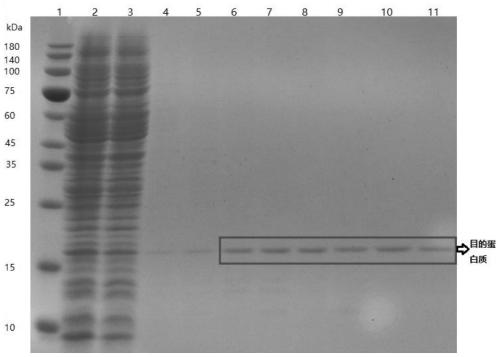
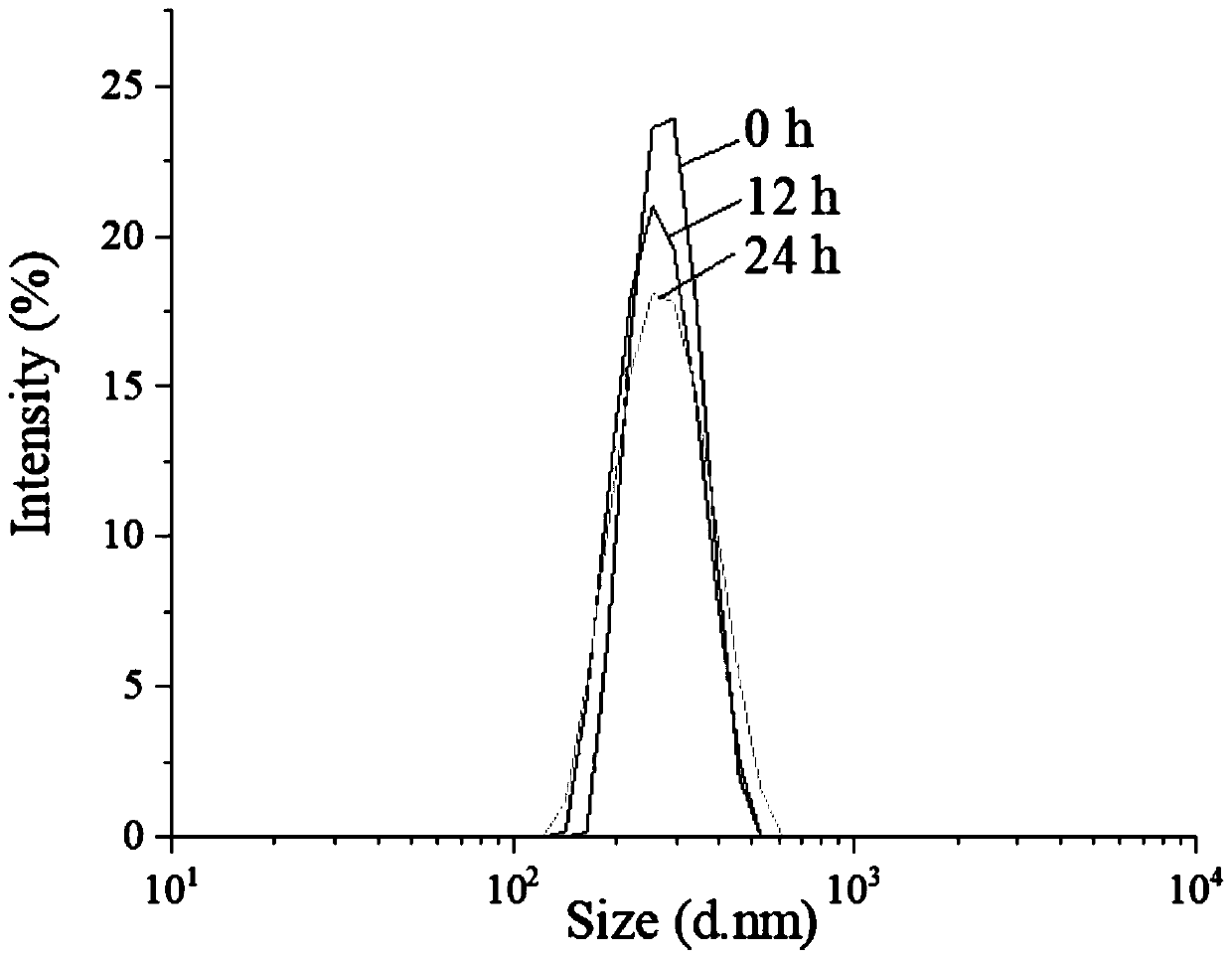
Patents
Literature
246 results about "Polyhistidine-tag" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A polyhistidine-tag is an amino acid motif in proteins that typically consists of at least six histidine (His) residues, often at the N- or C-terminus of the protein. It is also known as hexa histidine-tag, 6xHis-tag, His6 tag, by the US trademarked name HIS TAG (US Trademark serial number 74242707), and most commonly as His-Tag. The tag was invented by Roche, although the use of histidines and its vectors are distributed by Qiagen. Various purification kits for histidine-tagged proteins are available from Qiagen, Sigma, Thermo Scientific, GE Healthcare, Macherey-Nagel, Cube Biotech, Clontech, Bio-Rad, and others.
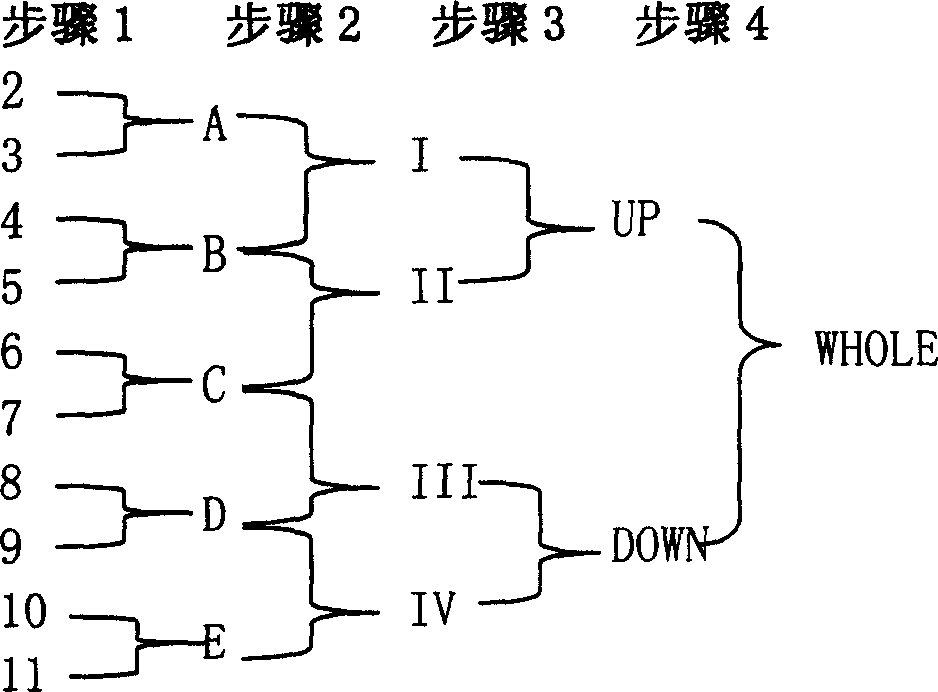
Recombining single chained three specific antibodies of anti CCA, anti CD 3, anti CD 28 through genetic engineering
InactiveCN1563092AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntiendomysial antibodiesSingle-Chain Antibodies
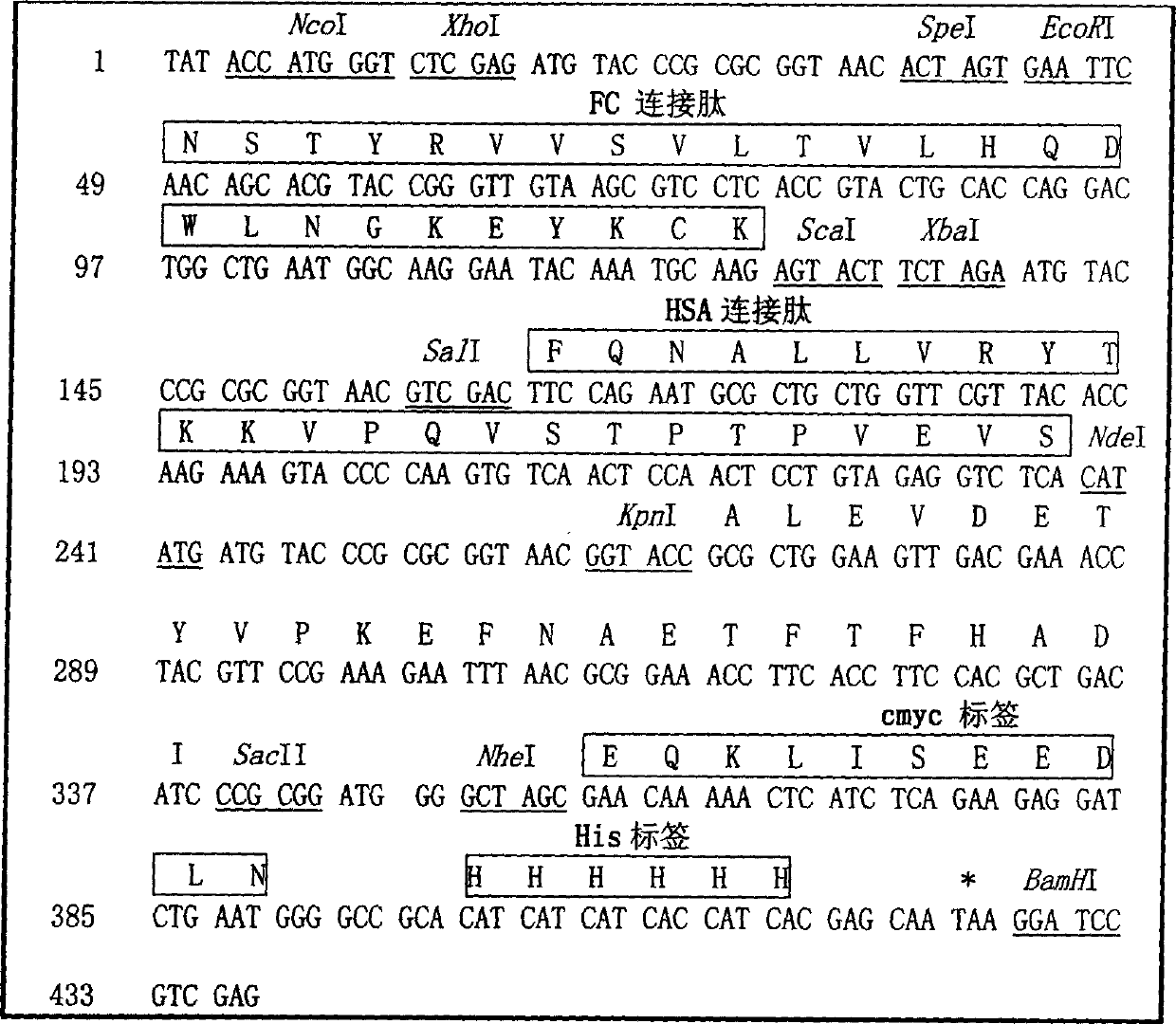
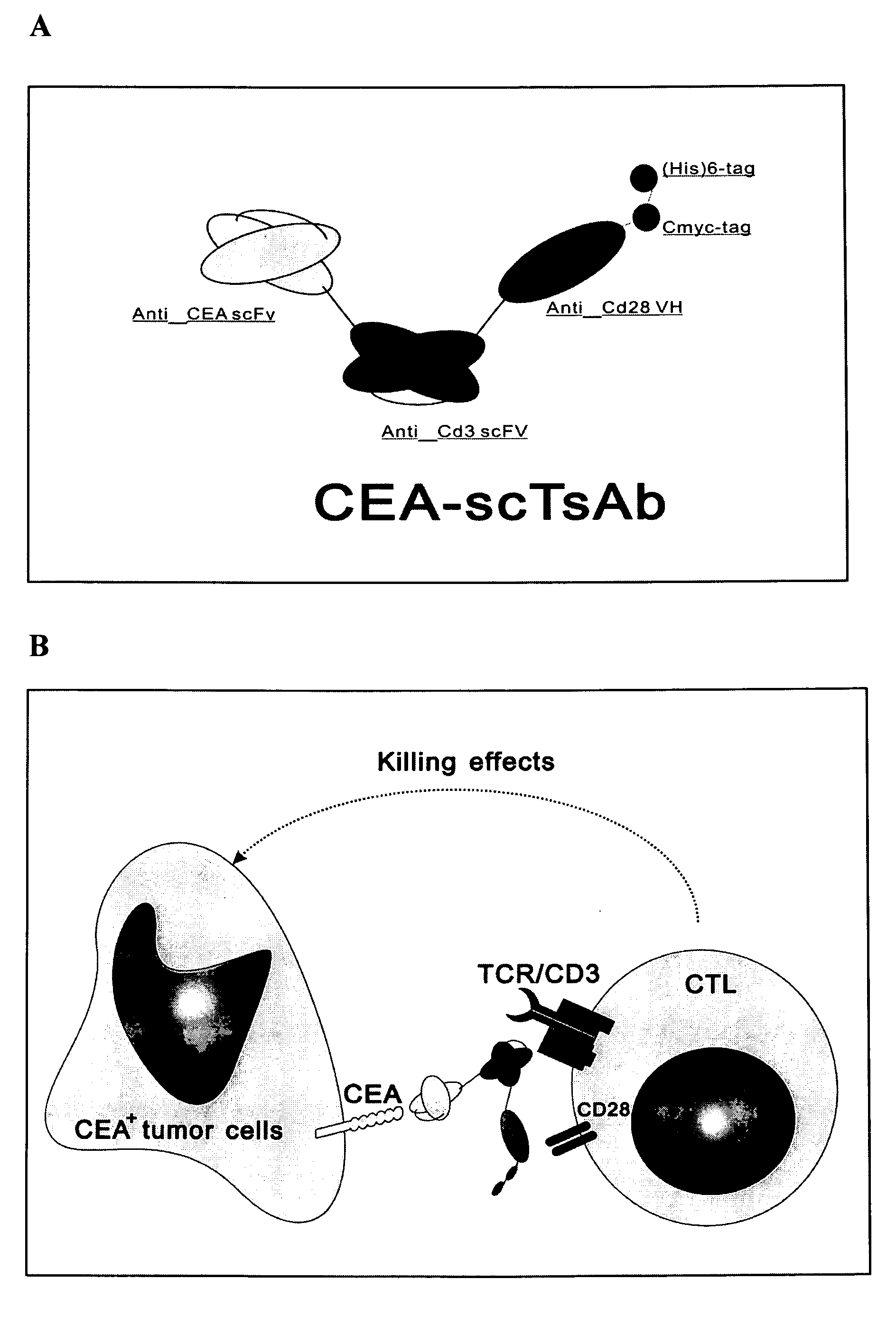
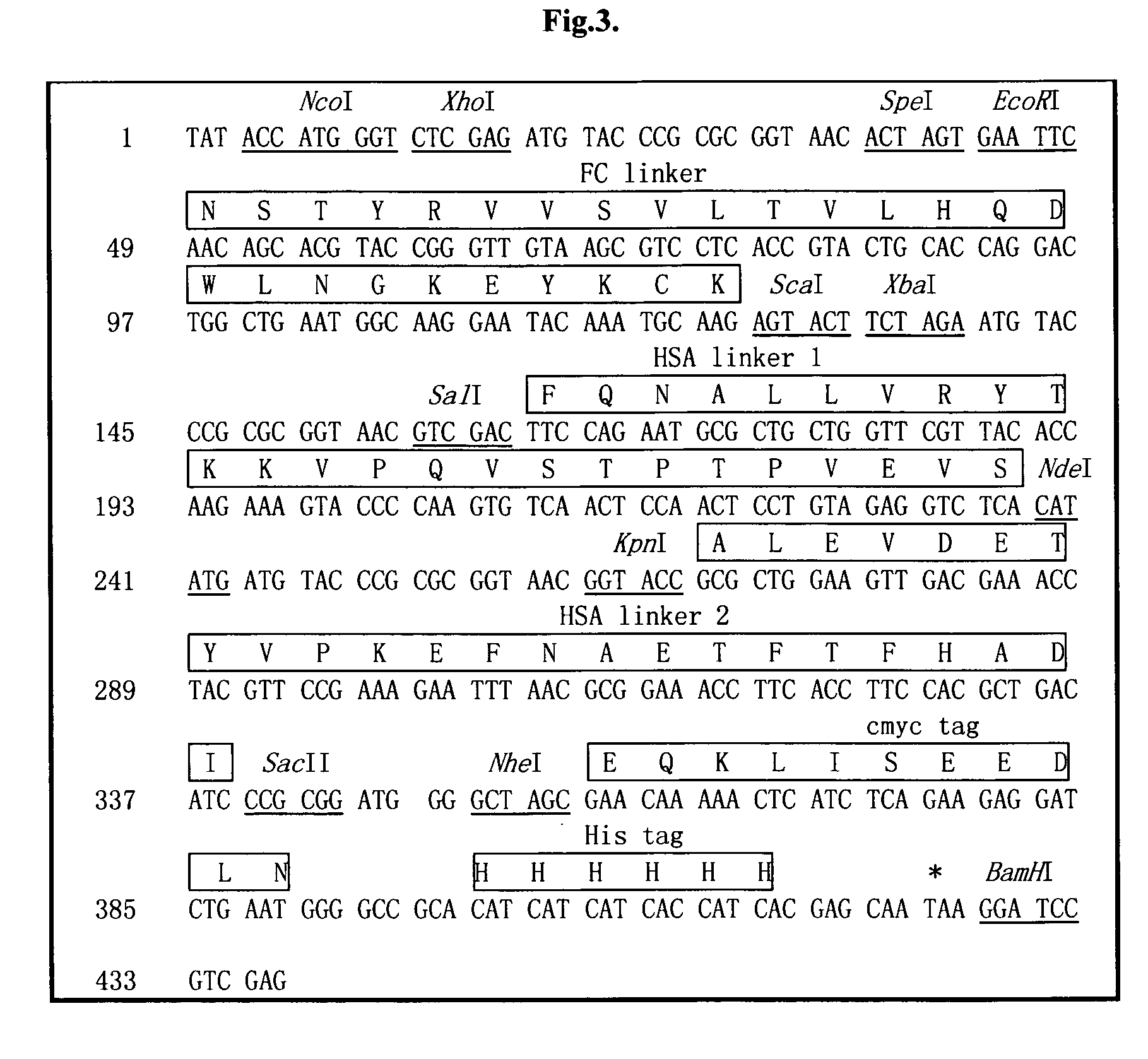
The invention relates to a recombinant single chain tri-specific antibody, and it is characterized by that it is made by successively connecting antibody of antineoplastic related antigen, FC onnecting peptide, antihuman CD3 antibody, HSA connecting peptide and antihuman CD28 antibody. More specifically, said invention relates to a recombinant single chain tri-specific antibody; CEA-TsAb of anti-CEA, anti-CD3 and anti-CD28. Said antibody is made up by using three antibody fragments (unti-CEA single chain antibody, anti-CD3 single chain antibody and anti-CD28 single chain antibody), utilizing two connecting peptides (FC connecting peptide and HSA connecting peptide) and series-connecting them together, and on its C end C-myc tag and (His)6-tag are added. Said invention also relates to a method for constructing expressing and purifying said antibody, including exonic DNA sequence of said antibody, expression vector and host cell containing said vector.
