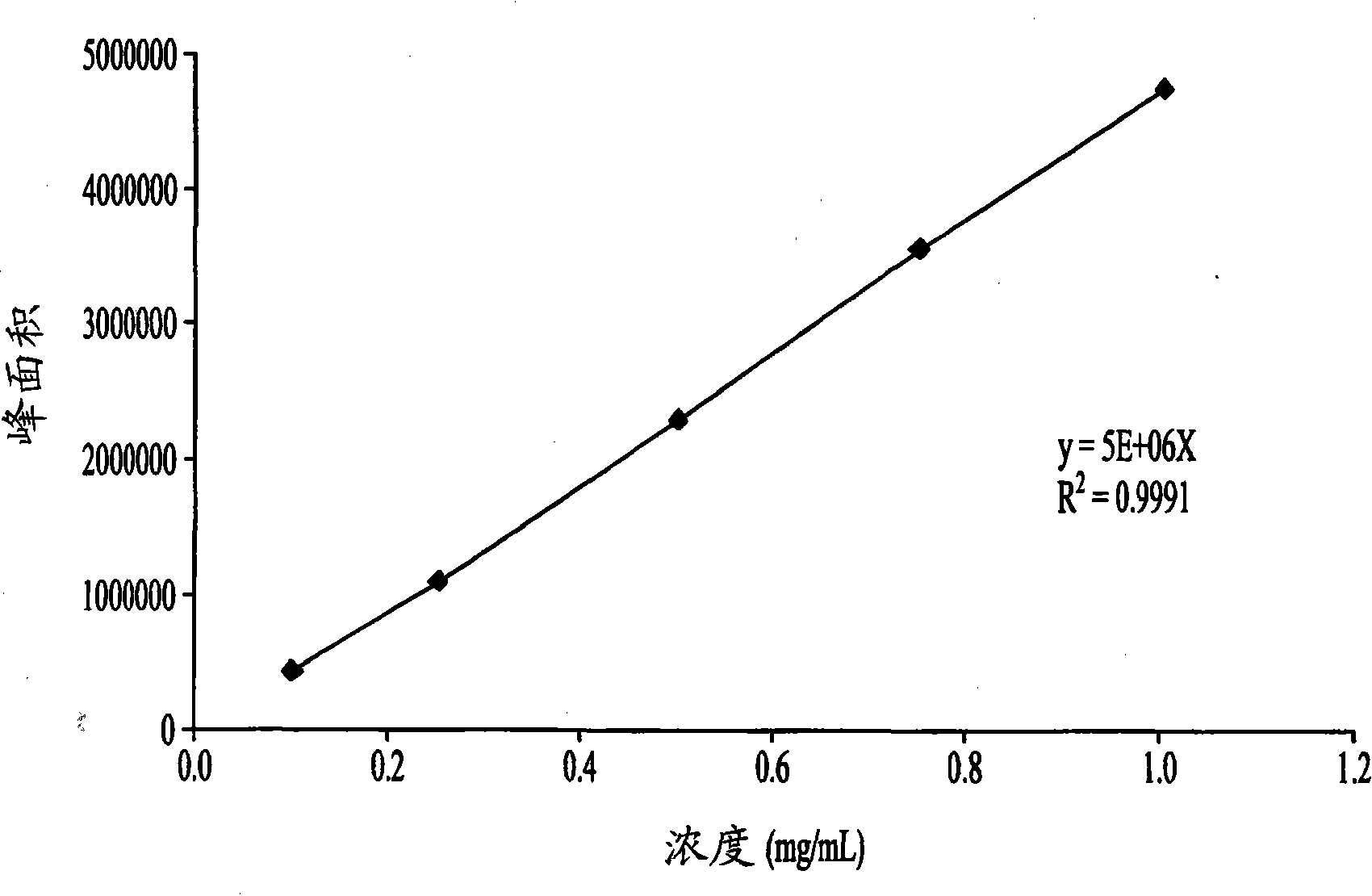
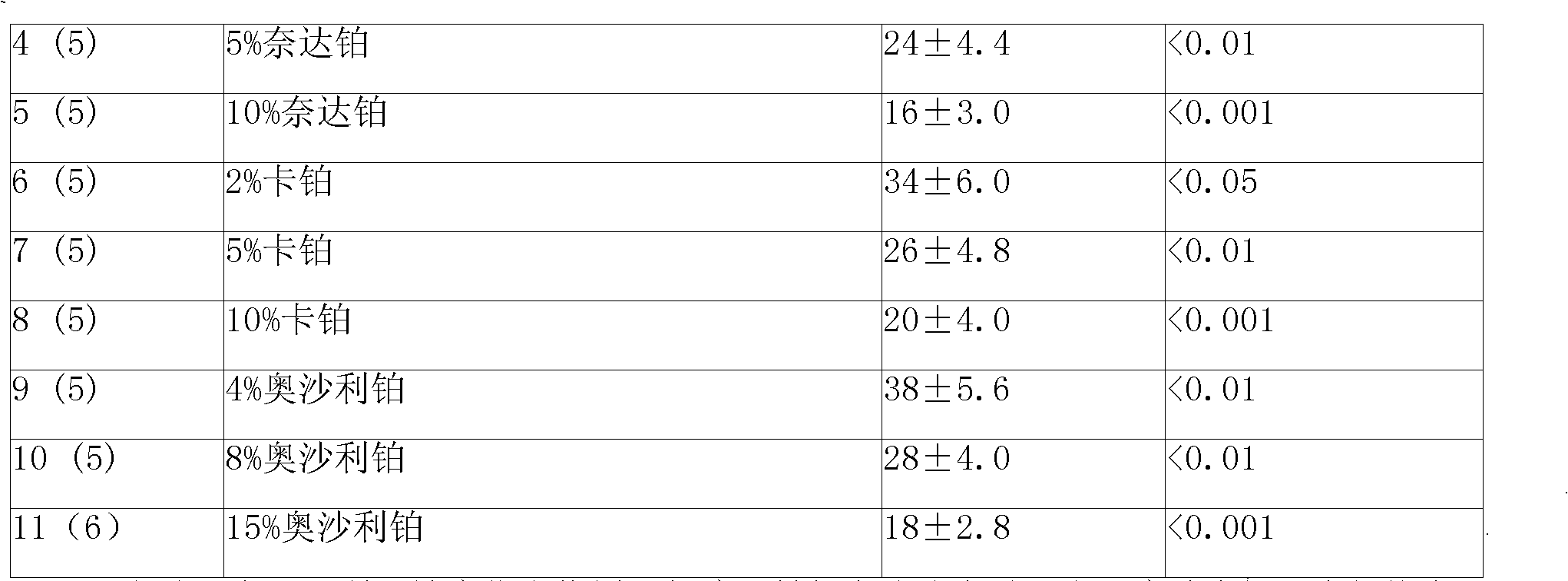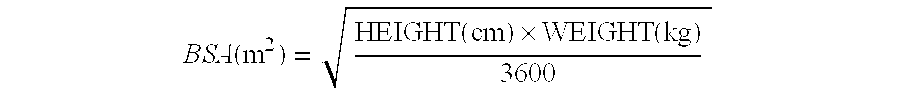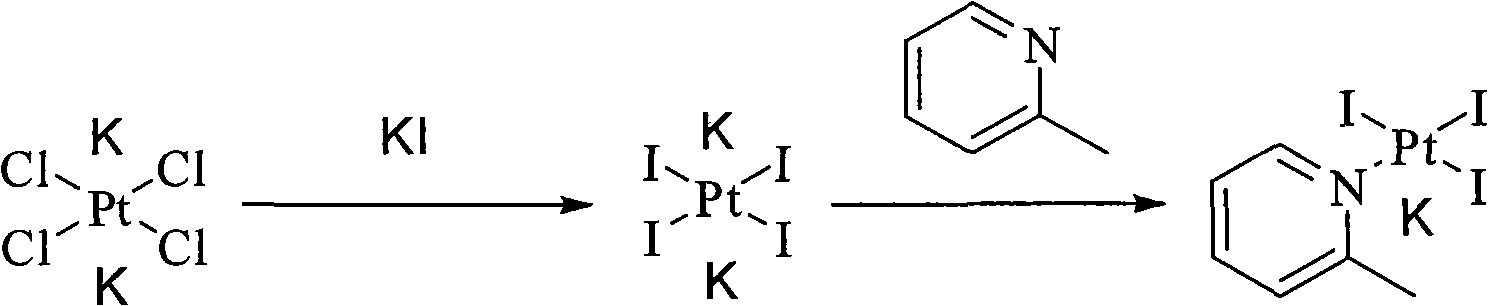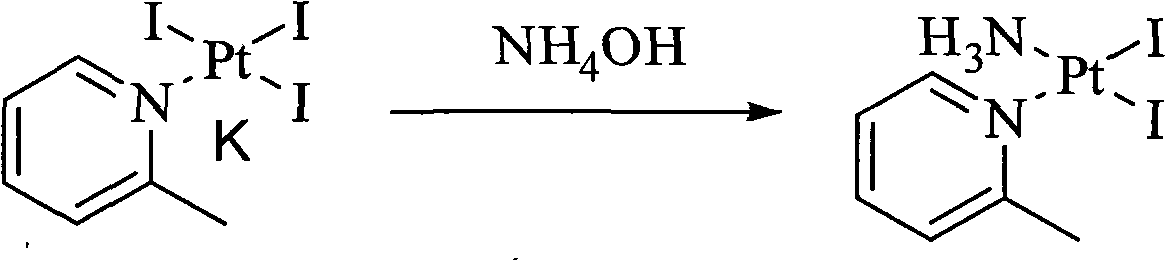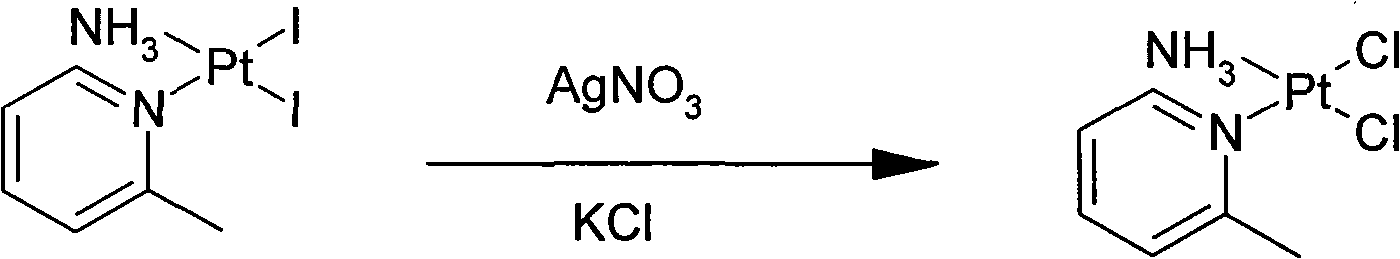Patents
Literature
44 results about "Picoplatin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
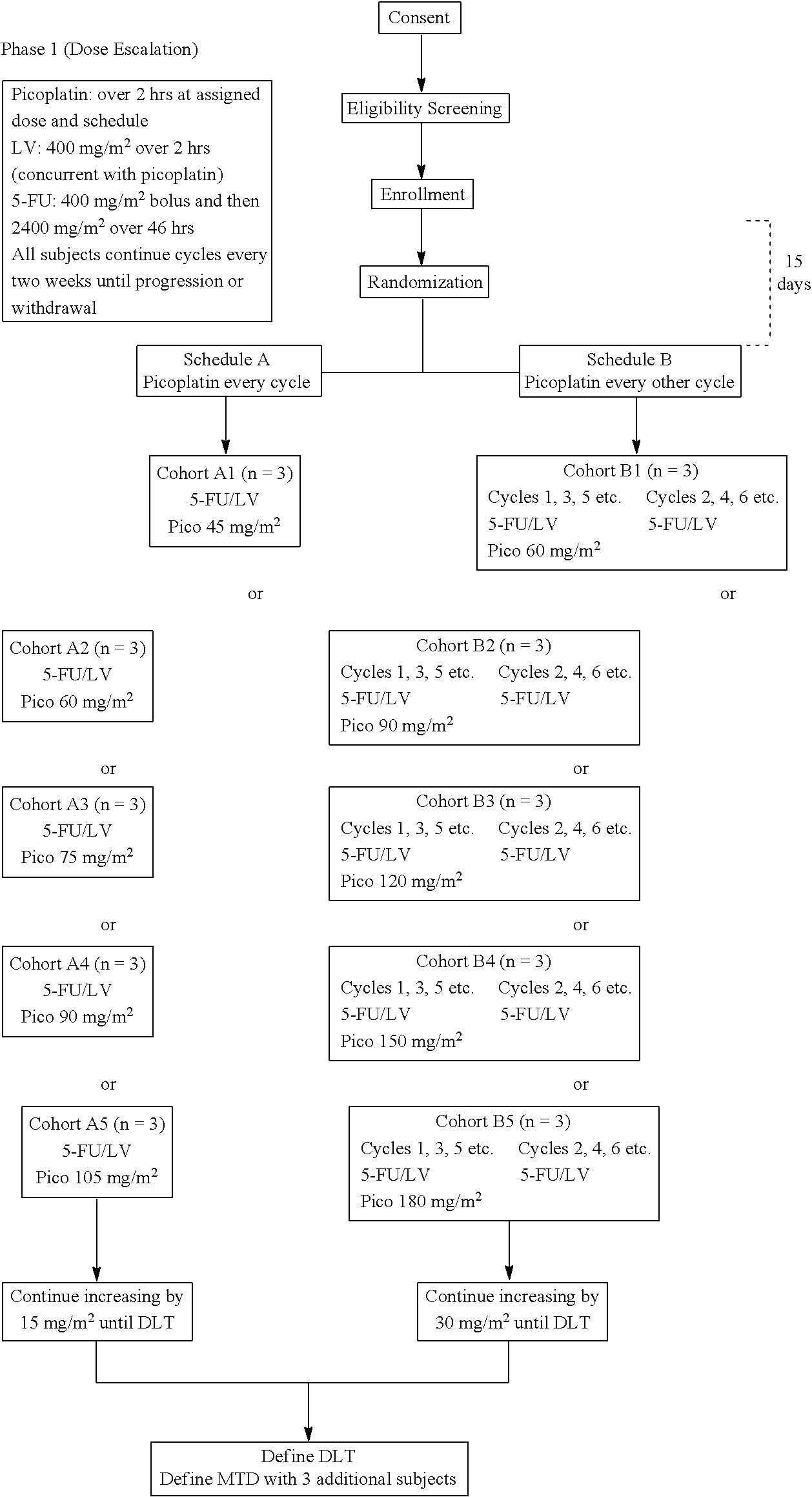
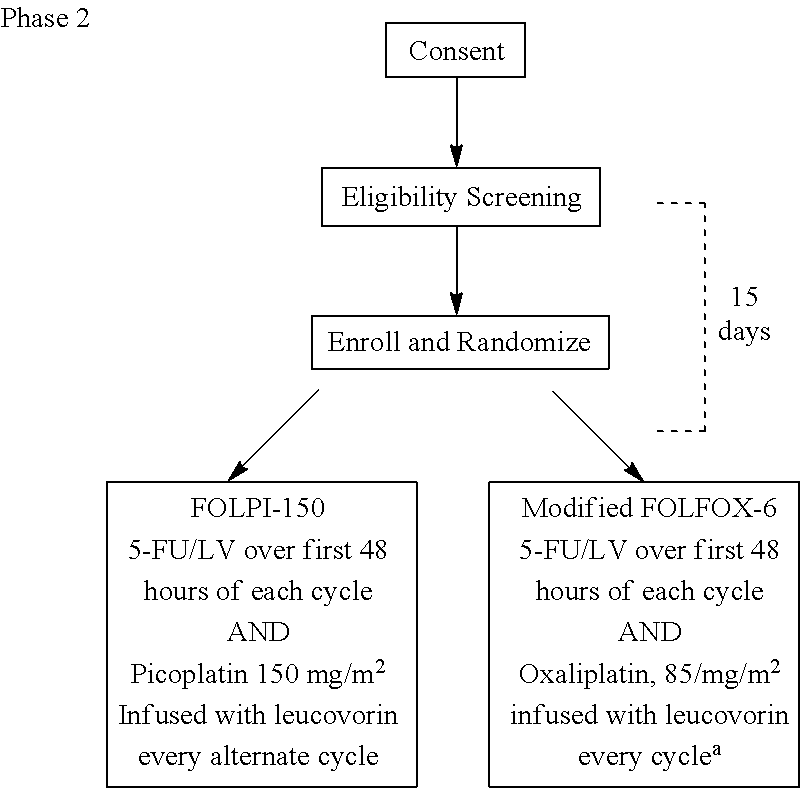
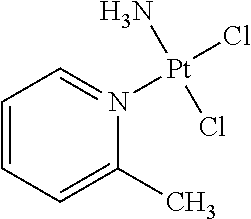
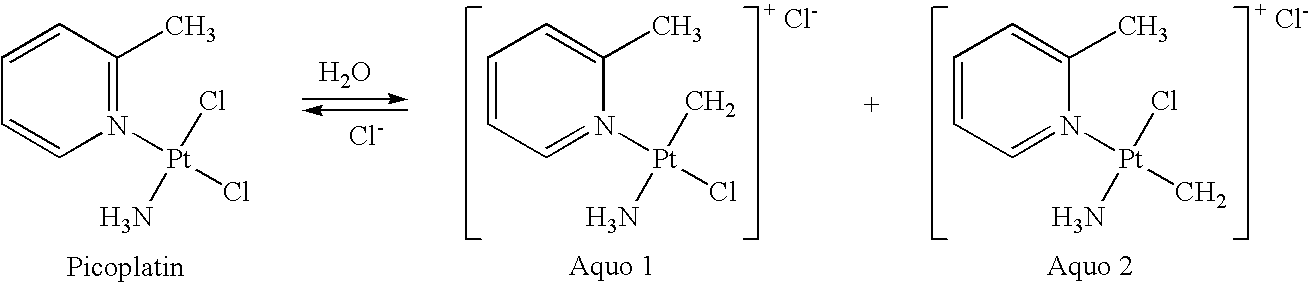
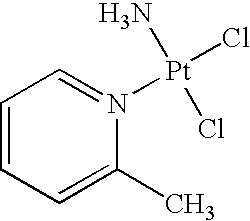
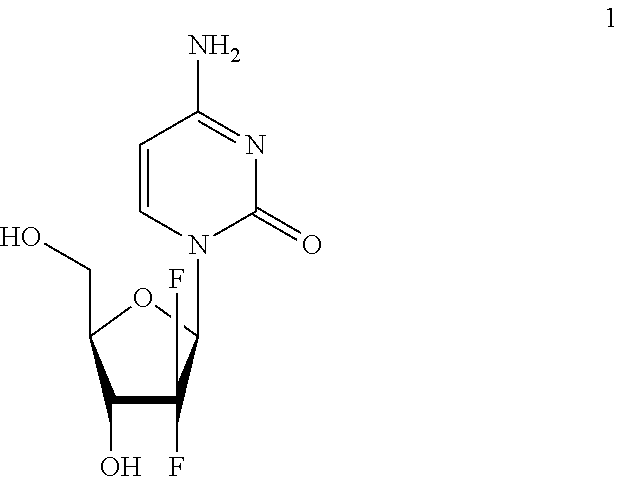
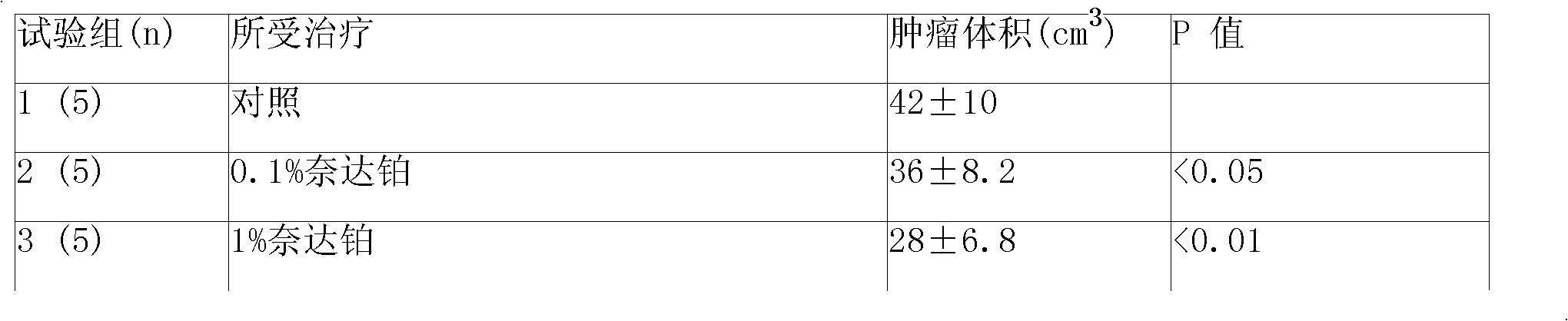
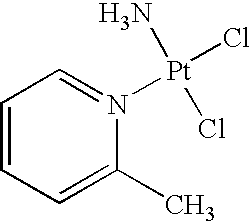
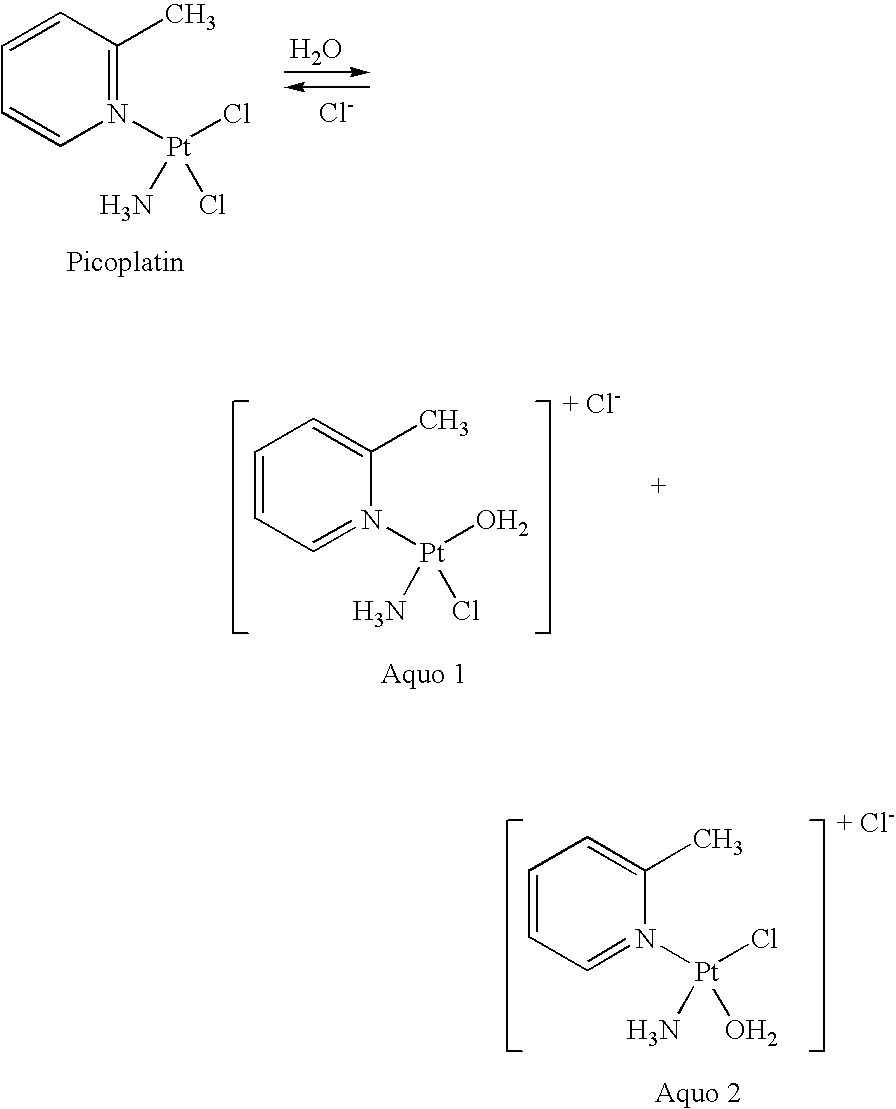
