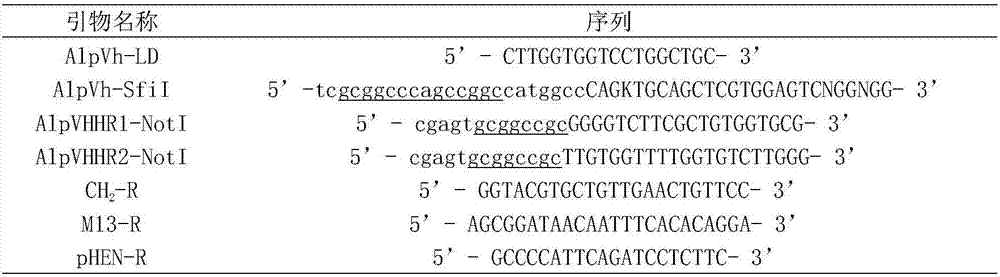
Patents
Literature
64 results about "Myc-tag" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A myc tag is a polypeptide protein tag derived from the c-myc gene product that can be added to a protein using recombinant DNA technology. It can be used for affinity chromatography, then used to separate recombinant, overexpressed protein from wild type protein expressed by the host organism. It can also be used in the isolation of protein complexes with multiple subunits.
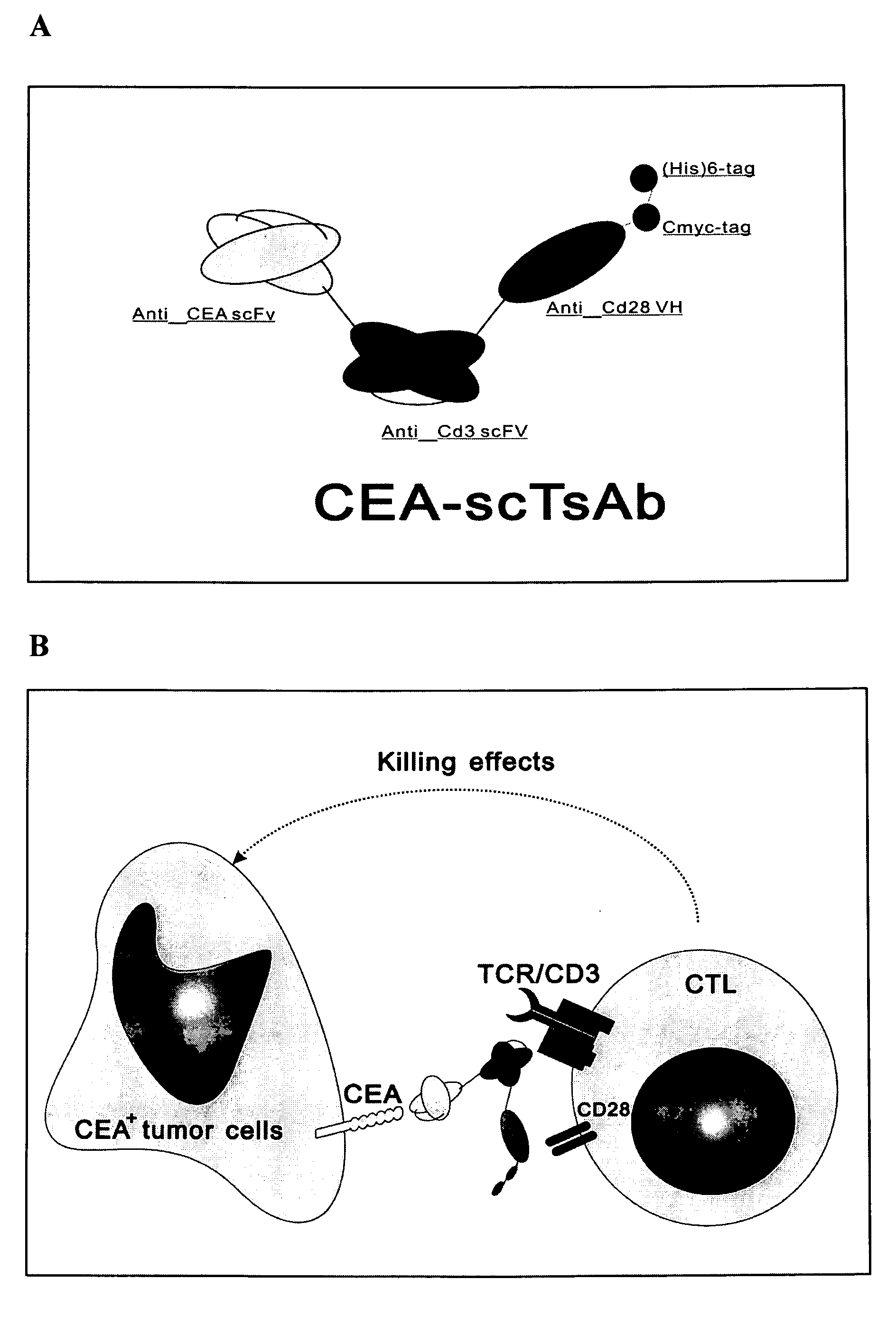
Recombining single chained three specific antibodies of anti CCA, anti CD 3, anti CD 28 through genetic engineering
InactiveCN1563092AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntiendomysial antibodiesSingle-Chain Antibodies
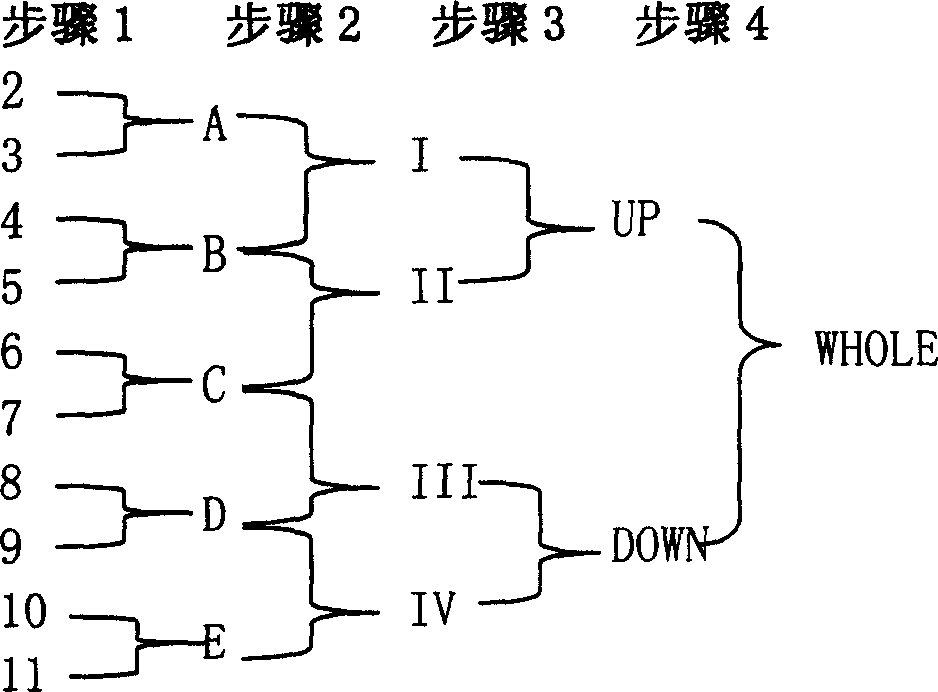
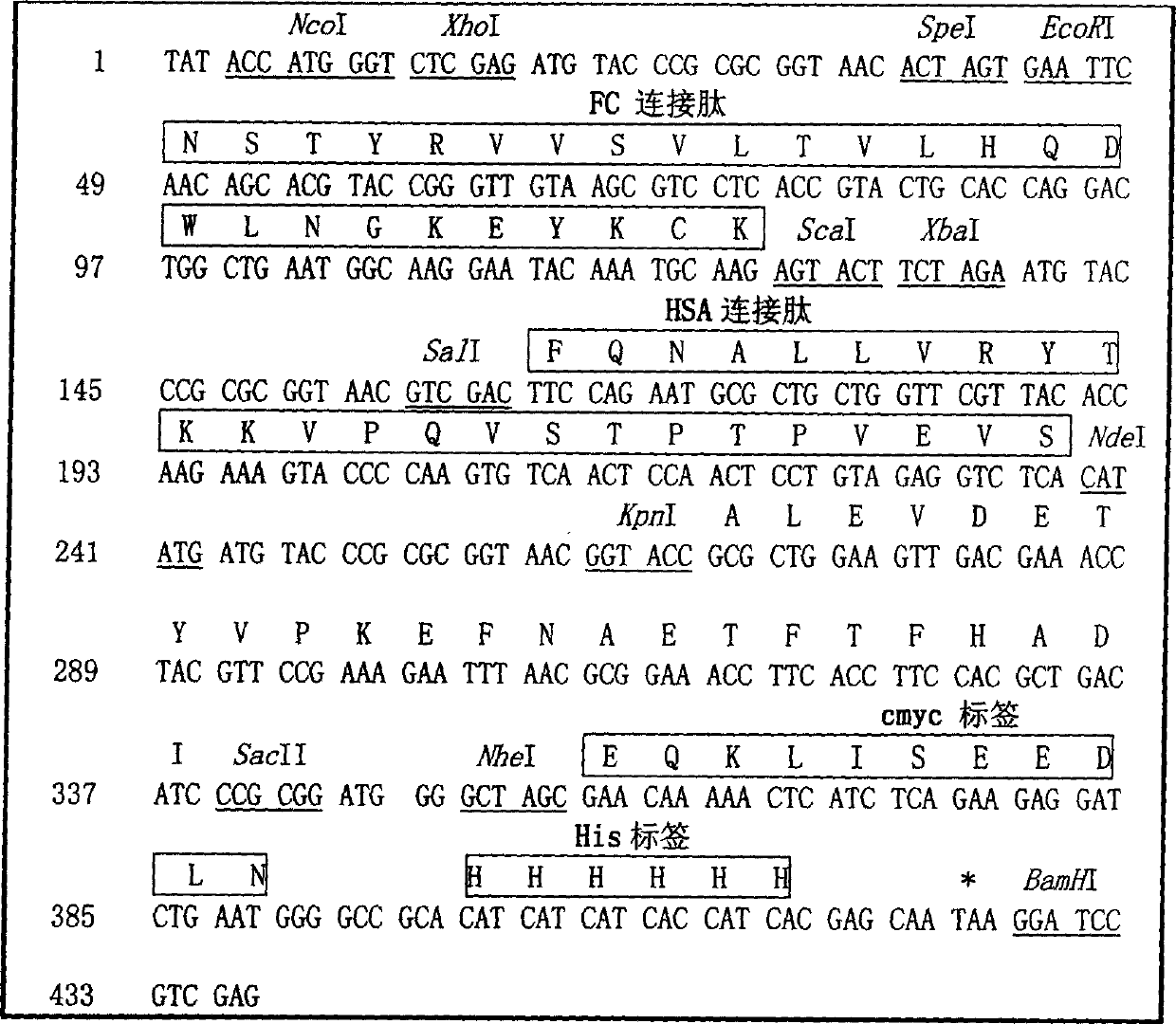
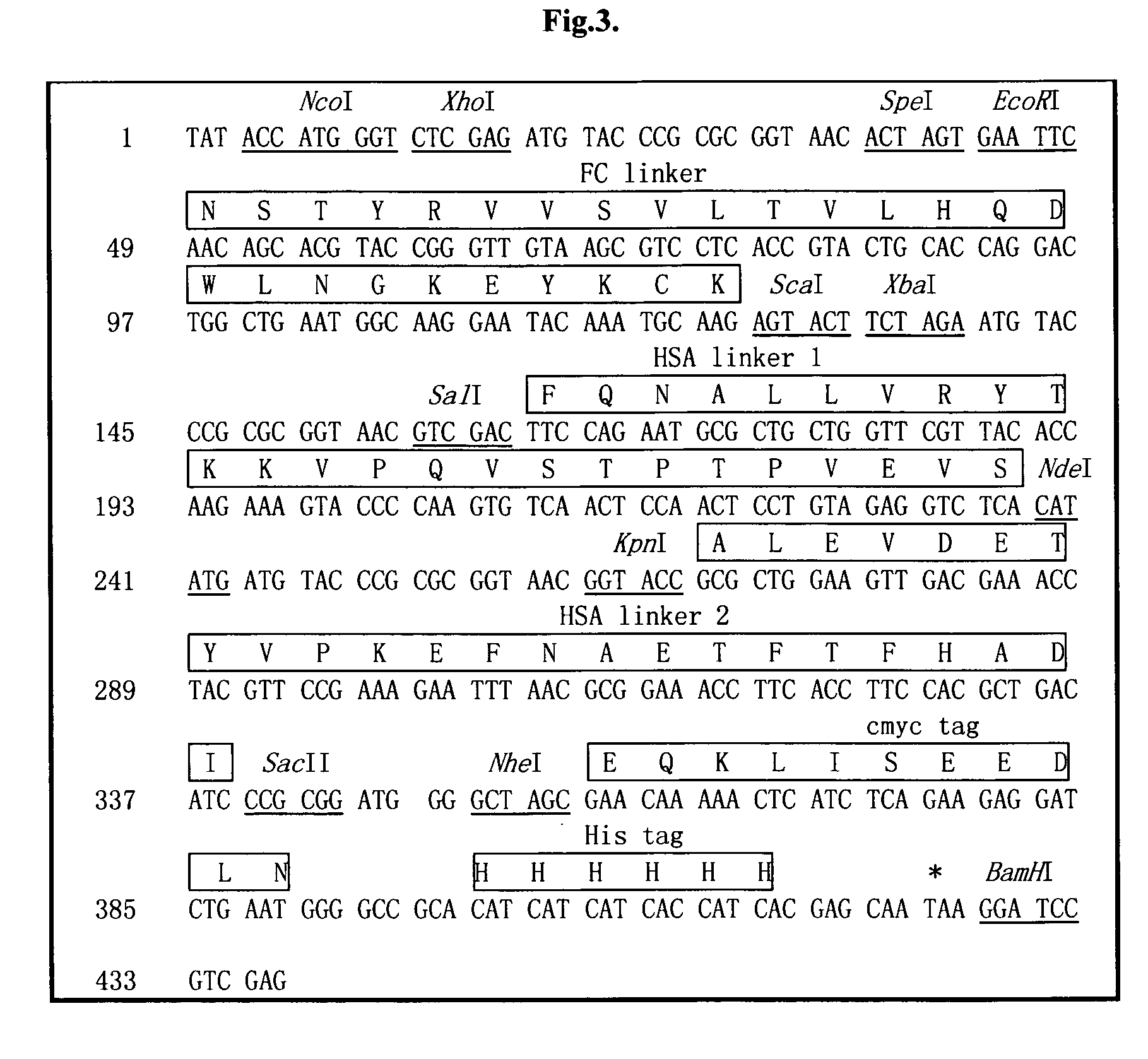
The invention relates to a recombinant single chain tri-specific antibody, and it is characterized by that it is made by successively connecting antibody of antineoplastic related antigen, FC onnecting peptide, antihuman CD3 antibody, HSA connecting peptide and antihuman CD28 antibody. More specifically, said invention relates to a recombinant single chain tri-specific antibody; CEA-TsAb of anti-CEA, anti-CD3 and anti-CD28. Said antibody is made up by using three antibody fragments (unti-CEA single chain antibody, anti-CD3 single chain antibody and anti-CD28 single chain antibody), utilizing two connecting peptides (FC connecting peptide and HSA connecting peptide) and series-connecting them together, and on its C end C-myc tag and (His)6-tag are added. Said invention also relates to a method for constructing expressing and purifying said antibody, including exonic DNA sequence of said antibody, expression vector and host cell containing said vector.
