14-3-3 Antagonists for the Prevention and Treatment of Arthritis
a technology of 1433 proteins and antagonists, applied in the field of arthritis prevention and treatment, can solve the problems of permanent structural damage, swelling of the joint area, and substantial loss of mobility, and achieve the effect of reducing the effectors of arthritis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
14-3-3 Eta Immunogen Sequences and Anti-14-3-3 Eta Antibodies
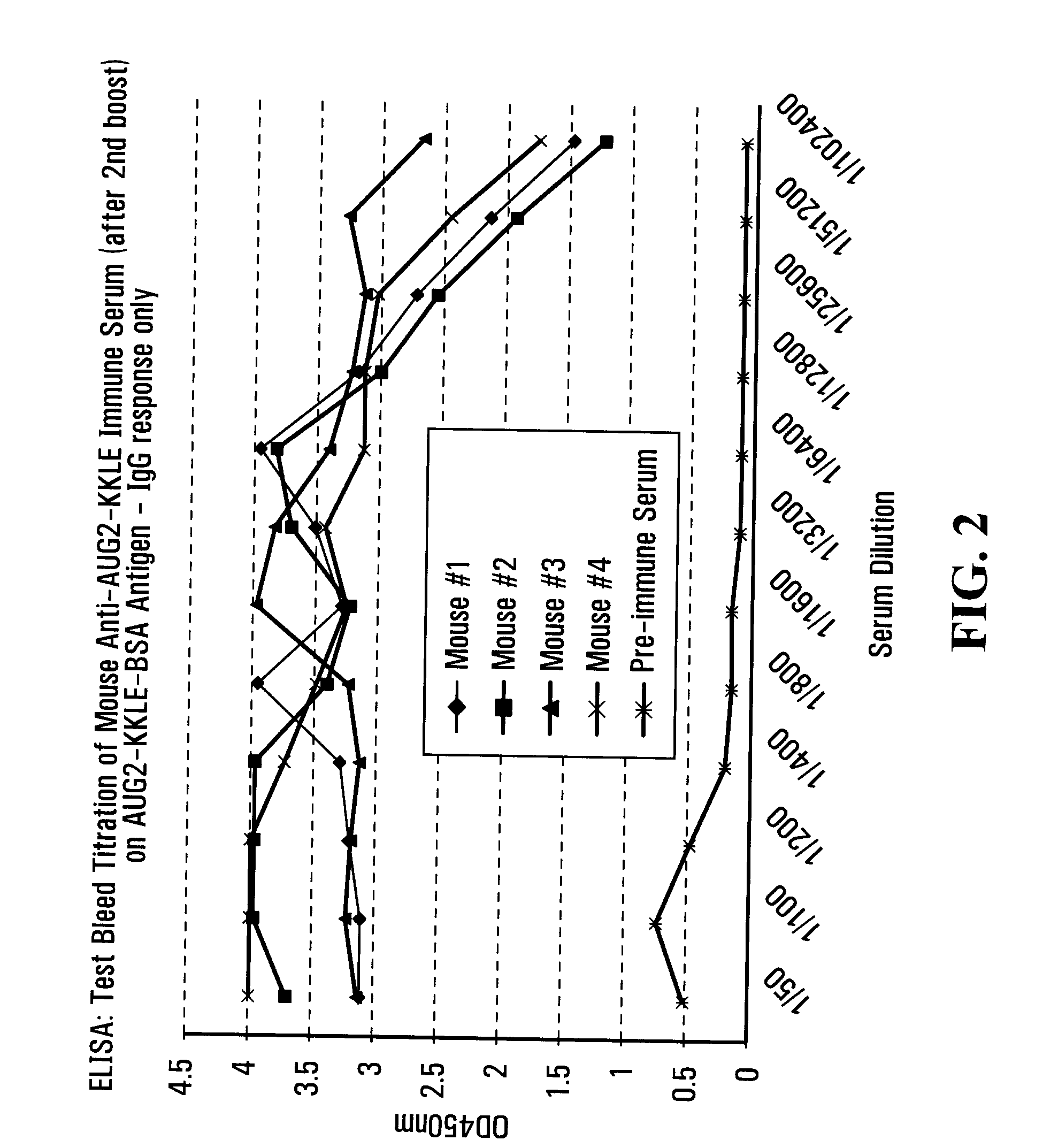
[0238]To prepare monospecific anti-14-3-3 eta antibodies, various peptides, 8 to 15 amino acids in length, were selected based on our own criteria. These peptides, as well as full-length recombinant native (untagged) 14-3-3 eta were used as immunogens in the production of monoclonal antibodies. A protein sequence alignment for the 7 isoforms of 14-3-3 is shown in FIG. 4 (14-3-3 gamma (SEQ ID NO: 64, 14-3-3 eta (SEQ ID NO:63), 14-3-3 alpha / beta (SEQ ID NO: 71), 14-3-3 zeta (SEQ ID NO:72), 14-3-3 theta (SEQ ID NO:73), 14-3-3 sigma (SEQ ID NO:74), and 14-3-3 epsilon (SEQ ID NO:75)).
