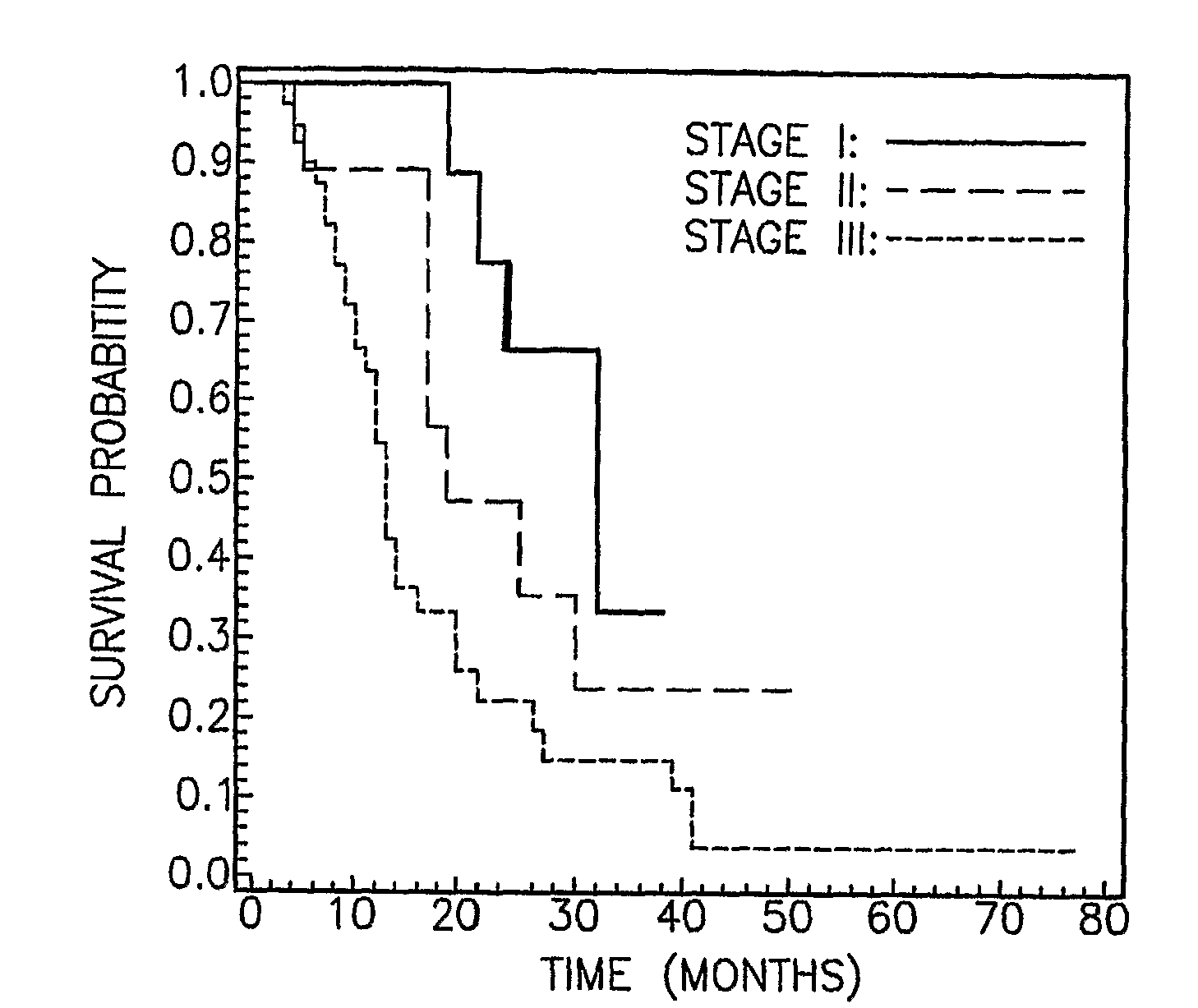
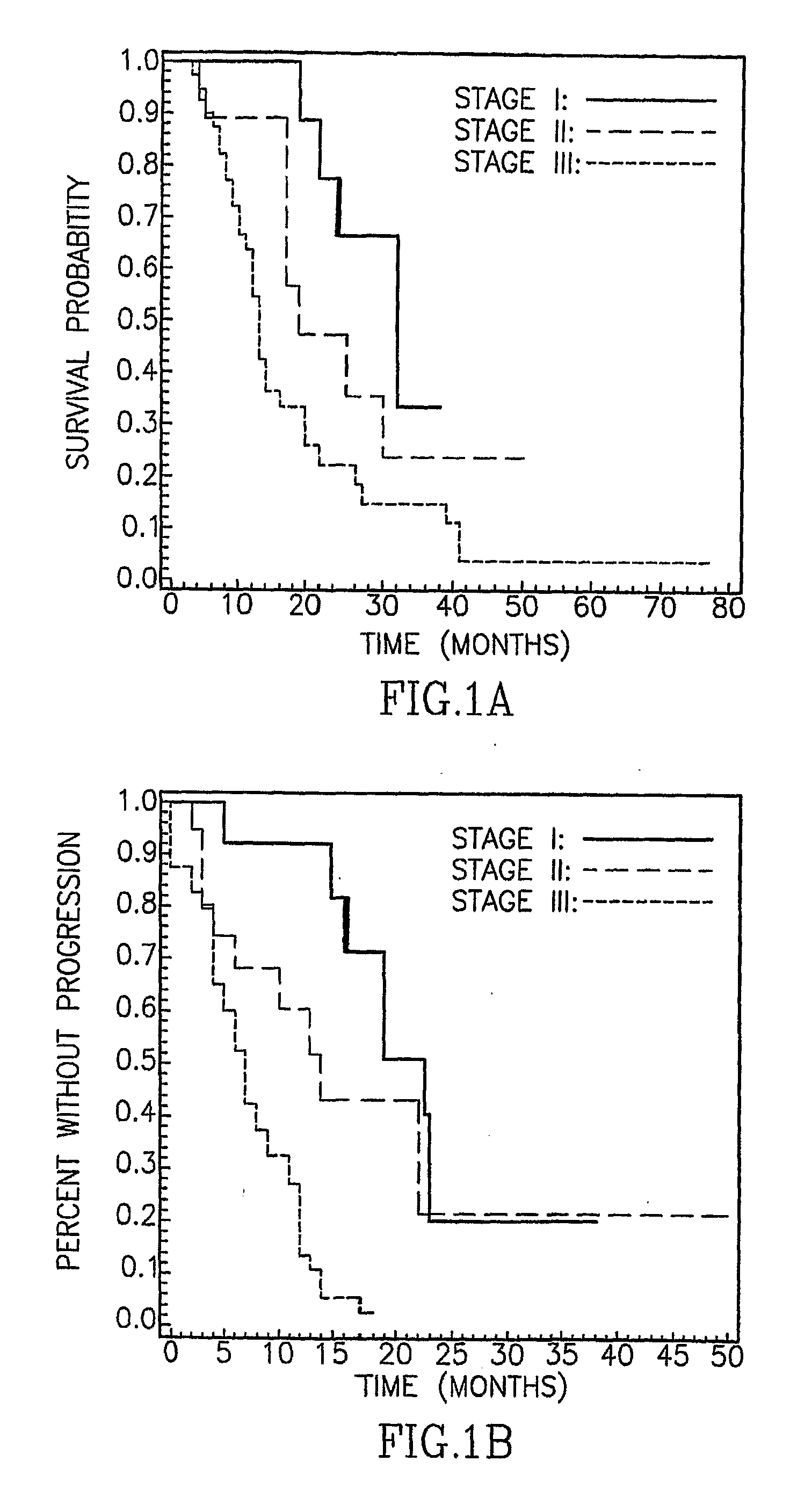
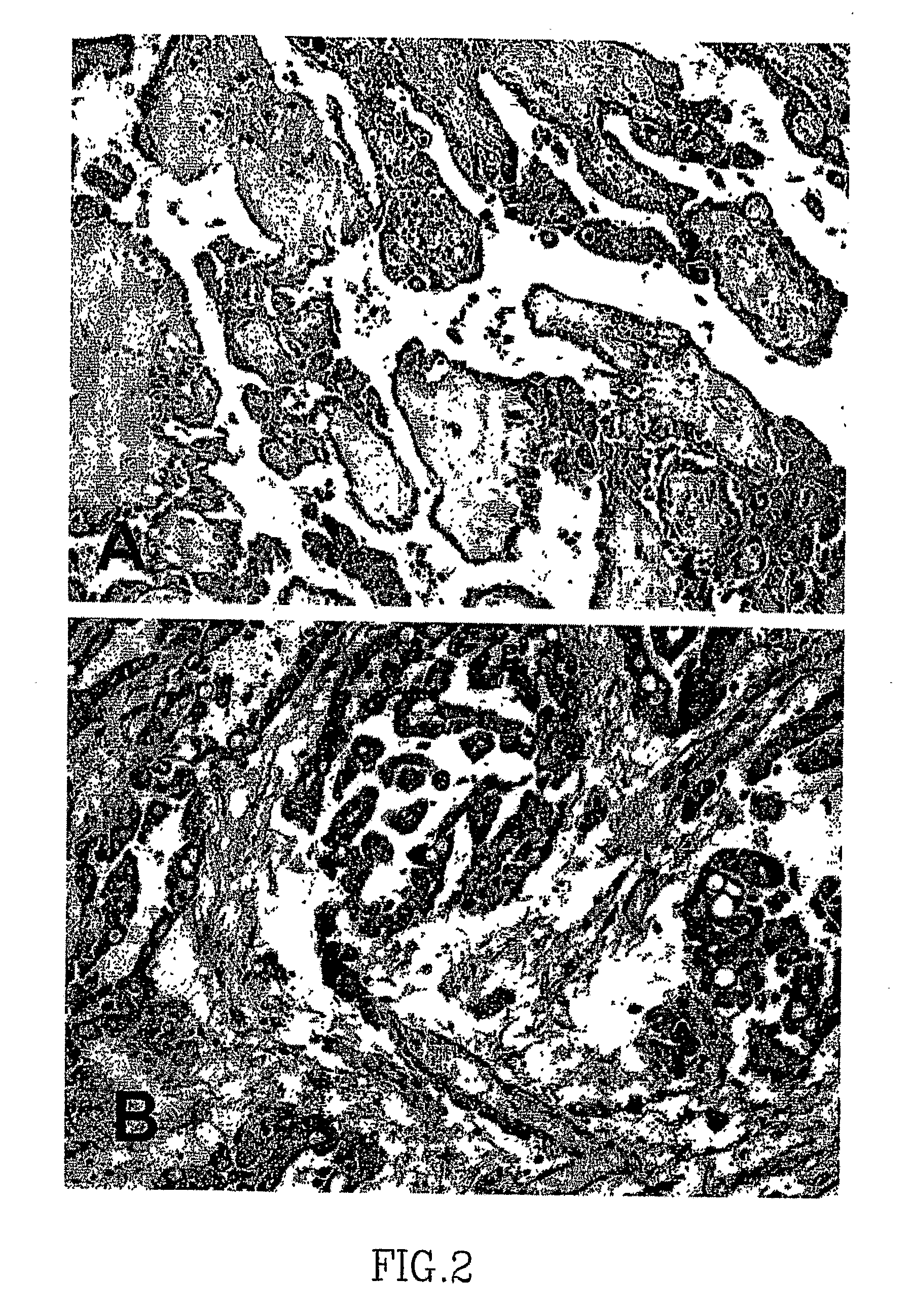
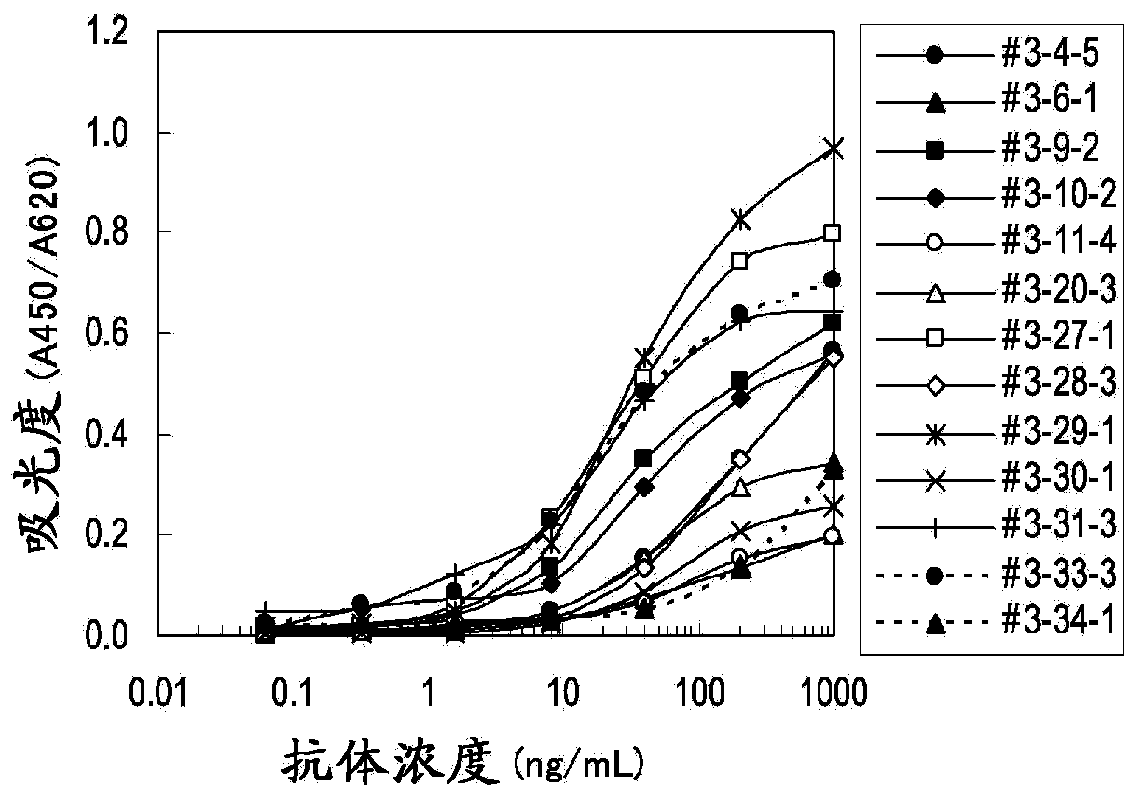
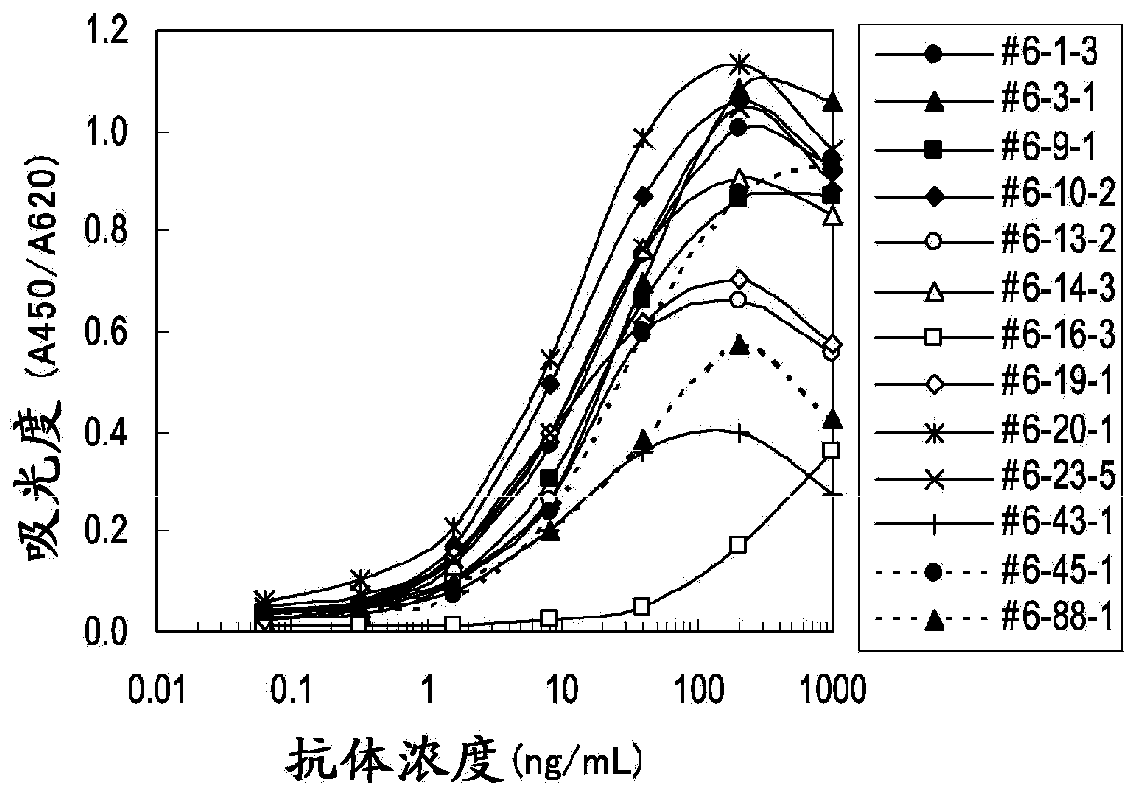
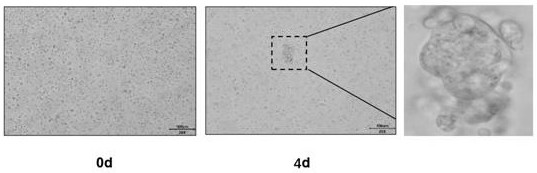
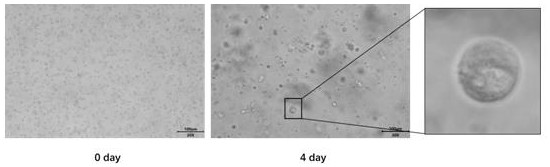
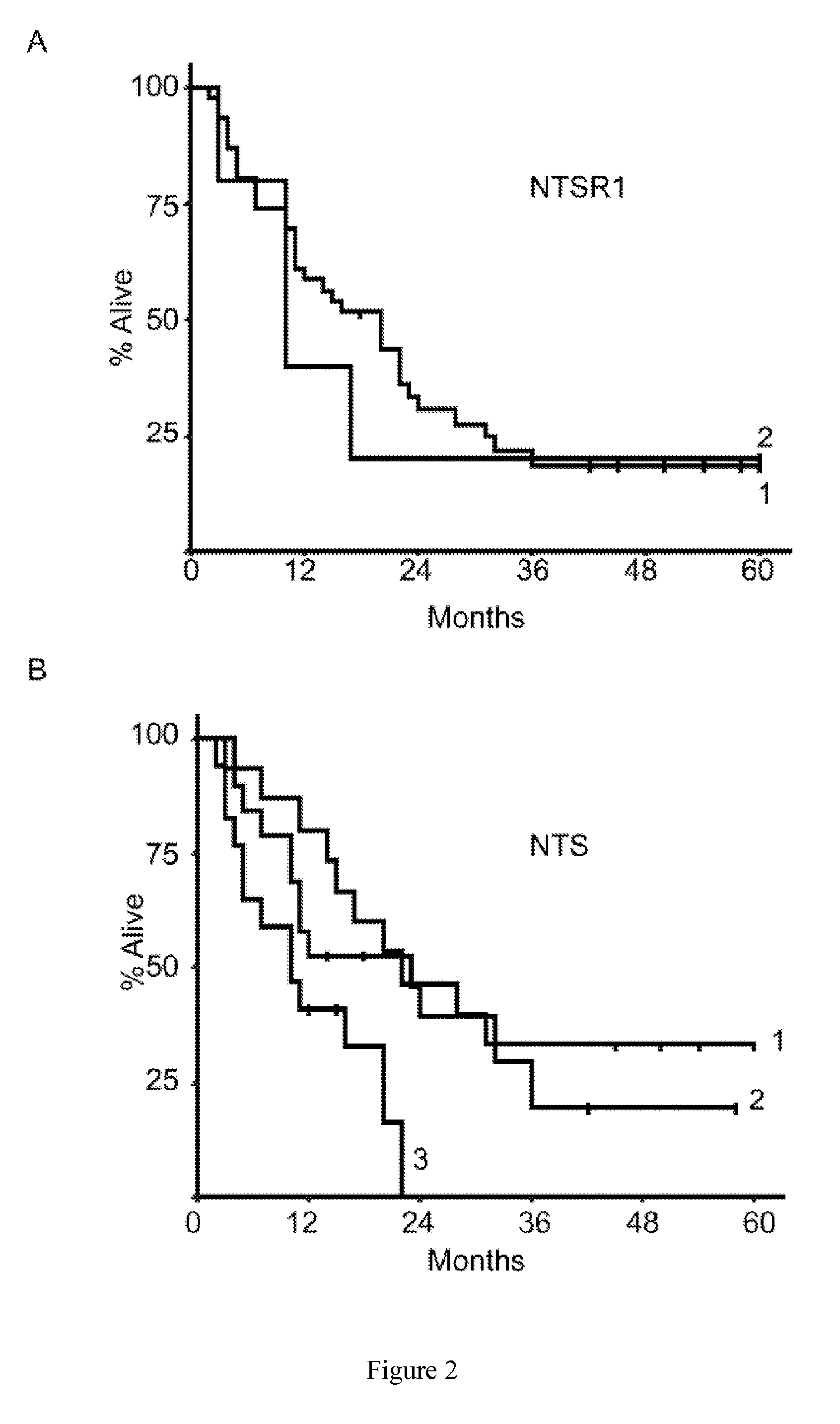
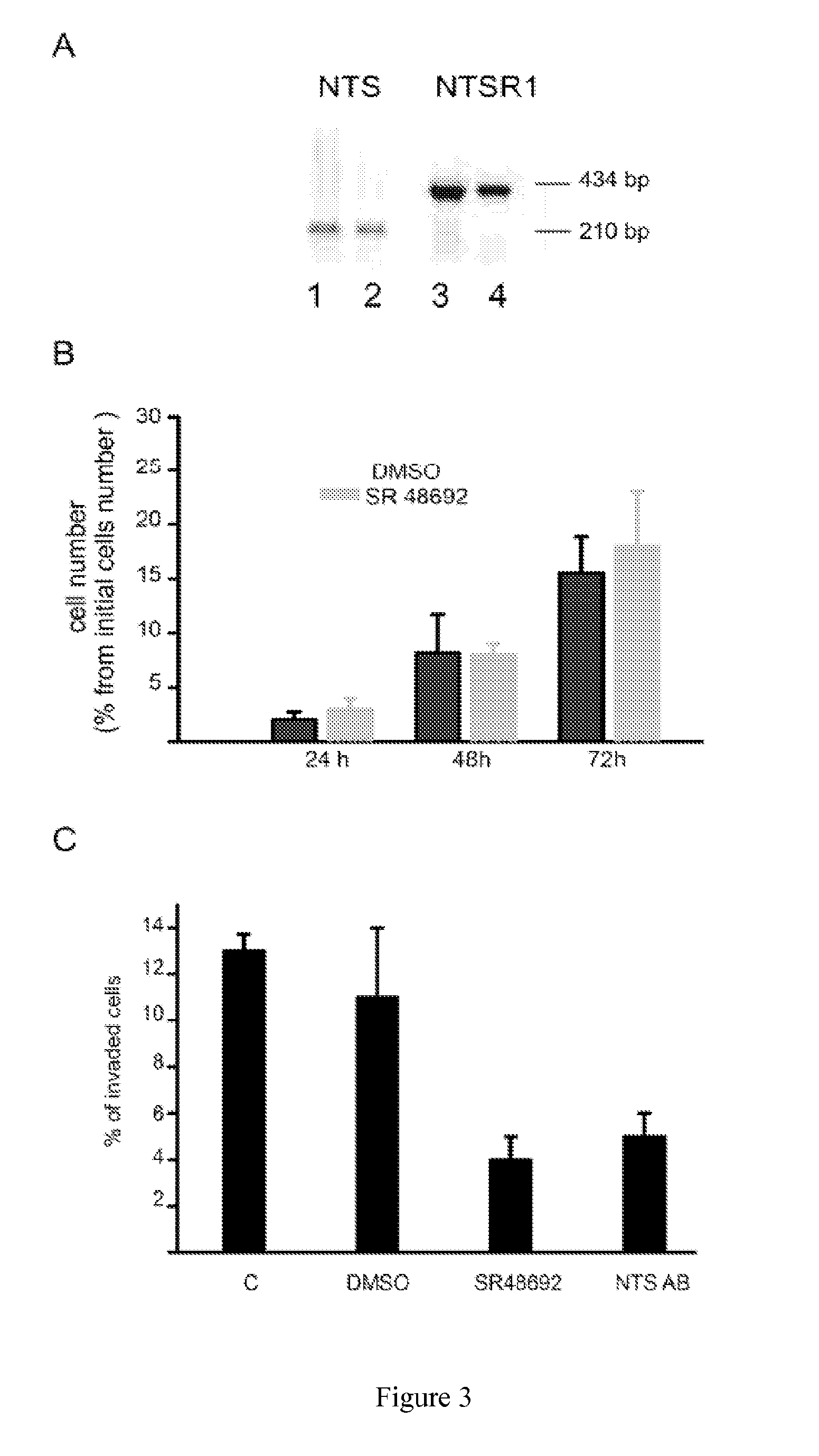
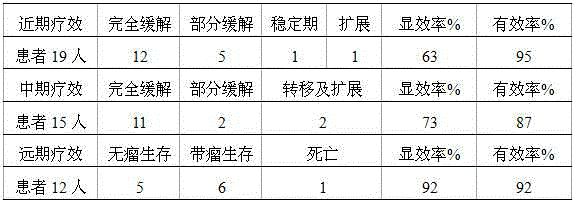
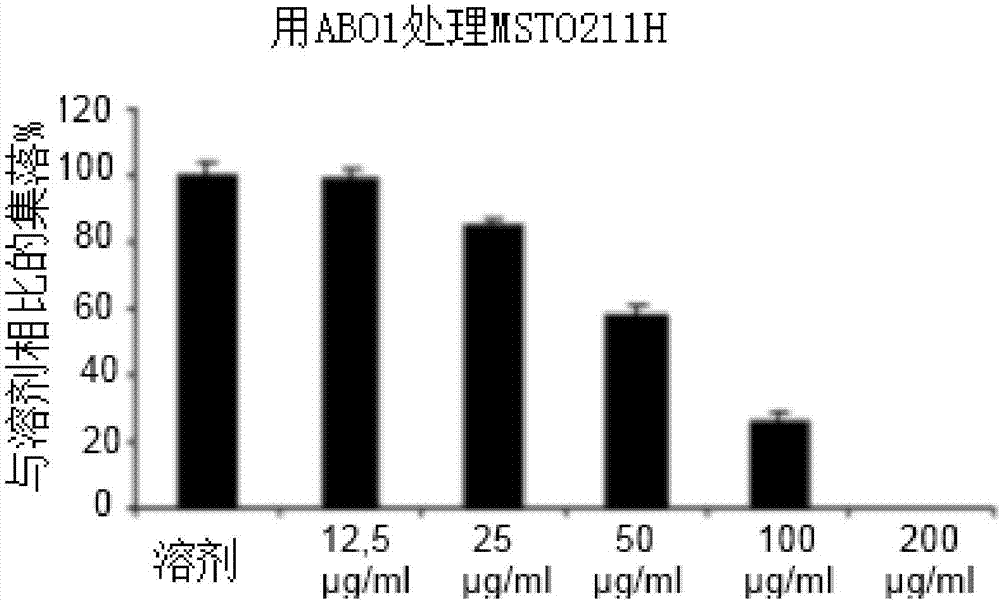
Patents
Literature
32 results about "Pleural mesothelioma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
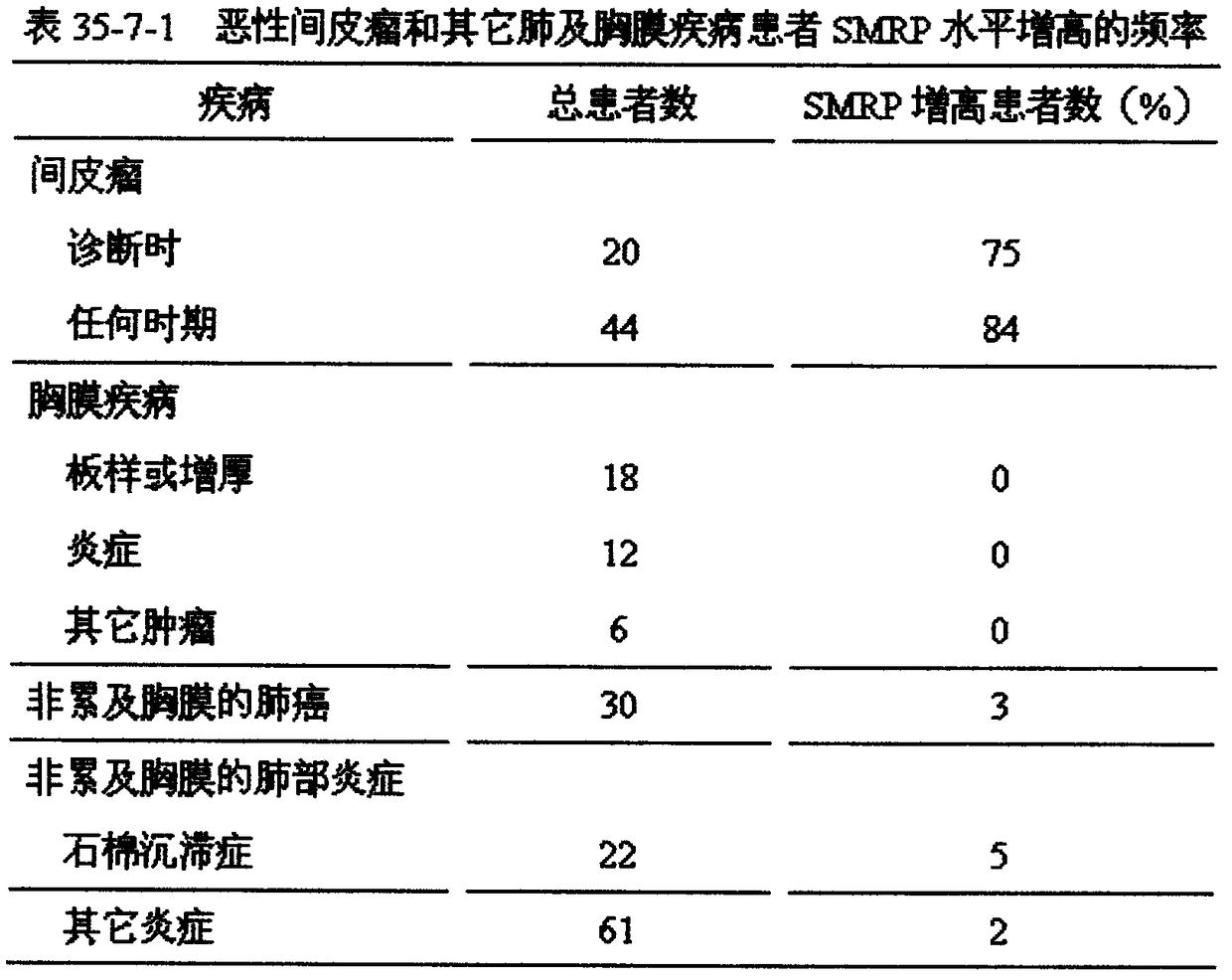
Pleural mesothelioma is a rare cancer of the pleura, the lining of the lungs. It is the most common form of cancer caused by asbestos.
Diagnostic and Prognostic Tests
InactiveUS20090104617A1Simple powerEasy to adaptMicrobiological testing/measurementMaterial analysisTissue sampleNormal tissue
