Patents
Literature
49 results about "Protein isoform" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
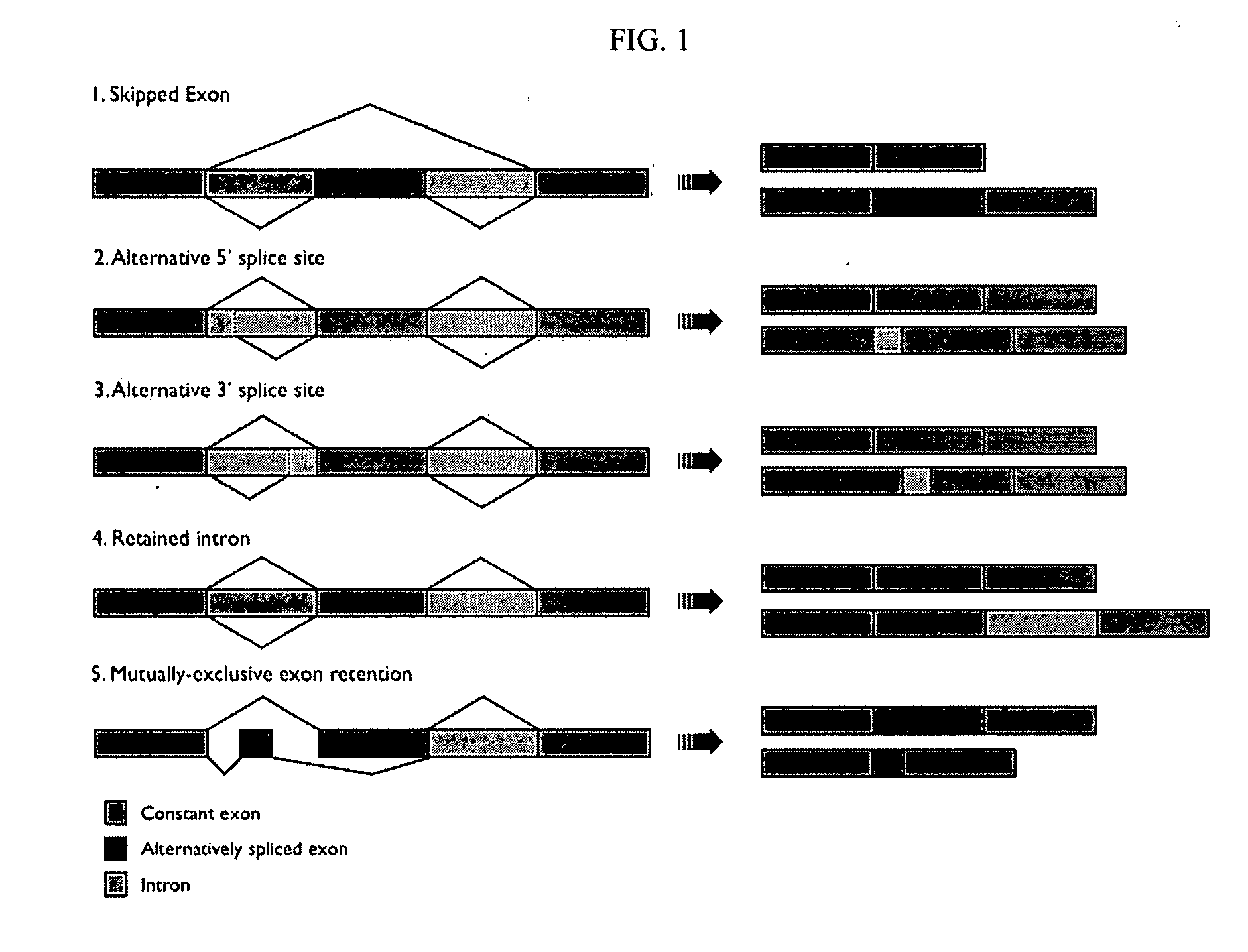
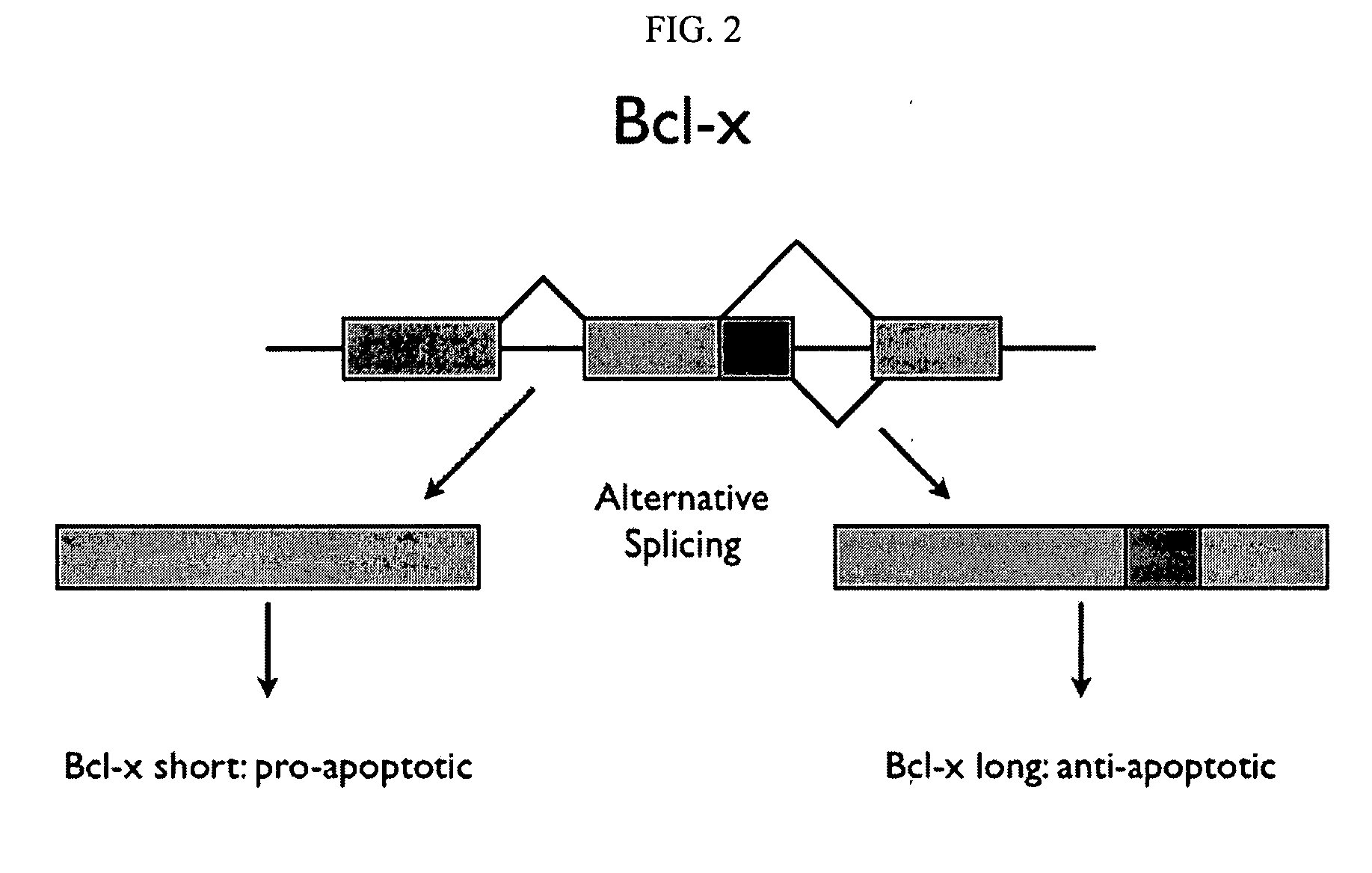
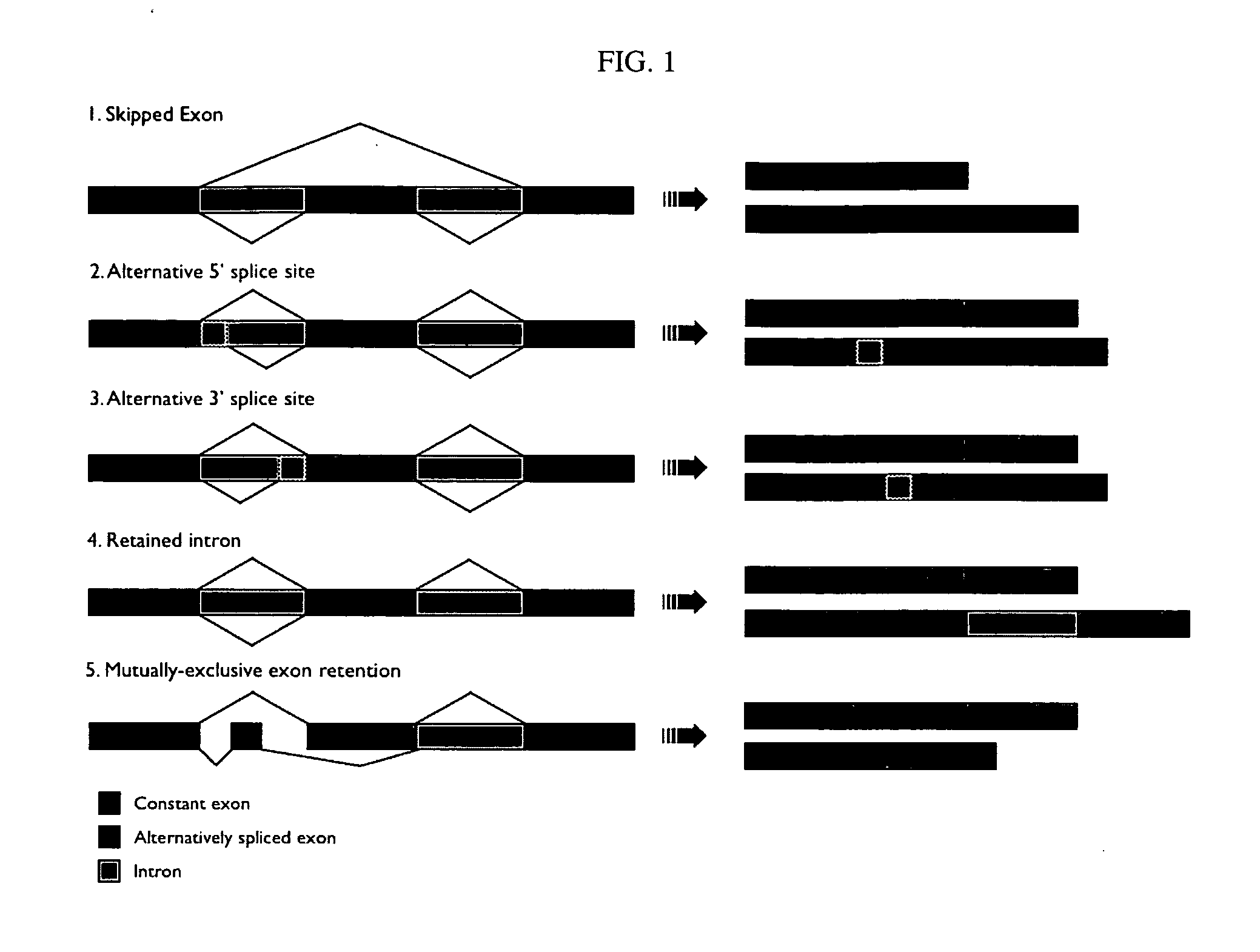
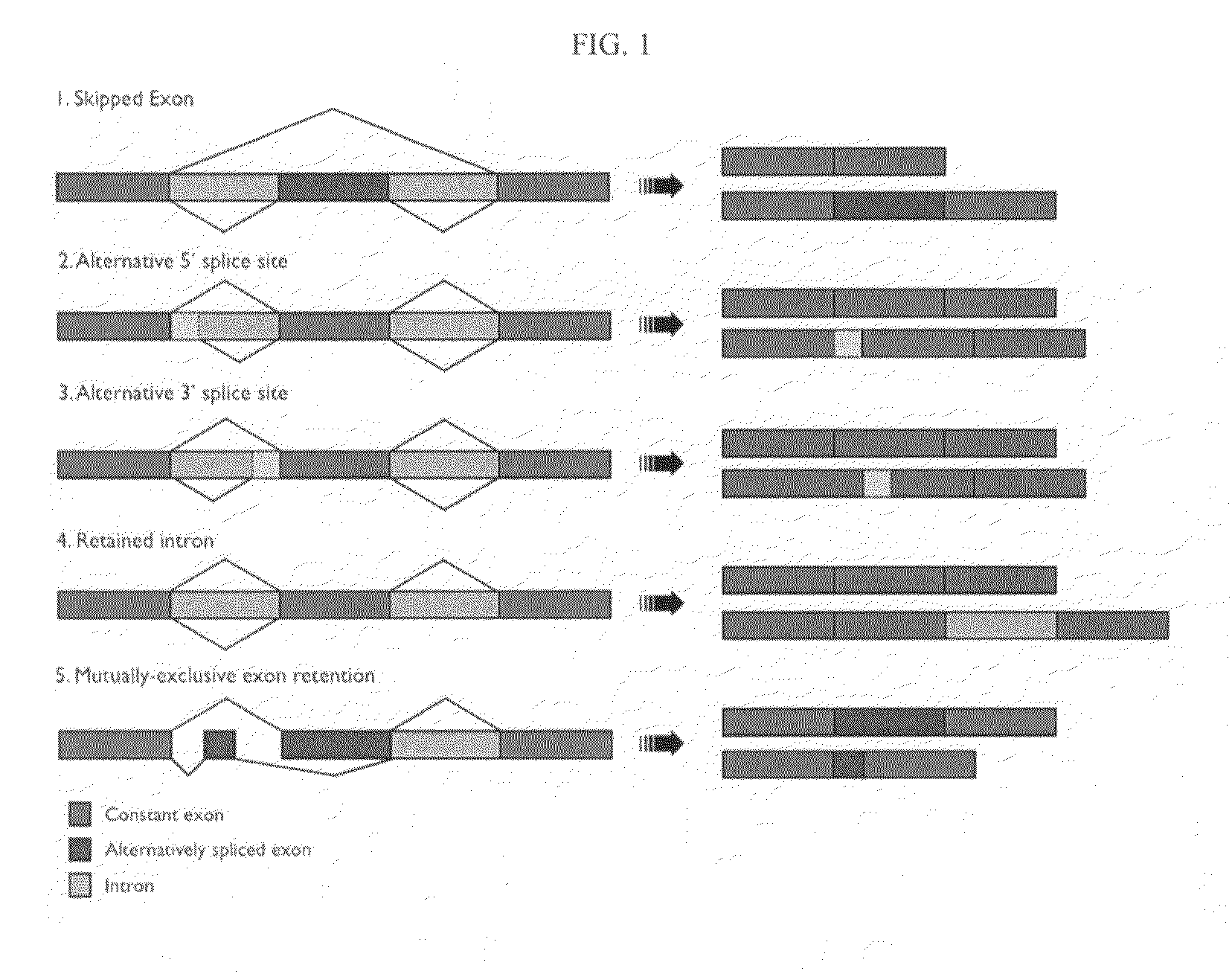
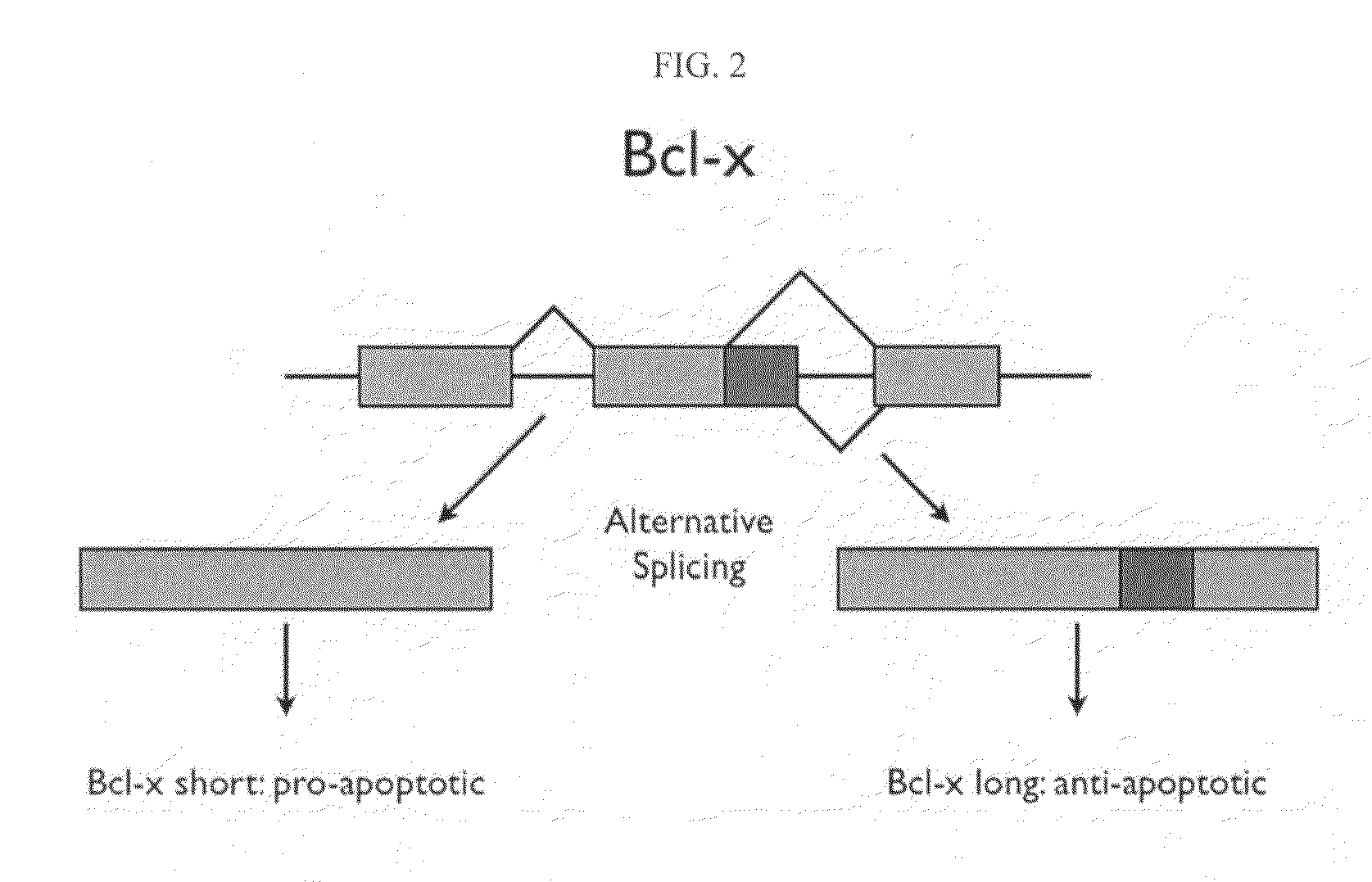
A protein isoform, or "protein variant", is a member of a set of highly similar proteins that originate from a single gene or gene family and are the result of genetic differences. While many perform the same or similar biological roles, some isoforms have unique functions. A set of protein isoforms may be formed from alternative splicings, variable promoter usage, or other post-transcriptional modifications of a single gene; post-translational modifications are generally not considered. Through RNA splicing mechanisms, mRNA has the ability to select different protein-coding segments (exons) of a gene, or even different parts of exons from RNA to form different mRNA sequences. Each unique sequence produces a specific form of a protein.
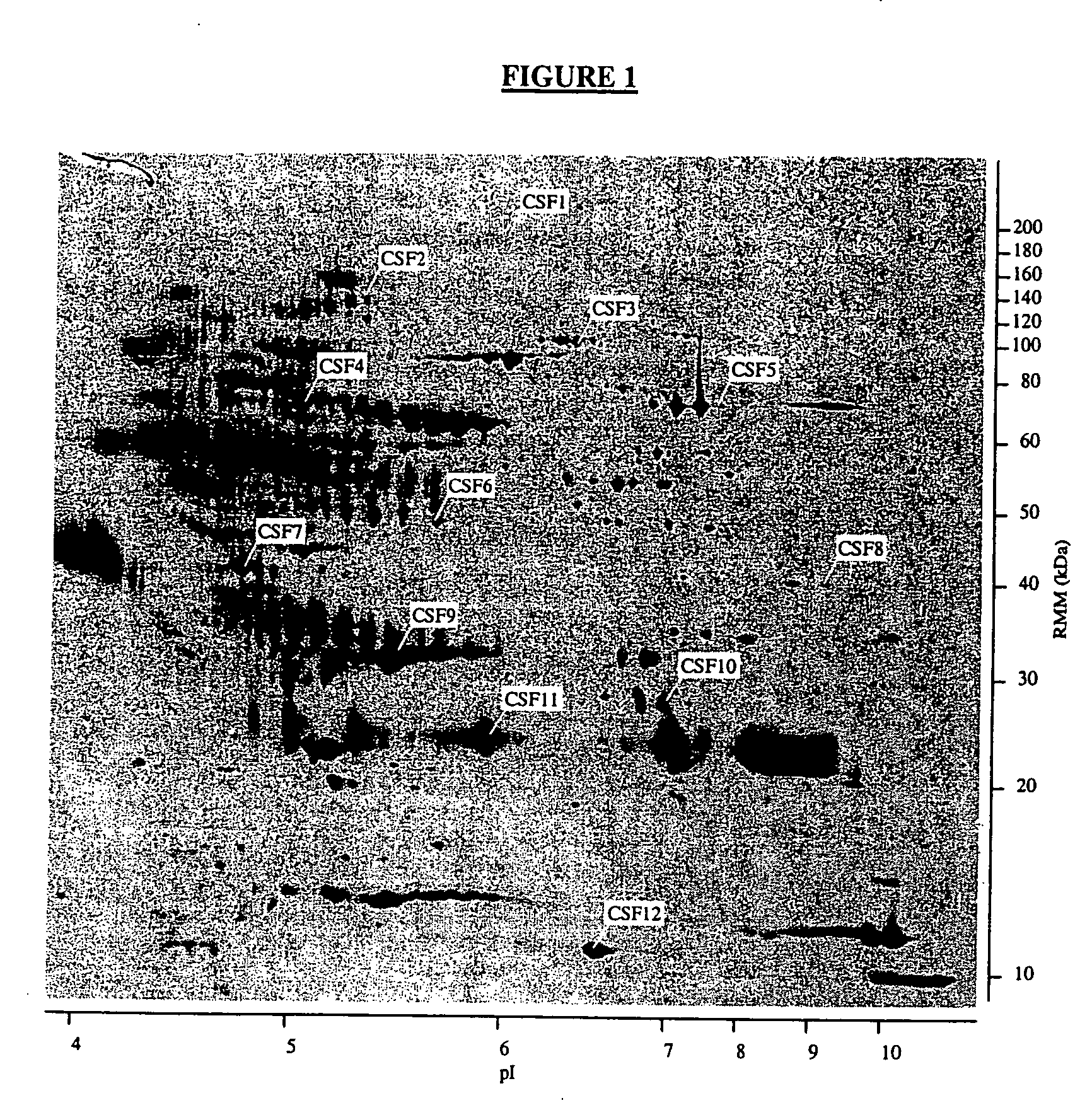
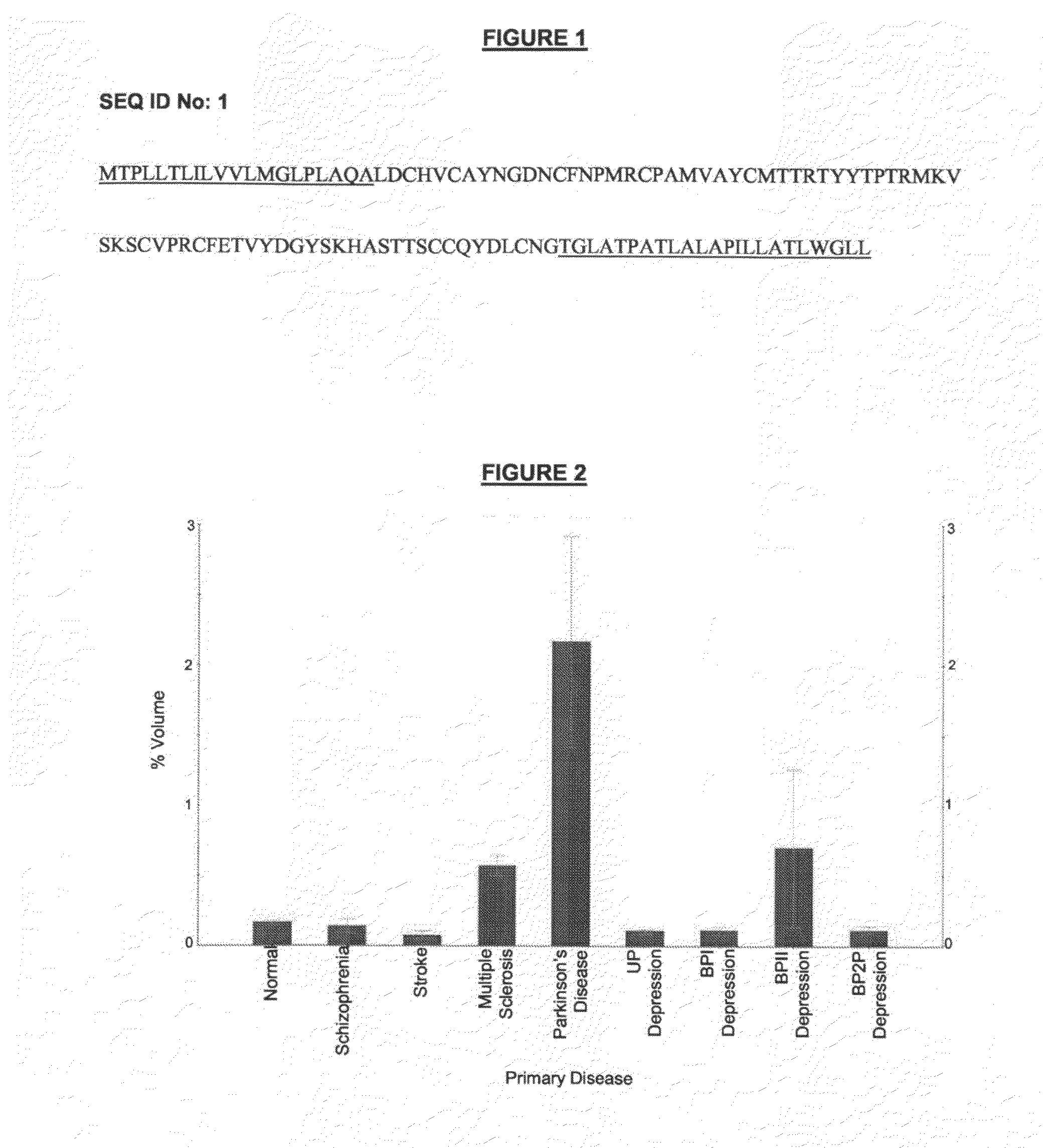
Proteins, genes and their use for diagnosis and treatment of schizophrenia
InactiveUS20040110938A1Peptide/protein ingredientsDisease diagnosisDrug developmentTherapeutic treatment
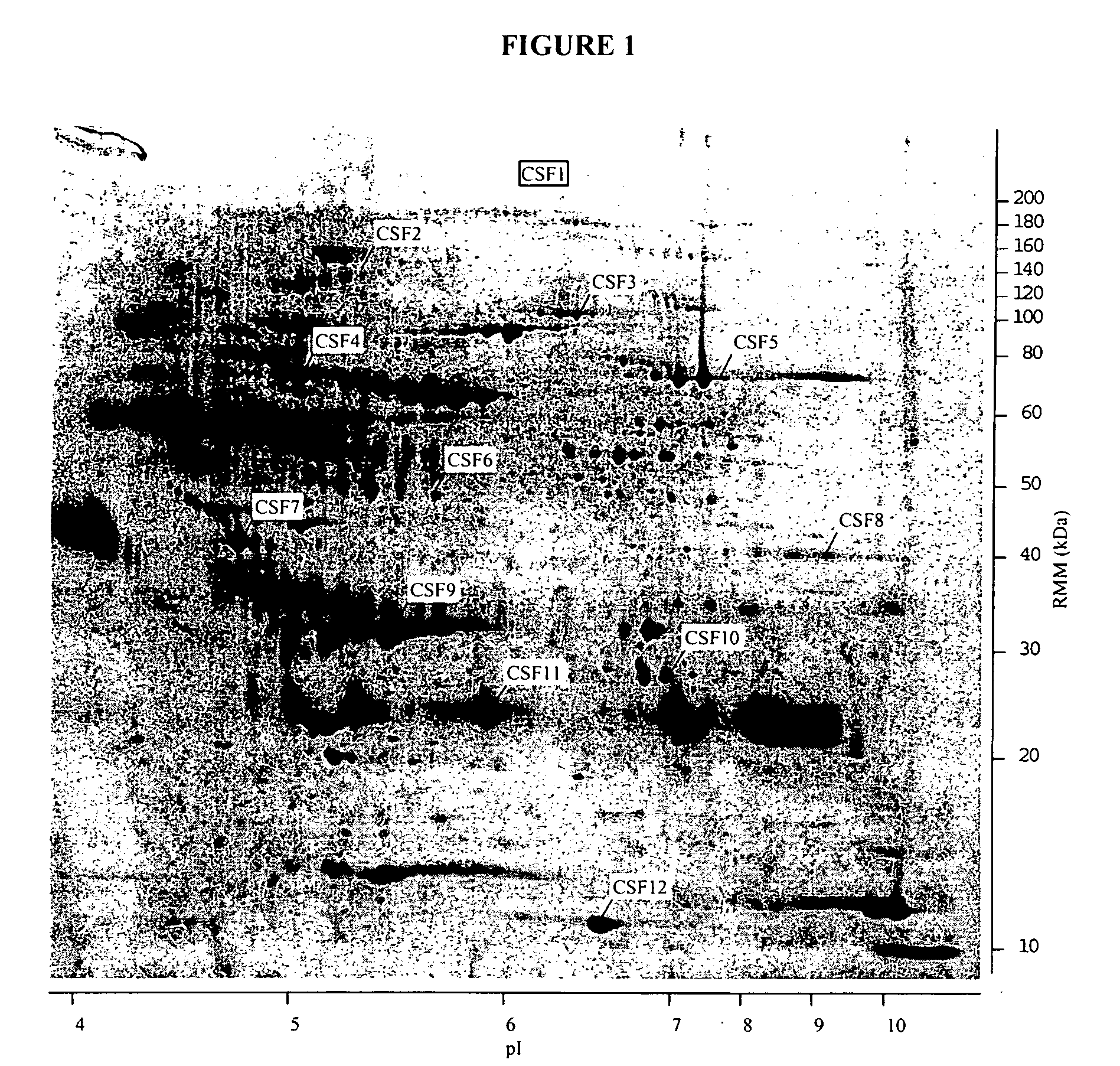
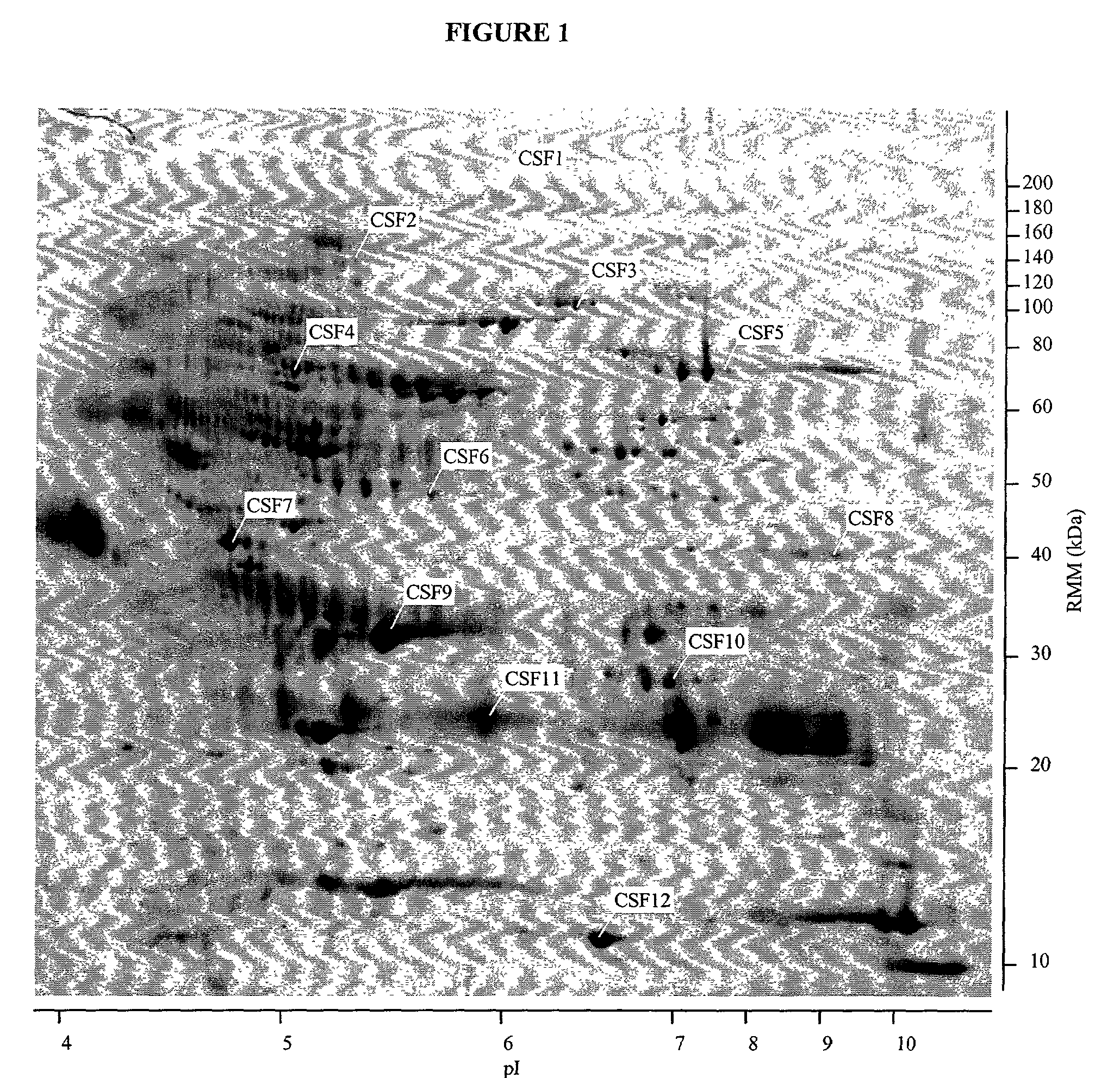
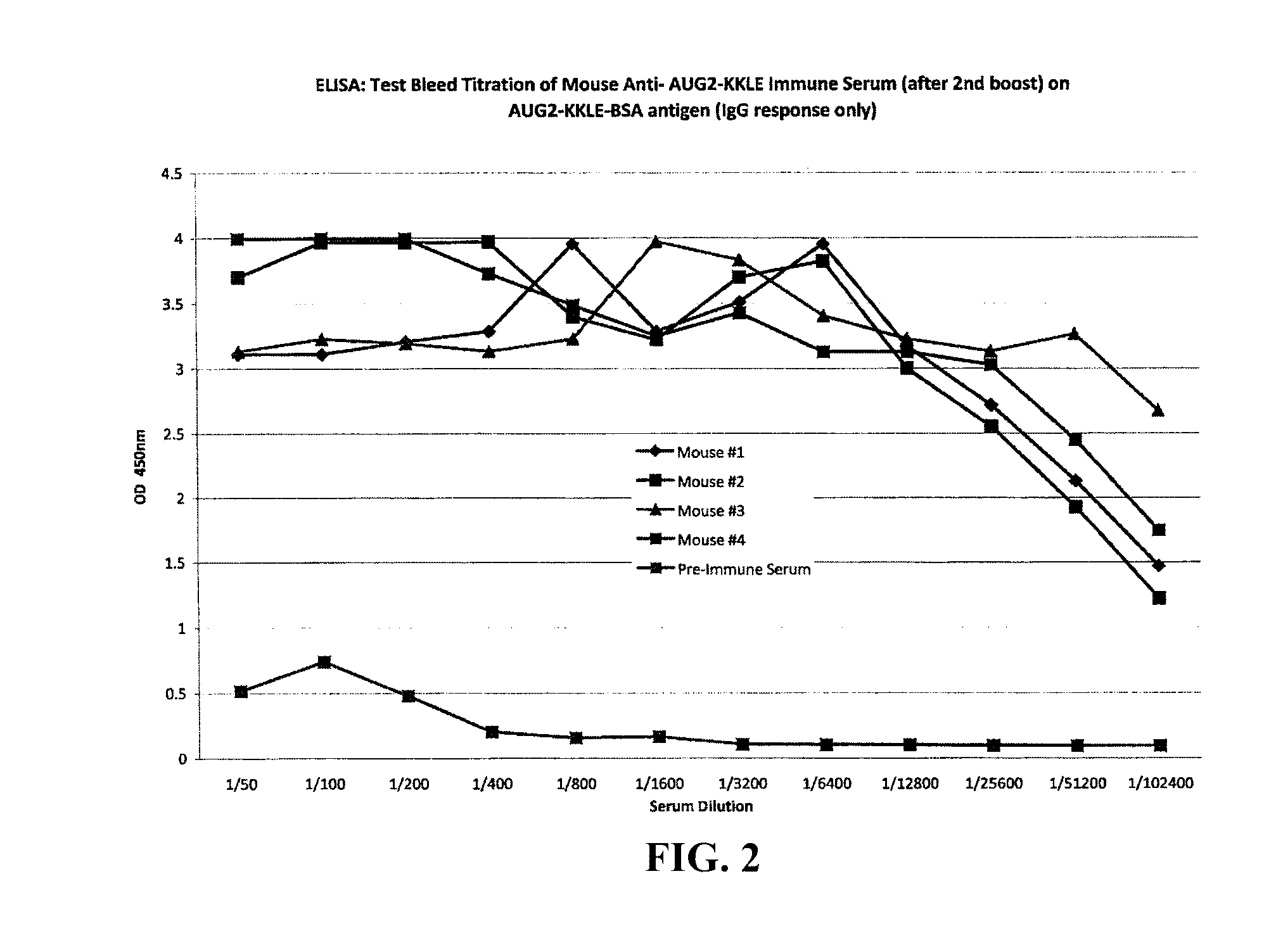
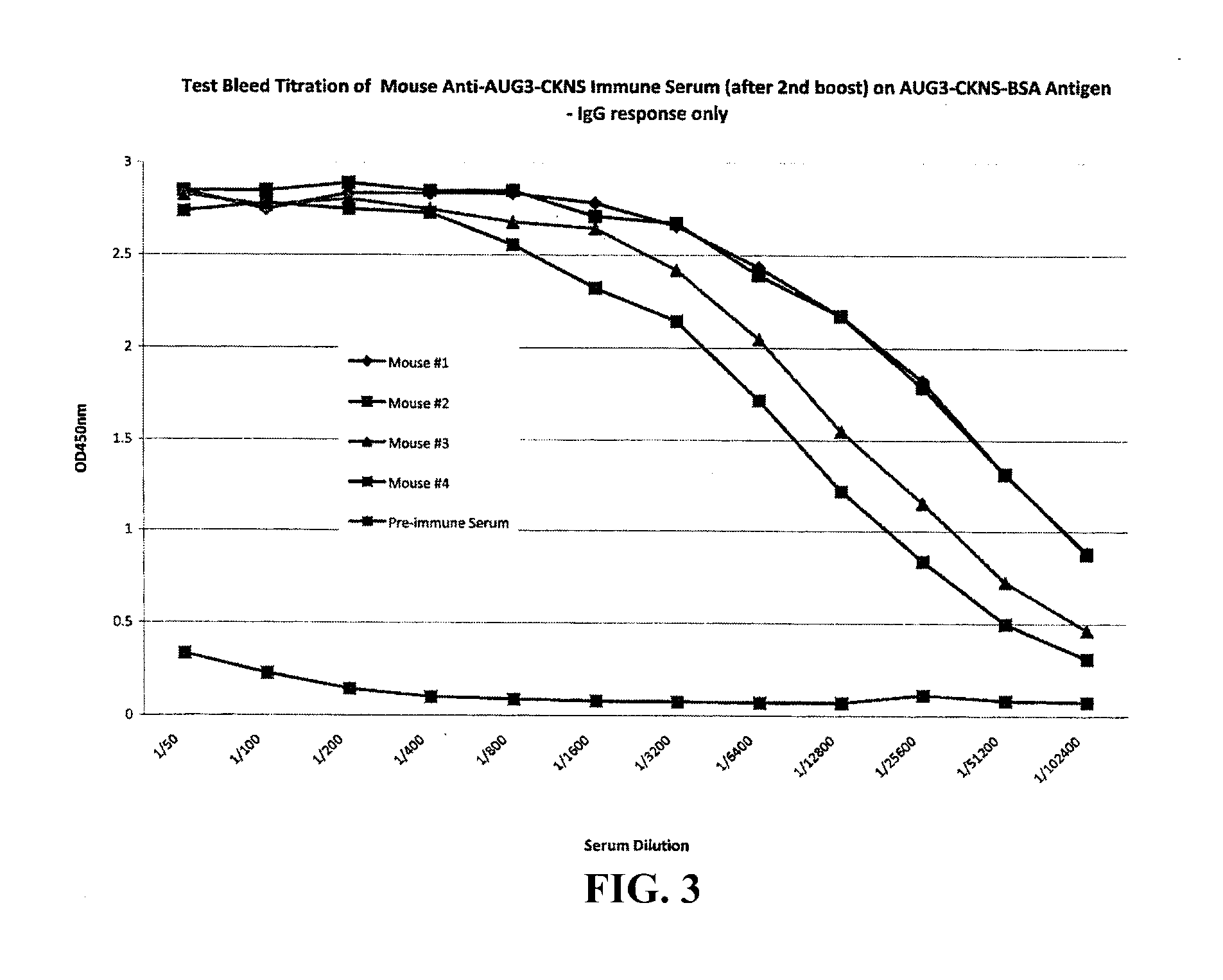
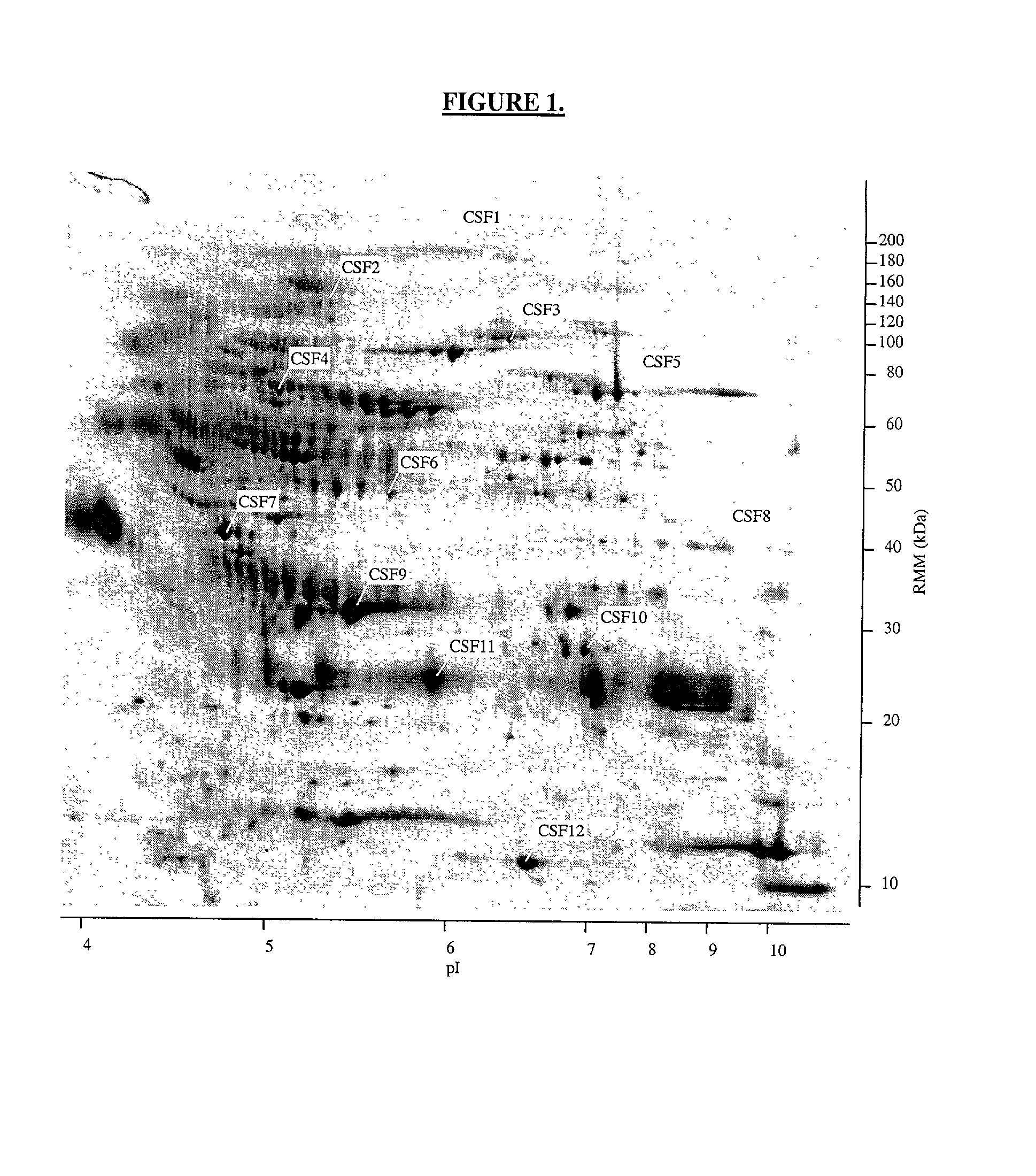
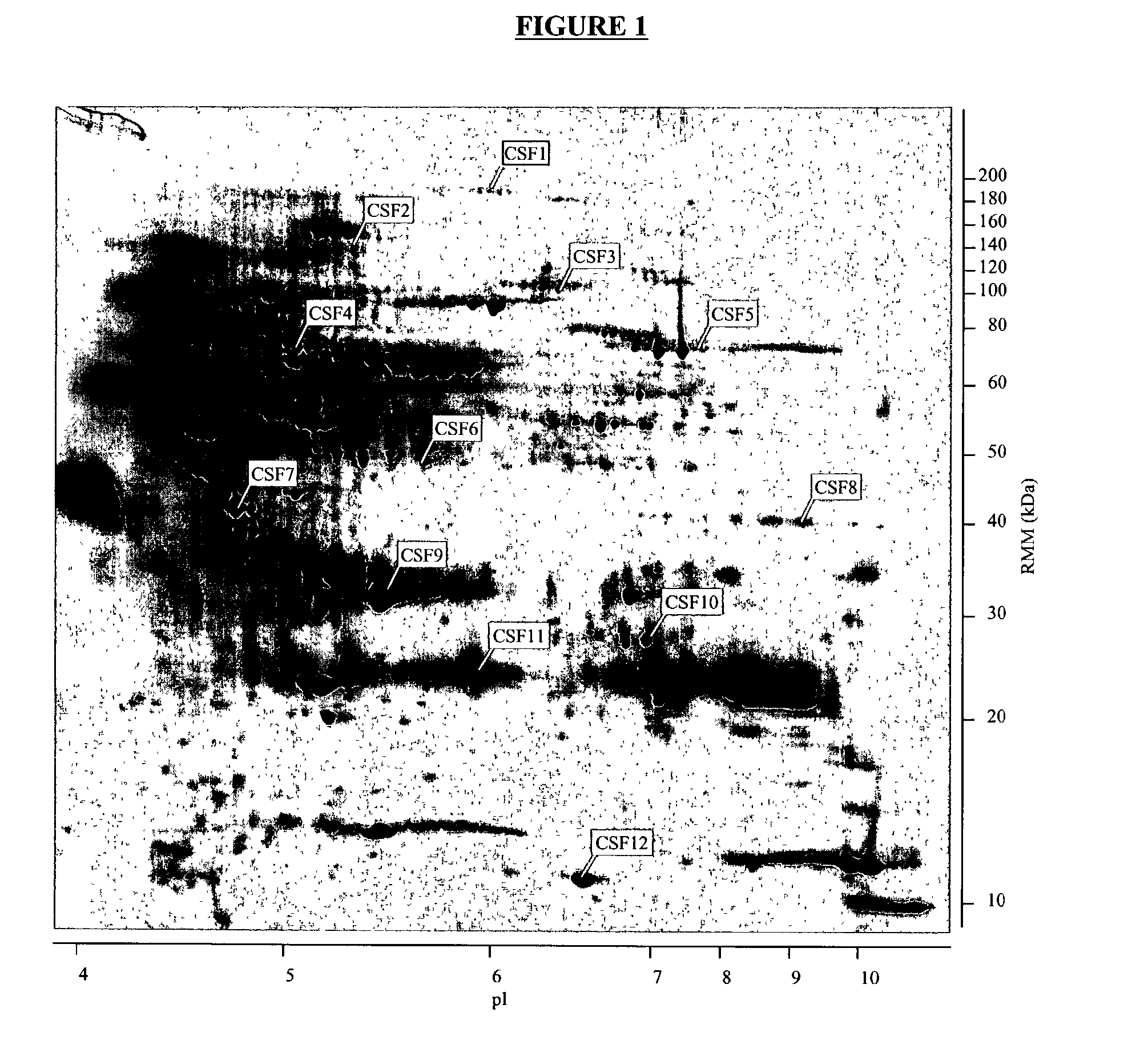
The present invention provides methods and compositions for screening, diagnosis and prognosis of Schizophrenia, for monitoring the effectiveness of Schizophrenia treatment, identifying patients most likely to respond to a particular therapeutic treatment and for drug development. Schizophrenia-Associated Features (SFs), detectable by two-dimensional electrophoresis of cerebrospinal fluid, serum or plasma are described. The invention further provides Schizophrenia-Associated Protein Isoforms (SPIs) detectable in cerebrospinal fluid, serum or plasma, preparations comprising isolated SPIs, antibodies immunospecific for SPIs, and kits comprising the aforesaid.
Owner:OXFORD GLYCOSCI UK
