Molecular marker for the early detection of malignant pleural mesothelioma and the methods of its expression analysis using blood and pleural effusion samples
a mesothelioma and pleural effusion technology, applied in the field of mesothelioma detection methods, can solve the problems of belated diagnosis, poor prognosis, and unfavorable prognosis of malignant pleural mesothelioma, and achieve the effects of low cost, high reliability, and simple structur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of Anti-Periostin Antibody
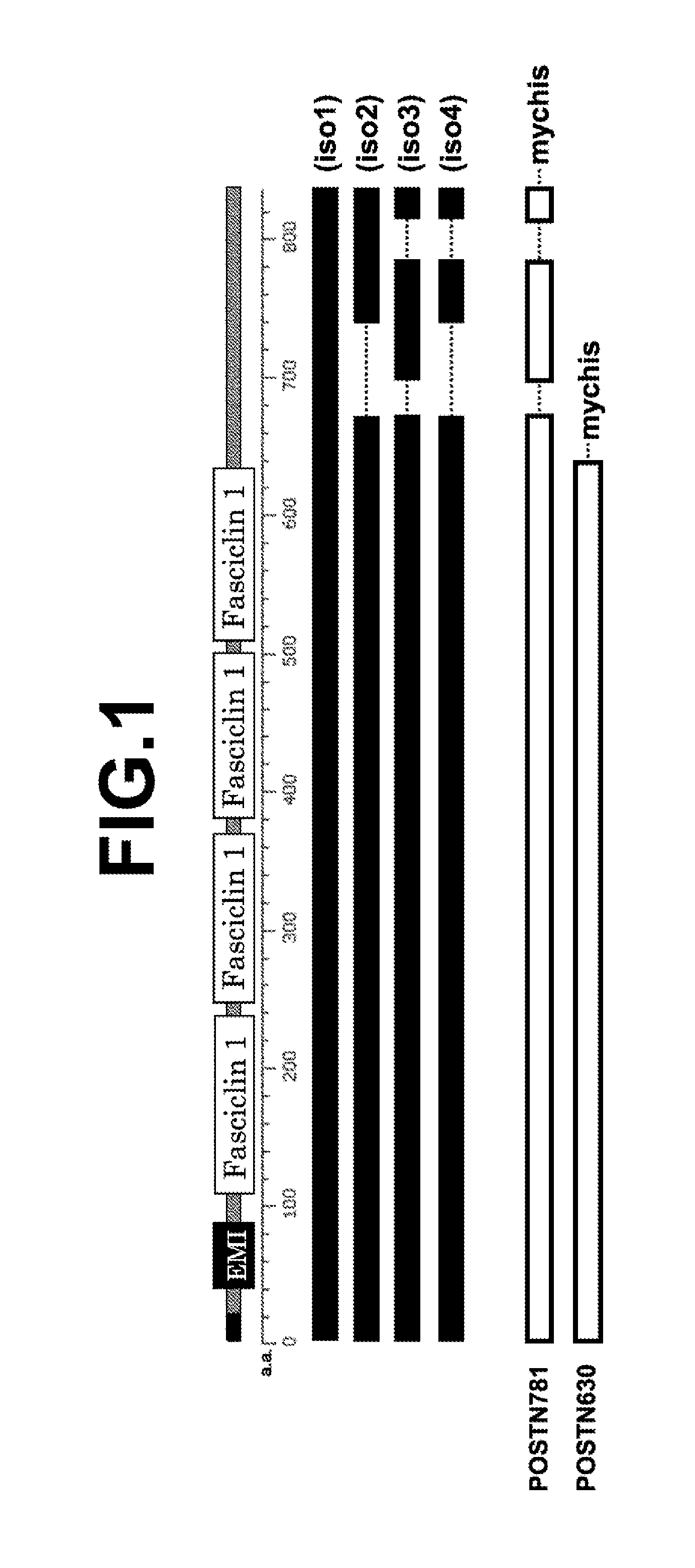
[0066](1) Structure of Human Periostin Protein
[0067]FIG. 1 shows a schematic view illustrating relation among positions of functional domains of a human periostin protein, the structure of each isoform, and the structure of the immunogen used for producing the periostin antibody of the present invention. In FIG. 1, EMI and Fasciclin 1 represent an EMILIN homologous domain and a fasciclin homologous domain, respectively. “iso1” to “iso4” represent isoforms 1 to 4 of human periostin, respectively. POSTN781 represents a recombinant protein obtained by fusing myc tag and his tag (represented by “mychis”) to a carboxyl terminal of a polypeptide having 781 amino acid residues of a human periostin protein isoform 3. POSTN630 represents a recombinant protein obtained by fusing myc tag and his tag to a carboxyl terminal of a polypeptide having 630 amino acid residues that are common to all isoforms of the human periostin protein covering four fasciclin do...