Owner:弘业新创抗体技术股份有限公司
Gene Engineering Recombinant Anti-CEA, Anti-CD3, And Anti-CD28 Single-Chain Tri-Specific Antibody
InactiveUS20090117108A1Increase production costHigh expressionBacteriaSugar derivativesAntibody fragmentsSingle-domain antibody
The invention is related to a recombinant single-chain tri-specific antibody made from anti-Tumor Associated Antigen (TAA) antibody, FC interlinker, anti-CD3 antibody, HSA interlinker and anti-CD28 antibody in turn. Particularly, the invention relates to an anti-CEA, anti-CD3, anti-CD28 recombinant single-chain tri-specific antibody, CEA-scTsAb, which was constructed with three tandem antibody fragments (anti-CEA scFv, anti-CD3 scFv and anti-CD28 single-domain antibody) linked by two interlinkers (FC interlinker, HSA interlinker), and could be appended by C myc tag or histidine tag ((His)6-tag) at the C terminal. It also concerns a method for construction, expression and purification of the antibody. It also offers the encoded DNA sequence of the antibody, expression vectors and host cells for the vectors.
Owner:WANG XIANGBIN +5
Monoclonal antibody blocking enzyme-linked immunosorbent assay (ELISA) kit and method for detecting nonstructural protein (NSP) antibody of foot-and-mouth disease virus (FMDV)
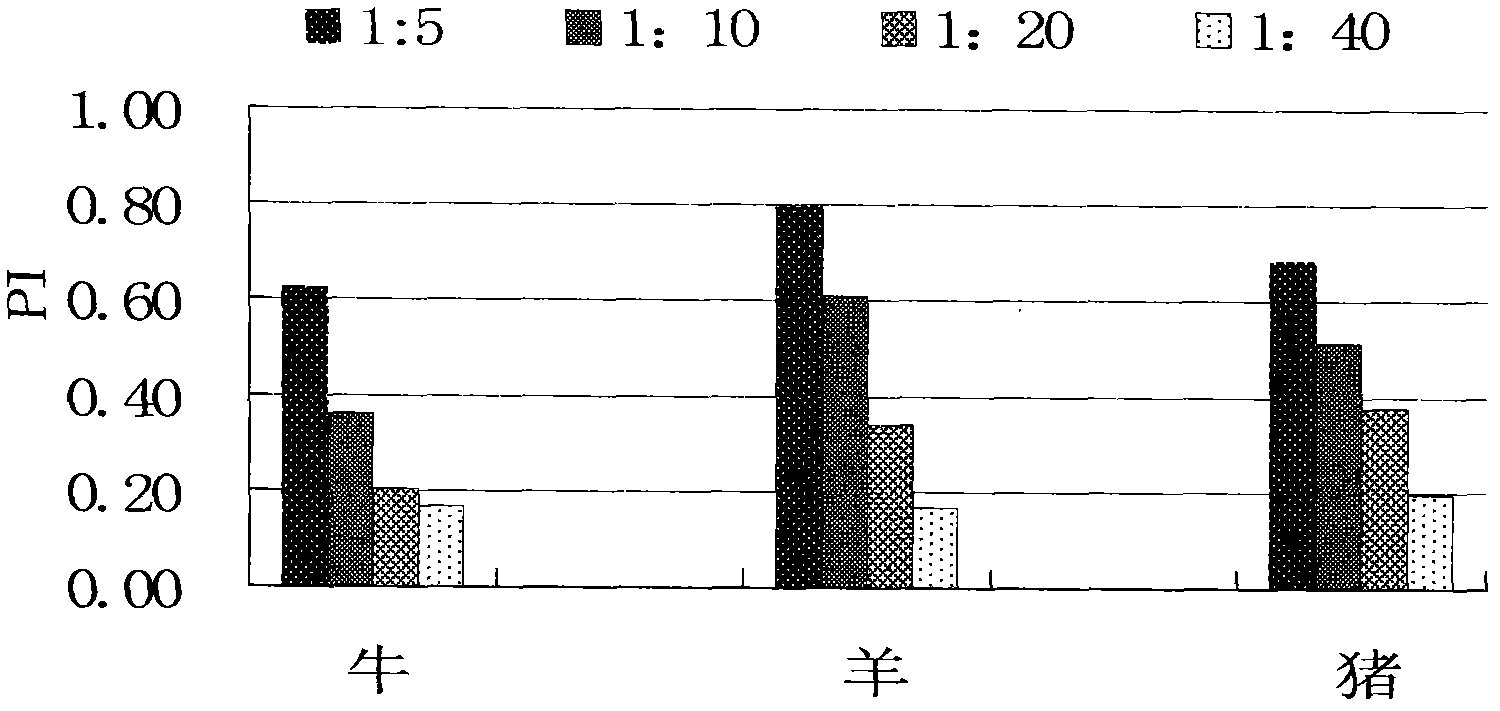
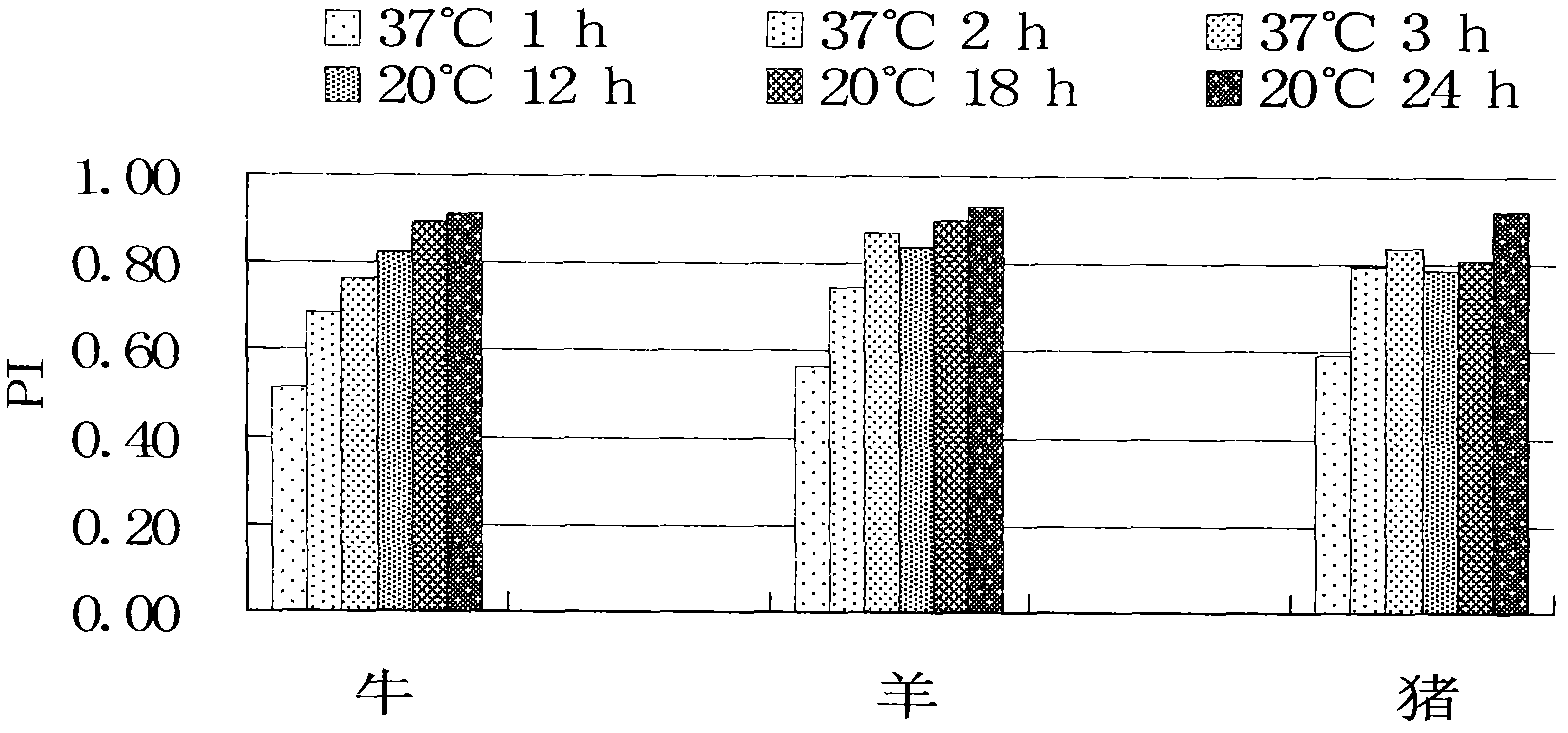
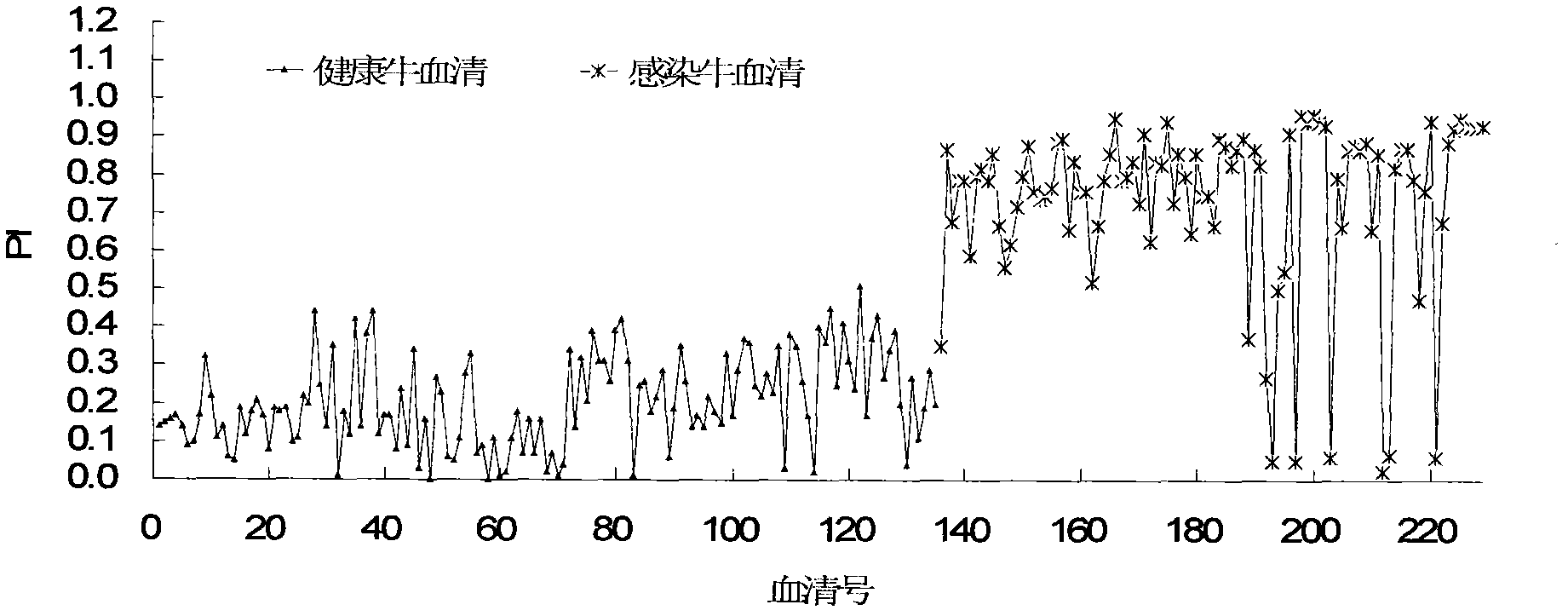
The invention discloses a monoclonal antibody blocking enzyme-linked immunosorbent assay (ELISA) kit and a method for detecting the nonstructural protein (NSP) antibody of a foot-and-mouth disease virus (FMDV) (FMD NSP B-ELISA); the kit comprises ELISA reaction plates, serum diluent, 25 times concentrated detergent, substrate solution, 100* concentrated ELISA detecting antibody, stop buffer, positive control serum and negative control serum; the ELISA reaction plates are two 96-pore high-affinity ELISA reaction plates, firstly 6* groups of amino acid monoclonal antibody or NSP 2C polyclonal antibody, and then FMDV 3ABC or 2C3AB NSP which is expressed by pronucleus and is provided with 6* groups of amino acid labels is captured through the monoclonal antibody or the polyclonal antibody; and compared with other similar kits, the method has higher coincidence rate and higher positive serum detection rate, and is applicable to detecting the serum of cattle, sheep, pigs and other susceptible animals.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Preparation of magnetic silicon dioxide microsphere with metallic ion chelated surface and use thereof
InactiveCN101323454APrevent leakageStrong forceSilicaPeptide preparation methodsProtein targetMicrosphere
The invention relates to a method for preparing magnetic silicon dioxide microspheres with the surfaces chelated with metal ions; the method of the inventioncomprises the following steps of preparation of the magnetic silicon dioxide microspheres, epoxidation of the magnetic silicon dioxide microspheres, carboxylation of the magnetic silicon dioxide microspheres and chelation technique of metal ions. Compared with the existing preparing method, the method of the invention has the advantages of reasonable design, simple operation and large amount of chelation of metal, etc. The magnetic silicon dioxide microspheres with the surfaces chelated with the metal ions, which are prepared by adopting the method, contain cobalt ions, have stronger bonding force with the modified microspheres so as to lead the cobalt ions to be not easy to leak out, and have higher selectivity and proper bonding strength with histidine on the protein, thus leading the magnetic silicon dioxide microspheres with the surfaces chelated with the metal ions to have high selectivity to hexameric histidine tag protein, and being easy to realize the separation and the purification of the target protein; the magnetic silicon dioxide microspheres with the surfaces chelated with the metal ions, which are prepared by adopting the method, can be used for separating the hexameric histidine tag protein.