Quantitative analysis of protein isoforms using matrix-assisted laser desorption/ionization time of flight mass spectrometry
InactiveUS20040119010A1Particle separator tubesMicrobiological testing/measurementMass Spectrometry-Mass SpectrometryMatrix assisted laser desorption ionization time of flight
The present invention provides for methods of quantitating the amounts of proteins or peptides, including those that are closely related isoforms, using matrix-assisted laser desorption / ionization time of flight mass spectrometry (MALDI-TOF-MS). Measurement of protein concentrations in vivo has been extremely difficult and problematic, and protein concentrations have not been shown to correlate well with mRNA levels, the standard used in the past. The present invention overcomes the deficiencies of prior methodologies by taking advantage of MALDI-TOF-MS technology and applying it to proteins and peptides in a way that allows for accurate, quantitative measurement in vivo of protein or peptide concentrations.
Owner:UNIV OF COLORADO THE REGENTS OF
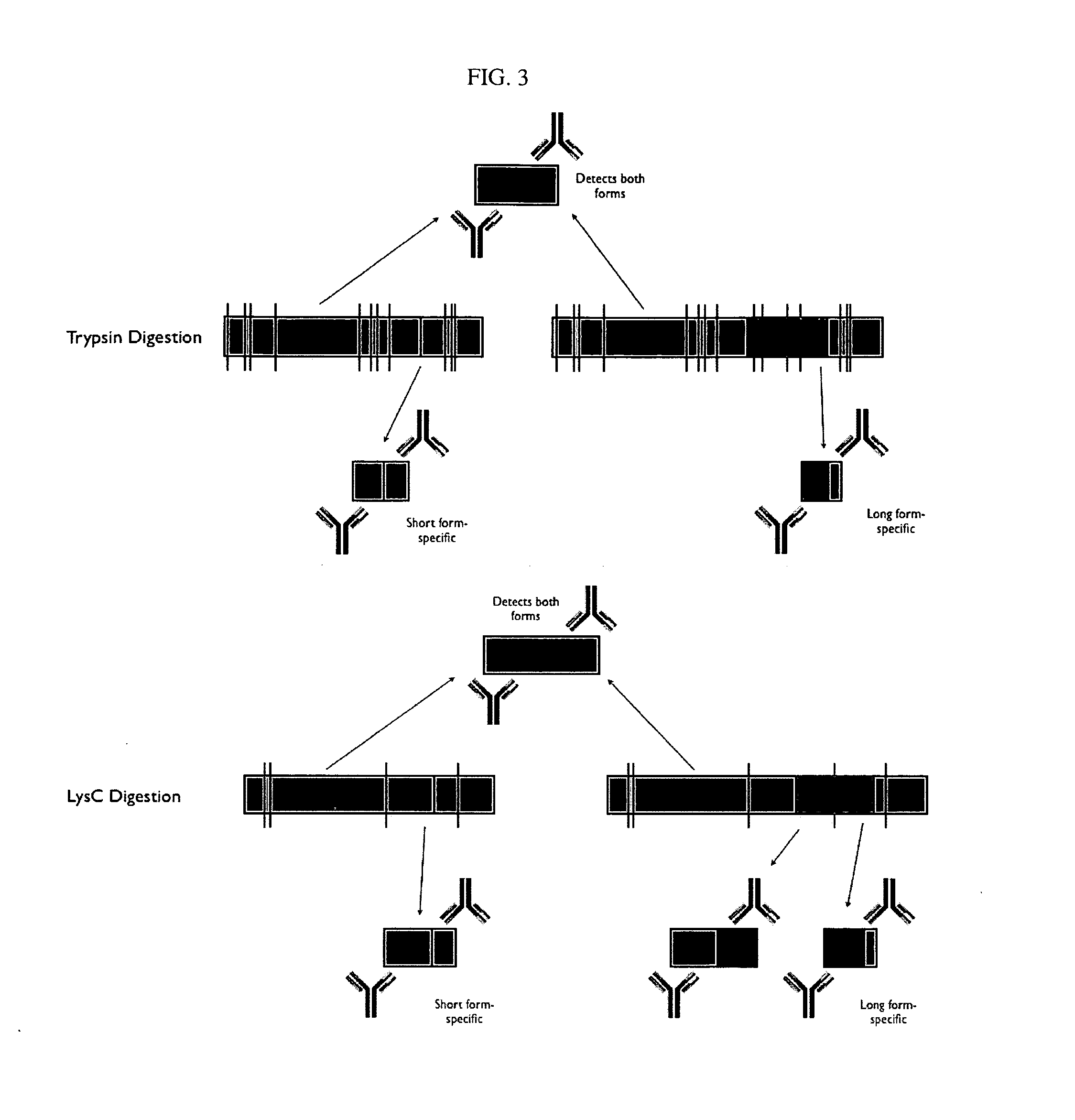
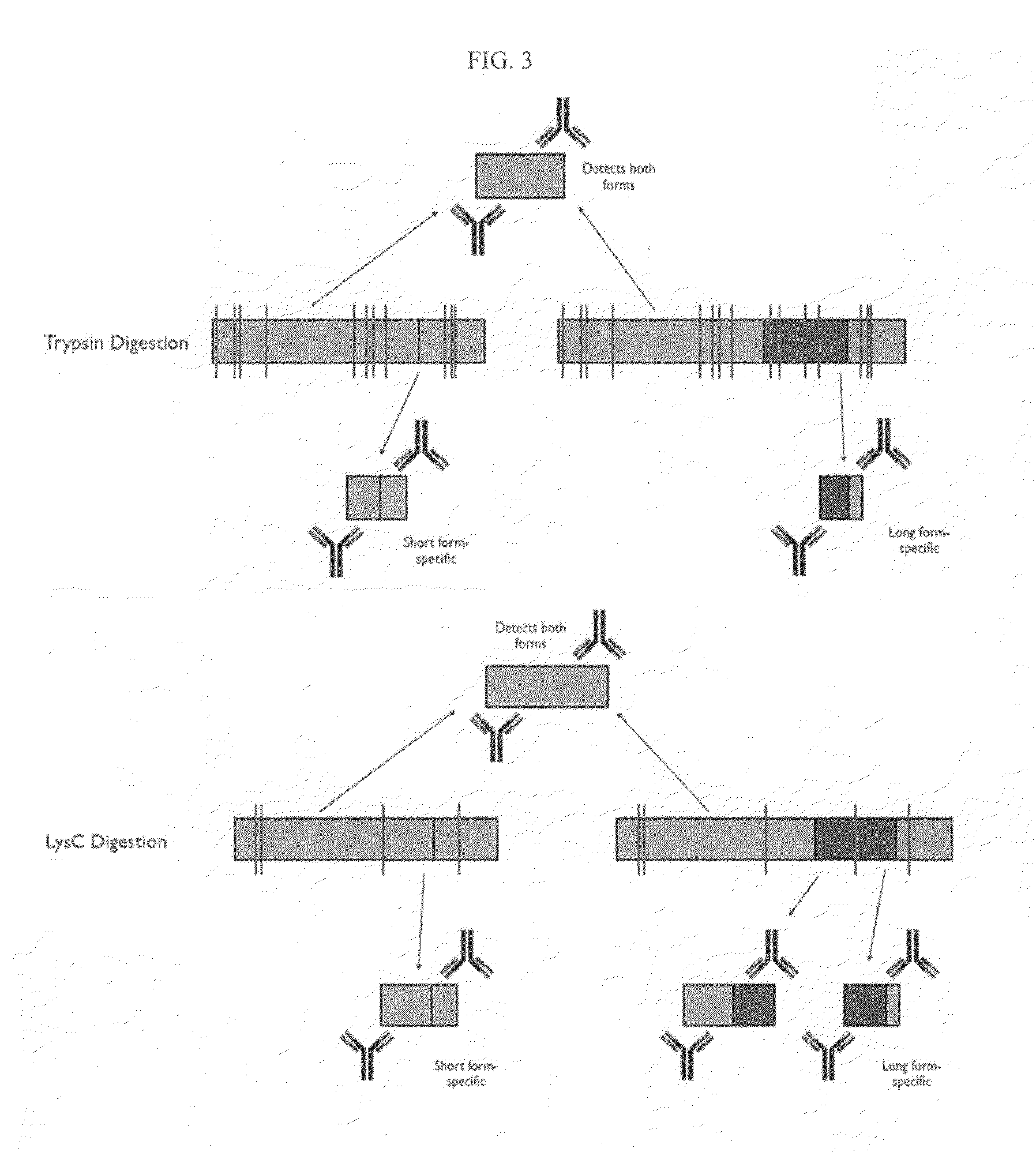
Protein isoform discrimination and quantitative measurements thereof
InactiveUS20070224628A1Reliably retrievedMicrobiological testing/measurementBiological testingAlternative splicingBiology
The invention relates to methods, reagents and apparatus for detecting protein isoforms (e.g., those due to alternative splicing, or different disease protein isoforms or degradation products) in a sample, including using combinations of capture agents to identify the isoforms to be detected / measured.
Owner:MILLIPORE CORP
Methods and compositions for manipulating translation of protein isoforms from alternative initiation of start sites
InactiveUS20140296321A1High sensitivityImprove translationSugar derivativesPolymorphism usesStart codonStart site
Provided herein are antisense oligonucleotides, compositions comprising antisense oligonucleotides, and methods for the use of antisense oligonucleotides in manipulating translation. Expression of isoforms of proteins expressed from different start codons of the same transcript are inhibited by antisense oligonucleotides, which may also enhance expression of non-target isoforms.
