Novel preparation technology of targeting antitumor fusion protein LPO (lipid peroxidation)
A technology for fusing proteins and gene fragments, which is applied in the field of genetic engineering and can solve problems such as low renaturation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
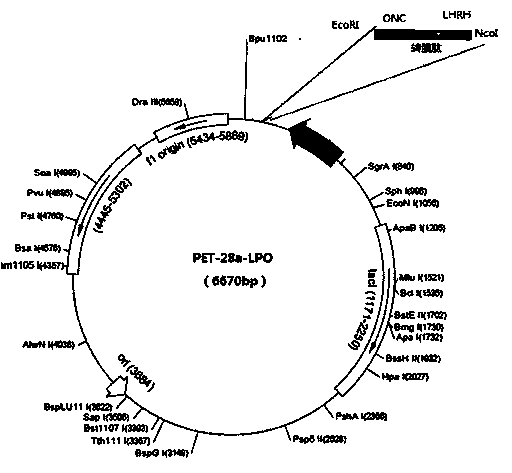
[0056] Embodiment 1: the preparation of LPO expression vector and engineering bacterium
[0057] Construction and Identification of Recombinant Expression Plasmids
[0058] a. Gene synthesis: In the present invention, according to the above-mentioned design concept, the restriction site and the LPO whole gene sequence are designed and the following sequence is artificially synthesized in vitro:
[0059] NcoI endonuclease recognition sequence (CCATGG)+GC+LHRH polypeptide gene+PEA transmembrane peptide+ONC sequence+stop codon (TAA)+EcoRI endonuclease recognition sequence (GAATTC), the gene sequence is shown in SEQ ID NO:1, The synthetic sequence was directly cloned into the T vector provided by Dalian Takara Company, and transformed into Escherichia coli JM109 bacteria, positive clones were confirmed by PCR identification, positive clones were expanded and cultured, and plasmid DNA was extracted by conventional molecular cloning techniques, using NcoI and EcoRI double enzymes ...
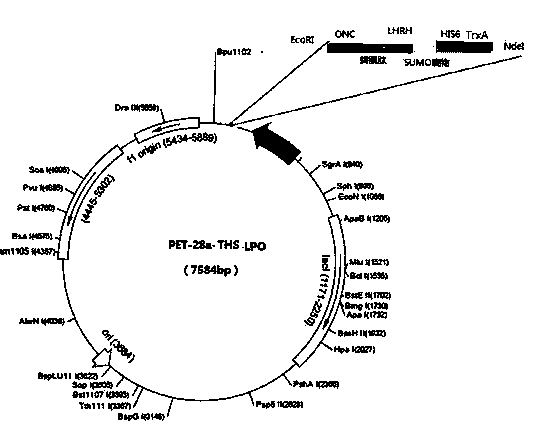
Embodiment 2
[0062] Example 2: Expression of LPO fusion protein and purification of product:
[0063] Escherichia coli BL21 (DE3) (containing T 7 RNA polymerase gene) were cultured on LB agar plates containing kanamycin (50 μg / ml). After culturing, select kanamycin-resistant colonies and culture them in LB medium containing kanamycin (50 μg / ml) at 37°C. When A 600 When it reaches about 0.4~0.6, add 1mM isopropylthio-β-D-galactoside (IPTG) (final concentration 1mM), and continue culturing at 37°C for 3-4 hours to induce the expression of the target product. Then centrifuge the cells and the medium, and add buffer components to the cells containing the target protein, the final concentration reaches 50mM Tris-HCl, pH8.0, 1mM EDTA, sonicate, and centrifuge at 4°C (20,000g, 30 minutes ), and the precipitate (insoluble part) is the crude extract of the fusion protein.
[0064] The crude extract was washed, denatured and refolded, and the obtained refolded protein was purified by ion-excha...
Embodiment 3
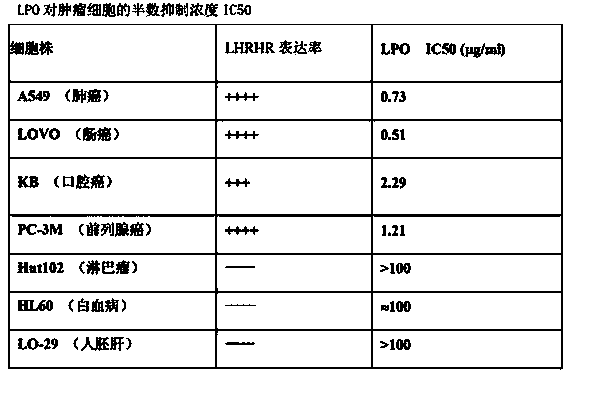
[0065] Example 3: The biological activity of the fusion protein was determined by the tumor cell inhibition method cultured in vitro (for specific operations, refer to Appendix XC of Part III of the Chinese Pharmacopoeia 2010 Edition).
[0066] Cytopathic Inhibition Assay:
[0067] The cultured human tumor cell monolayer was digested with trypsin, the cell suspension was collected by blowing and blowing, counted by a cell counting plate, the number of cells was adjusted to 60000 / ml, and 80 μl / well was added to a 96-well culture plate (per Well 5000 cells), 5% CO 2 , and cultured at 37°C for 4h. Adjust the concentration of the prepared LPO protein sample to 1 mg / ml, the quantitative sample is filtered and sterilized, and different amounts of samples are added to each cell well according to the equal dilution method, and then the culture medium is supplemented to make the total volume 100 μl, 5% CO 2 , and cultured at 37°C for 24h. Then discard the supernatant in the cell ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com