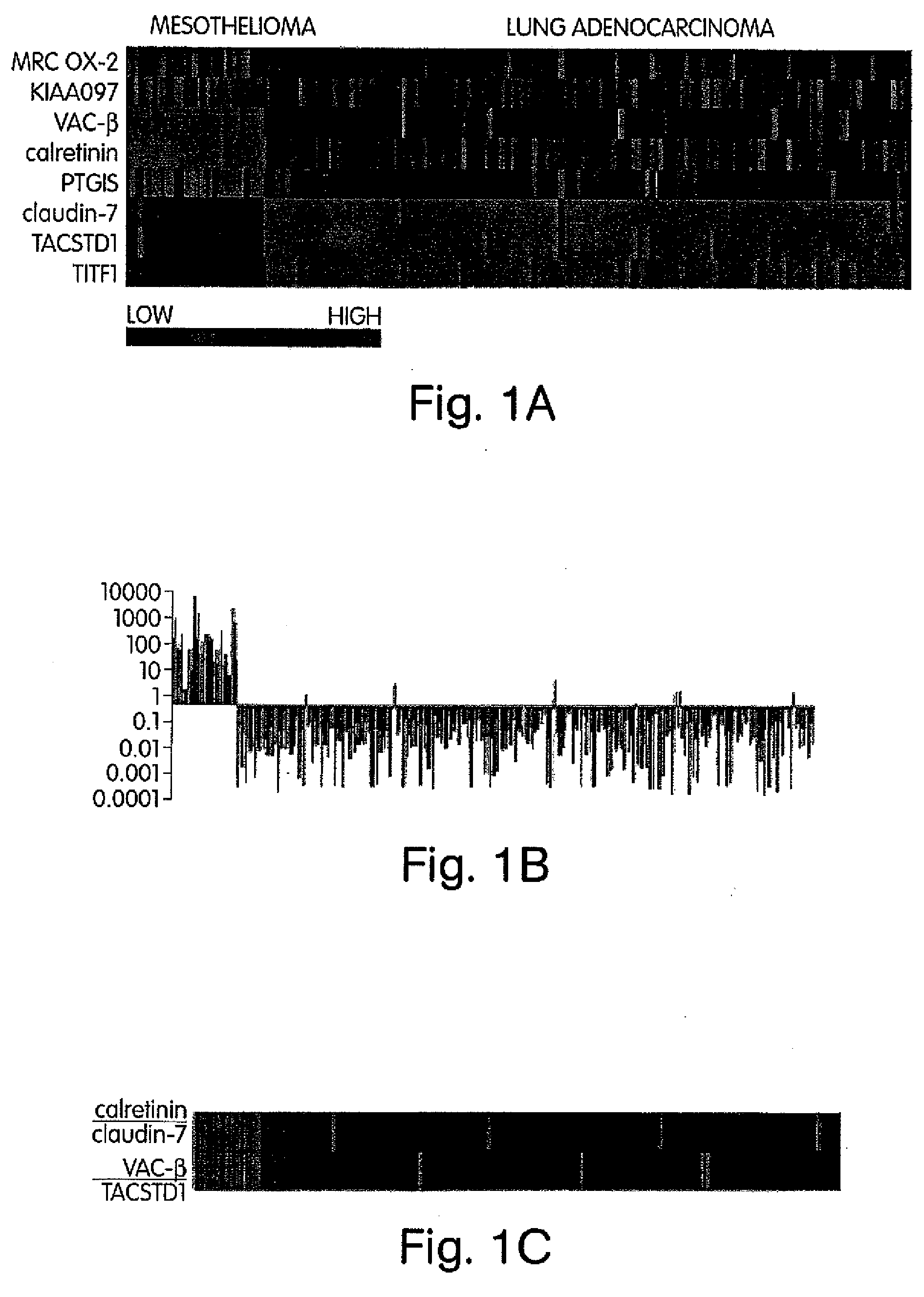
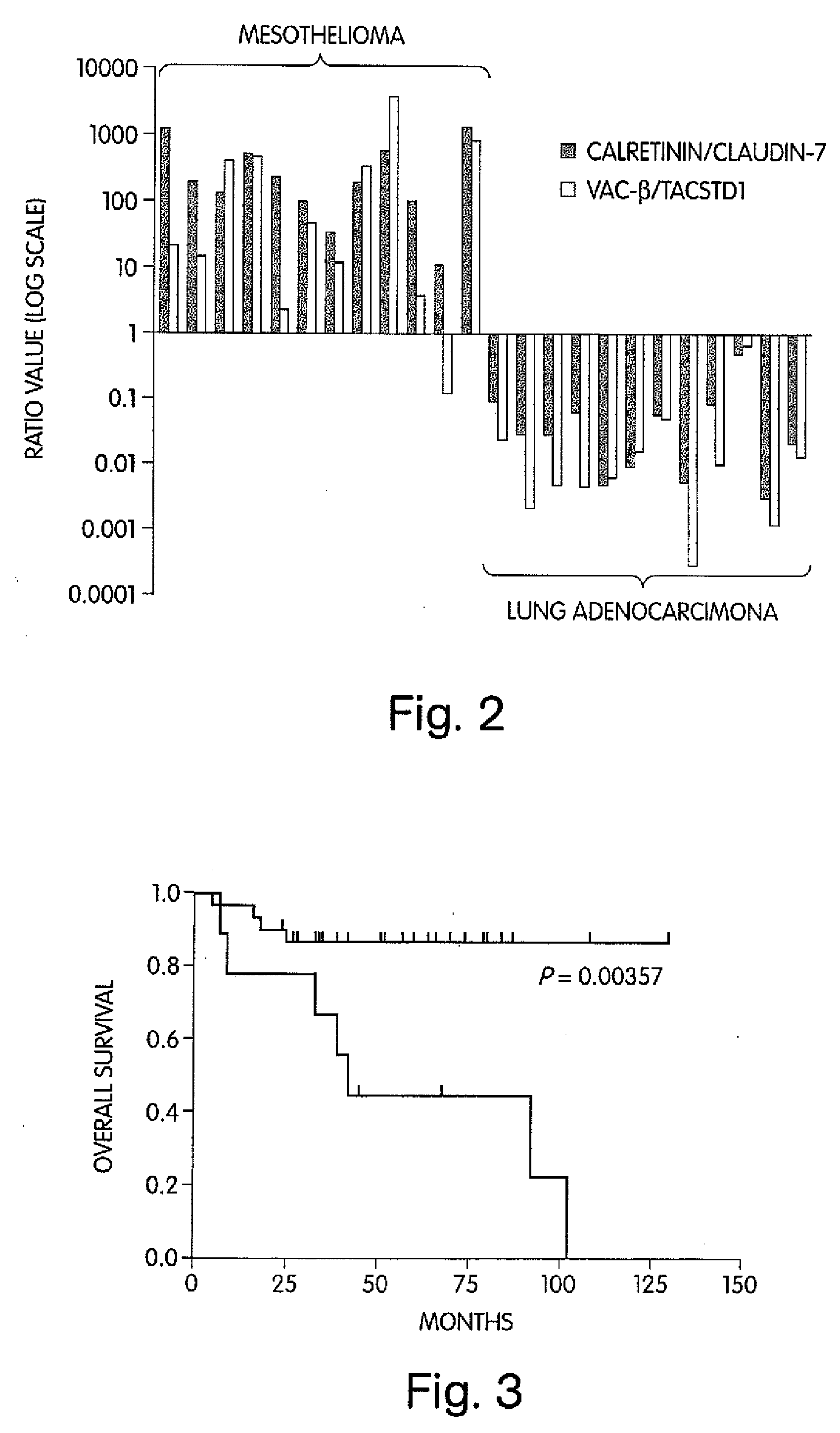
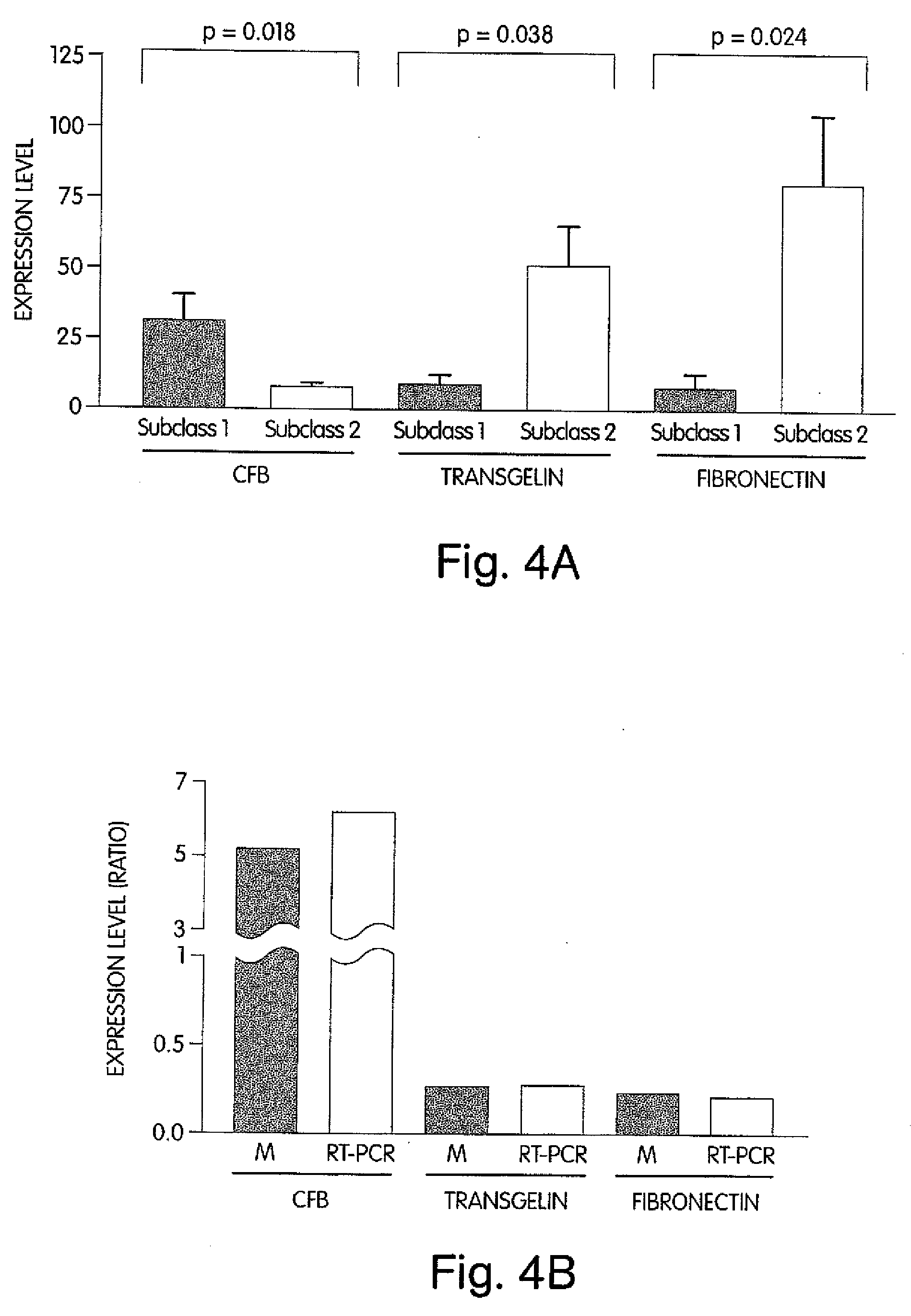
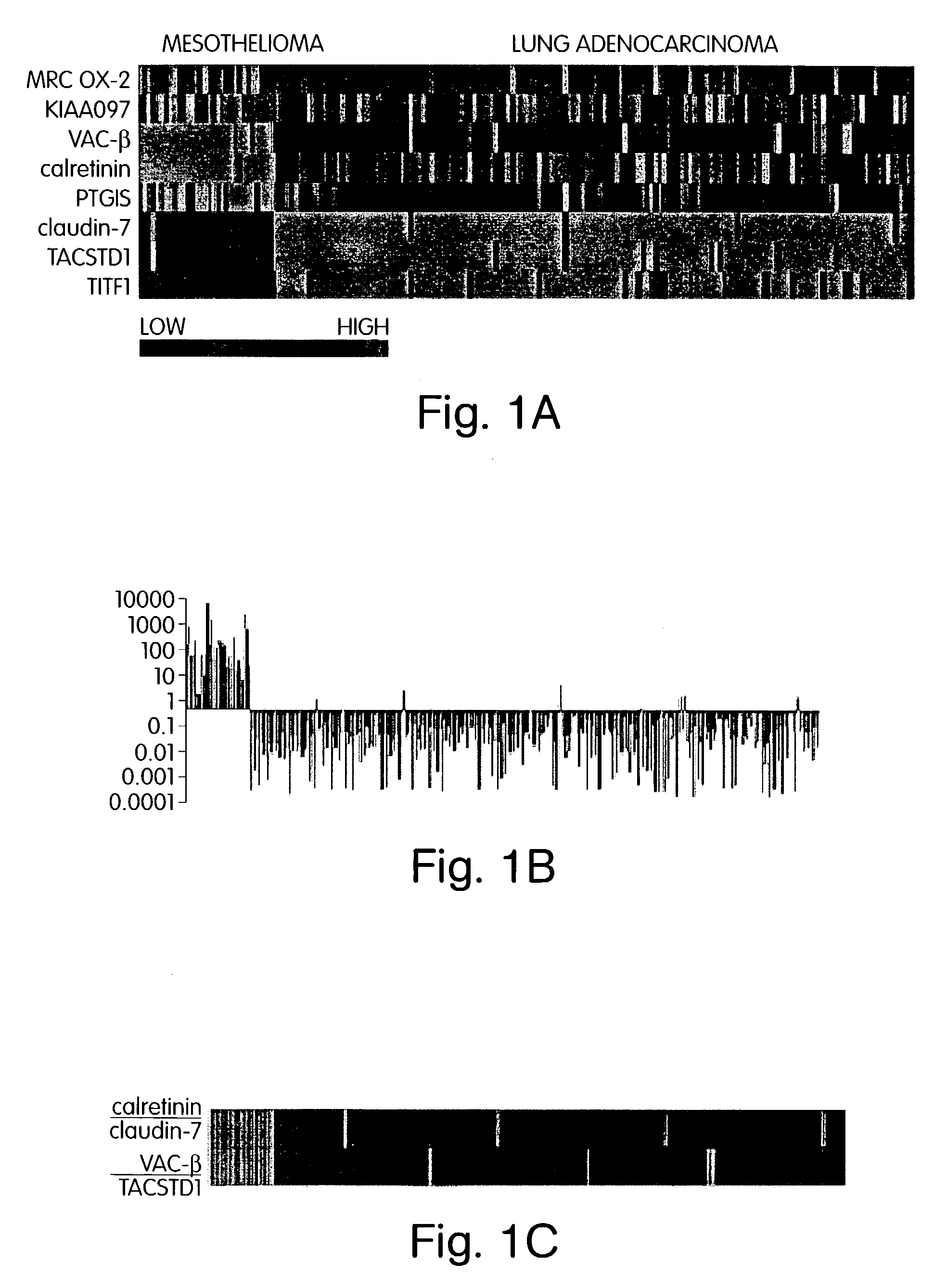
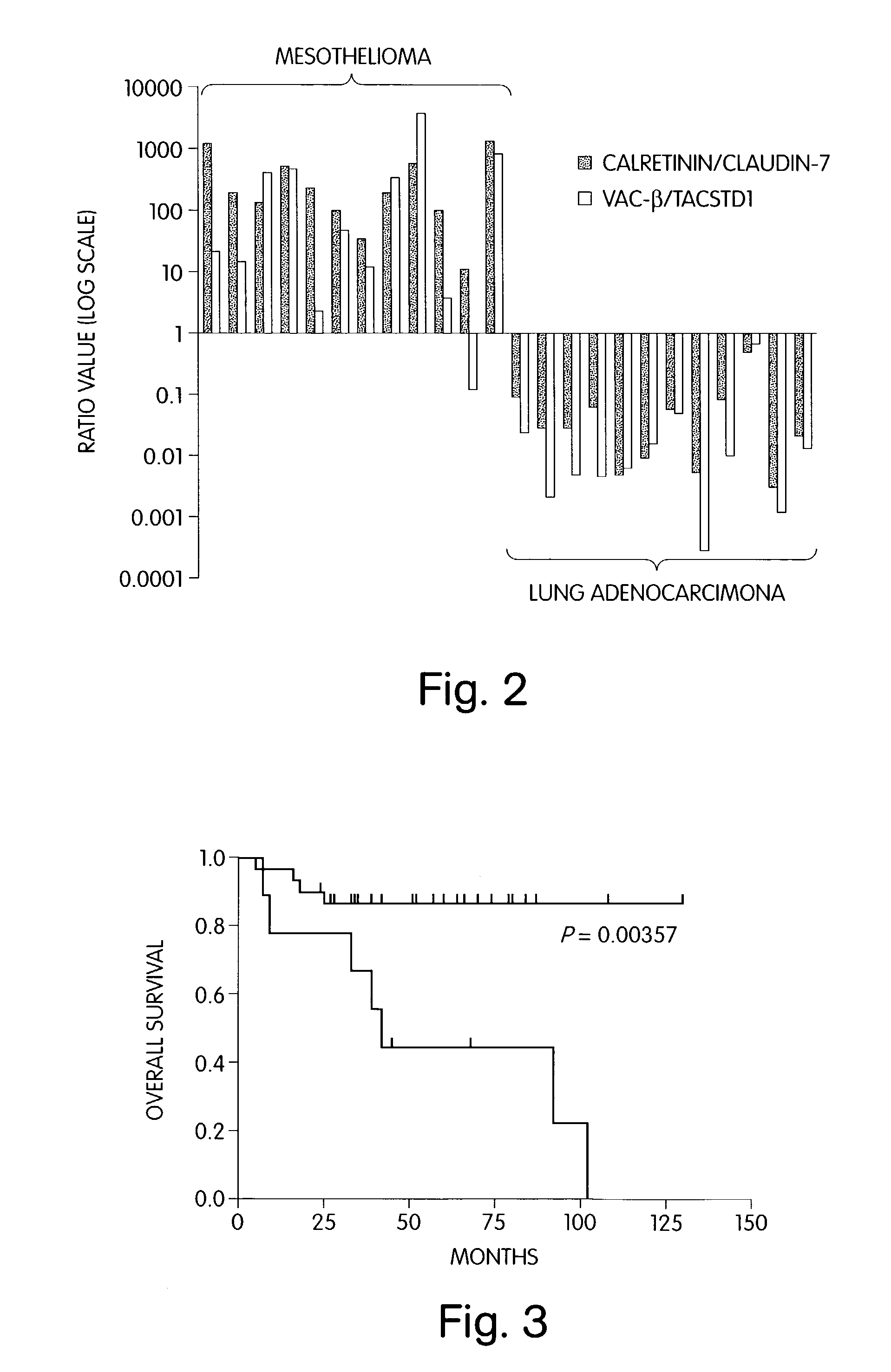
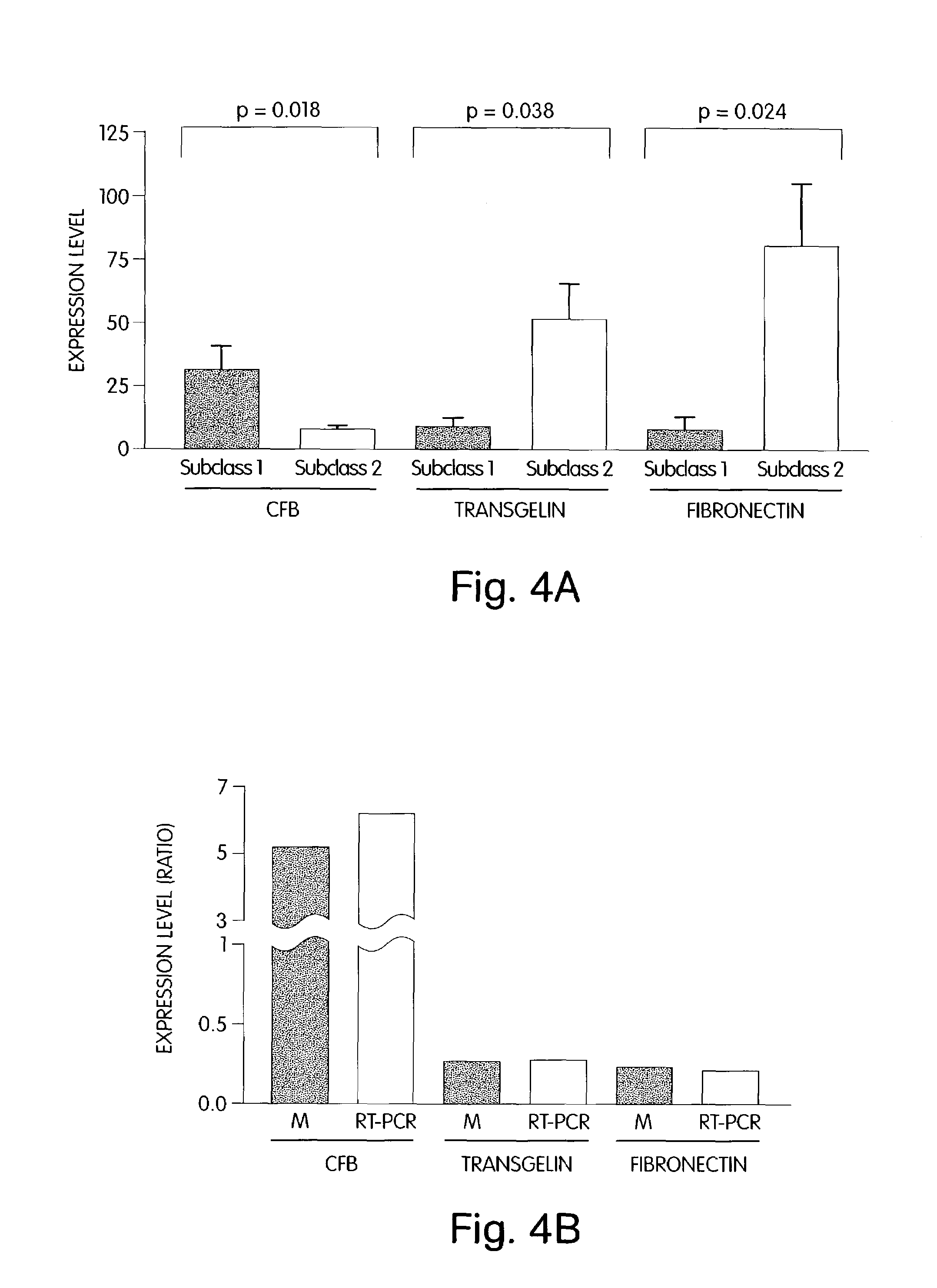
The invention provides methods for diagnosing biological states or conditions based on ratios of gene expression data from tissue samples, such as cancer tissue samples. The invention also provides sets of genes that are expressed differentially in malignant pleural mesothelioma. These sets of genes can be used to discriminate between normal and malignant tissues, and between classes of malignant tissues. Accordingly, diagnostic assays for classification of tumors, prediction of tumor outcome, selecting and monitoring treatment regimens and monitoring tumor progression / regression also are provided.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
Diagnostic and prognostic tests
InactiveUS7622260B2Simple powerEasy to adaptMicrobiological testing/measurementBiological testingTissue sampleExpression gene
The invention provides methods for diagnosing biological states or conditions based on ratios of gene expression data from tissue samples, such as cancer tissue samples. The invention also provides sets of genes that are expressed differentially in malignant pleural mesothelioma. These sets of genes can be used to discriminate between normal and malignant tissues, and between classes of malignant tissues. Accordingly, diagnostic assays for classification of tumors, prediction of tumor outcome, selecting and monitoring treatment regimens and monitoring tumor progression / regression also are provided.