Owner:SAREPTA THERAPEUTICS INC
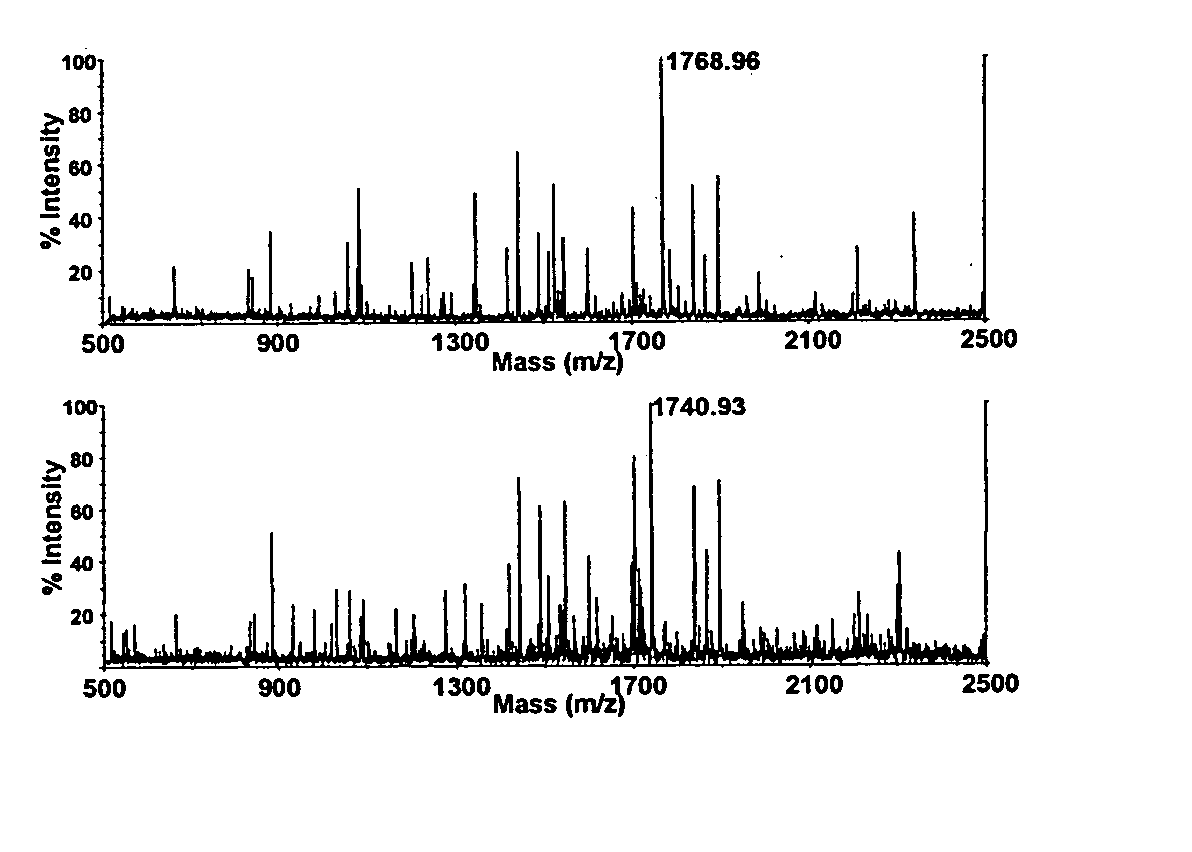
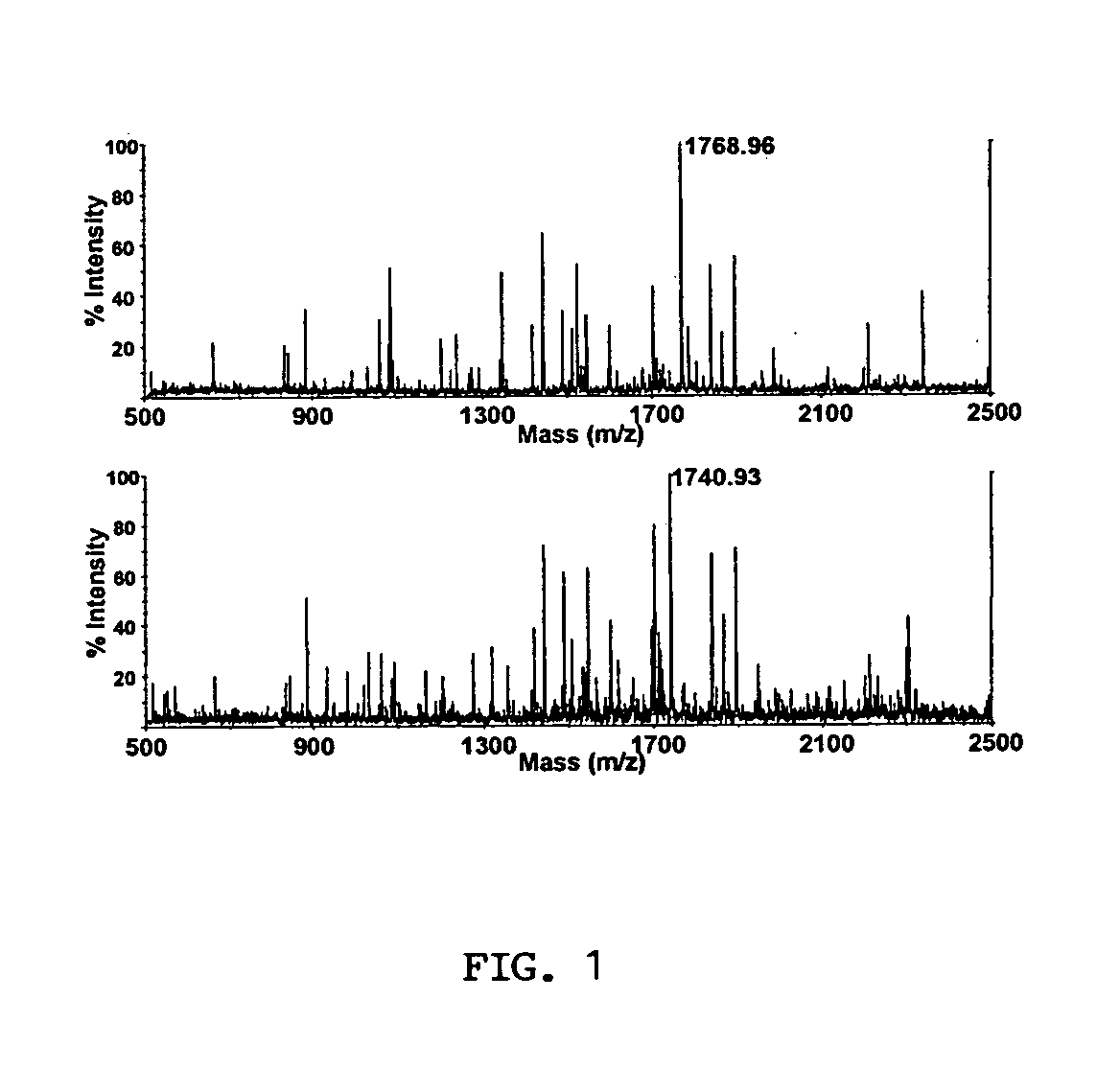
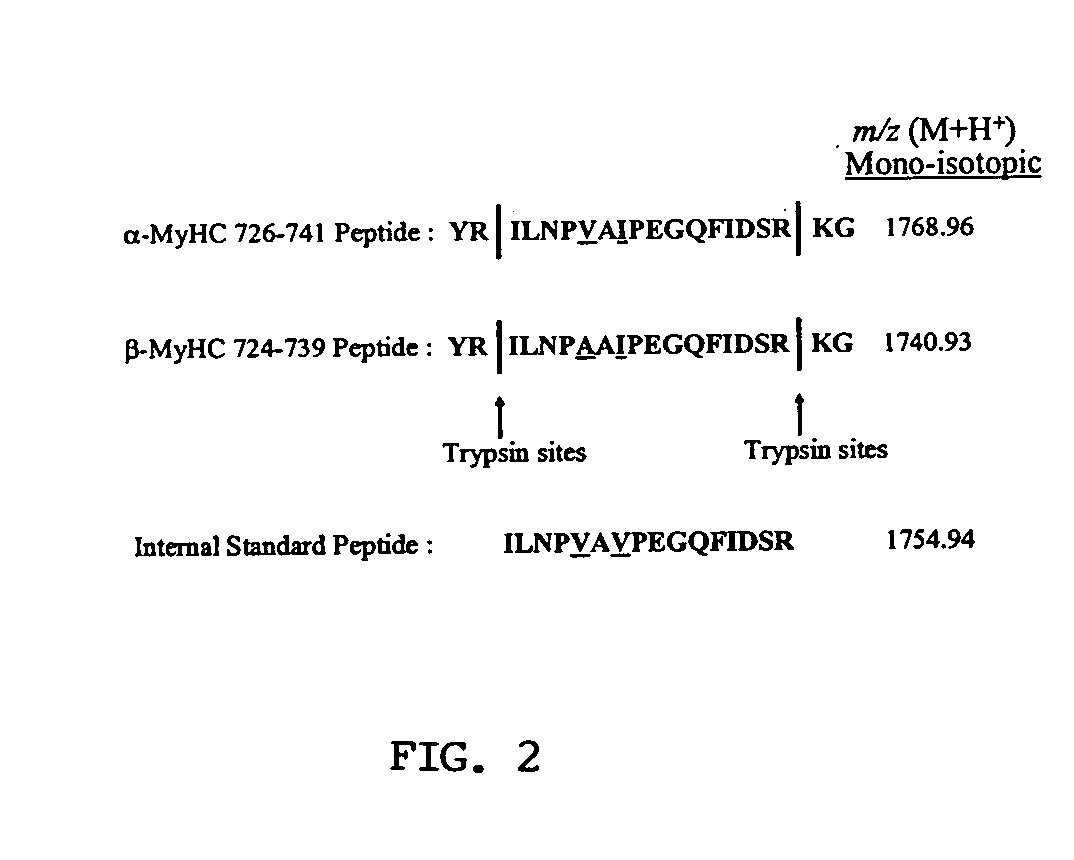
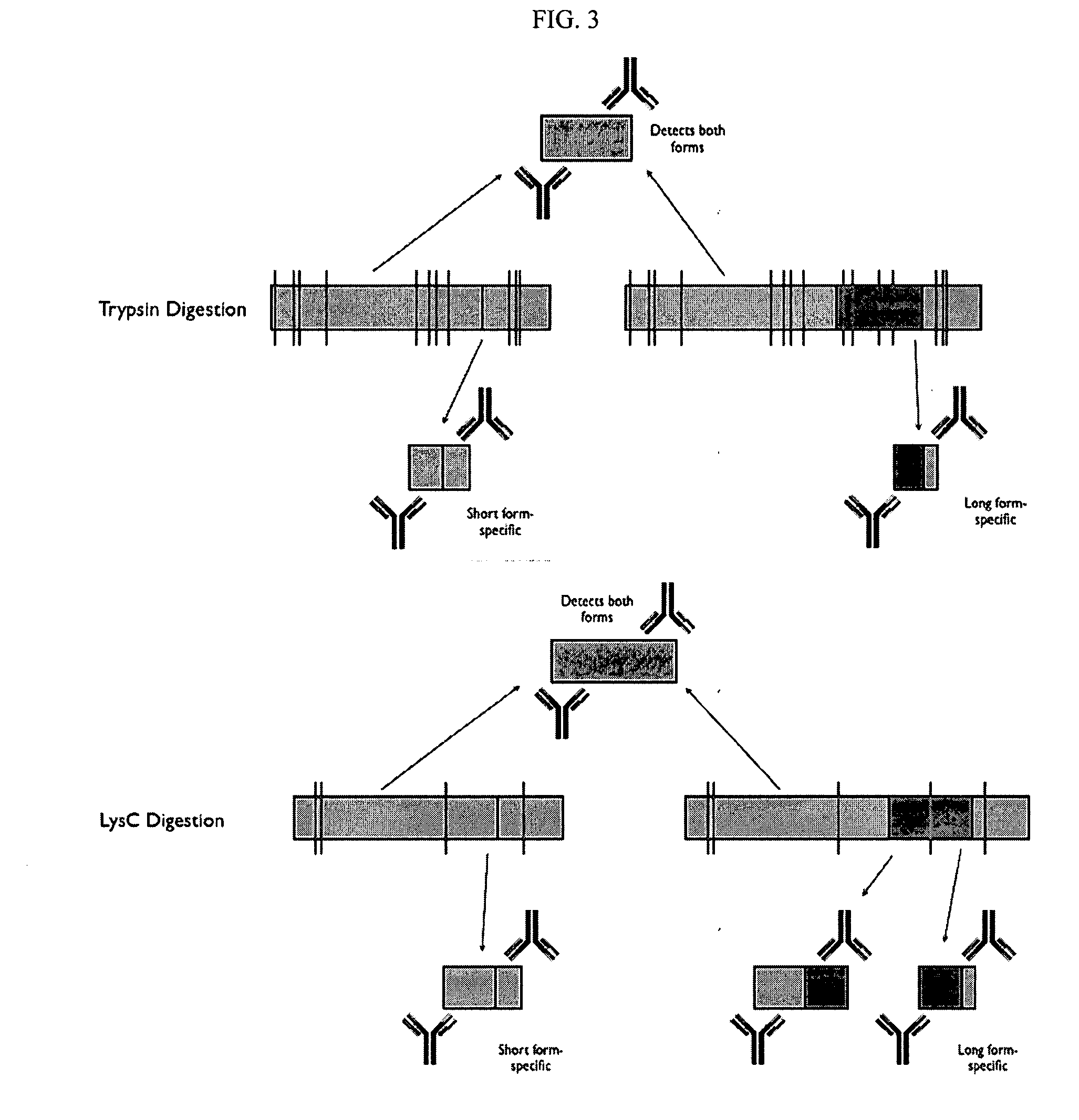
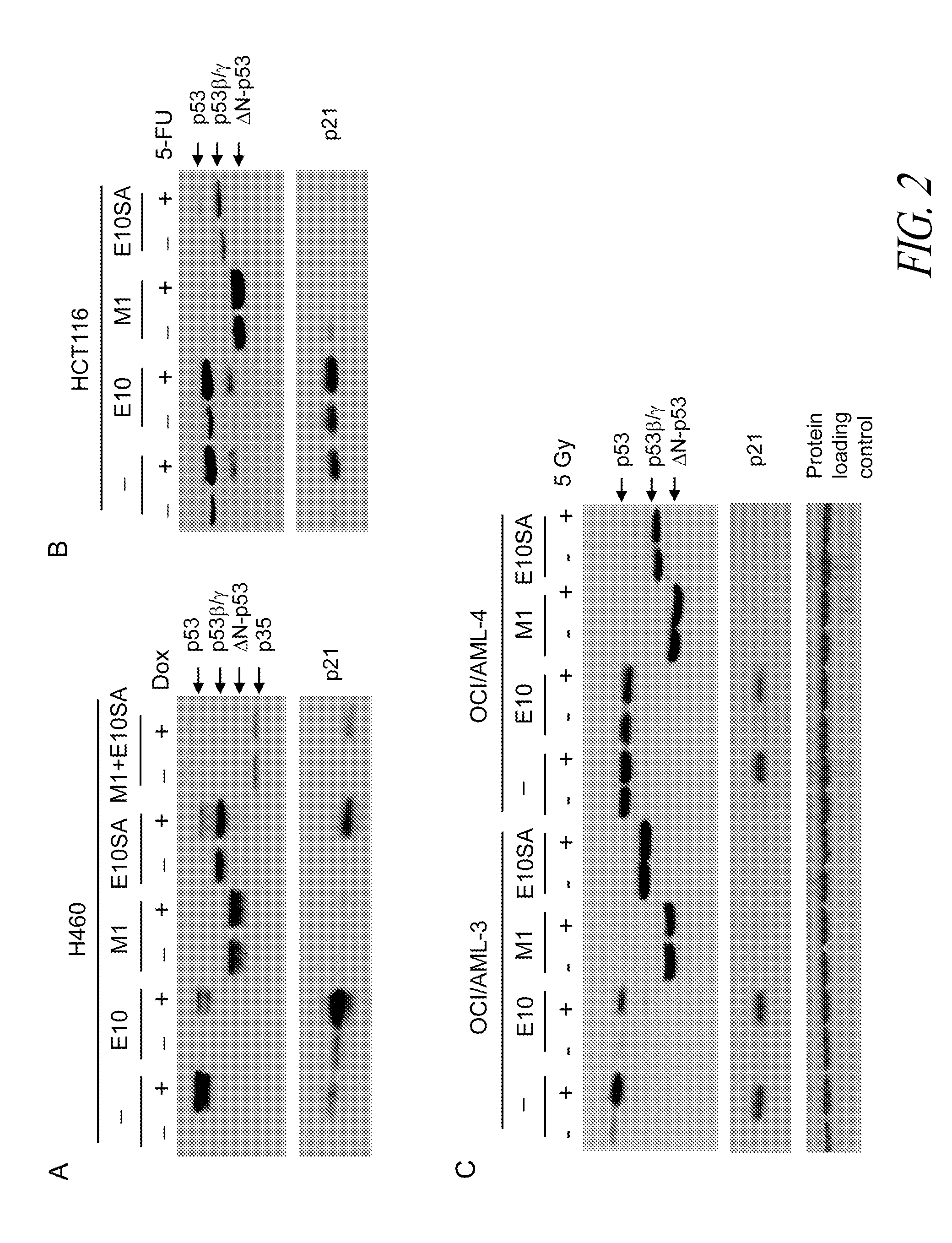
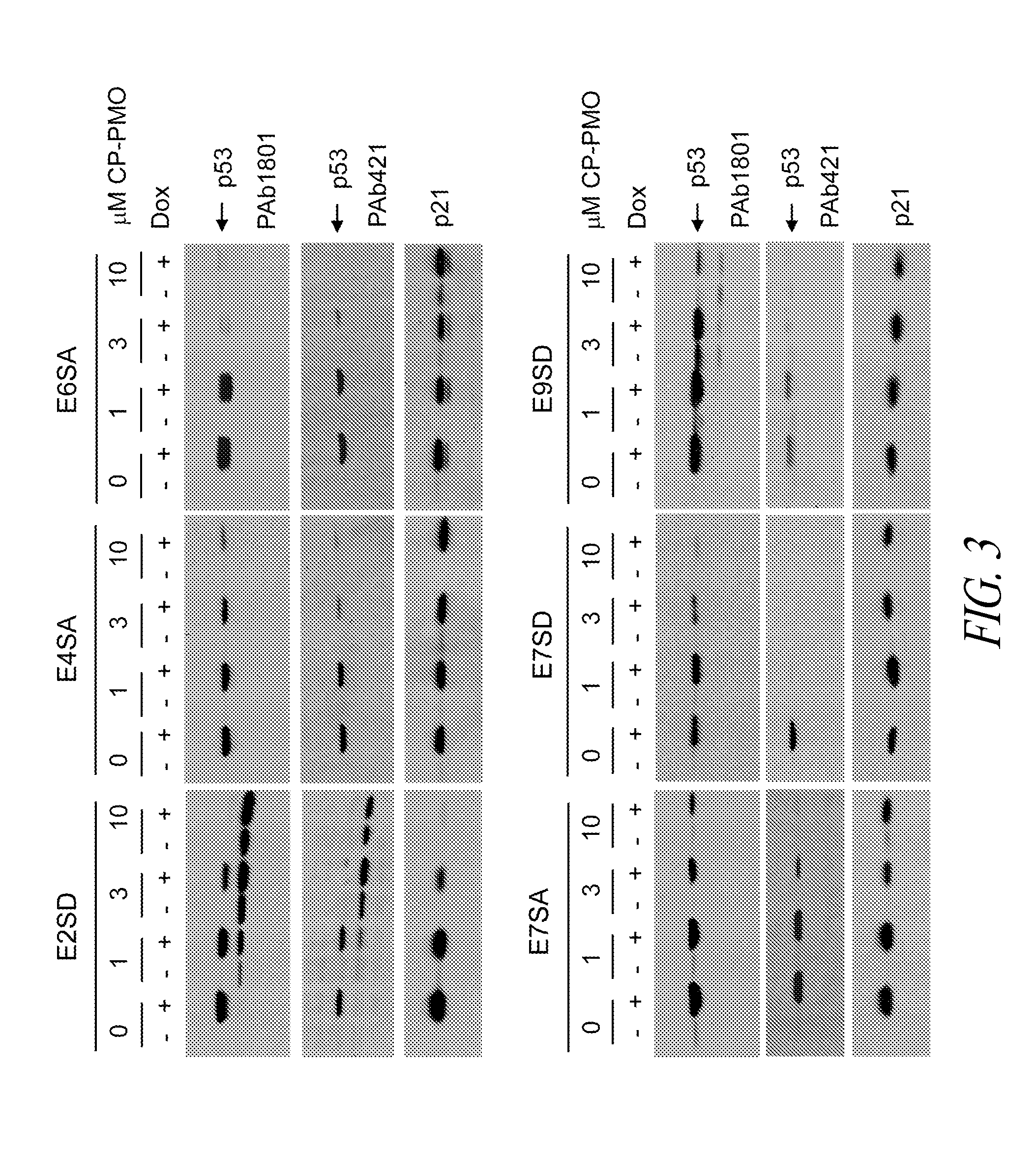
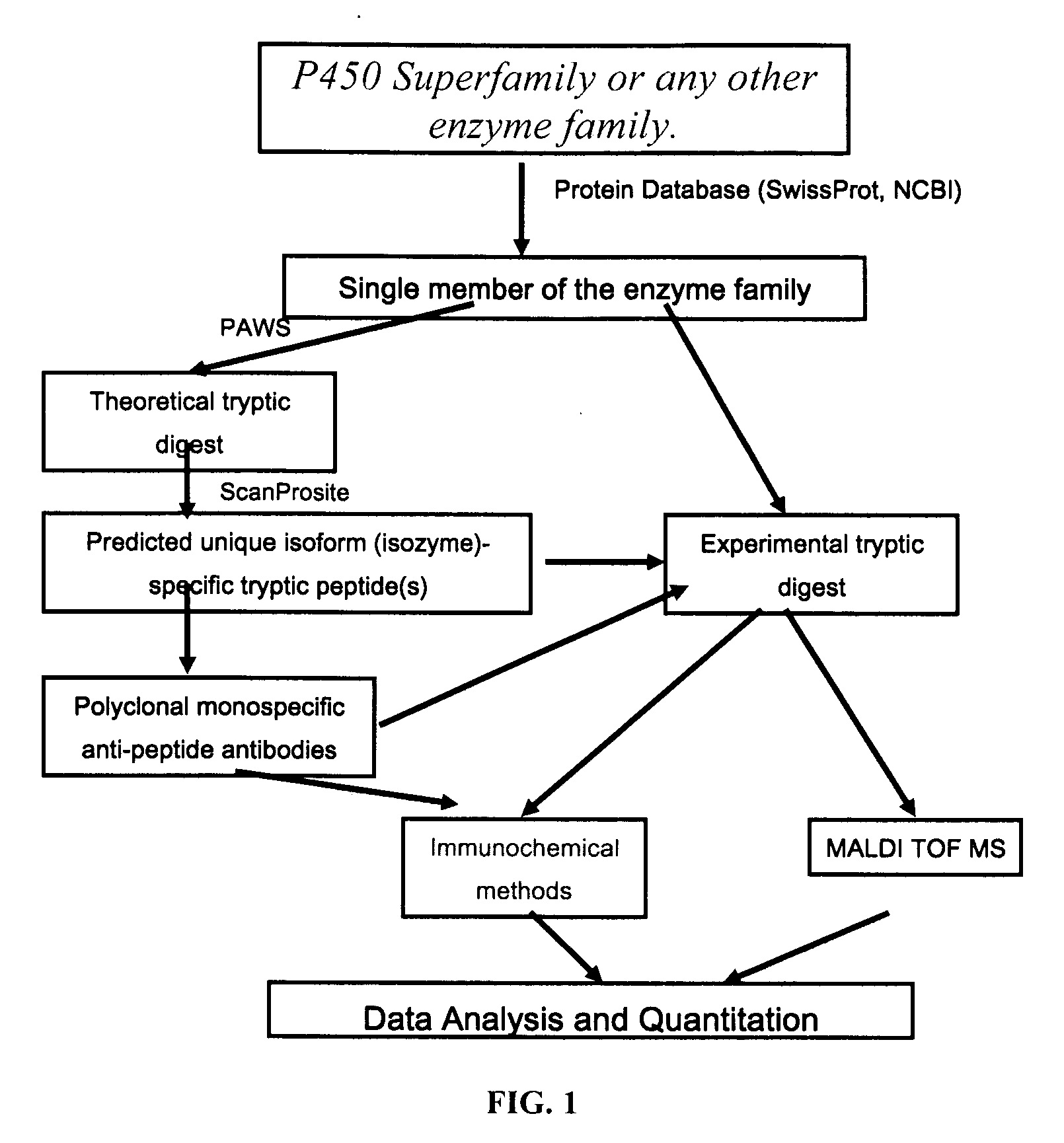
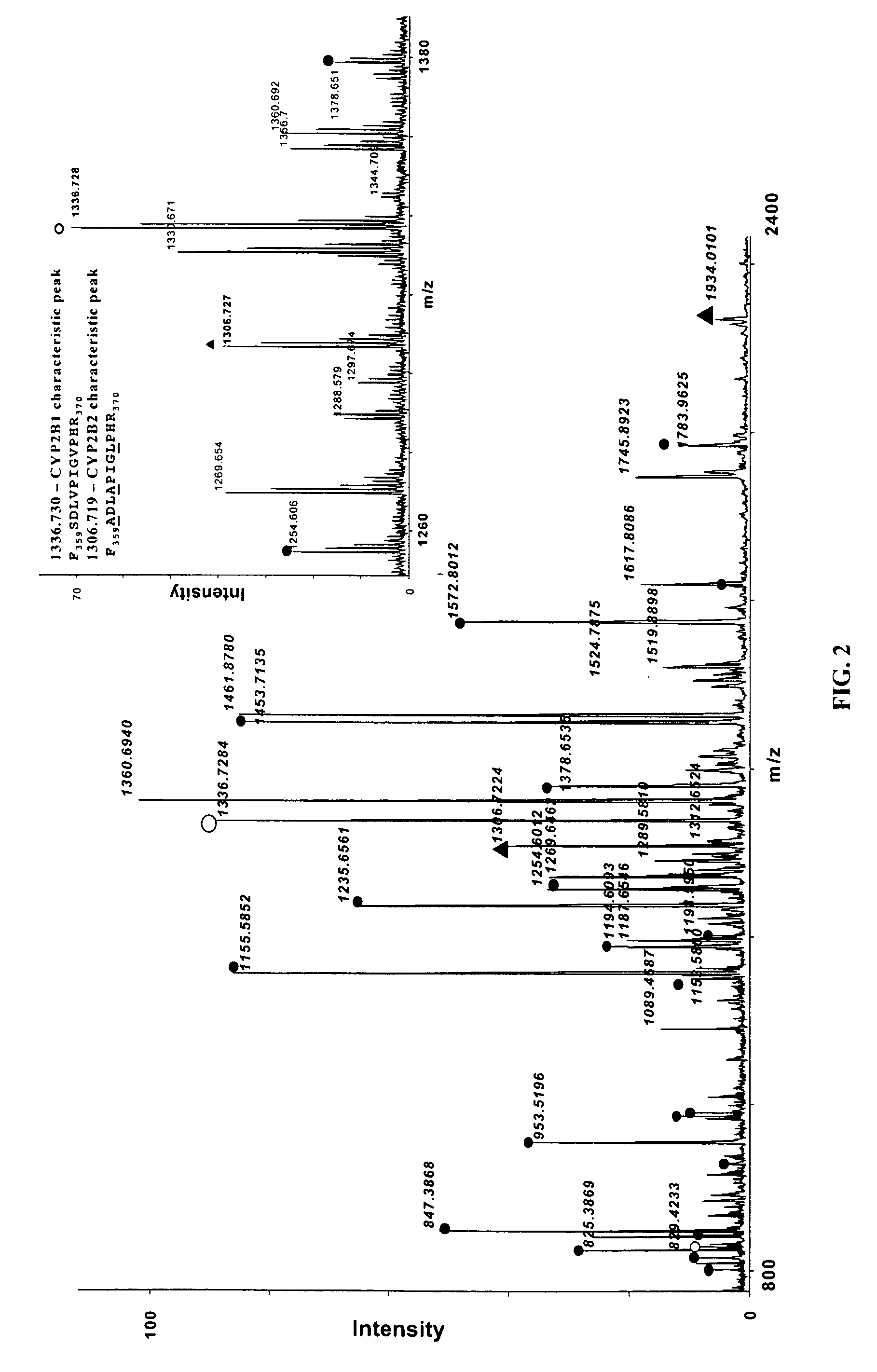
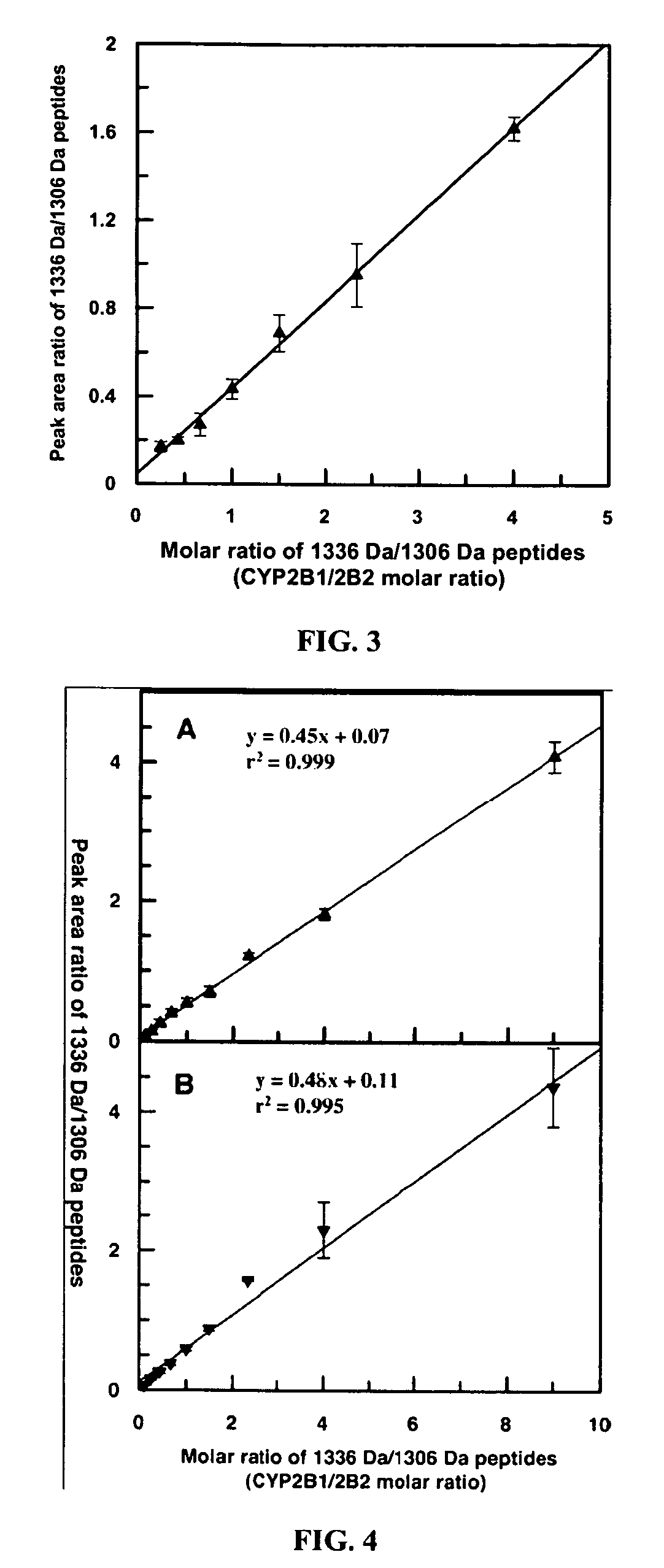
Analysis of protein isoforms using unique tryptic peptides by mass spectrometry and immunochemistry
InactiveUS20070092926A1Accurate measurementReliable detectionMicrobiological testing/measurementBiological material analysisIsozymeMass Spectrometry-Mass Spectrometry
A method for qualitatively and quantitatively detecting a protein isoform (p450 isozyme) in a sample using MALDI-TOF mass spectrometry or immunochemistry using a unique proteolytic peptide for the isoform. Relative and absolute quantitation can be performed using calibration curves with P450 isozyme-specific peptide standards.