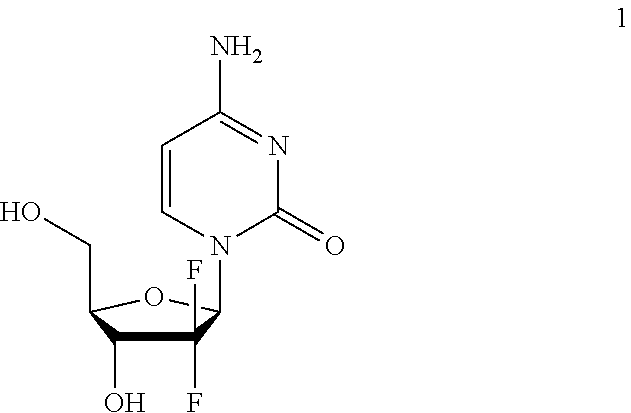
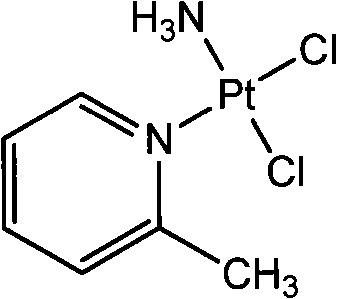
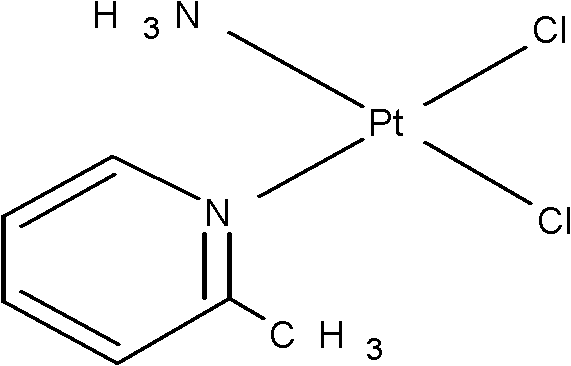
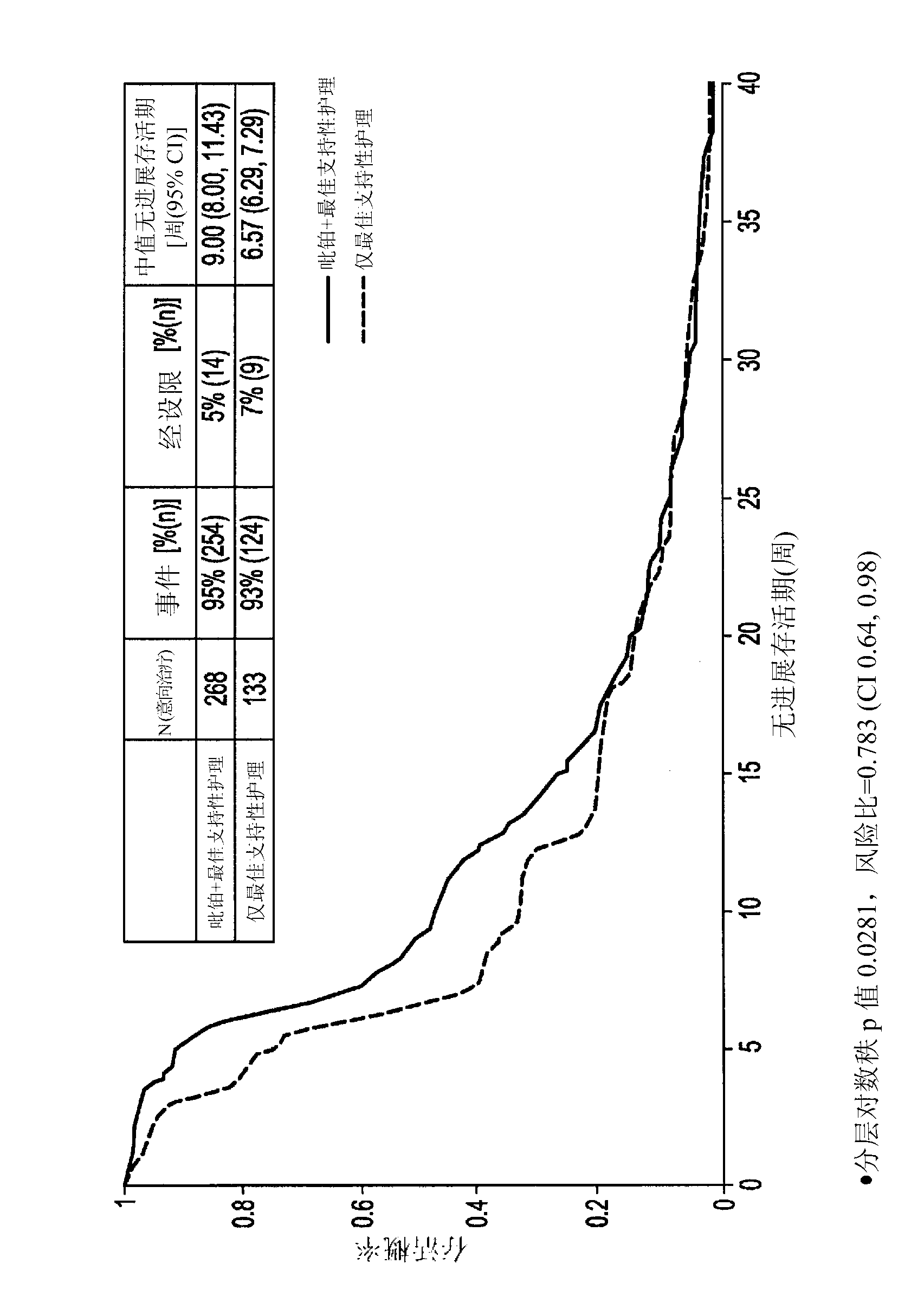
Picoplatin is a platinum-based antineoplastic agent in clinical development by Poniard Pharmaceuticals (previously NeoRx) for the treatment of patients with solid tumors. In Phase I and Phase II clinical trials, picoplatin demonstrated activity in a variety of solid tumors, including lung, ovarian, colorectal and hormone-refractory prostate cancer. However, in Phase III trials, picoplatin failed to hit its primary endpoint for advanced small cell lung cancer. Hopes are now pinned on its use for metastatic colorectal cancer.