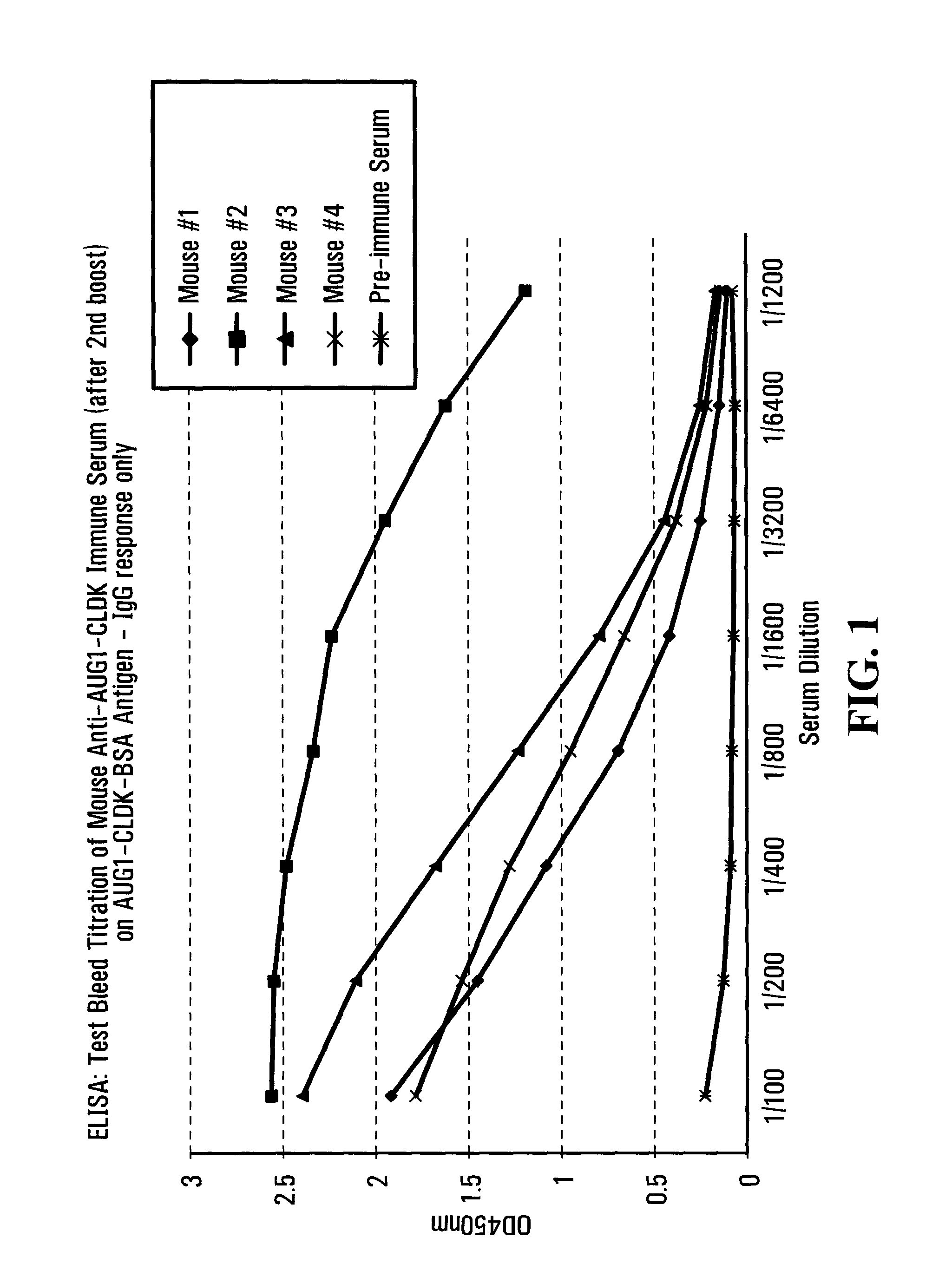
[0239]Immunogen #1: C-LDKFLIKNSNDF (SEQ ID NO:76) (Amino Acid Sequence 104-115; “AUG1-CLDK”). A peptide corresponding to a segment of human 14-3-3 eta residues 104-115 was modified by addition of an N-terminal cysteine moiety for conjugation to carrier, and replacement of internal cysteine-112 moiety to avoid formation of internal disulphide bonds....