Owner:KANSAS UNIV OF
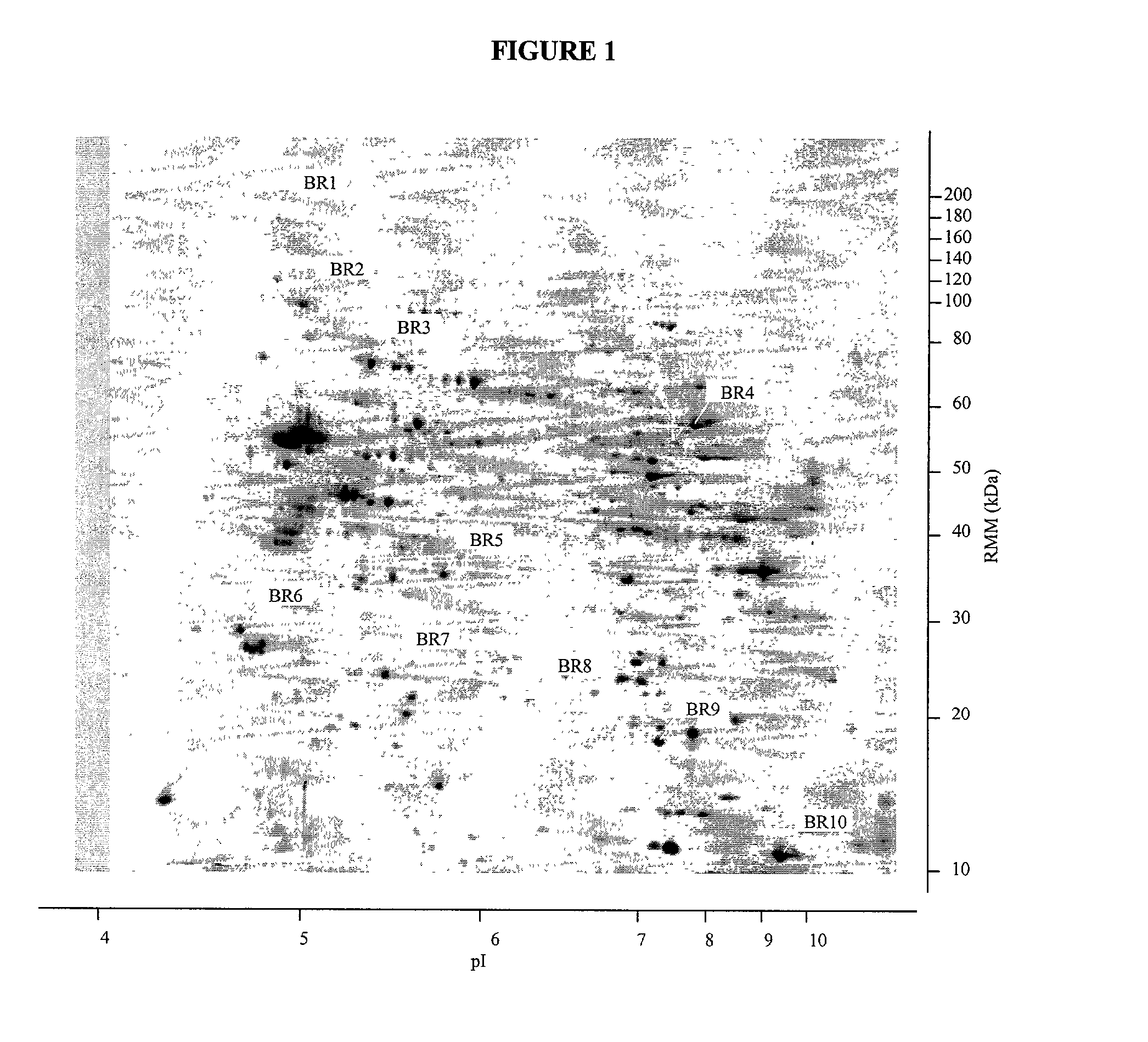
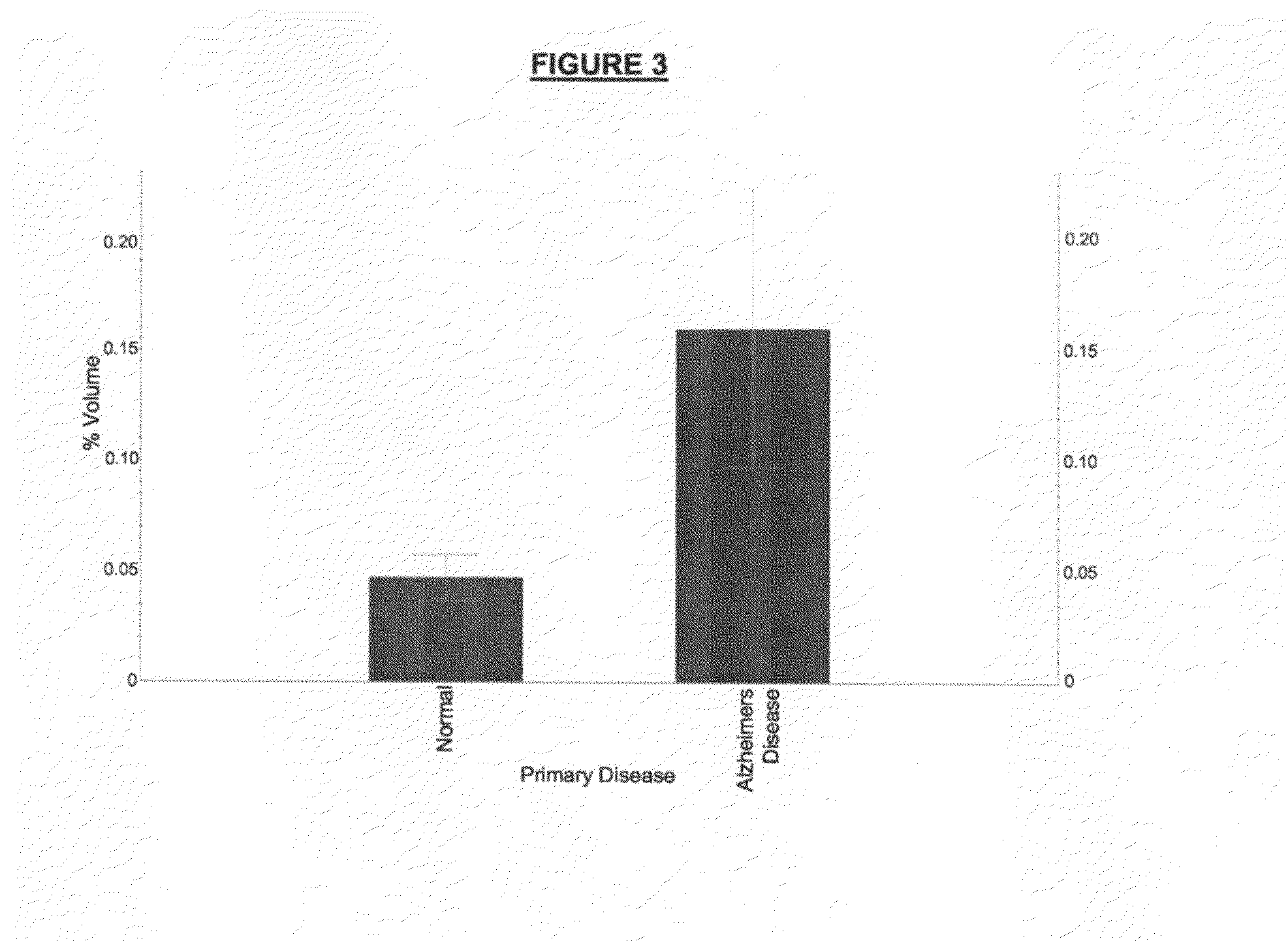
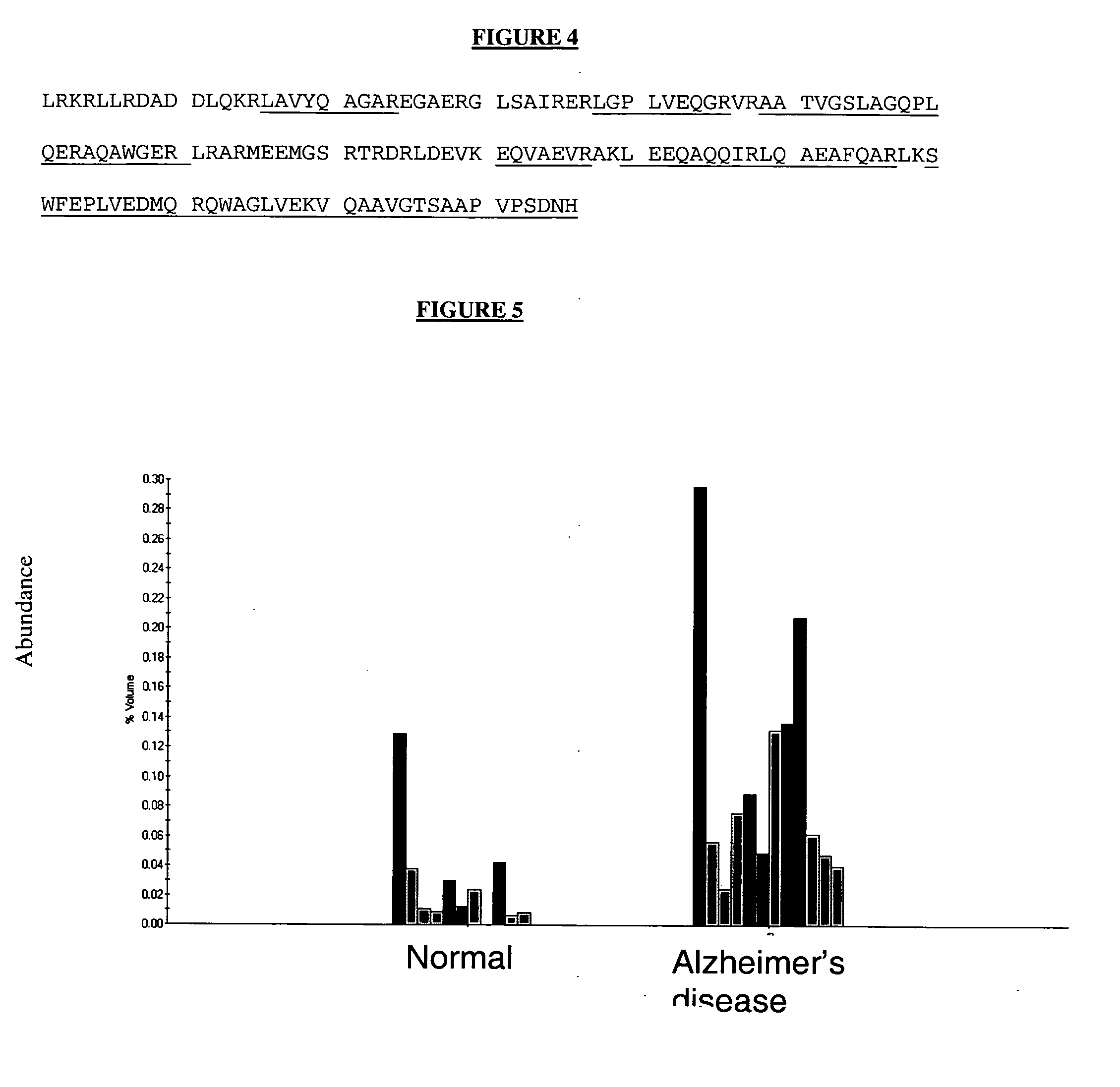
Nucleic acid molecules, polypeptides and uses therefor, including diagnosis and treatment of Alzheimer's disease
InactiveUS20030064411A1Peptide/protein ingredientsDisease diagnosisAlzheimer's disease treatmentDrug development
The present invention provides methods and compositions for screening, diagnosis and prognosis of Alzheimer's disease, for monitoring the effectiveness of Alzheimer's disease treatment and for drug development. Alzheimer's disease-Associated Features (ADFs) detectable by two-dimensional electrophoresis of brain tissue are described. The invention further provides Alzheimer's disease-Associated Protein Isoforms (ADPIs) detectable in brain tissue, preparations comprising isolated ADPIs, antibodies specific for ADPIs and kits comprising the aforesaid.
Owner:OXFORD GLYCOSCI UK
Nucleic acid molecules, polypeptides and uses therefor, including diagnosis and treatment of Alzheimer's disease
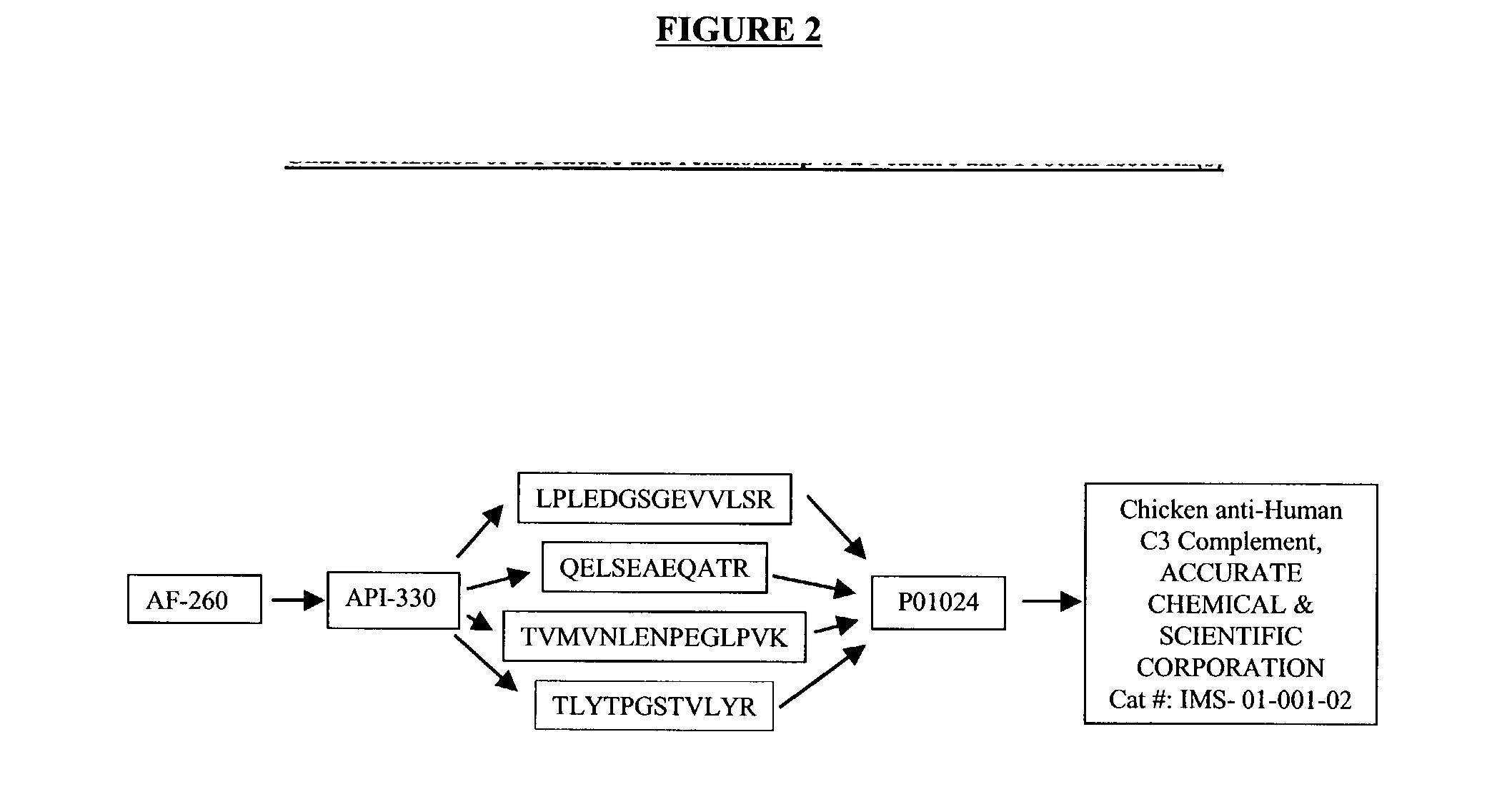
The present invention provides methods and compositions for screening, diagnosis and prognosis of Alzheimer's disease, for monitoring the effectiveness of Alzheimer's disease treatment, and for drug development. Alzheimer's Disease-Associated Features (AFs), detectable by two-dimensional electrophoresis of cerebrospinal fluid, serum or plasma are described. The invention further provides Alzheimer's Disease-Associated Protein Isoforms (APIs) detectable in cerebrospinal fluid, serum or plasma, preparations comprising isolated APIs, antibodies, pharmaceutical compositions, diagnostic and therapeutic methods, and kits comprising or based on the same.
Owner:DURHAM L KATHRYN +12
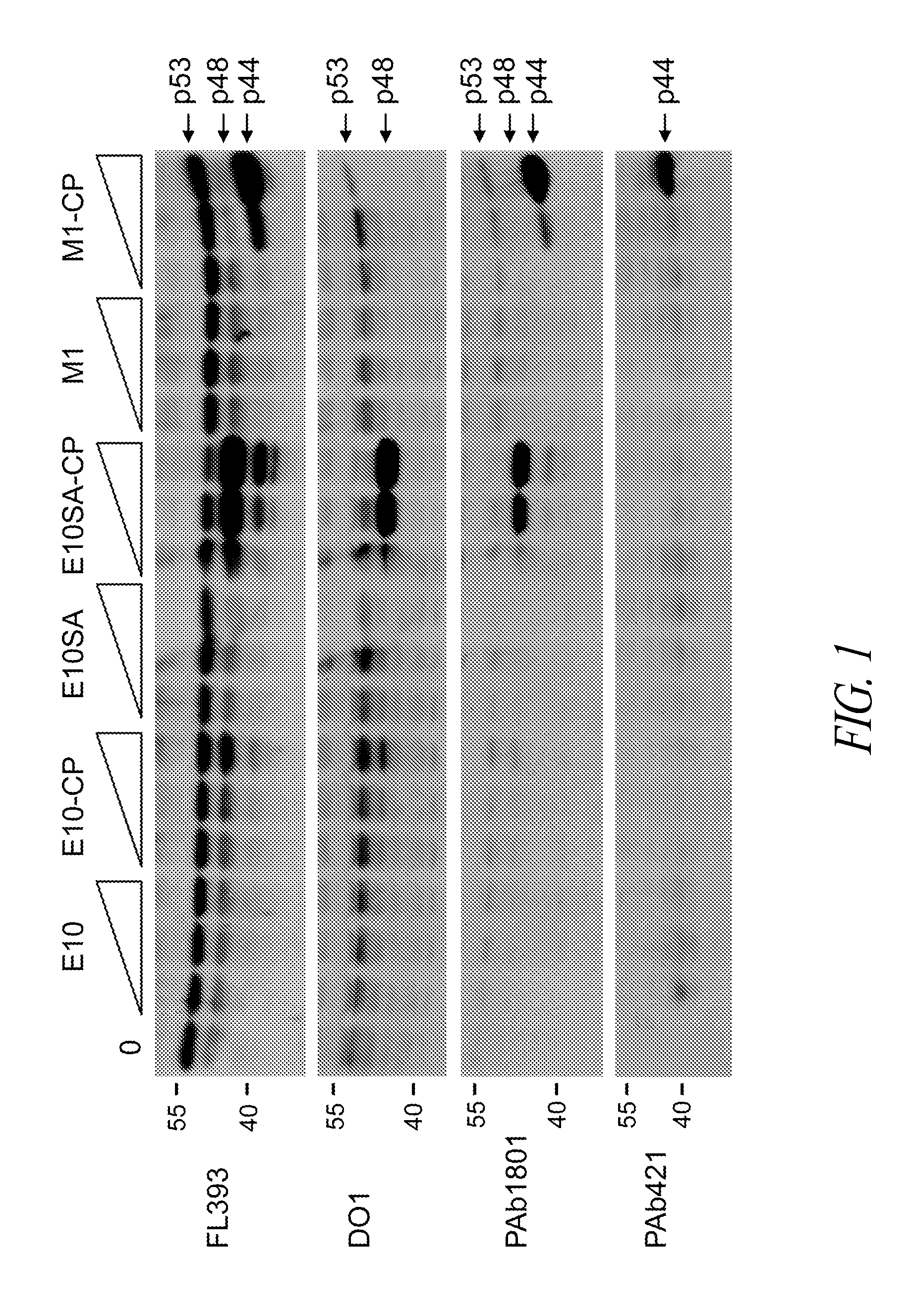
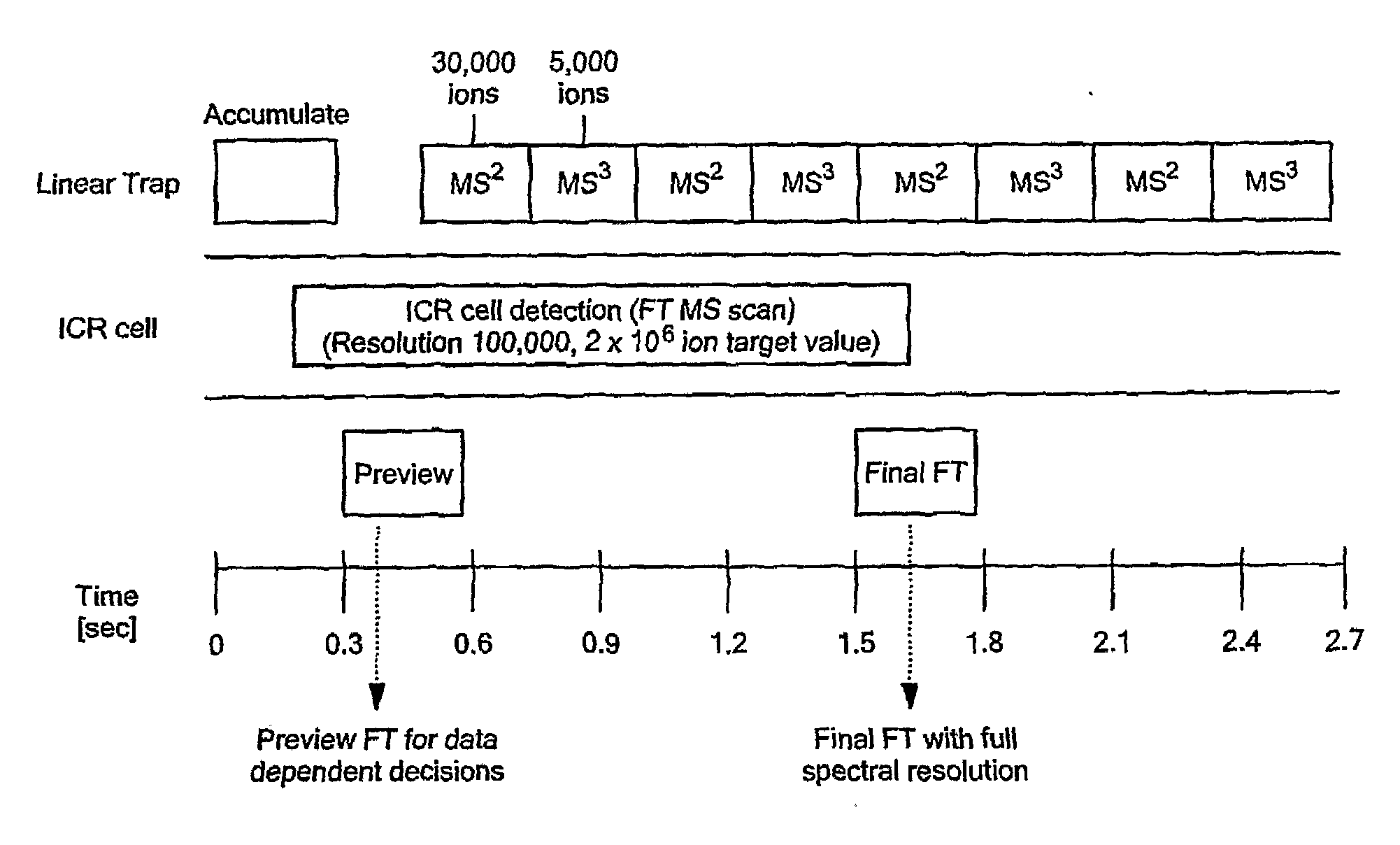
Comprehensive Characterization Of Complex Proteins At Trace Levels
InactiveUS20080280317A1High sequence coverageHigh molecular weightElectrolysis componentsMicrobiological testing/measurementConjugated proteinData acquisition
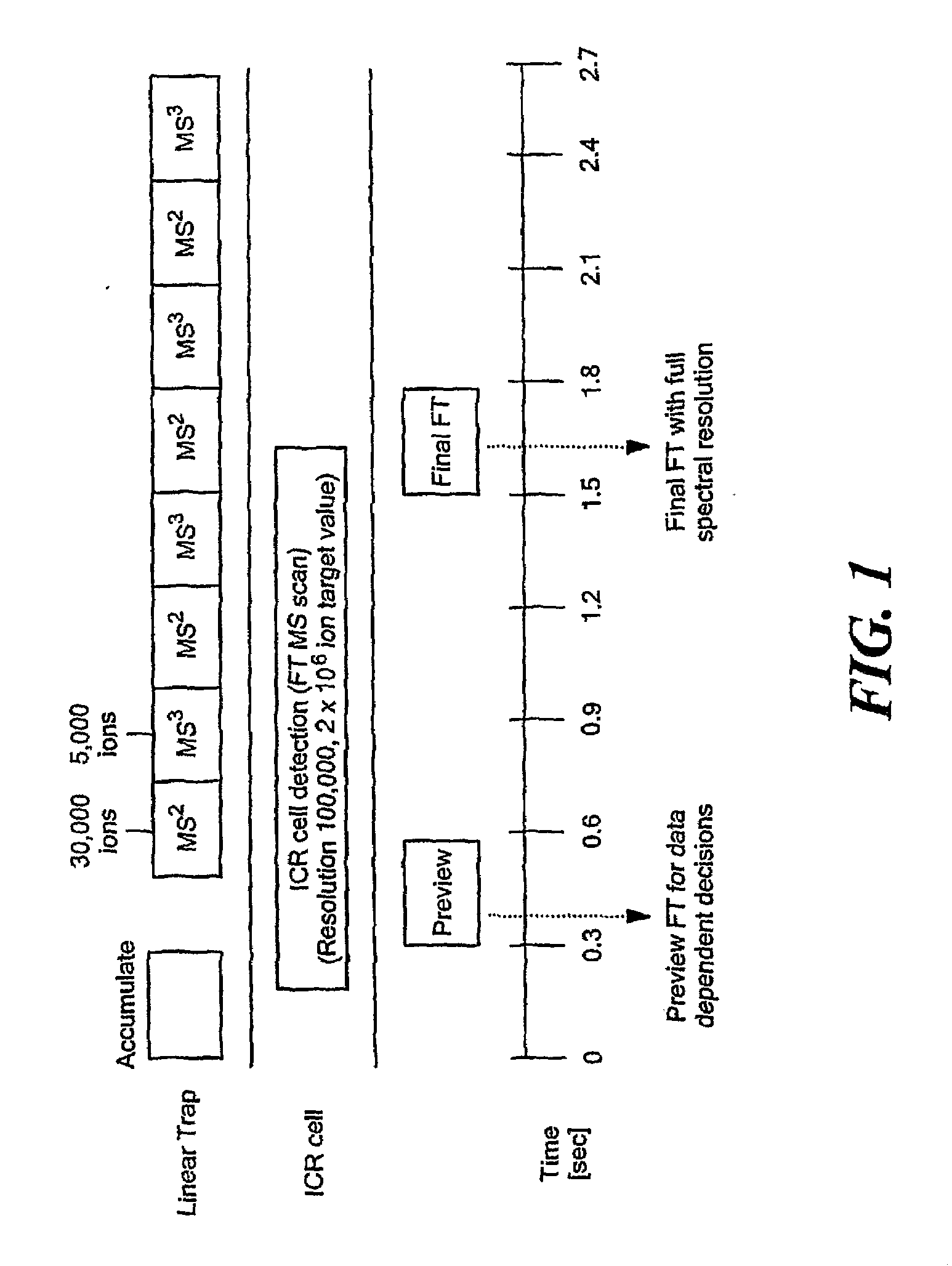
A combination of “bottom up” and “top down” MS analysis of posttranslational modifications in complex proteins is described. The method comprises digestion of the protein with an enzyme that forms larger peptide fragments than trypsin (>3000 D), performing HPLC with the fragments and applying a new data acquisition strategy using on-line coupling with e.g. LTQ-FTMS, a hybrid mass spectrometer that couples a linear ion trap with a Fourier transform ion cyclotron resonance (FTICR) cell. The method is applied to analysis of posttranslational modifications of protein isoforms.