Owner:WESLEYAN UNIVERSITY +1
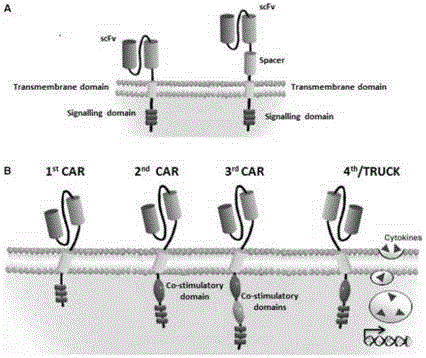
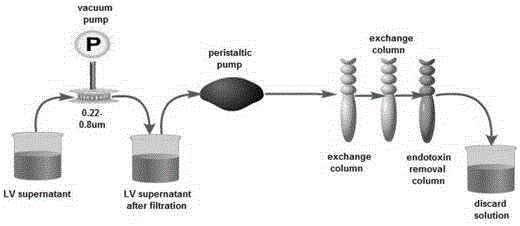
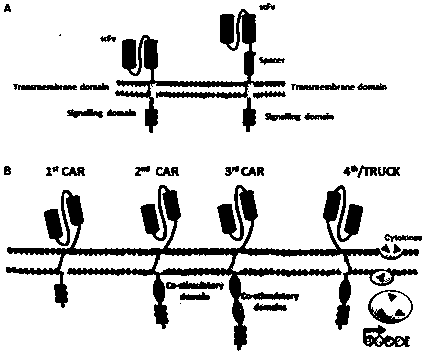
Mesothelin-targeted replication-defective recombinant lentivirus CAR-T transgenic carrier as well as establishment method and application thereof
ActiveCN105969805APromote secretionImprove in vitro killing effectMammal material medical ingredientsNucleic acid vectorEucaryotic cellFluorescence
The invention discloses a Mesothelin-targeted replication-defective recombinant lentivirus CAR-T transgenic carrier which comprises a pronucleus replicon pUC Ori sequence for plasmid replication, an amicillin resistance gene AmpR containing sequence for mass amplification of target strain, virus replicon SV40Ori sequence for enhancing replication in eukaryocyte, a lentivirus packaging cis-element for lentivirus packaging, a ZsGreen1 green fluorescent protein for green fluorescence expression of eukaryocyte, an IRES ribosome combination sequence for joint transcriptional expression of protein, a human EF1(alpha) promoter for the eukaryotic transcription of chimeric antigen receptor gene, a chimeric antigen receptor for forming a second-generation CAR or third-generation CAR integrating identification, transfer and start, and an eWPRE element for improving the transgenic expression efficiency. Moreover, the invention also discloses an establishment method and application of the carrier. In the invention, the secretion of cell factors and the in-vitro killing effect of CAR-T cells can be remarkably enhanced, and the effect of clinical treatment of malignant pleural mesothelioma and pancreatic cancer is outstanding.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
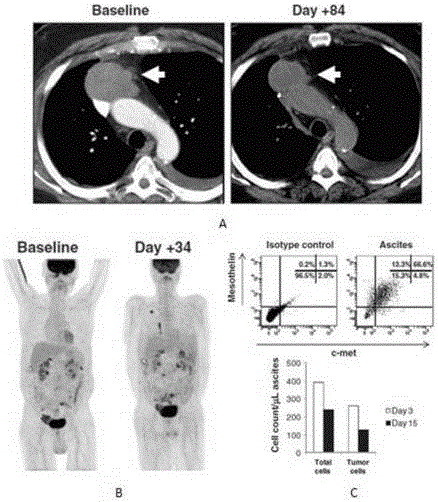
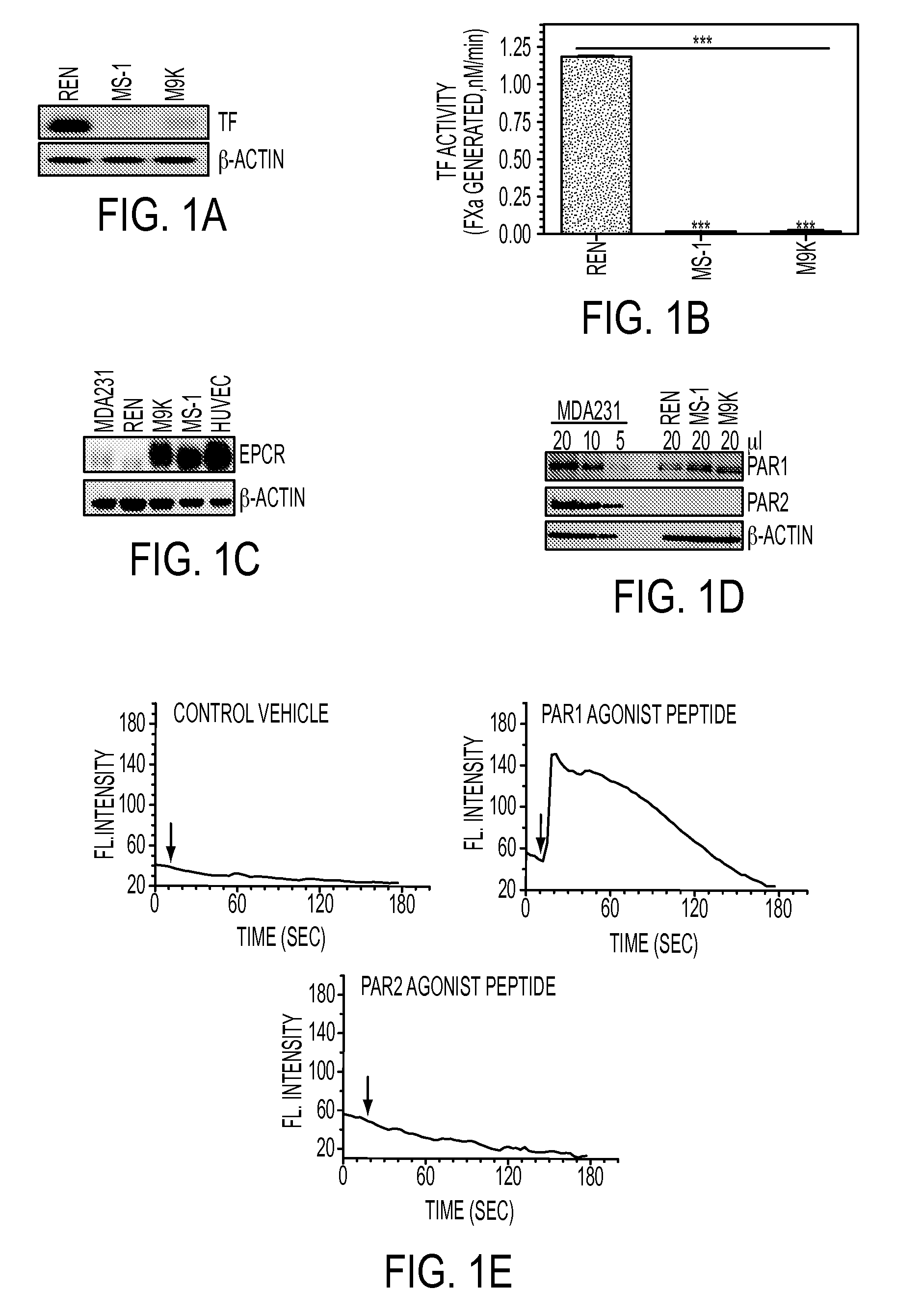
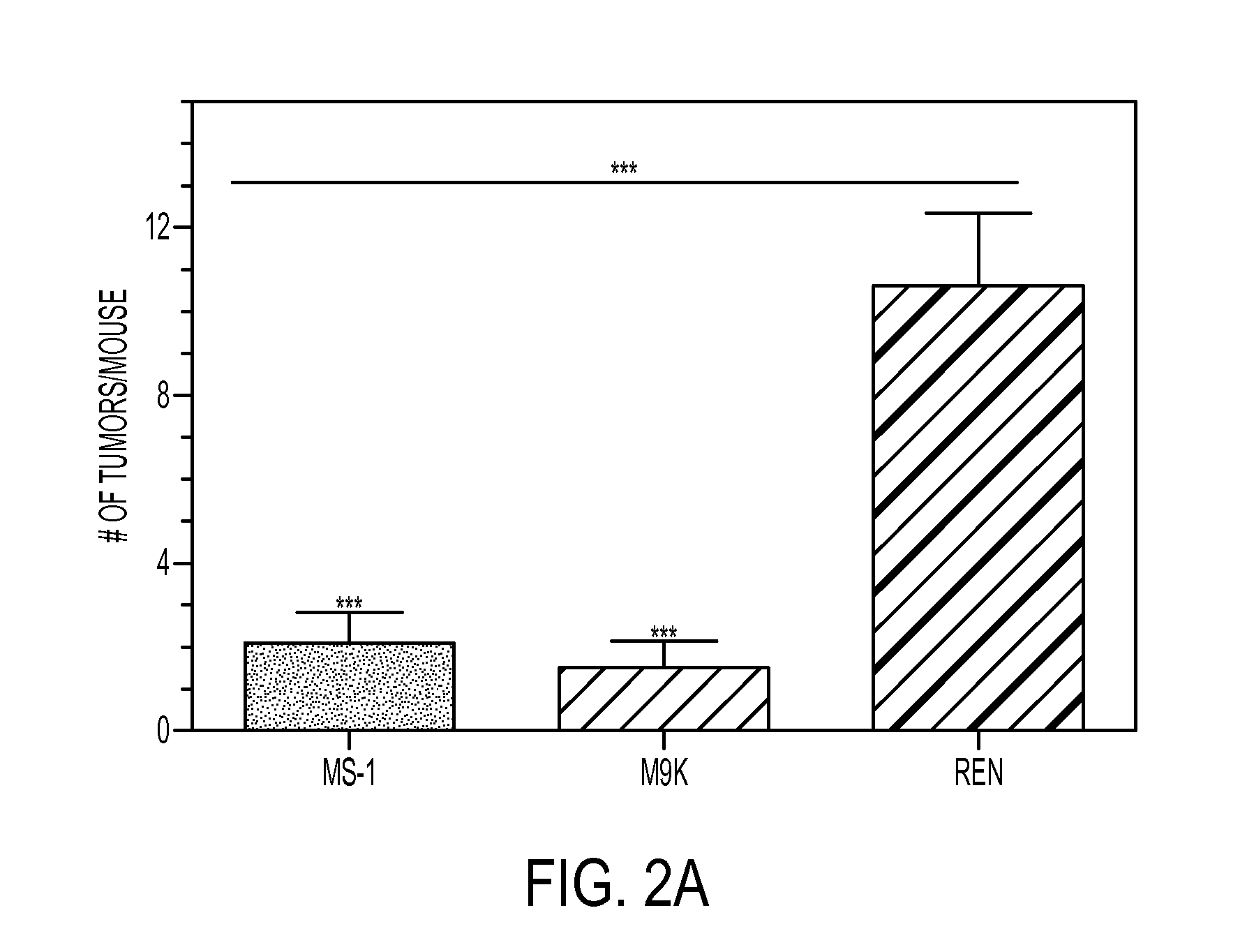
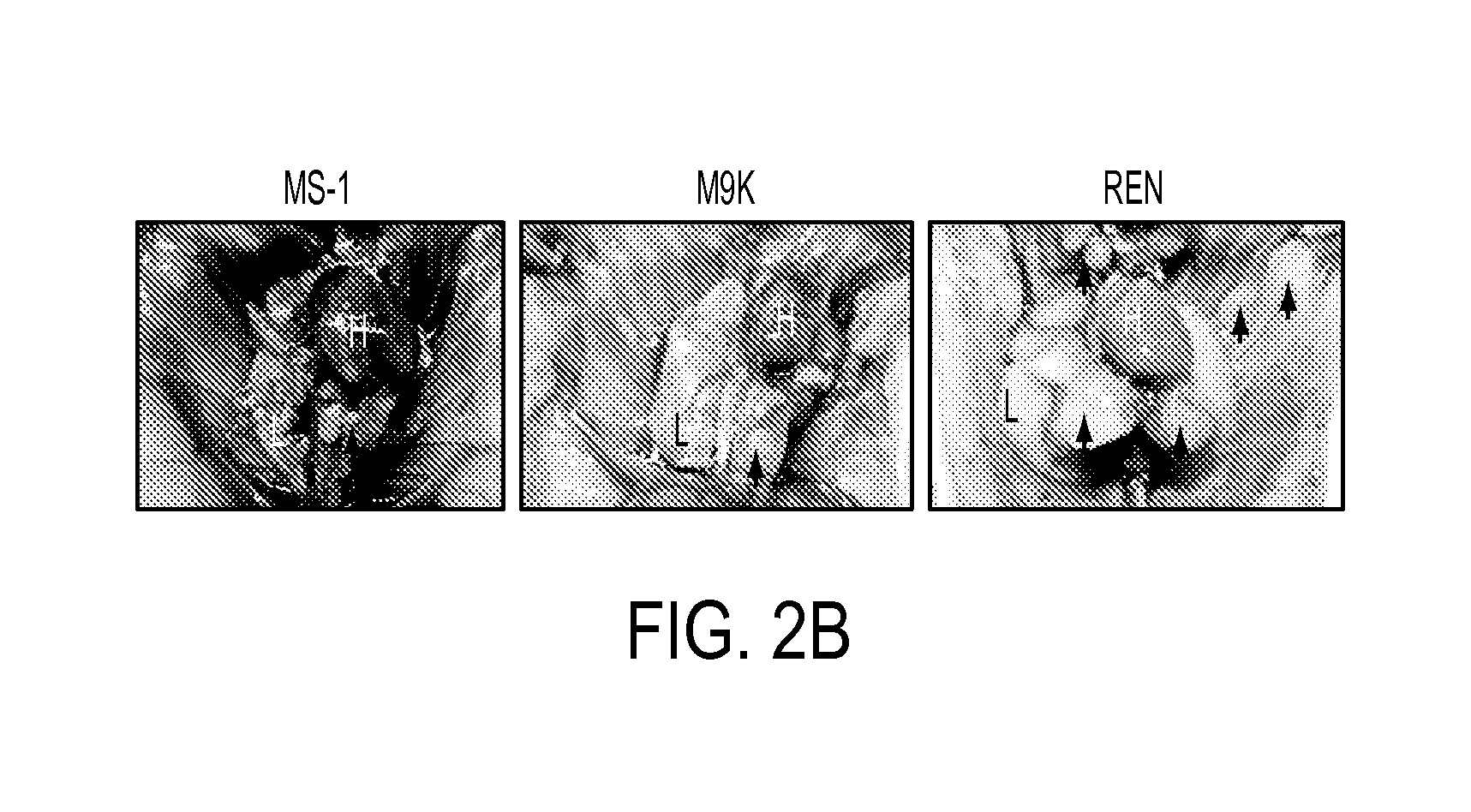
Suppression of malignant mesothelioma by overexpression or stimulation of endothelial protein c receptors (EPCR)
ActiveUS20150366939A1Slow tumor growthInduce an aggressive phenotypeBiocidePeptide/protein ingredientsPleural cavityEndothelial Cell Protein C Receptor
The influence of TF, endothelial cell protein C receptor (EPCR) and protease activated receptor-1 (PAR1) on tumor growth of malignant pleural mesothelioma (MPM) is disclosed. MPM cells that lack or express TF, EPCR or PAR1 and a murine orthotopic model of MPM led to the discovery that intrapleural administration into nude mice of REN MPM cells expressing TF and PAR1 but lacking EPCR and PAR2 generated large pleural cavity tumors. Suppression of TF or PAR1 expression markedly reduced tumor growth. Overexpression of TF in non-aggressive MPM cells expressing EPCR and PAR1 but exhibiting minimal levels of TF failed to alter their tumorigenicity. Introduction of EPCR expression in aggressive MPM cells attenuated tumor growth whereas EPCR silencing in non-aggressive MPM cells overexpressing TF increased tumorigenicity of non-aggressive cells. Expression of EPCR by MPM cells suppresses tumor growth and treats MPM.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Conjugates for the treatment of mesothelioma
InactiveUS20110263512A1Doubled progression free survivalSuccessful usePeptide/protein ingredientsTumor necrosis factorCell-Extracellular MatrixMedicine
The present invention provides conjugates of cytokines and targeting peptides that is able to bind to a receptor expressed on tumor-associated vessels or to a component of the extracellular matrix associated to the tumor vessels, for treatment of malignant pleural mesothelioma. In particular, the invention provides conjugates comprising the cytokine TNF linked to a peptide containing the NGR motif. The invention further provides pharmaceutical compositions comprising such conjugate and pharmaceutical formulations comprising conjugates dissolved in appropriate buffers.
