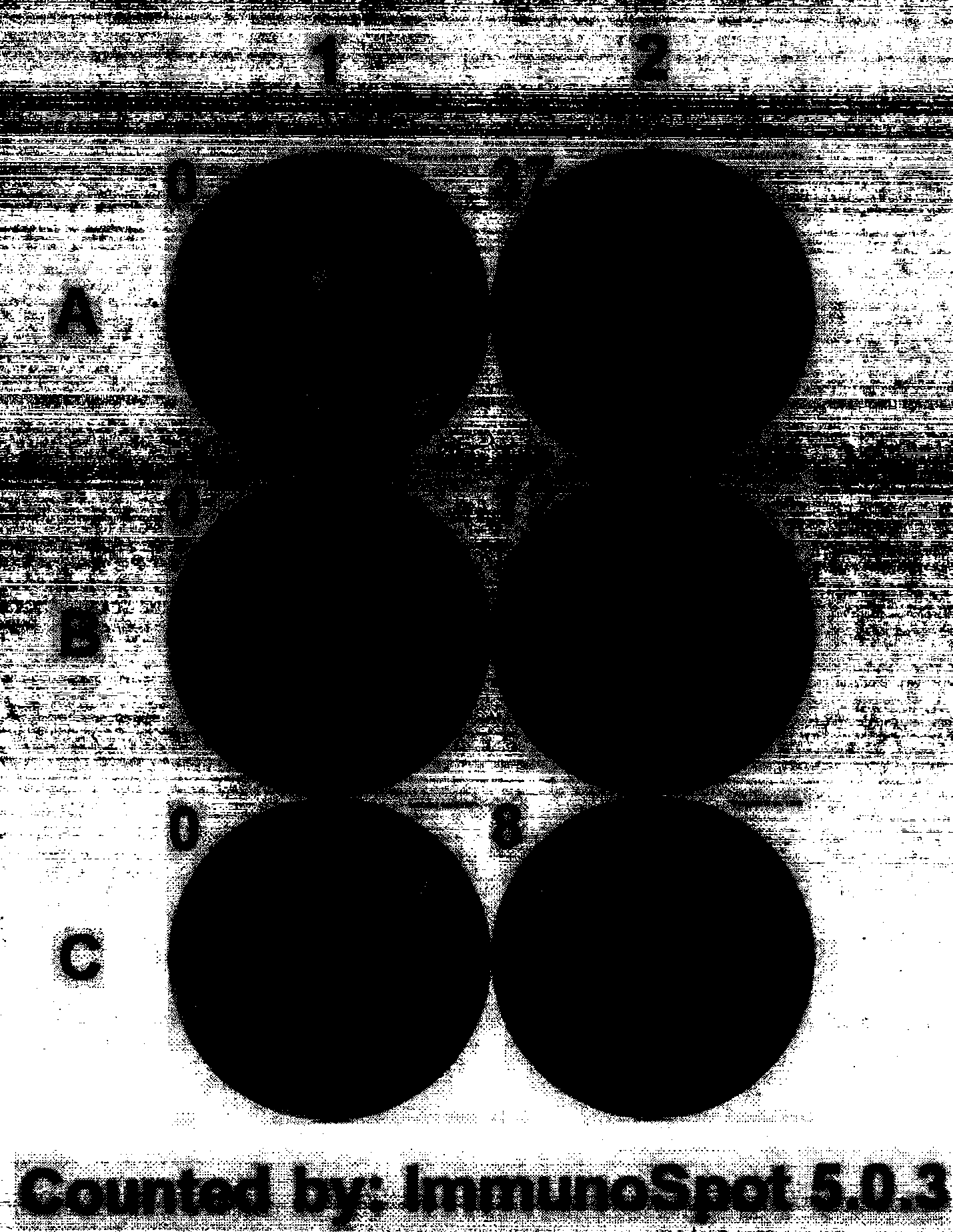Patents
Literature
70 results about "Recombinant Interleukin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Formulated therapeutic analogs of one of a number of endogenous cytokine interleukins. Produced by T cells, macrophages, and other cells, interleukins bind to a specific surface receptor on immunohematopoietic cells, thereby inducing a multitude of biologic effects including stimulation of growth, differentiation, and proliferation of lymphocytes and eosinophils; activation of lymphocytes and macrophages; enhancement of mast cell activity; activation of the acute phase response; and stimulation of hematopoiesis. Some interleukins may enhance the host's immune response to malignant cells by stimulating lymphokine-activated killer (LAK) cells and tumor-infiltrating lymphocytes (TIL), which are capable of lysing some tumor cells. (NCI04)
Methods for treatment of cutaneous T-cell lymphoma
InactiveUS7052685B1Relieve symptomsPeptide/protein ingredientsWhite blood cellCutaneous T-cell lymphoma
A method and composition for treatment of advanced cutaneous T cell lymphoma is provided which involves administration of recombinant interleukin-12.
Owner:UNIV OF PENNSYLVANIA THE TRUSTEES OF THE
Recombinant human interleukin-2 and polyethylene coupling compound
InactiveCN101104077APeptide/protein ingredientsPharmaceutical non-active ingredientsPolyethylene glycolOligopeptide
The invention relates to a coupling compound formed by the recombinant human interleukin-2 connecting with the monomethyl polyethylene glycol through the amino acid or the oligopeptide; the coupling compound has rather good plasma stability, and can release and recompose the recombinant human interleukin slowly to actively play the pharmacological action.
Owner:北京紫辰医药生物技术研究所 +1
Application of regulatory T cells in preparing drug for treating autoimmune diabetes and expanding cultivation solution and method thereof
ActiveCN107349219AGood curative effectTherapy is safeMetabolism disorderCulture processRegulatory T cellPenicillin
The invention discloses application of regulatory T cells in preparing a drug for treating autoimmune diabetes and an expanding cultivation solution and method thereof. The expanding cultivation solution of the regulatory T cells is prepared from, by volume, 35.274-42.69 parts of serum-free cultivation solutions, 1.25-2.5 parts of 4-(2-hydroxyerhyl)piperazine-1-erhanesulfonic acid buffer solution, 0.5-1 part of penicillin-streptomycin solution, 0.5-1 part of L-glutamine, 0.05-0.1 part of 2-mercaptoethanol, 0.00769-0.01154 part of recombinant interleukin-2, 0.001-0.01 part of rapamycin and 5-10 parts of AB serum. According to the application of the regulatory T cells in preparing the drug for treating the autoimmune diabetes, through targeted research on the regulatory T cells and the pathogenesis of the autoimmune diabetes, applicants find that the regulatory T cells has relatively good therapeutic effects at the aspect of treating the autoimmune diabetes.
Owner:CENT SOUTH UNIV
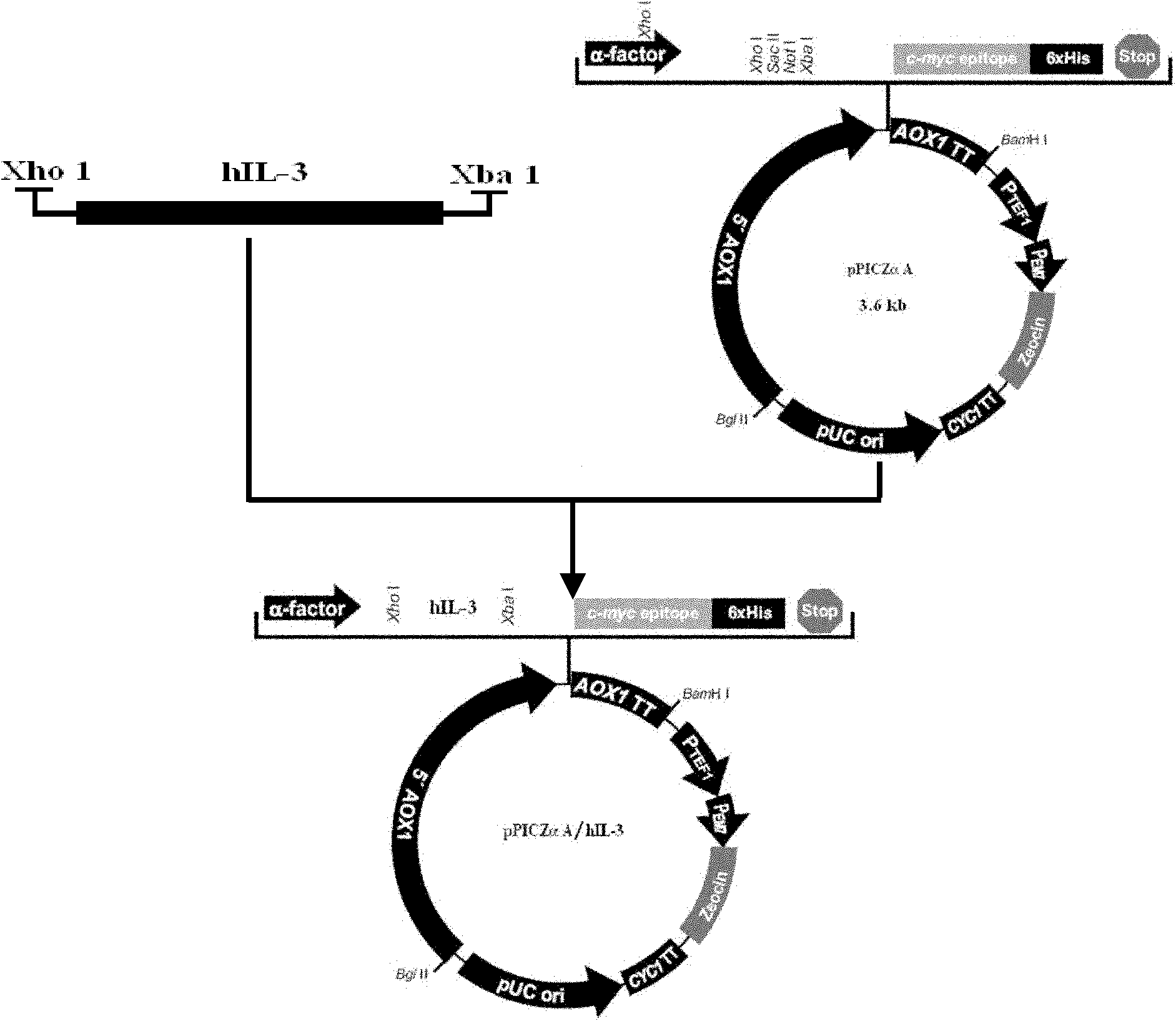
Method for expressing and purifying human recombinant interleukin-3
ActiveCN102146413AAvoid degradationLow toxicityPeptide preparation methodsFermentationPichia pastorisMass spectrometric
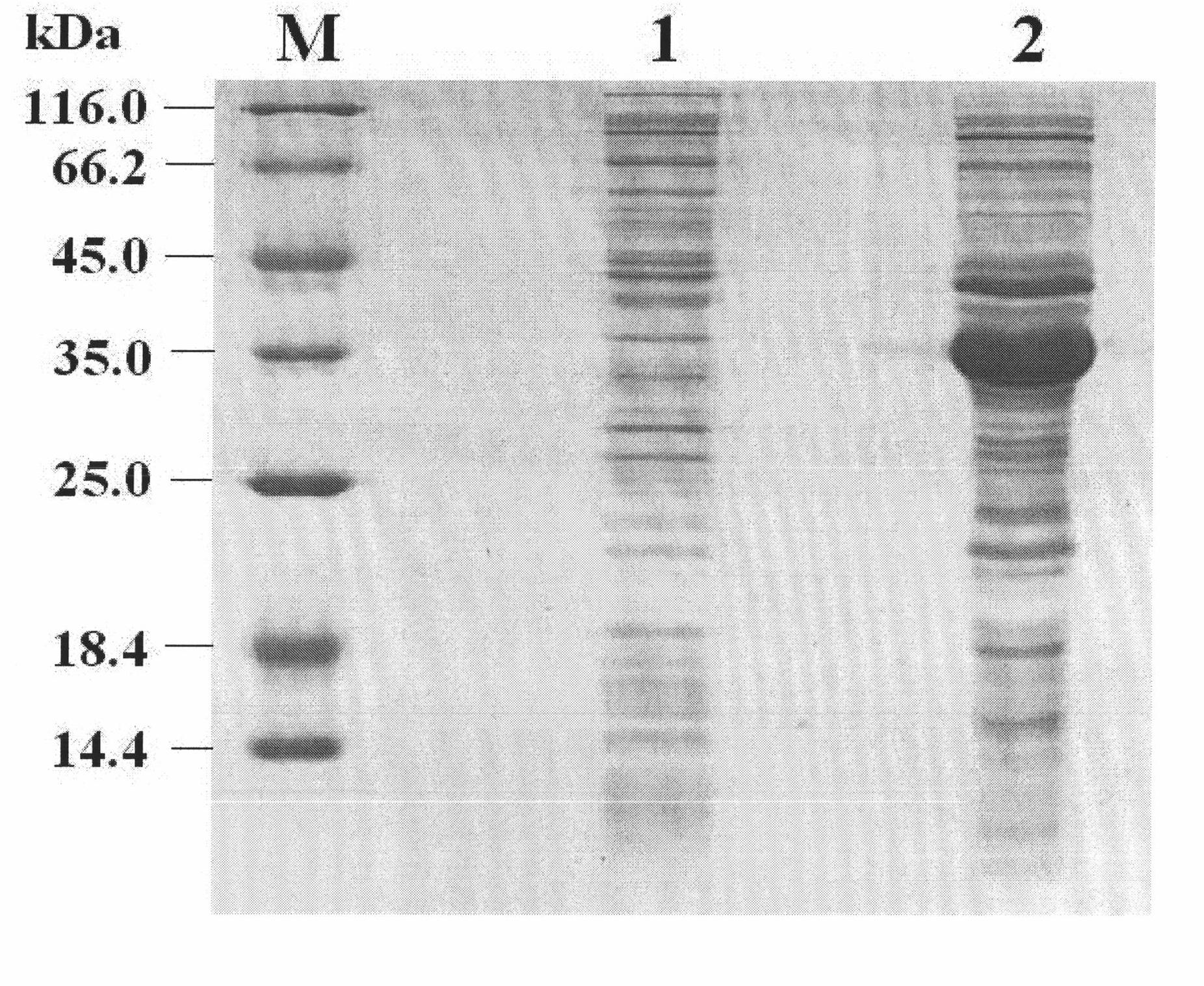
The invention discloses a Pichia pastoris transformant capable of expressing human recombinant interleukin-3 (rhIL-3) with high efficiency and a purification method thereof. In the method, the eukaryotic host is pichia pastorisX-33. The purification method comprises the following steps: cloning a human IL-3 gene; establishing a eukaryotic expression vector, and transforming the eukaryotic expression vector into the eukaryotic yeast host; obtaining a yeast transformant for high-level secretory expression by screening, wherein the IL-3 expressed by the yeasts are available in a glycosylated mode and a non-glycosylated module; and performing amplified culture by using a shake flask, and subjecting the supernate of the culture solution to dialysis, nickel affinity purification and further purification by diethylaminoethanol (DEAE) anion column. The purified product is subjected to mass spectrometric identification and analysis, and the result of the mass spectometric identification and analysis indicates that the expressed IL-3 is modified by different glycosyls and that the IL-3 has an his*6 tag and a C-MYC tag and is easy for purification and detection of expression product. In the invention, different from the conventional method using a prokaryotic host to express the rhIL-3, the method for expressing a large amount of rhIL-3 by using a Pichia pastoris expression system is adopted for the first time, quick purification is realized by using a His-tag protein, the purified rhIL-3 is high-activity rhIL-3 protein which is glycosylated to different extents and of which the molecular weight is 19kDa and 22kDa. The method ensures that the high-activity rhIL-3 recombinant protein is obtained quickly.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Construction and application of recombinant human interleukin 12 eukaryotic expression vector
InactiveCN101638657AFully extendedFully foldedGenetic material ingredientsAntibody medical ingredientsLaboratory mouseClinical trial
The invention provides a construction and an application of recombinant human interleukin 12 eukaryotic expression vector and relates to the development of recombinant human interleukin 12 (rhIL-12) eukaryotic expression vector, the screening of stable expression cell lines and the adjuvant function of nucleic acid vaccine gene and the therapeutical effect of tumor gene. Ten hydrophobic flexible amino acid joints are used to connect P40 and P35 to obtain fusion gene to construct rhIL-12 eukaryotic expression plasmid (pCDNA6-IL-12), then the expression plasmid is transferred to CHO cell and finally stable expression cell lines are screened. The expression product has good biological activity. Nucleic acid vaccine and pCDNA6-IL-12 are used for coimmune so as to significantly increase the immune effect. Strong antitumor effect can be exerted when the expression vector of the invention is directly injected inside the tumor of laboratory mouse tumor model or is first used to perform gene modification to tumor cells and then implanted in tumor. The expression vector of the invention can be used in the search of clinical trial stage after further perfecting.