Owner:弘业新创抗体技术股份有限公司
Gene Engineering Recombinant Anti-CEA, Anti-CD3, And Anti-CD28 Single-Chain Tri-Specific Antibody
InactiveUS20090117108A1Increase production costHigh expressionBacteriaSugar derivativesAntibody fragmentsSingle-domain antibody
The invention is related to a recombinant single-chain tri-specific antibody made from anti-Tumor Associated Antigen (TAA) antibody, FC interlinker, anti-CD3 antibody, HSA interlinker and anti-CD28 antibody in turn. Particularly, the invention relates to an anti-CEA, anti-CD3, anti-CD28 recombinant single-chain tri-specific antibody, CEA-scTsAb, which was constructed with three tandem antibody fragments (anti-CEA scFv, anti-CD3 scFv and anti-CD28 single-domain antibody) linked by two interlinkers (FC interlinker, HSA interlinker), and could be appended by C myc tag or histidine tag ((His)6-tag) at the C terminal. It also concerns a method for construction, expression and purification of the antibody. It also offers the encoded DNA sequence of the antibody, expression vectors and host cells for the vectors.
Owner:WANG XIANGBIN +5
Indirect ELISA detection kit of sheep echinococciosis Eg95 protein antibody
InactiveCN107831319AStrong specificityIncreased sensitivityBiological testingPichia pastorisPositive control
The invention discloses an indirect ELISA detection kit of sheep echinococciosis Eg95 protein antibody. The invention is characterized in that the kit comprises an echinococcus granulosus recombinantantigen Eg95 protein precoated ELISA plate, a serum diluent, an enzyme-labeled conjugate diluent, a positive control solution, a negative control solution, an enzyme-labeled conjugate, a washing solution, a substrate developing solution and a stopping solution. During the preparation process of the echinococcus granulosus recombinant antigen, a His tag sequence and a Myc tag sequence are respectively connected to the front and back of the Eg95 gene; then, EcoR I and Xho I are adopted for simultaneous digestion of a PCR product and an eukaryotic expression vector pPIC9K, the target fragment andthe vector are connected after gel extraction, recombinant plasmid pPIC9K-(His-Eg95-Myc) is constructed, the recombinant plasmid is transformed into pichia pastoris GS115 strain, and the recombinantantigen Eg95 is expressed by the induction of methanol and purified by the use of Ni-NTA. The sheep echinococciosis antibody detection kit obtained by the adoption of the dual-tag sequence and eukaryotic expression vector system has strong specificity, high sensitivity and good repeatability, and is easy to operate.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
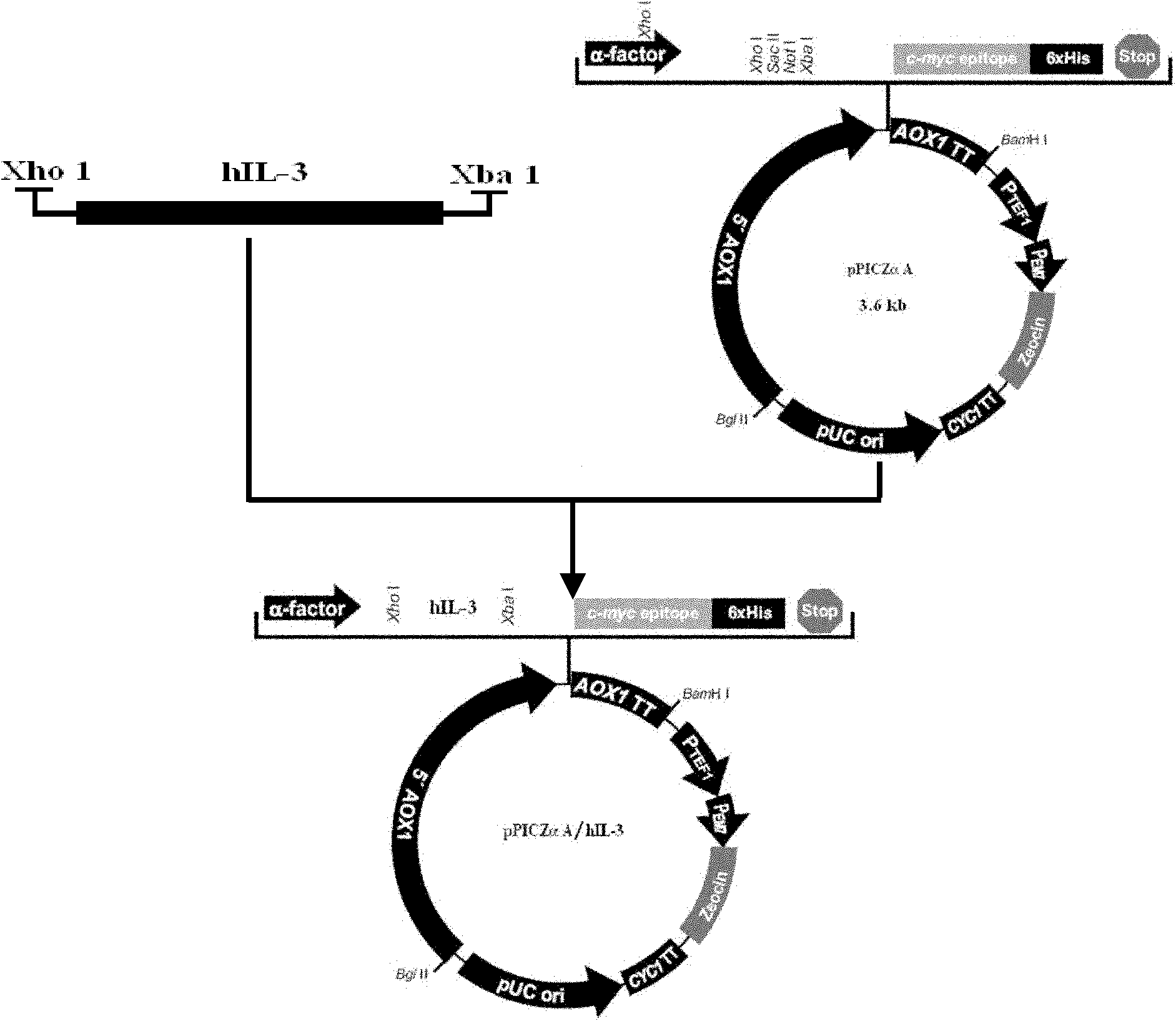
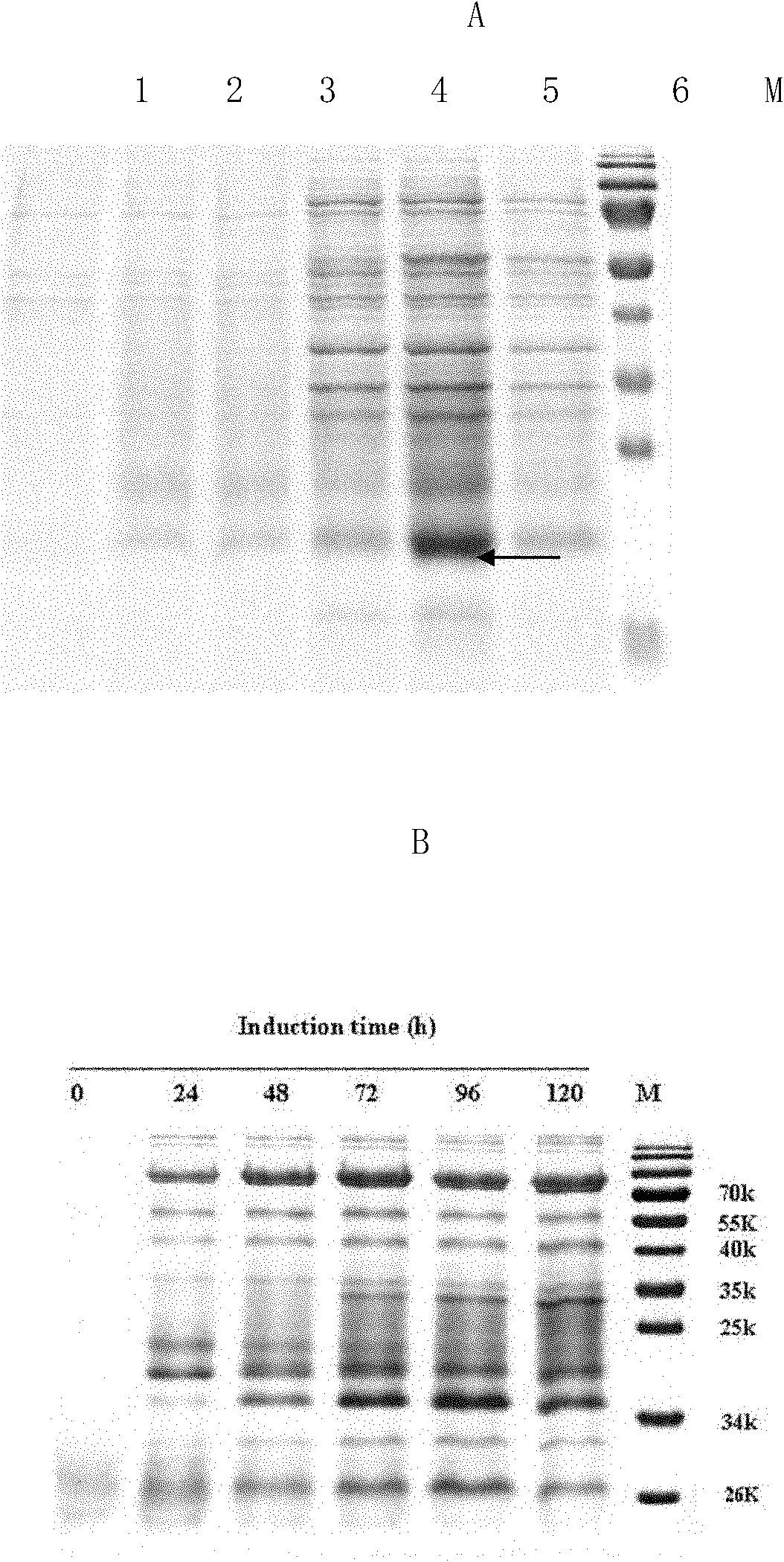
Method for expressing and purifying human recombinant interleukin-3
ActiveCN102146413AAvoid degradationLow toxicityPeptide preparation methodsFermentationPichia pastorisMass spectrometric