Owner:NORTHEASTERN UNIV
Proteins, genes and their use for diagnosis and treatment of Schizophrenia
InactiveUS20020142303A1Monitor effectivenessCell receptors/surface-antigens/surface-determinantsSugar derivativesDrug developmentTherapeutic treatment
The present invention provides methods and compositions for screening, diagnosis and prognosis of Schizophrenia, for monitoring the effectiveness of Schizophrenia treatment, identifying patients most likely to respond to a particular therapeutic treatment and for drug development. Schizophrenia-Associated Features (SFs), detectable by two-dimensional electrophoresis of cerebrospinal fluid, serum or plasma are described. The invention further provides Schizophrenia-Associated Protein Isoforms (SPIs) detectable in cerebrospinal fluid, serum or plasma, preparations comprising isolated SPIs, antibodies immunospecific for SPIs, and kits comprising the aforesaid.
Owner:OXFORD GLYCOSCI UK
Protein splice variant / isoform discrimination and quantitative measurements thereof
The invention relates to methods, reagents and apparatus for detecting protein isoforms (e.g., those due to alternative splicing, or different disease protein isoforms or degradation products) in a sample, including using combinations of capture agents, each combination being unique to the splicing variant to be detected / measured.
Owner:MILLIPORE CORP
Autism gene
InactiveUS20060194201A1Efficient use ofPeptide/protein ingredientsMicrobiological testing/measurementDrug developmentGenes proteins
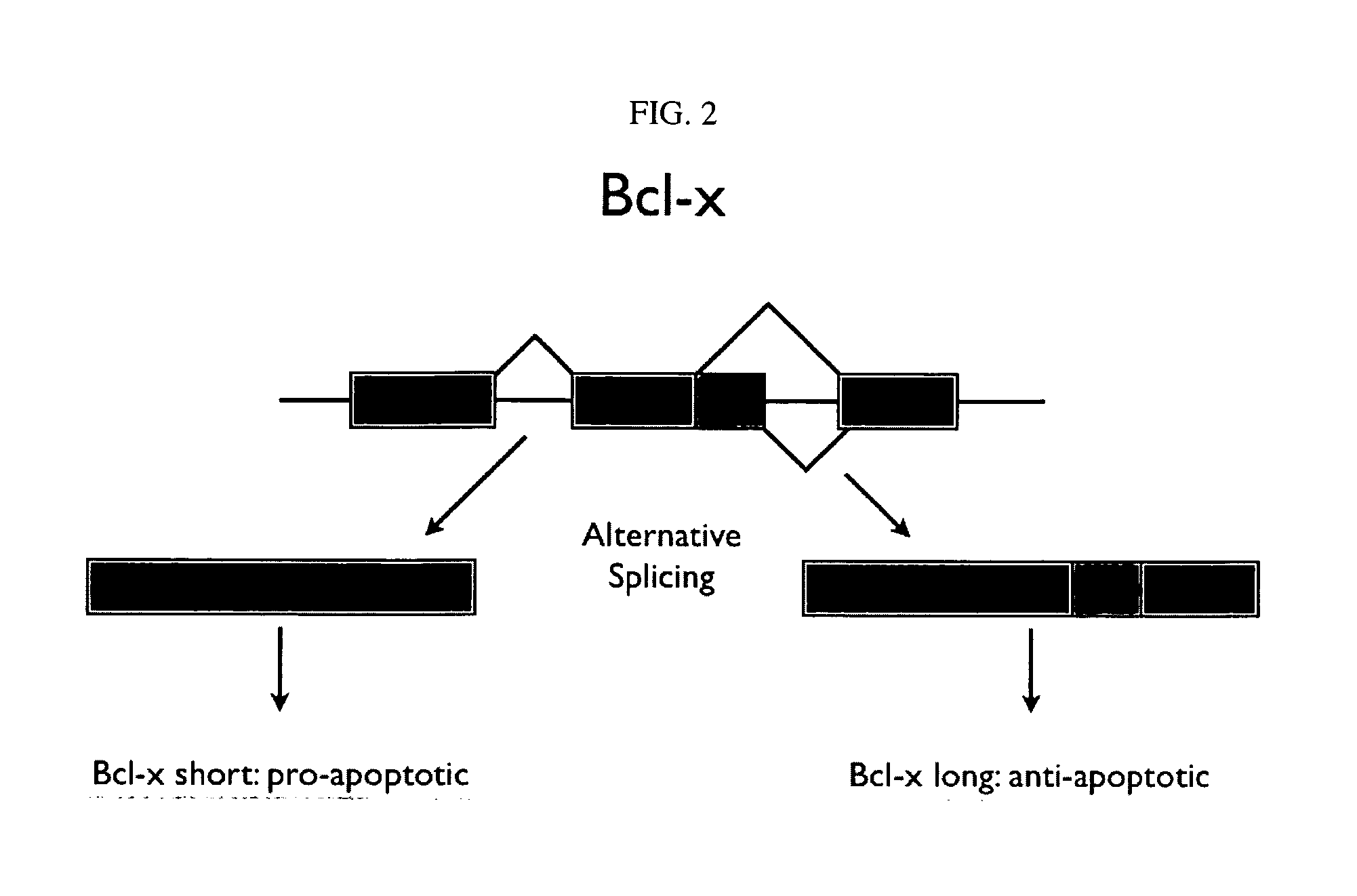
The present invention concerns genes containing mutations associated with autism its onset and development and also to the encoded proteins of said genes associated with autism, its onset and development and the use of said genes, proteins or protein isoforms. The invention thus also relates to methods of screening for, diagnosis and treatment of autism in human subjects e.g., clinical screening, diagnosis, prognosis, therapy and prophylaxis, as will as for drug screening and drug development.
Owner:K U LEUVEN RES & DEV
14-3-3 Antagonists for the Prevention and Treatment of Arthritis
InactiveUS20110027269A1Reduce effectorReduce the effectors of arthritisPeptide/protein ingredientsAntipyreticArthritisMatrix metalloproteases
Methods for treating arthritis comprising 14-3-3 antagonists that are capable of specifically binding to extracellularly localized 14-3-3 eta and / or 14-3-3 gamma protein isoforms are provided. In preferred embodiments, the 14-3-3 antagonist is an inhibitory peptide or an anti-14-3-3 antibody. The 14-3-3 antagonists are also formulated in a pharmaceutical composition and used in a method for reducing matrix metalloprotease (MMP) expression in the synovial fluid of a patient, wherein the MMP is MMP-1 or MMP-3.
Owner:THE UNVERSITY OF BRITISH OFFICE
Protein isoform discrimination and quantitative measurements thereof
InactiveUS7645586B2Reliably retrievedMicrobiological testing/measurementBiological testingDiseaseAlternative splicing
The invention relates to methods, reagents and apparatus for detecting protein isoforms (e.g., those due to alternative splicing, or different disease protein isoforms or degradation products) in a sample, including using combinations of capture agents to identify the isoforms to be detected / measured.
Owner:MILLIPORE CORP
Protein Isoforms and Uses Thereof
InactiveUS20090311180A1Modulate activityModulate expressionOrganic active ingredientsNervous disorderTest sampleCvd risk
A method for screening for or diagnosis or prognosis of a neurological disorder in a subject, for determining the stage or severity of such a neurological disorder in a subject, for identifying a subject at risk of developing such a neurological disorder, or for monitoring the effect of therapy administered to a subject having such a neurological disorder, said method comprising: (a) analyzing a test sample of body fluid or tissue from the subject said sample comprising at least one Protein Isoform selected from the Protein Isoform Nos 1-6 listed in Table 1; and (b) comparing the abundance of said Protein Isoform(s) in the test sample with the abundance of said Protein Isoform(s) in a test sample from one or more persons free from neurological disorder, or with a previously determined reference range for that Protein Isoform in subjects free from neurological disorder, wherein a diagnosis of or a positive result in screening for or a prognosis of a more advanced condition of said neurological disorder is indicated by increased abundance of said Protein Isoform(s) in the test sample relative to the abundance of said Protein Isoform(s) in the test sample from one or more persons free from neurological disorder, or with the previously determined reference range for that Protein Isoform in subjects free from neurological disorder.
Owner:OXFORD GENOME SCI UK
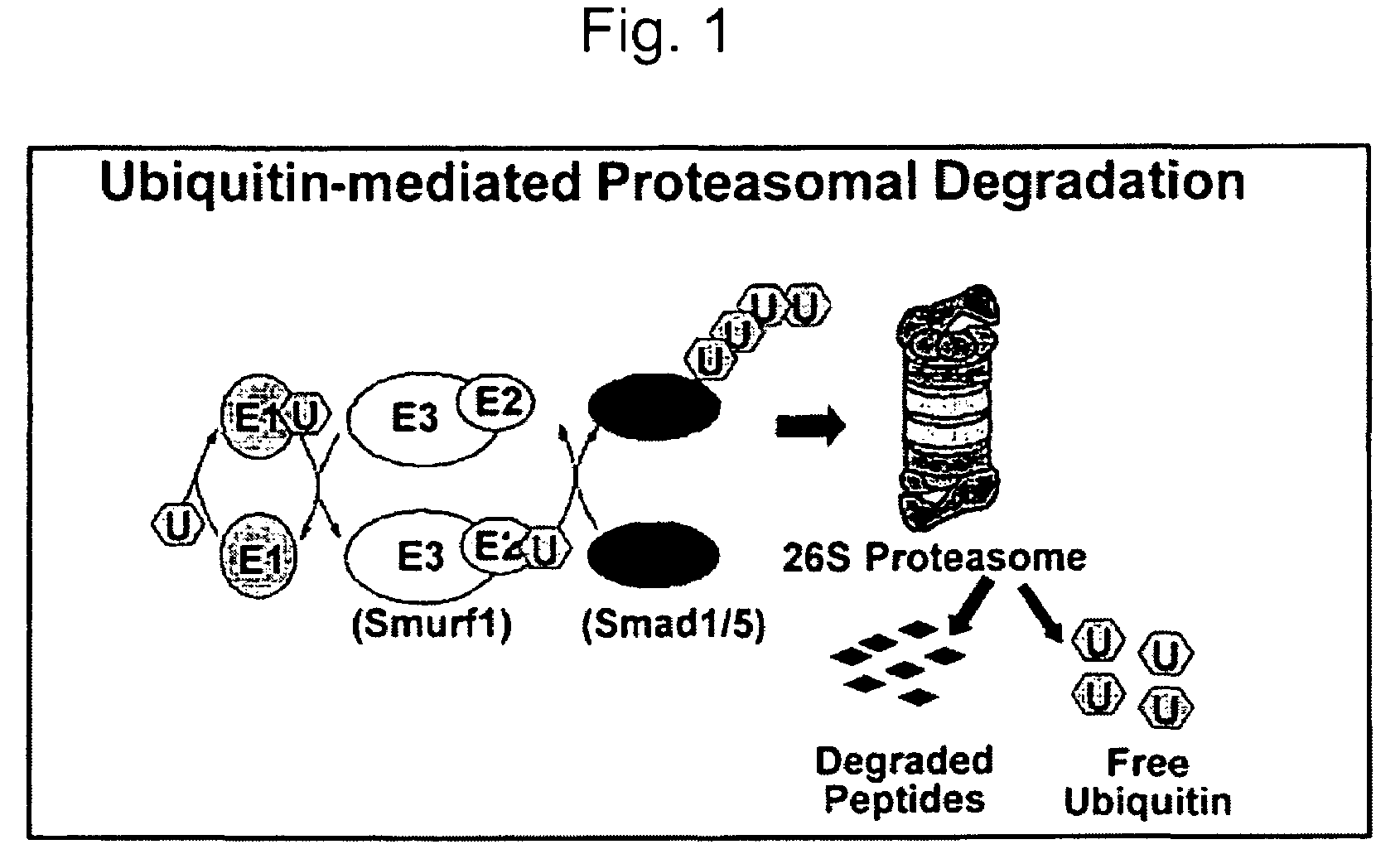
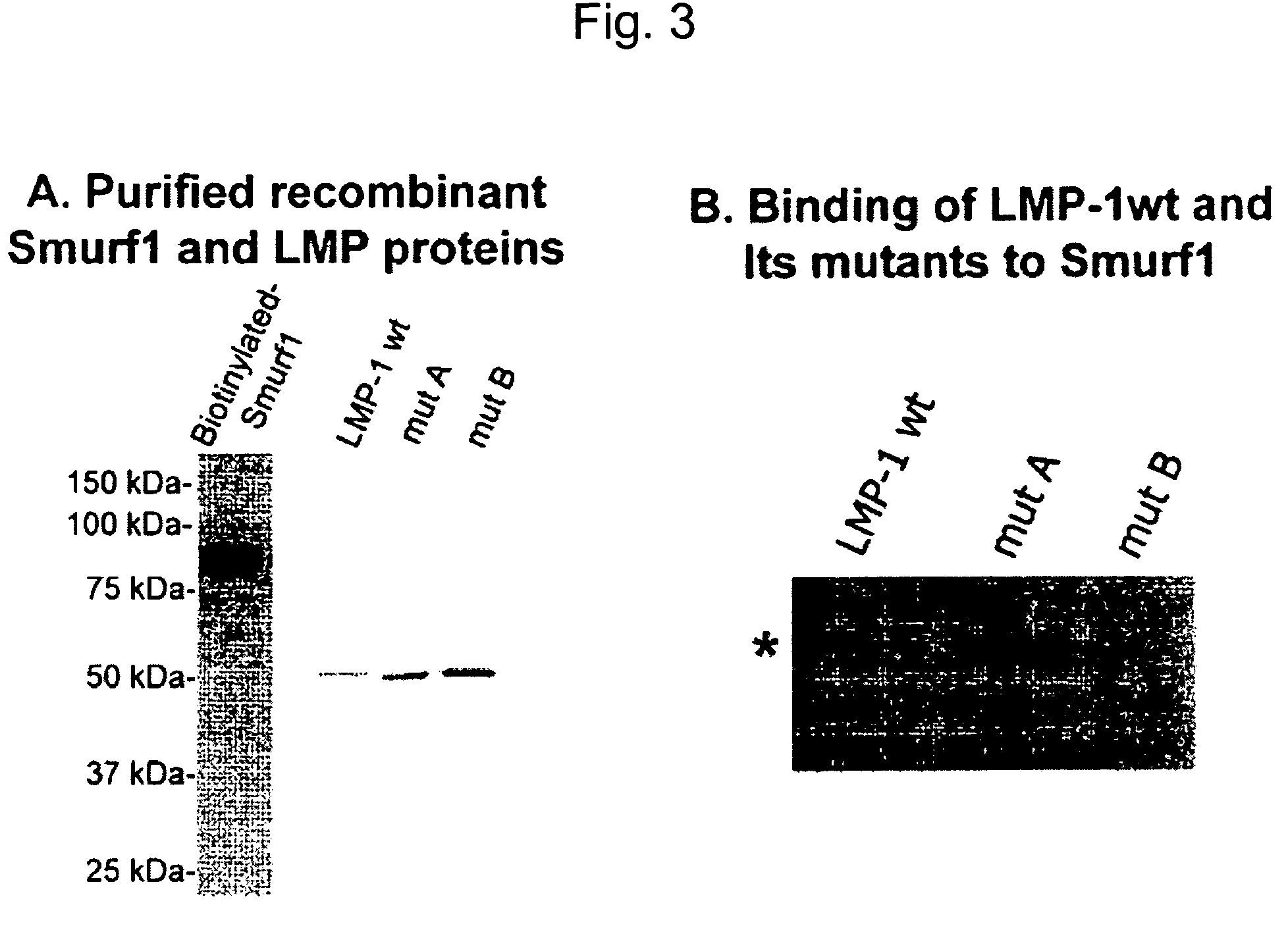
Methods and kits using a molecular interaction between a Smurf-1 WW domain and LIM mineralization protein isoforms
The instant application provides kits and methods for identifying agents which induce or inhibit the osteogenic effect of LMP or BMP proteins. The kits are directed to methods which measure either an amount of a complex between a Smurf protein or a fragment thereof and an LMP protein or a fragment thereof. Alternatively, the kits are directed to methods of measuring an amount of the ubiquitinated Smad protein or a fragment thereof.
Owner:EMORY UNIVERSITY
14-3-3 ETA Antibodies and Uses Thereof for the Diagnosis and Treatment of Arthritis
The invention provides anti-14-3-3 eta antibodies that specifically bind to the human 14-3-3 eta protein isoform in its natural configuration while exhibiting selectivity over human 14-3-3 alpha, beta, delta, epsilon, gamma, tau, and zeta protein isoforms. Methods, kits and pharmaceutical compositions comprising said specific anti-14-3-3 eta antibodies are further provided for the diagnosis and treatment of arthritis.
Owner:THE UNIV OF BRITISH COLUMBIA
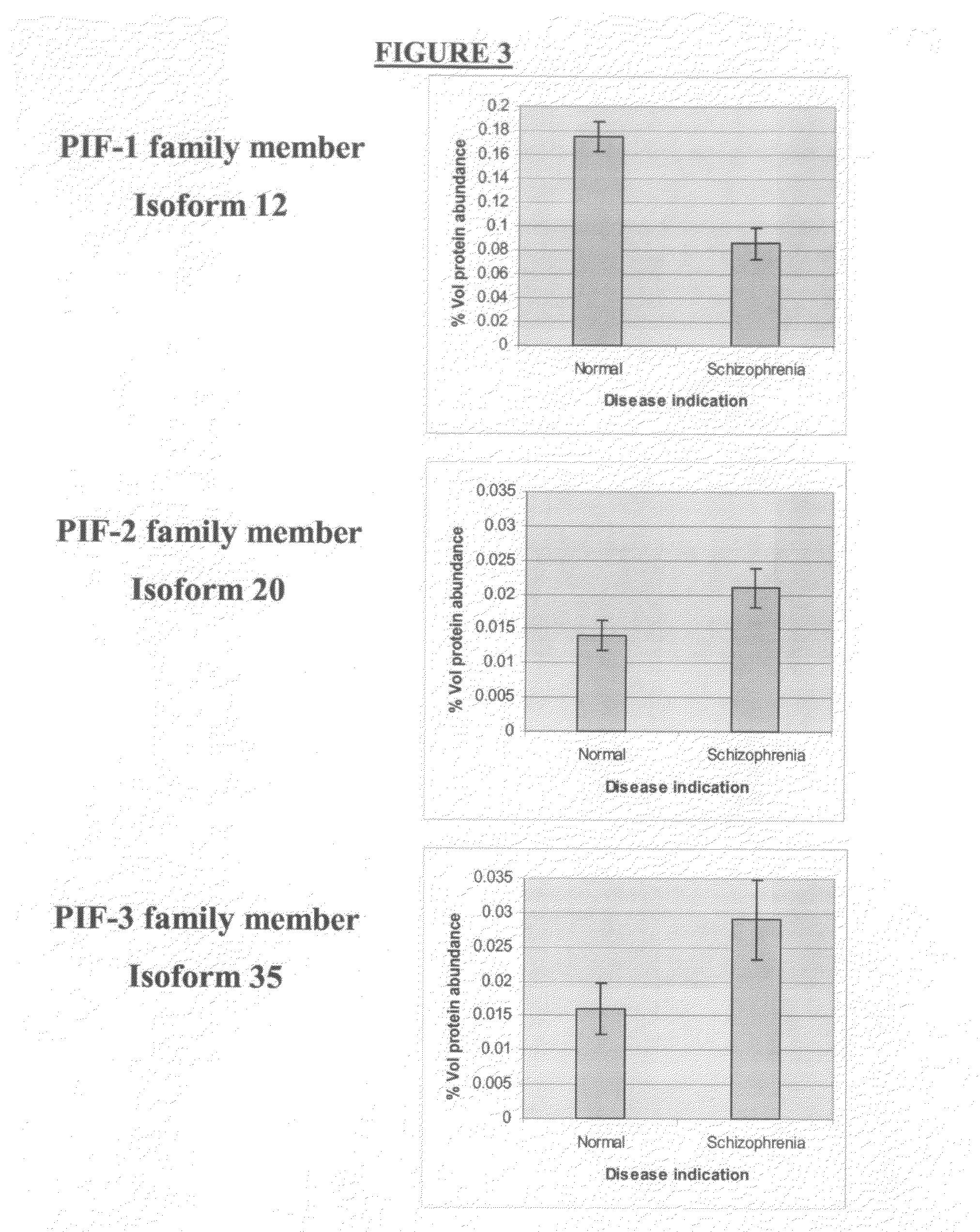
Protein isoforms and uses thereof
InactiveUS20070166765A1Modulate activityModulate expressionDisease diagnosisBiological testingDiseaseNervous system
There is provided a method for screening for or, diagnosis or prognosis of a neurological disorder in a subject, for determining the stage or severity of such a neurological disorder in a subject, for identifying a subject at risk of developing such a neurological disorder, or for monitoring the effect of therapy administered to a subject having such a neurological disorder, said method comprising: (a) analyzing a test sample of body fluid or tissue from the subject said sample comprising at least one Protein Isoform selected from one of the following Protein Isoform Families: PIF-1 and PIF-2; and (b) comparing the abundance of said Protein Isoform(s) in the test sample with the abundance of said Protein Isoform(s) in a test sample from one or more persons free from neurological disorder, or with a previously determined reference range for that Protein Isoform in subjects free from neurological disorder, wherein a diagnosis of or a positive result in screening for or a prognosis of a more advanced condition of said neurological disorder is indicated by an increased abundance of said Protein Isoform(s) in the test sample relative to the abundance of said Protein Isoform(s) in the test sample from one or more persons free from neurological disorder, or with the previously determined reference range for that Protein Isoform in subjects free from neurological disorder.
Owner:OXFORD GENOME SCI UK
Proteins, genes and their use for diagnosis and treatment of bipolar affective disorder (BAD) and unipolar depression
The present invention provides methods and compositions for screening, diagnosis and prognosis of BAD, for monitoring the effectiveness of BAD treatment, and for drug development. BAD-Associated Features (DFs), detectable by two-dimensional electrophoresis of cerebrospinal fluid, serum or plasma are described. The invention further provides BAD-Associated Protein Isoforms (DPIs) detectable in cerebrospinal fluid, serum or plasma, preparations comprising isolated DPIs, antibodies immunospecific for DPIs, and kits comprising the aforesaid.
Owner:OXFORD GLYCOSCI UK
Discovery and validation of cancer biomarkers using a protein analysis methodology to analyze specimens
InactiveUS20100261224A1Good choiceMicrobiological testing/measurementOmicsSequence analysisPost translational
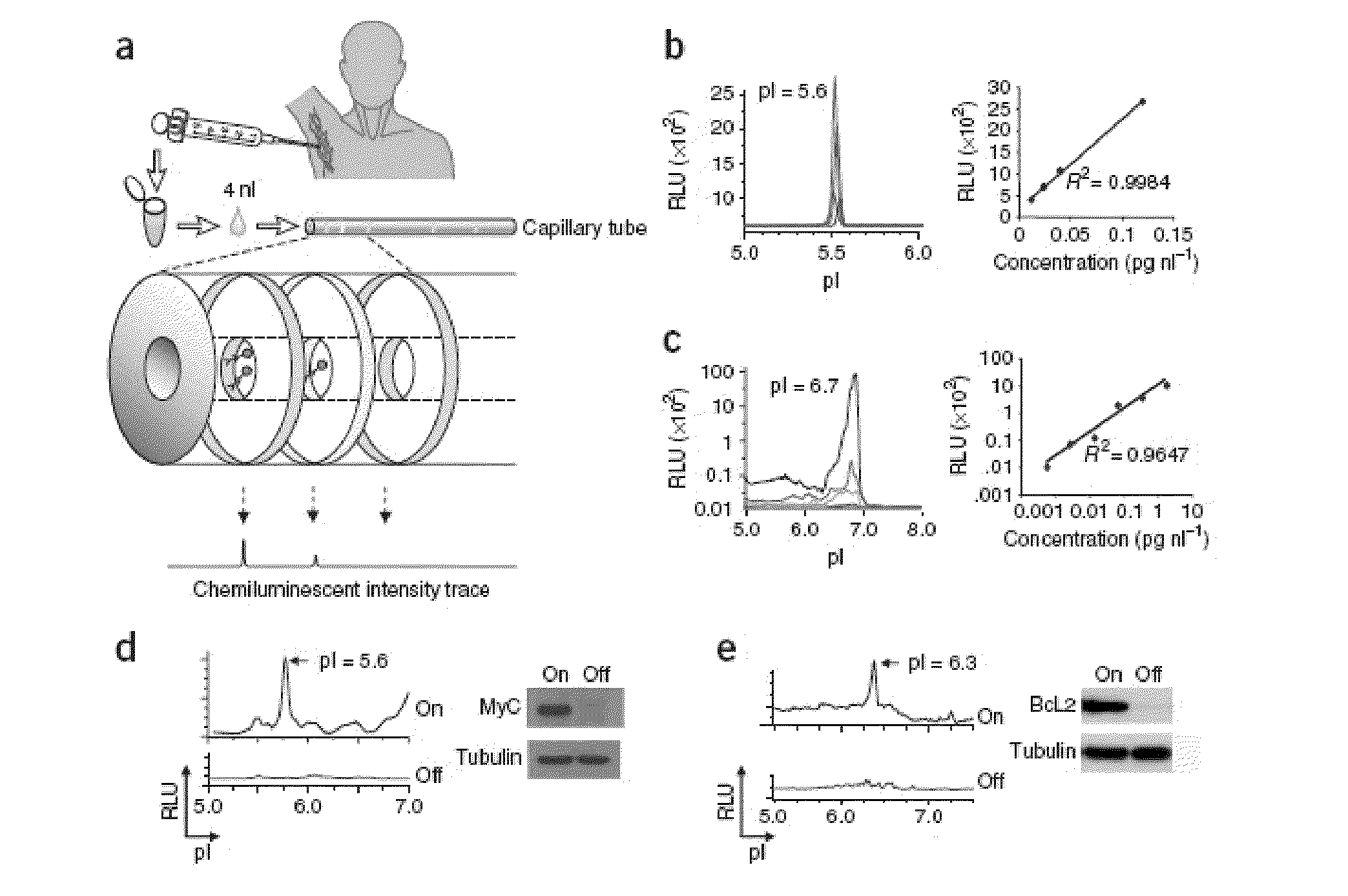
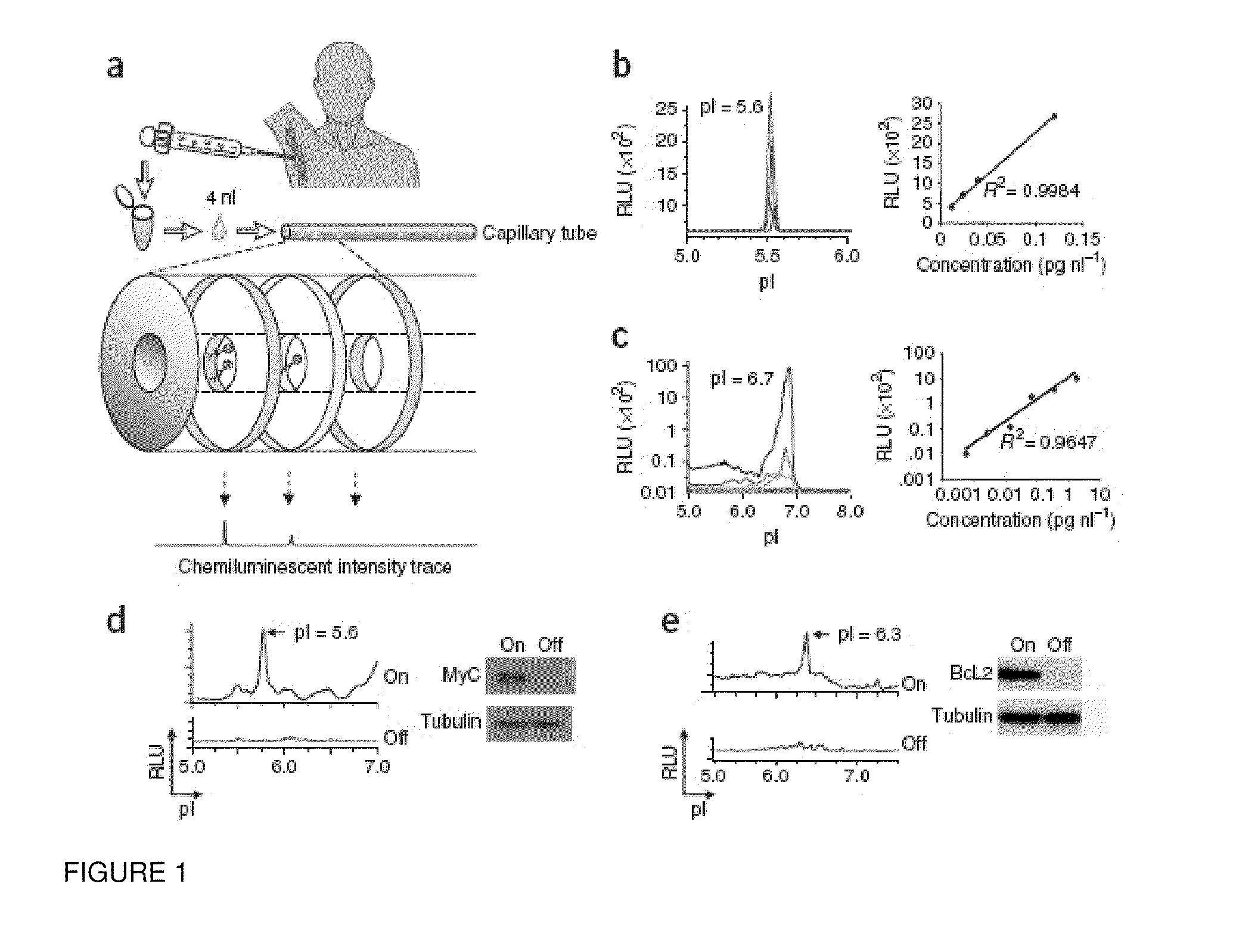
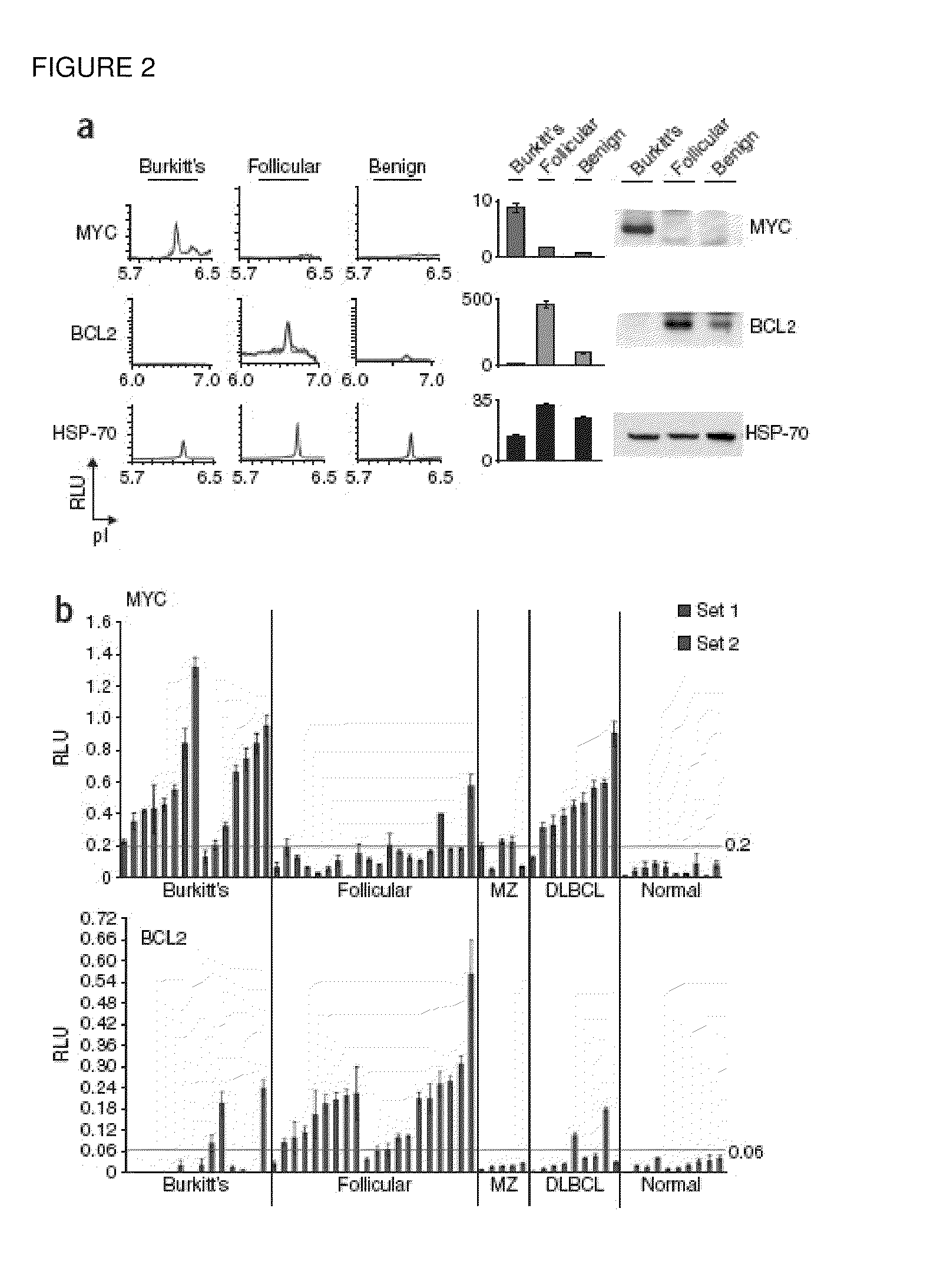
Methods are provided for the analysis, including the serial analysis, of very small samples of tissue. The methods utilize a nanofluidic proteomic immunoassay (NIA) to quantify total and low-abundance protein isoforms in a small amount of lysate. NIA detection accurately measure oncoprotein expression and activation in limited clinical specimens, including isoforms that differ in post-translational modifications, such as phosphorylation, and the like. The NIA detection method combines isoelectric protein focusing and antibody detection in a nanofluidic system.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Nucleic acid molecules, polypeptides and uses therefor, including diagnosis and treatment of Alzheimer's disease
InactiveUS20050163789A9Electrophoretic profilingMicrobiological testing/measurementDrug developmentBlood plasma
The present invention provides methods and compositions for screening, diagnosis and prognosis of Alzheimer's disease, for monitoring the effectiveness of Alzheimer's disease treatment, and for drug development. Alzheimer's Disease-Associated Features (AFs), detectable by two-dimensional electrophoresis of cerebrospinal fluid, serum or plasma are described. The invention further provides Alzheimer's Disease-Associated Protein Isoforms (APIs) detectable in cerebrospinal fluid, serum or plasma, preparations comprising isolated APIs, antibodies immunospecific for APIs, pharmaceutical compositions, diagnostic and therapeutic methods, and kits comprising or based on the same.