Owner:张文卿
Recombination interleukin-2 frozen-dried preparation, and its preparing method
InactiveCN101002735AFor long-term storageNo change in potencyPowder deliveryPeptide/protein ingredientsFreeze-dryingAdditive ingredient
A freeze-dried recombinant interleukin-2 able to be stored at ordinary temp for 5 years is prepared from recombinant interleukin-2, buffer, stabilizer, non-ionic surfactant and freeze-drying excipient through mixing said auxiliary components, diluting with the water for injection, proportionally adding interleukin-2, sterilizing, filtering, filling it in bottles, and freeze-drying.
Owner:SHANGHAI HUAXIN HIGH BIOTECH
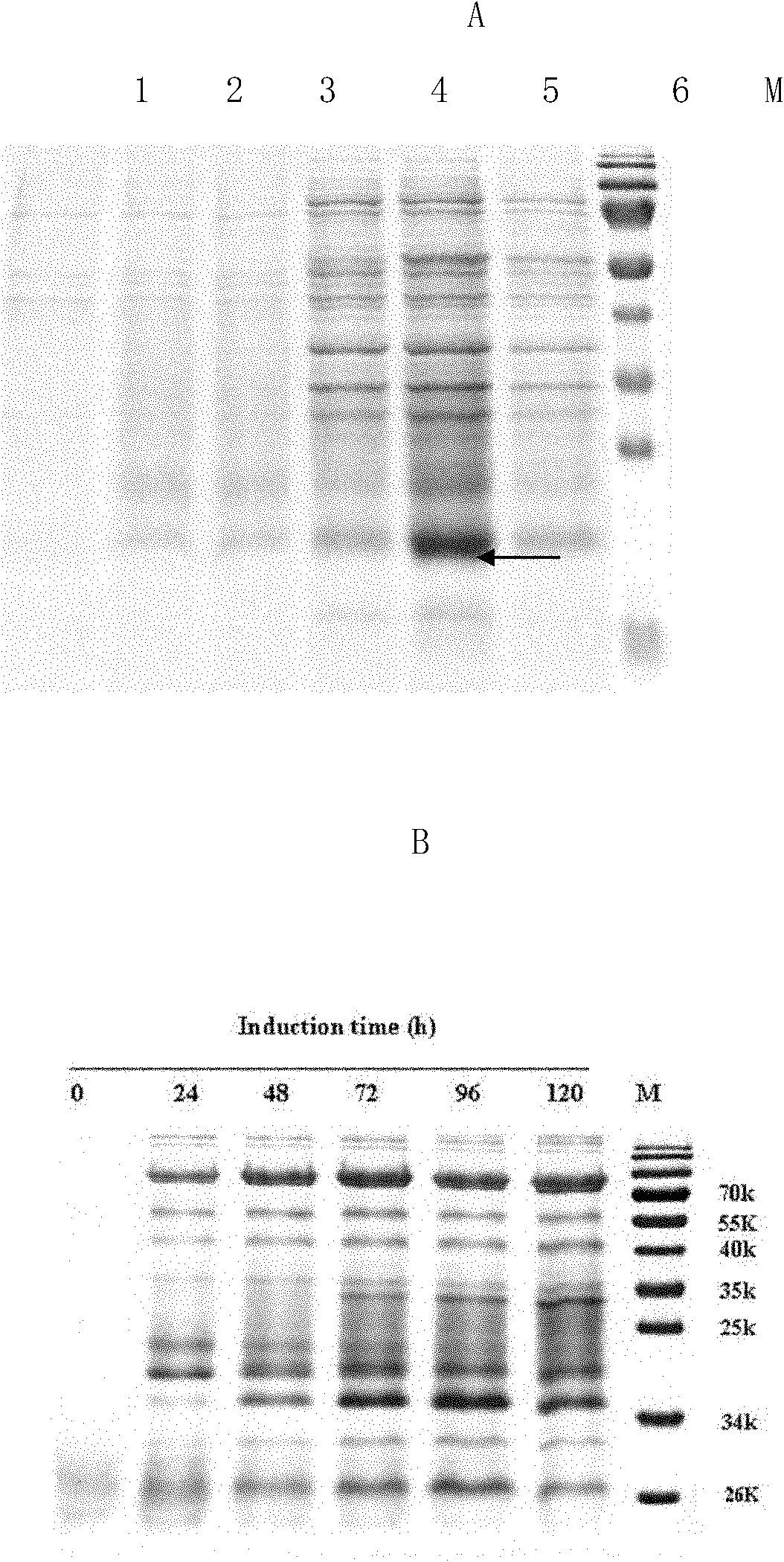
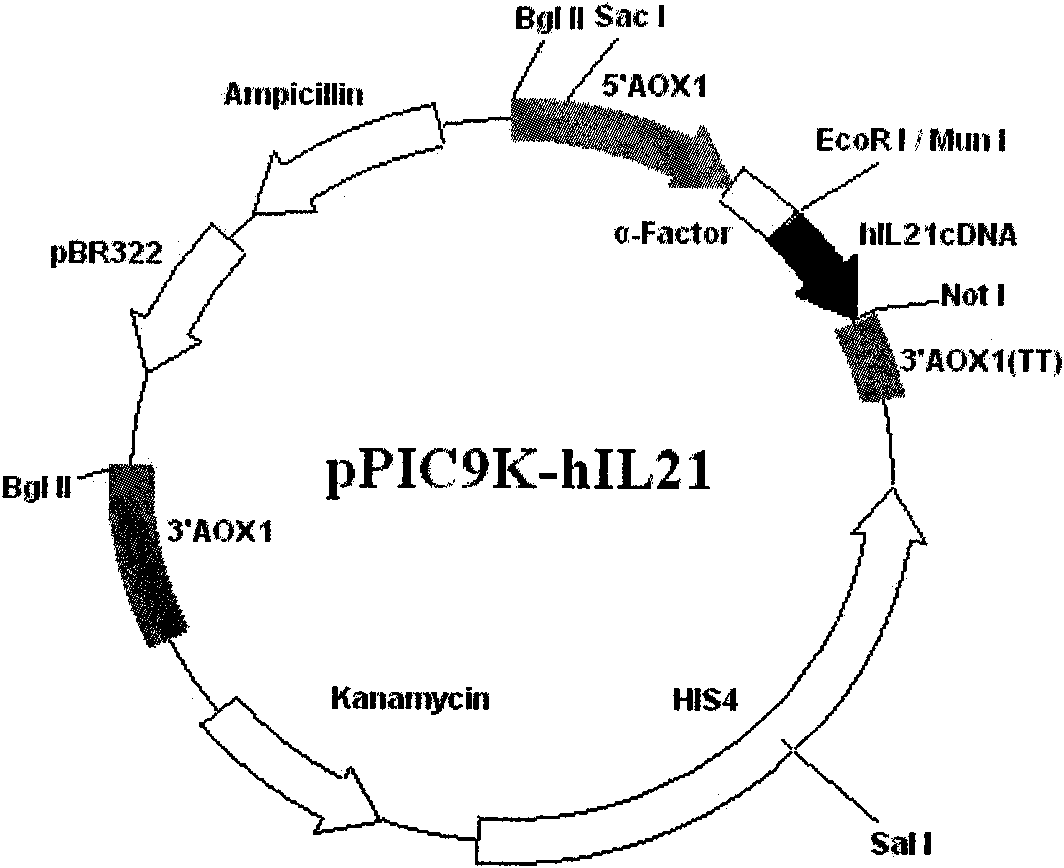
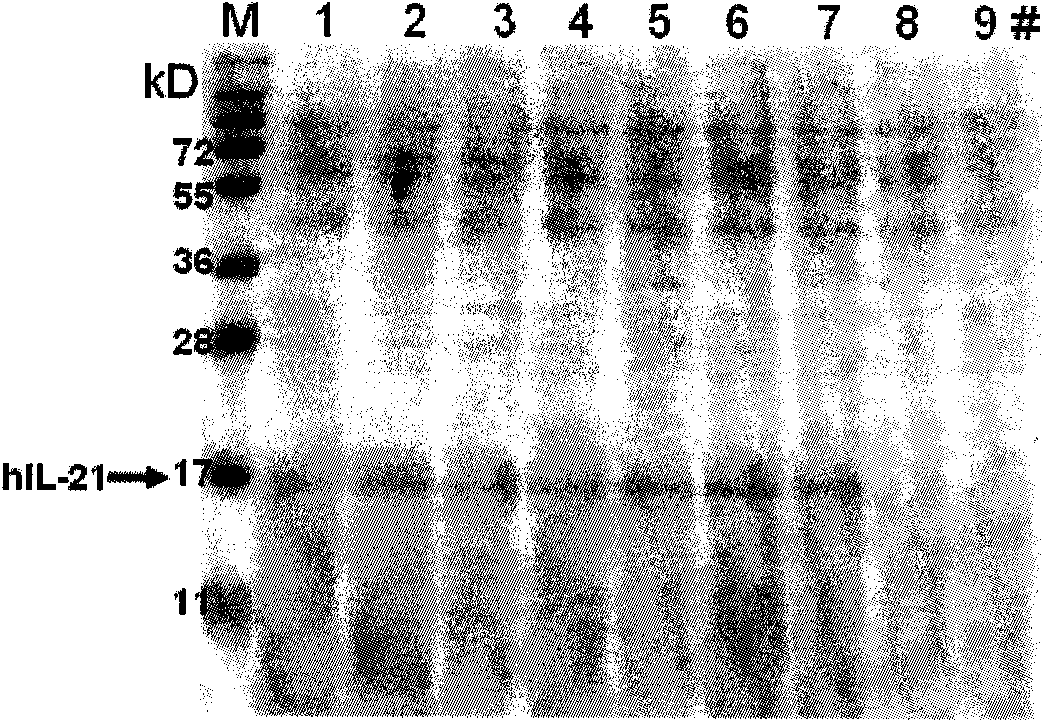
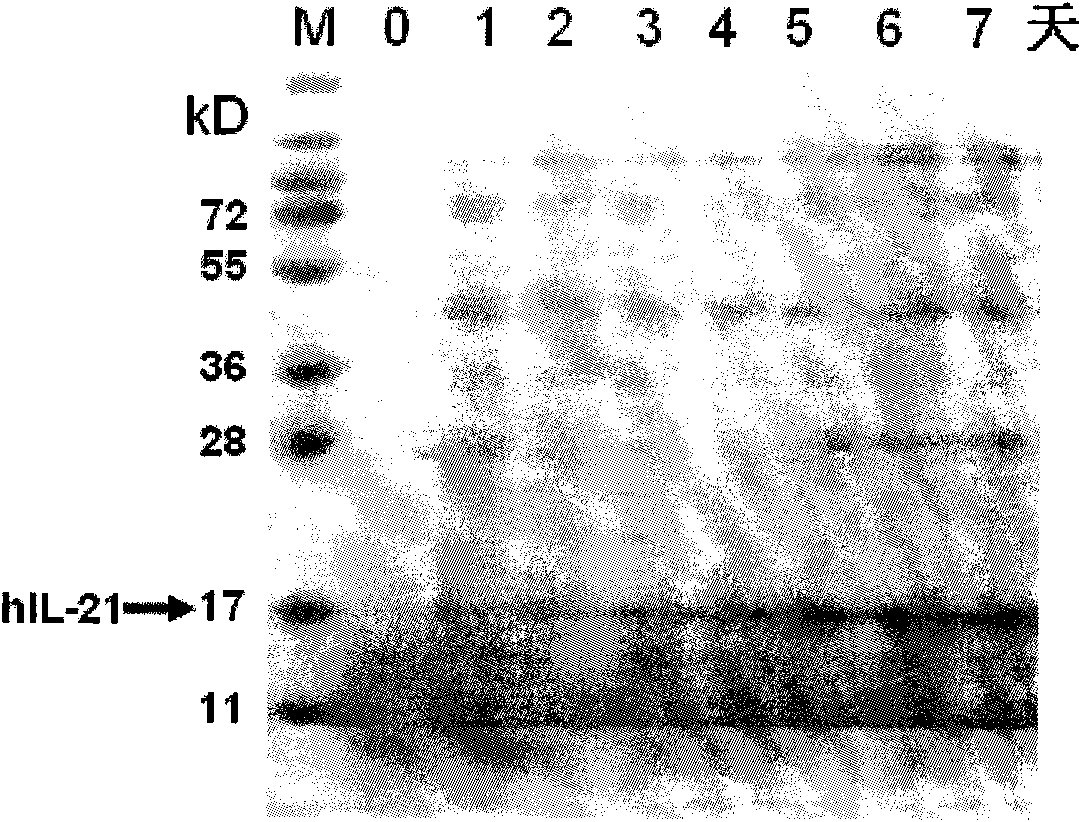
Method for producing recombinant human interleukin-21 by using Pichia pastoris
InactiveCN102021196AHigh expressionEasy to operateFungiMicroorganism based processesInterleukin 21Recombinant Interleukin
The invention provides a method for producing recombinant human interleukin-21 by using Pichia pastoris, in particular to a production method of the recombinant human interleukin-21 expressed by the Pichia pastoris, which comprises the following steps: firstly, the reverse transcription PCR (polymerase chain reaction) is carried out in lymphocytes of healthy people to obtain encoding genes of rhIL-21, and the encoding genes are fused in expressional regulatory elements of the Pichia pastoris to construct Pichia pastoris high-level-expression engineering bacteria; and secondly, the Pichia pastoris high-level-expression engineering bacteria are induced to produce a large number of recombinant human interleukins-21 by adding methanol. The Pichia pastoris is very easy to achieve high-density fermentation and has the characteristics of hypersecretion, so that a large number of recombinant human interleukins-21 can be industrially produced easily at low cost.