The invention discloses a Pichia pastoris transformant capable of expressing human recombinant interleukin-3 (rhIL-3) with high efficiency and a purification method thereof. In the method, the eukaryotic host is pichia pastorisX-33. The purification method comprises the following steps: cloning a human IL-3 gene; establishing a eukaryotic expression vector, and transforming the eukaryotic expression vector into the eukaryotic yeast host; obtaining a yeast transformant for high-level secretory expression by screening, wherein the IL-3 expressed by the yeasts are available in a glycosylated mode and a non-glycosylated module; and performing amplified culture by using a shake flask, and subjecting the supernate of the culture solution to dialysis, nickel affinity purification and further purification by diethylaminoethanol (DEAE) anion column. The purified product is subjected to mass spectrometric identification and analysis, and the result of the mass spectometric identification and analysis indicates that the expressed IL-3 is modified by different glycosyls and that the IL-3 has an his*6 tag and a C-MYC tag and is easy for purification and detection of expression product. In the invention, different from the conventional method using a prokaryotic host to express the rhIL-3, the method for expressing a large amount of rhIL-3 by using a Pichia pastoris expression system is adopted for the first time, quick purification is realized by using a His-tag protein, the purified rhIL-3 is high-activity rhIL-3 protein which is glycosylated to different extents and of which the molecular weight is 19kDa and 22kDa. The method ensures that the high-activity rhIL-3 recombinant protein is obtained quickly.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
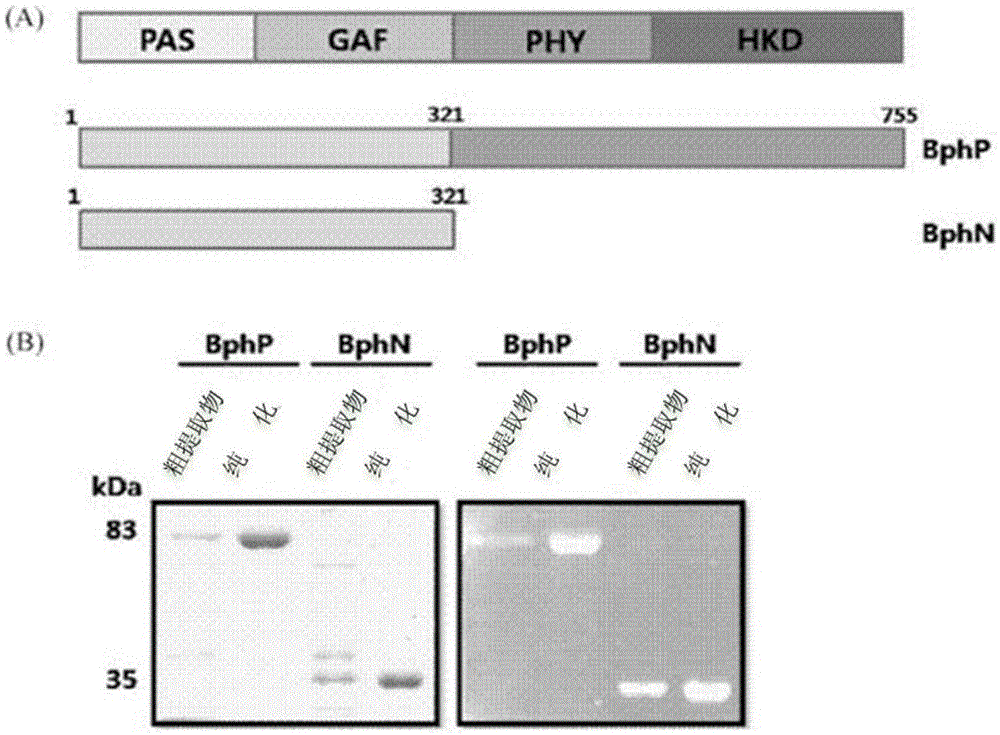
Novel peptide tag and uses thereof
InactiveCN105636976AEliminate non-specific reactionsEfficient detectionAntibody mimetics/scaffoldsImmunoglobulins against bacteriaEpitopePlant photoreceptors
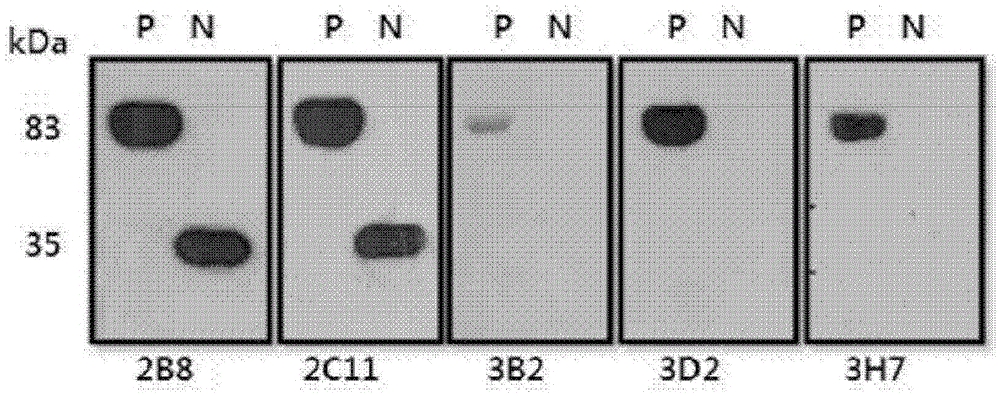
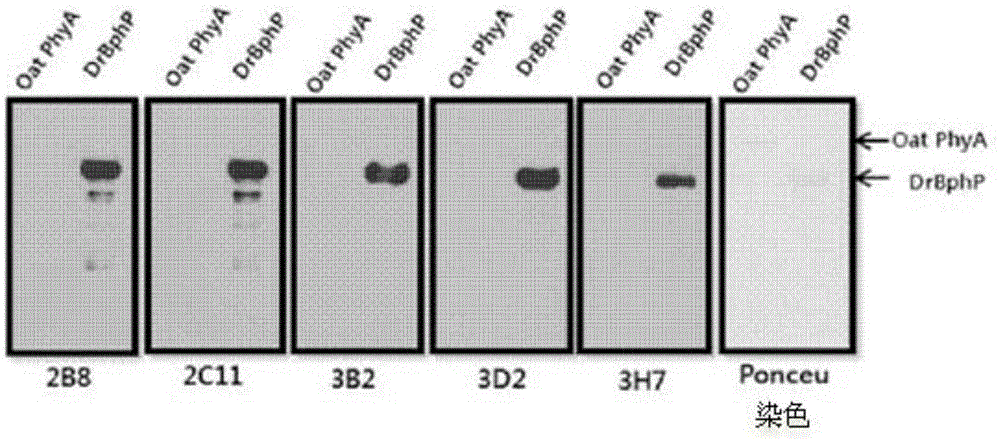
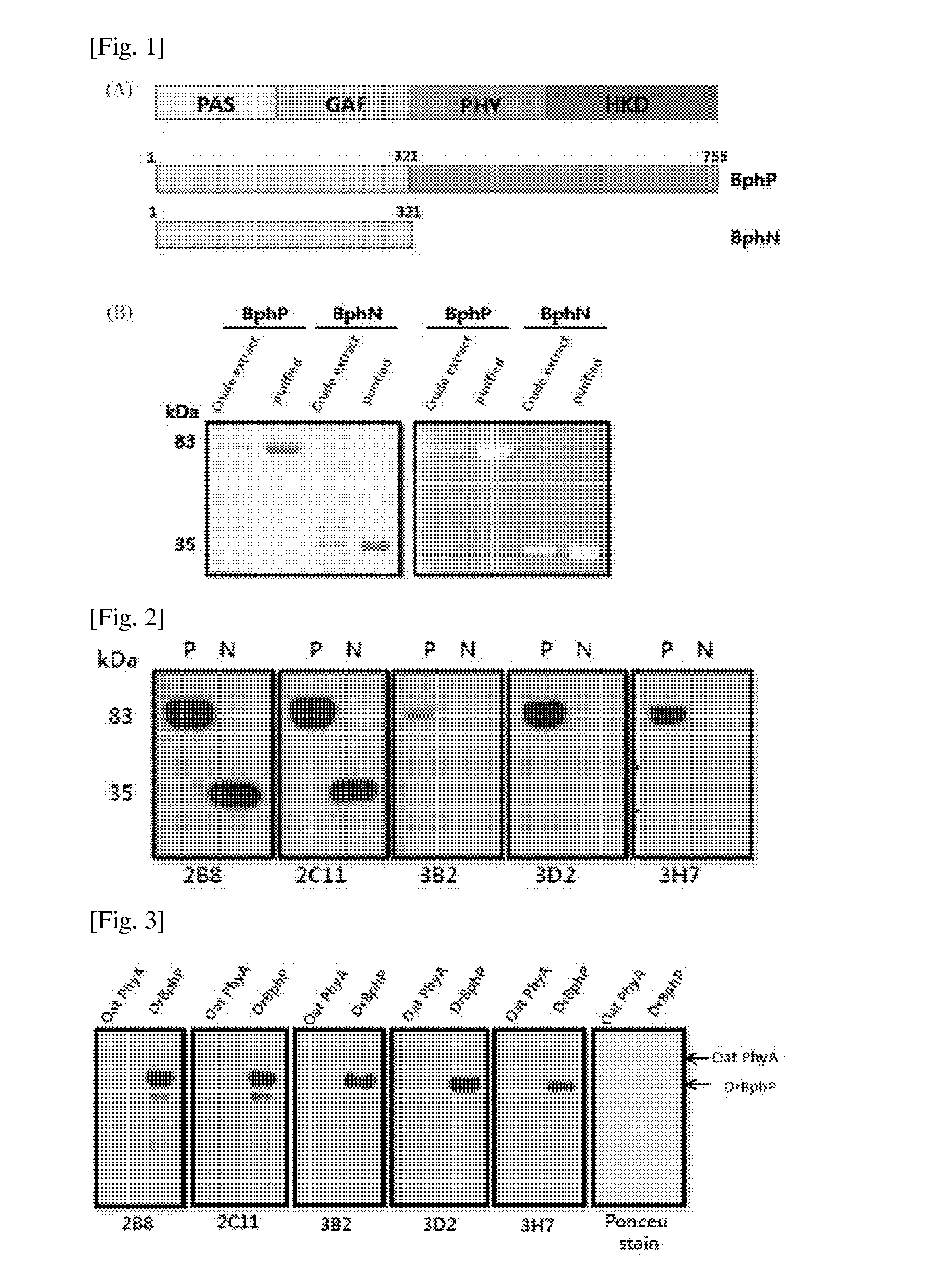
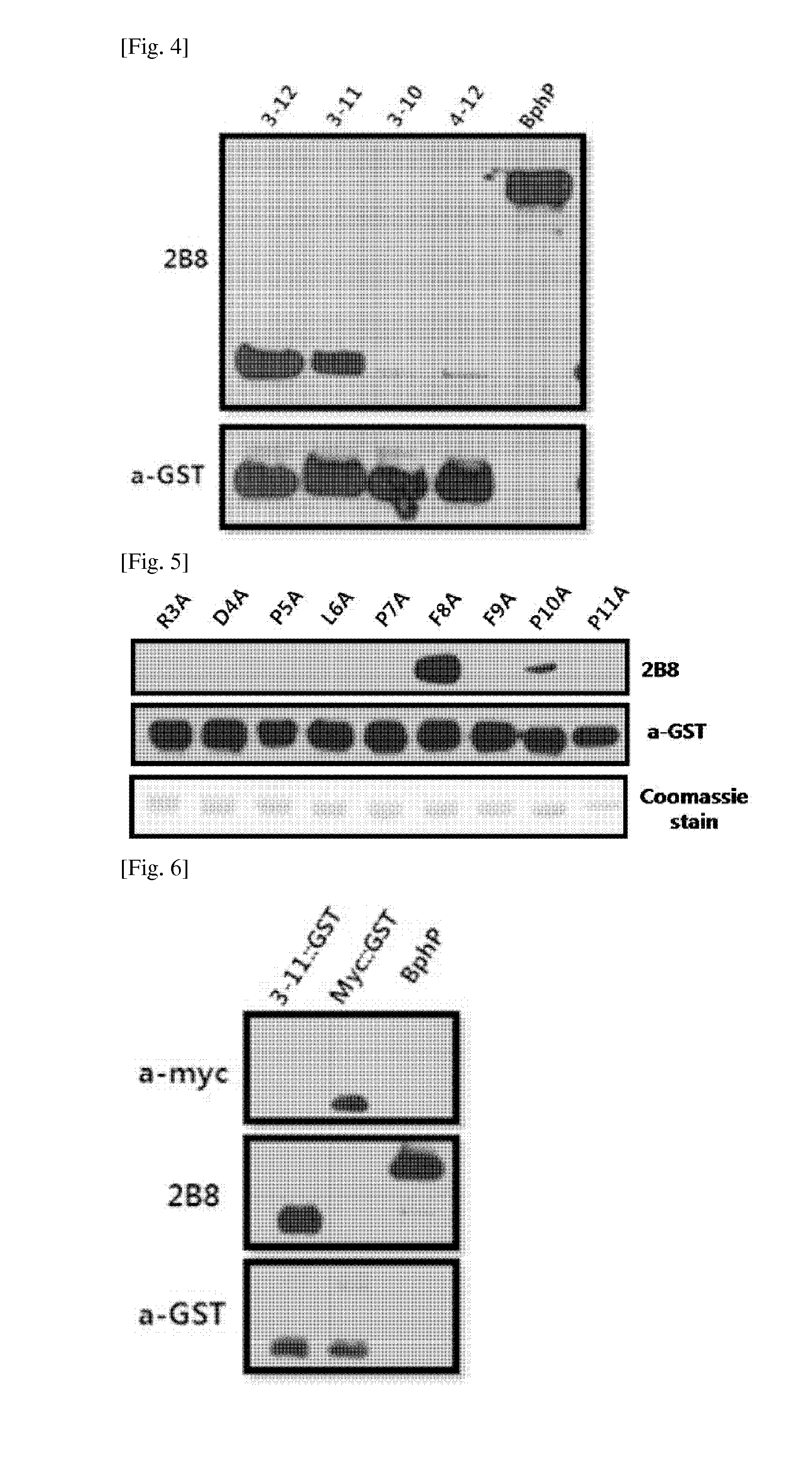
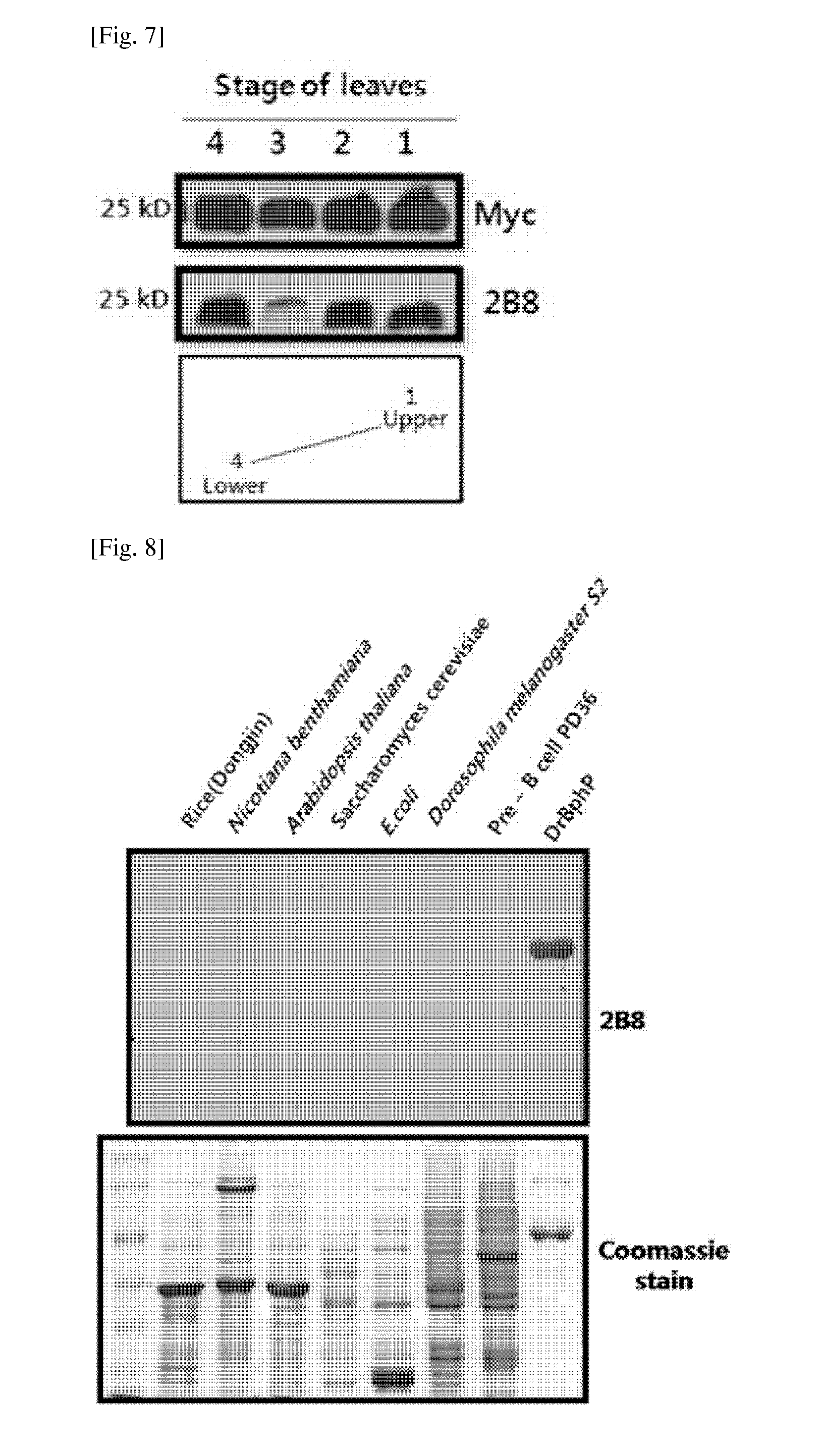
There are provided peptide tags derived from bacteriophytochrome (BphP) that is photoreceptor protein of Deinococcus radiodurans, an antibody capable of specifically recognizing the peptide tags, hybridoma cell lines capable of producing the antibody, and uses thereof. The novel peptide tag has advantages in that it has a short length and can remove a non-specific reaction of the conventional c-myc tag and FLAG tag. Therefore, in the case of using the novel peptide tag and antibody thereto, the fusion protein expressed in a recombinant cell can be very effectively detected or purified. In addition, an epitope tagging system including the novel peptide tag and antibody thereto can be applied in various fields such as a determination of an intracellular site, a confirmation of functionality, detection and purification of specific protein, and researches on interaction between proteins.