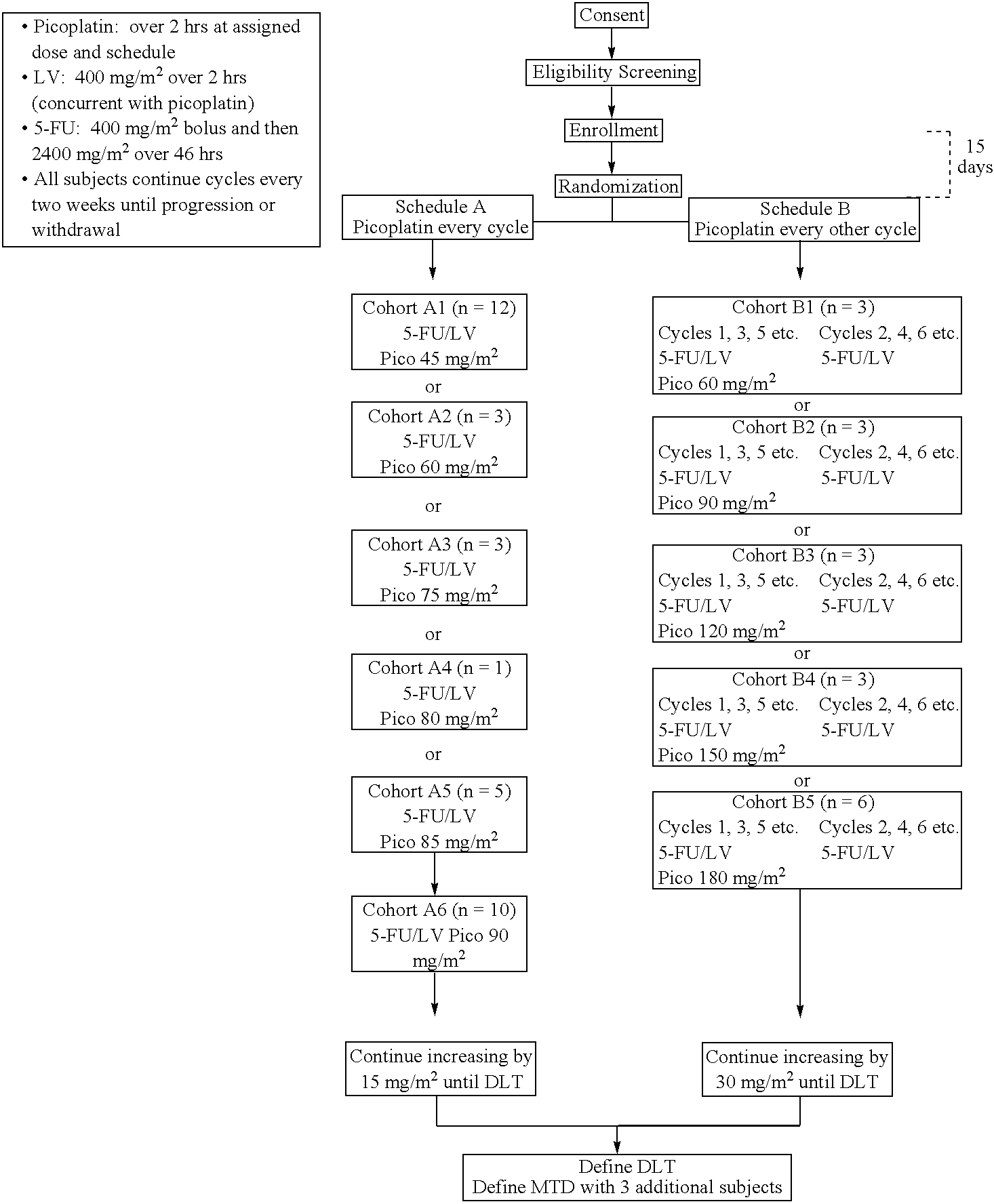
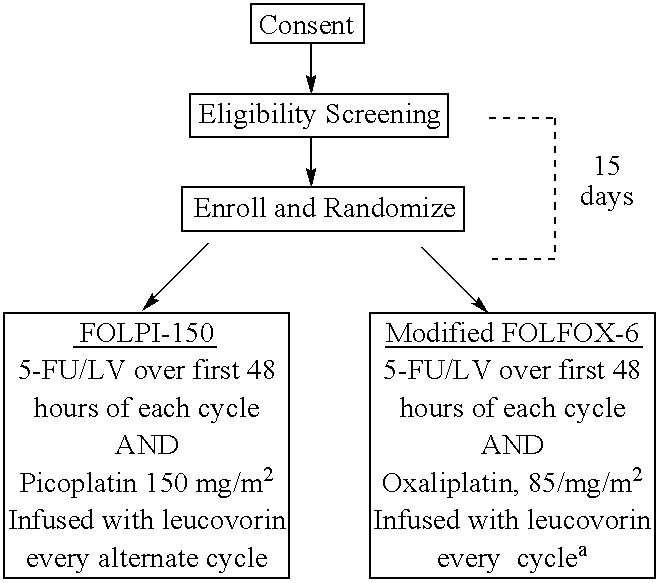
Use of picoplatin and cetuximab to treat colorectal cancer
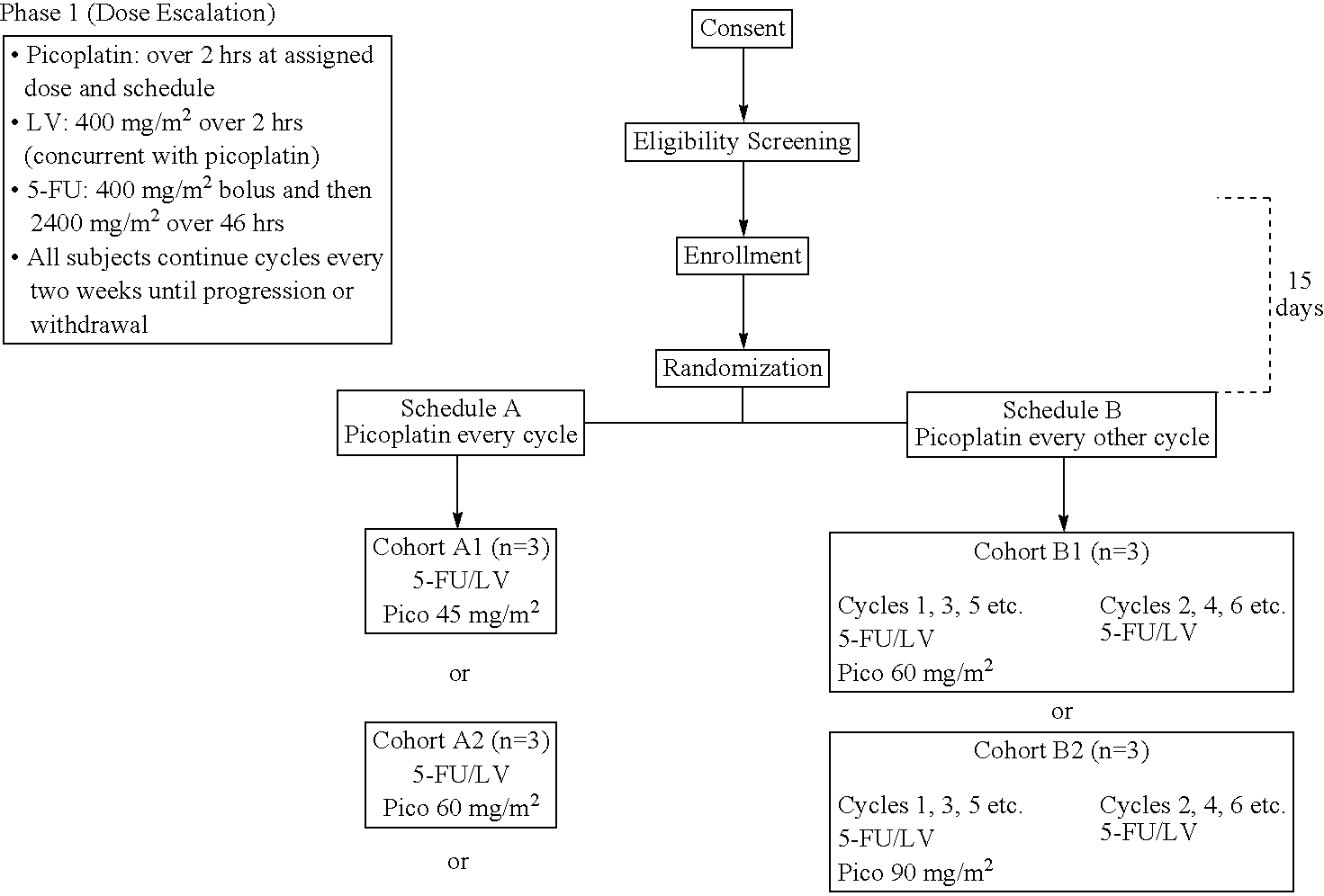
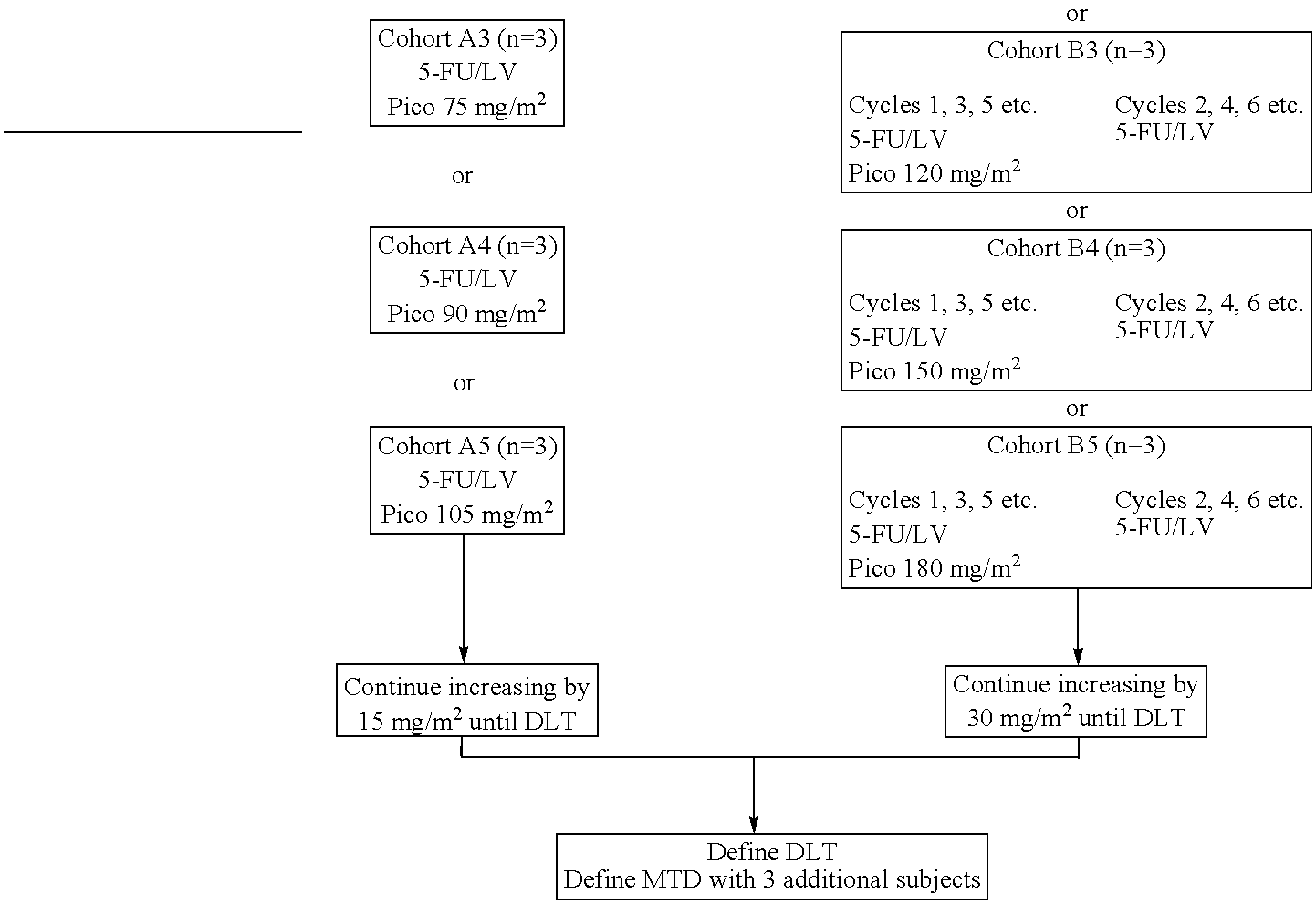
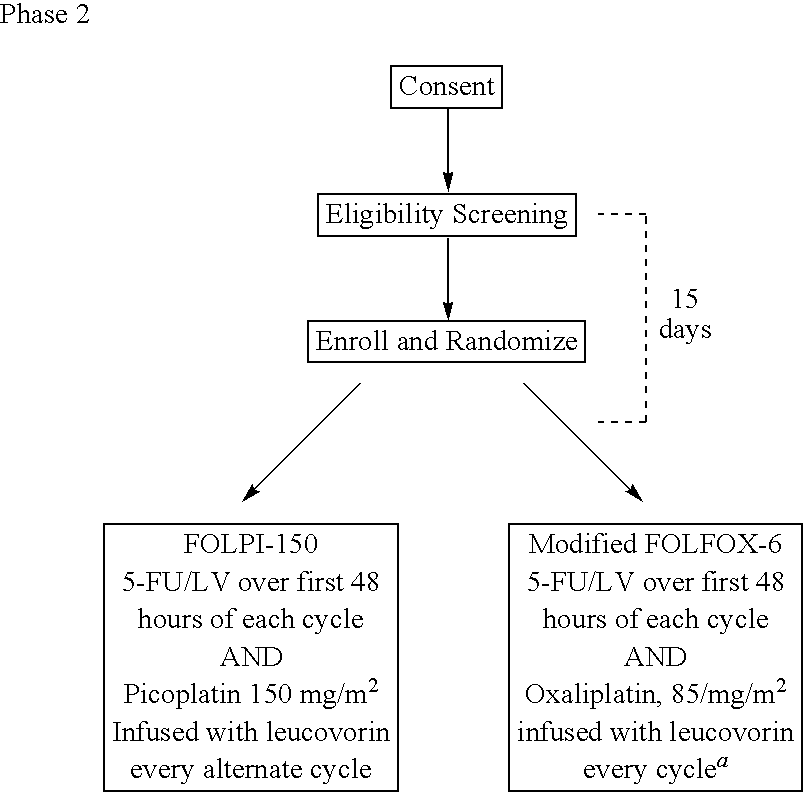
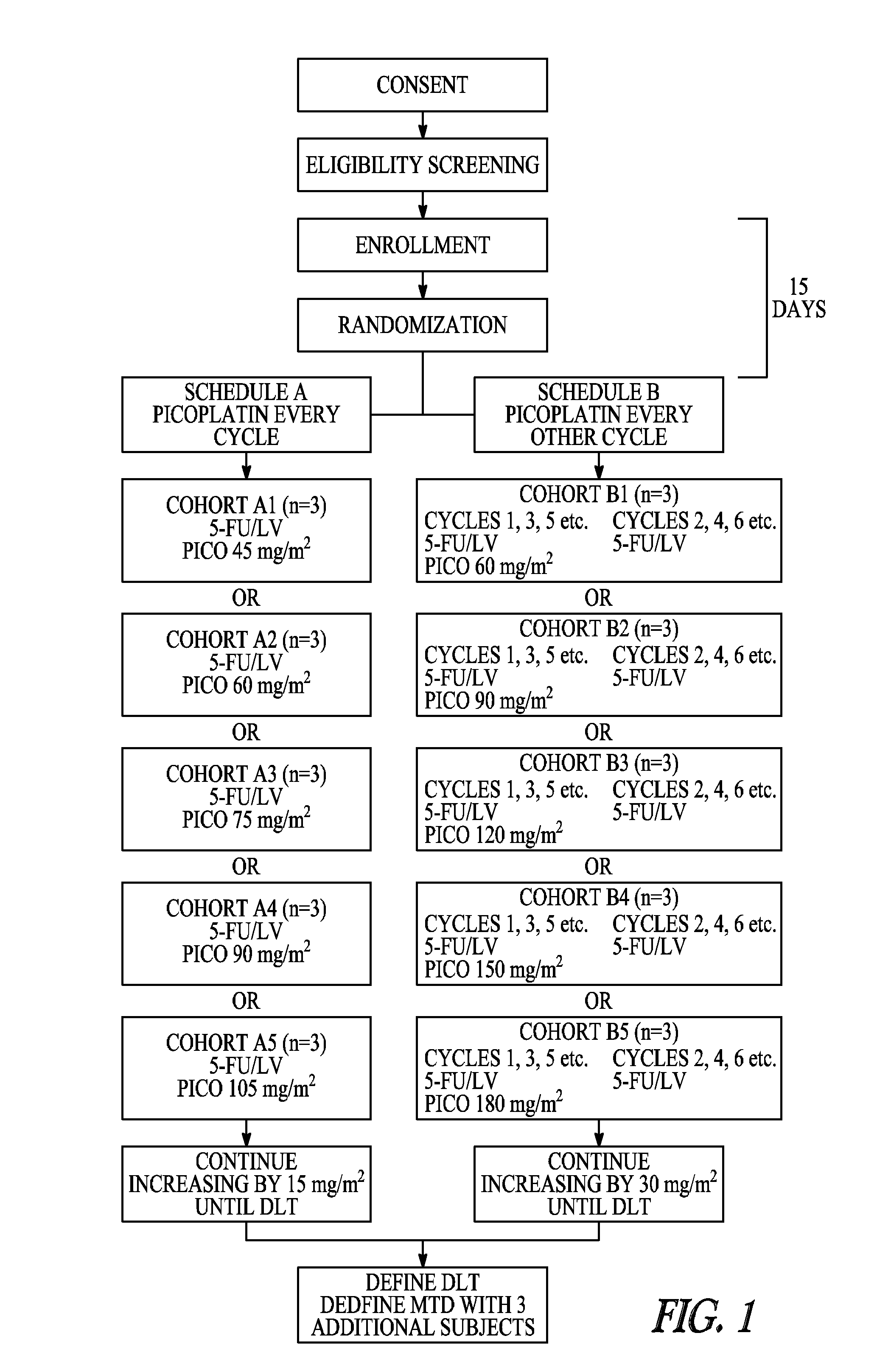
The invention provides a method of treatment of metastatic colorectal cancer by administration of the anti-cancer platinum drug picoplatin in conjunction with cetuximab, 5-FU, and leucovorin in a variety of treatment regimens. The invention also provides a use of picoplatin in conjunction with cetuximab, 5-FU, and leucovorin for treatment of metastatic colorectal cancer. The invention further provides kits adapted for administration of picoplatin in conjunction with cetuximab. Also, methods for determining dosage regimens for patients afflicted with a cancer comprising EGFR are provided.
