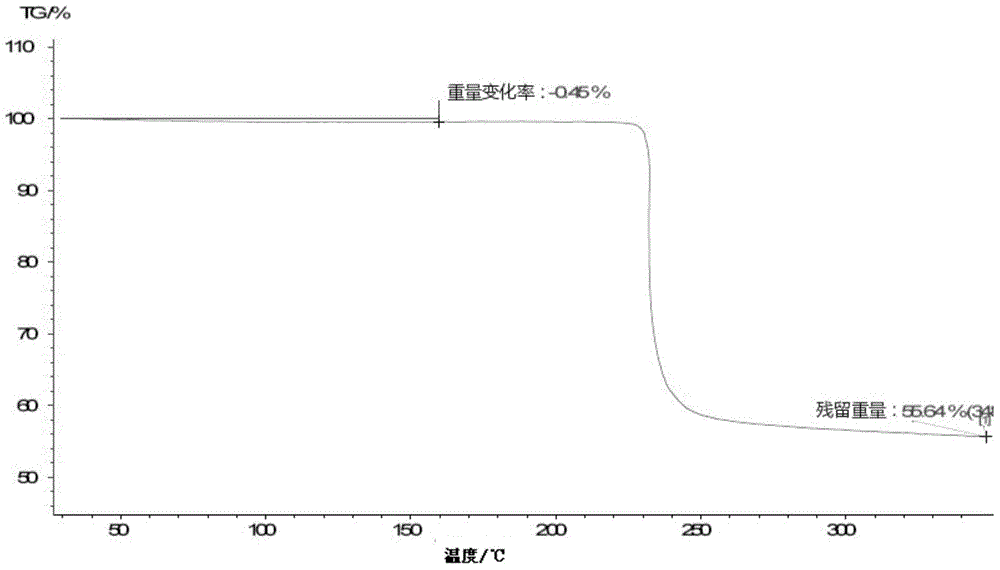
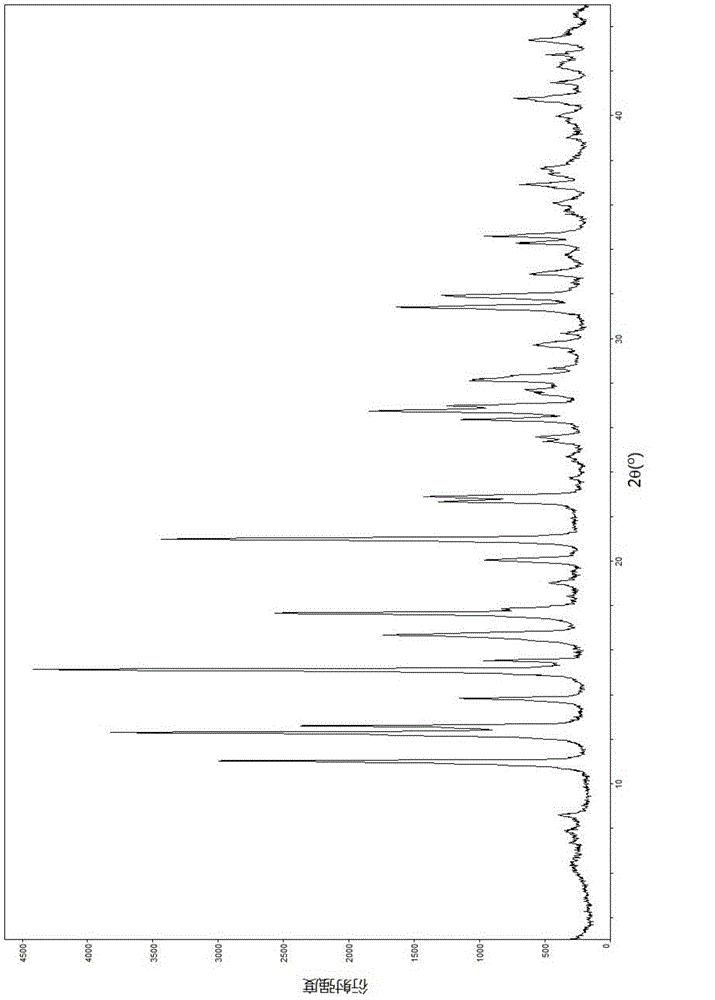
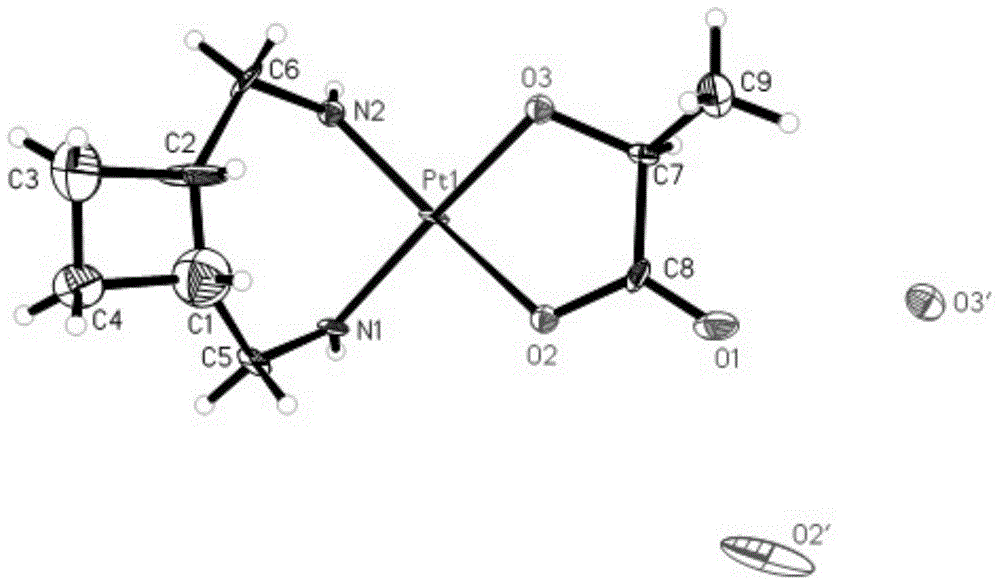
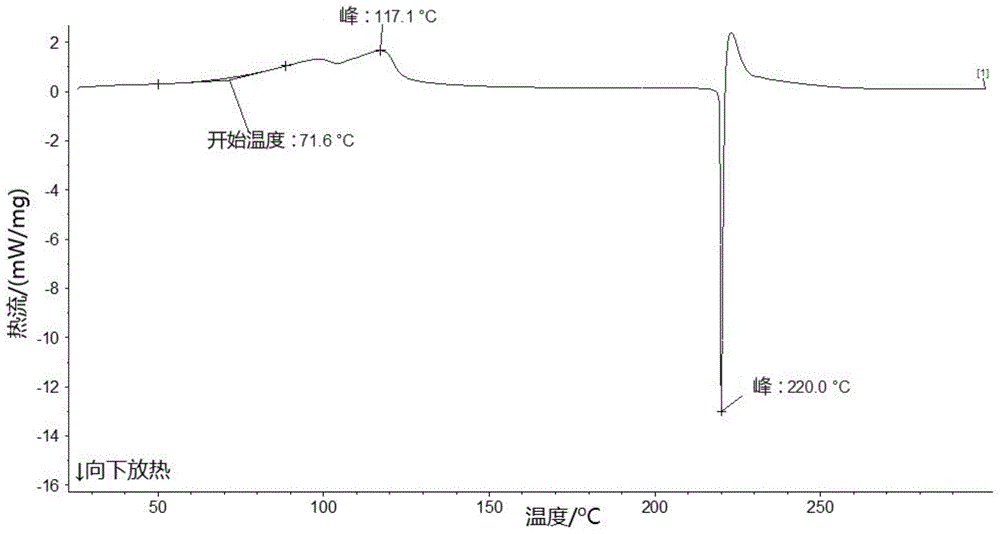
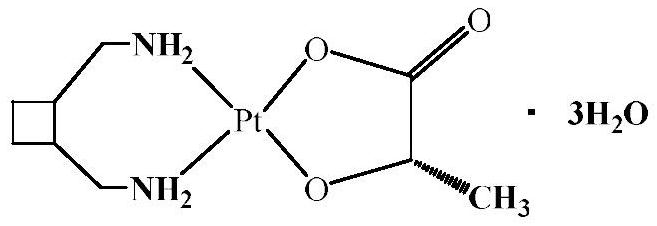
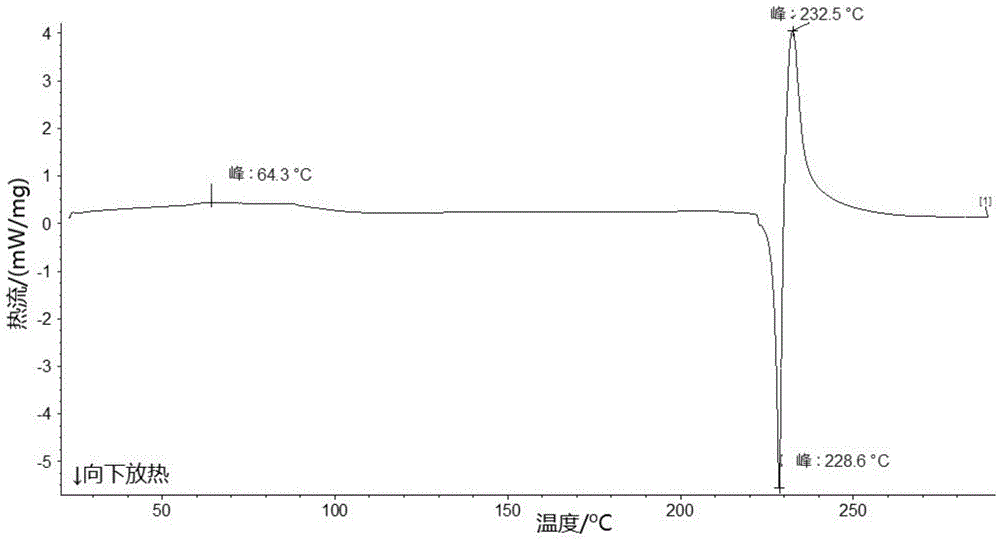
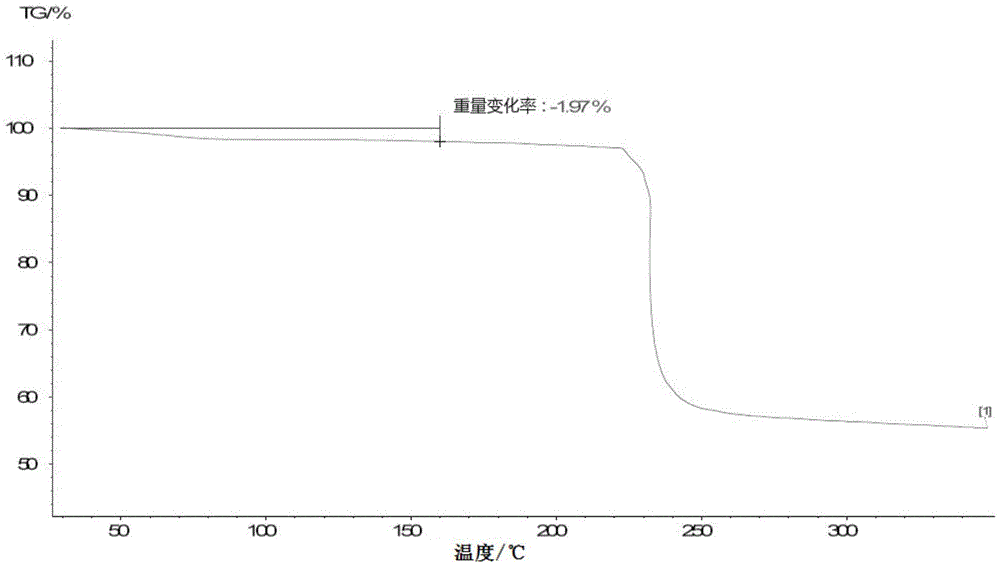
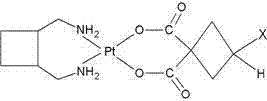
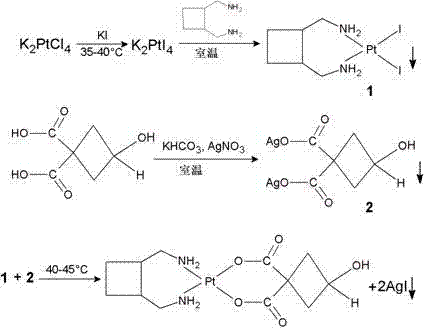
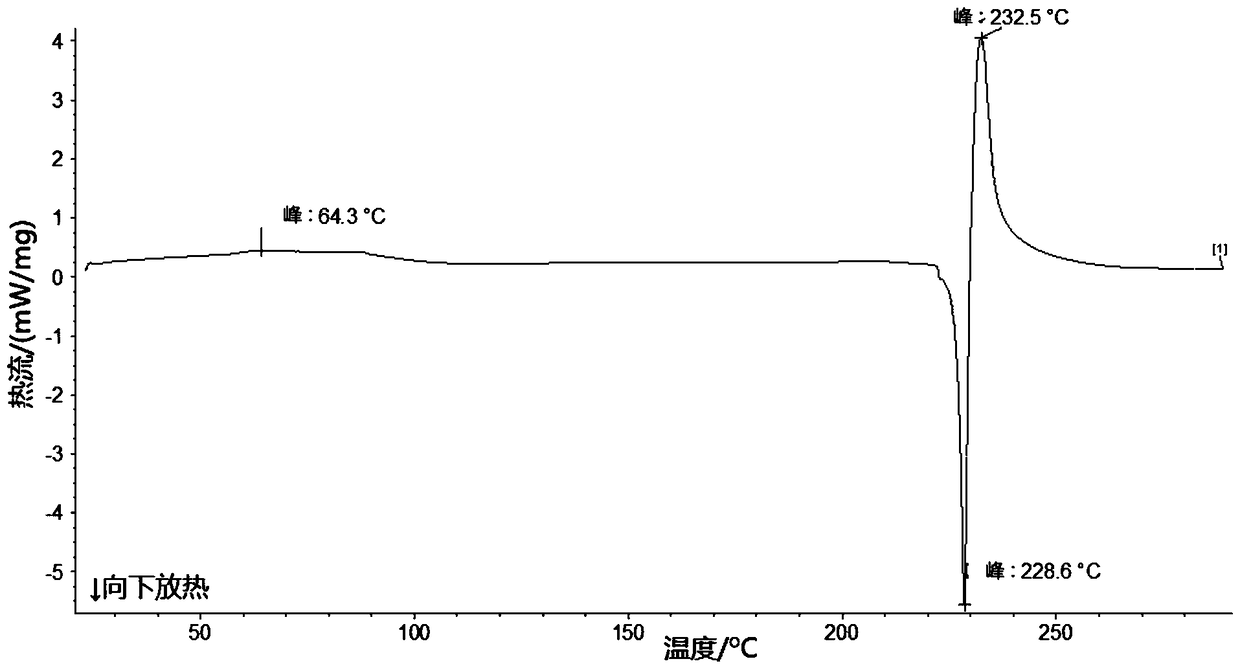
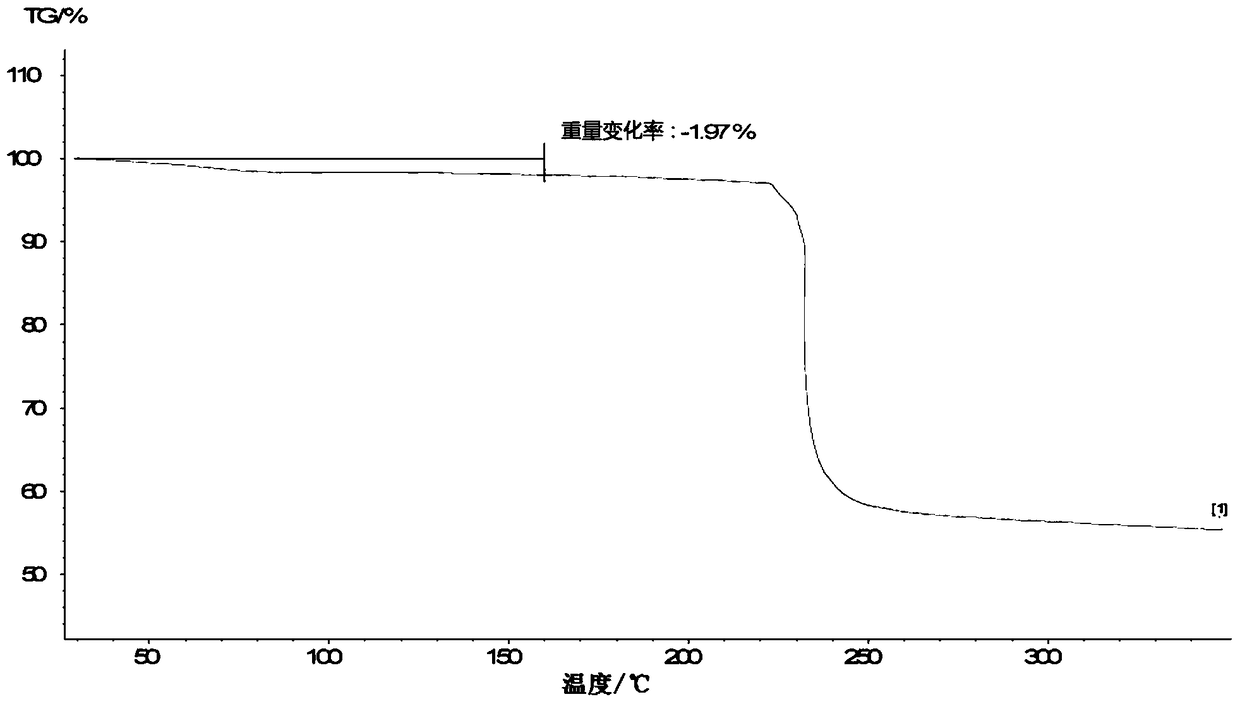
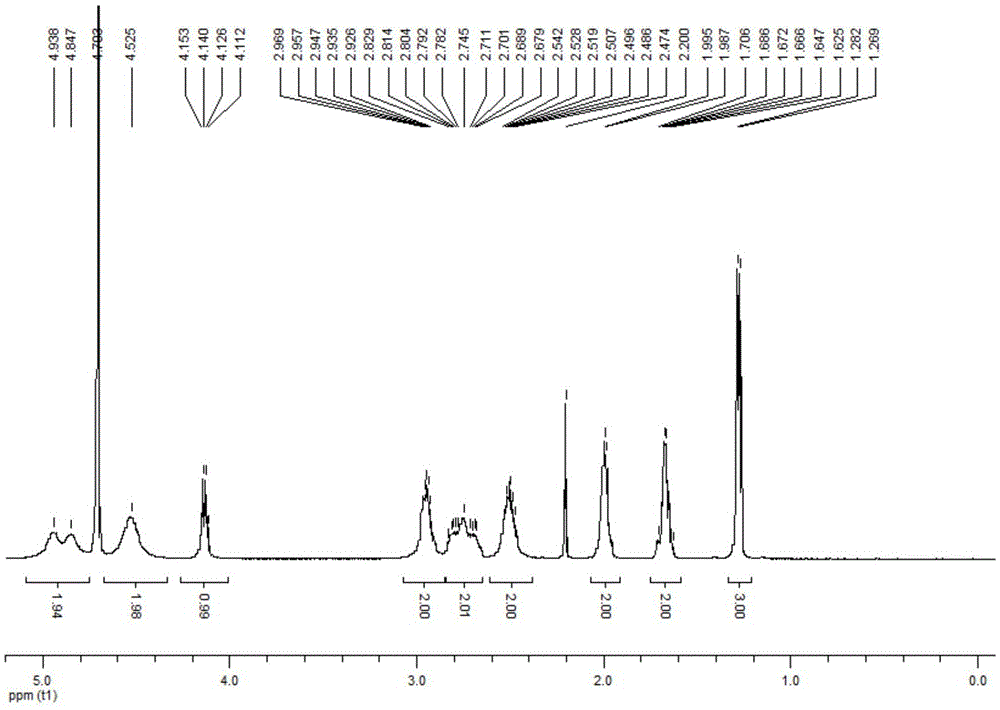
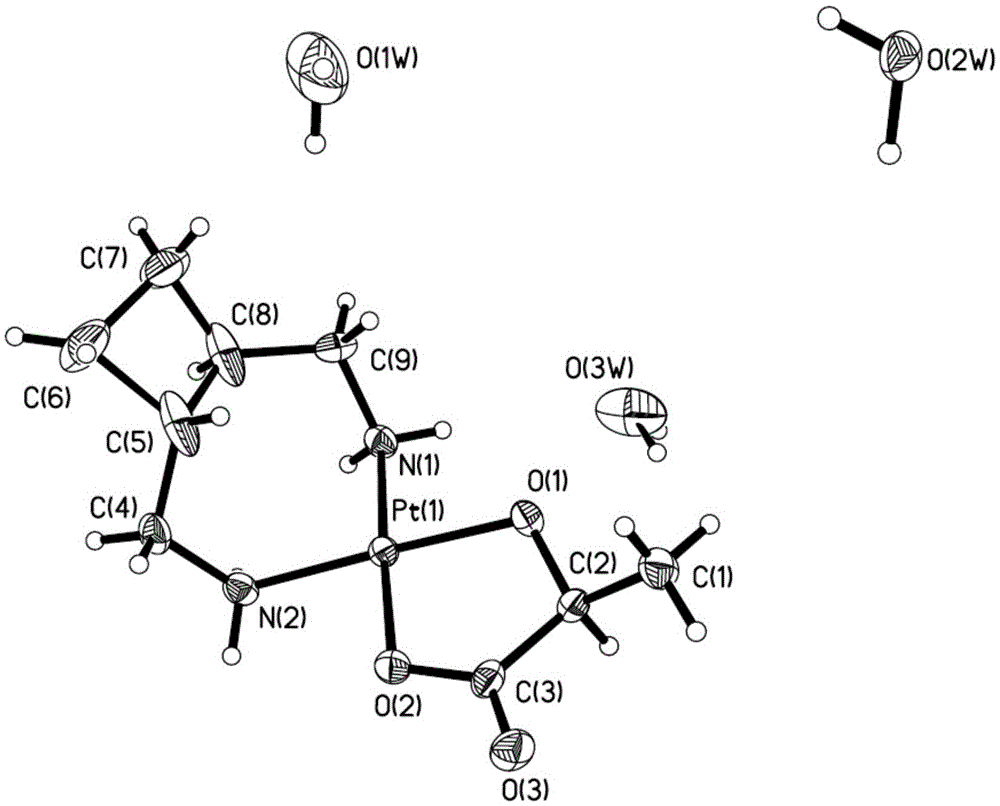
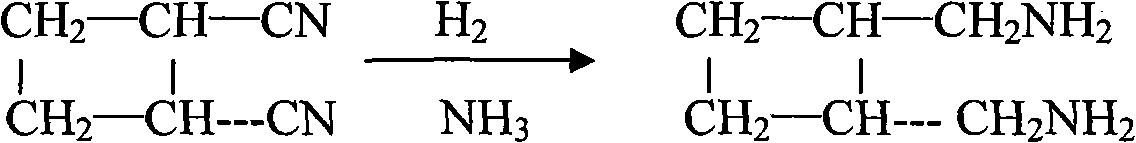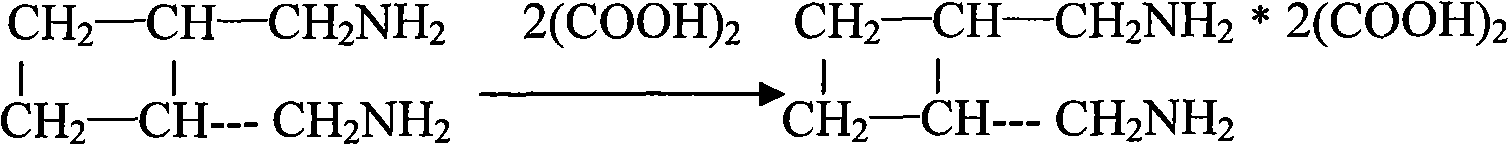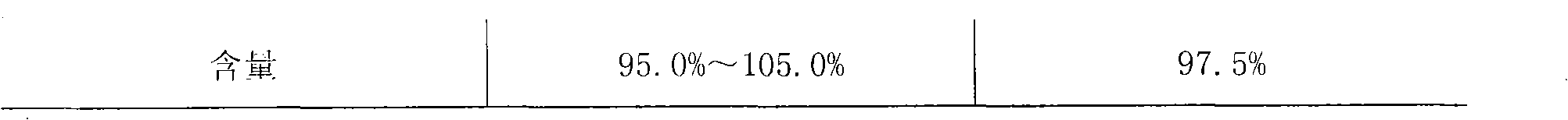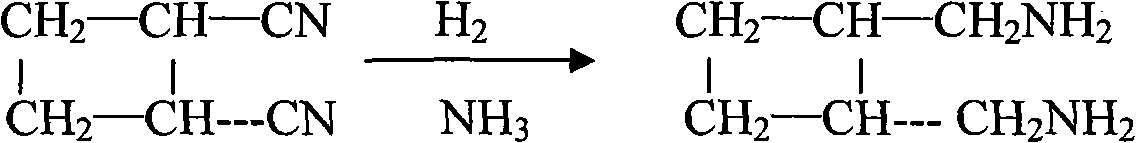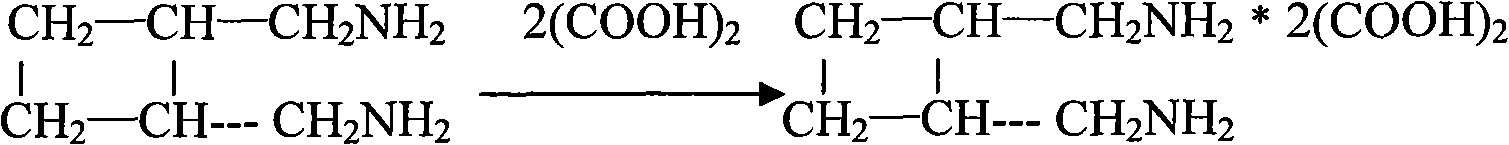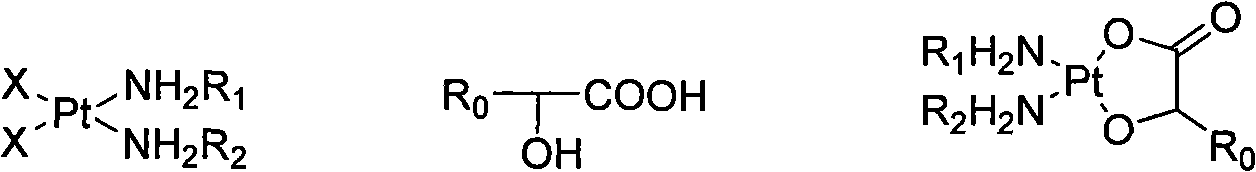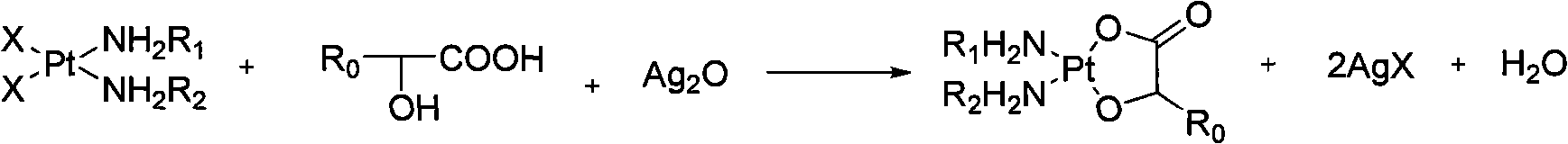Patents
Literature
41 results about "Lobaplatin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
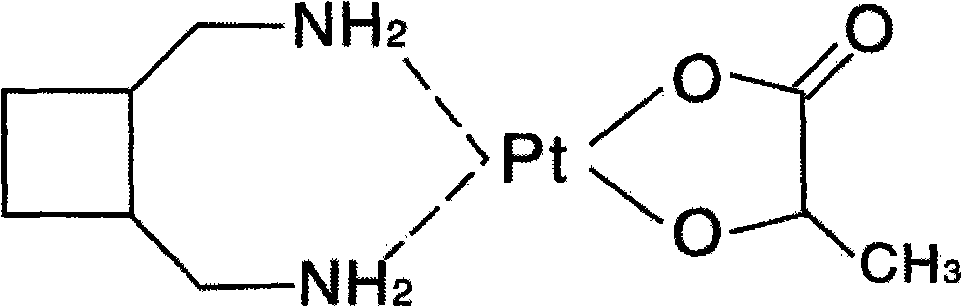
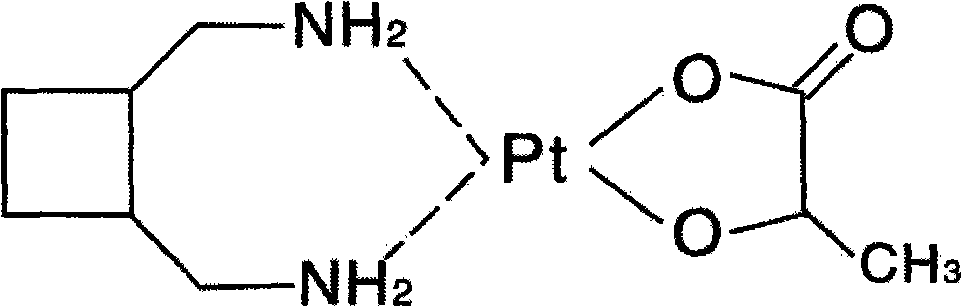
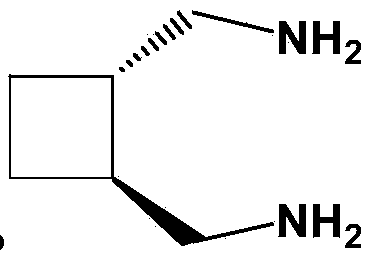
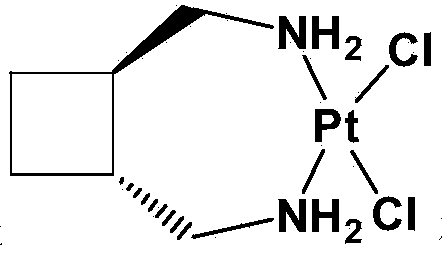
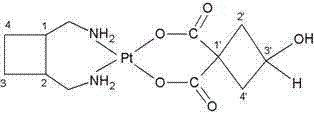
