Preparation method of lobaplatin
A technology of lobaplatin and chloroplatinite, which is applied in the field of preparation of lobaplatin, can solve the problems of poor product purity and content repeatability, difficulty in making preparations, and increased production costs, so as to shorten the reaction time and reduce production costs. The effect of low cost and short production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: the preparation method of lobaplatin and trihydrate
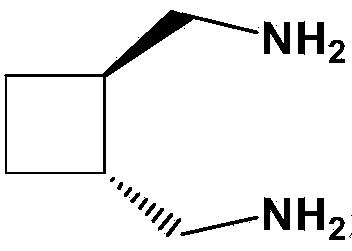
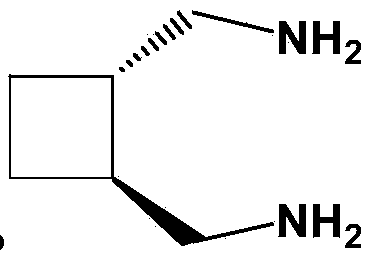
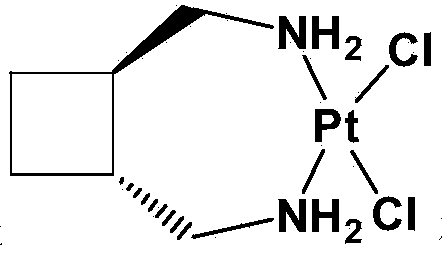
[0043] Add 20.7g (0.05mol) of potassium chloroplatinite into 200mL of purified water, stir and dissolve until clear, add 5.7g (0.05mol) of trans-diaminomethylcyclobutane under nitrogen protection, and stir at 20°C in the dark for reaction 8 hours, filtered, washed the filter cake with an appropriate amount of purified water, absolute ethanol, and ether in sequence, and dried the filter cake in vacuum at 50°C for 4 hours to obtain 18.1 g of dichloro-1,2-diaminomethylcyclobutane platinum, Yield 95.3%.
[0044] Disperse 17.1g (0.045mol) of dichloro-1,2-diaminomethylcyclobutane platinum in 170mL of purified water, stir until evenly mixed, add 15.2g (0.09mol) of silver nitrate, and then add 21mL of acetone , 35 ° C dark stirring reaction for 1.5 hours.
[0045] Cool the reaction solution at -5°C for 3 hours, filter the resulting silver chloride precipitate, and wash the filter cake with an appropriate amou...
Embodiment 2
[0047] Embodiment 2: the preparation method of lobaplatin and trihydrate
[0048] Add 41.5g (0.10mol) of potassium chloroplatinite into 600mL of purified water, stir and dissolve until clear, add 11.4g (0.10mol) of trans-diaminomethylcyclobutane under nitrogen protection, and stir at 25°C in the dark for 10 hours, filtered, washed the filter cake successively with appropriate amount of purified water, absolute ethanol, and ether, and dried the filter cake in vacuum at 40°C for 5 hours to obtain 36.5 g of dichloro-1,2-diaminomethylcyclobutane platinum, The yield is 96.1%.
[0049] Disperse 34.2g (0.09mol) of dichloro-1,2-diaminomethylcyclobutane platinum in 500mL of purified water, stir until evenly mixed, add 30.5g (0.18mol) of silver nitrate, and then add 50mL of acetone , 40 ° C dark stirring reaction for 2 hours.
[0050] Cool the reaction solution at 0°C for 4 hours, filter the resulting silver chloride precipitate, and wash the filter cake with an appropriate amount of ...
Embodiment 3
[0052] Embodiment 3: the preparation method of lobaplatin and trihydrate
[0053] Add 103.8g (0.25mol) of potassium chloroplatinite into 2000mL of purified water, stir and dissolve until clear, add 28.5g (0.25mol) of trans-diaminomethylcyclobutane under nitrogen protection, and stir at 30°C in the dark for 16 hours, filtered, washed the filter cake with an appropriate amount of purified water, absolute ethanol, and ether in sequence, and dried the filter cake in vacuum at 30°C for 6 hours to obtain 91.5 g of dichloro-1,2-diaminomethylcyclobutane platinum, Yield 96.3%.
[0054] Disperse 91.2g (0.24mol) of dichloro-1,2-diaminomethylcyclobutane platinum in 1800mL of purified water, stir until evenly mixed, add 81.5g (0.48mol) of silver nitrate, and then add 150mL of acetone , 45°C and stirred in the dark for 3 hours.
[0055] Cool the reaction solution at 5°C for 6 hours, filter the resulting silver chloride precipitate, and wash the filter cake with an appropriate amount of pu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com