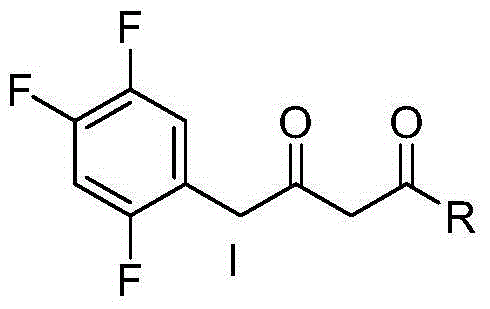
Immobilized transaminase and application of immobilized transaminase in synthesis of sitagliptin intermediate
A technique for immobilizing transaminase and transaminase, which is applied in the asymmetric synthesis of sitagliptin intermediates. In the field of immobilized transaminase, it can solve the problems of difficult mass transfer, large loss of enzyme activity, and difficult separation of transaminase, and achieve simple separation. , less loss of enzyme activity, environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The preparation of embodiment 1 immobilized carrier A
[0051] Under stirring, slowly add 100 grams of amino resin of the type D301 of Shanghai Huazhen Company to 200 mL of 5% (v / v) ethylene glycol diglyceryl ether in toluene solution, stir and react at 60°C for 1 hour, and use Wash with toluene and water, then place in 350mL of 100mM iminodiacetic acid (IDA), 10mM triethanolamine, pH 9.0 aqueous solution, react at 60°C for 2 hours, filter and wash with water, and reconstitute with 200mL of 10mM cobalt chloride solution Suspended, stirred at room temperature for 30 min, filtered and washed with water to obtain immobilized carrier A.
Embodiment 2
[0052] Embodiment 2 Preparation of immobilized carrier B
[0053] Add 100 grams of ion chelating resin model HZD401 from Shanghai Huazhen Company under stirring to 200 mL of 10 mM cobalt chloride solution for resuspension, stir at room temperature for 30 min, filter and wash with water to obtain immobilized carrier B.
Embodiment 3
[0054] Embodiment 3 Preparation of recombinant transaminase expression transformant
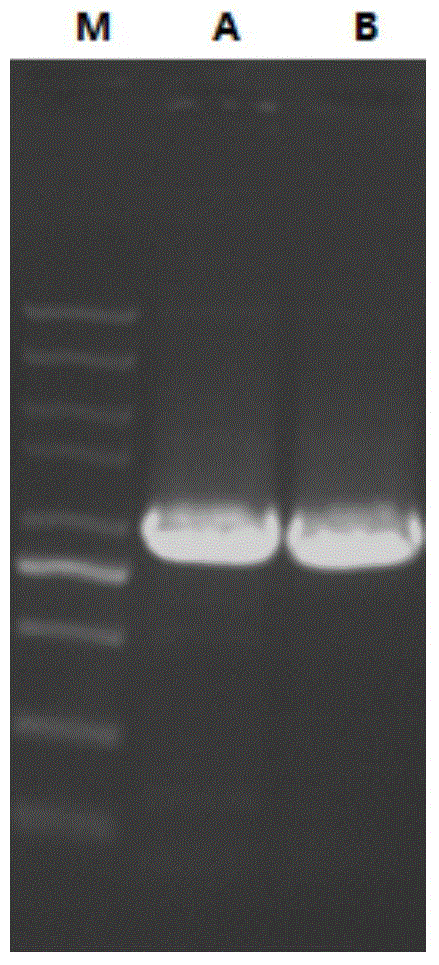
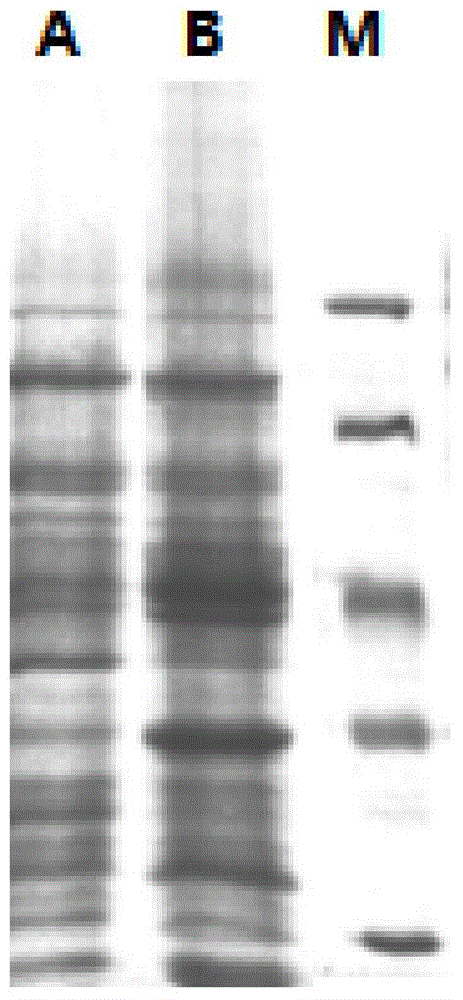
[0055] According to Chinese patent application number 201410169882.4, the recombinant expression vector pET21a-MvAT containing SEQNo.1 was constructed, digested with restriction endonucleases NdeI and EcoRI at 37°C for 8h, purified by agarose gel electrophoresis, and used agarose gel DNA The recovery kit recovers the target fragment. Under the action of T4 DNA ligase, the target fragment was ligated with the plasmid pET28a digested by NdeI and EcoRI at 16°C overnight to obtain the recombinant expression plasmid pET28a-MvAT. The recombinant expression plasmid was transformed into Escherichia coli (E.coli) DH5α competent cells, the transformation conditions were 45°C, heat shock for 90 seconds, and the positive recombinants were screened on the resistance plate containing kanamycin, Pick a single clone and verify the positive clone by colony PCR (see figure 1 A). Cultivate the recombinant ba...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com