Preparation method and application of recombination tumor necrosis factor relevant cell apoptosis induction ligand protein
A technology for recombining bacteria and proteins, which is applied in the field of preparation of recombinant tumor necrosis factor-related apoptosis-inducing ligand proteins, can solve the problems of poor stability, easy precipitation, and low renaturation rate of inclusion bodies of recombinant TRAIL proteins, and achieves easy purification. and detection, high solubility, and the effect of inducing apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
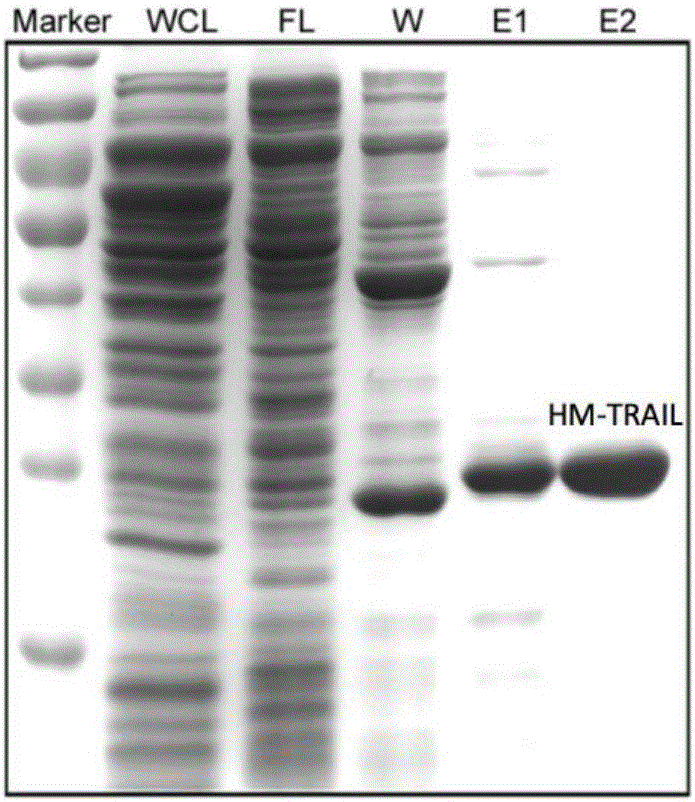
[0043] Example 1: Preparation of recombinant human HM-TRAIL protein
[0044] 1. Construction of pQE16-HM-TRAIL expression vector
[0045] will have His 6 -The amino acid sequence of the Myc tag (MRGSHHHHHHGSEQKLISEEDLNLQ) is inserted behind the 95-281 amino acid sequence of the human TRAIL protein (sequence such as SEQ ID NO.1), PCR amplifies the nucleotide sequence encoding the above-mentioned recombinant human HM-TRAIL protein ( The sequence is shown as SEQ ID NO.2), and inserted into the multiple cloning site of the pQE16 expression vector by double restriction digestion, and the sequence was verified by sequencing.
[0046] 2. Transformation of pQE16-HM-TRAIL expression vector (construction of recombinant bacteria)
[0047] (1) Take out the E.coli TOP10 competent bacteria (100ul) stored at -80°C and put them on ice to thaw.
[0048] (2) Add the ligation product (about 20ul) into the competent bacteria, flick the bottom of the EP tube, mix well, and then ice-bath for 30m...
Embodiment 2
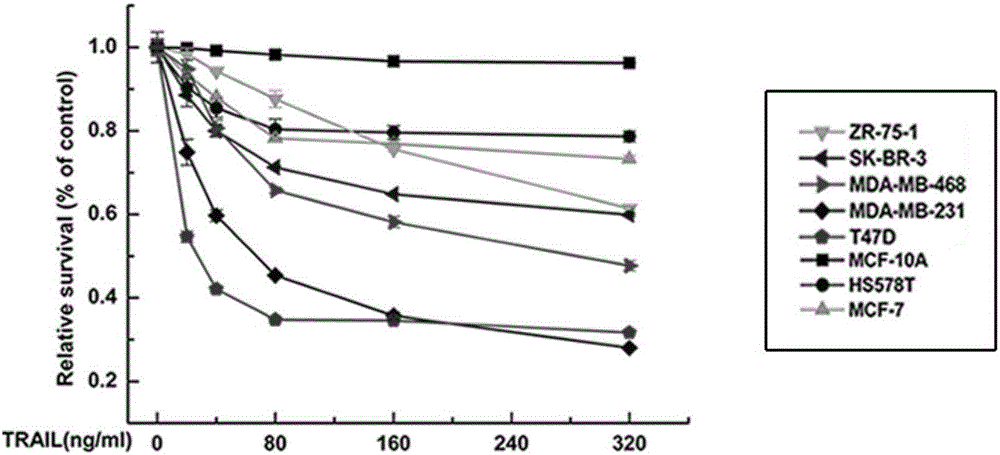
[0100] Embodiment 2: in vitro experiment
[0101] In order to study the induction effect of recombinant human HM-TRAIL protein on tumor cell apoptosis in vitro, we used MTT method to detect the effect of various cell lines on HM-TRAIL in many breast cancer cell lines and normal breast epithelial cell line MCF-10A. sensitivity.
[0102] 1. Cell culture and culture conditions
[0103] Human breast cancer cell lines MCF-7, MDA-MB-231 and MDA-MB-468 were cultured in DMEM containing 10% fetal bovine serum, 100 U / ml penicillin and 100 μg / ml streptomycin (Dulbecco's modified MEM medium ) in high glucose medium. Human breast cancer cell lines SK-BR-3 and ZR-75-1 were cultured in RPMI 1640 medium containing 10% fetal bovine serum and antibiotics. T47D breast cancer cell lines were cultured in RPMI 1640 medium containing 10 μg / ml bovine insulin and 10% fetal bovine serum. All cells were kept at 37°C and 5% CO 2 cultured under normal conditions.
[0104] 2. MTT method to detect the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com