Owner:MOLMED SPA
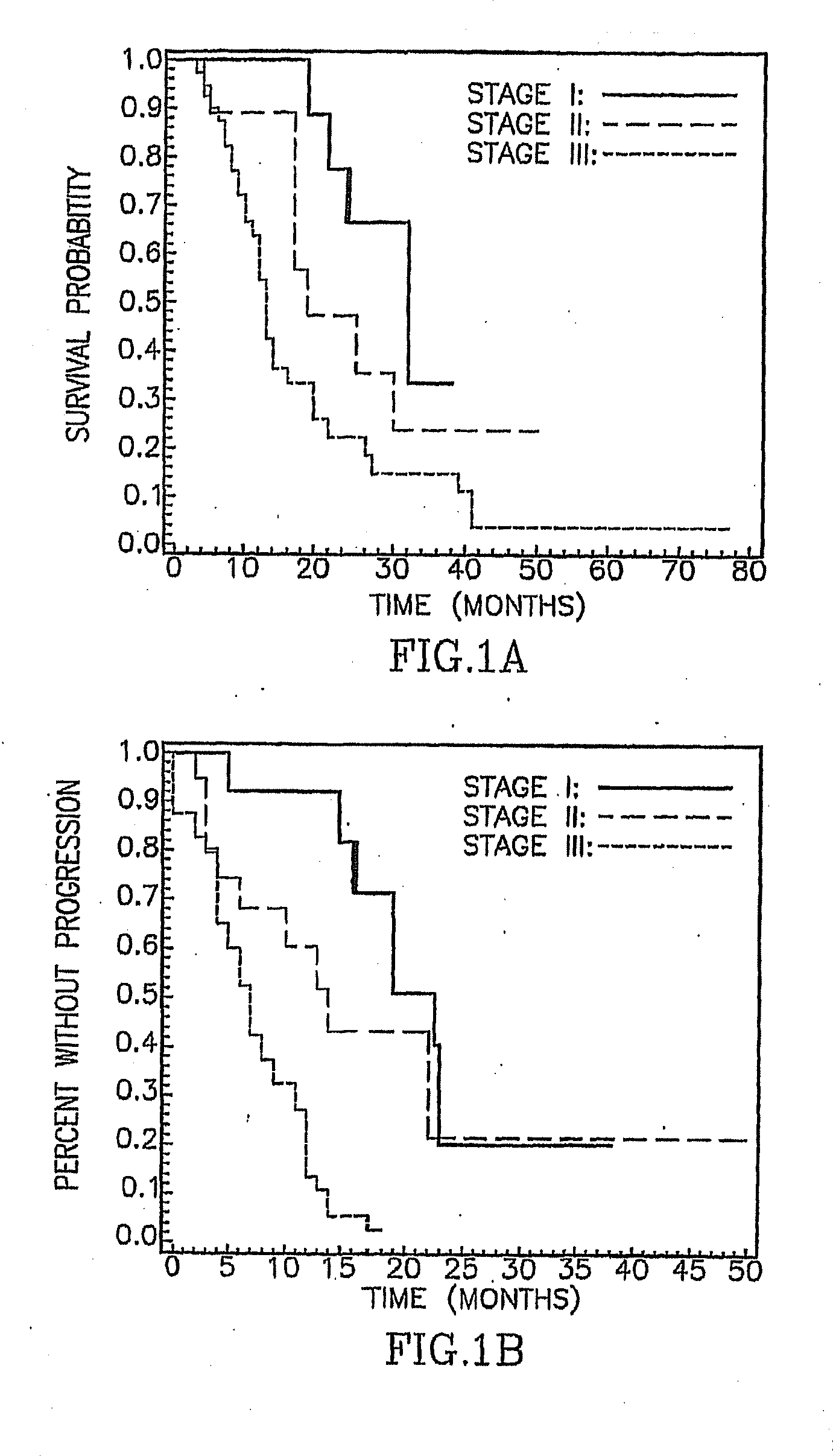
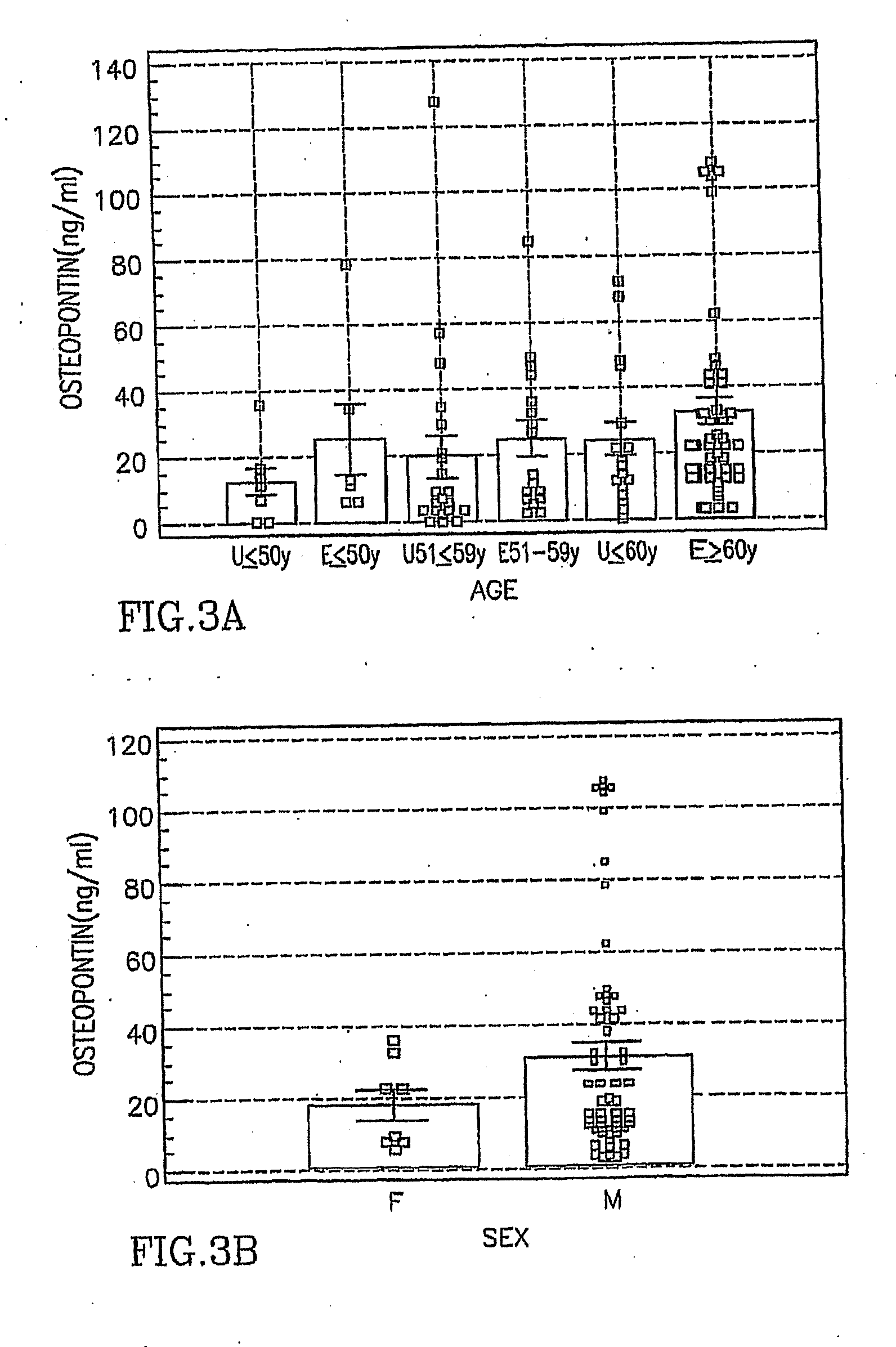
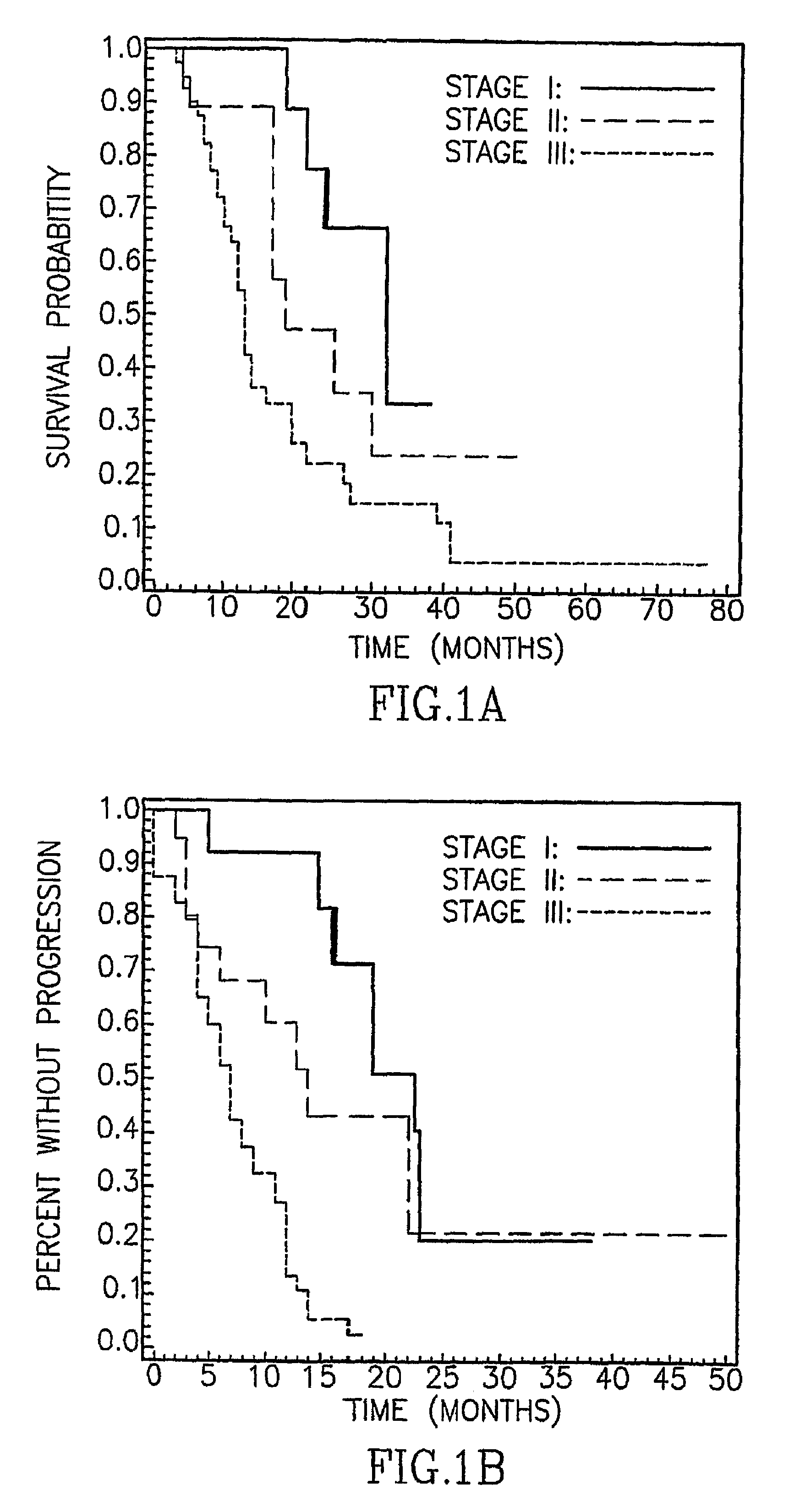
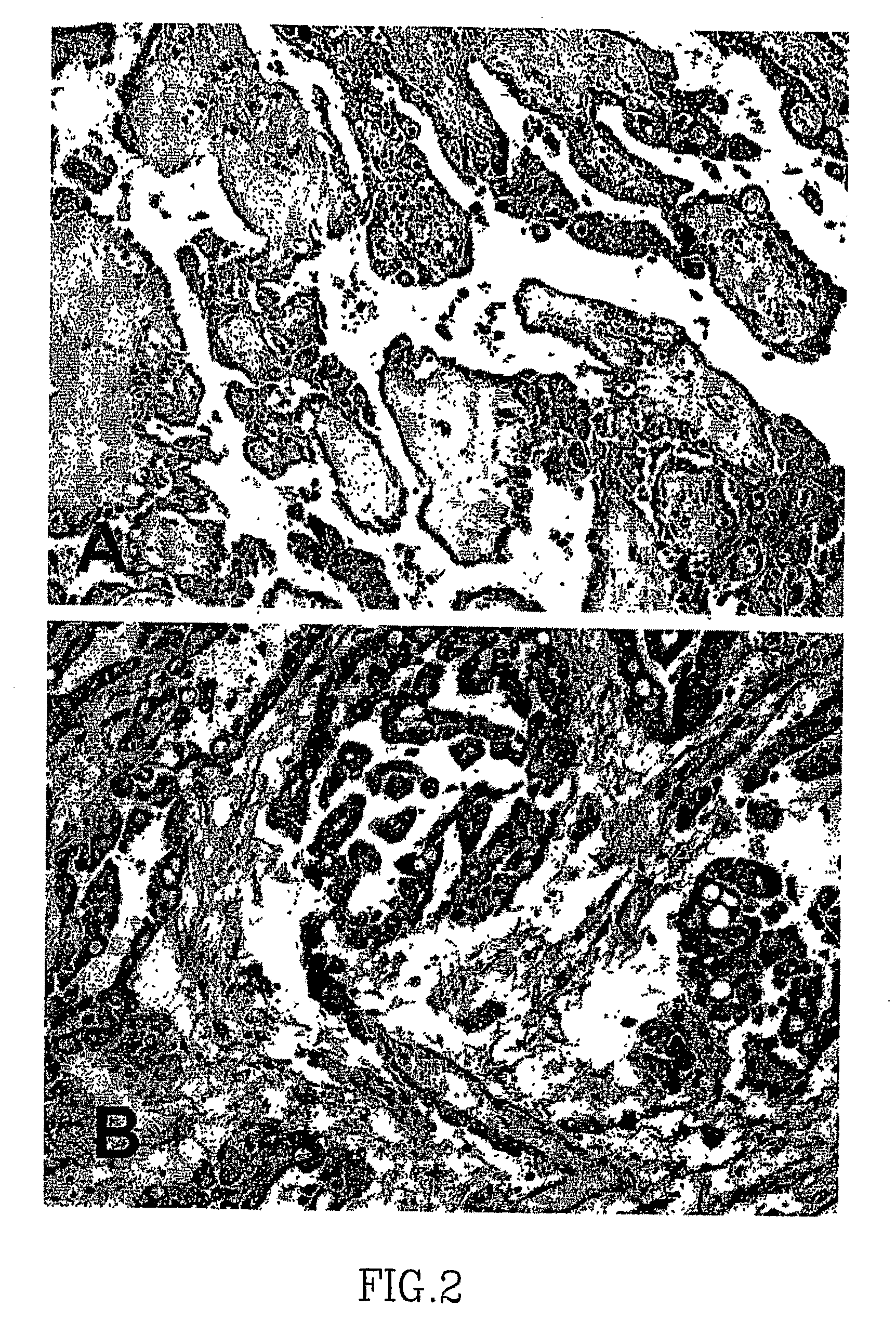
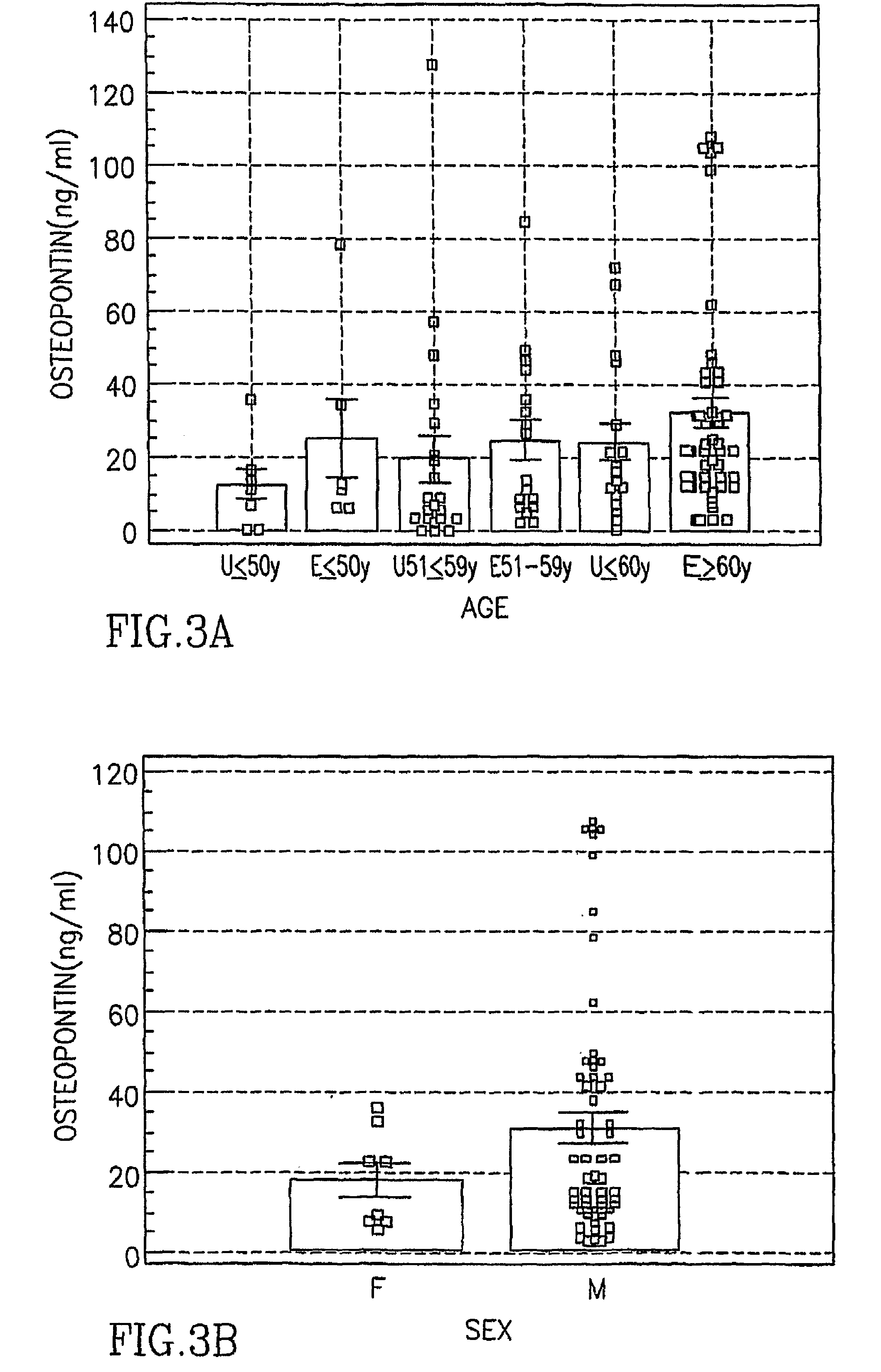
Asbestos exposure, pleural mesothelioma, and serum osteopontin levels
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +2
Novel mouse in-situ pleural mesothelioma model and establishment method thereof
InactiveCN113599010ARestore invasive abilityRestore the effect of tumor metastasisAnimal fetteringSurgical veterinaryAnterior axillaryTumor transplantation
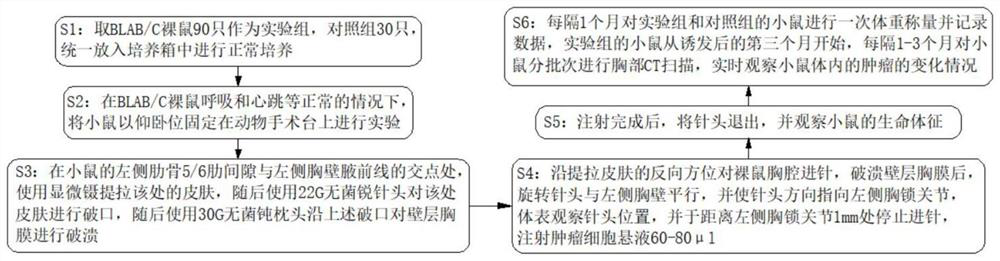
The invention discloses a novel mouse in-situ pleural mesothelioma model and an establishment method thereof. The establishment method comprises the following steps of 1, taking 90 BLAB / C nude mice as an experimental group and 30 control groups, and uniformly putting the experimental group and the control groups into an incubator for normal culture; 2, under the condition that BLAB / C nude mice breathe, heartbeat and the like are normal, anesthetizing the mice and fixing the mice on an animal operating table in a supine position for experiment; and 3, at the intersection point of the left rib 5 / 6 intercostal space and the left chest wall anterior axillary line of the mouse, using microscopic forceps to lift and pull the skin at the intersection point, then using a 22G sterile sharp needle head to break the skin at the intersection point, then using a 30G sterile blunt pillow to break the wall layer pleura along the broken opening. The tumor transplantation method provided by the invention is applicable to orthotopic transplantation of pleural tumors. The growth mode, the invasion ability and the tumor metastasis effect of the pleural tumor can be restored to the maximum extent, and a good mouse model is constructed for studying the pleural tumor.
Owner:FIRST AFFILIATED HOSPITAL OF KUNMING MEDICAL UNIV
Liquid pharmaceutical composition comprising pemetrexed
InactiveUS20180235969A1Improve long-term stabilityReduce solubilityOrganic active ingredientsPharmaceutical delivery mechanismAntioxidantSolvent
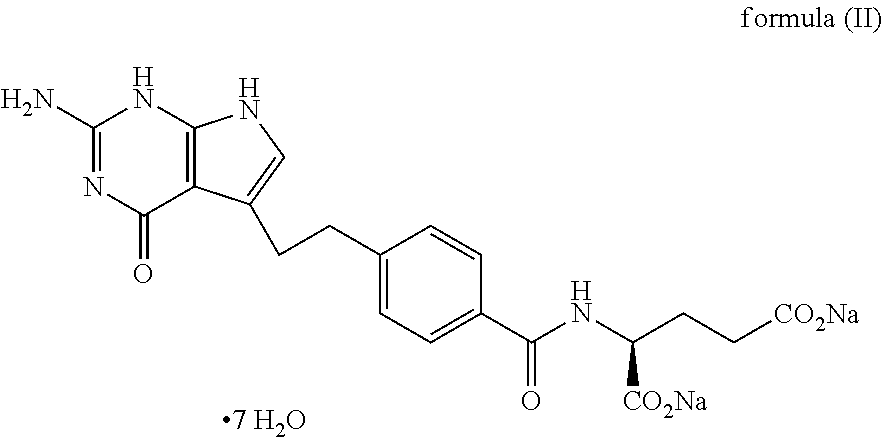
The present invention relates to a liquid pharmaceutical composition suitable for parenteral administration comprising: a) pemetrexed diacid; b) at least one organic amine; c) at least one antioxidant; d) 10-200 mg / ml of propylene glycol; and e) one or more parenteral solvents, wherein the preparation thereof is conducted in an atmosphere of inert gas and wherein the organic amine(s) is present in an amount sufficient to reach a pH of the composition in the range from 8.3 to 9.1 The invention further relates to the use of said liquid pharmaceutical composition as medicament in the treatment of malignant pleural mesothelioma and non-small cell lung cancer.
Owner:SYNTHON BV
Stable liquid compositions of pemetrexed
InactiveUS20200246263A1Improve stabilityOrganic active ingredientsPharmaceutical delivery mechanismCyclodextrinMalignancy
The present invention relates to a stable liquid pharmaceutical composition of pemetrexed for parenteral administration. The invention provides composition comprising pemetrexed diacid, an organic amine and cyclodextrin. The composition may further comprise an inert gas. The composition can be ready to use infusion solution of pemetrexed diacid or liquid concentrate formulation to be diluted before administration to the patient. The present invention further relates to a process for manufacturing the compositions as well as use of the compositions of the invention for the treatment of malignant pleural mesothelioma and non-small cell lung cancer.