Owner:PONIARD PHARMA INC
Combination therapy for ovarian cancer
The present invention provides a method to treat ovarian cancer by the administration of effective amounts of picoplatin and pegylated liposomal doxorubicin.
Owner:PONIARD PHARMA INC
Stabilized picoplatin oral dosage form
InactiveUS20110033528A1Prevent and minimize amountDelayed photolysisBiocideOrganic active ingredientsParticulatesWater dispersible
The invention provides an oral dosage form for the anti-cancer drug picoplatin comprising a core and a coating, the dosage form being free of redox-active metal salts. The core of the tablet is a substantially dry powder comprising about 10 to 60 wt % picoplatin wherein the picoplatin is a particulate of less than about 10 microns average particle diameter, about 40-80 wt % of a filler comprising a substantially water-soluble, water-dispersible, or water-absorbing carbohydrate, and an effective amount of up to about 5 wt % of a lubricant. The dosage form can further include a dispersant.
Owner:GENZYME CORP +1
Stabilized picoplatin dosage form
Methods for stabilizing aqueous solutions of picoplatin are provided. Such stable, preferably aseptic solutions are particularly useful for preparing unit dosages of picoplatin for oral or intravenous administration, preferably in combination with at least one additional non-platinum anti-cancer agent.
Owner:PONIARD PHARMA INC
Encapsulated picoplatin
InactiveUS20100062056A1Maximize and to saturate blood plasma levelRapidly “ spike ”BiocideHeavy metal active ingredientsParticulatesOral medication
The invention provides an encapsulated unit dosage form for picoplatin that is adapted for oral administration of the picoplatin containing a substantially dry powder with about 20 to 55 wt % picoplatin in the physical form of a picoplatin particulate wherein an average picoplatin particle diameter is less than about 10 microns. The picoplatin particles are dispersed within the powder of the formulation which includes a substantially water-soluble, water-dispersible, or water-absorbing carbohydrate and an effective amount of up to about 5 wt % of a lubricant.
Owner:PONIARD PHARMA INC
Use of picoplatin to treat colorectal cancer
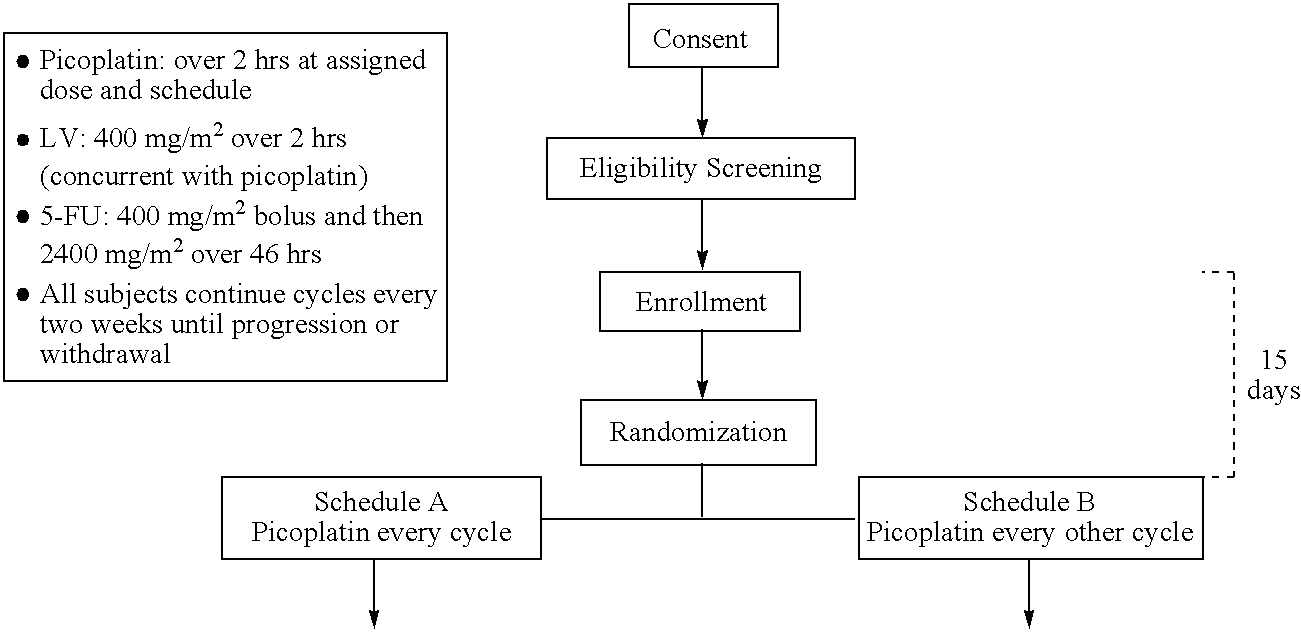
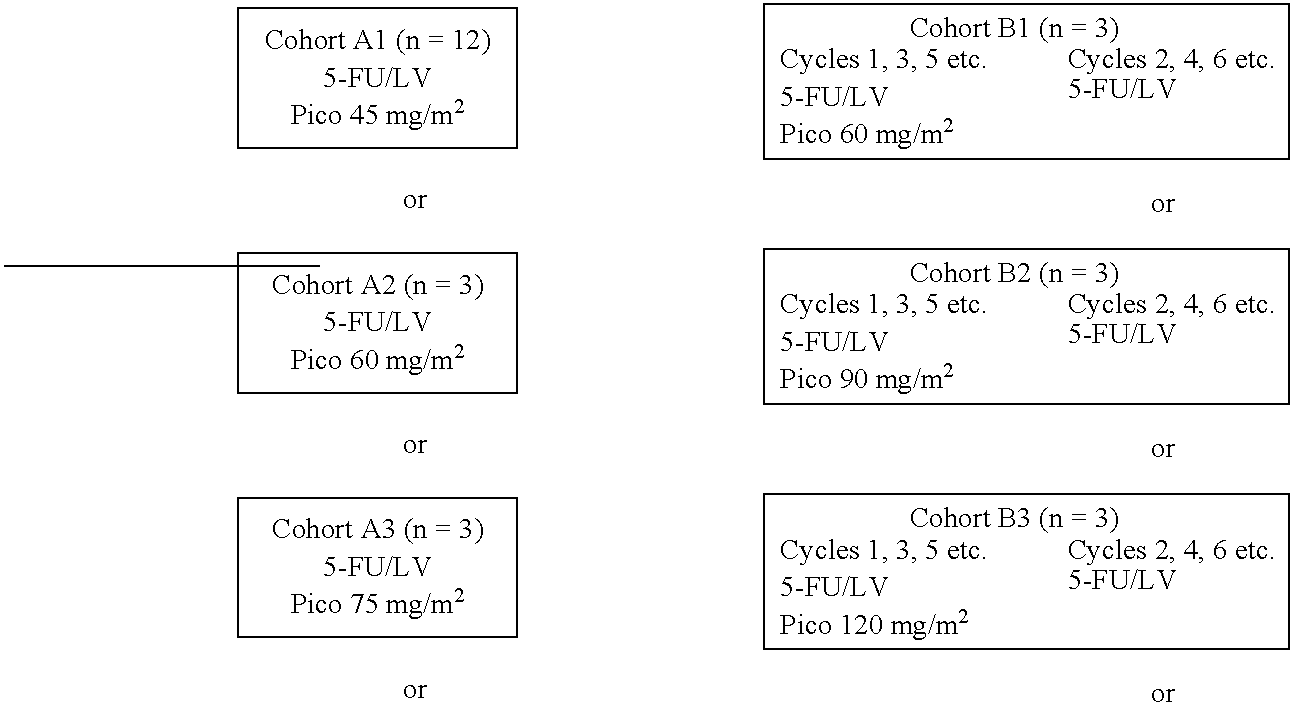
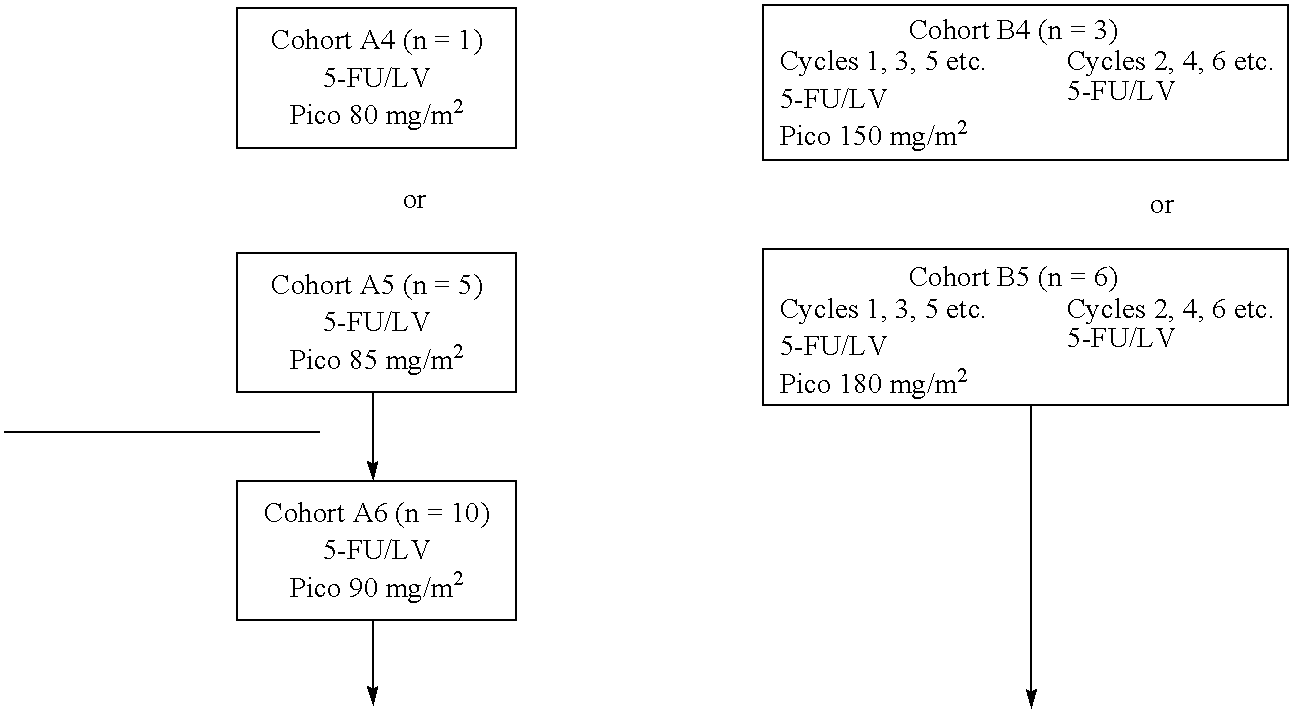
The invention provides a method of treatment of colorectal cancer by administration of the anti-cancer platinum drug picoplatin in conjunction with 5-FU and leucovorin in a variety of treatment regimens. Dosages, dosing schedules, and ancillary treatments are described.
Owner:ACCELERATED PHARMA
Use of picoplatin to treat prostate cancer
InactiveUS20120122825A1Improve the quality of lifeLower Level RequirementsBiocideOrganic active ingredientsRegimenDocetaxel-PNP
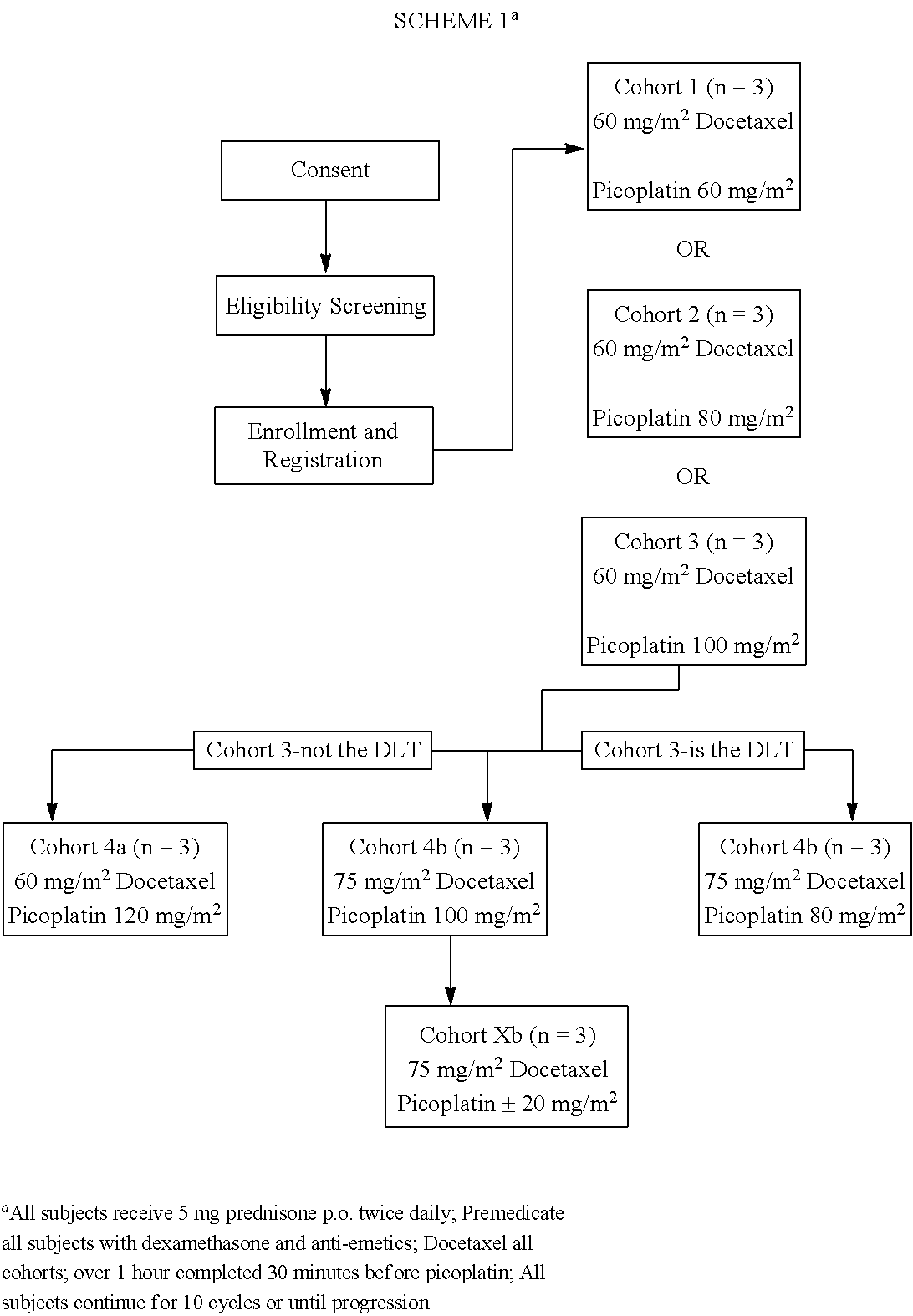
The invention provides a method of treatment of metastatic hormone-refractory prostate cancer involving substantially as concurrent administration of picoplatin and docetaxel. Prednisone may also be administered. Dosages and dosing regimens are provided.