Owner:SHAANXI NORMAL UNIV
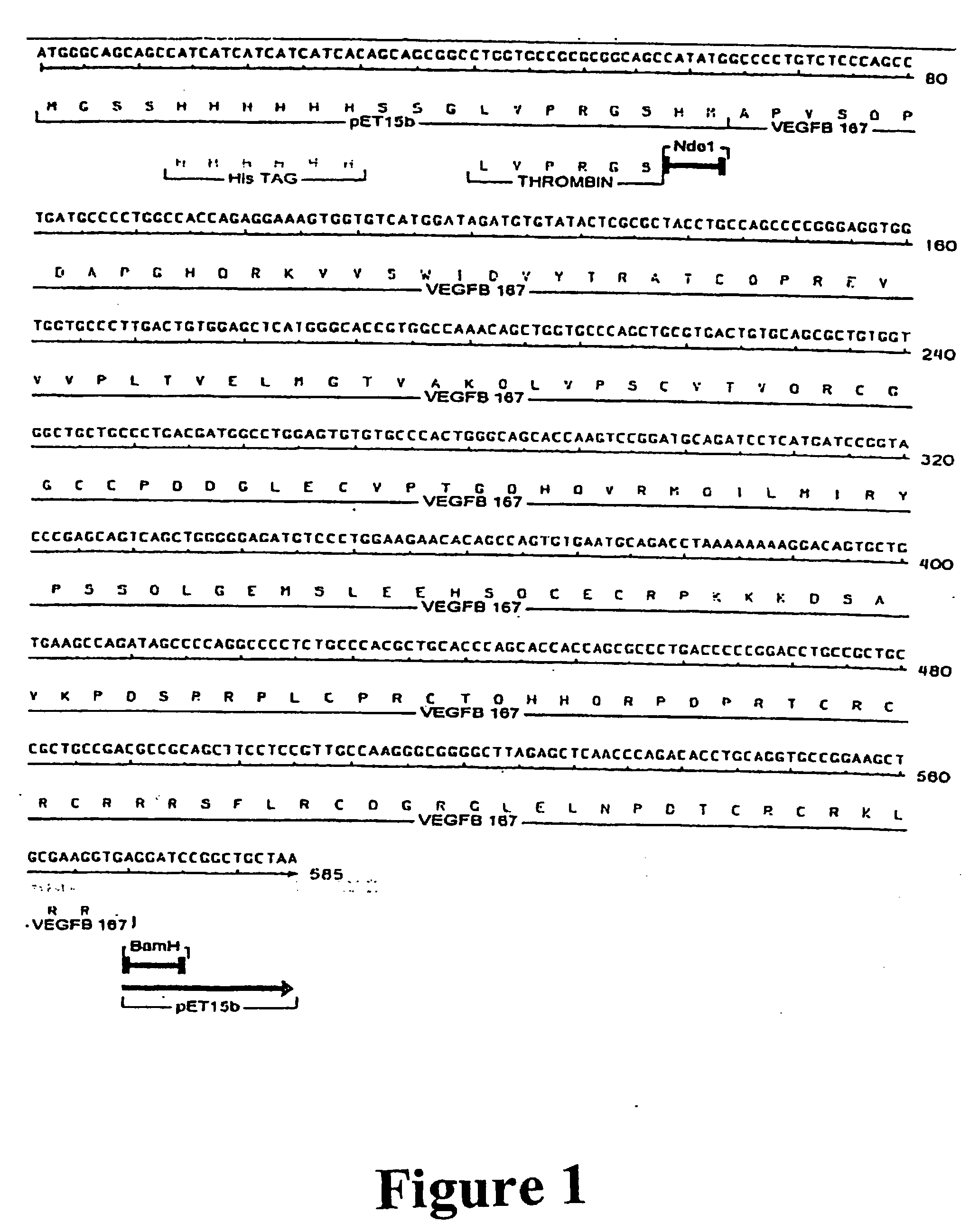
Purification of vascular endothelial growth factor-B
InactiveUS20070111944A1Peptide/protein ingredientsPeptide preparation methodsHEXARecombinant peptide
The present invention provides a method for purifying recombinant peptides, polypeptides or proteins away from truncated or other full-length forms of these molecules. In particular the invention contemplates a method of purifying a vascular endothelial growth factor (VEGF) molecule by subjecting a biological sample containing the molecule to be purified to affinity chromatography under conditions sufficient for the full length molecules to bind and not the truncated or clipped forms. In the preferred embodiment there are two columns, the first is based on affinity for a poly his tag, the second column based on heparin binding affinity. Particularly preferred VEGF molecules are untagged VEGF-B167, hexa-His-tagged VEGF-B167, hexa-His-tagged VEGF-B186 and hexa-His-tagged VEGF-B10-108.
Owner:AMRAD OPERATIONS PTY LTD
Immobilized transaminase and application of immobilized transaminase in synthesis of sitagliptin intermediate
The invention provides immobilized transaminase. The immobilized transaminase is obtained by fixing transaminase, with histidine tag, derived from Mycobacterium vanbaalenii PYR-1 onto an enzyme immobilization carrier; the enzyme immobilization carrier is obtained by derivatization of epoxy resin through IDA (imino diethyl acetic acid) and cobalt chloride or derivatization of ionic chelate resin through cobalt chloride. The immobilized transaminase has the advantages of firmness in combination, low enzyme activity loss, simplicity in separation and high reusability; low cost of an asymmetric conversion process, mild reaction conditions, environment friendliness, simplicity and convenience in operation, easiness in industrial expanding and promising industrial application prospect are realized.
Owner:ABIOCHEM BIOTECH CO LTD
Beta-mannanase, gene, preparation method, vector and host cell
InactiveCN101392241AMeet the need for heat resistanceEasy to purifyBacteriaHydrolasesAmino acid substitutionProkaryotic expression
The invention provides a Beta-mannose which has amino acid sequences shown in SEQ ID: NO.2, SEQ ID: NO.4, SEQ ID: NO.6 and SEQ ID: NO.8. Or substitution, depletion or addition of one or multiple amino acids is carried out to the amino acid sequences shown in the SEQ ID: NO.2, SEQ ID: NO.4, SEQ ID: NO.6 or SEQ ID: NO.8 to obtain amino acid sequences of Beta-mannose with the same activity. The invention also provides a gene for coding the Beta-mannose, a recombinant vector containing the gene and a host cell containing the recombinant vector. The invention further provides a preparation method of the Beta-mannose, including the culture of the host cell provided by the invention. The Beta-mannose provided by the invention has heat-resisting property and acid resistance and a prokaryotic expression system established by the invention can be utilized for production. Furthermore, six histidine tags can be utilized for purification.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Immobilized metal ion affinity chromatograph (IMAC) material, and preparation and application thereof
ActiveCN106622181AAchieve specific captureAchieve releaseOther chemical processesSolid sorbent liquid separationComplete proteinPhysical chemistry
The invention relates to a novel immobilized metal ion affinity chromatograph (IMAC) material, and a preparation and application thereof. According to the novel IMAC material, the surface of the traditional IMAC material is subjected to coating modification, the naked tail end of protein enters the coating through the selectivity of the surface coating pore sieving action to be captured by metal ions, and other integrated protein has high mass transfer resistance and enters the coating difficultly. The material combines the strong affinity of the IMAC material and the high selectivity of the surface coating pore sieving action, and is further applied to specific capture, release and purification of histidine label protein in the technical fields of biological analysis, biological analysis and biology.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Enzyme immobilization method and application
InactiveCN105969757AEasy to getGood repeatabilityChemical industryOxidoreductasesAlcoholPolyhistidine-tag
The invention discloses a simple and convenient method for recombinase immobilization. According to the method, the characteristic that a histidine label of recombinant protein and metal ion affinity chromatography resin are combined specifically is utilized, and therefore recombinase is immobilized on the resin, and the immobilized recombinase is prepared. Immobilized carbonyl reductase is used for continuous production of chiral alcohol, and the space time yield is greatly increased.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
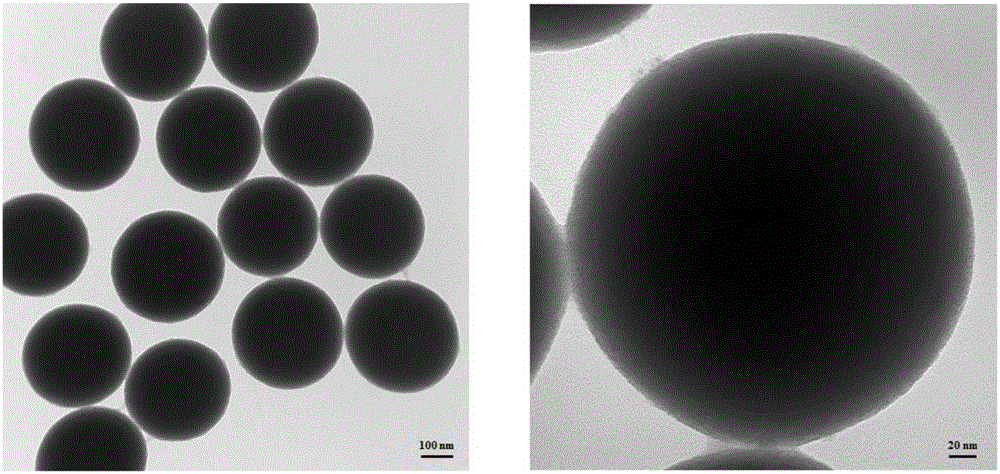
Magnetic microsphere as well as preparation and preparation method thereof used for purifying histidine tag protein
ActiveCN101745353AGuaranteed high purification efficiencyQuick separation characteristicsPeptide preparation methodsMicroballoon preparationMicrosphereMagnetic response
Owner:SHANGHAI JIAO TONG UNIV
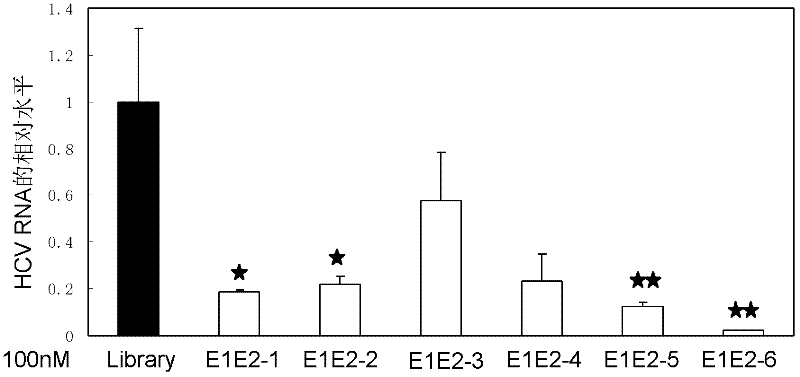
Nucleic acid aptamer capable of identifying HCV E1E2 (hepatitis C virus E1E2), nucleic acid aptamer derivatives and screening method and application thereof
InactiveCN102229932AHigh affinityLow costGenetic material ingredientsMicrobiological testing/measurementProtein detectionNucleotide
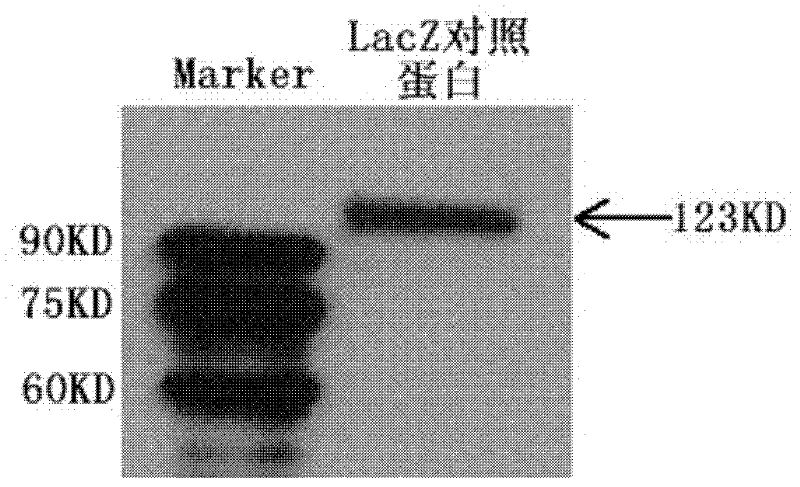
The invention discloses a nucleic acid aptamer capable of identifying HCV E1E2 (hepatitis C virus E1E2), which is a DNA fragment having a sequence shown in any one of SEQ ID No.1 to SEQ ID No.5. Derivatives of the nucleic acid aptamer include nucleotide-substituted RNA nucleic acid aptamers, skeleton-modified derivatives, peptide nucleic acid derivatives and derivatives of nucleic acid aptamer conjugated with radioactive molecules. A method for screening the nucleic acid aptamer comprises the following steps: firstly preparing HCV E1E2 proteins with histidine tags and then preparing pET200 / D / LacZ proteins with histidine tags, then immobilizing the proteins and designing a random nucleic acid library, and finally screening nucleic acid aptamers. Specifically, the screening step comprises the following steps: pre-treating the DNA library, performing reverse screening, performing forward screening, and repeatedly screening, to obtain the nucleic acid aptamer with strong competitiveness and optimized sequence length. The nucleic acid aptamer and derivatives thereof can be applied to preparing HCV E1E2 protein detection kits or diagnostic kits as well as agents for suppressing HCV infection in liver cells.
