Application of lobaplatin in preparing medicines for treating malignant pleural mesothelioma
A malignant and pleural technology, applied in the application field of lobaplatin in the preparation of drugs for the treatment of malignant pleural mesothelioma, which can solve the problems of no treatment effect on malignant pleural mesothelioma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
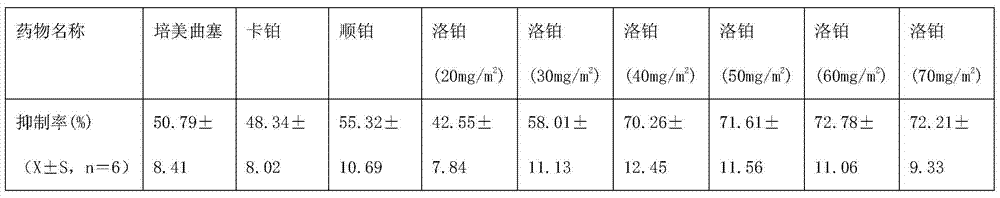
[0018] Example 1: Study on the activity of lobaplatin against pleural mesothelioma in vitro
[0019] 1.1 Materials and reagents
[0020] 1.1.1 Drug name and source: Lobaplatin is white freeze-dried powder, Hainan Changan International Pharmaceutical Co., Ltd.; carboplatin is white powder, Kunming Guiyan Pharmaceutical Co., Ltd.; pemetrexed, Nanjing Xiansheng Dongyuan Pharmaceutical Co., Ltd. ; Cisplatin is yellow powder, Jiangsu Hansoh Pharmaceutical Co., Ltd.
[0021] Preparation method: the above drugs are formulated with corresponding concentrations in serum-free medium.
[0022] 1.1.2 Cell lines
[0023] Pleural mesothelioma NCL-H2452 cells were purchased from the American Standard Biological Collection (ATCC) and cultured according to the instructions provided.
[0024] 1.1.3 Reagents and instruments
[0025] DMEM / F12 (1:1) medium, fetal bovine serum (Gibco, USA), penicillin (100,000 u / L), streptomycin (100 mg / L), CO 2 Incubator, ELx800 microplate reader (Bio-Tek, US...
example 2
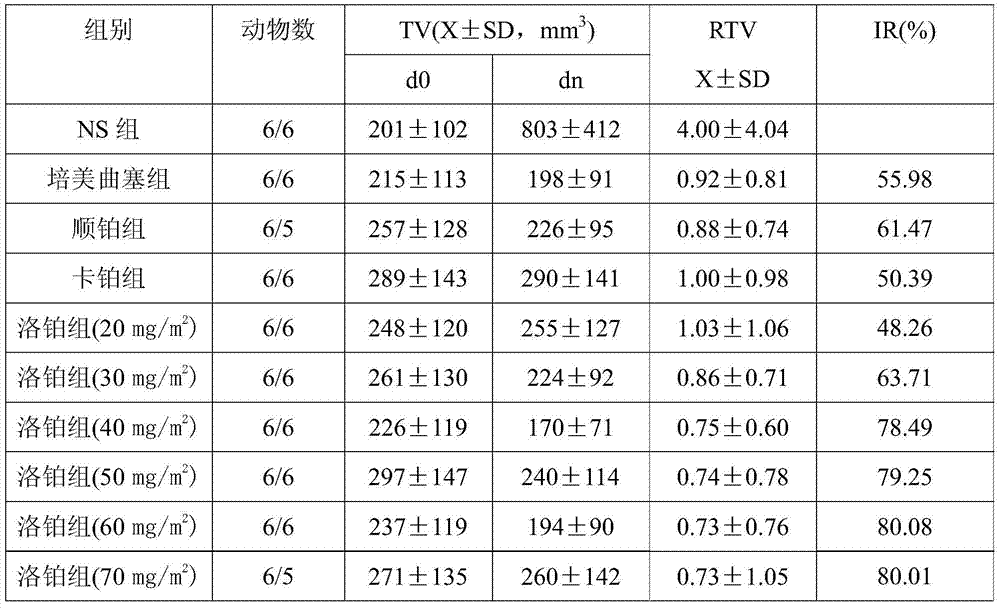
[0042] Trial Example 2: In vivo curative effect study of lobaplatin on pleural mesothelioma
[0043] 2.1 Materials and reagents:
[0044] 2.1.1 Drug name and source: Lobaplatin is white freeze-dried powder, Hainan Changan International Pharmaceutical Co., Ltd.; carboplatin is white powder, Kunming Guiyan Pharmaceutical Co., Ltd.; pemetrexed, Nanjing Xiansheng Dongyuan Pharmaceutical Co., Ltd. ; Cisplatin is yellow powder, Jiangsu Hansoh Pharmaceutical Co., Ltd.
[0045] 2.1.2 Experimental animals and cells
[0046] Clean-grade Kunming mice (4-6 weeks old) were provided by the Department of Animals, Kunming Medical College; pleural mesothelioma NCL-H2452 cells were purchased from the American Standard Biological Collection (ATCC).
[0047] 2.1.3 Reagents and instruments
[0048] DMEM / F12 (1:1) medium, fetal bovine serum (Gibco, USA), penicillin (100,000 u / L), streptomycin (100 mg / L), inverted microscope, CO 2 Incubator, pipette, suction ear ball, alcohol lamp.
[0049] 2.2...
Embodiment 3
[0066] Example 3: Clinical trials of lobaplatin in the treatment of pleural mesothelioma
[0067] 3.1 Case selection: According to the CT diagnosis of pleural mesothelioma in the literature (Liu Zhisong; Jing Suining; Wang Jianhong), the application of ultrasonography in the diagnosis of pleural mesothelioma (Shi Linshu; Yang Wenlan; Tao Juwei), thoracoscopy in the diagnosis of pleural mesothelioma Applications in diagnosis and treatment (Wang Yongliang) were screened according to UICC TNM staging. Screened out 19 patients with definite diagnosis of pleural mesothelioma, and all patients had KPS scores greater than 60. 19 patients were randomly divided into two groups, 10 cases in the treatment group, 5 males and 5 females, aged 50-64 years, with an average age of 58.6 years, 2 cases in stage III, 8 cases in stage IV; 9 cases in the control group, male 5 cases, 4 females, aged 52-60 years old, average 56.9 years old, 2 cases in stage III, 7 cases in stage IV. The two groups ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com