example 2
Measurement of Clinical Specimen
[0083](1) Reactivity to Clinical Specimen
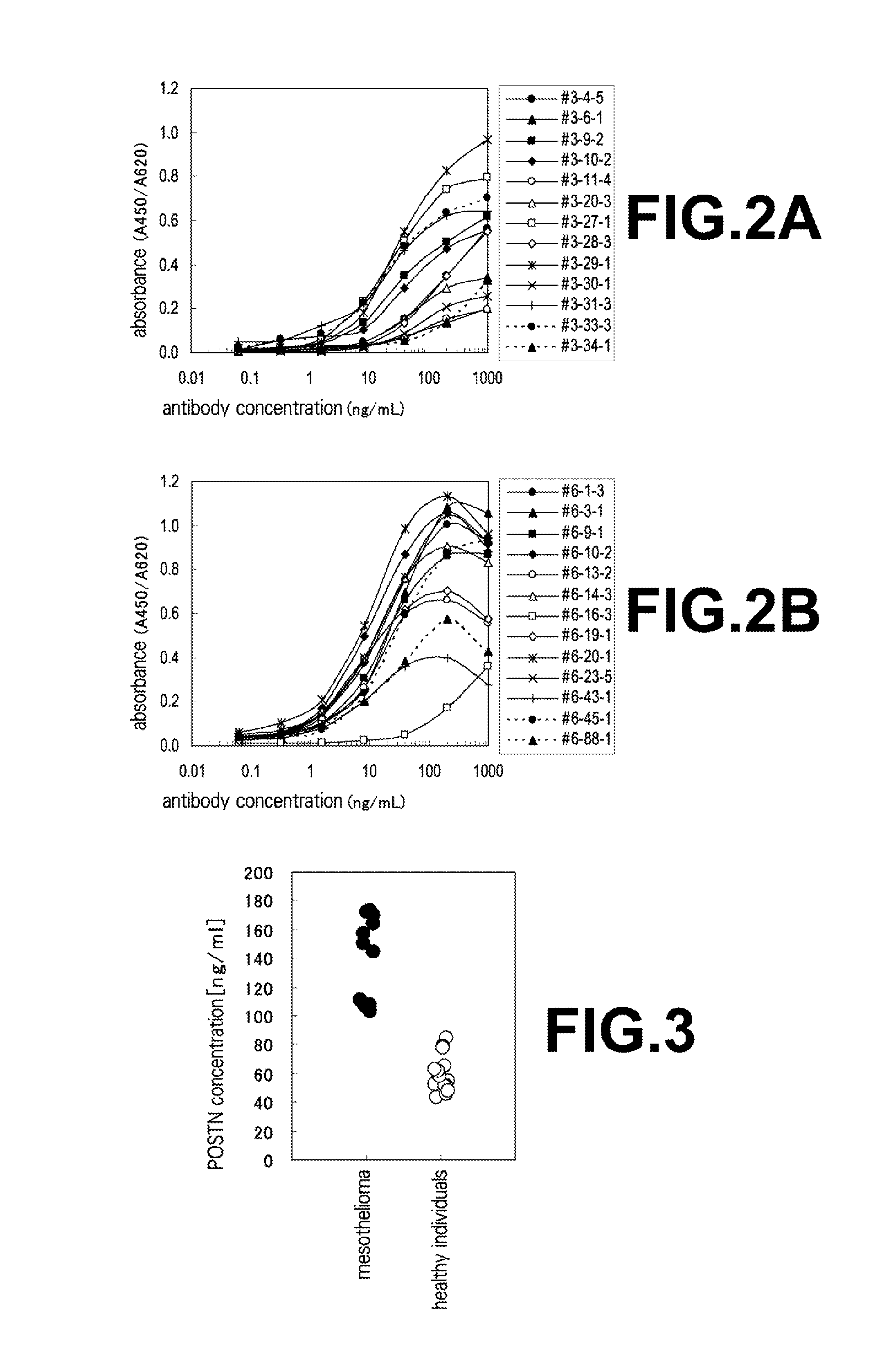
[0084]With regard to the specimens from healthy individuals, an approval by Ethics Committee Organization of Medical & Biological Laboratories Co., Ltd.; MBL ethics review board (Project number: 022; Date of Approval: Mar. 27, 2007) was obtained and then specimens from healthy volunteers were solicited. Written consent for the measurement was obtained beforehand from each healthy individual who donated the specimen. With regard to specimens from mesothelioma patients, the aforementioned plasma specimens diluted 1,000 fold were used in place of human periostin purified recombinant proteins for the sandwich ELISA method explained in Example 1, section (7) which had been requested and obtained from BMR (Bio Medical Resources (integrated with Sera Care Life Sciences Milford, Mass., at the time of application)). Results of measurements in connection with the combinations of antibodies shown in Table 1 on the plasma ...