Owner:FRESENIUS KABI ONCOLOGY LTD
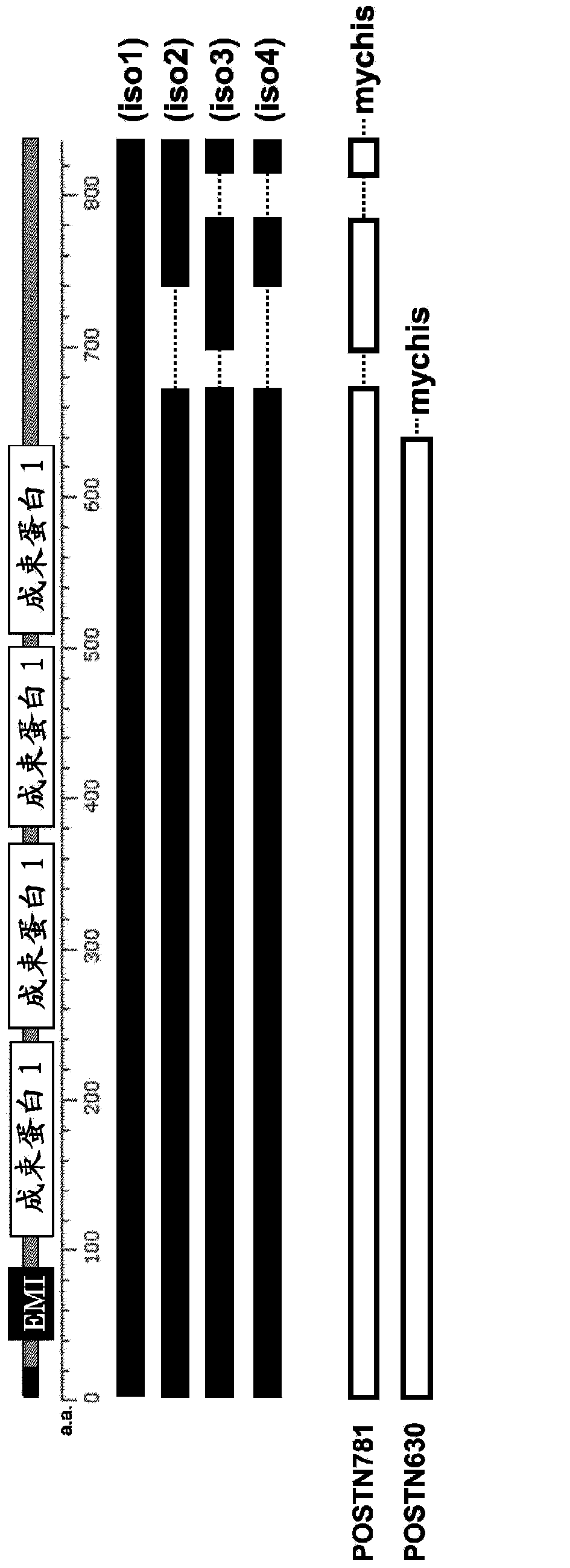
Molecular marker for the early detection of malignant pleural mesothelioma and the methods of its expression analysis using blood and pleural effusion samples
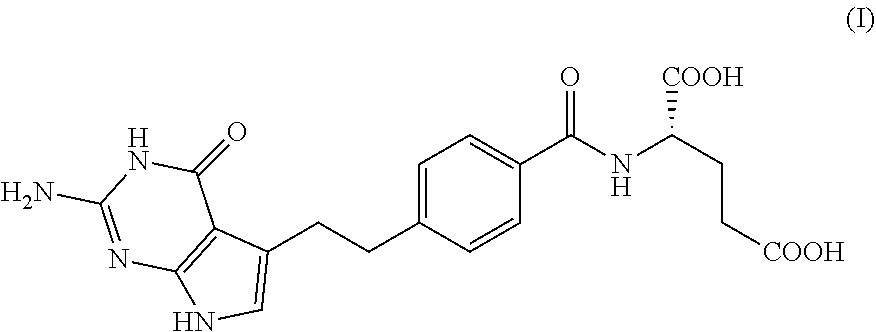
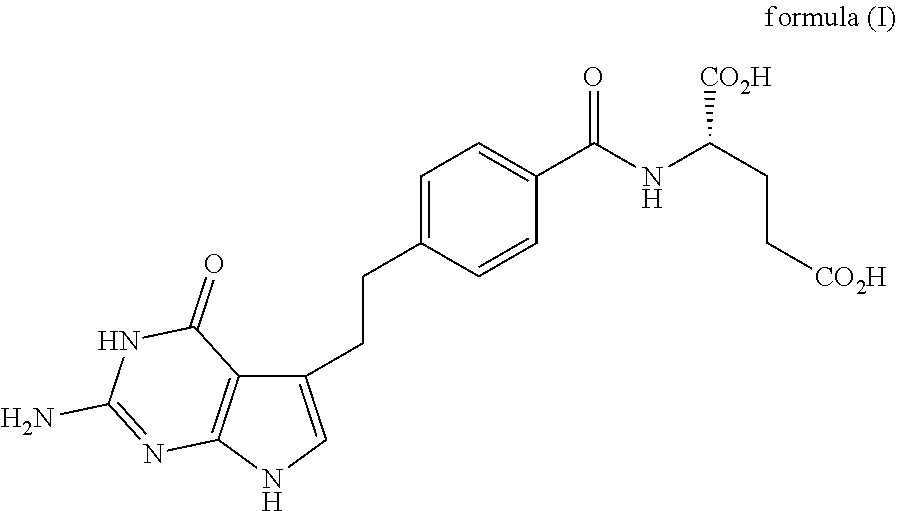
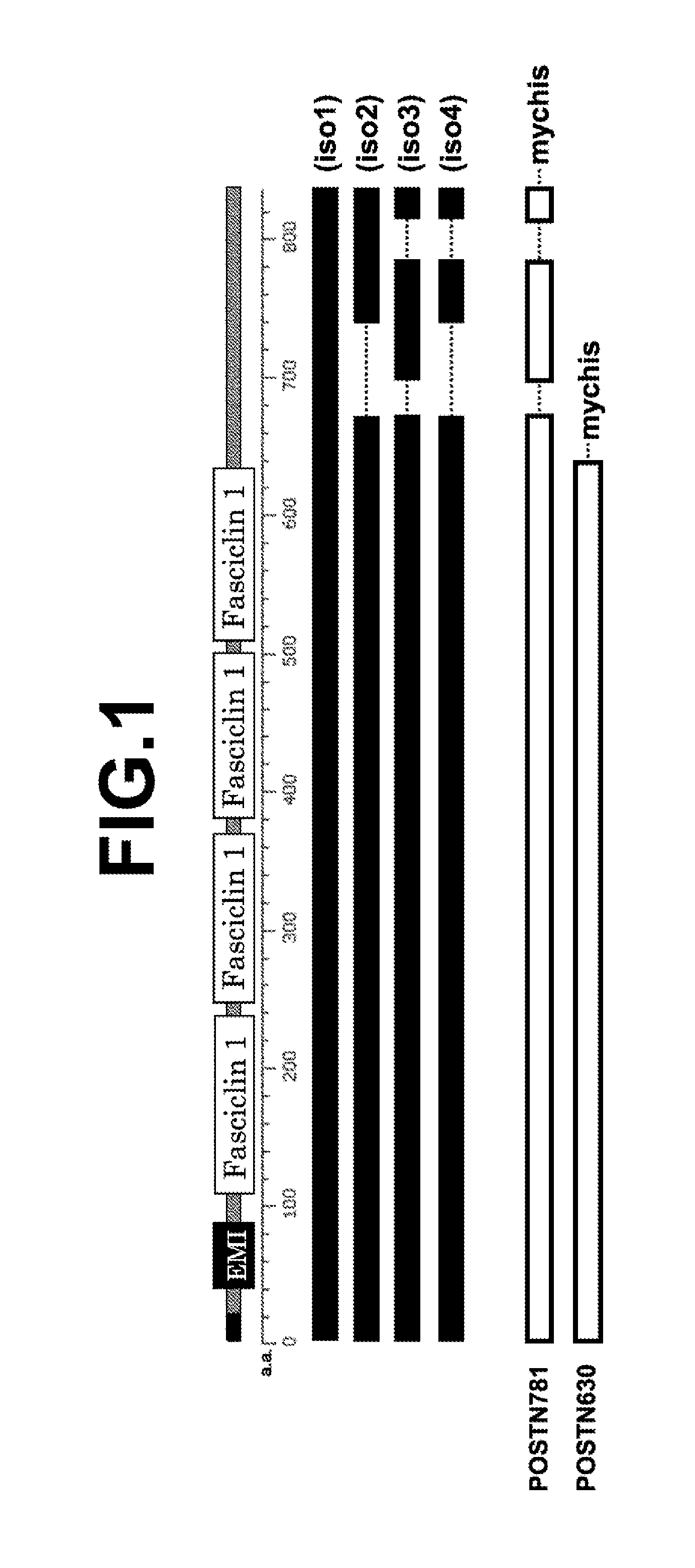
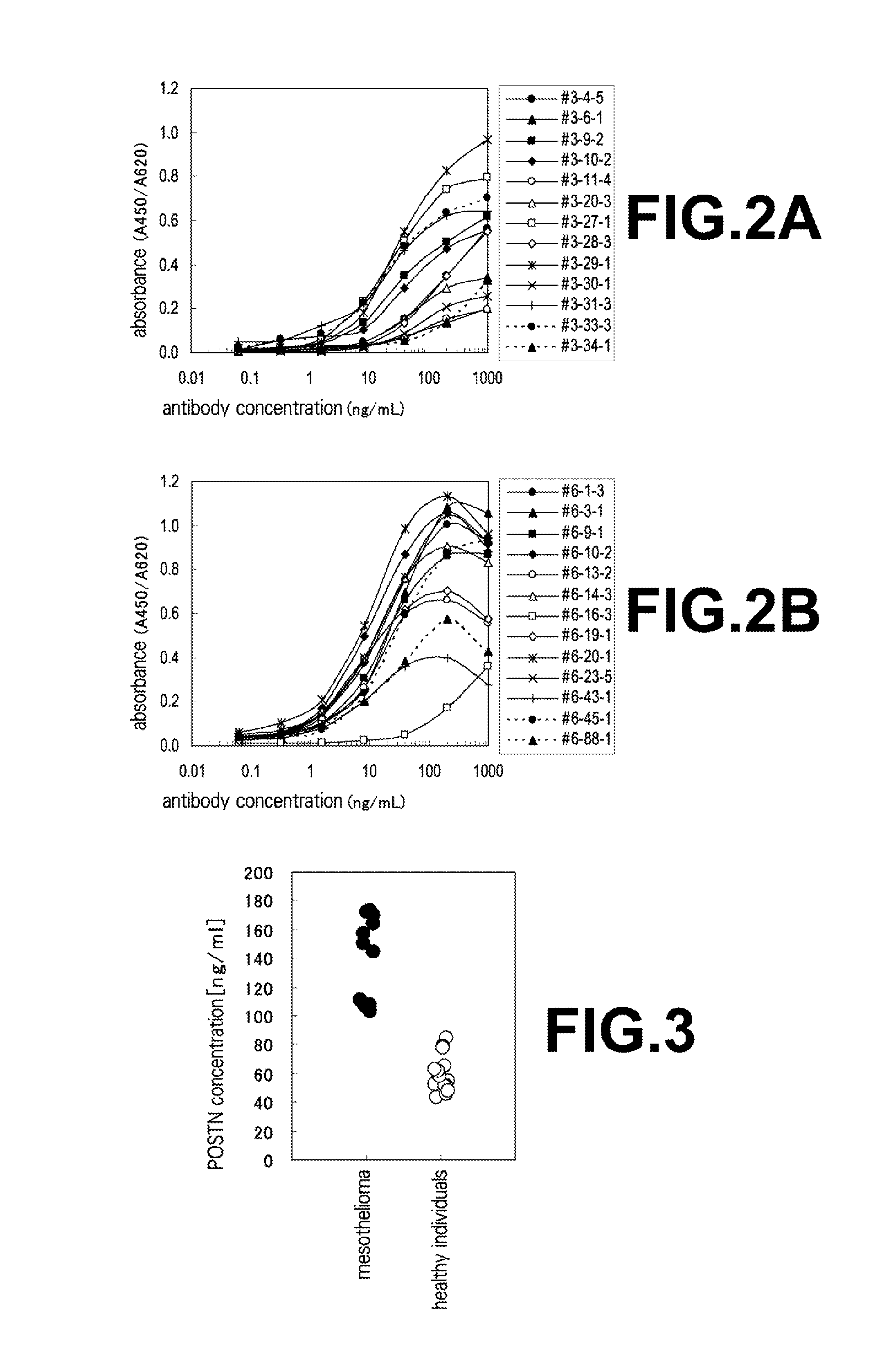
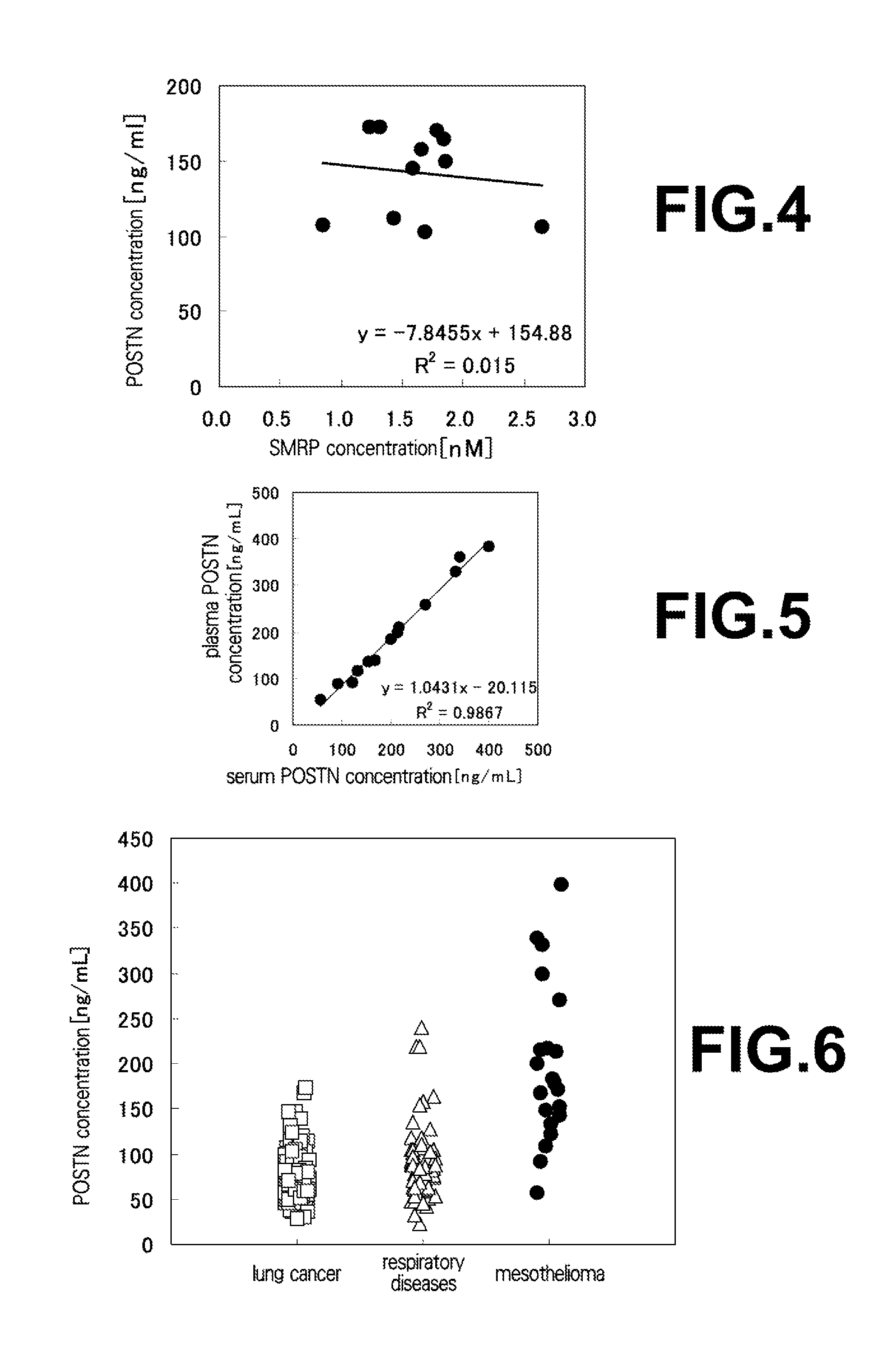
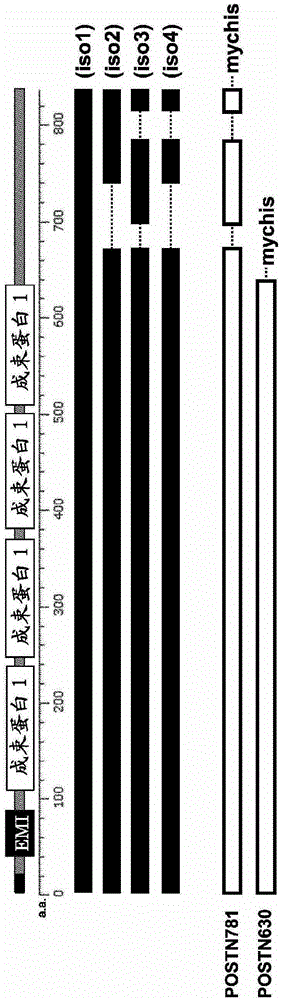
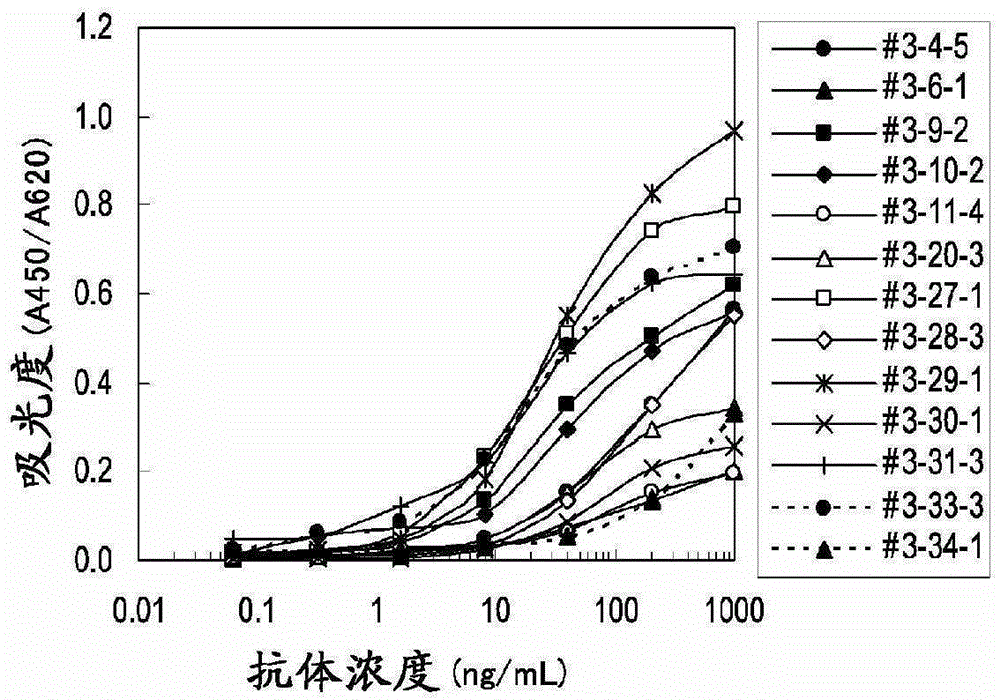
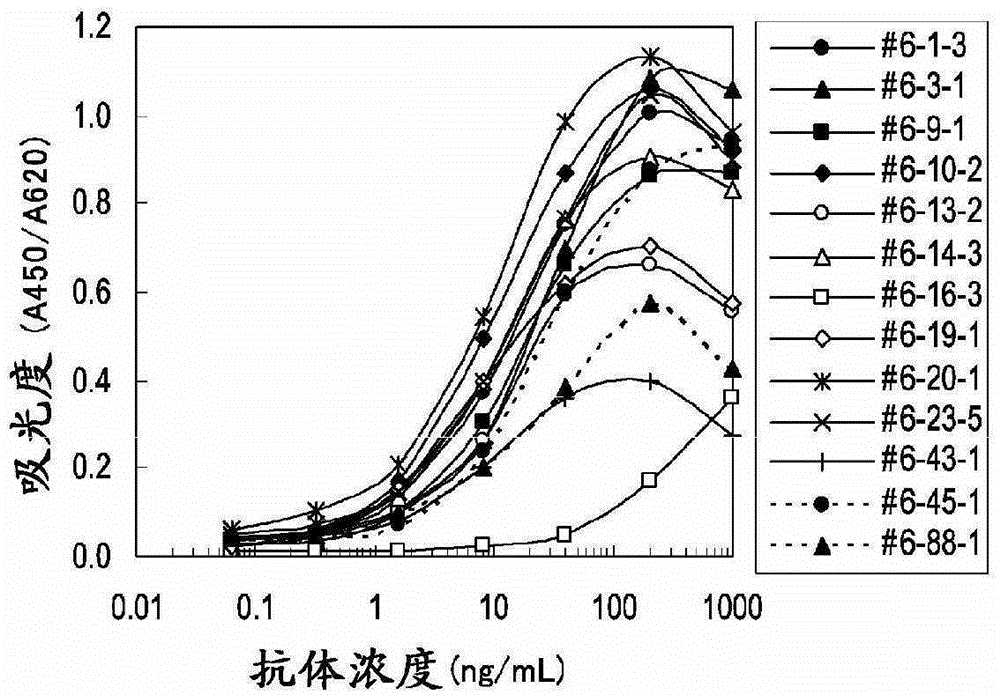
The present invention provides a method for testing mesothelioma comprising a step of determining a concentration of a human periostin protein in at least one type of sample of blood or pleural fluid of a subject. In the step of determining the concentration of human periostin protein, an antibody directed against human periostin protein may be used. The present invention further provides a kit for diagnosing mesothelioma, said kit comprising an antibody directed against human periostin protein. In the kit for diagnosing mesothelioma, the antibody directed against a human periostin protein may be an antibody that binds to a polypeptide consisting of an amino acid sequence set out in SE ID NO: 2.
Owner:NAGOYA UNIVERSITY +2
Methods for the treatment and the prognostic assessment of malignant pleural mesothelioma
InactiveUS8771691B2Reduce negative impactImprove rendering capabilitiesBiocideOrganic active ingredientsPathologyPleural mesothelioma
The present invention relates to methods for the treatment and the prognostic assessment of malignant pleural mesothelioma.