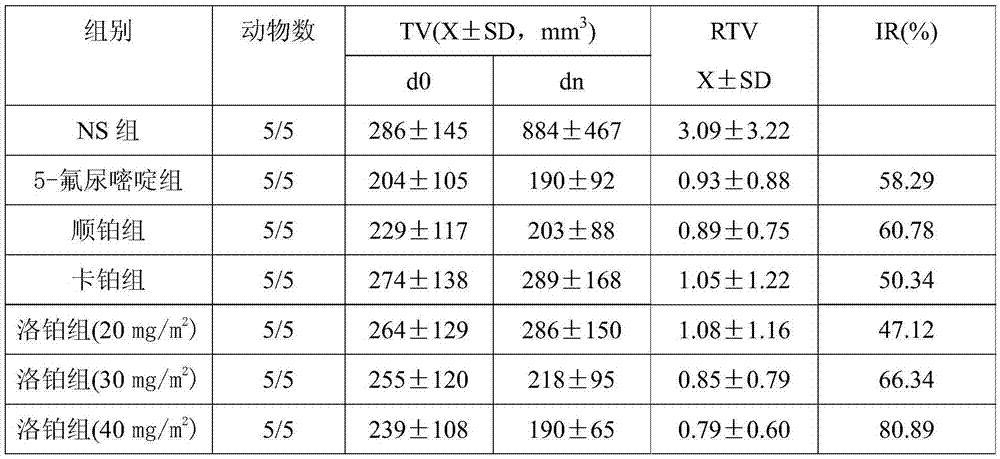
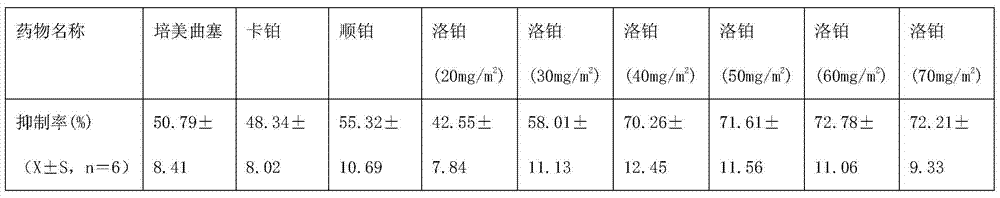
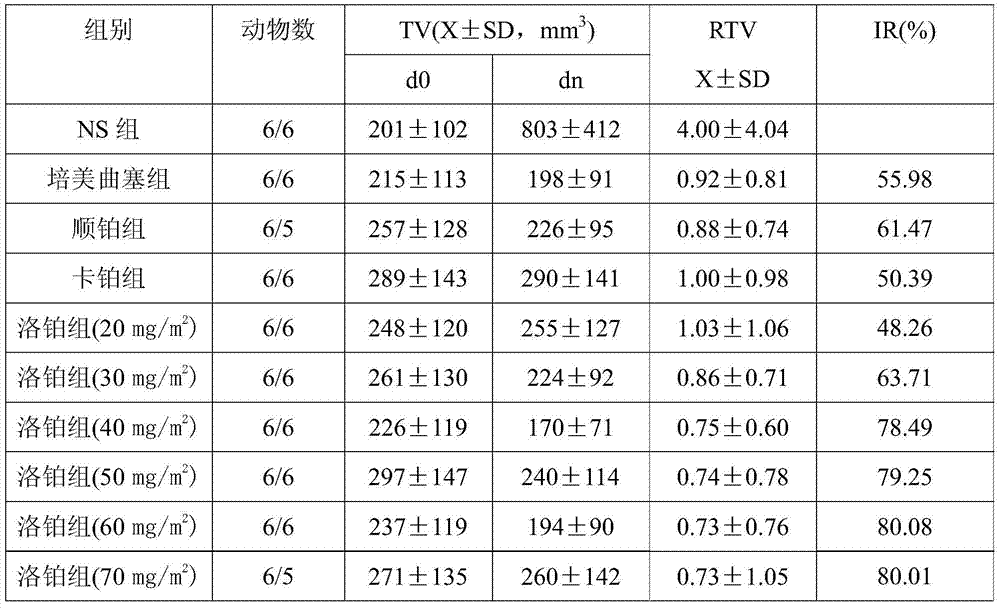
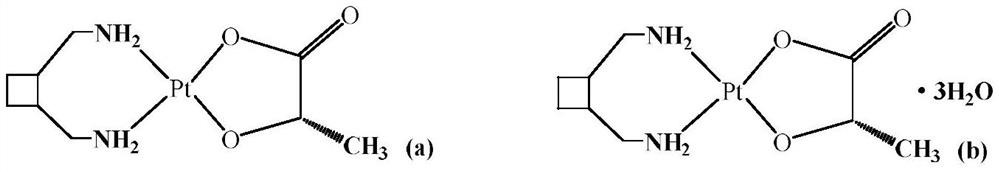
A third-generation, water-soluble platinum compound with potential antineoplastic activity. Lobaplatin forms highly reactive, charged, platinum complexes that bind to nucleophilic groups such as GC- and AG-rich sites in DNA, inducing intrastrand DNA cross-links. These cross-links will ultimately result in induction of apoptosis and cell growth inhibition. Compared to first and second generation platinum compounds, lobaplatin appears to be more stable, less toxic, have a better therapeutic index and may overcome tumor resistance.
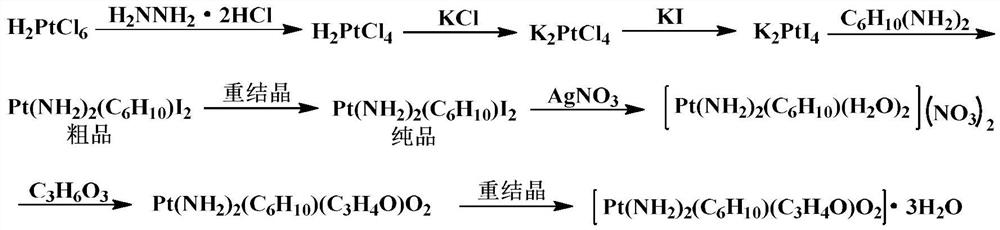
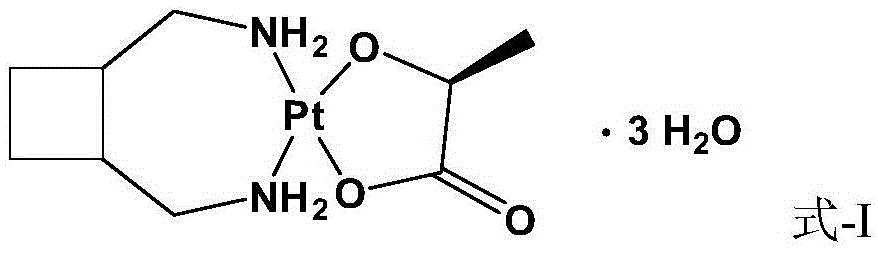
Method for preparing lobaplatin trihydrate by usingoxalate
ActiveCN102020679AHigh activitySuitable for industrialized mass productionGroup 8/9/10/18 element organic compoundsSilver iodidePotassium tetrachloroplatinate
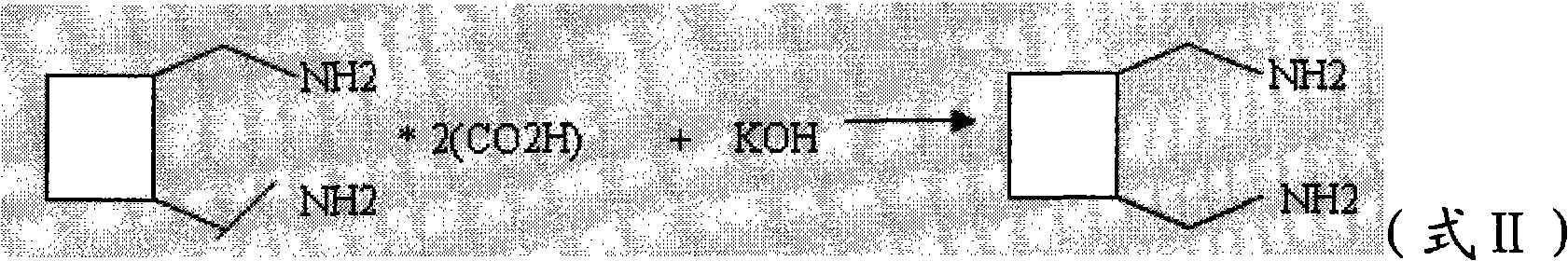
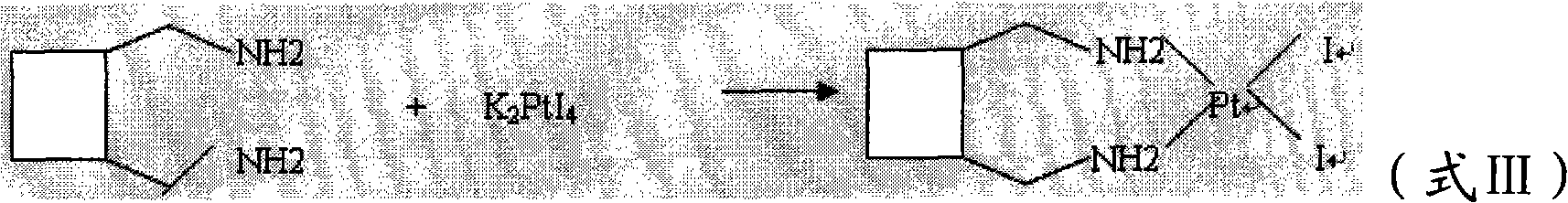
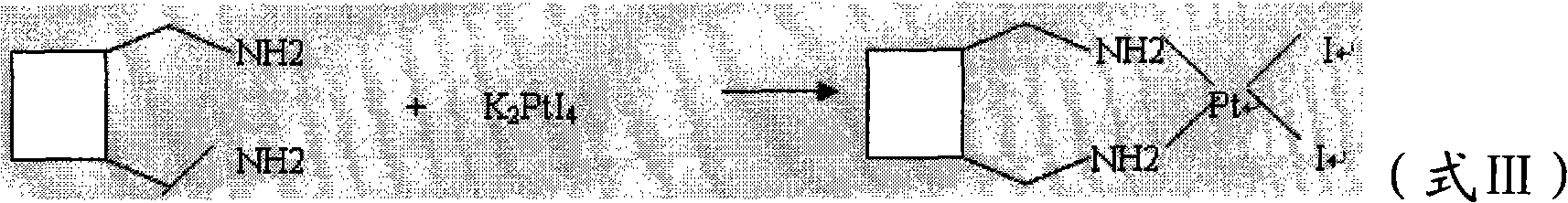
The invention relates to a method for preparing a lobaplatin trihydrate by using oxalate, diaminomethyl cyclobutane oxalate, potassium chloroplatinite and potassium iodide are taken as raw materials, and the method comprises the following steps: firstly replacing the potassium chloroplatinite to potassium iodoplatinite, neutralizing the oxalic acid in diaminomethyl cyclobutane under alkaline condition, enabling the diaminomethyl cyclobutane to be freed, and then reacting the diaminomethyl cyclobutane with K2PtI4 (potassium iodoplatinate) for synthesizing a diiodide; enabling the diiodide to carry out replacement reaction with silver nitrate to generate silver iodide precipitate, enabling reactants after filtering out the precipitate to carry out ion exchange with anion exchange resin, then synthesizing a lobaplatin anhydride with lactic acid, re-crystallizing the anhydride in water / acetone mixed solution, and finally obtaining a lobaplatin trihydrate product. The raw materials used in the method have stable performances, the reaction is fuller and more complete, and the yield of the lobaplatin trihydrate is high.