Owner:SHANGHAI GENON BIOENG +1
Recombinant porcine interleukin 2, and encoding gene and expression method thereof
The invention provides a recombinant porcine interleukin 2, and an encoding gene, an expression method, a purification method and an inclusion body renaturation method of the recombinant porcine interleukin 2, and belongs to the field of biological genetic engineering. The interleukin 2 plays an important part in immune regulation in a consequence of disease, thereby being widely used in animal disease treatments. In order to obtain a large amount of porcine interleukin 2, the escherichia coli expression system is used for performing heterologous expression to the recombinant porcine interleukin 2 gene of which the codon is optimized. In addition, because the porcine interleukin 2 in a prokaryotic expression is mostly expressed in form of inclusion body, the invention also provides an inclusion body purification method of the recombinant interleukin 2 and screens the inclusion body renaturation method. Finally, the activity of the recombinant interleukin 2 obtained according to the invention is 1.05*10<7>IU / ml and is 1.05 folds of the activity of the internationally recognized porcine interleukin 2 standard, thus the standard of industrial production is completely achieved.
Owner:GENSUN INST OF BIOMEDICINE
Preparation method for poly(ethylene glycol) modified recombinant human interleukin-2
InactiveCN103193879AGood molecular weight uniformityStrong antiviral activityDepsipeptidesPeptide preparation methodsPolyethylene glycolP-Toluenesulfonic acid
The invention provides a preparation method for poly(ethylene glycol) modified recombinant human interleukin-2, belonging to the field of biological medicine. The method comprises the following steps: activating mPEG with an activator so as to obtain an activated mPEG molecule; and reacting the activated mPEG molecule with recombinant human interleukin-2, adding a glycine solution to terminate a reaction and separating and purifying an obtained product; wherein the activator is one selected from the group consisting of succinic anhydride and N-hydroxysuccinimide, p-toluenesulfonic acid-chlorine, N,N'-carbonyl diimidazole, N,N'-disuccinimidocarbonate, p-nitrophenyl carbonate, benzotriazole carbonate, phenylsuccinimide carbonate and N-acetoxysuccinimide. The preparation method provided by the invention is simple and convenient to operate and is easy for quality control and enlarged production.
Owner:SHENZHEN YATAIXING IND
Method for detecting activity of recombinant human interleukin 12 (rhIL-12) protein
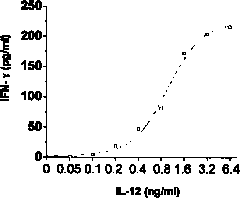
InactiveCN101799474AEasy to trainUnlimited number of passagesMicrobiological testing/measurementBiological testingWhite blood cellInterferon alpha
The invention discloses a method for detecting the activity of the recombinant human interleukin 12 (rhIL-12) protein, comprising the following steps: (1) stimulating the natural killer cell (NKC) which have the collection number of 2901 in the China General Microbiological Culture Collection Center (CGMCC) to genera Gamma interferon by using the standard rhIL-12 protein of different concentration and detecting the amount of the Gamma interferon to obtain a standard curve; (2) doing the experiment with the rhIL-12 protein to be detected in the activity by using the same method adopted in the step (1) so as to obtain the concentration of the rhIL-12 protein to be detected in the activity, which corresponds to half of the maximum amount of the Gamma interferon; and (3) calculating the activity of the rhIL-12 protein. The method for detecting the activity of the rhIL-12 protein can be operated easily, rapidly and stably and has low cost and high stability, repeatability, sensitivity and specificity, thereby having broad application prospect in the field of the detection of the activity of the rhIL-12 protein and laying a good foundation for the clinical use and the medicinal detection of the rhIL-12 protein.
Owner:UNIV OF SCI & TECH OF CHINA
Oral care compositions containing human recombinant interleukin-1
InactiveUS20120219511A1Cosmetic preparationsPeptide/protein ingredientsRecombinant Interleukin-1-alphaWhite blood cell
The present invention relates to oral care compositions comprising human recombinant interleukin-1 and methods thereof for keeping the oral cavity in a good condition, reducing oral malodor, and / or preventing or treating periodontal diseases. Preferably, the human recombinant interleukin-1 is human recombinant interleukin-1 alpha or beta.
Owner:UNITED TECH UT
Preparation method for monkshood polysaccharide-induced nature killer T (NKT) cell proliferation and application thereof
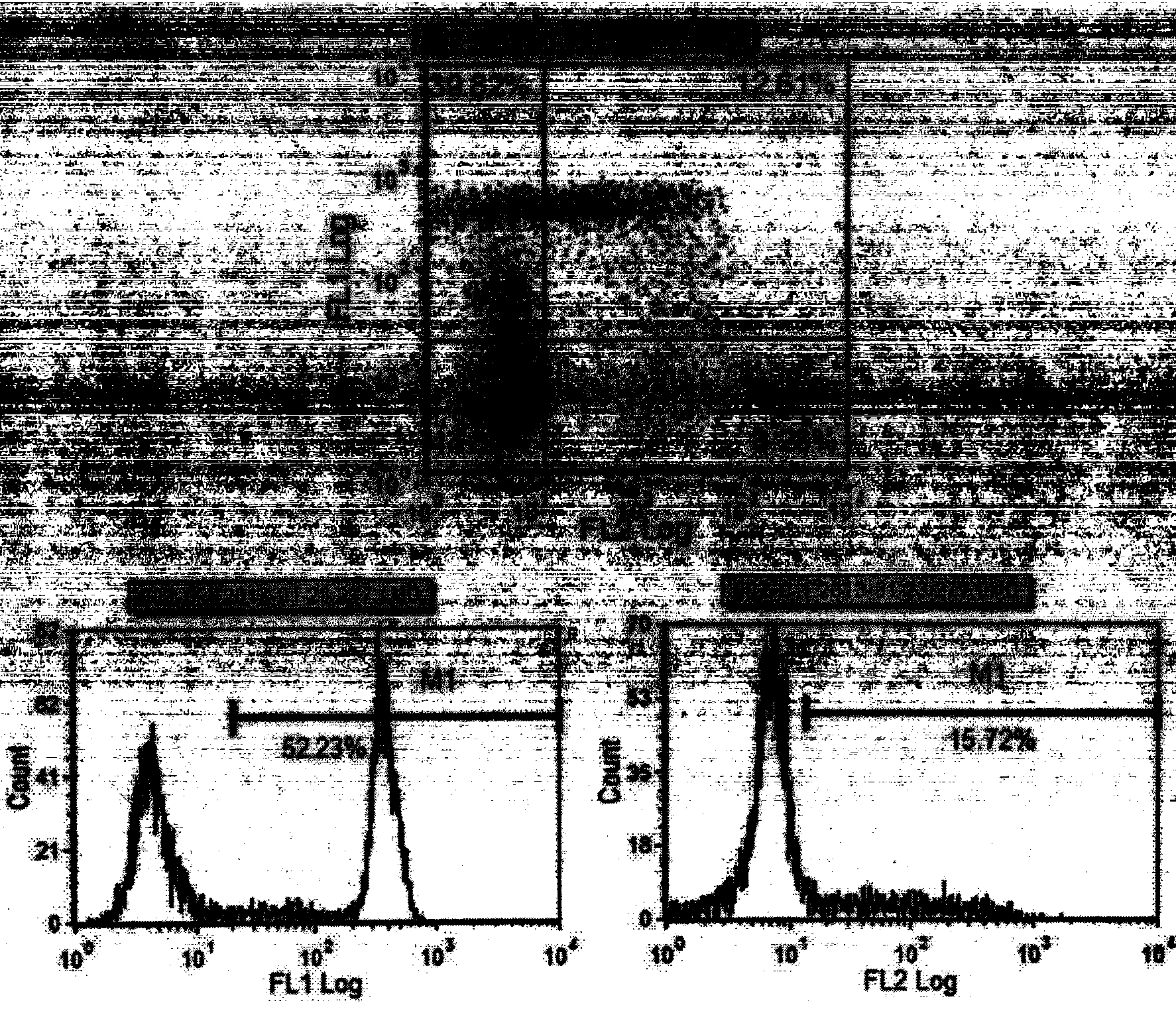
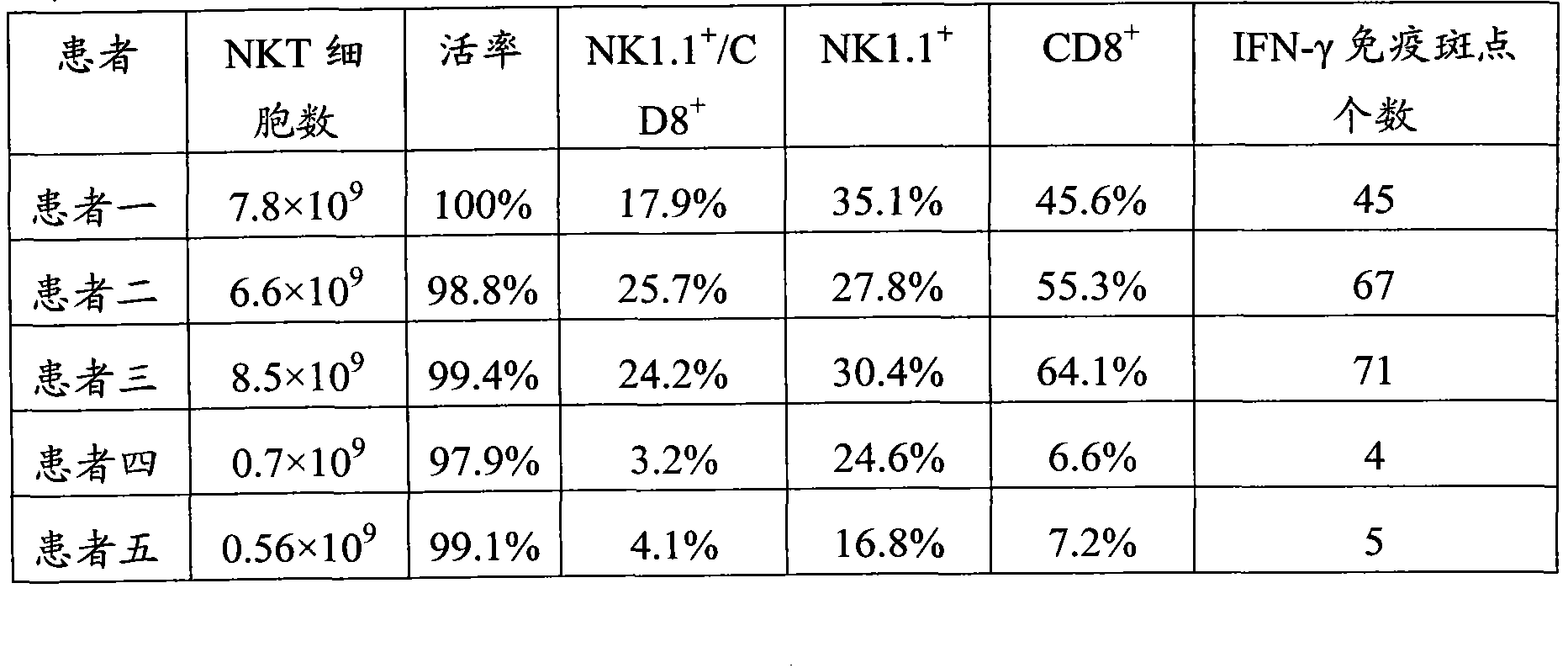
The invention provides a culture method for monkshood polysaccharide-induced nature killer T cell (Nature Killer T cell, NKT) proliferation. The method comprises the characteristics as follows: human peripheral blood, marrow or a mononuclear cell with an umbilical cord blood source are continuously cultivated by traditional Chinese medicine monkshood polysaccharide and recombinant human interleukin 2, a lot of nature kill T cells are obtained, NK<1.1+> and CD<8+> are expressed, interferon gamma is secreted, the ratio in lymphocyte at the 14th day is more than 16.15%, the order of magnitude can be up to over 5*10<9>, and the nature kill T cell have effective anti-tumor and anti-virus functions. A clinical application of the NKT cell obtained by the method is also provided by the invention.
