Recombinant interleukin-15 analogue
An analogue, il-15-xa-yb-zc technology, applied in the field of molecular biology, can solve problems such as short half-life, difficult purification, and difficult industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1 Expression of wild-type IL-15 and IL-15 analogues in Escherichia coli
[0077] 1.1 Construction of expression vector
[0078] The wild-type IL-15 nucleotide sequence was synthesized by Shanghai Sangong Company.
[0079] (1) Primer design
[0080] The C-terminal sequence of IL-15 was changed by introducing the base sequence of different amino acids to be added in the reverse primer. The amino acid sequence, nucleotide sequence and primer sequences used during construction of wild-type IL-15 and the constructed IL-15 analogs are shown in Table 2:
[0081] The amino acid sequence of table 2 wild-type IL-15, IL-15 analogue, nucleotide sequence and the primer sequence used when constructing
[0082]
[0083]
[0084]
[0085]
[0086]
[0087]
[0088]
[0089] Analogs 34-43 are formed by introducing mutations into wild-type IL-15 to form IL-15 mutants, and then adding the sequence GSLPETGGSGGSHHHHHH to the C-terminus of the IL-15 mutants. A...
Embodiment 2
[0155] Example 2 In vitro cell activity experiment of IL-15 analogues
[0156] 2.1 Preparation of IL-15 analogs
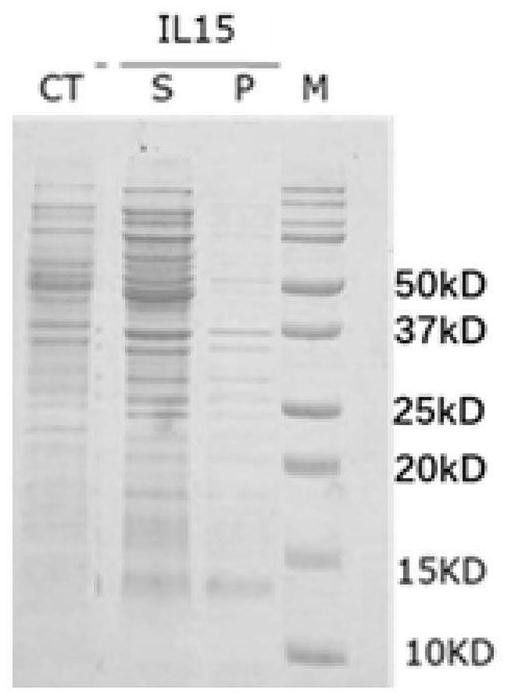
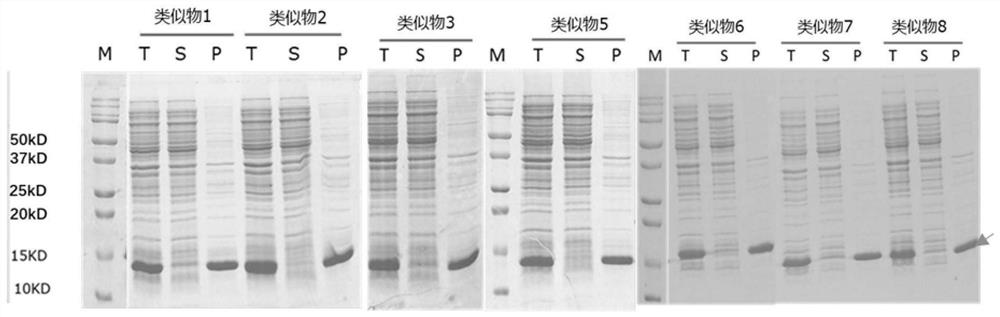
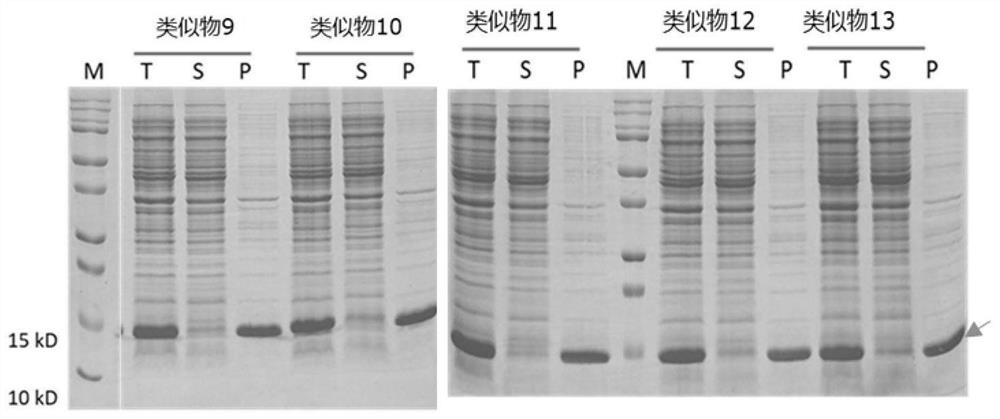
[0157] The IL-15 analog 11, analog 18, analog 21 and analog 28 expressed by inclusion bodies were dissolved in 8M urea solution, and purified by ion exchange and reversed-phase chromatography (see: Yunier et al, Preparative Biochemistry and Biotechnology, 47:9,889-900), purified to obtain a relatively pure protein, SDS-PAGE electrophoresis as shown in Figure 4 shown.
[0158] 2.2 CTLL-2 cell experiment
[0159] The proliferation test of CTLL-2 cells is a commonly used test to measure the activity of interleukin-stimulated immune cells at the cell level. Therefore, the biological activity of IL-15 analogues was examined here by wild-type IL-15 and the proliferative effect of IL-15 analogues on CTLL-2 cells.
[0160] 1) CTLL-2 cell preparation: the cells were resuspended in culture medium containing FBS and Rat-T-Stim.
[0161] 2) Adding samples: inoculate the...
Embodiment 3I
[0167] Preparation of conjugates of embodiment 3 IL-15 analogs (enzymatic reaction method)
[0168] Utilizing the purified IL-15 analog 21 (IL-15-GS-LPETG-GSGGSHHHHHH), the fatty acid chain (816366) with GGG at the N-terminus (816366) and the IL-15-GS -LPETG-GSGGSHHHHHH is connected. The reaction was carried out according to the Sortase A:IL-15:fatty acid chain ratio of 1:6:30, and the reaction buffer was 50mM Tris-HCl, 1mM CaCl 2 , 150 mM NaCl pH 8.0 at room temperature for 3 hours before purification. Purification uses reverse phase chromatography C8 (Saifen Technology) to separate the uncoupled IL-15 analog 21, unreacted fatty acid chain and the coupled product, and the final product is tested for purity by UPLC (such as Figure 5 ) and LC-MS ( Figure 6 ) identification showed that IL-15 analogue 21 has been coupled to fatty acid chains to form IL-15 analogue 21-816366.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com