Immobilized metal ion affinity chromatograph (IMAC) material, and preparation and application thereof
A technology for immobilizing metals and chromatographic materials, applied in release and purification, functional biological materials and their preparation, and in the field of protein-specific capture, can solve the problems of large steric resistance of intact proteins and inability to enter the polymer network, and achieve the realization of Specific capture, reduction of metal ion leakage, and reduction of non-specific adsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Based on NTA and Ni 2+ Preparation of Chelating Silica IMAC Surface Coating Material (SC-IMAC1)
[0022] 3 g of amino-modified silica nanoparticles (particle size: 350 nm) were dispersed in 60 mL of PB buffer, 2 mL of glutaraldehyde was added, reacted at room temperature for 6 h, and washed twice with water to remove unreacted glutaraldehyde. Resuspend the material with 90mL PB buffer, add 200mg NTA, adjust the pH to 8.0, react overnight at room temperature, wash twice with water to remove unreacted NTA. Disperse the resulting material in 30 mL of 10 mg / mL NiSO 4 In aqueous solution, Ni was immobilized on the surface 2+ IMAC material. Subsequently, 100 mg of particles were redispersed in 20 mL of acetonitrile, 10 mg of acrylamide (AAm), 10 mg of methylenebisacrylamide (MBA), 0.5 mg of AIBN, and N 2 After 20 min, 65 ° C magnetic stirring reaction for 12 h, centrifuged to collect the product, washed 3 times with ultra-pure water, vacuum dried to obtain NTA and Ni-base...
Embodiment 2
[0026] Based on NTA and Ni 2+ Chelating Silica IMAC Surface Coating Material (SC-IMAC1) for Purification of Histidine-Tagged Proteins
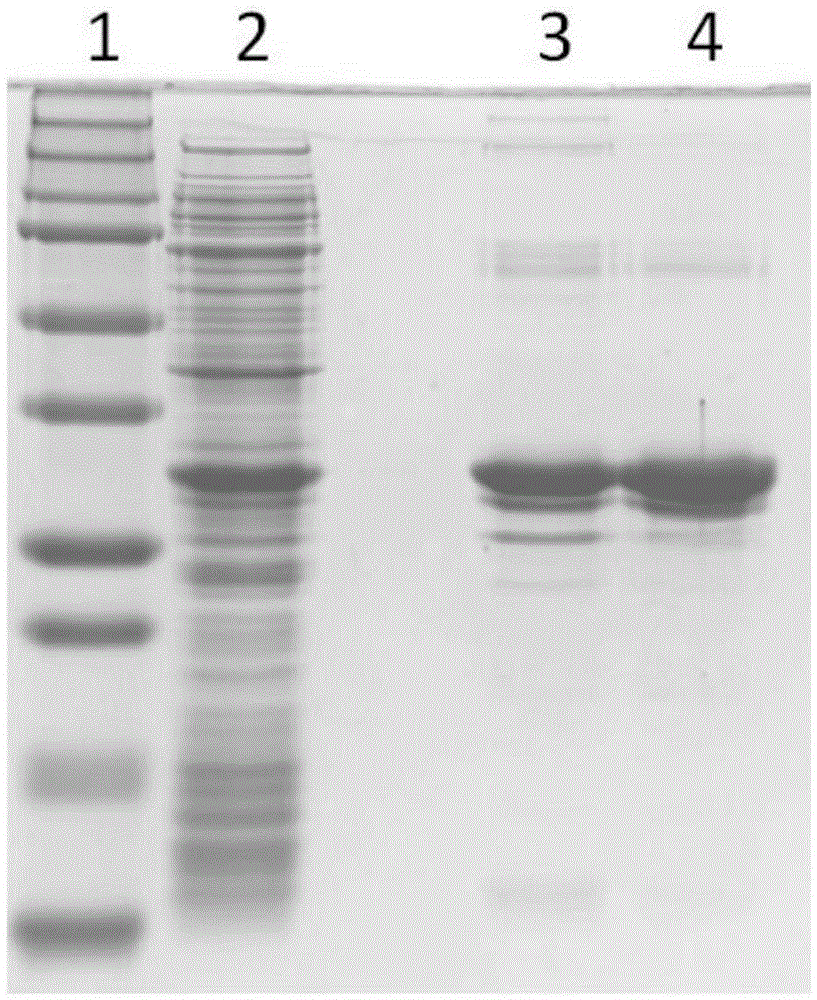
[0027] 4 mg of SC-IMAC was washed twice with 80 μL of loading buffer containing 10 mM imidazole at 4°C to keep SC-IMAC1 in the loading environment. Dilute the recombinant protein extract to 4 mg / mL with loading buffer, add 200 μL to the pre-balanced SC-IMAC1, and incubate at 4°C for 30 min with shaking. The material was collected by centrifugation and washed once with 100 μL of loading buffer to remove unbound protein. Wash twice with 30 μL of washing buffer containing 25 mM imidazole, 30 min each time, to remove weakly bound foreign proteins. 30 μL of elution buffer containing 250 mM imidazole was eluted four times, 30 min each time, to elute the histidine-tagged protein, and the purified product was analyzed by SDS-PAGE.
[0028] As a reference group, under the same operating conditions, IMAC was used as the affinity purification material...