Owner:ASSISTANCE PUBLIQUE HOPITAUX DE PARIS
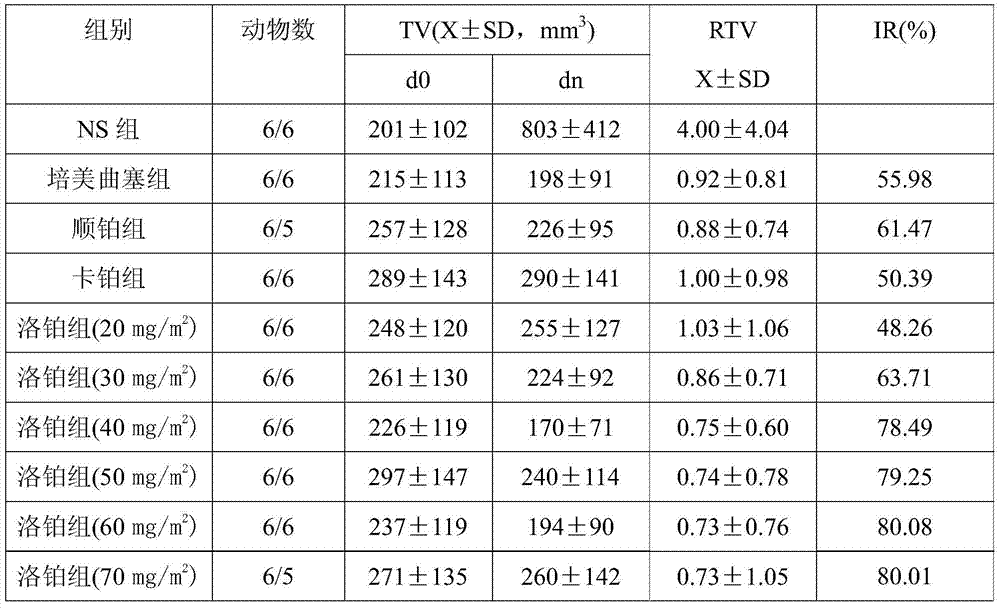
Application of lobaplatin in preparing medicines for treating malignant pleural mesothelioma
InactiveCN106974902AAntineoplastic agentsHeavy metal compound active ingredientsTreatment effectCancer research
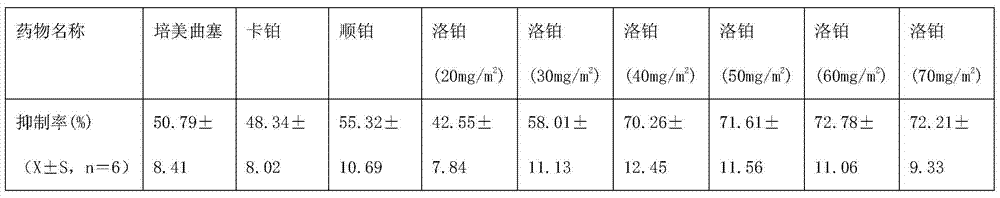
The invention discloses an application of lobaplatin in preparing medicines for treating malignant pleural mesothelioma. The lobaplatin is represented in dosage forms of lyophilized powder for injection, a small -volume injection or a large-volume injection, with an effective therapeutic dose of 30-60mg / m<2> body surface area. The lobaplatin and a preparation thereof are relatively strong in action of resisting the malignant pleural mesothelioma; and the lobaplatin and the preparation thereof are significant in therapeutic effect, low in toxicity and safe; therefore, a novel effective means is provided for the treatment of the malignant pleural mesothelioma and clinical application of the lobaplatin and the preparation thereof is widened.
Owner:GUIZHOU YIBAI PHARMA CO LTD
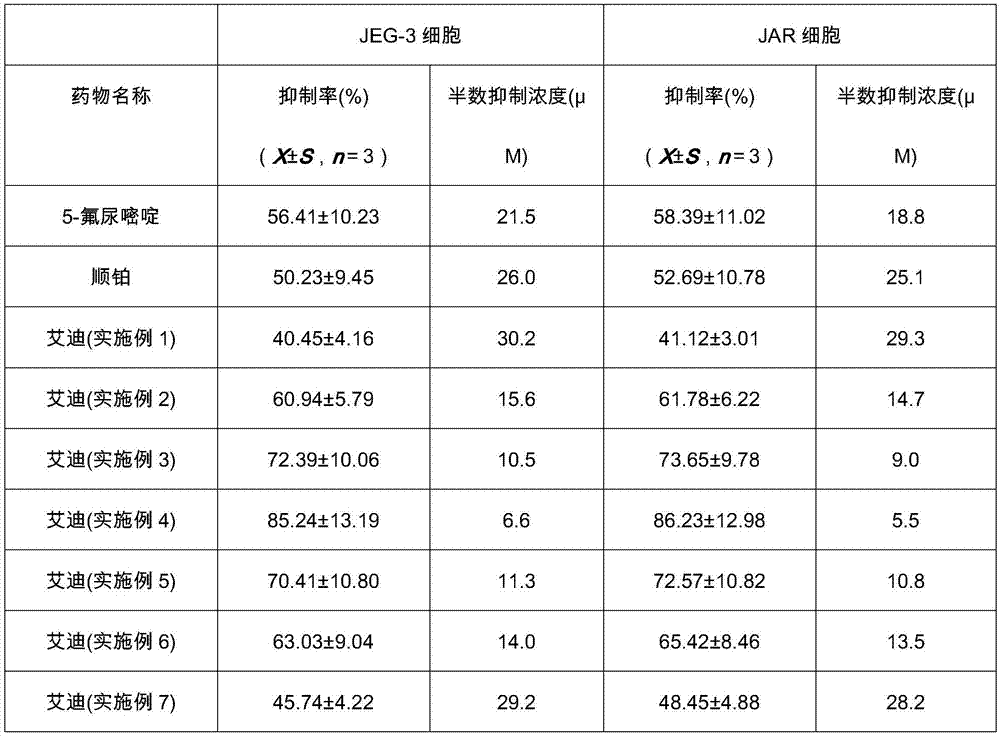
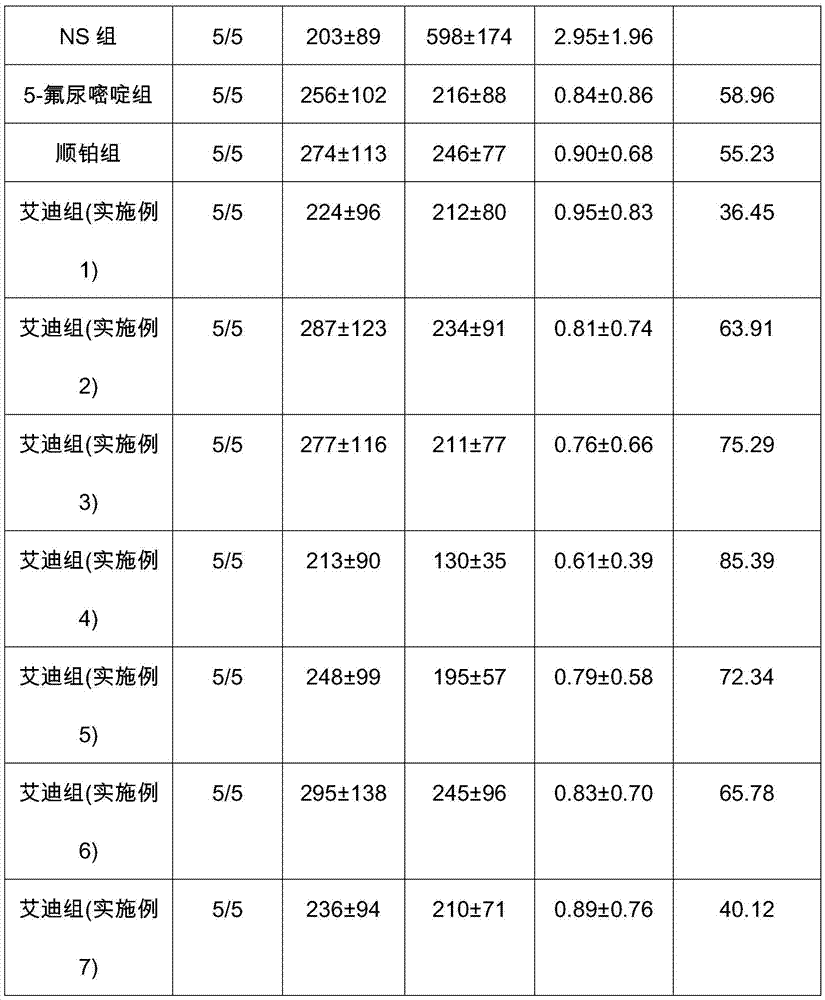
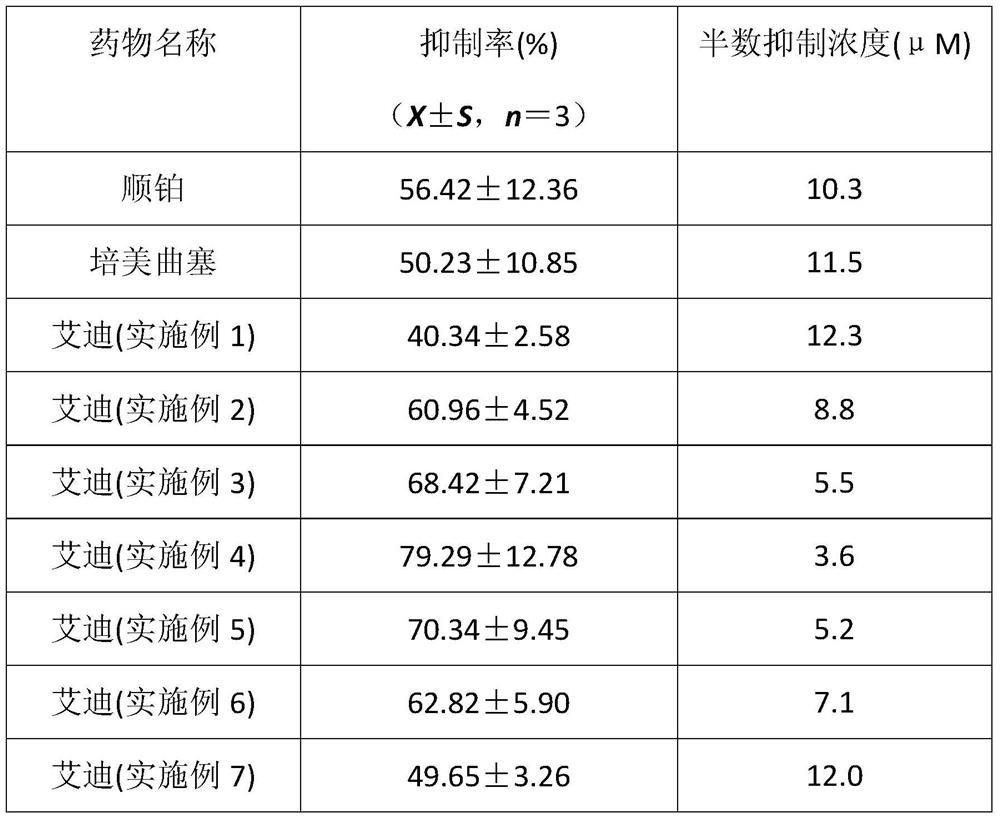
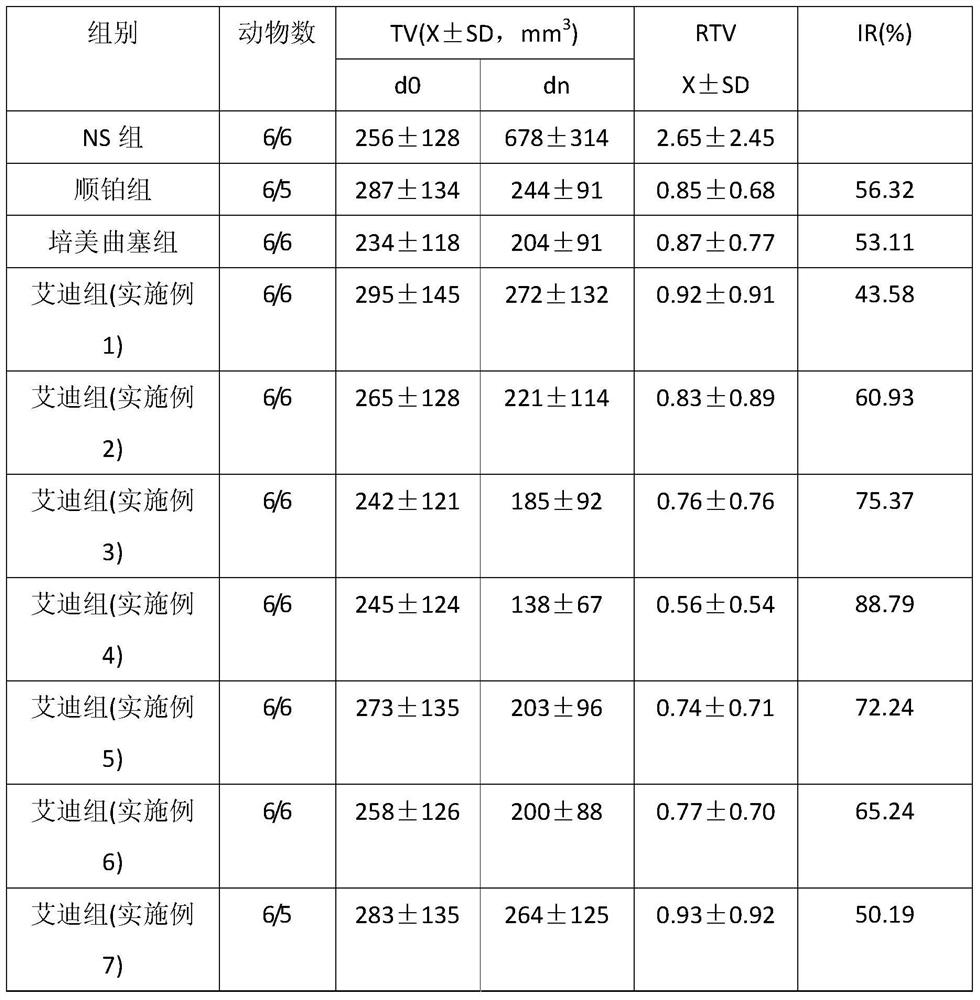
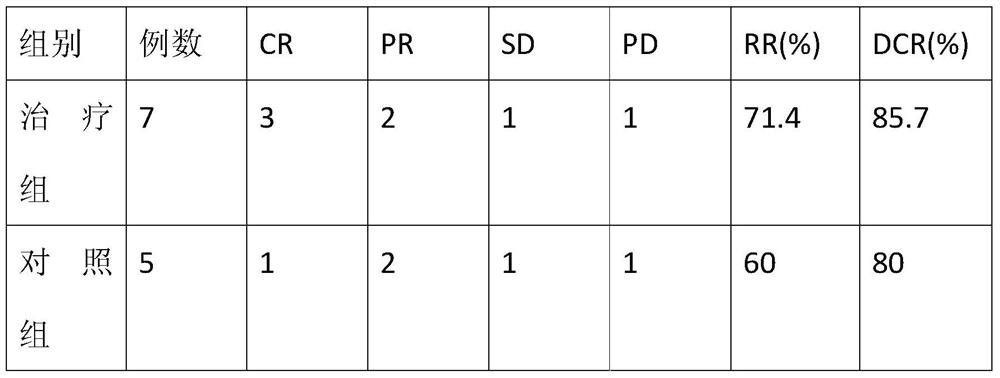
Application of Aidi preparation in preparing medicines for treating malignant pleural mesothelioma
ActiveCN106924330ASignificant effectImprove the quality of lifeAnthropod material medical ingredientsPharmaceutical delivery mechanismSide effectMedicine
The invention discloses an application of an Aidi preparation in preparing medicines for treating malignant pleural mesothelioma. The Aidi preparation consists of four medicines, namely Chinese blister beetles, ginseng, radix astragali and radix acanthopanacis semticosi. The Aidi preparation provided by the invention is relatively strong in effect on treating the malignant pleural mesothelioma; and the medicine (the Aidi preparation) is obvious in curative effect, low in side effects and safer in clinical administration; therefore, a novel therapeutic approach is provided for the treatment of the malignant pleural mesothelioma and clinical application of the Aidi preparation is widened.
Owner:GUIZHOU YIBAI PHARMA CO LTD
Asbestos Exposure, Pleural Mesothelioma and Osteopontin Levels
The present invention provides diagnostic and disease progression or recurrence prediction methods based on osteopontin levels in a biological sample.
Owner:WAYNE STATE UNIV +1
Molecular marker for early indentification of pleural mesothelioma patients, and expression analysis method for same
InactiveCN103842823AImmunoglobulins against animals/humansRecombinant DNA-technologyPleural fluidRespiratory disease
To develop a simple, low-cost and highly reliable testing method for mesothelioma, and a kit used for said testing method. The present invention provides a testing method for mesothelioma comprising a step in which the concentration of human periostin protein is measured in at least one type of sample from among samples of the blood or pleural fluid of a subject. The step in which the concentration of human periostin protein is measured may use an antibody directed against human periostin protein. The present invention further provides a kit for diagnosing mesothelioma, said kit including the antibody directed against human periostin protein. In one kit for diagnosing mesothelioma, the diagnosis of mesothelioma determines if a subject that may have mesothelioma has mesothelioma or is healthy. In another kit for diagnosing mesothelioma, the diagnosis of mesothelioma determines if a subject that may have mesothelioma has mesothelioma or has a respiratory illness other than mesothelioma.
Owner:NAGOYA UNIVERSITY
Culture medium and culture method of pleural mesothelioma organ
InactiveCN113881635AEffective trainingLow costCell dissociation methodsEpidermal cells/skin cellsPenicillinVascular Endothelial Growth Factor Receptor
The invention discloses a culture medium and a culture method of a pleural mesothelioma organ. The culture medium comprises a basic culture medium Advanced DMEM / F12 and a specific addition factor, the specific addition factor is prepared from the following components: B27 which does not contain vitamin A, N2, N-acetylcysteine, EGF (Epidermal Growth Factor), Noggin, R-spondin 1, Wnt3a, CHIR99021, Thiazovivin, VEGFR (Vascular Endothelial Growth Factor Receptor), PDGFR, Penicillin and Streptomycin mixed liquor and Primocin. The culture medium contains the least components required by pleural mesothelioma organoid culture, can effectively culture mesothelial cell-derived tumor tissues, does not contain FBS, saves the cost, and reduces the cytotoxicity and inhibitors caused by FBS at the same time. The pleural mesothelioma organoid cultured by the invention maintains the morphological structure and gene characteristics of the primary tissue.
Owner:ZHEJIANG CANCER HOSPITAL
Molecular markers and their expression analysis methods for early detection of patients with pleural mesothelioma
InactiveCN103842823BImmunoglobulins against animals/humansRecombinant DNA-technologyPleural fluidRespiratory disease
An object of the present invention is to develop a simple, inexpensive, and highly reliable method for examining mesothelioma, and a kit used in the method. As a solution to the problem, the present invention provides a method for examining mesothelioma, comprising the step of measuring the concentration of human periostin protein in at least one sample of blood or pleural fluid of a subject. In some cases, the step of measuring the concentration of the human periostin protein uses an antibody against the human periostin protein. The present invention provides a kit for the diagnosis of mesothelioma, comprising an antibody against human periostin protein. In the kit for diagnosing mesothelioma of the present invention, the diagnosis of mesothelioma may be to determine whether a subject who may have mesothelioma is a mesothelioma patient or a normal person. In the kit for diagnosing mesothelioma of the present invention, the diagnosis of mesothelioma may be to determine whether a subject who may suffer from mesothelioma is a patient with mesothelioma or a respiratory organ disease other than mesothelioma of patients.
Owner:NAGOYA UNIVERSITY
Methods for the Treatment and the Prognostic Assessment of Malignant Pleural Mesothelioma
InactiveUS20110300161A1Reduce negative impactImprove rendering capabilitiesOrganic active ingredientsPeptide/protein ingredientsPathologyPleural mesothelioma
The present invention relates to methods for the treatment and the prognostic assessment of malignant pleural mesothelioma.
Owner:ASSISTANCE PUBLIQUE HOPITAUX DE PARIS
Preparation method of bio-modified castor protein and application of bio-modified castor protein to preparation of drug for treating malignant pleural mesothelioma
InactiveCN104628816AThe content can be detectedClear structureAnthropod material medical ingredientsPeptide/protein ingredientsMicropore FilterChromatographic separation
The invention discloses a preparation method of a bio-modified castor protein and application of the bio-modified castor protein to preparation of a drug for treating malignant pleural mesothelioma. The preparation method of the bio-modified castor protein comprises the following steps of puffing and smashing a bio-modified castor insect body in a normal-temperature puffing device; then, adding the smashed bio-modified castor insect body into an expanding solvent system extracting device to extract a crude bio-modified castor protein; collecting a solution containing the crude bio-modified castor protein, and filtering by using a micropore filter to remove impurities; carrying out ultrafiltration treatment by using an ultrafilter, and collecting a bio-modified castor protein ultrafiltration solution; dissolving the ultrafiltration solution into acetonitrile, separating by using a solid-phase extraction column, and collecting an eluted component; dissolving the eluted component into a solvent system, and separating and purifying by using an ultrasonic stationary wave preparative liquid chromatography; and preparing freeze-dried protein powder by using a vacuum freeze-drying machine, wherein the freeze-dried protein powder can be used for preparing the drug for treating malignant pleural mesothelioma. A bio-modified castor protein preparation prepared by using the preparation method is stable in quality, clear in structure, distinct in pharmacology and remarkable in curative effect.