Owner:UNIV IND COOP GRP OF KYUNG HEE UNIV +1
Manufacturing method and application of insect in-vitro protein interaction detecting system
InactiveCN104991072AEfficient transfectionSimple methodBiological testingFluorescence/phosphorescenceProtein insertionPolyhedral virus
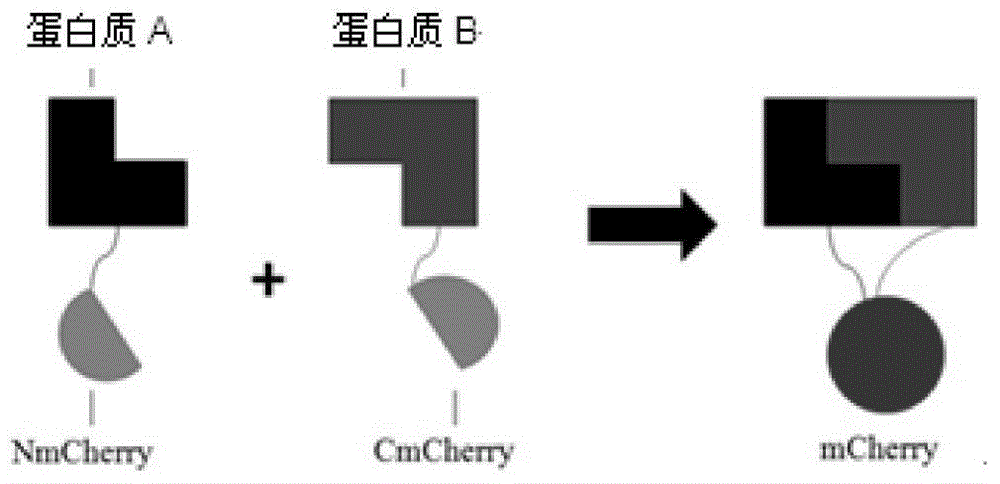
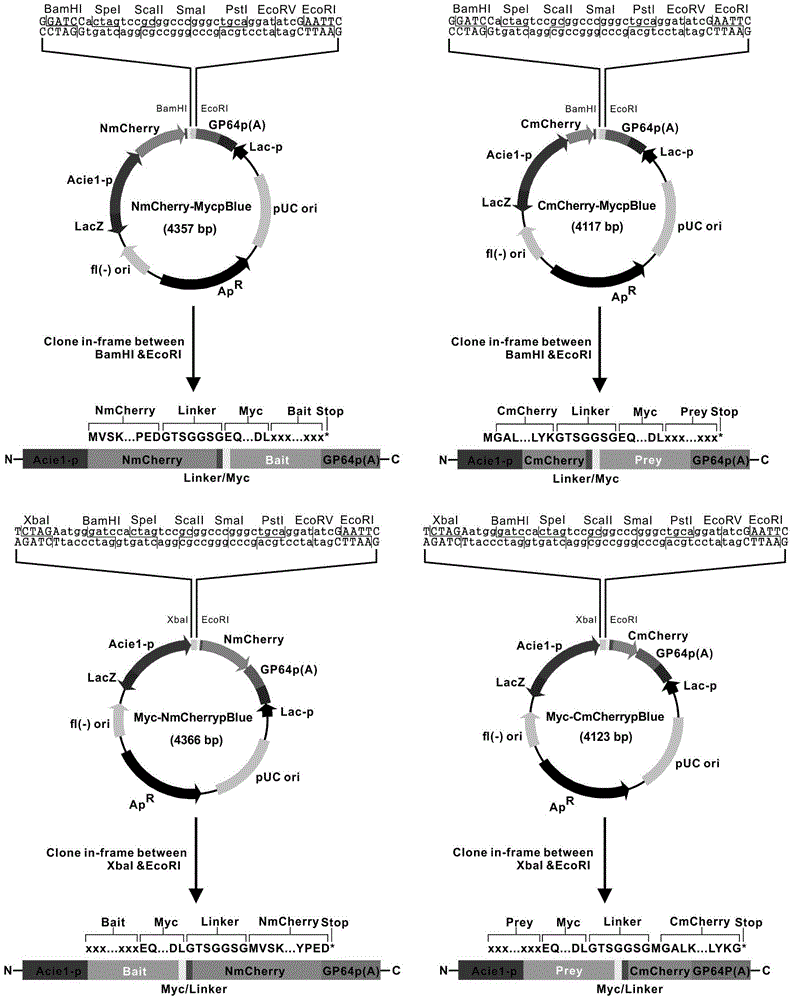
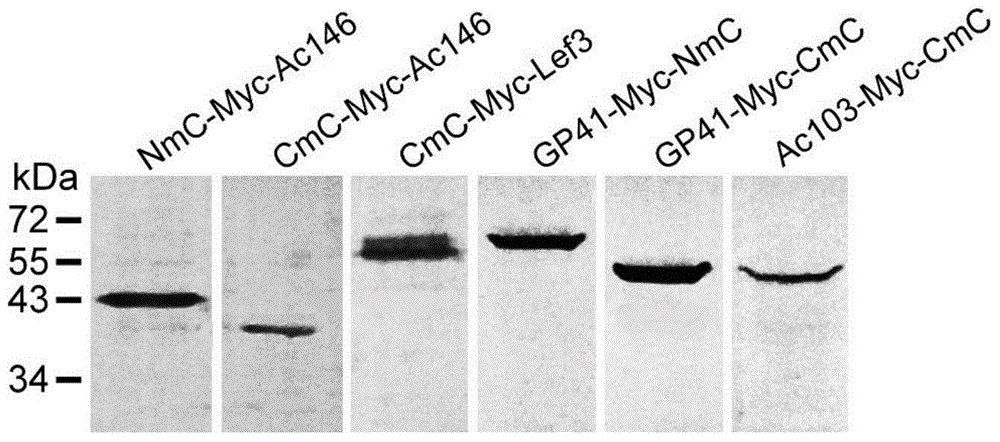
The invention discloses a manufacturing method and application of an insect in-vitro protein interaction detecting system. At the positions of amino acid residues at the 159th bit and the 160th bit, mCherry is divided into a segment NmC and a segment CmC through a PCR method, and then the segment NmC and the segment CmC are each fused with a connecting zone sequence, a c-Myc label and a poly(A) sequence to form four fused segments; the four fused segments are each connected with a pIE-MCS plasmid with an autographa californica multiple nuclear polyhedrosis virus ie1 gene promoter and a gp64 gene poly(A) sequence to establish expression plasmids; the expression plasmids are combined to form an insect cell dimolecular fluorescent complementary detecting system. The method is simple and easy to implement, and the established dimolecular fluorescent complementary detecting system expands the application of an insect cell expression system and provides a convenient and effective technological means for insect proteomics and research on protein interaction between insects and pathogenic microorganisms of the insects.
Owner:NORTHWEST A & F UNIV
Plasmid vector for building overexpression stable system on basis of transposase and application thereof
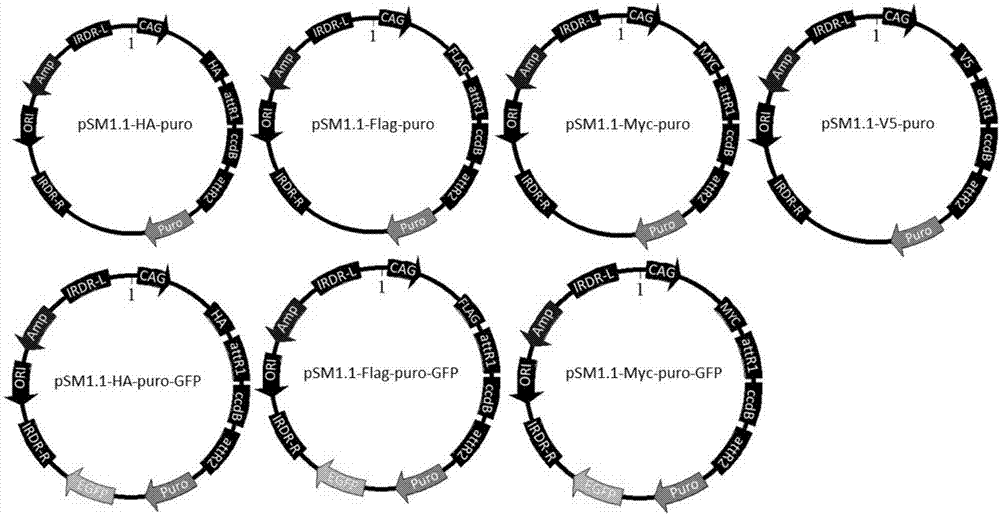
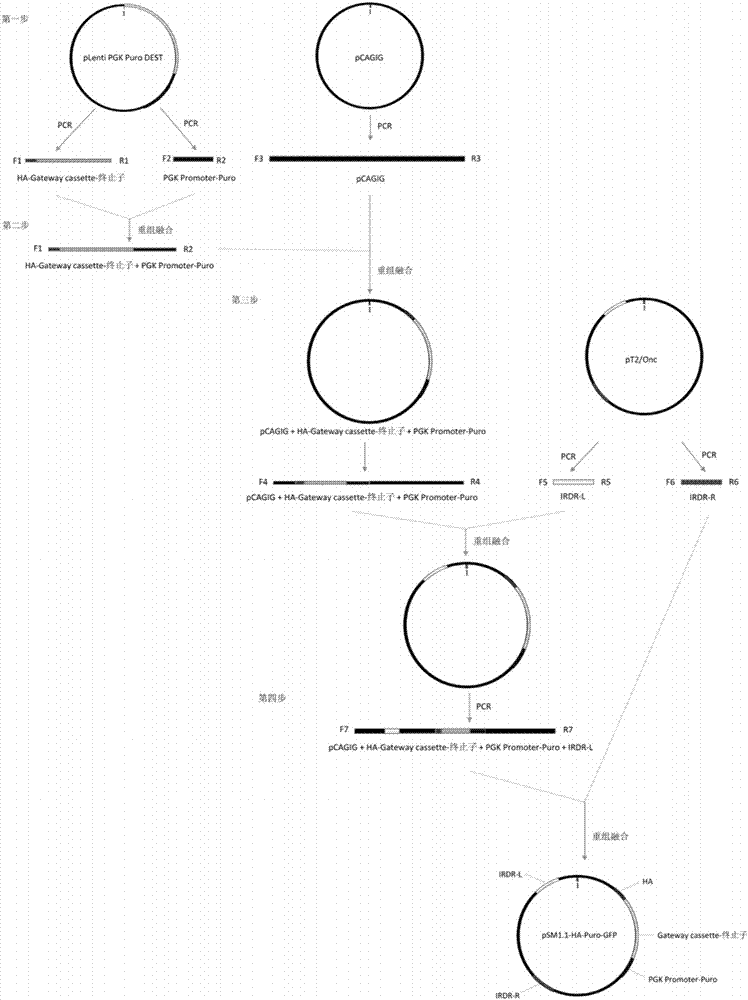
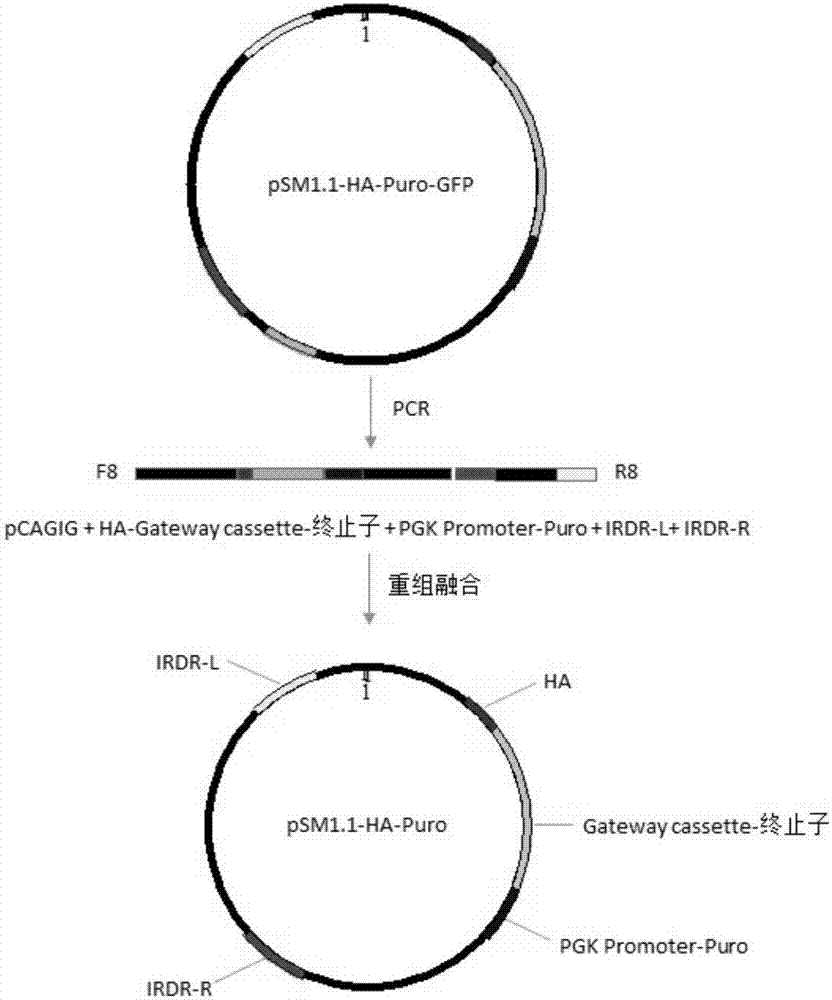
InactiveCN107400679ASize is not affectedShorten the timeAntibody mimetics/scaffoldsNucleic acid vectorMyc-tagDouble chain
The invention discloses a series of plasmid vector for building an overexpression stable system on the basis of transposase and application thereof. The plasmid vector is a double-chain annular plasmid containing an IRDR-L-IRDR-R kit; the IRDR-L-IRDR-R kit comprises an IRDR-L sequence, a promotor, a tag sequence, an attR1 sequence, a reverse selective tag gene sequence ccdB, an attR2sequence, a screening gene sequence and an IRDR-R sequence; the tag is an HA tag, an Myc tag, a Flag tag or a V5 tag. The plasmid vector has a Sleeping Beauty transposon tail end reverse repeated sequence and a Gateway Cassette sequence; the overexpression stable system can be fast and efficiently built on the basis of the transposase method; various different tag sequences Tag and screening genes GFP exist, so that the conventional requirement of expressing the target gene in eukaryotic cells can be met.
