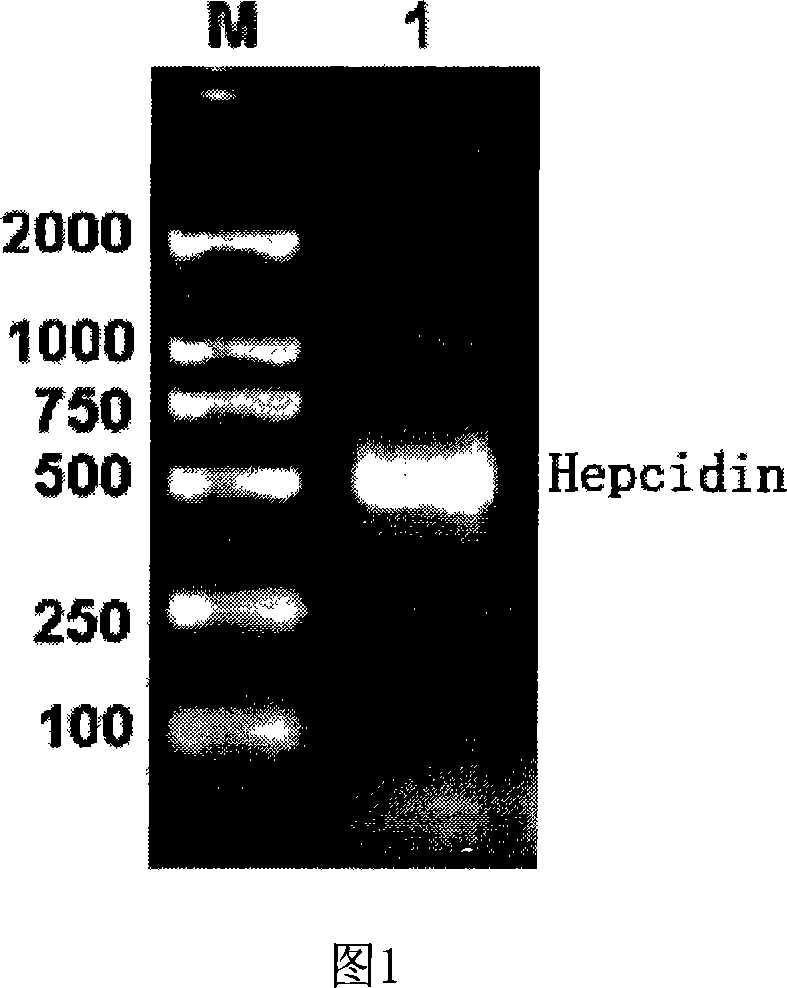
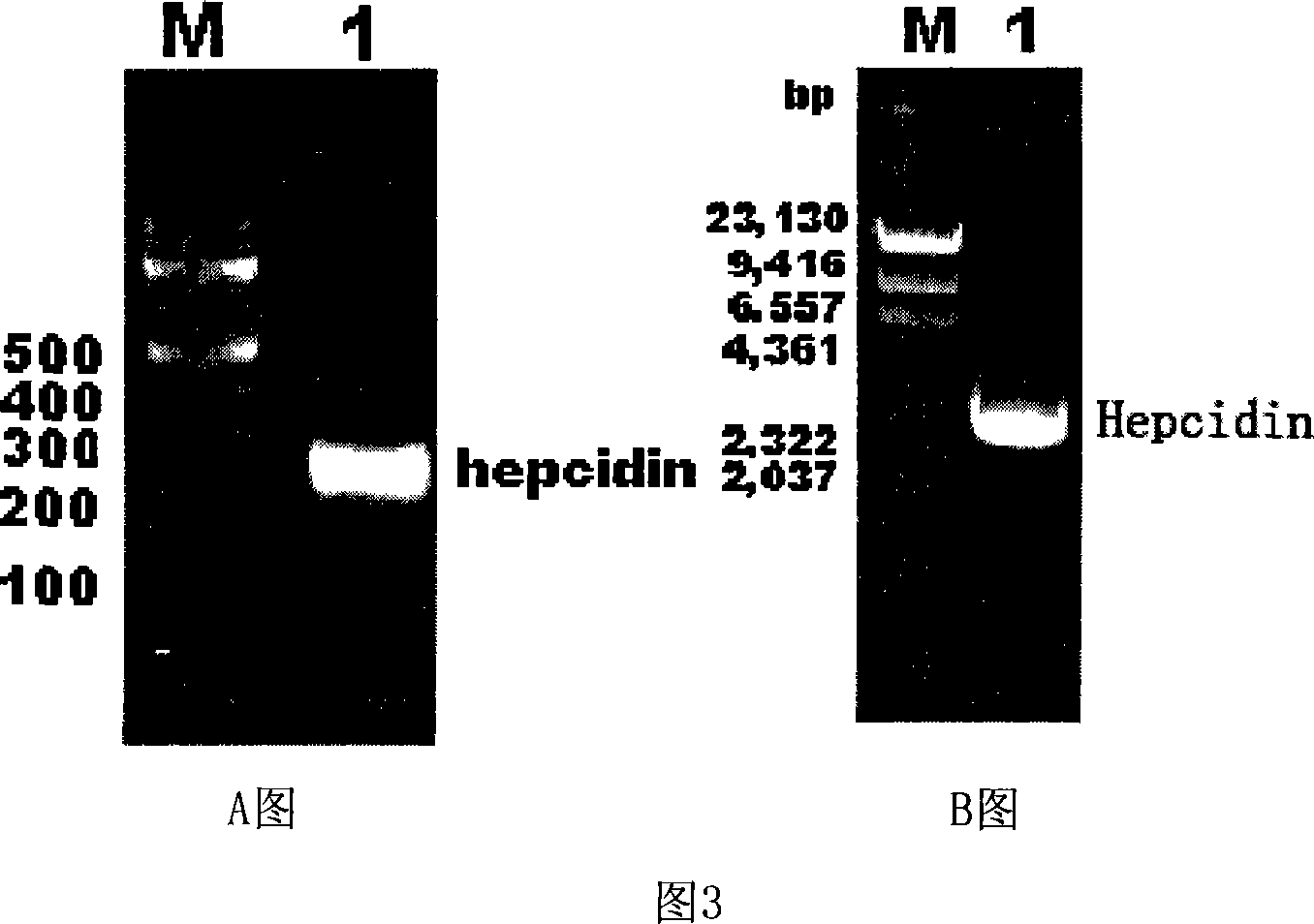
Expression carrier for black porgy antibiotic peptide Hepcidin and expression product and constructing preparation method
A technology for expression vectors and expression products, which is applied in the field of construction and preparation of expression vectors, and can solve problems such as inability to meet industrialization requirements, few research reports, and complicated processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0071] 1. Construction of recombinant expression vector pTrc-CKS / hepcidin
[0072] 1) Obtaining the recombinant pPMD18-T positive plasmid containing black sea bream hepcidin (cDNA):
[0073] (1) Trizol kit (purchased from Invitrogen) was used to extract total RNA from the liver of juvenile black sea bream. ,
[0074] (2) According to the black sea bream Hepcidin gene (Genbank accession number: AY669377 ) Synthetic specific primer: S1 (upstream primer: 26 bp (including ATG) before the initiation codon ATG sequence): TGAAGCAGTGGAAGCCTCCTAAGATG.
[0075] (3) 3′RACE amplification of the black sea bream hepcidin gene: According to the TaKaRa company 3′-Full RACE Core Set instructions for cDNA first-strand synthesis: take 1μl RNA (1-3μg) into a 0.2ml thin-walled tube, and incubate at 70°C 10min, immediately placed on ice. Add the following reagents in order to prepare the reaction solution: 10×RNA PCR Buffer 2μl, MgCl2 (25mM) 4μl, RNAasin (40U / μl) 0.5μl, dNTPs (10mM each) 2μl, O...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com