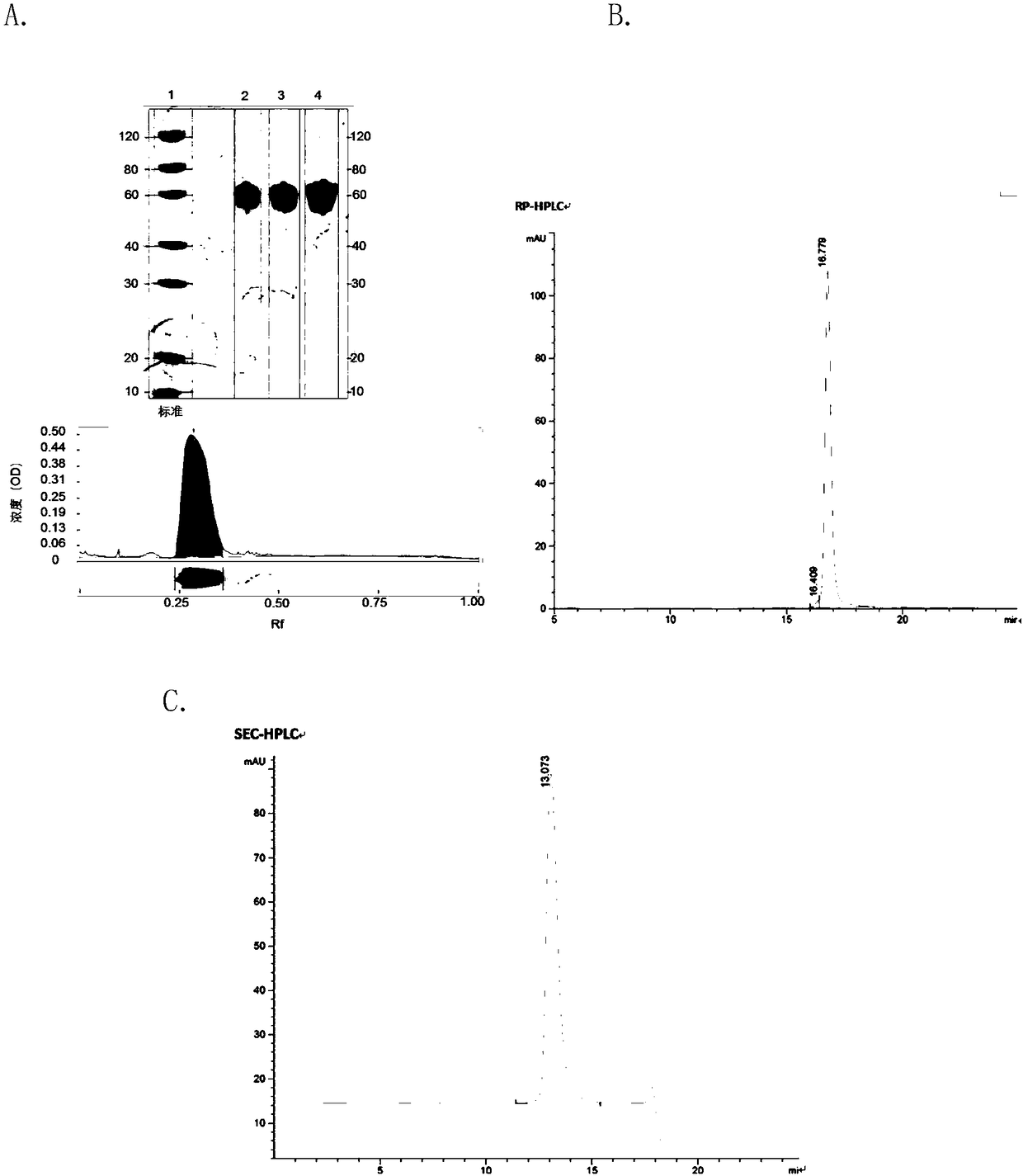
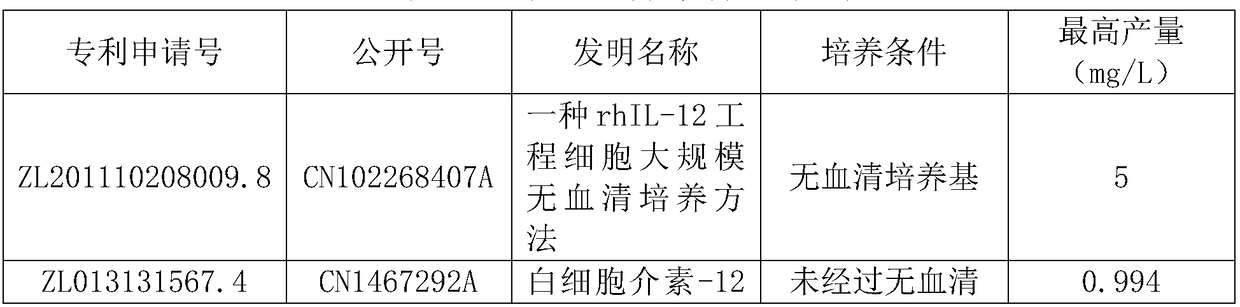
Method for fermentative production of recombinant human interleukin-12
A technology of human interleukin and seed cells, applied in biochemical equipment and methods, fermentation, microorganisms, etc., can solve the problem of low expression of recombinant human interleukin-12, and achieve the cost saving of culture medium, high survival rate and time saving. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、5
[0029] Embodiment 1, 5L scale cell culture process development
[0030] According to the previous experiments, CD OptiCHO medium was selected as the basic medium, CHO CD EfficientFeed B was used as the feed medium, and a 5L (tank volume, working volume 3L) Sartorius fermenter fed-batch fermentation culture was carried out to establish a fermenter The technological process of cultivation, and the influence of different rotation speeds of the stirring paddles in the 5L fermenter on the growth, production and metabolic parameters of cells were compared and analyzed, and the parameter values with better performance were selected.
[0031] The feed addition scheme is to add feed FeedB once every 2-3 days, and control the total amount of FeedB added to be 40% of the final volume of culture; according to the detection of Nova Bioprofile400 biochemical analyzer, add glutamine (Gln) and glucose (Gluc ), control the Gluc concentration above 2g / L, and the Gln content between 1.5-5mM....
Embodiment 2、7
[0062] Example 2, 7L pilot scale cell culture process optimization
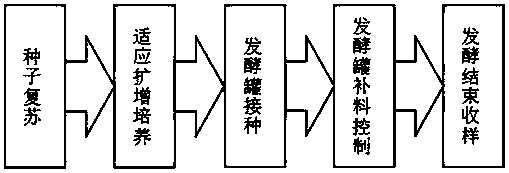
[0063] Based on the previous 5L Sartorius fermenter fed-batch fermentation process, the 7L (10L tank volume, 7L actual culture volume) scale process was explored according to the 5L fermenter process, and the 7L pilot scale fermentation process was determined. Production process such as figure 1 shown.