Owner:SUN YAT SEN MEMORIAL HOSPITAL SUN YAT SEN UNIV
Non-radioactive label immunoprecipitation method for detecting specific self-antibody MDA5 of inflammatory myopathy
InactiveCN105974124ASmall molecular weightIncreased sensitivityHydrolasesNucleic acid vectorAntigenElisa kit
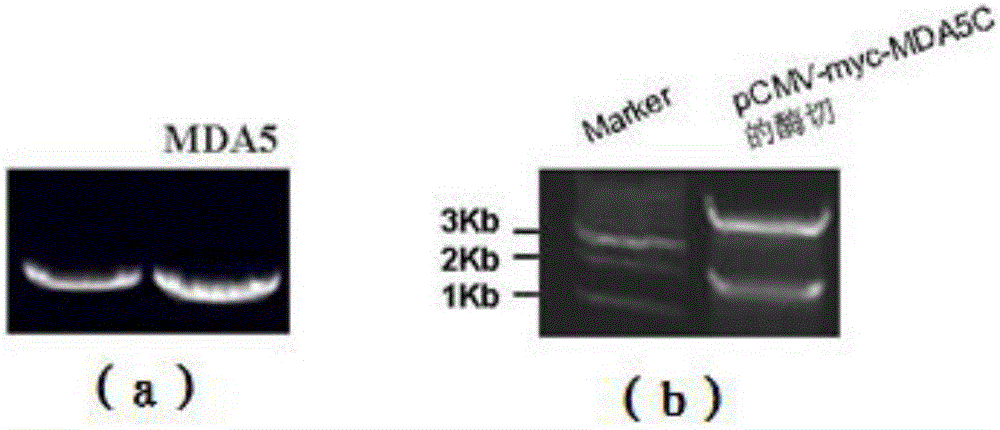
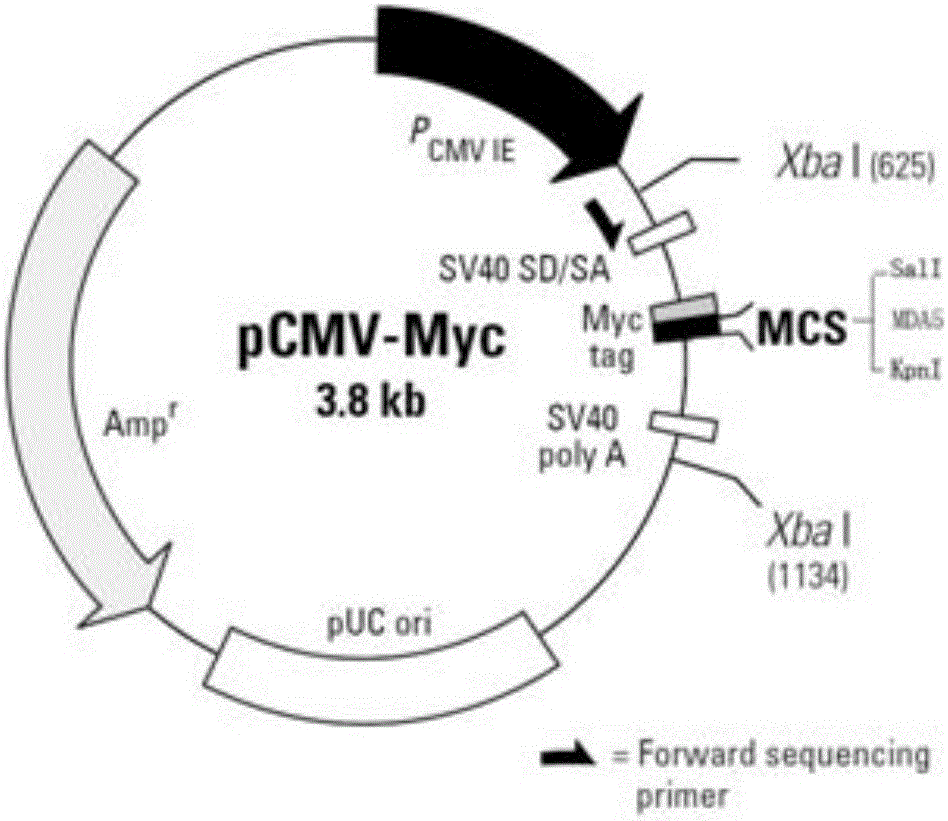
This application discloses a non-radiolabeled immunoprecipitation method for detecting inflammatory myopathy-specific autoantibody MDA5. The present invention provides the following method: constructing a plasmid containing the MDA5 C-terminus (447‑1025aa) and the N-terminus with a Myc tag HEK293 cells were transiently transfected with pCMV‑myc‑MDA5C, and serum from patients with inflammatory myopathy was immunoprecipitated with HEK293 cell lysates overexpressing the MDA5 C-terminus, followed by SDS‑PAGE gel electrophoresis and detection with c‑myc antibody Precipitated MDA5C-terminus solves the problem that there are no commercial ELISA kits for detecting MDA5 antibodies and western blot strips in China, as well as the high cost, high false positive rate and radiolabeling of ELISA kits that self-coat MDA5 antigens The safety of immunoprecipitation and other issues.
Owner:CENT SOUTH UNIV
Recombinant human interleukin 15 long peptide fragment and production method thereof
ActiveCN104212808ALow toxicityAvoid degradationPeptide preparation methodsFermentationPichia pastorisRecombinant Human Interleukin-15
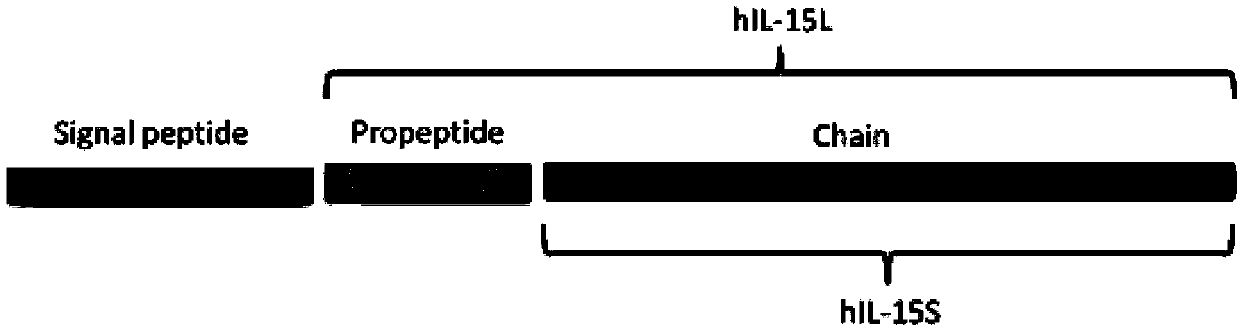
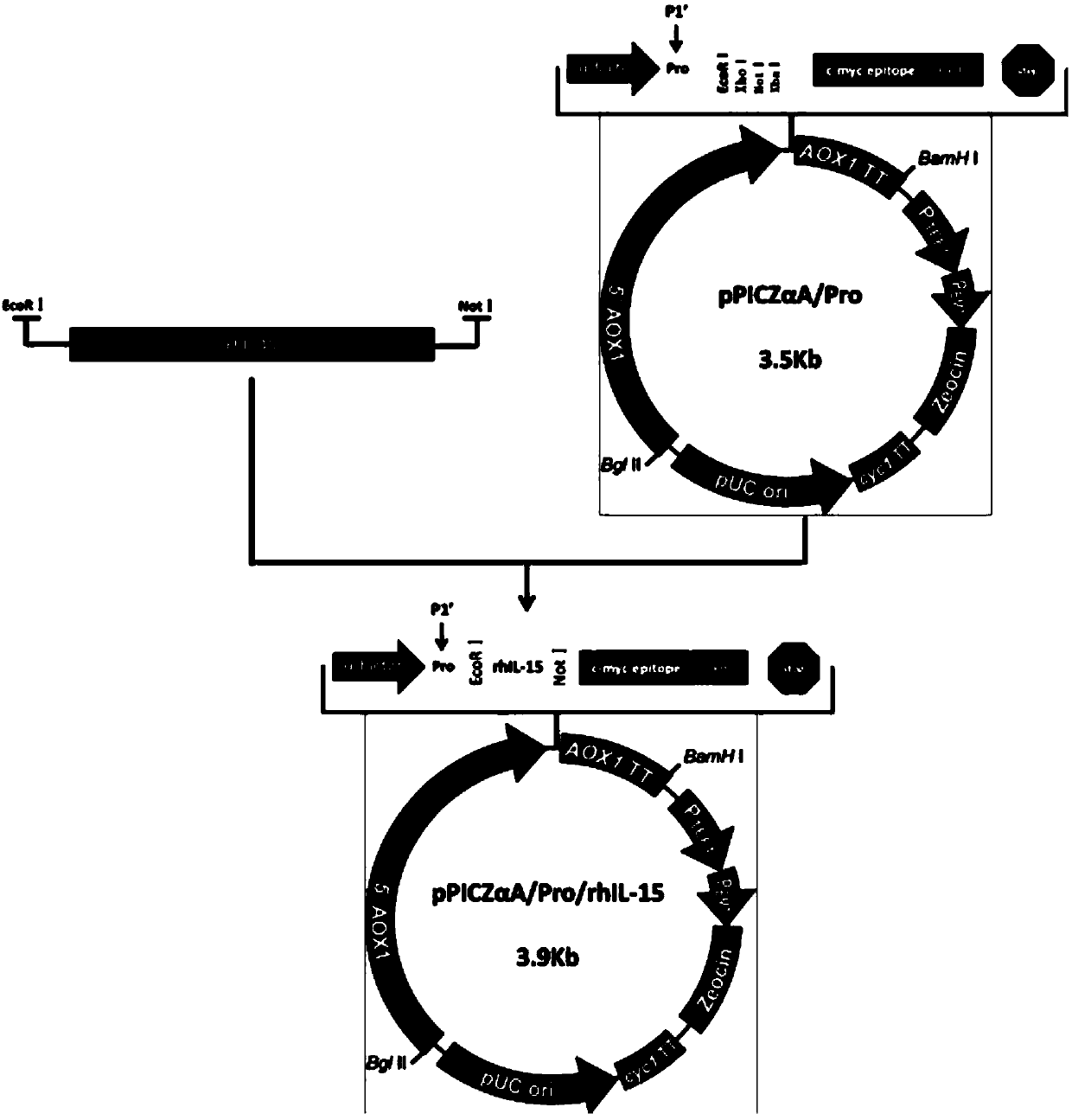
The invention discloses a recombinant human interleukin (IL) 15 long peptide fragment and a production method thereof. The production method comprises the following steps: obtaining a human IL-15 long peptide fragment gene; establishing an eukaryotic expression vector and transforming the vector into Pichia pastoris X-33; performing resistance screening on high-level secretory-expressed yeast transformants; performing induced expression, purifying the supernate through a nickel column and an ion exchange column to obtain rhIL-15L. The production method is characterized in that the amino acid residue Ala of the site Kex2P1' of the vector pPICZAlphaA is mutated into Pro and the Pichia pastoris X-33 is taken as host bacteria, and therefore, mass high-efficiency stable expression of the rhIL-15L is realized; the recombinant protein rhIL-15L produced by the method is marked with HIS and C-MYC tags and prone to protein purification and detection; besides, the recombinant protein rhIL-15L is a glycoprotein which is modified through glycosylation to a certain extent, and therefore, the recombinant protein rhIL-15L has excellent bioactivity and is capable of effectively maintaining the proliferation and the bioactivity of human NK (Natural killer) cells in vitro and of a mouse in vivo.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Preparation of double-labeled virus-like particles (VLPs) of PRRSV
The invention discloses a method for preparing and identifying double-labeled virus-like particles (VLPs) of PRRSV, and relates to the field of biological research technology; a pMD18T recombinant plasmid containing GP5, M and N protein coding genes ORF5, ORF6 and ORF7 is prepared as an amplification template, a Myc tag is introduced at the end of the ORF5, a His tag is introduced into the end ofthe ORF6, a recombinant plasmid is obtained by recombination to PpH and Pp10 promoters of a binary vector, the recombinant plasmid is transferred into DH10Bac E. coli to obtain a recombinant bacmid; and the ORF7 and a pFastBacHTB vector are recombined to obtain a recombinant bacmid. The two recombinant bacmids are inoculated into SF9 cells, and Myc-GP5, His-M and His-N proteins are successfully expressed and automatically assembled into the VLPs. The method lays a foundation for studying the function of the GP5 and M and N major structural proteins and developing VLP vaccines that can distinguish between infection and immunity.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Affinity adsorption material based on c-Myc label nano antibody
ActiveCN106890622AEasy to getAvoid cumbersome production methods such as artificial antibodiesComponent separationOther chemical processesGenetic engineeringMyc-tag
The invention provides an affinity adsorption material based on a c-Myc label nano antibody, belongs to the field of genetic engineering and particularly provides a c-Myc label-oriented single-domain heavy-chain antibody (also known as a nano antibody). The affinity adsorption material has an amino acid sequence shown as SEQ ID NO.1. The amino acid sequence can serve as a precursor, a mutant having better properties (affinity, specificity, stability and the like) can be obtained by improving with a random or site-specific mutagenesis technology, the nano antibody having the high specificity can serve as the c-Myc-oriented immuno-affinity adsorption material, and the recombinant protein containing c-Myc label is purified.