Owner:HUNAN UNIV
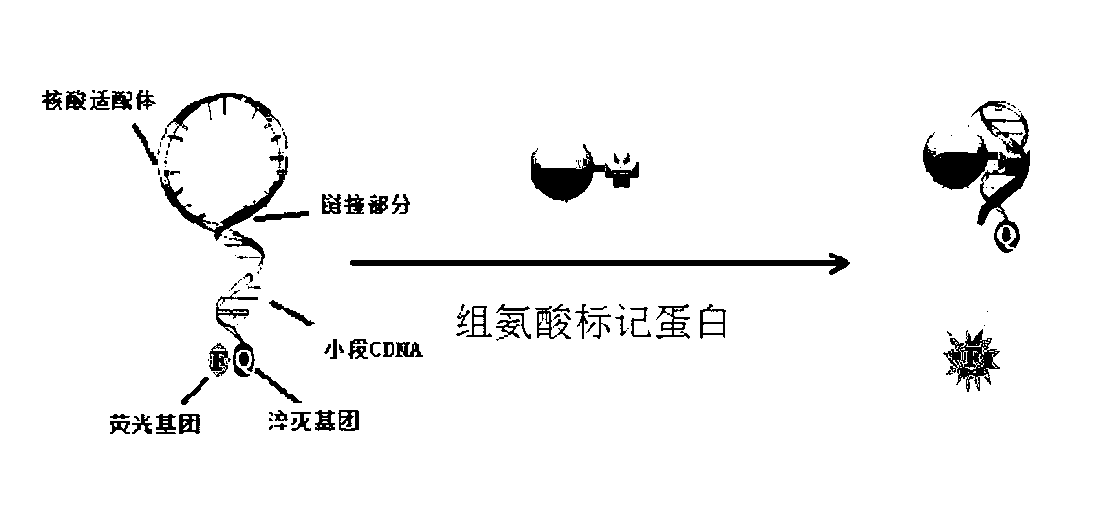
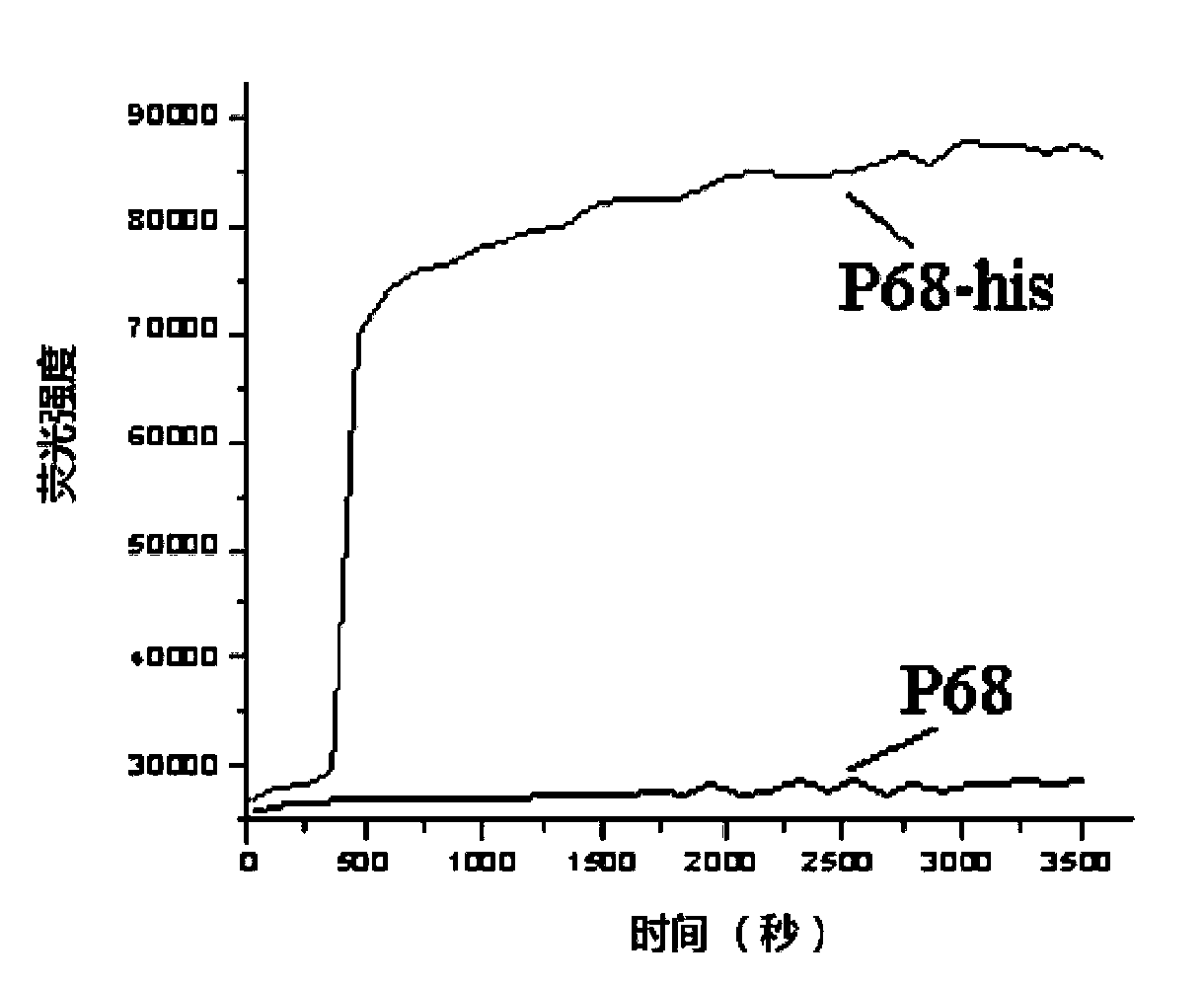
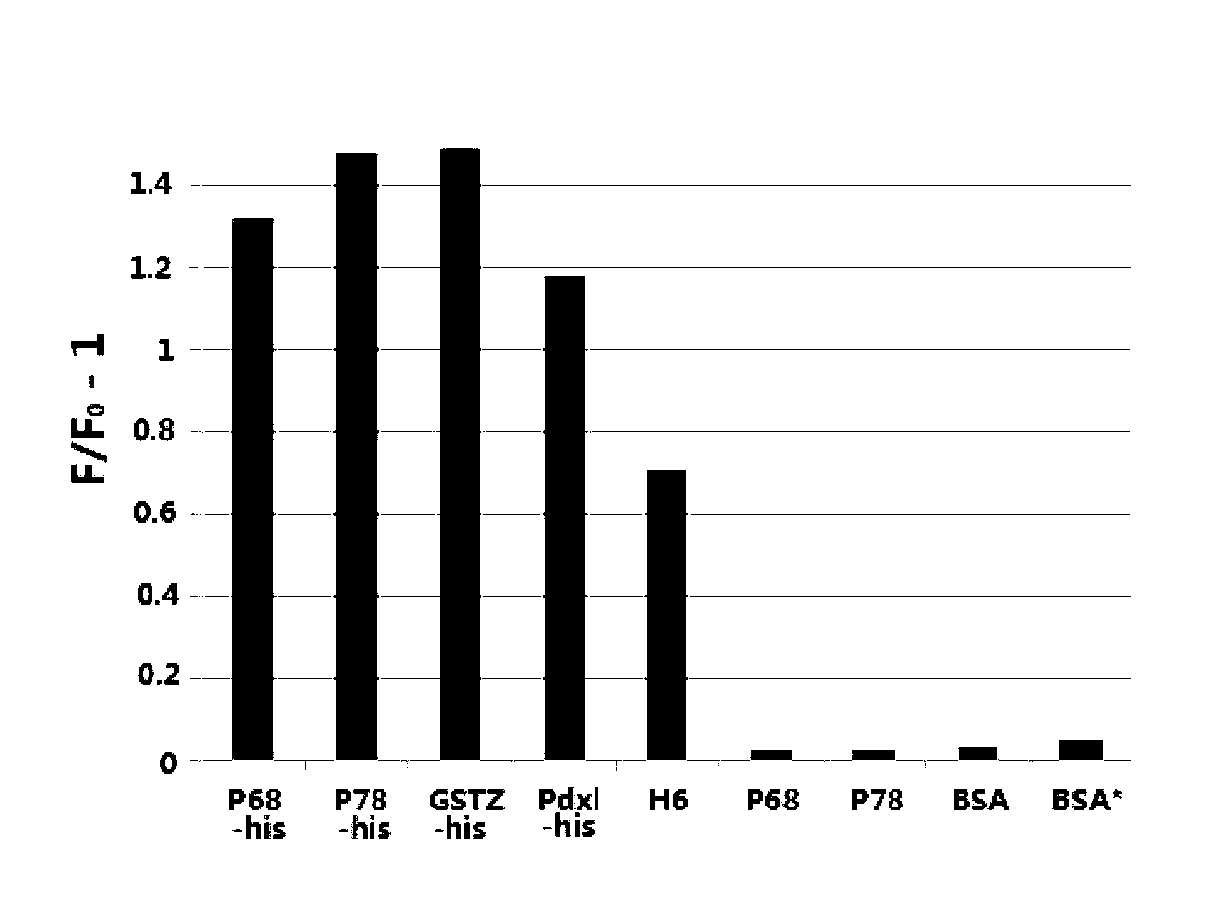
Nucleic acid aptamer molecular beacon probe for detecting histidine-tag recombinant proteins and detection method thereof
ActiveCN102719430ARapid detection and quantitative analysisHigh affinityMicrobiological testing/measurementFluorescence/phosphorescenceProtein markersPolyhistidine-tag
The invention discloses a nucleic acid aptamer molecular beacon probe for detecting histidine-tag recombinant proteins and a direct general method for detecting the histidine-tag recombinant proteins in cell lysates by the probe. The nucleic acid aptamer probe comprises sequences shown in SEQIDNO.1 and SEQIDNO.2; the end 3' of the sequence shown in the SEQIDNO.1 is bridged with the end 5' of the sequence shown in the SEQIDNO.2 through PEG36; the end 5' of the sequence shown in the SEQIDNO.1 is modified with fluorescein FAM; and the end 3' of the sequence shown in the SEQIDNO.2 is modified with a quenching group Dabcy1. According to the nucleic acid aptamer molecular beacon probe, the expression of the histidine-tag recombinant proteins in the cell lysates can be quickly and quantificationally analyzed without protein tagging, and the method disclosed by the invention has the advantages of universality and low cost, and the advantage that the histidine-tag recombinant proteins can be simply, quickly and quantificationally analyzed.
Owner:HUNAN UNIV
Histidine tag protein affinity purification material and application thereof
InactiveCN106492770AFull binding affinityReduce distractionsIon-exchange process apparatusOther chemical processesPolyhistidine-tagMolecular imprinting
The invention relates to a histidine tag protein affinity purification material. The histidine tag protein affinity purification material is prepared from a molecular imprinting material based on a metal chelation function, an immobilized metal chelation affinity chromatography material (IMAC) utilized as a matrix material and a histidine tag utilized as a template module by using a molecular imprinting technique. The histidine tag protein affinity purification material combines the good affinity of the metal chelation function and the high selectivity of the molecular imprinting material, and can be further applied to high-purity purification of the histidine tag protein.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
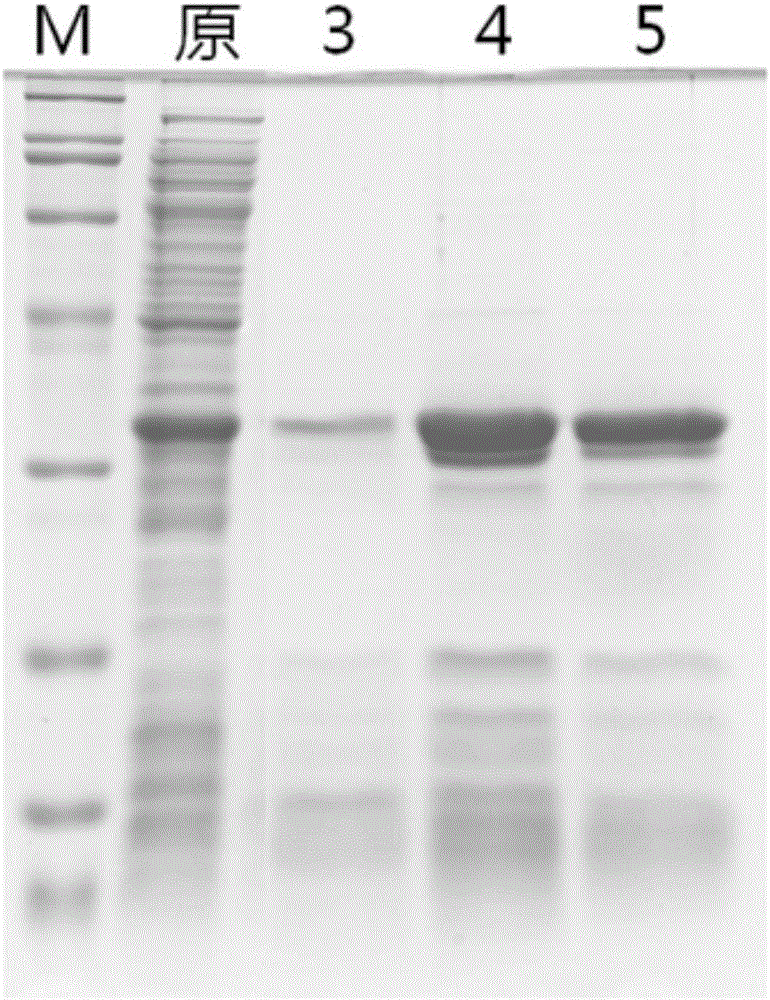
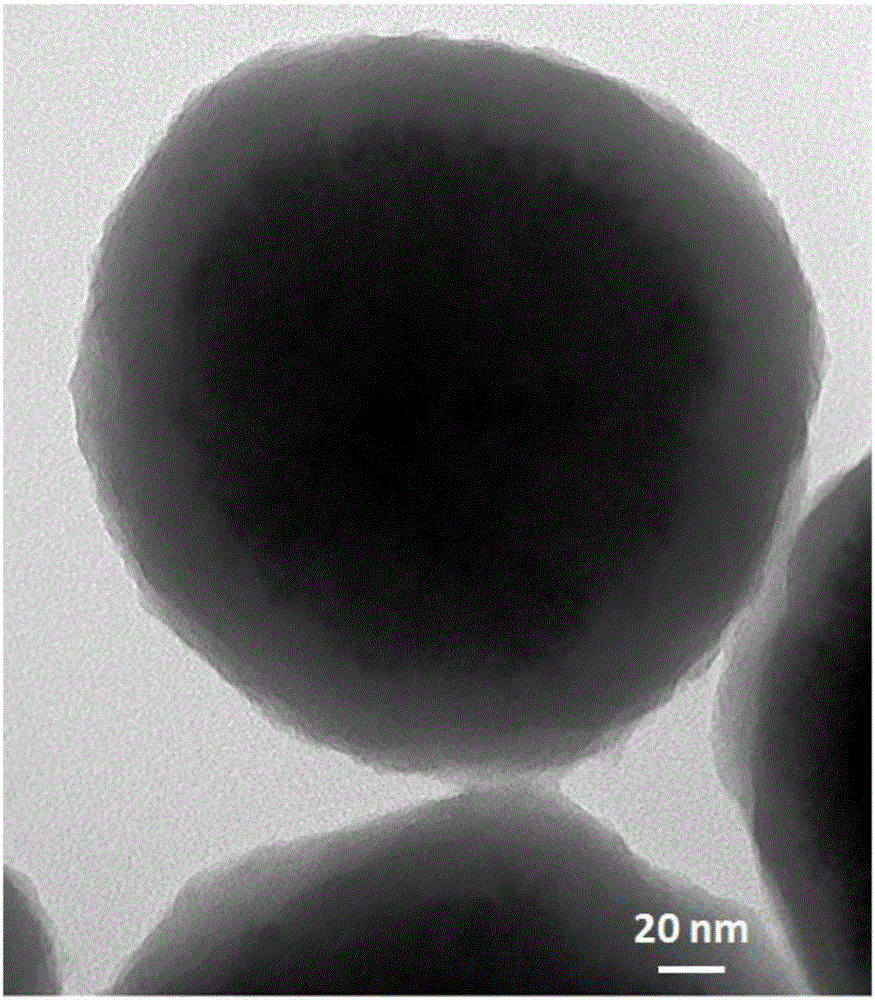
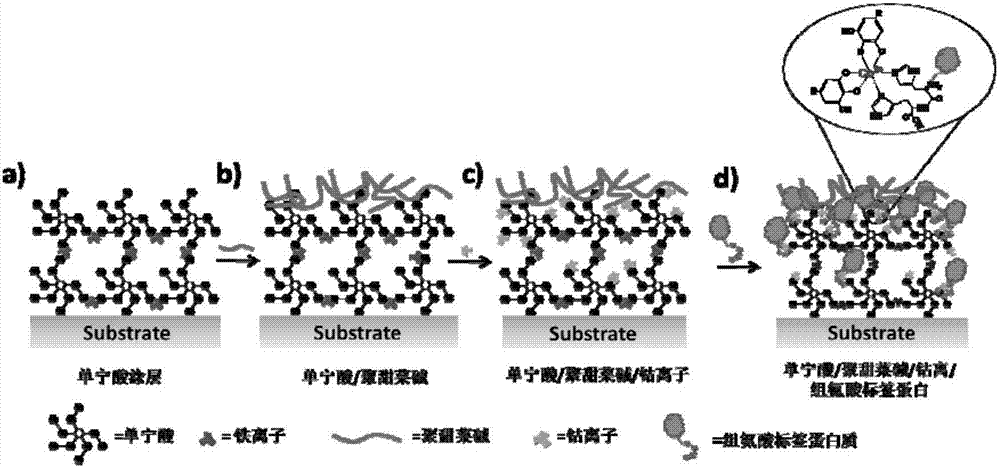
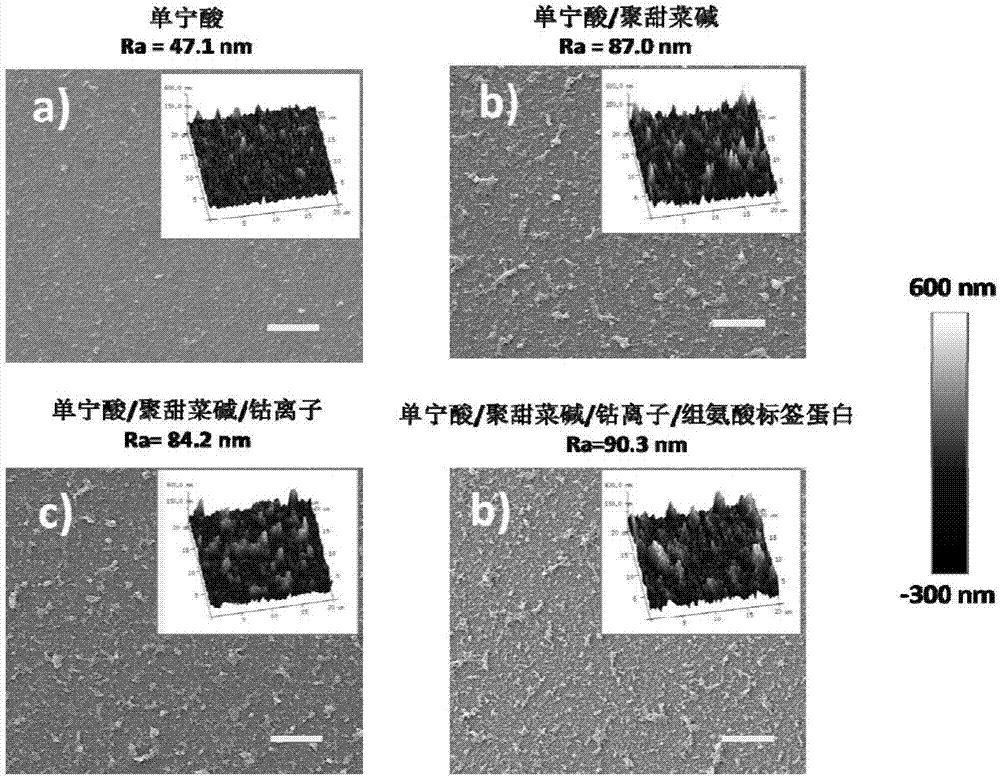
Method for purifying and directionally immobilizing histidine tag protein and application
ActiveCN107446916ARapid purificationEasy to purifyOther chemical processesOn/in organic carrierPhysical chemistryPolyhistidine-tag
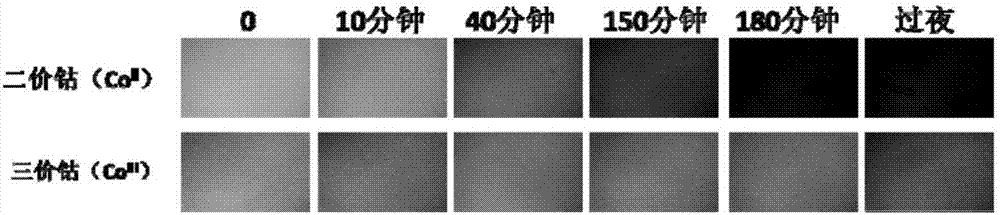