Owner:李金珍
Fusion protein of human serum albumin and interleukin 2 and application thereof
The invention relates to a fusion protein of human serum albumin (especially wild-type human serum albumin) and human interleukin-2 (especially mutant human interleukin-2). The fusion protein has a significantly prolonged in vivo half-life relative to recombinant interleukin-2, so that the fusion protein can be used alone for the treatment of tumors. Moreover, the fusion protein can significantly improve the anti-tumor efficacy of an anti-PD-1 antibody or an anti-PD-L1 antibody.
Owner:TIANJIN LINDA SINOBIOTECH CO LTD +3
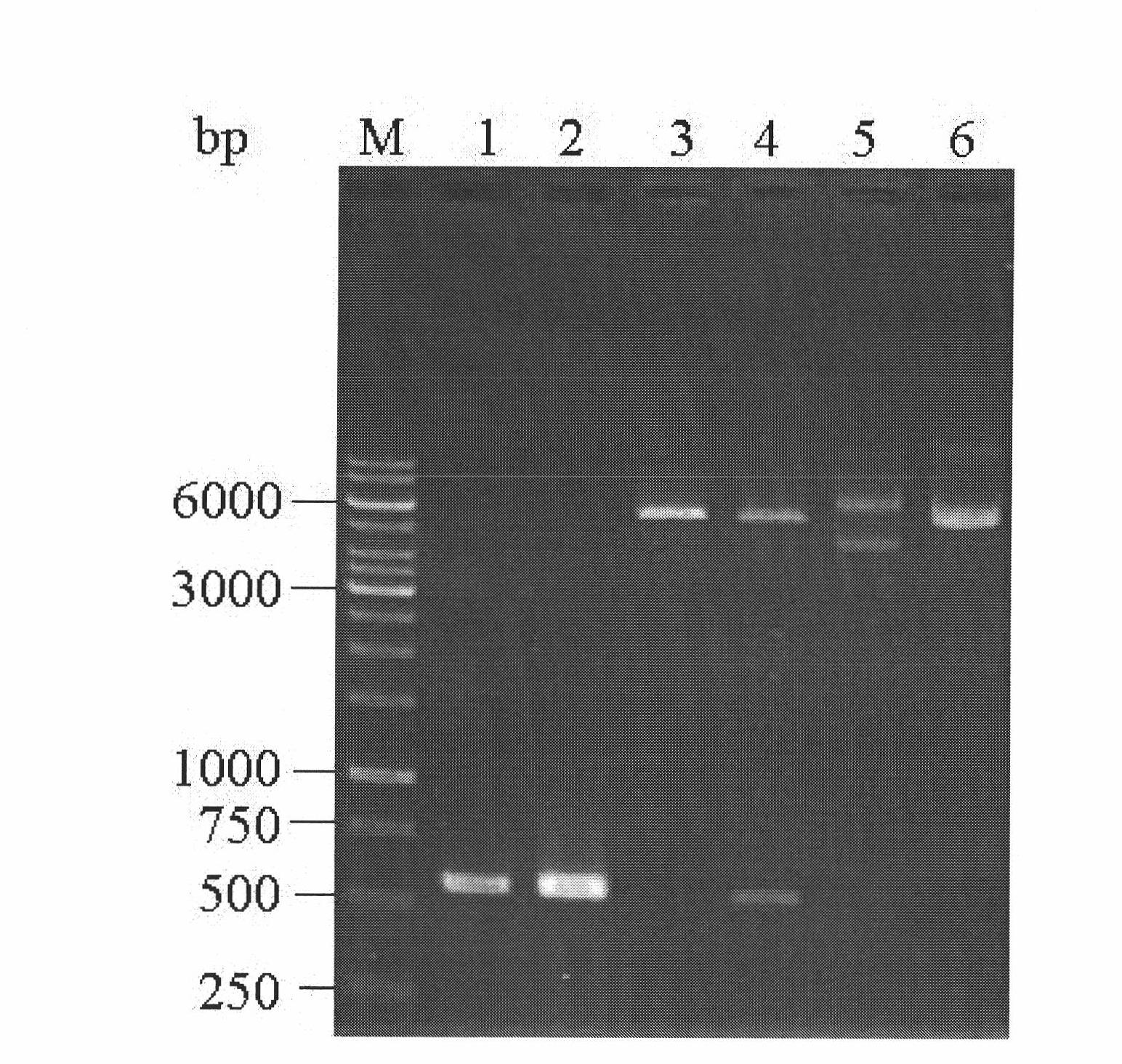
Pichiapastoris expression strain for recombinant duck interleukin 2 and construction method and application thereof
InactiveCN101892168AAvoid lossEasy to operateFungiMicroorganism based processesInterleukin IIMicrobiology
The invention discloses a Pichiapastoris expression strain for recombinant duck interleukin 2, and a construction method and application thereof. The chromosome of the strain integrates duck interleukin 2 genes. The invention has the characteristics of convenient operation, high conversion rate, simple production process, high product purity, high activity, contribution to simplifying the subsequent purification process and contribution to large scale production.
Owner:JIMEI UNIV
Purification method for recombinant human interleukin-12
InactiveCN105924514ARetain natural activitySimple processPeptide preparation methodsInterleukinsPurification methodsWhite blood cell
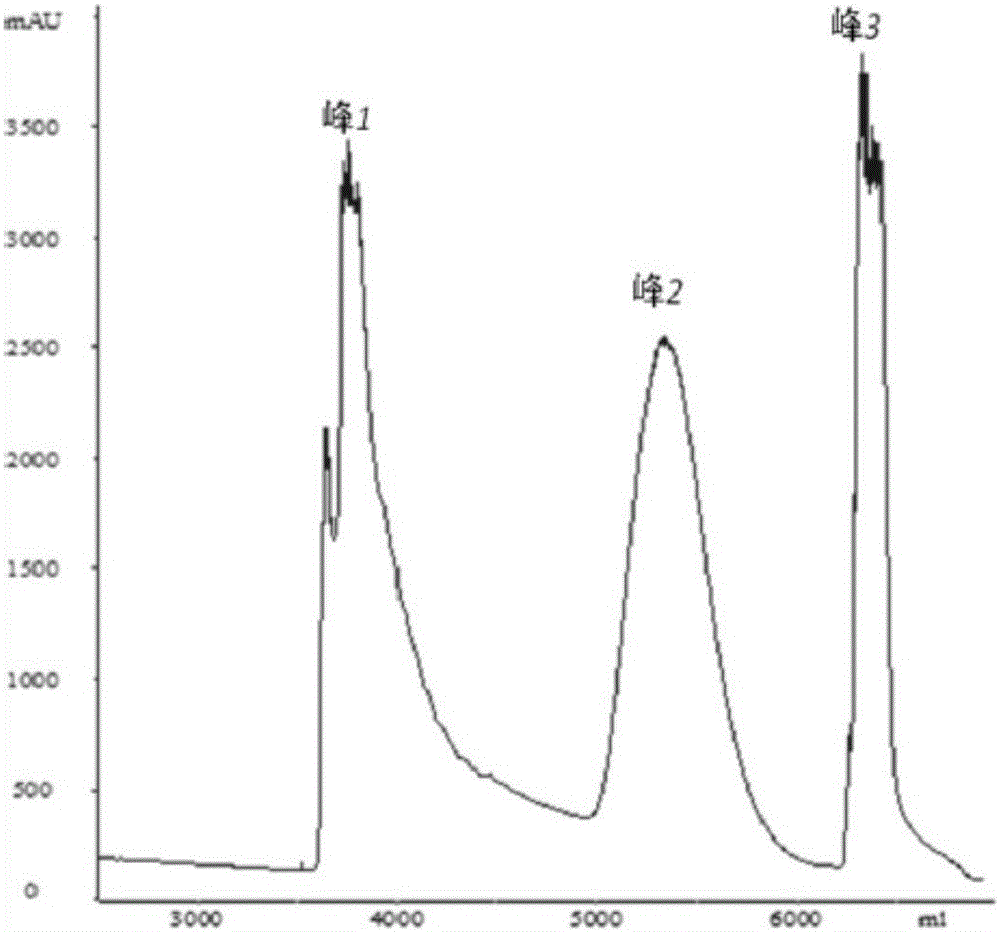
The invention relates to a purification method for recombinant human interleukin-12. The method includes the steps that cell culture fluid containing recombinant human interleukin-12 is clarified and filtered through a deep filtering system; 2, Q Sepharose HP purification is conducted; 3, Phenyl Sepharose 6FF Highsub purification is conducted; 4, SP Sepharose HP purification is conducted; 5, Superdex 200 purification is conducted. The method has the advantages of being simple in process, good in stability, uniform in product, low in production cost, environmentally friendly and high in activity and is suitable for industrial production.
Owner:KANGLITAI BIOMEDICAL (QINGDAO) CO LTD
Method for preparing recombinant human interleukin-11
InactiveCN102140487ATreatment safetySafe and effective treatmentPeptide preparation methodsFermentationWhite blood cellGradient elution
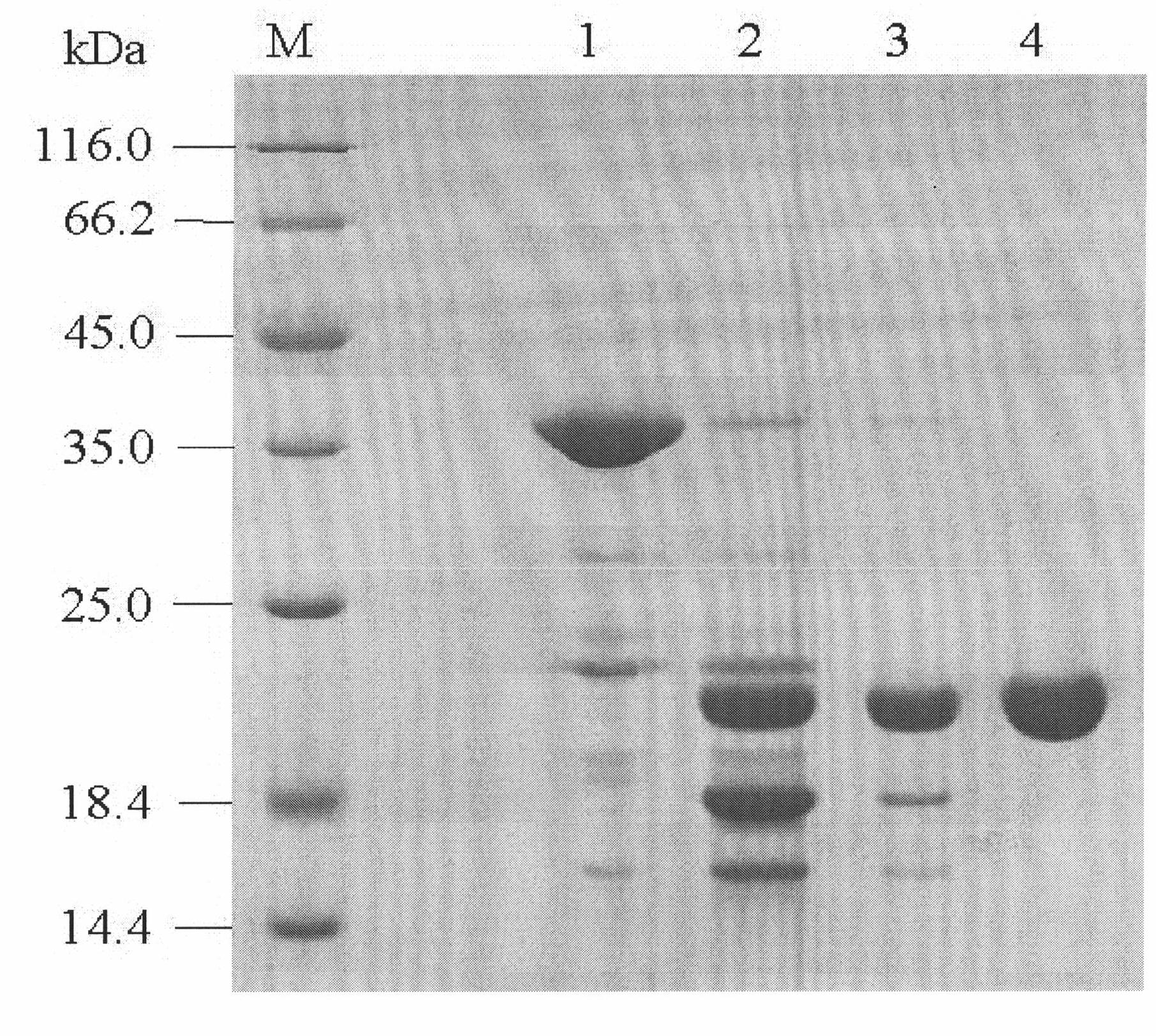
The invention provides a method for preparing recombinant human interleukin-11 (rhIL). The method comprises the following steps: 1) providing a fusion protein, wherein from the N-end to the C-end, the fusion protein is thioredoxin-(His)6-proteolytic enzyme recognition site-rhIL-11 or (His)6-thioredoxin-proteolytic enzyme recognition site-rhIL-11; 2) using the proteolytic enzyme to perform enzyme cutting to the fusion protein and obtain an enzyme cutting product, wherein the enzyme cutting product contains thioredoxin and rhIL-11; and 3) using the nickel ion chelate affinity column chromatography to purify the enzyme cutting product, and performing gradient elution to the column to obtain rhIL-11, wherein the thioredoxin and the rhIL-11 are both adsorbed on the column. By adopting the method provided by the invention, the rhIL-11 can be purified rapidly, conveniently and efficiently and the recovery rate can be greatly increased.