Owner:NANCHANG DAJIA TECH
Preparation method and application of recombination tumor necrosis factor relevant cell apoptosis induction ligand protein
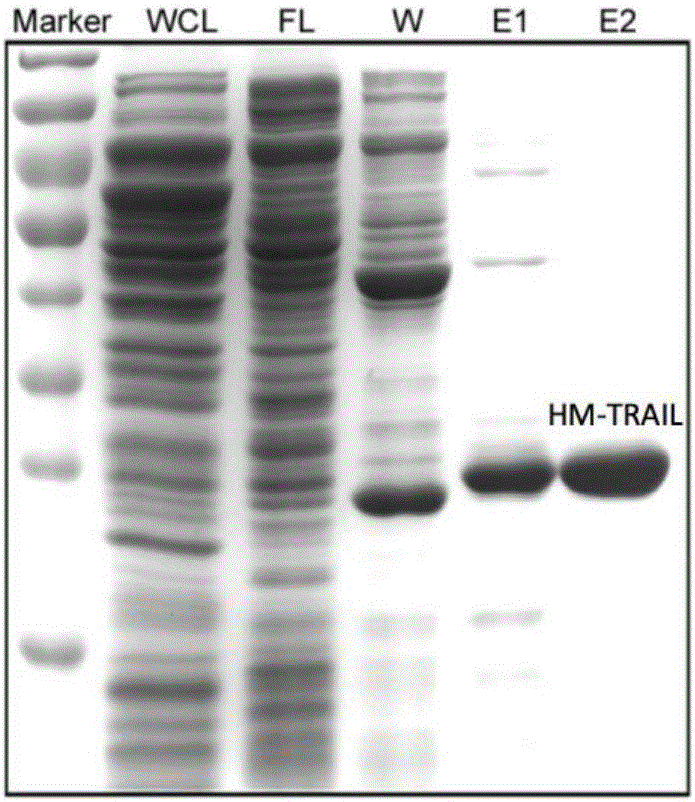
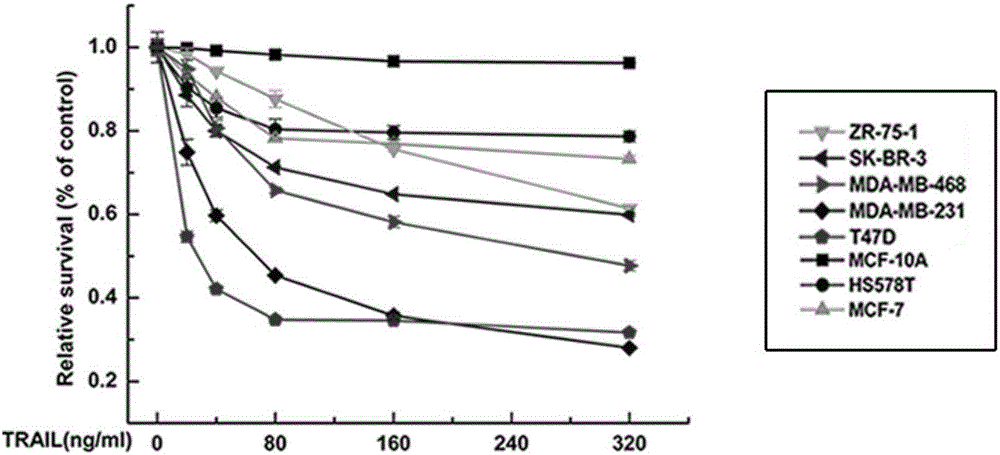
ActiveCN106188311AImprove stabilityImprove solubilityBacteriaPeptide/protein ingredientsTRAIL ProteinWilms' tumor
The invention discloses recombination human HM-TRALL protein with an amino acid sequence shown in the SEQ ID NO.1. The method comprises the steps that a human recombination HM-TRALL protein expression carrier containing a His6-Myc label is built at first, recombination bacteria for expressing human recombination HM-TRALL protein are built through the carrier, recombinant expression is carried out on the recombination human HM-TRALL protein through IPTG induction, extraction and purification are carried out, and high-purity HM-TRAIL protein is obtained. The recombination human HM-TRALL protein can selectively induce apoptosis of breast cancer cells in vitro, and will not cause normal breast cell apoptosis. The prepared recombination human HM-TRALL protein has the advantages of being good in stability and high in dissolving degree, can obviously induce apoptosis of tumor cells and is good in application prospect.
Owner:SHANDONG UNIV QILU HOSPITAL
Nanobody resisting to c-Myc labels
ActiveCN106831991AHigh affinityImprove featuresOther chemical processesBiological material analysisAntigenGenetic engineering
The invention belongs to the field of genetic engineering and particularly provides a single-domain heavy chain antibody targeted at c-Myc labels. The antibody has an amino acid sequence shown in SEQ ID NO.:1 and can be used in fields such as immunodetection, antigen enrichment and purification and the like. The amino acid sequence can serve as a precursor and be improved with a random or site-specific mutagenesis technique, so that a mutant with better properties including compatibility, specificity, stability and the like can be obtained and used for developing protein or polypeptides for medicine, industry and agriculture.
Owner:NANCHANG UNIV
Nanobody capable of being specifically bound with c-Myc tag
ActiveCN106831990AHigh affinityImprove featuresOther chemical processesBiological material analysisAntigenSite-directed mutagenesis
The invention belongs to the field of genetic engineering and particularly provides a single-domain and heavy-chain antibody specific to a c-Myc tag. The single-domain and heavy-chain antibody has an amino acid sequence represented as SEQ ID NO.:1 and can be applied to fields of immunodetection, antigen enrichment and purification and the like. Theamino acid sequence can be taken as a precursor and is modified with a random or site-directed mutagenesis technology, so that mutants with better properties such as affinity, specificity, stability and the like can be obtained and are used for developing protein or polypeptides for medicine, industry and agriculture.
Owner:深圳晶蛋生物医药科技有限公司
A kind of multi-label antigen and its preparation method and application
ActiveCN105296478BMeet the needs of setting positive controlsMeet the needs of positive controlsMicroorganism based processesBiological testingBlotMyc-tag
The invention discloses a multi-tag antigen. The multi-tag antigen comprises the following nine tags: a GST tag, an HA tag, a T7 tag, a V5 tag, a Myc tag, a StrepII tag, a Flag tag, an EGFP tag and a His tag which are connected in series according to a sequence of GST-HA-T7-V5-Myc-StrepII-Flag-EGFP-His. The invention also discloses a preparation method for the multi-tag antigen and application of the multi-tag antigen in immunoblotting. The multi-tag antigen disclosed in the invention can meet demand for setting of positive contrast in most immunoblotting reactions and has good compatibility and strong immunoblotting specificity.
Owner:YEASEN BIOTECHNOLOGY (SHANGHAI) CO LTD
Immunoaffinity adsorbing material based on specifically recognized c-Myc label nano-antibody
InactiveCN107042091AEasy to getAvoid cumbersome production methods such as artificial antibodiesOther chemical processesSolid sorbent liquid separationSite-directed mutagenesisMutant
The invention belongs to the field of gene engineering, and in particular discloses a single-domain heavy-chain antibody (also known as nano-antibody) aiming at a c-Myc label, and the antibody has an amino acid sequence as shown in SEQ ID NO. 1. The amino acid sequence provided by the invention can be used as a precursor, and by conducting reconstruction through a random or fixed-point mutation technique, a mutant with better properties (affinity, specificity, stability and the like) can be obtained. The nano-antibody with high specificity can be used as an immunoaffinity adsorbing material aiming at c-Myc and used for purifying recombinant protein containing the c-Myc label.
Owner:NANCHANG UNIV
Method for efficiently expressing foreign protein
InactiveCN102816758ABiologically activeHigh purityDepsipeptidesPeptide preparation methodsNicotiana tabacumProtein target
The invention discloses a method for efficiently expressing foreign protein. The method comprises the steps of cloning a foreign protein gene onto a pMYC2 carrier, enabling the foreign protein gene and an encoding genes of six MYC tags on the pMYC2 carrier to form a fused gene, and then utilizing an agrobacterium infection transformation method to express the foreign protein in tobacco leaves instantly and efficiently; utilizing affinity media carried with corresponding tag antibodies to efficiently purify the protein expressed by tobacco according to MYC affinity tags carried by recombinant protein and finally performing competitive elution of the affinity media to obtain purified target protein. The purified protein can be used for further biological function research. The method achieves protein purification in one step and is simple and quick, and the protein purity is high. The foreign recombinant protein can be used for biological safety evaluation and biological function research.
Owner:TSINGHUA UNIV
Fusion protein of chemoattracting small peptide and dual specific antibodies
InactiveCN1958614AHybrid immunoglobulinsSugar derivativesSecondary Lymphoid-Tissue ChemokineSmall peptide
This invention relates to a fusion protein of chenotactic peptide and bispecific antibody against ovarian carcinoma and CD3 molecules. The fusion protein is constructed by sequentially connecting N-terminated 18-peptides of secondary lymphoid tissue chemokine, (GGGGS)2 linking peptide, and the bispecific antibody against ovarian carcinoma and CD3 molecules. C-myc label and His6 label are connected at the C-terminal. This invention also relates to the method for constructing, expressing and purifying the fusion protein, its coding sequence, its relevant expression vector, and the host cells containing the expression vector.
Owner:BEIJING ABT GENETIC ENG TECH
TCR gene targeted to CD19, preparation method, plasmid with TCR gene, kit and application
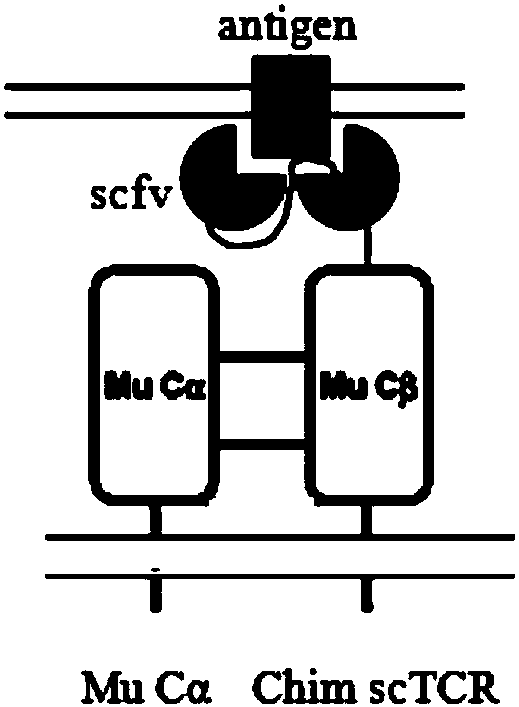
ActiveCN107916269AImprove effectivenessImprove securityImmunoglobulin superfamilyAntibody mimetics/scaffoldsGene targetsNucleotide sequencing
The invention discloses a safe and efficient TCR gene targeted to CD19. The TCR gene is characterized in that a nucleotide sequence of the TCR gene is SEQ ID NO.1, and the TCR gene is formed by CD8leader, CD19scFv, TRBC, T2A, CD8leader, myc-tag and TRAC which are sequentially connected in series. The invention also discloses a preparation method of the TCR gene, a plasmid with the TCR gene, a kitand an application. The TCR gene disclosed by the invention has the advantages that clinical effectiveness and safety of a TCR technology are improved, and fussy experimental operations are also avoided.
Owner:上海兴瑞一达生物科技有限公司
Affinity adsorbing material based on specifically recognized c-Myc label single-domain heavy-chain antibody
InactiveCN107042092AEasy to getAvoid cumbersome production methods such as artificial antibodiesOther chemical processesSolid sorbent liquid separationMutantGene engineering
The invention belongs to the field of gene engineering, and in particular discloses a single-domain heavy-chain antibody (also known as nano-antibody) aiming at a c-Myc label, and the antibody has an amino acid sequence as shown in SEQ ID NO. 1. The amino acid sequence provided by the invention can be used as a precursor, and by conducting reconstruction through a random or fixed-point mutation technique, a mutant with better properties (affinity, specificity, stability and the like) can be obtained. The nano-antibody with high specificity can be used as an immunoaffinity adsorbing material aiming at c-Myc and used for purifying recombinant protein containing the c-Myc label.
Owner:NANCHANG UNIV
Antibody For Epitope Tagging, Hybridoma Cell Line and Uses Thereof
ActiveUS20150191532A1Shorten the lengthRemove non-specific reactionAnimal cellsImmunoglobulins against bacteriaEpitopePlant photoreceptors
There are provided peptide tags derived from bacteriophytochrome (BphP) that is photoreceptor protein of Deinococcusradiodurans, an antibody capable of specifically recognizing the peptide tags, hybridoma cell lines capable of producing the antibody, and uses thereof. The novel peptide tag has advantages in that it has a short length and can remove a non-specific reaction of the conventional c-myc tag and FLAG tag. Therefore, in the case of using the novel peptide tag and antibody thereto, the fusion protein expressed in a recombinant cell can be very effectively detected or purified. In addition, an epitope tagging system including the novel peptide tag and antibody thereto can be applied in various fields such as a determination of an intracellular site, a confirmation of functionality, detection and purification of specific protein, and researches on interaction between proteins.
Owner:UNIV IND COOP GRP OF KYUNG HEE UNIV +1
Nano-antibody aiming to c-Myc tag
ActiveCN106946990AHigh affinityImprove featuresOther chemical processesBiological material analysisAntigenSite-directed mutagenesis
The invention belongs to the field of gene engineering and particularly provides a single-domain heavy-chain antibody aiming to a c-Myc tag. The amino acid sequence of the antibody is represented as the SEQ ID No.1. The antibody can be used in the fields of immunodetection, enrichment and purification of antigens, etc. The amino acid sequence, as a precursor, can be modified through random or site-directed mutation technology, so that a mutant having better properties (e.g. affinity, specificity and stability) can be produced and can be further applied to proteins or polypeptides in the fields of medicines, industry and agriculture.