The invention discloses a method for purifying and directionally immobilizing histidine tag protein and application. According to the method disclosed by the invention, tannic acid, metal ions and a hydrophilic anti-protein adhesion polymer are self-assembled and modified on the surface of a carrier to obtain a material surface which is efficiently chelated with cobalt ions, so that one-step purification and immobilization of the histidine tag protein is realized; a ligand of trivalent cobalt has inertia and divalent cobalt ions are oxidized into trivalent cobalt ions so that the stability of the immobilized protein can be improved. The method disclosed by the invention has the advantages of simple process and low price; a separation and purification or immobilization operation process for the histidine tag protein of the method is simple and effective, so that the technology has a wide application prospect.
Owner:DALIAN UNIV OF TECH
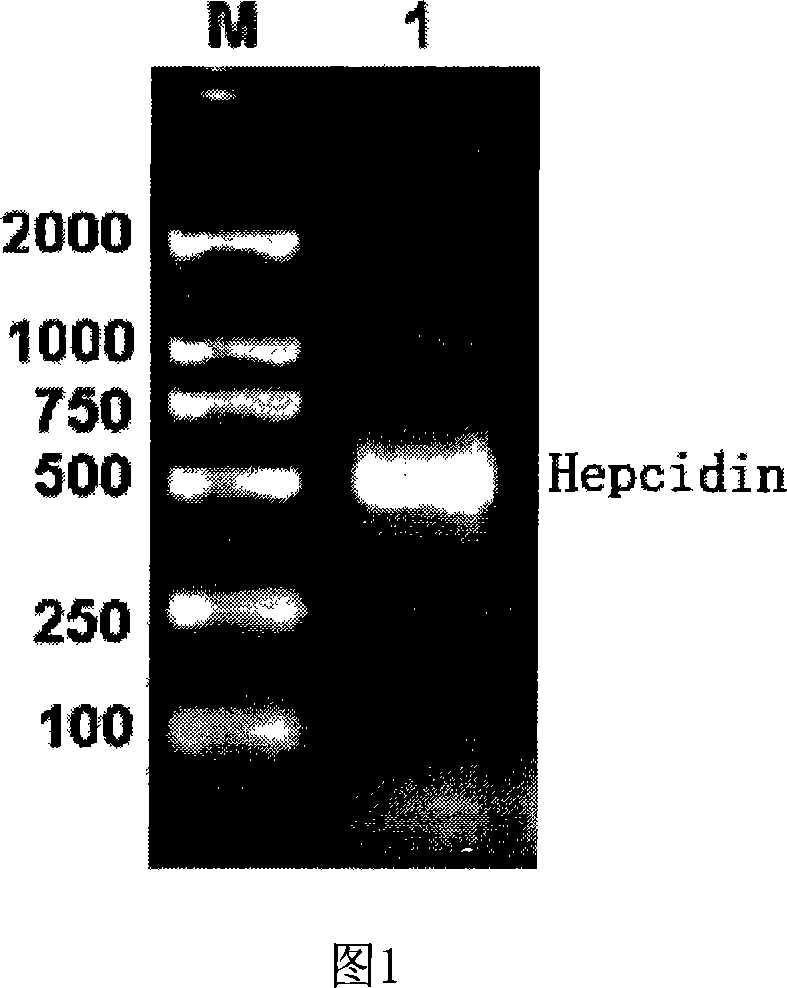
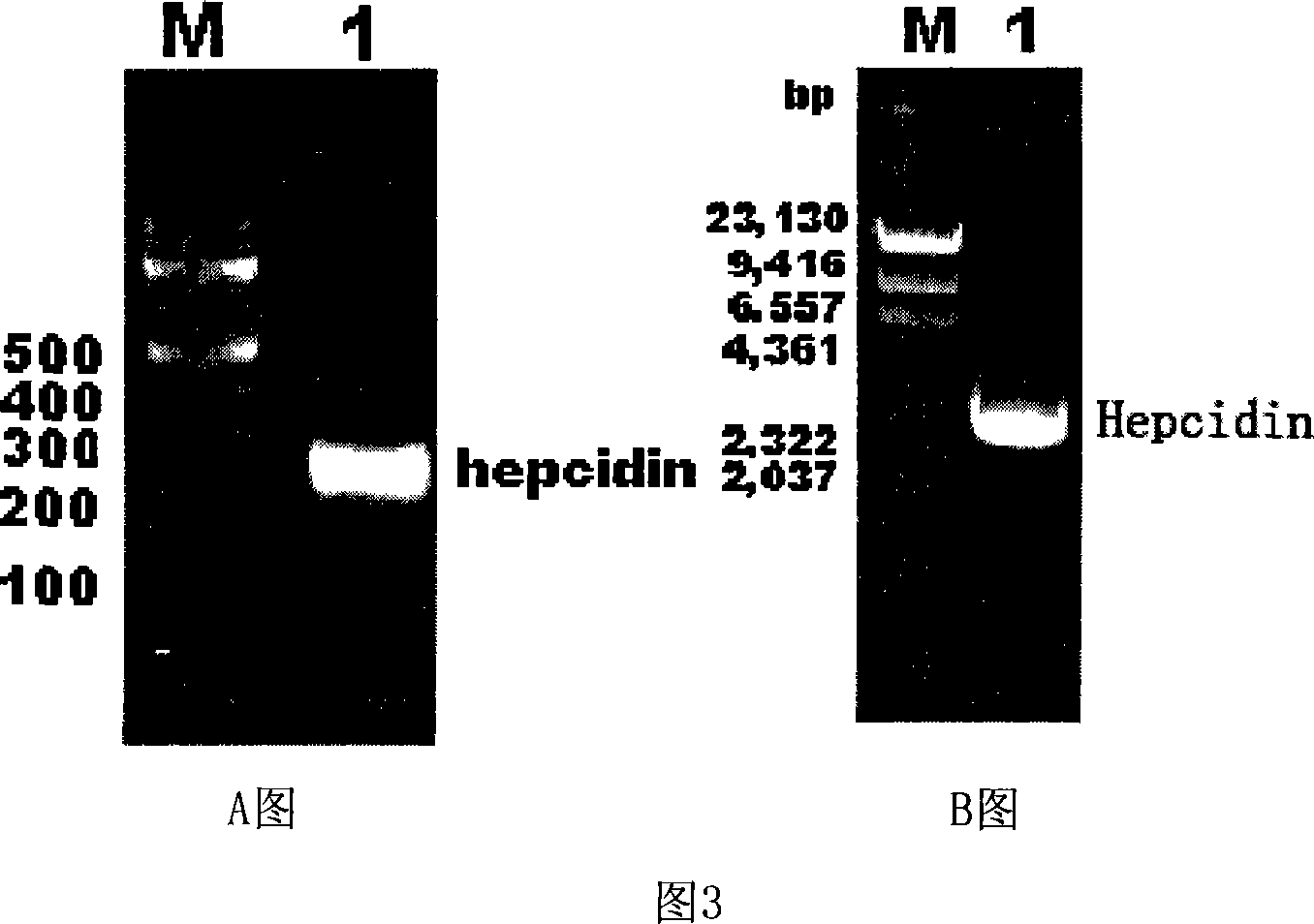
Expression carrier for black porgy antibiotic peptide Hepcidin and expression product and constructing preparation method
The invention discloses an expressing carrier of black porgy antibiotic peptide Hepcidin, expressing product and preparing method, which is characterized by the following: incorporating E. coliTrc promoter, protein project improving CKS gene and procaryotic expressing carrier pTrc-CKS of histidine tag (His-Tag); connecting to black porgy Hepcidin gene; constructing pTrc-CKS / hepcidin expressing plasmid; possessing P3C enzyme cutting site; fusing six histidine on C end; getting CKS-hepcidin; purifying through C end His-Tag affinity chromatography; getting the purified product; cutting CKS with P3C enzyme in fuse protein; getting the purified Hepcidin.
Owner:XIAMEN UNIV
Production of antimicrobial proteins in fusion proteins
InactiveUS7785828B1Polypeptide with affinity tagMicrobiological testing/measurementCysteine thiolatePolyhistidine-tag
The invention relates to a method of producing cysteine containing polypeptides in fusion proteins by recombinantly expressing in a host cell sequences encoding an antifungal polypeptide, a maltose binding protein, and a histidine tag. The method is carried out in the presence of a reducing agent to prevent misfolding of the fusion proteins.
Owner:PIONEER HI BRED INT INC
Protein chip and preparation and detection method thereof
InactiveCN103197081AFunction does not affectEasy to separate and purifyPreparing sample for investigationScattering properties measurementsHigh fluxProtein insertion
The invention provides a protein chip. The affinity of histidine labels and cations is utilized to fix protein to be fixed on a chip solid phase carrier on a chip carrier. The combined mode basically does not generate influences to functions of protein; meanwhile the carrying of the histidine labels simplifies the separation purification of protein as well as a fixing method of an SPR chip; the preparation of the protein chip is simple and is easy to implement; and the protein chip is low in cost, and high in throughput.