Owner:DONGGUAN TAILI BIOTECH
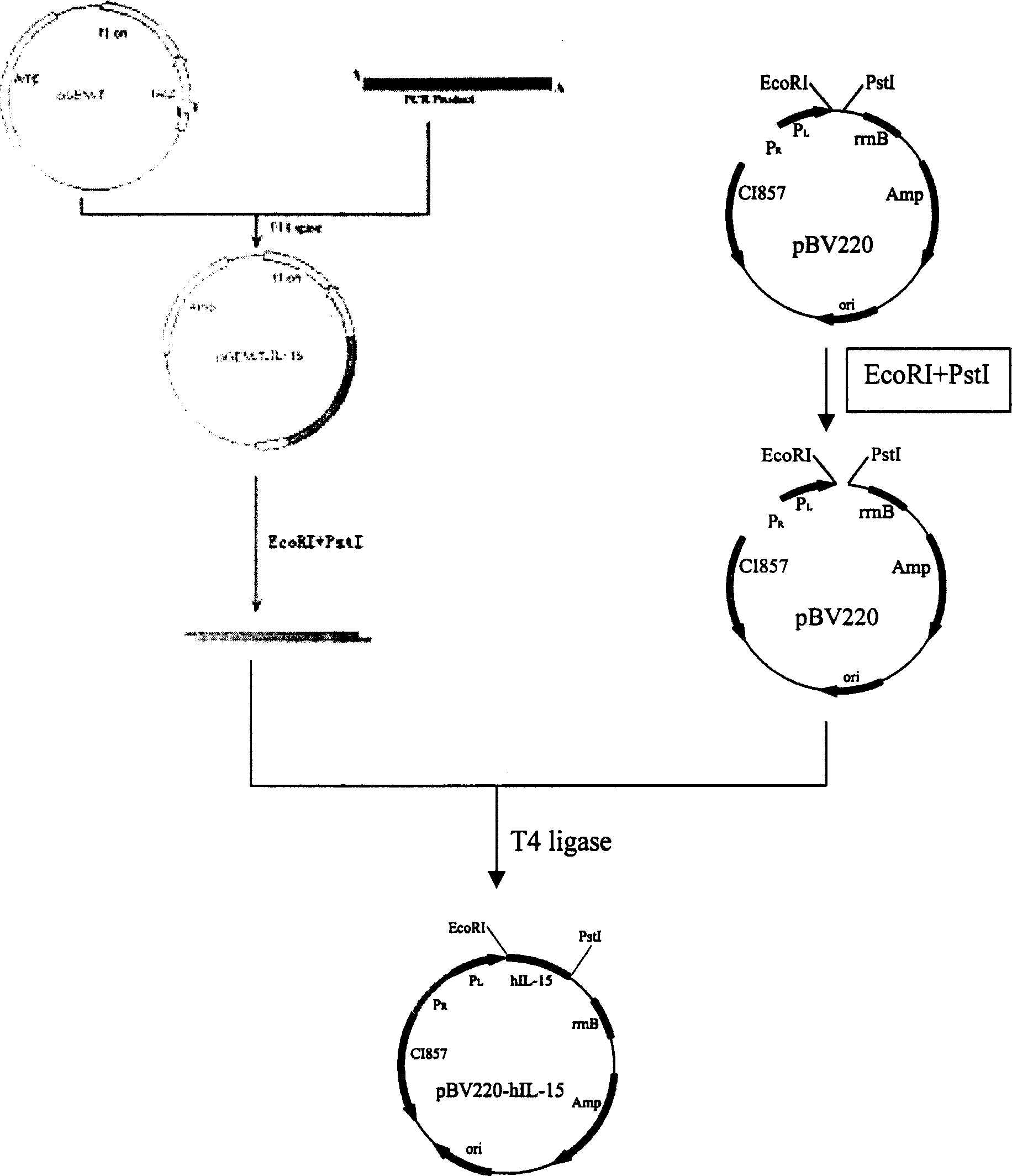
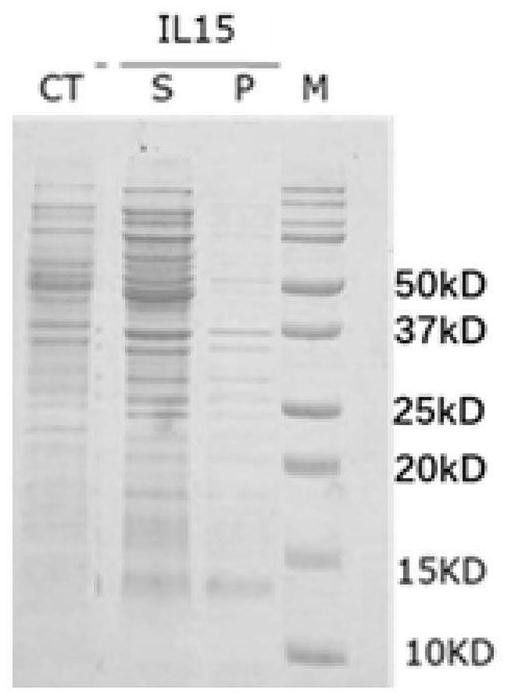
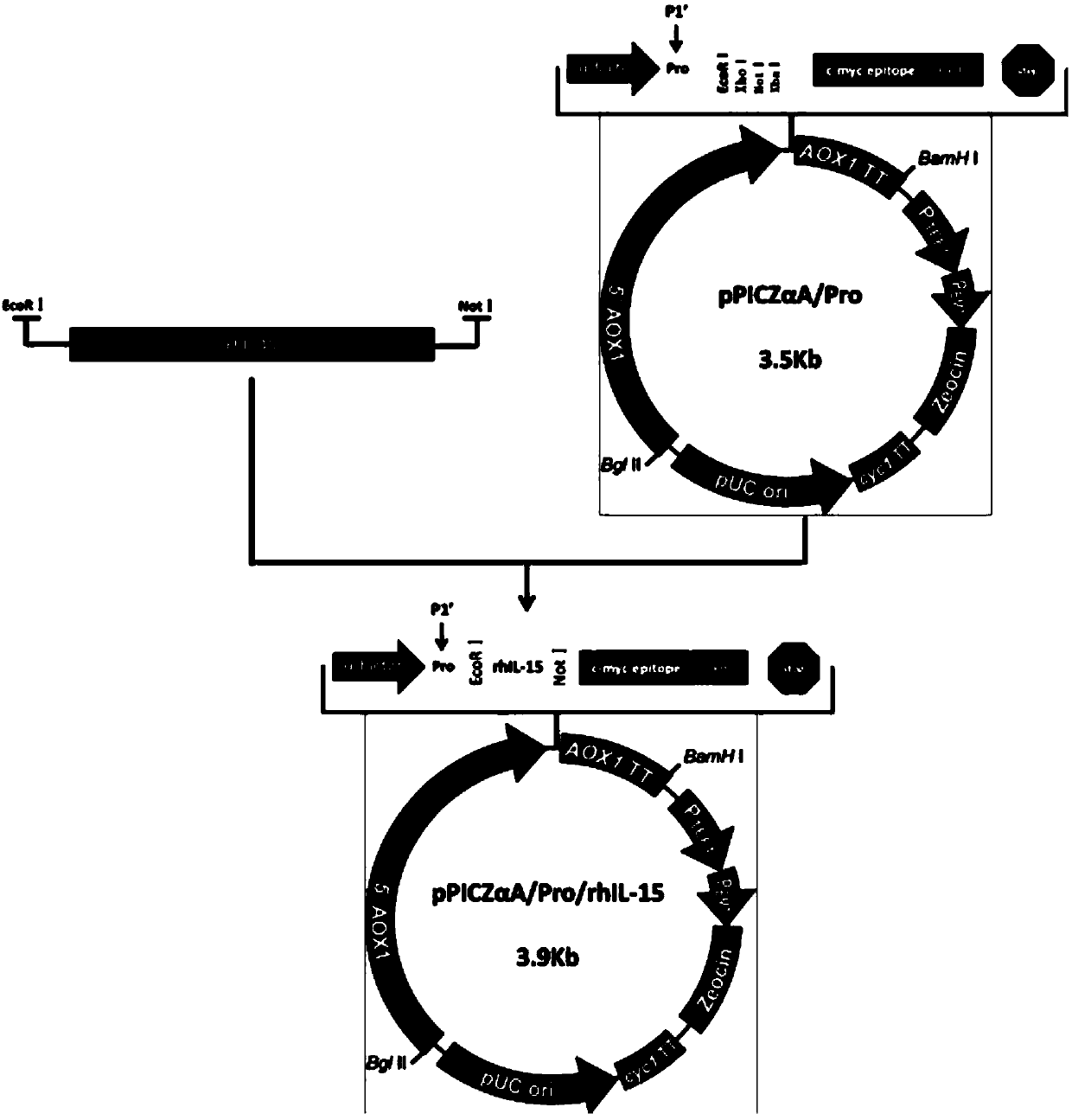
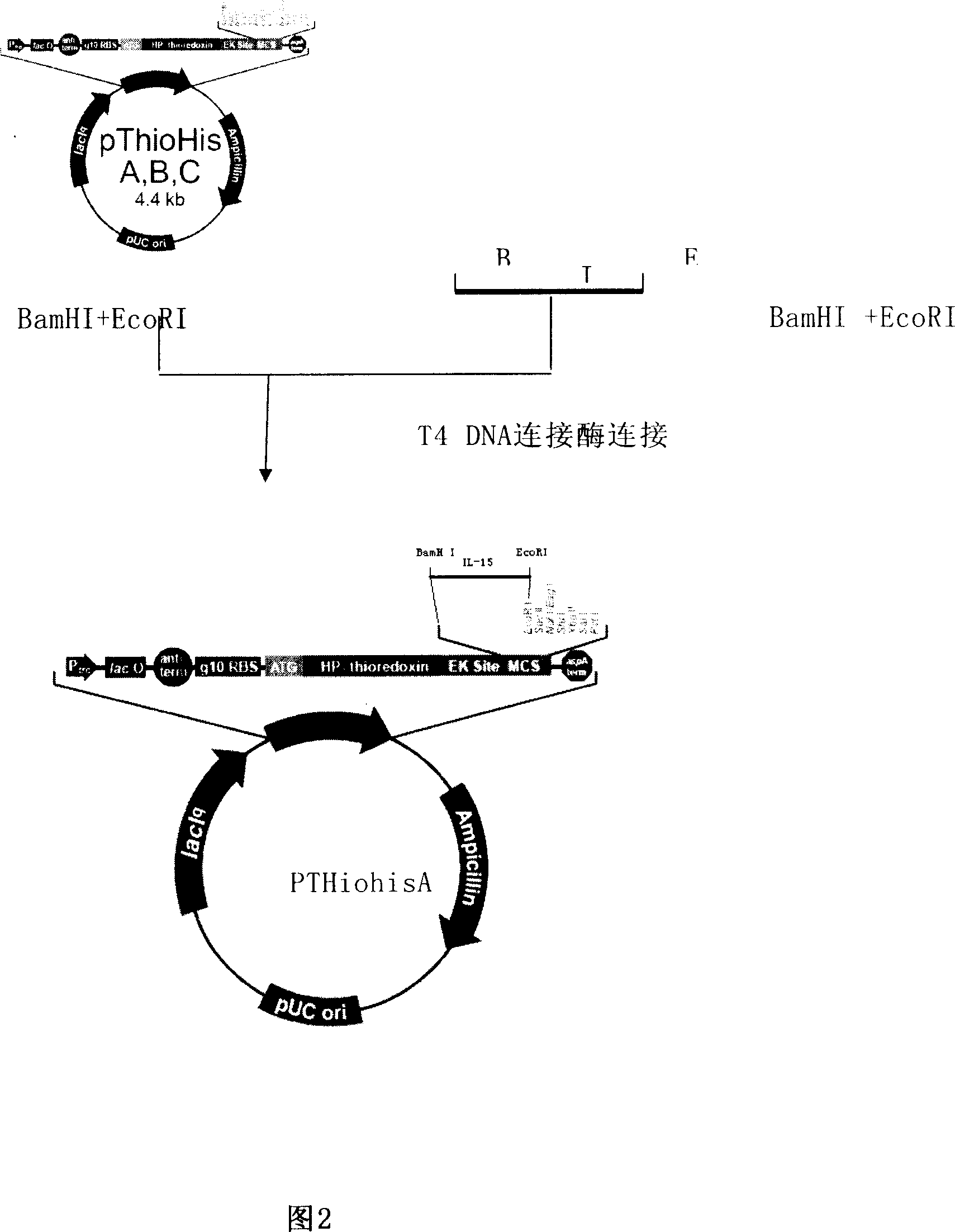
Prokaryotic expression engineering bacteria for producing human recombinant interleukin-15 and its purifying method
The present invention relates to one kind of prokaryotic system expression and purification technology consists of being used in large scale production of recombinant human interleukin-15. specifically, the technology of the present invention includes one kind of engineering bacterian and its completely new expression and purification technology. The engineering bacterium contains one completely new recombinant expression vector with one completely new synthesized DNA segment.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
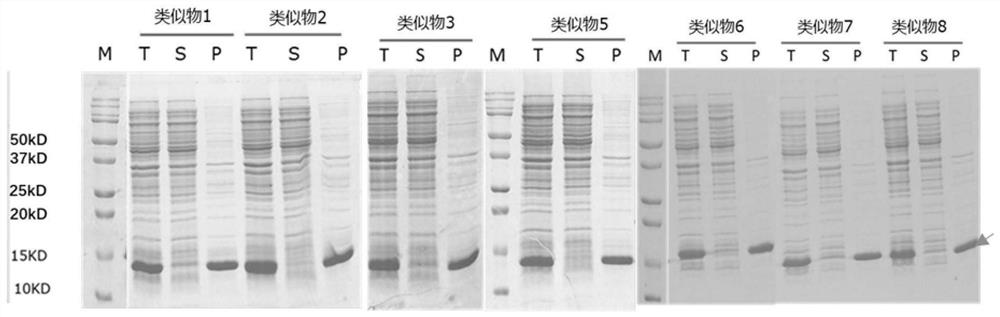
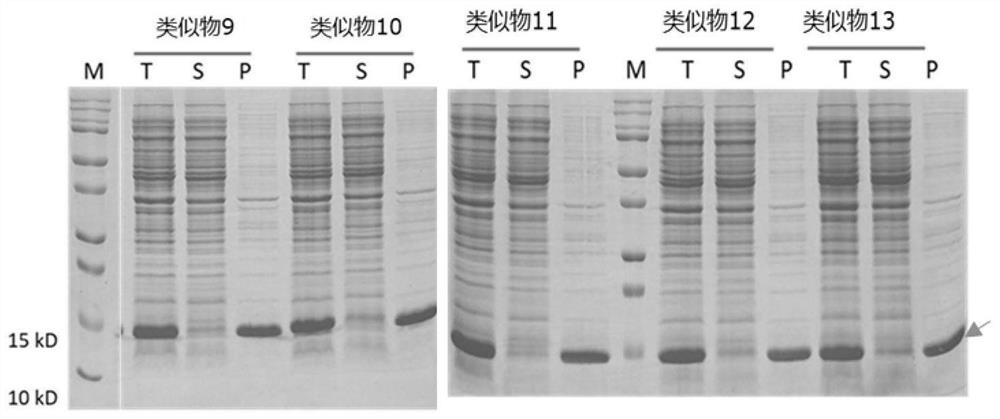
Recombinant interleukin-15 analogue
PendingCN112552391AHigh molecular weightEscape kidney filtrationBacteriaMicroorganism based processesCell activityPharmaceutical drug
The invention discloses an recombinant interleukin-15 (IL-15) analogue. The IL-15 analogue has the amino acid sequence of the IL-15, as well as one or more amino acids added at the C terminal of the IL-15 amino acid sequence; and the one or more amino acids comprise positively charged amino acids. The IL-15 analogue disclosed by the invention is highly expressed in escherichia coli that the expression level is about 20 times that of wild IL-15; and the in-vitro cell activity of the IL-15 analogue has no obvious difference. The invention further discloses a conjugate of the IL-15 analogue. Theconjugate of the IL-15 analogue can prolong half life of the IL-15 analogue by coupling a fatty acid chain so as to improve long-acting property of the IL-15 analogue. Thus, a foundation for industrialization of IL-15 protein drugs is laid.