Owner:SCHWEGMAN LUNDBERG& WOESSNER P A
Aqueous solution injection for picoplatin
ActiveCN101804025AImprove solubilitySolve the dosageOrganic active ingredientsInorganic non-active ingredientsPicoplatinPolyethylene glycol
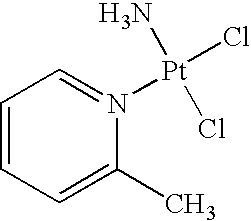
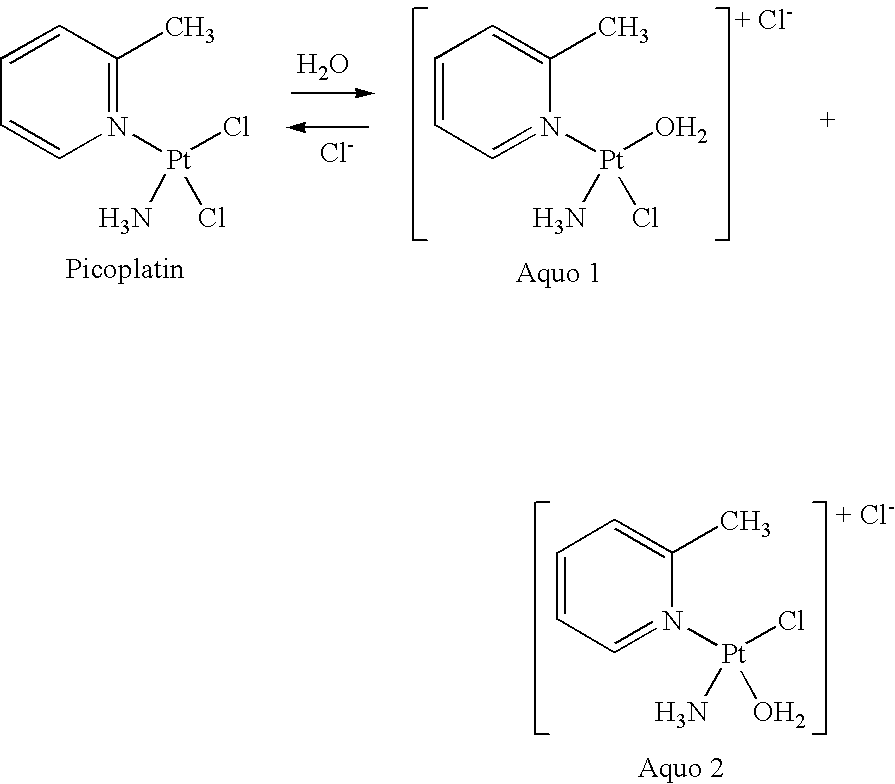
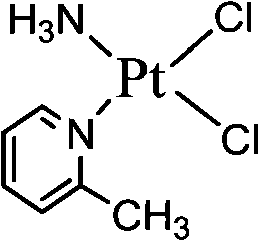
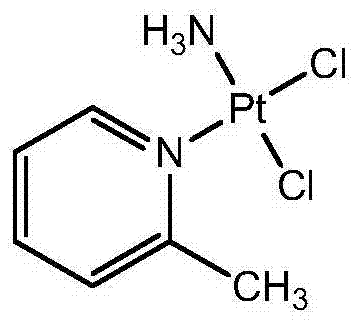
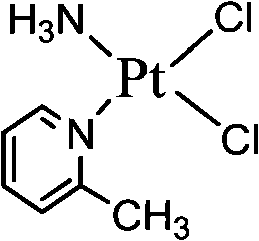
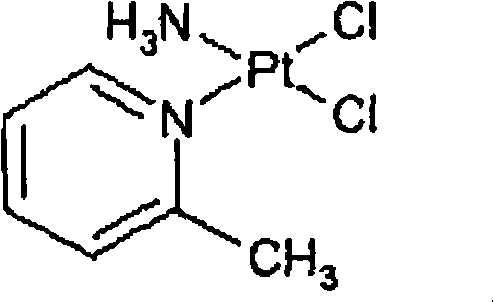
The invention provides picoplatin, which is a new generation of platinum group metal anti-tumor medicament with steric hinderance effect and is hardly dissolved in pure water, and relates to a preparation formula of the picoplatin (C6H10N2Pt). The injection is prepared by dissolving the picoplatin with mixed solution consisting of polyethylene glycol and solution of hydrochloride sodium chloride in a certain proportion serving as a solvent. The mixed solution obviously improves the dissolvability of the picoplatin up to 15mg / ml; and the injection has easy preparation, stable process and convenient clinical application, and is a perfect picoplatin preparation.
Owner:KUNMING GUIYAN PHARMA
Anti-cancer medicinal composition
ActiveCN102309493AEasy to useImprove complianceAntineoplastic agentsHeavy metal compound active ingredientsCarboplatinPicoplatin
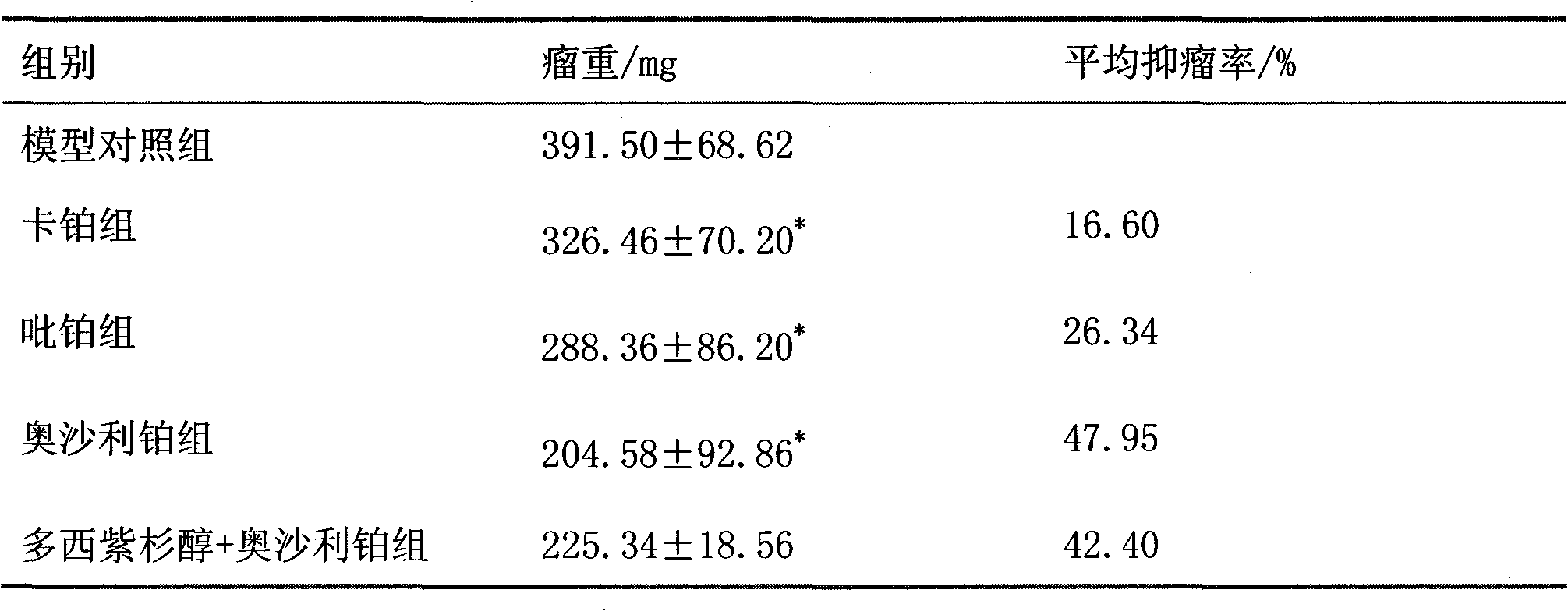
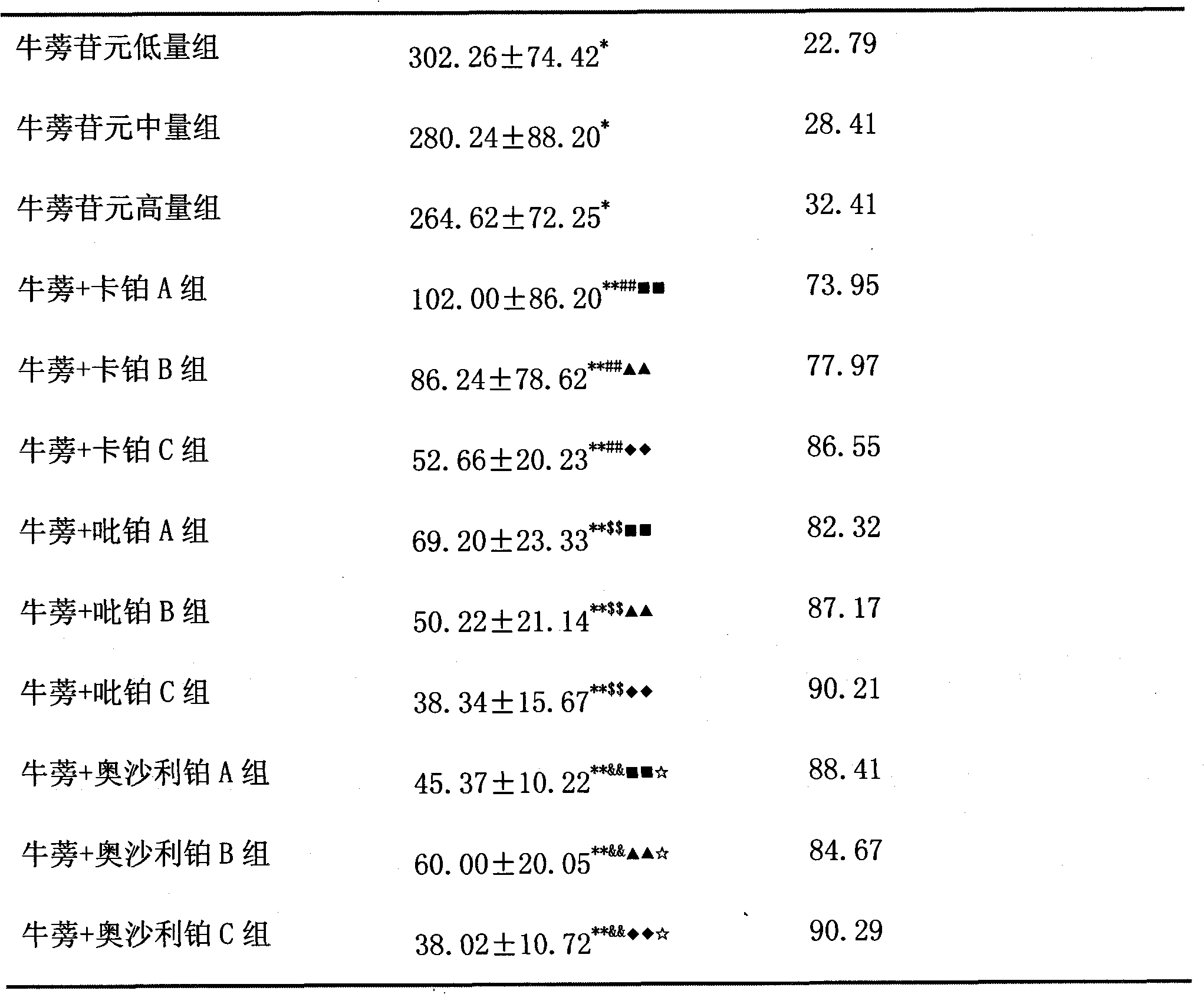
The invention belongs to the field of medicines, and particularly relates to an anti-cancer medicinal composition. The composition comprises arctigenin and a platinum anti-cancer medicament, wherein the platinum medicament is carboplatin, picoplatin or oxaliplatin. Compared with a single medicine, the medicinal composition has a synergistic effect in the aspect of inhibiting non-small cell lung cancers.
Owner:鲁南新时代生物技术有限公司
Use of picoplatin to treat colorectal cancer
The invention provides a method of treatment of colorectal cancer by administration of the anti-cancer platinum drug picoplatin in conjunction with 5-FU and leucovorin in a variety of treatment regimens.
Owner:ACCELERATED PHARMA
Combination therapy
InactiveUS20190022117A1Improve survival rateGood synergistic effectHeavy metal active ingredientsPharmaceutical delivery mechanismBladder cancerPicoplatin
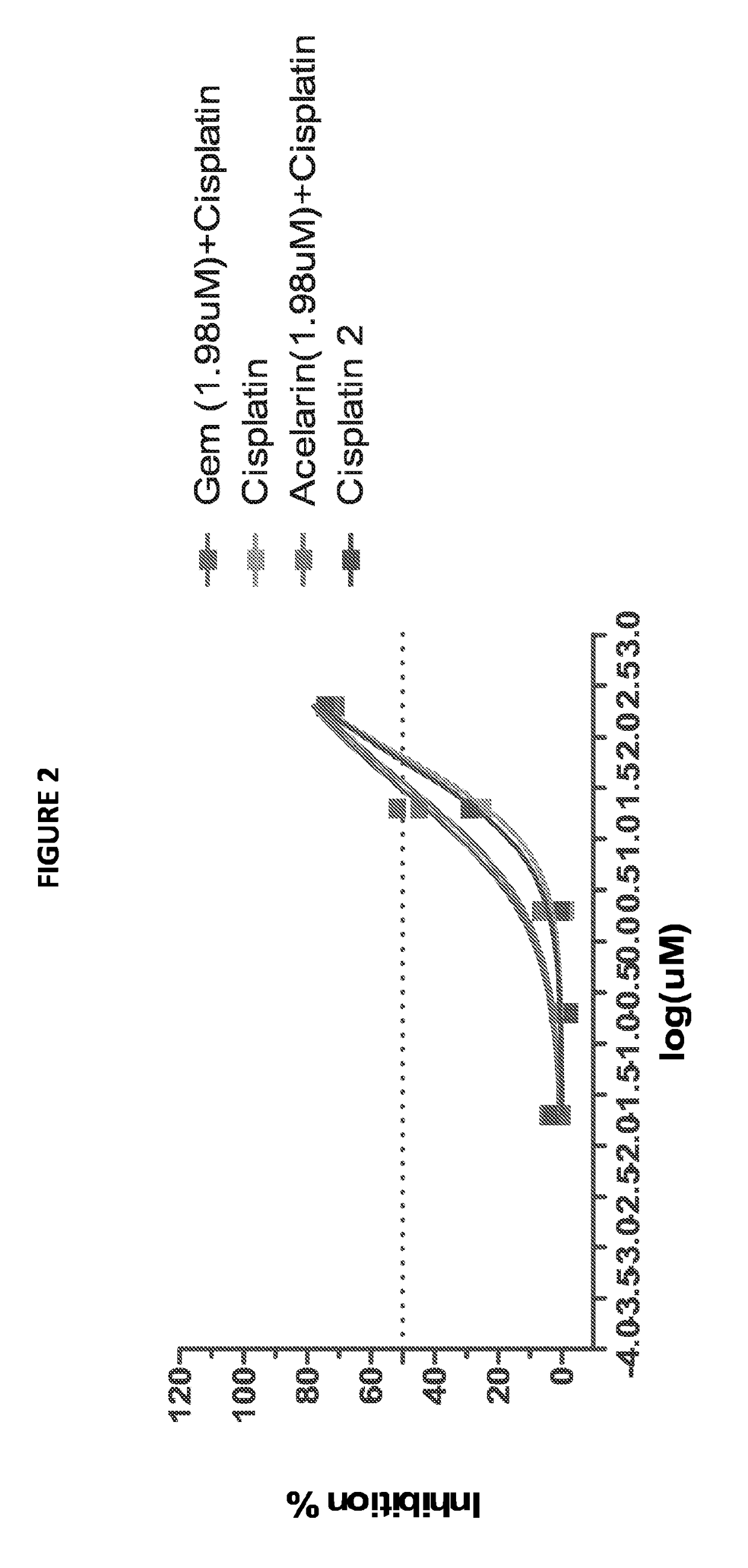
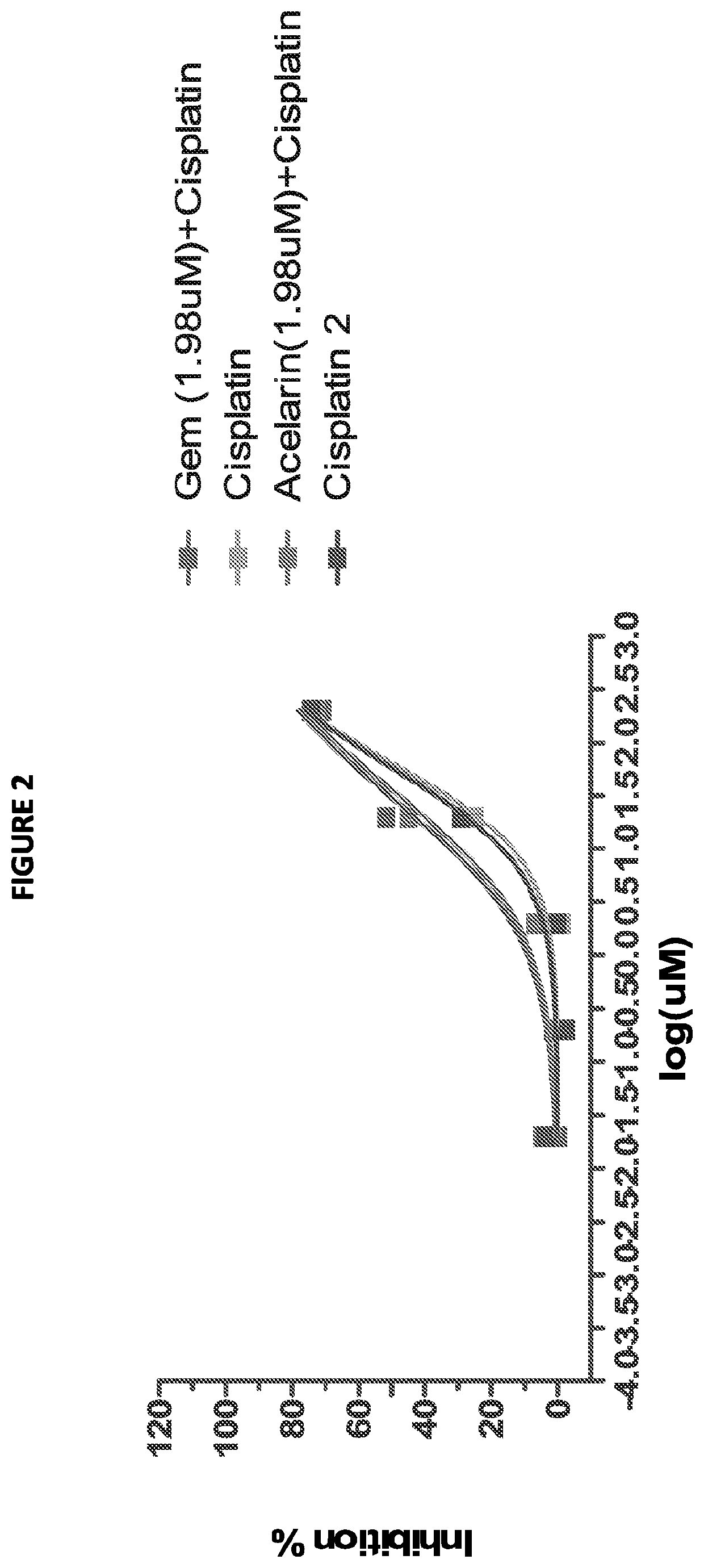
This invention relates to a combination of gemcitabine-[phenyl-(benzoxy-L-alaninyl)]-phosphate (chemical name: 2′-Deoxy-2′,2′-difluoro-D-cytidine-5′-O-[phenyl (benzoxy-L-alaninyl)] phosphate) (NUC-1031) and a platinum-based anticancer agent selected from cisplatin, picoplatin, lipoplatin and triplatin. The combinations are useful in the treatment of cancer, particularly biliary tract and bladder cancer.