Owner:SHENZHEN QIANHAI ICECOLD IT CO LTD
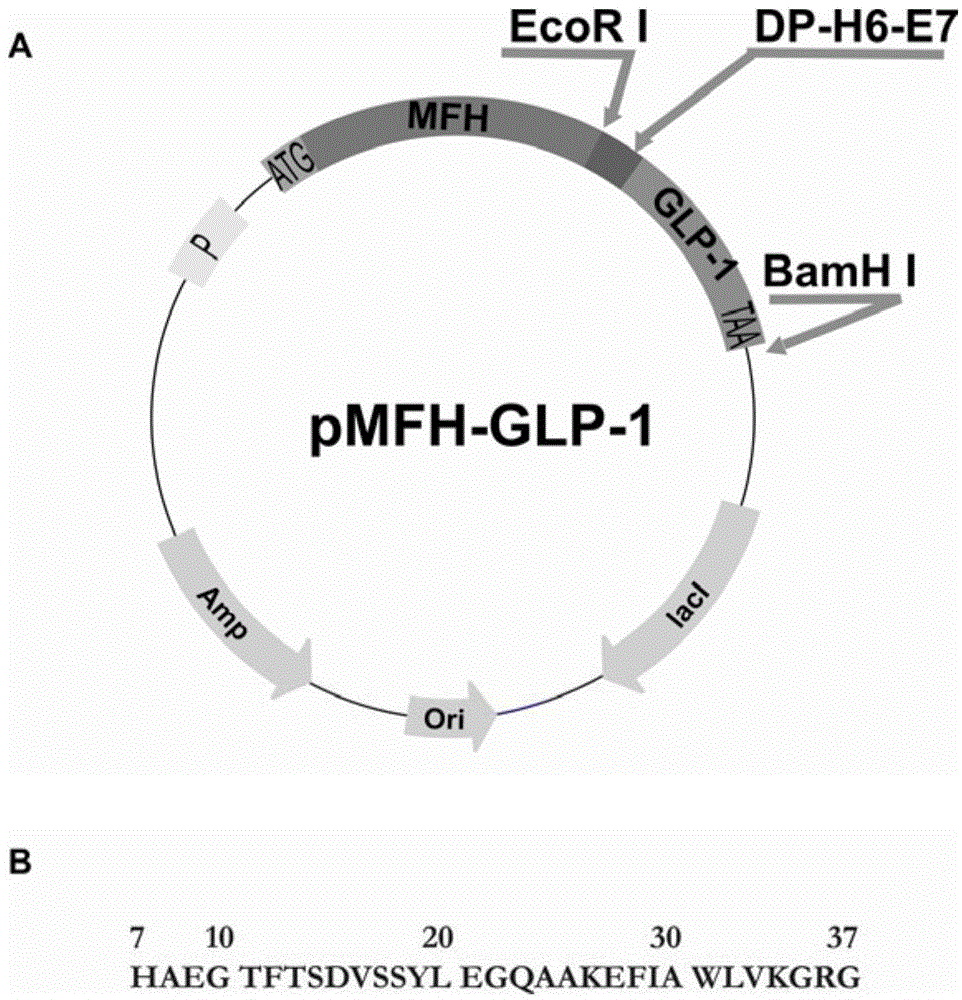
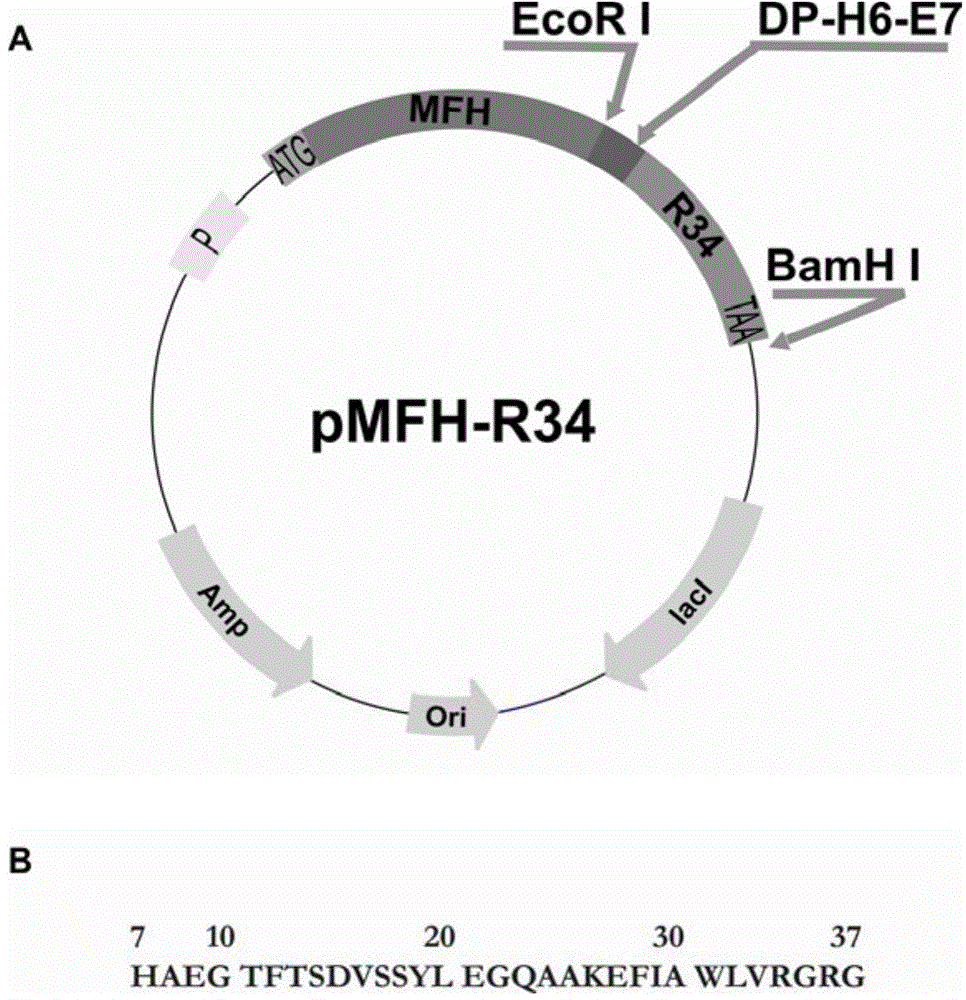
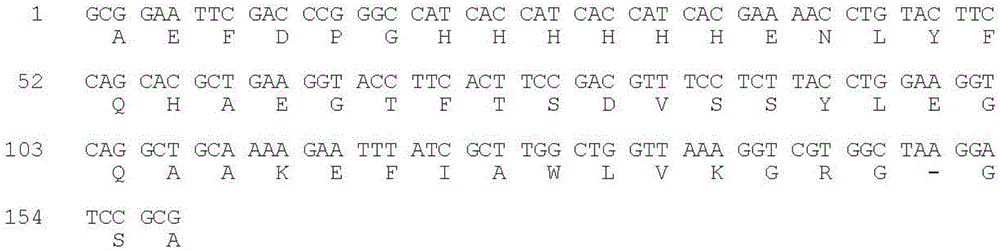
Method for preparation of GLP-1 polypeptide or analogue thereof through MFH fusion protein and application of GLP-1 polypeptide or analogue thereof
ActiveCN104098702AAvoid insolubleAvoid the disadvantages of multiple impuritiesPeptide/protein ingredientsMetabolism disorderEscherichia coliStructural formula
The invention discloses a method for preparation of GLP-1 polypeptide or an analogue thereof through MFH fusion protein and application of the GLP-1 polypeptide or the analogue thereof, and belongs to the field of biotechnology. The structural formula of the MFH fusion protein of the GLP-1 polypeptide or the analogue thereof is MFH-DP-H6-E7-PEP, wherein the MFH is a protein fusion vector, DP is a formic acid hydrolysis site, H6 is a histidine label, E7 is a special protease cutting site and PEP is the GLP-1 polypeptide or the analogue thereof. The DNA of the code DP-H6-E7-PEP is connected to pMFH plasmid to obtain pMFH-PEP plasmid and then the pMFH-PEP plasmid is transferred into escherichia coli, MFH-DP-H6-E7-PEP fusion protein is obtained through prokaryotic expression, and formic acid hydrolysis, special protease hydrolysis and affinity chromatography are performed, so that GLP-1 polypeptide or the analogue thereof is obtained. According to the invention, the purposes of large-scale efficient expression of polypeptide, less steps of a peptide release process, mild condition and low production cost are achieved, and the GLP-1 polypeptide or the analogue thereof is suitable for industrial production.
Owner:HUBEI UNIV OF TECH
Substrate and method for producing the substrate
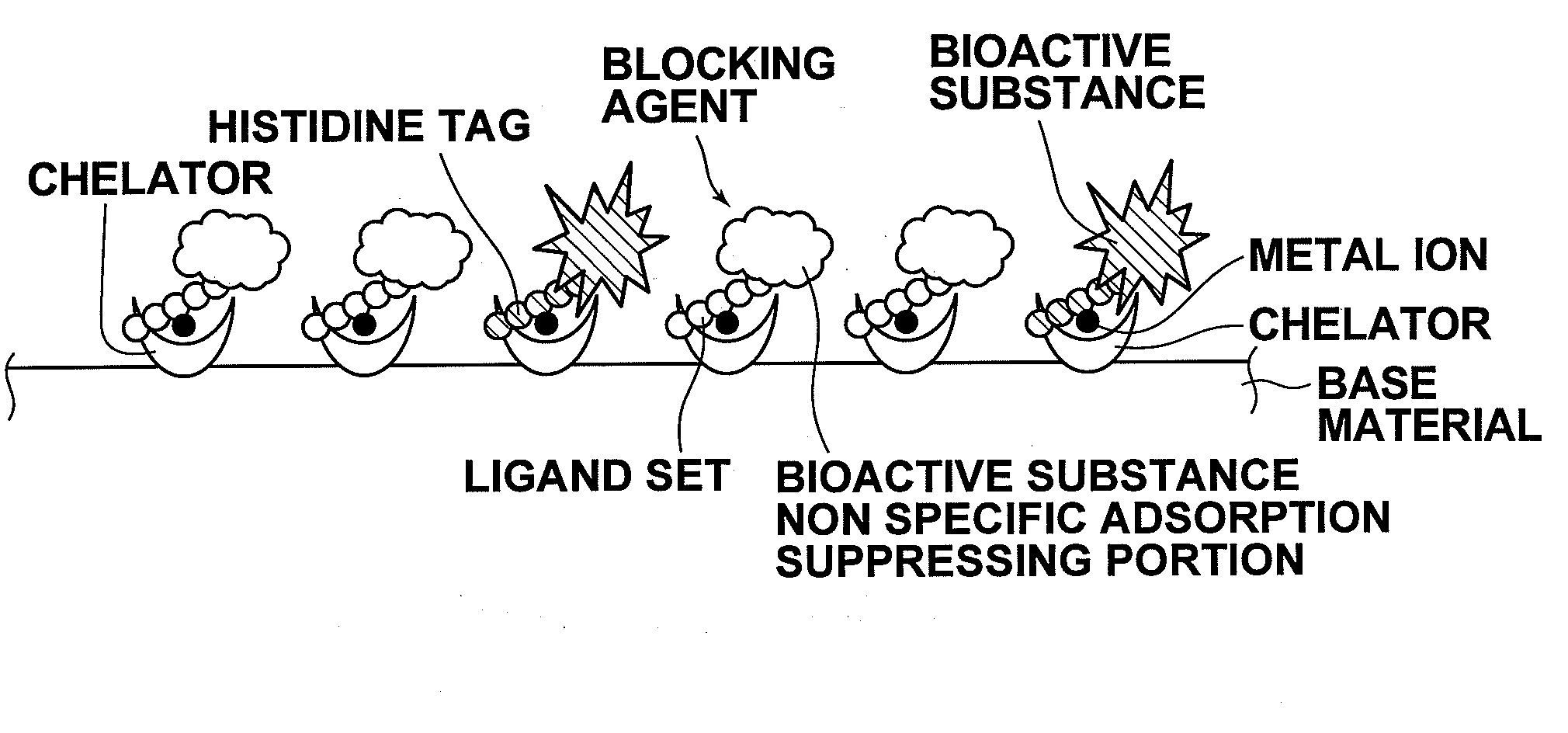
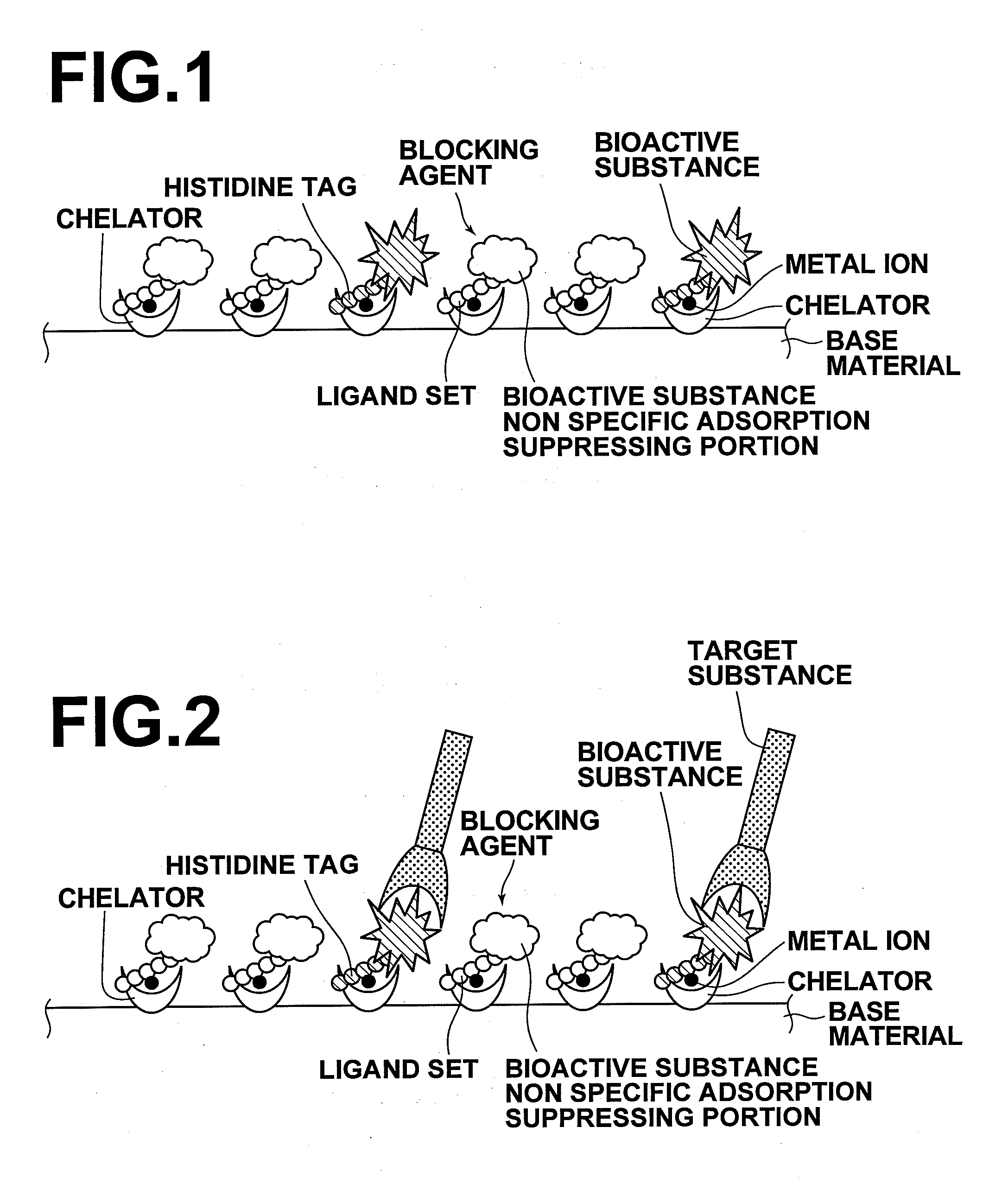
InactiveUS20090081371A1Improve signal-to-noise ratioBioreactor/fermenter combinationsBiological substance pretreatmentsVolumetric Mass DensityPolyhistidine-tag
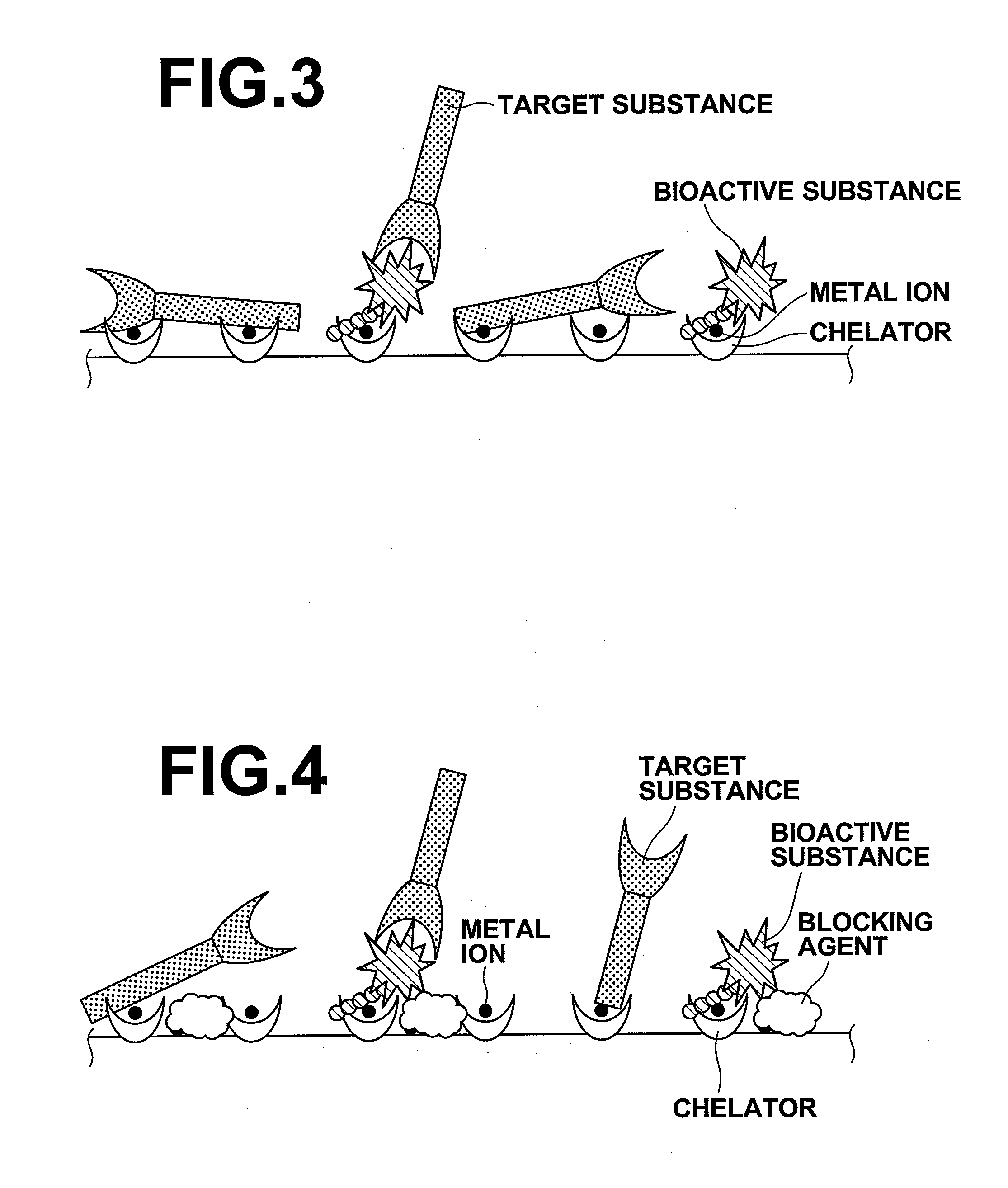
A substrate includes: a base material; and chelators which are three dimensionally covalently bound to the base material at a density of 7.8×1015 / mm3 or greater and 4.5×1017 / mm3 or less, or two dimensionally covalently bound to the base material at a planar density of 2.0×1011 / mm2 or greater and 1.0×1013 / mm2 or less. Metal ions are coordinately bound to the chelators, and a bioactive substance having histidine tags are coordinately bound to the metal ions. A blocking agent having ligand sets are coordinately bound to the metal ions to which the bioactive substance is not coordinately bound.
