Immune affinity adsorption material for specifically identifying c-Myc labeled nano antibody
An adsorption material and immunoaffinity technology, applied in the field of immunoaffinity adsorption materials, can solve the problems of reduced antibody activity and non-reusable use, and achieve the effects of easy acquisition, avoiding production methods, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Construction of an immune library of anti-c-Myc tag single domain heavy chain antibody (i.e. against c-Myc tag single domain heavy chain antibody)
[0023] The c-Myc tag was covalently coupled to bovine serum albumin (BSA) to obtain the c-Myc artificial antigen c-Myc-BSA. After emulsifying 300 μg of c-Myc-BSA with Freund's complete adjuvant, Alpacas (Lamapacos) were immunized by subcutaneous multipoint injection. For booster immunization, 150 μg c-Myc-BSA was emulsified with Freund's incomplete adjuvant at intervals of 2 weeks. Blood was collected from the vein 7 days after each immunization, and the serum titer was determined by indirect ELISA method. The sample with the highest serum titer was selected to separate lymphocytes. cells, RNA was extracted.
[0024] The extraction of RNA was carried out according to the instruction manual of RNAiso reagent from TAKARA company. Using RNA as a template and oligo dT as a primer, the first strand of cDNA was synthesized acco...
Embodiment 2
[0033] Panning and Identification of Anti-c-Myc Tag Single Domain Heavy Chain Antibody
[0034] The method of solid-phase affinity panning was used to pan the single domain heavy chain antibody against the c-Myc tag from the anti-c-Myc tag single domain heavy chain antibody immune library obtained in Example 1. Add 120 μL of Myc-GST fusion protein (the fusion protein of Myc tag and glutathione) diluted with PBS to each enzyme-labeled well, coat at 4°C overnight, and the coating concentration of each round of panning is 100 , 75, 50 μg / mL; aspirate the coating solution, wash the plate 5 times with PBS, add 300 μL 3% BSA-PBS to each well, block for 2 hours at 37°C; wash the plate 5 times with PBS, add 100 μL phage antibody library (containing about 1× 10 11 CFU), 37°C, incubate for 2.0 h; aspirate unbound phage, wash the plate with PBST (containing 0.5% Tween-20) for 3-5 times (increase 5 times for each round), and then wash the plate with PBS for 15-25 times; Use 100 μL eluen...
Embodiment 3
[0044] Scale Production of Anti-c-Myc Tag Single Domain Heavy Chain Antibody
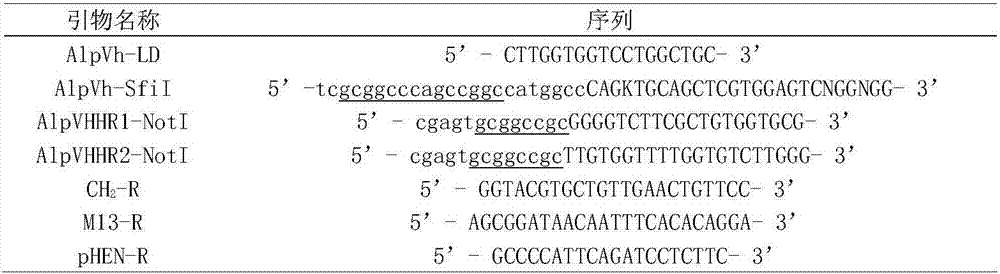
[0045] Acquisition of the DNA fragment encoding the anti-c-Myc tag single domain heavy chain antibody: 1. Using restriction endonuclease SfiI / NotI, double digestion of the phagemid pHEN-anti-c-Myc single domain heavy chain antibody gene, agar Glycogel electrophoresis to recover the anti-c-Myc tag single domain heavy chain antibody gene; 2. Directly send the anti-c-Myc tag single domain heavy chain antibody coding sequence to a biotechnology service company for chemical synthesis; 3. Design specific primers, through PCR technology amplifies from a cDNA library derived from alpaca (Lama pacos).
[0046] The obtained anti-c-Myc tag single-domain heavy chain antibody gene fragment was cloned into the expression vector pET25-flag (the c-Myc tag carried by the vector itself was replaced with the Flag tag: DYKDDDDK), identified by PCR and enzyme digestion, and constructed Complete the E. coli expression p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com