Owner:NUCANA PLC
Temperature controlled sustained-release injection containing platinum compound
InactiveCN101273964AHeavy metal active ingredientsPharmaceutical delivery mechanismPolyesterPicoplatin
The invention relates to a temperature-controlled sustained-release injection containing a platinum compound and a preparation method thereof, the temperature-controlled sustained-release injection comprises effective anti-cancer amount of the platinum compound, an amphiphilic block copolymer and a certain amount of drug release regulator, wherein, the amphiphilic block copolymer is composed of polyethylene glycol and polyester, the mixture of the amphiphilic block copolymer and a solvent without organic solvent has the temperature-sensitive gelatinization feature, which is flowable liquid in the environment that is lower than the body temperature and can be automatically converted to the water-insoluble gel that can not flow and be biodegradable for absorption in an endotherm, and the water-insoluble gel can allow the contained platinum compound to have the local sustained release in a tumor and maintain the effective drug concentration for a plurality of weeks to a plurality of months; the anti-cancer sustained-release gel injection can be injected in the tumor or the tumor periphery or be arranged in the postoperative tumor cavity, thus being used for the treatment of the tumors in different stages. The platinum compound is selected from cisplatin, carboplatin, cycloplatin, eptaplatin, denaplatin, zeniplatin, enloplatin, sulfatodiaminocyclohexane platin, cis-spiroplatin, dexormaplatin, iproplatin, lobaplatin, miboplatin, picoplatin, nedaplatin, ormaplatin, oxaliplatin and so on.
Owner:SHANDONG LANJIN PHARMA +1
Use of picoplatin to treat colorectal cancer
The invention provides a method of treatment of colorectal cancer by administration of the anti-cancer platinum drug picoplatin in conjunction with 5-FU and leucovorin in a variety of treatment regimens.
Owner:ACCELERATED PHARMA
Anticancer sustained-release gel injection containing platinum compound
InactiveCN101283978APharmaceutical delivery mechanismPharmaceutical non-active ingredientsPicoplatinSolvent
An anticancer sustained-release gel injection sustained-release microspheres containing platinum-based compound, amphiphilic block copolymer, solvent and release regulator, the mixture of amphiphilic block copolymer and solvent has a temperature sensitive gelatinization characteristic, and can automatically become non-flowing degradable water insoluble gel, which can locally and slowly release drug at tumor foci for several weeks to several months, after injection into body. The adjuvant in the sustained-release microspheres is poly(lactic acid)-glycollic acid copolymer; and the amphiphilic block copolymer is PLGA-PEG-PLGA copolymer. The preparation can be injected into artery, inside a tumor or around a tumor with remarkably reduced systemic toxicity of drug, and can be used for solid tumors at different stages. The platinum-based compound is selected from nedaplatin, carboplatin, cisplatin, enloplatin, ormaplatin, sulfato-1,2-diaminocyclohezane-platinum (SHP), picoplatin, cyclopentylamine platium, zeniplatin, spiroplatin, lobaplatin, iproplatin, oxaliplatin, cycloplatin, and dexormaplatin. The preparation can be used with radioactive particles and can enhance chemotherapy effect.
Owner:济南基福医药科技有限公司
Anti-cancer medicine sustained-released injection loaded with platinum compound and synergist thereof
InactiveCN101380303AEasy to operateGood repeatabilityOrganic active ingredientsPharmaceutical delivery mechanismEptaplatinPicoplatin
A anticarcinogenic slow release injection carrying platinum compounds and a synergist thereof is composed of slow release microspheres and a dissolvant, wherein, the slow release microspheres comprise anticancer active components and a slow release adjuvant, and the dissolvant is a special dissolvant containing a suspending agent. The anticancer active component comprises platinum compounds such as sunpla, dicycloplatin, eptaplatin, (cis-amminedichloro(2-methylpyridine) platinum, camphoramine chloroacetic platinum or picoplatin, and the like, and a cytotoxic drug selected from a phosphoinositide 3-kinase inhibitor, pyrimidine analogue and / or a DNA repair enzyme inhibitor; the slow release adjuvant is biocompatible macromolecules such as polylactic acid and copolymer thereof, polyethylene glycol, carboxyl end polylactic acid copolymer, copolymer of dienoic fatty acid and sebacic acid, poly (erucic acid dimer-sebacic acid), poly (fumaric acid-sebacic acid), polifeprosan, polylactic acid, EVAc, and the like, and the suspending agent has the viscosity of 100cp-3,000cp (at the temperature of 20-30 DEG C) and is selected from sodium carboxymethyl cellulose, and the like. The slow release microspheres can also be made into a slow release implant. The slow release injection is injected or placed in tumors or around the tumors, which can improve the curative effects of non-operative therapies such as radiotherapy, chemotherapy, and the like.
Owner:SHANDONG LANJIN PHARMA +1
Purification method of Picoplatin
ActiveCN107778331AEfficient removalHigh purityPlatinum organic compoundsPurification methodsOrganic solvent
The invention discloses a purification method of an anti-tumor drug Picoplatin. The technological process comprises the following steps: dissolving a Picoplatin crude product with an organic solvent at a certain temperature; adding a crystallization solvent into the solution for crystallization at a certain temperature; filtering and drying, so as to obtain a Picoplatin highly-finished product. The purification method of the Picoplatin is simple in operation and comparatively high in yield, the purity of the highly-finished product can reach 99.9% or above, a single impurity can be controlledunder 0.1%, and total impurities can be controlled within 0.2%.
Owner:LUNAN BETTER PHARMA
Synthesis method of potassium trichloroammine platinate
ActiveCN113173607ASolve difficultySolve the costRuthenium/rhodium/palladium/osmium/iridium/platinum compounds preparationSatraplatinPicoplatin
In order to solve the problems of high synthesis difficulty and high cost of potassium trichloroammine platinate in the prior art, the invention discloses a synthesis method of potassium trichloroammine platinate. The method comprises the following steps: by taking chloroplatinous acid or chloroplatinite, ammonium chloride, alkali chloride and carbonate as raw materials, adjusting the pH value of a system to prepare a trichloroammine complexed platinic acid solution, and thenpreparing trichlorine complex potassium platinatefrom the trichlorine complex platinum acid and potassium chloride. According to the synthesis method of the potassium trichloroammine platinate, the required raw materials are simple and easy to obtain, the cost is low, the synthesis conditions are mild, the yield of the potassium trichloroammine platinate is 78% or above, and the potassium trichloroammine platinate can be used as an intermediate for synthesizing anti-cancer drugs such as picoplatin and satraplatin and is suitable for industrial production.
Owner:内江洛伯尔材料科技有限公司
Combination therapy for ovarian cancer
InactiveUS20100260832A1Reduce non-hematologic side effectExhibit synergistic efficacyBiocideCarbohydrate active ingredientsPicoplatinOncology
Owner:PONIARD PHARMA INC
Use of picoplatin to treat colorectal cancer
The invention provides a method of treatment of colorectal cancer by administration of the anti-cancer platinum drug picoplatin in conjunction with 5-FU and leucovorin in a variety of treatment regimens.
Owner:ACCELERATED PHARMA
Oral formulations for picoplatin
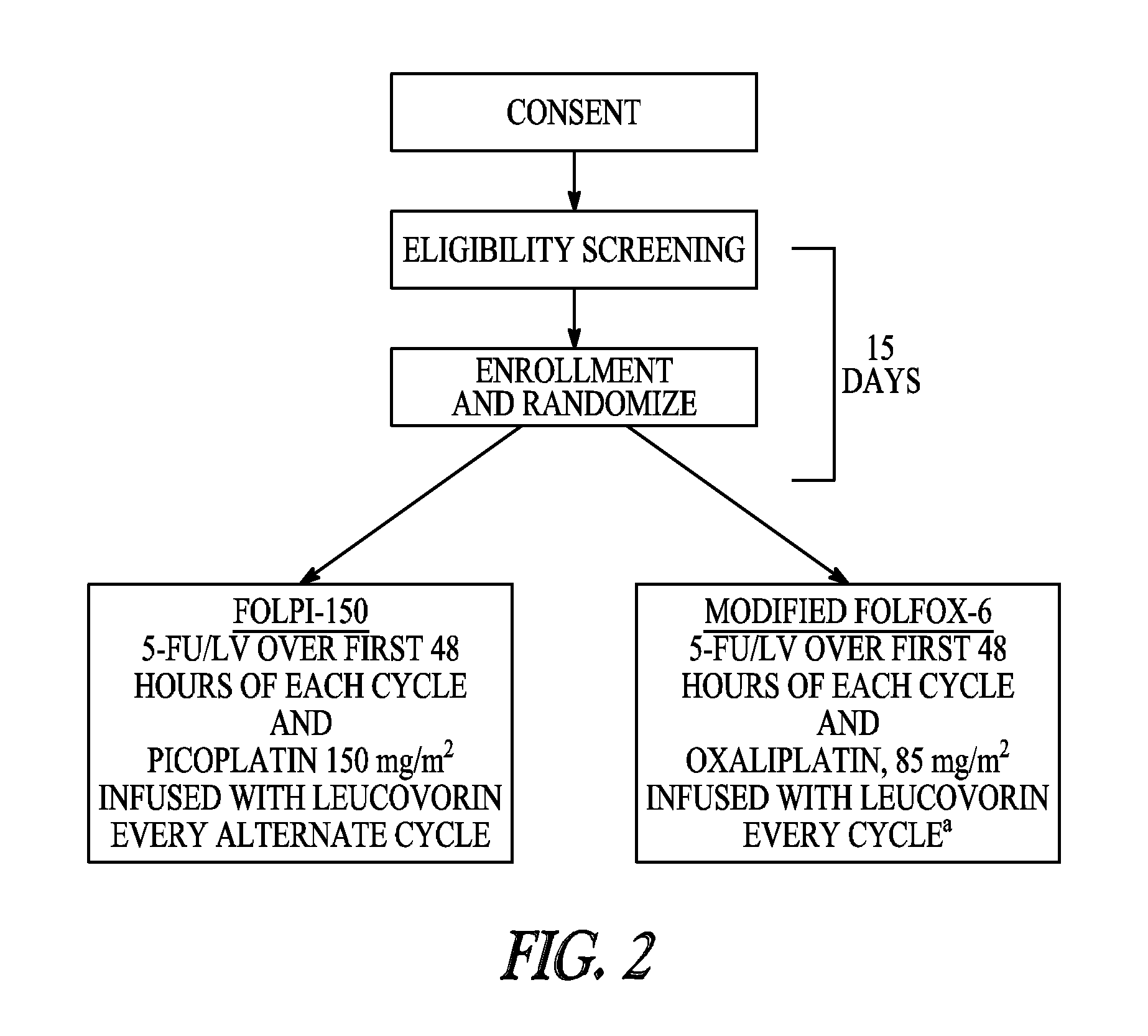
The invention provides formulations for the organoplatinum anticancer drug picoplatin. Self emulsifying compositions, stabilized nanoparticulate compositions, solid dispersions, and nanoparticulate suspensions in oils are provided, along with methods for preparation of the formulations. The formulations can provide improved oral availability of picoplatin relative a to a simple solution of picoplatin such as in water or normal saline solution and can be used in combination therapy.