Owner:PFIZER INC +1
Methods and compositions for manipulating translation of protein isoforms from alternative initiation start sites
InactiveUS20190241890A1High sensitivityImprove translationPolymorphism usesScreening processStart codonStart site
Provided herein are antisense oligonucleotides, compositions comprising antisense oligonucleotides, and methods for the use of antisense oligonucleotides in manipulating translation. Expression of isoforms of proteins expressed from different start codons of the same transcript are inhibited by antisense oligonucleotides, which may also enhance expression of non-target isoforms.
Owner:SAREPTA THERAPEUTICS INC
Methods and kits for diagnosing sjogren's syndrome
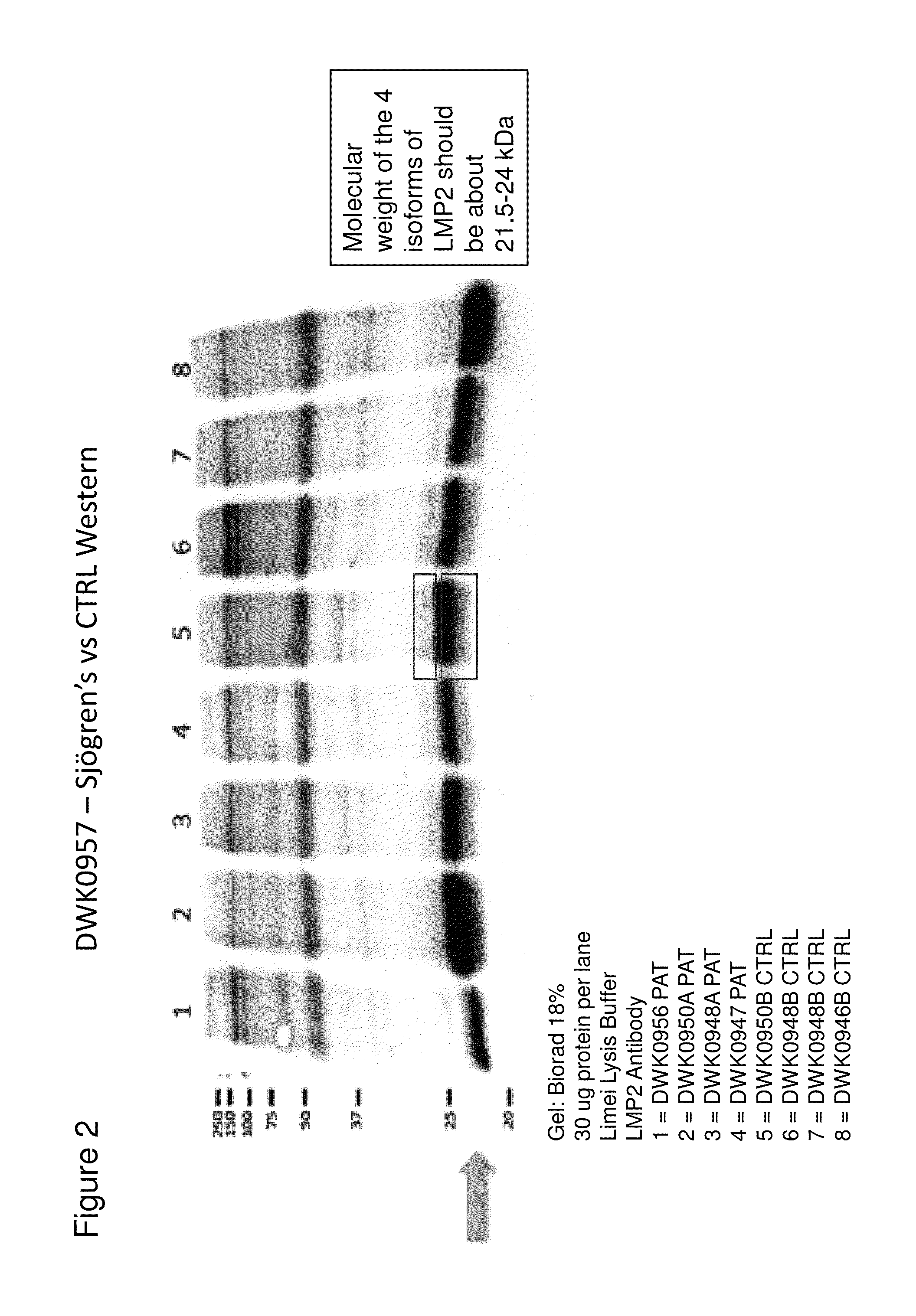
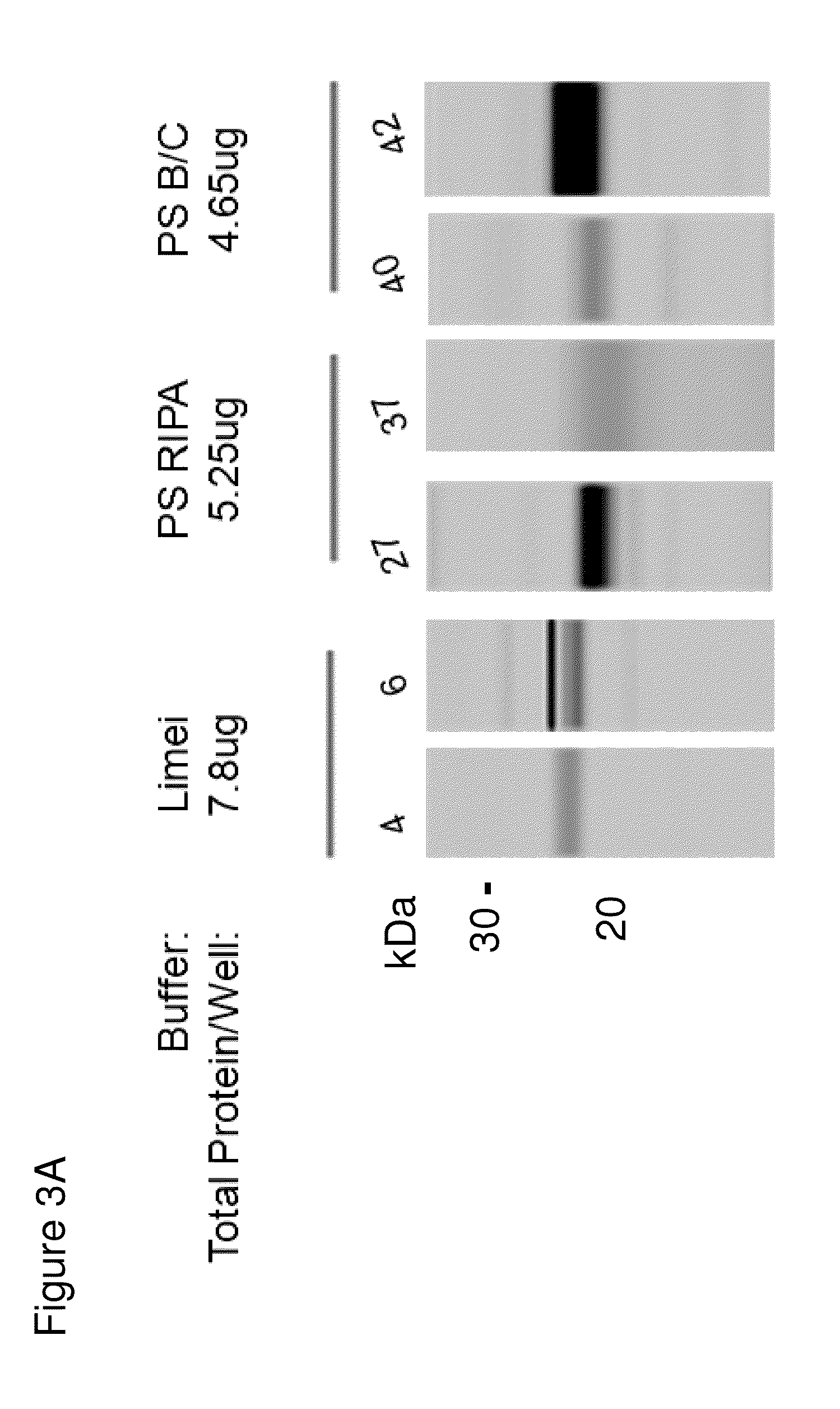
InactiveUS20140134644A1Reduce the amount requiredDisease diagnosisDetection of post translational modificationsPhosphorylationSjoegren's syndrome
The invention features methods and kits for determining the presence of, or a predisposition to develop, Sjögren's syndrome in humans. The invention features methods to detect changes in the levels of one or more LMP-2 protein isoforms, in particular phosphorylated isoforms of LMP-2, and to detect changes in cellular protein phosphorylation and ubiquitination in samples from Sjögren's patients.
Owner:THE GENERAL HOSPITAL CORP
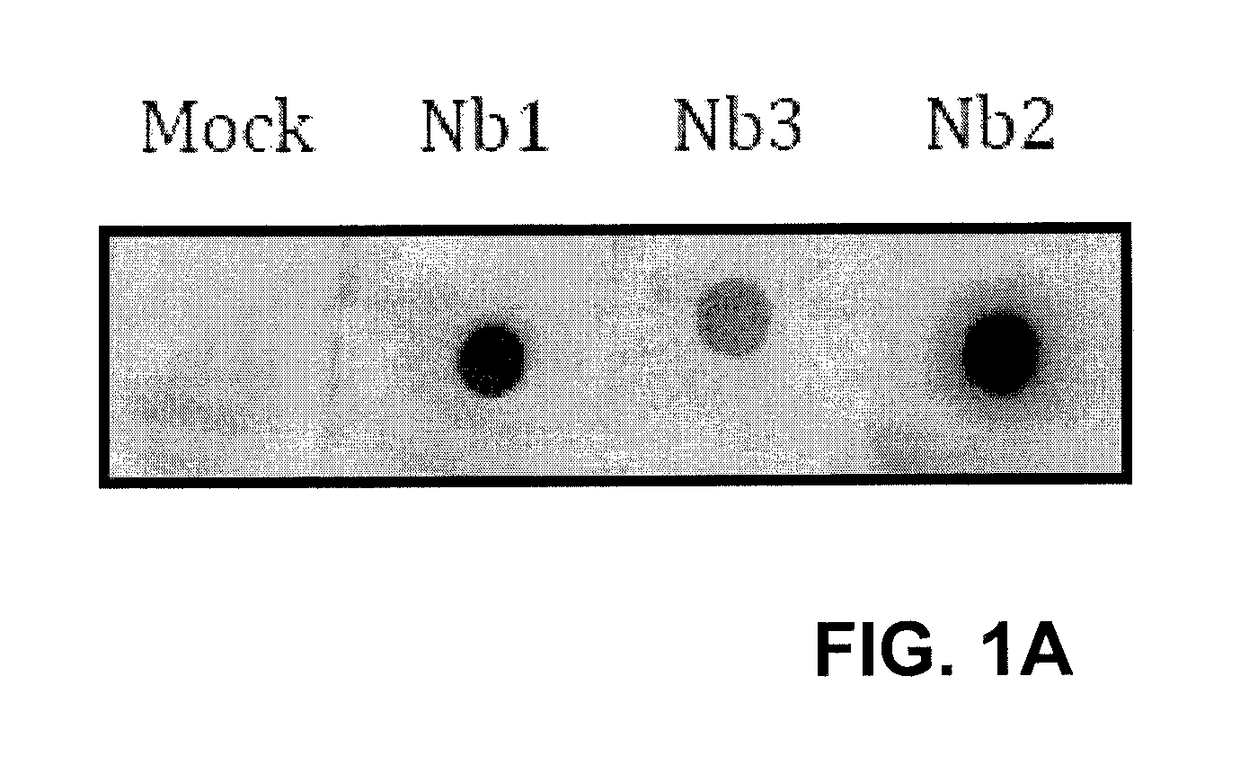
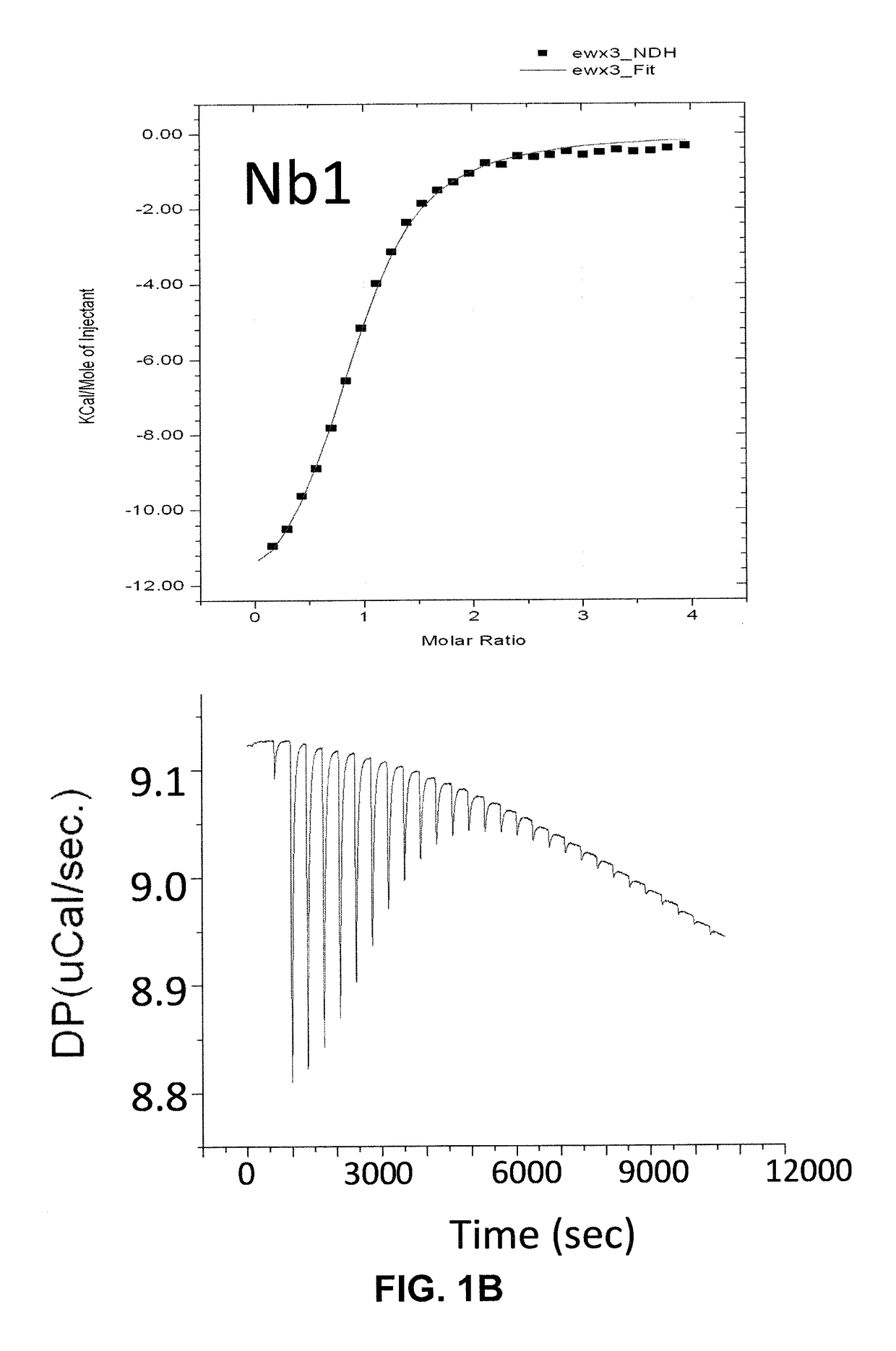
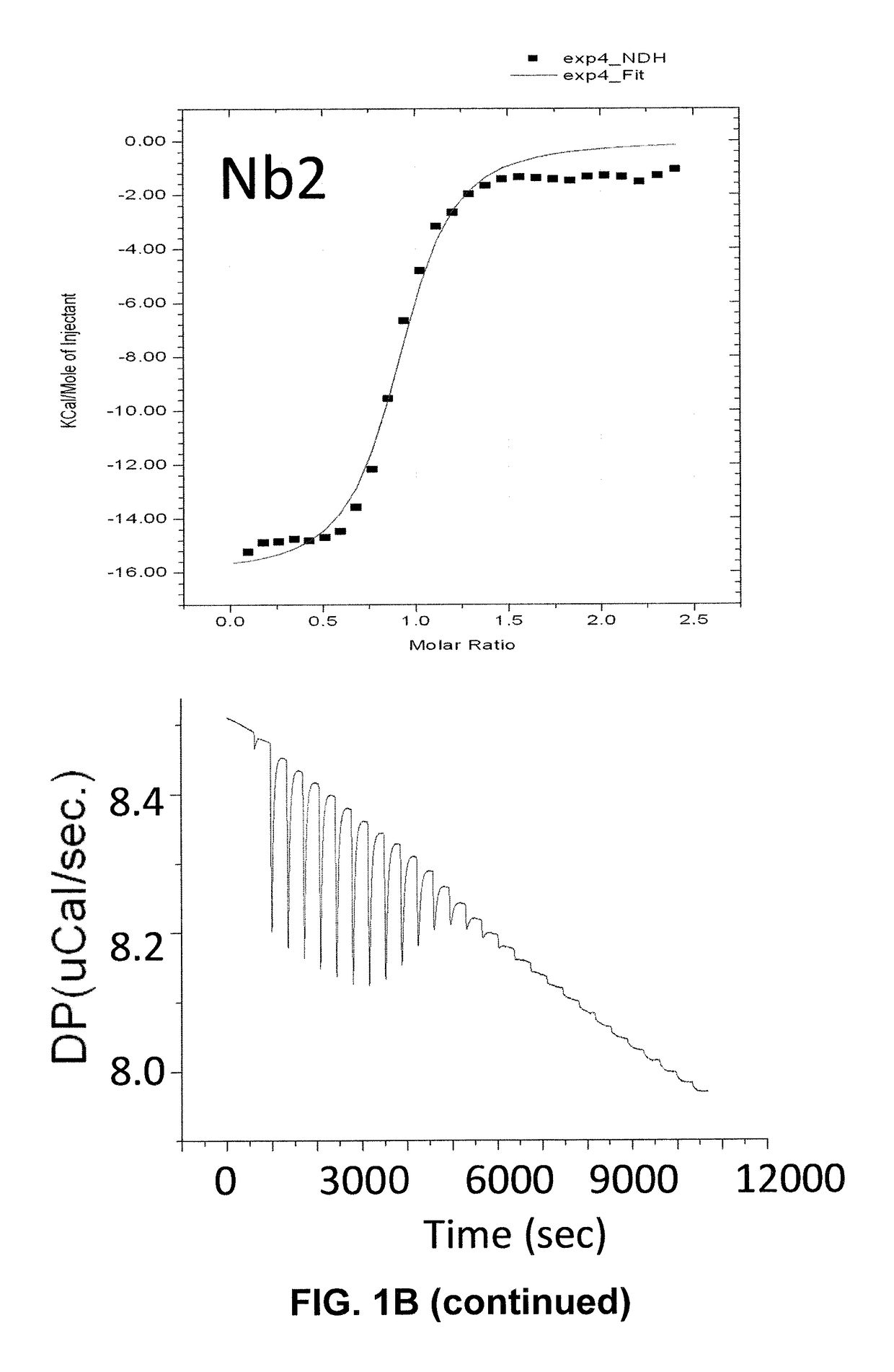
Single domain antibodies against SOD1 and their use in medicine
InactiveUS9862777B2High affinityLower Level RequirementsAntibody mimetics/scaffoldsAntibody ingredientsDiseaseSOD1
The present application relates to the field of single-domain antibodies (also called nanobodies), more particularly single-domain antibodies against SOD1 protein isoforms. It also relates to the use of these nanobodies in medicine. Accordingly, methods to treat a disease using these nanobodies are provided herein. The single-domain antibodies are particularly envisaged for treatment of ALS.
