Histidine tag protein affinity purification material and application thereof
A histidine tag and protein technology, which is applied in the preparation of functional biological materials and in the field of protein recognition and purification, can solve the problems of lower purification purity of histidine tag, increase of purification cost, and low purification recovery rate, and achieve reduction The interference of strong non-specific adsorption, the effect of reducing the leakage of metal ions and improving the purification purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
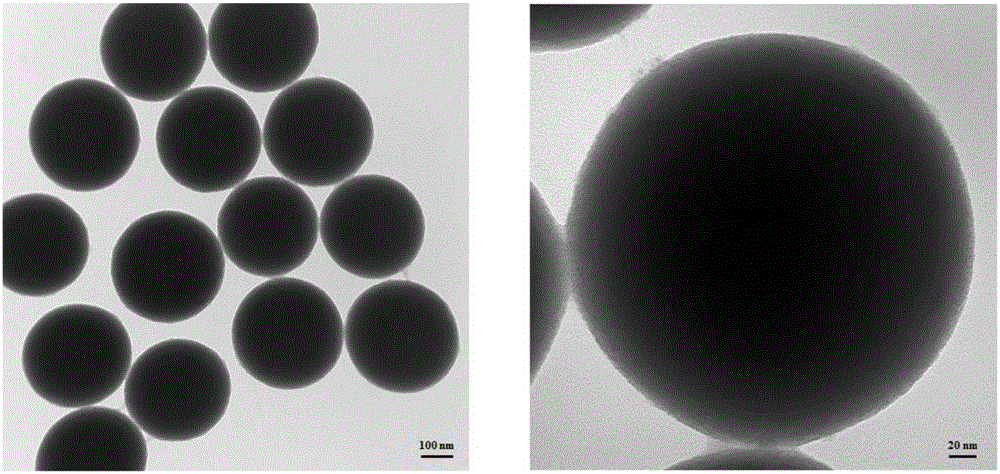
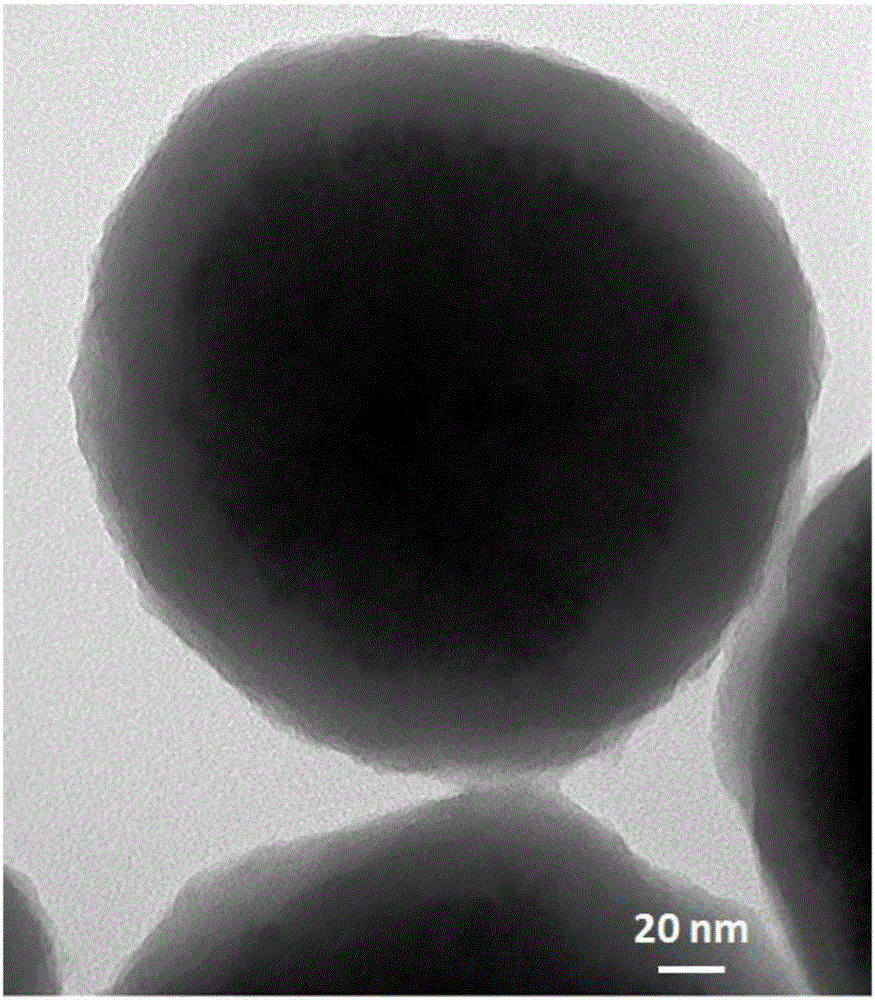
[0037] Based on IDA and Ni 2+ Preparation of histidine-tagged molecularly imprinted silica material (MIP1) for chelation
[0038] use Monodisperse silica nanoparticles were prepared by method, and surface-modified IDA and Ni were prepared by reference method 2+ The matrix material (L.Zhang, et al.Chem.Commun., 2011,47,3969-3971; L.Zhang, et al.Anal.Chem., DOI:10.1021 / ac5047246): 5g silica nanoparticles were added In 100mL GLYMO-IDA solution, react at 65°C for 24 hours to modify the IDA group on the surface of the silica material. 5 g of the above materials were added to 150 mL of methanol, followed by 10 mL of γ-MAPS, and refluxed at 90° C. for 15 hours to modify γ-MAPS on the surface of the matrix material so as to be imprinted as a matrix material. Then 100 mg of the above material was redispersed in 1 mL of 12 mg / mL template molecule histidine tag (HHHHHH) aqueous solution (the mass ratio of template molecule to the above matrix material was 120:1000), and incubated at ...
Embodiment 2
[0040] The non-imprinted material 1 (NIP1) was prepared by the same method as in Example 1, but without adding a template molecule histidine tag (HHHHHH).
Embodiment 3
[0042] Using the same method as in Example 1, only metal ions were immobilized on the matrix material without subsequent imprinting process to prepare non-imprinted material 2 (NIP2).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com