Owner:PONIARD PHARMA INC
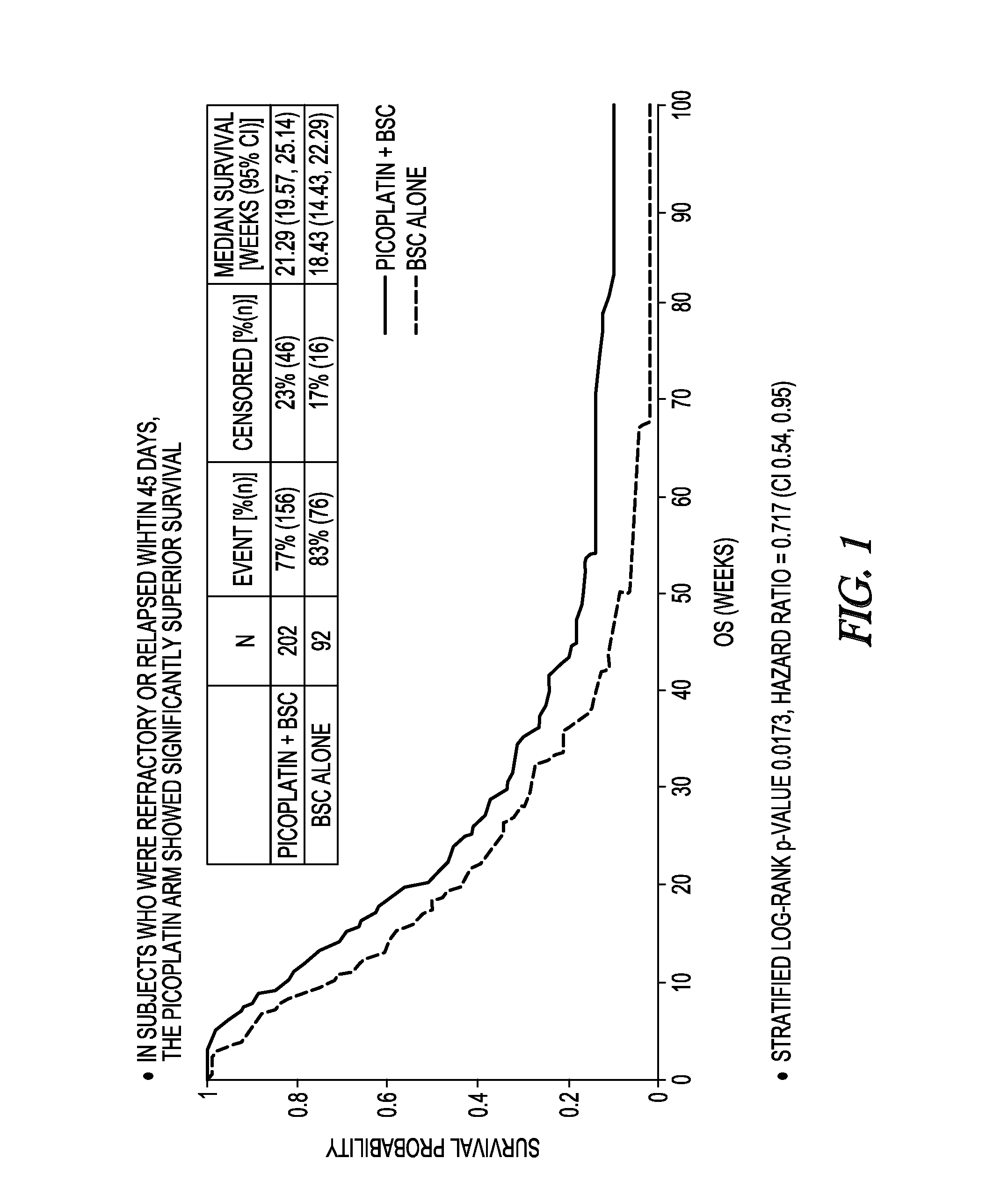
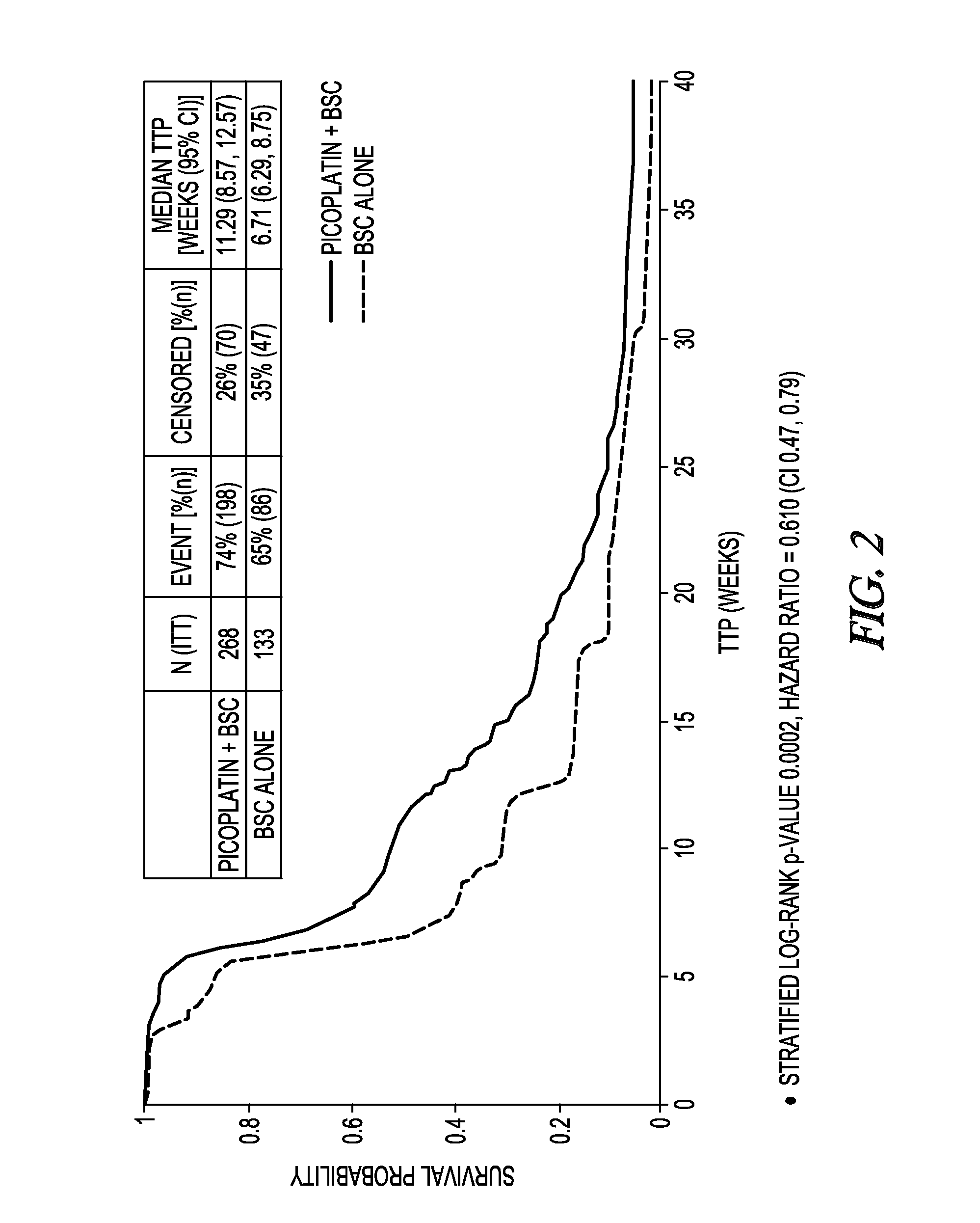
Method to treat small cell lung cancer
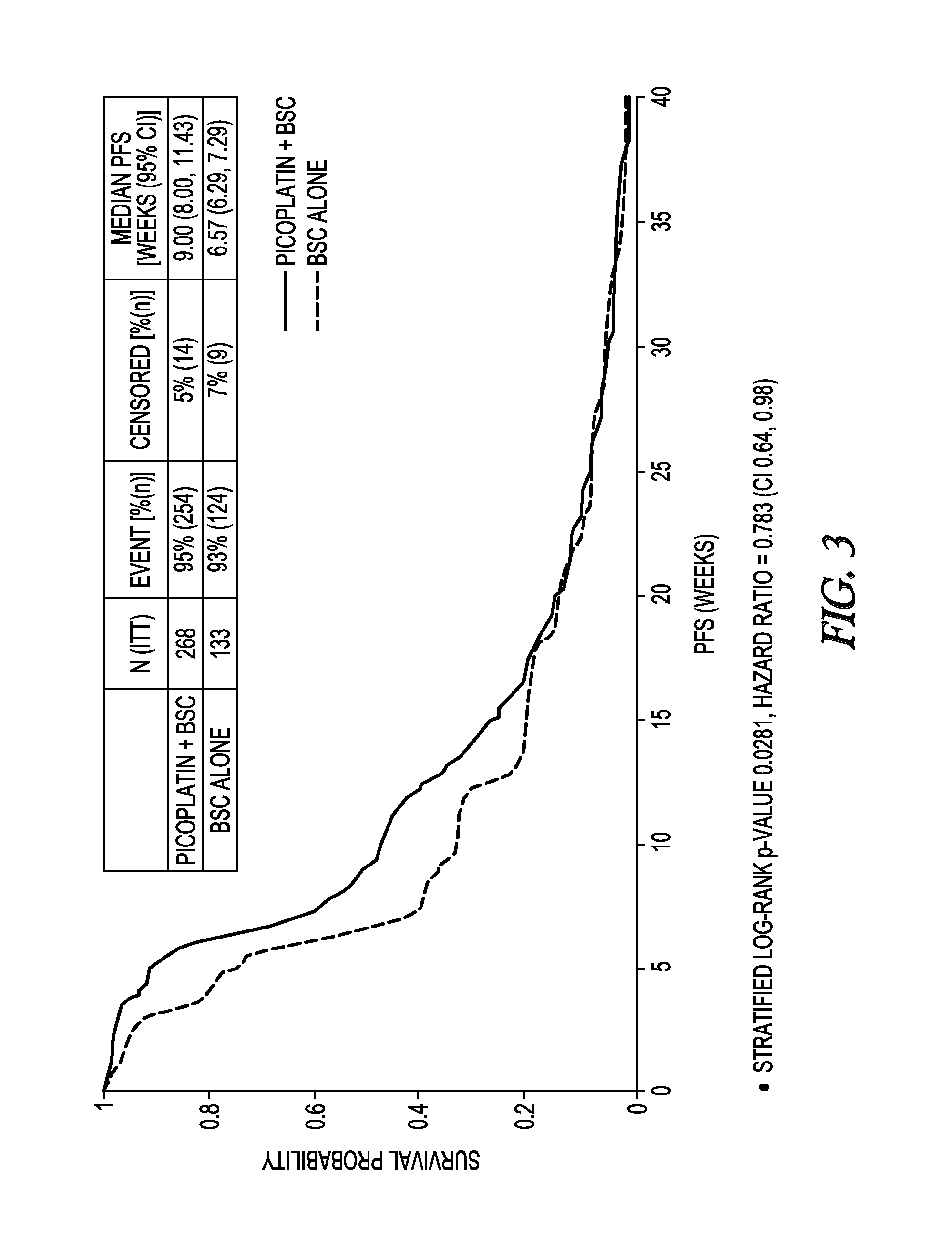
InactiveUS20130203725A1Extend your lifeProlong lifeBiocideHeavy metal active ingredientsRegimenPicoplatin
A method for treatment of small cell lung cancer (SCLC) that does not respond to first-line treatment or that progresses following cessation of first-line organoplatinum chemotherapy is provided that includes the administration of picoplatin, optionally in conjunction with a regimen of best supportive care. Multiple doses of picoplatin can be administered. The picoplatin can also treat SCLC that has metastacized to the brain.
Owner:TALLIKUT PHARMA
Stabilized picoplatin oral dosage form
InactiveCN104288119ADosage increase or decreaseImprove complianceHeavy metal active ingredientsCapsule deliveryPicoplatinPharmaceutical Substances
The invention provides an oral dosage form for the anti-cancer drug picoplatin comprising a core and a coating, the dosage form being free of redox-active metal salts. The core of the tablet is a substantially dry powder comprising about 10 to 60 wt% picoplatin wherein the picoplatin is a particulate of less than about 10 microns average particle diameter, about 40-80 wt% of a filler comprising a substantially water-soluble, water-dispersible, or water-absorbing carbohydrate, and an effective amount of up to about 5 wt% of a lubricant. The dosage form can further include a dispersant.
Owner:ENCARTA INC
Combination therapy
InactiveUS20190381084A1Good synergistic effectReduce riskHeavy metal active ingredientsPharmaceutical delivery mechanismBladder cancerCombined Modality Therapy
This invention relates to a combination of gemcitabine-[phenyl-(benzoxy-L-alaninyl)]-phosphate (chemical name: 2′-Deoxy-2′,2′-difluoro-D-cytidine-5′-O-[phenyl (benzoxy-L-alaninyl)] phosphate) (NUC-1031) and a platinum-based anticancer agent selected from cisplatin, picoplatin, lipoplatin and triplatin. The combinations are useful in the treatment of cancer, particularly biliary tract and bladder cancer.
Owner:NUCANA PLC
Stabilized picoplatin oral dosage form
InactiveCN101678046ADosage increase or decreaseImprove complianceBiocideHeavy metal active ingredientsParticulatesWater dispersible
The invention provides an oral dosage form for the anti-cancer drug picoplatin comprising a core and a coating, the dosage form being free of redox-active metal salts. The core of the tablet is a substantially dry powder comprising about 10 to 60 wt% picoplatin wherein the picoplatin is a particulate of less than about 10 microns average particle diameter, about 40-80 wt% of a filler comprising asubstantially water-soluble, water-dispersible, or water-absorbing carbohydrate, and an effective amount of up to about 5 wt% of a lubricant. The dosage form can further include a dispersant.
Owner:PONIARD PHARMA INC +1
Use of picoplatin to treat colorectal cancer
The invention provides a method of treatment of colorectal cancer by administration of the anti-cancer platinum drug picoplatin in conjunction with 5-FU and leucovorin in a variety of treatment regimens. Dosages, dosing schedules, and ancillary treatments are described.