Owner:GUIZHOU YIBAI PHARMA CO LTD
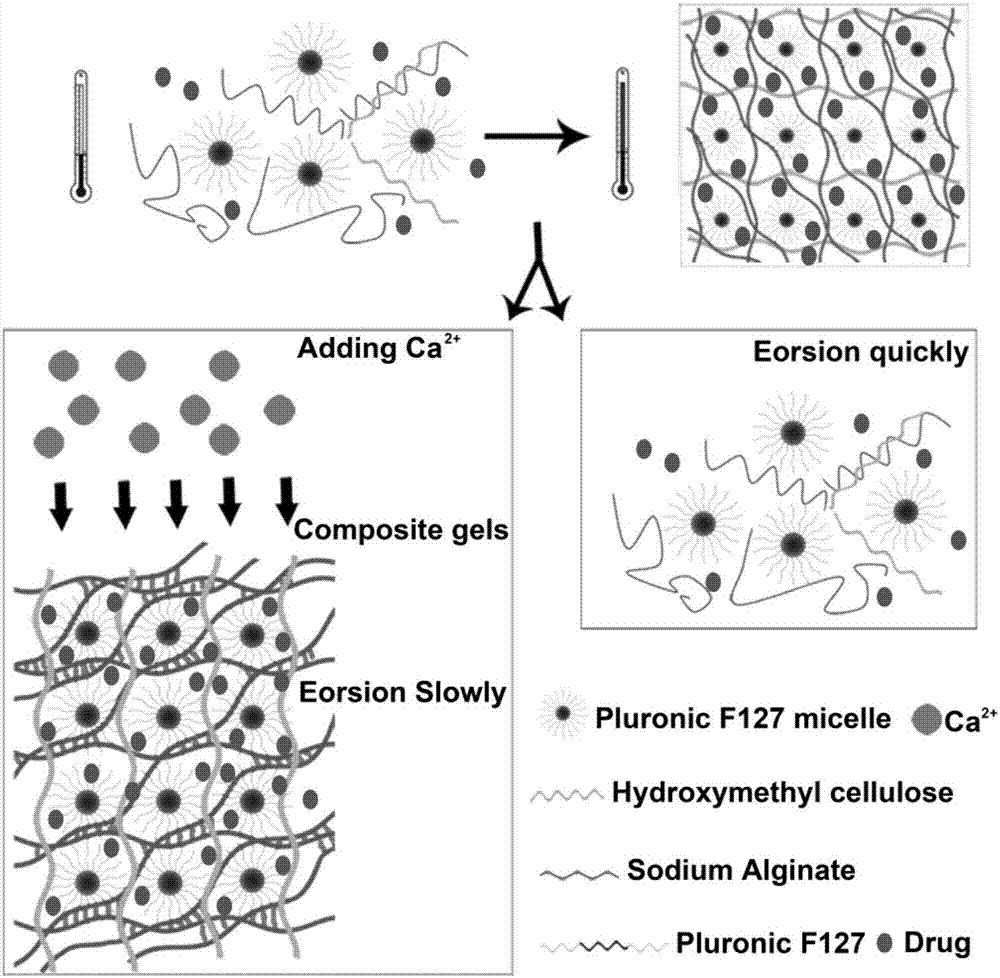
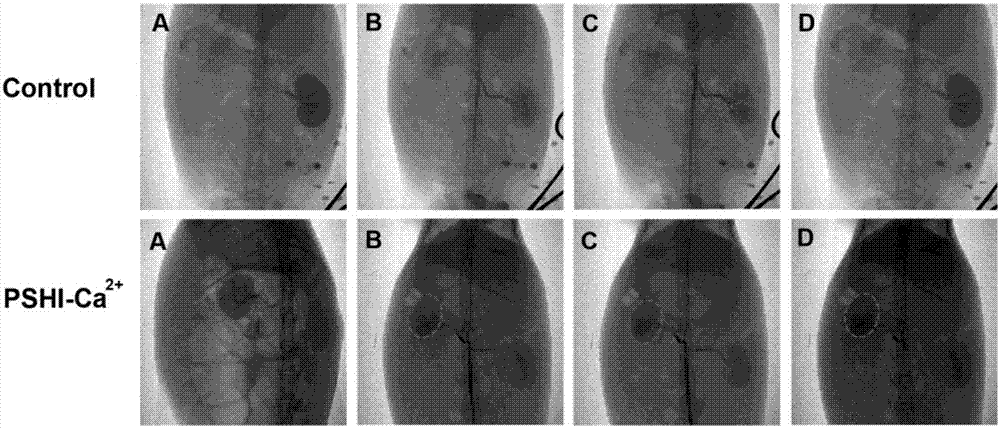
Composite developing thermosensitive gel embolizing agent as well as preparation method and application thereof
ActiveCN107281502AIncrease success rateReduce chanceOrganic active ingredientsHeavy metal active ingredientsCarboplatinMiriplatin
The invention relates to a composite developing thermosensitive gel embolizing agent as well as a preparation method and application thereof. The preparation method comprises the following steps: firstly, preparing a mixed aqueous solution of an anticancer active substance, a thermosensitive material and a developing agent into composite developing thermosensitive gel; secondly, forming a composite developing thermosensitive gel embolizing agent by the composite developing thermosensitive gel and a coagulant on the scene, wherein the thermosensitive material is hydroxyl C1-4 alkyl cellulose, Pluronic, alginate or a mixture of the substances; the anticancer active substance is arsenic trioxide, docetaxel, cisplatin, carboplatin, nedaplatin, oxaliplatin, lobaplatin, miriplatin, siRNA or a mixture of the substances; the developing agent is a water-soluble developing agent of iodixanol, ioversol or iohexol and the like. The preparation method disclosed by the invention is simple and convenient, is suitable for industrial large-scale production, is particularly suitable for preparing an embolizing agent which is biodegradable and good in biocompatibility and is used for hemorrhagic diseases, and is especially suitable for preparing the composite developing thermosensitive gel embolizing agent for treating liver cancer, kidney cancer, lung cancer, prostate cancer, uterine myoma or splenic tumor and the like.
Owner:苏州申润医疗科技有限公司 +1
Method for preparing lobaplatin trihydrate by usingoxalate
ActiveCN102020679BHigh yieldGroup 8/9/10/18 element organic compoundsSilver iodidePotassium tetrachloroplatinate
The invention relates to a method for preparing a lobaplatin trihydrate by using oxalate, diaminomethyl cyclobutane oxalate, potassium chloroplatinite and potassium iodide are taken as raw materials, and the method comprises the following steps: firstly replacing the potassium chloroplatinite to potassium iodoplatinite, neutralizing the oxalic acid in diaminomethyl cyclobutane under alkaline condition, enabling the diaminomethyl cyclobutane to be freed, and then reacting the diaminomethyl cyclobutane with K2PtI4 (potassium iodoplatinate) for synthesizing a diiodide; enabling the diiodide to carry out replacement reaction with silver nitrate to generate silver iodide precipitate, enabling reactants after filtering out the precipitate to carry out ion exchange with anion exchange resin, then synthesizing a lobaplatin anhydride with lactic acid, re-crystallizing the anhydride in water / acetone mixed solution, and finally obtaining a lobaplatin trihydrate product. The raw materials used in the method have stable performances, the reaction is fuller and more complete, and the yield of the lobaplatin trihydrate is high.
Owner:GUIZHOU YIBAI PHARMA CO LTD
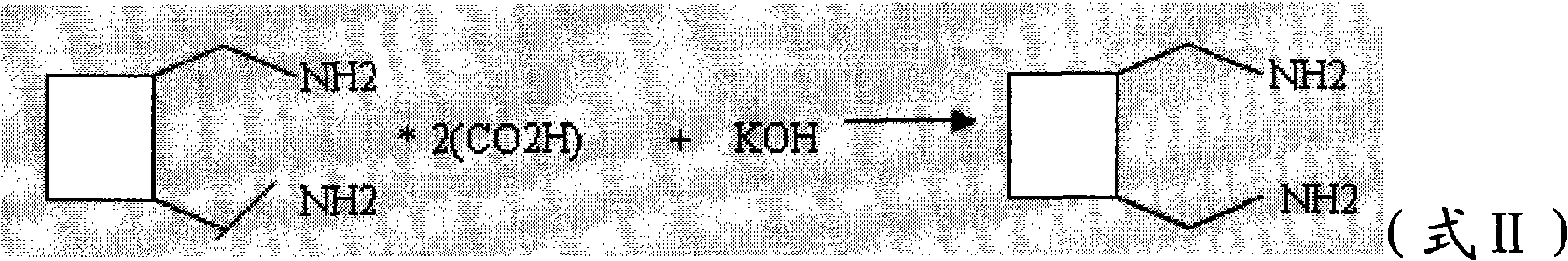
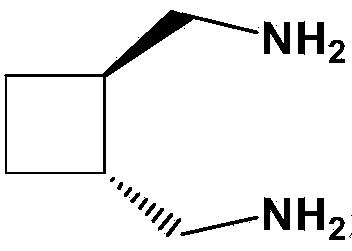
Preparation method of diaminomethyl cyclobutane oxalate
The invention relates to a preparation method of diaminomethyl cyclobutane oxalate, which comprises the following steps: carrying out dimerization on acrylonitrile used as a raw material to generate a cis-dinitrile cyclobutane / trans-dinitrile cyclobutane mixture, carrying out distillation separation on the generated cis-dinitrile cyclobutane / trans-dinitrile cyclobutane mixture, and converting into trans-dinitrile cyclobutane by reduction; by using active nickel as a catalyst, introducing ammonia, and pressurizing with hydrogen to react, thereby obtaining trans-diaminomethyl cyclobutane; adding oxalic acid to obtain a crude diaminomethyl cyclobutane oxalate product; and finally, purifying with alcohol to obtain the finished diaminomethyl cyclobutane oxalate product. By using the method provided by the invention, the raw material is accessible, the operation is easy to control, the obtained product can be used as an intermediate to participate in the synthesis of Lobaplatin, and the obtained Lobaplatin has more stable properties and is more suitable for industrial production.