Owner:FUJIFILM CORP
High-load agarose chromatography media and preparation method thereof
ActiveCN105498701AIncrease the number of sitesImprove abilitiesOther chemical processesMetal chelateIon exchange
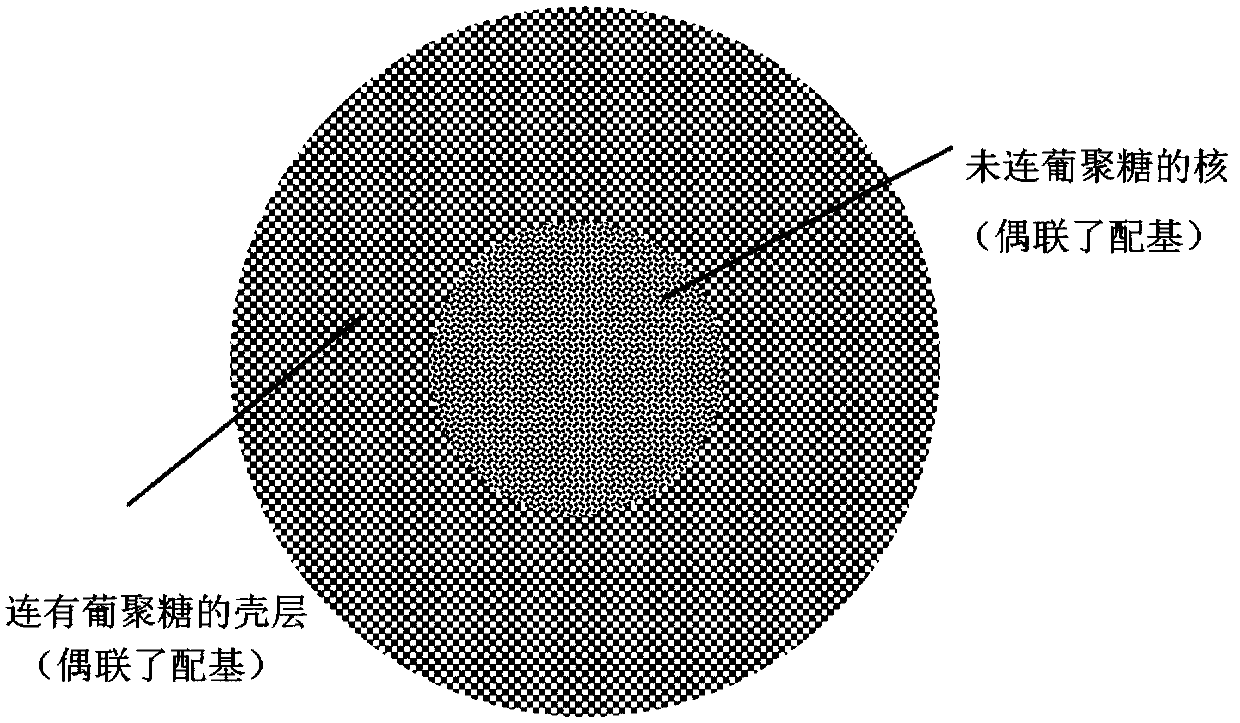
The present invention relates to a high-load agarose chromatography media comprising a shell and an inner core, the shell portion is covalently bonded with linear macromolecules such as glucan and the like, the shell portion and the inner core portion are coupled with ligands, and ligand species include metal ions, hydrophobic ligands, ion exchange groups and proteins, and the like. The present invention also includes a preparation method of the high-load agarose chromatography media. For example, the load of histidine tagged (His-tagged) recombinant protein of a metal chelated chromatography media prepared by the method is about 2.5 times of that of conventional media. The high-load agarose chromatography media has good prospects in the fields of biological separation and purification.
Owner:SENHUI MICROSPHERE TECH SUZHOU CO LTD
Nano antibody for specifically identifying histidine label
InactiveCN106046152AHigh affinityImprove featuresMaterial nanotechnologyOther chemical processesAntigenMutant
The invention belongs to the field of genetic engineering and particularly relates to a single-domain heavy chain antibody specific to a histidine label, having an amino acid sequence shown as in SEQ ID NO: 1 and applicable to the fields such as immunodetection and antigen enrichment and purification. The amino acid sequence provided herein may function as a precursor, and by modifying through random or fixed-point mutation technology, it is possible to obtain a mutant with better properties (affinity, specificity, stability and the like), and the mutant is useful to develop proteins or polypeptides applied to medicine, industry and agriculture.
Owner:NANCHANG UNIV
Metal chelating magnetic microspheres and preparation method thereof
ActiveCN110215900AAbility to isolate proteins with high selectivityProtection resistanceOther chemical processesPeptide preparation methodsEpoxyProtein target
The invention provides metal chelating magnetic microspheres and a preparation method thereof. According to the metal chelating magnetic microspheres, superparamagnetic nano ferroferric oxide is takenas a core, the surface of the superparamagnetic nano ferroferric oxide is wrapped with a layer of silicon dioxide, and then a ligand formed by a silane coupling agent, epoxy chloropropane and iminodiacetic acid is adopted to modify the surface of silicon dioxide. The silane coupling agent and the epoxy chloropropane in the ligand can be used as connecting arms to prolong the distance between metal ions and a magnetic core, nonspecific adsorption is reduced, and thus specific adsorption of the magnetic beads to label proteins is improved. Space hindrance of combination of the target protein and the magnetic beads is reduced, so that relatively high protein extraction efficiency is achieved. The iminodiacetic acid in the ligand can chelate the metal ions with strong binding force with histidine tag protein, so that the metal chelating magnetic microspheres disclosed by the invention have relatively high capability for selectively separating proteins.
Owner:WUHAN UNIV OF TECH
Recombinant pichiapastoris for producing FAD-dependent glucose dehydrogenase as well as construction method and application thereof
InactiveCN107460138AIncrease enzyme activityFungiMicroorganism based processesPichia pastorisRestriction site
The invention provides recombinant pichiapastoris for producing FAD-dependent glucose dehydrogenase as well as a construction method and application thereof and belongs to the technical field of genetic engineering. The invention provides a recombinant plasmid pMD-GDH containing FAD-dependent glucose dehydrogenase genes. After the FAD-dependent glucose dehydrogenase genes are subjected to codon preference adjustment, EcoRI restriction sites and NotI restriction sites are added at two ends of the sequence, and six histidine tags are added at the 3' end. The recombinant pichiapastoris takes Pichia pastoris as an expression host and comprises the recombinant plasmid. The invention further provides application of the recombinant pichiapastoris for producing FAD-dependent glucose dehydrogenase in fermentation production of the FAD-dependent glucose dehydrogenase. The embodiments show that when the recombinant pichiapastoris is subjected to induction culture for 136 hours during culture in a 10L of fermentation tank, the enzyme activity reaches 257600U / L and is 5.3 times that of the enzyme activity reported in the prior art.
Owner:河北省微生物研究所有限公司
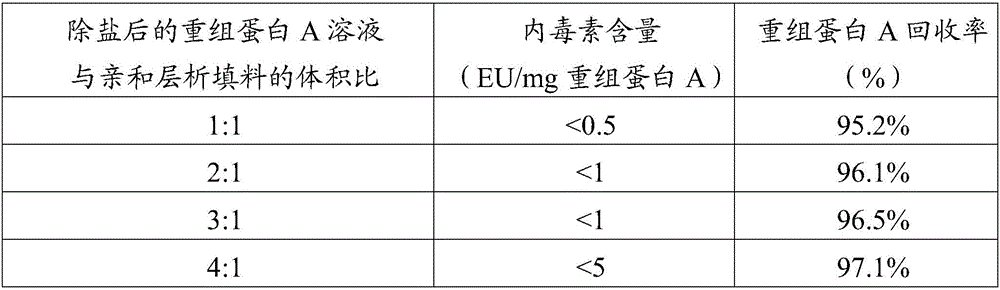
Method for removing endotoxin in recombinant protein A solution
InactiveCN106397552ALow adsorption capacityHigh recovery rateDepsipeptidesPeptide preparation methodsEndotoxin removalChromatography column
The invention provides a method for removing endotoxin in a recombinant protein A solution. The method is good in endotoxin removal effect, high in the recovery rate of recombinant protein A and greatly reduced in cost. The method comprises the following steps: (1) heating a bacterial suspension containing recombinant protein A with a histidine tag so as to fully fragment bacteria; (2) adding polyethyleneimine and successively carrying out uniform mixing and centrifugation so as to obtain supernatant containing the recombinant protein A, wherein a weight ratio of the bacterial suspension containing recombinant protein A to polyethyleneimine is 10000: 1 to 100: 1; (3) allowing the supernatant to pass through a metal ion chelated chromatography column and flushing a medium by using a PBS buffer solution so as to obtain a pretreated recombination protein A solution; (4) removing a salt so as to obtain a desalted recombination protein A solution; and (5) purifying the desalted recombination protein A solution with an affinity chromatographic filling material so as to obtain the recombinant protein A solution without endotoxin, wherein the affinity chromatographic filling material can specifically bind to endotoxin.
Owner:湖北中创医疗用品有限公司
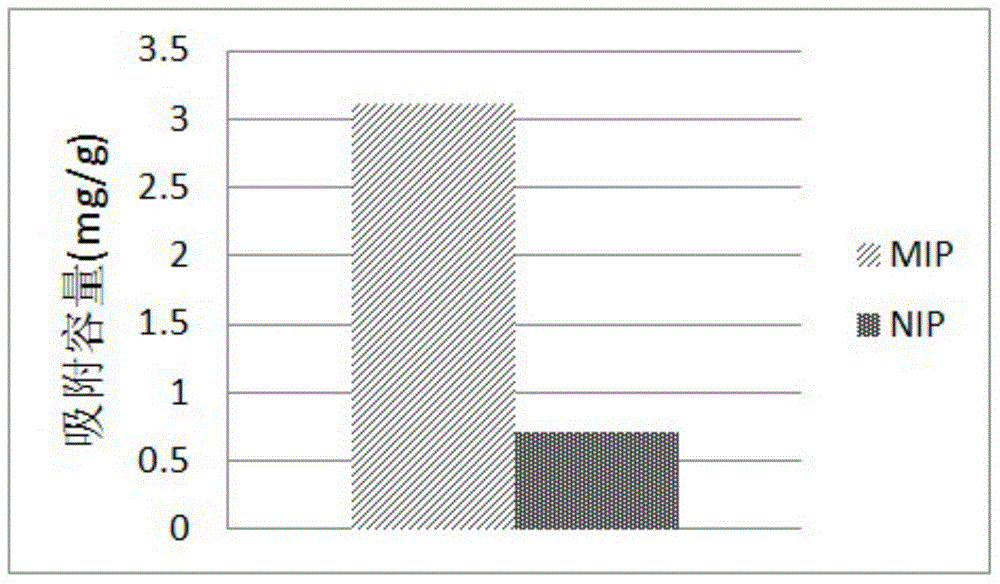
Protein antigen determinant molecularly imprinted material as well as preparation and application thereof
InactiveCN104941602AConsistent loading efficiencySimple methodOther chemical processesBiological testingFunctional monomerProtein target
The invention relates to an antigen determinant molecularly imprinted material capable of carrying out selective recognition on a target protein and a preparation process of the antigen determinant molecularly imprinted material. The molecularly imprinted material is prepared by the following steps: by utilizing a section of specific peptide fragment corresponding to the target protein as a template molecule, fixing the specific peptide fragment on a matrix material by employing a histidine tag as a connecting arm; and polymerizing on the matrix material through a functional monomer. The imprinted material is applied to selective recognition of a standard protein.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
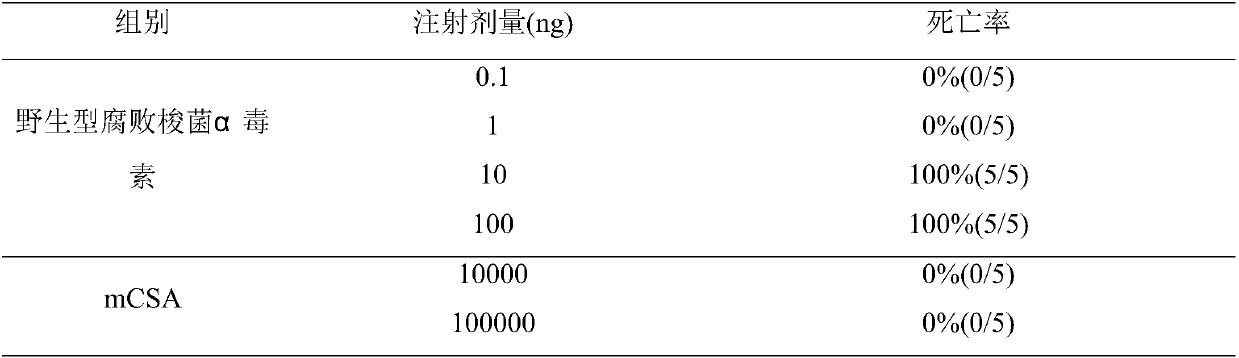
Clostridium septicum alpha toxin recombinant subunit vaccine and production method thereof
ActiveCN107812183AReduce Biosecurity RisksEasy to purifyAntibacterial agentsBacterial antigen ingredientsAntigenAlpha-toxin
The invention relates to a clostridium septicum alpha toxin recombinant subunit vaccine and a production method thereof. According to the production method, 54-site cysteine of wild clostridium septicum alpha mature toxin is mutated into leucine, 264-site asparaginate is mutated into alanine, 269-site histidine is mutated into alanine, and 310-site tryptophan is mutated into alanine, so that an alpha toxin mutant which is nontoxic to animals is obtained; and 6 groups of histidine tags are added to a C end of a mutant protein so as to obtain an antigen for preparing vaccines. The invention simultaneously discloses a recombinant expression vector and a recombinant host cell which contain an encoding gene of a nontoxic alpha toxin mutant of clostridium septicum. The efficacies of the preparedclostridium septicum alpha toxin recombinant subunit vaccine are far higher than the efficacies of existing vaccines, and the bio-safety risk in the production process of the vaccine is greatly reduced. Besides, by virtue of the superiority that a semi-finished product of the vaccine is high in protein concentration, a mixed vaccine can be prepared without increasing the dosage of the vaccine.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Preparation method of nano magnetic beads for purifying histidine tagged protein
ActiveCN109758989ARapid purificationEasy to separateMicroballoon preparationMicrocapsule preparationCross-linkMagnetic bead
The invention discloses a preparation method of nano magnetic beads for purifying histidine tagged protein. The method comprises the following steps: taking sodium alginate, FeCl3.6H2O, NaAc.3H2O andPEG as raw materials, taking ethylene glycol and deionized water as solvent, reacting through a solvothermal method to generate monodispersed sodium alginate / Fe3O4 nano magnetic beads, subsequently further carrying out an epoxy chloropropane cross-linking reaction and activation on the nano magnetic beads and the newly added sodium alginate, then introducing an iminodiacetic acid (IDA) chelating agent so as to obtain water-stable nano magnetic beads efficiently chelated with metal nickel or cobalt ions; and efficient, rapid, simple and low-cost separation and purification of the histidine tagged protein are realized. The method disclosed by the invention is simple, rapid and stable to operate and low in cost; the synthesized nano magnetic beads are large in specific surface area and stablein a water phase and not prone to agglomeration and precipitation; the purification efficiency of the histidine tagged protein is greatly improved; and the large-batch industrial production of the nano magnetic beads can be realized.