Owner:LETO LAB CO LTD
Method for purifying recombinant interleukin 12
InactiveCN106349384AHigh purityGuaranteed yieldPeptide preparation methodsInterleukinsProtein targetFiltration
The invention relates to the technical field of protein purification, in particular to a method for purifying recombinant interleukin 12. The method comprises the following steps: pre-treating the cell culture medium of recombinant human interleukin 12, and then performing cation exchange column chromatography, ammonium sulfate fractionation, hydrophobic chromatography, anion exchange column chromatography and gel filtration chromatography, so as to obtain the recombinant interleukin 12. According to the method, the degradation of target protein by protease can be effectively reduced through the pretreatment on the cell culture medium. During the cation exchange column chromatography, impurities which are combined with the target protein by hydrophobic force and difficult to remove and noncovalently conjugated degraded fragments of the target protein can be removed through the washing of a buffer solution containing 20 percent absolute ethyl alcohol, so that the purpose of purification is achieved. The method is high in protein recovery rate, simple and rapid to operate and low in cost.
Owner:GUANGDONG COOWAY BIOTECH CO LTD
Recombinant porcine interleukin 4, and encoding gene and expression method thereof
ActiveCN103012577AImprove expression efficiencyBacteriaMicroorganism based processesDiseaseBiotechnology
The invention provides a recombinant porcine interleukin 4, and an encoding gene, an expression method, a purification method and an inclusion body renaturation method of the recombinant porcine interleukin 4, and belongs to the field of biological genetic engineering. The interleukin 4 plays an important part in immune regulation in a consequence of disease, thereby being widely used in animal disease treatments. In order to obtain a large amount of porcine interleukin 4, the escherichia coli expression system is used for performing heterologous expression to the recombinant porcine interleukin 4 gene of which the codon is optimized. In addition, because the porcine interleukin 4 in a prokaryotic expression is mostly expressed in form of inclusion body, the invention also provides an inclusion body purification method of the recombinant interleukin 4 and screens the inclusion body renaturation method. Finally, the activity of the recombinant interleukin 4 obtained according to the invention is 5*10<5>IU / ml to 2*10<6> IU / ml and is 2 folds of the activity of the internationally recognized porcine interleukin 4 standard, thus the standard of industrial production is completely achieved.
Owner:GENSUN INST OF BIOMEDICINE
Preparation method and application of recombinant canine interleukin-2
InactiveCN104120143ASimple methodSimple procedurePeptide/protein ingredientsDepsipeptidesPichia pastorisBiotechnology
The invention belongs to the technical field of molecular immunology, and in particular relates to a preparation method and an application of recombinant canine interleukin-2. The preparation method comprises the following steps: amplifying mature protein genes of the canine interleukin-2 from canine peripheral blood lymphocytes via a PCR method and connecting the genes onto a pichia pastoris expression vector pPICZaA so as to construct a recombinant vector of the mature protein genes of the recombinant canine interleukin-2; integrating the recombinant vector into a specific position in pichia pastoris in an electrical transformation manner. Thus, the high-efficiency expression of the canine interleukin-2 in the pichia pastoris is realized, and the expressed recombinant protein is good in biological activity. The method is low in production cost and high in production efficiency, and the recombinant canine interleukin-2 is good in biological activity. Thus, the method is suitable for enterprise production.