Owner:海南长安国际制药有限公司
Preparation method of lobaplatin
ActiveCN103467528AShort reaction timeShorten the production cycleGroup 8/9/10/18 element organic compoundsFiltrationSolvent
The invention provides a preparation method of lobaplatin and lobaplatin trihydrate. Trans-diamine methyl cyclobutane reacts and synthesizes with chloroplatinite in water at the atmosphere of inert gas to generate dichloride, wherein the trans-diamine methyl cyclobutane and the chloroplatinite are taken as raw materials, and the water serves as a solvent; replacement reaction is performed on the dichloride and silver nitrate, so that silver chloride sediments are generated after filtration; filter liquor reacts with L-lactic acid and / or L-lactate under a certain pH value condition so as to generate the lobaplatin; then water / acetone recrystallization is performed on the lobaplatin, so that high-purity lobaplatin trihydrate is obtained. The preparation method provided by the invention includes few reaction steps, and is short in reaction time, high in reaction efficiency, easy and convenient to operate and suitable for industrial production.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Method for preparing antitumor medical preparation
The invention belongs to the technical field of chemical pharmacy and in particular relates to a method for preparing an antitumor medical preparation. The method comprises the following steps of: (1) preparing solution by taking Lobaplatin as a raw material and adding a proper amount of cooled water for injection; (2) adding a proper amount of active carbon with needle uses and adding the water for injection until full content, stirring while preserving heat and then performing aseptic filtration; (3) bulking filtrate and half pressing the plug, collecting the filtrate to a free-drying plate; putting into a freeze fryer cooling chamber, prefreezing, vacuumizing and heating, performing sublimation drying and desorbtion drying and performing vacuum breaking and free-drying; (4) introducing dried and degermed high-purity nitrogen, taking the obtained product out of the freezer and rolling the cover to obtain Lobaplatin for injection.
Owner:海南长安国际制药有限公司
Preparation method of diaminomethyl cyclobutane oxalate
The invention relates to a preparation method of diaminomethyl cyclobutane oxalate, which comprises the following steps: carrying out dimerization on acrylonitrile used as a raw material to generate a cis-dinitrile cyclobutane / trans-dinitrile cyclobutane mixture, carrying out distillation separation on the generated cis-dinitrile cyclobutane / trans-dinitrile cyclobutane mixture, and converting into trans-dinitrile cyclobutane by reduction; by using active nickel as a catalyst, introducing ammonia, and pressurizing with hydrogen to react, thereby obtaining trans-diaminomethyl cyclobutane; adding oxalic acid to obtain a crude diaminomethyl cyclobutane oxalate product; and finally, purifying with alcohol to obtain the finished diaminomethyl cyclobutane oxalate product. By using the method provided by the invention, the raw material is accessible, the operation is easy to control, the obtained product can be used as an intermediate to participate in the synthesis of Lobaplatin, and the obtained Lobaplatin has more stable properties and is more suitable for industrial production.
Owner:海南长安国际制药有限公司
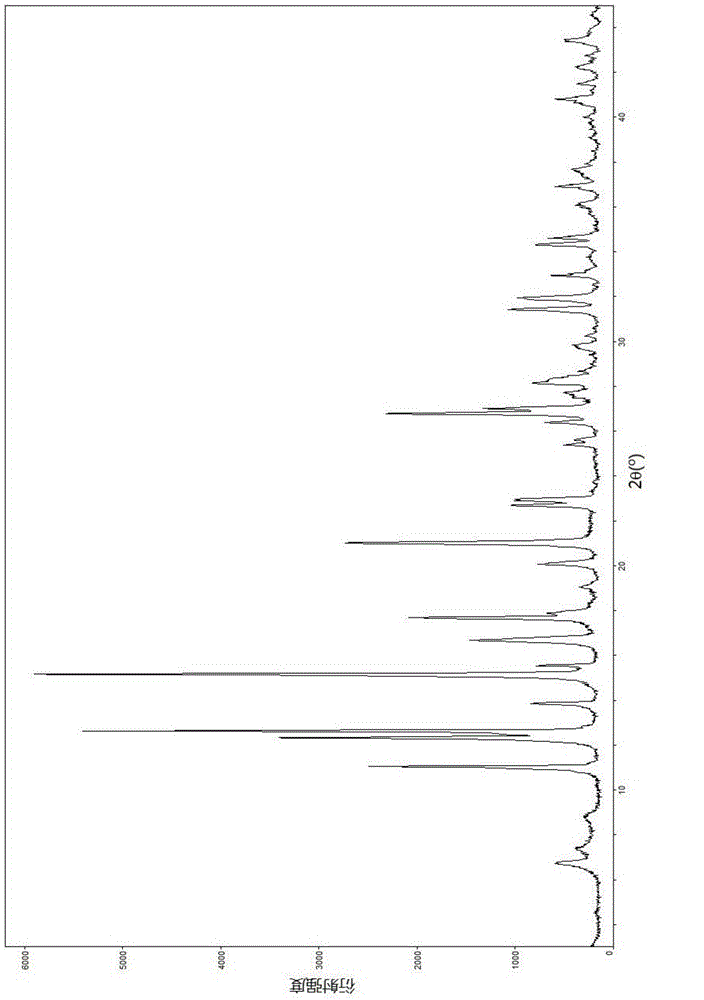
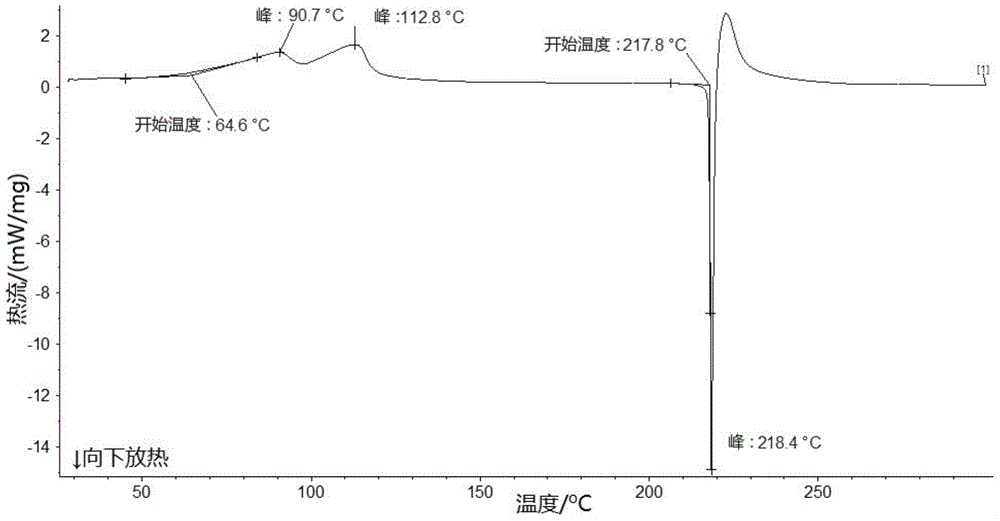
Lobaplatin crystal, and preparation method thereof and pharmaceutical application thereof
ActiveCN105218587AImprove bioavailabilityNot easy to absorb moisture and become stickyGroup 8/9/10/18 element organic compoundsAntineoplastic agentsSolubilityX-ray
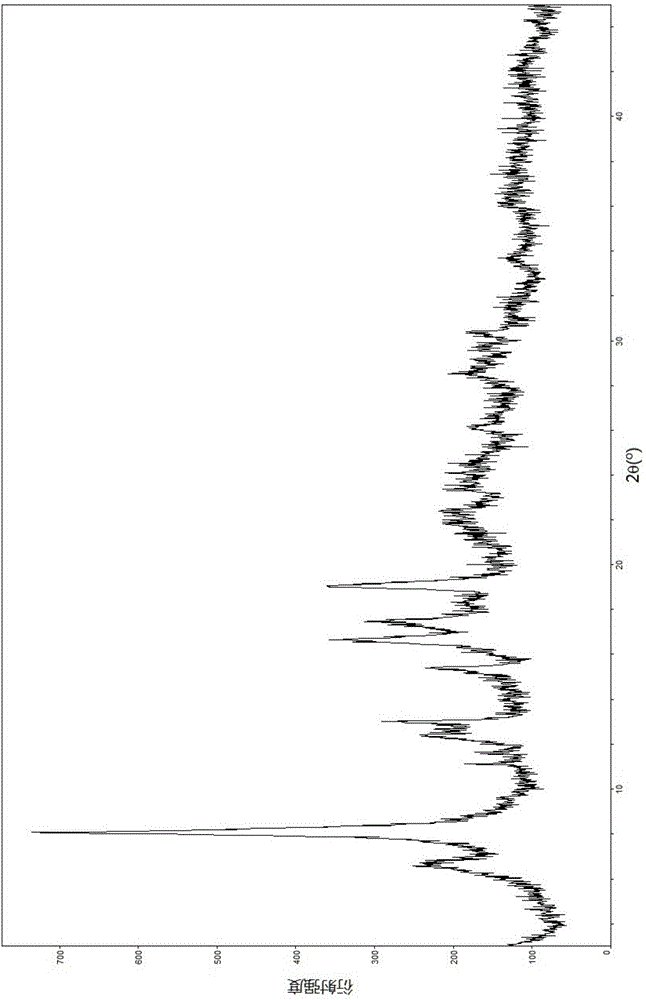
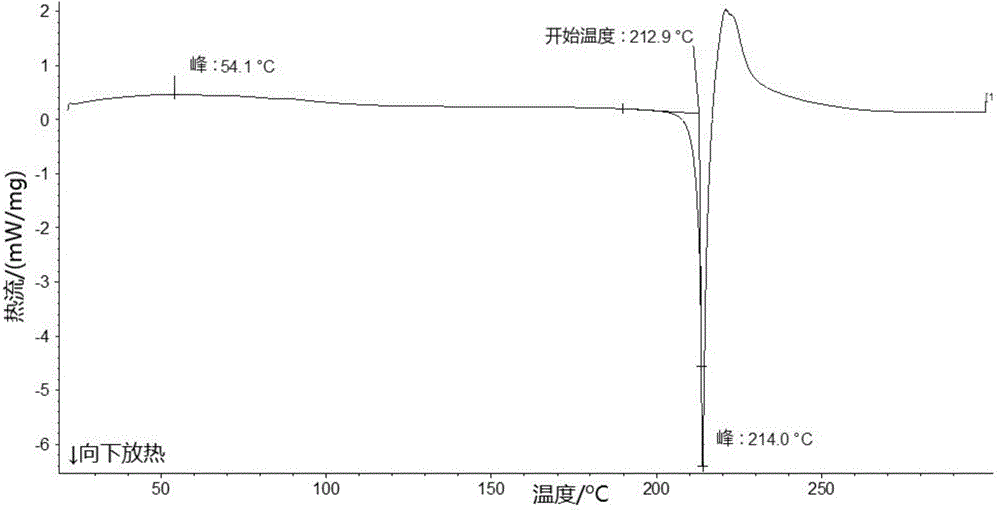
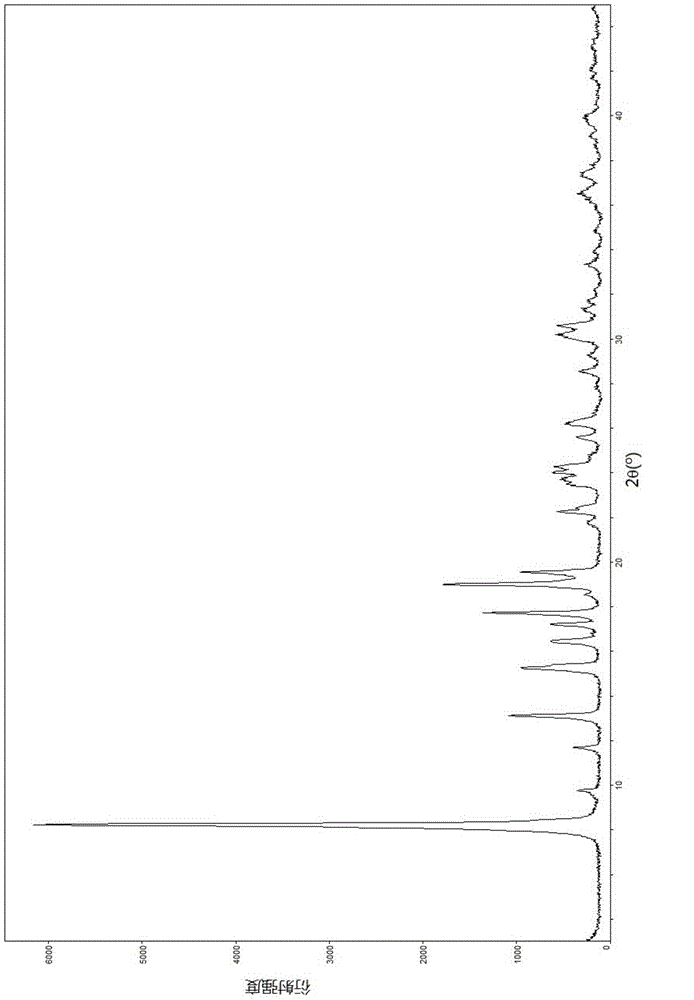
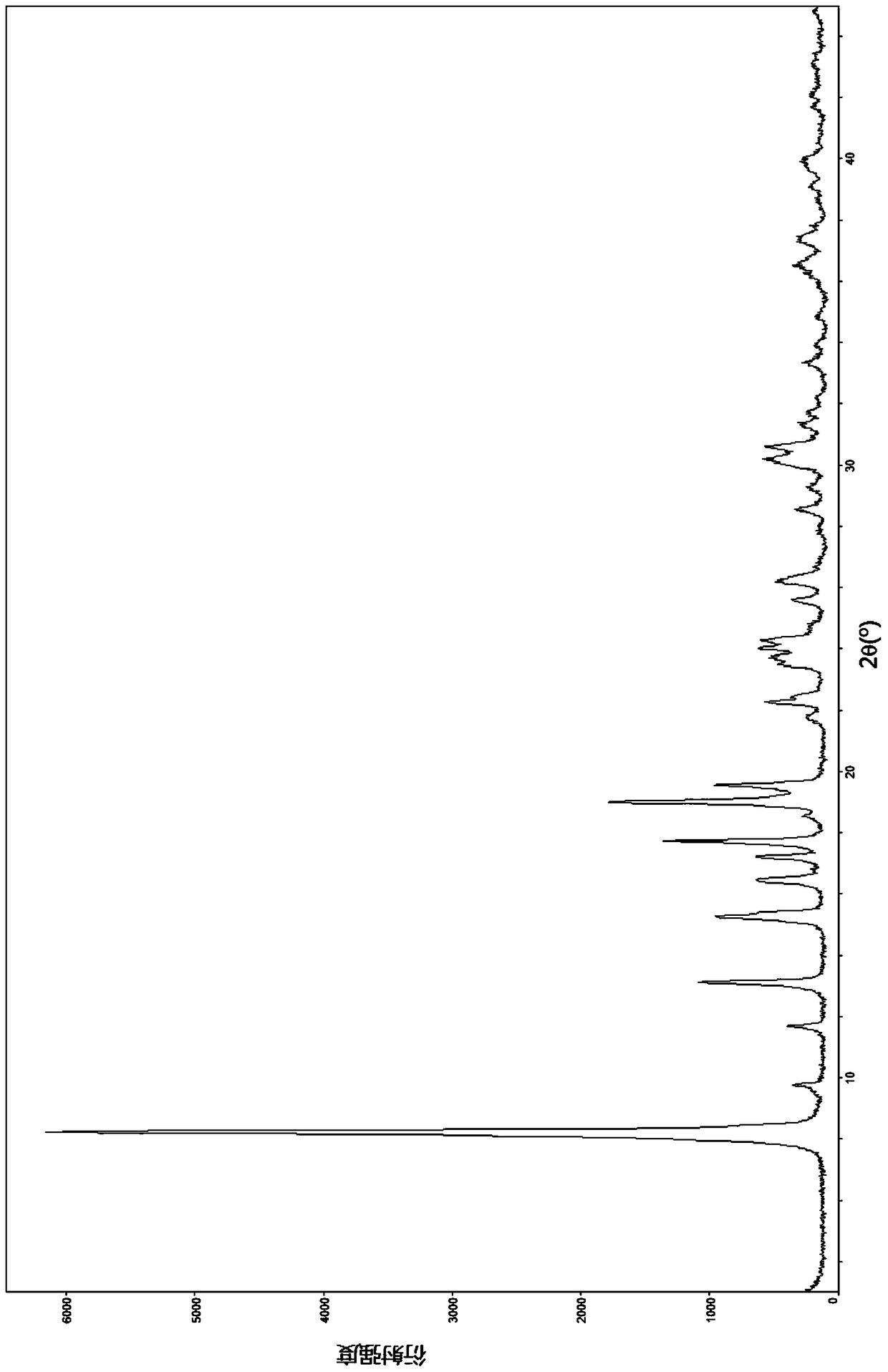