example 3
Measurement of Clinical Specimens
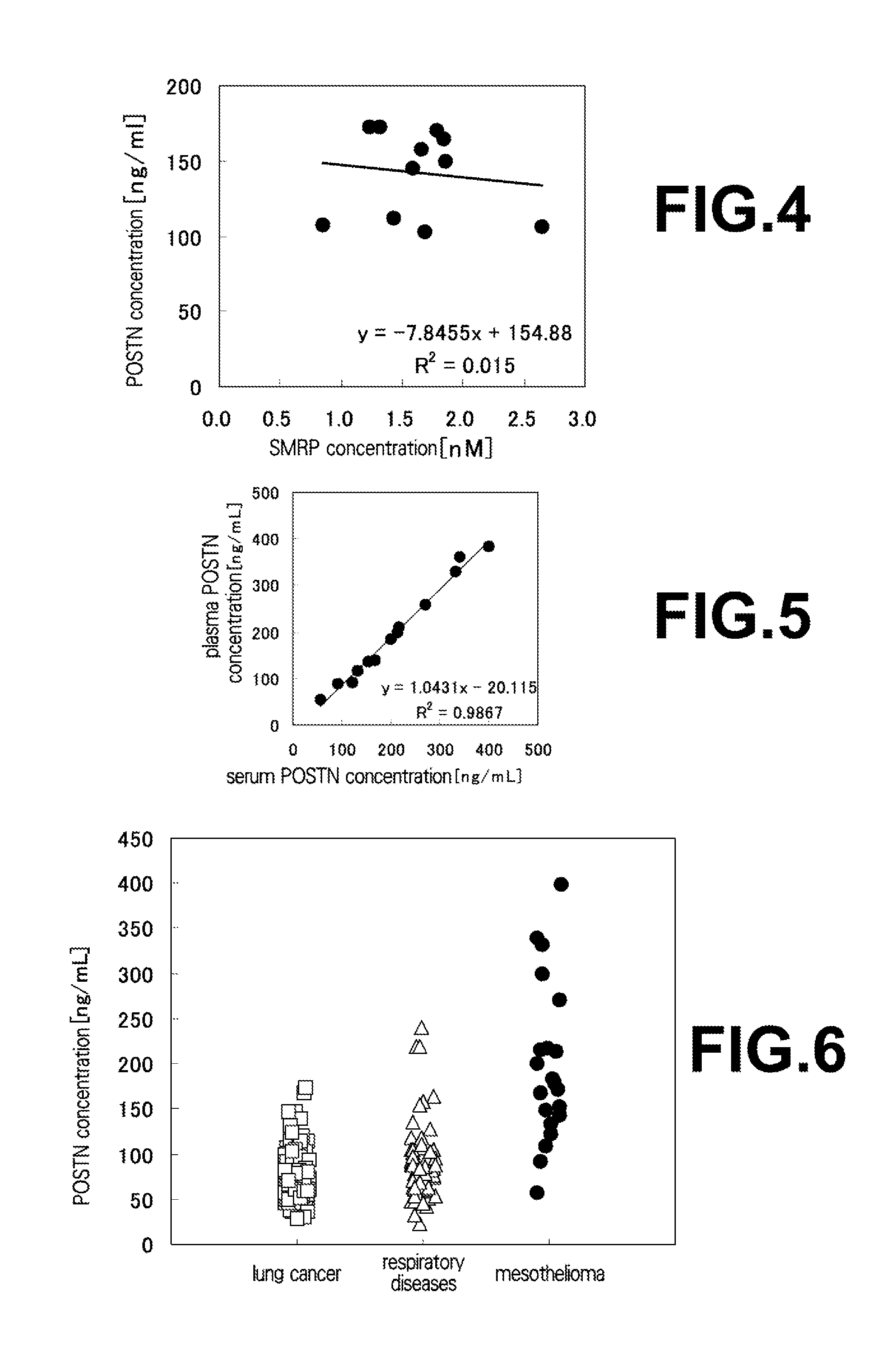
[0088](1) Measurement of Concentration of Periostin in Plasma Samples of Mesothelioma Patients and Healthy Individuals
[0089]Each 50 μL of the anti-human periostin monoclonal antibody #6-14-3 diluted to 10 μg / mL with a solution of 0.1 M NaHCO3, 0.1 M Na CO3 and 0.15% Proclin 150 (SUPELCO) was coated on a MaxiSorp 96-well plate (NUNC, Thermo Fisher Scientific K.K.), and left to stand at 4° C. overnight or at room temperature for 2 hours to allow the antibody to be immobilized. After the solution of the antibody was eliminated, each 150 μL of a blocking buffer (PBS supplemented with 1% BSA (Proliant, Veritas Corporation), 0.15% Proclin 150 and 5% sucrose) was dispensed, followed by leaving to stand at 4° C. overnight or at room temperature for 2 hours. After the blocking buffer solution was eliminated, using PBS supplemented with 1% BSA, 0.1% Tween20, 0.15% Proclin 150 and 50 μg / mL MAK-33 as a dilution liquid, a periostin standard substance (a series of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com