Owner:VLAAMS INTERUNIVERSITAIR INST VOOR BIOTECHNOLOGIE VZW +3
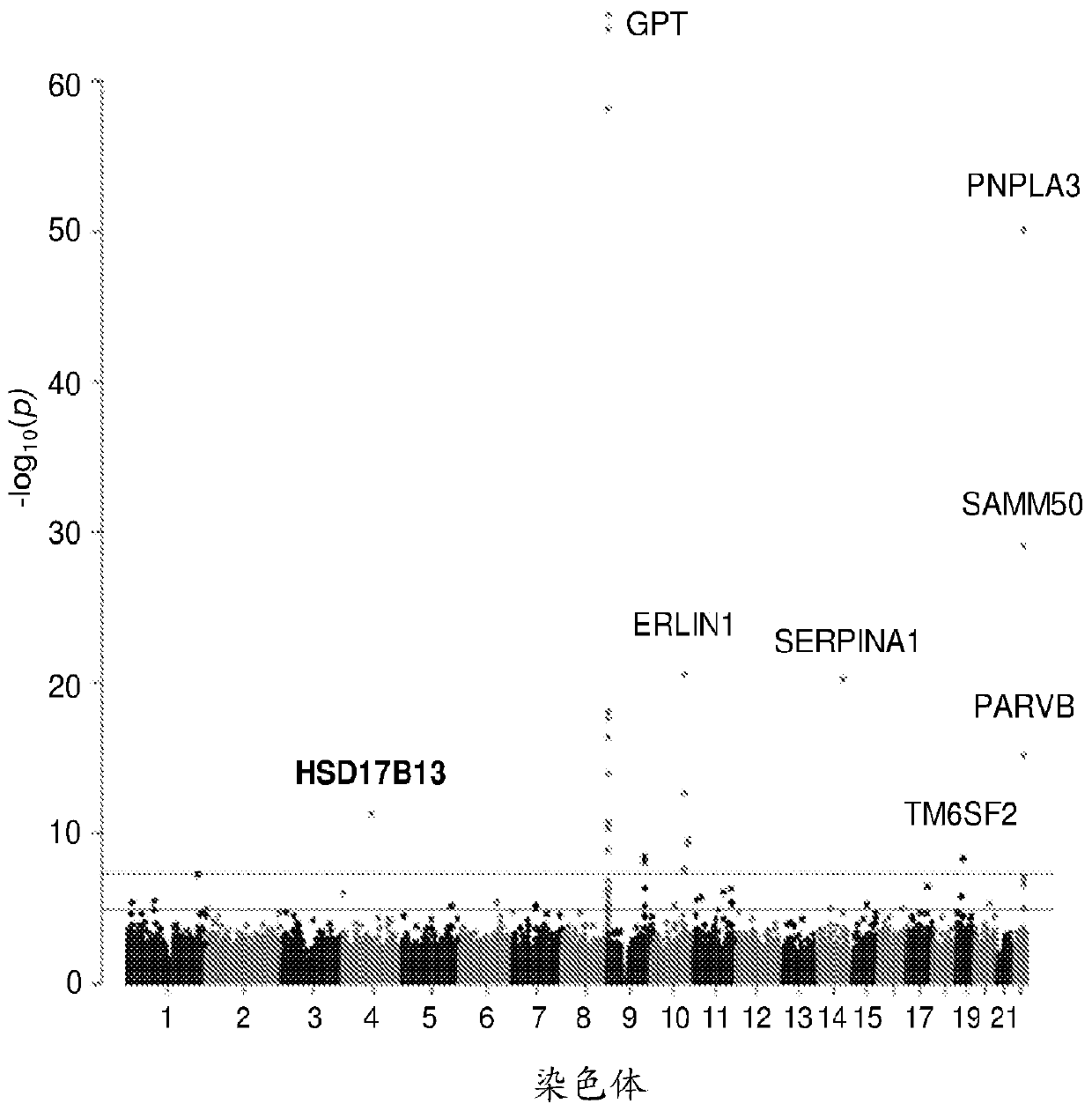
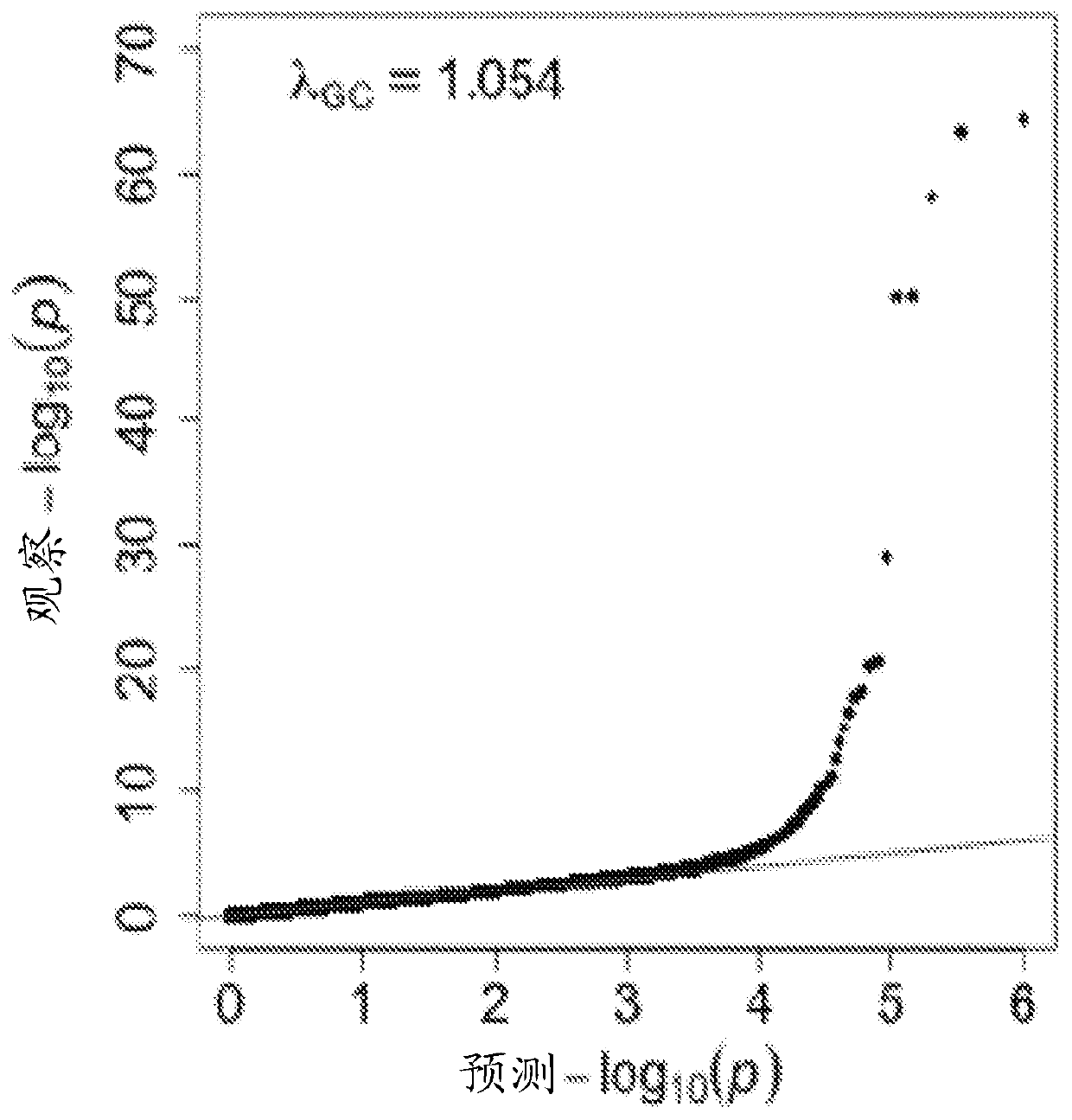
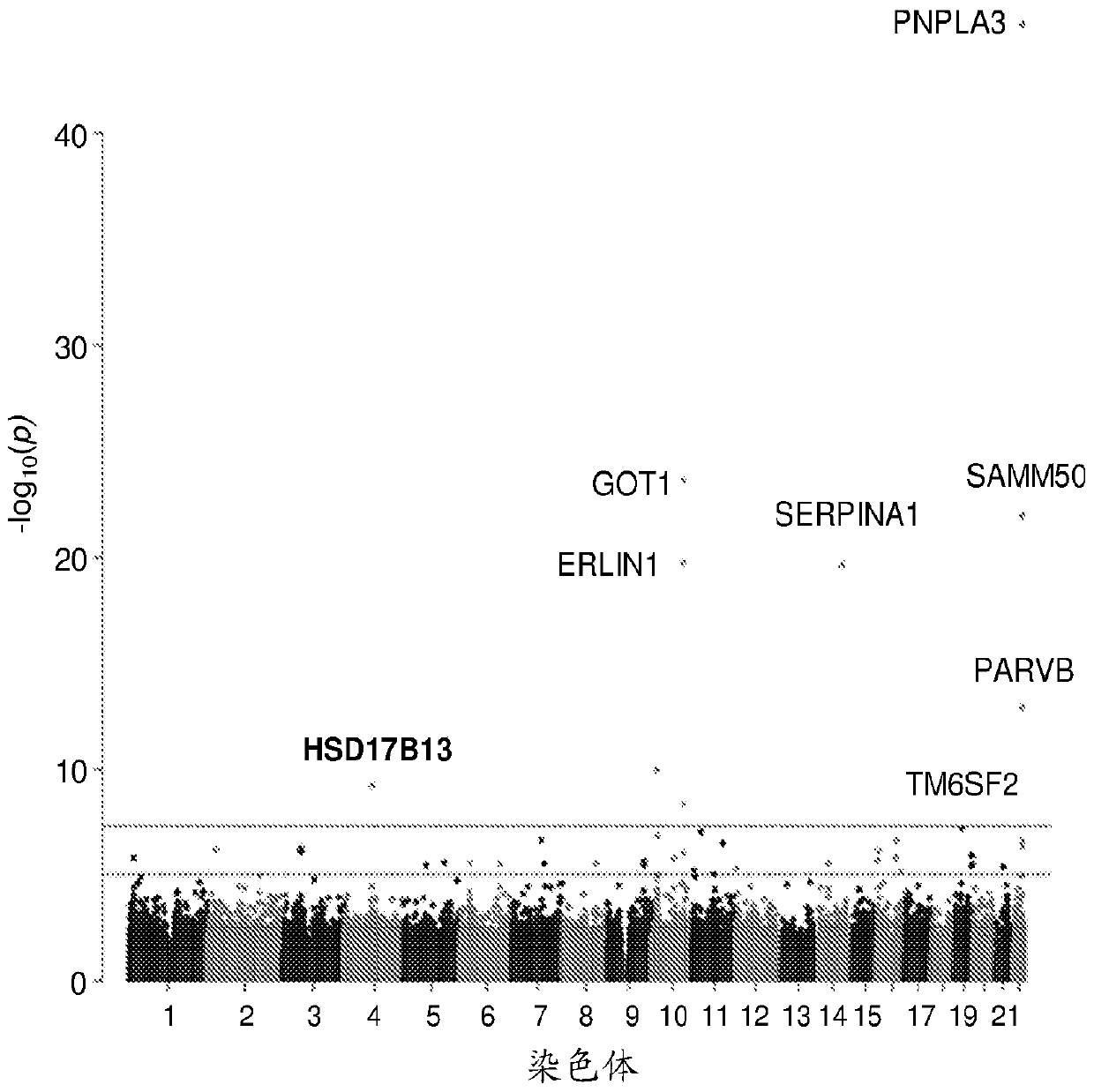
Hydroxysteroid 17-beta dehydrogenase 13 (hsd17b13) variants and uses thereof
Provided are compositions related to HSD17B13 variants, including nucleic acid molecules and polypeptides related to variants of HSD17B13, and cells comprising those nucleic acid molecules and polypeptides. Also provided are methods related to HSD17B13 variants. Such methods include methods for detecting the presence of the HSD17B13 rs72613567 variant in a biological sample comprising genomic DNA,for detecting the presence or levels of any one of variant HSD17B13 Transcripts C, D, E, F, G, and H, and particularly D, in a biological sample comprising mRNA or cDNA, or for detecting the presenceor levels of any one of variant HSD17B13 protein Isoforms C, D, E, F, G, or H, and particularly D, in a biological sample comprising protein. Also provided are methods for determining a subject's susceptibility to developing a liver disease or of diagnosing a subject with liver disease.
Owner:REGENERON PHARM INC
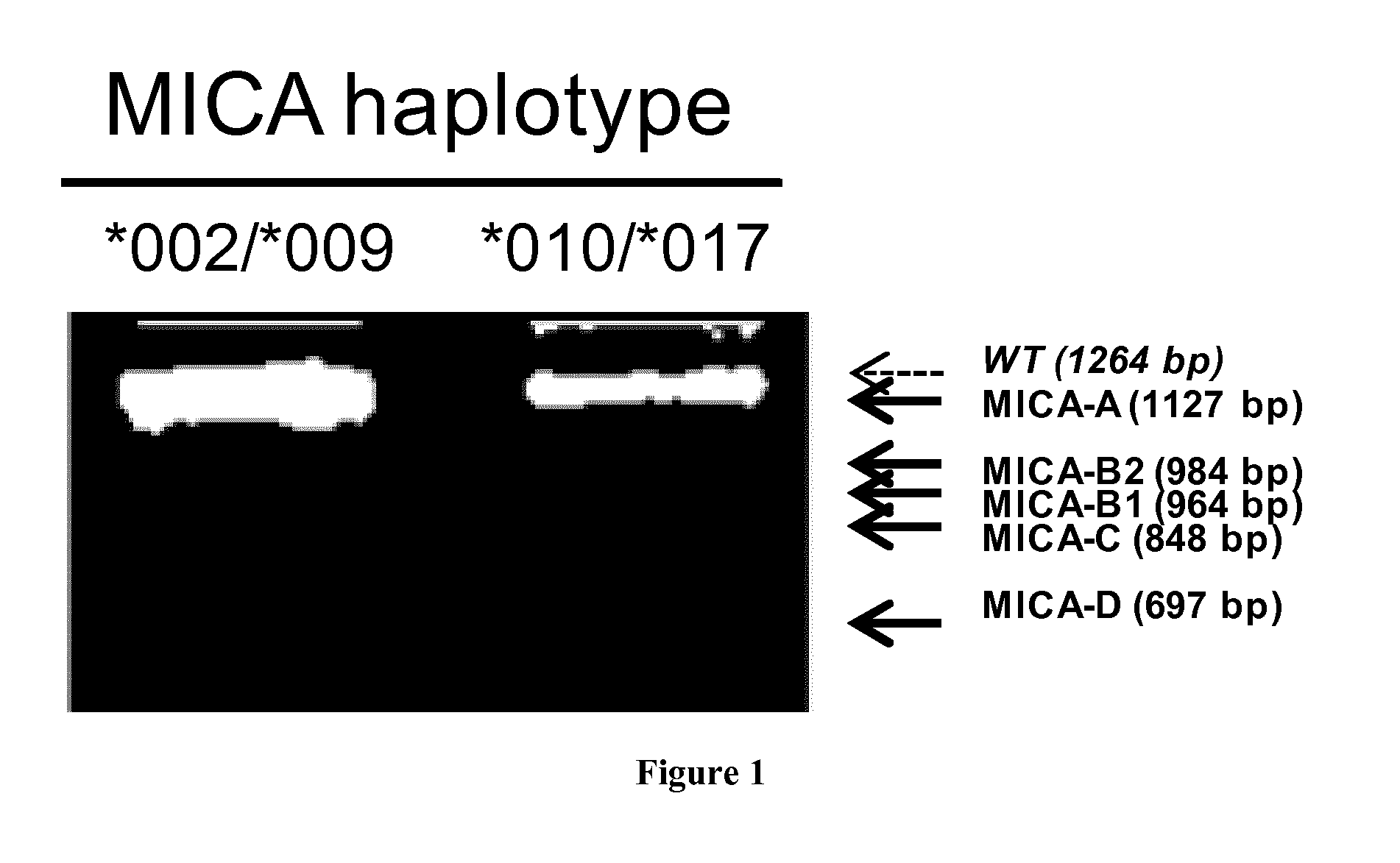
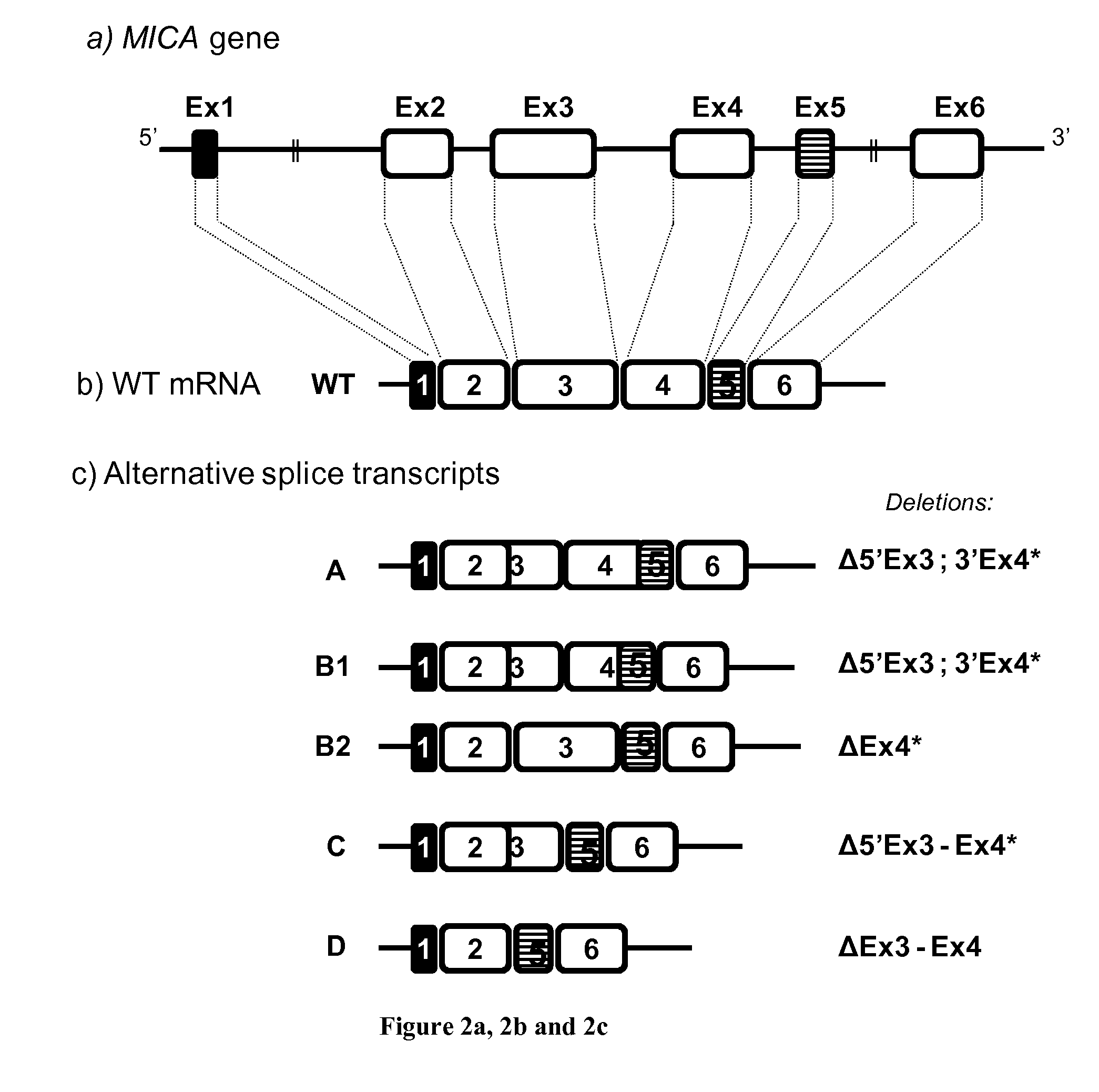
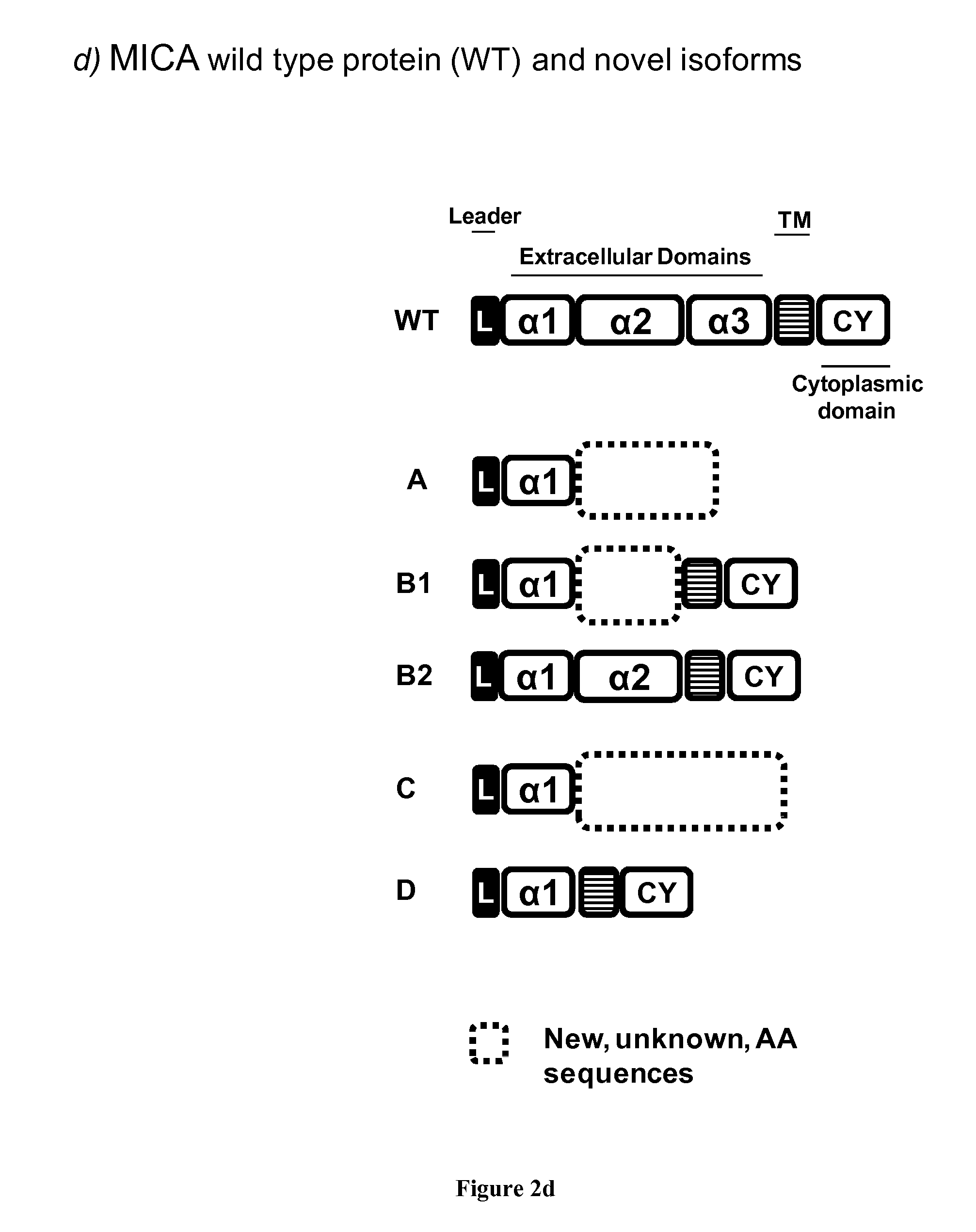
Novel alternative splice transcripts for mhc class i related chain alpha (MICA) and uses thereof
InactiveUS20160176943A1Improve purification effectStable duplexAntibody mimetics/scaffoldsTissue cultureMHC class IAlternative splicing
The present invention relates to novel alternative splice transcripts (AST) for MICA (MHC class I related chain alpha) encoding novel MICA protein isoforms and uses thereof. In particular, the present invention relates to an isolated polypeptide at least 80% of identity with a sequence selected from the group consisting of SEQ ID NO:1 (MICA-A), SEQ ID NO:2 (MICA-B1), SEQ ID NO:3 (MICA-B2); SEQ ID NO:4 (MICA-C) and SEQ ID NO: (MICA-D).
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
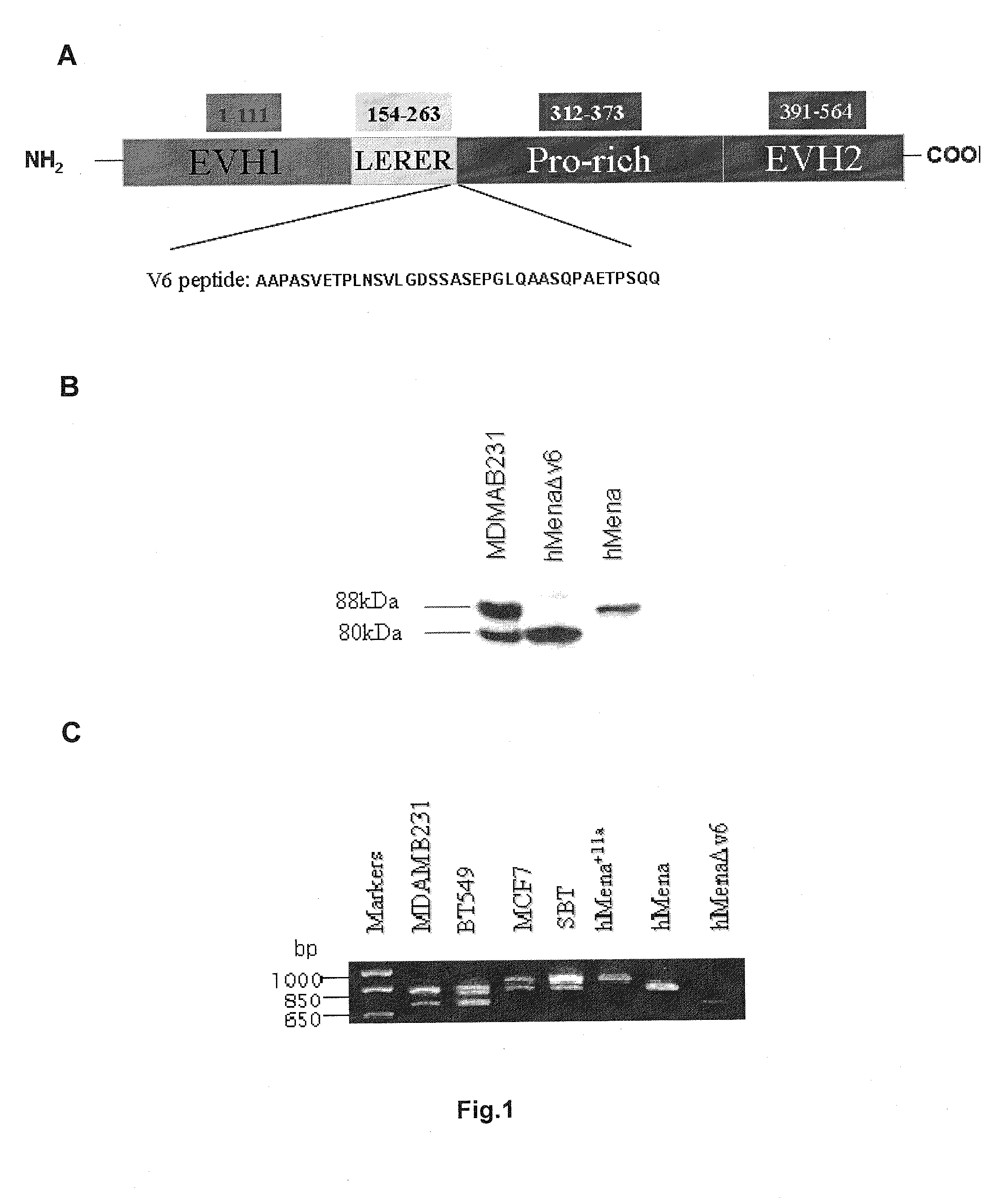
New markers for the epithelial and proliferative or mesenchymal invasive phenotype of human neoplasias
InactiveUS20130183673A1Accurate predictionSugar derivativesMicrobiological testing/measurementMesenchymal phenotypeInvasive phenotype
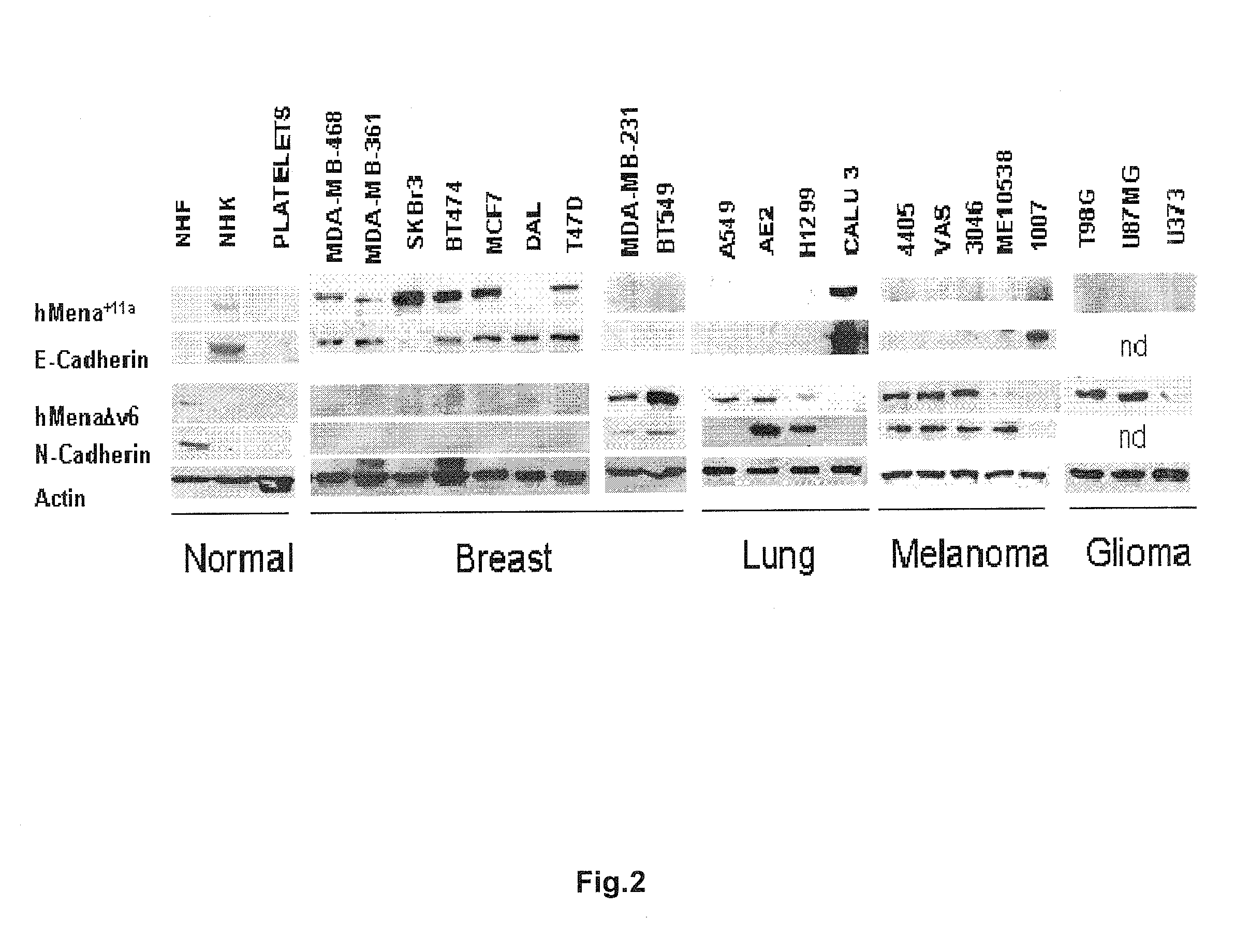
The present invention relates to a new Ena / VASP protein isoform, uses thereof, diagnostic methods and kits comprising the same.