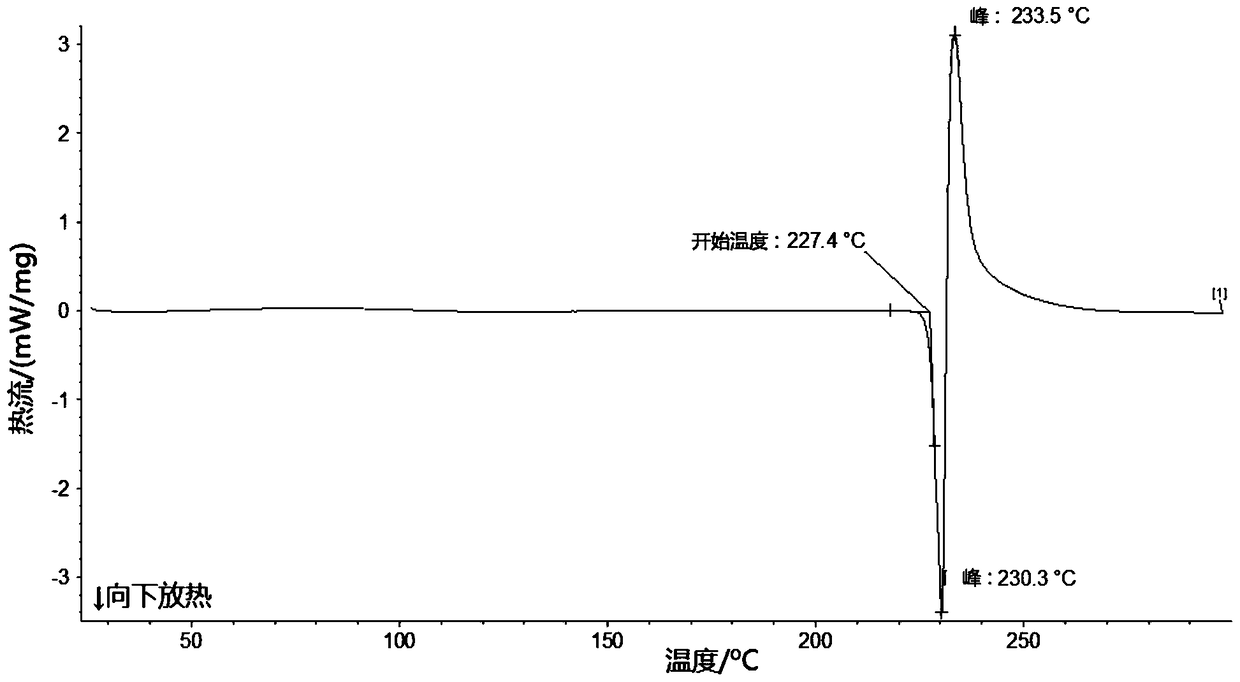
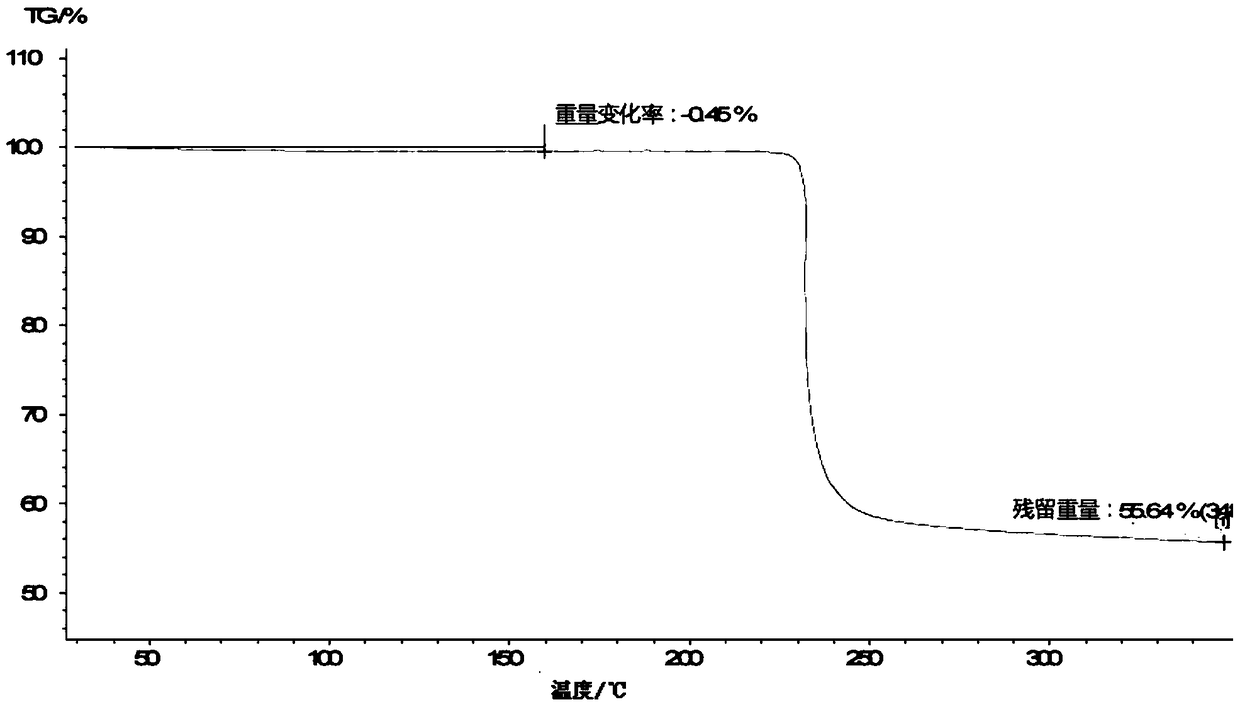
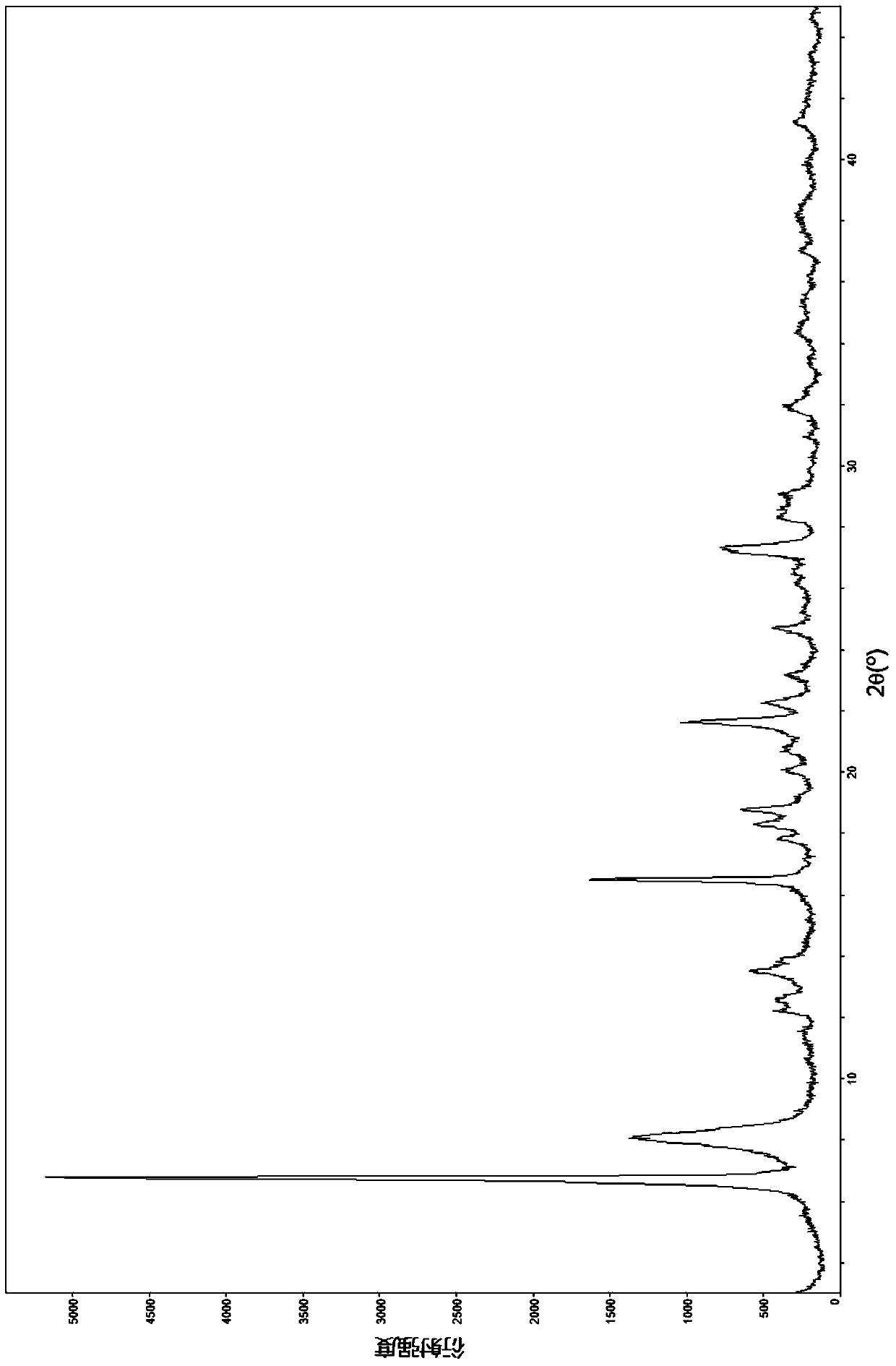
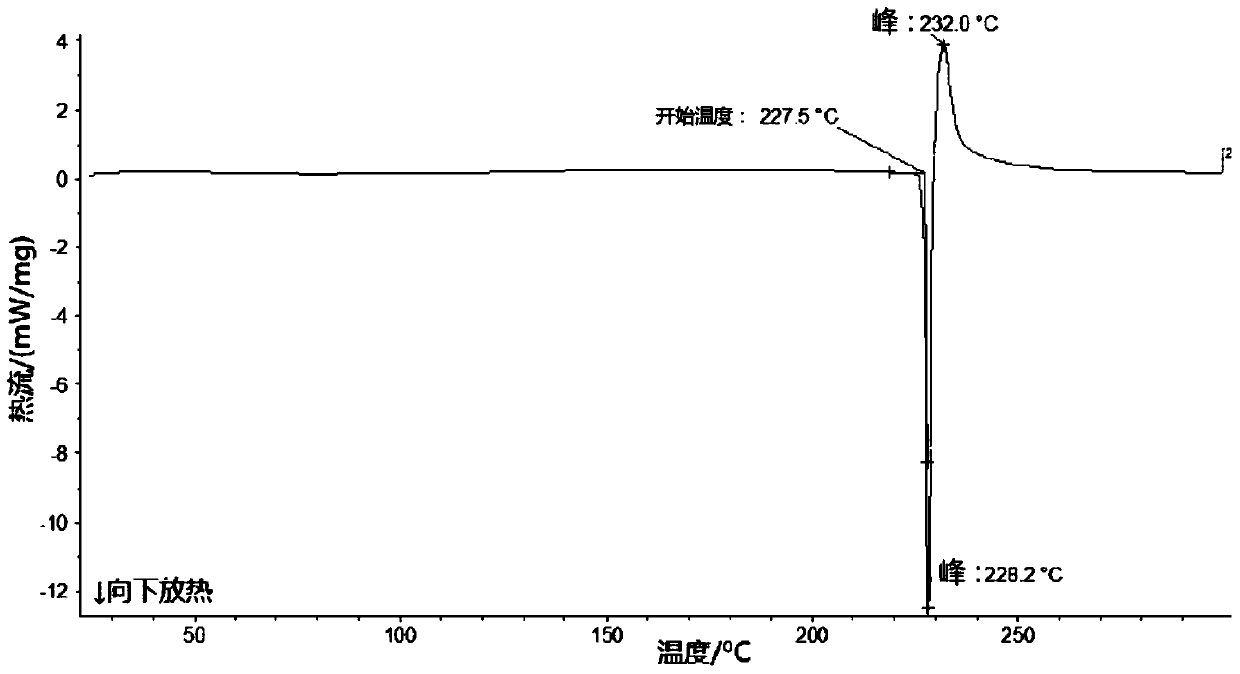
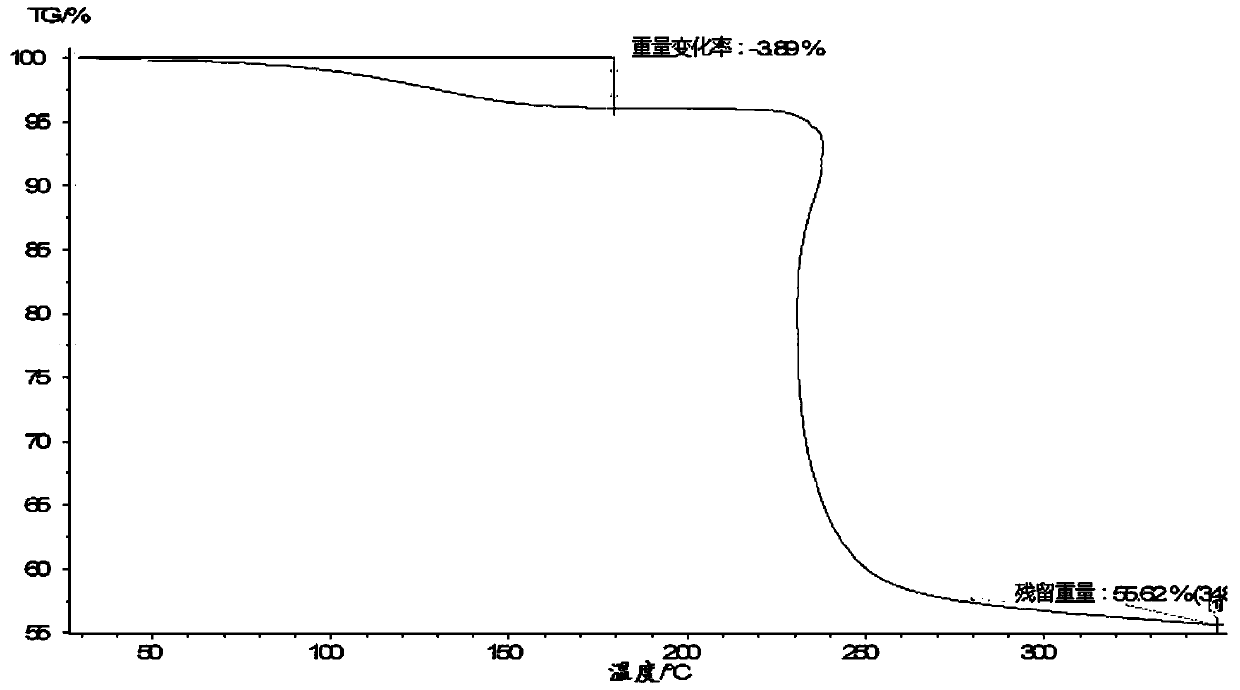
The invention relates to a lobaplatin crystal, and a preparation method thereof and a pharmaceutical application thereof. The crystal has a crystal form of E, and has a melting point Tm.p.. of 214+ / -5 DEG C. In an X-ray powder diffraction PXRD pattern of the crystal, diffraction peaks appear when 2theta angle values are 6.61, 8.09, 12.38, 13.03, 15.40, 16.66, 17.47 and 19.07, wherein an 2theta value error range is 0.2. The crystal form E is obtained through the following steps: glycol dimethyl ether is added into lobaplatin dihydrate; suspension stirring is carried out, and crystals are precipitated; the crystals are separated and dried, such that white powder is obtained. Therefore, the novel crystal form E of lobaplatin is obtained. Compared with existing lobaplatin trihydrate, the crystal form provided by the invention has better stability and solubility. The crystal form is suitable to be used for preparing various forms of pharmaceutical preparations, and is suitable for storage and application. The crystal can be better used for treating cancers such as breast cancer, small-cell lung cancer or chronic myelogenous leukemia.
Owner:GUIZHOU YIBAI PHARMA CO LTD
Lobaplatin crystal and preparation method and drug application thereof
ActiveCN105440083AImprove bioavailabilityNot easy to absorb moisture and become stickyGroup 8/9/10/18 element organic compoundsAntineoplastic agentsSolubilityChronic granulocytic leukemia
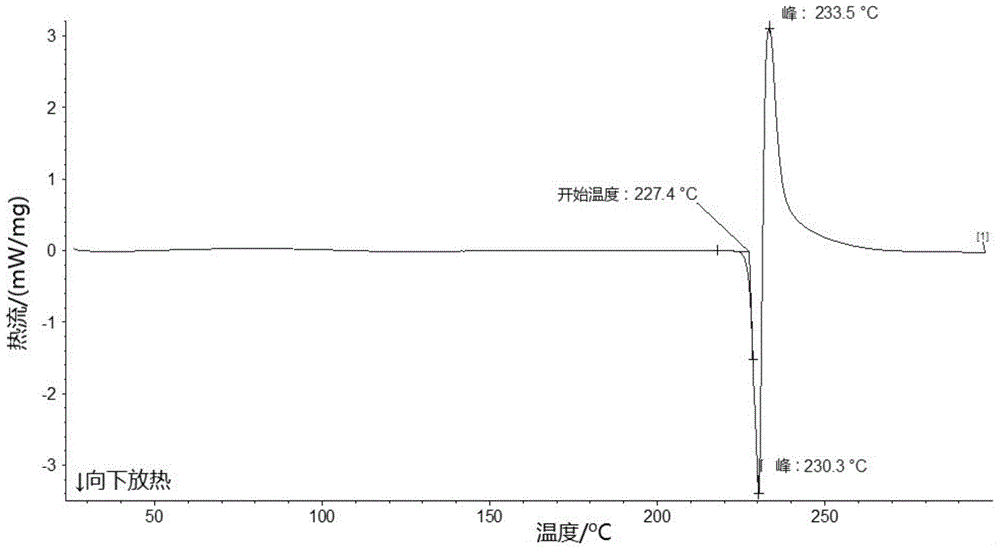
The present invention relates to a lobaplatin crystal and a preparation method and drug application thereof, the lobaplatin crystal form is B, the melting point Tm. p. . is 230 + / -5 DEG C, diffraction peaks exist at the 2 theta angle values of 8.25, 9.77, 11.70, 13.13, 15.28, 16.48, 17.22, 17. 74, 19.01, 19.56, 22.28, 23.72, 24.04, 24.30, 25.62, 26.20, 28.57, 30.22 and 30.61 in an X-ray powder diffraction PXRD spectrum, wherein the 2 theta angle value error is in the range of 0.2. The crystal form B is obtained by solvent evaporation of lobaplatin trihydrate, or volatilization at room temperature after addition of a solvent in lobaplatin dihydrate or solvent-out crystallization, and drying. Compared with lobaplatin and the lobaplatin trihydrate in the prior art, the lobaplatin crystal in the crystal form B has better stability and solubility, is more suitable for the preparation of various forms of pharmaceutical preparations and storage and use, and can be better used to treat cancers such as breast cancer, small cell lung cancer or chronic granulocytic leukemia.
Owner:GUIZHOU YIBAI PHARMA CO LTD
Lobaplatin dihydrate, preparation method and drug application
ActiveCN105198932ANot easy to deliquescenceDeliquescence becomes stickyGroup 8/9/10/18 element organic compoundsAntineoplastic agentsSolubilityMyeloid leukemia
The invention relates to lobaplatin dihydrate, a preparation method and drug application. The melting point Tm.p.. of the lobaplatin dihydrate is 220+ / -5 DEG C, and the crystal form of the lobaplatin dihydrate is A. An X-ray powder diffraction PXRD pattern has diffraction peaks when the 2theta value is 11.04, 12.32, 12.61, 13.85, 15.14, 15.55, 16.68, 17.67, 17.86, 19.03, 20.06, 21.00, 22.68, 22.92, 23.76, 25.39, 25.58, 26.37, 26.77, 27.00, 27.71, 28.13, 29.71, 31.42, 31.94, 32.89, 34.29, 34.60, 36.10, 36.93, 37.66, 40.78 and 43.41, wherein the error range of the 2theta value is 0.2. The lobaplatin dihydrate is obtained by adding lobaplatin trihydrate into suspension crystallization solvent and performing suspension crystallization. Compared with the prior art lobaplatin and lobaplatin trihydrate, the lobaplatin dihydrate has better stability and solubility, is more suitable for preparation of drug preparations of different forms, storage and usage and can be better used for treating breast cancer or small cell lung cancer or chronic myeloid leukemia or the like.