Owner:南京青柠生物科技有限公司
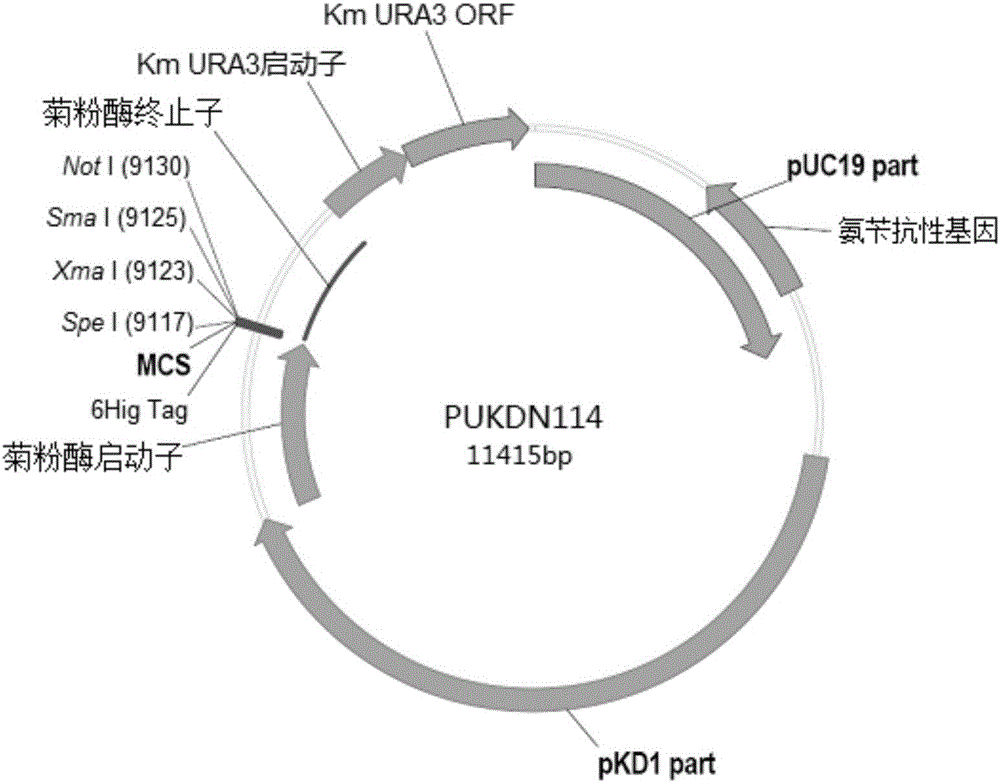
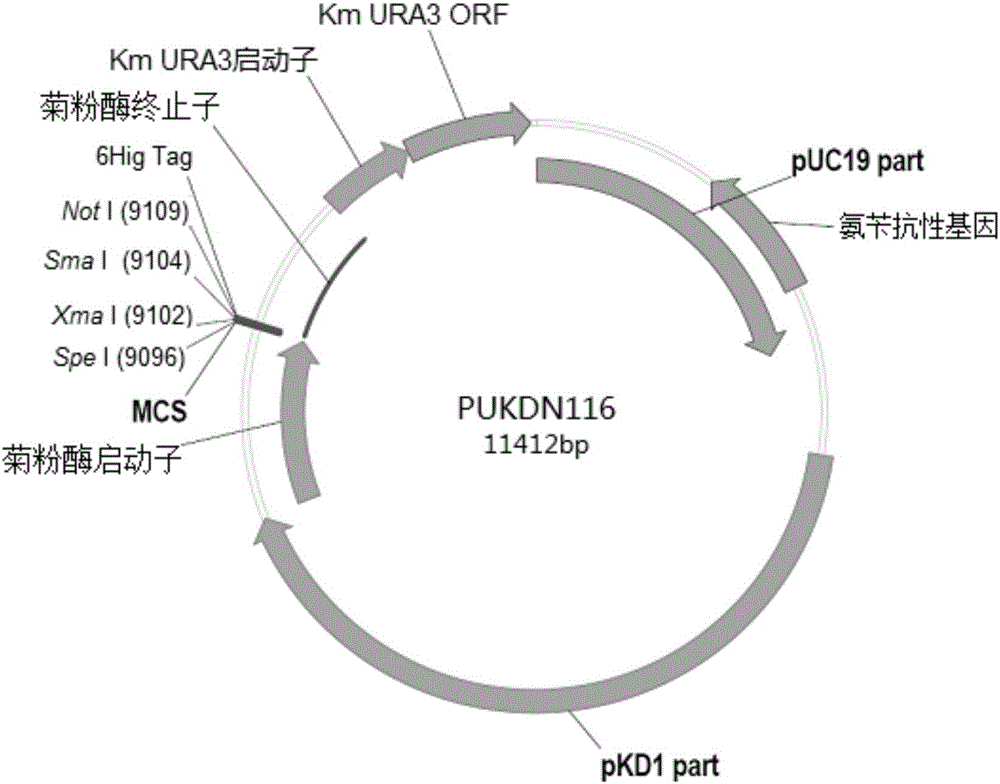
Recombinant vector for expressing histidine-tag-fused foreign gene in Kluyveromyces marxianus nutritional deficient strain
The invention provides a recombinant vector for expressing a histidine-tag-fused foreign gene in a Kluyveromyces marxianus nutritional deficient strain, and a preparation method and application of the recombinant vector. The recombinant vector sequentially comprises an ampicillin resistance gene, a PKD1 vector sequence, an inulase promotor, multiple cloning sites, an inulase terminator, a nutritional gene promotor and a nutritional gene open reading frame, and further comprises histidine tags located at C ends or N ends of the multiple cloning sites. The recombinant vector provided by the invention can be used for building a transformant to realize expression of the foreign gene.
Owner:FUDAN UNIV
Novel preparation technology of targeting antitumor fusion protein LPO (lipid peroxidation)
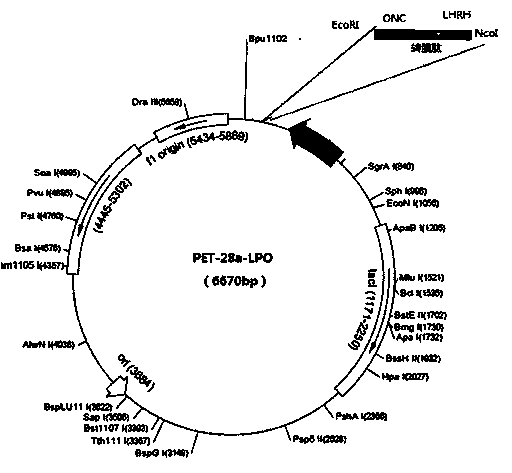
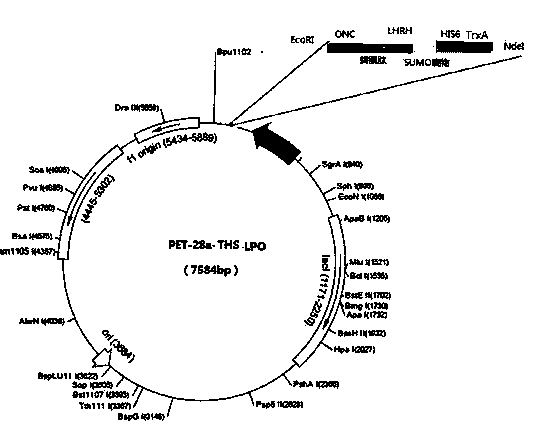
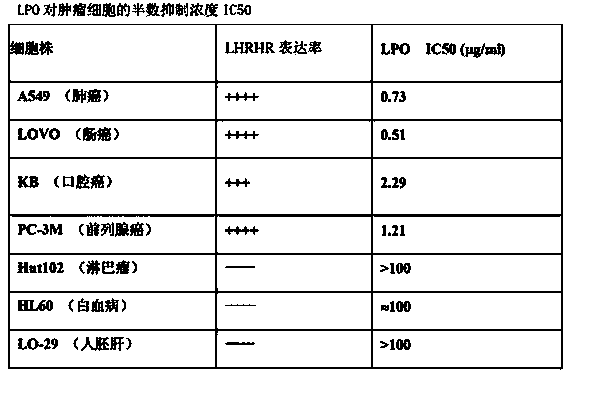
InactiveCN103805621AEnhanced inhibitory effectHybrid peptidesAntineoplastic agentsHistidineLipid peroxidation
The invention discloses a novel preparation technology of targeting antitumor fusion protein (lipid peroxidation). The novel preparation method comprises the steps of building a fusion expression protein which takes the glutathione S-transferase A (TrxA)-His-tag (His6-Tag)-SUMO protease recognition substrate as a protein soluble expression auxiliary fragment and takes LHRH (luteinizing hormone releasing hormone)-PEA (phenylethylamine) trans-cell penetrating peptides-ONC (LPO for short) as a target segment, connecting the two proteins with each other by a correct read frame and carrier positive sequence and converting to enter into expression bacteria, finally building fusion protein which is connected with six expression substances in series, crudely extracting, carrying out metal chelating medium purification in the presence of imidazole, and carrying out SUMO protease digestion. Compared with the LPO prepared by a conventional method, the LPO prepared by the novel preparation technology disclosed by the invention can obviously improve the inhibiting effect on the tumor cell lines such as colon cancer HT-29 cells, ovarian cancer OVCAR3 cells, cervical adenocarcinoma HeLa cells and liver cancer HepG-2 cells.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
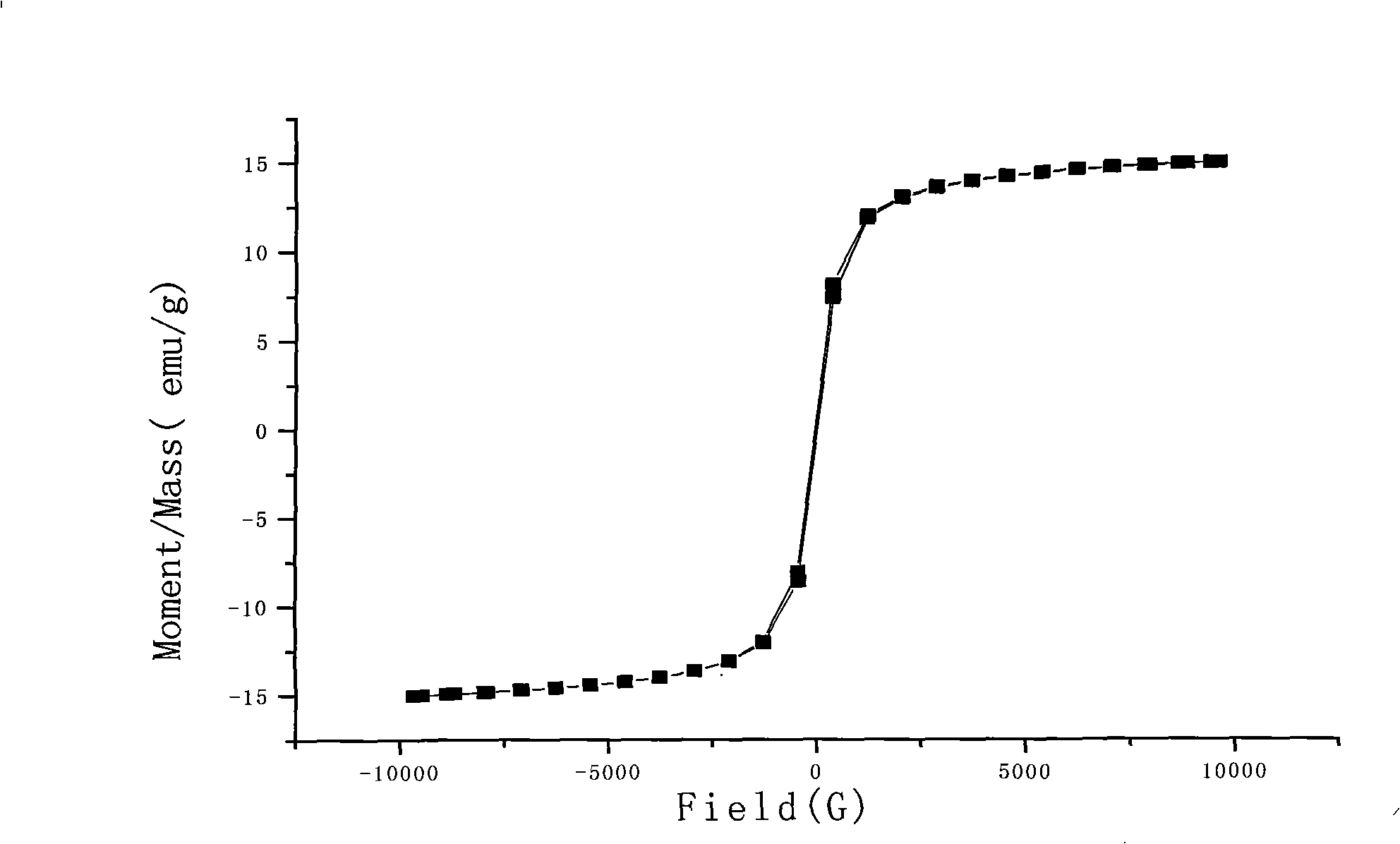
Composite magnetic nanoparticles Fe3O4/MPS/PAA/NTA-Ni<2+> and preparation method and application thereof in separation and purification of histidine-tagged proteins
InactiveCN105837766AStrong magnetismImprove stabilityPeptide preparation methodsMg compositeMagnetite Nanoparticles
The invention relates to composite magnetic nanoparticles Fe3O4 / MPS / PAA / NTA-Ni<2+> and a preparation method and an application thereof in separation and purification of histidine-tagged proteins, and belongs to the technical fields of nano magnetic materials and biological analysis. Fe3O4 magnetic nanoparticles with an average hydration particle size of 100-400 nm are used as a core, MPS surface modification is adopted for making the surface rich in double bonds, polycarboxyl-structure magnetic nanoparticles with an average hydration particle size of 100-600 nm are obtained through PAA coating, and then furthermore, the polycarboxyl-structure magnetic nanoparticles are coupled with NTA-Ni<2+> to obtain the composite magnetic nanoparticles Fe3O4 / MPS / PAA / NTA-Ni<2+> having fast magnetic field response. The prepared composite magnetic nanoparticles enable the thickness of a coating layer to be controlled in nanometer scale; the prepared composite magnetic nanoparticles have the advantages of uniform size distribution, strong magnetic and low cost, have higher ability of enrichment and separation purification of the histidine-tagged proteins, and have the purification efficiency of the histidine-tagged proteins as high as 40-70 [mu]g / mg composite magnetic nanoparticles; and the composite magnetic nanoparticles can be repeatedly used.
Owner:HUBEI UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com