Owner:HARBIN INST OF TECH
Titled extracts of cynara scolymus for use in the treatment of mesothelioma
The present invention relates to a titrated extract of Cynara scolymus, to titrated fractions of extract of Cynara scolymus or titrated mixtures of said extract with one or more of said titrated fractions or mixtures of said fractions and to compositions and kits that comprise them for the prevention and / or the treatment of malignant pleural mesothelioma.
Owner:ABOCA S P A SOC AGRI
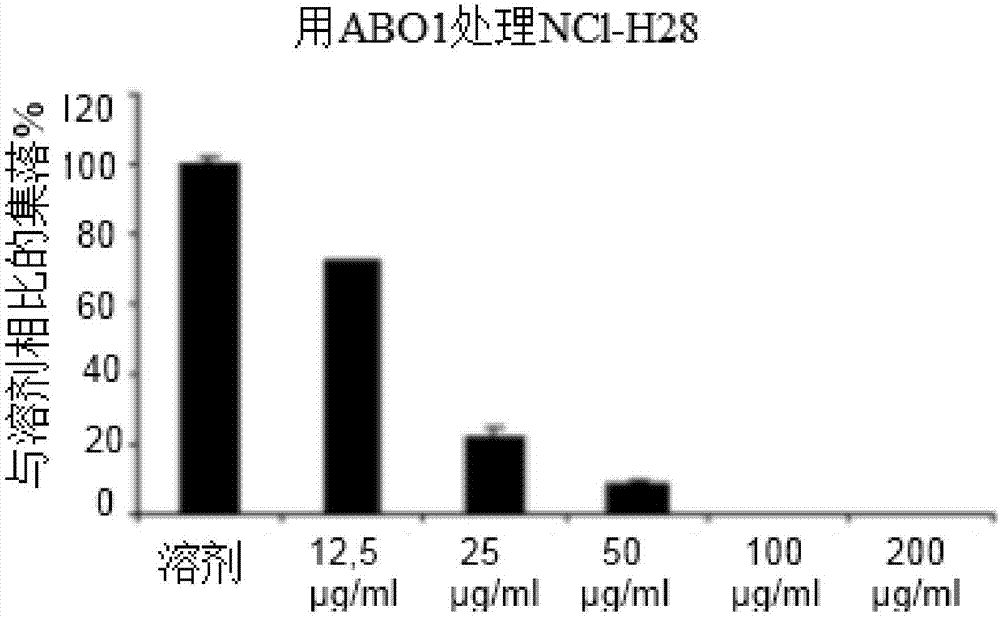
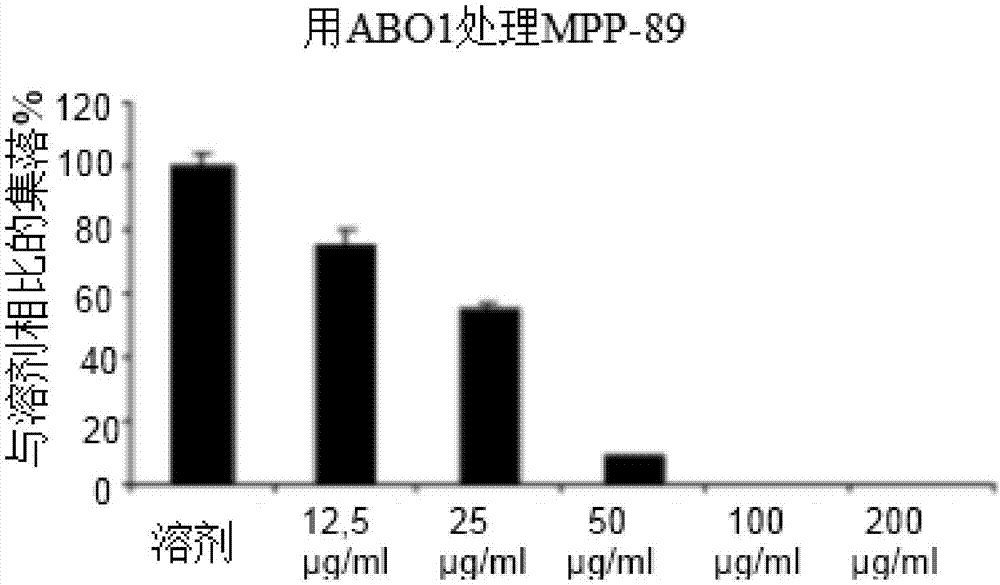
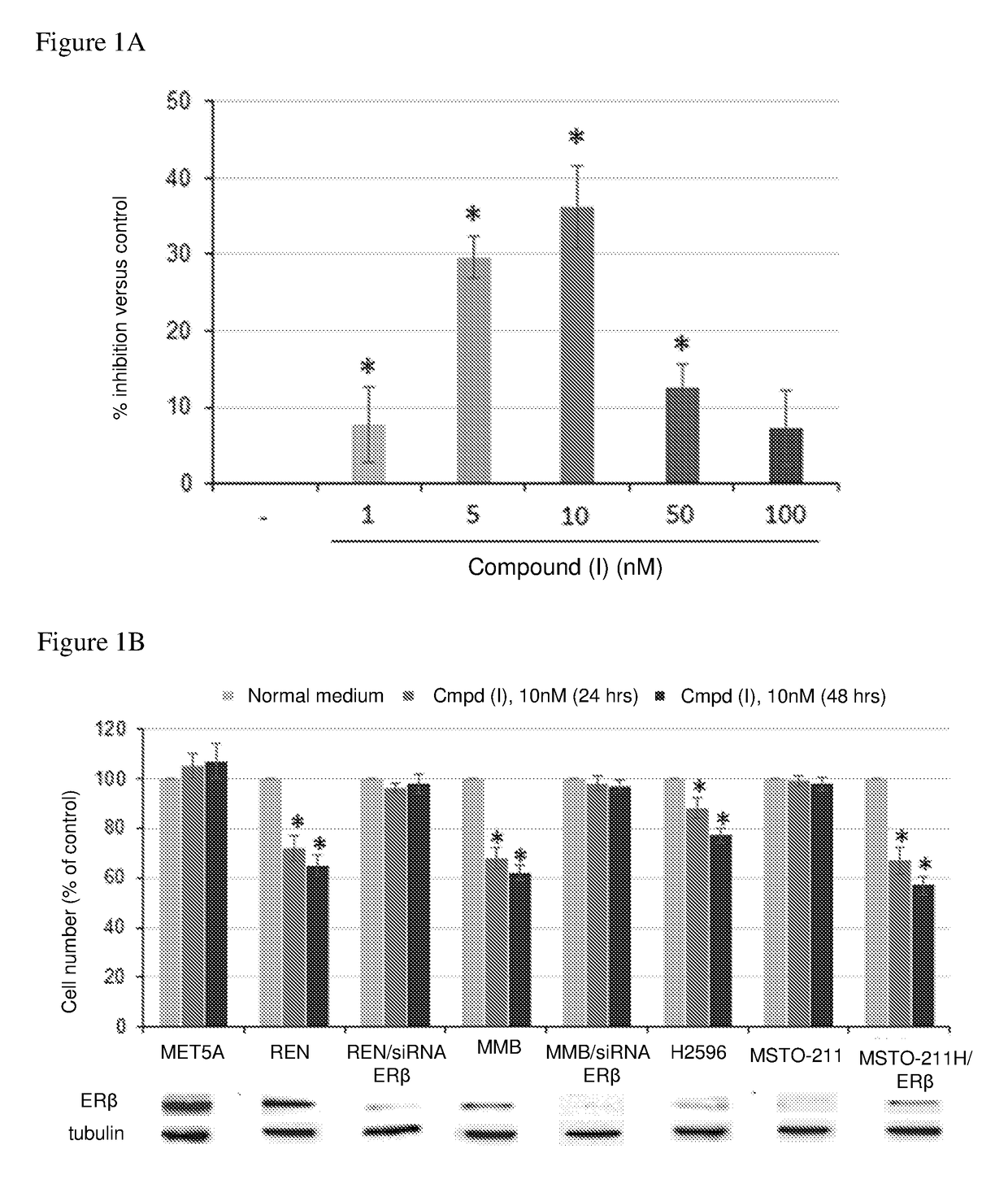
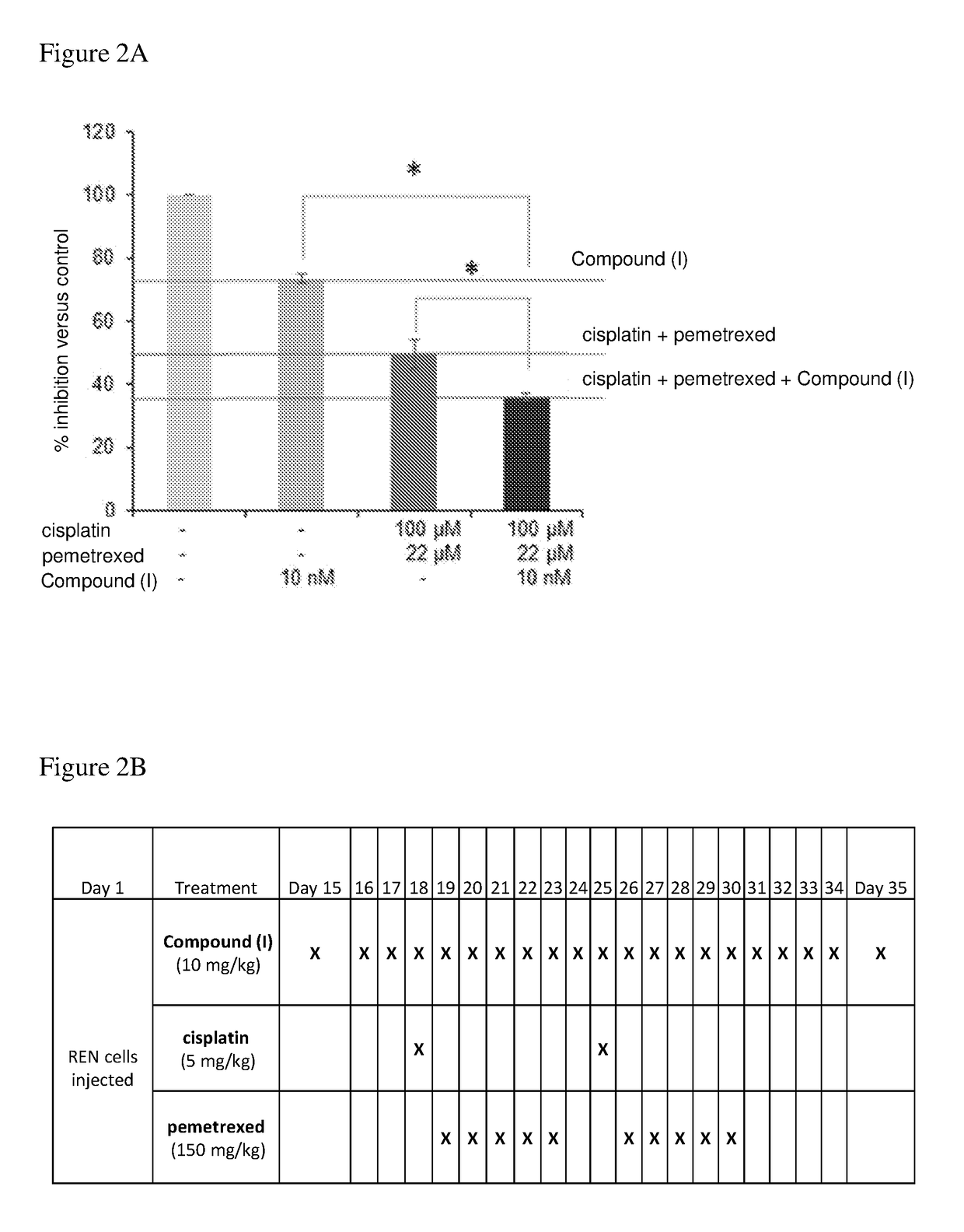
Estrogen receptor beta agonists for use in treating mesothelioma
The invention provides a treatment of mesothelioma, especially malignant pleural mesothelioma, using an estrogen receptor β subtype (ERβ) agonist, wherein the treatment comprises administering the ERβ agonist to the patient, and then after a time, t, of up to 24 hours; administering a platinum-containing anti-cancer drug to the patient. The invention also provides an ERβ agonist and a platinum-containing anti-cancer drug for use in the treatment of mesothelioma in a patient, wherein the treatment comprises administering the ERβ agonist to the patient, and then after a time, t, of up to 24 hours, administering the platinum-containing anti-cancer drug to the patient; and a kit comprising a platinum-containing anti-cancer drug and an ERβ agonist.
Owner:OASMIA PHARMA AB
A replication-defective recombinant lentiviral car-t transgene vector targeting mesothelin and its construction method and application
ActiveCN105969805BPromote secretionImprove in vitro killing effectMammal material medical ingredientsNucleic acid vectorEucaryotic cellTranscriptional expression
The invention discloses a Mesothelin-targeted replication-defective recombinant lentivirus CAR-T transgenic carrier which comprises a pronucleus replicon pUC Ori sequence for plasmid replication, an amicillin resistance gene AmpR containing sequence for mass amplification of target strain, virus replicon SV40Ori sequence for enhancing replication in eukaryocyte, a lentivirus packaging cis-element for lentivirus packaging, a ZsGreen1 green fluorescent protein for green fluorescence expression of eukaryocyte, an IRES ribosome combination sequence for joint transcriptional expression of protein, a human EF1(alpha) promoter for the eukaryotic transcription of chimeric antigen receptor gene, a chimeric antigen receptor for forming a second-generation CAR or third-generation CAR integrating identification, transfer and start, and an eWPRE element for improving the transgenic expression efficiency. Moreover, the invention also discloses an establishment method and application of the carrier. In the invention, the secretion of cell factors and the in-vitro killing effect of CAR-T cells can be remarkably enhanced, and the effect of clinical treatment of malignant pleural mesothelioma and pancreatic cancer is outstanding.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
FKBP5 gene methylation detection primer and kit based on pyrosequencing technology
InactiveCN113462767AQuick checkLow costMicrobiological testing/measurementDNA/RNA fragmentationCpG siteSystemic vasculitis
The invention belongs to the technical field of methylation detection, and particularly relates to an FKBP5 gene methylation detection primer and kit based on a pyrosequencing technology. According to the FKBP5 gene methylation detection primer based on the pyrosequencing technology, provided by the invention, a methylated CpG site which is remarkably related to the expression level of the FKBP5 gene can be obtained through amplification; and the detection primer is prepared into the detection kit for detecting the methylation of the FKBP5 gene based on the pyrosequencing technology, and the methylation of the FKBP5 gene can be simply, conveniently and quickly detected at low cost, so that the precise evaluation of the methylation level of the FKBP5 gene is realized. The kit can be used for effectively detecting the methylation of the cg03546163 site of the FKBP5 gene, so that the methylation degree of related genes of a sample to be detected is obtained, and judgment on the death risk of coronary heart disease and malignant pleural mesothelioma and the onset risk of systemic vasculitis is facilitated.
Owner:新开源晶锐广州生物医药科技有限公司 +1
microRNA-based method for treating malignant pleural mesothelioma
InactiveCN108421038AGood effectHeavy metal active ingredientsOrganic active ingredientsStimulantHumanized antibody
The invention discloses a microRNA-based method for treating malignant pleural mesothelioma, wherein the traditional Chinese medicine raw materials comprise, by mass, an active component and a microRNA simulant, the active component is a ganglioside GM2 conjugated antibody or a fragment thereof, the microRNA simulant contains a mature sequence and a lagging strand, the antibody is a recombinant antibody, and the recombinant antibody is one selected from a chimeric antibody, a humanized antibody and a human antibody. According to the present invention, the microRNA-based method can be combinedwith radiotherapy, thermotherapy and photodynamics; and the ganglioside GM2 conjugated antibody or the fragment thereof is used as the active component, the microRNA-based microRNA stimulant is used as the main treatment means, and surgery therapy, chemotherapy, radiotherapy, biological therapy, thermotherapy and photodynamic therapy are used as the auxiliary treatment ways, such that the effectsare significant by combining various treatments.
Owner:范兴龙
Application of Aidi preparation in preparation of anti-malignant pleural mesothelioma medicine
ActiveCN106924330BSignificant effectImprove the quality of lifeAnthropod material medical ingredientsPharmaceutical delivery mechanismSide effectEfficacy
Owner:GUIZHOU YIBAI PHARMA CO LTD
Conjugates for the treatment of mesothelioma
InactiveUS20130315856A1Speed upImproved profilePeptide/protein ingredientsTumor necrosis factorCell-Extracellular MatrixReceptor
The present invention provides conjugates of cytokines and targeting peptides that is able to bind to a receptor expressed on tumor-associated vessels or to a component of the extracellular matrix associated to the tumor vessels, for treatment of malignant pleural mesothelioma. In particular, the invention provides conjugates comprising the cytokine TNF linked to a peptide containing the NGR motif. The invention further provides pharmaceutical compositions comprising such conjugate and pharmaceutical formulations comprising conjugates dissolved in appropriate buffers.
Owner:MOLMED SPA
Asbestos exposure, pleural mesothelioma, and osteopontin levels
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +2
Liquid pharmaceutical composition comprising pemetrexed
ActiveUS20180271872A1Reduce solubilityImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismAntioxidantSolvent
The present invention relates to a liquid pharmaceutical composition suitable for parenteral administration comprising:a) pemetrexed diacid;b) at least one organic amine;c) at least one antioxidant;d) 10-200 mg / ml of propylene glycol; ande) one or more parenteral solvents,wherein the preparation thereof is conducted in an atmosphere of inert gas and wherein the organic amine(s) is present in an amount sufficient to reach a pH of the composition in the range from 8.3 to 9.1The invention further relates to the use of said liquid pharmaceutical composition as medicament in the treatment of malignant pleural mesothelioma and non-small cell lung cancer.
Owner:SYNTHON BV
Application of Yiganle tablets in preparing medicine for inhibiting cell proliferation of malignant pleural mesothelioma cell SMC-1
The invention belongs to the technical field of traditional Chinese medicines and particularly relates to an application of Yiganle tablets in preparing a medicine for inhibiting the cell proliferation of the malignant pleural mesothelioma cell SMC-1 and a preparation method of Yiganle tablets. The Yiganle tablets are prepared from the active pharmaceutical ingredients including 200g of paederia scandens, 160g of polygonum cuspidatum, 200g of verveine officinale, 200g of serissa japonica, 200g of herba kalimeridis, 200g of rubus parvifolius, 170g of pink reineckea herb, 100g of caulis mahoniae and 70g of pilea notata by supercritical extraction, and the content of emodin is greatly increased.
Owner:JINAN XINSHIDAI MEDICINE SCI & TECH
Stable liquid compositions of pemetrexed
PendingUS20220151923A1Improve stabilityOrganic active ingredientsPharmaceutical delivery mechanismCyclodextrinPharmaceutical drug
The present invention relates to a stable liquid pharmaceutical composition of pemetrexed for parenteral administration. The invention provides composition comprising pemetrexed diacid, an organic amine and cyclodextrin. The composition may further comprise an inert gas. The composition can be ready to use infusion solution of pemetrexed diacid or liquid concentrate formulation to be diluted before administration to the patient. The present invention further relates to a process for manufacturing the compositions as well as use of the compositions of the invention for the treatment of malignant pleural mesothelioma and non-small cell lung cancer.
Owner:FRESENIUS KABI ONCOLOGY LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com