example 2
Testing the Cross-Reactivity of Tissue Culture (TC) Supernatants from Hybridoma Clones Using Biotinylated 14-3-3 Isoforms as Bait in a Capture ELISA
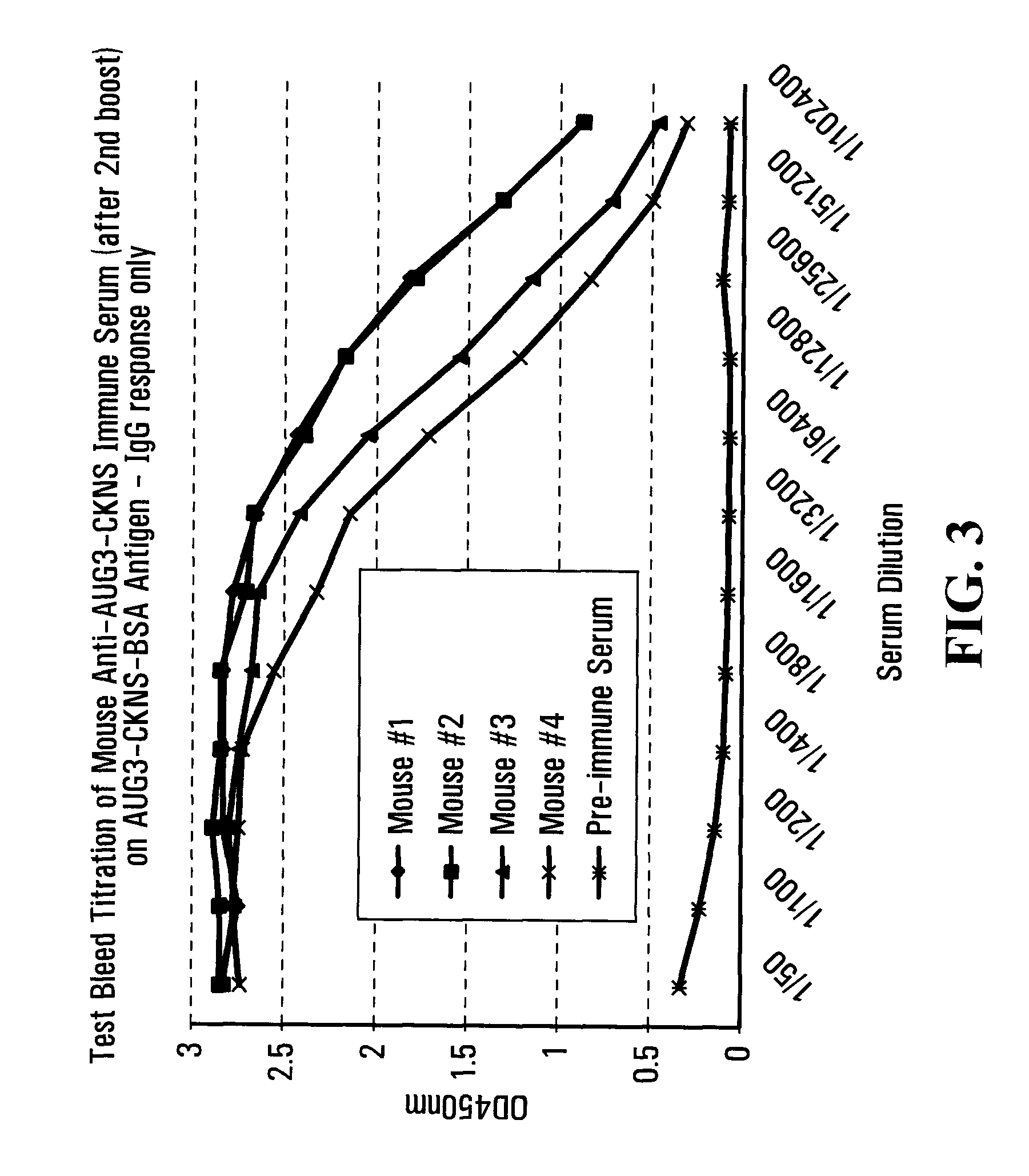
[0250]We have utilized a custom capture ELISA using the seven 14-3-3 isoforms as “bait” to determine whether any of the hybridoma clones that we have produced cross-react or recognize any of the six isoforms other than 14-3-3 Eta (η). As is evidenced by the representative data presented in Table 4, four of the selected hybridoma clones (AUG3-CKNS-2D5, AUG3-CKNS-7F8, AUG3-CKNS-7H8, AUG4-ETA-8F10) bind to and recognize 14-3-3 Eta at two serial dilutions, but do not bind with or cross-react with any of the other 14-3-3 isoforms, even at the lower dilution tested. This data clearly demonstrates that these clones are highly specific for 14-3-3 Eta (η). By contrast, one clone, AUG3-CKNS-4F10, binds with or cross-reacts with three other 14-3-3 isoforms, mainly 14-3-3 gamma, beta and zeta respectively. Taken together, these data indicate that ou...
example 3
14-3-3 Expression in Synovial Fluid and Serum of RA Affected Patients
[0252]The levels of the different isoforms of 14-3-3 proteins—β, γ, ε, η, τσ and ξ—in pooled patient synovial fluid (SF) and serum (PS) samples were analyzed by western analysis using keratinocyte cell lysate (K) as a positive control. Only the η and γ isoforms were detected in SF samples, and stained with greater intensity compared to PS. Articular joint synovial fluid samples from 17 RA patients who presented with active synovitis, but had not yet received anti-TNF therapies also exhibited consistent expression of the η isoform of 14-3-3 (data not shown). All patients had a disease activity score (DAS) greater than 6.0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com