Owner:GUIZHOU YIBAI PHARMA CO LTD
Method for preparing hydroxy carboxylic acid platinum complexes
InactiveCN101787052AThe synthesis method is simpleShort processGroup 8/9/10/18 element organic compoundsFiltrationCarboxylic acid
The invention relates to a method for preparing hydroxy carboxylic acid platinum complexes, in particular to a method for preparing platinum anticancer drugs of nedaplatin and lobaplatin. The preparation method includes that: compounds (I) and (II) and silver oxide in molar ratio of 1:1: 1, and certain quantity of water are added to a reactor and stirred to conduct light-shielding reaction for 1-20 hours at the temperature of 0-80 DEG C. The filtrate after filtration is concentrated to be dry, is cooled and rinsed by water, filtrated and rinsed by ethanol and is finally pumped out to obtain the product (III) after vacuum drying for 2-3 hours at the temperature of 40-45 DEG C. The invention obtains the product water solution through one-step reaction, concentrates the water solution to obtain the final product, has no need to adjust the pH value in the synthesis process, avoids the Na<+>, NO<3->, SO4<2->, Ba<2+> interference reaction, and accordingly realizes high productivity and good purity.
Owner:HANGZHOU MINSHENG PHARM CO LTD
Lobaplatin crystal, preparation method and medical application thereof
ActiveCN105330702AImprove bioavailabilityNot easy to absorb moisture and become stickyGroup 8/9/10/18 element organic compoundsAntineoplastic agentsSolubilityChronic granulocytic leukemia
The invention relates to a lobaplatin crystal, a preparation method and a medical application thereof. A crystal form of the crystal is D, its melting point Tm.p..is 218+ / -5 DEG C, in a PXRD pattern of the lobaplatin crystal, diffraction peaks exist when the 2theta value is 6.76, 11.07, 12.35, 12.65, 13.88, 15.18, 15.56, 16.68, 17.70, 17.90, 20.08, 21.02, 22.70, 22.92, 25.41, 25.64, 26.41, 26.79, 27.02, 28.15, 31.44, 31.96, 32.96, 34.34, 34.62, 36.93, 40.82 and 43.46, and the error range of the 2theta value is 0.2. The preparing method for the crystal form D includes the steps that 1,2-dichloroethane is added into lobaplatin dihydrate, a mixture is stirred in a suspension mode, and the crystal is dissolved out, separated out, dried and then obtained. Compared with existing lobaplatin and lobaplatin trihydrate, better stability and better solubility are achieved, and the lobaplatin crystal can be better suitable for preparing medicine preparations in various forms, can be better stored and used and can be better used for treating cancers such as the breast cancer, the small cell lung cancer and the chronic granulocytic leukemia.
Owner:海南长安国际制药有限公司
Preparation method of lobaplatin trihydrate
ActiveCN112225757ASpend less timeHigh purityPlatinum organic compoundsBulk chemical productionSodium lactateSilver iodide
The invention discloses a preparation method of lobaplatin trihydrate, which comprises the following steps: by using potassium chloroplatinite, potassium iodide and trans-1,2-diaminomethylcyclobutanehydrochloride as raw materials and water as a solvent, replacing potassium chloroplatinite with potassium iodo-platinite, and reacting potassium iodo-platinite with trans-1,2-diaminomethylcyclobutanewhich is dissociated from the 1,2-diaminomethylcyclobutane hydrochloride under an alkaline condition through neutralization to generate a diiodide; and refining the diiodide, carrying out hydrolysis reaction on the refined diiodide and silver nitrate, filtering out generated silver iodide precipitate, reacting filtrate with sodium lactate to generate a lobaplatin anhydrous substance, and recrystallizing the lobaplatin anhydrous substance in an acetone-water mixed solvent to obtain the lobaplatin trihydrate. The method has the advantages of short synthesis time and simple operation, the precursor chemical acetone involved in the reaction process can be recycled, and the obtained lobaplatin trihydrate has the advantages of high purity, high yield and good stability, and is suitable for industrial production.
Owner:KUNMING GUIYAN PHARMA
Novel synthesis method of lobaplatin intermediate
ActiveCN113416150ALow priceReduce manufacturing costOrganic compound preparationOrganic chemistry methodsHydrolysisReaction step
The invention relates to the technical field of medicine synthesis, in particular to a novel synthesis method of a lobaplatin intermediate, the method comprises the following steps: taking low-toxicity dimethyl malonate as an initial raw material, carrying out coupling, bromination, cyclization, hydrolysis, amidation and dehydration reaction to synthesize high-purity trans-1, 2-dicyanocyclobutane. In the whole route synthesis, all adopted raw and auxiliary materials are easy to purchase, low in price and low in toxicity, the reaction conditions are not harsh and uncontrollable, the reaction conditions of each step can be suitable for amplified production, although the reaction steps are increased, the yield of the product is greatly improved, so that the cost is greatly reduced, and the purity of the obtained trans-1,2-dicyanocyclobutane is improved and completely meets the subsequent use requirements, and the market competitiveness is greatly improved.
Owner:上海寻科生物医药科技有限公司
Lobaplatin crystal and preparation method and drug application thereof
ActiveCN105440082AImprove bioavailabilityNot easy to absorb moisture and become stickyGroup 8/9/10/18 element organic compoundsAntineoplastic agentsSolubilityChronic granulocytic leukemia
The present invention relates to a lobaplatin crystal and a preparation method and drug application thereof, the lobaplatin crystal form is F, the melting point Tm. p. . is 229 + / -5 DEG C, diffraction peaks exist at the 2 theta angle values of 8.21, 11.60, 12.99, 15.24, 16.44, 17.11, 17.55, 18.42, 19.01, 19.20, 19.42, 21.81, 22.17, 22.42, 23.33, 23.85, 24.18, 24.40, 24.77, 25.46, 25.98, 26.13, 27.89, 28.42, 29.03, 30.32, 31.17, 31.94, 33.30, 36.20, 37.62 and 39.66 in an X-ray powder diffraction PXRD spectrum, wherein the 2 theta angle value error is in the range of 0.2. The crystal form F is obtained by adding methanol or ethanol into lobaplatin dihydrate, stirring at room temperature until the solid is dissolved, filtering insoluble matters out, adding dropwise slowly an organic solvent, after precipitation of a crystal, separating the crystal, and drying. Compared with lobaplatin and lobaplatin trihydrate in the prior art, the lobaplatin crystal in the crystal form F has better stability and solubility, is more suitable for the preparation of various forms of pharmaceutical preparations and storage and use, and can be better used to treat cancers such as breast cancer, small cell lung cancer or chronic granulocytic leukemia.
Owner:GUIZHOU YIBAI PHARMA CO LTD
Water-soluble stable lobaplatin derivative
InactiveCN103772435AEnhanced inhibitory effectGroup 8/9/10/18 element organic compoundsAntineoplastic agentsAnticarcinogenic EffectMethyl palmoxirate
The invention discloses a water-soluble stable lobaplatin derivative, namely, shionlobaplatin. The chemical name of the shionlobaplatin is cis-[trans-1,2-bis(aminomethyl) cyclobutane 3-hydroxy-1,1-cyclobutane dicarboxylate platinum as shown in the specification. The preparation of the water-soluble stable derivative shionlobaplatin comprises the steps of by taking K2[PtCl4] as a starting raw material in water, adding KI to the K2[PtCl4] so that K2[PtCl4) is transformed into K2PtI4, reacting the K2PtI4 with trans-1,2-bis(aminomethyl) cyclobutane to prepare a corresponding diiodine intermediate, and then carrying out an equimolar quantitative reaction between the diiodine intermediate and 3-hydroxy-1,1-cyclobutane dicarboxylic acid to obtain the target compound. The compound shionlobaplatin has the characteristics of good water solubility, high stability and high anti-cancer effect, and can be prepared into a freeze-dried powder or an aqueous solution for clinical treatment of cancers.
Owner:KUNMING INST OF PRECIOUS METALS +1
Lobaplatin pharmaceutical composition for injection and preparation method thereof
ActiveCN103599079AGood lookingSubstance reductionPowder deliveryPharmaceutical non-active ingredientsPolyolAlcohol
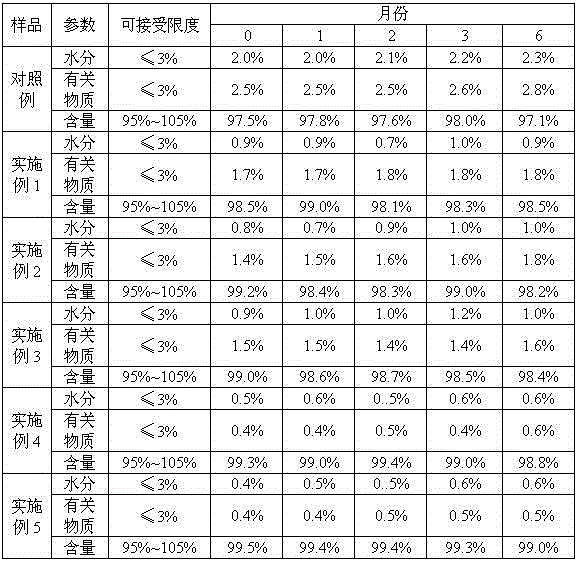
The invention belongs to the chemical pharmaceuticals field, and specifically provides a lobaplatin pharmaceutical composition for injection and a preparation method thereof. The lobaplatin pharmaceutical composition comprises lobaplatin and an excipient in a mass ratio of 1:(0.1-5), wherein the excipient can be selected from monosaccharide, disaccharide, polysaccharide, polyhydric alcohols, amino acids and the like, and preferably selected from polysaccharide. The lobaplatin pharmaceutical composition for injection provided by the invention is less in content of related substances, low in water content and good in stability, and compared with the quality of a finished product prepared in the prior art, the quality is improved to a great extent.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Pharmaceutical composition for treating small cell lung cancer and application, kit and package thereof
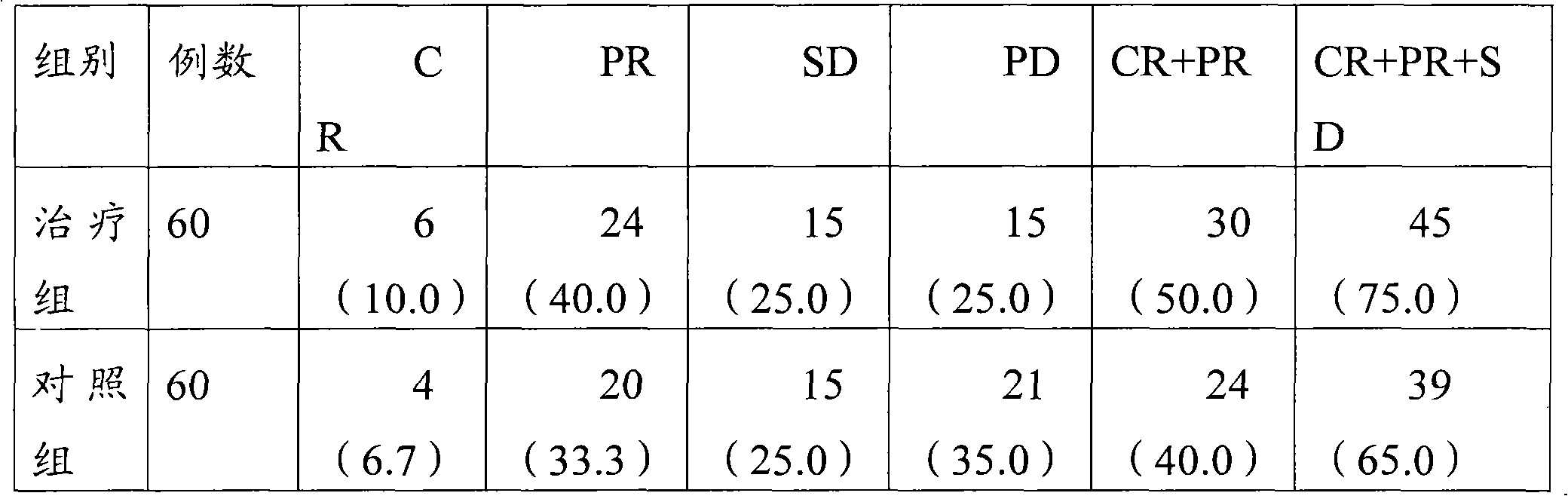
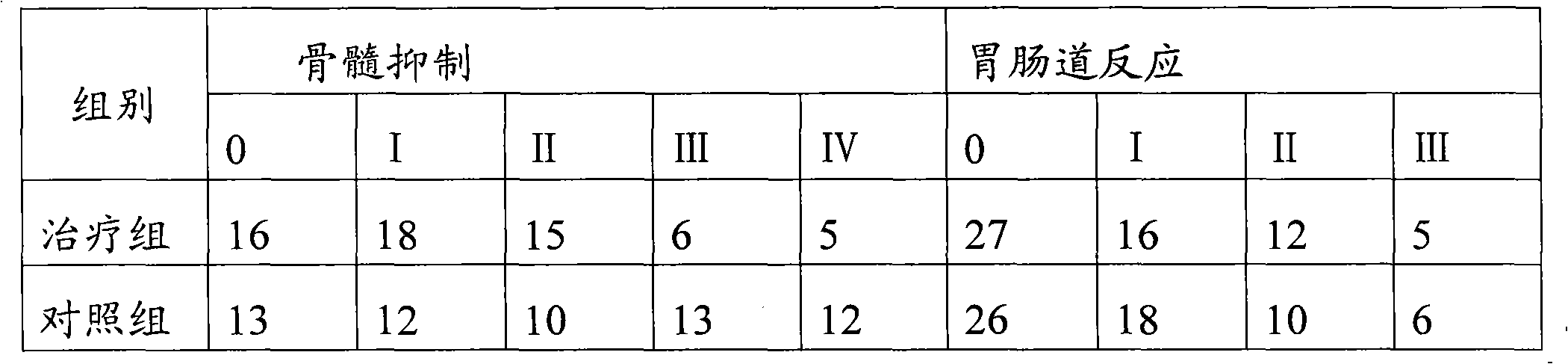
ActiveCN102172349ASmall side effectsGood treatment effectPill deliveryCapsule deliveryTreatment effectSide effect
The invention relates to a pharmaceutical composition for treating small cell lung cancer and application, a kit and package thereof. The pharmaceutical composition, kit and package contain a treatment effective dose of lobaplatin and a treatment effective dose of etoposide as a combination for simultaneous, separated or sequential use. When used for treating the small cell lung cancer, the pharmaceutical composition, kit and package provided by the invention can obtain excellent treatment effect and have small toxic side effect.