Owner:NANJING POLICE DOG RES INST OF MINIST OF PUBLIC SECURITY
Method for amplification of gamma delta T cells by phosphate and application thereof
InactiveCN104651308AEnhance killing activityLow costMammal material medical ingredientsBlood/immune system cellsPeripheral blood mononuclear cellPhosphate
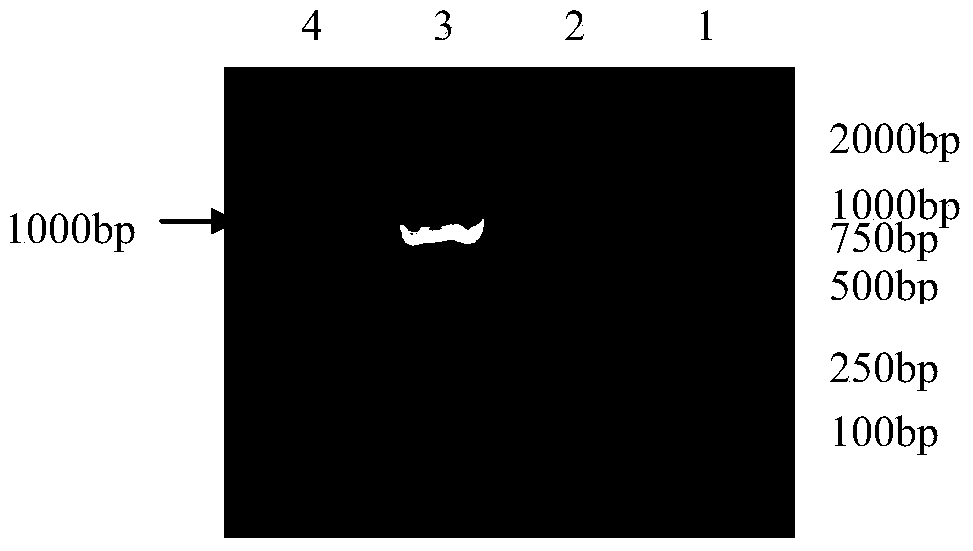
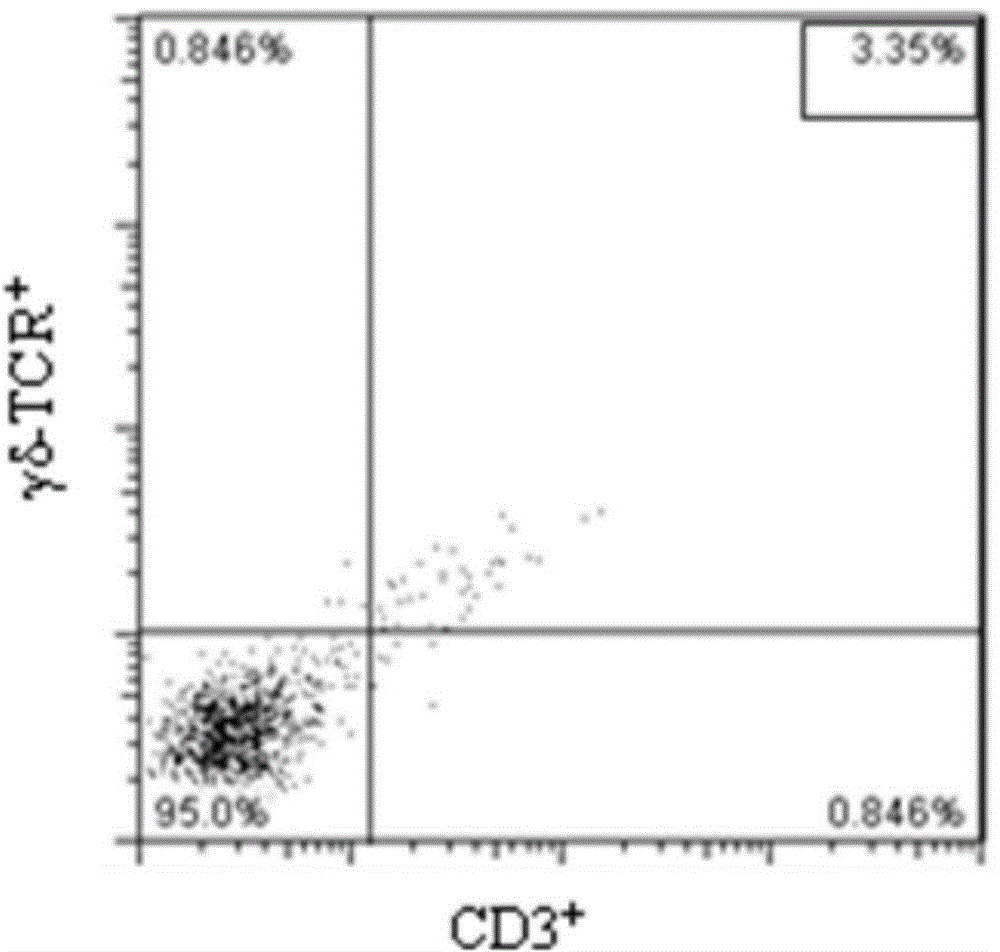
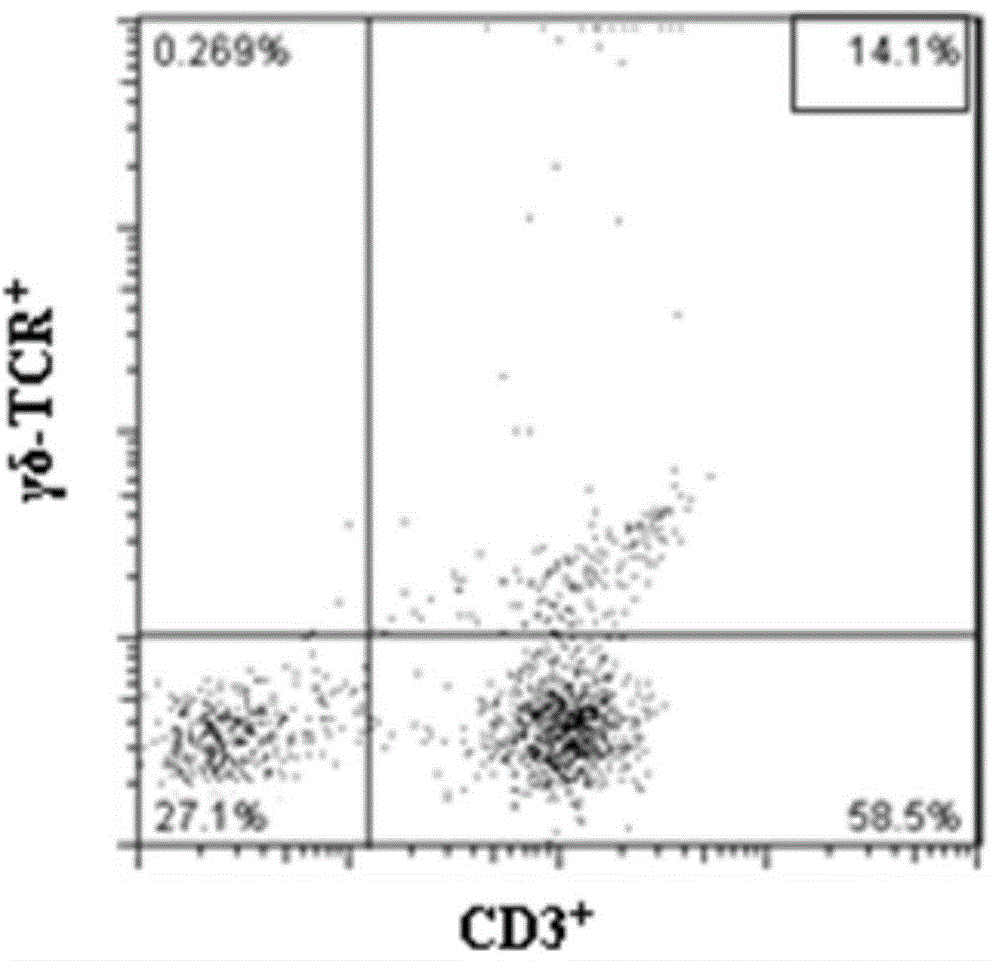
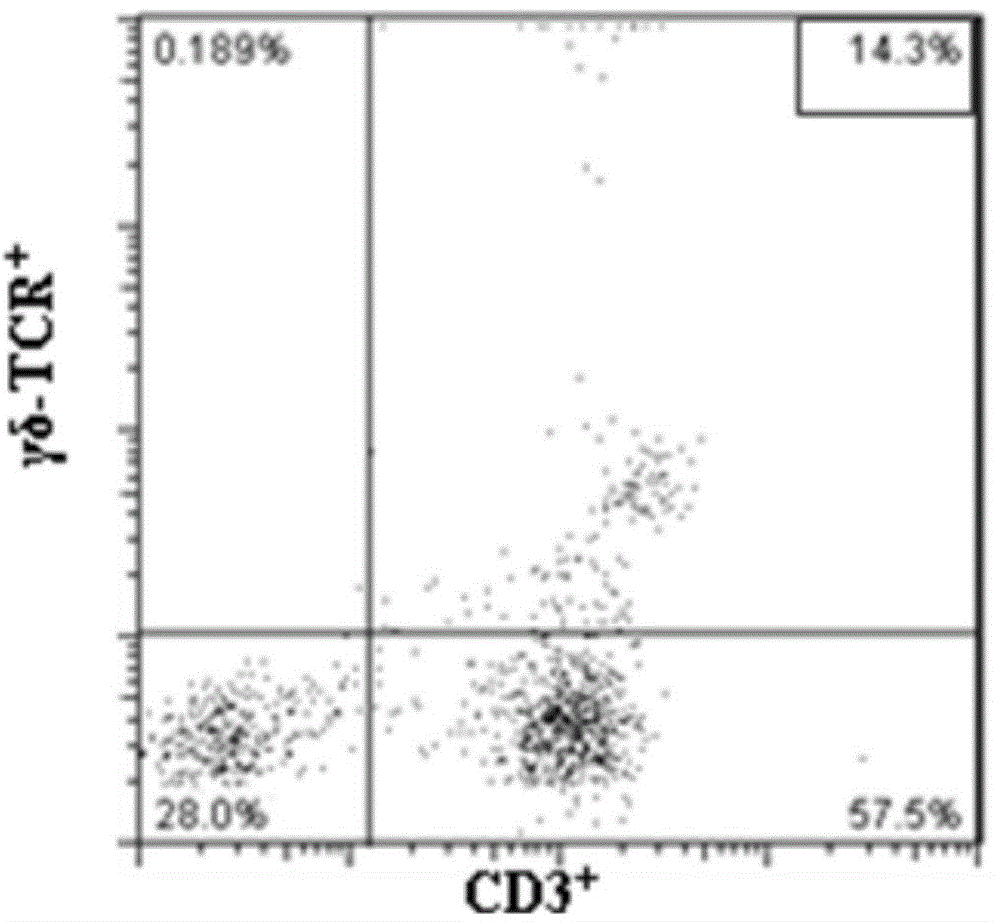
The invention provides a method for in vitro amplification of human gamma delta T cells and application thereof. The method for in vitro amplification of human gamma delta T cells includes the steps of: (1) preparing or providing mononuclear cell; (2) adding the mononuclear cell into a cell culture liquid, and placing the cell culture liquid into a cell incubator to conduct culture; and (3) acquiring the cells. The cell culture liquid contains (1-hydroxy-3-(methyl pentyl amino)propylidene)diphosphonic acid monosodium salt (IBA) and human recombinant interleukin rhIL-2. According to the invention, the method of combining rhIL-2 and IBA to stimulate human peripheral blood mononuclear cells (PBMC) is employed to amplify human gamma delta T cells in vitro. The method has the characteristics of low cost, simple preparation and high amplification efficiency, and can obtain gamma delta T cells with strong tumor lethality and TCR (T cell receptor) cloning diversity.
Owner:SHENZHEN INST OF ADVANCED TECH
Vagina injection agent for treating uterine prolapse and preparation method of vagina injection agent
PendingCN110522716AHigh purityQuality improvementPeptide/protein ingredientsPharmaceutical delivery mechanismDamages tissueTissue repair
The invention discloses a vagina injection agent for treating uterine prolapse. The vagina injection agent is prepared from the following components in parts by weight: 5-20 parts of beta-glucan, 1-10parts of lycium barbarum polysaccharide, 1-10 parts of chitosan oligosaccharide, 5-15 parts of recombinant human serum albumin, 0.05-0.5 part of recombinant human interleukin-22, 0.1-0.6 part of recombinant human interleukin-4, 0.3-1.5 parts of a recombinant human epidermal growth factor, 10-15 parts of active lactobacillus powder, 0.1-1 part of a penetration enhancer, 1-5 parts of a bio-adhesiveagent, 40-60 parts of a buffer solution and 50-100 parts of normal saline. The vagina injection agent directly acts on lesion parts, local drug concentration is high, the vagina micro-environment canbe effectively improved, the micro-ecological balance in a vagina and the normal micro-environment of a cervix are maintained, and local and body immune functions are improved; and the vagina injection agent can further promote cell regeneration, promote collagen synthesis, improve skin elasticity, accelerate repair of damaged tissue, promote tension recovery of pelvic floor muscles, fascia and uterus ligament, and can effectively improve uterine prolapse; and recurring is not prone to appearing after cure, using is convenient and safe, and the effect is fast.
Owner:广东圆康再生医学科技开发有限公司
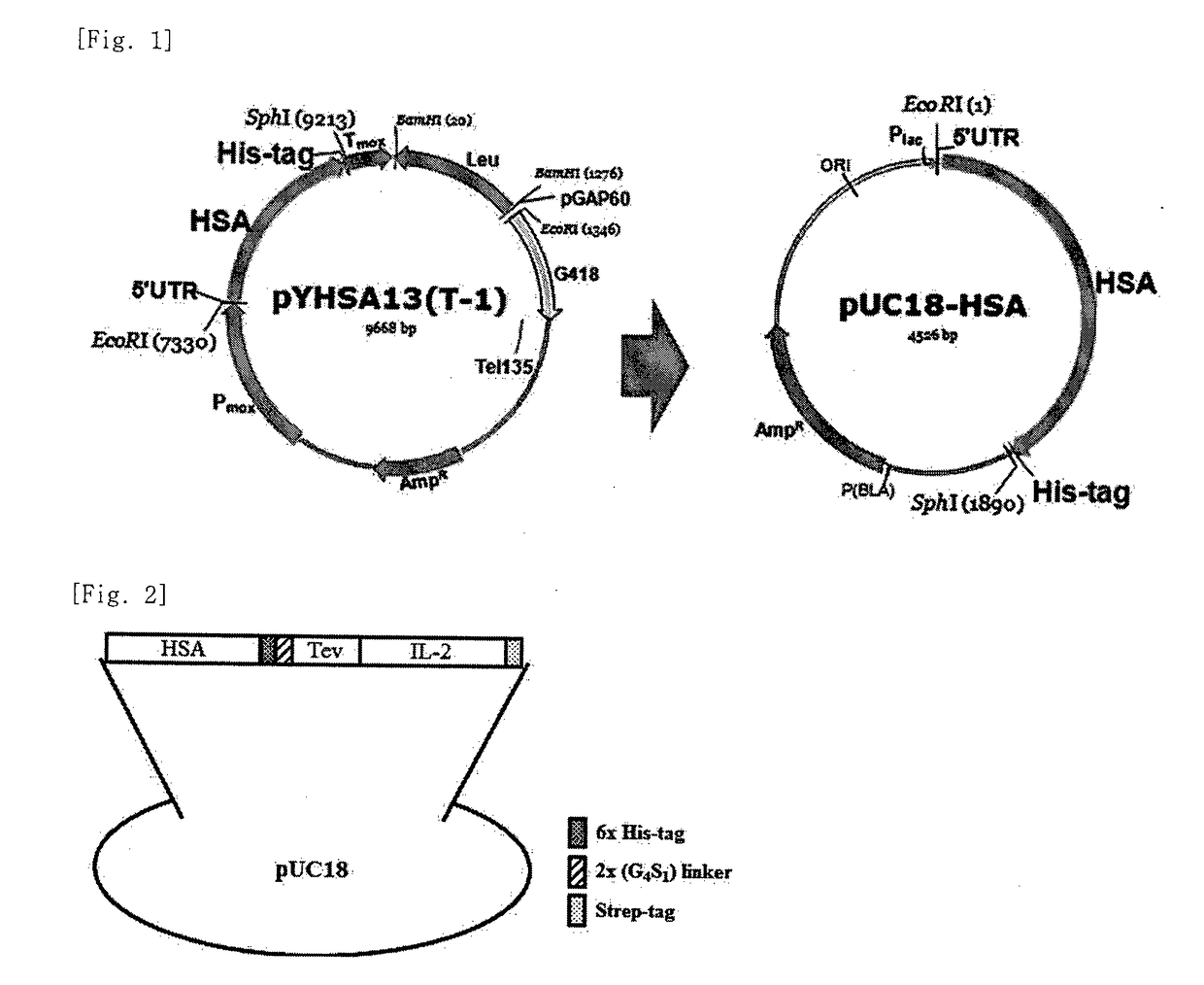
Interleukin-2 expression construct using human serium albumin
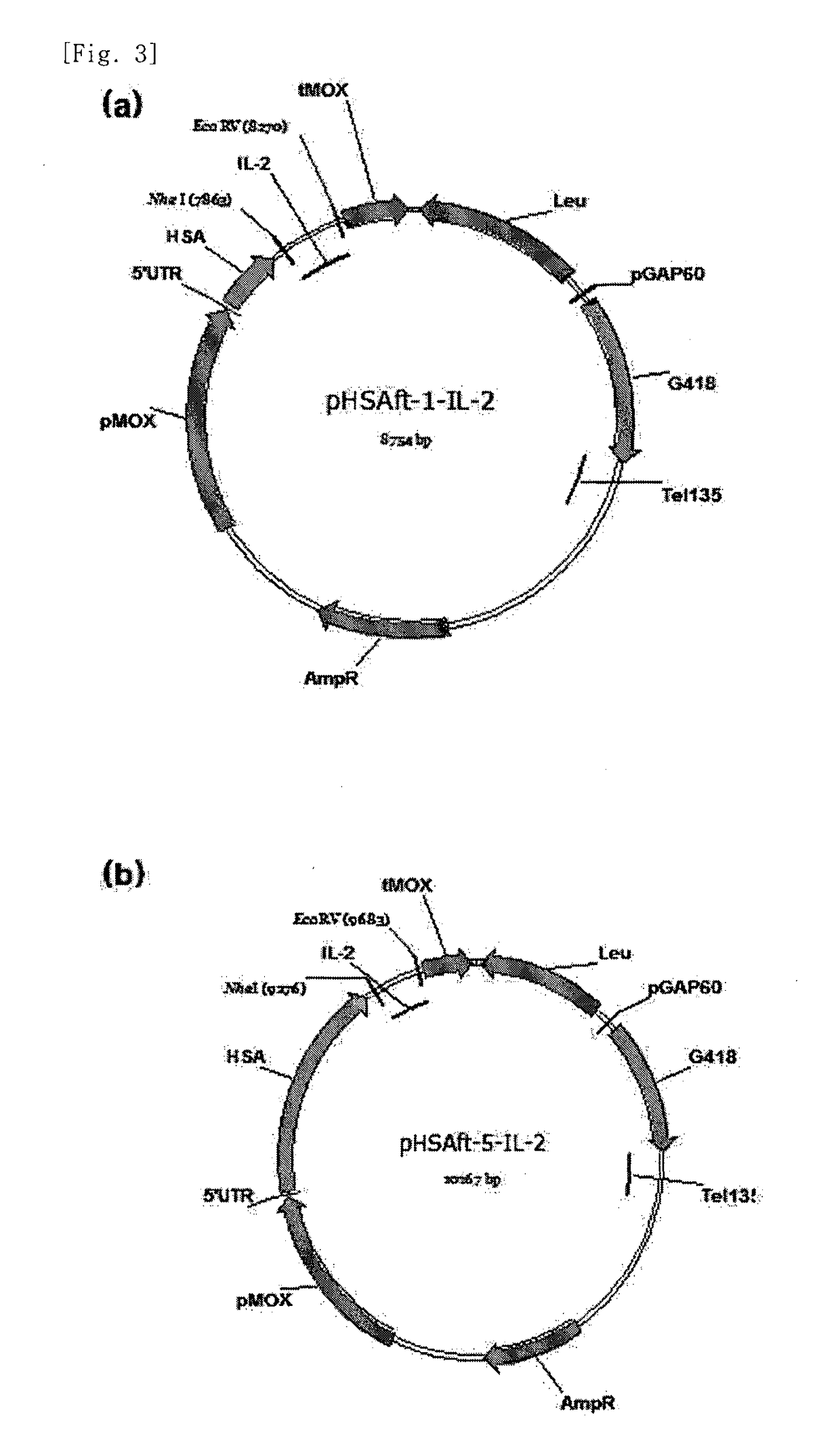
The present invention relates to an interleukin-2 expression construct for yeast, comprising a methanol oxidase (MOX) promoter; a human serum albumin gene or a fragment thereof; and an interleukin-2 (IL-2) gene, and to a yeast comprising the expression construct. The interleukin-2 expression construct for yeast according to the present invention makes it possible to produce an expressed and secreted fusion protein of human serum albumin (HSA) and interleukin-2 at low costs and easily separate recombinant interleukin-2 from the fusion protein. Thus, the interleukin-2 expression construct for yeast may be effectively used to produce a large amount of recombinant interleukin-2 with high purity.