[0064] With a preheated CD OptiCHO TM AGT + 0.18% F68 + 8mM L-glutamine medium for recovery of rhIL-12 production cells, the density of recovered cultured cells is 4.0-5.0×10 5 individual / mL. The culture conditions are: T-125 shake flask, rotation speed 110-120rpm, temperature 37°C, 5% CO 2 incubator. Cells were subcultured every 2 days and adapted to 2-3 passages until the cell doubling time returned to normal. After the cells grow normally, the cell expansion is performed, and the inoculation is performed when the cells expand to the required volume. Inoculate the cell density at 5.0×10 5 Individu...
Embodiment 3
[0071] Example 3. 7L three batches of continuous production
[0072]The Sartorius fermenter (BIOSTAT B plus 10L) used in the three batches of 7L continuous production tests in this embodiment. Rinse the fermenter with tap water until there is no visible foreign matter, and then soak in 0.2M sodium hydroxide for 6-24 hours to soften or dissolve the attachment; scrub the soaked fermenter with tap water at least 3 times, and rinse until it is invisible to the naked eye. Remove any residue; rinse 3 times with pure water; finally wash 3 times with Mill-Q ultrapure water. After the tank body is assembled, prepare 1 / 3 tank volume of PBS solution and add it to the fermenter tank. After completing the electrode calibration, connect it to the tank body. Sterilize at 121°C for 35 minutes according to the tank body manual and sterilizer verification parameters.
[0073] To seed CHO cells, use pre-warmed CD OptiCHO TM Culture medium + 0.18% F68 + 8mM L-glutamine medium for resuscitat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com