Owner:NANCHANG DAJIA TECH
Preparation method and application of MYC tag fusion expression vector
PendingCN114107369AMaintain protein epitopeMaintain structureAntibody mimetics/scaffoldsVector-based foreign material introductionEnzyme digestionLigation
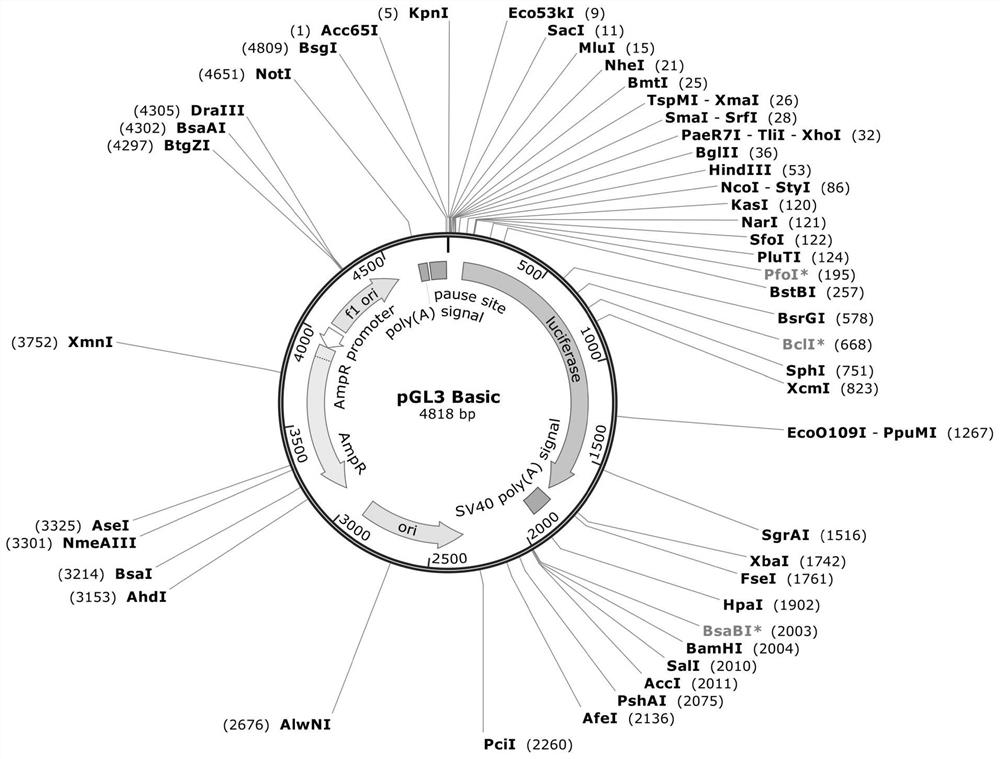
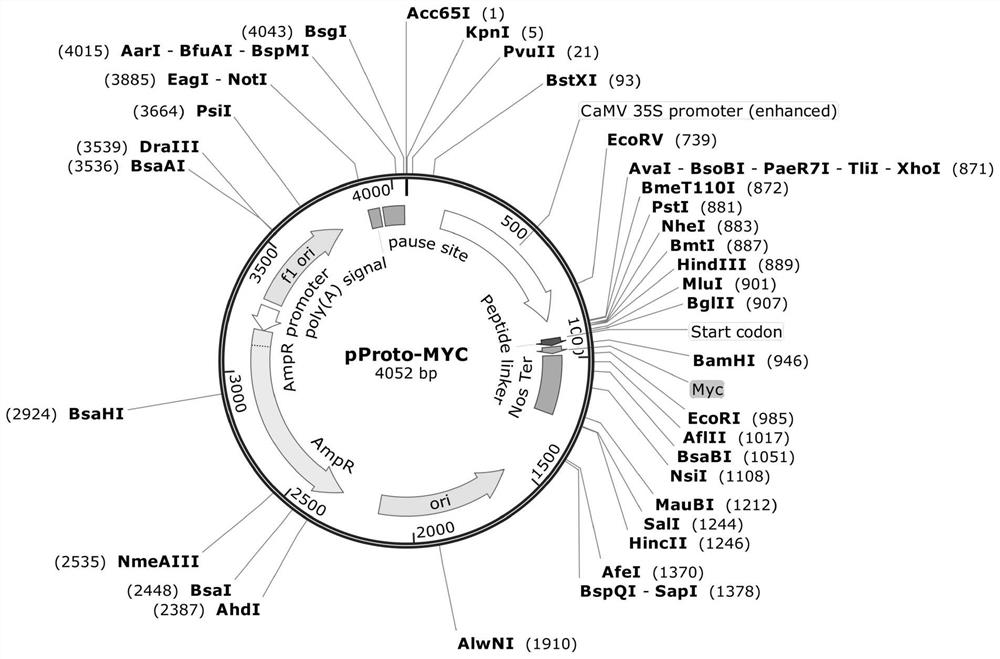
The invention provides a preparation method of an MYC tag fusion expression vector, which comprises the following steps: using a KpnI / XhoI double-enzyme digestion pGL3 Basic vector to obtain a recovered product of the KpnI / XhoI double-enzyme digestion pGL3 Basic vector, using a pRGEB32Bar-Cas9 plasmid as a template to obtain a 2 * 35S promoter PCR recovered product, carrying out a ligation reaction to obtain an intermediate vector pGL-35S, carrying out double-enzyme digestion, connecting an MCS annealing primer to obtain an intermediate vector pGL-35S-MCS, carrying out double-enzyme digestion, connecting a Nos Ter sequence, carrying out double-enzyme digestion, and carrying out purification to obtain the MYC tag fusion expression vector. The MYC tag fusion expression vector is characterized in that an intermediate vector pGL-35S-MCS-Nos is obtained by using an MYC tag as a template, the intermediate vector pGL-35S-MCS-Nos is connected with a connecting peptide Linker primer subjected to denaturation annealing after double enzyme digestion to obtain an intermediate vector pGL-35S-MCS-Linker-Nos, and the intermediate vector pGL-35S-MCS-Linker-Nos is connected with an MYC tag annealing primer after double enzyme digestion to obtain the MYC tag The MYC tag fusion expression vector prepared by the invention has a small framework, has a full length of 4052bp, and is suitable for plant protoplast transformation.
Owner:HENAN AGRICULTURAL UNIVERSITY
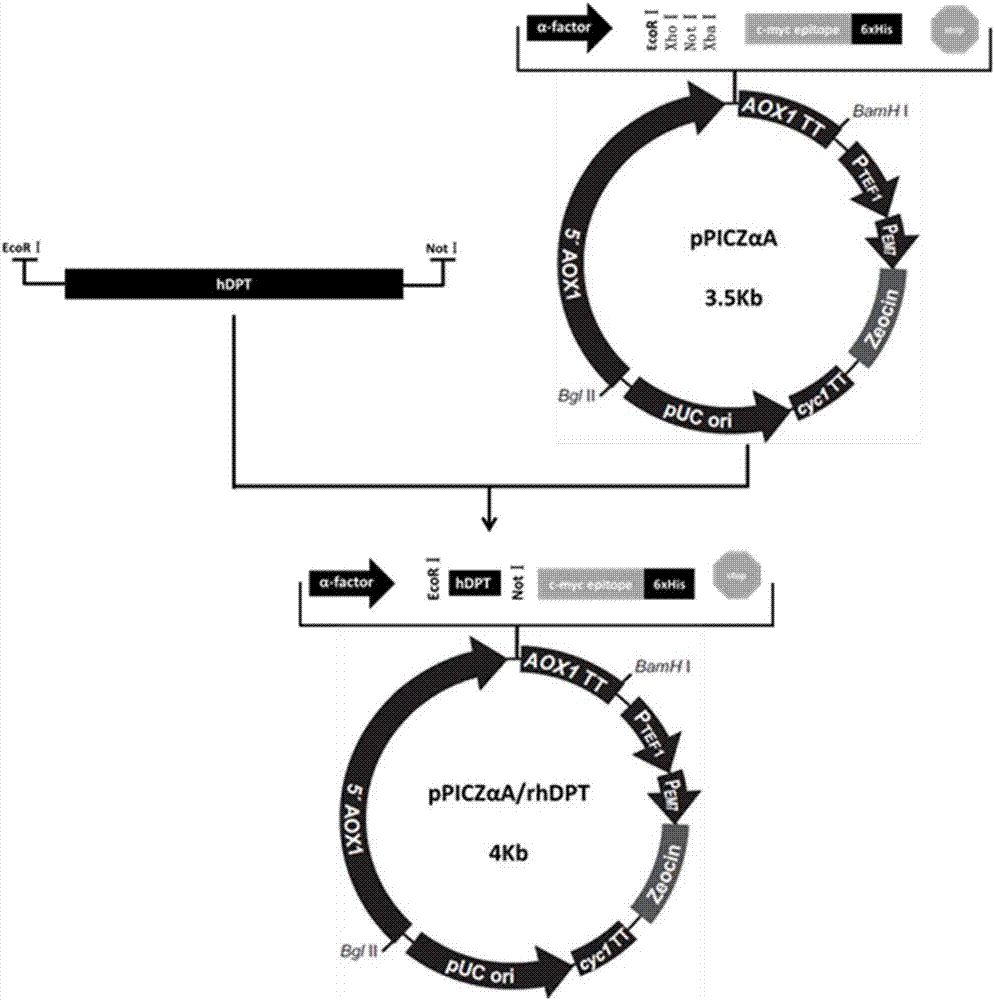
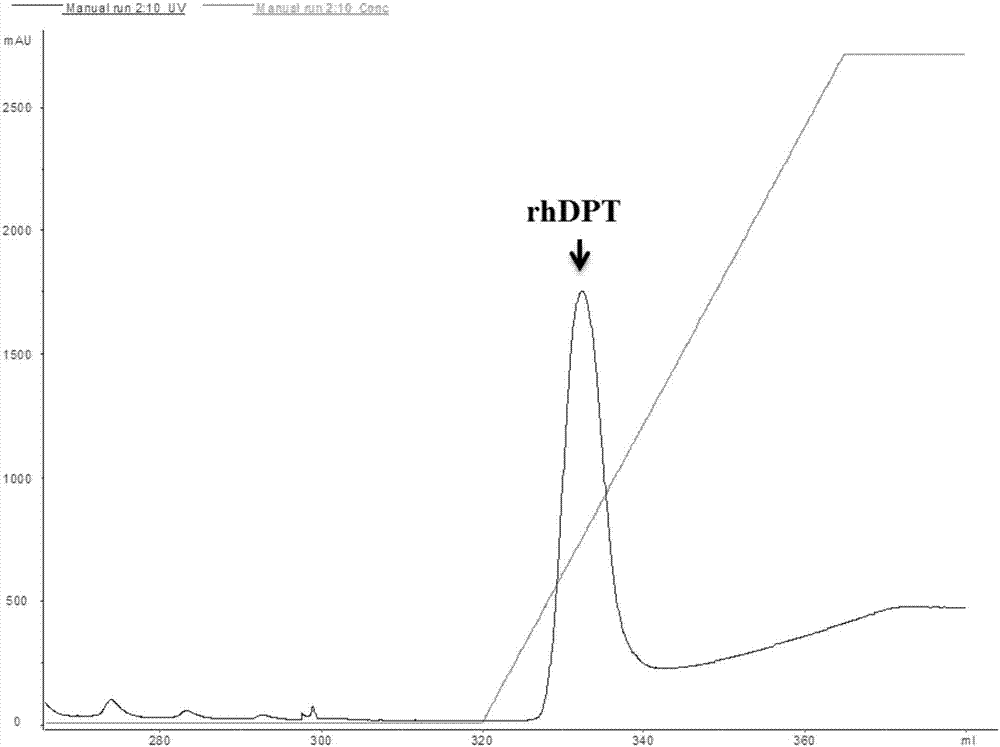
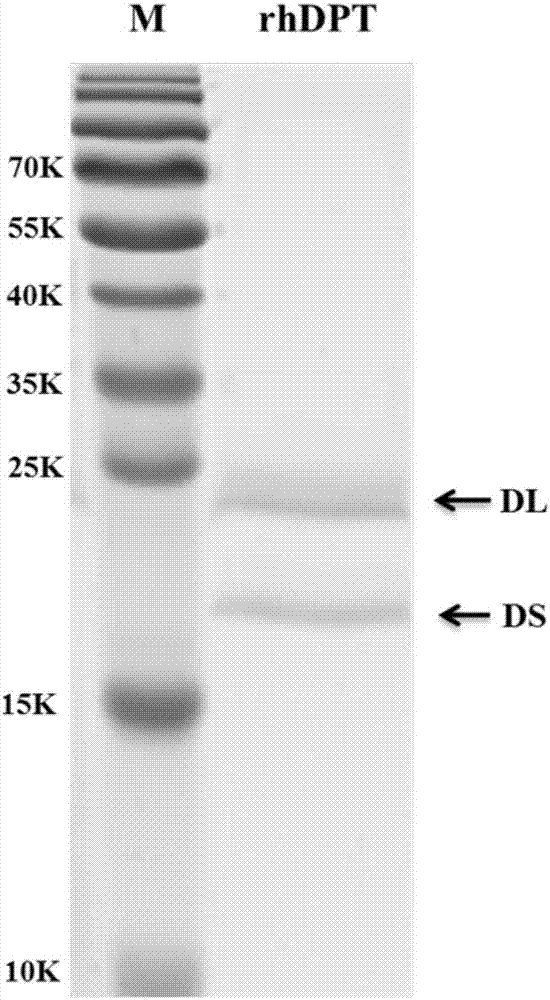
Mature peptide of recombination human DPT (dermatopontin) and production method thereof
InactiveCN107058369AEfficient and stable expressionAvoid degradationPeptide preparation methodsAnimals/human peptidesPichia pastorisBiotechnology
The invention discloses mature peptide of recombination human DPT (dermatopontin) and a production method thereof. The production method comprises the following steps of obtaining a segment gene of the mature peptide of the human DPT; establishing an eukaryotic expression carrier, and converting into pichia pastoris X-33; performing resistance screening to obtain a yeast transformant with high-level excretion expression; inducing to express supernatant, and purifying by a nickel column, so as to obtain the rhDPT. The recombination protein rhDPT prepared by the method has the advantages that the HIS and C-MYC labels are carried, and the protein purification and detection are easy; in the in-vitro experiment, the rhDPT can effectively inhibit the growth of fat cells, and the biological activity is good.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Anti-c-Myc label single domain heavy chain antibody
The invention belongs to the field of gene engineering and in particular relates to a single domain heavy chain antibody for a c-Myc label. The antibody has an amino acid sequence as shown in SEQ ID NO.1 and can be used in the fields of immunodetection, antigen enrichment and purification and the like. The amino acid sequence provided by the invention can be used as a precursor which is transformed by a random or fixed mutation technology, so that a mutant which is better in properties (affinity, specificity, stability and the like) can be obtained for developing proteins or polypeptides which are further used for medicines, industries and agriculture.