Owner:SOONCHUNYANG UNIV IND ACAD COOP FOUND
Recombinant human interleukin 15 long peptide fragment and production method thereof
ActiveCN104212808ALow toxicityAvoid degradationPeptide preparation methodsFermentationPichia pastorisRecombinant Human Interleukin-15
The invention discloses a recombinant human interleukin (IL) 15 long peptide fragment and a production method thereof. The production method comprises the following steps: obtaining a human IL-15 long peptide fragment gene; establishing an eukaryotic expression vector and transforming the vector into Pichia pastoris X-33; performing resistance screening on high-level secretory-expressed yeast transformants; performing induced expression, purifying the supernate through a nickel column and an ion exchange column to obtain rhIL-15L. The production method is characterized in that the amino acid residue Ala of the site Kex2P1' of the vector pPICZAlphaA is mutated into Pro and the Pichia pastoris X-33 is taken as host bacteria, and therefore, mass high-efficiency stable expression of the rhIL-15L is realized; the recombinant protein rhIL-15L produced by the method is marked with HIS and C-MYC tags and prone to protein purification and detection; besides, the recombinant protein rhIL-15L is a glycoprotein which is modified through glycosylation to a certain extent, and therefore, the recombinant protein rhIL-15L has excellent bioactivity and is capable of effectively maintaining the proliferation and the bioactivity of human NK (Natural killer) cells in vitro and of a mouse in vivo.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
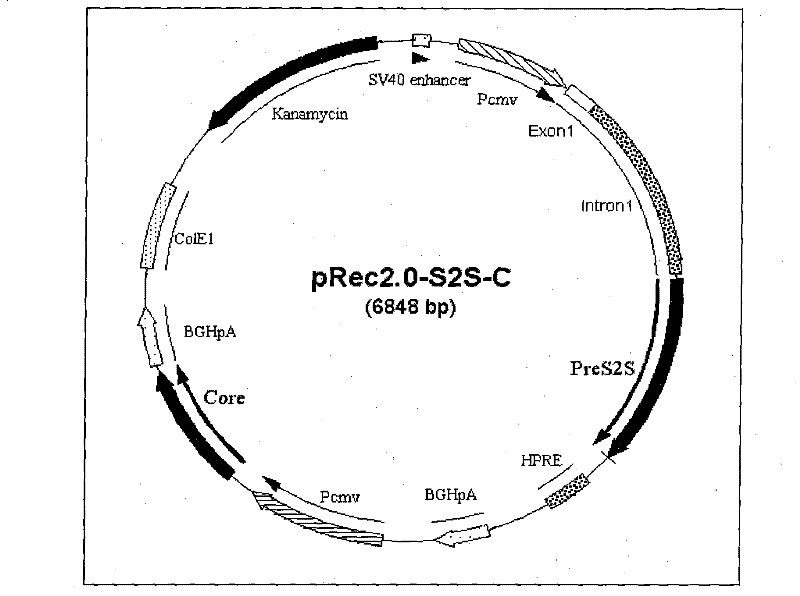
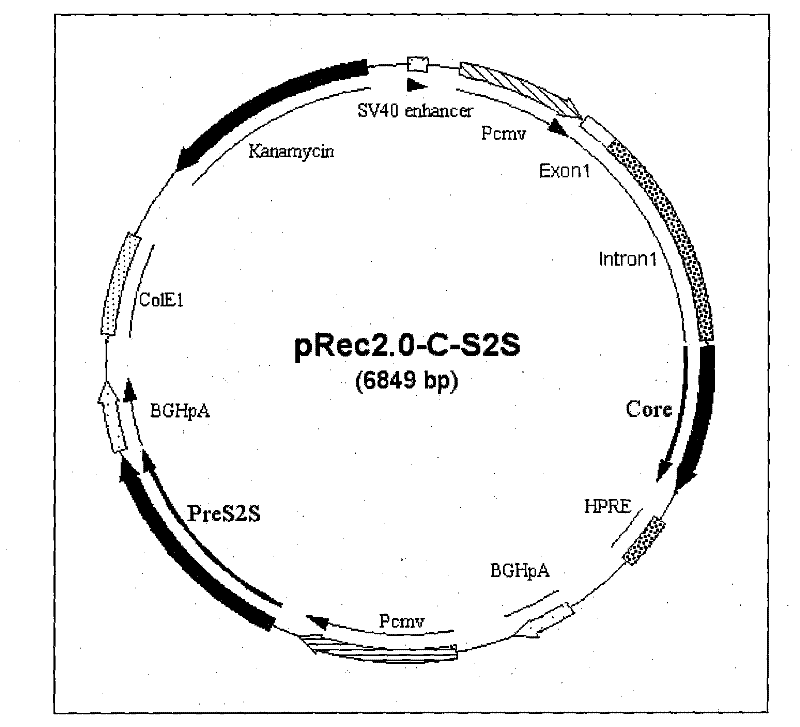
Recombinant plasmid DNA vaccine composition for treating Hepatitis B
The invention relates to a recombinant plasmid DNA vaccine composition for treating Hepatitis B. The composition contains a recombinant vaccine plasmid which simultaneously carries Hepatitis B middle protein S2S coding gene and Hepatitis B Core protein coding gene as well as a recombinant adjuvant plasmid, wherein the adjuvant plasmid is the adjuvant plasmid of recombinant interleukin 2 or recombinant human interferon gamma.
Owner:BEIJING KAWIN TECH SHARE HLDG
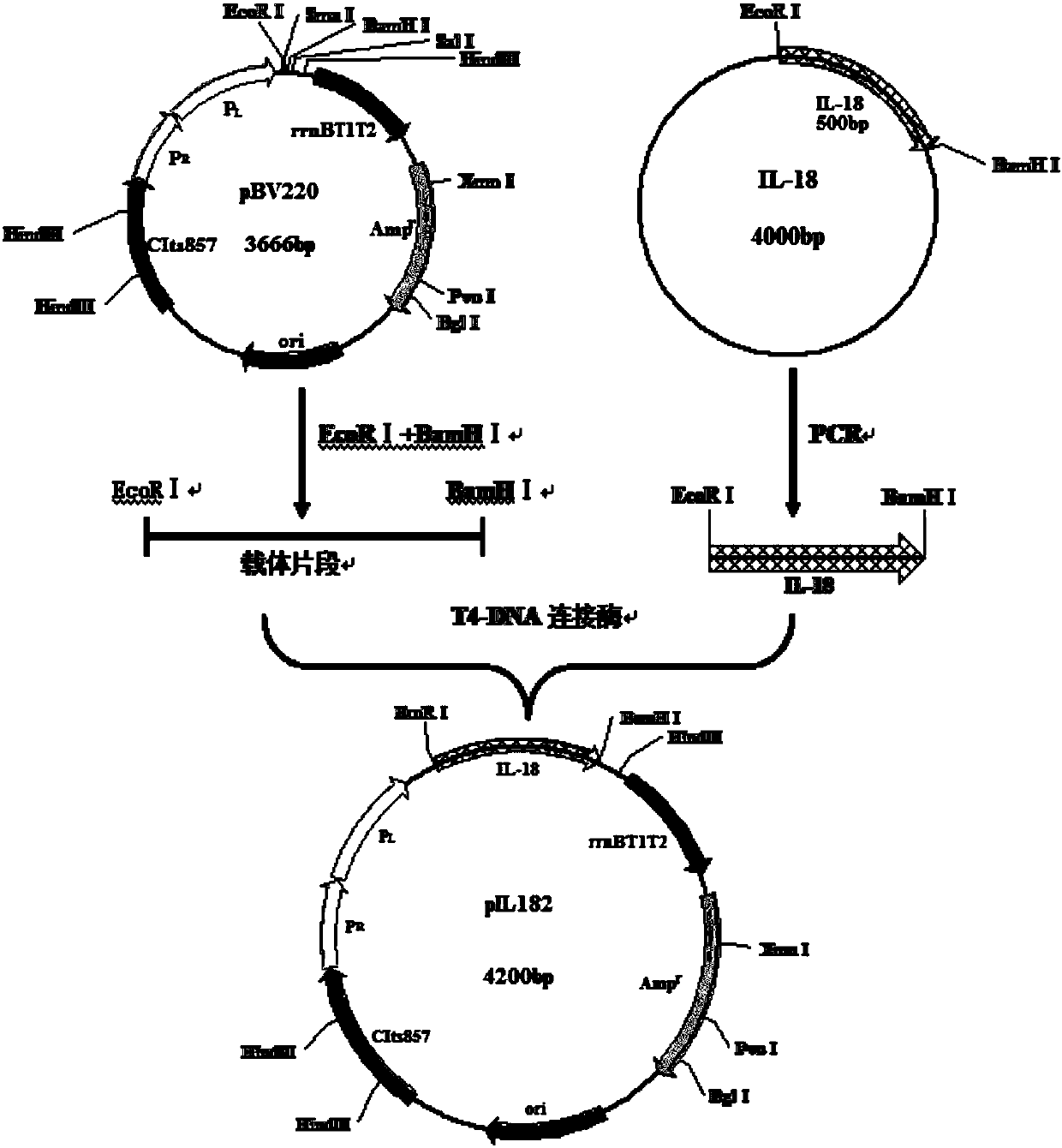
Recombinant interleukin 18 as well as preparation method and application thereof
The invention relates to the field of biotechnology and particularly relates to a recombinant interleukin 18 as well as a preparation method and application thereof. The rhIL-18 provided in the invention is mutated from a serine to an amino acid residue capable of being hydroxylated at a 10 site of N-terminus; hrIL-18 shown in SEQ ID NO: 1 is used for experiments and solves problems of low solubility, poor stability, low bioactivity and the like of wild IL-18; the hrIL-18 shown in SEQ ID NO: 1 is easier to purify and is suitable for industrial production. The rhIL-18 provided in the invention can inhibit generation of microtubules in human endothelial cells, can promote activity of NK cells and promote formation of IFN-gamma, but does not affect the activity of human retinal pigment epithelial cells, so as to indicate that the rhIL-18 can be used as a medicine for treating neovascularization diseases and / or malignant tumors.
Owner:HE EYE HOSPITAL SHENYANG
Method for improving yield of recombinant human interleukin-2 (rhIL-2)
InactiveCN109721652ABiologically activeHigh yieldDepsipeptidesPeptide preparation methods2-MercaptoethanolDrug biological activity
The invention discloses a method for improving the yield of recombinant human interleukin-2 (rhIL-2). The method comprises the steps of purification, eluent recovery after the purification, reductionof rhIL-2 dimer and wrong ligand by using 2-mercaptoethanol, reoxidation renaturation, and purification. The method performs reduction of rhIL-2 dimer and wrong ligand by using the 2-mercaptoethanol,opens disulfide bonds, and performs reoxidation renaturation and purification, so that correctly-paired disulfide bonds with biological activity can be formed so as to obtain the rhIL-2 with the biological activity, and the yield is improved by about 20 percent.
Owner:江苏金丝利药业股份有限公司
Secretory expression method for recombinant human IL-15
InactiveCN101134956AOptimize the coding sequenceBacteriaRecombinant DNA-technologyEscherichia coliRecombinant Human Interleukin-15
The present invention provides the coding sequence of recombinant human interleukin-15 (IL-15), the method of constituting engineering cell for high expressing recombinant human IL-15 protein, and the constitution of corresponding expression vector. Through optimizing human IL-15 gene and constituting to proper expression vector, the IL-15 is suitable for secreting expression in colibacillus. Through screening, high expressing recombinant human IL-15 engineering cell is obtained.
Owner:SHANGHAI NEWSUMMIT BIOPHARMA +1
Method for fermentative production of recombinant human interleukin-12
ActiveCN108251375AImprove survival rateImprove biological activityGenetically modified cellsCulture processBiotechnologyHamster
The invention provides a method for fermentative production of recombinant human interleukin-12, and belongs to the field of biological product production processes. The method comprises: 1) resuscitating and activating Chinese hamster ovary cells (CHO) containing a recombinant human interleukin-12 expression gene by using a serum-free culture medium, and carrying out enlarging culture to obtain seed cells; 2) after the seed cells normally grow, carrying out adaptive enlarging culture on the seed cells; 3) inoculating the obtained seed cells into a bioreactor, and culturing; 4) periodically adding a serum-free supplemented culture medium and glutamine during the culturing, and monitoring cell growth; and 5) terminating the culturing and harvesting the cells when the cell viability is lessthan 80%. With the method of the present invention, a large amount of the recombinant human interleukin-12 can be expressed, and the downstream purification is easily performed.
Owner:KANGLITAI BIOMEDICAL (QINGDAO) CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com