Embodiment 3
[0031] Based on IDA and Ni 2+ Preparation of Chelating Magnetic IMAC Surface Coating Material (SC-IMAC2)
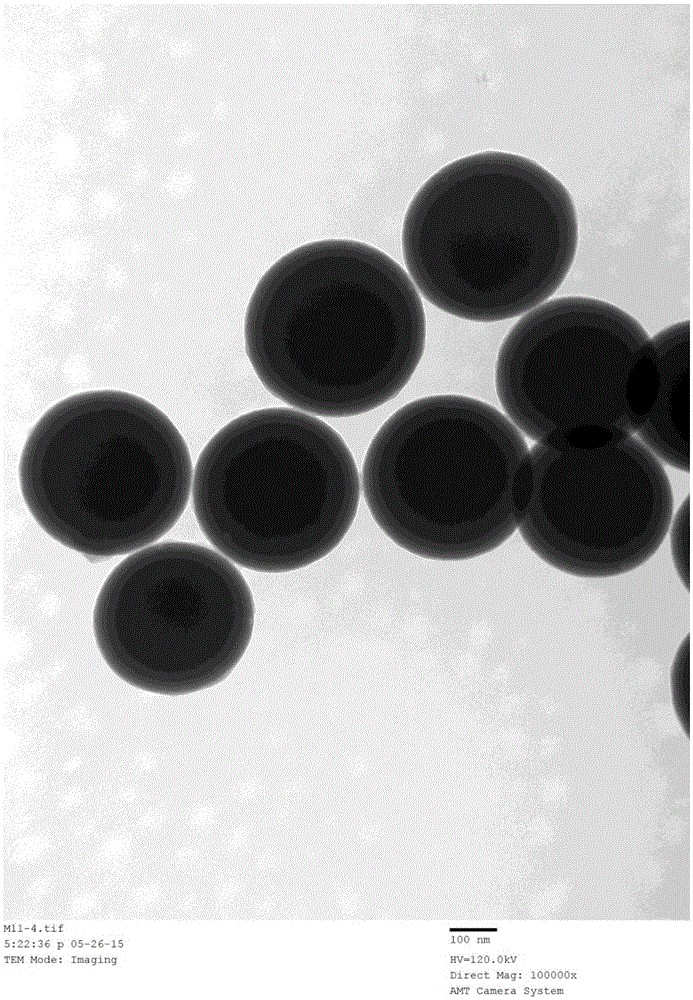
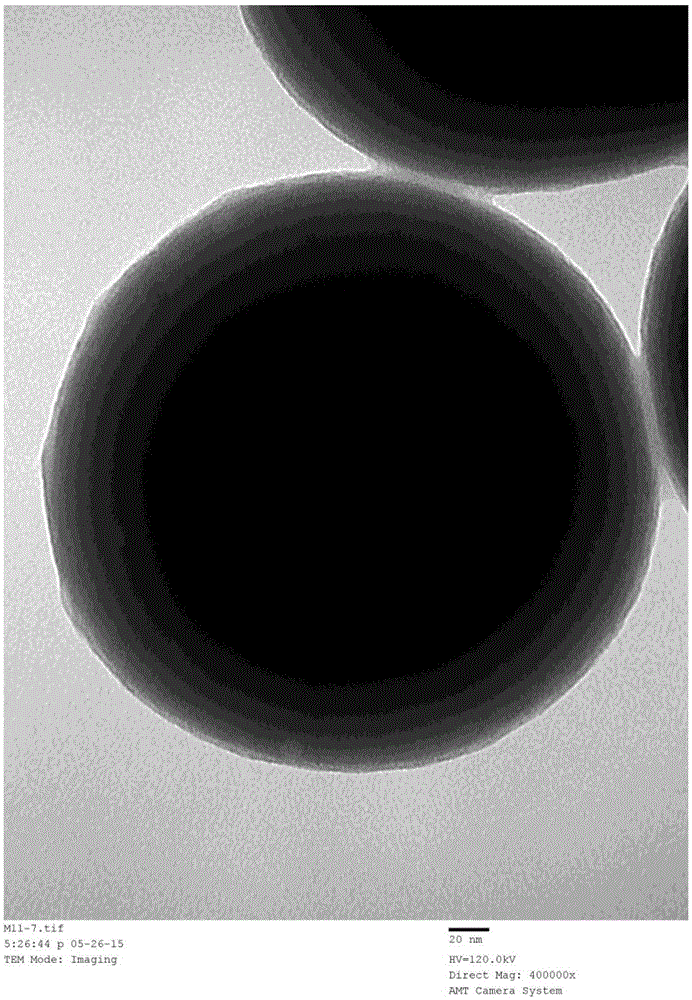
[0032] Add 5g of silica nanoparticles into 100mL of GLYMO-IDA solution, react at 65°C for 24 hours, and modify the IDA group on the surface of the silica material. Add 5 g of the above materials into 150 mL of methanol, then add 10 mL of γ-MAPS, reflux at 90° C. for 15 hours, and then modify γ-MAPS on the surface of the matrix material by methanol reflux. With embodiment 1 method, can make based on IDA and Ni 2+ Chelating magnetic IMAC surface coating material (SC-IMAC2), such as image 3 shown. Same as Example 2, using SC-IMAC2 to purify histidine-tagged protein, the purification result is as follows Figure 4 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com