Owner:INSTI FISIOTERAPICI OSPITALERI IFO INST REGINA ELENA PER LO STUDIO E LA CURA DEI TUMORI
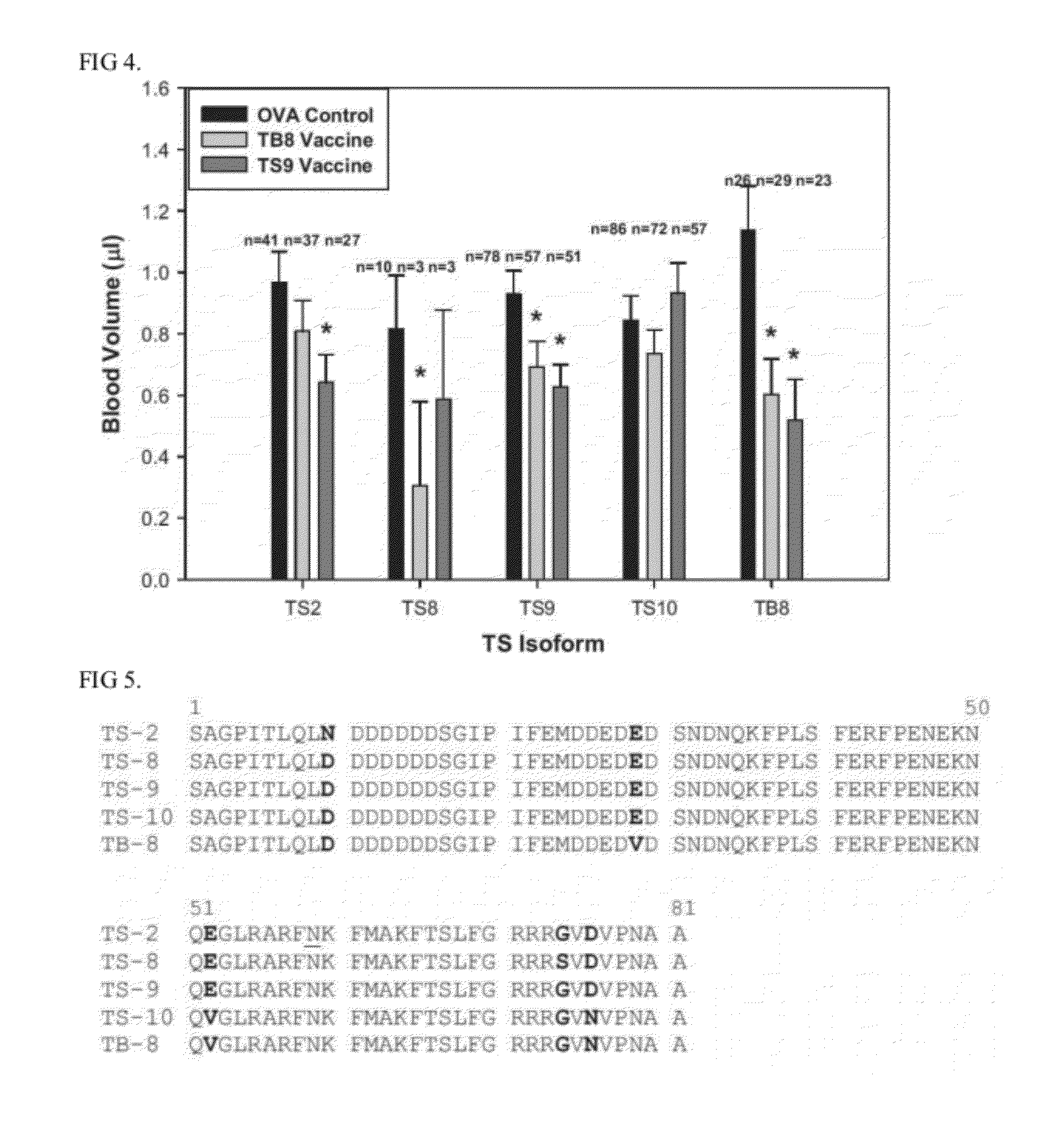
Combination of Protein Forms for Hornfly Vaccination
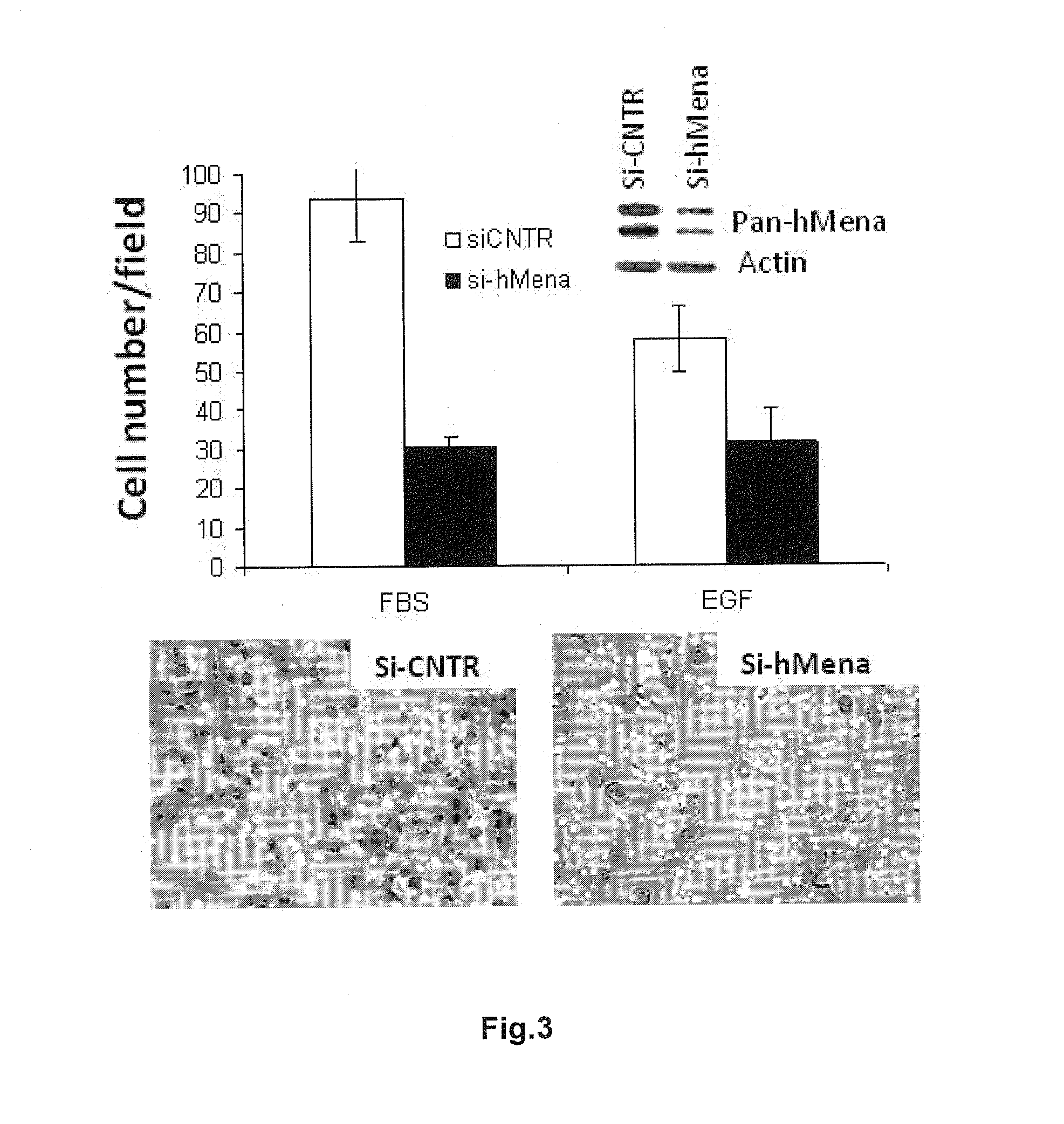
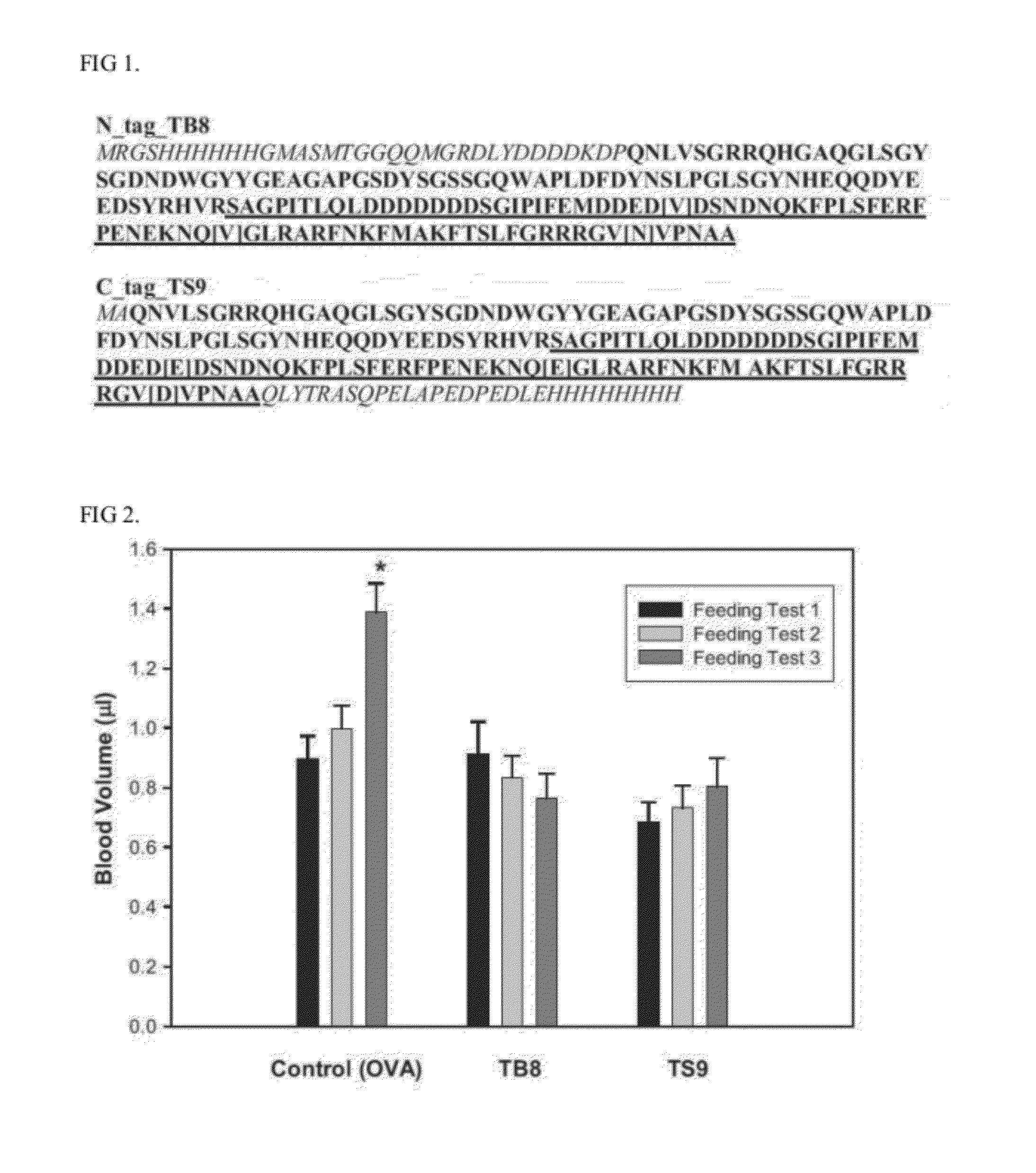
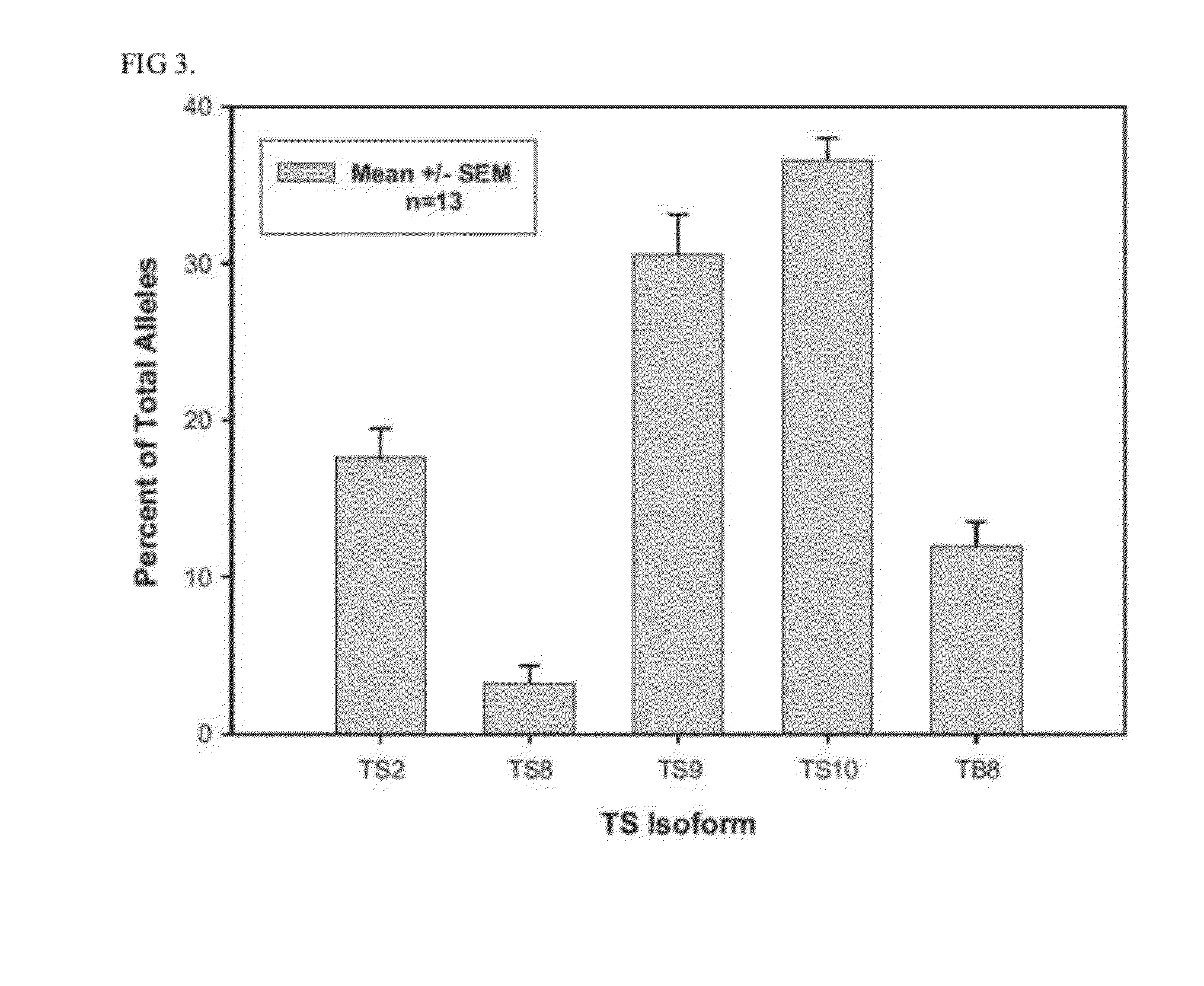
Thrombostasin is an anti-clotting protein found in saliva of Haematobia irritans. Disclosed herein are studies testing blood uptake of horn flies feeding on cattle which confirm the association of ts genotype with blood uptake of horn flies. Blood uptake volumes of homozygous ts10 horn flies were lower than those of other ts genotypes when fed on control cattle. Cattle vaccinated with recombinant protein isoforms rTS9 or rTB8 resisted horn fly feeding by yielding lower blood volumes compared to flies feeding on control cattle. The impact of vaccination varied by ts genotype of flies. Cattle vaccinated with isoforms rTS9 resisted flies of ts2, ts9, and tb8 genotype. Vaccination with isoforms rTB8 produced resistance to ts8, ts9 and tb8 genotype flies. Horn flies of genotype ts10 were not affected with either TS isoforms and fed well on rTS9 and rTB9 vaccinated as on control-vaccinated cattle.
Owner:AUBURN UNIV
Proteins, genes and their use for diagnosis and treatment of vascular response
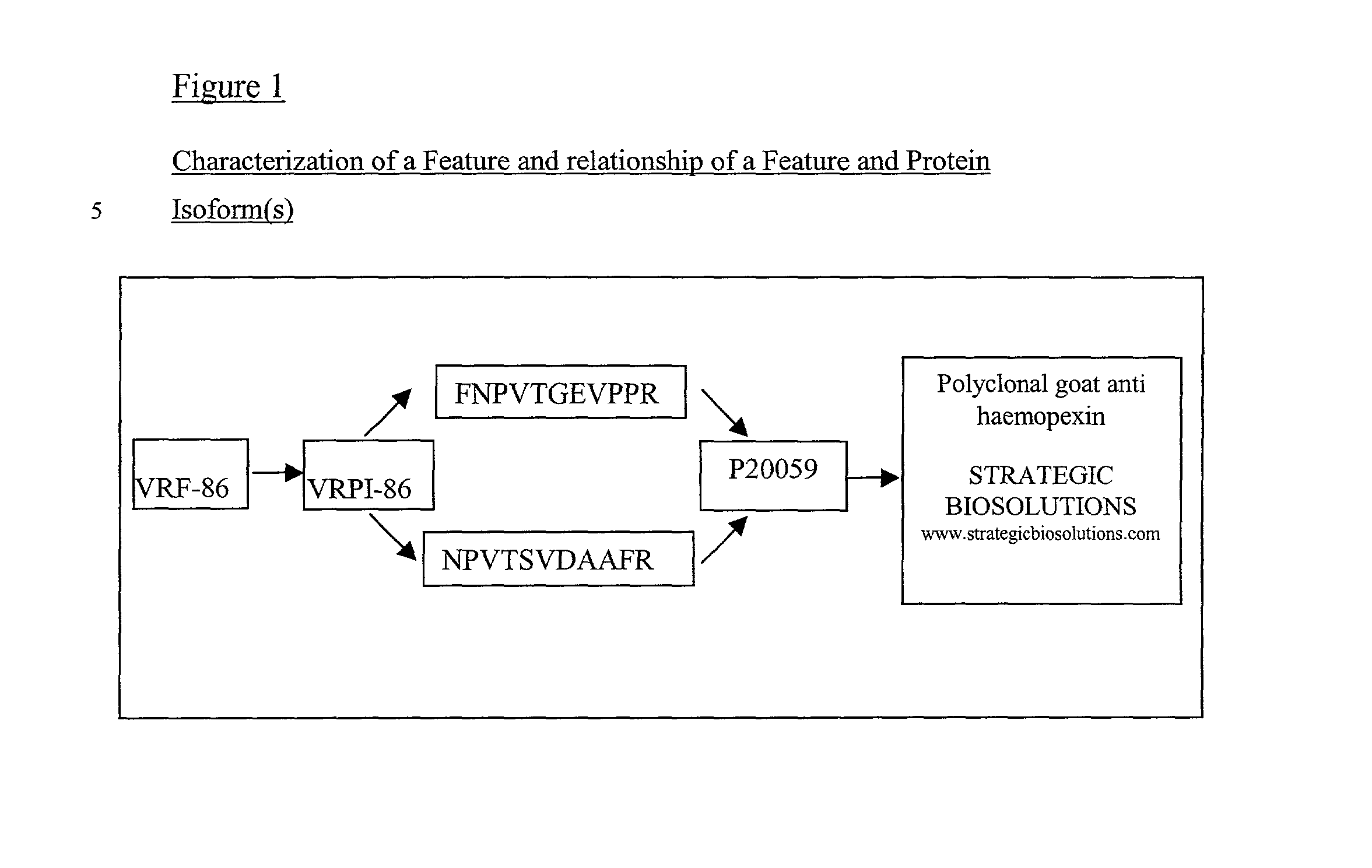
InactiveUS20030049640A1Increase costLow costPeptide/protein ingredientsMicrobiological testing/measurementTherapeutic treatmentBlood plasma
The present invention provides methods and compositions for screening, diagnosis and prognosis of vascular response, for monitoring the effectiveness of vascular response treatment, identifying patients most likely to respond to a particular therapeutic treatment and for drug development. In particular, the screening of drug candidates for their ability to induce a vascular response. Vascular Response-Associated Features (VRFs), detectable by two-dimensional electrophoresis of blood, serum or plasma are described. The invention further provides Vascular Response-Associated Protein Isoforms (VRPIs) detectable in blood, serum or plasma, preparations comprising isolated VRPIs, antibodies immunospecific for VRPIs, and kits comprising the aforesaid.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Protein isoforms of the pif-family and uses thereof
InactiveUS20100223678A1Modulate activityModulate expressionOrganic active ingredientsNervous disorderComparative testTest sample
A method for screening for or diagnosis or prognosis of a neurological disorder associated with de-regulated glutamate signalling in a subject, for determining the stage or severity of such a neurological disorder in a subject, for identifying a subject at risk of developing such a neurological disorder, or for monitoring the effect of therapy administered to a subject having such a neurological disorder, said method comprising:(a) analyzing a test sample of body fluid or tissue from the subject said sample comprising at least one Protein Isoform selected from the following Protein Isoform Families: PIF-1, PIF-2, and PIF-3 in a detectable amount; and(b) comparing the abundance of said Protein Isoform(s) in the test sample or the abundance of said Protein Isoform(s) relative to another Protein Isoform with the abundance or relative abundance of said Protein Isoform(s) in a test sample from one or more persons free from neurological disorder, or with a previously determined reference range for that Protein Isoform in subjects free from neurological disorder, wherein a diagnosis of or a positive result in screening for or a prognosis of a more advanced condition of said neurological disorder is indicated by (i) decreased abundance or relative abundance of PIF-1 and / or (ii) increased abundance or relative abundance of PIF-2 and / or (iii) decreased abundance or relative abundance of PIF-3.
Owner:OXFORD GENOME SCI UK
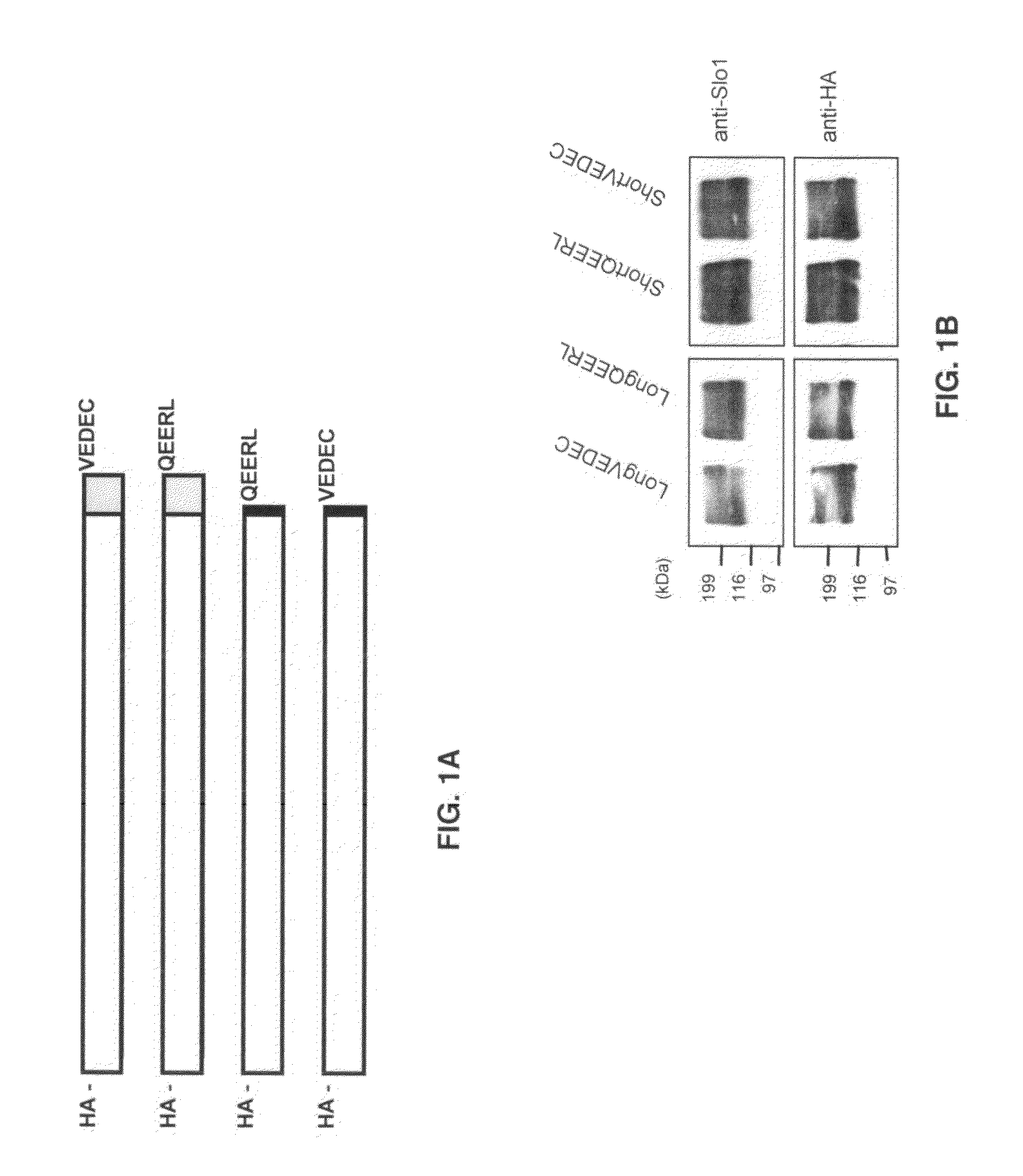
Small peptide modulators of potassium channel trafficking
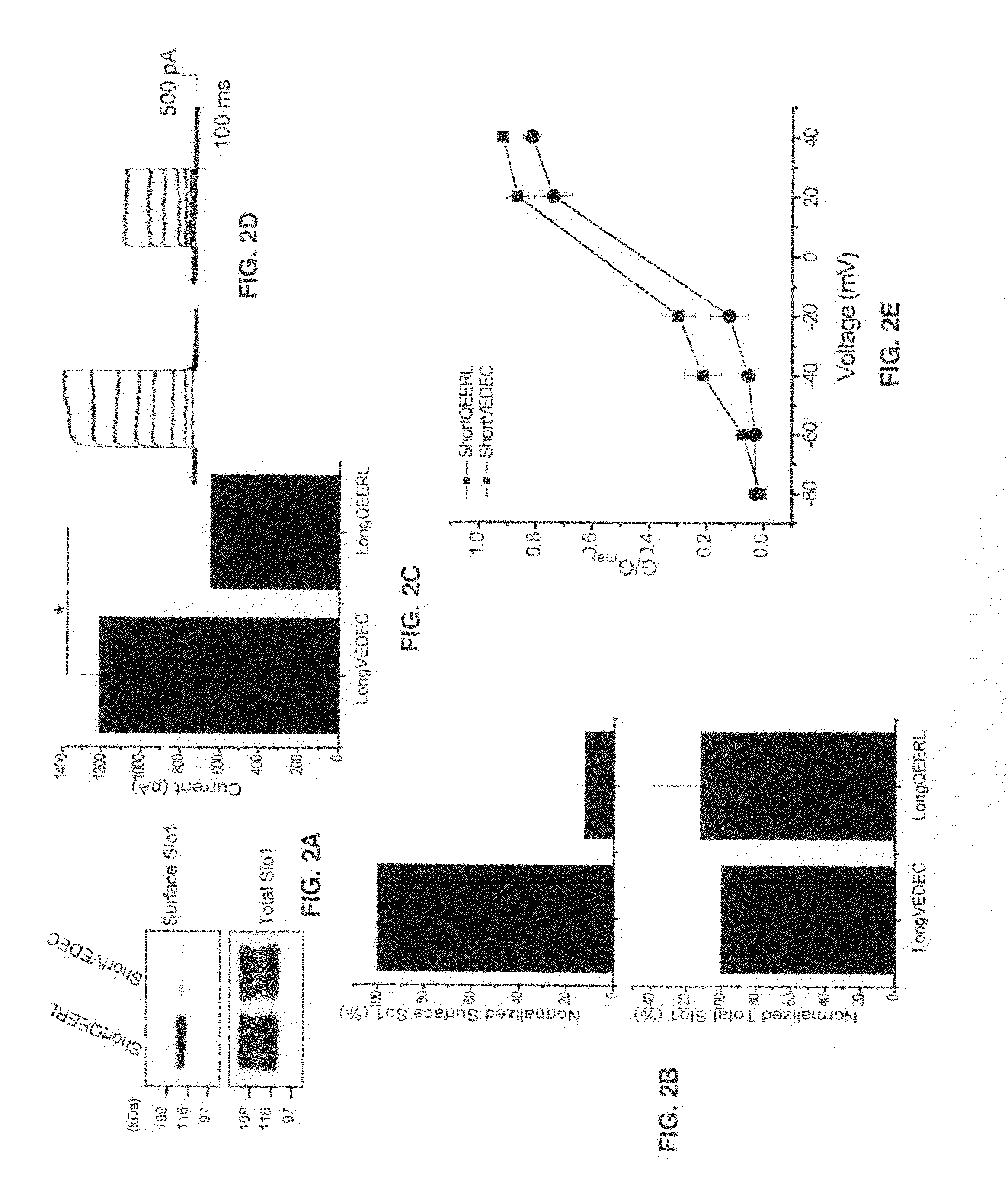
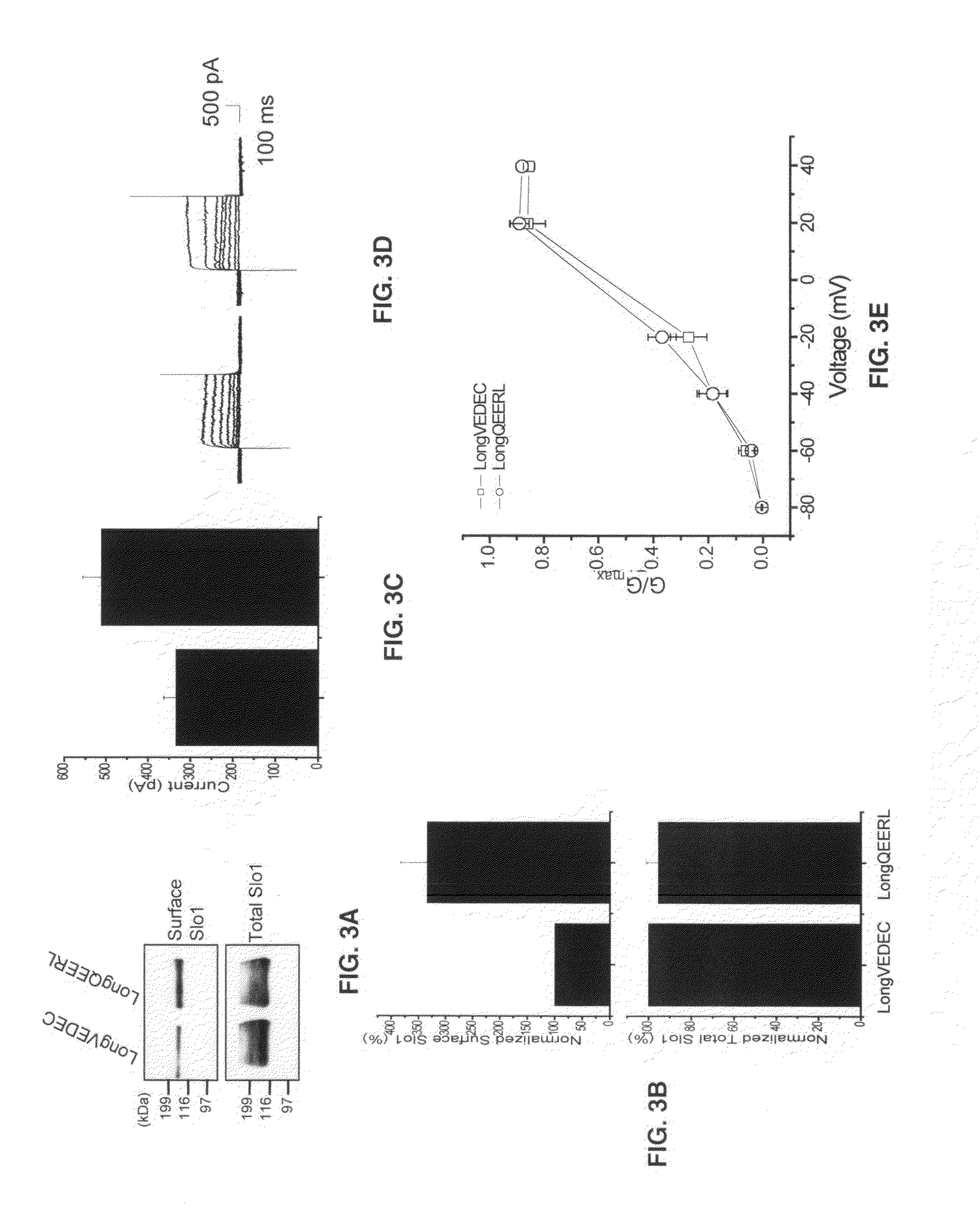
Provided herein are peptide modulators of ion channels. Specifically, the peptide modulators comprise the amino acid sequence VEDEC wherein V is valine, E is glutamate, D is aspartate, C is cysteine. In certain embodiments, the modulator is attached to the C-terminal end of Slo1 protein isoform. The present invention also claims conjugations of the first valine that make the peptide modulator more membrane permeable, such as myristoyl moieties and arginine-rich cell penetrating peptides. The present invention contemplates use of the peptide modulators in the treatment of diseases / malfunctions such as epilepsy, chronic pain, migraine, asthma, chronic obstructive pulmonary disease, urinary incontinence, hypertension, erectile dysfunction, irritable bowel syndrome, renal disorders of electrolyte imbalance, and possibly in certain kinds of cancer.
Owner:UNIV HOUSTON SYST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com