Owner:ACCELERATED PHARMA
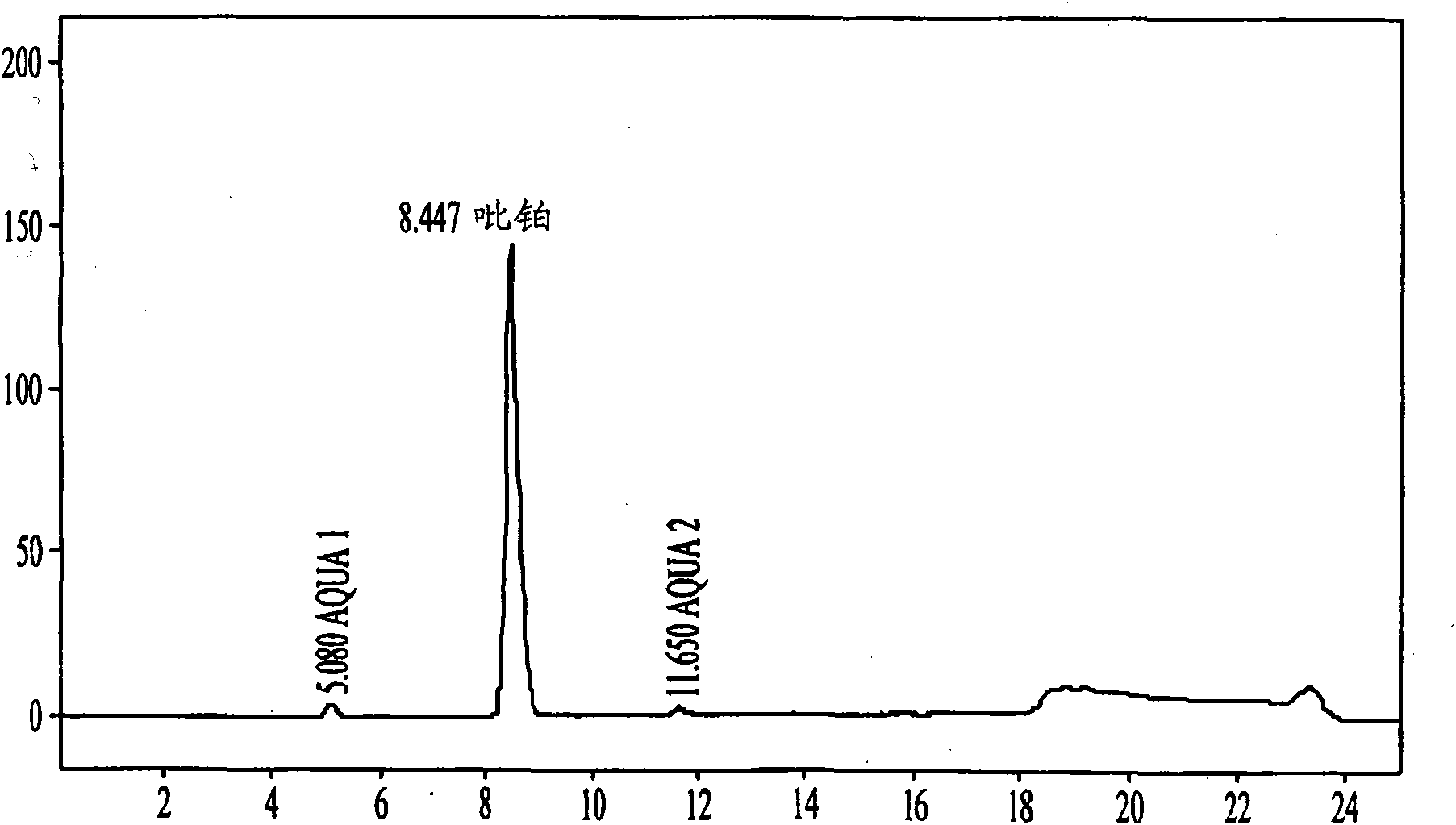
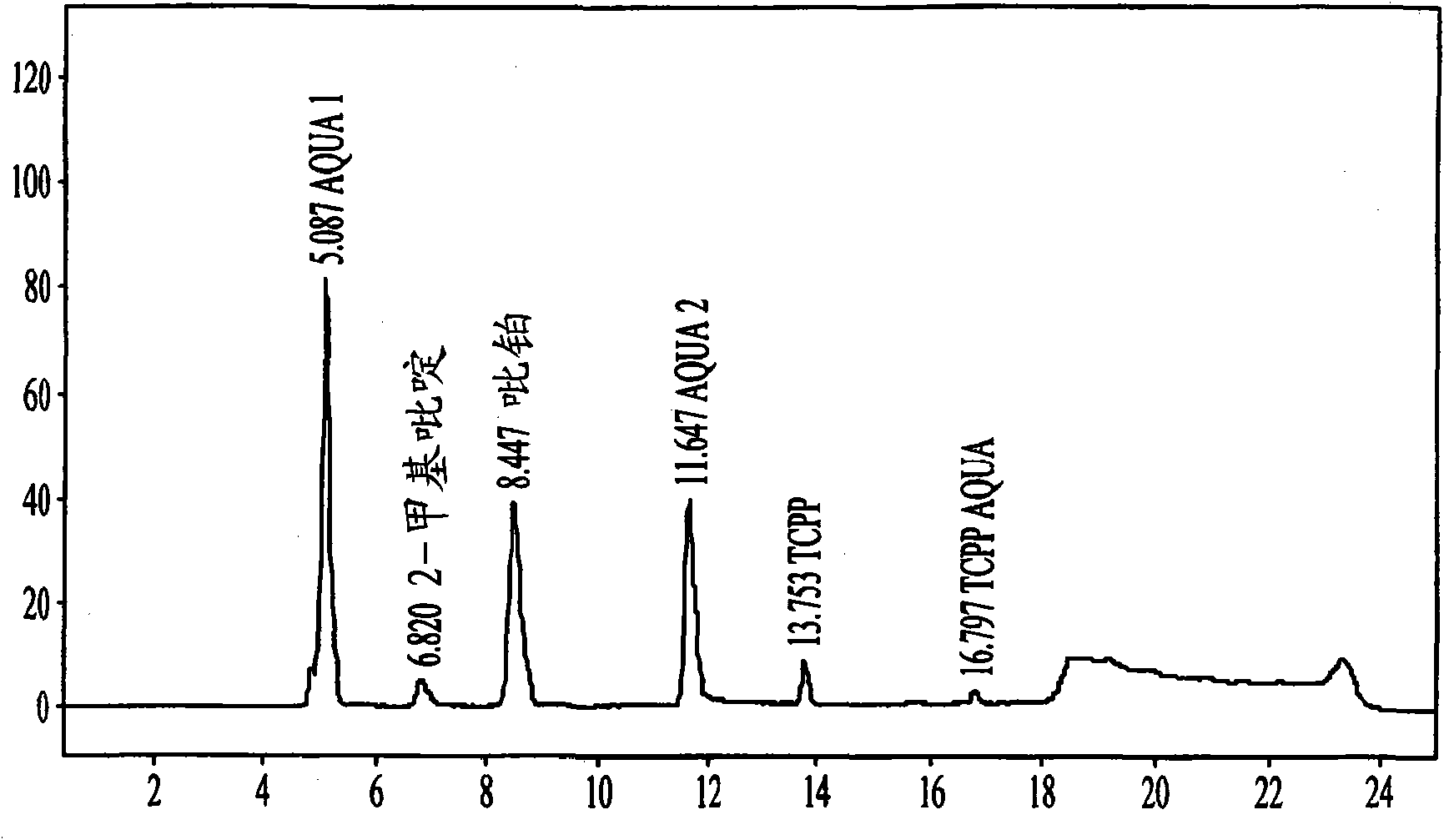
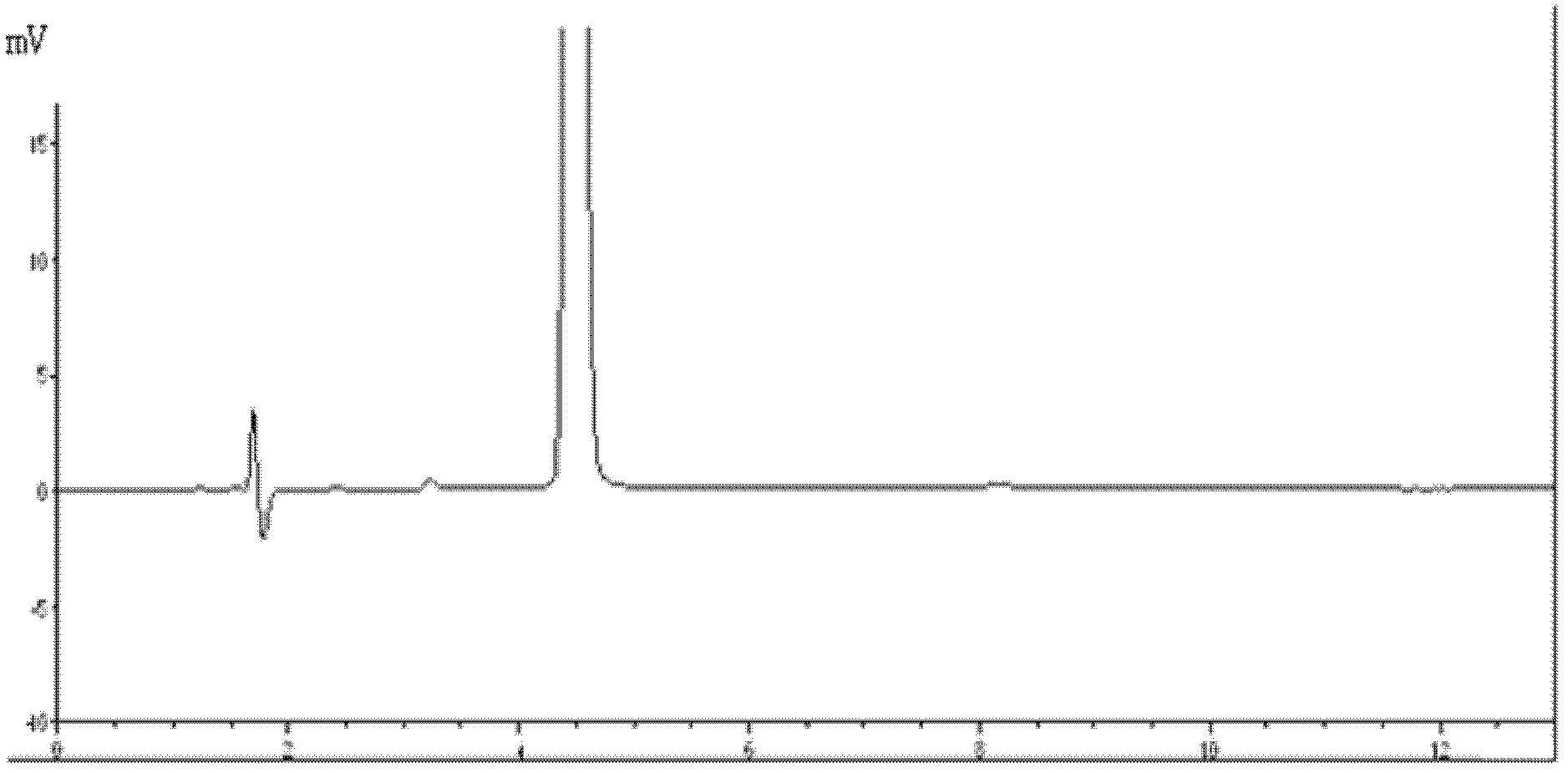
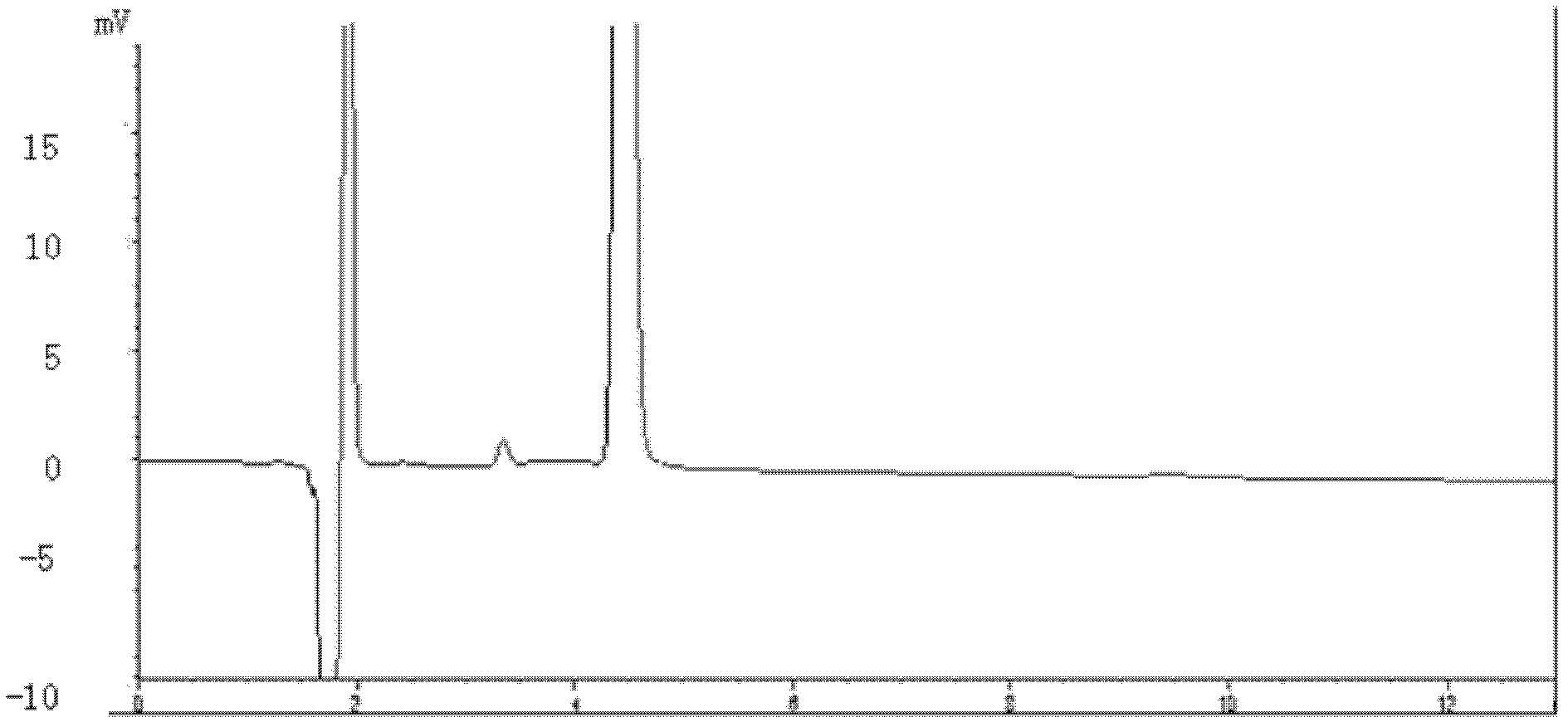
Method for detecting picoplatin and impurities thereof
The invention relates to a method for detecting an antitumor medicine, namely a picoplatin bulk medicine and impurities thereof. The method comprises the following specific steps of: 1, adopting high performance liquid chromatography, wherein the chromatographic conditions are that: octadecylsilane chemically bonded silica is used as filler; a flowing phase is a mixed solution of a buffer salt solution (pH is 4.0 to 6.0) of ocane sulfonic acid sodium, sodium heptanesulfonate or monopotassium phosphate and acetonitrile or methanol; the detection wavelength is 200 to 260nm; the column temperature is 25 to 45 DEG C; the flow speed of the flowing phase is 0.5 to 2.0ml / min; 2, preparing a sample solution, namely preparing a 0.1 to 0.3mg / ml solution containing picoplatin by using pure water or normal saline, and keeping in a dark place; and 3, determining, namely injecting 5 to 20 mu L of the sample solution into a high performance liquid chromatograph, recording a chromatogram and analyzing. By the method, the picoplatin can be detected, and the impurities in the picoplatin bulk medicine can be detected easily, quickly and flexibly.
Owner:KUNMING GUIYAN PHARMA
New method for preparing picoplatin
ActiveCN101775040ASolubility product is smallEasy to removeGroup 8/9/10/18 element organic compoundsSilver iodidePicoplatin
The invention relates to a novel method for preparing picoplatin. The technical scheme adopts following steps of: firstly generating potassium tetraiodoplatinate by reacting potassium tetrachloro platinum with potassium iodide to improve the activity of reactants; generating triiodo (2-picoline) platinum (II) potassium by reacting the potassium tetraiodoplatinate with 2- picoline; generating Cis-2 iodine-ammonia and (2-picoline) platinum (II) by reacting triiodo (2-picoline) platinum (II) potassium with ammonia water; adding the Cis-2 iodine-ammonia and the (2-picoline) platinum (II) together to deionized water; stirring the deionized water for reaction at the room temperature; filtering silver iodide precipitate when the reaction is completed; and adding potassium chloride so as to gradually separate out crystallized products of the picoplatin. In the invention, the reaction is more complete, the utilization of the platinum and the yield are enhanced, the products do not have silver irons, and the problem that the silver irons are exceeded needs not to be worried.
Owner:NANJING CHENGONG PHARM CO LTD
Method to treat small cell lung cancer
InactiveCN103260415ABSC extensionExtend your lifeBiocideInorganic active ingredientsRegimenPicoplatin
A method for treatment of small cell lung cancer (SCLC) that does not respond to first-line treatment or that progresses following cessation of first-line organoplatinum chemotherapy is provided that includes the administration of picoplatin, optionally in conjunction with a regimen of best supportive care. Multiple doses of picoplatin can be administered. The picoplatin can also treat SCLC that has metastacized to the brain.
Owner:PONIARD PHARMA INC
Aqueous solution injection for picoplatin
ActiveCN101804025BAvoid hydrolysisGuaranteed stabilityOrganic active ingredientsInorganic non-active ingredientsPicoplatinPolyethylene glycol
The invention provides picoplatin, which is a new generation of platinum group metal anti-tumor medicament with steric hinderance effect and is hardly dissolved in pure water, and relates to a preparation formula of the picoplatin (C6H10N2Pt). The injection is prepared by dissolving the picoplatin with mixed solution consisting of polyethylene glycol and solution of hydrochloride sodium chloride in a certain proportion serving as a solvent. The mixed solution obviously improves the dissolvability of the picoplatin up to 15mg / ml; and the injection has easy preparation, stable process and convenient clinical application, and is a perfect picoplatin preparation.
Owner:KUNMING GUIYAN PHARMA
Picoplatin and amrubicin to treat lung cancer
A method for treatment of lung cancer comprising administration of picoplatin and amrubicin, or comprising radiation therapy and picoplatin is provided. A use of picoplatin in conjunction with amrubicin for treatment of lung cancer is provided. The lung cancer can be SCLC or NSCLC. The cancer can be resistant or refractory to treatment or that progresses following cessation of first-line organoplatinum chemotherapy. The treatment can include the administration of picoplatin and amrubicin, optionally in conjunction with a regimen of best supportive care. Multiple doses of the drug or drug combination can be administered.
Owner:PONIARD PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com