Owner:NANCHANG UNIV
Nonradioactive labeling immunoprecipitation method for detecting NXP2 autoantibody of inflammatory myopathies and application
InactiveCN106443017AAvoid defectsTumor rejection antigen precursorsTumor specific antigensAntigenElisa kit
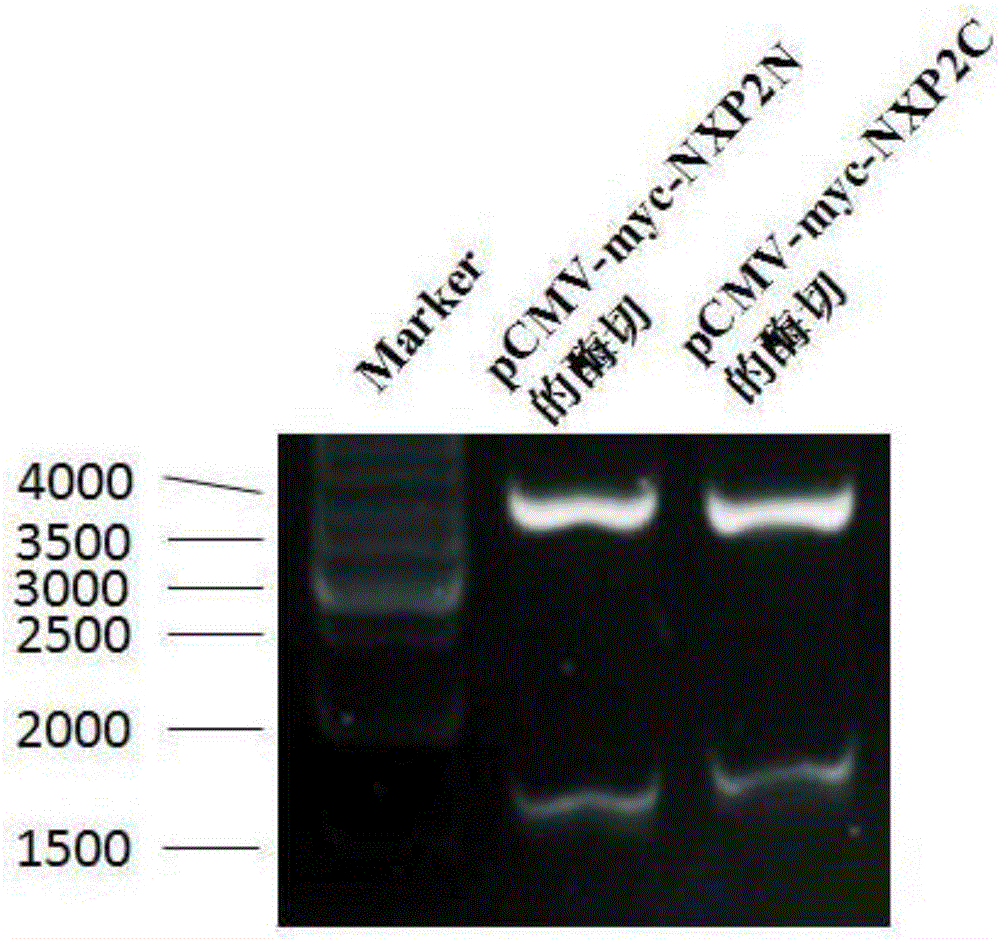
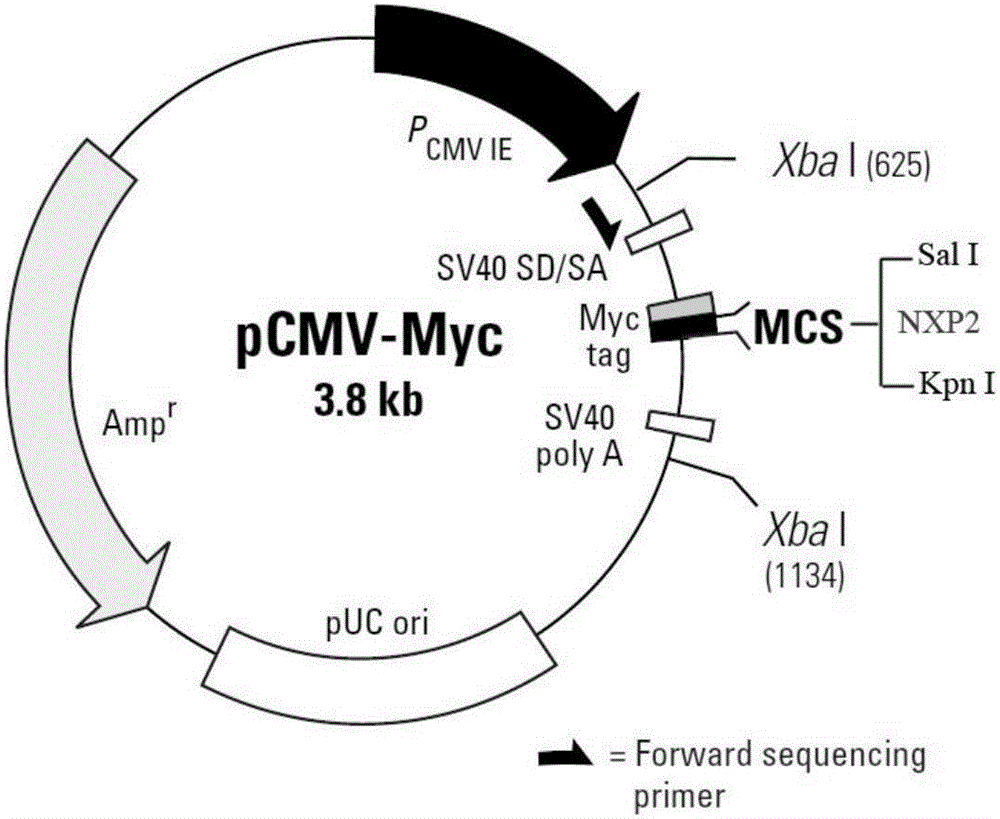
The invention of the application discloses a nonradioactive labeling immunoprecipitation method for detecting an NXP2 autoantibody of inflammatory myopathies and application. A plasma pCMV-myc-NXP2N or pCMV-myc-NXP2C which comprises an NXP2 N end (1-500aa) or an NXP2 C end (400-939aa) and has a Myc tag is constructed, an HEK293 cell is transiently transfected, a serum of an inflammatory myopathies patient with positive NXP2 autoantibody is subjected to immunoprecipitation with an HEK293 cell lysis buffer with an overexpressed NXP2 N end or an NXP2 C end, the NXP2 N end or the NXP2 C end is found to be precipitated by the NXP2 autoantibody, the to-be-detected serum of the inflammatory myopathies patient is subjected to immunoprecipitation with the HEK293 cell lysis buffer with the overexpressed NXP2 C end, then SDS-PAGE gel electrophoresis is implemented, and the precipitated NXP2 C end is detected by a c-myc antibody to determine whether the NXP2 autoantibody exists in the serum of the inflammatory myopathies patient. The problem that no immunoblotting membrane strip or ELISA (Enzyme-Linked Immuno Sorbent Assay) kit for commercially detecting the NXP2 autoantibody exists in the domestic at present is solved, and the problems of high cost of the ELISA kit autonomously coating an NXP2 antigen, high false positive rate, safety of a radioactive labeling immunoprecipitation method and the like are solved.
Owner:CENT SOUTH UNIV
Immune affinity adsorption material for specifically identifying c-Myc labeled nano antibody
InactiveCN107051397AEasy to getAvoid cumbersome production methodsOther chemical processesSolid sorbent liquid separationGenetic engineeringMyc-tag
The invention belongs to the field of genetic engineering and in particular to a single domain heavy chain antibody (also called as a nano antibody) for a c-Myc label. The single domain heavy chain antibody has an amino acid sequence as shown in SEQ ID NO. 1. The amino acid sequence provided by the invention can be transformed as a precursor by means of a random or site-specific mutagenesis technology, and a mutant with better properties (affinity, specificity, stability and etc.) is obtained. The nano antibody which is high in specificity can be used as an immune affinity adsorption material for c-Myc and can purify a recombinant protein containing the c-Myc label.
Owner:NANCHANG UNIV
Affinity absorption material based on anti-c-Myc label single-domain heavy chain antibody
InactiveCN107081136AEasy to getAvoid cumbersome production methods such as artificial antibodiesOther chemical processesSolid sorbent liquid separationSite-directed mutagenesisMutant
The invention belongs to the field of genetic engineering and discloses a single-domain heavy chain antibody aiming at a c-Myc label. The single-domain heavy chain antibody is named as a nano-antibody and has an amino acid sequence shown in the formula of SEQ ID NO. 1. Through a random or site-directed mutagenesis technique, a mutant has better properties (such as affinity, specificity and stability) is obtained based on the amino acid sequence as a precursor. The high-specificity nanometer antibody can be used as an immuno-affinity absorption material aiming at c-Myc for purification of a c-Myc label-containing recombinant protein.
Owner:NANCHANG UNIV
Immunoaffinity material specifically adsorbing c-Myc label nano antibody
InactiveCN107042098AEasy to getAvoid cumbersome production methods such as artificial antibodiesOther chemical processesPeptide preparation methodsSite-directed mutagenesisMutant
The invention belongs to the field of genetic engineering and in particular relates to a single domain heavy chain antibody (also known as a nano antibody) specific to a c-Myc label, and the single domain heavy chain antibody has an amino acid sequence shown in SEQ ID NO.1. The amino acid sequence provided by the invention can be taken as a precursor, modification is carried out by virtue of a random or site-directed mutagenesis technology, a mutant with better properties (affinity, specificity, stability and the like) can be obtained, and the high-specificity nano antibody can serve as an immunoaffinity adsorption material specific to c-Myc and purify recombinant proteins containing the c-Myc label.
Owner:NANCHANG UNIV
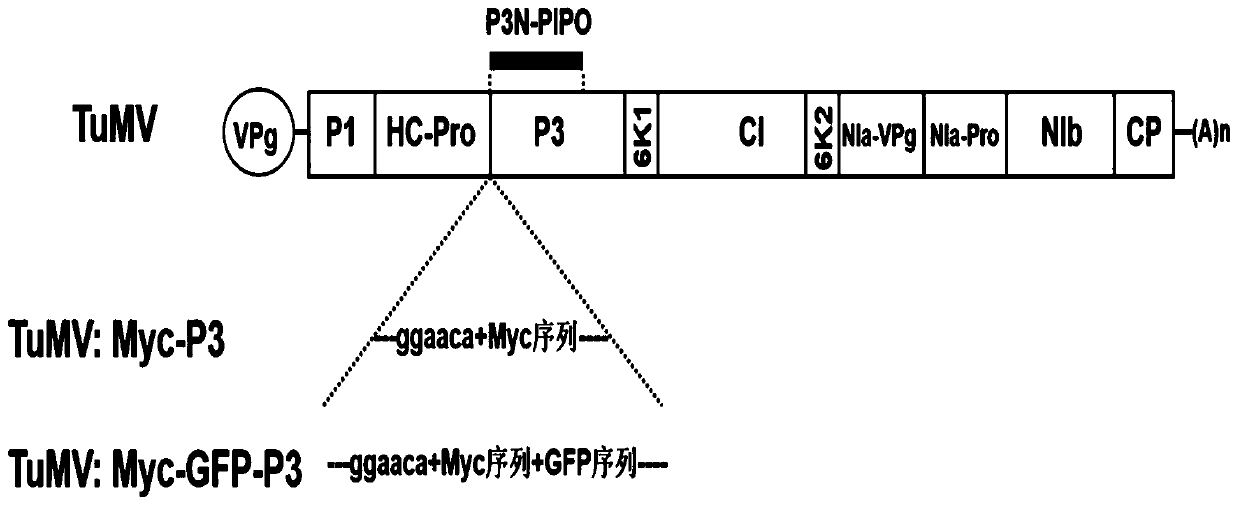
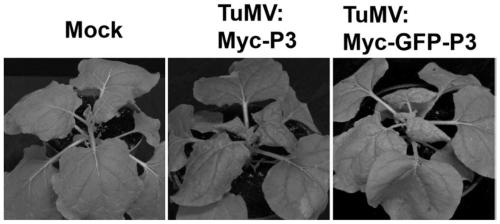
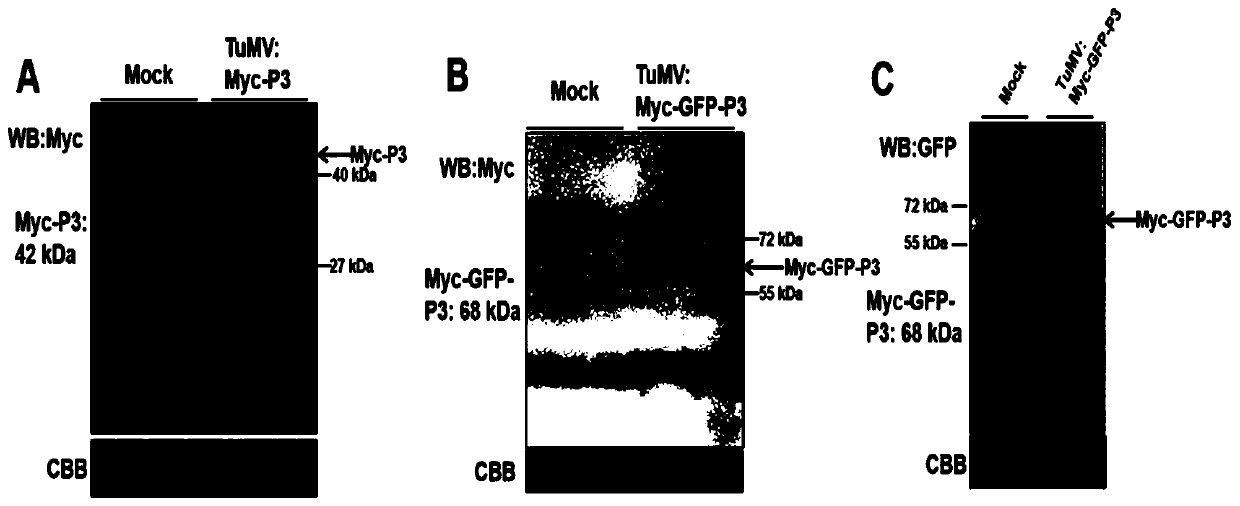
Method for inserting turnip mosaic virus (TuMV) P3 protein into labels and recombinant vector and application of (TuMV) P3 protein
ActiveCN110129362ASpecific tag fusion proteinObserve the real functionSsRNA viruses positive-senseVirus peptidesFluorescenceTurnip yellow mosaic virus
The invention discloses a method for inserting turnip mosaic virus (TuMV) P3 protein into labels and a recombinant vector and application of the TuMV P3 protein. In the method, an Myc label and an Myc-GFP label are inserted into the N-terminal of P3 position in TuMV infected clone pCambia2300-TuMV, and Myc-P3 protein and Myc-GFP-P3 protein with the labels can be expressed; the constructed recombinant vectors pCambia2300-TuMV:Myc-P3 and pCambia2300-TuMV:Myc-GFP-P3 have the characteristics and advantages of infectivity, and the inserted labels do not affect the infectivity of the TuMV; the vectors can express specific label fusion protein and can be used for subsequent analyse of TuMV P3 protein; due to high expression of the fusion protein, P3 is proved to be the important functional gene of the TuMV, immunoprecipitation can be carried out by label antibody, and host proteins interacting with P3 are screened out; the P3 protein inserted into GFP fluorescence labels, and the real function of the TuMV P3 protein infected by virus can be observed.
Owner:INST OF PLANT PROTECTION CHINESE ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com