Owner:海南长安国际制药有限公司
Application of lobaplatin in preparation of drugs used for treating prostate cancer
ActiveCN103505450AAntineoplastic agentsHeavy metal compound active ingredientsHormone refractory prostate cancerHormone dependence
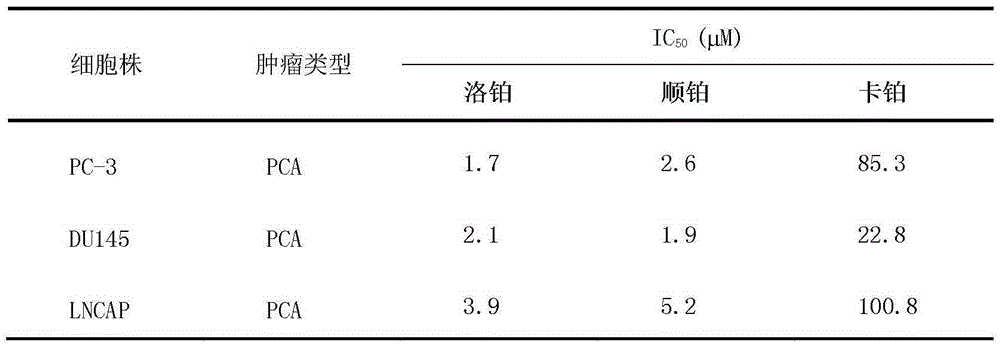
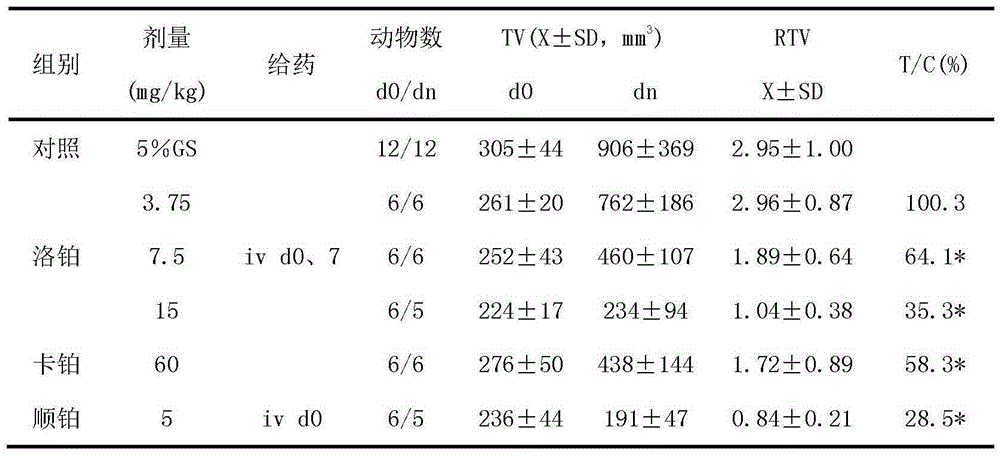
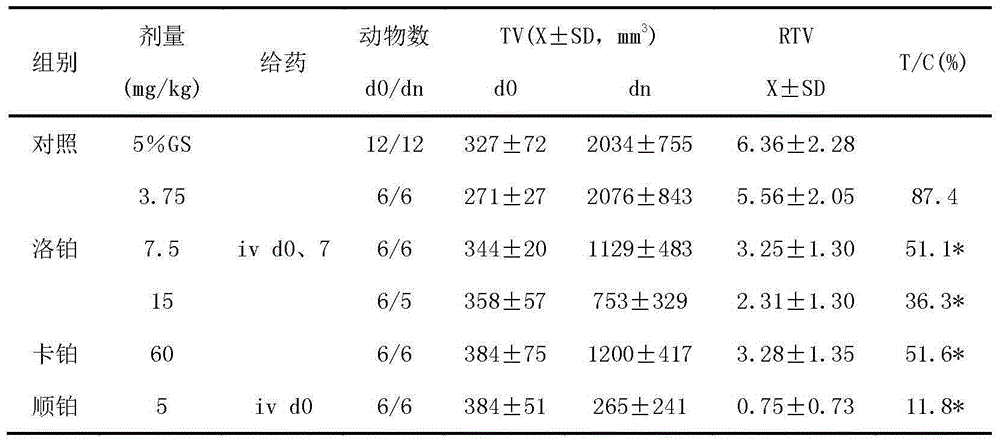
The invention relates to application of lobaplatin in preparation of drugs used for treating prostate cancer, especially to application of lobaplatin in preparation of drugs used for treating hormone refractory prostate cancer. An effective therapeutic dose of lobaplatin is 20 to 50 mg per square of a body surface area. According to the invention, a novel therapeutic drug is provided for treatment of prostate cancer, and clinical application of lobaplatin and a preparation thereof is broadened.
Owner:GUIZHOU YIBAI PHARMA CO LTD
Application of lobaplatin in the preparation of drugs for treating prostate cancer
ActiveCN103505450BAntineoplastic agentsHeavy metal compound active ingredientsHormone refractory prostate cancerHormone dependence
The present invention relates to the application of lobaplatin in the preparation of drugs for treating prostate cancer, especially the application in the preparation of drugs for treating non-hormone-dependent prostate cancer. The effective therapeutic dose of lobaplatin is 20-50 mg / m2 body surface area. The present invention It provides new therapeutic drugs for prostate cancer and expands the clinical application of lobaplatin and its preparations.
Owner:GUIZHOU YIBAI PHARMA CO LTD
Anticancer composition containing both platinum compound and Sirolimus
InactiveCN1973824AAvoid damageGrowth inhibitionPharmaceutical delivery mechanismPharmaceutical non-active ingredientsCarboplatinMicrosphere
The anticancer composition containing both platinum compound and sirolimus is one kind of slow releasing injection or slow releasing implant. The slow releasing injection consists of slow released microsphere and special solvent containing suspending agent. The platinum compound is selected from cisplatin, carboplatin, lobaplatin, etc; the slow releasing supplementary material is selected from polylactic acid, lactic acid copolymer, polifeprosan, etc; and the suspending agent is selected from sodium carboxymethyl cellulose, iodine glycerin, etc. The slow releasing injection and the slow releasing implant are injected or set into solid tumor to diffuse and produce synergistic curative effect.
Owner:SHANDONG LANJIN PHARMA +1
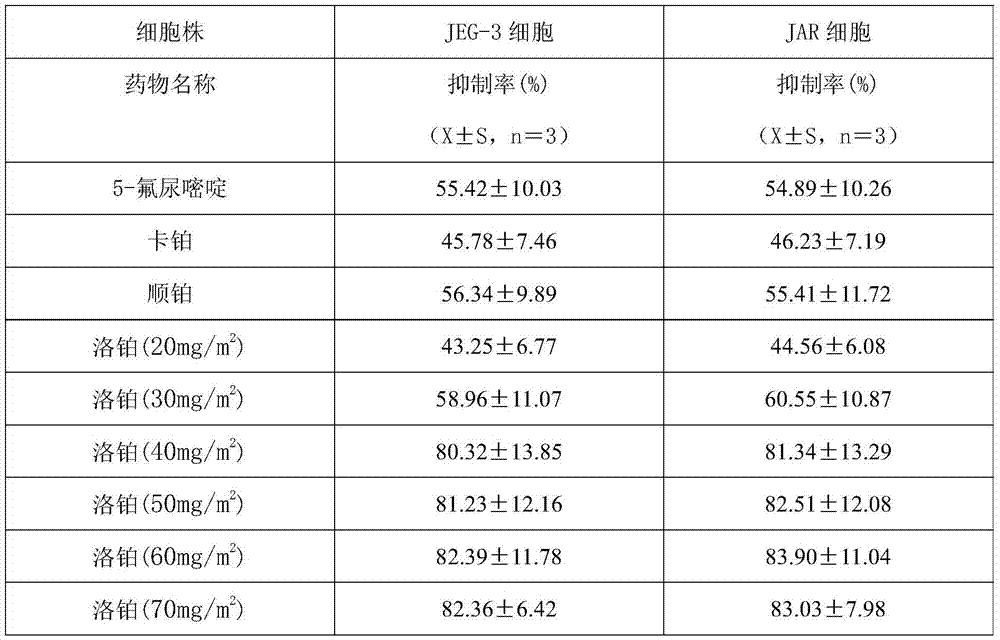
Application of lobaplatin in preparing medicines for treating malignant trophoblastic tumor
The invention discloses an application of lobaplatin in preparing medicines for treating malignant trophoblastic tumor. The lobaplatin is represented in dosage forms of lyophilized powder for injection, a small -volume injection or a large-volume injection, with an effective therapeutic dose of 30-60mg / m<2> body surface area. The lobaplatin and a preparation thereof are relatively strong in action of resisting the malignant trophoblastic tumor; and the lobaplatin and the preparation thereof are significant in therapeutic effect, low in toxicity and safe; therefore, a novel effective means is provided for the treatment of the malignant trophoblastic tumor and clinical application of the lobaplatin and the preparation thereof is widened.
Owner:GUIZHOU YIBAI PHARMA CO LTD
Application of lobaplatin in preparing medicines for treating malignant pleural mesothelioma
InactiveCN106974902AAntineoplastic agentsHeavy metal compound active ingredientsTreatment effectCancer research
The invention discloses an application of lobaplatin in preparing medicines for treating malignant pleural mesothelioma. The lobaplatin is represented in dosage forms of lyophilized powder for injection, a small -volume injection or a large-volume injection, with an effective therapeutic dose of 30-60mg / m<2> body surface area. The lobaplatin and a preparation thereof are relatively strong in action of resisting the malignant pleural mesothelioma; and the lobaplatin and the preparation thereof are significant in therapeutic effect, low in toxicity and safe; therefore, a novel effective means is provided for the treatment of the malignant pleural mesothelioma and clinical application of the lobaplatin and the preparation thereof is widened.
Owner:GUIZHOU YIBAI PHARMA CO LTD
Purification method of high-purity lobaplatin trihydrate for preparing antitumor drugs
ActiveCN113173953AShorten the timeLow pricePlatinum organic compoundsAntineoplastic agentsDiethyl etherSolvent
The invention relates to a purification method of a high-purity lobaplatin trihydrate for preparing an antitumor drug. The method comprises the following steps: 1, dissolving a lobaplatin crude product in a high-temperature aqueous solution, filtering, crystallizing at low temperature, re-filtering, and drying to obtain the lobaplatin trihydrate; and 2, dissolving the lobaplatin crude product in a high-temperature aqueous solution, filtering, adding diethyl ether into the filtrate, crystallizing at low temperature, filtering, and drying to obtain the lobaplatin trihydrate. According to the invention, the purification can be repeated multiple times until the high-purity lobaplatin trihydrate is obtained; according to the purification method, the lobaplatin crude product with the content of 55-95% is purified, the purity of the lobaplatin crude product can be improved to 98% or above, the used purification solvent is water or diethyl ether, and after the sample is fully washed with water, the obtained product is high in safety; purification belongs to conventional operation, and preparation is easy; the prepared high-purity lobaplatin trihydrate can be applied to antitumor drugs; and the preparation method is short in preparation time, simple to operate, low in production cost and suitable for industrial production.
Owner:KUNMING GUIYAN PHARMA
A kind of lobaplatin crystal, preparation method and pharmaceutical application
ActiveCN105440083BImprove bioavailabilityNot easy to absorb moisture and become stickyGroup 8/9/10/18 element organic compoundsAntineoplastic agentsSolubilityChronic granulocytic leukemia
The invention relates to a lobaplatin crystal, a preparation method and a drug application. The lobaplatin crystal form is B, its melting point Tm.p..=230±5°C, and its X-ray powder diffraction PXRD pattern has a 2θ angle value of There are diffraction peaks at 8.25, 9.77, 11.70, 13.13, 15.28, 16.48, 17.22, 17.74, 19.01, 19.56, 22.28, 23.72, 24.04, 24.30, 25.62, 26.20, 28.57, 30.22, 30.61, and the error range of 2θ is 0. The crystal form is obtained by solvent volatilization of lobaplatin trihydrate, or adding a solvent to lobaplatin dihydrate and volatilizing at room temperature or drying after elution and crystallization. Compared with the existing lobaplatin and lobaplatin trihydrate, the lobaplatin compound with crystal form B has better stability and solubility, is more suitable for the preparation of various forms of pharmaceutical preparations, storage and use, and can be more It is preferably used in the treatment of cancer such as the treatment of breast cancer, small cell lung cancer or chronic myelogenous leukemia.
Owner:海南长安国际制药有限公司
A kind of synthetic method of lobaplatin intermediate
ActiveCN113416150BLow priceReduce manufacturing costOrganic compound preparationOrganic chemistry methodsMalonic acidDrugs synthesis
The invention relates to the technical field of drug synthesis, in particular to a method for synthesizing a lobaplatin intermediate. The method uses low-toxicity dimethyl malonate as a starting material, and undergoes coupling, bromination, cyclization, hydrolysis, Synthesis of high-purity trans-1,2-dicyanocyclobutane after amidation and dehydration reactions. In the synthesis of the whole route, all the raw and auxiliary materials used are easy to purchase, cheap and low in toxicity. The reaction conditions are not harsh and uncontrollable. The reaction conditions of each step can be applied to scale-up production. Although the reaction steps are increased, the yield of the product However, the yield has been greatly improved, thereby greatly reducing the cost, and the obtained trans-1,2-dicyanocyclobutane has improved purity, fully meets the subsequent use requirements, and greatly improves market competitiveness.
Owner:上海寻科生物医药科技有限公司
A kind of lobaplatin crystal, preparation method and pharmaceutical application
ActiveCN105440082BImprove bioavailabilityNot easy to absorb moisture and become stickyGroup 8/9/10/18 element organic compoundsAntineoplastic agentsSolubilityOrganosolv
Owner:海南长安国际制药有限公司
A kind of preparation method of lobaplatin
ActiveCN103467528BShort reaction timeShorten the production cycleGroup 8/9/10/18 element organic compoundsPlatinum saltsCyclobutane
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
A kind of lobaplatin crystal, preparation method and pharmaceutical application
ActiveCN105198933BImprove solubilityImprove liquidityGroup 8/9/10/18 element organic compoundsAntineoplastic agentsIsobutanolSolubility
The invention relates to a lobaplatin crystal, a preparing method and a medicine application. The crystal form of the lobaplatin crystal is C, and the melting point Tm.p.. is 228+ / -5 DEG C; in a PXRD pattern of the lobaplatin crystal, diffraction peaks exist when the 2theta value is 6.79, 8.07, 12.24, 12.61, 13.50, 16.50, 17.83, 18.32, 18.79, 20.09, 21.64, 22.27, 23.19, 24.73, 27.34, 28.35, 29.12 and 31.92, and the error range of the 2theta value is 0.2. The preparing method for the crystal form C includes the steps that isobutanol or normal propyl alcohol is added into lobaplatin dihydrate, the mixture is stirred in a suspension mode, and the crystal is dissolved out, separated out, dried and then obtained. Compared with existing lobaplatin and lobaplatin trihydrate, better stability and better solubility are achieved, and the lobaplatin crystal can be better suitable for preparing medicine preparations in various forms, can be better stored and used and can be better used for treating cancers such as the breast cancer, the small cell lung cancer and the chronic granulocytic leukemia.
Owner:海南长安国际制药有限公司
Application of lobaplatin in preparation of medicine for treating bladder cancer
PendingCN111012775AStrong anti-cystoma effectGood treatment effectPharmaceutical delivery mechanismAntineoplastic agentsPharmaceutical drugTherapeutic effect
Owner:吉林大学中日联谊医院
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com